Epidemiological studies have revealed an inverse correlation
|
|
|
- Amy Hart
- 5 years ago
- Views:
Transcription
1 LXR-Induced Redistribution of ABCG1 to Plasma Membrane in Macrophages Enhances Cholesterol Mass Efflux to HDL Nan Wang, Mollie Ranalletta, Fumihiko Matsuura, Felix Peng, Alan R. Tall Objectives This study examines the ABCG1-mediated cholesterol efflux and intracellular cholesterol transport by studying the ABCG1 localization and function in macrophages. Methods and Results HEK 293 cell overexpressing ABCG1, RNA interference, or macrophages from ABCG1 or ABCG4 knockout mice were used. ABCG1 but not ABCG4 had a major role in the increased cholesterol mass efflux produced by treatment of macrophages with LXR activators. In 293 cells, ABCG1 was found in the plasma membrane, Golgi, and recycling endosomes. In contrast, in basal macrophages, ABCG1 was predominantly intracellular, and redistributed to the plasma membrane after LXR activation. LXR activation increased macrophage cholesterol efflux to high-density lipoprotein (HDL), low-density lipoprotein (LDL), and cyclodextrin in an ABCG1-dependent fashion. Suppression of ABCG1 expression increased cholesteryl ester formation and decreased SREBP2 target gene expression in macrophages, even in the absence of HDL acceptors. Conclusions LXR activation induces redistribution of ABCG1 from intracellular sites to the plasma membrane and increases cholesterol mass efflux to HDL in an ABCG1-dependent fashion. ABCG1 acts in the macrophage plasma membrane to increase the availability of cholesterol to a variety of lipoprotein and nonlipoprotein acceptors while limiting the accumulation of cholesterol in the endoplasmic reticulum. (Arterioscler Thromb Vasc Biol. 2006;26: ) Key Words: ABCG1 cholesterol efflux high-density lipoprotein liver X receptor macrophage Epidemiological studies have revealed an inverse correlation between plasma high-density lipoprotein (HDL) cholesterol levels and atherosclerosis 1,2 and low HDL is the most common lipoprotein abnormality in men with coronary heart disease. 3 Reverse cholesterol transport, ie, the transport of excess cholesterol from macrophage foam cells in the arterial wall to the liver, has been proposed as a major mechanism to explain for the protective effect of HDL. 4 However, the molecular and cellular mechanisms responsible for the various steps of reverse cholesterol transport are not fully understood. Several different pathways have been implicated in cholesterol efflux from macrophage foam cells to HDL or its apolipoproteins. 5,6 ABCA1 is a membrane transporter that binds apoa-1 and acts in the plasma membrane and endosomal system to promote cellular cholesterol efflux to lipid-poor apolipoproteins, such as apoa-i and apoe. 7,8 However, ABCA1 interacts poorly with HDL2 and HDL3 that constitute the bulk of plasma HDL. 7 Recently, ABCG1 has been identified as a membrane transporter that promotes macrophage cholesterol efflux to HDL Overexpression of ABCG1 increased cholesterol efflux to HDL but not lipidpoor apoa-1, 9 whereas suppression of ABCG1 expression, by RNA interference or by targeted disruption of the ABCG1 gene, reduced the efflux of radioactive cholesterol from cholesterolloaded macrophages to HDL. 9,11 In macrophages, LXR activation promotes isotopic cholesterol efflux to HDL particles, 9,11 and this induction is almost absent in ABCG1 / macrophages. 11 Together these studies suggested a major role of ABCG1 in promoting isotopic cholesterol efflux from macrophage foam cells to HDL. However, because isotopic efflux to HDL can arise either from net mass movement or from isotopic exchange, these studies do not disclose the role of the ABCG1 pathway in promoting mass cholesterol efflux to HDL. See page 1198 In addition to ABCG1, we found that ABCG4, a close relative of ABCG1, could also promote cholesterol efflux to HDL when overexpressed in HEK293 cells. 9 Knockdown of ABCG4 expression by sirna suggested a possible lesser role of ABCG4 in cholesterol efflux from macrophages, but studies were inconclusive. 9 In this study, we assessed the roles of ABCG1 and ABCG4 in cholesterol efflux to HDL from macrophages with or without LXR activation. We previously reported that ABCG1 overexpression in 293 cells led to increased cholesterol efflux to a variety of Original received November 21, 2005; final version accepted March 9, From the Division of Molecular Medicine, Department of Medicine, Columbia University, New York. Correspondence to Nan Wang, Division of Molecular Medicine, Department of Medicine, PS 8-401, Columbia University, 630 West 168th Street, New York, NY nw30@columbia.edu 2006 American Heart Association, Inc. Arterioscler Thromb Vasc Biol. is available at DOI: /01.ATV
2 Wang et al Cellular Distribution and Function of ABCG lipoprotein and nonlipoprotein acceptors, including HDL, low-density lipoprotein (LDL), and cyclodextrin, but did not increase cell association of HDL, suggesting that ABCG1 acts to increase availability of cholesterol in the plasma membrane. 9 Consistent with this concept, Vaughan and Oram recently used biotinylation to show plasma membrane localization of an epitope-tagged ABCG1 in a baby hamster kidney (BHK) cell overexpression system, and demonstrated an increase in availability of cholesterol to cholesterol oxidase. 12 However, the possible localization of ABCG1 in different intracellular pools and cellular localization of endogenous ABCG1 in macrophages or other cells have not been reported. Materials and Methods Reagents and Chemicals Polyclonal anti-abcg1 antibodies, H-65 (for immunofluorescence microscopy) and E-20 (for Western analysis) were from Santa Cruz Biotechnology (Santa Cruz, Calif). Plasmid Constructs and Cell Transfection The plasmid constructs expressing mouse ABCG1 and transfection were described previously. 9 ABCG1-Flag or ABCG1-GFP expression plasmid was prepared by tagging the epitope at C-terminus of mouse ABCG1. Cellular Lipid Efflux Assays Cholesterol efflux assay using [ 3 H]cholesterol labeled cells were performed as described before. 9 For mass cholesterol efflux, macrophages were treated with or without the indicated sirna for 48 hours in the presence or absence of 3 mol/l TO During the last 16 hours of treatment, acetyl-ldl (50 g/ml acetyl-ldl protein) was added. Cells were then washed and cholesterol mass efflux was initiated by adding the indicated amount of HDL2 and proceeded for 8 hours. The lipid fraction was extracted from the media or cell lysates with hexane:isopropanol (3:2, v:v) and -sitosterol (5 g/sample) was added as the internal standard. After drying under nitrogen gas, the mass of cholesterol dissolved in chloroform was determined using gas chromatography. Small Interfering RNA (sirna)-mediated Macrophage RNA Interference The ABCG1 target sequence selected for sirna synthesis was 5 - TCGTATCTTATCTGTAGAGAA-3. Transfection of macrophages with sirna and cholesterol efflux assay were performed as described previously. 9 Levels of ABCG1 or ABCG4 mrnas normalized against -actin mrna were determined using Taqman real-time quantitative reverse-transcriptase (RT) polymerase chain reaction (PCR). Animals ABCG1 / and ABCG4 / mice were derived from ABCG1 / or ABCG4 / mice obtained from Deltagen Inc. A stretch of 26 nucleotides encoding 9 amino acids in ATP-binding Walker A motif of ABCG4 was deleted in these knockout mice. RT-PCR using total RNA from the brain or macrophages of ABCG4 / mice failed to detect ABCG4 mrna, whereas a robust ABCG4 RT-PCR product was generated from the wild-type tissues (not shown). Subcellular Fractionation Peritoneal macrophages were cultured for 24 hours in 10% FBS and DMEM and then scraped and collected by centrifugation in ice cold phosphate buffered saline. The cells were processed and fractionated according to a procedure by Li et al, 13 except that a continuous to 1.1-mol/L sucrose density gradient centrifugation was used. Limited Proteolysis Macrophages were treated with indicated amount of trypsin in DMEM medium at 37 C or room temperature for the indicated period, washed 5 times with DMEM containing 0.2% bovine serum albumin, and lysed with lysis buffer containing a protease inhibitor cocktail and soybean trypsin inhibitor. The cell lysates were subjected to Western analysis. Cell Surface Protein Biotinylation Cell surface protein biotinylation, Western analysis, and quantification of the total cellular and cell surface ABCG1 were performed similarly as described previously. 14 Results Role of ABCG1 and ABCG4 in Mass Cholesterol Efflux From Macrophages LXR activation significantly increased cholesterol mass efflux to HDL2 from wild-type macrophages (Figure 1A). This increased cholesterol mass efflux was markedly reduced in ABCG1-deficient macrophages. In contrast, the basal cholesterol mass efflux was only slightly decreased by ABCG1 deficiency (Figure 1A). These findings show the essential role of ABCG1 in promoting cholesterol mass efflux to HDL in LXR-activated macrophages but also indicate a residual component of mass efflux to HDL in the basal state that is not attributable to ABCG1. We also examined the effect of acute suppression of ABCG1 expression on macrophage cholesterol mass efflux using a synthetic interfering RNA (sirna) targeting mouse ABCG1 as described previously. 9 On LXR activation by TO901317, ABCG1 protein levels were increased by 4-fold and ABCG1 mrna levels were increased by 10-fold (not shown) and an sirna selected previously to effectively downregulate ABCG1 mrna levels 9 reduced ABCG1 protein levels by 70% (Figure 1B). Consistent with the results using ABCG1 / macrophages, acute suppression of ABCG1 expression also reduced cholesterol mass efflux to HDL2 in LXR-activated macrophages (Figure 1B). ABCG1-facilitated cholesterol efflux to HDL would be expected to lead to decreased cholesterol mass accumulation in macrophages. In scrambled sirna-treated, cholesterol-loaded, LXR-activated macrophages, HDL2-promoted cholesterol efflux reduced cellular cholesterol mass by 25% (P 0.01) (Figure 1C). In contrast, HDL2 decreased cholesterol mass by 12% (P 0.05) in macrophages transfected with sirna targeting ABCG1. Together, these data indicate an essential role of ABCG1 in promoting cholesterol efflux to HDL and thereby regulating macrophage cholesterol content, especially after LXR activation. To directly evaluate the role of ABCG4 in cholesterol efflux to HDL, we isolated macrophages from wild-type and ABCG4 / mice. ABCG4 deficiency did not affect isotopic cholesterol efflux from macrophages to HDL2 with or without LXR activation (Figure 1D), indicating that ABCG4 does not have a role in the exchange or net efflux of macrophage cholesterol to HDL2. The small reduction of cholesterol efflux to HDL2 previously observed in macrophages treated with sirna targeting ABCG4 might be caused by an offtarget inhibition of ABCG1 translation. 15 Together, these data
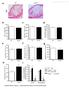
3 1312 Arterioscler Thromb Vasc Biol. June 2006 Figure 1. ABCG1 but not ABCG4 promotes cholesterol mass efflux to HDL and decreases cellular cholesterol mass. A, Peritoneal macrophages from ABCG1 / and wild-type mice were cholesterol loaded with 50 g/ml acetyl-ldl protein and treated with or without 3 mol/l TO for 16 hours. Cholesterol mass efflux was initiated by adding HDL2 (25 g/ml HDL protein) and proceeded for 8 hours. Free cholesterol mass in media was extracted and determined using gas chromatography. HDL added to the media without cells was used as the blank control. The change in cholesterol mass in media is relative to this control. *P 0.05 in a Student t test for WT vs ABCG1 /. The error bars for this and the following experiments represent standard deviation. B and C, Macrophages were cholesterol-loaded with acetyl-ldl and treated with TO and sirna according to the Experimental Procedures; 160 nmol of either scrambled or ABCG1-targeting sirna was used. Cholesterol efflux was performed as in (A). Free cholesterol mass in media (B) or total cholesterol mass in cells (C) were determined. *P 0.05 for test vs control (ABCG1 versus scrambled sirna in [B], HDL2 vs no HDL in [C]). #P 0.05 for ABCG1 vs scrambled sirna in the presence of HDL2 (C). A representative of 2 experiments is shown. D, Peritoneal macrophages from ABCG4 / and wild-type mice were labeled with [ 3 H]cholesterol in 10% fetal bovine serum and DMEM medium and treated with or without 3 mol/l TO for 16 hours. Cholesterol efflux was initiated by adding HDL2 (25 g/ml HDL protein) and proceeded for 4 hours. indicate that ABCG1 but not ABCG4 is essential for cholesterol efflux from LXR-activated macrophages. Subcellular Localization of ABCG1 in Transfected HEK293 Cell To understand how ABCG1 promotes cellular cholesterol efflux, we used ABCG1 antibodies to determine the cellular distribution of ABCG1 in transiently transfected 293 cells. 9 Confocal immunofluorescence microscopy showed prominent plasma membrane as well as intracellular localization of ABCG1 (Figure 2A and 2B). Partial colocalization of ABCG1 with a Golgi marker, Golgi 97, and with transferrin was observed (Figure 2A and 2B), suggesting the presence of ABCG1 in Golgi and endocytic recycling compartments. In contrast, coimmunostaining with antibodies to calnexin, an endoplasmic reticulum marker, or antibodies to Lamp-1, a lysosomal marker, did not show significant colocalization with ABCG1 (not shown). The primary antibody to ABCG1 used in immunofluorescence studies showed a high specificity in Western analysis (Figure 2C). However, this antibody generated specific immunofluorescent signals only in 293 cells overexpressing ABCG1 but not in mouse peritoneal macrophages even after induction of ABCG1 expression by LXR activation (not shown), suggesting high specificity but low affinity of the antibody to ABCG1. We also tested GFP-tagged or Flag-tagged ABCG1 cdna constructs. However, transient transfection of 293 cells with these tagged constructs failed to increase cholesterol efflux to HDL (Figure 2D), even though the expression of ABCG1-GFP or ABCG1-Flag was readily detectable by immunofluorescence microscopy (not shown). Attempts to insert a hemagglutinin tag on the predicted sixth extracellular/luminal loop again failed to generate a functional ABCG1 (not shown). LXR Activation Increases ABCG1 at the Cell Surface in Macrophages To evaluate the distribution of endogenous ABCG1 in mouse macrophages, we used subcellular fractionation by continuous sucrose density gradient centrifugation and Western analysis. We examined the distribution of ABCG1 in mouse macrophages with and without treatment with the LXR agonist, TO901317, Surprisingly, in basal macrophages, ABCG1 appeared to be primarily in intracellular fractions (Figure 3A), colocalizing with the Golgi marker, but not with the plasma membrane marker ( 1-integrin) or endosomal marker (Rab5). In contrast, after LXR activation, ABCG1 distribution closely paralleled the distribution of 1-integrin (Figure 3B), indicating a predominant cell surface localization. To confirm the cell surface localization of ABCG1 in LXR-activated macrophages, we used limited proteolysis of cell surface proteins by treating intact cells with trypsin. ABCG1 protein levels in LXR-activated macrophages were substantially decreased by trypsin treatment at 37 C in a dose-dependent fashion (Figure 4A). The protein levels of -actin, an intracellular protein, showed no change (Figure
4 Wang et al Cellular Distribution and Function of ABCG Figure 2. ABCG1 is present at cell surface and in Golgi and endocytic recycling compartment in HEK293 cells. A, 293 cells transiently transfected with pcdna/abcg1 were examined using confocal immunofluorescence microscopy after cell permeabilization and immunostaining with anti-abcg1 antibody (red) and anti-golgi 97 antibody (green). B, Similar to (A), except that cells were incubated with Alexa488-labeled transferrin (green) at 37 C for 15 minutes before immunostaining with anti-abcg1 antibody (red). Note the specific staining of Golgi (A) and transferrin signal (B) in cells with or without ABCG1 overexpression. The images shown represent 1 mol/l confocal slices. Scale bar, 10 mol/l. C, Western analysis of 293 cells transfected with empty vector (lane 1) or pcdna3.1/abcg1 (lane 2) with ABCG1 antibody. D, Cells transfected with the indicated plasmid construct were labeled with [ 3 H]cholesterol in 10% fetal bovine serum and DMEM medium and cholesterol efflux to HDL2 for 4 hours was determined. 4A). To confirm that trypsin activity was effectively blocked by trypsin inhibitors added at the end of the assay, we mixed lysates of control cells with lysates of trypsin-treated cells. ABCG1 protein levels showed no further change once the digestion was stopped by adding trypsin inhibitors (not shown). Therefore, the digestion of ABCG1 by trypsin occurred during the brief incubation of trypsin with intact cells and no further digestion occurred during washing and lysis of cells in the presence of trypsin inhibitors. These results are consistent with a predominant cell surface localization of ABCG1 in LXR-activated macrophages. We also examined the limited proteolysis of ABCG1 in intact cells by trypsin at room temperature ( 25 C) using human macrophages derived from THP-1 cells in the presence or absence of LXR activation (Figure 4 and 4C). The lower temperature would likely limit any potential entry of trypsin into cells by endocytosis during incubation at 37 C. Unlike mouse peritoneal macrophage, these human macrophages expressed a moderate amount of ABCG1 protein even in the absence of LXR activation. LXR activation only moderately increased ABCG1 protein levels (Figure 4B and 4C). On trypsin treatment, ABCG1 protein levels in these LXR activated cells were reduced to a level similar to that of the control cells (without TO treatment). Further, trypsin treatment did Figure 3. ABCG1 is present at the cell surface in LXR-activated macrophages. A, Subcellular fractionation of mouse macrophages in the basal state by a 0.58 to 1.1 mol/l continuous sucrose density gradient centrifugation was described in the Experimental Procedures and the results of Western analysis of each individual fraction are shown. B, Subcellular fractionation of LXR-activated mouse macrophages with a procedure similar to A. Figure 4. LXR activation increases cell surface ABCG1 protein levels. A, Mouse macrophages treated with TO (3 mol/l, 16 hour) were incubated at 37 C for 20 minutes with the indicated amount of trypsin followed by washing and cell lysis in the presence of trypsin inhibitors. Western analysis is shown. B, THP-1 macrophages treated with or without TO (3 mol/l, 16 hour) were incubated at 25 C for 30 minutes with 0.5 mg/ml trypsin and shown is the Western analysis. C, ABCG1 protein levels normalized against -actin in B were determined by densitometry.
5 1314 Arterioscler Thromb Vasc Biol. June 2006 Figure 5. LXR activation redistributes ABCG1 from the intracellular pool to the cell surface. Human THP-1 macrophages were treated with or without 3 mol/l TO for 16 hours, and the cell surface proteins were then biotinylated. The total or biotinylated cellular proteins were subjected to Western analysis (A) and the ABCG1 proteins were quantified by densitometry (B) and normalized against an arbitrary unit. *P not alter ABCG1 protein levels in the control cells. Together, these data also support a mobilization of ABCG1 to the cell surface on LXR activation in both human and mouse macrophages. We also used cell surface biotinylation of ABCG1 as an independent confirmation of the LXR-induced redistribution of ABCG1 to the cell surface. We used human THP-1 macrophages, because we were unable to successfully biotinylate mouse ABCG1 even under overexpression conditions. In the basal state, cell surface ABCG1 was barely detectable by biotinylation. However, LXR activation increased cell surface ABCG1 by 6 fold (Figure 5A and 5B). These data provide an independent confirmation that LXR activation induces a redistribution of ABCG1 from intracellular sites to the plasma membrane. Whereas the LXR activation in these studies relied on a synthetic LXR agonist, upregulation of ABCG1 expression in vivo by cholesterol loading is likely through generation of endogenous LXR ligands. Therefore, we also tested the effect of cholesterol loading on ABCG1 distribution in macrophages. In THP-1 macrophages, cholesterol loading by acetyl-ldl increased total ABCG1 protein mass by fold, whereas the cell surface ABCG1, as determined by biotinylation, was increased by fold (P 0.05). These results support the idea that LXR activation induces an increase in the amount and a redistribution of ABCG1 to the cell surface as a physiological response to cholesterol accumulation. LXR Activation Increases Cholesterol Efflux to HDL, LDL, and Cyclodextrin in an ABCG1-Dependent Fashion in Macrophages ABCG1 overexpression in 293 cells increased cholesterol efflux not only to HDL but also to LDL and cyclodextrin, 9 suggesting the possibility that ABCG1 activity increased the amount of cell surface cholesterol that was accessible to a variety of extracellular acceptors. To see whether similar results would be obtained in macrophages, we induced ABCG1 expression by LXR activation, then measured cholesterol efflux to the various acceptors. LXR activation significantly increased cholesterol efflux from macrophages to HDL, LDL, and cyclodextrin (Figure 6A). LXR activation also increased cholesterol efflux to apoa-i (Figure 6A), likely reflecting the upregulation of ABCA1 expression. 16 To test the specific role of ABCG1, cholesterol efflux was determined from macrophages with or without suppression of ABCG1 expression by sirna. Suppression of ABCG1 expression reduced cholesterol efflux from macrophages to HDL, LDL and cyclodextrin, but not apoa-1 (Figure 6B). These results suggest that LXR activation enhances ABCG1 expression and promotes cholesterol availability to a variety of lipoprotein acceptors and to cyclodextrin at the macrophage plasma membrane, but that this is not sufficient to promote cholesterol efflux to apoa-1. Suppression of ABCG1 Expression Increases Cholesteryl Ester Formation and Decreases SREBP2 Target Gene Expression To test the potential role of ABCG1 in regulation of ER cholesterol metabolism, we determined cholesteryl ester (CE) formation after acute suppression of ABCG1 expression in macrophages. When [ 14 C]oleate was used as a substrate for ACAT-catalyzed CE formation, suppression of ABCG1 expression markedly increased CE formation in macrophages activated by 2 mol/l TO and loaded with cholesterol by incubation with acetyl-ldl (Supplement 1A). Intriguingly, this increased CE formation occurred in the absence of HDL-promoted cholesterol efflux. As a control, sirna targeting ABCA7, another ABC transporter, showed no effect on CE formation (supplemental Figure IA, see ahajournals.org). Decreased ABCG1 activity did not affect uptake of [ 14 C]oleate by macrophages (not shown). The increased CE formation could be a result of an increased FC accessibility to ACAT as substrate in ER or a consequence of increased ACAT activity. Using real-time quantitative RT-PCR, we determined the mrna levels of ACAT1, the major ACAT form in macrophage. 17 ACAT1 mrna levels did not show significant change on suppression of ABCG1 expression in macrophages (supplemental Figure IB). Similarly, downregulation of ABCG1 expression in mouse macrophages cholesterol loaded with acetyl-ldl but without TO treatment also increased CE formation without alteration of ACAT1 mrna levels (supplemental Figure IC and ID). Together, these findings suggest that ABCG1 activity alters the subcellular distribution of cholesterol, even in the absence of cholesterol efflux. Decreased ABCG1 activity may lead to an increased accumulation of FC in the ER, which subsequently serves as substrate for ACAT-catalyzed CE formation. To further test this idea, we determined the expression of 3-hydroxy-3-methylglutaryl (HMG) coenzyme A (CoA) reductase and LDL receptor, 2 SREBP2 target genes. Downregulation of ABCG1 expression by RNA interference decreased HMG CoA reductase mrna levels by 50% in cholesterol-loaded mouse peritoneal macrophages in the presence or absence of TO (P 0.01). The mrna levels of LDL receptor were already low in these cholesterol-loaded
6 Wang et al Cellular Distribution and Function of ABCG Figure 6. LXR activation increases cholesterol efflux to HDL, LDL, and CD, but not apoa-i in macrophages in an ABCG1-dependent fashion. A, Mouse peritoneal macrophages were labeled with [ 3 H]cholesterol in 10% FBS and DMEM medium in the presence or absence of 3 mol/l TO for 16 hours. Cholesterol efflux was initiated by adding HDL2 (25 g/ml HDL protein), LDL (25 g/ml LDL protein), or 2-hydroxypropyl- -cyclodextrin (0.4 mmol/l) and proceeded at 37 C for 2 hours. B, Macrophages were transfected with 160 nmol scrambled or ABCG1 sirna in the presence of 3 mol/l TO and labeled with [ 3 H]cholesterol as described in the Experimental Procedures. Cholesterol efflux similar to A was determined. *P 0.05 for vehicle vs TO in (A) or scambled sirna versus ABCG1 sirna in (B) for the indicated acceptor. macrophages. Although reduced LDL receptor mrna levels ( 35%) were detected on suppression of ABCG1 expression, the data did not reach statistical significance (not shown). Overall, the data are consistent with the idea that suppression of ABCG1 expression leads to an increased FC accumulation in the ER, which inhibits SREBP2 processing and activation while increasing CE formation by serving as substrates for ACAT. Discussion By using macrophages with targeted disruption of the ABCG1 or ABCG4 genes, we show that ABCG1 but not ABCG4 plays a pivotal role in macrophage cholesterol mass efflux to HDL after LXR activation. The results with ABCG4 are not surprising, because it is expressed primarily in the brain, where it could potentially play a role in promoting cholesterol efflux to HDL in the cerebrospinal fluid (CSF). 18 ABCG1 had only a limited contribution to cholesterol efflux in the basal state without LXR activation or cholesterol loading, even though protein expression was readily detectable. A potential explanation for this finding was the novel observation that LXR activation in addition to increasing the amount of ABCG1 also resulted in a redistribution of ABCG1 from intracellular sites to the plasma membrane. Thus, LXRs in addition to inducing expression of target genes may lead to altered distribution of induced protein products within cells, leading to enhanced function. ABCG1 localizes in the plasma membrane of LXR-activated macrophages and increases the availability of cholesterol to a variety of particles that have direct capacity to accept cholesterol, but not to lipid-poor apoa-i. ABCG1 activity also appears to limit accumulation of FC in the ER, decreasing CE formation and expression of SREBP2 target genes, even in the absence of HDL. These findings provide a mechanism to explain the accumulation of CE in macrophage foam cells in ABCG1-deficient mice. 11 In LXR-activated or cholesterol-loaded macrophages, predominant cell surface localization of ABCG1 was shown by cellular fractionation, limited proteolysis and biotinylation experiments. Although LXR activation or cholesterol loading moderately increased total ABCG1 protein in human macrophages derived from THP-1 cells, surprisingly, a more substantial increase in ABCG1 protein at the cell surface was observed. Also subcellular fractionation suggested a major LXR-induced redistribution of ABCG1 from the Golgi to the
7 1316 Arterioscler Thromb Vasc Biol. June 2006 plasma membrane (Figure 3). This finding could explain the lack of a contribution of ABCG1 to cholesterol efflux in the basal state, despite readily detectable ABCG1 in cell lysates (Figure 3). The mechanism for this increased ABCG1 presentation at the cell surface is unknown. Previous studies have shown several different isoforms of ABCG1 caused by alternative promoter usage or splicing, 10,19 and some isoforms appeared to be more responsive to LXR activation than others. 10 It is possible that different isoforms of ABCG1 have distinct preferences for cell surface localization. Alternatively, LXR activation may regulate ABCG1 cellular distribution by an undefined posttranscriptional mechanism. The predominant cell surface localization of ABCG1 in LXR-activated macrophages argues for the plasma membrane as the primary site for ABCG1 to promote cellular cholesterol efflux to HDL. Conceivably, ABCG1 may translocate cholesterol from the inner to the outer leaflet of the plasma membrane, which subsequently is available for efflux, by diffusional or collisional mechanisms, 6 to extracellular cholesterol acceptors. LXR activation promoted macrophage cholesterol efflux to HDL, LDL, and CD in an ABCG1-dependent fashion, consistent with our earlier findings in overexpressing 293 cells. 9 Together these data suggest that ABCG1 activity redistributes cellular cholesterol to the cell surface, where it is accessible for removal by extracellular cholesterol acceptors. It is intriguing that this apparent increase in cell surface cholesterol content is not sufficient to promote cholesterol efflux to lipid-poor apoa-1. Whereas some have argued that ABCA1 acts to form cholesterol-enriched microdomains that are subsequently solubilized by apoa-1, the findings with ABCG1 suggest that the ability of ABCA1 to bind apoa-1 may be key to its ability to promote lipid efflux to apoa-1. Interestingly, suppression of ABCG1 expression in cholesterol-loaded macrophages with or without TO treatment markedly increased CE formation without change in ACAT mrna level and reduced expression of SREBP-2 target genes. Importantly, these changes occurred in the absence of HDL in the medium. This suggests that activity of ABCG1 in the plasma membrane and possibly at intracellular sites promotes a redistribution of cholesterol away from regulatory pools in the ER. Surprisingly, we were not able to show colocalization of ABCG1 with ER markers. It is conceivable that activity of ABCG1 in plasma membrane or Golgi indirectly leads to a reduction in the ER cholesterol regulatory pool. Although the function of ABCG1 in plasma membrane is likely linked to HDL-mediated cholesterol efflux, its potential role in the Golgi is unknown. An interesting possibility is that Golgi ABCG1 is involved in the formation of cholesterol-rich lipid microdomains that are eventually transported to the cell surface. Our findings showing increased ACAT activity in ABCG1 deficient cells are consistent with the observation that ABCG1- deficient mice accumulate macrophage foam cells in various organs 11 but inconsistent with a recent report that ABCG1 overexpression increased CE accumulation in transfected BHK cells. 12 Perhaps this discrepancy is related to very high overexpression of ABCG1 in the latter study. Increased CE accumulation in ABCG1-deficient macrophages is likely the result of increased free cholesterol in ER that is subsequently converted to CE by ACAT activity. References 1. Castelli WP, Garrison RJ, Wilson PW, Abbott RD, Kalousdian S, Kannel WB. Incidence of coronary heart disease and lipoprotein cholesterol levels. The Framingham Study. JAMA. 1986;256: Rhoads GG, Gulbrandsen CL, Kagan A. Serum lipoproteins and coronary heart disease in a population study of Hawaii Japanese men. N Engl J Med. 1976;294: Genest JJ, McNamara JR, Salem DN, Schaefer EJ. Prevalence of risk factors in men with premature coronary artery disease. Am J Cardiol. 1991;67: Glomset JA. High-density lipoproteins in human health and disease. Adv Intern Med. 1980;25: Tall AR, Costet P, Wang N. Regulation and mechanisms of macrophage cholesterol efflux. J Clin Invest. 2002;110: Yancey PG, Bortnick AE, Kellner-Weibel G, de la Llera-Moya M, Phillips MC, Rothblat GH. Importance of different pathways of cellular cholesterol efflux. Arterioscler Thromb Vasc Biol. 2003;23: Wang N, Tall AR. Regulation and mechanisms of ATP-binding cassette transporter A1-mediated cellular cholesterol efflux. Arterioscler Thromb Vasc Biol. 2003;23: Chen W, Wang N, Tall AR. A PEST Deletion Mutant of ABCA1 Shows Impaired Internalization and Defective Cholesterol Efflux from Late Endosomes. J Biol Chem. 2005;280: Wang N, Lan D, Chen W, Matsuura F, Tall AR. ATP-binding cassette transporters G1 and G4 mediate cellular cholesterol efflux to high-density lipoproteins. Proc Natl Acad Sci U S A. 2004;101: Nakamura K, Kennedy MA, Baldan A, Bojanic DD, Lyons K, Edwards PA. Expression and regulation of multiple murine ATP-binding cassette transporter G1 mrnas/isoforms that stimulate cellular cholesterol efflux to high density lipoprotein. J Biol Chem. 2004;279: Kennedy MA, Barrera GC, Nakamura K, Baldan A, Tarr P, Fishbein MC, Frank J, Francone OL, Edwards PA. ABCG1 has a critical role in mediating cholesterol efflux to HDL and preventing cellular lipid accumulation. Cell Metab. 2005;1: Vaughan AM, Oram JF. ABCG1 redistributes cell cholesterol to domains removable by high density lipoprotein but not by lipid-depleted apolipoproteins. J Biol Chem. 2005;280: Li Y, Ge M, Ciani L, Kuriakose G, Westover EJ, Dura M, Covey DF, Freed JH, Maxfield FR, Lytton J, Tabas I. Enrichment of endoplasmic reticulum with cholesterol inhibits sarcoplasmic-endoplasmic reticulum calcium ATPase-2b activity in parallel with increased order of membrane lipids: implications for depletion of endoplasmic reticulum calcium stores and apoptosis in cholesterol-loaded macrophages. J Biol Chem. 2004; 279: Wang N, Chen W, Linsel-Nitschke P, Martinez LO, Agerholm-Larsen B, Silver DL, Tall AR. A PEST sequence in ABCA1 regulates degradation by calpain protease and stabilization of ABCA1 by apoa-i. J Clin Invest. 2003;111: Scacheri PC, Rozenblatt-Rosen O, Caplen NJ, Wolfsberg TG, Umayam L, Lee JC, Hughes CM, Shanmugam KS, Bhattacharjee A, Meyerson M, Collins FS. Short interfering RNAs can induce unexpected and divergent changes in the levels of untargeted proteins in mammalian cells. Proc Natl Acad Sci U S A. 2004;101: Costet P, Luo Y, Wang N, Tall AR. Sterol-dependent transactivation of the ABC1 promoter by the liver X receptor/retinoid X receptor. J Biol Chem. 2000;275: Chang TY, Chang CC, Lin S, Yu C, Li BL, Miyazaki A. Roles of acyl-coenzyme A:cholesterol acyltransferase-1 and -2. Curr Opin Lipidol. 2001;12: Demeester N, Castro G, Desrumaux C, De Geitere C, Fruchart JC, Santens P, Mulleners E, Engelborghs S, De Deyn PP, Vandekerckhove J, Rosseneu M, Labeur C. Characterization and functional studies of lipoproteins, lipid transfer proteins, and lecithin:cholesterol acyltransferase in CSF of normal individuals and patients with Alzheimer s disease. J Lipid Res. 2000;41: Kennedy MA, Venkateswaran A, Tarr PT, Xenarios I, Kudoh J, Shimizu N, Edwards PA. Characterization of the human ABCG1 gene: liver X receptor activates an internal promoter that produces a novel transcript encoding an alternative form of the protein. J Biol Chem. 2001;276:
Supplemental Material. Results
 Supplemental Material Results Fractionation of mouse plasma by high-resolution SEC. APOA1 eluted as a single major peak in fractions 16 of plasma (the apparent size of mature, lipidated HDL) when it was
Supplemental Material Results Fractionation of mouse plasma by high-resolution SEC. APOA1 eluted as a single major peak in fractions 16 of plasma (the apparent size of mature, lipidated HDL) when it was
2.5. AMPK activity
 Supplement Fig. A 3 B phos-ampk 2.5 * Control AICAR AMPK AMPK activity (Absorbance at 45 nm) 2.5.5 Control AICAR Supplement Fig. Effects of AICAR on AMPK activation in macrophages. J774. macrophages were
Supplement Fig. A 3 B phos-ampk 2.5 * Control AICAR AMPK AMPK activity (Absorbance at 45 nm) 2.5.5 Control AICAR Supplement Fig. Effects of AICAR on AMPK activation in macrophages. J774. macrophages were
High density lipoprotein metabolism
 High density lipoprotein metabolism Lipoprotein classes and atherosclerosis Chylomicrons, VLDL, and their catabolic remnants Pro-atherogenic LDL HDL Anti-atherogenic Plasma lipid transport Liver VLDL FC
High density lipoprotein metabolism Lipoprotein classes and atherosclerosis Chylomicrons, VLDL, and their catabolic remnants Pro-atherogenic LDL HDL Anti-atherogenic Plasma lipid transport Liver VLDL FC
SR-BI inhibits ABCG1-stimulated net cholesterol efflux from cells to plasma HDL
 SR-BI inhibits ABCG1-stimulated net cholesterol efflux from cells to plasma HDL Laurent Yvan-Charvet, 1, * Tamara A. Pagler,* Nan Wang,* Takafumi Senokuchi,* May Brundert, Hongna Li,* Franz Rinninger,
SR-BI inhibits ABCG1-stimulated net cholesterol efflux from cells to plasma HDL Laurent Yvan-Charvet, 1, * Tamara A. Pagler,* Nan Wang,* Takafumi Senokuchi,* May Brundert, Hongna Li,* Franz Rinninger,
Summary and concluding remarks
 Summary and concluding remarks This thesis is focused on the role and interaction of different cholesterol and phospholipid transporters. Cholesterol homeostasis is accomplished via a tightly regulated
Summary and concluding remarks This thesis is focused on the role and interaction of different cholesterol and phospholipid transporters. Cholesterol homeostasis is accomplished via a tightly regulated
TFEB-mediated increase in peripheral lysosomes regulates. Store Operated Calcium Entry
 TFEB-mediated increase in peripheral lysosomes regulates Store Operated Calcium Entry Luigi Sbano, Massimo Bonora, Saverio Marchi, Federica Baldassari, Diego L. Medina, Andrea Ballabio, Carlotta Giorgi
TFEB-mediated increase in peripheral lysosomes regulates Store Operated Calcium Entry Luigi Sbano, Massimo Bonora, Saverio Marchi, Federica Baldassari, Diego L. Medina, Andrea Ballabio, Carlotta Giorgi
ANSC/NUTR 618 LIPIDS & LIPID METABOLISM The LDL Receptor, LDL Uptake, and the Free Cholesterol Pool
 ANSC/NUTR 618 LIPIDS & LIPID METABOLISM The, LDL Uptake, and the Free Cholesterol Pool I. Michael Brown and Joseph Goldstein A. Studied families with familial hypercholesterolemia. B. Defined the relationship
ANSC/NUTR 618 LIPIDS & LIPID METABOLISM The, LDL Uptake, and the Free Cholesterol Pool I. Michael Brown and Joseph Goldstein A. Studied families with familial hypercholesterolemia. B. Defined the relationship
MCB130 Midterm. GSI s Name:
 1. Peroxisomes are small, membrane-enclosed organelles that function in the degradation of fatty acids and in the degradation of H 2 O 2. Peroxisomes are not part of the secretory pathway and peroxisomal
1. Peroxisomes are small, membrane-enclosed organelles that function in the degradation of fatty acids and in the degradation of H 2 O 2. Peroxisomes are not part of the secretory pathway and peroxisomal
Mechanisms of high density lipoprotein-mediated efflux of cholesterol from cell plasma membranes
 Atherosclerosis 137 Suppl. (1998) S13 S17 Mechanisms of high density lipoprotein-mediated efflux of cholesterol from cell plasma membranes Michael C. Phillips *, Kristin L. Gillotte, M. Page Haynes, William
Atherosclerosis 137 Suppl. (1998) S13 S17 Mechanisms of high density lipoprotein-mediated efflux of cholesterol from cell plasma membranes Michael C. Phillips *, Kristin L. Gillotte, M. Page Haynes, William
G. Chinetti-Gbaguidi and B. Staels, UR 545 INSERM, Institut Pasteur de Lille and Université de Lille 2, Lille, France
 LIVER X RECEPTORS (LXRS): TRANSCRIPTIONAL REGULATORS OF MACROPHAGE CHOLESTEROL METABOLISM G. Chinetti-Gbaguidi and B. Staels, UR 545 INSERM, Institut Pasteur de Lille and Université de Lille 2, Lille,
LIVER X RECEPTORS (LXRS): TRANSCRIPTIONAL REGULATORS OF MACROPHAGE CHOLESTEROL METABOLISM G. Chinetti-Gbaguidi and B. Staels, UR 545 INSERM, Institut Pasteur de Lille and Université de Lille 2, Lille,
Unit IV Problem 3 Biochemistry: Cholesterol Metabolism and Lipoproteins
 Unit IV Problem 3 Biochemistry: Cholesterol Metabolism and Lipoproteins - Cholesterol: It is a sterol which is found in all eukaryotic cells and contains an oxygen (as a hydroxyl group OH) on Carbon number
Unit IV Problem 3 Biochemistry: Cholesterol Metabolism and Lipoproteins - Cholesterol: It is a sterol which is found in all eukaryotic cells and contains an oxygen (as a hydroxyl group OH) on Carbon number
Lipoproteins Metabolism Reference: Campbell Biochemistry and Lippincott s Biochemistry
 Lipoproteins Metabolism Reference: Campbell Biochemistry and Lippincott s Biochemistry Learning Objectives 1. Define lipoproteins and explain the rationale of their formation in blood. 2. List different
Lipoproteins Metabolism Reference: Campbell Biochemistry and Lippincott s Biochemistry Learning Objectives 1. Define lipoproteins and explain the rationale of their formation in blood. 2. List different
 Acetyl CoA HMG CoA Mevalonate (C6) Dimethylallyl Pyrophosphate isopentenyl Pyrophosphate (C5) Geranyl Pyrophosphate (C10) FarnesylPyrophosphate (C15) Squalene (C30) Lanosterol (C30) 7 Dehydrocholesterol
Acetyl CoA HMG CoA Mevalonate (C6) Dimethylallyl Pyrophosphate isopentenyl Pyrophosphate (C5) Geranyl Pyrophosphate (C10) FarnesylPyrophosphate (C15) Squalene (C30) Lanosterol (C30) 7 Dehydrocholesterol
Cholesterol metabolism. Function Biosynthesis Transport in the organism Hypercholesterolemia
 Cholesterol metabolism Function Biosynthesis Transport in the organism Hypercholesterolemia - component of all cell membranes - precursor of bile acids steroid hormones vitamin D Cholesterol Sources: dietary
Cholesterol metabolism Function Biosynthesis Transport in the organism Hypercholesterolemia - component of all cell membranes - precursor of bile acids steroid hormones vitamin D Cholesterol Sources: dietary
Supplementary Figure 1. PAQR3 knockdown inhibits SREBP-2 processing in CHO-7 cells CHO-7 cells were transfected with control sirna or a sirna
 Supplementary Figure 1. PAQR3 knockdown inhibits SREBP-2 processing in CHO-7 cells CHO-7 cells were transfected with control sirna or a sirna targeted for hamster PAQR3. At 24 h after the transfection,
Supplementary Figure 1. PAQR3 knockdown inhibits SREBP-2 processing in CHO-7 cells CHO-7 cells were transfected with control sirna or a sirna targeted for hamster PAQR3. At 24 h after the transfection,
review Cell cholesterol efflux: integration of old and new observations provides new insights
 Cell cholesterol efflux: integration of old and new observations provides new insights review George H. Rothblat, 1, * Margarita de la Llera-Moya,* Veronique Atger, Ginny Kellner-Weibel,* David L. Williams,
Cell cholesterol efflux: integration of old and new observations provides new insights review George H. Rothblat, 1, * Margarita de la Llera-Moya,* Veronique Atger, Ginny Kellner-Weibel,* David L. Williams,
Companion to Biosynthesis of Ketones & Cholesterols, Regulation of Lipid Metabolism Lecture Notes
 Companion to Biosynthesis of Ketones & Cholesterols, Regulation of Lipid Metabolism Lecture Notes The major site of acetoacetate and 3-hydorxybutyrate production is in the liver. 3-hydorxybutyrate is the
Companion to Biosynthesis of Ketones & Cholesterols, Regulation of Lipid Metabolism Lecture Notes The major site of acetoacetate and 3-hydorxybutyrate production is in the liver. 3-hydorxybutyrate is the
Cellular control of cholesterol. Peter Takizawa Department of Cell Biology
 Cellular control of cholesterol Peter Takizawa Department of Cell Biology Brief overview of cholesterol s biological role Regulation of cholesterol synthesis Dietary and cellular uptake of cholesterol
Cellular control of cholesterol Peter Takizawa Department of Cell Biology Brief overview of cholesterol s biological role Regulation of cholesterol synthesis Dietary and cellular uptake of cholesterol
Chapter VIII: Dr. Sameh Sarray Hlaoui
 Chapter VIII: Dr. Sameh Sarray Hlaoui Lipoproteins a Lipids are insoluble in plasma. In order to be transported they are combined with specific proteins to form lipoproteins: Clusters of proteins and lipids.
Chapter VIII: Dr. Sameh Sarray Hlaoui Lipoproteins a Lipids are insoluble in plasma. In order to be transported they are combined with specific proteins to form lipoproteins: Clusters of proteins and lipids.
Supplementary information. MARCH8 inhibits HIV-1 infection by reducing virion incorporation of envelope glycoproteins
 Supplementary information inhibits HIV-1 infection by reducing virion incorporation of envelope glycoproteins Takuya Tada, Yanzhao Zhang, Takayoshi Koyama, Minoru Tobiume, Yasuko Tsunetsugu-Yokota, Shoji
Supplementary information inhibits HIV-1 infection by reducing virion incorporation of envelope glycoproteins Takuya Tada, Yanzhao Zhang, Takayoshi Koyama, Minoru Tobiume, Yasuko Tsunetsugu-Yokota, Shoji
ABCA1 and ABCG1 or ABCG4 act sequentially to remove cellular cholesterol and generate cholesterol-rich HDL
 ABCA1 and ABCG1 or ABCG4 act sequentially to remove cellular cholesterol and generate cholesterol-rich HDL Ashley M. Vaughan 1 and John F. Oram Department of Medicine, Division of Metabolism, Endocrinology,
ABCA1 and ABCG1 or ABCG4 act sequentially to remove cellular cholesterol and generate cholesterol-rich HDL Ashley M. Vaughan 1 and John F. Oram Department of Medicine, Division of Metabolism, Endocrinology,
Supplementary Figure S I: Effects of D4F on body weight and serum lipids in apoe -/- mice.
 Supplementary Figures: Supplementary Figure S I: Effects of D4F on body weight and serum lipids in apoe -/- mice. Male apoe -/- mice were fed a high-fat diet for 8 weeks, and given PBS (model group) or
Supplementary Figures: Supplementary Figure S I: Effects of D4F on body weight and serum lipids in apoe -/- mice. Male apoe -/- mice were fed a high-fat diet for 8 weeks, and given PBS (model group) or
Cholesterol Metabolism
 Cholesterol Metabolism Lippincott s Illustrated Review Chapter 18 Steroid Nucleus 1 2 Cholesterol was isolated from gall bladder stones in 1774 3 Sources and Elimination of Cholesterol Synthesis: 1000
Cholesterol Metabolism Lippincott s Illustrated Review Chapter 18 Steroid Nucleus 1 2 Cholesterol was isolated from gall bladder stones in 1774 3 Sources and Elimination of Cholesterol Synthesis: 1000
Project report October 2012 March 2013 CRF fellow: Principal Investigator: Project title:
 Project report October 2012 March 2013 CRF fellow: Gennaro Napolitano Principal Investigator: Sergio Daniel Catz Project title: Small molecule regulators of vesicular trafficking to enhance lysosomal exocytosis
Project report October 2012 March 2013 CRF fellow: Gennaro Napolitano Principal Investigator: Sergio Daniel Catz Project title: Small molecule regulators of vesicular trafficking to enhance lysosomal exocytosis
A PEST sequence in ABCA1 regulates degradation by calpain protease and stabilization of ABCA1 by apoa-i
 A PEST sequence in ABCA1 regulates degradation by calpain protease and stabilization of ABCA1 by apoa-i Nan Wang, Wengen Chen, Patrick Linsel-Nitschke, Laurent O. Martinez, Birgit Agerholm-Larsen, David
A PEST sequence in ABCA1 regulates degradation by calpain protease and stabilization of ABCA1 by apoa-i Nan Wang, Wengen Chen, Patrick Linsel-Nitschke, Laurent O. Martinez, Birgit Agerholm-Larsen, David
Supplementary Figure 1.TRIM33 binds β-catenin in the nucleus. a & b, Co-IP of endogenous TRIM33 with β-catenin in HT-29 cells (a) and HEK 293T cells
 Supplementary Figure 1.TRIM33 binds β-catenin in the nucleus. a & b, Co-IP of endogenous TRIM33 with β-catenin in HT-29 cells (a) and HEK 293T cells (b). TRIM33 was immunoprecipitated, and the amount of
Supplementary Figure 1.TRIM33 binds β-catenin in the nucleus. a & b, Co-IP of endogenous TRIM33 with β-catenin in HT-29 cells (a) and HEK 293T cells (b). TRIM33 was immunoprecipitated, and the amount of
HDL from CETP-deficient subjects shows enhanced ability to promote cholesterol efflux from macrophages in an apoe- and ABCG1-dependent pathway
 Related Commentary, page 1226 Research article HDL from CETP-deficient subjects shows enhanced ability to promote cholesterol efflux from macrophages in an apoe- and ABCG1-dependent pathway Fumihiko Matsuura,
Related Commentary, page 1226 Research article HDL from CETP-deficient subjects shows enhanced ability to promote cholesterol efflux from macrophages in an apoe- and ABCG1-dependent pathway Fumihiko Matsuura,
Supplementary Figure S1. Venn diagram analysis of mrna microarray data and mirna target analysis. (a) Western blot analysis of T lymphoblasts (CLS)
 Supplementary Figure S1. Venn diagram analysis of mrna microarray data and mirna target analysis. (a) Western blot analysis of T lymphoblasts (CLS) and their exosomes (EXO) in resting (REST) and activated
Supplementary Figure S1. Venn diagram analysis of mrna microarray data and mirna target analysis. (a) Western blot analysis of T lymphoblasts (CLS) and their exosomes (EXO) in resting (REST) and activated
Cholesterol Transport and Regulation in the Mammary Gland
 J Mammary Gland Biol Neoplasia (2014) 19:43 58 DOI 10.1007/s10911-014-9316-x Cholesterol Transport and Regulation in the Mammary Gland Edgar C. Ontsouka & Christiane Albrecht Received: 10 September 2013
J Mammary Gland Biol Neoplasia (2014) 19:43 58 DOI 10.1007/s10911-014-9316-x Cholesterol Transport and Regulation in the Mammary Gland Edgar C. Ontsouka & Christiane Albrecht Received: 10 September 2013
THE ROLE OF ALTERED CALCIUM AND mtor SIGNALING IN THE PATHOGENESIS OF CYSTINOSIS
 Research Foundation, 18 month progress report THE ROLE OF ALTERED CALCIUM AND mtor SIGNALING IN THE PATHOGENESIS OF CYSTINOSIS Ekaterina Ivanova, doctoral student Elena Levtchenko, MD, PhD, PI Antonella
Research Foundation, 18 month progress report THE ROLE OF ALTERED CALCIUM AND mtor SIGNALING IN THE PATHOGENESIS OF CYSTINOSIS Ekaterina Ivanova, doctoral student Elena Levtchenko, MD, PhD, PI Antonella
Nature Medicine doi: /nm.3150
 Nature Medicine doi:10.1038/nm.3150 Supplementary Table 1 Primer sequences used for q-pcr analysis Gene Forward primer Reverse primer Abca1 CAGCTTCCATCCTCCTTGTC CCACATCCACAACTGTCTGG Abcg1 GTACCATGACATCGCTGGTG
Nature Medicine doi:10.1038/nm.3150 Supplementary Table 1 Primer sequences used for q-pcr analysis Gene Forward primer Reverse primer Abca1 CAGCTTCCATCCTCCTTGTC CCACATCCACAACTGTCTGG Abcg1 GTACCATGACATCGCTGGTG
Live cell imaging of trafficking of the chaperone complex vaccine to the ER. BMDCs were incubated with ER-Tracker Red (1 M) in staining solution for
 Live cell imaging of trafficking of the chaperone complex vaccine to the ER. BMDCs were incubated with ER-Tracker Red (1 M) in staining solution for 15 min at 37 C and replaced with fresh complete medium.
Live cell imaging of trafficking of the chaperone complex vaccine to the ER. BMDCs were incubated with ER-Tracker Red (1 M) in staining solution for 15 min at 37 C and replaced with fresh complete medium.
Regulating Hepatic Cellular Cholesterol
 Under circumstances of cholesterol deficiency, Sterol Regulatory Element Binding Proteins (SREBPs) via binding to DNA nuclear response elements set off genomic production of proteins and enzymes that induce
Under circumstances of cholesterol deficiency, Sterol Regulatory Element Binding Proteins (SREBPs) via binding to DNA nuclear response elements set off genomic production of proteins and enzymes that induce
Macrophage cholesterol efflux and the active domains of serum amyloid A 2.1
 Macrophage cholesterol efflux and the active domains of serum amyloid A 2.1 Robert Kisilevsky 1, *,, and Shui Pang Tam, Departments of Pathology* and Biochemistry, Queen s University, and The Syl and Molly
Macrophage cholesterol efflux and the active domains of serum amyloid A 2.1 Robert Kisilevsky 1, *,, and Shui Pang Tam, Departments of Pathology* and Biochemistry, Queen s University, and The Syl and Molly
SUPPLEMENTARY FIGURES
 SUPPLEMENTARY FIGURES Supplementary Figure 1. (A) Left, western blot analysis of ISGylated proteins in Jurkat T cells treated with 1000U ml -1 IFN for 16h (IFN) or left untreated (CONT); right, western
SUPPLEMENTARY FIGURES Supplementary Figure 1. (A) Left, western blot analysis of ISGylated proteins in Jurkat T cells treated with 1000U ml -1 IFN for 16h (IFN) or left untreated (CONT); right, western
genome edited transient transfection, CMV promoter
 Supplementary Figure 1. In the absence of new protein translation, overexpressed caveolin-1-gfp is degraded faster than caveolin-1-gfp expressed from the endogenous caveolin 1 locus % loss of total caveolin-1-gfp
Supplementary Figure 1. In the absence of new protein translation, overexpressed caveolin-1-gfp is degraded faster than caveolin-1-gfp expressed from the endogenous caveolin 1 locus % loss of total caveolin-1-gfp
Cells and reagents. Synaptopodin knockdown (1) and dynamin knockdown (2)
 Supplemental Methods Cells and reagents. Synaptopodin knockdown (1) and dynamin knockdown (2) podocytes were cultured as described previously. Staurosporine, angiotensin II and actinomycin D were all obtained
Supplemental Methods Cells and reagents. Synaptopodin knockdown (1) and dynamin knockdown (2) podocytes were cultured as described previously. Staurosporine, angiotensin II and actinomycin D were all obtained
SUPPLEMENTARY FIGURE LEGENDS
 SUPPLEMENTARY FIGURE LEGENDS Supplemental FIG. 1. Localization of myosin Vb in cultured neurons varies with maturation stage. A and B, localization of myosin Vb in cultured hippocampal neurons. A, in DIV
SUPPLEMENTARY FIGURE LEGENDS Supplemental FIG. 1. Localization of myosin Vb in cultured neurons varies with maturation stage. A and B, localization of myosin Vb in cultured hippocampal neurons. A, in DIV
Lysosomes and endocytic pathways 9/27/2012 Phyllis Hanson
 Lysosomes and endocytic pathways 9/27/2012 Phyllis Hanson General principles Properties of lysosomes Delivery of enzymes to lysosomes Endocytic uptake clathrin, others Endocytic pathways recycling vs.
Lysosomes and endocytic pathways 9/27/2012 Phyllis Hanson General principles Properties of lysosomes Delivery of enzymes to lysosomes Endocytic uptake clathrin, others Endocytic pathways recycling vs.
ABCA1 mutants reveal an interdependency between lipid export function, apoa-i binding activity, and Janus kinase 2 activation
 ABCA1 mutants reveal an interdependency between lipid export function, apoa-i binding activity, and Janus kinase 2 activation Ashley M. Vaughan, Chongren Tang, and John F. Oram 1 Division of Metabolism,
ABCA1 mutants reveal an interdependency between lipid export function, apoa-i binding activity, and Janus kinase 2 activation Ashley M. Vaughan, Chongren Tang, and John F. Oram 1 Division of Metabolism,
Plasma lipoproteins & atherosclerosis by. Prof.Dr. Maha M. Sallam
 Biochemistry Department Plasma lipoproteins & atherosclerosis by Prof.Dr. Maha M. Sallam 1 1. Recognize structures,types and role of lipoproteins in blood (Chylomicrons, VLDL, LDL and HDL). 2. Explain
Biochemistry Department Plasma lipoproteins & atherosclerosis by Prof.Dr. Maha M. Sallam 1 1. Recognize structures,types and role of lipoproteins in blood (Chylomicrons, VLDL, LDL and HDL). 2. Explain
Hmgcoar AGCTTGCCCGAATTGTATGTG TCTGTTGTAACCATGTGACTTC. Cyp7α GGGATTGCTGTGGTAGTGAGC GGTATGGAATCAACCCGTTGTC
 Supplement Table I: primers for Real Time RT-PCR Gene Foward Reverse Hmgcoar AGCTTGCCCGAATTGTATGTG TCTGTTGTAACCATGTGACTTC Cyp7α GGGATTGCTGTGGTAGTGAGC GGTATGGAATCAACCCGTTGTC Cyp27a1 GTGGTCTTATTGGGTACTTGC
Supplement Table I: primers for Real Time RT-PCR Gene Foward Reverse Hmgcoar AGCTTGCCCGAATTGTATGTG TCTGTTGTAACCATGTGACTTC Cyp7α GGGATTGCTGTGGTAGTGAGC GGTATGGAATCAACCCGTTGTC Cyp27a1 GTGGTCTTATTGGGTACTTGC
Supporting Information
 Supporting Information Chen et al. 10.1073/pnas.0807991106 SI Methods Sucrose Gradient Fractionation. Fractionation by sucrose gradient was performed as described by Gasparini et al. [(2001) J Neurosci
Supporting Information Chen et al. 10.1073/pnas.0807991106 SI Methods Sucrose Gradient Fractionation. Fractionation by sucrose gradient was performed as described by Gasparini et al. [(2001) J Neurosci
Chapter 6. Antigen Presentation to T lymphocytes
 Chapter 6 Antigen Presentation to T lymphocytes Generation of T-cell Receptor Ligands T cells only recognize Ags displayed on cell surfaces These Ags may be derived from pathogens that replicate within
Chapter 6 Antigen Presentation to T lymphocytes Generation of T-cell Receptor Ligands T cells only recognize Ags displayed on cell surfaces These Ags may be derived from pathogens that replicate within
Sestrin2 and BNIP3 (Bcl-2/adenovirus E1B 19kDa-interacting. protein3) regulate autophagy and mitophagy in renal tubular cells in. acute kidney injury
 Sestrin2 and BNIP3 (Bcl-2/adenovirus E1B 19kDa-interacting protein3) regulate autophagy and mitophagy in renal tubular cells in acute kidney injury by Masayuki Ishihara 1, Madoka Urushido 2, Kazu Hamada
Sestrin2 and BNIP3 (Bcl-2/adenovirus E1B 19kDa-interacting protein3) regulate autophagy and mitophagy in renal tubular cells in acute kidney injury by Masayuki Ishihara 1, Madoka Urushido 2, Kazu Hamada
The relationship between coronary artery disease and low
 Cellular Phospholipid and Cholesterol Efflux in High-Density Lipoprotein Deficiency Michel Marcil, PhD; Rachel Bissonnette, MSc; Jérôme Vincent, DEA; Larbi Krimbou, DES; Jacques Genest, MD Background Prospective
Cellular Phospholipid and Cholesterol Efflux in High-Density Lipoprotein Deficiency Michel Marcil, PhD; Rachel Bissonnette, MSc; Jérôme Vincent, DEA; Larbi Krimbou, DES; Jacques Genest, MD Background Prospective
Novel Reduction of PCSK9 Expression: Mechanistic Insights into the Anti-Atherosclerotic & Hypolipidemic Effects of Heat Shock Protein 27
 Novel Reduction of PCSK9 Expression: Mechanistic Insights into the Anti-Atherosclerotic & Hypolipidemic Effects of Heat Shock Protein 27 Ed O Brien, Jean-Claude Bakala-N Goma, Chunhua Shi Cumming School
Novel Reduction of PCSK9 Expression: Mechanistic Insights into the Anti-Atherosclerotic & Hypolipidemic Effects of Heat Shock Protein 27 Ed O Brien, Jean-Claude Bakala-N Goma, Chunhua Shi Cumming School
HDL and Its Galaxy of Proteins: What Do they Do? Jay Heinecke, University of Washington
 HDL and Its Galaxy of Proteins: What Do they Do? Jay Heinecke, University of Washington Disclosures NIH AHA Insilicos Consultant Merck Research Support GSK Research Support Pfizer Research Support Corcept
HDL and Its Galaxy of Proteins: What Do they Do? Jay Heinecke, University of Washington Disclosures NIH AHA Insilicos Consultant Merck Research Support GSK Research Support Pfizer Research Support Corcept
Behind LDL: The Metabolism of ApoB, the Essential Apolipoprotein in LDL and VLDL
 Behind LDL: The Metabolism of ApoB, the Essential Apolipoprotein in LDL and VLDL Sung-Joon Lee, PhD Division of Food Science Institute of Biomedical Science and Safety Korea University Composition of Lipoproteins:
Behind LDL: The Metabolism of ApoB, the Essential Apolipoprotein in LDL and VLDL Sung-Joon Lee, PhD Division of Food Science Institute of Biomedical Science and Safety Korea University Composition of Lipoproteins:
Supplemental Information
 Supplemental Information Tobacco-specific Carcinogen Induces DNA Methyltransferases 1 Accumulation through AKT/GSK3β/βTrCP/hnRNP-U in Mice and Lung Cancer patients Ruo-Kai Lin, 1 Yi-Shuan Hsieh, 2 Pinpin
Supplemental Information Tobacco-specific Carcinogen Induces DNA Methyltransferases 1 Accumulation through AKT/GSK3β/βTrCP/hnRNP-U in Mice and Lung Cancer patients Ruo-Kai Lin, 1 Yi-Shuan Hsieh, 2 Pinpin
Figure S1. Western blot analysis of clathrin RNA interference in human DCs Human immature DCs were transfected with 100 nm Clathrin SMARTpool or
 Figure S1. Western blot analysis of clathrin RNA interference in human DCs Human immature DCs were transfected with 100 nm Clathrin SMARTpool or control nontargeting sirnas. At 90 hr after transfection,
Figure S1. Western blot analysis of clathrin RNA interference in human DCs Human immature DCs were transfected with 100 nm Clathrin SMARTpool or control nontargeting sirnas. At 90 hr after transfection,
The clathrin adaptor Numb regulates intestinal cholesterol. absorption through dynamic interaction with NPC1L1
 The clathrin adaptor Numb regulates intestinal cholesterol absorption through dynamic interaction with NPC1L1 Pei-Shan Li 1, Zhen-Yan Fu 1,2, Ying-Yu Zhang 1, Jin-Hui Zhang 1, Chen-Qi Xu 1, Yi-Tong Ma
The clathrin adaptor Numb regulates intestinal cholesterol absorption through dynamic interaction with NPC1L1 Pei-Shan Li 1, Zhen-Yan Fu 1,2, Ying-Yu Zhang 1, Jin-Hui Zhang 1, Chen-Qi Xu 1, Yi-Tong Ma
Nature Genetics: doi: /ng.3561
 Supplementary Figure 1 Pedigrees of families with APOB p.gln725* mutation and APOB p.gly1829glufs8 mutation (a,b) Pedigrees of families with APOB p.gln725* mutation. (c) Pedigree of family with APOB p.gly1829glufs8
Supplementary Figure 1 Pedigrees of families with APOB p.gln725* mutation and APOB p.gly1829glufs8 mutation (a,b) Pedigrees of families with APOB p.gln725* mutation. (c) Pedigree of family with APOB p.gly1829glufs8
SUPPLEMENTARY INFORMATION
 doi:10.1038/nature11700 Figure 1: RIP3 as a potential Sirt2 interacting protein. Transfected Flag-tagged Sirt2 was immunoprecipitated from cells and eluted from the Sepharose beads using Flag peptide.
doi:10.1038/nature11700 Figure 1: RIP3 as a potential Sirt2 interacting protein. Transfected Flag-tagged Sirt2 was immunoprecipitated from cells and eluted from the Sepharose beads using Flag peptide.
Cholesterol loading in macrophages stimulates formation of ER-derived vesicles with elevated ACAT1 activity
 Cholesterol loading in macrophages stimulates formation of ER-derived vesicles with elevated ACAT1 activity Naomi Sakashita, 1, *, Catherine C. Y. Chang, Xiaofeng Lei, * Yukio Fujiwara, * Motohiro Takeya,
Cholesterol loading in macrophages stimulates formation of ER-derived vesicles with elevated ACAT1 activity Naomi Sakashita, 1, *, Catherine C. Y. Chang, Xiaofeng Lei, * Yukio Fujiwara, * Motohiro Takeya,
Supplementary Materials for
 www.sciencesignaling.org/cgi/content/full/6/283/ra57/dc1 Supplementary Materials for JNK3 Couples the Neuronal Stress Response to Inhibition of Secretory Trafficking Guang Yang,* Xun Zhou, Jingyan Zhu,
www.sciencesignaling.org/cgi/content/full/6/283/ra57/dc1 Supplementary Materials for JNK3 Couples the Neuronal Stress Response to Inhibition of Secretory Trafficking Guang Yang,* Xun Zhou, Jingyan Zhu,
Cholesterol is an essential component of mammalian cell
 Brief Reviews Intracellular Cholesterol Transport Raymond E. Soccio, Jan L. Breslow Abstract Intracellular cholesterol transport is essential for the maintenance of cholesterol homeostasis. Many aspects
Brief Reviews Intracellular Cholesterol Transport Raymond E. Soccio, Jan L. Breslow Abstract Intracellular cholesterol transport is essential for the maintenance of cholesterol homeostasis. Many aspects
Metabolism and Atherogenic Properties of LDL
 Metabolism and Atherogenic Properties of LDL Manfredi Rizzo, MD, PhD Associate Professor of Internal Medicine Faculty of Medicine, University of Palermo, Italy & Affiliate Associate Professor of Internal
Metabolism and Atherogenic Properties of LDL Manfredi Rizzo, MD, PhD Associate Professor of Internal Medicine Faculty of Medicine, University of Palermo, Italy & Affiliate Associate Professor of Internal
Supplementary Figure 1
 Supplementary Figure 1 Constitutive EGFR signaling does not activate canonical EGFR signals (a) U251EGFRInd cells with or without tetracycline exposure (24h, 1µg/ml) were treated with EGF for 15 minutes
Supplementary Figure 1 Constitutive EGFR signaling does not activate canonical EGFR signals (a) U251EGFRInd cells with or without tetracycline exposure (24h, 1µg/ml) were treated with EGF for 15 minutes
SUPPLEMENTAL MATERIALS AND METHODS. Puromycin-synchronized metabolic labelling - Transfected HepG2 cells were depleted of
 SUPPLEMENTAL MATERIALS AND METHODS Puromycin-synchronized metabolic labelling - Transfected HepG2 cells were depleted of cysteine and methionine and then treated with 10 μm puromycin in depletion medium
SUPPLEMENTAL MATERIALS AND METHODS Puromycin-synchronized metabolic labelling - Transfected HepG2 cells were depleted of cysteine and methionine and then treated with 10 μm puromycin in depletion medium
Supplemental Figure 1 ELISA scheme to measure plasma total, mature and furin-cleaved
 1 Supplemental Figure Legends Supplemental Figure 1 ELISA scheme to measure plasma total, mature and furin-cleaved PCSK9 concentrations. 4 Plasma mature and furin-cleaved PCSK9s were measured by a sandwich
1 Supplemental Figure Legends Supplemental Figure 1 ELISA scheme to measure plasma total, mature and furin-cleaved PCSK9 concentrations. 4 Plasma mature and furin-cleaved PCSK9s were measured by a sandwich
Visualizing caveolin-1 and HDL in cholesterol-loaded aortic endothelial cells
 Visualizing caveolin-1 and HDL in cholesterol-loaded aortic endothelial cells W. T. Chao,* S. S. Fan,* J. K. Chen, and V. C. Yang 1,* Department of Biology and Life Science Research Center,* Tunghai University,
Visualizing caveolin-1 and HDL in cholesterol-loaded aortic endothelial cells W. T. Chao,* S. S. Fan,* J. K. Chen, and V. C. Yang 1,* Department of Biology and Life Science Research Center,* Tunghai University,
Progesterone blocks intracellular translocation of free cholesterol derived from cholesteryl ester in macrophages
 Progesterone blocks intracellular translocation of free cholesterol derived from cholesteryl ester in macrophages Theodore Mazzone, Madhuri Krishna, and Yvonne Lange Departments of Medicine, Biochemistry,
Progesterone blocks intracellular translocation of free cholesterol derived from cholesteryl ester in macrophages Theodore Mazzone, Madhuri Krishna, and Yvonne Lange Departments of Medicine, Biochemistry,
SUPPLEMENTARY INFORMATION
 SUPPLEMENTARY INFORMATION FOR Liver X Receptor α mediates hepatic triglyceride accumulation through upregulation of G0/G1 Switch Gene 2 (G0S2) expression I: SUPPLEMENTARY METHODS II: SUPPLEMENTARY FIGURES
SUPPLEMENTARY INFORMATION FOR Liver X Receptor α mediates hepatic triglyceride accumulation through upregulation of G0/G1 Switch Gene 2 (G0S2) expression I: SUPPLEMENTARY METHODS II: SUPPLEMENTARY FIGURES
The investigation of serum lipids and prevalence of dyslipidemia in urban adult population of Warangal district, Andhra Pradesh, India
 eissn: 09748369, www.biolmedonline.com The investigation of serum lipids and prevalence of dyslipidemia in urban adult population of Warangal district, Andhra Pradesh, India M Estari, AS Reddy, T Bikshapathi,
eissn: 09748369, www.biolmedonline.com The investigation of serum lipids and prevalence of dyslipidemia in urban adult population of Warangal district, Andhra Pradesh, India M Estari, AS Reddy, T Bikshapathi,
SUPPLEMENTARY INFORMATION
 doi:10.1038/nature12652 Supplementary Figure 1. PRDM16 interacts with endogenous EHMT1 in brown adipocytes. Immunoprecipitation of PRDM16 complex by flag antibody (M2) followed by Western blot analysis
doi:10.1038/nature12652 Supplementary Figure 1. PRDM16 interacts with endogenous EHMT1 in brown adipocytes. Immunoprecipitation of PRDM16 complex by flag antibody (M2) followed by Western blot analysis
SUPPLEMENTARY INFORMATION
 Supplementary Figures Supplementary Figure S1. Binding of full-length OGT and deletion mutants to PIP strips (Echelon Biosciences). Supplementary Figure S2. Binding of the OGT (919-1036) fragments with
Supplementary Figures Supplementary Figure S1. Binding of full-length OGT and deletion mutants to PIP strips (Echelon Biosciences). Supplementary Figure S2. Binding of the OGT (919-1036) fragments with
Supplemental information contains 7 movies and 4 supplemental Figures
 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 Supplemental information contains 7 movies and 4 supplemental Figures Movies: Movie 1. Single virus tracking of A4-mCherry-WR MV
1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 Supplemental information contains 7 movies and 4 supplemental Figures Movies: Movie 1. Single virus tracking of A4-mCherry-WR MV
SUPPLEMENTARY FIGURES AND TABLE
 SUPPLEMENTARY FIGURES AND TABLE Supplementary Figure S1: Characterization of IRE1α mutants. A. U87-LUC cells were transduced with the lentiviral vector containing the GFP sequence (U87-LUC Tet-ON GFP).
SUPPLEMENTARY FIGURES AND TABLE Supplementary Figure S1: Characterization of IRE1α mutants. A. U87-LUC cells were transduced with the lentiviral vector containing the GFP sequence (U87-LUC Tet-ON GFP).
Niacin Metabolism: Effects on Cholesterol
 Niacin Metabolism: Effects on Cholesterol By Julianne R. Edwards For Dr. William R. Proulx, PhD, RD Associate Professor of Nutrition and Dietetics In partial fulfillments for the requirements of NUTR342
Niacin Metabolism: Effects on Cholesterol By Julianne R. Edwards For Dr. William R. Proulx, PhD, RD Associate Professor of Nutrition and Dietetics In partial fulfillments for the requirements of NUTR342
Differential inhibition of macrophage foam-cell formation and atherosclerosis in mice by PPARα, β/δ, and γ
 Research article Related Commentary, page 1538 Differential inhibition of macrophage foam-cell formation and atherosclerosis in mice by PPARα, β/δ, and γ Andrew C. Li, 1 Christoph J. Binder, 2 Alejandra
Research article Related Commentary, page 1538 Differential inhibition of macrophage foam-cell formation and atherosclerosis in mice by PPARα, β/δ, and γ Andrew C. Li, 1 Christoph J. Binder, 2 Alejandra
Reviewers' comments: Reviewer #1 (Remarks to the Author):
 Reviewers' comments: Reviewer #1 (Remarks to the Author): In this manuscript, Song et al. identified FBXW7 as a new positive regulator for RIG-Itriggered type I IFN signaling pathway. The authors observed
Reviewers' comments: Reviewer #1 (Remarks to the Author): In this manuscript, Song et al. identified FBXW7 as a new positive regulator for RIG-Itriggered type I IFN signaling pathway. The authors observed
Lipid Metabolism in Familial Hypercholesterolemia
 Lipid Metabolism in Familial Hypercholesterolemia Khalid Al-Rasadi, BSc, MD, FRCPC Head of Biochemistry Department, SQU Head of Lipid and LDL-Apheresis Unit, SQUH President of Oman society of Lipid & Atherosclerosis
Lipid Metabolism in Familial Hypercholesterolemia Khalid Al-Rasadi, BSc, MD, FRCPC Head of Biochemistry Department, SQU Head of Lipid and LDL-Apheresis Unit, SQUH President of Oman society of Lipid & Atherosclerosis
Lipid metabolism in familial hypercholesterolemia
 Lipid metabolism in familial hypercholesterolemia Khalid Al-Rasadi, BSc, MD, FRCPC Head of Biochemistry Department, SQU Head of Lipid and LDL-Apheresis Unit, SQUH President of Oman society of Lipid & Atherosclerosis
Lipid metabolism in familial hypercholesterolemia Khalid Al-Rasadi, BSc, MD, FRCPC Head of Biochemistry Department, SQU Head of Lipid and LDL-Apheresis Unit, SQUH President of Oman society of Lipid & Atherosclerosis
Curriculum Vitae. Research Officer in Baker IDI Heart & Diabetes Institute from Aug
 Curriculum Vitae Yi Fu ( 付毅 ) (PhD, MBBS) Baker IDI Heart and Diabetes Institute 75 Commercial Road, Prahran, Melbourne, VIC 3004 Australia Phone: +61 3 8532 1288 Email: yi.fu@bakeridi.edu.au PROFILE Research
Curriculum Vitae Yi Fu ( 付毅 ) (PhD, MBBS) Baker IDI Heart and Diabetes Institute 75 Commercial Road, Prahran, Melbourne, VIC 3004 Australia Phone: +61 3 8532 1288 Email: yi.fu@bakeridi.edu.au PROFILE Research
Potential Atheroprotective Effects of Ixmyelocel-T Cellular Therapy. Kelly J. Ledford, Nikki Murphy, Frank Zeigler, Ronnda L.
 Potential Atheroprotective Effects of Ixmyelocel-T Cellular Therapy Kelly J. Ledford, Nikki Murphy, Frank Zeigler, Ronnda L. Bartel 1 Ixmyelocel-T, an expanded, autologous multicellular therapy cultured
Potential Atheroprotective Effects of Ixmyelocel-T Cellular Therapy Kelly J. Ledford, Nikki Murphy, Frank Zeigler, Ronnda L. Bartel 1 Ixmyelocel-T, an expanded, autologous multicellular therapy cultured
cholesterol structure Cholesterol FAQs Cholesterol promotes the liquid-ordered phase of membranes Friday, October 15, 2010
 cholesterol structure most plasma cholesterol is in the esterified form (not found in cells or membranes) cholesterol functions in all membranes (drives formation of lipid microdomains) cholesterol is
cholesterol structure most plasma cholesterol is in the esterified form (not found in cells or membranes) cholesterol functions in all membranes (drives formation of lipid microdomains) cholesterol is
Many G protein-coupled receptors (GPCRs) 2 are rapidly endocytosed after agonist binding, but the pathway of postendocytic
 THE JOURNAL OF BIOLOGICAL CHEMISTRY VOL. 282, NO. 40, pp. 29646 29657, October 5, 2007 2007 by The American Society for Biochemistry and Molecular Biology, Inc. Printed in the U.S.A. Hepatocyte Growth
THE JOURNAL OF BIOLOGICAL CHEMISTRY VOL. 282, NO. 40, pp. 29646 29657, October 5, 2007 2007 by The American Society for Biochemistry and Molecular Biology, Inc. Printed in the U.S.A. Hepatocyte Growth
TRAF6 ubiquitinates TGFβ type I receptor to promote its cleavage and nuclear translocation in cancer
 Supplementary Information TRAF6 ubiquitinates TGFβ type I receptor to promote its cleavage and nuclear translocation in cancer Yabing Mu, Reshma Sundar, Noopur Thakur, Maria Ekman, Shyam Kumar Gudey, Mariya
Supplementary Information TRAF6 ubiquitinates TGFβ type I receptor to promote its cleavage and nuclear translocation in cancer Yabing Mu, Reshma Sundar, Noopur Thakur, Maria Ekman, Shyam Kumar Gudey, Mariya
04_polarity. The formation of synaptic vesicles
 Brefeldin prevents assembly of the coats required for budding Nocodazole disrupts microtubules Constitutive: coatomer-coated Selected: clathrin-coated The formation of synaptic vesicles Nerve cells (and
Brefeldin prevents assembly of the coats required for budding Nocodazole disrupts microtubules Constitutive: coatomer-coated Selected: clathrin-coated The formation of synaptic vesicles Nerve cells (and
IP: anti-gfp VPS29-GFP. IP: anti-vps26. IP: anti-gfp - + +
 FAM21 Strump. WASH1 IP: anti- 1 2 3 4 5 6 FAM21 Strump. FKBP IP: anti-gfp VPS29- GFP GFP-FAM21 tail H H/P P H H/P P c FAM21 FKBP Strump. VPS29-GFP IP: anti-gfp 1 2 3 FKBP VPS VPS VPS VPS29 1 = VPS29-GFP
FAM21 Strump. WASH1 IP: anti- 1 2 3 4 5 6 FAM21 Strump. FKBP IP: anti-gfp VPS29- GFP GFP-FAM21 tail H H/P P H H/P P c FAM21 FKBP Strump. VPS29-GFP IP: anti-gfp 1 2 3 FKBP VPS VPS VPS VPS29 1 = VPS29-GFP
PCSK9 and its Role in LDL Receptor Regulation Muscat, Oman - 9 February 2019
 PCSK9 and its Role in LDL Receptor Regulation Muscat, Oman - 9 February 2019 Professor Gilles Lambert, PhD LaboratoireInserm U1188 Universitéde la Réunion Faculté de Médecine Saint Denis de la Réunion,
PCSK9 and its Role in LDL Receptor Regulation Muscat, Oman - 9 February 2019 Professor Gilles Lambert, PhD LaboratoireInserm U1188 Universitéde la Réunion Faculté de Médecine Saint Denis de la Réunion,
Figure S1. PMVs from THP-1 cells expose phosphatidylserine and carry actin. A) Flow
 SUPPLEMENTARY DATA Supplementary Figure Legends Figure S1. PMVs from THP-1 cells expose phosphatidylserine and carry actin. A) Flow cytometry analysis of PMVs labelled with annexin-v-pe (Guava technologies)
SUPPLEMENTARY DATA Supplementary Figure Legends Figure S1. PMVs from THP-1 cells expose phosphatidylserine and carry actin. A) Flow cytometry analysis of PMVs labelled with annexin-v-pe (Guava technologies)
Supplementary Information. MicroRNA-33b knock-in mice for an intron of sterol regulatory
 Supplementary Information MicroRNA-33b knock-in mice for an intron of sterol regulatory element-binding factor 1 (Srebf1) exhibit reduced HDL-C in vivo Takahiro Horie, Tomohiro Nishino, Osamu Baba, Yasuhide
Supplementary Information MicroRNA-33b knock-in mice for an intron of sterol regulatory element-binding factor 1 (Srebf1) exhibit reduced HDL-C in vivo Takahiro Horie, Tomohiro Nishino, Osamu Baba, Yasuhide
Lipoproteins Metabolism
 Lipoproteins Metabolism LEARNING OBJECTIVES By the end of this Lecture, the student should be able to describe: What are Lipoproteins? Describe Lipoprotein Particles. Composition of Lipoproteins. The chemical
Lipoproteins Metabolism LEARNING OBJECTIVES By the end of this Lecture, the student should be able to describe: What are Lipoproteins? Describe Lipoprotein Particles. Composition of Lipoproteins. The chemical
Cholesterol and its transport. Alice Skoumalová
 Cholesterol and its transport Alice Skoumalová 27 carbons Cholesterol - structure Cholesterol importance A stabilizing component of cell membranes A precursor of bile salts A precursor of steroid hormones
Cholesterol and its transport Alice Skoumalová 27 carbons Cholesterol - structure Cholesterol importance A stabilizing component of cell membranes A precursor of bile salts A precursor of steroid hormones
Supplementary Figure 1
 Supplementary Figure 1 YAP negatively regulates IFN- signaling. (a) Immunoblot analysis of Yap knockdown efficiency with sh-yap (#1 to #4 independent constructs) in Raw264.7 cells. (b) IFN- -Luc and PRDs
Supplementary Figure 1 YAP negatively regulates IFN- signaling. (a) Immunoblot analysis of Yap knockdown efficiency with sh-yap (#1 to #4 independent constructs) in Raw264.7 cells. (b) IFN- -Luc and PRDs
ab SREBP-2 Translocation Assay Kit (Cell-Based)
 ab133114 SREBP-2 Translocation Assay Kit (Cell-Based) Instructions for Use For analysis of translocation of SREBP-2 into nuclei. This product is for research use only and is not intended for diagnostic
ab133114 SREBP-2 Translocation Assay Kit (Cell-Based) Instructions for Use For analysis of translocation of SREBP-2 into nuclei. This product is for research use only and is not intended for diagnostic
Chapter 3. Expression of α5-megfp in Mouse Cortical Neurons. on the β subunit. Signal sequences in the M3-M4 loop of β nachrs bind protein factors to
 22 Chapter 3 Expression of α5-megfp in Mouse Cortical Neurons Subcellular localization of the neuronal nachr subtypes α4β2 and α4β4 depends on the β subunit. Signal sequences in the M3-M4 loop of β nachrs
22 Chapter 3 Expression of α5-megfp in Mouse Cortical Neurons Subcellular localization of the neuronal nachr subtypes α4β2 and α4β4 depends on the β subunit. Signal sequences in the M3-M4 loop of β nachrs
Bioluminescence Resonance Energy Transfer (BRET)-based studies of receptor dynamics in living cells with Berthold s Mithras
 Bioluminescence Resonance Energy Transfer (BRET)-based studies of receptor dynamics in living cells with Berthold s Mithras Tarik Issad, Ralf Jockers and Stefano Marullo 1 Because they play a pivotal role
Bioluminescence Resonance Energy Transfer (BRET)-based studies of receptor dynamics in living cells with Berthold s Mithras Tarik Issad, Ralf Jockers and Stefano Marullo 1 Because they play a pivotal role
ANSC/NUTR 618 LIPIDS & LIPID METABOLISM Lipoprotein Metabolism
 ANSC/NUTR 618 LIPIDS & LIPID METABOLISM Lipoprotein Metabolism I. Chylomicrons (exogenous pathway) A. 83% triacylglycerol, 2% protein, 8% cholesterol plus cholesterol esters, 7% phospholipid (esp. phosphatidylcholine)
ANSC/NUTR 618 LIPIDS & LIPID METABOLISM Lipoprotein Metabolism I. Chylomicrons (exogenous pathway) A. 83% triacylglycerol, 2% protein, 8% cholesterol plus cholesterol esters, 7% phospholipid (esp. phosphatidylcholine)
Supplementary Figure 1. Confocal immunofluorescence showing mitochondrial translocation of Drp1. Cardiomyocytes treated with H 2 O 2 were prestained
 Supplementary Figure 1. Confocal immunofluorescence showing mitochondrial translocation of Drp1. Cardiomyocytes treated with H 2 O 2 were prestained with MitoTracker (red), then were immunostained with
Supplementary Figure 1. Confocal immunofluorescence showing mitochondrial translocation of Drp1. Cardiomyocytes treated with H 2 O 2 were prestained with MitoTracker (red), then were immunostained with
MTC-TT and TPC-1 cell lines were cultured in RPMI medium (Gibco, Breda, The Netherlands)
 Supplemental data Materials and Methods Cell culture MTC-TT and TPC-1 cell lines were cultured in RPMI medium (Gibco, Breda, The Netherlands) supplemented with 15% or 10% (for TPC-1) fetal bovine serum
Supplemental data Materials and Methods Cell culture MTC-TT and TPC-1 cell lines were cultured in RPMI medium (Gibco, Breda, The Netherlands) supplemented with 15% or 10% (for TPC-1) fetal bovine serum
T H E J O U R N A L O F C E L L B I O L O G Y
 Supplemental material Chairoungdua et al., http://www.jcb.org/cgi/content/full/jcb.201002049/dc1 T H E J O U R N A L O F C E L L B I O L O G Y Figure S1. Expression of CD9 and CD82 inhibits Wnt/ -catenin
Supplemental material Chairoungdua et al., http://www.jcb.org/cgi/content/full/jcb.201002049/dc1 T H E J O U R N A L O F C E L L B I O L O G Y Figure S1. Expression of CD9 and CD82 inhibits Wnt/ -catenin
Macrophages are the major source of apolipoprotein E
 Distinct Cellular Loci for the ABCA1-Dependent and ABCA1-Independent Lipid Efflux Mediated by Endogenous Apolipoprotein E Expression Zhi H. Huang, Michael L. Fitzgerald, Theodore Mazzone Objective Macrophage
Distinct Cellular Loci for the ABCA1-Dependent and ABCA1-Independent Lipid Efflux Mediated by Endogenous Apolipoprotein E Expression Zhi H. Huang, Michael L. Fitzgerald, Theodore Mazzone Objective Macrophage
T H E J O U R N A L O F C E L L B I O L O G Y
 Supplemental material Jewell et al., http://www.jcb.org/cgi/content/full/jcb.201007176/dc1 T H E J O U R N A L O F C E L L B I O L O G Y Figure S1. IR Munc18c association is independent of IRS-1. (A and
Supplemental material Jewell et al., http://www.jcb.org/cgi/content/full/jcb.201007176/dc1 T H E J O U R N A L O F C E L L B I O L O G Y Figure S1. IR Munc18c association is independent of IRS-1. (A and
endomembrane system internal membranes origins transport of proteins chapter 15 endomembrane system
 endo system chapter 15 internal s endo system functions as a coordinated unit divide cytoplasm into distinct compartments controls exocytosis and endocytosis movement of molecules which cannot pass through
endo system chapter 15 internal s endo system functions as a coordinated unit divide cytoplasm into distinct compartments controls exocytosis and endocytosis movement of molecules which cannot pass through
Intracellular MHC class II molecules promote TLR-triggered innate. immune responses by maintaining Btk activation
 Intracellular MHC class II molecules promote TLR-triggered innate immune responses by maintaining Btk activation Xingguang Liu, Zhenzhen Zhan, Dong Li, Li Xu, Feng Ma, Peng Zhang, Hangping Yao and Xuetao
Intracellular MHC class II molecules promote TLR-triggered innate immune responses by maintaining Btk activation Xingguang Liu, Zhenzhen Zhan, Dong Li, Li Xu, Feng Ma, Peng Zhang, Hangping Yao and Xuetao
Cell Quality Control. Peter Takizawa Department of Cell Biology
 Cell Quality Control Peter Takizawa Department of Cell Biology Cellular quality control reduces production of defective proteins. Cells have many quality control systems to ensure that cell does not build
Cell Quality Control Peter Takizawa Department of Cell Biology Cellular quality control reduces production of defective proteins. Cells have many quality control systems to ensure that cell does not build
Scavenger receptor class B type I affects cholesterol homeostasis by magnifying cholesterol flux between cells and HDL
 Scavenger receptor class B type I affects cholesterol homeostasis by magnifying cholesterol flux between cells and HDL Margarita de la Llera-Moya, 1, * Margery A. Connelly, Denise Drazul,* Seth M. Klein,
Scavenger receptor class B type I affects cholesterol homeostasis by magnifying cholesterol flux between cells and HDL Margarita de la Llera-Moya, 1, * Margery A. Connelly, Denise Drazul,* Seth M. Klein,
