Short- and Long-Range Attraction of Cortical GABAergic Interneurons by Neuregulin-1
|
|
|
- Cecil Lane
- 6 years ago
- Views:
Transcription
1 Neuron, Vol. 44, , October 14, 2004, Copyright 2004 by Cell Press Short- and Long-Range Attraction of Cortical GABAergic Interneurons by Neuregulin-1 Nuria Flames, 1 Jason E. Long, 2 Alistair N. Garratt, 3 Tobias M. Fischer, 4 Martin Gassmann, 5 Carmen Birchmeier, 3 Cary Lai, 4 John L.R. Rubenstein, 2 and Oscar Marín 1, * 1 Instituto de Neurociencias de Alicante Consejo Superior de Investigaciones Científicas and Universidad Miguel Hernández San Joan d Alacant Spain 2 Department of Psychiatry Nina Ireland Laboratory of Developmental Neurobiology th Street University of California, San Francisco San Francisco, California Max-Delbrueck-Centrum Robert-Roessle-Strasse 10 D Berlin-Buch Germany 4 The Scripps Research Institute La Jolla, California Department of Physiology Biozentrum/Pharmazentrum University of Basel Klingelbergstrasse 50 CH-4056 Basel Switzerland Summary Most cortical interneurons arise from the subcortical telencephalon, but the molecules that control their migration remain largely unidentified. Here, we show that different isoforms of Neuregulin-1 are expressed in the developing cortex and in the route that migrating interneurons follow toward the cortex, whereas a pop- ulation of the migrating interneurons express ErbB4, a receptor for Neuregulin-1. The different isoforms of Neuregulin-1 act as short- and long-range attractants for migrating interneurons, and perturbing ErbB4 func- tion in vitro decreases the number of interneurons that tangentially migrate to the cortex. In vivo, loss of Neuregulin-1/ErbB4 signaling causes an alteration in the tangential migration of cortical interneurons and a reduction in the number of GABAergic interneurons in the postnatal cortex. These observations provide evidence that Neuregulin-1 and its ErbB4 receptor directly control neuronal migration in the nervous system. Introduction The function of the cerebral cortex requires the coordinated action of two major neuronal types, the glutamater- *Correspondence: o.marin@umh.es gic projection neurons and the GABAergic interneurons. Whereas it is well established that projection neurons of the cerebral cortex derive from the progenitor zones of the pallium, research over the past few years has provided compelling evidence that a large number of cortical GABAergic interneurons are born in the subpallial telencephalon (reviewed in Corbin et al., 2001; Marín and Rubenstein, 2001). This conclusion is based on a number of different experimental approaches, including analysis of cell dispersion using chimeras (Tan and Breen, 1993; Tan et al., 1995), neuronal migration in slice cultures (Anderson et al., 1997; De Carlos et al., 1996; Lavdas et al., 1999; Wichterle et al., 1999), genetic manipulations (Anderson et al., 1997; Casarosa et al., 1999; Sussel et al., 1999), and in vivo fate mapping of cortical GABAergic interneurons (Anderson et al., 2002; Nery et al., 2002; Wichterle et al., 2001). GABAergic interneurons born in the subpallium migrate tangentially to populate the entire cortex, including the piriform cortex, isocortex (e.g., neocortex), and hippocampus (Anderson et al., 1997; Lavdas et al., 1999; Marín et al., 2001; Pleasure et al., 2000; Sussel et al., 1999; Wichterle et al., 1999). Most tangentially migrating interneurons appear to derive from the medial ganglionic eminence (MGE), although the caudal ganglionic eminence (CGE) and, to some extent, the lateral ganglionic eminence (LGE) also give rise to cortical GABAergic interneurons (Anderson et al., 2001; Ang et al., 2003; Jiménez et al., 2002; Lavdas et al., 1999; Nery et al., 2002; Sussel et al., 1999; Wichterle et al., 1999, 2001). Despite the detailed picture that is emerging on the origin and migratory routes followed by cortical interneurons, the mechanisms that control their migration from the subpallium to the developing cortex are still poorly characterized. Neurotrophins that activate the TrkB receptor (brain-derived neurotrophic factor [BDNF] and neurotrophin-4 [NT-4/5]), as well as hepatocyte growth factor (HGF), can acutely induce the motility of MGE-derived cells and therefore are thought to act as motogenic factors for migrating interneurons (Polleux et al., 2002; Powell et al., 2001). In addition, directional guidance of GABAergic interneurons from the MGE to the cortex appears to require the simultaneous activity of chemorepulsive and chemoattractive factors (Marín et al., 2001, 2003; Wichterle et al., 2003). Thus, MGE- derived cells actively avoid the preoptic area and the developing striatum in their way toward the cortex, whereas the developing cortex appears to produce an activity that attracts migrating interneurons. Although it is known that the repulsion of cortical interneurons by the striatal mantle is mediated by semaphorin/neuropilin interactions (Marín et al., 2001), the molecules responsi- ble for the other activities have not been identified. In an attempt to determine the molecular nature of the attractive activity present in the developing cortex, we have focused our analysis on one candidate, Neuregulin-1 (NRG1), a member of the neuregulin family of proteins. Neuregulins contain an epidermal growth factor (EGF)-like motif that activates membrane-associated tyrosine kinases related to the EGF receptor (also known
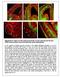
2 Neuron 252 as ErbB1). NRG1 directly binds to ErbB3 and ErbB4 receptors, which alone or in combination with ErbB2 mediate a large range of functions (Buonanno and Fischbach, 2001; Falls, 2003). In the central and peripheral nervous system, NRG1 signaling is of central importance for glial cell survival, proliferation, and differentiation (Adlkofer and Lai, 2000; Buonanno and Fischbach, 2001; Garratt et al., 2000). In neurons, NRG1 functions range from the control of proliferation to late differentiation events such as the regulation of ligand- and voltagegated channels expression (reviewed in Buonanno and Fischbach, 2001). In cortical structures such as the cerebellum and the neocortex, NRG1 signaling is important for the formation and maintenance of radial glial cells, which in turn are required to support neuronal migration (Anton et al., 1997; Rio et al., 1997; Schmid et al., 2003). Interestingly, NRG1 directly promotes the motility of Schwann cells (Mahanthappa et al., 1996; Meintanis et al., 2001), raising the possibility that it may also play a similar role for some neuronal types. Here, we present evidence that NRG1 is a chemoattractive factor for MGE-derived neurons and that NRG1/ErbB4 signaling plays a prominent role in the directional guidance of GABAergic interneurons from the subpallium to the developing cortex. Results Figure 1. Complementary Expression of Nrg1 Isoforms and Their ErbB4 Receptor in the Developing Telencephalon during the Period of Interneuron Migration to the Cortex Serial coronal sections through the telencephalon of E13.5 embryos showing mrna expression for ErbB4, Nrg1-CRD, Nrg1-Ig, Sema3A, and Sema3F. (A and H) ErbB4 mrna expression in immature interneurons migrating toward the cortex (NCx; solid arrowheads). (D) Lhx6 expression in immature interneurons migrating toward the cortex (NCx; solid arrowheads). (B, G, and H) Nrg1-CRD expression in the route of interneuron migration toward the cortex, in the devel- oping striatum (Str) and in the anlagen of the hippocampus (H). (C) Nrg1-Ig expression in the cortex (NCx). (E) Sema3A expression in the striatum. (F and G) Sema3F expression in the striatum. ErbB4- expressing interneurons reach the cortex through a cellular corridor expressing Nrg1-CRD (G, H, and I), avoiding the striatal mantle due to Sema3A/3F-mediated chemorepulsion (for details, see Marín et al., 2001). Double in situ images (G and H) were composed from adjacent sections using Photoshop software. LGE, lateral ganglionic eminence; MGE, medial ganglionic eminence. Scale bars equal 200 m. 1E 1I and Supplemental Figure S1 at org/cgi/content/full/44/2/251/dc1/). In addition, the developing cortex the target of the migrating ErbB4 interneurons specifically expressed the diffusible form of the Nrg1 gene, Nrg1-Ig (Figure 1C and Supplemental Figure S1). At the stage when migrating interneurons first enter the cortex (approximately at E13), Nrg1-Ig expression is stronger in lateral than medial regions of the cortex throughout the whole rostrocaudal extent of telencephalon (Supplemental Figure S1). Nrg1-Ig ex- pression, however, expands to more medial regions of Expression of Nrg1 Isoforms in the Developing Telencephalon Previous studies have shown that ErbB4 is expressed in a subpopulation of interneurons migrating from the MGE to the cortex (Yau et al., 2003) (Figure 1A), raising the possibility that ErbB4 and its high-affinity ligand, NRG1, might be involved in controlling cortical interneuron migration. The Nrg1 gene is subject to differential promoter usage and alternative splicing, resulting in the expression of distinct protein isoforms (Buonanno and Fischbach, 2001; Falls, 2003). Three major classes of NRG1 proteins can be distinguished on the basis of their domain architecture. Types I and II comprise secreted isoforms that contain an extracellular immunoglobulin (Ig)-like domain (NRG1-Ig). Type III, on the other hand, corresponds to membrane bound isoforms (NRG1- CRD), which contain an extracellular cysteine-rich do- main (CRD). The three types of NRG1 proteins share high sequence homology in their EGF-like domain, which is sufficient to elicit ErbB receptor dimerization, tyrosine phosphorylation, and the activation of downstream signaling pathways. Using isoform-specific RNA probes, we found that Nrg1-CRD is expressed throughout the LGE, from the subventricular zone to the developing striatal mantle (Figures 1B, 1G, and 1H). As previously reported, Semaphorin3A (Sema3A) and Semaphorin3F (Sema3F) were found to be expressed in the striatal mantle (Figures 1E 1G), where they create an inhibitory territory that migrating cortical interneurons avoid in their way toward the cortex (Marín et al., 2001). Interestingly, the analysis of adjacent sections revealed that the tangentially migrating ErbB4-expressing cells follow a corridor through the LGE that is Nrg1-CRD and that lacks Semaphorin3A/3F (Sema3A/3F) expression (Figures 1A, 1B, and
3 NRG1 and Cortical Interneuron Tangential Migration 253 Figure 2. MGE-Derived Cells Prefer a Nrg1- CRD-Expressing Substrate in the Stripe Choice Assay (A) Schematic of the experimental paradigm used in the stripe choice assay. Slides were coated with alternating stripes of nontransfected COS cells (dark gray dots) and either mock-transfected or Nrg1-CRD-transfected COS cells (red dots). Dissociated E13.5 MGE cells (green dots) were plated on top of the stripe carpets, and their distribution was studied 24 hr later. (B and E) Distribution of GFP MGE-derived cells in control (B) and experimental stripe carpets. (C and F) Homogeneous distribution of nontransfected and transfected COS cells as visualized with the fluorescent nuclear stain DAPI. The dotted lines indicate the boundary between nontransfected and transfected COS cells. (D and G) Quantification of the percentage of MGE cells on nontransfected and mock-transfected COS cells (D), or over nontransfected and Nrg1-CRD-transfected COS cells (G). F 21.65; *p (data from three independent experiments). Scale bar equals 50 m. the cortex as development proceeds, in parallel with the cells were directed toward the source of NRG1 (n 38; progressive advance of migrating interneurons in medial Figures 3A 3D). NRG1-Ig did not affect the mitotic index direction (Supplemental Figure S1). in the explants (Supplemental Figure S2 at neuron.org/cgi/content/full/44/2/251/dc1/), suggesting NRG1-CRD Is a Permissive Factor that the increased migration induced by NRG1-Ig was for MGE-Derived Neurons not caused by a rise in the number of cells produced in The pattern of expression of the different Nrg1 isoforms the MGE explant. Thus, NRG1-Ig is a chemoattractive and their ErbB4 receptor is consistent with a model in factor for immature interneurons migrating from the which ErbB4-expressing interneurons preferentially MGE. use Nrg1-CRD-expressing cells as substrate en route To test whether interneurons migrating to the cortex through the LGE toward the cortex. To test this hypothesis, respond to NRG1-Ig in a more physiologically relevant we performed a stripe choice assay in which E13.5 context, we placed aggregates of Nrg1-Ig-expressing MGE-derived cells were given the possibility to migrate on top of COS cells transfected with a control plasmid or with Nrg1-CRD (Figure 2A). In brief, we plated alternative stripes of COS cells that were either nontransfected or transfected with dsred alone (control) or dsred and Nrg1-CRD (experimental cases). Dissociated MGE cells that were obtained from green fluorescence protein (GFP)-expressing mice (Hadjantonakis et al., 1998) were plated on top of the stripes, and their final location was assayed 24 hr later. MGE cells showed no preference for either nontransfected or mock-transfected stripes in control experiments (Figures 2B 2D) but displayed an 2-fold preference for Nrg1-CRD-expressing cells compared to nontransfected stripes (n 3 independent experiments, 20 fields examined per experiment; Figures 2E 2G). Thus, MGE-derived cells preferred a Nrg1-CRDexpressing substrate, suggesting that NRG1-CRD may play a permissive role in the guidance of interneurons toward the cortex. NRG1-Ig Is a Chemoattractant for MGE-Derived Neurons Figure 3. NRG1-Ig Is a Chemoattractant for MGE-Derived Cells Guidance of interneurons requires not only the existence (A and B) Medial ganglionic eminence (MGE) explants from the of a permissive corridor toward the cortex but also the telencephalon of E13.5 embryos were cultured in Matrigel adjacent existence of a diffusible attractive activity that confers to mock-transfected (A) or Nrg1-Ig-transfected (B) COS cell aggredirectionality to the migration (Marín et al., 2003; Wichfication of the attraction of MGE cells by Nrg1-Ig-transfected COS gates. (C) Scoring scheme modified from Zhu et al. (1999). (D) Quanti- terle et al., 2003). To test whether secreted NRG1 atcell aggregates (n 29 explants). In addition to directing the migratracts cortical interneurons, we cocultured E13.5 MGE tion of MGE-derived cells, NRG1-Ig enhanced the length of their explants with aggregates of COS cells expressing the leading process. Mean length of leading processes was secreted Nrg1-Ig isoform in a three-dimensional matrix. m (average SEM) in controls and m in experimental In coculture experiments, the majority of MGE-derived cases. F 6.41; p Scale bar equals 300 m.
4 Neuron 254 Figure 4. Ectopic Expression of NRG1-Ig Redirects the Migration of MGE-Derived Cells in Slice Cultures (A) Schematic of the experimental paradigm used to analyze the migration of MGE-derived cells in slice cultures in the presence of control and Nrg1-Ig-transfected COS cells. (B) Coronal slice through the telencephalon with cell aggregates formed with control (left) and Nrg1-Ig (right) transfected COS cells. DiI-labeled cells (arrowheads) from both the ipsilateral and contralateral MGE (asterisk) migrate ectopically in a ventrolateral direction toward the COS cell aggregate expressing NRG1-Ig. (C) Higher magnification of the experimental side in (B). Arrowheads, DiI-labeled cells. Dotted lines, slice outline. Scale bars equal 200 m (B) and 50 m (C). COS cells in the ventrolateral telencephalon in slice cul- 1 and 4 increased to roughly 90 ( [average tures (Anderson et al., 1997) and followed the migration SE]; n 5; Figures 5A and 5B), because many neurons of DiI-labeled E13.5 MGE cells (Figure 4A). As a control, derived from the MGE were attracted toward the cortical aggregates of GFP-expressing COS cells were placed explant (Marín et al., 2003; Wichterle et al., 2003). In on the opposite side of the slices (Figure 4A). Cell aggre- contrast, MGE cells expressing dnerbb4 became largely gates expressing Nrg1-Ig attracted migrating interneurons unresponsive to the cortical attractant, as revealed by toward the ventrolateral telencephalon (Figures 4B the roughly 45 angle of migration adopted by the elec- and 4C), diverting them from their normal route of migration troporated cells in the presence of a cortical explant toward the cortex (n 25). Similar results were ob- ( [average SE]; n 13; Figures 5A and 5C). tained when heparin beads soaked in recombinant NRG1 It is of note that the rate of migration of GFP/dnErbB4- protein were used instead of cell aggregates (Supplemen- expressing cells was indistinguishable from that observed tal Figure S3 at in cells electroporated with GFP alone (data not 2/251/DC1/). Control beads did not modify the migration shown), suggesting that expression of dnerbb4 does not of DiI-labeled MGE-derived cells toward the cortex (n nonspecifically impair cell migration. Thus, ErbB4 appears 25; Supplemental Figure S3B), whereas many MGE- to mediate, at least in part, the response of interneurons derived cells abnormally migrated toward a NRG1-coated to the cortical attractive activity present in the devel- bead located in the ventrolateral telencephalon (n 17/ oping cortex. 20; Supplemental Figure S3B). Thus, MGE-derived cells We next analyzed the pattern of migration of MGE migrating toward the cortex are attracted by secreted cells expressing the dnerbb4 in slice cultures. For this NRG1. set of experiments, we focally electroporated the MGE of slices obtained from E13.5 brains and analyzed the ErbB4 Function Is Required for Interneuron distribution of migrating cells after 36 hr in culture (Marín Migration In Vitro et al., 2001) (Figure 5D). In control experiments, GFPexpressing To determine if the attractive effect of NRG1 on MGEderived cells largely migrated through the Nrg1- cells was mediated by its high-affinity receptor, CRD /Sema3A/F LGE corridor deep to the striatum on ErbB4, we next performed loss-of-function experiments. their way to the cortex (at least 200 cells in the cortex To this aim, we first analyzed the migration of MGE- in 24 of 24 slices; Figures 5E and 5E ). In contrast, in derived interneurons expressing a dominant-negative slices electroporated with both Gfp and dnerbb4, most form of ErbB4 (dnerbb4) (Jones et al., 1999). In a three- GFP-expressing cells failed to migrate to the neocortex dimensional matrix, cells derived from an isolated E13.5 and hippocampus (fewer than ten cells in the cortex in MGE explant migrated uniformly in all directions (see, 16 of 19 slices) and instead accumulated in the basal for example, Figure 3A). Thus, when an explant was telencephalon (Figures 5F and 5F ). These results sug- subdivided into eight sectors of equal size, migrating gest that decreased ErbB4 signaling interferes with the cells within each of these sectors dispersed with an normal pattern of interneuron migration to the cortex angle of roughly 45 ( [average SE]; n 6; and are in agreement with the hypothesis that NRG1 Supplemental Figure S4 at signaling through ErbB4 receptors mediates the migracontent/full/44/2/251/dc1/). When MGE explants were co- tion of at least a population of cortical interneurons. cultured along with a piece of embryonic cortex, the maximum To test this, we examined cortical interneurons in mice angle adopted by neurons migrating within sectors lacking either ErbB4 or Nrg1.
5 NRG1 and Cortical Interneuron Tangential Migration 255 Figure 5. Loss of ErbB4 Function Perturbs Interneuron Migration to the Cortex (A) Schematic of the experimental paradigm used to analyze the migration of MGE-derived cells expressing Gfp or Gfp dnerbb4. Expression vectors were pressure injected focally into the MGE of coronal slice cultures, the slices were electroporated, and the electroporated MGE explants were then cultured in Matrigel adjacent to a piece of embryonic cortex. Explants were subdivided into eight sectors, and the migration angle adopted by MGE cells was quantified in sectors 1 and 4. (B and C) Migration of MGE-derived cells electroporated with Gfp (B) or Gfp and dnerbb4 (C). Arrowheads point to cells directed toward the cortical explant (to the right in both [B] and [C]). Vector diagrams represent the orientation of migrating cells in three different cases of control (B) or experimental (C) explants, and the numbers illustrate the maximum angle range adopted by migrating cells in each case. Note that many MGE-derived cells in control experiments (B) migrated within the angle range expected for cells not attracted by the cortex (45 ; Supplemental Figure S4 at a substantial number of MGE-derived cells do not migrate to the cortex in vivo but instead remain within the basal telencephalon (reviewed in Marı n and Rubenstein, 2003). This suggests that a number of MGE-derived cells are normally unresponsive to the cortical attractant(s). (D) Schematic of the experimental paradigm used to analyze the migration of MGE-derived cells expressing Gfp or Gfp and dnerbb4 in slice cultures. Dotted lines, slice outline. (E and F) Migration of cells electroporated with a Gfp expression vector alone (E) or with Gfp and dnerbb4 expression vectors (F). Arrowheads point to a few cells that have reached the cortex in the experimental case. (E and F ) Schematic representation of migratory routes in the slices shown in (E) and (F), respectively. Scale bars equal 50 m (B and C) and 200 m (E and F). Reduced Numbers of Cortical GABAergic Interneurons in ErbB4 and Nrg1 Mutants Targeted inactivation of ErbB4 results in midembryonic lethality due to failed development of myocardial trabeculae (Gassmann et al., 1995), precluding the analysis of interneuron migration in these animals. To circumvent this problem, we examined ErbB4 mouse mutants in which the heart defects have been rescued by the expression of human ErbB4 (HER4) under a cardiac-specific myosin promoter (Tidcombe et al., 2003). Consistent with our in vitro experiments, ErbB4 mutant interneurons largely failed to enter the NRG1-CRD corridor in the LGE (see Figure 1G) as they migrate toward the cortex (Figures 6A and 6B). As expected from these initial migratory defects, the embryonic cortex of ErbB4 / HER4heart mutant embryos contained significantly fewer migrating interneurons than controls. This was observed by immunohistochemistry against Calbindin (control, ; mutant, [cells, average SD]; p 0.026; n 3; Figures 6C and 6D), a well-established marker for migrating embryonic GABAergic interneurons (Anderson et al., 1997), or by in situ hybridization with other markers of cortical interneurons, such as Lhx6 (data not shown). In agreement with our previous experiments suggesting that NRG1 largely mediates the attraction of
6 Neuron 256 Figure 6. Loss of ErbB4/NRG1 Signaling Decreases the Number of GABAergic Interneurons in the Embryonic Cortex (A and B) Coronal sections through the telencephalon of control (A) and ErbB4 mutant (B) E14.5 embryos, showing Lhx6 mrna expression. Arrowheads point to migrating cells entering the LGE NRG1 corridor in controls, which are disseminated in mutant embryos (empty arrowheads). Very few Lhx6 neurons enter the LGE corridor at this early stage of the migration in ErbB4 mutants (asterisk). (C and D) Coronal sections through the cortex of control (C) and ErbB4 mutant (D) E14.5 embryos, showing Calbindin immunohistochemistry. (C and D ) Details of the boxed areas in (C) and (D), respectively. Arrowheads point to cells migrating through the marginal zone or the subventricular zone in controls, most of which are missing in mutant embryos (empty arrowheads). (E and F) Coronal sections through the cortex of control and Nrg1 mutant E13 embryos, showing Lhx6 RNA expression. (E and F ) Details of the boxed areas in (E) and (F), respectively. Arrowheads point to migrating cells entering the cortex in controls, which are absent in mutant embryos. (E and F ) Schemas depict the migratory route followed by Lhx6 cells in control (E) and Nrg1 mutants (F). H, hippocampus; LGE, lateral ganglionic eminence; MGE, medial ganglionic eminence; NCx, neocortex; Str, striatum. Scale bars equal 50 m (C and D ) and 100 m (A, B, E, F, E, and F ). ErbB4 cortical interneurons, tangentially migrating the cerebral cortex in postnatal mice. Gross anatomical cells also failed to enter the LGE corridor and reach the observation of Nissl-stained coronal sections through cortex in Nrg1 conditional mutant embryos at the onset the telencephalon of rescued ErbB4 mutant mice did of migration (n 3; Figures 6E and 6F). Moreover, similar not reveal gross morphological defects in 3-week-old to ErbB4 / HER4 heart mutants, the number of migrating mice (Figures 7A and 7B). Nevertheless, immunohistochemical interneurons in the cortex of Nrg1 conditional mutants analysis using anti-gaba antibodies demoninterneurons was also reduced at E14.5 (data not shown). Thus, the strated a significant decrease in the number of GABA embryonic phenotypes of the two mutant lines fully support cells in the postnatal cortex of rescued ErbB4 mutant the in vitro evidence that ErbB4/Nrg1 signaling par- mice compared to controls (n 3; Figures 7C and 7D). ticipates in controlling the tangential migration of interneurons Quantification of these defects at different rostrocaudal to the cortex. levels of the cerebral cortex showed that the number Although Nrg1 conditional mutants die perinatally of GABA interneurons was normal at rostral cortical (A.N.G. and C.B., unpublished data), ErbB4 / HER4 heart levels, but there was a significant reduction at midcortical mice develop to adulthood, allowing the examination of and caudal cortical levels (Figure 7E). The decrease
7 NRG1 and Cortical Interneuron Tangential Migration 257 migrating toward the cortex expresses ErbB4. In the LGE, these cells move through a corridor that expresses a membrane bound form of NRG1, which may act as a permissive cue for their migration. Moreover, the developing cortex expresses a diffusible isoform of NRG1, which in vitro acts as a potent attractant for MGEderived cells. Furthermore, loss of ErbB4 or NRG1 function perturbs interneuron migration to the cortex and alters the number of GABAergic interneurons in the postnatal cortex. Consequently, we suggest that NRG1/ ErbB4 interactions mediate both short- and long-range attraction for tangentially migrating interneurons at different stages of their journey and that this signaling system is required for the normal development of a subpopulation of cortical interneurons. ErbB Receptors and Neuronal Migration in the Nervous System NRG1 directly binds to ErbB3 and ErbB4 receptors, which alone or in combination with ErbB2 mediate the large range of functions attributed to this factor during the development of the nervous system (Buonanno and Fischbach, 2001; Falls, 2003). In the brain, NRG1 is required for the normal development of radial glial cells, and this function appears to depend on either ErbB2 or ErbB4 receptors (Anton et al., 1997; Schmid et al., 2003). In the cerebral cortex, for example, disrupting ErbB2 function leads to abnormal radial glia formation, indirectly perturbing the radial migration of projection neurons (Anton et al., 1997; Schmid et al., 2003). In the cerebellum, NRG1 induces astrocytes to adopt a radial glia phenotype in vitro, which is in turn required to support neuronal migration (Rio et al., 1997). Thus, ErbB2 and ErbB4 appear to have only an indirect role in the process of radial migration in the developing central Figure 7. Loss of ErbB4 Signaling Decreases the Number of nervous system, contributing to the development of the GABAergic Interneurons in the Postnatal Cortex supporting elements necessary for the radial migration Coronal sections through the parietal cortex of P20 control (A and of neocortical projection neurons and cerebellar gran- C) and ErbB4 mutant (B and D) mice. Roman numerals designate ule cells. cortical layers. (A and B) Nissl staining. (C and D) GABA immunohis- In contrast to the non-cell-autonomous effects of tochemistry. (E) Quantification of the number of GABA interneurons ErbB2 and ErbB4 signaling on radial migration, our exat different rostrocaudal levels of the cortex in control (light gray) periments demonstrate that ErbB4 cell-autonomously and ErbB4 mutant (dark gray) mice. F 1.88, p 0.20 (Bregma 0.98); F 68.29, *p (Bregma 0.94); F 10.03, *p 0.02 directs the tangential migration of MGE-derived in- (Bregma 1.70); F 10.15, *p 0.02 (Bregma 2.80). Scale bar equals 200 m. has been disrupted failed to reach their target, demon- strating that the expression of this receptor confers on migrating interneurons the ability to respond to che- moattractive cues secreted by the cortex. Previous studies have also shown that a closely related receptor, the epidermal growth factor receptor (EGFR or ErbB1), can cell-autonomously induce chemotaxis of migrating neurons (Caric et al., 2001), probably in response to ligands such as heparin binding epidermal growth factor (HB-EGF) or transforming growth factor (TGF ). ErbB1 signaling, however, appears to exclusively influence radially migrating cortical neurons but not tangentially migrating interneurons. Since ErbB4 is also expressed in tangentially migrating neurons in other regions of the developing brain, including the pontine region or the cerebellar anlagen (N.F. and O.M., unpublished data), in the number of GABAergic neurons appeared to be uniform across all cortical layers. In addition, the number of GABAergic cells was also significantly reduced in the hippocampus of rescued ErbB4 mutant mice compared to controls (Supplemental Figure S5 at org/cgi/content/full/44/2/251/dc1/). Thus, ErbB4/NRG1 signaling appears to be required for normal development of a subpopulation of cortical GABAergic interneurons in vivo. Discussion Our results demonstrate that NRG1 is a chemoattractant for a population of cortical interneurons and that this effect is mediated, at least in part, through ErbB4 receptors. First, a subpopulation of MGE-derived interneurons terneurons toward the NRG1 neocortex and thus directly implicate this signaling system in the control of neuronal migration in the developing brain. In slice cultures, migrating interneurons in which ErbB4 function
8 Neuron 258 it appears that ErbB1 and ErbB4 receptors may have tion (Powell et al., 2001), whereas both upa and upar specialized in regulating different types of migration in appear to mediate the cell invasive behavior that is in- the developing brain. Further analysis on the role of duced by NRG1 in cancer cells (Mazumdar et al., 2001). these members of the ErbB family of tyrosine kinase Thus, it would be interesting to determine how these receptors is required to resolve this question. different signaling systems could interact at the molecular level to generate an integrated cellular response. Molecular Guidance of Cortical GABAergic Interneurons NRG1, GABAergic Interneurons, The migration of immature GABAergic interneurons from and the Etiology of Schizophrenia the subpallium to the developing cortex involves both The role of NRG1/ErbB4 signaling in the development chemorepulsive and chemoattractive factors. Chemoreas of cortical GABAergic interneurons is of further interest, pulsive cues appear to be required to prevent the migraphrenia NRG1 has been implicated in susceptibility to schizo- tion of cortical interneurons to telencephalic structures (Corvin et al., 2004; Harrison and Owen, 2003; other than the cortex. This is illustrated, for example, Li et al., 2004; Stefansson et al., 2002, 2003; Tang et al., by the role of semaphorin/neuropilin interactions in rein 2004; Williams et al., 2003; Yang et al., 2003) (reviewed stricting the access of migrating cortical interneurons Corfas et al., 2004). Given the evidence that schizo- to the developing striatum (Marín et al., 2001). A similar phrenia may result from abnormal neurodevelopment mechanism may restrict the ventral migration of cortical (reviewed in Lewis and Levitt, 2002) and that defective interneurons into the preoptic area (Marín et al., 2003; GABA neurotransmission may underlie its pathophysiol- Wichterle et al., 2003), although the molecule(s) respon- ogy (Benes and Berretta, 2001; Keverne, 1999; Lewis, sible for this process has not been determined. 2000), a defect in the development of cortical GABAergic Several molecules have been described that promote interneurons could cause this disorder. Thus, the evithe migration of cortical GABAergic interneurons, such dence presented herein that implicates both NRG1 and as NT4/5 and HGF (Polleux et al., 2002; Powell et al., ErbB4 in cortical interneuron development provides a 2001). To our knowledge, however, NRG1-Ig is the first coherent biological context whereby NRG1 may confer factor that has been described to have a chemoattracis susceptibility to some forms of schizophrenia. Since it tive effect on cortical interneurons. Interestingly, nonsethe likely that NRG1 contributes to the pathogenesis of creted forms of NRG1 also influence the migration of disorder in only a subset of the patients, it will be cortical interneurons by creating a permissive corridor important to test the existence of a correlation between used by GABAergic interneurons in their way toward the an abnormal NRG1 genotype and migration defects in cortex. Since all isoforms of the Nrg1 gene contain an human cases. Moreover, since ErbB4 expression is EGF-like domain that appears to mediate all its biologiin maintained in a subpopulation of cortical interneurons cal functions, it is remarkable that the different membe the postnatal cortex (Yau et al., 2003), it remains to brane and secreted isoforms of Nrg1 are expressed in tested whether NRG1 may also influence later stages such a coordinated pattern to control the guidance of in the development of GABAergic interneurons, includ- ErbB4 interneurons. As cells leave the MGE, repellent ing the elaboration of axon arbors or synapse formation, signals largely govern the guidance of migrating inthis which may also contribute to the pathophysiology of terneurons by delineating the territories that are permisrole disorder (Woo et al., 1998). In view of the important sive to their migration. This is the case for Sema3A/3F that the expression of different isoforms of Nrg1 expression in the striatal mantle or the uncharacterized has in the development of cortical interneurons, it is activity present in the preoptic area. However, Nrg1- tempting to speculate that an alteration in the expres- CRD expression appears to be crucial to channel sion pattern of the different isoforms of the Nrg1 gene ErbB4 interneurons through the LGE subventricular in humans may lead to abnormal cortical GABAergic zone toward the pallial-subpallial boundary. This is interneurons function due to defects in this neuronal clearly illustrated in our experiments by the permissive population at different stages of development. role of NRG1-CRD in vitro and the inability of migrating cells to enter the LGE corridor in ErbB4 and Nrg1 mutant Experimental Procedures embryos. In addition to the channeling role of NRG1- Animals CRD, NRG1 function also relies on secreted isoforms Wild-type and GFP-expressing transgenic mice (Hadjantonakis et (NRG1-Ig), which establish the required chemoattractive al., 1998), maintained in a CD1 background, were used for expression gradient that directs GABAergic interneurons toward analysis and cell and tissue culture experiments. HER4 heart transgradient the cortex. genic mice, which express a human ErbB4 (HER4) cdna under It is worth noting that ErbB4 is only expressed in a the control of the cardiac-specific -HMC (myosin heavy chain) subpopulation of interneurons that migrates toward the promoter, were maintained in a mixed C57b/6 and 129/SvJ back- ground. HER4 heart transgenic mice were mated to ErbB4 heterozycortex, suggesting that different types of cortical in- gous mice to eventually generate ErbB4 / HER4 heart and ErbB4 / terneurons may be guided by distinct factors. In addi- HER4 heart littermate mice, which were used in our experiments as tion, it is possible that the same population of interneuare control and ErbB4 mutants, respectively. ErbB4 / HER4 heart mice rons respond to several migration-promoting factors at null for the ErbB4 gene except in the heart, due to the expression the same time, like HGF and NRG1, which will contribute of the HER4 transgene (Tidcombe et al., 2003). The generation of ErbB4 mutant mice (Gassmann et al., 1995) and HER4 heart transgenic to ensure proper guidance of interneurons toward the mice (Tidcombe et al., 2003) has been previously described in detail. cortex. Of note, the urokinase plasminogen activator Null and floxed alleles for the Nrg1 gene have also been described (upa) and its receptor (upar) appear to be involved at elsewhere (Meyer and Birchmeier, 1995). Foxg1 Cre/ (Hebert and some level in the role of HGF in promoting MGE migra- McConnell, 2000) mice were used to obtain telencephalic Nrg1 mu-
9 NRG1 and Cortical Interneuron Tangential Migration 259 In Vitro Focal Electroporation Coronal slice cultures were obtained as described previously (An- derson et al., 1997). Gfp was cloned into pcaggs, a chicken -actin promoter expression vector. The expression vector was pressure injected focally into the MGE of coronal slice cultures by a Pneumatic PicoPump (Narishige) through a glass micropipette. Slices were electroporated within a setup of two horizontally oriented platinum electrodes (Protech International Inc.) powered by a T820 Electro Square Porator (BXT). Quantification of Migration Angle Telencephalic slices from 13.5 embryos were electroporated into the subventricular zone of the MGE with either Gfp expression vector (control) or a mix of Gfp and dnerbb4 expression vectors. Immediately after electroporation, MGE explants were dissected out and cultured adjacent to cortical explants in Matrigel for 24 hr. Each MGE explant was subdivided into eight equal sectors. The orientation of migrating cells was estimated by drawing a vector with its origin in the nucleus and the direction of the leading process. To study the range of angles in which cells migrated out of the explant sector, the vectors of each experiment were pooled together in its origin, and the angle between the two most differently oriented cells was measured. Quantification of GABAergic Interneurons in the Cortex For the quantification of Calbindin interneurons in the embryonic cortex of ErbB4 / HER4 heart mutants and controls, a 200 m profile of the dorsal cortex at intermediate rostrocaudal levels was selected for each hemisphere, and migrating interneurons were counted throughout the thickness of the developing pallium, for three different brains from each genotype. For the quantification of GABAergic interneurons in the postnatal cortex of ErbB4 / HER4 heart mutants and controls, a 300 m profile of the lateral pallial wall, from the lateral ventricle to the pial surface, was counted in four different rostrocaudal levels (Bregma levels 0.98, 0.94, 1.70, and 2.80), for three different brains from each genotype. Acknowledgments We thank I. Gosp, M. Pérez, C. Griffel, and P. Krause for technical assistance; J.L. Weber for precious help during early stages of the study; S. Viniegra, F. Moya, and S. Martínez for sharing lab space; A. Nieto, B. Rico, and G. López-Bendito for comments on the manuscript; V. Pachnis, M. Tessier-Lavigne, B. Condie, and D.F. Stern for plasmids and reagents; and J.A. del Rio and F. de Castro for tips on the preparation of COS cell aggregates. N.F. is the recipient of a FPU predoctoral fellowship from the Spanish MEC. This work was supported by grants from the Spanish MCyT (BMC ), GVA (GRUPOS03/053), and NARSAD to O.M.; and by Nina Ireland, NIDA R01DA12462, and NIMH grants RO1MH49428, RO1MH51561, and K02MH01046 to J.L.R.R. O.M. is an EMBO Young Investigator and a NARSAD Young Investigator. Received: September 8, 2004 Revised: September 16, 2004 Accepted: September 27, 2004 Published: October 13, 2004 References tant embryos. It should be noted that, in Foxg1 Cre mice, Cre was knocked in the Foxg1 locus, and therefore mice expressing Cre are also heterozygous for the Foxg1 gene. To distinguish the effect of the Nrg1 deletion from any possible phenotype present in the absence of one copy of the Foxg1 gene, we systematically used Foxg1 Cre/ as controls in our experiments. Animals were kept at the Instituto de Neurociencias under Spanish and EU regulation. The Ethics Review Committee of the University Miguel Hernández approved all animal protocols that were used in the present study. In Situ Hybridization and Immunohistochemistry Twenty micrometer frozen sections were hybridized with digoxi- genin-labeled probes as described before (Schaeren-Wiemers and Gerfin-Moser, 1993). The following cdna probes were used in this study: ErbB4, Nrg1-CRD, Nrg1-Ig, and Lhx6 (kindly provided by V. Pachnis); Sema3A and Sema3F (kindly provided by M. Tessier- Lavigne); and Gad67 (kindly provided by B. Condie). Nrg1-Ig spans sequence number in accession number NM_031588, one of the rat Nrg1-Ig domain-containing isoforms, whereas Nrg1-CRD spans sequence number in accession number AF194438, a rat Nrg1 SMDF isoform. Double in situ images shown in Figure 1 were composed from adjacent sections using Photoshop software. For immunohistochemistry in postnatal brains, mice were anesthetized and perfused with 4% PFA. Brains were removed, cryoprotected in 30% sucrose in PBS, and cut frozen in the transverse plane on a sliding microtome at 40 m. Free-floating sections were then incubated in 1% BSA and 0.1% TX in PBS for 30 min at room temperature and were subsequently incubated with rabbit anti- GABA antibodies (Sigma) for 36 hr at 4 C in 0.5% BSA and 0.1% TX in PBS. Sections were then incubated in biotinylated secondary antibodies (Vector), diluted 1:200, and processed by the ABC histo- chemical method (Vector). In each experiment, sections from homo- zygous mutants and their wild-type or heterozygous littermates were processed together. For immunohistochemistry in MGE explants, Matrigel pads were fixed overnight in 4% PFA, incubated in 1% BSA and 1% TX in PBS for 30 min at room temperature, and subsequently incubated with primary antibodies (GFP, diluted 1:1000, Molecular Probes; Phospho-Histone H3, diluted 1:200, Upstate) for 36 hr at 4 C in 0.5% BSA and 1% TX in PBS. After washing, Alexa 488 or 595 secondary antibodies (Molecular Probes) diluted 1:200 were used. COS Cells COS7 cell aggregates expressing Gfp, Nrg1-Ig, or both were prepared by diluting transfected cells with Matrigel in a 1:1 proportion. After jellification, COS cell aggregates were cut with a scalpel in small cubes with dimensions of approximately 400 m. In control experiments, a control vector was transfected together with Gfp into COS7 cells. The sequences of the cdnas used for expression of type I NRG1 (Nrg1-Ig) and type III NRG1 (NRG1-CRD) correspond to Nrg1-typeI 1a (accession number AY648976) and Nrg1- typeiii 1a (accession number AY648975), respectively. Bead Experiments Beads were loaded with recombinant NRG1 protein as described before (Martínez et al., 1999). The recombinant NRG1 protein was constructed by expressing the EGF-like domain of NRG-1 as a C-terminal GST fusion protein. Adlkofer, K., and Lai, C. (2000). Role of neuregulins in glial cell Stripe Choice Assay development. Glia 29, COS cells were plated in a two-well chamber slide (Lab-Tek Cham- Anderson, S.A., Eisenstat, D.D., Shi, L., and Rubenstein, J.L.R. ber Slide System Permanox Slide ), transfected at approxi- (1997). Interneuron migration from basal forebrain to neocortex: demately 70% confluence, and incubated overnight. To produce the pendence on Dlx genes. Science 278, stripes, transfected cells were removed with a pipette tip (one line Anderson, S.A., Marín, O., Horn, C., Jennings, K., and Rubenstein, every 2 mm), and nontransfected cells were plated on top ( J.L.R. (2001). Distinct cortical migrations from the medial and lateral cells per well). Slides were subsequently incubated for 30 min to ganglionic eminences. Development 128, allow cells attachment to the empty stripes. The excess of cells was then washed out with three rinses of PBS, new media was added, Anderson, S.A., Kaznowski, C.E., Horn, C., Rubenstein, J.L., and and after 12 hr, GFP-expressing dissociated cells from the MGE McConnell, S.K. (2002). Distinct origins of neocortical projection were plated on top (10 5 cells per well). Analysis was performed after neurons and interneurons in vivo. Cereb. Cortex 12, hr. Ang, E.S., Jr., Haydar, T.F., Gluncic, V., and Rakic, P. (2003). Four-
10 Neuron 260 dimensional migratory coordinates of GABAergic interneurons in the developing mouse cortex. J. Neurosci. 23, Anton, E.S., Marchionni, M.A., Lee, K.F., and Rakic, P. (1997). Role of GGF/neuregulin signaling in interactions between migrating neurons and radial glia in the developing cerebral cortex. Development 124, Benes, F.M., and Berretta, S. (2001). GABAergic interneurons: implications for understanding schizophrenia and bipolar disorder. Neuropsychopharmacology 25, Buonanno, A., and Fischbach, G.D. (2001). Neuregulin and ErbB receptor signaling pathways in the nervous system. Curr. Opin. Neurobiol. 11, Caric, D., Raphael, H., Viti, J., Feathers, A., Wancio, D., and Lillien, L. (2001). EGFRs mediate chemotactic migration in the developing telencephalon. Development 128, Steinthorsdottir, V., Januel, D., Gudnadottir, V.G., Petursson, H., et al. (2004). Identification of a novel neuregulin 1 at-risk haplotype in Han schizophrenia Chinese patients, but no association with the Icelandic/Scottish risk haplotype. Mol. Psychiatry 9, Mahanthappa, N.K., Anton, E.S., and Matthew, W.D. (1996). Glial growth factor 2, a soluble neuregulin, directly increases Schwann cell motility and indirectly promotes neurite outgrowth. J. Neurosci. 16, Marín, O., and Rubenstein, J.L.R. (2001). A long, remarkable journey: tangential migration in the telencephalon. Nat. Rev. Neurosci. 2, Marín, O., and Rubenstein, J.L. (2003). Cell migration in the forebrain. Annu. Rev. Neurosci. 26, Marín, O., Yaron, A., Bagri, A., Tessier-Lavigne, M., and Rubenstein, J.L. (2001). Sorting of striatal and cortical interneurons regulated by semaphorin/neuropilin interactions. Science 293, Marín, O., Plump, A.S., Flames, N., Sanchez-Camacho, C., Tessier- Lavigne, M., and Rubenstein, J.L. (2003). Directional guidance of interneuron migration to the cerebral cortex relies on subcortical Slit1/2-independent repulsion and cortical attraction. Development 130, Martínez, S., Crossley, P.H., Cobos, I., Rubenstein, J.L., and Martin, G.R. (1999). FGF8 induces formation of an ectopic isthmic organizer and isthmocerebellar development via a repressive effect on Otx2 expression. Development 126, Casarosa, S., Fode, C., and Guillemot, F. (1999). Mash1 regulates neurogenesis in the ventral telencephalon. Development 126, Corbin, J.G., Nery, S., and Fishell, G. (2001). Telencephalic cells take a tangent: non-radial migration in the mammalian forebrain. Nat. Neurosci. 4, Corfas, G., Roy, K., and Buxbaum, J.D. (2004). Neuregulin 1-erbB signaling and the molecular/cellular basis of schizophrenia. Nat. Neurosci. 7, Corvin, A.P., Morris, D.W., McGhee, K., Schwaiger, S., Scully, P., Quinn, J., Meagher, D., Clair, D.S., Waddington, J.L., and Gill, M. Mazumdar, A., Adam, L., Boyd, D., and Kumar, R. (2001). Heregulin (2004). Confirmation and refinement of an at-risk haplotype for regulation of urokinase plasminogen activator and its receptor: schizophrenia suggests the EST cluster, Hs.97362, as a potential human breast epithelial cell invasion. Cancer Res. 61, susceptibility gene at the Neuregulin-1 locus. Mol. Psychiatry 9, Meintanis, S., Thomaidou, D., Jessen, K.R., Mirsky, R., and Matsas, R. (2001). The neuron-glia signal beta-neuregulin promotes De Carlos, J.A., López-Mascaraque, L., and Valverde, F. (1996). Schwann cell motility via the MAPK pathway. Glia 34, Dynamics of cell migration from the lateral ganglionic eminence in Meyer, D., and Birchmeier, C. (1995). Multiple essential functions of the rat. J. Neurosci. 16, neuregulin in development. Nature 378, Falls, D.L. (2003). Neuregulins: functions, forms, and signaling strateminence Nery, S., Fishell, G., and Corbin, J.G. (2002). The caudal ganglionic egies. Exp. Cell Res. 284, is a source of distinct cortical and subcortical cell popula- Garratt, A.N., Britsch, S., and Birchmeier, C. (2000). Neuregulin, a tions. Nat. Neurosci. 5, factor with many functions in the life of a Schwann cell. Bioessays Pleasure, S.J., Anderson, S., Hevner, R., Bagri, A., Marín, O., Low- 22, enstein, D.H., and Rubenstein, J.L. (2000). Cell migration from the Gassmann, M., Casagranda, F., Orioli, D., Simon, H., Lai, C., Klein, ganglionic eminences is required for the development of hippocam- R., and Lemke, G. (1995). Aberrant neural and cardiac development pal GABAergic interneurons. Neuron 28, in mice lacking the ErbB4 neuregulin receptor. Nature 378, Polleux, F., Whitford, K.L., Dijkhuizen, P.A., Vitalis, T., and Ghosh, Hadjantonakis, A.K., Gertsenstein, M., Ikawa, M., Okabe, M., and A. (2002). Control of cortical interneuron migration by neurotrophins Nagy, A. (1998). Generating green fluorescent mice by germline and PI3-kinase signaling. Development 129, transmission of green fluorescent ES cells. Mech. Dev. 76, Powell, E.M., Mars, W.M., and Levitt, P. (2001). Hepatocyte growth factor/scatter factor is a motogen for interneurons migrating from Harrison, P.J., and Owen, M.J. (2003). Genes for schizophrenia? the ventral to dorsal telencephalon. Neuron 30, Recent findings and their pathophysiological implications. Lancet 361, Rio, C., Rieff, H.I., Qi, P., Khurana, T.S., and Corfas, G. (1997). Neuregulin and erbb receptors play a critical role in neuronal migration. Hebert, J.M., and McConnell, S.K. (2000). Targeting of cre to the Neuron 19, Foxg1 (BF-1) locus mediates loxp recombination in the telencephalon and other developing head structures. Dev. Biol. 222, Schaeren-Wiemers, N., and Gerfin-Moser, A. (1993). A single proto- col to detect transcripts of various types and expression levels in Jiménez, D., Lopez-Mascaraque, L.M., Valverde, F., and De Carlos, neural tissue and cultured cells: in situ hybridization using digoxi- J.A. (2002). Tangential migration in neocortical development. Dev. genin-labelled crna probes. Histochemistry 100, Biol. 244, Schmid, R.S., McGrath, B., Berechid, B.E., Boyles, B., Marchionni, Jones, F.E., Welte, T., Fu, X.Y., and Stern, D.F. (1999). ErbB4 signal- M., Sestan, N., and Anton, E.S. (2003). Neuregulin 1-erbB2 signaling ing in the mammary gland is required for lobuloalveolar development is required for the establishment of radial glia and their transformaand Stat5 activation during lactation. J. Cell Biol. 147, tion into astrocytes in cerebral cortex. Proc. Natl. Acad. Sci. USA Keverne, E.B. (1999). GABA-ergic neurons and the neurobiology of 100, schizophrenia and other psychoses. Brain Res. Bull. 48, Stefansson, H., Sigurdsson, E., Steinthorsdottir, V., Bjornsdottir, S., Lavdas, A.A., Grigoriou, M., Pachnis, V., and Parnavelas, J.G. (1999). The medial ganglionic eminence gives rise to a population of early neurons in the developing cerebral cortex. J. Neurosci. 19, 7881 to schizophrenia. Am. J. Hum. Genet. 71, Sigmundsson, T., Ghosh, S., Brynjolfsson, J., Gunnarsdottir, S., Ivarsson, O., Chou, T.T., et al. (2002). Neuregulin 1 and susceptibility Stefansson, H., Sarginson, J., Kong, A., Yates, P., Steinthorsdottir, Lewis, D.A. (2000). GABAergic local circuit neurons and prefrontal V., Gudfinnsson, E., Gunnarsdottir, S., Walker, N., Petursson, H., cortical dysfunction in schizophrenia. Brain Res. Brain Res. Rev. Crombie, C., et al. (2003). Association of neuregulin 1 with schizo- 31, phrenia confirmed in a Scottish population. Am. J. Hum. Genet. Lewis, D.A., and Levitt, P. (2002). Schizophrenia as a disorder of 72, neurodevelopment. Annu. Rev. Neurosci. 25, Li, T., Stefansson, H., Gudfinnsson, E., Cai, G., Liu, X., Murray, R.M., Sussel, L., Marín, O., Kimura, S., and Rubenstein, J.L. (1999). Loss of Nkx2.1 homeobox gene function results in a ventral to dorsal
ErbB4 migrazione II parte
 ErbB4 migrazione II parte Control SVZ cells prefer to migrate on the NRG1 type III substrate the substrate preference of the neuroblasts migrating out of the SVZ explant was evaluated SVZ cells had a strong
ErbB4 migrazione II parte Control SVZ cells prefer to migrate on the NRG1 type III substrate the substrate preference of the neuroblasts migrating out of the SVZ explant was evaluated SVZ cells had a strong
During Brain Development Final Destinations for Neurons and Glia Get Separated from Germinal Niches
 During Brain Development Final Destinations for Neurons and Glia Get Separated from Germinal Niches Progenitors are Contained within Unique Domains and Tangentially Fixed. EMBRYO ADULT Migratory Behavior
During Brain Development Final Destinations for Neurons and Glia Get Separated from Germinal Niches Progenitors are Contained within Unique Domains and Tangentially Fixed. EMBRYO ADULT Migratory Behavior
ErbB4 migrazione I parte. 3- ErbB4- NRG1
 ErbB4 migrazione I parte 3- ErbB4- NRG1 1 In rodent brains postnatal neuronal migration is evident in three main areas: the cerebellum (CB), the hippocampus (Hipp) and the rostral migratory stream (RMS).
ErbB4 migrazione I parte 3- ErbB4- NRG1 1 In rodent brains postnatal neuronal migration is evident in three main areas: the cerebellum (CB), the hippocampus (Hipp) and the rostral migratory stream (RMS).
Cell Migration II: CNS Cell Migration. Steven McLoon Department of Neuroscience University of Minnesota
 Cell Migration II: CNS Cell Migration Steven McLoon Department of Neuroscience University of Minnesota 1 Hey! The major concepts discussed relative to neural crest cell migration apply to cell migration
Cell Migration II: CNS Cell Migration Steven McLoon Department of Neuroscience University of Minnesota 1 Hey! The major concepts discussed relative to neural crest cell migration apply to cell migration
During Brain Development Final Destinations for Neurons and Glia Get Separated from Germinal Niches
 During Brain Development Final Destinations for Neurons and Glia Get Separated from Germinal Niches Two mayor forms of neuronal migration: Radial and Tangential Leber & Sanes, 95 How do young neurons actually
During Brain Development Final Destinations for Neurons and Glia Get Separated from Germinal Niches Two mayor forms of neuronal migration: Radial and Tangential Leber & Sanes, 95 How do young neurons actually
Cell Migration II: CNS Cell Migration. Steven McLoon Department of Neuroscience University of Minnesota
 Cell Migration II: CNS Cell Migration Steven McLoon Department of Neuroscience University of Minnesota 1 Course News Coffee Hour Wednesday (Oct 18) 9:00-10:00am Surdyk s Café in Northrop Auditorium Stop
Cell Migration II: CNS Cell Migration Steven McLoon Department of Neuroscience University of Minnesota 1 Course News Coffee Hour Wednesday (Oct 18) 9:00-10:00am Surdyk s Café in Northrop Auditorium Stop
Emx2 patterns the neocortex by regulating FGF positional signaling
 Emx2 patterns the neocortex by regulating FGF positional signaling Tomomi Fukuchi-Shimogori and Elizabeth A Grove Presented by Sally Kwok Background Cerebral cortex has anatomically and functionally distinct
Emx2 patterns the neocortex by regulating FGF positional signaling Tomomi Fukuchi-Shimogori and Elizabeth A Grove Presented by Sally Kwok Background Cerebral cortex has anatomically and functionally distinct
Young neurons born in the developing or adult brain have to
 Permissive corridor and diffusible gradients direct medial ganglionic eminence cell migration to the neocortex Hynek Wichterle*, Manuel Alvarez-Dolado, Lynda Erskine, and Arturo Alvarez-Buylla *The Rockefeller
Permissive corridor and diffusible gradients direct medial ganglionic eminence cell migration to the neocortex Hynek Wichterle*, Manuel Alvarez-Dolado, Lynda Erskine, and Arturo Alvarez-Buylla *The Rockefeller
Development and specification of GABAergic cortical interneurons
 Kelsom and Lu Cell & Bioscience 2013, 3:19 Cell & Bioscience REVIEW Development and specification of GABAergic cortical interneurons Corey Kelsom and Wange Lu * Open Access Abstract GABAergic interneurons
Kelsom and Lu Cell & Bioscience 2013, 3:19 Cell & Bioscience REVIEW Development and specification of GABAergic cortical interneurons Corey Kelsom and Wange Lu * Open Access Abstract GABAergic interneurons
Receptor tyrosine kinase ErbB4 modulates neuroblast migration and placement in the adult forebrain
 Receptor tyrosine kinase ErbB4 modulates neuroblast migration and placement in the adult forebrain E S Anton 1,8, H T Ghashghaei 1,8, Janet L Weber 2, Corey McCann 1, Tobias M Fischer 2, Isla D Cheung
Receptor tyrosine kinase ErbB4 modulates neuroblast migration and placement in the adult forebrain E S Anton 1,8, H T Ghashghaei 1,8, Janet L Weber 2, Corey McCann 1, Tobias M Fischer 2, Isla D Cheung
Prss56, a novel marker of adult neurogenesis in the mouse brain. - Supplemental Figures 1 to 5- Brain Structure and Function
 Prss56, a novel marker of adult neurogenesis in the mouse brain - Supplemental Figures 1 to 5- Brain Structure and Function Alexandre Jourdon 1,2, Aurélie Gresset 1, Nathalie Spassky 1, Patrick Charnay
Prss56, a novel marker of adult neurogenesis in the mouse brain - Supplemental Figures 1 to 5- Brain Structure and Function Alexandre Jourdon 1,2, Aurélie Gresset 1, Nathalie Spassky 1, Patrick Charnay
Distinct Origins of Neocortical Projection Neurons and Interneurons In Vivo
 Distinct Origins of Neocortical Projection Neurons and Interneurons In Vivo Stewart A. Anderson 2, Christine E. Kaznowski 1, Carrie Horn, John L.R. Rubenstein and Susan K. McConnell 1 Department of Psychiatry,
Distinct Origins of Neocortical Projection Neurons and Interneurons In Vivo Stewart A. Anderson 2, Christine E. Kaznowski 1, Carrie Horn, John L.R. Rubenstein and Susan K. McConnell 1 Department of Psychiatry,
The neuron. Biocytin labeled pyramidal neuron recorded in piriform cortex
 The neuron Biocytin labeled pyramidal neuron recorded in piriform cortex Discovery of the neuron (A) Reticularist Doctrine (B) Neuron Doctrine Exception.GAP JUNCTIONS between neurons Neuronal shape Dendrites
The neuron Biocytin labeled pyramidal neuron recorded in piriform cortex Discovery of the neuron (A) Reticularist Doctrine (B) Neuron Doctrine Exception.GAP JUNCTIONS between neurons Neuronal shape Dendrites
Supplementary Figures
 Supplementary Figures Supplementary Figure 1. nrg1 bns101/bns101 embryos develop a functional heart and survive to adulthood (a-b) Cartoon of Talen-induced nrg1 mutation with a 14-base-pair deletion in
Supplementary Figures Supplementary Figure 1. nrg1 bns101/bns101 embryos develop a functional heart and survive to adulthood (a-b) Cartoon of Talen-induced nrg1 mutation with a 14-base-pair deletion in
Telencephalic cells take a tangent: non-radial migration in the mammalian forebrain
 review Telencephalic cells take a tangent: non-radial migration in the mammalian forebrain Joshua G. Corbin, Susana Nery and Gord Fishell Developmental Genetics Program and the Department of Cell Biology,
review Telencephalic cells take a tangent: non-radial migration in the mammalian forebrain Joshua G. Corbin, Susana Nery and Gord Fishell Developmental Genetics Program and the Department of Cell Biology,
Neurodevelopment II Structure Formation. Reading: BCP Chapter 23
 Neurodevelopment II Structure Formation Reading: BCP Chapter 23 Phases of Development Ovum + Sperm = Zygote Cell division (multiplication) Neurogenesis Induction of the neural plate Neural proliferation
Neurodevelopment II Structure Formation Reading: BCP Chapter 23 Phases of Development Ovum + Sperm = Zygote Cell division (multiplication) Neurogenesis Induction of the neural plate Neural proliferation
Genesis of cerebellar interneurons and the prevention of neural DNA damage require XRCC1.
 Genesis of cerebellar interneurons and the prevention of neural DNA damage require XRCC1. Youngsoo Lee, Sachin Katyal, Yang Li, Sherif F. El-Khamisy, Helen R. Russell, Keith W. Caldecott and Peter J. McKinnon.
Genesis of cerebellar interneurons and the prevention of neural DNA damage require XRCC1. Youngsoo Lee, Sachin Katyal, Yang Li, Sherif F. El-Khamisy, Helen R. Russell, Keith W. Caldecott and Peter J. McKinnon.
Zhu et al, page 1. Supplementary Figures
 Zhu et al, page 1 Supplementary Figures Supplementary Figure 1: Visual behavior and avoidance behavioral response in EPM trials. (a) Measures of visual behavior that performed the light avoidance behavior
Zhu et al, page 1 Supplementary Figures Supplementary Figure 1: Visual behavior and avoidance behavioral response in EPM trials. (a) Measures of visual behavior that performed the light avoidance behavior
Supplementary Figure 1
 Supplementary Figure 1 Kif1a RNAi effect on basal progenitor differentiation Related to Figure 2. Representative confocal images of the VZ and SVZ of rat cortices transfected at E16 with scrambled or Kif1a
Supplementary Figure 1 Kif1a RNAi effect on basal progenitor differentiation Related to Figure 2. Representative confocal images of the VZ and SVZ of rat cortices transfected at E16 with scrambled or Kif1a
Primary Mouse Cerebral Cortex Neurons V: 80% TE: 70%
 Primary Mouse Cerebral Cortex Neurons V: 80% TE: 70% Pictures: 9 days after electroporation Red: MAP2 Blue: GFAP Green: GFP The cells were from Embryonic Day 14 Mouse Cerebral Cortex Primary Mouse Hippocampal
Primary Mouse Cerebral Cortex Neurons V: 80% TE: 70% Pictures: 9 days after electroporation Red: MAP2 Blue: GFAP Green: GFP The cells were from Embryonic Day 14 Mouse Cerebral Cortex Primary Mouse Hippocampal
Supplemental Information. Otic Mesenchyme Cells Regulate. Spiral Ganglion Axon Fasciculation. through a Pou3f4/EphA4 Signaling Pathway
 Neuron, Volume 73 Supplemental Information Otic Mesenchyme Cells Regulate Spiral Ganglion Axon Fasciculation through a Pou3f4/EphA4 Signaling Pathway Thomas M. Coate, Steven Raft, Xiumei Zhao, Aimee K.
Neuron, Volume 73 Supplemental Information Otic Mesenchyme Cells Regulate Spiral Ganglion Axon Fasciculation through a Pou3f4/EphA4 Signaling Pathway Thomas M. Coate, Steven Raft, Xiumei Zhao, Aimee K.
The neuron. Biocytin labeled pyramidal neuron recorded in piriform cortex
 The neuron Biocytin labeled pyramidal neuron recorded in piriform cortex Discovery of the neuron (A) Reticularist Doctrine (B) Neuron Doctrine Exception.GAP JUNCTIONS between neurons Neuronal shape Dendrites
The neuron Biocytin labeled pyramidal neuron recorded in piriform cortex Discovery of the neuron (A) Reticularist Doctrine (B) Neuron Doctrine Exception.GAP JUNCTIONS between neurons Neuronal shape Dendrites
Origin and Molecular Specification of Striatal Interneurons
 The Journal of Neuroscience, August 15, 2000, 20(16):6063 6076 Origin and Molecular Specification of Striatal Interneurons Oscar Marín, Stewart A. Anderson, and John L. R. Rubenstein Department of Psychiatry,
The Journal of Neuroscience, August 15, 2000, 20(16):6063 6076 Origin and Molecular Specification of Striatal Interneurons Oscar Marín, Stewart A. Anderson, and John L. R. Rubenstein Department of Psychiatry,
Brain Development III
 Brain Development III Neural Development In the developing nervous system there must be: 1. The formation of different regions of the brain. 2. The ability of a neuron to differentiate. 3. The ability
Brain Development III Neural Development In the developing nervous system there must be: 1. The formation of different regions of the brain. 2. The ability of a neuron to differentiate. 3. The ability
4/18/2011. Physiology 67 Lecture on Neural Development
 Physiology 67 Lecture on Neural Development 1 2 3 4 5 6 Neural cell categories After the ectodermal tissue has folded into the neural tube, another series of signaling interactions determine the type of
Physiology 67 Lecture on Neural Development 1 2 3 4 5 6 Neural cell categories After the ectodermal tissue has folded into the neural tube, another series of signaling interactions determine the type of
glial cells missing and gcm2 Cell-autonomously Regulate Both Glial and Neuronal
 glial cells missing and gcm2 Cell-autonomously Regulate Both Glial and Neuronal Development in the Visual System of Drosophila Carole Chotard, Wendy Leung and Iris Salecker Supplemental Data Supplemental
glial cells missing and gcm2 Cell-autonomously Regulate Both Glial and Neuronal Development in the Visual System of Drosophila Carole Chotard, Wendy Leung and Iris Salecker Supplemental Data Supplemental
Carl P. Wonders* and Stewart A. Anderson
 The origin and specification of cortical interneurons Carl P. Wonders* and Stewart A. Anderson Abstract GABA-containing interneurons are crucial to both the development and function of the cerebral cortex.
The origin and specification of cortical interneurons Carl P. Wonders* and Stewart A. Anderson Abstract GABA-containing interneurons are crucial to both the development and function of the cerebral cortex.
Nature Neuroscience: doi: /nn Supplementary Figure 1. MADM labeling of thalamic clones.
 Supplementary Figure 1 MADM labeling of thalamic clones. (a) Confocal images of an E12 Nestin-CreERT2;Ai9-tdTomato brain treated with TM at E10 and stained for BLBP (green), a radial glial progenitor-specific
Supplementary Figure 1 MADM labeling of thalamic clones. (a) Confocal images of an E12 Nestin-CreERT2;Ai9-tdTomato brain treated with TM at E10 and stained for BLBP (green), a radial glial progenitor-specific
Cell Birth and Death. Chapter Three
 Cell Birth and Death Chapter Three Neurogenesis All neurons and glial cells begin in the neural tube Differentiated into neurons rather than ectoderm based on factors we have already discussed If these
Cell Birth and Death Chapter Three Neurogenesis All neurons and glial cells begin in the neural tube Differentiated into neurons rather than ectoderm based on factors we have already discussed If these
Supplementary Figure S1: Pial and periventricular vascular networks and the dual GABA neuron streams of the early embryonic mouse telencephalon
 Supplementary Figure S1: Pial and periventricular vascular networks and the dual GABA neuron streams of the early embryonic mouse telencephalon a) The profile of GABA neurons arriving at the pallial-subpallial
Supplementary Figure S1: Pial and periventricular vascular networks and the dual GABA neuron streams of the early embryonic mouse telencephalon a) The profile of GABA neurons arriving at the pallial-subpallial
Plasticity of Cerebral Cortex in Development
 Plasticity of Cerebral Cortex in Development Jessica R. Newton and Mriganka Sur Department of Brain & Cognitive Sciences Picower Center for Learning & Memory Massachusetts Institute of Technology Cambridge,
Plasticity of Cerebral Cortex in Development Jessica R. Newton and Mriganka Sur Department of Brain & Cognitive Sciences Picower Center for Learning & Memory Massachusetts Institute of Technology Cambridge,
Preferential Origin and Layer Destination of GAD65-GFP Cortical Interneurons
 Preferential Origin and Layer Destination of GAD65-GFP Cortical Interneurons Guillermina López-Bendito 1, Katherine Sturgess 1, Ferenc Erdélyi 2, Gábor Szabó 2, Zoltán Molnár 1 and Ole Paulsen 3 1 Department
Preferential Origin and Layer Destination of GAD65-GFP Cortical Interneurons Guillermina López-Bendito 1, Katherine Sturgess 1, Ferenc Erdélyi 2, Gábor Szabó 2, Zoltán Molnár 1 and Ole Paulsen 3 1 Department
Embryonic MGE Cells as a Treatment for Epilepsy December 1, 2012
 Embryonic MGE Cells as a Treatment for Epilepsy December 1, 2012 Scott C. Baraban, PhD University of California, San Francisco American Epilepsy Society Annual Meeting Disclosure Name of Commercial Interest
Embryonic MGE Cells as a Treatment for Epilepsy December 1, 2012 Scott C. Baraban, PhD University of California, San Francisco American Epilepsy Society Annual Meeting Disclosure Name of Commercial Interest
Supplemental Information. Induction of Expansion and Folding. in Human Cerebral Organoids
 Cell Stem Cell, Volume 20 Supplemental Information Induction of Expansion and Folding in Human Cerebral Organoids Yun Li, Julien Muffat, Attya Omer, Irene Bosch, Madeline A. Lancaster, Mriganka Sur, Lee
Cell Stem Cell, Volume 20 Supplemental Information Induction of Expansion and Folding in Human Cerebral Organoids Yun Li, Julien Muffat, Attya Omer, Irene Bosch, Madeline A. Lancaster, Mriganka Sur, Lee
Department of Cognitive Science UCSD
 Department of Cognitive Science UCSD Verse 1: Neocortex, frontal lobe, Brain stem, brain stem, Hippocampus, neural node, Right hemisphere, Pons and cortex visual, Brain stem, brain stem, Sylvian fissure,
Department of Cognitive Science UCSD Verse 1: Neocortex, frontal lobe, Brain stem, brain stem, Hippocampus, neural node, Right hemisphere, Pons and cortex visual, Brain stem, brain stem, Sylvian fissure,
Broad Integration of Expression Maps and Co-Expression Networks Compassing Novel Gene Functions in the Brain
 Supplementary Information Broad Integration of Expression Maps and Co-Expression Networks Compassing Novel Gene Functions in the Brain Yuko Okamura-Oho a, b, *, Kazuro Shimokawa c, Masaomi Nishimura b,
Supplementary Information Broad Integration of Expression Maps and Co-Expression Networks Compassing Novel Gene Functions in the Brain Yuko Okamura-Oho a, b, *, Kazuro Shimokawa c, Masaomi Nishimura b,
Development of the Nervous System. Leah Militello, class of 2018
 Development of the Nervous System Leah Militello, class of 2018 Learning Objectives 1. Describe the formation and fate of the neural tube and neural crest including timing and germ layer involved. 2. Describe
Development of the Nervous System Leah Militello, class of 2018 Learning Objectives 1. Describe the formation and fate of the neural tube and neural crest including timing and germ layer involved. 2. Describe
CXCR4 and CXCR7 Have Distinct Functions in Regulating Interneuron Migration
 Article CXCR4 and CXCR7 Have Distinct Functions in Regulating Interneuron Migration Yanling Wang, 1, * Guangnan Li, 2,5 Amelia Stanco, 1,5 Jason E. Long, 3 Dianna Crawford, 4 Gregory B. Potter, 1 Samuel
Article CXCR4 and CXCR7 Have Distinct Functions in Regulating Interneuron Migration Yanling Wang, 1, * Guangnan Li, 2,5 Amelia Stanco, 1,5 Jason E. Long, 3 Dianna Crawford, 4 Gregory B. Potter, 1 Samuel
Multidirectional and multizonal tangential migration of GABAergic interneurons in the developing cerebral cortex
 RESEARCH ARTICLE 2167 Development 133, 2167-2176 (2006) doi:10.1242/dev.02382 Multidirectional and multizonal tangential migration of GABAergic interneurons in the developing cerebral cortex Daisuke H.
RESEARCH ARTICLE 2167 Development 133, 2167-2176 (2006) doi:10.1242/dev.02382 Multidirectional and multizonal tangential migration of GABAergic interneurons in the developing cerebral cortex Daisuke H.
Lhx6 and Lhx8 Coordinately Induce Neuronal Expression of Shh that Controls the Generation of Interneuron Progenitors
 Article Lhx6 and Lhx8 Coordinately Induce Neuronal Expression of Shh that Controls the Generation of Interneuron Progenitors Pierre Flandin, 1 Yangu Zhao, 2 Daniel Vogt, 1 Juhee Jeong, 1 Jason Long, 1,3
Article Lhx6 and Lhx8 Coordinately Induce Neuronal Expression of Shh that Controls the Generation of Interneuron Progenitors Pierre Flandin, 1 Yangu Zhao, 2 Daniel Vogt, 1 Juhee Jeong, 1 Jason Long, 1,3
Shh signaling guides spatial pathfinding of raphespinal tract axons by multidirectional repulsion
 ORIGINAL ARTICLE Cell Research (2012) 22:697-716. 2012 IBCB, SIBS, CAS All rights reserved 1001-0602/12 $ 32.00 www.nature.com/cr npg Shh signaling guides spatial pathfinding of raphespinal tract axons
ORIGINAL ARTICLE Cell Research (2012) 22:697-716. 2012 IBCB, SIBS, CAS All rights reserved 1001-0602/12 $ 32.00 www.nature.com/cr npg Shh signaling guides spatial pathfinding of raphespinal tract axons
SUPPLEMENTARY INFORMATION
 SUPPLEMENTARY INFORMATION doi:10.1038/nature11306 Supplementary Figures Supplementary Figure 1. Basic characterization of GFP+ RGLs in the dentate gyrus of adult nestin-gfp mice. a, Sample confocal images
SUPPLEMENTARY INFORMATION doi:10.1038/nature11306 Supplementary Figures Supplementary Figure 1. Basic characterization of GFP+ RGLs in the dentate gyrus of adult nestin-gfp mice. a, Sample confocal images
Dlx1&2-Dependent Expression of Zfhx1b (Sip1, Zeb2) Regulates the Fate Switch between Cortical and Striatal Interneurons
 Article Dlx1&2-Dependent Expression of Zfhx1b (Sip1, Zeb2) Regulates the Fate Switch between Cortical and Striatal Interneurons Gabriel L. McKinsey, 1,2 Susan Lindtner, 1 Brett Trzcinski, 3 Axel Visel,
Article Dlx1&2-Dependent Expression of Zfhx1b (Sip1, Zeb2) Regulates the Fate Switch between Cortical and Striatal Interneurons Gabriel L. McKinsey, 1,2 Susan Lindtner, 1 Brett Trzcinski, 3 Axel Visel,
NIH Public Access Author Manuscript Science. Author manuscript; available in PMC 2011 September 1.
 NIH Public Access Author Manuscript Published in final edited form as: Science. 2010 February 26; 327(5969): 1145 1148. doi:10.1126/science.1183962. Cortical Plasticity Induced by Inhibitory Neuron Transplantation
NIH Public Access Author Manuscript Published in final edited form as: Science. 2010 February 26; 327(5969): 1145 1148. doi:10.1126/science.1183962. Cortical Plasticity Induced by Inhibitory Neuron Transplantation
Nature Neuroscience: doi: /nn Supplementary Figure 1. Splenic atrophy and leucopenia caused by T3 SCI.
 Supplementary Figure 1 Splenic atrophy and leucopenia caused by T3 SCI. (a) Gross anatomy of representative spleens from control and T3 SCI mice at 28 days post-injury. (b and c) Hematoxylin and eosin
Supplementary Figure 1 Splenic atrophy and leucopenia caused by T3 SCI. (a) Gross anatomy of representative spleens from control and T3 SCI mice at 28 days post-injury. (b and c) Hematoxylin and eosin
Supplementary information. Nkx2.1 regulates the generation of telencephalic astrocytes during embryonic
 Supplementary information Nkx2.1 regulates the generation of telencephalic astrocytes during embryonic development Shilpi Minocha 1*, Delphine Valloton 1*, Yvan Arsenijevic 2, Jean-René Cardinaux 3, Raffaella
Supplementary information Nkx2.1 regulates the generation of telencephalic astrocytes during embryonic development Shilpi Minocha 1*, Delphine Valloton 1*, Yvan Arsenijevic 2, Jean-René Cardinaux 3, Raffaella
EGFRs mediate chemotactic migration in the developing telencephalon
 Development 128, 4203-4216 (2001) Printed in Great Britain The Company of Biologists Limited 2001 DEV9789 4203 EGFRs mediate chemotactic migration in the developing telencephalon Damira Caric, Heather
Development 128, 4203-4216 (2001) Printed in Great Britain The Company of Biologists Limited 2001 DEV9789 4203 EGFRs mediate chemotactic migration in the developing telencephalon Damira Caric, Heather
The Zinc Finger Transcription Factor Sp8 Regulates the Generation and Diversity of Olfactory Bulb Interneurons
 Neuron 49, 503 516, February 16, 2006 ª2006 Elsevier Inc. DOI 10.1016/j.neuron.2006.01.018 The Zinc Finger Transcription Factor Sp8 Regulates the Generation and Diversity of Olfactory Bulb Interneurons
Neuron 49, 503 516, February 16, 2006 ª2006 Elsevier Inc. DOI 10.1016/j.neuron.2006.01.018 The Zinc Finger Transcription Factor Sp8 Regulates the Generation and Diversity of Olfactory Bulb Interneurons
Synapse Formation. Steven McLoon Department of Neuroscience University of Minnesota
 Synapse Formation Steven McLoon Department of Neuroscience University of Minnesota 1 Course News Midterm Exam Monday, Nov 13 9:30-11:30am Bring a #2 pencil!! 2 Course News Lecture schedule: Mon (Oct 31)
Synapse Formation Steven McLoon Department of Neuroscience University of Minnesota 1 Course News Midterm Exam Monday, Nov 13 9:30-11:30am Bring a #2 pencil!! 2 Course News Lecture schedule: Mon (Oct 31)
Supplementary Figure 1. Electroporation of a stable form of β-catenin causes masses protruding into the IV ventricle. HH12 chicken embryos were
 Supplementary Figure 1. Electroporation of a stable form of β-catenin causes masses protruding into the IV ventricle. HH12 chicken embryos were electroporated with β- Catenin S33Y in PiggyBac expression
Supplementary Figure 1. Electroporation of a stable form of β-catenin causes masses protruding into the IV ventricle. HH12 chicken embryos were electroporated with β- Catenin S33Y in PiggyBac expression
Development of the Nervous System 1 st month
 Development of the Nervous System 1 st month day 1 - fertilization of egg day 6 - uterine implantation day 18 - trilaminar (3-layered) disc (blastoderm, embryo) ectoderm (dorsal) - nervous system and skin
Development of the Nervous System 1 st month day 1 - fertilization of egg day 6 - uterine implantation day 18 - trilaminar (3-layered) disc (blastoderm, embryo) ectoderm (dorsal) - nervous system and skin
Journal of Cell Science. Research Article 2797
 Research Article 2797 MET signaling in GABAergic neuronal precursors of the medial ganglionic eminence restricts GDNF activity in cells that express GFR 1 and a new transmembrane receptor partner Maurice
Research Article 2797 MET signaling in GABAergic neuronal precursors of the medial ganglionic eminence restricts GDNF activity in cells that express GFR 1 and a new transmembrane receptor partner Maurice
Evidence of Common Progenitors and Patterns of Dispersion in Rat Striatum and Cerebral Cortex
 The Journal of Neuroscience, May 15, 2002, 22(10):4002 4014 Evidence of Common Progenitors and Patterns of Dispersion in Rat Striatum and Cerebral Cortex Christopher B. Reid 1,2 and Christopher A. Walsh
The Journal of Neuroscience, May 15, 2002, 22(10):4002 4014 Evidence of Common Progenitors and Patterns of Dispersion in Rat Striatum and Cerebral Cortex Christopher B. Reid 1,2 and Christopher A. Walsh
Investigating the role of EphAl ephrin-a signalling during trigeminal ganglion axon guidance
 Investigating the role of EphAl ephrin-a signalling during trigeminal ganglion axon guidance A thesis submitted for the degree of Doctor of Philosophy Molecular and Biomedical Science (Discipline of Genetics),
Investigating the role of EphAl ephrin-a signalling during trigeminal ganglion axon guidance A thesis submitted for the degree of Doctor of Philosophy Molecular and Biomedical Science (Discipline of Genetics),
SUPPLEMENTARY INFORMATION
 DOI: 10.1038/ncb2566 Figure S1 CDKL5 protein expression pattern and localization in mouse brain. (a) Multiple-tissue western blot from a postnatal day (P) 21 mouse probed with an antibody against CDKL5.
DOI: 10.1038/ncb2566 Figure S1 CDKL5 protein expression pattern and localization in mouse brain. (a) Multiple-tissue western blot from a postnatal day (P) 21 mouse probed with an antibody against CDKL5.
Radial Glial Dependent and Independent Dynamics of Interneuronal Migration in the Developing Cerebral Cortex
 Radial Glial Dependent and Independent Dynamics of Interneuronal Migration in the Developing Cerebral Cortex Yukako Yokota 1, H. T. Ghashghaei 1, Christine Han 1, Hannah Watson 1, Kenneth J. Campbell 2,
Radial Glial Dependent and Independent Dynamics of Interneuronal Migration in the Developing Cerebral Cortex Yukako Yokota 1, H. T. Ghashghaei 1, Christine Han 1, Hannah Watson 1, Kenneth J. Campbell 2,
mir-7a regulation of Pax6 in neural stem cells controls the spatial origin of forebrain dopaminergic neurons
 Supplemental Material mir-7a regulation of Pax6 in neural stem cells controls the spatial origin of forebrain dopaminergic neurons Antoine de Chevigny, Nathalie Coré, Philipp Follert, Marion Gaudin, Pascal
Supplemental Material mir-7a regulation of Pax6 in neural stem cells controls the spatial origin of forebrain dopaminergic neurons Antoine de Chevigny, Nathalie Coré, Philipp Follert, Marion Gaudin, Pascal
SUPPLEMENTARY INFORMATION
 doi:10.1038/nature10495 WWW.NATURE.COM/NATURE 1 2 WWW.NATURE.COM/NATURE WWW.NATURE.COM/NATURE 3 4 WWW.NATURE.COM/NATURE WWW.NATURE.COM/NATURE 5 6 WWW.NATURE.COM/NATURE WWW.NATURE.COM/NATURE 7 8 WWW.NATURE.COM/NATURE
doi:10.1038/nature10495 WWW.NATURE.COM/NATURE 1 2 WWW.NATURE.COM/NATURE WWW.NATURE.COM/NATURE 3 4 WWW.NATURE.COM/NATURE WWW.NATURE.COM/NATURE 5 6 WWW.NATURE.COM/NATURE WWW.NATURE.COM/NATURE 7 8 WWW.NATURE.COM/NATURE
Are Both Embryonic Migratory Pathways Preserved in the Adult Brain Cerebral Cortex?
 Prague Medical Report / Vol. 107 (2006) No. 1, p. 71 80 71) Are Both Embryonic Migratory Pathways Preserved in the Adult Brain Cerebral Cortex? Šimonová Z., Dutt J. Department of Neuroscience of the Institute
Prague Medical Report / Vol. 107 (2006) No. 1, p. 71 80 71) Are Both Embryonic Migratory Pathways Preserved in the Adult Brain Cerebral Cortex? Šimonová Z., Dutt J. Department of Neuroscience of the Institute
Contralateral migration of oculomotor neurons is regulated by Slit/Robo signaling
 Bjorke et al. Neural Development (2016) 11:18 DOI 10.1186/s13064-016-0073-y RESEARCH ARTICLE Open Access Contralateral migration of oculomotor neurons is regulated by Slit/Robo signaling Brielle Bjorke
Bjorke et al. Neural Development (2016) 11:18 DOI 10.1186/s13064-016-0073-y RESEARCH ARTICLE Open Access Contralateral migration of oculomotor neurons is regulated by Slit/Robo signaling Brielle Bjorke
Figure S1. Generation of inducible PTEN deficient mice and the BMMCs (A) B6.129 Pten loxp/loxp mice were mated with B6.
 Figure S1. Generation of inducible PTEN deficient mice and the BMMCs (A) B6.129 Pten loxp/loxp mice were mated with B6.129-Gt(ROSA)26Sor tm1(cre/ert2)tyj /J mice. To induce deletion of the Pten locus,
Figure S1. Generation of inducible PTEN deficient mice and the BMMCs (A) B6.129 Pten loxp/loxp mice were mated with B6.129-Gt(ROSA)26Sor tm1(cre/ert2)tyj /J mice. To induce deletion of the Pten locus,
Regional Distribution of Cortical Interneurons and Development of Inhibitory Tone Are Regulated by Cxcl12/ Cxcr4 Signaling
 The Journal of Neuroscience, January 30, 2008 28(5):1085 1098 1085 Development/Plasticity/Repair Regional Distribution of Cortical Interneurons and Development of Inhibitory Tone Are Regulated by Cxcl12/
The Journal of Neuroscience, January 30, 2008 28(5):1085 1098 1085 Development/Plasticity/Repair Regional Distribution of Cortical Interneurons and Development of Inhibitory Tone Are Regulated by Cxcl12/
CNTF/LIF/gp130 receptor complex signaling maintains a VZ precursor differentiation gradient in the developing ventral forebrain
 Research article 565 CNTF/LIF/gp130 receptor complex signaling maintains a VZ precursor differentiation gradient in the developing ventral forebrain Christopher Gregg and Samuel Weiss* Genes and Research
Research article 565 CNTF/LIF/gp130 receptor complex signaling maintains a VZ precursor differentiation gradient in the developing ventral forebrain Christopher Gregg and Samuel Weiss* Genes and Research
Department of Cell and Developmental Biology, Vanderbilt University, Nashville, TN
 UNC-6/Netrin mediates dendritic self-avoidance Cody J. Smith 1, Joseph D. Watson 1,4,5, Miri K. VanHoven 2, Daniel A. Colón-Ramos 3 and David M. Miller III 1,4,6 1 Department of Cell and Developmental
UNC-6/Netrin mediates dendritic self-avoidance Cody J. Smith 1, Joseph D. Watson 1,4,5, Miri K. VanHoven 2, Daniel A. Colón-Ramos 3 and David M. Miller III 1,4,6 1 Department of Cell and Developmental
Supplementary Figure S1. Monolayer differentiation of mouse ESCs into telencephalic neural precursors. (a) Schematic representation of the protocols
 Supplementary Figure S1. Monolayer differentiation of mouse ESCs into telencephalic neural precursors. (a) Schematic representation of the protocols used to differentiate mouse ESCs. (b) Representative
Supplementary Figure S1. Monolayer differentiation of mouse ESCs into telencephalic neural precursors. (a) Schematic representation of the protocols used to differentiate mouse ESCs. (b) Representative
Supporting Online Material for
 www.sciencemag.org/cgi/content/full/1171320/dc1 Supporting Online Material for A Frazzled/DCC-Dependent Transcriptional Switch Regulates Midline Axon Guidance Long Yang, David S. Garbe, Greg J. Bashaw*
www.sciencemag.org/cgi/content/full/1171320/dc1 Supporting Online Material for A Frazzled/DCC-Dependent Transcriptional Switch Regulates Midline Axon Guidance Long Yang, David S. Garbe, Greg J. Bashaw*
Jonas Rose bird brains
 Jonas Rose bird brains Bird brains BIRDS? Who are they What tricks can they do What do their brains look like What don t we know yet What are the implications Bird evolution Birds are dinosaurs Henry Huxley
Jonas Rose bird brains Bird brains BIRDS? Who are they What tricks can they do What do their brains look like What don t we know yet What are the implications Bird evolution Birds are dinosaurs Henry Huxley
Radial glia in the ventral telencephalon
 REVIEW ARTICLE Radial glia in the ventral telencephalon Miguel Turrero Garcıa and Corey C. Harwell Department of Neurobiology, Harvard Medical School, Boston, MA, USA Correspondence C. C. Harwell, Department
REVIEW ARTICLE Radial glia in the ventral telencephalon Miguel Turrero Garcıa and Corey C. Harwell Department of Neurobiology, Harvard Medical School, Boston, MA, USA Correspondence C. C. Harwell, Department
Supplemental Data. Wnt/β-Catenin Signaling in Mesenchymal Progenitors. Controls Osteoblast and Chondrocyte
 Supplemental Data Wnt/β-Catenin Signaling in Mesenchymal Progenitors Controls Osteoblast and Chondrocyte Differentiation during Vertebrate Skeletogenesis Timothy F. Day, Xizhi Guo, Lisa Garrett-Beal, and
Supplemental Data Wnt/β-Catenin Signaling in Mesenchymal Progenitors Controls Osteoblast and Chondrocyte Differentiation during Vertebrate Skeletogenesis Timothy F. Day, Xizhi Guo, Lisa Garrett-Beal, and
Title: Chapter 5 Recorded Lecture. Speaker: Amit Dhingra Created by: (remove if same as speaker) online.wsu.edu
 Title: Chapter 5 Recorded Lecture Speaker: Title: What Anthony is the title Berger/Angela of this lecture? Williams Speaker: Amit Dhingra Created by: (remove if same as speaker) online.wsu.edu Chapter
Title: Chapter 5 Recorded Lecture Speaker: Title: What Anthony is the title Berger/Angela of this lecture? Williams Speaker: Amit Dhingra Created by: (remove if same as speaker) online.wsu.edu Chapter
Signaling Vascular Morphogenesis and Maintenance
 Signaling Vascular Morphogenesis and Maintenance Douglas Hanahan Science 277: 48-50, in Perspectives (1997) Blood vessels are constructed by two processes: vasculogenesis, whereby a primitive vascular
Signaling Vascular Morphogenesis and Maintenance Douglas Hanahan Science 277: 48-50, in Perspectives (1997) Blood vessels are constructed by two processes: vasculogenesis, whereby a primitive vascular
Gene co-expression networks in the mouse, monkey, and human brain July 16, Jeremy Miller Scientist I
 Gene co-expression networks in the mouse, monkey, and human brain July 16, 2013 Jeremy Miller Scientist I jeremym@alleninstitute.org Outline 1. Brief introduction to previous WGCNA studies in brain 2.
Gene co-expression networks in the mouse, monkey, and human brain July 16, 2013 Jeremy Miller Scientist I jeremym@alleninstitute.org Outline 1. Brief introduction to previous WGCNA studies in brain 2.
a 0,8 Figure S1 8 h 12 h y = 0,036x + 0,2115 y = 0,0366x + 0,206 Labeling index Labeling index ctrl shrna Time (h) Time (h) ctrl shrna S G2 M G1
 (GFP+ BrdU+)/GFP+ Labeling index Labeling index Figure S a, b, y =,x +, y =,x +,,,,,,,, Time (h) - - Time (h) c d S G M G h M G S G M G S G h Time of BrdU injection after electroporation (h) M G S G M
(GFP+ BrdU+)/GFP+ Labeling index Labeling index Figure S a, b, y =,x +, y =,x +,,,,,,,, Time (h) - - Time (h) c d S G M G h M G S G M G S G h Time of BrdU injection after electroporation (h) M G S G M
( neural progeni2 tor cell), ,,,, , (neural crest) (radial glial cell) mrna [2 ]
![( neural progeni2 tor cell), ,,,, , (neural crest) (radial glial cell) mrna [2 ] ( neural progeni2 tor cell), ,,,, , (neural crest) (radial glial cell) mrna [2 ]](/thumbs/92/110608454.jpg) 246, 1999 6, 51 (3), 246 252 Acta Physiologica Sinica 3 P19 3 3 (, 200031 ;, 200031) (nestin),, RA P19,, (neural precursor cell) BMP4, (NF160), ( neural progeni2 tor cell),, : ; P19 ; BMP4 ; : Q71,,,,
246, 1999 6, 51 (3), 246 252 Acta Physiologica Sinica 3 P19 3 3 (, 200031 ;, 200031) (nestin),, RA P19,, (neural precursor cell) BMP4, (NF160), ( neural progeni2 tor cell),, : ; P19 ; BMP4 ; : Q71,,,,
MIDTERM EXAM 1 COGNITIVE SCIENCE 107A
 MIDTERM EXAM 1 COGNITIVE SCIENCE 107A FALL 2011 Name: Points: / 100 PID: I. SHORT ANSWERS (6 points each for a total of 30 points) 1. Describe two contributions made by Ramon y Cajal (1852-1934) in terms
MIDTERM EXAM 1 COGNITIVE SCIENCE 107A FALL 2011 Name: Points: / 100 PID: I. SHORT ANSWERS (6 points each for a total of 30 points) 1. Describe two contributions made by Ramon y Cajal (1852-1934) in terms
CNS Developmental. Anke van Eekelen, PhD. Telethon Institute for Child Health Research
 CNS Developmental Anke van Eekelen, PhD Telethon Institute for Child Health Research (Some slides are modified versions of Prof. Alan Harvey s Neuroscience lecture at ANHB and Dr. Joanne Britto s Dev Neuroscience
CNS Developmental Anke van Eekelen, PhD Telethon Institute for Child Health Research (Some slides are modified versions of Prof. Alan Harvey s Neuroscience lecture at ANHB and Dr. Joanne Britto s Dev Neuroscience
Discovery of Schizophrenia Drug Targets from DISC1 Mechanisms Atsushi Kamiya M.D., Ph.D.
 Discovery of Schizophrenia Drug Targets 1 Assistant Professor Department of Psychiatry and Behavioral Sciences Johns Hopkins University School of Medicine akamiya1@jhmi.edu How does neurobiology offer
Discovery of Schizophrenia Drug Targets 1 Assistant Professor Department of Psychiatry and Behavioral Sciences Johns Hopkins University School of Medicine akamiya1@jhmi.edu How does neurobiology offer
Neuronal migration in the adult brain: are we there yet?
 Neuronal migration in the adult brain: are we there yet? H. Troy Ghashghaei*, Cary Lai and E. S. Anton* Abstract The generation and targeting of appropriate numbers and types of neurons to where they are
Neuronal migration in the adult brain: are we there yet? H. Troy Ghashghaei*, Cary Lai and E. S. Anton* Abstract The generation and targeting of appropriate numbers and types of neurons to where they are
SUPPLEMENTARY FIGURES
 SUPPLEMENTARY FIGURES 1 Supplementary Figure 1, Adult hippocampal QNPs and TAPs uniformly express REST a-b) Confocal images of adult hippocampal mouse sections showing GFAP (green), Sox2 (red), and REST
SUPPLEMENTARY FIGURES 1 Supplementary Figure 1, Adult hippocampal QNPs and TAPs uniformly express REST a-b) Confocal images of adult hippocampal mouse sections showing GFAP (green), Sox2 (red), and REST
Development of the Central Nervous System
 Development of the Central Nervous System an ongoing process, through adolescence and maybe even adult hood? the nervous system is plastic Experience plays a key role Dire consequences when something goes
Development of the Central Nervous System an ongoing process, through adolescence and maybe even adult hood? the nervous system is plastic Experience plays a key role Dire consequences when something goes
Dlx5 and Dlx6 Regulate the Development of Parvalbumin-Expressing Cortical Interneurons
 5334 The Journal of Neuroscience, April 14, 2010 30(15):5334 5345 Development/Plasticity/Repair Dlx5 and Dlx6 Regulate the Development of Parvalbumin-Expressing Cortical Interneurons Yanling Wang, 1 *
5334 The Journal of Neuroscience, April 14, 2010 30(15):5334 5345 Development/Plasticity/Repair Dlx5 and Dlx6 Regulate the Development of Parvalbumin-Expressing Cortical Interneurons Yanling Wang, 1 *
Communicated by Dominick P. Purpura, Albert Einstein College of Medicine, Bronx, NY, September 30, 2002 (received for review July 8, 2002)
 Differential modulation of BMP signaling promotes the elaboration of cerebral cortical GABAergic neurons or oligodendrocytes from a common sonic hedgehog-responsive ventral forebrain progenitor species
Differential modulation of BMP signaling promotes the elaboration of cerebral cortical GABAergic neurons or oligodendrocytes from a common sonic hedgehog-responsive ventral forebrain progenitor species
Src-INACTIVE / Src-INACTIVE
 Biology 169 -- Exam 1 February 2003 Answer each question, noting carefully the instructions for each. Repeat- Read the instructions for each question before answering!!! Be as specific as possible in each
Biology 169 -- Exam 1 February 2003 Answer each question, noting carefully the instructions for each. Repeat- Read the instructions for each question before answering!!! Be as specific as possible in each
The contribution of GABAergic dysfunction to neurodevelopmental disorders
 Review The contribution of GABAergic dysfunction to neurodevelopmental disorders Kartik Ramamoorthi 1,2,3 and Yingxi Lin 1,2 1 McGovern Institute for Brain Research, Massachusetts Institute of Technology,
Review The contribution of GABAergic dysfunction to neurodevelopmental disorders Kartik Ramamoorthi 1,2,3 and Yingxi Lin 1,2 1 McGovern Institute for Brain Research, Massachusetts Institute of Technology,
SUPPLEMENTARY INFORMATION
 Figure S1 Treatment with both Sema6D and Plexin-A1 sirnas induces the phenotype essentially identical to that induced by treatment with Sema6D sirna alone or Plexin-A1 sirna alone. (a,b) The cardiac tube
Figure S1 Treatment with both Sema6D and Plexin-A1 sirnas induces the phenotype essentially identical to that induced by treatment with Sema6D sirna alone or Plexin-A1 sirna alone. (a,b) The cardiac tube
Tlx3 and Tlx1 are post-mitotic selector genes determining glutamatergic over GABAergic cell fates
 Tlx3 and Tlx1 are post-mitotic selector genes determining glutamatergic over GABAergic cell fates L. Cheng, A. Arata, R. Mizuguchi, Y. Qian, A. Karunaratne, P.A. Gray, S. Arata, S. Shirasawa, M. Bouchard,
Tlx3 and Tlx1 are post-mitotic selector genes determining glutamatergic over GABAergic cell fates L. Cheng, A. Arata, R. Mizuguchi, Y. Qian, A. Karunaratne, P.A. Gray, S. Arata, S. Shirasawa, M. Bouchard,
Supplementary Fig. 1 Blocking shh function at the protein level confirms its role as a guidance cue for postcommissural axons.
 Supplementary Fig. 1 Blocking shh function at the protein level confirms its role as a guidance cue for postcommissural axons. As an alternative method to demonstrate the role of shh as a guidance cue
Supplementary Fig. 1 Blocking shh function at the protein level confirms its role as a guidance cue for postcommissural axons. As an alternative method to demonstrate the role of shh as a guidance cue
Terminology. Terminology. Terminology. Terminology. Terminology. Bromodeoxyuridine
 Kateřina Náměstková, Zuzana Šimonová, Eva Syková Behavioural Brain Research Bromodeoxyuridine : Doublecortin : DCX Glial Fibrillary Acidic Protein : GFAP Trace eye blink conditioning 1 Volume 163 : pp.
Kateřina Náměstková, Zuzana Šimonová, Eva Syková Behavioural Brain Research Bromodeoxyuridine : Doublecortin : DCX Glial Fibrillary Acidic Protein : GFAP Trace eye blink conditioning 1 Volume 163 : pp.
Olfactory ensheathing glia
 Olfactory ensheathing glia From Wikipedia, the free encyclopedia Neuroglia of the brain shown by Golgi's method. Olfactory ensheathing glia (OEG), also known as olfactory ensheathing cells (OECs) or olfactory
Olfactory ensheathing glia From Wikipedia, the free encyclopedia Neuroglia of the brain shown by Golgi's method. Olfactory ensheathing glia (OEG), also known as olfactory ensheathing cells (OECs) or olfactory
Supplementary Figure 1 Expression of Crb3 in mouse sciatic nerve: biochemical analysis (a) Schematic of Crb3 isoforms, ERLI and CLPI, indicating the
 Supplementary Figure 1 Expression of Crb3 in mouse sciatic nerve: biochemical analysis (a) Schematic of Crb3 isoforms, ERLI and CLPI, indicating the location of the transmembrane (TM), FRM binding (FB)
Supplementary Figure 1 Expression of Crb3 in mouse sciatic nerve: biochemical analysis (a) Schematic of Crb3 isoforms, ERLI and CLPI, indicating the location of the transmembrane (TM), FRM binding (FB)
Neural stem cells and the neurobiology of ageing. Chen Siyun 1, Dawe G.S. 2
 ABSTRACT Neural stem cells and the neurobiology of ageing Chen Siyun 1, Dawe G.S. 2 Department of Physics, Faculty of Science, National University of Singapore 10 Kent Ridge Road, Singapore 117546 The
ABSTRACT Neural stem cells and the neurobiology of ageing Chen Siyun 1, Dawe G.S. 2 Department of Physics, Faculty of Science, National University of Singapore 10 Kent Ridge Road, Singapore 117546 The
Inner ear development Nervous system development
 Upcoming Sessions April 22: Nervous System Development Lecture April 24: Reviews of Axonal Pathfinding in Sensory Systems April 29: Inner Ear Development Lecture May 1: May 6: May 8: Auditory System Pathfinding
Upcoming Sessions April 22: Nervous System Development Lecture April 24: Reviews of Axonal Pathfinding in Sensory Systems April 29: Inner Ear Development Lecture May 1: May 6: May 8: Auditory System Pathfinding
NIH Public Access Author Manuscript Trends Mol Med. Author manuscript; available in PMC 2012 August 1.
 NIH Public Access Author Manuscript Published in final edited form as: Trends Mol Med. 2011 August ; 17(8): 452 462. doi:10.1016/j.molmed.2011.03.003. The Contribution of GABAergic Dysfunction to Neurodevelopmental
NIH Public Access Author Manuscript Published in final edited form as: Trends Mol Med. 2011 August ; 17(8): 452 462. doi:10.1016/j.molmed.2011.03.003. The Contribution of GABAergic Dysfunction to Neurodevelopmental
Dopamine receptor activation modulates GABA neuron migration from the basal forebrain to the cerebral cortex
 University of Massachusetts Medical School escholarship@umms Open Access Articles Open Access Publications by UMMS Authors 4-6-2007 Dopamine receptor activation modulates GABA neuron migration from the
University of Massachusetts Medical School escholarship@umms Open Access Articles Open Access Publications by UMMS Authors 4-6-2007 Dopamine receptor activation modulates GABA neuron migration from the
Citation for published version (APA): Martina-Mamber, C. E. (2014). GFAP as an understudy in adult neurogenesis. 's-hertogenbosch: Boxpress.
 UvA-DARE (Digital Academic Repository) GFAP as an understudy in adult neurogenesis Mamber, C.E. Link to publication Citation for published version (APA): Martina-Mamber, C. E. (2014). GFAP as an understudy
UvA-DARE (Digital Academic Repository) GFAP as an understudy in adult neurogenesis Mamber, C.E. Link to publication Citation for published version (APA): Martina-Mamber, C. E. (2014). GFAP as an understudy
BIOL2005 WORKSHEET 2008
 BIOL2005 WORKSHEET 2008 Answer all 6 questions in the space provided using additional sheets where necessary. Hand your completed answers in to the Biology office by 3 p.m. Friday 8th February. 1. Your
BIOL2005 WORKSHEET 2008 Answer all 6 questions in the space provided using additional sheets where necessary. Hand your completed answers in to the Biology office by 3 p.m. Friday 8th February. 1. Your
Divergent functions of the proneural genes Mash1 and Ngn2 in the specification of neuronal subtype identity
 Divergent functions of the proneural genes Mash1 and Ngn2 in the specification of neuronal subtype identity Carlos M. Parras, 1,4 Carol Schuurmans, 1,3,4 Raffaella Scardigli, 1 Jaesang Kim, 2 David J.
Divergent functions of the proneural genes Mash1 and Ngn2 in the specification of neuronal subtype identity Carlos M. Parras, 1,4 Carol Schuurmans, 1,3,4 Raffaella Scardigli, 1 Jaesang Kim, 2 David J.
Supplementary Materials for
 advances.sciencemag.org/cgi/content/full/1/10/e1500775/dc1 Supplementary Materials for Structural-functional connectivity deficits of neocortical circuits in the Fmr1 /y mouse model of autism Matthias
advances.sciencemag.org/cgi/content/full/1/10/e1500775/dc1 Supplementary Materials for Structural-functional connectivity deficits of neocortical circuits in the Fmr1 /y mouse model of autism Matthias
Mammalian Cerebral Cortex: Embryonic Development and Cytoarchitecture
 Mammalian Cerebral Cortex: Embryonic Development and Cytoarchitecture 2 The prenatal developmental of the mammalian cerebral cortex, including that of humans, is characterized by two sequential and interrelated
Mammalian Cerebral Cortex: Embryonic Development and Cytoarchitecture 2 The prenatal developmental of the mammalian cerebral cortex, including that of humans, is characterized by two sequential and interrelated
SUPPLEMENTARY INFORMATION
 SUPPLEMENTARY INFORMATION Human cerebral cortex development from pluripotent stem cells to functional excitatory synapses Yichen Shi 1,2, Peter Kirwan 1,2, James Smith 1,2, Hugh P.C. Robinson 3 and Frederick
SUPPLEMENTARY INFORMATION Human cerebral cortex development from pluripotent stem cells to functional excitatory synapses Yichen Shi 1,2, Peter Kirwan 1,2, James Smith 1,2, Hugh P.C. Robinson 3 and Frederick
