The Ras GTPases are membrane-associated binary switches that
|
|
|
- Jonah Peters
- 6 years ago
- Views:
Transcription
1 Plasma Membrane Localization Is Required for RasA-Mediated Polarized Morphogenesis and Virulence of Aspergillus fumigatus Jarrod R. Fortwendel, f Praveen R. Juvvadi, a Luise E. Rogg, a Yohannes G. Asfaw, c Kimberlie A. Burns, d Scott H. Randell, d,e and William J. Steinbach a,b Department of Pediatrics, Division of Pediatric Infectious Diseases, Duke University Medical Center, Durham, North Carolina, USA a ; Department of Molecular Genetics and Microbiology, Duke University Medical Center, Durham, North Carolina, USA b ; Division of Laboratory Animal Resources, Duke University Medical Center, Durham, North Carolina, USA c ; Cystic Fibrosis/Pulmonary Research and Treatment Center, University of North Carolina, Chapel Hill, North Carolina, USA d ; Department of Cell and Molecular Physiology, University of North Carolina, Chapel Hill, North Carolina, USA e ; and Department of Microbiology and Immunology, University of South Alabama, Mobile, Alabama, USA f Ras is a highly conserved GTPase protein that is essential for proper polarized morphogenesis of filamentous fungi. Localization of Ras proteins to the plasma membrane and endomembranes through posttranslational addition of farnesyl and palmitoyl residues is an important mechanism through which cells provide specificity to Ras signal output. Although the Aspergillus fumigatus RasA protein is known to be a major regulator of growth and development, the membrane distribution of RasA during polarized morphogenesis and the role of properly localized Ras signaling in virulence of a pathogenic mold remain unknown. Here we demonstrate that Aspergillus fumigatus RasA localizes primarily to the plasma membrane of actively growing hyphae. We show that treatment with the palmitoylation inhibitor 2-bromopalmitate disrupts normal RasA plasma membrane association and decreases hyphal growth. Targeted mutations of the highly conserved RasA palmitoylation motif also mislocalized RasA from the plasma membrane and led to severe hyphal abnormalities, cell wall structural changes, and reduced virulence in murine invasive aspergillosis. Finally, we provide evidence that proper RasA localization is independent of the Ras palmitoyltransferase homolog, encoded by erfb, but requires the palmitoyltransferase complex subunit, encoded by erfd. Our results demonstrate that plasma membrane-associated RasA is critical for polarized morphogenesis, cell wall stability, and virulence in A. fumigatus. The Ras GTPases are membrane-associated binary switches that control the activity of a multitude of signal transduction pathways. Specificity of Ras signal output occurs in part via subcellular localization of the Ras protein to various membranes, as well as through compartmentalization to specific regions with the membrane (23, 37). Differential localization of Ras proteins is accomplished through posttranslational modification of the hypervariable domain, the region in which Ras homologs are most divergent. The hypervariable domains of nascent Ras proteins contain an extreme C-terminal CAAX motif that serves as a recognition sequence for cytoplasmic farnesyltransferase (5). The farnesyltransferase enzyme modifies the CAAX motif by addition of a farnesyl lipid moiety to the cysteine residue. Farnesyl modification has been shown to be necessary and sufficient for direction of the Ras protein to the endoplasmic reticulum (ER) and Golgi membranes (5). Once localized to the endomembrane system, Ras proteins are further processed by the activity of a Ras-converting enzyme (Rce), which catalyzes the proteolytic cleavage of the CAAX residues from the farnesylated CAAX motif (43). The farnesylated cysteine is then carboxymethylated by action of an isoprenylcysteine carboxymethyltransferase (19, 40). For Ras to complete transit via the exocytosis machinery of the ER and Golgi complex and become associated with the plasma membrane, another ER resident protein complex, the palmitoyltransferase, must modify the Ras protein through addition of a palmitoyl lipid moiety (1). This modification occurs at conserved cysteine residues located just upstream of the CAAX motif, within the hypervariable domain (42). Palmitoylation leads to translocation of the Ras protein to the plasma membrane, as this modification is proposed to impart greater affinity for cholesterol-rich membrane domains (17). Ras palmitoyltransferase complexes have been identified and characterized in humans as well as in the yeast Saccharomyces cerevisiae (27, 46). Ras protein palmitoylation is reversible, and plasma membrane-localized Ras has been shown to cycle back to the ER and Golgi membranes through the activity of thioesterase enzymes at the plasma membrane (2, 15). One thioesterase, APT1, which is responsible for depalmitoylation of Ras at the plasma membrane, has been identified (8). The importance of subcellular localization of Ras proteins for function in specific morphological processes has been explored in several fungi. Early evidence for this is found in the model yeast Schizosaccharomyces pombe. When the Ras1 palmitoylation motif of S. pombe is ablated, Ras1 localization is restricted to endomembranes, where it interacts with the guanine nucleotide exchange factor (GEF) Efc25p and signals through a Cdc42-mediated pathway to control cell morphology (34). In contrast, S. pombe Ras1p restricted to the plasma membrane is activated by a different GEF, Ste6p, and signals through a mitogen-activated protein (MAP) kinase pathway to mediate the mating response (34). Ras membrane distribution in the fungal pathogen Cryptococcus neoformans is also important for differential activation of morphology and mating pathways. In C. neoformans, plasma membrane-localized Ras1 is required for morphology, high-temperature growth, Received 20 March 2012 Accepted 24 April 2012 Published ahead of print 4 May 2012 Address correspondence to Jarrod R. Fortwendel, jfortwendel@jaguar1.usouthal.edu. Copyright 2012, American Society for Microbiology. All Rights Reserved. doi: /ec ec.asm.org Eukaryotic Cell p August 2012 Volume 11 Number 8
2 Ras Regulates A. fumigatus Growth from Plasma Membrane and virulence (32). Mutation of the highly conserved dualpalmitoylation motif of C. neoformans Ras1 results in a mislocalized Ras protein that cannot support morphology and high-temperature growth yet is still able to support the mating response (32). Most recently, the importance of Ras membrane localization has also been explored in the yeast pathogen Candida albicans. Cells expressing a palmitoylation-deficient C. albicans RAS1 are able to produce hyphae and grow at wild type-levels in rich media, although the mutant hyphae are more variable in length (36). However, polarized morphogenesis of the palmitoylation-deficient mutant is drastically reduced when cells are embedded in a matrix, a condition requiring Ras signaling for hyphal induction (36). In addition, expression of a constitutively activated RAS1 overcomes the embedded filamentation defect, arguing that mislocalization of C. albicans Ras1 may negatively affect activity (36). Together, these studies support the hypothesis that fungal Ras signal transduction from different cellular locations can lead to unique biological outputs, a general paradigm of Ras protein regulation that is apparent in many organisms. The conservation of this paradigm among the yeast organisms described above leads to questions about the role of this form of Ras regulation in pathogenic molds, in which polarized morphogenesis is required for normal growth and virulence. In the human pathogen Aspergillus fumigatus, rasa is not essential, but it plays a critical role in hyphal morphogenesis and cell wall integrity (10, 12). From what membrane(s) does Ras mediate hyphal morphogenesis, and therefore likely virulence, in filamentous fungal organisms? Despite the emerging data describing its role in growth and development, the subcellular localization of Ras proteins in a mold has not been explored. Here, we show for the first time that RasA localizes to the A. fumigatus plasma membrane. Mutation of the highly conserved palmitoylation motif resulted in mislocalization of RasA from the plasma membrane to endomembrane compartments, leading to defects in hyphal morphogenesis, cell wall formation, and virulence. In addition, the putative RasA palmitoyltransferase subunit genes were identified and deleted: erfb, the homolog of S. cerevisiae ERF2, and erfd, the homolog of S. cerevisiae ERF4. Our data establish the importance of erfd for RasA plasma membrane localization in A. fumigatus, whereas erfb is not required. Our findings provide novel insights into the regulation of Ras signal transduction in A. fumigatus and may potentially be extrapolated for studies with other filamentous fungal pathogens. MATERIALS AND METHODS Strains, culture conditions, and growth rate analyses. Aspergillus fumigatus wild-type strain H237, the rasa strain (10), and each of the green fluorescent protein (GFP)-RasA fusion strains were maintained on glucose minimal medium (GMM) (10). For quantification of growth rates, a 10- l drop containing 10,000 conidia was inoculated onto GMM agar. The colony diameter of each strain was measured daily over the first 72 h of growth, and images were taken at the end of day 3. Live-cell microscopy was employed for analysis of morphogenesis during germination, as previously described (11). Briefly, conidia from each strain were preincubated in GMM for 4hat37 C in a glass-bottom tissue culture plate. Plates were subsequently used for live-cell analysis on a Zeiss Axio Observer inverted microscope equipped with a 37 C environmental chamber. For analysis of hyphal morphogenesis during branch formation, 100 conidia from each strain were inoculated onto a thin layer of GMM agar solidified on a glass-bottom petri dish. Cultures were incubated at 37 C for the indicated amount of time, and images were captured on an inverted Nikon Diaphot microscope mounted with a Nikon D50 digital camera. To analyze the effects of 2-bromopalmitate (2-BP) (Sigma) treatment, 10 6 conidia of the GFP-RasA strain were inoculated into GMM broth supplemented with the indicated concentrations of 2-BP and incubated for 24 h at 37 C. Mycelia from each treatment were collected by centrifugation, and excess water was expressed onto filter paper using a sterile spatula. Hyphal mats were then allowed to dry for6hatroom temperature before weights were recorded. To assess the effect of 2-BP on RasA localization, 100- l aliquots were removed from each culture prior to collection of mycelia and mounted for fluorescence microscopy. Construction of GFP-RasA fusion strains. For construction of a vector expressing a green fluorescent protein (GFP)-RasA fusion, the rasa coding sequence was PCR amplified from pooled A. fumigatus cdna using primers incorporating NotI restriction sites to both 5= and 3= ends. The amplified rasa cdna was restriction digested with NotI and cloned into expression vector pagrp. Vector pagrp was generated by cloning enhanced GFP (egfp), amplified as an N-terminal fusion from pucgh (22), into vector parp (11) as a BamHI and NotI restriction fragment. The GFP-RasA expression vector was then introduced into the rasa mutant via protoplast transformation, as previously described (10). Eight transformants positive for fluorescence were found to have identical localization of GFP signal to the hyphal periphery. To introduce mutations in the dual-palmitoylation motif, the rasa cdna was PCR amplified using reverse primers that incorporate the C206S, C207S, or C206S C207S mutations. The rasa palmitoylation mutated cdnas were then cloned into pagrp and verified by sequence analysis. Each GFP-RasA palmitoylation mutant vector was transformed into the rasa background, and multiple transformants were analyzed for RasA localization patterns. To generate the GFP-DARasA C206/207S strain, the previously constructed DArasA cdna (12) was amplified using the same primers employed for the C206S C207S mutation described above. The DArasA and DArasA C206/207S cdnas were then cloned, sequenced, and transformed into the rasa mutant. All strains were analyzed by Southern blot analysis to verify that a single, ectopic integration event occurred. Western blot analysis. Western blot analysis for the GFP-RasA and GFP-RasA C206/207S mutant was performed as previously described (22). In short, each strain was cultured in liquid GMM for 24 h at 37 C with shaking at 250 rpm. Total protein extracts were prepared by homogenizing mycelia in liquid nitrogen, followed by addition of extraction buffer (50 mm Tris-HCl [ph 7.5], 150 mm NaCl, 50 mm KCl, 0.01% Triton X-100, 1 mm phenylmethylsulfonyl fluoride [PMSF], and 1:100 protease cocktail inhibitor). Lysates were centrifuged at 4,000 rpm for 8 min to remove debris. Supernatants were removed, and protein content was quantitated by Bradford assay. Fifty micrograms of total protein from each strain was resolved on a 12% SDS-polyacrylamide gel using a Miniprotean tetracell (Bio-Rad). Proteins were then electroblotted to a polyvinylidene difluoride membrane (Bio-Rad) and probed with a polyclonal rabbit anti-gfp antibody. A horseradish peroxidase-conjugated anti-rabbit IgG was used as a secondary antibody, and detection was performed using SuperSignal West Pico chemiluminescent substrate (Thermo Scientific). Identification and deletion of A. fumigatus erfb and erfd. The A. fumigatus ERF2 homolog, erfb (Afu3G06470), was previously identified (14). The A. fumigatus ERF4 homolog, erfd (Afu5G7920), was identified using BLAST search analysis of the A. fumigatus genome database with the S. cerevisiae Erf4p sequence as a query. To delete erfb and erfd, 1.5 kb of upstream and downstream flanking genomic sequence was PCR amplified for each gene. Flanking sequences were cloned into vector phygr, carrying a hygromycin resistance cassette, generating a deletion construct for each gene. The erfb and erfd deletion constructs were PCR amplified, and 2 g of each purified product was used for transformation into A. fumigatus protoplasts, as previously described (10). Hygromycin-resistant transformants were screened for integration by PCR and homologous August 2012 Volume 11 Number 8 ec.asm.org 967
3 Fortwendel et al. recombination confirmed by Southern blot analysis. To generate strains expressing GFP-RasA in either the erfb or erfd background, the construct generated for GFP-RasA expression (vector pagrp) was transformed into the erfb and erfd mutants as described above. Multiple phleomycin-resistant transformants were isolated for both strains, and RasA localization patterns were analyzed in each. Six erfb GFP-RasA isolates were identified and found to have unaltered RasA plasma membrane localization patterns. Five erfb GFP-RasA strains were isolated, and each displayed similar mislocalization of RasA to endomembrane structures. Fluorescence microscopy for localization analysis and cell wall staining. Ten thousand conidia of each strain were inoculated in 3 ml of GMM broth and incubated in a petri dish on sterile coverslips. After 16 h of growth at 37 C, the strains were observed for fluorescence using an Axioskop 2 Plus microscope (Zeiss) equipped with a digital camera. Images were captured using AxioVision 4.6 imaging software. Calcofluor white and aniline blue staining was performed as previously described (10, 25). Calcofluor white staining of the wild-type and RasAC206/207S strains was performed by culturing conidia on coverslips immersed in 5 ml GMM broth and grown for 16 h at 37 C. The coverslips were briefly rinsed in sterile water and inverted onto a 500- l drop of 0.4- g/ml fluorescent brightener 28 (Sigma) for 5 min at room temperature. Mycelia were then rinsed twice for 5 min each in sterile water and observed by fluorescence microscopy. -Glucan staining with aniline blue was performed by rinsing coverslips in sterile water and inverting them onto a 500- l drop of aniline blue (0.1 mg/ml) for 5 min at room temperature. Stained mycelia were directly observed by fluorescence microscopy. Electron microscopy. For transmission electron microscopy (TEM) of the wild type and the Ras C206/207S mutant, 10,000 conidia of each strain were inoculated into 10 ml GMM broth and grown for 48 h at 37 C. Mycelia were collected by centrifugation and washed three times in distilled water. Washed mycelia were then fixed in 5% glutaraldehyde in 0.1 M cacodylate buffer. The mycelia were embedded in low-viscosity Spurr s resin, ultrathin sectioned, and then stained with uranyl acetate and lead citrate. Images were obtained on Zeiss EM900 electron microscope, and negatives were scanned for image analysis. For scanning electron microscopy (SEM), coverslip cultures of 10,000 conidia/strain were grown in 10 ml of GMM in a petri dish containing sterile 22- by 22-mm (no. 2) coverslips. The wild type was grown for 24 h at 37 C, and the RasA C206/207S mutant was grown for 48 h at 37 C due to reduced hyphal growth. The mycelia on the coverslips were briefly rinsed in 50 mm PIPES [piperazine-n,n=-bis(2-ethanesulfonic acid)] buffer (ph 7) and then fixed with 50 mm PIPES buffer (ph 7) containing 3.5% formaldehyde for 60 min. Following fixation, coverslips were washed three times for 10 min each in 50 mm PIPES buffer (ph 7). The fixed mycelia were then dehydrated with 50, 70, and 90% ethanol for 10 min each, followed by 99.5% ethanol three times for 10 min each. The coverslips were then allowed to dry overnight. Samples were then sputter coated with gold and observed on a field emission SEM (FE-SEM) (FEI model XL-30) using an accelerating voltage of 7 kv. Virulence studies. A transient-immunosuppression model of invasive aspergillosis was used as previously described (38). Six-week-old CD-1 male mice (Charles River) (20 per group) were immunosuppressed with a single dose of triamcinolone acetonide (40 mg/kg of body weight injected subcutaneously) on day 1 of infection. The mice were anesthetized with 3.5% isoflurane and inoculated intranasally with conidia in 20 l of sterile saline on day 0. Mortality was monitored twice daily for the next 15 days, and mice that appeared moribund were sacrificed by CO 2 euthanasia. The lungs of all mice were plated onto inhibitory mold agar (Becton Dickinson), and the phenotypes of recovered isolates were analyzed to confirm infection with the appropriate strain. Survival was plotted on a Kaplan-Meier curve for each group, and a log rank test was used for pairwise comparison of survival, as previously described (45). RESULTS RasA localizes to the plasma membranes of germlings and actively growing hyphae. To investigate the distribution of RasA during A. fumigatus growth and development, a GFP-RasA fusion was generated and expressed in the rasa background. To limit artifactual cellular distribution, the endogenous rasa promoter was used to drive GFP-RasA expression (11). Expression of GFP- RasA in the rasa mutant fully complemented the compact colony morphology and conidiation defects associated with loss of rasa (10), suggesting that the GFP-RasA fusion protein is functional (Fig. 1A and B). During early growth, GFP-RasA was visible at the cell periphery of swollen conidia (Fig. 1C, inset at upper left) and distributed around the periphery of nascent germlings (Fig. 1C). During fully polarized growth, GFP-RasA was visible at the outer edge of hyphae, with strong localization to septa, and no GFP signal was associated with internal compartments (Fig. 1D). These findings are consistent with RasA localization to the A. fumigatus plasma membrane, with the strong septal localization likely stemming from plasma membrane apposition along each side of the septum. Palmitoylation of the Ras protein on conserved residues is required for plasma membrane localization and full function of Ras signaling in various organisms (4, 7, 32, 36, 49). However, nothing is known about the importance of Ras membrane distribution during invasive growth and virulence of a filamentous pathogen, such as A. fumigatus. To investigate, we cultured the GFP-RasA strain in the presence of increasing concentrations of 2-bromopalmitate (2-BP) (1 to 50 M), a nonreversible inhibitor of palmitoylation (20), and assayed hyphal mass. 2-BP inhibited hyphal growth in a dose-dependent manner (Fig. 1E), whereas dimethyl sulfoxide (DMSO) vehicle controls produced no effect (data not shown). To examine the effects of increasing concentrations of 2-BP on RasA localization, aliquots were removed from each culture and hyphae were observed using fluorescence microscopy. Although 1 M 2-BP treatment reduced growth, no detectable disruption of RasA localization was noted (Fig. 1F). However, higher concentrations of 2-BP (5, 10, and 50 M) caused mislocalization of GFP-RasA to internal patches (Fig. 1F). These results suggested that, similar to the case in C. albicans and C. neoformans, palmitoylation may be important for proper RasA localization during polarized growth and that mislocalization of RasA may contribute to decreased hyphal growth. Mutation of the conserved RasA palmitoylation motif inhibits hyphal growth and mislocalizes RasA. Nichols et al. recently characterized the importance of Ras palmitoylation to C. neoformans growth and virulence, describing a dual-palmitoylation motif that is conserved among the filamentous fungal Ras homologs (32). In C. neoformans, mutation of both cysteine residues to serine completely blocked palmitoylation of the Ras1 protein and caused mislocalization to internal membranes (32). Further, mutation of the single conserved palmitoylation residue of C. albicans Ras1 causes mislocalization from the plasma membrane to endomembrane structures (36). To investigate the role of the palmitoylation motif in RasA localization and function during A. fumigatus polarized morphogenesis, mutations were introduced to replace each conserved cysteine residue of the dual-palmitoylation motif with serine, either singly (RasA C206S and RasA C207S )or in tandem (RasA C206/207S ). Using the same vector constructed for GFP-RasA, each of the new RasA palmitoylation motif mutants 968 ec.asm.org Eukaryotic Cell
4 Ras Regulates A. fumigatus Growth from Plasma Membrane FIG 1 RasA localizes to the plasma membrane and is disrupted by 2-bromopalmitate. (A) Colony morphology comparison of the wild-type (WT), rasa, and GFP-RasA strains. Ten thousand conidia were inoculated onto glucose minimal media (GMM) and incubated for 98 h at 37 C. (B) Quantification of the average colony diameter for each strain at 24, 48, 72, and 98 h postinoculation. (C and D) Two hundred conidia from the GFP-RasA strain were inoculated into GMM broth and cultured on coverslips at 37 C. Coverslips were removed from culture at defined time points, and GFP fluorescence in swollen conidia (C, inset), germlings (C), and hyphae (D) was analyzed. (E) Conidia (10 6 ) of the GFP-RasA strain were inoculated into 50 ml of GMM broth with the indicated concentration of 2-bromopalmitate and incubated for 24 h at 37 C and 250 rpm. Hyphae were harvested by centrifugation and dried on Miracloth for 6 h before weighing. Each culture was performed in triplicate, and the graph represents the mean hyphal mass standard deviation (SD). (F) Aliquots (100 l) were removed from each culture from panel E, and hyphae were analyzed by fluorescence microscopy. Arrowheads indicate the presence of GFP-RasA patches in the tips and subapical compartments of hyphae treated with 5, 10, or 50 M 2-bromopalmitate. was expressed in the rasa background. Expression of either GFP-RasA C206S or GFP-RasA C207S was able to fully complement the hyphal growth defects associated with rasa, indicating that only one of the conserved cysteines is sufficient for RasA function in hyphal growth (Fig. 2A and B). However, expression of GFP- RasA C206/207S, representing complete blockade of RasA palmitoylation, only partially restored hyphal growth of the rasa mutant (Fig. 2A and B). Because mutation of the palmitoylation domain in C. albicans Ras1 caused reduced protein abundance (36), we compared steady-state levels of the RasA protein in the GFP-RasA and GFP-RasA C206/207S strains. Western blot analysis of whole-cell protein lysates revealed the expected band size for a GFP-RasA fusion at 51 kda in both strains (Fig. 2C). In contrast to the C. albicans data, RasA protein levels in the GFP-RasA C206/207S mutant were more abundant than those in the GFP-RasA strain (Fig. 2C), suggesting that the RasA C206/207S mutation does not negatively affect protein abundance in A. fumigatus. These data suggest that the RasA C206/207S mutant phenotypes are not due to decreased protein abundance and are more directly attributable to mislocalization of RasA. Under fluorescence microscopy, the GFP-RasA C206S mutant displayed no alteration in plasma membrane association in the subapical sections of hyphae, whereas the apical hyphal sections showed internal patches of GFP fluorescence (Fig. 2D, arrows). In contrast, the GFP-RasA C207S mutant displayed mislocalized GFP signal in both the subapical and apical regions (Fig. 2D). Although GFP-RasA C206S was associated with internal patches only in apical compartments whereas GFP-RasA C207S was found on internal patches in both the apical and subapical sections, both were still associated with the plasma membrane, as evidenced by clear lo- August 2012 Volume 11 Number 8 ec.asm.org 969
5 Fortwendel et al. FIG 2 The A. fumigatus RasA dual-palmitoylation motif is required for hyphal growth and RasA localization. (A) Colony morphology of the GFP-RasA palmitoylation mutants. Ten thousand conidia from each strain were inoculated onto GMM agar and incubated for 72 h at 37 C. (B) Quantification of radial growth of the GFP-RasA palmitoylation mutants. Data points represent the mean colony diameter of each strain after 24, 48, and 72 h of growth at 37 C. (C) Western blot analysis of total protein extracts from the GFP-RasA and GFP-RasA C206/207S strains using an anti-gfp antibody. A Coomassie blue-stained protein gel is shown (lower panel) for a loading control. (D) Fluorescence microscopy images of the GFP-RasA palmitoylation mutant strains. Two hundred conidia from each strain were cultured on glass coverslips immersed in GMM broth for 24 h at 37 C. Arrowheads denote positions of septa. Note that the GFP-RasA C206/207S fluorescence pattern does not appear along the hyphal periphery or septa. Arrows denote internal patches of GFP fluorescence in apical regions of the GFP-RasA C206S mutant. calization of GFP signal to septa (Fig. 2D, arrowheads). In contrast to either single mutation, simultaneous mutation of both residues within the dual-palmitoylation motif (GFP-RasA C206/207S ) resulted in a dramatic reduction of GFP signal at the plasma membrane. The GFP-RasA C206/207S mutant was mislocalized from the hyphal periphery and septa to the cytosol and internal patches, presumably to compartments of the endomembrane system (Fig. 2D). These data suggest that whereas a single palmitoylation site is sufficient for RasA function in supporting polarized morphogenesis, complete blockade of RasA palmitoylation through ablation of the entire dual-palmitoylation motif disrupts plasma membrane association and causes decreased hyphal growth. However, the radial growth difference between the RasA C206/207S and rasa mutants suggests that the palmitoylation-deficient mutant is partially functional. It is possible that the aberrant growth phenotypes noted in the GFP-RasA C206/207S strain were due to accumulation of RasA on the endomembranes and not to loss of RasA at the plasma membrane. To test this, we generated a strain expressing the rasa C206/207S allele in the wild-type A. fumigatus background. As with the GFP-RasA C206/207S mutant, this new strain (GFP-RasA C206/207S WT) exhibited an enhanced GFP-RasA signal in the cytosol and on the endomembranes (data not shown). However, no alteration in growth or morphology was observed (data not shown). Taken together, these data argue that aberrant growth in the GFP-RasA C206/207S mutant was due to loss of RasA localization at the plasma membrane. 970 ec.asm.org Eukaryotic Cell
6 Ras Regulates A. fumigatus Growth from Plasma Membrane FIG 3 Plasma membrane-localized RasA controls the timing and morphogenesis of germination. Conidia from the wild-type (WT), rasa, and GFP-RasA C206/207S strains were inoculated into GMM broth in a 6-well glass-bottom culture dish and preincubated for 4 h at37 C. Following preincubation, plates were used for live-cell analysis on a Zeiss Axio Observer inverted microscope equipped with a 37 C environmental chamber and a digital camera. Images from time points that represent similar germination stages for each strain were used for comparison. The arrowheads denote the swollen initial conidium in the GFP-RasA C206/207S strain. Images of each strain were acquired at identical magnifications. Plasma membrane-localized RasA signaling controls early polarized growth and branch emergence. Normal vegetative growth of Aspergillus species progresses from resting conidia through germination to fully polarized hyphae. Deletion of rasa in A. fumigatus causes delayed germination and subsequent formation of wide, blunted hyphae that continually switch polarized growth axes (10). To examine the contribution of plasma membrane-localized Ras activity to these important morphogenetic processes, we compared polarization phenotypes during germination and early hyphal growth of the GFP-RasA C206/207S mutant to those of the wild-type and rasa strains. Live-cell microscopy analysis revealed nearly identical growth defects in the rasa and GFP-RasA C206/207S mutants during germination. Both mutants displayed low germination rates and formed stunted, highly branched germlings over the initial 15 h of culture (Fig. 3), suggesting a nonfunctional Ras protein. The only notable difference between the mutant strains throughout the initial growth stages was the exaggerated swelling of the conidium during germ tube extension in the GFP-RasA C206/207S strain (Fig. 3, arrowheads). In contrast, the germination rates and morphologies of the GFP- RasA C206S and GFP-RasA C207S mutants were similar to those of the GFP-RasA control strain (data not shown). However, after 24 h of growth, the highly branched, stunted hyphae of the GFP-RasA C206/207S mutant developed into morphologically normal hyphae (Fig. 4B, arrows). In contrast, the rasa mutant grew only as stunted, meandering hyphae at all stages (data not shown). Although the GFP-RasA C206/207S mutant developed grossly normal hyphae after the initial 24 h of culture, subsequent branching resulted in reversion to the early growth phenotype (Fig. 4C). The altered morphology of germination and branch formation in the GFP-RasA C206/207S mutant mimicked the phenotype of the rasa strain (10). Taken together, these results further suggest partial Ras functionality of the GFP-RasA C206/207S mutant during invasive growth. Although exogenous 2-BP inhibited hyphal growth (Fig. 1E and F), 2-BP treatment did not phenocopy the RasA C206/207S phenotypes noted in Fig. 4. These findings are likely due to the inability of 2-BP to completely inhibit RasA palmitoylation and therefore to perfectly mimic the rasa C206/207S mutation. Plasma membrane localization is required for full Ras function in cell wall integrity, -glucan deposition, and virulence. Deletion of RasA causes decreased cell wall integrity as evidenced by increased susceptibility to cell wall-destabilizing agents (10). To examine the contribution of plasma membrane-localized RasA activity to cell wall integrity, we first compared the susceptibilities of the wild type and the GFP-RasA C206/207S mutant to cell wall-damaging agents. The GFP-RasA C206/207S mutant displayed greater sensitivity to the chitin-perturbing compounds nikkomycin Z and calcofluor white than the wild-type strain. Nikkomycin Z (0.25 g/ml) treatment yielded 10% versus 52% inhibition (P 0.01) of radial growth rates for the wild-type and GFP-RasA C206/207S strains, respectively. Furthermore, growth in the presence of calcofluor white (3 g/ml) yielded 3% inhibition of the wild-type strain versus 32% inhibition of the GFP-RasA C206/207S strain (P 0.01). The GFP-RasA C206S and August 2012 Volume 11 Number 8 ec.asm.org 971
7 Fortwendel et al. FIG 4 Plasma membrane-localized RasA regulates morphology of branch formation. Conidia of the GFP-RasA C206/207S strain were inoculated onto a thin layer of GMM agar solidified on a glass-bottom petri dish and incubated at 37 C. Bright-field microscopy images were taken during germination (A) and at 24 h (B) and 48 h (C) of hyphal growth. Arrows indicate the appearance of morphologically normal hyphae after 24 h of growth. Arrowheads denote formation of branches after 48 h of growth that revert to the early growth phenotype. GFP-RasA C207S mutants displayed no increase in sensitivity to either cell wall-damaging agent compared to the wild type (data not shown). Because increased sensitivity to cell wall-disruptive agents was apparent in the GFP-RasA C206/207S mutant, cell wall organizational differences were examined using scanning electron microscopy (SEM) and transmission electron microscopy (TEM). SEM analysis revealed the presence of an extracellular matrix associated with cultures of the GFP-Ras C206/207S mutant that was not evident in the wild FIG 5 RasA contributes to cell wall structure and integrity from the plasma membrane. (A) Twenty-four-hour coverslip cultures of the wild-type (WT) and GFP-RasA C206/207S strains were used for scanning electron microscopy analysis. The lower panels are magnified images of the areas indicated in the upper panels by dashed boxes. Arrowheads indicate extracellular matrix produced by the GFP-RasA C206/207S mutant. (B) Twenty-four-hour coverslip cultures of each strain were utilized for transmission electron microscopy analysis. Note the disorganized outer layer and translucent middle layer of the GFP-RasA C206/207S cell wall. (C) Conidia of the wild-type (WT), rasa, and GFP-RasA C206/207S strains were grown on glass coverslips for 16 h at 37 C. Following incubation, coverslips were rinsed briefly and hyphae stained in 0.1% aniline blue. Arrowheads indicate low staining of hyphal tips in the rasa and GFP-RasA C206/207S mutants. 972 ec.asm.org Eukaryotic Cell
8 Ras Regulates A. fumigatus Growth from Plasma Membrane FIG 6 RasA mislocalization affects virulence in a murine model of invasive aspergillosis. Groups of 20 6-week-old CD1 male mice (20 to 26 g) were immunosuppressed with a single dose of triamcinolone acetonide (40 mg kg 1 ) on day 1 and inoculated intranasally with conidia from each strain. Survival was assessed over a 15-day period. The rasa and GFP-RasA C206/207S mutants displayed statistically significantly reduced virulence compared to the wild type. type (Fig. 5A, arrowheads). Subsequent TEM analysis revealed a GFP-RasA C206/207S cell wall that was disorganized compared to that of the wild type, with fibrillar material protruding from the outer layers of hyphae (Fig. 5B). We hypothesized that the extracellular fibrillar matrix was likely to be cell wall material that was inadequately incorporated at the growing hyphal tip. To visualize the distribution of chitin and -glucan, major cell wall components of A. fumigatus, hyphae from each strain were stained with calcofluor white or aniline blue. Although calcofluor white staining of the rasa and GFP- RasA C206/207S hyphae revealed slightly brighter fluorescence than for the wild type, all strains displayed similar patterns of chitin distribution (data not shown). This finding coincided with the TEM analysis, which revealed that the innermost portion of the cell wall, consisting mostly of chitin, was relatively unaltered in the mutant strain (Fig. 5B). In contrast, hyphal staining with aniline blue revealed an altered distribution of -1,3-glucan in the rasa and GFP-RasA C206/207S cell walls. After 16 h of culture, the wild-type strain stained intensely at the hyphal tip, whereas the subapical hyphal walls stained at relatively low levels (Fig. 5C). In contrast, both the rasa and RasA C206/207S mutants displayed reduced aniline blue staining at the hyphal tips accompanied by intense staining along the subapical hyphal walls (Fig. 5C). Thus, normal -glucan deposition appears to be dependent on plasma membrane-associated RasA. In C. neoformans, the deletion of Ras1 and mutation of the Ras1 palmitoylation domain both result in avirulence, due to lack of growth at 37 C (32). To determine the contribution of plasma membrane localization of RasA to A. fumigatus virulence, the rasa and GFP-RasA C206/207S mutants were compared to the wild-type strain in a murine model of invasive aspergillosis. This model utilizes a single dose of triamcinolone acetonide to induce transient immunosuppression (38). Virulence of both the rasa and GFP-RasA C206/207S mutants was significantly attenuated (P ), with infected mice displaying 25% and 20% mortality at 15 days postinoculation, respectively (Fig. 6). This was in contrast to the wild-type and complemented ( rasa rasa) strains, both of which caused higher mortality (Fig. 6), consistent with previously published results using wild-type A. fumigatus in the same mouse model (38). Proper RasA localization to the plasma membrane is therefore essential for full virulence in A. fumigatus infection. Plasma membrane localization is required for full induction of the activated RasA phenotypes of the DArasA1 mutant. We recently reported novel phenotypes caused by the expression of constitutively activated A. fumigatus RasA, including unchecked vacuolar expansion, hyphal swelling, and lysis of hyphal compartments (11). Recent studies with C. albicans revealed that constitutive activation of CaRas1p can overcome the effects of mislocalization, suggesting that CaRas1p localization at the plasma membrane may be more critical for full Ras protein activity than for engagement of the proper effector proteins (36). To test this hypothesis in A. fumigatus, we mutated the dual-palmitoylation motif of the previously generated DArasA1 cdna to generate a palmitoylation-deficient DArasA1 mutant (DArasA1 C206/207S ). The DArasA1 and DArasA1 C206/207S alleles were then expressed as GFP fusion proteins in the rasa background using the pagrp vector. Expression of GFP-DArasA1 produced the same phenotypes as previously reported for the untagged DARasA1 strain (11), including development of large, vacuolated subapical hyphal segments intermingled with normal apical compartments (Fig. 7A). Similarly to the wild-type RasA protein, GFP-DARasA1 localized primarily to the plasma membrane, along the hyphal periphery, and at septa (Fig. 7A). In contrast, mutation of the dual-palmitoylation motif caused the accumulation of GFP- DARasA1 C206/207S in the cytosol and on punctate endomembrane structures but not at the plasma membrane (Fig. 7B). Phenotypic analysis revealed that the GFP-DARasA1 C206/207S mutant developed hyphae that were stunted and highly branched (Fig. 7B). These hyphal phenotypes were more similar to those of the GFP- RasA C206/207S mutant (Fig. 3 and Fig. 4A) than to those of the GFP-DARasA1 mutant (Fig. 7A) (11), suggesting that constitutively activated RasA does not overcome the defects caused by RasA mislocalization. Although the hyphal morphology was more similar to that of the GFP-RasA C206/207S strain, the radial growth and colony morphology of the GFP-DARasA1 C206/207S mutant resembled that of the DARasA1 strain. Both strains formed more compact, slowly growing colonies, compared to the more substantial radial outgrowth generated by the GFP-RasA C206/207S mutant (Fig. 7C). Together, these data show that constitutively active RasA mislocalized to the endomembranes only partially induces activated Ras phenotypes. Therefore, although plasma membrane localization of RasA may be required for full induction of RasA activity, our studies do not rule out the lack of proper effector engagement by RasA restricted to the endomembrane system as a plausible scenario in A. fumigatus. The A. fumigatus Erf4p homolog, ErfD, is important for proper RasA localization, whereas the Erf2p homolog, ErfB, is not required. In S. cerevisiae, the Ras palmitoyltransferase complex is known to consist of two proteins, Erf2p and Erf4p (27, 50). Erf2p is a DHHC palmitoyltransferase enzyme responsible for transferring the palmitoyl moiety onto the Ras protein (1, 30, 31). The second subunit, Erf4p, was originally copurified with Erf2p and later found to be a peripheral ER membrane protein (1, 21). Although Erf4p does not contain the DHHC domain required for palmitoyl transfer, deletion of either subunit causes mislocalization of Ras2p from the plasma membrane (50). The A. fumigatus ERF2 homolog, erfb, was previously identified in a study of fungal DHHC palmitoyltransferases (14). To explore the importance of the palmitoyltransferase complex to RasA localization, we first identified the putative Erf4p homolog in A. fumigatus. The S. cerevisiae Erf4p sequence was used in a BLAST search of the A. August 2012 Volume 11 Number 8 ec.asm.org 973
9 Fortwendel et al. deletion of erfd ( erfd) led to a minor, but statistically significant (P 0.01), reduction in radial growth rate (Fig. 8B and C). No alteration in hyphal morphology was noted in the mutant strains (data not shown). Furthermore, expression of GFP-RasA in the mutant backgrounds revealed punctate RasA localization in the erfd strain, whereas erfb deletion caused no disruption in RasA localization (Fig. 8D). Mislocalization of the GFP-RasA signal to internal patches, resembling that in the GFP-RasA C206/207S and 2-BP-treated strains, was evident in the erfd mutant background (Fig. 8D). These data suggest a role for erfd in RasA localization during hyphal growth. FIG 7 Plasma membrane association is required for full development of the DARasA1 phenotype. (A and B) The DArasA or DArasA C206/207S cdna was cloned into vector pagrp and expressed in the rasa background. Conidia from each strain were inoculated onto coverslips immersed in GMM broth and incubated at 37 C for 16 h. Left panels, differential interference contrast (DIC) micrographs. Right panels, UV micrographs. Arrows indicate positions of septa. Note the grossly normal apical compartment (a.c.) mixed with swollen subapical sections in the GFP-DARasA1 mutant, which is not seen in the DARasA1 C206/207S strain. Plasma membrane localization was evident throughout the GFP-DARasA strain, whereas mutation of both C206 and C207 mislocalized DARasA to endomembranes. (C) Colony morphologies of the GFP- RasA C206/207S, GFP-DARasA1, and GFP-DARasA1 C206/207S strains. Ten thousand conidia of each strain were inoculated onto GMM agar and incubated for 3 days at 37 C. Note the compact colony morphology induced by expression of activated RasA, seen in both palmitoylated and palmitoylationdeficient mutants. fumigatus genome database ( This search identified a single A. fumigatus open reading frame (ORF) (Afu5G7920) which contains a Golgin subfamily A member 7/Erf4 superfamily domain. This protein shares 24% identity with S. cerevisiae Erf4p and was named ErfD. A Clustal V alignment of the Erf4 superfamily domain, with the highest identity between the S. cerevisiae and A. fumigatus proteins, is shown in Fig. 8A.To investigate whether the putative Ras-palmitoyltransferase complex is required for filamentous growth and/or RasA localization, strains with either erfb or erfd deleted were generated. Loss of erfb ( erfb), the DHHC palmitoyltransferase enzyme homolog, caused no alteration in colony morphology or growth rate compared to those of the wild-type strain (Fig. 8B and C). In contrast, DISCUSSION In the present study, mutations of the conserved Ras palmitoylation motif were introduced into A. fumigatus to delineate the importance of this domain to RasA localization and to understand the role of properly localized RasA in polarized morphogenesis. We found that RasA localized primarily to the plasma membrane, including at septa. Mislocalization of RasA from the plasma membrane caused defects in germination and hyphal morphogenesis, aberrant cell wall formation, and decreased virulence. The majority of these phenotypes were shared by the rasa mutant (10), suggesting a mostly nonfunctional RasA C206/207S protein. However, hyphal growth of the RasA C206/207S mutant was significantly greater than that seen in the complete absence of RasA, suggesting that mislocalized RasA remains partially competent to mediate some aspects of fungal growth and/or morphogenesis. This partial functionality was not attributable to reduced protein abundance, as was seen with a similar mutation in C. albicans Ras1 (36), because steady-state RasA protein levels were not negatively affected by the palmitoylation mutation in our model. Although temporal regulation of RasA protein activity is also important for RasAmediated growth (12, 35, 44), our findings support the hypothesis that spatial regulation of RasA through proper localization to the plasma membrane is required for engagement of RasA signaling pathways controlling polarized morphogenesis. At present, three possible models for spatial regulation of Ras signal transduction in fungi are as follows: (i) fungal Ras proteins employ the plasma membrane and endomembranes as separate signaling platforms, engaging distinct effectors at each membrane; (ii) fungal Ras proteins can potentially interact with all effectors equally from either membrane, but plasma membrane localization is required for activation and, therefore, function; or (iii) Ras activity is not affected by membrane distribution, and the partial functionality of the RasA C206/207S mutant could be explained by the presence of a pool of Ras that is still active at or near the plasma membrane. Each of these models is at least partially supported by the current literature, and some combination of the three is likely to provide a more complete picture of spatiotemporal control of Ras signaling in fungi. Differential localization of human Ras proteins to the plasma membrane and endomembrane propagates distinct signal transduction events through the utilization of distinct effector proteins in human and yeast (3, 4). Our data, along with recent reports for the yeasts, provide some support for a model of spatially segregated Ras-effector interactions in fungal organisms as well. For example, the utilization of specific membrane platforms for Ras signaling to morphogenetic programs appears to be conserved for C. neoformans, C. albicans, S. pombe, and, as our data show, A. fumigatus. At the plasma membrane, C. neoformans Ras1 controls 974 ec.asm.org Eukaryotic Cell
10 Ras Regulates A. fumigatus Growth from Plasma Membrane FIG 8 The A. fumigatus ERF4 homolog, erfd, is required for proper RasA localization. (A) Partial alignment of the Erf4 superfamily domain of S. cerevisiae (Sc) Erf4p and the putative protein sequence of A. fumigatus (Af) ErfD. Conserved residues are highlighted in gray. Amino acid position numbers represent the A. fumigatus ErfD sequence. (B) Colony morphologies of the erfb and erfd mutants. Ten thousand conidia from each strain were inoculated into GMM agar and incubated for 4 days at 37 C. (C) Quantitative comparison of radial growth for the wild-type (WT) and mutant strains. Data points are the mean colony diameter in millimeters from triplicate growth experiments for each time point. (D) Localization of GFP-RasA in the erfb and erfd mutant backgrounds. Coverslip cultures of each strain were utilized for fluorescence microscopy. Note the increased presence of endomembrane GFP-RasA signal in the erfd mutant background. cell shape and high-temperature growth, while C. albicans Ras1 regulates the yeast-to-hypha transition (32). Our data similarly suggest that A. fumigatus RasA may utilize the plasma membrane as a platform to interact with distinct effectors mediating polarized growth and hyphal morphogenesis. Further, C. neoformans Ras1 restricted to endomembranes is functional for the support of the mating response (32). In contrast, S. pombe utilizes plasma membrane-localized Ras1 for the mating response, whereas endomembrane-localized Ras1 mediates proper morphology (4, 32). Although the specific membrane platforms utilized for Ras signal output differ, the current data argue that both C. neoformans and S. pombe may be able to spatially segregate the activation of Rasmediated morphology and mating pathways through activation of distinct effectors (4, 32). As a sexual cycle for A. fumigatus has recently been described, it will be of interest to analyze the ability of a RasA C206/207S mutant to mate (33). Such studies would reveal whether activation of the mating pathway in a mold can be accomplished when Ras localization or activity is not concentrated at the plasma membrane. In addition to morphogenesis, -glucan display properties and cell wall integrity were altered in both the rasa and RasA C206/207S mutants, again suggesting that plasma membrane localization is important for these roles. Host recognition of exposed -glucan of the A. fumigatus cell wall is an important mediator of host-pathogen interactions (25, 28). Future work in this area will focus on quantitative measurement of -glucan exposure and the subsequent immune response generated by RasA mutant strains. Although differential activation of distinct effectors from each membrane is an attractive model, we cannot yet rule out the possibility that mislocalization of RasA results in greatly decreased activation due to the inability of Ras to interact with its cognate GEF. Data from studies with C. albicans seem to support this alternative, as expression of a constitutively activated palmitoylation-deficient Ras1 can overcome the embedded filamentation defect of the Ras1 palmitoylation mutant (36). However, in S. cerevisiae, where expression of activated Ras2p results in hypersensitivity to heat shock and nutrient stress, loss of Ras2p palmitoylation reverses activated Ras phenotypes (6). This supports a model in which Ras must be localized to the plasma membrane to engage proper effectors. Our data suggest that regulation of A. fumigatus RasA may involve a combination of these two scenarios. Expression of DARasA1 C026/207S caused loss of some hyphal phenotypes associated with DARasA1 expression, suggesting that the constitutively activated RasA was unable to interact with needed effectors. However, mislocalization of DARasA1 did not cause complete reversion to the RasA C206/207S phenotype, as colony morphology was compact, similar to that of the DARasA1 mutant. A specific, biochemical analysis of Ras activity will be required to decipher this question. Similarly, we cannot rule out the potential for a pool of active RasA near the plasma membrane that may be able to propagate the signals needed to produce the limited hyphal growth observed in the RasA C206/207S mutant. This caveat was also noted in recent studies with the Ras1 protein of C. neoformans (32). Current models of human Ras protein trafficking suggest a August 2012 Volume 11 Number 8 ec.asm.org 975
SUPPLEMENTARY INFORMATION
 Supplementary Figures Supplementary Figure S1. Binding of full-length OGT and deletion mutants to PIP strips (Echelon Biosciences). Supplementary Figure S2. Binding of the OGT (919-1036) fragments with
Supplementary Figures Supplementary Figure S1. Binding of full-length OGT and deletion mutants to PIP strips (Echelon Biosciences). Supplementary Figure S2. Binding of the OGT (919-1036) fragments with
Luminescent platforms for monitoring changes in the solubility of amylin and huntingtin in living cells
 Electronic Supplementary Material (ESI) for Molecular BioSystems. This journal is The Royal Society of Chemistry 2016 Contents Supporting Information Luminescent platforms for monitoring changes in the
Electronic Supplementary Material (ESI) for Molecular BioSystems. This journal is The Royal Society of Chemistry 2016 Contents Supporting Information Luminescent platforms for monitoring changes in the
Factors influencing the Aspergillus fumigatus survival into the host mediated by the calcineurin pathway
 Factors influencing the Aspergillus fumigatus survival into the host mediated by the calcineurin pathway Gustavo H. Goldman Universidade de São Paulo, Brazil Calcineurin is a calmodulin/ca +2 dependent
Factors influencing the Aspergillus fumigatus survival into the host mediated by the calcineurin pathway Gustavo H. Goldman Universidade de São Paulo, Brazil Calcineurin is a calmodulin/ca +2 dependent
HCC1937 is the HCC1937-pcDNA3 cell line, which was derived from a breast cancer with a mutation
 SUPPLEMENTARY INFORMATION Materials and Methods Human cell lines and culture conditions HCC1937 is the HCC1937-pcDNA3 cell line, which was derived from a breast cancer with a mutation in exon 20 of BRCA1
SUPPLEMENTARY INFORMATION Materials and Methods Human cell lines and culture conditions HCC1937 is the HCC1937-pcDNA3 cell line, which was derived from a breast cancer with a mutation in exon 20 of BRCA1
TFEB-mediated increase in peripheral lysosomes regulates. Store Operated Calcium Entry
 TFEB-mediated increase in peripheral lysosomes regulates Store Operated Calcium Entry Luigi Sbano, Massimo Bonora, Saverio Marchi, Federica Baldassari, Diego L. Medina, Andrea Ballabio, Carlotta Giorgi
TFEB-mediated increase in peripheral lysosomes regulates Store Operated Calcium Entry Luigi Sbano, Massimo Bonora, Saverio Marchi, Federica Baldassari, Diego L. Medina, Andrea Ballabio, Carlotta Giorgi
Conventional or molecular measurement of Aspergillus load. Dr Karl Clemons California Institute for Medical Research
 Conventional or molecular measurement of Aspergillus load Dr Karl Clemons California Institute for Medical Research Everyone quantifies burdens or numbers of organisms, so what s the big deal?? Why does
Conventional or molecular measurement of Aspergillus load Dr Karl Clemons California Institute for Medical Research Everyone quantifies burdens or numbers of organisms, so what s the big deal?? Why does
Supplemental Data. Hiruma et al. Plant Cell. (2010) /tpc Col-0. pen2-1
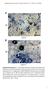 A Ch B Col-0 Cg pen2-1 Supplemental Figure 1. Trypan Blue Staining of Leaves Inoculated with Adapted and Nonadapted Colletotrichum Species.(A) Conidial suspensions of C. higginsianum MAFF305635 (Ch) were
A Ch B Col-0 Cg pen2-1 Supplemental Figure 1. Trypan Blue Staining of Leaves Inoculated with Adapted and Nonadapted Colletotrichum Species.(A) Conidial suspensions of C. higginsianum MAFF305635 (Ch) were
ab CytoPainter Golgi/ER Staining Kit
 ab139485 CytoPainter Golgi/ER Staining Kit Instructions for Use Designed to detect Golgi bodies and endoplasmic reticulum by microscopy This product is for research use only and is not intended for diagnostic
ab139485 CytoPainter Golgi/ER Staining Kit Instructions for Use Designed to detect Golgi bodies and endoplasmic reticulum by microscopy This product is for research use only and is not intended for diagnostic
Procaspase-3. Cleaved caspase-3. actin. Cytochrome C (10 M) Z-VAD-fmk. Procaspase-3. Cleaved caspase-3. actin. Z-VAD-fmk
 A HeLa actin - + + - - + Cytochrome C (1 M) Z-VAD-fmk PMN - + + - - + actin Cytochrome C (1 M) Z-VAD-fmk Figure S1. (A) Pan-caspase inhibitor z-vad-fmk inhibits cytochrome c- mediated procaspase-3 cleavage.
A HeLa actin - + + - - + Cytochrome C (1 M) Z-VAD-fmk PMN - + + - - + actin Cytochrome C (1 M) Z-VAD-fmk Figure S1. (A) Pan-caspase inhibitor z-vad-fmk inhibits cytochrome c- mediated procaspase-3 cleavage.
Newer Combination Therapies
 Newer Combination Therapies William J. Steinbach, MD Associate Professor of Pediatrics, Molecular Genetics & Microbiology Pediatric Infectious Diseases Duke University Medical Center Combination Therapy
Newer Combination Therapies William J. Steinbach, MD Associate Professor of Pediatrics, Molecular Genetics & Microbiology Pediatric Infectious Diseases Duke University Medical Center Combination Therapy
Supplementary Information
 Supplementary Information Supplementary Figure 1. CD4 + T cell activation and lack of apoptosis after crosslinking with anti-cd3 + anti-cd28 + anti-cd160. (a) Flow cytometry of anti-cd160 (5D.10A11) binding
Supplementary Information Supplementary Figure 1. CD4 + T cell activation and lack of apoptosis after crosslinking with anti-cd3 + anti-cd28 + anti-cd160. (a) Flow cytometry of anti-cd160 (5D.10A11) binding
1. endoplasmic reticulum This is the location where N-linked oligosaccharide is initially synthesized and attached to glycoproteins.
 Biology 4410 Name Spring 2006 Exam 2 A. Multiple Choice, 2 pt each Pick the best choice from the list of choices, and write it in the space provided. Some choices may be used more than once, and other
Biology 4410 Name Spring 2006 Exam 2 A. Multiple Choice, 2 pt each Pick the best choice from the list of choices, and write it in the space provided. Some choices may be used more than once, and other
Islet viability assay and Glucose Stimulated Insulin Secretion assay RT-PCR and Western Blot
 Islet viability assay and Glucose Stimulated Insulin Secretion assay Islet cell viability was determined by colorimetric (3-(4,5-dimethylthiazol-2-yl)-2,5- diphenyltetrazolium bromide assay using CellTiter
Islet viability assay and Glucose Stimulated Insulin Secretion assay Islet cell viability was determined by colorimetric (3-(4,5-dimethylthiazol-2-yl)-2,5- diphenyltetrazolium bromide assay using CellTiter
The Allergens of Cladosporium herbarum and Alternaria alternata
 Breitenbach M, Crameri R, Lehrer SB (eds): Fungal Allergy and Pathogenicity. Chem Immunol. Basel, Karger, 2002, vol 81, pp 48 72 The Allergens of Cladosporium herbarum and Alternaria alternata Michael
Breitenbach M, Crameri R, Lehrer SB (eds): Fungal Allergy and Pathogenicity. Chem Immunol. Basel, Karger, 2002, vol 81, pp 48 72 The Allergens of Cladosporium herbarum and Alternaria alternata Michael
(A) PCR primers (arrows) designed to distinguish wild type (P1+P2), targeted (P1+P2) and excised (P1+P3)14-
 1 Supplemental Figure Legends Figure S1. Mammary tumors of ErbB2 KI mice with 14-3-3σ ablation have elevated ErbB2 transcript levels and cell proliferation (A) PCR primers (arrows) designed to distinguish
1 Supplemental Figure Legends Figure S1. Mammary tumors of ErbB2 KI mice with 14-3-3σ ablation have elevated ErbB2 transcript levels and cell proliferation (A) PCR primers (arrows) designed to distinguish
SUPPLEMENTARY INFORMATION
 DOI: 10.1038/ncb2988 Supplementary Figure 1 Kif7 L130P encodes a stable protein that does not localize to cilia tips. (a) Immunoblot with KIF7 antibody in cell lysates of wild-type, Kif7 L130P and Kif7
DOI: 10.1038/ncb2988 Supplementary Figure 1 Kif7 L130P encodes a stable protein that does not localize to cilia tips. (a) Immunoblot with KIF7 antibody in cell lysates of wild-type, Kif7 L130P and Kif7
Dental Research Institute, Faculty of Dentistry, University of Toronto, Toronto, Canada *For correspondence:
 Zymogram Assay for the Detection of Peptidoglycan Hydrolases in Streptococcus mutans Delphine Dufour and Céline M. Lévesque * Dental Research Institute, Faculty of Dentistry, University of Toronto, Toronto,
Zymogram Assay for the Detection of Peptidoglycan Hydrolases in Streptococcus mutans Delphine Dufour and Céline M. Lévesque * Dental Research Institute, Faculty of Dentistry, University of Toronto, Toronto,
Instructions for Use. APO-AB Annexin V-Biotin Apoptosis Detection Kit 100 tests
 3URGXFW,QIRUPDWLRQ Sigma TACS Annexin V Apoptosis Detection Kits Instructions for Use APO-AB Annexin V-Biotin Apoptosis Detection Kit 100 tests For Research Use Only. Not for use in diagnostic procedures.
3URGXFW,QIRUPDWLRQ Sigma TACS Annexin V Apoptosis Detection Kits Instructions for Use APO-AB Annexin V-Biotin Apoptosis Detection Kit 100 tests For Research Use Only. Not for use in diagnostic procedures.
Supplementary Information POLO-LIKE KINASE 1 FACILITATES LOSS OF PTEN-INDUCED PROSTATE CANCER FORMATION
 Supplementary Information POLO-LIKE KINASE 1 FACILITATES LOSS OF PTEN-INDUCED PROSTATE CANCER FORMATION X. Shawn Liu 1, 3, Bing Song 2, 3, Bennett D. Elzey 3, 4, Timothy L. Ratliff 3, 4, Stephen F. Konieczny
Supplementary Information POLO-LIKE KINASE 1 FACILITATES LOSS OF PTEN-INDUCED PROSTATE CANCER FORMATION X. Shawn Liu 1, 3, Bing Song 2, 3, Bennett D. Elzey 3, 4, Timothy L. Ratliff 3, 4, Stephen F. Konieczny
Supplementary Figure 1. AdipoR1 silencing and overexpression controls. (a) Representative blots (upper and lower panels) showing the AdipoR1 protein
 Supplementary Figure 1. AdipoR1 silencing and overexpression controls. (a) Representative blots (upper and lower panels) showing the AdipoR1 protein content relative to GAPDH in two independent experiments.
Supplementary Figure 1. AdipoR1 silencing and overexpression controls. (a) Representative blots (upper and lower panels) showing the AdipoR1 protein content relative to GAPDH in two independent experiments.
Work-flow: protein sample preparation Precipitation methods Removal of interfering substances Specific examples:
 Dr. Sanjeeva Srivastava IIT Bombay Work-flow: protein sample preparation Precipitation methods Removal of interfering substances Specific examples: Sample preparation for serum proteome analysis Sample
Dr. Sanjeeva Srivastava IIT Bombay Work-flow: protein sample preparation Precipitation methods Removal of interfering substances Specific examples: Sample preparation for serum proteome analysis Sample
SUPPLEMENTARY INFORMATION
 SUPPLEMENTARY INFORMATION FOR Liver X Receptor α mediates hepatic triglyceride accumulation through upregulation of G0/G1 Switch Gene 2 (G0S2) expression I: SUPPLEMENTARY METHODS II: SUPPLEMENTARY FIGURES
SUPPLEMENTARY INFORMATION FOR Liver X Receptor α mediates hepatic triglyceride accumulation through upregulation of G0/G1 Switch Gene 2 (G0S2) expression I: SUPPLEMENTARY METHODS II: SUPPLEMENTARY FIGURES
(Stratagene, La Jolla, CA) (Supplemental Fig. 1A). A 5.4-kb EcoRI fragment
 SUPPLEMENTAL INFORMATION Supplemental Methods Generation of RyR2-S2808D Mice Murine genomic RyR2 clones were isolated from a 129/SvEvTacfBR λ-phage library (Stratagene, La Jolla, CA) (Supplemental Fig.
SUPPLEMENTAL INFORMATION Supplemental Methods Generation of RyR2-S2808D Mice Murine genomic RyR2 clones were isolated from a 129/SvEvTacfBR λ-phage library (Stratagene, La Jolla, CA) (Supplemental Fig.
Supporting Information
 Supporting Information Dauvillée et al. 10.1073/pnas.0907424106 Fig. S1. Iodine screening of the C. cohnii mutant bank. Each single colony was grown on rich-medium agar plates then vaporized with iodine.
Supporting Information Dauvillée et al. 10.1073/pnas.0907424106 Fig. S1. Iodine screening of the C. cohnii mutant bank. Each single colony was grown on rich-medium agar plates then vaporized with iodine.
SUPPLEMENTARY INFORMATION. Supplementary Figures S1-S9. Supplementary Methods
 SUPPLEMENTARY INFORMATION SUMO1 modification of PTEN regulates tumorigenesis by controlling its association with the plasma membrane Jian Huang 1,2#, Jie Yan 1,2#, Jian Zhang 3#, Shiguo Zhu 1, Yanli Wang
SUPPLEMENTARY INFORMATION SUMO1 modification of PTEN regulates tumorigenesis by controlling its association with the plasma membrane Jian Huang 1,2#, Jie Yan 1,2#, Jian Zhang 3#, Shiguo Zhu 1, Yanli Wang
Supplementary Figure 1.
 Supplementary Figure 1. Visualization of endoplasmic reticulum-mitochondria interaction by in situ proximity ligation assay. A) Illustration of targeted proteins in mitochondria (M), endoplasmic reticulum
Supplementary Figure 1. Visualization of endoplasmic reticulum-mitochondria interaction by in situ proximity ligation assay. A) Illustration of targeted proteins in mitochondria (M), endoplasmic reticulum
SUPPORTING MATREALS. Methods and Materials
 SUPPORTING MATREALS Methods and Materials Cell Culture MC3T3-E1 (subclone 4) cells were maintained in -MEM with 10% FBS, 1% Pen/Strep at 37ºC in a humidified incubator with 5% CO2. MC3T3 cell differentiation
SUPPORTING MATREALS Methods and Materials Cell Culture MC3T3-E1 (subclone 4) cells were maintained in -MEM with 10% FBS, 1% Pen/Strep at 37ºC in a humidified incubator with 5% CO2. MC3T3 cell differentiation
Structural vs. nonstructural proteins
 Why would you want to study proteins associated with viruses or virus infection? Receptors Mechanism of uncoating How is gene expression carried out, exclusively by viral enzymes? Gene expression phases?
Why would you want to study proteins associated with viruses or virus infection? Receptors Mechanism of uncoating How is gene expression carried out, exclusively by viral enzymes? Gene expression phases?
a b G75 G60 Sw-2 Sw-1 Supplementary Figure 1. Structure predictions by I-TASSER Server.
 a b G75 2 2 G60 Sw-2 Sw-1 Supplementary Figure 1. Structure predictions by I-TASSER Server. a. Overlay of top 10 models generated by I-TASSER illustrates the potential effect of 7 amino acid insertion
a b G75 2 2 G60 Sw-2 Sw-1 Supplementary Figure 1. Structure predictions by I-TASSER Server. a. Overlay of top 10 models generated by I-TASSER illustrates the potential effect of 7 amino acid insertion
Legionella pneumophila: an intracellular pathogen of phagocytes Prof. Craig Roy
 an intracellular pathogen of phagocytes Section of Microbial Pathogenesis, Yale University School of Medicine 1 Legionella pneumophila Gram-negative bacterium Facultative intracellular pathogen Protozoa
an intracellular pathogen of phagocytes Section of Microbial Pathogenesis, Yale University School of Medicine 1 Legionella pneumophila Gram-negative bacterium Facultative intracellular pathogen Protozoa
(a) Significant biological processes (upper panel) and disease biomarkers (lower panel)
 Supplementary Figure 1. Functional enrichment analyses of secretomic proteins. (a) Significant biological processes (upper panel) and disease biomarkers (lower panel) 2 involved by hrab37-mediated secretory
Supplementary Figure 1. Functional enrichment analyses of secretomic proteins. (a) Significant biological processes (upper panel) and disease biomarkers (lower panel) 2 involved by hrab37-mediated secretory
Expression of acid base transporters in the kidney collecting duct in Slc2a7 -/-
 Supplemental Material Results. Expression of acid base transporters in the kidney collecting duct in Slc2a7 -/- and Slc2a7 -/- mice. The expression of AE1 in the kidney was examined in Slc26a7 KO mice.
Supplemental Material Results. Expression of acid base transporters in the kidney collecting duct in Slc2a7 -/- and Slc2a7 -/- mice. The expression of AE1 in the kidney was examined in Slc26a7 KO mice.
Mammalian Membrane Protein Extraction Kit
 Mammalian Membrane Protein Extraction Kit Catalog number: AR0155 Boster s Mammalian Membrane Protein Extraction Kit is a simple, rapid and reproducible method to prepare cellular protein fractions highly
Mammalian Membrane Protein Extraction Kit Catalog number: AR0155 Boster s Mammalian Membrane Protein Extraction Kit is a simple, rapid and reproducible method to prepare cellular protein fractions highly
Supplemental Data. Wang et al. (2013). Plant Cell /tpc
 Supplemental Data. Wang et al. (2013). Plant Cell 10.1105/tpc.112.108993 Supplemental Figure 1. 3-MA Treatment Reduces the Growth of Seedlings. Two-week-old Nicotiana benthamiana seedlings germinated on
Supplemental Data. Wang et al. (2013). Plant Cell 10.1105/tpc.112.108993 Supplemental Figure 1. 3-MA Treatment Reduces the Growth of Seedlings. Two-week-old Nicotiana benthamiana seedlings germinated on
SUPPLEMENTAL INFORMATION
 SUPPLEMENTAL INFORMATION EXPERIMENTAL PROCEDURES Tryptic digestion protection experiments - PCSK9 with Ab-3D5 (1:1 molar ratio) in 50 mm Tris, ph 8.0, 150 mm NaCl was incubated overnight at 4 o C. The
SUPPLEMENTAL INFORMATION EXPERIMENTAL PROCEDURES Tryptic digestion protection experiments - PCSK9 with Ab-3D5 (1:1 molar ratio) in 50 mm Tris, ph 8.0, 150 mm NaCl was incubated overnight at 4 o C. The
Supplemental Data. Wu et al. (2010). Plant Cell /tpc
 Supplemental Figure 1. FIM5 is preferentially expressed in stamen and mature pollen. The expression data of FIM5 was extracted from Arabidopsis efp browser (http://www.bar.utoronto.ca/efp/development/),
Supplemental Figure 1. FIM5 is preferentially expressed in stamen and mature pollen. The expression data of FIM5 was extracted from Arabidopsis efp browser (http://www.bar.utoronto.ca/efp/development/),
Materials and Methods , The two-hybrid principle.
 The enzymatic activity of an unknown protein which cleaves the phosphodiester bond between the tyrosine residue of a viral protein and the 5 terminus of the picornavirus RNA Introduction Every day there
The enzymatic activity of an unknown protein which cleaves the phosphodiester bond between the tyrosine residue of a viral protein and the 5 terminus of the picornavirus RNA Introduction Every day there
Immature organoids appear after hours.
 THE ESSENTIALS OF LIFE SCIENCE RESEARCH GLOBALLY DELIVERED Allison Ruchinskas, B.S., and James Clinton, Ph.D. ATCC Cell Systems, Gaithersburg, MD INTRODUCTION Figure 1. Mouse small intestinal organoid
THE ESSENTIALS OF LIFE SCIENCE RESEARCH GLOBALLY DELIVERED Allison Ruchinskas, B.S., and James Clinton, Ph.D. ATCC Cell Systems, Gaithersburg, MD INTRODUCTION Figure 1. Mouse small intestinal organoid
Ultrastructure of Mycoplasmatales Virus laidlawii x
 J. gen. Virol. (1972), I6, 215-22I Printed in Great Britain 2I 5 Ultrastructure of Mycoplasmatales Virus laidlawii x By JUDY BRUCE, R. N. GOURLAY, AND D. J. GARWES R. HULL* Agricultural Research Council,
J. gen. Virol. (1972), I6, 215-22I Printed in Great Britain 2I 5 Ultrastructure of Mycoplasmatales Virus laidlawii x By JUDY BRUCE, R. N. GOURLAY, AND D. J. GARWES R. HULL* Agricultural Research Council,
Protocol for Gene Transfection & Western Blotting
 The schedule and the manual of basic techniques for cell culture Advanced Protocol for Gene Transfection & Western Blotting Schedule Day 1 26/07/2008 Transfection Day 3 28/07/2008 Cell lysis Immunoprecipitation
The schedule and the manual of basic techniques for cell culture Advanced Protocol for Gene Transfection & Western Blotting Schedule Day 1 26/07/2008 Transfection Day 3 28/07/2008 Cell lysis Immunoprecipitation
Annexin V-PE Apoptosis Detection Kit
 Annexin V-PE Apoptosis Detection Kit Catalog Number KA0716 100 assays Version: 02 Intended for research use only www.abnova.com Table of Contents Introduction... 3 Background... 3 General Information...
Annexin V-PE Apoptosis Detection Kit Catalog Number KA0716 100 assays Version: 02 Intended for research use only www.abnova.com Table of Contents Introduction... 3 Background... 3 General Information...
Nature Structural & Molecular Biology: doi: /nsmb Supplementary Figure 1. Generation and validation of mtef4-knockout mice.
 Supplementary Figure 1 Generation and validation of mtef4-knockout mice. (a) Alignment of EF4 (E. coli) with mouse, yeast and human EF4. (b) Domain structures of mouse mtef4 compared to those of EF4 (E.
Supplementary Figure 1 Generation and validation of mtef4-knockout mice. (a) Alignment of EF4 (E. coli) with mouse, yeast and human EF4. (b) Domain structures of mouse mtef4 compared to those of EF4 (E.
SUPPLEMENTARY INFORMATION
 SUPPLEMENTARY INFORMATION doi:10.1038/nature11429 S1a 6 7 8 9 Nlrc4 allele S1b Nlrc4 +/+ Nlrc4 +/F Nlrc4 F/F 9 Targeting construct 422 bp 273 bp FRT-neo-gb-PGK-FRT 3x.STOP S1c Nlrc4 +/+ Nlrc4 F/F casp1
SUPPLEMENTARY INFORMATION doi:10.1038/nature11429 S1a 6 7 8 9 Nlrc4 allele S1b Nlrc4 +/+ Nlrc4 +/F Nlrc4 F/F 9 Targeting construct 422 bp 273 bp FRT-neo-gb-PGK-FRT 3x.STOP S1c Nlrc4 +/+ Nlrc4 F/F casp1
MCB130 Midterm. GSI s Name:
 1. Peroxisomes are small, membrane-enclosed organelles that function in the degradation of fatty acids and in the degradation of H 2 O 2. Peroxisomes are not part of the secretory pathway and peroxisomal
1. Peroxisomes are small, membrane-enclosed organelles that function in the degradation of fatty acids and in the degradation of H 2 O 2. Peroxisomes are not part of the secretory pathway and peroxisomal
Supplementary Figure 1. SC35M polymerase activity in the presence of Bat or SC35M NP encoded from the phw2000 rescue plasmid.
 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 Supplementary Figure 1. SC35M polymerase activity in the presence of Bat or SC35M NP encoded from the phw2000 rescue plasmid. HEK293T
1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 Supplementary Figure 1. SC35M polymerase activity in the presence of Bat or SC35M NP encoded from the phw2000 rescue plasmid. HEK293T
RayBio Annexin V-Cy5 Apoptosis Detection Kit
 RayBio Annexin V-Cy5 Apoptosis Detection Kit User Manual Version 1.0 Mar 20, 2014 (Cat#: 68C5-AnnV-S) RayBiotech, Inc. We Provide You With Excellent Support And Service Tel:(Toll Free)1-888-494-8555 or
RayBio Annexin V-Cy5 Apoptosis Detection Kit User Manual Version 1.0 Mar 20, 2014 (Cat#: 68C5-AnnV-S) RayBiotech, Inc. We Provide You With Excellent Support And Service Tel:(Toll Free)1-888-494-8555 or
Probe. Hind III Q,!?R'!! /0!!!!D1"?R'! vector. Homologous recombination
 Supple-Zhang Page 1 Wild-type locus Targeting construct Targeted allele Exon Exon3 Exon Probe P1 P P3 FRT FRT loxp loxp neo vector amh I Homologous recombination neo P1 P P3 FLPe recombination Q,!?R'!!
Supple-Zhang Page 1 Wild-type locus Targeting construct Targeted allele Exon Exon3 Exon Probe P1 P P3 FRT FRT loxp loxp neo vector amh I Homologous recombination neo P1 P P3 FLPe recombination Q,!?R'!!
04_polarity. The formation of synaptic vesicles
 Brefeldin prevents assembly of the coats required for budding Nocodazole disrupts microtubules Constitutive: coatomer-coated Selected: clathrin-coated The formation of synaptic vesicles Nerve cells (and
Brefeldin prevents assembly of the coats required for budding Nocodazole disrupts microtubules Constitutive: coatomer-coated Selected: clathrin-coated The formation of synaptic vesicles Nerve cells (and
THE ROLE OF ALTERED CALCIUM AND mtor SIGNALING IN THE PATHOGENESIS OF CYSTINOSIS
 Research Foundation, 18 month progress report THE ROLE OF ALTERED CALCIUM AND mtor SIGNALING IN THE PATHOGENESIS OF CYSTINOSIS Ekaterina Ivanova, doctoral student Elena Levtchenko, MD, PhD, PI Antonella
Research Foundation, 18 month progress report THE ROLE OF ALTERED CALCIUM AND mtor SIGNALING IN THE PATHOGENESIS OF CYSTINOSIS Ekaterina Ivanova, doctoral student Elena Levtchenko, MD, PhD, PI Antonella
Chapter 3. Expression of α5-megfp in Mouse Cortical Neurons. on the β subunit. Signal sequences in the M3-M4 loop of β nachrs bind protein factors to
 22 Chapter 3 Expression of α5-megfp in Mouse Cortical Neurons Subcellular localization of the neuronal nachr subtypes α4β2 and α4β4 depends on the β subunit. Signal sequences in the M3-M4 loop of β nachrs
22 Chapter 3 Expression of α5-megfp in Mouse Cortical Neurons Subcellular localization of the neuronal nachr subtypes α4β2 and α4β4 depends on the β subunit. Signal sequences in the M3-M4 loop of β nachrs
Nature Immunology: doi: /ni.3631
 Supplementary Figure 1 SPT analyses of Zap70 at the T cell plasma membrane. (a) Total internal reflection fluorescent (TIRF) excitation at 64-68 degrees limits single molecule detection to 100-150 nm above
Supplementary Figure 1 SPT analyses of Zap70 at the T cell plasma membrane. (a) Total internal reflection fluorescent (TIRF) excitation at 64-68 degrees limits single molecule detection to 100-150 nm above
Recipes for Media and Solution Preparation SC-ura/Glucose Agar Dishes (20mL/dish, enough for 8 clones)
 Protocol: 300 ml Yeast culture preparation Equipment and Reagents needed: Autoclaved toothpicks Shaker Incubator set at 30 C Incubator set at 30 C 60 mm 2 sterile petri dishes Autoclaved glass test tubes
Protocol: 300 ml Yeast culture preparation Equipment and Reagents needed: Autoclaved toothpicks Shaker Incubator set at 30 C Incubator set at 30 C 60 mm 2 sterile petri dishes Autoclaved glass test tubes
Problem Set 8 Key 1 of 8
 7.06 2003 Problem Set 8 Key 1 of 8 7.06 2003 Problem Set 8 Key 1. As a bright MD/PhD, you are interested in questions about the control of cell number in the body. Recently, you've seen three patients
7.06 2003 Problem Set 8 Key 1 of 8 7.06 2003 Problem Set 8 Key 1. As a bright MD/PhD, you are interested in questions about the control of cell number in the body. Recently, you've seen three patients
McWilliams et al., http :// /cgi /content /full /jcb /DC1
 Supplemental material JCB McWilliams et al., http ://www.jcb.org /cgi /content /full /jcb.201603039 /DC1 THE JOURNAL OF CELL BIOLOGY Figure S1. In vitro characterization of mito-qc. (A and B) Analysis
Supplemental material JCB McWilliams et al., http ://www.jcb.org /cgi /content /full /jcb.201603039 /DC1 THE JOURNAL OF CELL BIOLOGY Figure S1. In vitro characterization of mito-qc. (A and B) Analysis
HiPer Western Blotting Teaching Kit
 HiPer Western Blotting Teaching Kit Product Code: HTI009 Number of experiments that can be performed: 5/20 Duration of Experiment: ~ 2 days Day 1: 6-8 hours (SDS- PAGE and Electroblotting) Day 2: 3 hours
HiPer Western Blotting Teaching Kit Product Code: HTI009 Number of experiments that can be performed: 5/20 Duration of Experiment: ~ 2 days Day 1: 6-8 hours (SDS- PAGE and Electroblotting) Day 2: 3 hours
Onion (Allium cepa) genotoxicity test
 Onion (Allium cepa) genotoxicity test 1. Purpose The Allium cepa assay is an efficient test for chemical screening and in situ monitoring for genotoxicity of environmental contaminants. The test has been
Onion (Allium cepa) genotoxicity test 1. Purpose The Allium cepa assay is an efficient test for chemical screening and in situ monitoring for genotoxicity of environmental contaminants. The test has been
Introduction. Study of fungi called mycology.
 Fungi Introduction Study of fungi called mycology. Some fungi are beneficial: ex a) Important in production of some foods, ex: cheeses, bread. b) Important in production of some antibiotics, ex: penicillin
Fungi Introduction Study of fungi called mycology. Some fungi are beneficial: ex a) Important in production of some foods, ex: cheeses, bread. b) Important in production of some antibiotics, ex: penicillin
TSH Receptor Monoclonal Antibody (49) Catalog Number MA3-218 Product data sheet
 Website: thermofisher.com Customer Service (US): 1 800 955 6288 ext. 1 Technical Support (US): 1 800 955 6288 ext. 441 TSH Receptor Monoclonal Antibody (49) Catalog Number MA3-218 Product data sheet Details
Website: thermofisher.com Customer Service (US): 1 800 955 6288 ext. 1 Technical Support (US): 1 800 955 6288 ext. 441 TSH Receptor Monoclonal Antibody (49) Catalog Number MA3-218 Product data sheet Details
Figure S1. (A) SDS-PAGE separation of GST-fusion proteins purified from E.coli BL21 strain is shown. An equal amount of GST-tag control, LRRK2 LRR
 Figure S1. (A) SDS-PAGE separation of GST-fusion proteins purified from E.coli BL21 strain is shown. An equal amount of GST-tag control, LRRK2 LRR and LRRK2 WD40 GST fusion proteins (5 µg) were loaded
Figure S1. (A) SDS-PAGE separation of GST-fusion proteins purified from E.coli BL21 strain is shown. An equal amount of GST-tag control, LRRK2 LRR and LRRK2 WD40 GST fusion proteins (5 µg) were loaded
Problem Set #5 4/3/ Spring 02
 Question 1 Chloroplasts contain six compartments outer membrane, intermembrane space, inner membrane, stroma, thylakoid membrane, and thylakoid lumen each of which is populated by specific sets of proteins.
Question 1 Chloroplasts contain six compartments outer membrane, intermembrane space, inner membrane, stroma, thylakoid membrane, and thylakoid lumen each of which is populated by specific sets of proteins.
Influenza virus exploits tunneling nanotubes for cell-to-cell spread
 Supplementary Information Influenza virus exploits tunneling nanotubes for cell-to-cell spread Amrita Kumar 1, Jin Hyang Kim 1, Priya Ranjan 1, Maureen G. Metcalfe 2, Weiping Cao 1, Margarita Mishina 1,
Supplementary Information Influenza virus exploits tunneling nanotubes for cell-to-cell spread Amrita Kumar 1, Jin Hyang Kim 1, Priya Ranjan 1, Maureen G. Metcalfe 2, Weiping Cao 1, Margarita Mishina 1,
Evaluation of an alternative slide culture technique for the morphological identification of fungal species
 Research Article Evaluation of an alternative slide culture technique for the morphological identification of fungal species Abstract M H Wijedasa 1, L V C Liyanapathirana 1. Sri Lanka Journal of Infectious
Research Article Evaluation of an alternative slide culture technique for the morphological identification of fungal species Abstract M H Wijedasa 1, L V C Liyanapathirana 1. Sri Lanka Journal of Infectious
Supplemental Information. Autophagy in Oncogenic K-Ras. Promotes Basal Extrusion. of Epithelial Cells by Degrading S1P. Current Biology, Volume 24
 Current Biology, Volume 24 Supplemental Information Autophagy in Oncogenic K-Ras Promotes Basal Extrusion of Epithelial Cells by Degrading S1P Gloria Slattum, Yapeng Gu, Roger Sabbadini, and Jody Rosenblatt
Current Biology, Volume 24 Supplemental Information Autophagy in Oncogenic K-Ras Promotes Basal Extrusion of Epithelial Cells by Degrading S1P Gloria Slattum, Yapeng Gu, Roger Sabbadini, and Jody Rosenblatt
Supplementary Figure S1. Venn diagram analysis of mrna microarray data and mirna target analysis. (a) Western blot analysis of T lymphoblasts (CLS)
 Supplementary Figure S1. Venn diagram analysis of mrna microarray data and mirna target analysis. (a) Western blot analysis of T lymphoblasts (CLS) and their exosomes (EXO) in resting (REST) and activated
Supplementary Figure S1. Venn diagram analysis of mrna microarray data and mirna target analysis. (a) Western blot analysis of T lymphoblasts (CLS) and their exosomes (EXO) in resting (REST) and activated
Biol403 MAP kinase signalling
 Biol403 MAP kinase signalling The mitogen activated protein kinase (MAPK) pathway is a signalling cascade activated by a diverse range of effectors. The cascade regulates many cellular activities including
Biol403 MAP kinase signalling The mitogen activated protein kinase (MAPK) pathway is a signalling cascade activated by a diverse range of effectors. The cascade regulates many cellular activities including
Supplemental Information. Helicobacter pylori Employs a Unique Basolateral. Type IV Secretion Mechanism for CagA Delivery
 Cell Host & Microbe, Volume 22 Supplemental Information Helicobacter pylori Employs a Unique Basolateral Type IV Secretion Mechanism for CagA Delivery Nicole Tegtmeyer, Silja Wessler, Vittorio Necchi,
Cell Host & Microbe, Volume 22 Supplemental Information Helicobacter pylori Employs a Unique Basolateral Type IV Secretion Mechanism for CagA Delivery Nicole Tegtmeyer, Silja Wessler, Vittorio Necchi,
Human Urokinase / PLAU / UPA ELISA Pair Set
 Human Urokinase / PLAU / UPA ELISA Pair Set Catalog Number : SEK10815 To achieve the best assay results, this manual must be read carefully before using this product and the assay is run as summarized
Human Urokinase / PLAU / UPA ELISA Pair Set Catalog Number : SEK10815 To achieve the best assay results, this manual must be read carefully before using this product and the assay is run as summarized
(a) Schematic diagram of the FS mutation of UVRAG in exon 8 containing the highly instable
 Supplementary Figure 1. Frameshift (FS) mutation in UVRAG. (a) Schematic diagram of the FS mutation of UVRAG in exon 8 containing the highly instable A 10 DNA repeat, generating a premature stop codon
Supplementary Figure 1. Frameshift (FS) mutation in UVRAG. (a) Schematic diagram of the FS mutation of UVRAG in exon 8 containing the highly instable A 10 DNA repeat, generating a premature stop codon
PRODUCT INFORMATION & MANUAL
 PRODUCT INFORMATION & MANUAL Mitochondrial Extraction Kit NBP2-29448 Research use only. Not for diagnostic or therapeutic procedures www.novusbio.com P: 303.760.1950 P: 888.506.6887 F: 303.730.1966 technical@novusbio.com
PRODUCT INFORMATION & MANUAL Mitochondrial Extraction Kit NBP2-29448 Research use only. Not for diagnostic or therapeutic procedures www.novusbio.com P: 303.760.1950 P: 888.506.6887 F: 303.730.1966 technical@novusbio.com
Supplementary Materials for
 www.sciencesignaling.org/cgi/content/full/6/283/ra57/dc1 Supplementary Materials for JNK3 Couples the Neuronal Stress Response to Inhibition of Secretory Trafficking Guang Yang,* Xun Zhou, Jingyan Zhu,
www.sciencesignaling.org/cgi/content/full/6/283/ra57/dc1 Supplementary Materials for JNK3 Couples the Neuronal Stress Response to Inhibition of Secretory Trafficking Guang Yang,* Xun Zhou, Jingyan Zhu,
Supplemental Figure S1. Expression of Cirbp mrna in mouse tissues and NIH3T3 cells.
 SUPPLEMENTAL FIGURE AND TABLE LEGENDS Supplemental Figure S1. Expression of Cirbp mrna in mouse tissues and NIH3T3 cells. A) Cirbp mrna expression levels in various mouse tissues collected around the clock
SUPPLEMENTAL FIGURE AND TABLE LEGENDS Supplemental Figure S1. Expression of Cirbp mrna in mouse tissues and NIH3T3 cells. A) Cirbp mrna expression levels in various mouse tissues collected around the clock
SUPPLEMENTARY MATERIAL. Sample preparation for light microscopy
 SUPPLEMENTARY MATERIAL Sample preparation for light microscopy To characterize the granulocytes and melanomacrophage centers, cross sections were prepared for light microscopy, as described in Material
SUPPLEMENTARY MATERIAL Sample preparation for light microscopy To characterize the granulocytes and melanomacrophage centers, cross sections were prepared for light microscopy, as described in Material
MALDI-TOF Mass Spectrometry: A New Rapid ID Method in Clinical Microbiology
 MALDI-TOF Mass Spectrometry: A New Rapid ID Method in Clinical Microbiology Patrick R. Murray, PhD WW Director, Scientific Affairs BD Diagnostic Systems Outline MALDI-TOF is the most important innovation
MALDI-TOF Mass Spectrometry: A New Rapid ID Method in Clinical Microbiology Patrick R. Murray, PhD WW Director, Scientific Affairs BD Diagnostic Systems Outline MALDI-TOF is the most important innovation
Influenza B Hemagglutinin / HA ELISA Pair Set
 Influenza B Hemagglutinin / HA ELISA Pair Set Catalog Number : SEK11053 To achieve the best assay results, this manual must be read carefully before using this product and the assay is run as summarized
Influenza B Hemagglutinin / HA ELISA Pair Set Catalog Number : SEK11053 To achieve the best assay results, this manual must be read carefully before using this product and the assay is run as summarized
KEY CONCEPT QUESTIONS IN SIGNAL TRANSDUCTION
 Signal Transduction - Part 2 Key Concepts - Receptor tyrosine kinases control cell metabolism and proliferation Growth factor signaling through Ras Mutated cell signaling genes in cancer cells are called
Signal Transduction - Part 2 Key Concepts - Receptor tyrosine kinases control cell metabolism and proliferation Growth factor signaling through Ras Mutated cell signaling genes in cancer cells are called
1. This is the location where N-linked oligosaccharide is initially synthesized and attached to glycoproteins.
 Biology 4410 Name Spring 2006 Exam 2 A. Multiple Choice, 2 pt each Pick the best choice from the list of choices, and write it in the space provided. Some choices may be used more than once, and other
Biology 4410 Name Spring 2006 Exam 2 A. Multiple Choice, 2 pt each Pick the best choice from the list of choices, and write it in the space provided. Some choices may be used more than once, and other
Protocol for protein SDS PAGE and Transfer
 Protocol for protein SDS PAGE and Transfer According to Laemmli, (1970) Alaa El -Din Hamid Sayed, Alaa_h254@yahoo.com Serum Selection of a protein source cell cultures (bacteria, yeast, mammalian, etc.)
Protocol for protein SDS PAGE and Transfer According to Laemmli, (1970) Alaa El -Din Hamid Sayed, Alaa_h254@yahoo.com Serum Selection of a protein source cell cultures (bacteria, yeast, mammalian, etc.)
Supporting Information File S2
 Pulli et al. Measuring Myeloperoxidase Activity in Biological Samples Page 1 of 6 Supporting Information File S2 Step-by-Step Protocol Reagents: Sucrose (Sigma, S3089) CaCl 2 (Sigma, C5770) Heparin Sodium
Pulli et al. Measuring Myeloperoxidase Activity in Biological Samples Page 1 of 6 Supporting Information File S2 Step-by-Step Protocol Reagents: Sucrose (Sigma, S3089) CaCl 2 (Sigma, C5770) Heparin Sodium
ab SREBP-2 Translocation Assay Kit (Cell-Based)
 ab133114 SREBP-2 Translocation Assay Kit (Cell-Based) Instructions for Use For analysis of translocation of SREBP-2 into nuclei. This product is for research use only and is not intended for diagnostic
ab133114 SREBP-2 Translocation Assay Kit (Cell-Based) Instructions for Use For analysis of translocation of SREBP-2 into nuclei. This product is for research use only and is not intended for diagnostic
Laboratory 10 Factors Controlling Phagocytosis in Tetrahymena,
 BIO354: Cell Biology Laboratory 1 Laboratory 1 Factors Controlling Phagocytosis in Tetrahymena, I. Introduction A characteristic feature of all eukaryotic cells is the ability to pinch off portions of
BIO354: Cell Biology Laboratory 1 Laboratory 1 Factors Controlling Phagocytosis in Tetrahymena, I. Introduction A characteristic feature of all eukaryotic cells is the ability to pinch off portions of
Protein Trafficking in the Secretory and Endocytic Pathways
 Protein Trafficking in the Secretory and Endocytic Pathways The compartmentalization of eukaryotic cells has considerable functional advantages for the cell, but requires elaborate mechanisms to ensure
Protein Trafficking in the Secretory and Endocytic Pathways The compartmentalization of eukaryotic cells has considerable functional advantages for the cell, but requires elaborate mechanisms to ensure
Determination of the temporal pattern and importance of BALF1 expression in Epstein-Barr viral infection
 Determination of the temporal pattern and importance of BALF1 expression in Epstein-Barr viral infection Melissa Mihelidakis May 6, 2004 7.340 Research Proposal Introduction Apoptosis, or programmed cell
Determination of the temporal pattern and importance of BALF1 expression in Epstein-Barr viral infection Melissa Mihelidakis May 6, 2004 7.340 Research Proposal Introduction Apoptosis, or programmed cell
SUPPLEMENTARY INFORMATION
 Supplementary Figure 1. Normal AMPAR-mediated fepsp input-output curve in CA3-Psen cdko mice. Input-output curves, which are plotted initial slopes of the evoked fepsp as function of the amplitude of the
Supplementary Figure 1. Normal AMPAR-mediated fepsp input-output curve in CA3-Psen cdko mice. Input-output curves, which are plotted initial slopes of the evoked fepsp as function of the amplitude of the
Supplemental Figure 1. Intracranial transduction of a modified ptomo lentiviral vector in the mouse
 Supplemental figure legends Supplemental Figure 1. Intracranial transduction of a modified ptomo lentiviral vector in the mouse hippocampus targets GFAP-positive but not NeuN-positive cells. (A) Stereotaxic
Supplemental figure legends Supplemental Figure 1. Intracranial transduction of a modified ptomo lentiviral vector in the mouse hippocampus targets GFAP-positive but not NeuN-positive cells. (A) Stereotaxic
Supplementary Information
 Supplementary Information Archaeal Elp3 catalyzes trna wobble uridine modification at C5 via a radical mechanism Kiruthika Selvadurai, Pei Wang, Joseph Seimetz & Raven H Huang* Department of Biochemistry,
Supplementary Information Archaeal Elp3 catalyzes trna wobble uridine modification at C5 via a radical mechanism Kiruthika Selvadurai, Pei Wang, Joseph Seimetz & Raven H Huang* Department of Biochemistry,
High resolution structural evidence suggests the Sarcoplasmic Reticulum forms microdomains with Acidic Stores (lyososomes) in the heart.
 High resolution structural evidence suggests the Sarcoplasmic Reticulum forms microdomains with Acidic Stores (lyososomes) in the heart. Daniel Aston, Rebecca A. Capel, Kerrie L. Ford, Helen C. Christian,
High resolution structural evidence suggests the Sarcoplasmic Reticulum forms microdomains with Acidic Stores (lyososomes) in the heart. Daniel Aston, Rebecca A. Capel, Kerrie L. Ford, Helen C. Christian,
Chromatin IP (Isw2) Fix soln: 11% formaldehyde, 0.1 M NaCl, 1 mm EDTA, 50 mm Hepes-KOH ph 7.6. Freshly prepared. Do not store in glass bottles.
 Chromatin IP (Isw2) 7/01 Toshi last update: 06/15 Reagents Fix soln: 11% formaldehyde, 0.1 M NaCl, 1 mm EDTA, 50 mm Hepes-KOH ph 7.6. Freshly prepared. Do not store in glass bottles. 2.5 M glycine. TBS:
Chromatin IP (Isw2) 7/01 Toshi last update: 06/15 Reagents Fix soln: 11% formaldehyde, 0.1 M NaCl, 1 mm EDTA, 50 mm Hepes-KOH ph 7.6. Freshly prepared. Do not store in glass bottles. 2.5 M glycine. TBS:
Identification of Yeasts. Medical Mycology Training Network 15 November 2018 Dr Tan Ai Ling Department of Microbiology, Singapore General Hospital
 Identification of Yeasts Medical Mycology Training Network 15 November 2018 Dr Tan Ai Ling Department of Microbiology, Singapore General Hospital Definition of Yeasts Eukaryote cells have defined nucleus
Identification of Yeasts Medical Mycology Training Network 15 November 2018 Dr Tan Ai Ling Department of Microbiology, Singapore General Hospital Definition of Yeasts Eukaryote cells have defined nucleus
SUPPLEMENTARY INFORMATION
 SUPPLEMENTARY INFORMATION doi:1.138/nature9814 a A SHARPIN FL B SHARPIN ΔNZF C SHARPIN T38L, F39V b His-SHARPIN FL -1xUb -2xUb -4xUb α-his c Linear 4xUb -SHARPIN FL -SHARPIN TF_LV -SHARPINΔNZF -SHARPIN
SUPPLEMENTARY INFORMATION doi:1.138/nature9814 a A SHARPIN FL B SHARPIN ΔNZF C SHARPIN T38L, F39V b His-SHARPIN FL -1xUb -2xUb -4xUb α-his c Linear 4xUb -SHARPIN FL -SHARPIN TF_LV -SHARPINΔNZF -SHARPIN
Nature Methods: doi: /nmeth Supplementary Figure 1
 Supplementary Figure 1 Subtiligase-catalyzed ligations with ubiquitin thioesters and 10-mer biotinylated peptides. (a) General scheme for ligations between ubiquitin thioesters and 10-mer, biotinylated
Supplementary Figure 1 Subtiligase-catalyzed ligations with ubiquitin thioesters and 10-mer biotinylated peptides. (a) General scheme for ligations between ubiquitin thioesters and 10-mer, biotinylated
SUPPLEMENT. Materials and methods
 SUPPLEMENT Materials and methods Cell culture and reagents Cell media and reagents were from Invitrogen unless otherwise indicated. Antibiotics and Tet-certified serum were from Clontech. In experiments
SUPPLEMENT Materials and methods Cell culture and reagents Cell media and reagents were from Invitrogen unless otherwise indicated. Antibiotics and Tet-certified serum were from Clontech. In experiments
Supplementary Figures
 Supplementary Figures a miel1-2 (SALK_41369).1kb miel1-1 (SALK_978) b TUB MIEL1 Supplementary Figure 1. MIEL1 expression in miel1 mutant and S:MIEL1-MYC transgenic plants. (a) Mapping of the T-DNA insertion
Supplementary Figures a miel1-2 (SALK_41369).1kb miel1-1 (SALK_978) b TUB MIEL1 Supplementary Figure 1. MIEL1 expression in miel1 mutant and S:MIEL1-MYC transgenic plants. (a) Mapping of the T-DNA insertion
Supplementary materials
 Supplementary materials Chemical library from ChemBridge 50,240 structurally diverse small molecule compounds dissolved in DMSO Hits Controls: No virus added μ Primary screening at 20 g/ml of compounds
Supplementary materials Chemical library from ChemBridge 50,240 structurally diverse small molecule compounds dissolved in DMSO Hits Controls: No virus added μ Primary screening at 20 g/ml of compounds
7.06 Cell Biology EXAM #3 April 24, 2003
 7.06 Spring 2003 Exam 3 Name 1 of 8 7.06 Cell Biology EXAM #3 April 24, 2003 This is an open book exam, and you are allowed access to books and notes. Please write your answers to the questions in the
7.06 Spring 2003 Exam 3 Name 1 of 8 7.06 Cell Biology EXAM #3 April 24, 2003 This is an open book exam, and you are allowed access to books and notes. Please write your answers to the questions in the
DEVELOPMENTAL VALIDATION OF SPERM HY-LITER EXPRESS Jennifer Old, Chris Martersteck, Anna Kalinina, Independent Forensics, Lombard IL
 DEVELOPMENTAL VALIDATION OF SPERM HY-LITER EXPRESS Jennifer Old, Chris Martersteck, Anna Kalinina, Independent Forensics, Lombard IL Background and Introduction SPERM HY-LITER is the first immunofluorescent
DEVELOPMENTAL VALIDATION OF SPERM HY-LITER EXPRESS Jennifer Old, Chris Martersteck, Anna Kalinina, Independent Forensics, Lombard IL Background and Introduction SPERM HY-LITER is the first immunofluorescent
T H E J O U R N A L O F C E L L B I O L O G Y
 Supplemental material Beck et al., http://www.jcb.org/cgi/content/full/jcb.201011027/dc1 T H E J O U R N A L O F C E L L B I O L O G Y Figure S1. Membrane binding of His-tagged proteins to Ni-liposomes.
Supplemental material Beck et al., http://www.jcb.org/cgi/content/full/jcb.201011027/dc1 T H E J O U R N A L O F C E L L B I O L O G Y Figure S1. Membrane binding of His-tagged proteins to Ni-liposomes.
PAF Acetylhydrolase Assay Kit
 PAF Acetylhydrolase Assay Kit Catalog Number KA1354 96 assays Version: 04 Intended for research use only www.abnova.com Table of Contents Introduction... 3 Background... 3 Principle of the Assay... 3 General
PAF Acetylhydrolase Assay Kit Catalog Number KA1354 96 assays Version: 04 Intended for research use only www.abnova.com Table of Contents Introduction... 3 Background... 3 Principle of the Assay... 3 General
Insulin Resistance. Biol 405 Molecular Medicine
 Insulin Resistance Biol 405 Molecular Medicine Insulin resistance: a subnormal biological response to insulin. Defects of either insulin secretion or insulin action can cause diabetes mellitus. Insulin-dependent
Insulin Resistance Biol 405 Molecular Medicine Insulin resistance: a subnormal biological response to insulin. Defects of either insulin secretion or insulin action can cause diabetes mellitus. Insulin-dependent
NF-κB p65 (Phospho-Thr254)
 Assay Biotechnology Company www.assaybiotech.com Tel: 1-877-883-7988 Fax: 1-877-610-9758 NF-κB p65 (Phospho-Thr254) Colorimetric Cell-Based ELISA Kit Catalog #: OKAG02015 Please read the provided manual
Assay Biotechnology Company www.assaybiotech.com Tel: 1-877-883-7988 Fax: 1-877-610-9758 NF-κB p65 (Phospho-Thr254) Colorimetric Cell-Based ELISA Kit Catalog #: OKAG02015 Please read the provided manual
SUPPLEMENTAL MATERIAL. Supplementary Methods
 SUPPLEMENTAL MATERIAL Supplementary Methods Culture of cardiomyocytes, fibroblasts and cardiac microvascular endothelial cells The isolation and culturing of neonatal rat ventricular cardiomyocytes was
SUPPLEMENTAL MATERIAL Supplementary Methods Culture of cardiomyocytes, fibroblasts and cardiac microvascular endothelial cells The isolation and culturing of neonatal rat ventricular cardiomyocytes was
