Pax2 regulates neuronal glial cell fate choice in the embryonic optic nerve
|
|
|
- Kelley Arnold
- 5 years ago
- Views:
Transcription
1 Developmental Biology 303 (2007) Genomes & Developmental Control Pax2 regulates neuronal glial cell fate choice in the embryonic optic nerve Chadi Soukkarieh, Eric Agius, Cathy Soula, Philippe Cochard Centre de Biologie du Développement, CNRS UMR 5547, Institut d'exploration Fonctionnelle des Génomes, Université Paul Sabatier, 118 Route de Narbonne, Toulouse Cedex, France Received for publication 25 July 2006; revised 6 November 2006; accepted 7 November 2006 Available online 11 November 2006 Abstract During development, neural cell fate in the vertebrate optic nerve is restricted to the astroglial lineage. However, when isolated from the embryo and explanted in vitro, optic nerve progenitors generate neurons instead of astrocytes, suggesting that neuronal potentialities exist and are repressed in progenitors in vivo. Here we have investigated the mechanisms controlling cell fate in the optic nerve. The optic nerve is characterized by expression of the homeodomain transcription factor Pax2 which is maintained in differentiated astrocytes. We have observed that Pax2 is rapidly down-regulated in explanted optic nerves that generate neurons, and that its overexpression by electroporation in the optic nerve, or ectopically in the neural tube, is sufficient to block neuronal differentiation and allow glial development, showing that Pax2 plays a major role in controlling cell fate in the optic nerve. In vitro and ex vivo experiments further show that a signaling cascade that involves successively Sonic hedgehog and FGF is required to maintain Pax2 expression in optic nerve precursors whereby inhibiting the neuronal fate and promoting astroglial differentiation Elsevier Inc. All rights reserved. Keywords: Optic nerve; Cell fate determination; Sonic hedgehog; FGF; Pax2; Neural tube; Glia Introduction The large variety of neuronal and macroglial cell types that populate the vertebrate central nervous system (CNS) develop from initially multipotent stem cells and/or progenitor cells of the neuroepithelium (Temple, 2001). In most CNS areas, the neural cell types locally generated are quite diverse and comprise several classes of neurons and glial cells (for reviews, see (Bertrand et al., 2002; Pearson and Doe, 2004). In a few CNS areas, however, neural cell production is restricted to a single cell type. Such is the case for a major component of the forebrain, the optic nerve, a purely glial territory. In the adult, the optic nerve entirely lacks neuronal cell bodies and comprises astrocytes and oligodendrocytes that wrap retinal ganglion cell axons on their way to the tectum. During development, however, oligodendrocytes are not generated locally but arise from extrinsic sources and migrate into the nerve in a proximodistal direction (Small et al., 1987; Ono et al., 1997). Thus, in contrast to the neighboring retina, where neural progenitors Corresponding author. Fax: address: cochard@cict.fr (P. Cochard). generate a diverse progeny, including six types of neurons and one type of glia, optic nerve progenitors only produce astrocytes. This has lead to the assumption that cell fate in this region could be, from the onset, restricted to this particular glial cell type (Small et al., 1987). However, it has been shown that optic nerve precursors are not initially restricted to the astroglial fate. In particular, when isolated from influences of the surrounding tissues, optic nerve cells have the ability to generate neurons (Omlin and Waldmeyer, 1989; Giess et al., 1990), indicating that their developmental repertoire is broader than their normal fate in vivo and that neuronal potentialities must be repressed in this territory to allow only astrocytes to develop. The optic nerve arises from the optic stalk, a constriction of the neuroepithelium connecting the optic vesicle to the diencephalon. This neuroepithelial domain is characterized from early stages by the specific expression of two homeodomain transcription factors, Pax2 and Vax1 (Nornes et al., 1990; Hallonet et al., 1998; Bertuzzi et al., 1999). Initial expression of both factors is under the control of the Shh signaling pathway (Chiang et al., 1996; Take-uchi et al., 2003). Overexpression of Shh at early stages of eye formation expands /$ - see front matter 2006 Elsevier Inc. All rights reserved. doi: /j.ydbio
2 C. Soukkarieh et al. / Developmental Biology 303 (2007) the Pax2 and Vax1 expression domains, and reduces that of Pax6 in the retina, resulting in severe malformations of the eye (Ekker et al., 1995; Macdonald et al., 1995; Zhang and Yang, 2001; Take-uchi et al., 2003). Mutually repressive influences between Pax2 and Pax6 have been well established, which progressively contribute to the definition of sharp boundaries between the retinal and optic nerve fields (Schwarz et al., 2000). FGF signaling is another essential component in optic system morphogenesis (de Iongh and McAvoy, 1993; Pittack et al., 1997). FGF8, expressed in the retina (Vogel-Hopker et al., 2000) and in the optic stalk (Crossley et al., 2001), has been shown to direct retinal neuron specification (Martinez-Morales et al., 2005). FGF signaling can also promote Vax gene expression in the optic system (Take-uchi et al., 2003). However, the role of FGF signaling in the development of the optic stalk has not been investigated. Pax2 and Vax1 play critical roles in eye morphogenesis, by defining the identity of the optic stalk field. In mutant mice for either factor, the retina optic nerve boundaries are lost, leading to the expansion the retinal field at the expense of the optic stalk territory. In addition, the optic fissure does not close properly, resulting in optic nerve coloboma (Favor et al., 1996; Torres et al., 1996; Otteson et al., 1998; Bertuzzi et al., 1999; Hallonet et al., 1999). In the Pax2 mutant, most optic stalk cells degenerate, while a few differentiate into pigment cells (Schwarz et al., 2000), whereas in the Vax1 mutant optic nerve glia seem to develop normally, but fail to associate with axons, leading to defects in retinal axon trajectories (Bertuzzi et al., 1999; Hallonet et al., 1999). Beyond initial morphogenesis, factors regulating cell fate in the optic system have been well studied in the retina. For example, Pax6 has been shown to control neuronal identity of retinal precursors, since in conditional Pax6 mutant mice, in which Pax6 is inactivated after initial retinal morphogenesis, only amacrine cells develop at the expense of the other neuronal types (Marquardt et al., 2001). In comparison, little is known about cell fate regulation in the optic nerve. Loss of Shh activity in the early chick and mouse optic system from its retinal source modifies cell fate in the optic disk and distal part of the optic nerve, leading either to neuronal development of optic stalk neural precursors (Zhang and Yang, 2001) or to cell death and pigment cell differentiation (Dakubo et al., 2003). Possible functions for Pax2 and Vax1 in cell specification in the optic nerve once morphogenesis is completed have not been characterized. In the present study, we have defined some of the factors which repress neuronal differentiation in the embryonic chick and mouse optic nerve and lead to glial fate restriction of optic nerve precursors. We have focused on Pax2, and show that this transcription factor is able to regulate neuronal/glial cell choice in the optic nerve. Pax2 expression is lost in explanted optic nerves that generate neurons. It is maintained by Shh activity which in parallel totally blocks neuronal differentiation. However, Shh effects are indirect and are mediated by FGF signaling, suggesting that ongoing FGF activity is required to inhibit neuronal differentiation and promote astroglial development. Pax2 overexpression in optic nerve explants is sufficient to block neuronal differentiation and allow glial development. Furthermore, ectopic expression of Pax2 in the trunk neural tube also strongly inhibits neuronal development and induces glial cells. We propose that a regulatory cascade involving successively Shh, FGF and Pax2 regulates neuronal glial cell fate choice in the optic nerve. Materials and methods Fertilized White Leghorn chick eggs obtained from commercial source were incubated at 38 C and staged according to the series of Hamburger and Hamilton (Hamburger and Hamilton, 1992). Pax6 Neu mutant mice (Small Eye allele) were a gift of Pr. Dr. P. Gruss. Dissections, cultures and treatments Optic nerves of embryos were dissected free of surrounding tissues at different stages (E4.5, E6 and E8) in sterile PBS. In all experiments, the whole optic nerve was excised, but without contamination by retinal or diencephalic tissues (see Results). At E4.5, optic nerves were grown as single explants. At later stages, they were subdivided into two to three explants. Cultures were grown on collagen gel-coated 12-mm plastic coverslips placed in 14-mm fourwell dishes (Nunc). The culture medium was Neurobasal supplemented with 1% of B27 supplement (Gibco). The recombinant N-terminal fragment of the human Shh protein (Biogen) was used at concentrations of 4, 12, 24, and 50 nm. The recombinant mouse FGF8b and recombinant human FGF4 (R&D systems) were used at 100 ng/ml. The recombinant human BMP protein (R&D Systems) was used at a final concentration of 20 ng/ml. Cyclopamine, a gift of Dr. Frederic Rosa (Ecole Normale Supérieure, Paris, France), was used at a concentration of 4 μm. SU5402 (Calbiochem) was used at a concentration of 50 μm. Some experiments aiming to block the Shh signaling pathway were performed exvivo. The head was dissected at E5, opened along the dorsal midline, leaving the mesenchyme surrounding the optic nerve. These explants were then grown for 12 h with or without cyclopamine. Staining procedures For immunohistochemical analyses, heads of embryos were fixed in 3.7% formaldehyde (Sigma) in PBS overnight at 4 C. Tissues were then sectioned at 80 μm on a vibratome (Leica) before being processed. Optic nerve explants were fixed in the same fixative for 30 min at room temperature. O4 immunohistochemistry was performed as previously described (Giess et al., 1992). For the detection of intracellular antigens, fixed cultures and sections were first permeabilized using Triton-X-100 (0.5% in PBS). Then they were blocked using BSA (1% in PBS) and primary antibodies were applied at the appropriate dilution in 0.1% Triton-X-100/BSA 1% in PBS and incubated overnight at 4 C. After rinsing, they were incubated for 30 min with biotinylated secondary antibody directed against either mouse or rabbit IG (Amersham, 1:50), followed either by FITC or TRITC conjugates coupled to streptavidin (Amersham, 1:50). In some cases, they were revealed for 1 h using goat anti-mouse or goat antirabbit antibodies coupled to Alexa 488, Alexa 546, or Alexa 647 (Molecular Probes). Apoptosis was detected by TUNEL staining using Cell Death Detection Kit (Roche) as recommended by the manufacturer. Sections and optic nerve explants were analyzed with either a Zeiss LSM-410 or a Leica SP2 confocal microscope. Apoptosis was quantified by measuring mean pixel intensity in optic nerve explants using image-j software. Antibodies Neurons were identified either with Tuj1 monoclonal antibody (Babco), which recognizes neuron-specific β3-tubulin, used at 1/2000 or with NeuN antibody (Chemicon), used at 1/500 or with anti HuC/D (Molecular Probes) used at 1/500. Astroglial cells were evidenced by a rabbit antiserum directed against the glutamate aspartate transporter (GLAST), a gift from Dr. Masahiko Watanabe used at 1/500. The anti-pax2 antiserum (Babco) was used at 1/500 dilution. Mouse antibodies against Pax6, (used at 1:2) and HNK1 (used at 1:500)
3 802 C. Soukkarieh et al. / Developmental Biology 303 (2007) were obtained from the Developmental studies Hybridoma Bank. Mouse monoclonal O4 antibody (O4 mab) (used at 1:4) was obtained from culture supernatant of O4 hybridoma cells, a gift of Dr. R. Bansal. The AA3 antibody directed against the DM20/PLP protein (used at 1:10) was provided by Dr. B. Zalc. In situ hybridization Transcripts were analyzed using antisense digoxigenin RNA probe. The chick cdnas were Ngn2 (gift of Dr. D.J. Anderson), FGF8 (gift of Dr. S. Martinez), Sprouty1 (gift of Dr. F. Pituello), Shh and Patched (gift of Dr. C. Tabin), Slug (gift of Dr. M. Catala). The mouse cdnas were Patched (gift of Dr. M.P. Scott) and FGF8 (gift of Dr. S. Martinez). In situ hybridization was performed as described in Wilkinson (1992), with minor modifications. Hybridization of optic nerve explants cultures were carried out manually, whereas hybridization of vibratome sections was performed with an in situ hybridization robot (InsituPro, AbiMed). In ovo electroporation Complementary DNA for chick Pax2 was cloned into the two expression vectors pires and pcig. To generate Pax2-VP16 and Pax2-EnR fusion constructs, PCR-amplified cdna fragments that encode the transcriptional activation domain of the viral protein VP16 (80 amino acids) (Triezenberg et al., 1988), and the repressor domain of Drosophila Engrailed (330 amino acids) (Smith and Jaynes, 1996) respectively, were fused to the N-terminus of the homeodomain of chick Pax2. For neural tube electroporation, plasmidic DNA solution (3 μg/μl) was injected into the lumen of HH stage 10 to 14 and of HH stage 24 (E4.5) chick embryo neural tube. Electroporation was performed as described (Nakamura and Funahashi, 2001). The pcs2-vp16 and pcs2-enr plasmids were also electroporated in control embryos. Embryos were harvested and analyzed at HH stage 16 to 30 (E2.5 to E6.5). For electroporation into the optic nerve, heads of E4 chick embryos were dissected to reveal the optic nerves which were carefully separated from contaminating tissues. A 3 μg/μl DNA solution was then injected into the lumen of the optic nerve. A pair of electrodes was placed on either side of the optic nerve and 20 V of DC pulses were then applied for 50 ms, 6 8 times by an Intercel electroporator. Optic nerves were then separated from head tissues and cultivated on collagen coated dishes as described above. In both optic nerve and neural tube electroporations, control experiments with a GFP plasmid lacking the Pax2 construct were systematically performed but never showed any perturbation in the development of the neural structures or in cell differentiation. Results Pax2 expression is inversely correlated with neuronal genesis in the chick optic nerve The optic nerve is purely gliogenic in vivo, but generates neurons when isolated in vitro (Giess et al., 1990), providing a simple paradigm to study neuronal versus glial cell fate choice in this territory. To address the question of a possible function of Pax2 in optic nerve cell specification, we first examined its expression in intact and cultivated optic nerves. Between E4 and E6 in vivo, Pax2 was expressed throughout the optic nerve up to the optic nerve head (Fig. 1A), together with the glial marker GLAST (Shibata et al., 1997; Hartfuss et al., 2001) (Fig. 1D). In contrast, the retinal marker Pax6 (Fig. 1A), the proneural gene ngn2 expressed in the retina (Fig. 1B), and the pan-neuronal markers NeuN and HuC/D (Fig. 1C) were all undetectable in the entire nerve, indicating that neuroblasts or neurons do not pre-exist in the Pax2+ nerve territory in vivo. Explantation of E4.5 chick optic nerves in vitro elicited rapid neuronal development, as shown by strong ngn2 expression after 2 days in vitro (n=5/5; Fig. 1E) and extensive neuronal differentiation after 6 days, monitored through TuJ1 or HuC/D expression (n=12/12; Figs. 1F, G and 2O). Control RT-PCR experiments using two retinal specific markers, Pax6 and Chx10, showed that optic nerve explants were not contaminated by retinal tissue (not shown). In contrast to the optic nerve in vivo, astroglial differentiation, monitored using the GLAST marker, was much reduced in all cases (n=9/9), restricted to small areas of the explants (Fig. 1F). Interestingly, in these experimental conditions Pax2 expression was rapidly and strongly down-regulated. After 2 days in vitro, some cells had already lost Pax2 expression and double-staining experiments showed that they had started to express Pax6 (Fig. 1H). After 6 days in vitro, only 16% of cells expressed Pax2 (n=6/6; Figs. 1G and 2O) whereas Pax6 expression became prominent (Fig. 1I). In addition, Tuj1 labeling indicated that all Pax6+ cells were neurons (n=3/3; Fig. 1J). These results indicate that Pax2 expression in optic nerve cells is labile and is not maintained when the nerve is isolated from its normal tissular environment. We also analyzed Pax2 expression in optic nerves explanted at later developmental stages, which display reduced neuronal potentialities (Giess et al., 1990). Most cells of the explants (97%) expressed Pax2 (n=3/3; Fig. 1K), in correlation with widespread expression of the astroglial maker GLAST (n = 6/6; Fig. 1L), whereas, as expected, very few neurons (3%) differentiated in optic nerves explanted at E8 (Figs. 1K, L, compare to Figs. 1F, G). Together, these results indicate that optic nerve cells isolated from surrounding influences differentiate into neurons at the expense of glial cell formation and that Pax2 expression correlates positively with glial differentiation and negatively with neuronal differentiation in the chick optic nerve. Shh and FGF abolish neuronal differentiation in the optic nerve and maintain Pax2 expression We next analyzed signaling factors that could be involved in vivo in the inhibition of neuronal differentiation and maintenance of Pax2 expression and that could be lacking in explanted optic nerves. We first examined the effect of Shh, required for optic disc and stalk neuroepithelial cell differentiation and which modulates Pax2 expression (Dakubo et al., 2003). The entire nerve is under the influence of hedgehog signaling as shown by uniform expression of Patched, the Shh receptor and a direct response gene of this signaling system (Dakubo et al., 2003 and not shown). However, Shh is not expressed within the nerve itself. It is initially expressed in the basal diencephalon and later on in retinal ganglion cells as they differentiate (Jensen and Wallace, 1997). Consequently, Shh signaling is lost de facto in optic nerves isolated in culture. Strikingly, neuronal differentiation was strongly reduced in E4.5 nerve explants cultivated in the presence of 4 nm Shh and was practically abolished at 12 or 24 nm Shh (n=6/6; Figs. 2C, D, O), compared to control explants (Figs. 2A, B, O). In
4 C. Soukkarieh et al. / Developmental Biology 303 (2007) Fig. 1. Neuronal and glial differentiation and Pax2 expression in explanted embryonic chick optic nerves. (A D) Frontal sections of the E6 E7 chick optic system. (A) Double immunostaining for Pax2 (green) and Pax6 (red) shows that in vivo all cells of the optic nerve (on) express Pax2, whereas retinal ganglion cells (ret) express Pax6. (B) In situ hybridization shows that ngn2 is not expressed in the E6 optic nerve, although it is prominently expressed in the retina. (C) Immunostaining for HuC/D. Developing neurons are found in the retina, but not in the optic nerve. (D) Immunostaining for GLAST shows generalized expression of the glial marker at E7. (E J) Optic nerves were explanted from chicken embryos at E4.5 and cultivated for 2 days (E, H) or for 6 days (F, G, I, J). (E) ngn2 becomes rapidly expressed in optic nerve explants 2 days after explantation. (F) Nerve explants display extensive neuronal differentiation as shown by Tuj1 staining (red) of numerous cell bodies and profuse neurite outgrowth outside of the explant. GLAST staining (green), in contrast, is very limited. (G) Pax2 is down-regulated and restricted to a very small subset of cells (green), while the vast majority of optic nerve cells have differentiated into HuC/D+ neurons (red). (H) Two days after explantation, Pax2 expression (green) decreases and Pax6 expression (red) starts in optic nerve cells. Note that some cells co-express both transcription factors (arrowheads). (I) After 6 days, most of the explant contains numerous Pax6+ cells (red), while Pax2 (green) is only maintained in a small region. (J) In an E4.5 optic nerve explanted for 6 days, most Pax6+ cells express the neuronal marker Tuj1. Note, however, that some neurons do not express Pax6 (arrowheads). (K, L) Optic nerves were explanted from chicken embryos at E8 and cultivated for 6 days. Neuronal differentiation (Tuj1 staining, red) is less extensive and Pax2 (green) remains expressed in most optic nerve cells (K), while glial differentiation (L) monitored though GLAST expression (green) is more extensive, compared to the previous stage (see (F)). (Scale bars, 50 μm in J, 100 μm in all other micrographs). contrast, strong expression of GLAST was maintained throughout the explant (Fig. 2C), indicating generalized astrocyte differentiation. Interestingly, Shh treatment also maintained Pax2 expression in virtually all optic nerve cells (Figs. 2D, O, compare with Fig. 2B). We also examined the role of FGF8, a positive regulator of Pax2 expression in the isthmus (Shamim et al., 1999), shown to be expressed in the developing optic stalk (Crossley et al., 2001; Aoto et al., 2002), but which function in this territory has not been investigated. We found fgf8 mrna expression in the proximal part of the optic stalk as soon as at E2, which was maintained at least up to E7 in the nerve (Fig. 2E). FGF signaling was not restricted to the proximal optic nerve, since we found expression of the response gene sprouty1 throughout the nerve (supplementary Fig. S1). However, fgf8 and sprouty1 expressions were not maintained in explanted E4.5 optic nerves (n = 4/4; Fig. 2F and supplementary Fig. S1), indicating that factors extrinsic to the nerve are required to maintain this signaling activity. We thus examined the effect of FGF8 treatment on explanted optic nerves. As with Shh, FGF8, or the related FGF4 (not shown), abolished neuronal differentiation (n=6/6; Figs. 2G, H, O) and maintained strong GLAST (Fig. 2G) and Pax2 (Figs. 2H, O) expressions. Together, these results suggest that ongoing activities of both Shh and FGF signaling pathways may be required in vivo to prevent neuronal differentiation and maintain Pax2 expression.
5 804 C. Soukkarieh et al. / Developmental Biology 303 (2007) Fig. 2. Control of the differentiation of optic nerve explants by morphogens. Chick optic nerves were explanted at E4.5 and cultivated for 6 days (A D, G, H, K, L), 2 days (F, I, J) or for 3 days (M, N). (A D) Compared to control explants (A, B), Shh treatment inhibits neuronal differentiation monitored by Tuj1 staining and maintains strong GLAST (C) and Pax2 (D) expressions. (E, F) FGF8 is expressed in the proximal part of the E6 optic nerve (on, E). This expression is not maintained after 2 days in E4.5 explanted optic nerves (F). (G, H) FGF8 treatment also inhibits neuronal differentiation (Tuj1 staining) and maintains GLAST (G) and Pax2 (H) expressions. (I) Shh treatment maintains strong FGF8 expression in E4.5 nerves explanted for 2 days. (J) Conversely, FGF8 treatment is unable to promote Shh expression in similar explants. (K, L) Compared to explants treated with Shh alone (C, D) or with FGF8 alone (G, H), co-treatment with Shh and the FGF-signaling blocking agent Su 5402 restores neuronal differentiation and leads to reduced GLAST (K) and Pax2 (L) expressions, as in control conditions (A and B). This indicates that the effect of Shh is mediated through FGF signaling. (M, N) TUNEL staining shows limited cell death in control (M) and Shh-treated (N) explants. (O) Quantification of the relative proportions of neurons and Pax2+ cells developing in optic nerve explants in various conditions. Counts were performed on at least 3 different explants in each condition, double-stained with a neuronal marker (TuJ1 or HuC/D) and Pax2. Note that both Shh and FGF8 treatments practically abolish neuronal differentiation, while co-treatment with Shh and SU 5402 restores this proportion to the level found in control conditions (Scale bars, 100 μm in all micrographs).
6 C. Soukkarieh et al. / Developmental Biology 303 (2007) BMP4 induces apoptosis and pigmentation in optic nerve precursors Members of the TGFβ superfamily (BMPs and activin) are expressed in the dorsal retina (Trousse et al., 2001) and in the head mesenchyme (Stern et al., 1995). We thus tested the effect of BMP signaling, using BMP4 protein. BMP4 treatment (20 ng/ml) of E4.5 optic nerve explants for 6 days was clearly deleterious for the survival of optic nerve precursors, as all explants (5 to 7 explants in 4 different experiments) were hypocellular. Analysis of apoptosis using the TUNEL assay confirmed this observation and showed massive cell death in BMP4-treated explants compared to either untreated or Shh-treated explants, which displayed few apoptotic cells distributed around their border (supplementary Fig. S2A D). Strikingly, BMP4 also induced the formation of pigment cells in large areas of the explant (supplementary Fig. S2E, F). Very few neurons formed and some of the remaining non-pigmented cells expressed Pax2 and GLAST (supplementary Fig. S2G, H). Thus, in contrast to Shh and FGF8, BMP4 is not able to maintain the normal phenotype of the optic nerve in vivo. Epistatic relationships between Shh and FGF8 to maintain Pax2 expression and to block neuronal differentiation From the above results, we can infer that Shh and FGF8 could either work synergistically, or act upstream one of the other. To distinguish between these possibilities, we analyzed epistatic relationships between Shh and FGF expression in explants. Shh treatment (12 nm) maintained robust fgf8 (n =6/ 6) and sprouty1 (n = 3/3) expressions in E4.5 optic nerves explanted for 2 days (Fig. 2I and supplementary Fig. S1), whereas FGF8 was totally unable to induce Shh expression (n=3/3; Fig. 2J). The loss of fgf8 and sprouty1 expressions in control conditions and their maintenance by Shh was not related to cell death, since apoptosis, evidenced after 3 days in vitro using the TUNEL assay was restricted to a few cells in the explant and was similar in control and Shh-treated explants (Figs. 2M, N and supplementary Fig. S2). Thus, Shh effect on Pax2 expression could possibly be indirect and be mediated via positive regulation of FGF8. To test this possibility, E4.5 optic nerve explants exposed to Shh were treated with SU5402, a FGF-signaling blocking agent (Mohammadi et al., 1997). SU5402 treatment completely reversed the effect of Shh in its Fig. 3. A Shh FGF pathway maintains Pax2 expression in the optic nerve in vivo. Dissected E5 chick heads were maintained ex vivo for 12 h (A and B) or 2 days (C J). (A) In control conditions, fgf8 is expressed in the distal part of the optic nerves (dashed lines), whereas it is totally blocked after cyclopamine treatment (B). (C F) In control conditions, Pax2 is expressed normally throughout the nerve (C). Remark that in these ex vivo conditions, Pax2 (D) and Pax6 (E) are not coexpressed at the optic nerve/retina boundary, as shown in the overlay (F). (G J) Su5402 treatment leads to drastic down regulation of Pax2 expression (G). Furthermore, coexpression of Pax2 (H) and Pax6 (I) is observed in some cells (arrowheads in (H J)). (Scale bars, 200 μm in A and B, 50 μm in C and G, 10 μm in D F and H J).
7 806 C. Soukkarieh et al. / Developmental Biology 303 (2007) ability to maintain Pax2 (n=6/6; Figs. 2L, O) and GLAST (n = 7/7; Fig. 2K) expressions, leading to the typical extensive neuronal differentiation found in control explants (Figs. 2K, L, O). Conversely, treatment with cyclopamine, a potent inhibitor of Shh signaling pathway (Incardona et al., 1998), was without effect on the FGF-mediated maintenance of Pax2 expression (n=3; not shown). Together, these results show that Shh activity is a positive regulator of FGF8, which in turn maintains Pax2 expression in optic nerve cells. To confirm whether a Shh FGF pathway is responsible for the permanence of Pax2 expression in the normal optic nerve environment, we blocked either signaling pathway in vivo in partially dissected heads of E5 embryos which maintain normal expression of fgf8 and Pax2 (Figs. 3A, C). First, treatment for 12 h with cyclopamine to block Shh signaling totally inhibited fgf8 expression (n = 4/4; Fig. 3B), showing that endogenous Shh activity is required to maintain FGF expression in the optic nerve. Second, treatment of similar preparations for 2 days with SU5402 caused a strong reduction in Pax2 expression in optic nerve cells (n=4/4; Fig. 3G), showing that endogenous FGF signaling is required to maintain Pax2 expression. Interestingly, in SU5402-treated head explants, we observed Pax2 Pax6 double-labeled cells in the optic nerve (Figs. 3H J) but not in control head explants (Figs. 3D F), indicating replacement of Pax2 by Pax6, as observed in optic nerves isolated in vitro. Together, these data show that in the developing optic nerve, Shh activity is required to maintain FGF8 expression which in turn controls Pax2 expression. Pax2 inhibits neurogenesis in the optic nerve In all the above experiments, Pax2 expression was inversely correlated to the neurogenic potential of optic nerve cells, suggesting that Pax2 itself could be responsible for inhibiting the neuronal fate and promoting the glial fate in the optic nerve. To directly address this question, we experimentally maintained Pax2 expression in explanted nerves. A Pax2-GFP plasmid was electroporated in E4.5 optic nerves just prior to explantation and we analyzed the consequences on neuronal differentiation 4 days later. Electroporation of a control GFP plasmid did not modify neuronal differentiation (n=8;figs. 4A, B). In contrast, Pax2-GFP electroporation resulted in all cases (n = 6) in marked inhibition of neuronal differentiation in the regions of the explants successfully electroporated (Figs. 4C, D). Furthermore, none of the GFP+ electroporated cells expressed neuronal markers (Figs. 4E, F), while many neurons differentiated from non-electroporated cells. Most electroporated cells also expressed the astroglial marker GLAST (Figs. 4G, H). Together, this shows that forced expression of Pax2 in optic nerve precursors results in cell autonomous inhibition of the neuronal fate and induction of the glial fate. As shown above, in isolated optic nerves Pax2 expression is rapidly replaced by Pax6 in cells that differentiate along the neuronal lineage. The mutual transcriptional repression activities of Pax2 and Pax6 (Schwarz et al., 2000), and the role of Pax6 in neuronal specification in the retina (Marquardt et al., 2001), raised the possibility that Pax2 function in inhibiting Fig. 4. Pax2 overexpression inhibits neuronal differentiation and promotes GLAST expression in explanted optic nerves. Optic nerves were electroporated at E4.5 with the plasmids indicated in the captions, immediately explanted for 4 days and stained with Tuj1 (A D), HuC/D (E, F) or GLAST (G, H) antibodies. Neuronal differentiation (red) is not modified with a control GFP plasmid (green) (A, B), while it is strongly reduced with a Pax2-GFP plasmid (C, D). At higher magnification, Pax2-GFP electroporated cells (green) do not differentiate into neurons, while neighboring non-electroporated cells express the neuronal marker HuC/D (red) (E, F). (G, H) Composite panel of 4 different electroporated cells which show expression of the glial marker GLAST. (Scale bars, 100 μm in A D, 25 μm in E H).
8 C. Soukkarieh et al. / Developmental Biology 303 (2007) neuronal development could result from repression of a neurogenic function of Pax6. We addressed this possibility in a direct manner by using the sey Neu mouse mutants, which carry a null allele of Pax6, and in which the development of the optic system is abortive (Grindley et al., 1995). If Pax6 activity was required for neuron formation, neurons would not develop from the mutant optic nerve. At E12.5 E13.5, Patched and FGF8 expressions, which were readily detected in wild type mice (Figs. 5A, C) were maintained in the abortive optic cup and optic nerve of sey/sey mutants (Fig. 5B, D), suggesting that Shh and FGF signalings are preserved in the mutant. Indeed, we also found Pax2 expression in the remnant optic system of the mutant (Figs. 5E, F), confirming previous observations (Grindley et al., 1995). We then examined neuronal potentialities in the mutant optic system by explanting the optic system rudiment carefully microdissected at E12.5. Explants of wild type embryonic optic nerves were used as controls. In all cases (n=4), explants from either mutant (Fig. 5H) or wild type (Fig. 5G) animals displayed extensive neuronal differentiation, accompanied by a strong down regulation of Pax2 expression (Fig. 5H). Thus, Pax6 activity does not seem required for neuronal development in explanted optic nerves, suggesting that Pax2 does not block neuronal development through Pax6 inhibition. Pax2 may exert its transcriptional functions not only through repression but also through activation of target genes (Dehbi et al., 1996; Brophy et al., 2001, 2003). To further analyze its transcriptional activity, we constructed fusion proteins of the pax2 gene with either the transcriptional repressor domain of Drosophila engrailed (Pax2-EnR) or with the herpes simplex virus protein 16 transactivation domain (Pax2-VP16). Each of these forms was again electroporated in the optic nerve just prior to explantation. Many cells electroporated with the Pax2- EnR fusion adopted a neuronal phenotype (n=8/8; Figs. 5I, J) showing that the repressive form of Pax2 had no effect on neuronal differentiation. In contrast, the Pax2-VP16 fusion phenocopied the wild type protein, reducing the number of Fig. 5. Analysis of transcriptional activities of Pax2 in the mouse and chick embryo. (A F) Frontal sections of E13.5 wild type (A, C, E) and sey/sey mutant (B, D, F) mice. (A D) In situ hybridization shows that Patched and FGF8, normally expressed in wild type embryos (A, C), are also expressed in the mutant abortive optic system (B, D). (E, F) Pax2 expression in the wild-type optic nerve (E) is also maintained throughout the mutant optic system (F). (G, H) Optic nerve of wild type mice (G) and optic system of Pax6 mutant mice (Sey/Sey) (H), were explanted at E12.5 and cultivated for 6 days. (G) As in the chick embryo, explanted wild type optic nerves generate numerous Tuj1+ neurons (red) and limited GLAST+ glial cells (green). (H) Similarly, the explanted mutant optic system generates numerous Tuj1+ neurons (red). Note that Pax2 expression (green) is almost totally absent from the explant. (I L) Electroporation in the chick optic nerve of Pax2-engrailed (Pax2-EnR) and Pax2- VP16 fusion plasmids. (I, J) Pax2-EnR fusion plasmid does not modify neuronal differentiation. Note that Pax2 electroporated cells have differentiated into neurons, as shown by coexpression of GFP (green) and HuC/D (red), leading to yellow cytoplasm (arrowheads). (K, L) In marked contrast, Pax2-VP16 fusion plasmid lowers the number of HuC/D+ neurons in the electroporated area. Note that none of the Pax2-VP16-GFP+ cells (green) have differentiated into HuC/D+ neurons (red). (Scale bars, 100 μm ina H, 25 μm ini L).
9 808 C. Soukkarieh et al. / Developmental Biology 303 (2007) neurons. In particular, none of the electroporated cells differentiated as neurons (n = 7/7; Figs. 5K, L). Together, these results indicate that maintaining Pax2 activity is sufficient to inhibit neuronal differentiation in the optic nerve territory and that Pax2 exerts this effect as a transcriptional activator. Ectopic expression of Pax2 in the neural tube inhibits neurogenesis and promotes gliogenesis through transcriptional activation To assess whether Pax2 can control neuronal and glial cell fate choice outside the particular tissular context of the optic system, we determined its effect when ectopically expressed in the trunk neural tube. In this region, Pax2 is never expressed in neural precursors and thus is not involved in neuronal versus glial cell choice. It is restricted to post-mitotic interneurons (Burrill et al., 1997) and plays a role in their differentiation (Cheng et al., 2004). The Pax2-GFP plasmid was electroporated in the trunk neural tube of stage embryos, which were fixed and examined 24 h to 4 days later. We first observed that Pax2 electroporation efficiently blocked Pax6 expression (n = 4/4; Figs. 6A, B), indicating that in the spinal cord as in the optic system and in the forebrain (Matsunaga et al., 2000; Schwarz et al., 2000), Pax2 inhibits Fig. 6. Pax2 ectopic expression in the neural tube down-regulates Pax6 and inhibits neuronal differentiation. Pax2-GFP bicistronic plasmid was electroporated in the E1.5 neural tube and embryos analyzed 24 (A F) or 48 (G, H) h later. (A, B) and (E H) are image pairs of the same section showing on the left GFP+ electroporated cells (green) superimposed to the marker analyzed (red), and on the right expression of the marker alone. The electroporated side is on the right in all micrographs. Pax2 down-regulated the expression of Pax6 (A, B). (D) Pax2 reduced expression of ngn2 on the electroporated side, compared to the control side or to a non-electroporated embryo (C), suggesting inhibition of neuronal specification. (E, F) Maximum projection of horizontal confocal sections of an electroporated embryo. Note the dramatic decrease in neuronal cell bodies evidenced through Tuj1 staining. (G, H) Transverse E4 spinal cord section stained with HuC/D antibody. Note the absence of neuronal cell bodies at the level of electroporated cells. (I) Mean number of neurons, stained with Tuj1 antibody, counted 24 h after electroporation on transverse spinal cord sections from control embryos (n=4), electroporated with the GFP plasmid and from experimental embryos (n=4), electroporated with the Pax2-GFP plasmid. Only GFP+ cell bodies were taken into account. Pax2 electroporation caused a 70% decrease in neuron formation. Error bars represent S.E.M. (Scale bars, 50 μm).
10 C. Soukkarieh et al. / Developmental Biology 303 (2007) Pax6 expression. We then analyzed neuron formation throughout the neural tube, using ngn2 as a marker of neuronal precursors and a battery of pan-neuronal markers. Pax2 electroporation considerably down-regulated the expression of ngn2 (n = 2/2; Figs. 6C, D) and reduced the number of neurons recognized by Tuj1 (n=6/6; Figs. 6E, F), HuC/D (n=4/4; Figs. 6G, H) or Neu- N (not shown), irrespective of their position along the dorsoventral axis of the neural tube. Quantification taking into account cells efficiently electroporated, monitored either through GFP expression or Pax2 immunoreactivity, indicated a 70% reduction in the number of differentiated neurons (Fig. 6I). This inhibition of neuronal development was accompanied by the systematic induction of glial markers including O4 (n=6/6; Figs. 7A, B), a marker of oligodendrocytes and Schwann cells and of the myelin marker PLP/DM20 (n=3/3; Figs. 7C, D), but not of astroglial markers GFAP and GLAST (not shown). Further analysis with lineage markers such as HNK1 (n=5/5; Figs. 7E, F), Slug (not shown) and Sox9 (n=4/4; Figs. 7G, H) suggested that Pax2 electroporated cells had evolved along a Schwann-type lineage fate. In all experiments, control embryos electroporated with a GFP plasmid never displayed modification in neuronal differentiation and induction of glial markers. Together, these results indicate that in ectopic situation, Pax2 is also able to inhibit the neuronal fate and to induce the glial fate. The transactivating and repressive constructs of Pax2, Pax2- VP16 and Pax2-EnR were also misexpressed in the neural tube. The Pax2-EnR fusion phenocopied the intact protein in its inhibitory effect on Pax6 expression (Fig. 8A), but had no effect on either neuronal or glial differentiation (Figs. 8B, C). In contrast, the Pax2-VP16 fusion did not inhibit Pax6 expression (Fig. 8D), but phenocopied all the other effects of Pax2, including strong inhibition of pan-neuronal markers (Fig. 8E) and induction of glial markers (Fig. 8F). Electroporation of control pcs2-enr and pcs2-vp16 plasmids were without effect (not shown). Thus, as in the optic nerve, Pax2 protein inhibits neuronal differentiation and promotes glial development from neural progenitors through activation of transcription. Discussion Precursor cells that populate the optic nerve only give rise to astrocytes in vivo but display an intrinsic neuronal potential when isolated in vitro. Here, we have investigated mechanisms regulating the neuronal versus glial cell fate choice in the optic nerve. We show that the homeodomain transcription factor Pax2, which characterizes the optic nerve territory and later on optic nerve astrocytes, represses the neuronal fate and promotes the glial fate. We also show that Pax2 expression is labile in optic nerve cells and is maintained in this CNS area by a signaling cascade involving successively Shh and FGF activities. The intrinsic neuronal potential of optic nerve precursors has already been observed in chick (Giess et al., 1990, 1992) and rodents (Juurlink and Fedoroff, 1980; Omlin and Waldmeyer, 1989). In the chick embryo, this potential is extremely high in optic nerves isolated at early developmental stages (e.g. E4 E5), most cells differentiating into neurons, and very few into Fig. 7. Pax2 ectopic expression in the neural tube induces differentiation of Schwann-like cells. (A H) Electroporations of Pax2/GFP plasmid in the E1.5 neural tube. Transverse spinal cord sections were stained 24 h (A, B, E H) or 4 to 5 days (C, D) later. (A, B) Double-staining for Pax2 (blue) and O4 (red). Pax2 induces O4 expression in a subset of neuroepithelial cells. Note that only cells expressing Pax2 also express O4, suggesting a cell autonomous effect. (C, D) At E5 (C) and E6 (D), GFP+ cells express PLP/DM20. Note that some cells have migrated in the marginal zone (C), while others remained in the neuroepithelium and formed a small aggregate (D). (E, F) GFP+ cells express the HNK1 epitope, irrespective of their dorso-ventral level in the spinal cord. (G, H) A subset of the GFP+ cells express Sox9, suggesting weak or transient induction of the expression of this transcription factor. (Scale bars, 50 μm in A, B and E, F; 25 μm inc,g,h;10μm in D).
11 810 C. Soukkarieh et al. / Developmental Biology 303 (2007) Fig. 8. Pax2 inhibits the neuronal fate and promotes the glial fate through transcriptional activation. Pax2-engrailed fusion (Pax2-EnR) and Pax2-VP16 fusion were electroporated in the E1.5 neural tube and transverse spinal cord sections were stained 24 h later. (A C) In the neural tube, Pax2-EnR down-regulated expression of Pax6 (A) but was without effect on neuronal differentiation (B) and on glial induction (C). (D F) In contrast, Pax2-VP16 did not affect Pax6 expression (D), but inhibited neuronal differentiation (E) and promoted formation of O4+ glial cells (F). Arrowheads delineate the neural tube areas electroporated. (Scale bar, 50 μm in all micrographs). astrocytes. It decreases with the age of the nerve, with a progressive increase in astroglial differentiation, suggesting that neuronal differentiation in the explants occurs at the expense of glial differentiation. Thus, presumably precursors are at least bipotential and progressively loose their ability to differentiate into neurons as they become engaged along the astroglial pathway. Interestingly, neural stem cells which can give rise to neurons have been observed in the adult rat optic nerve (Palmer et al., 1999), suggesting that neuron-competent precursors persist throughout life in this CNS region. Optic nerves isolated in vitro and deprived of Shh signaling generate neurons whereas exogenous Shh suppresses neuronal differentiation. This indicates that ongoing Shh signaling is required until late in development for correct cell differentiation in the nerve. In support of this, injections of Shh neutralizing antibodies in the early chick optic system cause neuronal differentiation in the most distal part of the optic stalk (Zhang and Yang, 2001). A role for Shh in controlling cell fate in the optic disk and optic stalk has also been observed in the mouse. Conditional Shh gene ablation in mouse retinal ganglion cells lead to the lack of astrocyte development in the optic disk and distal part of the optic stalk, showing the important role of Shh transported along retinal axons in controlling the fate of these neural precursors (Dakubo et al., 2003). However, in these Shhdeprived nerves, neuronal development was not reported. Instead, the nerve was hypocellular and was surrounded by pigment cells. One possible reason for the discrepancy between the results of Dakubo et al. (2003) and our own findings may be that in vivo, optic stalk precursors are the target of other signaling systems, including factors of TGFβ superfamily (BMPs and activin) expressed in the dorsal retina (Trousse et al., 2001) and in the head mesenchyme (Stern et al., 1995). The present data show that chick optic nerve explants cultivated in the presence of BMP4 form very few neurons and are hypocellular and pigmented, a phenotype reminiscent of that found in the mutant mice. Therefore, our data suggest that the intrinsic fate of optic nerve precursors is to form neurons. In the presence of Shh alone, neuronal specification is abolished, whereas in the absence of Shh and in the presence of BMPs, precursors are diverted from the neuronal fate, some undergoing apoptosis and the other becoming pigment cells. An important observation from the present study is that the function of Shh signaling on cell fate appears indirect and is relayed by the FGF signaling pathway. At least three lines of evidence support this view. First, Shh is required to maintain FGF8 expression in the optic nerve; second, FGF signaling, mediated by FGF8 (and also by FGF4), mimics Shh activity and is sufficient to block neuronal differentiation in isolated nerves. Finally, Shh-mediated suppression of neuronal differentiation in explanted nerves is abolished by blocking FGF signaling. Altogether, these data are consistent with the idea that Shh is required to maintain FGF activity within the nerve, which in turn is primarily responsible for inhibiting the neuronal fate. In zebrafish embryos, FGF3 and FGF17 in addition to FGF8 have also been identified in the optic stalk (Reifers et al., 2000; Walshe and Mason, 2003). Whether FGF8 is the only member
12 C. Soukkarieh et al. / Developmental Biology 303 (2007) of the FGF family active in the optic nerve in higher vertebrates remains to be determined. There are few examples of a positive control of FGF expression by Shh. A positive feed-back loop between FGF and Shh signaling systems has been shown in the limb bud, but little is known regarding similar relationships in the nervous system (see Wilson and Maden, 2005, for references). In Shh mutant mice, FGF8 expression is reduced in the forebrain, but it was unclear whether this was due to inhibition of expression or to apoptosis of FGF-producing cells (Ohkubo et al., 2002). In our system, explanted optic nerves grown without Shh displayed down regulation of FGF8 expression but negligible apoptosis. This indicates that the loss of FGF8 expression was not due to cell death and that most probably Shh maintains FGF8 expression at the level of its transcription. The mechanisms by which Shh maintains FGF8 expression remain to be elucidated. Analysis of Gli3 / ; Shh / double mutant mice show maintained FGF8 expression in the early neural tube, leading to the suggestion that FGF8 expression could be regulated independently of Shh (Aoto et al., 2002). This discrepancy could be explained by the fact that FGF8 expression becomes dependent on Shh signaling in the optic nerve at later developmental stages, possibly involving a balance between activator (Gli1/2) and repressor (Gli3) forms of Gli activity. Alternatively, Indian hedgehog, expressed along the optic stalk (Wallace and Raff, 1999), could complement for Shh loss of function. We observed that FGF activity was required to maintain Pax2 expression in optic nerve cells. Relationships between Pax2 and FGF8 have been well exemplified in the isthmus. Pax2, one of the earliest genes expressed in this structure, is required and sufficient to induce FGF8 expression (Ye et al., 2001), which itself, subsequently, is required to maintain Pax2 expression. In addition, FGF8 overexpression in the anterior neural tube induces ectopic expression of Pax2 (Crossley et al., 1996; Shamim et al., 1999). In FGF8 conditional mutants, Pax2 expression is reduced, possibly due to cell death (Chi et al., 2003). In such mutants, however, the phenotype of the optic nerve has not been studied (Meyers et al., 1998). Interestingly, Gli3 / mutant mice show up-regulation of FGF8, accompanied by an extension of Pax2 expression in the Pax6 retinal domain (Aoto et al., 2002). Together, these data indicate that one of the main roles of FGF signaling in the optic nerve is to maintain Pax2 expression. In the present experiments, performed well after eye morphogenesis has been completed, Pax2 function alone appears sufficient to inhibit the neuronal fate and promote astrocyte development in the optic nerve. The murine optic system is also characterized by expression of other homeodomain transcription factors, Vax1 in the optic stalk and Vax2 in the ventral retina (Hallonet et al., 1998; Barbieri et al., 1999). Combined inactivation of Vax1 and Vax2 leads to the transformation of the optic nerve into retina and it has been suggested that both factors play an early role in eye morphogenesis by repressing synergistically Pax6 expression in the optic nerve, allowing development of the retinal field (Mui et al., 2005). However, Vax1 does not seem to play a role in the specification of optic nerve cells, since in the single Vax1 mutant, despite formation of a coloboma and major defects in optic tract formation, optic nerve glia seems to form normally and Pax2 expression is maintained (Bertuzzi et al., 1999; Hallonet et al., 1999). The Drosophila homolog of Pax2, sparkling (D-Pax2), also plays important roles in eye development, where it is required for the proper specification of non-neuronal cells in late larval and pupal eye discs (Fu and Noll, 1997) and in the specification of support cells of mechanosensory organs in the drosophila peripheral nervous system (Kavaler et al., 1999). These data suggest a conserved function of Pax2 between invertebrates and vertebrates in the specification of non-neuronal cells. This is also strongly supported by the consequences of Pax2 ectopic expression in the neural tube where it inhibited neuron formation and induced the glial fate, both effects being mediated through transcriptional activation. Strikingly, in the particular context of the trunk neural tube, ectopic expression of Pax2 did not promote the astrocyte fate, but instead induced the formation of Schwann-like cells, identified as such by the expression of neural crest lineage and myelin markers (HNK1, Slug, Sox9, O4 and PLP/DM20). The mechanism underlying the induction of this particular cell type remains to be determined. Nevertheless, it is possible that it involves initially induction of Sox9 expression, as this gene, when ectopically expressed in the chick neural tube, has been shown to inhibit neuronal differentiation and to promote Schwann cell formation (Cheung and Briscoe, 2003). Interestingly, Sox9 loss of function leads to enhanced neuronal differentiation at the expense of oligodendroglial and astroglial development (Stolt et al., 2003). Irrespective of the mechanism involved, it should be stressed that during normal in vivo development of the trunk neural tube, Pax2 function is not related to the generation of glial cells but to the differentiation of neuronal phenotypes (Cheng et al., 2004). The mechanism of action of Pax2 in the optic nerve remains to be established. Our experiments with Pax6 mutant mice show that neurons develop readily from optic rudiment explants in the absence of Pax6. Thus Pax2 function cannot be simply explained by its known repression of the Pax6 gene (Schwarz et al., 2000). Furthermore, our molecular dissection using Pax2 chimeric proteins shows that inhibition of the neuronal fate results from transcriptional activation of target genes. Thus, other factors activated downstream of Pax2 are probably involved in this repression, for example Hes or Id genes, known to block neuronal specification and promote the glial fate (Morrison et al., 2000; see also Bertrand et al., 2002). In conclusion, our present results indicate that Pax2 can act both as an inhibitor of neurogenesis and as an inducer of gliogenesis in the optic nerve. They support the notion that in the optic system Pax2 may be able to control a switch mechanism between neuronal and glial fates. Acknowledgments We thank N. Escalas and B. Ronsin for expert technical assistance. We greatly acknowledge our colleagues who
Neuroepithelial Cells and Neural Differentiation
 Neuroepithelial Cells and Neural Differentiation Neurulation The cells of the neural tube are NEUROEPITHELIAL CELLS Neural crest cells migrate out of neural tube Neuroepithelial cells are embryonic stem
Neuroepithelial Cells and Neural Differentiation Neurulation The cells of the neural tube are NEUROEPITHELIAL CELLS Neural crest cells migrate out of neural tube Neuroepithelial cells are embryonic stem
Cell Birth and Death. Chapter Three
 Cell Birth and Death Chapter Three Neurogenesis All neurons and glial cells begin in the neural tube Differentiated into neurons rather than ectoderm based on factors we have already discussed If these
Cell Birth and Death Chapter Three Neurogenesis All neurons and glial cells begin in the neural tube Differentiated into neurons rather than ectoderm based on factors we have already discussed If these
Tlx3 and Tlx1 are post-mitotic selector genes determining glutamatergic over GABAergic cell fates
 Tlx3 and Tlx1 are post-mitotic selector genes determining glutamatergic over GABAergic cell fates L. Cheng, A. Arata, R. Mizuguchi, Y. Qian, A. Karunaratne, P.A. Gray, S. Arata, S. Shirasawa, M. Bouchard,
Tlx3 and Tlx1 are post-mitotic selector genes determining glutamatergic over GABAergic cell fates L. Cheng, A. Arata, R. Mizuguchi, Y. Qian, A. Karunaratne, P.A. Gray, S. Arata, S. Shirasawa, M. Bouchard,
Supplementary Figure 1. Electroporation of a stable form of β-catenin causes masses protruding into the IV ventricle. HH12 chicken embryos were
 Supplementary Figure 1. Electroporation of a stable form of β-catenin causes masses protruding into the IV ventricle. HH12 chicken embryos were electroporated with β- Catenin S33Y in PiggyBac expression
Supplementary Figure 1. Electroporation of a stable form of β-catenin causes masses protruding into the IV ventricle. HH12 chicken embryos were electroporated with β- Catenin S33Y in PiggyBac expression
Supplementary Fig. 1 Blocking shh function at the protein level confirms its role as a guidance cue for postcommissural axons.
 Supplementary Fig. 1 Blocking shh function at the protein level confirms its role as a guidance cue for postcommissural axons. As an alternative method to demonstrate the role of shh as a guidance cue
Supplementary Fig. 1 Blocking shh function at the protein level confirms its role as a guidance cue for postcommissural axons. As an alternative method to demonstrate the role of shh as a guidance cue
Bone morphogenetic proteins negatively control oligodendrocyte precursor specification in the chick spinal cord
 Development 129, 5117-5130 (2002) Printed in Great Britain The Company of Biologists Limited 2002 DEV1796 5117 Bone morphogenetic proteins negatively control oligodendrocyte precursor specification in
Development 129, 5117-5130 (2002) Printed in Great Britain The Company of Biologists Limited 2002 DEV1796 5117 Bone morphogenetic proteins negatively control oligodendrocyte precursor specification in
Regionalization of the nervous system. Paul Garrity 7.68J/9.013J February 25, 2004
 Regionalization of the nervous system Paul Garrity 7.68J/9.013J February 25, 2004 Patterning along: Rostral/Caudal (AP) axis Dorsal/Ventral (DV) axis Start with DV axial patterning in Spinal Cord Dorsal/Ventral
Regionalization of the nervous system Paul Garrity 7.68J/9.013J February 25, 2004 Patterning along: Rostral/Caudal (AP) axis Dorsal/Ventral (DV) axis Start with DV axial patterning in Spinal Cord Dorsal/Ventral
Developmental Biology
 Developmental Biology 362 (2012) 141 153 Contents lists available at SciVerse ScienceDirect Developmental Biology journal homepage: www.elsevier.com/developmentalbiology Dual function of suppressor of
Developmental Biology 362 (2012) 141 153 Contents lists available at SciVerse ScienceDirect Developmental Biology journal homepage: www.elsevier.com/developmentalbiology Dual function of suppressor of
Chapter 5. Summary and Future directions
 95 Chapter 5 Summary and Future directions 96 Much of our knowledge about glial development in the vertebrate CNS comes from studies of purified oligodendrocyte precursor cells (Raff 1989; Pfeiffer et
95 Chapter 5 Summary and Future directions 96 Much of our knowledge about glial development in the vertebrate CNS comes from studies of purified oligodendrocyte precursor cells (Raff 1989; Pfeiffer et
Supporting Online Material for
 www.sciencemag.org/cgi/content/full/1171320/dc1 Supporting Online Material for A Frazzled/DCC-Dependent Transcriptional Switch Regulates Midline Axon Guidance Long Yang, David S. Garbe, Greg J. Bashaw*
www.sciencemag.org/cgi/content/full/1171320/dc1 Supporting Online Material for A Frazzled/DCC-Dependent Transcriptional Switch Regulates Midline Axon Guidance Long Yang, David S. Garbe, Greg J. Bashaw*
Neurodevelopment II Structure Formation. Reading: BCP Chapter 23
 Neurodevelopment II Structure Formation Reading: BCP Chapter 23 Phases of Development Ovum + Sperm = Zygote Cell division (multiplication) Neurogenesis Induction of the neural plate Neural proliferation
Neurodevelopment II Structure Formation Reading: BCP Chapter 23 Phases of Development Ovum + Sperm = Zygote Cell division (multiplication) Neurogenesis Induction of the neural plate Neural proliferation
Supporting Information
 Supporting Information Creuzet 10.1073/pnas.0906072106 Fig. S1. Migrating CNC cells express Bmp inhibitors. (A) HNK1 immunolabeling of migrating CNC cells at 7 ss. (B) At this stage, Fgf8 is strongly expressed
Supporting Information Creuzet 10.1073/pnas.0906072106 Fig. S1. Migrating CNC cells express Bmp inhibitors. (A) HNK1 immunolabeling of migrating CNC cells at 7 ss. (B) At this stage, Fgf8 is strongly expressed
Supplemental Information. Otic Mesenchyme Cells Regulate. Spiral Ganglion Axon Fasciculation. through a Pou3f4/EphA4 Signaling Pathway
 Neuron, Volume 73 Supplemental Information Otic Mesenchyme Cells Regulate Spiral Ganglion Axon Fasciculation through a Pou3f4/EphA4 Signaling Pathway Thomas M. Coate, Steven Raft, Xiumei Zhao, Aimee K.
Neuron, Volume 73 Supplemental Information Otic Mesenchyme Cells Regulate Spiral Ganglion Axon Fasciculation through a Pou3f4/EphA4 Signaling Pathway Thomas M. Coate, Steven Raft, Xiumei Zhao, Aimee K.
SUPPLEMENTARY FIG. S2. Representative counting fields used in quantification of the in vitro neural differentiation of pattern of dnscs.
 Supplementary Data SUPPLEMENTARY FIG. S1. Representative counting fields used in quantification of the in vitro neural differentiation of pattern of anpcs. A panel of lineage-specific markers were used
Supplementary Data SUPPLEMENTARY FIG. S1. Representative counting fields used in quantification of the in vitro neural differentiation of pattern of anpcs. A panel of lineage-specific markers were used
Genesis of cerebellar interneurons and the prevention of neural DNA damage require XRCC1.
 Genesis of cerebellar interneurons and the prevention of neural DNA damage require XRCC1. Youngsoo Lee, Sachin Katyal, Yang Li, Sherif F. El-Khamisy, Helen R. Russell, Keith W. Caldecott and Peter J. McKinnon.
Genesis of cerebellar interneurons and the prevention of neural DNA damage require XRCC1. Youngsoo Lee, Sachin Katyal, Yang Li, Sherif F. El-Khamisy, Helen R. Russell, Keith W. Caldecott and Peter J. McKinnon.
Supplementary Figure 1
 Supplementary Figure 1 Kif1a RNAi effect on basal progenitor differentiation Related to Figure 2. Representative confocal images of the VZ and SVZ of rat cortices transfected at E16 with scrambled or Kif1a
Supplementary Figure 1 Kif1a RNAi effect on basal progenitor differentiation Related to Figure 2. Representative confocal images of the VZ and SVZ of rat cortices transfected at E16 with scrambled or Kif1a
CNS Developmental. Anke van Eekelen, PhD. Telethon Institute for Child Health Research
 CNS Developmental Anke van Eekelen, PhD Telethon Institute for Child Health Research (Some slides are modified versions of Prof. Alan Harvey s Neuroscience lecture at ANHB and Dr. Joanne Britto s Dev Neuroscience
CNS Developmental Anke van Eekelen, PhD Telethon Institute for Child Health Research (Some slides are modified versions of Prof. Alan Harvey s Neuroscience lecture at ANHB and Dr. Joanne Britto s Dev Neuroscience
Neocortex Zbtb20 / NFIA / Sox9
 Neocortex / NFIA / Sox9 Supplementary Figure 1. Expression of, NFIA, and Sox9 in the mouse neocortex at. The lower panels are higher magnification views of the oxed area. Arrowheads indicate triple-positive
Neocortex / NFIA / Sox9 Supplementary Figure 1. Expression of, NFIA, and Sox9 in the mouse neocortex at. The lower panels are higher magnification views of the oxed area. Arrowheads indicate triple-positive
mir-7a regulation of Pax6 in neural stem cells controls the spatial origin of forebrain dopaminergic neurons
 Supplemental Material mir-7a regulation of Pax6 in neural stem cells controls the spatial origin of forebrain dopaminergic neurons Antoine de Chevigny, Nathalie Coré, Philipp Follert, Marion Gaudin, Pascal
Supplemental Material mir-7a regulation of Pax6 in neural stem cells controls the spatial origin of forebrain dopaminergic neurons Antoine de Chevigny, Nathalie Coré, Philipp Follert, Marion Gaudin, Pascal
Supplementary Table 1. List of primers used in this study
 Supplementary Table 1. List of primers used in this study Gene Forward primer Reverse primer Rat Met 5 -aggtcgcttcatgcaggt-3 5 -tccggagacacaggatgg-3 Rat Runx1 5 -cctccttgaaccactccact-3 5 -ctggatctgcctggcatc-3
Supplementary Table 1. List of primers used in this study Gene Forward primer Reverse primer Rat Met 5 -aggtcgcttcatgcaggt-3 5 -tccggagacacaggatgg-3 Rat Runx1 5 -cctccttgaaccactccact-3 5 -ctggatctgcctggcatc-3
Supplementary Figure S1. Monolayer differentiation of mouse ESCs into telencephalic neural precursors. (a) Schematic representation of the protocols
 Supplementary Figure S1. Monolayer differentiation of mouse ESCs into telencephalic neural precursors. (a) Schematic representation of the protocols used to differentiate mouse ESCs. (b) Representative
Supplementary Figure S1. Monolayer differentiation of mouse ESCs into telencephalic neural precursors. (a) Schematic representation of the protocols used to differentiate mouse ESCs. (b) Representative
Sox9 is critical for suppression of neurogenesis but not initiation of gliogenesis in the cerebellum. Vong et al.
 Sox9 is critical for suppression of neurogenesis but not initiation of gliogenesis in the cerebellum Vong et al. Vong et al. Molecular Brain (2015) 8:25 DOI 10.1186/s13041-015-0115-0 Vong et al. Molecular
Sox9 is critical for suppression of neurogenesis but not initiation of gliogenesis in the cerebellum Vong et al. Vong et al. Molecular Brain (2015) 8:25 DOI 10.1186/s13041-015-0115-0 Vong et al. Molecular
Shh signaling guides spatial pathfinding of raphespinal tract axons by multidirectional repulsion
 ORIGINAL ARTICLE Cell Research (2012) 22:697-716. 2012 IBCB, SIBS, CAS All rights reserved 1001-0602/12 $ 32.00 www.nature.com/cr npg Shh signaling guides spatial pathfinding of raphespinal tract axons
ORIGINAL ARTICLE Cell Research (2012) 22:697-716. 2012 IBCB, SIBS, CAS All rights reserved 1001-0602/12 $ 32.00 www.nature.com/cr npg Shh signaling guides spatial pathfinding of raphespinal tract axons
A Cxcl12-Cxcr4 Chemokine Signaling Pathway Defines
 Supplemental Data A Cxcl12-Cxcr4 Chemokine Signaling Pathway Defines the Initial Trajectory of Mammalian Motor Axons Ivo Lieberam, Dritan Agalliu, Takashi Nagasawa, Johan Ericson, and Thomas M. Jessell
Supplemental Data A Cxcl12-Cxcr4 Chemokine Signaling Pathway Defines the Initial Trajectory of Mammalian Motor Axons Ivo Lieberam, Dritan Agalliu, Takashi Nagasawa, Johan Ericson, and Thomas M. Jessell
Patterning the Embryo
 Patterning the Embryo Anteroposterior axis Regional Identity in the Vertebrate Neural Tube Fig. 2.2 1 Brain and Segmental Ganglia in Drosophila Fig. 2.1 Genes that create positional and segment identity
Patterning the Embryo Anteroposterior axis Regional Identity in the Vertebrate Neural Tube Fig. 2.2 1 Brain and Segmental Ganglia in Drosophila Fig. 2.1 Genes that create positional and segment identity
Retinal ganglion cell-derived sonic hedgehog signaling is required for optic disc and stalk neuroepithelial cell development
 Development 130, 2967-2980 2003 The Company of Biologists Ltd doi:10.1242/dev.00515 2967 Retinal ganglion cell-derived sonic hedgehog signaling is required for optic disc and stalk neuroepithelial cell
Development 130, 2967-2980 2003 The Company of Biologists Ltd doi:10.1242/dev.00515 2967 Retinal ganglion cell-derived sonic hedgehog signaling is required for optic disc and stalk neuroepithelial cell
glial cells missing and gcm2 Cell-autonomously Regulate Both Glial and Neuronal
 glial cells missing and gcm2 Cell-autonomously Regulate Both Glial and Neuronal Development in the Visual System of Drosophila Carole Chotard, Wendy Leung and Iris Salecker Supplemental Data Supplemental
glial cells missing and gcm2 Cell-autonomously Regulate Both Glial and Neuronal Development in the Visual System of Drosophila Carole Chotard, Wendy Leung and Iris Salecker Supplemental Data Supplemental
Nature Neuroscience: doi: /nn Supplementary Figure 1. MADM labeling of thalamic clones.
 Supplementary Figure 1 MADM labeling of thalamic clones. (a) Confocal images of an E12 Nestin-CreERT2;Ai9-tdTomato brain treated with TM at E10 and stained for BLBP (green), a radial glial progenitor-specific
Supplementary Figure 1 MADM labeling of thalamic clones. (a) Confocal images of an E12 Nestin-CreERT2;Ai9-tdTomato brain treated with TM at E10 and stained for BLBP (green), a radial glial progenitor-specific
Supplemental Information. Tissue Myeloid Progenitors Differentiate. into Pericytes through TGF-b Signaling. in Developing Skin Vasculature
 Cell Reports, Volume 18 Supplemental Information Tissue Myeloid Progenitors Differentiate into Pericytes through TGF-b Signaling in Developing Skin Vasculature Tomoko Yamazaki, Ani Nalbandian, Yutaka Uchida,
Cell Reports, Volume 18 Supplemental Information Tissue Myeloid Progenitors Differentiate into Pericytes through TGF-b Signaling in Developing Skin Vasculature Tomoko Yamazaki, Ani Nalbandian, Yutaka Uchida,
SUPPLEMENTARY FIGURES
 SUPPLEMENTARY FIGURES 1 Supplementary Figure 1, Adult hippocampal QNPs and TAPs uniformly express REST a-b) Confocal images of adult hippocampal mouse sections showing GFAP (green), Sox2 (red), and REST
SUPPLEMENTARY FIGURES 1 Supplementary Figure 1, Adult hippocampal QNPs and TAPs uniformly express REST a-b) Confocal images of adult hippocampal mouse sections showing GFAP (green), Sox2 (red), and REST
Vertebrate Limb Patterning
 Vertebrate Limb Patterning What makes limb patterning an interesting/useful developmental system How limbs develop Key events in limb development positioning and specification initiation of outgrowth establishment
Vertebrate Limb Patterning What makes limb patterning an interesting/useful developmental system How limbs develop Key events in limb development positioning and specification initiation of outgrowth establishment
Fig. S1. RT-PCR analyses of the expression and distribution of Xdscr6 transcripts during early development.
 Fig. S1. RT-PCR analyses of the expression and distribution of Xdscr6 transcripts during early development. (A) Temporal expression of Xdscr6 at various stages (numbers on the top) and its distribution
Fig. S1. RT-PCR analyses of the expression and distribution of Xdscr6 transcripts during early development. (A) Temporal expression of Xdscr6 at various stages (numbers on the top) and its distribution
Development of the Nervous System 1 st month
 Development of the Nervous System 1 st month day 1 - fertilization of egg day 6 - uterine implantation day 18 - trilaminar (3-layered) disc (blastoderm, embryo) ectoderm (dorsal) - nervous system and skin
Development of the Nervous System 1 st month day 1 - fertilization of egg day 6 - uterine implantation day 18 - trilaminar (3-layered) disc (blastoderm, embryo) ectoderm (dorsal) - nervous system and skin
Polarity and Segmentation. Chapter Two
 Polarity and Segmentation Chapter Two Polarization Entire body plan is polarized One end is different than the other Head vs. Tail Anterior vs. Posterior Front vs. Back Ventral vs. Dorsal Majority of neural
Polarity and Segmentation Chapter Two Polarization Entire body plan is polarized One end is different than the other Head vs. Tail Anterior vs. Posterior Front vs. Back Ventral vs. Dorsal Majority of neural
(A) PCR primers (arrows) designed to distinguish wild type (P1+P2), targeted (P1+P2) and excised (P1+P3)14-
 1 Supplemental Figure Legends Figure S1. Mammary tumors of ErbB2 KI mice with 14-3-3σ ablation have elevated ErbB2 transcript levels and cell proliferation (A) PCR primers (arrows) designed to distinguish
1 Supplemental Figure Legends Figure S1. Mammary tumors of ErbB2 KI mice with 14-3-3σ ablation have elevated ErbB2 transcript levels and cell proliferation (A) PCR primers (arrows) designed to distinguish
Nature Neuroscience: doi: /nn Supplementary Figure 1. Splenic atrophy and leucopenia caused by T3 SCI.
 Supplementary Figure 1 Splenic atrophy and leucopenia caused by T3 SCI. (a) Gross anatomy of representative spleens from control and T3 SCI mice at 28 days post-injury. (b and c) Hematoxylin and eosin
Supplementary Figure 1 Splenic atrophy and leucopenia caused by T3 SCI. (a) Gross anatomy of representative spleens from control and T3 SCI mice at 28 days post-injury. (b and c) Hematoxylin and eosin
Tetrapod Limb Development
 IBS 8102 Cell, Molecular and Developmental Biology Tetrapod Limb Development February 11, 2008 Tetrapod Limbs Merlin D. Tuttle Vicki Lockard and Paul Barry Father Alejandro Sanchez Anne Fischer Limb Patterning
IBS 8102 Cell, Molecular and Developmental Biology Tetrapod Limb Development February 11, 2008 Tetrapod Limbs Merlin D. Tuttle Vicki Lockard and Paul Barry Father Alejandro Sanchez Anne Fischer Limb Patterning
100 mm Sucrose. +Berberine +Quinine
 8 mm Sucrose Probability (%) 7 6 5 4 3 Wild-type Gr32a / 2 +Caffeine +Berberine +Quinine +Denatonium Supplementary Figure 1: Detection of sucrose and bitter compounds is not affected in Gr32a / flies.
8 mm Sucrose Probability (%) 7 6 5 4 3 Wild-type Gr32a / 2 +Caffeine +Berberine +Quinine +Denatonium Supplementary Figure 1: Detection of sucrose and bitter compounds is not affected in Gr32a / flies.
Problem Set 5 KEY
 2006 7.012 Problem Set 5 KEY ** Due before 5 PM on THURSDAY, November 9, 2006. ** Turn answers in to the box outside of 68-120. PLEASE WRITE YOUR ANSWERS ON THIS PRINTOUT. 1. You are studying the development
2006 7.012 Problem Set 5 KEY ** Due before 5 PM on THURSDAY, November 9, 2006. ** Turn answers in to the box outside of 68-120. PLEASE WRITE YOUR ANSWERS ON THIS PRINTOUT. 1. You are studying the development
Nature Neuroscience: doi: /nn.2275
 Supplementary Figure S1. The presence of MeCP2 in enriched primary glial cultures from rat or mouse brains is not neuronal. Western blot analysis of protein extracts from (a) rat glial and neuronal cultures.
Supplementary Figure S1. The presence of MeCP2 in enriched primary glial cultures from rat or mouse brains is not neuronal. Western blot analysis of protein extracts from (a) rat glial and neuronal cultures.
Supplemental Data. Wnt/β-Catenin Signaling in Mesenchymal Progenitors. Controls Osteoblast and Chondrocyte
 Supplemental Data Wnt/β-Catenin Signaling in Mesenchymal Progenitors Controls Osteoblast and Chondrocyte Differentiation during Vertebrate Skeletogenesis Timothy F. Day, Xizhi Guo, Lisa Garrett-Beal, and
Supplemental Data Wnt/β-Catenin Signaling in Mesenchymal Progenitors Controls Osteoblast and Chondrocyte Differentiation during Vertebrate Skeletogenesis Timothy F. Day, Xizhi Guo, Lisa Garrett-Beal, and
Early Development of Neural Tube Development of Medulla Spinalis and Peripheral Nervous System. Assoc.Prof. E.Elif Güzel, M.D.
 Early Development of Neural Tube Development of Medulla Spinalis and Peripheral Nervous System Assoc.Prof. E.Elif Güzel, M.D. Third week of Embryogenesis Primitive streak/pit appears on the epiblast (day
Early Development of Neural Tube Development of Medulla Spinalis and Peripheral Nervous System Assoc.Prof. E.Elif Güzel, M.D. Third week of Embryogenesis Primitive streak/pit appears on the epiblast (day
Transduction of graded Hedgehog signaling by a combination of Gli2 and Gli3 activator functions in the developing spinal cord
 Research article 3593 Transduction of graded Hedgehog signaling by a combination of Gli2 and Gli3 activator functions in the developing spinal cord Qiubo Lei, Alice K. Zelman, Ed Kuang, Shike Li and Michael
Research article 3593 Transduction of graded Hedgehog signaling by a combination of Gli2 and Gli3 activator functions in the developing spinal cord Qiubo Lei, Alice K. Zelman, Ed Kuang, Shike Li and Michael
EMBO REPORT SUPPLEMENTARY SECTION. Quantitation of mitotic cells after perturbation of Notch signalling.
 EMBO REPORT SUPPLEMENTARY SECTION Quantitation of mitotic cells after perturbation of Notch signalling. Notch activation suppresses the cell cycle indistinguishably both within and outside the neural plate
EMBO REPORT SUPPLEMENTARY SECTION Quantitation of mitotic cells after perturbation of Notch signalling. Notch activation suppresses the cell cycle indistinguishably both within and outside the neural plate
Supplementary Figure 1
 Supplementary Figure 1 The average sigmoid parametric curves of capillary dilation time courses and average time to 50% peak capillary diameter dilation computed from individual capillary responses averaged
Supplementary Figure 1 The average sigmoid parametric curves of capillary dilation time courses and average time to 50% peak capillary diameter dilation computed from individual capillary responses averaged
Chapter 11 Development and Diseases
 Chapter 11 Development and Diseases Fertilization Development of the Nervous System Cleavage (Blastula, Gastrula) Neuronal Induction- Neuroblast Formation Cell Migration Axon Growth/Target innervation
Chapter 11 Development and Diseases Fertilization Development of the Nervous System Cleavage (Blastula, Gastrula) Neuronal Induction- Neuroblast Formation Cell Migration Axon Growth/Target innervation
Tetrapod Limb Development
 Biology 4361 Developmental Biology Tetrapod Limb Development July 29, 2009 Tetrapod Limbs Merlin D. Tuttle Vicki Lockard and Paul Barry Father Alejandro Sanchez Anne Fischer Limb Development - Overview
Biology 4361 Developmental Biology Tetrapod Limb Development July 29, 2009 Tetrapod Limbs Merlin D. Tuttle Vicki Lockard and Paul Barry Father Alejandro Sanchez Anne Fischer Limb Development - Overview
Biology 4361 Developmental Biology Gilbert Ch. 12. The Emergence of the Ectoderm: Central Nervous System and Epidermis November 30, 2006
 Biology 4361 Developmental Biology Gilbert Ch. 12. The Emergence of the Ectoderm: Central Nervous System and Epidermis November 30, 2006 Establishing the Neural Cells - neural plate - portion of the dorsal
Biology 4361 Developmental Biology Gilbert Ch. 12. The Emergence of the Ectoderm: Central Nervous System and Epidermis November 30, 2006 Establishing the Neural Cells - neural plate - portion of the dorsal
Supplementary Information
 Supplementary Information Title Degeneration and impaired regeneration of gray matter oligodendrocytes in amyotrophic lateral sclerosis Authors Shin H. Kang, Ying Li, Masahiro Fukaya, Ileana Lorenzini,
Supplementary Information Title Degeneration and impaired regeneration of gray matter oligodendrocytes in amyotrophic lateral sclerosis Authors Shin H. Kang, Ying Li, Masahiro Fukaya, Ileana Lorenzini,
SUPPLEMENTARY INFORMATION
 DOI: 10.1038/ncb2988 Supplementary Figure 1 Kif7 L130P encodes a stable protein that does not localize to cilia tips. (a) Immunoblot with KIF7 antibody in cell lysates of wild-type, Kif7 L130P and Kif7
DOI: 10.1038/ncb2988 Supplementary Figure 1 Kif7 L130P encodes a stable protein that does not localize to cilia tips. (a) Immunoblot with KIF7 antibody in cell lysates of wild-type, Kif7 L130P and Kif7
Tetrapod Limb Development
 Biology 4361 Developmental Biology Tetrapod Limb Development July 29, 2009 Tetrapod Limbs Merlin D. Tuttle Vicki Lockard and Paul Barry Father Alejandro Sanchez Anne Fischer Limb Development - Overview
Biology 4361 Developmental Biology Tetrapod Limb Development July 29, 2009 Tetrapod Limbs Merlin D. Tuttle Vicki Lockard and Paul Barry Father Alejandro Sanchez Anne Fischer Limb Development - Overview
Supplementary Figure 1. EC-specific Deletion of Snail1 Does Not Affect EC Apoptosis. (a,b) Cryo-sections of WT (a) and Snail1 LOF (b) embryos at
 Supplementary Figure 1. EC-specific Deletion of Snail1 Does Not Affect EC Apoptosis. (a,b) Cryo-sections of WT (a) and Snail1 LOF (b) embryos at E10.5 were double-stained for TUNEL (red) and PECAM-1 (green).
Supplementary Figure 1. EC-specific Deletion of Snail1 Does Not Affect EC Apoptosis. (a,b) Cryo-sections of WT (a) and Snail1 LOF (b) embryos at E10.5 were double-stained for TUNEL (red) and PECAM-1 (green).
( neural progeni2 tor cell), ,,,, , (neural crest) (radial glial cell) mrna [2 ]
![( neural progeni2 tor cell), ,,,, , (neural crest) (radial glial cell) mrna [2 ] ( neural progeni2 tor cell), ,,,, , (neural crest) (radial glial cell) mrna [2 ]](/thumbs/92/110608454.jpg) 246, 1999 6, 51 (3), 246 252 Acta Physiologica Sinica 3 P19 3 3 (, 200031 ;, 200031) (nestin),, RA P19,, (neural precursor cell) BMP4, (NF160), ( neural progeni2 tor cell),, : ; P19 ; BMP4 ; : Q71,,,,
246, 1999 6, 51 (3), 246 252 Acta Physiologica Sinica 3 P19 3 3 (, 200031 ;, 200031) (nestin),, RA P19,, (neural precursor cell) BMP4, (NF160), ( neural progeni2 tor cell),, : ; P19 ; BMP4 ; : Q71,,,,
SUPPLEMENTARY INFORMATION
 doi:10.1038/nature10188 Supplementary Figure 1. Embryonic epicardial genes are down-regulated from midgestation stages and barely detectable post-natally. Real time qrt-pcr revealed a significant down-regulation
doi:10.1038/nature10188 Supplementary Figure 1. Embryonic epicardial genes are down-regulated from midgestation stages and barely detectable post-natally. Real time qrt-pcr revealed a significant down-regulation
Investigating the role of EphAl ephrin-a signalling during trigeminal ganglion axon guidance
 Investigating the role of EphAl ephrin-a signalling during trigeminal ganglion axon guidance A thesis submitted for the degree of Doctor of Philosophy Molecular and Biomedical Science (Discipline of Genetics),
Investigating the role of EphAl ephrin-a signalling during trigeminal ganglion axon guidance A thesis submitted for the degree of Doctor of Philosophy Molecular and Biomedical Science (Discipline of Genetics),
Primary Mouse Cerebral Cortex Neurons V: 80% TE: 70%
 Primary Mouse Cerebral Cortex Neurons V: 80% TE: 70% Pictures: 9 days after electroporation Red: MAP2 Blue: GFAP Green: GFP The cells were from Embryonic Day 14 Mouse Cerebral Cortex Primary Mouse Hippocampal
Primary Mouse Cerebral Cortex Neurons V: 80% TE: 70% Pictures: 9 days after electroporation Red: MAP2 Blue: GFAP Green: GFP The cells were from Embryonic Day 14 Mouse Cerebral Cortex Primary Mouse Hippocampal
SUPPLEMENTARY INFORMATION
 DOI: 10.1038/ncb2566 Figure S1 CDKL5 protein expression pattern and localization in mouse brain. (a) Multiple-tissue western blot from a postnatal day (P) 21 mouse probed with an antibody against CDKL5.
DOI: 10.1038/ncb2566 Figure S1 CDKL5 protein expression pattern and localization in mouse brain. (a) Multiple-tissue western blot from a postnatal day (P) 21 mouse probed with an antibody against CDKL5.
Zhu et al, page 1. Supplementary Figures
 Zhu et al, page 1 Supplementary Figures Supplementary Figure 1: Visual behavior and avoidance behavioral response in EPM trials. (a) Measures of visual behavior that performed the light avoidance behavior
Zhu et al, page 1 Supplementary Figures Supplementary Figure 1: Visual behavior and avoidance behavioral response in EPM trials. (a) Measures of visual behavior that performed the light avoidance behavior
Sestrin2 and BNIP3 (Bcl-2/adenovirus E1B 19kDa-interacting. protein3) regulate autophagy and mitophagy in renal tubular cells in. acute kidney injury
 Sestrin2 and BNIP3 (Bcl-2/adenovirus E1B 19kDa-interacting protein3) regulate autophagy and mitophagy in renal tubular cells in acute kidney injury by Masayuki Ishihara 1, Madoka Urushido 2, Kazu Hamada
Sestrin2 and BNIP3 (Bcl-2/adenovirus E1B 19kDa-interacting protein3) regulate autophagy and mitophagy in renal tubular cells in acute kidney injury by Masayuki Ishihara 1, Madoka Urushido 2, Kazu Hamada
Supplementary Figure 1
 Supplementary Figure 1 Global TeNT expression effectively impairs synaptic transmission. Injection of 100 pg tent mrna leads to a reduction of vesicle mediated synaptic transmission in the spinal cord
Supplementary Figure 1 Global TeNT expression effectively impairs synaptic transmission. Injection of 100 pg tent mrna leads to a reduction of vesicle mediated synaptic transmission in the spinal cord
BRAIN DEVELOPMENT I: ESTABLISHMENT OF BASIC ARCHITECTURE. Thomas Marino, Ph.D.
 BRAIN DEVELOPMENT I: ESTABLISHMENT OF BASIC ARCHITECTURE Thomas Marino, Ph.D. Development of the Brain I. Competencies: Upon completion of this section of the course, the student must be able to: 1. Understand
BRAIN DEVELOPMENT I: ESTABLISHMENT OF BASIC ARCHITECTURE Thomas Marino, Ph.D. Development of the Brain I. Competencies: Upon completion of this section of the course, the student must be able to: 1. Understand
Brain Development III
 Brain Development III Neural Development In the developing nervous system there must be: 1. The formation of different regions of the brain. 2. The ability of a neuron to differentiate. 3. The ability
Brain Development III Neural Development In the developing nervous system there must be: 1. The formation of different regions of the brain. 2. The ability of a neuron to differentiate. 3. The ability
Embryology of the Nervous System. Steven McLoon Department of Neuroscience University of Minnesota
 Embryology of the Nervous System Steven McLoon Department of Neuroscience University of Minnesota In the blastula stage embryo, the embryonic disk has two layers. During gastrulation, epiblast cells migrate
Embryology of the Nervous System Steven McLoon Department of Neuroscience University of Minnesota In the blastula stage embryo, the embryonic disk has two layers. During gastrulation, epiblast cells migrate
Erzsebet Kokovay, Susan Goderie, Yue Wang, Steve Lotz, Gang Lin, Yu Sun, Badrinath Roysam, Qin Shen,
 Cell Stem Cell, Volume 7 Supplemental Information Adult SVZ Lineage Cells Home to and Leave the Vascular Niche via Differential Responses to SDF1/CXCR4 Signaling Erzsebet Kokovay, Susan Goderie, Yue Wang,
Cell Stem Cell, Volume 7 Supplemental Information Adult SVZ Lineage Cells Home to and Leave the Vascular Niche via Differential Responses to SDF1/CXCR4 Signaling Erzsebet Kokovay, Susan Goderie, Yue Wang,
Biology Developmental Biology Spring Quarter Midterm 1 Version A
 Biology 411 - Developmental Biology Spring Quarter 2013 Midterm 1 Version A 75 Total Points Open Book Choose 15 out the 20 questions to answer (5 pts each). Only the first 15 questions that are answered
Biology 411 - Developmental Biology Spring Quarter 2013 Midterm 1 Version A 75 Total Points Open Book Choose 15 out the 20 questions to answer (5 pts each). Only the first 15 questions that are answered
BIOL2005 WORKSHEET 2008
 BIOL2005 WORKSHEET 2008 Answer all 6 questions in the space provided using additional sheets where necessary. Hand your completed answers in to the Biology office by 3 p.m. Friday 8th February. 1. Your
BIOL2005 WORKSHEET 2008 Answer all 6 questions in the space provided using additional sheets where necessary. Hand your completed answers in to the Biology office by 3 p.m. Friday 8th February. 1. Your
Prss56, a novel marker of adult neurogenesis in the mouse brain. - Supplemental Figures 1 to 5- Brain Structure and Function
 Prss56, a novel marker of adult neurogenesis in the mouse brain - Supplemental Figures 1 to 5- Brain Structure and Function Alexandre Jourdon 1,2, Aurélie Gresset 1, Nathalie Spassky 1, Patrick Charnay
Prss56, a novel marker of adult neurogenesis in the mouse brain - Supplemental Figures 1 to 5- Brain Structure and Function Alexandre Jourdon 1,2, Aurélie Gresset 1, Nathalie Spassky 1, Patrick Charnay
Cell Type Nervous System I. Developmental Readout. Foundations. Stem cells. Organ formation. Human issues.
 7.72 10.11.06 Cell Type Nervous System I Human issues Organ formation Stem cells Developmental Readout Axes Cell type Axon guidance 3D structure Analysis Model + + organisms Foundations Principles 1 What
7.72 10.11.06 Cell Type Nervous System I Human issues Organ formation Stem cells Developmental Readout Axes Cell type Axon guidance 3D structure Analysis Model + + organisms Foundations Principles 1 What
TGF-β Signaling Regulates Neuronal C1q Expression and Developmental Synaptic Refinement
 Supplementary Information Title: TGF-β Signaling Regulates Neuronal C1q Expression and Developmental Synaptic Refinement Authors: Allison R. Bialas and Beth Stevens Supplemental Figure 1. In vitro characterization
Supplementary Information Title: TGF-β Signaling Regulates Neuronal C1q Expression and Developmental Synaptic Refinement Authors: Allison R. Bialas and Beth Stevens Supplemental Figure 1. In vitro characterization
What Cell Make Up the Brain and Spinal Cord
 What Cell Make Up the Brain and Spinal Cord Jennifer LaVail, Ph.D. (http://anatomy.ucsf.edu/pages/lavaillab/index.html) What kinds of cells are these?" Neuron?" Epithelial cell?" Glial cell?" What makes
What Cell Make Up the Brain and Spinal Cord Jennifer LaVail, Ph.D. (http://anatomy.ucsf.edu/pages/lavaillab/index.html) What kinds of cells are these?" Neuron?" Epithelial cell?" Glial cell?" What makes
The pathogenesis of nervous distemper
 Veterinary Sciences Tomorrow - 2004 The pathogenesis of nervous distemper Marc Vandevelde Canine distemper is a highly contagious viral disease of dogs and of all animals in the Canidae, Mustellidae and
Veterinary Sciences Tomorrow - 2004 The pathogenesis of nervous distemper Marc Vandevelde Canine distemper is a highly contagious viral disease of dogs and of all animals in the Canidae, Mustellidae and
Supplementary Figure 1.TRIM33 binds β-catenin in the nucleus. a & b, Co-IP of endogenous TRIM33 with β-catenin in HT-29 cells (a) and HEK 293T cells
 Supplementary Figure 1.TRIM33 binds β-catenin in the nucleus. a & b, Co-IP of endogenous TRIM33 with β-catenin in HT-29 cells (a) and HEK 293T cells (b). TRIM33 was immunoprecipitated, and the amount of
Supplementary Figure 1.TRIM33 binds β-catenin in the nucleus. a & b, Co-IP of endogenous TRIM33 with β-catenin in HT-29 cells (a) and HEK 293T cells (b). TRIM33 was immunoprecipitated, and the amount of
Nature Medicine: doi: /nm.4322
 1 2 3 4 5 6 7 8 9 10 11 Supplementary Figure 1. Predicted RNA structure of 3 UTR and sequence alignment of deleted nucleotides. (a) Predicted RNA secondary structure of ZIKV 3 UTR. The stem-loop structure
1 2 3 4 5 6 7 8 9 10 11 Supplementary Figure 1. Predicted RNA structure of 3 UTR and sequence alignment of deleted nucleotides. (a) Predicted RNA secondary structure of ZIKV 3 UTR. The stem-loop structure
Current Model for how cells become neural. Double Inhibition Model for Neural fate. Neural Development. How do cells become neurons?
 Current Model for how cells becoe neural 1) Default state is neural 2) Local secretion of BMPs by epideris inhibits neural fate 3) Local secretion of noggin, chordin by dorsal lip or esoder inhibits BMP
Current Model for how cells becoe neural 1) Default state is neural 2) Local secretion of BMPs by epideris inhibits neural fate 3) Local secretion of noggin, chordin by dorsal lip or esoder inhibits BMP
Sonic hedgehog signaling is required during the appearance of spinal cord oligodendrocyte precursors
 Development 126, 2419-2429 (1999) Printed in Great Britain The Company of Biologists Limited 1999 DEV1420 2419 Sonic hedgehog signaling is required during the appearance of spinal cord oligodendrocyte
Development 126, 2419-2429 (1999) Printed in Great Britain The Company of Biologists Limited 1999 DEV1420 2419 Sonic hedgehog signaling is required during the appearance of spinal cord oligodendrocyte
Fig.9.2. Structure of embryonic brain
 T Chapter 9 Development of Ectodermal Organs he ectoderm gives rise to 3 separate cell populations: neural(plate) ectoderm, neural crest cells, and epiderm (general body ectoderm). A primordium (anlage)
T Chapter 9 Development of Ectodermal Organs he ectoderm gives rise to 3 separate cell populations: neural(plate) ectoderm, neural crest cells, and epiderm (general body ectoderm). A primordium (anlage)
Supplementary Figure S1: Tanycytes are restricted to the central/posterior hypothalamus
 Supplementary Figure S1: Tanycytes are restricted to the central/posterior hypothalamus a: Expression of Vimentin, GFAP, Sox2 and Nestin in anterior, central and posterior hypothalamus. In the anterior
Supplementary Figure S1: Tanycytes are restricted to the central/posterior hypothalamus a: Expression of Vimentin, GFAP, Sox2 and Nestin in anterior, central and posterior hypothalamus. In the anterior
Gli2 is required for induction of floor plate and adjacent cells, but not most
 Development 125, 2759-2770 (1998) Printed in Great Britain The Company of Biologists Limited 1998 DEV9597 2759 Gli2 is required for induction of floor plate and adjacent cells, but not most ventral neurons
Development 125, 2759-2770 (1998) Printed in Great Britain The Company of Biologists Limited 1998 DEV9597 2759 Gli2 is required for induction of floor plate and adjacent cells, but not most ventral neurons
Supplementary figure 1
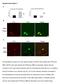 Supplementary figure 1 (A) Quantitative analysis of F-actin signal intensity in NIH3T3 cells treated with PTD4-myc- RBD. NIH3T3 cells were treated with PTD4-myc-RBD as described. Please note the increase
Supplementary figure 1 (A) Quantitative analysis of F-actin signal intensity in NIH3T3 cells treated with PTD4-myc- RBD. NIH3T3 cells were treated with PTD4-myc-RBD as described. Please note the increase
Identification of astrocyte- and oligodendrocyte-like cells of goldfish optic nerves in culture
 Neuroscience Letters, 101 (1989) 127-132 127 Elsevier Scientific Publishers Ireland Ltd. NSL 06139 Identification of astrocyte- and oligodendrocyte-like cells of goldfish optic nerves in culture M. Bastmeyer
Neuroscience Letters, 101 (1989) 127-132 127 Elsevier Scientific Publishers Ireland Ltd. NSL 06139 Identification of astrocyte- and oligodendrocyte-like cells of goldfish optic nerves in culture M. Bastmeyer
Specification of neuronal and glial subtypes from human pluripotent stem cells
 Cell. Mol. Life Sci. (2011) 68:3995 4008 DOI 10.1007/s00018-011-0770-y Cellular and Molecular Life Sciences REVIEW Specification of neuronal and glial subtypes from human pluripotent stem cells Huisheng
Cell. Mol. Life Sci. (2011) 68:3995 4008 DOI 10.1007/s00018-011-0770-y Cellular and Molecular Life Sciences REVIEW Specification of neuronal and glial subtypes from human pluripotent stem cells Huisheng
marker. DAPI labels nuclei. Flies were 20 days old. Scale bar is 5 µm. Ctrl is
 Supplementary Figure 1. (a) Nos is detected in glial cells in both control and GFAP R79H transgenic flies (arrows), but not in deletion mutant Nos Δ15 animals. Repo is a glial cell marker. DAPI labels
Supplementary Figure 1. (a) Nos is detected in glial cells in both control and GFAP R79H transgenic flies (arrows), but not in deletion mutant Nos Δ15 animals. Repo is a glial cell marker. DAPI labels
Chapter 1. Introduction
 1 Chapter 1 Introduction 2 The central nervous system (CNS) is one of the most important organs of an animal. It is also a most complicated one, comprised of hundreds of cell types and millions of cells
1 Chapter 1 Introduction 2 The central nervous system (CNS) is one of the most important organs of an animal. It is also a most complicated one, comprised of hundreds of cell types and millions of cells
Glia make up 10 20% of the cells in the Drosophila nervous system
 REVIEW doi:10.1038/nature09611 Developmental genetics of vertebrate glial-cell specification David H. Rowitch 1,2,3 & Arnold R. Kriegstein 4 Oligodendrocytes and astrocytes are macroglial cells of the
REVIEW doi:10.1038/nature09611 Developmental genetics of vertebrate glial-cell specification David H. Rowitch 1,2,3 & Arnold R. Kriegstein 4 Oligodendrocytes and astrocytes are macroglial cells of the
Receptor-interacting Protein Kinases Mediate Necroptosis In Neural Tissue Damage After Spinal Cord Injury
 Receptor-interacting Protein Kinases Mediate Necroptosis In Neural Tissue Damage After Spinal Cord Injury Haruo Kanno, M.D., Ph.D., Hiroshi Ozawa, M.D., Ph.D., Satoshi Tateda, M.D., Kenichiro Yahata, M.D.,
Receptor-interacting Protein Kinases Mediate Necroptosis In Neural Tissue Damage After Spinal Cord Injury Haruo Kanno, M.D., Ph.D., Hiroshi Ozawa, M.D., Ph.D., Satoshi Tateda, M.D., Kenichiro Yahata, M.D.,
Supplemental Figure 1. Intracranial transduction of a modified ptomo lentiviral vector in the mouse
 Supplemental figure legends Supplemental Figure 1. Intracranial transduction of a modified ptomo lentiviral vector in the mouse hippocampus targets GFAP-positive but not NeuN-positive cells. (A) Stereotaxic
Supplemental figure legends Supplemental Figure 1. Intracranial transduction of a modified ptomo lentiviral vector in the mouse hippocampus targets GFAP-positive but not NeuN-positive cells. (A) Stereotaxic
Emx2 patterns the neocortex by regulating FGF positional signaling
 Emx2 patterns the neocortex by regulating FGF positional signaling Tomomi Fukuchi-Shimogori and Elizabeth A Grove Presented by Sally Kwok Background Cerebral cortex has anatomically and functionally distinct
Emx2 patterns the neocortex by regulating FGF positional signaling Tomomi Fukuchi-Shimogori and Elizabeth A Grove Presented by Sally Kwok Background Cerebral cortex has anatomically and functionally distinct
Title: Chapter 5 Recorded Lecture. Speaker: Amit Dhingra Created by: (remove if same as speaker) online.wsu.edu
 Title: Chapter 5 Recorded Lecture Speaker: Title: What Anthony is the title Berger/Angela of this lecture? Williams Speaker: Amit Dhingra Created by: (remove if same as speaker) online.wsu.edu Chapter
Title: Chapter 5 Recorded Lecture Speaker: Title: What Anthony is the title Berger/Angela of this lecture? Williams Speaker: Amit Dhingra Created by: (remove if same as speaker) online.wsu.edu Chapter
Control of CNS Cell-Fate Decisions by SHP-2 and Its Dysregulation in Noonan Syndrome
 Article Control of CNS Cell-Fate Decisions by SHP-2 and Its Dysregulation in Noonan Syndrome Andrée S. Gauthier, 1,2,3 Olivia Furstoss, 1,2 Toshiyuki Araki, 5 Richard Chan, 5 Benjamin G. Neel, 5 David
Article Control of CNS Cell-Fate Decisions by SHP-2 and Its Dysregulation in Noonan Syndrome Andrée S. Gauthier, 1,2,3 Olivia Furstoss, 1,2 Toshiyuki Araki, 5 Richard Chan, 5 Benjamin G. Neel, 5 David
Lecture IV. Mechanisms of Neural. Neural Development
 Lecture IV. Mechanisms of Neural Bio 3411 Monday 1 Readings NEUROSCIENCE: 5 th ed, pp 477-506 (sorta) 4 th ed, pp 545-575 (sorta) References : Fainsod, A., Steinbeisser, H., & De Robertis, E. M. (1994).
Lecture IV. Mechanisms of Neural Bio 3411 Monday 1 Readings NEUROSCIENCE: 5 th ed, pp 477-506 (sorta) 4 th ed, pp 545-575 (sorta) References : Fainsod, A., Steinbeisser, H., & De Robertis, E. M. (1994).
SUPPLEMENTARY DATA. Supplementary Table 2. Antibodies used for Immunofluoresence. Supplementary Table 3. Real-time PCR primer sequences.
 Supplementary Table 2. Antibodies used for Immunofluoresence. Antibody Dilution Source Goat anti-pdx1 1:100 R&D Systems Rabbit anti-hnf6 1:100 Santa Cruz Biotechnology Mouse anti-nkx6.1 1:200 Developmental
Supplementary Table 2. Antibodies used for Immunofluoresence. Antibody Dilution Source Goat anti-pdx1 1:100 R&D Systems Rabbit anti-hnf6 1:100 Santa Cruz Biotechnology Mouse anti-nkx6.1 1:200 Developmental
SUPPLEMENTARY INFORMATION
 doi:10.1038/nature10353 Supplementary Figure 1. Mutations of UBQLN2 in patients with ALS and ALS/dementia. (a) A mutation, c.1489c>t (p.p497s), was identified in F#9975. The pedigree is shown on the left
doi:10.1038/nature10353 Supplementary Figure 1. Mutations of UBQLN2 in patients with ALS and ALS/dementia. (a) A mutation, c.1489c>t (p.p497s), was identified in F#9975. The pedigree is shown on the left
The functional investigation of the interaction between TATA-associated factor 3 (TAF3) and p53 protein
 THESIS BOOK The functional investigation of the interaction between TATA-associated factor 3 (TAF3) and p53 protein Orsolya Buzás-Bereczki Supervisors: Dr. Éva Bálint Dr. Imre Miklós Boros University of
THESIS BOOK The functional investigation of the interaction between TATA-associated factor 3 (TAF3) and p53 protein Orsolya Buzás-Bereczki Supervisors: Dr. Éva Bálint Dr. Imre Miklós Boros University of
ErbB4 migrazione II parte
 ErbB4 migrazione II parte Control SVZ cells prefer to migrate on the NRG1 type III substrate the substrate preference of the neuroblasts migrating out of the SVZ explant was evaluated SVZ cells had a strong
ErbB4 migrazione II parte Control SVZ cells prefer to migrate on the NRG1 type III substrate the substrate preference of the neuroblasts migrating out of the SVZ explant was evaluated SVZ cells had a strong
Supplementary Figures
 Supplementary Figures Supplementary Figure 1. nrg1 bns101/bns101 embryos develop a functional heart and survive to adulthood (a-b) Cartoon of Talen-induced nrg1 mutation with a 14-base-pair deletion in
Supplementary Figures Supplementary Figure 1. nrg1 bns101/bns101 embryos develop a functional heart and survive to adulthood (a-b) Cartoon of Talen-induced nrg1 mutation with a 14-base-pair deletion in
Inner ear development Nervous system development
 Upcoming Sessions April 22: Nervous System Development Lecture April 24: Reviews of Axonal Pathfinding in Sensory Systems April 29: Inner Ear Development Lecture May 1: May 6: May 8: Auditory System Pathfinding
Upcoming Sessions April 22: Nervous System Development Lecture April 24: Reviews of Axonal Pathfinding in Sensory Systems April 29: Inner Ear Development Lecture May 1: May 6: May 8: Auditory System Pathfinding
Supplementary Figures
 Supplementary Figures Supplementary Figure 1 Correlation between LKB1 and YAP expression in human lung cancer samples. (a) Representative photos showing LKB1 and YAP immunohistochemical staining in human
Supplementary Figures Supplementary Figure 1 Correlation between LKB1 and YAP expression in human lung cancer samples. (a) Representative photos showing LKB1 and YAP immunohistochemical staining in human
Supplemental Information. Ciliary Beating Compartmentalizes. Cerebrospinal Fluid Flow in the Brain. and Regulates Ventricular Development
 Current Biology, Volume Supplemental Information Ciliary Beating Compartmentalizes Cerebrospinal Fluid Flow in the Brain and Regulates Ventricular Development Emilie W. Olstad, Christa Ringers, Jan N.
Current Biology, Volume Supplemental Information Ciliary Beating Compartmentalizes Cerebrospinal Fluid Flow in the Brain and Regulates Ventricular Development Emilie W. Olstad, Christa Ringers, Jan N.
Supplementary Information
 Supplementary Information Astrocytes regulate adult hippocampal neurogenesis through ephrin-b signaling Randolph S. Ashton, Anthony Conway, Chinmay Pangarkar, Jamie Bergen, Kwang-Il Lim, Priya Shah, Mina
Supplementary Information Astrocytes regulate adult hippocampal neurogenesis through ephrin-b signaling Randolph S. Ashton, Anthony Conway, Chinmay Pangarkar, Jamie Bergen, Kwang-Il Lim, Priya Shah, Mina
Supplementary Figure 1. AdipoR1 silencing and overexpression controls. (a) Representative blots (upper and lower panels) showing the AdipoR1 protein
 Supplementary Figure 1. AdipoR1 silencing and overexpression controls. (a) Representative blots (upper and lower panels) showing the AdipoR1 protein content relative to GAPDH in two independent experiments.
Supplementary Figure 1. AdipoR1 silencing and overexpression controls. (a) Representative blots (upper and lower panels) showing the AdipoR1 protein content relative to GAPDH in two independent experiments.
