Cloning and characterization of small RNAs from Medicago truncatula reveals four novel legume-specific microrna families
|
|
|
- Bennett Heath
- 5 years ago
- Views:
Transcription
1 Research Cloning and characterization of small RNAs from Blackwell Oxford, NPH New X /j x June 085??? 98??? Original XXThe Phytologist Authors UK Article Publishing (2009). Ltd Journal compilation New Phytologist (2009) XX Medicago truncatula reveals four novel legume-specific microrna families Guru Jagadeeswaran 1 *, Yun Zheng 2 *, Yong-Fang Li 1, Lata I. Shukla 1, Jessica Matts 1, Peter Hoyt 1, Simone L. Macmil 3, Graham B. Wiley 3, Bruce A. Roe 3, Weixiong Zhang 2,4 and Ramanjulu Sunkar 1 1 Department of Biochemistry and Molecular Biology, Oklahoma State University, Stillwater, OK 74078, USA; 2 Department of Computer Science and Engineering, Washington University in St Louis, St Louis, MO 63130, USA; 3 Department of Chemistry and Biochemistry, University of Oklahoma, 101 David L. Boren Boulevard, Norman, OK 73019, USA; 4 Department of Genetics, Washington University School of Medicine, St Louis, MO 63110, USA Summary Author for correspondence: Ramanjulu Sunkar Tel: ramanjulu.sunkar@okstate.edu Received: 20 February 2009 Accepted: 22 April 2009 New Phytologist (2009) 184: doi: /j x Key words: legumes, Medicago truncatula, micrornas, posttranscriptional gene regulation, trans-acting sirnas. MicroRNAs (mirnas) and small-interfering RNAs (sirnas) have emerged as important regulators of gene expression in higher eukaryotes. Recent studies indicate that genomes in higher plants encode lineage-specific and species-specific mirnas in addition to the well-conserved mirnas. Leguminous plants are grown throughout the world for food and forage production. To date the lack of genomic sequence data has prevented systematic examination of small RNAs in leguminous plants. Medicago truncatula, a diploid plant with a near-completely sequenced genome has recently emerged as an important model legume. We sequenced a small RNA library generated from M. truncatula to identify not only conserved mirnas but also novel small RNAs, if any. Eight novel small RNAs were identified, of which four (mir1507, mir2118, mir2119 and mir2199) are annotated as legume-specific mirnas because these are conserved in related legumes. Three novel transcripts encoding TIR-NBS-LRR proteins are validated as targets for one of the novel mirna, mir2118. Small RNA sequence analysis coupled with the small RNA blot analysis, confirmed the expression of around 20 conserved mirna families in M. truncatula. Fifteen transcripts have been validated as targets for conserved mirnas. We also characterized Tas3-siRNA biogenesis in M. truncatula and validated three auxin response factor (ARF) transcripts that are targeted by tasirnas. These findings indicate that M. truncatula and possibly other related legumes have complex mechanisms of gene regulation involving specific and common small RNAs operating post-transcriptionally. Introduction There are two major classes of endogenous small RNAs in plants: micrornas (mirnas) and small-interfering RNAs (sirnas). Based on their origin, biogenesis, and potential targets, endogenous plant sirnas are further divided into trans-acting sirnas (tasirnas), natural antisense transcriptderived sirnas (nat-sirnas) and repeat-associated sirnas (rasirnas) (Vaucheret, 2006). *These authors contributed equally to this work. The mirnas are c. 21-nt long noncoding RNAs, which result from processing of imperfectly folded hairpin-like singlestranded RNAs by the Dicer-Like1complex ( Jones-Rhoades et al., 2006; Ramachandran & Chen, 2008). The 21- to 24-nt sirnas are processed by the other members of the Dicer family of proteins (DCL2, DCL3 and DCL4) from long, perfectly paired double-stranded RNAs (dsrnas). The dsrnas result from the transcription of inverted repeats, or convergent transcription of sense antisense gene pairs or due to activity of RNA-dependent RNA polymerases (RDRs) on aberrant transcripts (Allen et al., 2005; Vaucheret, 2006). The Authors (2009) New Phytologist (2009) 184: Journal compilation New Phytologist (2009) 85
2 86 Research Gene silencing occurs in both plants and animals when endonuclease complexes are guided by small RNAs to target RNAs (Bartel, 2004). The target genes are repressed posttranscriptionally when mirnas direct cleavage of target mrnas or prevent protein production ( Jones-Rhoades et al., 2006; Mallory & Vaucheret, 2006; Brodersen et al., 2008). Endogenous sirnas are capable of regulating gene expression at either transcriptional (heterochromatic sirnas) or posttranscriptional levels (tasirnas and nat-sirnas) (Arazi et al., 2005; Vaucheret, 2006). Identification of small RNAs and their target messenger RNAs are essential for understanding small RNA-mediated gene regulation of development and stress responses (Arazi et al., 2005; Jones-Rhoades et al., 2006; Sunkar et al., 2007). Accurate annotation of small RNAs into mirnas, tasirnas, nat-sirnas and rasirnas requires genome sequence information. As a result, plants with sequenced genomes such as Arabidopsis, rice (Oryza sativa), Populus and Physcomitrella, are the most intensively investigated plant systems for the discovery of small RNAs (Jones-Rhoades & Bartel, 2004; Arazi et al., 2005; C. Lu et al., 2005; Rajagopalan et al., 2006; Barakat et al., 2007; S. Lu et al., 2008). The common themes emerging from these studies are that c. 20 mirna families are highly conserved across monocots and dicots, but plants also express lineagespecific and species-specific mirnas (Arazi et al., 2005; Sunkar et al., 2005; Lu et al., 2006; Talmor-Neiman et al., 2006a; Axtell et al., 2007; Fattash et al., 2007; C. Lu et al., 2008; Moxon et al., 2008; Sunkar & Jagadeeswaran, 2008). Conserved mirnas appear to have conserved biological functions whereas lineage-specific mirnas and speciesspecific mirnas may have lineage-specific and species-specific roles, respectively. This observation means cataloging of small RNAs is required to accurately trace the evolution of lineagespecific and species-specific mirnas in plants. Currently, leguminous plants account for one-third of the world s primary crop production and are critical to meet large quantities of food and feed demands by humans and animals (Benedito et al., 2008). Legumes are also an interesting group of plants because they can fix atmospheric nitrogen. However, most cultivated legumes are polyploid with complex genomes and are therefore not amenable for genomic studies (Benedito et al., 2008). Most cultivated legumes are also resistant to common genetic manipulation tools such as transformation. Medicago truncatula, however, possesses a simple diploid genome, and is relatively easy to transform (Trinh et al., 1998; Chabaud et al., 2003; Crane et al., 2006). As a result, sequencing of the M. truncatula genome is nearly complete, and it has become a model legume for functional genomics research (Benedito et al., 2008). Sequence conservation has allowed scientists to predict mirnas in the legumes, M. truncatula and soybean (Glycine max) (Zhang et al., 2006; Sunkar & Jagadeeswaran, 2008). Recently, sequencing a small RNA library from soybean identified three novel mirnas (Subramanian et al., 2008). Complete genome information is needed to facilitate confident annotation of these and other small RNAs as mirnas or sirnas in soybean (Subramanian et al., 2008).The near-complete genome sequence of M. truncatula will allow more accurate characterization of small RNAs. In the present study, we have sequenced a M. truncatula small RNA library and identified 20 conserved mirna families. More importantly, eight novel small RNAs have been identified in M. truncatula. Four of these are annotated as legume-specific mirnas because these mirnas and their hairpin structures are conserved in related legumes. The remaining four appear to be candidates for M. truncatulaspecific mirnas. Three novel transcripts encoding TIR- NBS-LRR disease-resistance proteins are validated as targets for the novel mirna, mir2118 in M. truncatula. We also validated one representative target for most of the conserved mirna families using the 5 -rapid amplification of cdna ends (RACE) assay. Biogenesis and target gene validations were also determined for TAS3-siRNAs. Materials and Methods Cloning of M. truncatula small RNAs Total RNA was isolated from the frozen seedlings and flowers with TRIzol (Invitrogen) according to the manufacturer s instructions. Cloning of the mirnas was performed as described (Sunkar et al., 2008). The final PCR product was sequenced using a 454 sequencer (Roche) at the University of Oklahoma, USA. Plant materials and growth conditions Medicago truncatula Gaertner cv. Jemalong plants were grown in a controlled growth chamber (22 24 C) with a 16-h photoperiod and 300 µmol m 2 s 1 light intensity. Tissue samples from different organs were harvested and flash frozen and stored at 80 C. For stress treatments, 3-wk-old seedlings grown on hydroponic cultures were transferred to the same medium without sulfate, or phosphate or copper. Root and shoot tissues were harvested separately and stored. Method for identifying new candidate mirnas Our computational methods for analysing 454 small RNA libraries was reported previously (Sunkar et al., 2008). Briefly, all small RNA reads without perfect matches to the most proximal 11 nt of both adaptor sequences were first removed. Reads corresponding to repeats were removed using the einverted and etandem programs in the emboss (2000) package, respectively. The unique small RNAs were aligned to repbase (version 13.04, obtained from and known noncoding RNAs (rrnas, trnas, snrnas, snornas, etc., obtained from Rfam/ftp.shtml) with National Center for Biotechnology New Phytologist (2009) 184: The Authors (2009) Journal compilation New Phytologist (2009)
3 Research 87 Information (NCBI) blastn. Then the small RNAs were mapped to the reported mirnas in the mirbase (version 11, obtained from Small RNAs that matched known mirnas of M. truncatula or other plant species resulted in identification of conserved mirna homologs in M. truncatula. The unique small RNAs were aligned to the genome sequence of M. truncatula (downloaded from ( release 2.0), and for those sequences that matched with the genome, the fold-back structures were predicted using the rnafold program (Hofacker, 2003). This resulted in identification of 41 small RNAs with 145 loci. Most nonconserved and species-specific mirnas have single locus in Arabidopsis and rice (S. Lu et al., 2008; Fahlgren et al., 2006), and we applied this feature in our analysis. This resulted in identification of 21 candidate sequences. Out of these, only five were considered for further analysis, because these could be detected using small RNA blot analysis. The unique small RNAs that could not be mapped to the available M. truncatula genome were queried against the expressed sequences tag (EST) database of the NCBI to find homologs in other plants, including legumes. Surprisingly, three of the unique small RNAs that could not be mapped to the M. truncatula genome, were found to have perfect matches with the ESTs from M. truncatula and other related legumes. Fold-back structures could be predicted for these ESTs. This resulted in identification of novel legume-specific mirnas. mirna target prediction and validation The known M. truncatula open reading frames (ORFs), downloaded from the Medicago Genome Annotation Database, were used for mirna target predictions. For selecting putative mirna target pairs, only three mismatches were allowed between a mrna target and mirna in our prediction (Rhoades et al., 2002; Jones-Rhoades & Bartel, 2004). A modified 5 -RACE assay was performed using the GeneRacer Kit (Invitrogen) to validate the predicted targets. Briefly, the RNA was ligated with a 5 RNA adapter and a reverse transcription was performed. The resulting cdna was used as template for PCR amplification with GeneRacer 5 primer and a gene-specific primer. A second nested PCR was performed using nested primers (GeneRacer 5 nested primer and a genespecific nested primer). The amplified products were gel purified, cloned and sequenced. Gene specific primers used are provided as in the Supplementary Information, Table S3. Identification of TASi locus and tasirnas in M. truncatula To predict TAS genes and tasirnas in M. truncatula, we examined Hitsensor scores (Zheng & Zhang, 2008) and inter-site distances of mir390 binding sites of Arabidopsis TAS3 genes. AtTAS3 genes have two Hitsensor sites for mir390 (of which the 5 site with critical mismatches in position 10 11), with Hitsensor scores from 200 to 300. Based on these distances (228 nt for AtTAS3a, 201 nt for AtTAS3b and 181 nt for AtTAS3c) we examined 250 nt upstream and 250 nt downstream from M. truncatula unique RNA reads using Hitsensor to identify possible binding sites of mir390. Sequences without two mir390-binding sites were removed. Small RNA blot analysis Total RNA was isolated from different tissues of M. truncatula with TRIzol reagent following the manufacturer s instructions (Invitrogen). Low-molecular-weight (LMW) RNA was isolated from total RNA using polyethylene glycol (PEG) precipitation. Twenty micrograms of LMW RNA was used for detection of mirnas or candidate mirnas, whereas 50 µg was used for detection of mirna*. Small RNA blot analysis was performed as previously reported (Sunkar et al., 2008). Results Sequence analysis and annotation To identify mirnas and other endogenous small RNAs expressed in M. truncatula seedlings, we generated and sequenced a small RNA library using pooled RNA isolated from seedlings and flowers. After removal of the 5 and 3 adapter sequences, a total of raw sequence reads ranging in size between 18 nt and 26 nt were obtained. Of these, reads could be mapped to the existing M. truncatula genome, and the remaining sequences (c. 4237) that could not be mapped thus, were discarded. Of the genome-matched reads, redundant sequences were noted and only unique sequences were used for further analysis. Small RNAs matching the rrna, trna, snrna and snornas from these unique sequences were removed as reported previously (Sunkar et al., 2008). The cloning frequency of different sized (18 26 nt) small RNAs revealed two predominant sizes: 21 nt and 24 nt, which is consistent with previous reports (Lu et al., 2005, 2006; Sunkar et al., 2005; Fahlgren et al., 2007). Small RNAs of the 24-nt size class represented the largest category of sequence reads (9333 of reads, 42%). Most 24 nt sequences appeared only once in our sequence reads suggesting the 24 nt small RNAs are extremely diverse in M. truncatula. Similar results were found in Arabidopsis and rice (Lu et al., 2006; Nobuta et al., 2007). This study focuses on the 21-nt size class of small RNAs, representing mirnas and tasirnas. Identification of four novel legume-specific mirnas Annotating novel mirnas generally requires the dcl1 knockout mutant (Ambros et al., 2003). In addition, studies using Arabidopsis showed that dcl4 may also process certain mirna precursors (Rajagopalan et al., 2006), suggesting both the The Authors (2009) New Phytologist (2009) 184: Journal compilation New Phytologist (2009)
4 88 Research Table 1 Identified novel small RNAs (four legume-specific and four candidate mirnas) in Medicago truncatula srna_id Sequence (cloning frequency) Detected using blot analysis Conservation mir2118 UUACCGAUUCCACCCAUUCCUA (3) + Soybean, Chickpea, Peanut, Phaseolus, Vigna mir2199 UGAUACACUAGCACGGGUCAC (1) + Lotus japonicus mir1507 CCUCGUUCCAUACAUCAUCUA (1) + Peanut mir2119 UCAAAGGGAGUUGUAGGGGAA (1) + Soybean srna15993 (candidate) UAGAGUCACAUGGUCGGUAUCCC (2) + srna7556 (candidate) AGAUCGGUUGAUAGAGGAGGA (1) + srna19284 (candidate) UAGGUUUGAGAAAAUGGGCAG (1) + srna21166 (candidate) AUGAUGUAAGGGAUGAUGCAAAU (1) + The annotation of legume-specific mirnas was based on the detection of mirna and mirna* as well as their conservation in related legumes. dcl1 and dcl4 knockout mutants are needed for confident annotation of novel species-specific mirnas in plants. In the absence of these genetic tools, it was recently suggested that sequencing of a mirna* is required for annotating a small RNA as a new mirna in plants (Meyers et al., 2008). In addition, showing conservation as determined by bioinformatics or experimentation (e.g. small RNA blot analysis) strengthens confidence in annotating novel small RNAs as mirnas (Meyers et al., 2008). Using these guidelines, we assigned a small RNA as a novel mirna if: (1) a mirna* is detected using small RNA blot analysis; (2) its conservation is confirmed by using bioinformatics (small RNA sequence as well as predicted fold-back structure for the precursor sequence is conserved in related plant species); and (3), if the small RNA could be detected in M. truncatula and other related legumes using small RNA blot analysis. These criteria identified four small RNAs (mir2118, mir2199, mir1507 and mir2119) as novel mirna families in M. truncatula (Table 1, Fig. 1). To strengthen our annotation assignments, we analysed the expression of these M. truncatula mirna* sequences using small RNA blots. The antisense probes corresponding to the predicted mirna* gave discrete bands suggesting that the mirna* sequence accumulates to detectable levels (Fig. 2b). Attempts to hybridize the same blots with antisense probes from precursor sequences flanking the mature mirna showed no signal (data not shown). These findings suggest that only the mirna and mirna* are excised from their precursors of these four novel small RNAs in M. truncatula. Our sequence analysis resulted in identification of 21 candidate sequences, based on the genome matching and foldback structure predictions. Of these, only five were considered for further analysis based on accumulations detected using small RNA blot analysis. Among these, mir1507 is conserved in related legumes and thus is annotated as a legume-specific mirna, whereas the remaining four are regarded as candidate M. truncatula-specific mirnas. The fully sequenced M. truncatula genome is currently unavailable for analysis. We suspected that some of the unique small RNAs without matches to the available genome of M. truncatula could still be mirnas; these unique small RNAs were searched against the EST resources at NCBI to identify their precursors. Three of the unique small RNAs that could not be mapped to the M. truncatula genome, had perfect or nearly perfect matches (one or two mismatches) with the ESTs from M. truncatula and other related legumes. The blast analysis identified mir2118 homologues in soybean, pea (Phaseolus vulgaris) and black-eyed pea (Vigna unguiculata). Similarly, homologs for mir1507 and mir2199 are found in peanut (Arachis hypogaea) and Lotus japonicus, respectively (Table 1, Fig. 1). Another small RNA (mir2119) is conserved in soybean (Table 1, Fig. 1). Fold-back structures could be predicted using the genomic or EST sequences surrounding the novel small RNAs in the above mentioned leguminous plant species (Fig. 1). We then analysed the expression of these novel mirnas in related legumes such as soybean, chickpea (Cicer arietinum), peanut, black-eyed pea along with the Arabidopsis and rice (Fig. 2a). mir2118 and mir2199 could be detected in all five legumes tested (Fig. 2a), whereas mir1507 and mir2119 could be detected in three legumes of the five tested (Fig. 2a). None of these four small RNA sequences exist in completely sequenced genomes of Arabidopsis, rice, Populus or Physcomitrella. Accordingly, we annotated these four novel small RNAs (mir2118, mir2199, mir1507 and mir2119) as legume-specific mirnas based on detection of mirna and mirna* and conservation of small RNA sequence as well as their fold-back structure. These criteria have been used to categorize small RNA as a novel mirna (Sunkar et al., 2005, 2008; S. Lu et al., 2008). From the data, this study has provided compelling evidence for annotation of four novel small RNAs as legume-specific mirnas. One identified based on matching with the M. truncatula genome and the other three matching with the EST resources. The remaining four novel small RNA homologs (srna7556, srna19284, srna15993 and srna21166) were not found in any other plant species (Table 1). Fold-back structures are predicted for their precursor sequences (see the New Phytologist (2009) 184: The Authors (2009) Journal compilation New Phytologist (2009)
5 Research 89 Fig. 1 Predicted fold-back structures for the legume-specific mirnas identified in this study. (a) mir2118, (b) mir2199, (c) mir1507 and (d) mir2119. Ah, Arachis hypogaea; Gm, Glycine max; Lj, Lotus japonicus; Mt, Medicago truncatula; Pv, Phaseolus vulgaris; Vu, Vigna unguiculata. Supporting Information, Fig. S1) and some of them could be detected using small RNA blot analysis (Fig. 3). However, these characteristics do not meet the criteria to annotate them as Medicago-specific mirnas without cloning mirna* sequence (Meyers et al., 2008). Thus, these four small RNAs are regarded as candidate mirnas in M. truncatula. Recently, one of these novel small RNAs (srna19284) was also sequenced by another group (Szittya et al., 2008) and classified as a potential new mirna in M. truncatula, supporting our current results. Interestingly, more than 20 Toll/Interleukin 1 Receptornucleotide binding site-leucine-rich repeat (TIR-NBS-LRR) genes have been predicted as targets for the novel legumespecific mirna, mir2118 in M. truncatula (Table S1). However, at least two TIR-NBS-LRR genes in M. truncatula do not possess complementary sites for the mir2118 (Table S1). The TIR-NBS-LRR gene family is highly conserved among higher plants and homologs of this gene family possesses the complementary site for mir2118 in Arabidopsis and rice, but mir2118 homologs are absent in Arabidopsis, rice and Populus. Using 5 -RACE assays, three predicted targets (AC202360_18.1, AC203224_17.1 and AC143338_38.2) belonging to the TIR-NBS-LRR gene family have been validated as genuine targets for mirna, mir2118 (Fig. 2b). Identification of conserved mirnas from M. truncatula Higher plants have at least c. 20 conserved mirna families, and the latest mirbase release (11.0, September, 2008) lists 30 mirnas belonging to nine conserved mirna families in M. truncatula. Our small RNA sequence analysis identified mirnas belonging to 13 conserved mirna families, which includes nine families reported in the mirbase (Table 2). To detect the expression of the remaining conserved mirna families (mir162, mir393, mir394, mir395, mir397, mir398 and mir399), we performed small RNA blot analysis The Authors (2009) New Phytologist (2009) 184: Journal compilation New Phytologist (2009)
6 90 Research Fig. 2 Characterization of four novel legumespecific mirnas in Medicago truncatula. (a) Expression analysis of four novel mirnas (mir2118, mir2199, mir1507 and mir2119) in related legumes along with Arabidopsis and rice using 20 µg of low molecular weight RNA. Blots were stripped and rehybridized with the indicated mirna probes. The U6 probe served as a loading control. (b) Detection of mirna* sequence for the four of the novel mirnas (mir2118, mir2199, mir1507 and mir2119) using 50 µg of low molecular weight RNA for small RNA blot analysis. Small RNA blots from Medicago samples were hybridized with antisense probes of predicted mirna star sequence. The U6 probe served as a loading control. (c) Validation of three targets for the newly identified mirna, mir2118, by modified rapid amplification of cdna ends (RACE) assays in Medicago truncatula. The mrna target (top) and its corresponding mirna (bottom) are shown in each alignment; matches are indicated with straight lines, mismatches with colons and G U wobbles represented with a circle. Arrows show the site of cleavage and the fraction of cloned products that terminated at the mirna complementary sites is indicated above. using labeled antisense oligos. These experiments confirmed the expression of mir162, mir393 and mir398 in diverse tissues (Fig. 4). The other four mirna families (mir395, mir399 and mir397/398) are induced specifically under sulfate- or phosphate- or copper-deprived conditions, respectively (Chiou, 2007; Sunkar et al., 2007) and could not be detected using RNA isolated from the M. truncatula seedlings grown hydroponically with optimal levels of nutrients. Using small RNA blot analysis, mir395, mir398 and mir399 were detected in M. truncatula seedlings grown hydroponically but without sulfate or copper or phosphate respectively (Fig. 5a c). We also detected mir397 and mir408 (data not shown) in seedlings grown on medium without copper (Fig. 5c). When the mirna sequences in our library (Table 2) are combined with the mirna families detected in Figs 4 and 5, we confirmed the expression of 20 conserved mirna families in M. truncatula. While our manuscript was in review, two recent reports identified the conserved mirnas in M. truncatula (Szittya et al., 2008; Zhou et al., 2008). Using bioinformatics, Zhou et al. (2008) reported the identification of 11 conserved mirna families, whereas using an experimental approach Szittya et al. (2008) reported the identification of c. 20 conserved mirna families in M. truncatula. However, these reports do not analyse the expression patterns of conserved mirnas in different tissues or in response to limiting nutrient (sulfate or phosphate or copper) availability. The frequency of conserved mirnas varied between 1 and 734 reads in our library (Table 2). The mir172 family is the most abundant, represented by 734 reads, of which mir172b alone accounted for 444 (c. 60% of 734) reads. The second most-abundant mirna family was mir159, represented by 234 reads (Table 2). A very high count of mir172 reads was expected, because mir172 regulates the AP2 transcription factor implicated in flower development (Aukerman & Sakai, 2003; Chen, 2004), and our library was generated using pooled RNA isolated from M. truncatula seedlings and flowers. However, tissue-specific expression analysis indicated that mir172 is abundant in leaves, stem, root and flowers (Fig. 4). These results suggest that mir172 has developmental regulatory roles, in addition to roles in flower development. New Phytologist (2009) 184: The Authors (2009) Journal compilation New Phytologist (2009)
7 Research 91 Table 2 Identified conserved mirnas in Medicago truncatula using cloning approach and small RNA blot analysis mirna mirna sequence Cloning frequency Validated by small RNA blot analysis Validated targets Validated target gene family mir156/157 UGACAGAAGAGAGAGAGCACA 14 + mir159 UUGACAGAAGAUAGAGAGCAC mir160 UGCCUGGCUCCCUGUAUGCCA 4 + BQ148941, ES Auxin response factors mir162 UCGAUAAACCUCUGCAUCCAG + AC150443_32.2 Dicer Like-1 mir164 UGGAGAAGCAGGGCACGUGCA 2 + AC203553_1.1 NAC domain protein mir165/166 UCGGACCAGGCUUCAUCCCCC 5 + mir167 UGAAGCUGCCAGCAUGAUCUA 8 + AC144478_44.4, Auxin Response Factor CU326393_14.1 mir168 UCGCUUGGUGCAGGUCGGGAA 5 + AW Argonaute-1 mir169 CAGCCAAGGAUGACUUGCCGA 25 + mir-170/171 UGAUUGAGCCGUGUCAAUAUC 2 + AC121238_43.2 Scarecrow-like transcription factor mir172 AGAAUCUUGAUGAUGCUGCAU AL AP2 domain transcription factor mir319 UUGGACUGAAGGGAGCUCCC 30 mir390 AAGCUCAGGAGGGAUAGCGCC 2 + Noncoding RNA TAS3 pre-transcript mir393 UCCAAAGGGAUCGCAUUGAUCC + AC133780_22.2 F-box protein mir395 AUGAAGUGUUUGGGGGAACUC + AC146721_16.4 ATP sulfurylase mir396 UUCCACAGCUUUCUUGAACUU 6 mir397 UCAUUGAGUGCAGCGUUGAUG + AC203224_21.1, AC135467_30.2 Copper-resistance protein mir398 UGUGUUCUCAGGUCACCCCUU + mir399a UGCCAAAGGAGAUUUGCCCAG + AC159143_20.4 Ubiquitin-conjugating enzyme (E2 ligase) mir408 AUGCACUGCCUCUUCCCUGGC + BG Sequenced small RNA libraries often contained mir* sequences, although at much lower abundance compared with mirna (Lu et al., 2006; Rajagopalan et al., 2006). Not all conserved mirnas were detected at high abundance in this library. Many conserved mirna family members (mir160a, mir164b, mir167a, mir167b and mir167c) were represented by single reads (Table 2). Only mir396, mir160, mir169 and mir171 had their mir* sequences in our library. Fig. 3 Detection of novel candidate mirnas in Medicago truncatula. Expression analysis of newly identified candidate mirnas in roots and shoots of M. truncatula. Blots were stripped and rehybridized with the indicated mirna probes. The U6 probe served as a loading control. Expression analyses of conserved mirnas in M. truncatula Understanding the temporal and/or spatial expression of a mirna is an important initial step in probing its functional role in any organism. Many small RNAs are expressed only in certain tissues or cell types (Chen, 2004; Sunkar & Zhu, 2004; Combier et al., 2006; Boualem et al., 2008). Here, we determined the expression patterns of mirnas from diverse tissues of M. truncatula, such as leaves (young and old), stems, roots, flowers and in 3-wk-old seedlings (Fig. 4). Mostly independent blots were used for each probe, thus the expression levels of each microrna was monitored unambiguously. Although most mirnas were abundantly expressed in M. truncatula leaves, considerable variation in their abundance The Authors (2009) New Phytologist (2009) 184: Journal compilation New Phytologist (2009)
8 92 Research Fig. 4 Expression analysis of conserved mirnas in different tissues of the Medicago truncatula. Small RNA blots of low molecular weight RNA isolated from different tissues as indicated. The blots were probed with 32 P-end-labelled oligonucleotides complementary to the mirnas. Individual blots were used for each probe except for mir162, which was stripped and rehybridized with mir164; U6 served as a loading control. Fig. 5 Characterization of nutrient deprivation-induced mirnas in Medicago truncatula. (a c) Small RNA blots of low molecular weight RNA isolated from M. truncatula seedlings grown on hydroponic medium were grown continuously on the same medium (control) or transferred to medium lacking the indicated nutrient (a), sulfate, (b) phosphate and (c) copper. The blots were probed with 32 P-end-labelled oligonucleotides complementary to the mirnas. The blots were stripped and re-probed with U6, which served as a loading control. was noticeable. The exceptions to higher levels of expression in leaf tissues were mir160, mir168, mir170, mir390 and mir398, which showed reduced levels in leaves (Fig. 4). Similarly, flower tissue expressed all mirnas tested with notable exceptions being mir156 and mir393, where expression was the least compared with other tissues analysed (Fig. 4). By contrast, mir169, mir170 and mir398 showed elevated expression in flowers. mir169, was distinctly abundant in flower tissue, while other organs showed only a faint expression. Both mir393 and mir398 had relatively low expression in the M. truncatula stem, whereas the other mirnas showed strong expression (Fig. 4). Abundant expression of mir159, mir166 and mir167 was observed in roots (Fig. 4). The spatial expression pattern of mir398 differed greatly between M. truncatula and Arabidopsis. In Arabidopsis, mir398 is expressed abundantly in leaves (cauline and rosette) but not in inflorescence ( Jones-Rhoades & Bartel, 2004; Sunkar & Zhu, 2004; Sunkar et al., 2006). By contrast, mir398 in M. truncatula was expressed abundantly in flowers but only at lower levels in leaves (Fig. 4). These findings indicate that the expression patterns of conserved mirnas vary greatly across plant species. New Phytologist (2009) 184: The Authors (2009) Journal compilation New Phytologist (2009)
9 Research 93 Characterization of nutrient deprivation-induced mirnas in M. truncatula Studies show that mir395, mir399 and mir398 are induced in response to sulfate, phosphate and copper deficiency, respectively, in Arabidopsis (Sunkar et al., 2007). Copper deprivation has been also shown to induce the expression of mir397 and mir408 in Arabidopsis and Brassica (Yamasaki et al., 2007; Abdel-Ghany & Pilon, 2008; Buhtz et al., 2008) To compare these data with mir395, mir397, mir398, mir399 and mir408 in M. truncatula, 3-wk-old seedlings grown on optimal hydroponic medium were transferred to identical medium lacking sulfate or phosphate or copper. mir395 was upregulated in shoots and roots, with the upregulation being very strong in roots of M. truncatula seedlings grown on the medium without sulfate (Fig. 5a). Similarly, mir399 was induced when grown on medium without phosphate (Fig. 5b). As shown in Fig. 5c, mir398 was upregulated both in shoots and roots of M. truncatula grown on medium without copper, with the accumulation greater in roots than shoots. A similar pattern of induction was observed for mir397 in M. truncatula seedlings grown on copper-deficient medium (Fig. 5c). mirna target validations Plant mirnas and their targets are highly complementary (18 21 nt), which facilitates target prediction with the use of blastn search or patscan search (Rhoades et al., 2002; Jones- Rhoades & Bartel, 2004). To predict mirna-targeting mrnas in M. truncatula, we downloaded the currently annotated coding sequences from the Medicago genome project ( and used mirna sequences to search complimentary mrna sequences as suggested previously (Rhoades et al., 2002; Jones-Rhoades & Bartel, 2004). This analysis has identified c. 92 transcripts as potential targets in M. truncatula (Table S2). To date, only four mrnas have been validated as genuine targets of mirnas in M. truncatula (Combier et al., 2006; Boualem et al., 2008). The mir169-guided cleaved fragments were detected for the MtHAP2-1 transcript (Combier et al., 2006). Similarly, mir166-guided cleaved fragments were found for three transcripts, MtCNA1, MtCNA2 and MtHB-8 in M. truncatula (Boualem et al., 2008). In this study, a total of 15 mrnas (representing at least one target for most of the conserved mirna families) were confirmed as targets in M. truncatula (Table 2, Fig. 6). Most of the cleaved fragments were mapped exactly at the predicted cleavage sites (between nucleotides 10 and 11 from the 5 end). Some validated targets were cleaved at a slightly different position possibly owing to variations (5 or 3 shifts) in mature mirna species. ATP sulfurylases, UBC-like-E2-ligase and plantacyanin are predicted targets for mir395, mir399 and mir408, respectively (Table 2). Our attempts to validate these transcripts as mirna targets were unsuccessful using the Medicago seedlings grown on control medium. However, mirna-directed cleaved fragments for these transcripts could be detected in seedlings grown on medium without sulfate or phosphate or copper. This can be attributed to nutrient-specific induced transcription of the mirnas targeting these transcripts (Fig. 6). In Arabidopsis and rice, UBC transcript in its 5 -UTR (untranslated region) possesses four or five complementary sites of mir399 (Fujii et al., 2005; Bari et al., 2006). In M. truncatula, mir399 has five target sites located in the 5 -UTR of UBC and four of them were found to be cleaved (Fig. 6). These target validations are consistent with those of most conserved mirna targets in Arabidopsis, rice and Populus (Jones-Rhoades et al., 2006). Identification and characterization of TAS3-tasiRNAs in M. truncatula The tasirnas (trans-acting sirnas) are a class of endogenous 21 nt sirnas that downregulate target mrnas at the posttranscriptional level, as do mirnas (Peragine et al., 2004; Vazquez et al., 2004; Allen et al., 2005). They have been shown to regulate vegetative phase changes in Arabidopsis (Peragine et al., 2004; Vazquez et al., 2004; Allen et al., 2005; Gasciolli et al., 2005; Xie et al., 2005; Yoshikawa et al., 2005) and in moss (Arazi et al., 2005; Talmor-Neiman et al., 2006b). An initial DCL1-dependent, mirna-guided cleavage of tasirna primary transcript defines the 5 or 3 end of the transcript to be converted into dsrnas by RDR, and sets the phase for accurate formation of 21 nt tasirna by DCL4 in Arabidopsis (Peragine et al., 2004; Vazquez et al., 2004; Allen et al., 2005; Axtell et al., 2006). Thus, two DCLs, DCL1 and DCL4 and an RDR are required for tasirna biogenesis. In Arabidopsis, three mirnas mir173 mir390 and mir828 have been found to target primary tasirna transcripts (Allen et al., 2005; Rajagopalan et al., 2006). Of these, only mir390 is conserved from moss to higher plants and mir828 appears to be conserved between Arabidopsis and Populus, whereas mir173 homologs have not been found outside Arabidopsis. Since not all Arabidopsis mirnas that target tasirna primary transcripts are conserved, predicting computationally how many mirnas target tasirna precursors and how many tasirna loci exist in M. truncatula is difficult. Only sequencing can help identify tasirnas more confidently. To identify tasirna loci and tasirnas in Medicago, we searched for clusters of small RNAs that can be mapped to one locus but surrounded by two mir390 target sites. This resulted in identification of one authentic tasirna locus in Medicago (MtTAS3a) (Fig. 7a). In addition to the conserved dual mir390 target sites on the tasirna precursor, this transcript has another conserved region, which may be processed into a tas3-sirnas, and are complementary to auxin response factor (ARF) homologs in M. truncatula (Fig. 7a c). MtTAS3- tasirnas were in phase with the 3 target site (Fig. 7a) as found in Arabidopsis and rice (Allen et al., 2005; Liu et al., The Authors (2009) New Phytologist (2009) 184: Journal compilation New Phytologist (2009)
10 94 Research Fig. 6 Validation of conserved mirna targets in Medicago truncatula. Detection of mirna-guided cleavage sites on target transcripts using modified rapid amplification of cdna ends (RACE) assays in M. truncatula. Partial mrna sequences from target genes were aligned with the mirnas. In each alignment, the straight lines represent perfect matches, colons represent mismatches and circles represent G U wobbles. The fraction of cloned products that terminated at the predicted cleavage site is indicated. 2007). In Arabidopsis, only the 3 target site, not the 5 target site, is subjected to mir390-guided cleavage (Axtell et al., 2006; Howell et al., 2007). To verify whether the interaction between Medicago mir390 and the MtTAS3 precursor is similar to what was observed in Arabidopsis, 5 -RACE assay was performed to detect cleavage events at the 5 and 3 target sites on the TAS3 precursor. We failed to detect the cleavage at the 5 target site and these results are consistent with the previous suggestion that mir390 is unable to guide for a cleavage because of mismatches between 9 nt and 11 nt from the 5 end of the mir390 (Axtell et al., 2006). Surprisingly, only a small fraction of clones (3/40) confirmed the cleavage at the 3 target site using RNA isolated from seedlings. Instead, the sequenced PCR product corresponding to the 3 site mostly yielded a cleavage site 33 nt upstream from the predicted site (Fig. 7a). In Arabidopsis, detection of a cleavage at 33 nt upstream from the predicted site on TAS3 precursor was reported as a major cleavage event, although a minor cleavage event was also found at the predicted site (Allen et al., 2005). The upstream cleavage event in Arabidopsis has been attributed to the existence of a hypothetical sirna ( D2) generated from the TAS3 precursor (Allen et al., 2005), although the accumulation of such a sirna has not been examined. Because a similar cleavage event was also found in this study, we tested for the accumulation of the hypothetical D2 sirna using a small RNA blot and an antisense probe corresponding to the cleaved site. This analysis confirmed the accumulation of D2 sirna (Fig. 7d). Accumulation of sense (+D2) sirna could not be detected using a complementary probe, suggesting that D2 sirna corresponding to New Phytologist (2009) 184: The Authors (2009) Journal compilation New Phytologist (2009)
11 Research 95 Fig. 7 Tas3-siRNA biogenesis, expression analysis and validation of its targets in Medicago truncatula. (a) Nucleotide sequence of MtTAS3 locus. The 5 and 3 mir390 target sites are shown as alignments and the predicted cleavage site at the 3 target site is shown with red arrow. Predicted Dicer processing sites are shown with blue lines. MtTAs3 sirnas that are complementary to the auxin response factors are indicated with letters (+7TAS3 and +8TAS3). Detected mir390-guided cleavage fragments of the MtTAS3a precursor in M. truncatula are shown with a blue arrow. Detected D2 sirna-guided cleavage fragments of the MtTAS3a precursor in M. truncatula are shown with a red arrow. The fraction of cloned products that terminated at the cleaved site is indicated. (b) Sequence alignment of the TAS3 sirnas from Arabidopsis, rice and M. truncatula. (c) Expression analysis of TAS3 (+8 TAS3) sirnas in M. truncatula. (d) Expression analysis of D2 sirnas in M. truncatula. (e) Detection of TAS3- sirna-guided cleavage sites on three target transcripts by modified rapid amplification of cdna ends (RACE) assay in M. truncatula. Partial mrna sequences from target genes were aligned with the TAS3 sirna. The fraction of cloned products that terminated at the predicted cleavage site is indicated. the cleaved site has the ability to direct cleavage of TAS3 precursor. Cleavage at the 3 target site on the TAS3 precursor is essential to generate 21 nt phased, two tandemly arranged conserved TAS3 sirnas targeting auxin response factor (ARF)-like genes in plants (Allen et al., 2005; Axtell et al., 2006). However, a cleavage exactly 33 nt upstream from the 3 target site on TAS3 precursor was observed in this study and in the study of Allen et al. (2005). This cleavage would generate sirnas completely out of phase such that instead of generating active TAS3-tasiRNAs, the TAS3 precursor is degraded. A consistent cleavage in two different plant species (M. truncatula and Arabidopsis) further strengthens the proposal suggested by Allen et al. (2005) that the sirnas generated from the same locus will degrade the TAS3 precursor. The expression of TAS3-siRNAs in different tissues of M. truncatula was confirmed using a small RNA blot analysis (Fig. 7c). TAS3-siRNAs target three ARF genes (ARF2, ARF3 and ARF4) in Arabidopsis (Allen et al., 2005; Williams et al., 2005) and five ARF genes (four ARF3 homologs and one ARF2 homolog) in rice (Liu et al., 2007). Our computational prediction found four ARF genes as the targets of MtTAS3- sirnas (AC150891_17.2; AC152176_68.2; AC158497_40.2 and AC126794_50.2) in M. truncatula (Fig. 7e). Of these four genes, three (AC150891_17.2; AC152176_68.2 and AC158497_40.2) are close relatives of Arabidopsis ARF3. The Authors (2009) New Phytologist (2009) 184: Journal compilation New Phytologist (2009)
12 96 Research These three genes have two MtTAS3-siRNA complementary sites in their ORFs. Another gene, AC126794_50.2, is a homolog of the Arabidopsis ARF2 gene and it harbors only one TAS3-siRNA complementary site in its ORF. To verify whether MtTAS3-siRNAs directs cleavage of their target genes in M. truncatula, we used 5 -RACE assay to map the cleavage sites on four ARF3-like genes (Fig. 7e). This analysis confirmed two cleavages at the predicted sites on two ARF3- like genes (AC152176_68.2 and AC158497_40.2) while only one cleavage at the 5 target site was found for AC150891_17.2. In order to confirm the cleavages at two different sites on the same target gene (AC152176_68.2 and AC158497_40.2), we designed two independent primers downstream from each of the predicted target site (Table S3). We were unable to confirm the predicted cleavage site on ARF2-like gene because the mrna could not be amplified using RNA isolated from the seedlings. Nonetheless, these observations confirm that the MtTAS3-siRNAs are processed and can regulate multiple (3) ARF genes in M. truncatula. Discussion The present study has characterized eight novel small RNAs in M. truncatula (Table 1). Of these, four are annotated as legume-specific mirnas, because both mirna and mirna* are detected. These mirna sequences and predicted fold-back structures are conserved in leguminous plants, but these sequences are absent in the completely sequenced genomes of Arabidopsis, rice or Populus. This was further confirmed using small RNA blot analysis (Fig. 2). The remaining four novel small RNAs could be detected using small RNA blot analysis and consequently annotated as candidate mirnas in M. truncatula but their confident annotation as mirnas or sirnas requires deep sequencing. We have predicted more than 20 target transcripts encoding TIR1-NBS-LRR disease resistance proteins as targets for one of the newly identified legumespecific mirna (mir2118) and validated three of them in M. truncatula. In M. truncatula, TIR-NBS-LRR genes exist in an extensive cluster of R gene loci on the top arm of M. truncatula chromosome 4 (Ameline-Torregrosa et al., 2008). Recently, Yang et al. (2008) cloned RCT1 that encodes a TIR-NBS- LRR type R protein conferring broad-spectrum anthracnose resistance when transferred into the susceptible alfalfa lines. Interestingly, we validated RCT1 transcript (AC202360_18.1) as a target for one of the legume-specific mirnas (i.e. mir2118). In addition to the RCT1, we validated two other members (AC203224_17 and AC143338_38) of the TIR- NBS-LRR gene family as targets for the same mirna (mir2118). We predicted c. 20 genes belonging to this gene family as potential targets for this mirna in M. truncatula (Table S1). However, at least two genes belonging to this gene family are unlikely to be targeted by mir2118 (Table S1), despite the fact that the peptide sequence corresponding to the target site in all these proteins is highly conserved. A search of public databases showed a near-perfect target site in TIR- NBS-LRR genes of several other plant genomes including Arabidopsis and rice, but not the mir2118 homolog. Because mir2118 targets transcripts encoding TIR1-NBS-LRR proteins implicated in disease resistance, it will be interesting to see whether the expression of mir2118 is regulated during pathogen infection. Our experimental validation of three TIR-NBS-LRR genes as genuine targets and our prediction that 20 other related genes are potential targets of mir2118 provide opportunities to explore mirna-mediated plant defense responses in Medicago and other legumes. The mirna-dependent tasirna pathway is a plantspecific RNA silencing pathway that mimics the mirna pathway for mrna regulation. mir390 is conserved in all higher plants and in primitive land plants such as Physcomitrella and Selaginella (Arazi et al., 2005; Axtell et al., 2006, 2007). In Arabidopsis, mir390-guided cleavage occurs only at the 3 target site of the TAS3 precursor while the 5 target site is resistant to cleavage but is important for binding to the tasirna precursor transcript (Axtell et al., 2006; Howell et al., 2007). This characteristic feature is attributed to mismatches at nucleotides 9 12 from the 5 end of the mirna. These mismatches are conserved in the TAS3-primary transcript of M. truncatula and may be critical for the generation of TAS3-siRNAs in this species. The Arabidopsis TAS3 is expressed on the adaxial side of early leaf primordia (Adenot et al., 2006; Garcia et al., 2006), which possibly regulates the expression of ARF3 and ARF4 in the adaxial domain and determines the dorso-ventral leaf polarity (Garcia et al., 2006). A similar role has been reported for TAS3-tasiRNAs in maize that target ARF3 and ARF4 orthologs (Nogueira et al., 2007). The observation that M. truncatula mir390 targets TAS3 tasi-precursors and that the TAS3- tasirnas derived from these precursors in turn target four of the ARFs (three validated in this study) suggests that the TAS3-siRNA pathway indeed plays important regulatory roles in M. truncatula. Future experiments will address the functional aspects of TAS3-siRNA-guided ARF regulation in M. truncatula and other legumes. In summary, with a sequencing-depth of reads the present study has uncovered the existence of four legumespecific mirnas and four candidate mirnas in M. truncatula. By applying more robust deep sequencing technologies such as Sequencing-By-Synthesis (Illumina, Haywood, CA, USA), there is ample scope for the discovery of several additional novel mirnas. Indeed, while our manuscript was under review Szittya et al., reported eight new mirna families in M. truncatula by using a deep sequencing approach (Szittya et al., 2008). Interestingly, despite examining approx. 4 million reads there are only two small RNAs (one legume-specific mirna (mir1507) and one candidate mirna (srna19284)) that overlap between the study of Szittya et al. (2008) and this study. This suggests the identification of mirnas in New Phytologist (2009) 184: The Authors (2009) Journal compilation New Phytologist (2009)
13 Research 97 M. truncatula is far from being saturated. Importantly, we have uncovered legume-specific mirnas in this study which are not found in the deeply sequenced library (Szittya et al., 2008). Thus our study has complimented the published report (Szittya et al., 2008). Identification of small RNAs and their target genes is a highly useful resource for the large community of researchers working on gene regulation in M. truncatula and other legume crops. Further work is required to identify a near-complete set of mirnas and other novel small RNAs in M. truncatula. Acknowledgements Support for this research was provided by the Oklahoma Agricultural Experiment Station to R.S., and by the National Science Foundation grants IIS and DBI , a grant from the Alzheimer s Association and a grant from Monsanto to W.Z. We thank Drs Rao Uppalapati and Kiran Mysore (Samuel Roberts Noble Foundation, Ardmore) for providing us some of the tissues used in small RNA blot analysis. L.S. was a recipient of a BOYSCAST fellowship from the Department of Science and Technology, Government of India. References Abdel-Ghany SE, Pilon M MicroRNA-mediated systemic down-regulation of copper protein expression in response to low copper availability in arabidopsis. Journal of Biological Chemistry 283: Adenot X, Elmayan T, Lauressergues D, Boutet S, Bouche N, Gasciolli V, Vaucheret H DRB4-dependent TAS3 trans-acting sirnas control leaf morphology through AGO7. Current Biology 16: Allen E, Xie ZX, Gustafson AM, Carrington JC microrna-directed phasing during trans-acting sirna biogenesis in plants. Cell 121: Ambros V, Bartel B, Bartel DP, Burge CB, Carrington JC, Chen X, Dreyfuss G, Eddy SR, Griffiths-Jones S, Marshall M et al A uniform system for microrna annotation. RNA 9: Ameline-Torregrosa C, Wang BB, O Bleness MS, Deshpande S, Zhu H, Roe B, Young ND, Cannon SB Identification and characterization of nucleotide-binding site-leucine-rich repeat genes in the model plant Medicago truncatula. Plant Physiology 146: Arazi T, Talmor-Neiman M, Stav R, Riese M, Huijser P, Baulcombe DC Cloning and characterization of micro-rnas from moss. Plant Journal 43: Aukerman MJ, Sakai H Regulation of flowering time and floral organ identity by a microrna and its APETALA2-like target genes. Plant Cell 15: Axtell MJ, Jan C, Rajagopalan R, Bartel DP A two-hit trigger for sirna biogenesis in plants. Cell 127: Axtell MJ, Snyder JA, Bartell DP Common functions for diverse small RNAs of land plants. Plant Cell 19: Barakat A, Wall PK, Diloreto S, Depamphilis CW, Carlson JE Conservation and divergence of micrornas in Populus. BMC Genomics 8: 481. Bari R, Pant BD, Stitt M, Scheible WR PHO2, microrna399, and PHR1 define a phosphate-signaling pathway in plants. Plant Physiology 141: Bartel DP MicroRNAs: genomics, biogenesis, mechanism, and function. Cell 116: Benedito VA, Torres-Jerez I, Murray JD, Andriankaja A, Allen S, Kakar K, Wandrey M, Verdier J, Zuber H, Ott T et al A gene expression atlas of the model legume Medicago truncatula. Plant Journal 55: Boualem A, Laporte P, Jovanovic M, Laffont C, Plet J, Combier JP, Niebel A, Crespi M, Frugier F MicroRNA166 controls root and nodule development in Medicago truncatula. Plant Journal 54: Brodersen P, Sakvarelidze-Achard L, Bruun-Rasmussen M, Dunoyer P, Yamamoto YY, Sieburth L, Voinnet O Widespread translational inhibition by plant mirnas and sirnas. Science 320: Buhtz A, Springer F, Chappell L, Baulcombe DC, Kehr J Identification and characterization of small RNAs from the phloem of Brassica napus. Plant Journal 53: Chabaud M, de Carvalho-Niebel F, Barker DG Efficient transformation of Medicago truncatula cv. Jemalong using the hypervirulent Agrobacterium tumefaciens strain AGL1. Plant Cell Reports 22: Chen XM A microrna as a translational repressor of APETALA2 in Arabidopsis flower development. Science 303: Chiou TJ The role of micrornas in sensing nutrient stress. Plant, Cell & Environment 30: Combier JP, Frugier F, de Billy F, Boualem A, El-Yahyaoui F, Moreau S, Vernie T, Ott T, Gamas P, Crespi M et al MtHAP2-1 is a key transcriptional regulator of symbiotic nodule development regulated by microrna169 in Medicago truncatula. Genes & Development 20: Crane C, Dixon RA, Wang ZY Medicago truncatula transformation using root explants. Methods in Molecular Biology 343: Fahlgren N, Howell MD, Kasschau KD, Chapman EJ, Sullivan CM, Cumbie JS, Givan SA, Law TF, Grant SR, Dangl JL et al High-throughput sequencing of Arabidopsis micrornas: evidence for frequent birth and death of MIRNA genes. PLoS ONE 2: e219. Fahlgren N, Montgomery TA, Howell MD, Allen E, Dvorak SK, Alexander AL, Carrington JC Regulation of AUXIN RESPONSE FACTOR3 by TAS3 ta-sirna affects developmental timing and patterning in Arabidopsis. Current Biology 16: Fattash I, Voss B, Reski R, Hess WR, Frank W Evidence for the rapid expansion of microrna-mediated regulation in early land plant evolution. BMC Plant Biology 7: 13. Fujii H, Chiou TJ, Lin SI, Aung K, Zhu JK A mirna involved in phosphate-starvation response in Arabidopsis. Current Biology 15: Garcia D, Collier SA, Byrne ME, Martienssen RA Specification of leaf polarity in Arabidopsis via the trans-acting sirna pathway. Current Biology 16: Gasciolli V, Mallory AC, Bartel DP, Vaucheret H Partially redundant functions of Arabidopsis DICER-like enzymes and a role for DCL4 in producing trans-acting sirnas. Current Biology 15: Hofacker IL Vienna RNA secondary structure server. Nucleic Acids Research 31: Howell MD, Fahlgren N, Chapman EJ, Cumbie JS, Sullivan CM, Givan SA, Kasschau KD, Carrington JC Genome-wide analysis of the RNA-DEPENDENT RNA POLYMERASE6/DICER-LIKE4 pathway in Arabidopsis reveals dependency on mirna- and tasirnadirected targeting. Plant Cell 19: Jones-Rhoades MW, Bartel DP Computational identification of plant micrornas and their targets, including a stress-induced mirna. Molecular Cell 14: Jones-Rhoades MW, Bartel DP, Bartel B MicroRNAs and their regulatory roles in plants. Annual Review of Plant Biology 57: Liu B, Chen Z, Song X, Liu C, Cui X, Zhao X, Fang J, Xu W, Zhang H, Wang X et al Oryza sativa dicer-like4 reveals a key role for small interfering RNA silencing in plant development. Plant Cell 19: Lu C, Jeong DH, Kulkarni K, Pillay M, Nobuta K, German R, Thatcher SR, Maher C, Zhang L, Ware D et al Genome-wide analysis for discovery of rice micrornas reveals natural antisense The Authors (2009) New Phytologist (2009) 184: Journal compilation New Phytologist (2009)
Identification of mirnas in Eucalyptus globulus Plant by Computational Methods
 International Journal of Pharmaceutical Science Invention ISSN (Online): 2319 6718, ISSN (Print): 2319 670X Volume 2 Issue 5 May 2013 PP.70-74 Identification of mirnas in Eucalyptus globulus Plant by Computational
International Journal of Pharmaceutical Science Invention ISSN (Online): 2319 6718, ISSN (Print): 2319 670X Volume 2 Issue 5 May 2013 PP.70-74 Identification of mirnas in Eucalyptus globulus Plant by Computational
ANALYSIS OF SOYBEAN SMALL RNA POPULATIONS HLAING HLAING WIN THESIS
 ANALYSIS OF SOYBEAN SMALL RNA POPULATIONS BY HLAING HLAING WIN THESIS Submitted in partial fulfillment of the requirements for the degree of Master of Science in Bioinformatics in the Graduate College
ANALYSIS OF SOYBEAN SMALL RNA POPULATIONS BY HLAING HLAING WIN THESIS Submitted in partial fulfillment of the requirements for the degree of Master of Science in Bioinformatics in the Graduate College
Supplemental Figure 1. Small RNA size distribution from different soybean tissues.
 Supplemental Figure 1. Small RNA size distribution from different soybean tissues. The size of small RNAs was plotted versus frequency (percentage) among total sequences (A, C, E and G) or distinct sequences
Supplemental Figure 1. Small RNA size distribution from different soybean tissues. The size of small RNAs was plotted versus frequency (percentage) among total sequences (A, C, E and G) or distinct sequences
Cloning and Characterization of MicroRNAs from Rice W
 The Plant Cell, Vol. 17, 1397 1411, May 2005, www.plantcell.org ª 2005 American Society of Plant Biologists Cloning and Characterization of MicroRNAs from Rice W Ramanjulu Sunkar, Thomas Girke, Pradeep
The Plant Cell, Vol. 17, 1397 1411, May 2005, www.plantcell.org ª 2005 American Society of Plant Biologists Cloning and Characterization of MicroRNAs from Rice W Ramanjulu Sunkar, Thomas Girke, Pradeep
he micrornas of Caenorhabditis elegans (Lim et al. Genes & Development 2003)
 MicroRNAs: Genomics, Biogenesis, Mechanism, and Function (D. Bartel Cell 2004) he micrornas of Caenorhabditis elegans (Lim et al. Genes & Development 2003) Vertebrate MicroRNA Genes (Lim et al. Science
MicroRNAs: Genomics, Biogenesis, Mechanism, and Function (D. Bartel Cell 2004) he micrornas of Caenorhabditis elegans (Lim et al. Genes & Development 2003) Vertebrate MicroRNA Genes (Lim et al. Science
MicroRNAs as master regulators of the plant NB-LRR defense gene family via the production of phased, trans-acting sirnas
 RESOURCE/METHODOLOGY MicroRNAs as master regulators of the plant NB-LRR defense gene family via the production of phased, trans-acting sirnas Jixian Zhai, 1,2 Dong-Hoon Jeong, 1,2 Emanuele De Paoli, 1,2,7
RESOURCE/METHODOLOGY MicroRNAs as master regulators of the plant NB-LRR defense gene family via the production of phased, trans-acting sirnas Jixian Zhai, 1,2 Dong-Hoon Jeong, 1,2 Emanuele De Paoli, 1,2,7
IDENTIFICATION OF IN SILICO MIRNAS IN FOUR PLANT SPECIES FROM FABACEAE FAMILY
 Original scientific paper 10.7251/AGRENG1803122A UDC633:34+582.736.3]:577.2 IDENTIFICATION OF IN SILICO MIRNAS IN FOUR PLANT SPECIES FROM FABACEAE FAMILY Bihter AVSAR 1*, Danial ESMAEILI ALIABADI 2 1 Sabanci
Original scientific paper 10.7251/AGRENG1803122A UDC633:34+582.736.3]:577.2 IDENTIFICATION OF IN SILICO MIRNAS IN FOUR PLANT SPECIES FROM FABACEAE FAMILY Bihter AVSAR 1*, Danial ESMAEILI ALIABADI 2 1 Sabanci
Identification of novel and candidate mirnas in rice by high throughput sequencing. and J-K Zhu,
 Identification of novel and candidate mirnas in rice by high throughput sequencing Ramanjulu Sunkar 1,4*, Xuefeng Zhou 2* Yun Zheng 2, Weixiong Zhang 2, and Jian-Kang Zhu, 3,4 1 Department of Biochemistry
Identification of novel and candidate mirnas in rice by high throughput sequencing Ramanjulu Sunkar 1,4*, Xuefeng Zhou 2* Yun Zheng 2, Weixiong Zhang 2, and Jian-Kang Zhu, 3,4 1 Department of Biochemistry
Genome-Wide Identification of MicroRNAs in Medicago truncatula by High-Throughput Sequencing. Tian-Zuo Wang and Wen-Hao Zhang
 Chapter 6 Genome-Wide Identification of MicroRNAs in Medicago truncatula by High-Throughput Sequencing Abstract MicroRNAs (mirnas) are small, endogenous RNAs that play important regulatory roles in development
Chapter 6 Genome-Wide Identification of MicroRNAs in Medicago truncatula by High-Throughput Sequencing Abstract MicroRNAs (mirnas) are small, endogenous RNAs that play important regulatory roles in development
High AU content: a signature of upregulated mirna in cardiac diseases
 https://helda.helsinki.fi High AU content: a signature of upregulated mirna in cardiac diseases Gupta, Richa 2010-09-20 Gupta, R, Soni, N, Patnaik, P, Sood, I, Singh, R, Rawal, K & Rani, V 2010, ' High
https://helda.helsinki.fi High AU content: a signature of upregulated mirna in cardiac diseases Gupta, Richa 2010-09-20 Gupta, R, Soni, N, Patnaik, P, Sood, I, Singh, R, Rawal, K & Rani, V 2010, ' High
The Cornucopia of Small RNAs in Plant Genomes
 Rice (2008) 1:52 62 DOI 10.1007/s12284-008-9008-5 REVIEW The Cornucopia of Small RNAs in Plant Genomes Stacey A. Simon & Jixian Zhai & Jia Zeng & Blake C. Meyers Received: 10 June 2008 /Accepted: 3 July
Rice (2008) 1:52 62 DOI 10.1007/s12284-008-9008-5 REVIEW The Cornucopia of Small RNAs in Plant Genomes Stacey A. Simon & Jixian Zhai & Jia Zeng & Blake C. Meyers Received: 10 June 2008 /Accepted: 3 July
Circular RNAs (circrnas) act a stable mirna sponges
 Circular RNAs (circrnas) act a stable mirna sponges cernas compete for mirnas Ancestal mrna (+3 UTR) Pseudogene RNA (+3 UTR homolgy region) The model holds true for all RNAs that share a mirna binding
Circular RNAs (circrnas) act a stable mirna sponges cernas compete for mirnas Ancestal mrna (+3 UTR) Pseudogene RNA (+3 UTR homolgy region) The model holds true for all RNAs that share a mirna binding
In silico identification of mirnas and their targets from the expressed sequence tags of Raphanus sativus
 www.bioinformation.net Hypothesis Volume 8(2) In silico identification of mirnas and their targets from the expressed sequence tags of Raphanus sativus Charuvaka Muvva 1 *, Lata Tewari 2, Kasoju Aruna
www.bioinformation.net Hypothesis Volume 8(2) In silico identification of mirnas and their targets from the expressed sequence tags of Raphanus sativus Charuvaka Muvva 1 *, Lata Tewari 2, Kasoju Aruna
High-resolution identification and abundance profiling of cassava (Manihot esculenta Crantz) micrornas
 Khatabi et al. BMC Genomics (216) 17:85 DOI 1.1186/s12864-16-2391-1 RESEARCH ARTICLE High-resolution identification and abundance profiling of cassava (Manihot esculenta Crantz) micrornas Behnam Khatabi
Khatabi et al. BMC Genomics (216) 17:85 DOI 1.1186/s12864-16-2391-1 RESEARCH ARTICLE High-resolution identification and abundance profiling of cassava (Manihot esculenta Crantz) micrornas Behnam Khatabi
Bi 8 Lecture 17. interference. Ellen Rothenberg 1 March 2016
 Bi 8 Lecture 17 REGulation by RNA interference Ellen Rothenberg 1 March 2016 Protein is not the only regulatory molecule affecting gene expression: RNA itself can be negative regulator RNA does not need
Bi 8 Lecture 17 REGulation by RNA interference Ellen Rothenberg 1 March 2016 Protein is not the only regulatory molecule affecting gene expression: RNA itself can be negative regulator RNA does not need
Cross species analysis of genomics data. Computational Prediction of mirnas and their targets
 02-716 Cross species analysis of genomics data Computational Prediction of mirnas and their targets Outline Introduction Brief history mirna Biogenesis Why Computational Methods? Computational Methods
02-716 Cross species analysis of genomics data Computational Prediction of mirnas and their targets Outline Introduction Brief history mirna Biogenesis Why Computational Methods? Computational Methods
Criteria for Annotation of Plant MicroRNAs
 The Plant Cell, Vol. 20: 3186 3190, December 2008, www.plantcell.org ª 2008 American Society of Plant Biologists Criteria for Annotation of Plant MicroRNAs Blake C. Meyers, a,1 Michael J. Axtell, b,1 Bonnie
The Plant Cell, Vol. 20: 3186 3190, December 2008, www.plantcell.org ª 2008 American Society of Plant Biologists Criteria for Annotation of Plant MicroRNAs Blake C. Meyers, a,1 Michael J. Axtell, b,1 Bonnie
VirusDetect pipeline - virus detection with small RNA sequencing
 VirusDetect pipeline - virus detection with small RNA sequencing CSC webinar 16.1.2018 Eija Korpelainen, Kimmo Mattila, Maria Lehtivaara Big thanks to Jan Kreuze and Jari Valkonen! Outline Small interfering
VirusDetect pipeline - virus detection with small RNA sequencing CSC webinar 16.1.2018 Eija Korpelainen, Kimmo Mattila, Maria Lehtivaara Big thanks to Jan Kreuze and Jari Valkonen! Outline Small interfering
Widespread Long Noncoding RNAs as Endogenous Target Mimics for MicroRNAs in Plants 1[W]
![Widespread Long Noncoding RNAs as Endogenous Target Mimics for MicroRNAs in Plants 1[W] Widespread Long Noncoding RNAs as Endogenous Target Mimics for MicroRNAs in Plants 1[W]](/thumbs/96/127589351.jpg) Widespread Long Noncoding RNAs as Endogenous Target Mimics for MicroRNAs in Plants 1[W] Hua-Jun Wu 2, Zhi-Min Wang 2, Meng Wang, and Xiu-Jie Wang* State Key Laboratory of Plant Genomics, Institute of Genetics
Widespread Long Noncoding RNAs as Endogenous Target Mimics for MicroRNAs in Plants 1[W] Hua-Jun Wu 2, Zhi-Min Wang 2, Meng Wang, and Xiu-Jie Wang* State Key Laboratory of Plant Genomics, Institute of Genetics
MicroRNAs and Their Regulatory Roles in Plants
 Annu. Rev. lant Biol. 2006. 57:19 53 The Annual Review of lant Biology is online at plant.annualreviews.org doi: 10.1146/ annurev.arplant.57.032905.105218 opyright c 2006 by Annual Reviews. All rights
Annu. Rev. lant Biol. 2006. 57:19 53 The Annual Review of lant Biology is online at plant.annualreviews.org doi: 10.1146/ annurev.arplant.57.032905.105218 opyright c 2006 by Annual Reviews. All rights
Eukaryotic small RNA Small RNAseq data analysis for mirna identification
 Eukaryotic small RNA Small RNAseq data analysis for mirna identification P. Bardou, C. Gaspin, S. Maman, J. Mariette, O. Rué, M. Zytnicki INRA Sigenae Toulouse INRA MIA Toulouse GenoToul Bioinfo INRA MaIAGE
Eukaryotic small RNA Small RNAseq data analysis for mirna identification P. Bardou, C. Gaspin, S. Maman, J. Mariette, O. Rué, M. Zytnicki INRA Sigenae Toulouse INRA MIA Toulouse GenoToul Bioinfo INRA MaIAGE
Identification of mirna from Porphyra yezoensis by High-Throughput Sequencing and Bioinformatics Analysis
 Identification of mirna from Porphyra yezoensis by High-Throughput Sequencing and Bioinformatics Analysis Chengwei Liang 1., Xiaowen Zhang 2., Jian Zou 2, Dong Xu 2, Feng Su 1, Naihao Ye 2 * 1 Qingdao
Identification of mirna from Porphyra yezoensis by High-Throughput Sequencing and Bioinformatics Analysis Chengwei Liang 1., Xiaowen Zhang 2., Jian Zou 2, Dong Xu 2, Feng Su 1, Naihao Ye 2 * 1 Qingdao
MicroRNA and Male Infertility: A Potential for Diagnosis
 Review Article MicroRNA and Male Infertility: A Potential for Diagnosis * Abstract MicroRNAs (mirnas) are small non-coding single stranded RNA molecules that are physiologically produced in eukaryotic
Review Article MicroRNA and Male Infertility: A Potential for Diagnosis * Abstract MicroRNAs (mirnas) are small non-coding single stranded RNA molecules that are physiologically produced in eukaryotic
Table S1. Relative abundance of AGO1/4 proteins in different organs. Table S2. Summary of smrna datasets from various samples.
 Supplementary files Table S1. Relative abundance of AGO1/4 proteins in different organs. Table S2. Summary of smrna datasets from various samples. Table S3. Specificity of AGO1- and AGO4-preferred 24-nt
Supplementary files Table S1. Relative abundance of AGO1/4 proteins in different organs. Table S2. Summary of smrna datasets from various samples. Table S3. Specificity of AGO1- and AGO4-preferred 24-nt
MicroRNA in Cancer Karen Dybkær 2013
 MicroRNA in Cancer Karen Dybkær RNA Ribonucleic acid Types -Coding: messenger RNA (mrna) coding for proteins -Non-coding regulating protein formation Ribosomal RNA (rrna) Transfer RNA (trna) Small nuclear
MicroRNA in Cancer Karen Dybkær RNA Ribonucleic acid Types -Coding: messenger RNA (mrna) coding for proteins -Non-coding regulating protein formation Ribosomal RNA (rrna) Transfer RNA (trna) Small nuclear
Expression of mirnas confers enhanced tolerance to drought and salt stress in Finger millet (Eleusine coracona)
 Journal of Stress Physiology & Biochemistry, Vol. 9 No. 3 2013, pp. 220-231 ISSN 1997-0838 Original Text Copyright 2013 by Nageshbabu, Usha, Jyothi, Sharadamma, Rai, Devaraj ORIGINAL ARTICLE Expression
Journal of Stress Physiology & Biochemistry, Vol. 9 No. 3 2013, pp. 220-231 ISSN 1997-0838 Original Text Copyright 2013 by Nageshbabu, Usha, Jyothi, Sharadamma, Rai, Devaraj ORIGINAL ARTICLE Expression
Small RNA and PARE sequencing in flower bud reveal the involvement of srnas in endodormancy release of Japanese pear (Pyrus pyrifolia 'Kosui')
 Bai et al. BMC Genomics (2016) 17:230 DOI 10.1186/s12864-016-2514-8 RESEARCH ARTICLE Open Access Small RNA and PARE sequencing in flower bud reveal the involvement of srnas in endodormancy release of Japanese
Bai et al. BMC Genomics (2016) 17:230 DOI 10.1186/s12864-016-2514-8 RESEARCH ARTICLE Open Access Small RNA and PARE sequencing in flower bud reveal the involvement of srnas in endodormancy release of Japanese
MicroRNAs, RNA Modifications, RNA Editing. Bora E. Baysal MD, PhD Oncology for Scientists Lecture Tue, Oct 17, 2017, 3:30 PM - 5:00 PM
 MicroRNAs, RNA Modifications, RNA Editing Bora E. Baysal MD, PhD Oncology for Scientists Lecture Tue, Oct 17, 2017, 3:30 PM - 5:00 PM Expanding world of RNAs mrna, messenger RNA (~20,000) trna, transfer
MicroRNAs, RNA Modifications, RNA Editing Bora E. Baysal MD, PhD Oncology for Scientists Lecture Tue, Oct 17, 2017, 3:30 PM - 5:00 PM Expanding world of RNAs mrna, messenger RNA (~20,000) trna, transfer
sirna count per 50 kb small RNAs matching the direct strand Repeat length (bp) per 50 kb repeats in the chromosome
 Qi et al. 26-3-2564C Qi et al., Figure S1 sirna count per 5 kb small RNAs matching the direct strand sirna count per 5 kb small RNAs matching the complementary strand Repeat length (bp) per 5 kb repeats
Qi et al. 26-3-2564C Qi et al., Figure S1 sirna count per 5 kb small RNAs matching the direct strand sirna count per 5 kb small RNAs matching the complementary strand Repeat length (bp) per 5 kb repeats
RNA interference induced hepatotoxicity results from loss of the first synthesized isoform of microrna-122 in mice
 SUPPLEMENTARY INFORMATION RNA interference induced hepatotoxicity results from loss of the first synthesized isoform of microrna-122 in mice Paul N Valdmanis, Shuo Gu, Kirk Chu, Lan Jin, Feijie Zhang,
SUPPLEMENTARY INFORMATION RNA interference induced hepatotoxicity results from loss of the first synthesized isoform of microrna-122 in mice Paul N Valdmanis, Shuo Gu, Kirk Chu, Lan Jin, Feijie Zhang,
mirna Dr. S Hosseini-Asl
 mirna Dr. S Hosseini-Asl 1 2 MicroRNAs (mirnas) are small noncoding RNAs which enhance the cleavage or translational repression of specific mrna with recognition site(s) in the 3 - untranslated region
mirna Dr. S Hosseini-Asl 1 2 MicroRNAs (mirnas) are small noncoding RNAs which enhance the cleavage or translational repression of specific mrna with recognition site(s) in the 3 - untranslated region
Small RNAs and how to analyze them using sequencing
 Small RNAs and how to analyze them using sequencing RNA-seq Course November 8th 2017 Marc Friedländer ComputaAonal RNA Biology Group SciLifeLab / Stockholm University Special thanks to Jakub Westholm for
Small RNAs and how to analyze them using sequencing RNA-seq Course November 8th 2017 Marc Friedländer ComputaAonal RNA Biology Group SciLifeLab / Stockholm University Special thanks to Jakub Westholm for
NOVEL FUNCTION OF mirnas IN REGULATING GENE EXPRESSION. Ana M. Martinez
 NOVEL FUNCTION OF mirnas IN REGULATING GENE EXPRESSION Ana M. Martinez Switching from Repression to Activation: MicroRNAs can Up-Regulate Translation. Shoba Vasudevan, Yingchun Tong, Joan A. Steitz AU-rich
NOVEL FUNCTION OF mirnas IN REGULATING GENE EXPRESSION Ana M. Martinez Switching from Repression to Activation: MicroRNAs can Up-Regulate Translation. Shoba Vasudevan, Yingchun Tong, Joan A. Steitz AU-rich
Regulation of Gene Expression in Eukaryotes
 Ch. 19 Regulation of Gene Expression in Eukaryotes BIOL 222 Differential Gene Expression in Eukaryotes Signal Cells in a multicellular eukaryotic organism genetically identical differential gene expression
Ch. 19 Regulation of Gene Expression in Eukaryotes BIOL 222 Differential Gene Expression in Eukaryotes Signal Cells in a multicellular eukaryotic organism genetically identical differential gene expression
Novel and Stress-Regulated MicroRNAs and Other Small RNAs from Arabidopsis W
 The Plant Cell, Vol. 16, 2001 2019, August 2004, www.plantcell.org ª 2004 American Society of Plant Biologists Novel and Stress-Regulated MicroRNAs and Other Small RNAs from Arabidopsis W Ramanjulu Sunkar
The Plant Cell, Vol. 16, 2001 2019, August 2004, www.plantcell.org ª 2004 American Society of Plant Biologists Novel and Stress-Regulated MicroRNAs and Other Small RNAs from Arabidopsis W Ramanjulu Sunkar
Analysis of small RNAs from Drosophila Schneider cells using the Small RNA assay on the Agilent 2100 bioanalyzer. Application Note
 Analysis of small RNAs from Drosophila Schneider cells using the Small RNA assay on the Agilent 2100 bioanalyzer Application Note Odile Sismeiro, Jean-Yves Coppée, Christophe Antoniewski, and Hélène Thomassin
Analysis of small RNAs from Drosophila Schneider cells using the Small RNA assay on the Agilent 2100 bioanalyzer Application Note Odile Sismeiro, Jean-Yves Coppée, Christophe Antoniewski, and Hélène Thomassin
Genome-Wide Profiling and Analysis of Arabidopsis sirnas
 Genome-Wide Profiling and Analysis of Arabidopsis sirnas PLoS BIOLOGY Kristin D. Kasschau 1,2, Noah Fahlgren 1,2,3, Elisabeth J. Chapman 1,2,3, Christopher M. Sullivan 1,2, Jason S. Cumbie 1,2, Scott A.
Genome-Wide Profiling and Analysis of Arabidopsis sirnas PLoS BIOLOGY Kristin D. Kasschau 1,2, Noah Fahlgren 1,2,3, Elisabeth J. Chapman 1,2,3, Christopher M. Sullivan 1,2, Jason S. Cumbie 1,2, Scott A.
Genome-Wide Identification and Comparative Analysis of Conserved and Novel MicroRNAs in Grafted Watermelon by High-Throughput Sequencing
 Genome-Wide Identification and Comparative Analysis of Conserved and Novel MicroRNAs in Grafted Watermelon by High-Throughput Sequencing Na Liu 1,2, Jinghua Yang 1,2, Shaogui Guo 3, Yong Xu 3 *, Mingfang
Genome-Wide Identification and Comparative Analysis of Conserved and Novel MicroRNAs in Grafted Watermelon by High-Throughput Sequencing Na Liu 1,2, Jinghua Yang 1,2, Shaogui Guo 3, Yong Xu 3 *, Mingfang
Gynophore mirna analysis at different developmental stages in Arachis duranensis
 Gynophore mirna analysis at different developmental stages in Arachis duranensis Y. Shen 1,2 *, Y.H. Liu 1 *, X.J. Zhang 3, Q. Sha 1 and Z.D. Chen 1 1 Institute of Industrial Crops, Jiangsu Academy of
Gynophore mirna analysis at different developmental stages in Arachis duranensis Y. Shen 1,2 *, Y.H. Liu 1 *, X.J. Zhang 3, Q. Sha 1 and Z.D. Chen 1 1 Institute of Industrial Crops, Jiangsu Academy of
Phenomena first observed in petunia
 Vectors for RNAi Phenomena first observed in petunia Attempted to overexpress chalone synthase (anthrocyanin pigment gene) in petunia. (trying to darken flower color) Caused the loss of pigment. Bill Douherty
Vectors for RNAi Phenomena first observed in petunia Attempted to overexpress chalone synthase (anthrocyanin pigment gene) in petunia. (trying to darken flower color) Caused the loss of pigment. Bill Douherty
Human breast milk mirna, maternal probiotic supplementation and atopic dermatitis in offsrping
 Human breast milk mirna, maternal probiotic supplementation and atopic dermatitis in offsrping Melanie Rae Simpson PhD candidate Department of Public Health and General Practice Norwegian University of
Human breast milk mirna, maternal probiotic supplementation and atopic dermatitis in offsrping Melanie Rae Simpson PhD candidate Department of Public Health and General Practice Norwegian University of
Research Article Base Composition Characteristics of Mammalian mirnas
 Journal of Nucleic Acids Volume 2013, Article ID 951570, 6 pages http://dx.doi.org/10.1155/2013/951570 Research Article Base Composition Characteristics of Mammalian mirnas Bin Wang Department of Chemistry,
Journal of Nucleic Acids Volume 2013, Article ID 951570, 6 pages http://dx.doi.org/10.1155/2013/951570 Research Article Base Composition Characteristics of Mammalian mirnas Bin Wang Department of Chemistry,
RNA-Seq Atlas of Glycine max: A guide to the Soybean Transcriptome
 RNA-Seq Atlas of Glycine max: A guide to the Soybean Transcriptome How do novel structures or functions evolve? A more relevant example Symbiosis and Nitrogen Fixation Limpens & Bisseling (23) Curr. Opin.
RNA-Seq Atlas of Glycine max: A guide to the Soybean Transcriptome How do novel structures or functions evolve? A more relevant example Symbiosis and Nitrogen Fixation Limpens & Bisseling (23) Curr. Opin.
Small RNAs and how to analyze them using sequencing
 Small RNAs and how to analyze them using sequencing Jakub Orzechowski Westholm (1) Long- term bioinforma=cs support, Science For Life Laboratory Stockholm (2) Department of Biophysics and Biochemistry,
Small RNAs and how to analyze them using sequencing Jakub Orzechowski Westholm (1) Long- term bioinforma=cs support, Science For Life Laboratory Stockholm (2) Department of Biophysics and Biochemistry,
COMPUTATIONAL ANALYSIS OF SMALL RNAs IN MAIZE MUTANTS WITH DEFECTS IN DEVELOPMENT AND PARAMUTATION. Reza Hammond
 COMPUTATIONAL ANALYSIS OF SMALL RNAs IN MAIZE MUTANTS WITH DEFECTS IN DEVELOPMENT AND PARAMUTATION by Reza Hammond A thesis submitted to the Faculty of the University of Delaware in partial fulfillment
COMPUTATIONAL ANALYSIS OF SMALL RNAs IN MAIZE MUTANTS WITH DEFECTS IN DEVELOPMENT AND PARAMUTATION by Reza Hammond A thesis submitted to the Faculty of the University of Delaware in partial fulfillment
Characterization and comparison of flower bud micrornas from yellow-horn species
 Characterization and comparison of flower bud micrornas from yellow-horn species Y. Ao 1,2 1 Key Laboratory for Silviculture and Conservation, Ministry of Education, Beijing Forestry University, Beijing,
Characterization and comparison of flower bud micrornas from yellow-horn species Y. Ao 1,2 1 Key Laboratory for Silviculture and Conservation, Ministry of Education, Beijing Forestry University, Beijing,
Identification of aluminum-responsive micrornas in Medicago truncatula by genome-wide high-throughput sequencing
 Planta (2012) 235:375 386 DOI 10.1007/s00425-011-1514-9 ORIGINAL ARTICLE Identification of aluminum-responsive micrornas in Medicago truncatula by genome-wide high-throughput sequencing Lei Chen Tianzuo
Planta (2012) 235:375 386 DOI 10.1007/s00425-011-1514-9 ORIGINAL ARTICLE Identification of aluminum-responsive micrornas in Medicago truncatula by genome-wide high-throughput sequencing Lei Chen Tianzuo
Genome-Wide Analysis of leafbladeless1-regulated and Phased Small RNAs Underscores the Importance of the TAS3 ta-sirna Pathway to Maize Development
 Genome-Wide Analysis of leafbladeless1-regulated and Phased Small RNAs Underscores the Importance of the TAS3 ta-sirna Pathway to Maize Development Marcela C. Dotto 1, Katherine A. Petsch 1, Milo J. Aukerman
Genome-Wide Analysis of leafbladeless1-regulated and Phased Small RNAs Underscores the Importance of the TAS3 ta-sirna Pathway to Maize Development Marcela C. Dotto 1, Katherine A. Petsch 1, Milo J. Aukerman
Identification of UV-B-induced micrornas in wheat
 Identification of UV-B-induced micrornas in wheat B. Wang*, Y.F. Sun*, N. Song, X.J. Wang, H. Feng, L.L. Huang and Z.S. Kang College of Plant Protection and State Key Laboratory of Crop Stress Biology
Identification of UV-B-induced micrornas in wheat B. Wang*, Y.F. Sun*, N. Song, X.J. Wang, H. Feng, L.L. Huang and Z.S. Kang College of Plant Protection and State Key Laboratory of Crop Stress Biology
Genome-Wide Identification of mirnas Responsive to Drought in Peach (Prunus persica) by High-Throughput Deep Sequencing
 Genome-Wide Identification of mirnas Responsive to Drought in Peach (Prunus persica) by High-Throughput Deep Sequencing Vahap Eldem 1,2, Ufuk Çelikkol Akçay 3, Esma Ozhuner 1, Yakup Bakır 4, Serkan Uranbey
Genome-Wide Identification of mirnas Responsive to Drought in Peach (Prunus persica) by High-Throughput Deep Sequencing Vahap Eldem 1,2, Ufuk Çelikkol Akçay 3, Esma Ozhuner 1, Yakup Bakır 4, Serkan Uranbey
GENOME-WIDE COMPUTATIONAL ANALYSIS OF SMALL NUCLEAR RNA GENES OF ORYZA SATIVA (INDICA AND JAPONICA)
 GENOME-WIDE COMPUTATIONAL ANALYSIS OF SMALL NUCLEAR RNA GENES OF ORYZA SATIVA (INDICA AND JAPONICA) M.SHASHIKANTH, A.SNEHALATHARANI, SK. MUBARAK AND K.ULAGANATHAN Center for Plant Molecular Biology, Osmania
GENOME-WIDE COMPUTATIONAL ANALYSIS OF SMALL NUCLEAR RNA GENES OF ORYZA SATIVA (INDICA AND JAPONICA) M.SHASHIKANTH, A.SNEHALATHARANI, SK. MUBARAK AND K.ULAGANATHAN Center for Plant Molecular Biology, Osmania
Bacteria-responsive micrornas regulate plant innate immunity by modulating plant hormone networks
 Plant Mol Biol (2011) 75:93 105 DOI 10.1007/s11103-010-9710-8 Bacteria-responsive micrornas regulate plant innate immunity by modulating plant hormone networks Weixiong Zhang Shang Gao Xiang Zhou Padmanabhan
Plant Mol Biol (2011) 75:93 105 DOI 10.1007/s11103-010-9710-8 Bacteria-responsive micrornas regulate plant innate immunity by modulating plant hormone networks Weixiong Zhang Shang Gao Xiang Zhou Padmanabhan
Transcriptome-wide analysis of microrna expression in the malaria mosquito Anopheles gambiae
 Biryukova et al. BMC Genomics 2014, 15:557 RESEARCH ARTICLE Open Access Transcriptome-wide analysis of microrna expression in the malaria mosquito Anopheles gambiae Inna Biryukova 1*, Tao Ye 2 and Elena
Biryukova et al. BMC Genomics 2014, 15:557 RESEARCH ARTICLE Open Access Transcriptome-wide analysis of microrna expression in the malaria mosquito Anopheles gambiae Inna Biryukova 1*, Tao Ye 2 and Elena
micrornas (mirna) and Biomarkers
 micrornas (mirna) and Biomarkers Small RNAs Make Big Splash mirnas & Genome Function Biomarkers in Cancer Future Prospects Javed Khan M.D. National Cancer Institute EORTC-NCI-ASCO November 2007 The Human
micrornas (mirna) and Biomarkers Small RNAs Make Big Splash mirnas & Genome Function Biomarkers in Cancer Future Prospects Javed Khan M.D. National Cancer Institute EORTC-NCI-ASCO November 2007 The Human
WHITE PAPER. Increasing Ligation Efficiency and Discovery of mirnas for Small RNA NGS Sequencing Library Prep with Plant Samples
 WHITE PPER Increasing Ligation Efficiency and iscovery of mirns for Small RN NGS Sequencing Library Prep with Plant Samples ioo Scientific White Paper May 2017 bstract MicroRNs (mirns) are 18-22 nucleotide
WHITE PPER Increasing Ligation Efficiency and iscovery of mirns for Small RN NGS Sequencing Library Prep with Plant Samples ioo Scientific White Paper May 2017 bstract MicroRNs (mirns) are 18-22 nucleotide
Supplementary Figure 1 Transcription assay of nine ABA-responsive PP2C. Transcription assay of nine ABA-responsive PP2C genes. Total RNA was isolated
 Supplementary Figure 1 Transcription assay of nine ABA-responsive PP2C genes. Transcription assay of nine ABA-responsive PP2C genes. Total RNA was isolated from 7 day-old seedlings treated with or without
Supplementary Figure 1 Transcription assay of nine ABA-responsive PP2C genes. Transcription assay of nine ABA-responsive PP2C genes. Total RNA was isolated from 7 day-old seedlings treated with or without
Identification and Characterization of MicroRNAs from Barley (Hordeum vulgare L.) by High-Throughput Sequencing
 Int. J. Mol. Sci. 2012, 13, 2973-2984; doi:10.3390/ijms13032973 Article OPEN ACCESS International Journal of Molecular Sciences ISSN 1422-0067 www.mdpi.com/journal/ijms Identification and Characterization
Int. J. Mol. Sci. 2012, 13, 2973-2984; doi:10.3390/ijms13032973 Article OPEN ACCESS International Journal of Molecular Sciences ISSN 1422-0067 www.mdpi.com/journal/ijms Identification and Characterization
Supplementary Figure 1
 Supplementary Figure 1 Asymmetrical function of 5p and 3p arms of mir-181 and mir-30 families and mir-142 and mir-154. (a) Control experiments using mirna sensor vector and empty pri-mirna overexpression
Supplementary Figure 1 Asymmetrical function of 5p and 3p arms of mir-181 and mir-30 families and mir-142 and mir-154. (a) Control experiments using mirna sensor vector and empty pri-mirna overexpression
Identification of mirnas and Their Targets in Cotton Inoculated with Verticillium dahliae by High-Throughput Sequencing and Degradome Analysis
 Int. J. Mol. Sci. 2015, 16, 14749-14768; doi:10.3390/ijms160714749 Article OPEN ACCESS International Journal of Molecular Sciences ISSN 1422-0067 www.mdpi.com/journal/ijms Identification of mirnas and
Int. J. Mol. Sci. 2015, 16, 14749-14768; doi:10.3390/ijms160714749 Article OPEN ACCESS International Journal of Molecular Sciences ISSN 1422-0067 www.mdpi.com/journal/ijms Identification of mirnas and
Supplementary information
 Supplementary information Human Cytomegalovirus MicroRNA mir-us4-1 Inhibits CD8 + T Cell Response by Targeting ERAP1 Sungchul Kim, Sanghyun Lee, Jinwook Shin, Youngkyun Kim, Irini Evnouchidou, Donghyun
Supplementary information Human Cytomegalovirus MicroRNA mir-us4-1 Inhibits CD8 + T Cell Response by Targeting ERAP1 Sungchul Kim, Sanghyun Lee, Jinwook Shin, Youngkyun Kim, Irini Evnouchidou, Donghyun
Prediction of micrornas and their targets
 Prediction of micrornas and their targets Introduction Brief history mirna Biogenesis Computational Methods Mature and precursor mirna prediction mirna target gene prediction Summary micrornas? RNA can
Prediction of micrornas and their targets Introduction Brief history mirna Biogenesis Computational Methods Mature and precursor mirna prediction mirna target gene prediction Summary micrornas? RNA can
Alternative RNA processing: Two examples of complex eukaryotic transcription units and the effect of mutations on expression of the encoded proteins.
 Alternative RNA processing: Two examples of complex eukaryotic transcription units and the effect of mutations on expression of the encoded proteins. The RNA transcribed from a complex transcription unit
Alternative RNA processing: Two examples of complex eukaryotic transcription units and the effect of mutations on expression of the encoded proteins. The RNA transcribed from a complex transcription unit
Breast cancer. Risk factors you cannot change include: Treatment Plan Selection. Inferring Transcriptional Module from Breast Cancer Profile Data
 Breast cancer Inferring Transcriptional Module from Breast Cancer Profile Data Breast Cancer and Targeted Therapy Microarray Profile Data Inferring Transcriptional Module Methods CSC 177 Data Warehousing
Breast cancer Inferring Transcriptional Module from Breast Cancer Profile Data Breast Cancer and Targeted Therapy Microarray Profile Data Inferring Transcriptional Module Methods CSC 177 Data Warehousing
Rajesh Singh Tomar et al /J. Pharm. Sci. & Res. Vol. 9(5), 2017, Amity Institute of Biotechnology, Amity University Madhya Pradesh, Gwalior
 In-Silico identification of mirnas and their targets using Expressed Sequence Tags (ESTs) in Plants Rajesh Singh Tomar, Gagan Jyot Kaur, Shuchi Kaushik, Raghvendra Kumar Mishra* Amity Institute of Biotechnology,
In-Silico identification of mirnas and their targets using Expressed Sequence Tags (ESTs) in Plants Rajesh Singh Tomar, Gagan Jyot Kaur, Shuchi Kaushik, Raghvendra Kumar Mishra* Amity Institute of Biotechnology,
Regulation of Floral Organ Identity. Dr. Chloe Diamond Mara
 Regulation of Floral Organ Identity Dr. Chloe Diamond Mara Flower Development Angiosperms (flowering plants) are the most widespread group of land plants Flowers are the reproductive organs that consist
Regulation of Floral Organ Identity Dr. Chloe Diamond Mara Flower Development Angiosperms (flowering plants) are the most widespread group of land plants Flowers are the reproductive organs that consist
CELLULAR & MOLECULAR BIOLOGY LETTERS Received: 18 January 2013 Volume 18 (2013) pp
 CELLULAR & MOLECULAR BIOLOGY LETTERS http://www.cmbl.org.pl Received: 18 January 2013 Volume 18 (2013) pp 416-432 Final form accepted: 10 July 2013 DOI: 10.2478/s11658-013-0097-9 Published online: July
CELLULAR & MOLECULAR BIOLOGY LETTERS http://www.cmbl.org.pl Received: 18 January 2013 Volume 18 (2013) pp 416-432 Final form accepted: 10 July 2013 DOI: 10.2478/s11658-013-0097-9 Published online: July
Sequence variation and selection of small RNAs in domesticated rice
 RESEARCH ARTICLE Open Access Research article Sequence variation and selection of small RNAs in domesticated rice Yu Wang 1, Dan Shen 1, Shiping Bo 1, Huan Chen 1, Jian Zheng 1, Qian-Hao Zhu 2, Daguang
RESEARCH ARTICLE Open Access Research article Sequence variation and selection of small RNAs in domesticated rice Yu Wang 1, Dan Shen 1, Shiping Bo 1, Huan Chen 1, Jian Zheng 1, Qian-Hao Zhu 2, Daguang
reads observed in trnas from the analysis of RNAs carrying a 5 -OH ends isolated from cells induced to express
 Supplementary Figure 1. VapC-mt4 cleaves trna Ala2 in E. coli. Histograms representing the fold change in reads observed in trnas from the analysis of RNAs carrying a 5 -OH ends isolated from cells induced
Supplementary Figure 1. VapC-mt4 cleaves trna Ala2 in E. coli. Histograms representing the fold change in reads observed in trnas from the analysis of RNAs carrying a 5 -OH ends isolated from cells induced
Differentially expressed microrna cohorts in seed development may contribute to poor grain filling of inferior spikelets in rice
 Peng et al. BMC Plant Biology 2014, 14:196 RESEARCH ARTICLE Open Access Differentially expressed microrna cohorts in seed development may contribute to poor grain filling of inferior spikelets in rice
Peng et al. BMC Plant Biology 2014, 14:196 RESEARCH ARTICLE Open Access Differentially expressed microrna cohorts in seed development may contribute to poor grain filling of inferior spikelets in rice
Analysis of mirna expression under stress in Arabidopsis thaliana
 Analysis of mirna expression under stress in Arabidopsis thaliana Aida Hajdarpašić*, Pia Ruggenthaler Max F. Perutz Laboratories, Medical University of Vienna, Department of Medical Biochemistry, Dr. Bohrgasse
Analysis of mirna expression under stress in Arabidopsis thaliana Aida Hajdarpašić*, Pia Ruggenthaler Max F. Perutz Laboratories, Medical University of Vienna, Department of Medical Biochemistry, Dr. Bohrgasse
Small RNAs and Cross-Kingdom RNAi in Plant-Microbial Interaction Hailing Jin
 Small RNAs and Cross-Kingdom RNAi in Plant-Microbial Interaction Hailing Jin Department of Plant Pathology & Microbiology Institute for Integrative Genome Biology micrornas (mirnas) Small RNAs PolII PolIV
Small RNAs and Cross-Kingdom RNAi in Plant-Microbial Interaction Hailing Jin Department of Plant Pathology & Microbiology Institute for Integrative Genome Biology micrornas (mirnas) Small RNAs PolII PolIV
High coverage in planta RNA sequencing identifies Fusarium oxysporum effectors and Medicago truncatularesistancemechanisms
 High coverage in planta RNA sequencing identifies Fusarium oxysporum effectors and Medicago truncatularesistancemechanisms Louise Thatcher Gagan Garg, Angela Williams, Judith Lichtenzveig and Karam Singh
High coverage in planta RNA sequencing identifies Fusarium oxysporum effectors and Medicago truncatularesistancemechanisms Louise Thatcher Gagan Garg, Angela Williams, Judith Lichtenzveig and Karam Singh
Profiles of gene expression & diagnosis/prognosis of cancer. MCs in Advanced Genetics Ainoa Planas Riverola
 Profiles of gene expression & diagnosis/prognosis of cancer MCs in Advanced Genetics Ainoa Planas Riverola Gene expression profiles Gene expression profiling Used in molecular biology, it measures the
Profiles of gene expression & diagnosis/prognosis of cancer MCs in Advanced Genetics Ainoa Planas Riverola Gene expression profiles Gene expression profiling Used in molecular biology, it measures the
Obstacles and challenges in the analysis of microrna sequencing data
 Obstacles and challenges in the analysis of microrna sequencing data (mirna-seq) David Humphreys Genomics core Dr Victor Chang AC 1936-1991, Pioneering Cardiothoracic Surgeon and Humanitarian The ABCs
Obstacles and challenges in the analysis of microrna sequencing data (mirna-seq) David Humphreys Genomics core Dr Victor Chang AC 1936-1991, Pioneering Cardiothoracic Surgeon and Humanitarian The ABCs
RASA: Robust Alternative Splicing Analysis for Human Transcriptome Arrays
 Supplementary Materials RASA: Robust Alternative Splicing Analysis for Human Transcriptome Arrays Junhee Seok 1*, Weihong Xu 2, Ronald W. Davis 2, Wenzhong Xiao 2,3* 1 School of Electrical Engineering,
Supplementary Materials RASA: Robust Alternative Splicing Analysis for Human Transcriptome Arrays Junhee Seok 1*, Weihong Xu 2, Ronald W. Davis 2, Wenzhong Xiao 2,3* 1 School of Electrical Engineering,
Deep Annotation of Populus trichocarpa MicroRNAs from Diverse Tissue Sets
 Deep Annotation of Populus trichocarpa MicroRNAs from Diverse Tissue Sets The Harvard community has made this article openly available. Please share how this access benefits you. Your story matters Citation
Deep Annotation of Populus trichocarpa MicroRNAs from Diverse Tissue Sets The Harvard community has made this article openly available. Please share how this access benefits you. Your story matters Citation
Computational prediction of mirnas and their targets in Phaseolus vulgaris using simple sequence repeat signatures
 Nithin et al. BMC Plant Biology (2015) 15:140 DOI 10.1186/s12870-015-0516-3 RESEARCH ARTICLE Open Access Computational prediction of mirnas and their targets in Phaseolus vulgaris using simple sequence
Nithin et al. BMC Plant Biology (2015) 15:140 DOI 10.1186/s12870-015-0516-3 RESEARCH ARTICLE Open Access Computational prediction of mirnas and their targets in Phaseolus vulgaris using simple sequence
microrna Presented for: Presented by: Date:
 microrna Presented for: Presented by: Date: 2 micrornas Non protein coding, endogenous RNAs of 21-22nt length Evolutionarily conserved Regulate gene expression by binding complementary regions at 3 regions
microrna Presented for: Presented by: Date: 2 micrornas Non protein coding, endogenous RNAs of 21-22nt length Evolutionarily conserved Regulate gene expression by binding complementary regions at 3 regions
Supplemental Figure S1. Expression of Cirbp mrna in mouse tissues and NIH3T3 cells.
 SUPPLEMENTAL FIGURE AND TABLE LEGENDS Supplemental Figure S1. Expression of Cirbp mrna in mouse tissues and NIH3T3 cells. A) Cirbp mrna expression levels in various mouse tissues collected around the clock
SUPPLEMENTAL FIGURE AND TABLE LEGENDS Supplemental Figure S1. Expression of Cirbp mrna in mouse tissues and NIH3T3 cells. A) Cirbp mrna expression levels in various mouse tissues collected around the clock
V16: involvement of micrornas in GRNs
 What are micrornas? V16: involvement of micrornas in GRNs How can one identify micrornas? What is the function of micrornas? Elisa Izaurralde, MPI Tübingen Huntzinger, Izaurralde, Nat. Rev. Genet. 12,
What are micrornas? V16: involvement of micrornas in GRNs How can one identify micrornas? What is the function of micrornas? Elisa Izaurralde, MPI Tübingen Huntzinger, Izaurralde, Nat. Rev. Genet. 12,
MicroRNA-mediated incoherent feedforward motifs are robust
 The Second International Symposium on Optimization and Systems Biology (OSB 8) Lijiang, China, October 31 November 3, 8 Copyright 8 ORSC & APORC, pp. 62 67 MicroRNA-mediated incoherent feedforward motifs
The Second International Symposium on Optimization and Systems Biology (OSB 8) Lijiang, China, October 31 November 3, 8 Copyright 8 ORSC & APORC, pp. 62 67 MicroRNA-mediated incoherent feedforward motifs
MicroRNAs Modulate the Noncanonical NF- B Pathway by Regulating IKK Expression During Macrophage Differentiation
 MicroRNAs Modulate the Noncanonical NF- B Pathway by Regulating IKK Expression During Macrophage Differentiation Tao Li 1 *, Michael J. Morgan 1 *, Swati Choksi 1, Yan Zhang 1, You-Sun Kim 2#, Zheng-gang
MicroRNAs Modulate the Noncanonical NF- B Pathway by Regulating IKK Expression During Macrophage Differentiation Tao Li 1 *, Michael J. Morgan 1 *, Swati Choksi 1, Yan Zhang 1, You-Sun Kim 2#, Zheng-gang
(,, ) microrna(mirna) 19~25 nt RNA, RNA mirna mirna,, ;, mirna mirna. : microrna (mirna); ; ; ; : R321.1 : A : X(2015)
 35 1 Vol.35 No.1 2015 1 Jan. 2015 Reproduction & Contraception doi: 10.7669/j.issn.0253-357X.2015.01.0037 E-mail: randc_journal@163.com microrna ( 450052) microrna() 19~25 nt RNA RNA ; : microrna (); ;
35 1 Vol.35 No.1 2015 1 Jan. 2015 Reproduction & Contraception doi: 10.7669/j.issn.0253-357X.2015.01.0037 E-mail: randc_journal@163.com microrna ( 450052) microrna() 19~25 nt RNA RNA ; : microrna (); ;
Differential Expression of MicroRNAs Between 21A Genetic Male Sterile Line and Its Maintainer Line in Cotton (Gossypium hirsutum L.
 Journal of Plant Studies; Vol. 3, No. 1; 2014 ISSN 1927-0461 E-ISSN 1927-047X Published by Canadian Center of Science and Education Differential Expression of MicroRNAs Between 21A Genetic Male Sterile
Journal of Plant Studies; Vol. 3, No. 1; 2014 ISSN 1927-0461 E-ISSN 1927-047X Published by Canadian Center of Science and Education Differential Expression of MicroRNAs Between 21A Genetic Male Sterile
Identification of mirnas and mirna mediated regulatory pathways in Carica papaya
 Planta (2013) 238:739 752 DOI 10.1007/s00425-013-1929-6 ORIGINAL ARTICLE Identification of mirnas and mirna mediated regulatory pathways in Carica papaya Gang Liang Yang Li Hua He Fang Wang Diqiu Yu Received:
Planta (2013) 238:739 752 DOI 10.1007/s00425-013-1929-6 ORIGINAL ARTICLE Identification of mirnas and mirna mediated regulatory pathways in Carica papaya Gang Liang Yang Li Hua He Fang Wang Diqiu Yu Received:
Identification and profiling of novel micrornas in the Brassica rapa genome based on small RNA deep sequencing. Kim et al. novel mirna conserved mirna
 novel mirna conserved mirna Identification and profiling of novel micrornas in the Brassica rapa genome based on small RNA deep sequencing Kim et al. Kim et al. BMC Plant Biology 2012, 12:218 Kim et al.
novel mirna conserved mirna Identification and profiling of novel micrornas in the Brassica rapa genome based on small RNA deep sequencing Kim et al. Kim et al. BMC Plant Biology 2012, 12:218 Kim et al.
Improved annotation of C. elegans micrornas by deep sequencing reveals structures associated with processing by Drosha and Dicer
 BIOINFORMATICS Improved annotation of C. elegans micrornas by deep sequencing reveals structures associated with processing by Drosha and Dicer M. BRYAN WARF, 1 W. EVAN JOHNSON, 2 and BRENDA L. BASS 1
BIOINFORMATICS Improved annotation of C. elegans micrornas by deep sequencing reveals structures associated with processing by Drosha and Dicer M. BRYAN WARF, 1 W. EVAN JOHNSON, 2 and BRENDA L. BASS 1
RNA-Seq profiling of circular RNAs in human colorectal Cancer liver metastasis and the potential biomarkers
 Xu et al. Molecular Cancer (2019) 18:8 https://doi.org/10.1186/s12943-018-0932-8 LETTER TO THE EDITOR RNA-Seq profiling of circular RNAs in human colorectal Cancer liver metastasis and the potential biomarkers
Xu et al. Molecular Cancer (2019) 18:8 https://doi.org/10.1186/s12943-018-0932-8 LETTER TO THE EDITOR RNA-Seq profiling of circular RNAs in human colorectal Cancer liver metastasis and the potential biomarkers
Can Brachypodium distachyon provide insight into FHB? Paul Nicholson Disease and Stress Biology Department John Innes Centre
 Can Brachypodium distachyon provide insight into FHB? Paul Nicholson Disease and Stress Biology Department John Innes Centre Genetics and mechanisms of FHB resistance in wheat DON (mg kḡ 1 ) ET-silenced
Can Brachypodium distachyon provide insight into FHB? Paul Nicholson Disease and Stress Biology Department John Innes Centre Genetics and mechanisms of FHB resistance in wheat DON (mg kḡ 1 ) ET-silenced
Ch. 18 Regulation of Gene Expression
 Ch. 18 Regulation of Gene Expression 1 Human genome has around 23,688 genes (Scientific American 2/2006) Essential Questions: How is transcription regulated? How are genes expressed? 2 Bacteria regulate
Ch. 18 Regulation of Gene Expression 1 Human genome has around 23,688 genes (Scientific American 2/2006) Essential Questions: How is transcription regulated? How are genes expressed? 2 Bacteria regulate
38 Int'l Conf. Bioinformatics and Computational Biology BIOCOMP'16
 38 Int'l Conf. Bioinformatics and Computational Biology BIOCOMP'16 PGAR: ASD Candidate Gene Prioritization System Using Expression Patterns Steven Cogill and Liangjiang Wang Department of Genetics and
38 Int'l Conf. Bioinformatics and Computational Biology BIOCOMP'16 PGAR: ASD Candidate Gene Prioritization System Using Expression Patterns Steven Cogill and Liangjiang Wang Department of Genetics and
Nature Structural & Molecular Biology: doi: /nsmb Supplementary Figure 1. Differential expression of mirnas from the pri-mir-17-92a locus.
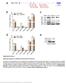 Supplementary Figure 1 Differential expression of mirnas from the pri-mir-17-92a locus. (a) The mir-17-92a expression unit in the third intron of the host mir-17hg transcript. (b,c) Impact of knockdown
Supplementary Figure 1 Differential expression of mirnas from the pri-mir-17-92a locus. (a) The mir-17-92a expression unit in the third intron of the host mir-17hg transcript. (b,c) Impact of knockdown
Role of Soybean Ecto-apyrase in Nodulation
 PAG XX Engineering NUE Workshop W248 January 14, 2012 Role of Soybean Ecto-apyrase in Nodulation Kiwamu Tanaka (Gary Stacey Lab) Division of Plant Sciences University of Missouri Legume-Rhizobium symbiotic
PAG XX Engineering NUE Workshop W248 January 14, 2012 Role of Soybean Ecto-apyrase in Nodulation Kiwamu Tanaka (Gary Stacey Lab) Division of Plant Sciences University of Missouri Legume-Rhizobium symbiotic
Arabidopsis thaliana small RNA Sequencing. Report
 Arabidopsis thaliana small RNA Sequencing Report September 2015 Project Information Client Name Client Company / Institution Macrogen Order Number Order ID Species Arabidopsis thaliana Reference UCSC hg19
Arabidopsis thaliana small RNA Sequencing Report September 2015 Project Information Client Name Client Company / Institution Macrogen Order Number Order ID Species Arabidopsis thaliana Reference UCSC hg19
SC-L-H shared(37) Specific (1)
 A. Brain (total 2) Tissue-specific (2) Brain-heart shared (8) Specific (2) CNS-heart shared(68) Specific () Heart (total 4) Tissue-specific () B-L-H shared (37) specific () B. Brain (total 2) Tissue-specific
A. Brain (total 2) Tissue-specific (2) Brain-heart shared (8) Specific (2) CNS-heart shared(68) Specific () Heart (total 4) Tissue-specific () B-L-H shared (37) specific () B. Brain (total 2) Tissue-specific
mirna Mediated Regulation of Rice (Oryza sativa) Genome
 Preprints of the 12th IFAC Symposium on Computer Applications in Biotechnology The International Federation of Automatic Control 16-18, 2013, December. Mumbai, India mirna Mediated Regulation of Rice (Oryza
Preprints of the 12th IFAC Symposium on Computer Applications in Biotechnology The International Federation of Automatic Control 16-18, 2013, December. Mumbai, India mirna Mediated Regulation of Rice (Oryza
Gene. Identification of conserved and novel micrornas in Aquilaria sinensis based on small RNA sequencing and transcriptome sequence data
 Gene 505 (2012) 167 175 Contents lists available at SciVerse ScienceDirect Gene journal homepage: www.elsevier.com/locate/gene Methods paper Identification of conserved and novel micrornas in Aquilaria
Gene 505 (2012) 167 175 Contents lists available at SciVerse ScienceDirect Gene journal homepage: www.elsevier.com/locate/gene Methods paper Identification of conserved and novel micrornas in Aquilaria
RECAP (1)! In eukaryotes, large primary transcripts are processed to smaller, mature mrnas.! What was first evidence for this precursorproduct
 RECAP (1) In eukaryotes, large primary transcripts are processed to smaller, mature mrnas. What was first evidence for this precursorproduct relationship? DNA Observation: Nuclear RNA pool consists of
RECAP (1) In eukaryotes, large primary transcripts are processed to smaller, mature mrnas. What was first evidence for this precursorproduct relationship? DNA Observation: Nuclear RNA pool consists of
Mature microrna identification via the use of a Naive Bayes classifier
 Mature microrna identification via the use of a Naive Bayes classifier Master Thesis Gkirtzou Katerina Computer Science Department University of Crete 13/03/2009 Gkirtzou K. (CSD UOC) Mature microrna identification
Mature microrna identification via the use of a Naive Bayes classifier Master Thesis Gkirtzou Katerina Computer Science Department University of Crete 13/03/2009 Gkirtzou K. (CSD UOC) Mature microrna identification
Journal of Agricultural Technology 2012 Vol. 8(4): Journal of Agricultural
 Journal of Agricultural Technology 2012 Vol. 8(4): 1389-1395 Journal of Agricultural Available Technology online http://www.ijat-aatsea.com 2012, Vol. 8(4): 1389-1395 ISSN 1686-9141 The effect of the decreased
Journal of Agricultural Technology 2012 Vol. 8(4): 1389-1395 Journal of Agricultural Available Technology online http://www.ijat-aatsea.com 2012, Vol. 8(4): 1389-1395 ISSN 1686-9141 The effect of the decreased
