Emerin inhibits Lmo7 binding to the Pax3 and MyoD promoters and expression of myoblast proliferation genes
|
|
|
- Jean Sharp
- 6 years ago
- Views:
Transcription
1 Research Article 1691 Emerin inhibits Lmo7 binding to the Pax3 and MyoD promoters and expression of myoblast proliferation genes Zinaida Dedeic 1, Maureen Cetera 1, Tatiana V. Cohen 2 and James M. Holaska 1,3,4, * 1 University of Chicago Committee on Developmental Biology, 920 East 58th Street, Chicago IL 60637, USA 2 Children s National Medical Center, Center for Genetic Medicine, 111 Michigan Avenue, Washington DC , USA 3 Department of Medicine, Section of Cardiology, The University of Chicago, 947 East 58th Street, Chicago, IL 60637, USA 4 Committee on Genetics, Genomics and Systems Biology, 5812 S. Ellis Street, Chicago IL 60637, USA *Author for correspondence (jholaska@uchicago.edu) Accepted 24 January , Published by The Company of Biologists Ltd doi: /jcs Summary X-linked Emery Dreifuss muscular dystrophy (X-EDMD) is caused by mutations in the inner nuclear membrane protein emerin. Previous studies have shown that emerin binds to and inhibits the activity of LIM domain only 7 (Lmo7), a transcription factor that regulates the expression of genes implicated in X-EDMD. Here, we analyzed Lmo7 function in C2C12 myoblast differentiation and its regulation by emerin. We found that Lmo7 was required for proper myoblast differentiation. Lmo7-downregulated myoblasts exhibited reduced expression of Pax3, Pax7, Myf5 and MyoD, whereas overexpression of GFP Lmo7 increased the expression of MyoD and Myf5. Upon myotube formation, Lmo7 shuttled from the nucleus to the cytoplasm, concomitant with reduced expression of MyoD, Pax3 and Myf5. Importantly, we show that Lmo7 bound the Pax3, MyoD and Myf5 promoters both in C2C12 myoblasts and in vitro. Because emerin inhibited Lmo7 activity, we tested whether emerin competed with the MyoD promoter for binding to Lmo7 or whether emerin sequestered promoter-bound Lmo7 to the nuclear periphery. Supporting the competition model, emerin binding to Lmo7 inhibited Lmo7 binding to and activation of the MyoD and Pax3 promoters. These findings support the hypothesis that the functional interaction between emerin and Lmo7 is crucial for temporally regulating the expression of key myogenic differentiation genes. Key words: Emerin, Lmo7, Emerin Dreifuss muscular dystrophy, Myogenic differentiation, Nuclear envelope Introduction The nuclear envelope is composed of two lipid bilayers, the outer nuclear membrane, which is contiguous with the endoplasmic reticulum, and the inner nuclear membrane. The inner nuclear membrane contains a variety of integral membrane proteins. Among the first inner nuclear membrane proteins identified were members of the LEM domain family of nuclear proteins, which are named for LAP2, emerin and MAN1 (Hetzer et al., 2005). The LEM domain mediates binding to the chromatin-associated protein barrier-to-autointegration factor (BAF) (Lee et al., 2001; Margalit et al., 2007; Shumaker et al., 2001). Emerin also binds directly to A-type lamins (Holaska et al., 2003; Lee et al., 2001). Lamins are nuclear intermediate filament proteins that form a nuclear-envelopeassociated lattice, which is required for nuclear envelope integrity (Vlcek and Foisner, 2007). A-type lamins are also required for localization of emerin to the inner nuclear membrane (Hetzer et al., 2005). Mutations in the gene encoding emerin cause X-linked Emery Dreifuss muscular dystrophy (X-EDMD), which is characterized by progressive skeletal muscle wasting, life-threatening irregular heart rhythms and contractures of major tendons (Vlcek and Foisner, 2007). Muscle biopsies from both X-EDMD patients and mice lacking emerin show increased expression of muscle regeneration pathway components (Bakay et al., 2006; Melcon et al., 2006), suggesting that emerin regulates the differentiation of muscle stem cells, also called satellite cells, during muscle regeneration. Further supporting a role for emerin in myogenic differentiation, emerin-downregulated myoblasts exhibit significant differentiation defects (Frock et al., 2006). These findings support a model in which the skeletal muscle phenotype of EDMD is caused by the inability of satellite cells to differentiate properly and repair the damaged muscle. Muscle regeneration is a multi-step process that repairs damaged muscle. Upon muscle injury, satellite cells are activated and begin proliferating. A small fraction of these cells will retain their original gene expression program and replenish the satellite cell niche. The remaining activated satellite cells, which express the transcription factors Pax3 and Myf5, will further differentiate into myoblasts that rapidly proliferate and express Pax3, Pax7, Myf5 and MyoD (Kuang and Rudnicki, 2008). Pax3 and Pax7 are satellite cell specification genes that help define the satellite cell niche (Kuang and Rudnicki, 2008) and directly regulate MyoD and Myf5 expression (Bajard et al., 2006; Brunelli et al., 2007; Maroto et al., 1997; Tajbakhsh et al., 1997). These proliferating myoblasts will then terminally differentiate and form myocytes, which express myogenin and other myocyte-specific genes. Myocytes do not express Pax3 or Myf5 (Kuang and Rudnicki, 2008). The committed myocytes will then fuse with the damaged myofiber and regenerate the muscle. The MyoD and Pax3 promoters bind many regulatory proteins including basal transcription factors (Leibham et al., 1994; Pedraza- Alva et al., 1994; Tapscott et al., 1992), myogenic transcription
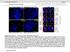
2 (10) factors (Kuang and Rudnicki, 2008; L Honore et al., 2007; Leibham et al., 1994; Pownall et al., 2002; Tapscott et al., 1992) and transcription repressors (Lee et al., 2006). There are three regulatory elements in the MyoD promoter: the proximal regulatory region (PRR; 275 to +20 nucleotides, relative to the transcription start site) (L Honore et al., 2007; Pownall et al., 2002; Tapscott et al., 1992), the distal regulatory region (DRR; 5.39 kb to 4.67 kb) (Tapscott et al., 1992) and the core enhancer (CER; 20 kb) (Goldhamer et al., 1992; Kucharczuk et al., 1999). The DRR and PRR primarily associate with transcription activators and the CER primarily associates with transcription repressors. The Pax3 promoter contains four known cis-regulatory elements, sites I, II, A and B. The POU transcription factors Oct1 and Brn2 bind sites I and II and activate Pax3 expression (Zhu and Pruitt, 2005). The TALE homeodomain transcription factors Pbx and Prep1 bind sites A and B and repress Pax3 expression (Zhu and Pruitt, 2005). However, the molecular mechanisms underlying the regulation of Pax3 expression by these transcription regulators during differentiation remain poorly understood. We previously identified Lmo7 as an emerin-binding protein by affinity chromatography of HeLa nuclear lysates (Holaska et al., 2006). Genomic deletion of Lmo7 plus eight exons of an upstream gene, Uchl3, causes myopathic phenotypes in mice (Semenova et al., 2003). Lmo7 regulates the expression of many muscle genes (Holaska et al., 2006), including components of the proposed emerin-regulated muscle regeneration pathway (Bakay et al., 2006; Holaska et al., 2006; Melcon et al., 2006). Interestingly, expression of wild-type emerin inhibits Lmo7 activation of predicted muscle regeneration genes and expression of an emerin mutant that fails to bind Lmo7 has no effect (Holaska et al., 2006). Thus we hypothesized that Lmo7 regulates myogenic differentiation and muscle regeneration. We further hypothesized that the interaction between emerin and Lmo7 is important for proper regulation of myogenic differentiation gene expression. There are three reported splice variants of Lmo7 (Fig. 1A) (Lindvall et al., 2005; Rozenblum et al., 2002; Semenova et al., 2003). Northern blotting revealed that skeletal muscle expresses only the largest Lmo7 isoform (Lmo7A; 7.2 kb), whereas the 6-kb isoform (Lmo7B) is expressed in all tissues except skeletal muscle (Rozenblum et al., 2002; Semenova et al., 2003). The heart expresses the 7.2-kb isoform in mice and a shorter 4.2-kb isoform in mice and humans (Lmo7C) (Rozenblum et al., 2002; Semenova et al., 2003). Lmo7A is the only isoform that contains the predicted transactivation domain (TAD; Fig. 1A). Because Lmo7A is the only isoform containing the predicted TAD and Lmo7A is expressed in skeletal muscle, we hypothesize that Lmo7A regulates musclespecific gene expression. Supporting this hypothesis, Lmo7A expression during development is coincident with the two waves of skeletal muscle differentiation and development at embryonic day (e) 11.5 and e15.5 (Kurihara et al., 2002; Rozenblum et al., 2002; Semenova et al., 2003). Collectively, these observations suggest that Lmo7A regulates genes important for myogenic differentiation and muscle development. Here, we analyzed C2C12 myoblast differentiation in Lmo7- downregulated myoblasts to test these hypotheses. C2C12 myotube formation was inhibited in Lmo7-downregulated myoblasts. Lmo7- Fig. 1. Lmo7 relocalizes from the nucleus to the cytoplasm during C2C12 myoblast differentiation. (A) Diagram of the domain structure of Lmo7 splice variants. The Lmo7 C-terminal fragment used for binding studies is also shown. The epitopes for Lmo7 sera 4001, 5688 and 5690 used in these studies is also shown. NES, nuclear export sequence; NLS, nuclear localization sequence; TAD, transactivation domain; PDZ, PDZ domain; LIM, LIM domain. (B) C2C12 myoblast differentiation was induced by addition of DM, and the localization of Lmo7 and emerin were monitored by immunofluorescence microscopy using antibodies against Lmo7 (serum 4001) or emerin. d, days post differentiation. Scale bars: 10 m. (C) Subcellular fractionation studies were performed on C2C12 myoblasts or differentiated myotubes. SDS-PAGE samples were run with whole cell lysates (L, Load), the nuclear fraction (N) and the cytosolic fraction (C), transferred onto nitrocellulose, and localization of Lmo7 was detected with Lmo7 antibodies (serum 5690). (D) Quadricep muscles from wildtype mice were stained with Lmo7 antibodies (serum 5688) or dystrophin antibodies (Dys), which marks the plasma membrane. Lmo7 localizes to the nuclei of putative myogenic precursors (arrows) and the plasma membrane of mature muscle (arrowhead).
3 Lmo7 regulates myogenic differentiation 1693 downregulated myoblasts also exhibited reduced expression of crucial myogenic differentiation markers, including Pax3, Pax7, Myf5 and MyoD. Interestingly, we found that Lmo7 binds to the promoters of Pax3, MyoD and Myf5, suggesting that Lmo7 directly regulates their expression. Lmo7 binding to and activation of these promoters was inhibited by emerin binding to Lmo7, providing a potential mechanism for how emerin inhibits Lmo7 activity. Results Lmo7 moves from the nucleus to the cytoplasm during myoblast differentiation We have shown previously that Lmo7 localizes to the nucleus, cytoplasm and cell surface in HeLa cells and actively shuttles between these compartments (Holaska et al., 2006). Thus we monitored Lmo7 localization during C2C12 myoblast differentiation. C2C12 myoblasts were induced to differentiate by the addition of differentiation medium (DM) and Lmo7 localization was monitored every day for 8 days by confocal immunofluorescence microscopy. Antibodies against emerin were used to determine whether Lmo7 and emerin colocalized at the nuclear envelope, as predicted from our previous work. Before differentiation Lmo7 localized primarily in the nucleoplasm of proliferating myoblasts, with a portion of Lmo7 localized at the nuclear envelope (Fig. 1B). By days 2 and 3 of differentiation a substantial portion of Lmo7 localized to the nuclear envelope, where it colocalized with emerin (Fig. 1B). Between day 4 and 6 of myogenic differentiation there was a shift in Lmo7 localization from the nucleus to the cytoplasm (Fig. 1B), which appeared to occur in a stepwise manner: at 4 days of differentiation the Lmo7 nucleoplasmic localization was lost and Lmo7 localized to the nuclear envelope and cytoplasm (Fig. 1B), a substantial amount of nuclear-envelope-associated Lmo7 was then lost by day five and Lmo7 localized exclusively to the cytoplasm by 6 days of differentiation (Fig. 1B). We next wanted to determine whether Lmo7 localized similarly in skeletal muscle. We performed immunofluorescence microscopy on mouse quadriceps and found that Lmo7 localized predominantly to the plasma membrane of mature fibers (Fig. 1D, arrowhead) and at the nuclear envelope of putative myogenic progenitors, which are located between the muscle fiber and the basal lamina (Fig. 1D, arrows). To confirm the relocalization of Lmo7 in differentiating C2C12 myoblasts we performed biochemical fractionation on C2C12 myoblasts and myotubes. In undifferentiated myoblasts, 96.4±5.7% of Lmo7 localized to the nucleus (Fig. 1C). In differentiated myotubes, 32.2±11.8% of Lmo7 was localized to the nucleus and 67.8±11.9% localized to the cytoplasm. A doublet was recognized by Lmo7 antibodies in the whole cell lysates (L) and the nuclear fractions (N), whereas only one band was seen in the cytoplasm (Fig. 1C). This suggests that the nucleus contains both post-translationally modified Lmo7 and unmodified Lmo7, whereas the cytoplasm contains only posttranslationally modified Lmo7. We next examined the expression of Lmo7 during myogenic differentiation. Expression of MyoD, myogenin and myosin heavy chain (MyHC) was also monitored to confirm proper myogenic differentiation. C2C12 myoblasts were incubated with DM for the indicated times and cells from each timepoint were suspended in sample buffer, separated by SDS-PAGE and subjected to western blotting with antibodies against the indicated proteins (Fig. 2A). Densitometry was then performed to quantify changes in protein expression. Lmo7 protein expression increased 1.6-fold after 1 day in DM and Lmo7 was upregulated 2.5-fold by two days after DM addition (Fig. 2A). Lmo7 remained at this level throughout the rest of differentiation. Emerin protein expression increased 1.3-fold after 2 days in DM, but returned to baseline by day 3. To determine whether mrna expression also showed a similar temporal expression pattern, we isolated RNA from C2C12 differentiating myoblasts everyday for 7 days and analyzed their expression by quantitative real-time PCR (qpcr). Expression of Lmo7, MyoD, myogenin and MyHC mrna was normalized to that of GAPDH. Similar to Lmo7 protein expression, Lmo7 mrna increased 2-fold after 1 day in DM (Fig. 2B). However, Lmo7 mrna levels continued to increase during differentiation with highest expression at day 3 (Fig. 2B). MyoD, myogenin and MyHC all had increased mrna expression during differentiation (Fig. 2C E). The highest expression of MyoD mrna and protein was seen after Lmo7 levels peaked, consistent with our hypothesis that Lmo7 activates MyoD expression. Importantly, the temporal activation of MyoD, myogenin and MyHC at both the protein and mrna levels is consistent with proper C2C12 myogenic differentiation. To confirm that only the largest muscle-specific Lmo7 isoform (Lmo7A) was expressed during C2C12 differentiation we reversetranscribed RNA from differentiating C2C12 myoblasts and used the resulting cdna for PCR using primers that spanned the region spliced out of Lmo7B and Lmo7C (Fig. 2F). Cloned Lmo7A plasmid DNA was used as a positive control. The majority product produced throughout differentiation was ~1100 bp (Fig. 2F), consistent with the presence of Lmo7A. A similar size band was seen in the positive control reaction. There was a faint band migrating at approximately 175 bp in samples from days 2 4 (Fig. 2F). The size of the predicted PCR product for Lmo7B or C is 101 bp. Thus we conclude that Lmo7A is the predominant Lmo7 isoform expressed throughout C2C12 differentiation. The relocalization of Lmo7 to the nuclear periphery coincides with increased emerin expression (Fig. 1B and Fig. 2A). Thus we tested whether increased emerin expression was able to recruit Lmo7 to the nuclear envelope by expressing GFP or GFP emerin in C2C12 myoblasts. Endogenous Lmo7 localized predominantly to the nucleoplasm in myoblasts expressing GFP (Fig. 2G), as expected. Expression of GFP emerin caused a dramatic relocalization of endogenous Lmo7 from the nucleoplasm to the nuclear envelope (Fig. 2G). These results suggest that increased emerin expression between 2 and 4 days of myoblast differentiation causes the relocalization of Lmo7 to the nuclear envelope at this time. Lmo7 is a transcription activator that regulates the expression of myoblast proliferation genes and myoblast differentiation To test whether Lmo7 regulates myoblast differentiation, Lmo7 short hairpin RNA (shrna) or control shrna plasmids were expressed in C2C12 myoblasts, and differentiation was induced by the addition of DM. The shrna plasmids express GFP from an internal ribosome entry site to mark the shrna containing myoblasts and myotubes. Myotube formation was assessed by counting the number of GFPpositive myotubes that had >3 nuclei and expressed myosin heavy chain after 5 days in DM. A total of GFP-positive cells were counted for each experiment (n 4); 53.0±12.9% of control myoblasts successfully formed myotubes after 5 days. Only 10.7±3.7% of Lmo7-downregulated myoblasts formed myotubes (Fig. 3A), demonstrating that Lmo7 is important for myotube formation. We
4 (10) also noticed that at 3 days after addition of DM 82±4.9% of control myoblasts became elongated, which is a hallmark of early myogenic differentiation. Interestingly, only 20.3±9.0% of Lmo7-downregulated myoblasts exhibited an elongated phenotype, suggesting Lmo7 downregulation delays the early steps in myoblast differentiation, which results in defective myotube differentiation. To test further whether Lmo7 downregulation caused differentiation delays, we examined the temporal expression of MyoD, myogenin and MyHC during differentiation of myoblasts expressing Lmo7 shrna or control shrna. Lmo7-downregulation in myoblasts caused a 64% decrease in Lmo7 expression. Lmo7 remained downregulated throughout differentiation (Fig. 3B), although Lmo7 expression increased after day 3 reaching a 33% decrease in Lmo7 expression by day 4. Importantly, proper activation and expression of MyoD, myogenin and MyHC were all delayed by 1 to 3 days (Fig. 3B). MyoD was reduced by 42% at 1 day of differentiation and did not reach wild-type expression levels until 4 days after differentiation. Myogenin and MyHC protein levels were reduced 83 89% at day 1 and 2, respectively. Myogenin and MyHC expression began to recover by day 4 (29% decreased), which is when Lmo7 expression begins to recover. Thus Lmo7 is required for proper temporal expression of myogenic factors important for differentiation. Collectively, the above data suggested that Lmo7 regulates the expression of genes important for myoblast proliferation or early steps in myogenic differentiation, or both. To test this hypothesis, we measured mrna levels of Pax3, Pax7, Myf5, MyoD and MyHC in C2C12 myoblasts expressing Lmo7 shrna or control shrna. RNA was isolated from Lmo7-downregulated or control myoblasts and measured by qpcr. Lmo7 downregulation resulted in a 60% decrease in Lmo7 mrna expression. Importantly, Lmo7- downregulated myoblasts had substantially reduced expression of Myf5 (50%), MyoD (60%), Pax3 (53%) and Pax7 (55%; Fig. 3C, n 5). There was no substantial change in MyHC (Fig. 3C) or myogenin (data not shown) expression in Lmo7-downregulated myoblasts. To confirm that Lmo7 activates the expression of myogenic proliferation and early differentiation genes, we expressed GFP or GFP Lmo7A in C2C12 myoblasts and measured the mrna expression of Myf5 and MyoD by qpcr. GFP Lmo7A expression increased expression of Myf5 6.2-fold and MyoD 4.1- fold (Fig. 3D), supporting our hypothesis that Lmo7 activates the expression of crucial myogenic differentiation genes. Thus, we conclude that the differentiation delay seen in Lmo7-downregulated myoblasts is caused by the failure to activate the expression of crucial myogenic genes. Lmo7 binds the promoters of myoblast proliferation genes We next tested whether Lmo7 binds the promoters of MyoD, Pax3 and Myf5. Chromatin immunoprecipitation (ChIP) assays were performed with anti-lmo7 antibodies (serum 5688; J.M.H. and K. Fig. 2. Lmo7 and emerin expression during differentiation. (A) C2C12 myoblasts were induced to differentiate by addition of DM, and samples were collected every day for 6 days. Samples were subjected to SDS-PAGE, transferred onto nitrocellulose and western blotted for the indicated proteins. -tubulin was used as a loading control. (B E) mrna expression was monitored during C2C12 myoblast differentiation by qpcr. Expression of each mrna was normalized to that of GADPH and plotted relative to the expression of each transcript in proliferating myoblasts. Results are means+s.e.m. (F) PCR was used to detect Lmo7 isoform expression during differentiation. cdna was generated from RNA isolated from differentiating myoblasts everyday for 7 days. Primers that lie outside the region spliced out of Lmo7B and Lmo7C were used for to test whether Lmo7A, Lmo7B or Lmo7C was present during differentiation. If Lmo7B or Lmo7C is expressed a 100 bp band would be expected; if Lmo7A is expressed an 1105 bp band would be expected. Lmo7A plasmid DNA was used as a positive control. (G) C2C12 myoblasts were transfected with GFP or GFP emerin and 36 hours later endogenous Lmo7 was localized using serum 4001.
5 Lmo7 regulates myogenic differentiation 1695 Wilson, unpublished results,) or pre-immune serum (negative control) to test whether Lmo7 binds to the Pax3, Myf5 or MyoD promoters in C2C12 myoblasts (n 4). Multiple sets of primers within the promoter region of each of these genes were used to test whether Lmo7 bound their promoters (Fig. 4A). The myogenin promoter was used as a negative control. Anti-Lmo7 antibodies only efficiently pulled down the region of the Pax3 promoter from 1135 to 722 nucleotides, which contains two known regulatory elements (sites B and II), and failed to interact with promoter regions downstream of these sites (Fig. 4A). Lmo7 efficiently pulled down the region of the MyoD promoter from 478 to 288 nucleotides (Fig. 4A), which overlaps with the PRR, a positive regulatory element, but failed to pull-down the DRR and CER, two other well-characterized regulatory elements (Fig. 4A). Lmo7 also efficiently immunoprecipitated the region of the Myf5 promoter from 269 to 111 nucleotides, which overlaps with the promoter region of Myf5, but failed to pull-down the enhancer region (Fig. 4A). Two different primer sets that span the known regulatory elements of the myogenin promoter were used to test whether Lmo7 bound the myogenin promoter. Lmo7 failed to immunoprecipitate myogenin, as expected (Fig. 4A). Pre-immune serum failed to pull-down Pax3, Myf5, MyoD or myogenin promoters. We conclude that Lmo7 interacts with the Pax3, Myf5 and MyoD promoters and preferentially targets active regulatory elements. We next tested whether Lmo7 bound directly to the MyoD or Pax3 promoters. Electrophoretic mobility-shift assays (EMSAs) were used to test whether Lmo7 bound to the Pax3 and MyoD promoters. Recombinant purified Lmo7 (5 M) from the C-terminal region (Lmo7 C-term; residues ) or GST Lmo7C1 (residues ; negative control) was incubated with 200 ng MyoD promoter (nucleotides 478 to 288) in EMSA binding buffer. Non-denaturing PAGE was performed on each sample and DNA was detected with SYBR Green. MyoD promoter incubation with Lmo7 C-term substantially retarded MyoD promoter migration (Fig. 4B, n 5), demonstrating that the Lmo7 C-term binds directly to the MyoD promoter. GST Lmo7C1 failed to bind the MyoD promoter, showing that the interaction between Lmo7 C-term and MyoD was specific. Lmo7 C-term (5 M) was incubated with 200 ng myogenin promoter (nucleotides 289 to +7) as a negative control. Incubation of Lmo7 C-term with the myogenin promoter failed to retard myogenin promoter migration (Fig. 4B), further confirming that the interaction of Lmo7 C-term with MyoD was promoter-specific. The Pax3 promoter (200 ng, nucleotides 1135 to 722) was also incubated with 5 M Lmo7 C-term or GST Lmo7C1 in EMSA binding buffer to test whether Lmo7 C-term bound directly to Pax3. Lmo7 C-term efficiently bound the Pax3 promoter (Fig. 4C), whereas GST-Lmo7C1 failed to bind the Pax3 promoter. Emerin binding inhibits Lmo7 binding to Pax3 and MyoD promoters Emerin inhibits the activity of a number of transcription regulators, including Lmo7, -catenin and GCL (Holaska et al., 2003; Holaska et al., 2006; Holaska and Wilson, 2006; Markiewicz et al., 2006). There are two models that can explain how emerin inhibits transcription regulator activity. The first is that emerin binds the transcription regulator at the nuclear envelope and inhibits its binding to DNA regulatory elements. The second model is that emerin binds to transcription regulators and recruits the regulators and the associated chromatin to the nuclear envelope. The repressive environment of the nuclear envelope would then be predicted to cause the associated chromatin to adopt a repressed structure. Because we had a transcription activator that bound emerin (Lmo7), and we had identified the promoter regions to which Lmo7 binds (in Pax3 and MyoD), we were able to directly test these models. The MyoD promoter (200 ng, 478 to 288) and Lmo7 C-term (1.5 M) was incubated in the presence or absence of 4.2 M wild-type emerin. These concentrations of emerin and Lmo7 C- term were used to ensure that >90% of Lmo7 C-term will be emerin-bound (Holaska et al., 2006). Incubation of Lmo7 C-term with wild-type emerin inhibited Lmo7 C-term binding to the MyoD promoter (Fig. 5A, n 7). To test whether emerin binding to Lmo7 C-term was required for inhibiting Lmo7 C-term binding to the MyoD promoter, we incubated an emerin mutant that fails to bind Lmo7 C-term (emerin m179) (Holaska et al., 2006) with Lmo7 C- Fig. 3. Downregulation of Lmo7 inhibits myoblast differentiation and expression of myoblast proliferation factors. (A) C2C12 myoblasts were transfected with control shrna or Lmo7 shrna, treated with differentiation medium for 5 days and the number of GFP-positive myotubes were counted. The shrna constructs express GFP from an internal ribosomal entry site, which was used to mark transfected cells. (B) Protein expression of Lmo7, MyoD, myogenin, MyHC and -tubulin were monitored throughout differentiation in C2C12 myoblasts transfected with Lmo7 shrna or control shrna. Levels of Lmo7, MyoD, myogenin and MyHC were normalized to -tubulin. Protein expression of myogenic factors is delayed by one to three days in Lmo7- downregulated cells. (C) The expression of Lmo7, Pax3, Pax7, Myf5, MyoD and MyHC were analyzed by qpcr 2 days after transfection with control shrna or Lmo7 shrna. Expression of each transcript was normalized to that of GAPDH. (D) GFP Lmo7 was transfected in C2C12 myoblasts and protein levels of MyoD and Myf5 were measured 48 hours after transfection to test whether Lmo7 activated their expression. MyoD and Myf5 protein levels increased 4- and 6-fold, respectively. Results in A, C and D are means+s.e.m.
6 (10) Fig. 4. Lmo7 binds myoblast proliferation gene promoters. (A) ChIP assays were performed on C2C12 myoblasts using 5 g anti-lmo7 antibodies (serum 5688). Regions of the Pax3, MyoD, Myf5 and myogenin promoters were amplified using primers specific for selected regions within each promoter, as denoted by the arrows. Pre, Preimmune sera; Im, Immune sera. (B) Lmo7 C-term (residues ) binds directly to the MyoD promoter. 5 M recombinant Lmo7 C-term or 5 M GST Lmo7C1 (residues ) was incubated for 30 minutes with the MyoD promoter (nucleotides 478 to 288) in EMSA binding buffer. Non-denaturing PAGE was performed and DNA was detected with SYBR Green. 5 M Lmo7 C-term was incubated with the myogenin promoter (nucleotides 289 to +7) as a negative control. (C) Lmo7 C-term binds the Pax3 promoter. EMSA assays were performed by incubating 200 ng Pax3 promoter (nucleotides 1135 to 722) with increasing concentrations of purified, recombinant Lmo7 C- term or GST Lmo7C1 in EMSA binding buffer. Non-denaturing PAGE was performed and DNA was detected with SYBR Green. The bar represents controls without Lmo7. term and the MyoD promoter. Incubation of Lmo7 C-term with emerin m179 failed to inhibit Lmo7 C-term binding to the MyoD promoter (Fig. 5A), demonstrating that emerin binding to Lmo7 C- term competes with Lmo7 C-term binding to the MyoD promoter. Importantly, neither wild-type emerin nor emerin m179 interacted with the MyoD promoter (Fig. 5A). We next tested whether wildtype emerin or emerin m179 inhibits Lmo7 C-term binding to the Pax3 promoter (nucleotides 1135 to 722). Addition of 1.7 M or 4.2 M wild-type emerin with 1.5 M Lmo7 C-term and 200 ng Pax3 promoter blocked Lmo7 C-term binding to the Pax3 promoter (Fig. 5B, n 3). Incubation of 1.5 M Lmo7 C-term with 1.7 M or 4.2 M emerin mutant m179 had no effect on Lmo7 C- term binding to the Pax3 promoter (Fig. 5B). Neither wild-type emerin nor emerin mutant m179 bound to the Pax3 promoter. We hypothesized that expression of wild-type emerin would inhibit MyoD and Myf5 expression because emerin inhibits Lmo7 promoter binding. Furthermore, we predicted that expression of an emerin mutant that fails to bind Lmo7 would have no effect on MyoD or Myf5 expression. Hence, GFP emerin or a GFP emerin disease-causing mutant (P183H), which fails to bind Lmo7 (Holaska et al., 2006), was expressed in C2C12 myoblasts and the levels of Myf5 and MyoD mrna were measured by qpcr. Expression of GFP emerin decreased Myf5 and MyoD mrna expression 1.8-fold (Fig. 5C, n 4). Importantly, expression of emerin P183H had no effect on Myf5 and MyoD expression, demonstrating that emerin binding to Lmo7 is necessary for inhibition of Lmo7 activity. Both GFP emerin and GFP emerin P183H were expressed at similar levels: 12.28±4.0- and 13.85±0.95-fold over endogenous emerin levels, respectively. Thus emerin binding to Lmo7 inhibits the binding of Lmo7 to myoblast proliferation promoters in vitro and the expression of myoblast proliferation genes in vivo. We next examined the expression of MyoD in C2C12 myoblasts downregulated for Lmo7, emerin, or both emerin and Lmo7, to confirm that emerin inhibits Lmo7 activity. Downregulation of Lmo7 decreased MyoD expression 42% (Fig. 5D), as expected. Emerin downregulation increased expression of MyoD 1.5-fold (Fig. 5D), consistent with emerin inhibiting MyoD expression. C2C12 myoblasts downregulated for both Lmo7 and emerin rescued the expression of MyoD (Fig. 5D). Increased MyoD expression was calculated in both emerin-downregulated and Lmo7-downregulated backgrounds and in both cases MyoD expression was at normal myoblast levels (Fig. 5D). To confirm these results we performed gene expression profiling on primary emerin-null mouse myoblasts. Expression of Pax7, Pax3 and Myf5 was increased 2.9-fold, 1.7-fold and 2.9-fold, respectively. We next
7 Lmo7 regulates myogenic differentiation 1697 Fig. 5. Emerin binding to Lmo7 inhibits Lmo7 binding to and activation of myoblast proliferation gene promoters. (A) Emerinbinding inhibits Lmo7 binding to the MyoD promoter. 1.5 M Lmo7 C-term was incubated with 200 ng MyoD promoter (nucleotides 478 to 288) in the absence or presence of either 4.2 M wild-type (WT) emerin (residues 1 222) or emerin mutant m179 (residues 1 222), which fails to bind Lmo7 (Holaska et al., 2006). Only wild-type emerin inhibited Lmo7 binding to the MyoD promoter, as evidenced by the shift in migration of the MyoD promoter (compare lane 3 with lane 2). 4.2 M wild-type emerin (1 222) or emerin mutant m179 (1 222) was also incubated with the MyoD promoter alone to confirm they do not bind the MyoD promoter. (B) Emerin-binding inhibits Lmo7 binding to the Pax3 promoter. 1.5 M Lmo7 C-term was incubated with 200 ng of the Pax3 promoter (nucleotides 1135 to 722) in the absence or presence of either 1.7 M (lane 3) or 4.2 M (lane 4) wild-type emerin (1 222) or 1.7 M (lane 5) or 4.2 M (lane 6) emerin mutant m179. Wild-type emerin and emerin m179 were incubated with the Pax3 promoter to confirm that they do not bind the Pax3 promoter. (C) Emerin binding to Lmo7 inhibits the expression of MyoD and Myf5. Plasmids encoding GFP, GFP emerin WT, or GFP emerin P183H, which fails to bind Lmo7 (Holaska et al., 2006), were transfected into C2C12 myoblasts. After 48 hours RNA was extracted and qpcr was performed using primers specific for MyoD and Myf5. mrna levels were normalized to the GAPDH mrna levels and compared with those in GFP transfected cells. (D) Reduction of both Lmo7 and emerin rescues the expression of MyoD in Lmo7-downregulated or emerin-downregulated myoblasts. Control shrna, Lmo7 shrna, psuper-emr or psuper-emr and Lmo7 shrna were transfected into C2C12 myoblasts. RNA was isolated 48 hours post-transfection and qpcr was performed to measure MyoD expression. mrna levels were normalized to that of GADPH and compared with MyoD expression in control shrnatransfected cells. Lmo7 shrna rescues MyoD expression in emerindownregulated myoblasts and emerin-downregulation rescues MyoD expression in Lmo7-downregulated myoblasts. (E) Emerin-null primary myoblasts were transfected with GFP, GFP emerin or GFP emerin P183H. RNA was extracted after 40 hours, qpcr was performed using primers specific for Pax3, Pax7, MyoD or Myf5 and expression of each transcript was normalized to GAPDH mrna levels. Results in C E are means±s.e.m. tested whether expression of wild-type emerin, but not the emerin mutant that cannot bind Lmo7, rescued expression of Pax3, Pax7 and Myf5 in the emerin-null myoblasts. We expressed GFP, GFP emerin or GFP emerin P183H in emerin-null myoblasts and measured expression of MyoD, Myf5, Pax3 and Pax7. The transfection efficiency of each construct was ~30 40% (data not shown). Similar to the findings upon overexpression of these constructs in C2C12 myoblasts, expression of GFP emerin caused a 50 66% decrease in expression of MyoD, Myf5, Pax3 and Pax7 (Fig. 5E), which restored their expression to wild-type levels. Expression of GFP emrin P183H failed to downregulate expression of MyoD, Myf5, Pax3 or Pax7 (Fig. 5E). Discussion The results presented here show that Lmo7 regulates C2C12 myoblast differentiation by activating the expression of crucial myoblast proliferation and differentiation genes. Lmo7 binds directly to the Pax3 and MyoD promoters, suggesting that Lmo7 regulates the expression of these genes in myoblasts by binding directly to their promoters and activating their expression. Interestingly, emerin binding to Lmo7 inhibits Lmo7 binding to, and expression of, the Pax3 and MyoD promoters. This is the first study to demonstrate that emerin binding to a transcription regulator inhibits the interaction of transcription regulators with their promoters. Collectively, these studies indicate that emerin regulates myogenic differentiation by inhibiting Lmo7 binding to, and activation of, crucial myogenic differentiation genes (Fig. 6). We propose that improper activation of Lmo7 caused by the loss or mutation of emerin contributes to the defective muscle regeneration seen in X-EDMD. Regulation of myogenic differentiation It has become increasingly clear that Pax3 and Pax7 are critical components for regulating satellite cell proliferation and
8 (10) Fig. 6. Model for Lmo7 regulation of myogenic differentiation. In myoblasts Lmo7 is predominantly nucleoplasmic, where it is free to bind the promoters of Pax3, Pax7, MyoD and Myf5 and activate their expression. Early in differentiation (0 2 days) Lmo7 expression increases and remains primarily nucleoplasmic. We propose that Lmo7 binds myogenic promoters early in differentiation and activates the expression of MyoD and other early differentiation genes, given that Lmo7 is predominantly nucleoplasmic. As differentiation proceeds, we propose that the affinity of emerin for Lmo7 increases and competes with Lmo7 binding to myogenic promoters. Localization of Lmo7 to the nuclear periphery and decreased expression of MyoD and Myf5 that occurs later in differentiation are both compatible with this model. Lmo7 is predominantly cytoplasmic in myotubes and we predict that there is a change in the nuclear transport dynamics that is at least partially responsible for this relocalization. This may also be coupled to increased affinity or expression of a cytoplasmic Lmo7-binding partner. differentiation (Kuang and Rudnicki, 2008). Pax3 activates MyoD and Myf5 expression (Bajard et al., 2006; Brunelli et al., 2007; Maroto et al., 1997) and is necessary for myogenic specification and muscle formation during development (Goulding et al., 1994; Relaix et al., 2004; Tajbakhsh and Buckingham, 2000; Tajbakhsh et al., 1997). Pax3 has also been implicated in adult muscle regeneration, as GFP Pax3-expressing satellite cells are able to regenerate damaged Pax3-null myofibers (Montarras et al., 2005). Importantly, the GFP Pax3-expressing satellite cells are able to repopulate the satellite cell niche (Montarras et al., 2005), a trait of bona fide satellite cells. Pax7, on the other hand, is required only for adult myogenesis and regeneration (Hutcheson et al., 2009; Oustanina et al., 2004; Relaix et al., 2006; Seale et al., 2000). Furthermore, Pax7-null mice exhibit a dramatic reduction in the number of satellite cells (Kuang et al., 2006; Oustanina et al., 2004; Relaix et al., 2006). Myf5 and MyoD are also implicated in satellite cell activation, myoblast proliferation and muscle regeneration. Satellite cells lacking Myf5 expression are able to repopulate the satellite cell niche, whereas Myf5-expressing satellite cells fail to do so (Kuang et al., 2007). Both populations are equally competent for muscle regeneration (Kuang et al., 2007; Seale et al., 2000), suggesting that Myf5 is integral for satellite cell activation, proliferation and commitment to a myogenic precursor lineage. MyoD and Myf5 regulate the expression of many myogenic differentiation genes, including that of each other, by binding to E-boxes within their promoters (Kuang and Rudnicki, 2008). MyoD is also expressed in a novel myogenic progenitor population that is sufficient to regenerate muscle in Pax7-null mice (Kuang et al., 2006). The regenerated muscle fibers in these mice derive from a rare myogenic progenitor cell population that expresses both Pax3 and MyoD, but not Pax7. These myogenic progenitor cells are probably distinct from satellite cells as they fail to express other known satellite cell markers (Kuang et al., 2006). Our results support Lmo7 as an important regulator of proliferation and early differentiation genes in myogenic progenitor cells. Furthermore, Lmo7 binding directly to and activation of Pax3 and MyoD promoters suggests that Lmo7 regulates the proliferation or differentiation of this newly discovered Pax3- and MyoD-positive myogenic progenitor cell population. Further studies are necessary to determine whether Lmo7 is expressed and transcriptionally active in satellite cells, myogenic progenitors (Myf5-postive) or these myogenic progenitors (Pax3- and MyoDpositive) in vivo. Regulation of the Pax3 and MyoD promoters How do we envision Lmo7 activating MyoD expression during differentiation? On the basis of our data we predict that Lmo7 localization to the nuclear envelope during differentiation, through binding to emerin, inhibits Lmo7 binding to the MyoD promoter and activation of MyoD expression. This is supported by our finding that emerin competes with Lmo7 binding to the MyoD promoter in vitro. Furthermore, MyoD expression begins to decrease between 4 and 6 days of myogenic differentiation (Rudnicki et al., 2008), which is concomitant with Lmo7 localization to the nuclear envelope and cytoplasm. This model also predicts that, upon satellite cell activation, the concentration of free nucleoplasmic Lmo7 would increase dramatically to activate Myf5, Pax3, and MyoD expression. Alternatively, there might be an increase in the expression or localization of an undefined Lmo7 transcriptional co-activator upon satellite cell activation. If the first model is correct, we predict that Lmo7 is either cytoplasmic or nuclear-envelope-localized (emerin-bound) in quiescent satellite cells and Lmo7 would move to the nucleoplasm upon satellite cell activation. Future studies will be needed to examine the expression and localization of Lmo7 in quiescent and activated satellite cells in vivo, as these studies were performed in proliferating myoblasts. In this study, we have shown that Lmo7 binds a region of the MyoD promoter that is juxtaposed to the PRR, which binds many transcription factors including AP-1, TBP and MEF2 (Leibham et al., 1994; Pedraza-Alva et al., 1994; Tapscott et al., 1992). The Lmo7-binding region of the MyoD promoter contains three sequences that are similar to the consensus E-box sequence. This region also contains putative binding sites for over 20 transcription factors, including AP-1, PPAR, IRF1 and IRF2. It will be interesting to determine whether Lmo7 binding to this region of the MyoD promoter competes with these transcription factors or whether they cooperatively bind to the promoter. The Pax3 promoter region that binds Lmo7 contains two known regulatory regions: site II and B. However, alignment of the MyoD and Pax3 promoter elements predicts Lmo7 binds site II of the Pax3 promoter (J.M.H., unpublished). Thus, we hypothesize that
9 Lmo7 regulates myogenic differentiation 1699 Lmo7 competes with Oct1 or Brn2 for binding to and activation of the Pax3 promoter during myogenic differentiation. Oct1 and Brn2 are POU transcription factors that bind this region of the Pax3 promoter and activate Pax3 expression (Zhu and Pruitt, 2005). Alternatively, Lmo7 might cooperatively bind with Oct1 and Brn2 on the Pax3 promoter to activate Pax3 expression. Determining how Lmo7 binding to, and the subsequent activation of, the Pax3 and MyoD promoters is regulated will be vital for understanding how Lmo7 regulates myogenic progenitor cell activation, proliferation, differentiation and muscle regeneration. Regulation of Lmo7 nuclear localization during myogenic differentiation Here, we demonstrate that Lmo7 moves from the nucleus to the cytoplasm upon myoblast differentiation. We have previously shown that Lmo7 actively shuttles between the nucleus and cytoplasm (Holaska et al., 2006). Thus we predict that increased cytoplasmic Lmo7 during differentiation results, at least in part, from changes in nuclear transport dynamics. Interestingly, increased Lmo7 cytoplasmic localization coincides with reduced expression of myogenic progenitor proliferation genes (Rudnicki et al., 2008) and we hypothesize that Lmo7 nuclear transport is regulated during differentiation to regulate access of Lmo7 to Pax3, Pax7, Myf5 and MyoD promoters. There are multiple mechanisms that could account for increased Lmo7 cytoplasmic localization. One probable mechanism, which is utilized by many proteins to regulate their nuclear localization, is phosphorylation of residues near a nuclear localization sequence (NLS) or nuclear export sequence (Sorokin et al., 2007). Serine 1191 of Lmo7 immediately follows the predicted NLS and it is phosphorylated in HeLa cells (Beausoleil et al., 2006; Dephoure et al., 2008). We are currently investigating whether phosphorylation of this residue alters Lmo7 localization. Increased cytoplasmic localization of Lmo7 might also result from increased expression of a cytoplasmic Lmo7-binding protein or loss of a nuclear Lmo7- binding protein. Although emerin is an ideal candidate for a nuclearsequestering factor, emerin expression increases during differentiation. Furthermore, if emerin were this factor, Lmo7 should localize exclusively to the nuclear envelope, whereas Lmo7 is clearly nucleoplasmic in C2C12 myoblasts. Finally, cytoplasmic localization of Lmo7 might result from newly translated protein that fails to be imported into the nucleus, as Lmo7 localization to the cytoplasm takes between 4 and 7 days. This is an attractive model, and would be coupled with increased expression of a cytoplasmic retention factor, which could occur during differentiation. We propose that all of these mechanisms might work in concert to ensure the proper localization of Lmo7 during differentiation. For example, if there is relatively low expression of a cytoplasmic Lmo7-binding partner (e.g. -actinin) in myoblasts that increases dramatically in myotubes, coupled with increased nuclear export because of phosphorylation of Lmo7 and enhanced binding to exportin 1, this would result in an increased cytoplasmic steady-state localization of Lmo7. Emerin regulates Lmo7 binding to and activation of myogenic proliferation promoters: implications for EDMD Emerin was previously implicated in muscle differentiation and regeneration, as muscle biopsies from both X-EDMD patients and mice lacking emerin show increased expression of muscle regeneration genes, including MyoD (Bakay et al., 2006; Melcon et al., 2006). Furthermore, emerin expression increases during myoblast differentiation (Chen et al., 2006; Squarzoni et al., 2005) and primary myoblasts downregulated for emerin exhibit differentiation defects (Frock et al., 2006). Here, we have shown that expression of exogenous emerin inhibits MyoD expression and emerin downregulation activates MyoD expression. Furthermore, emerin binding to Lmo7 inhibits Lmo7 binding to the MyoD promoter. We propose that changes in MyoD expression in these cells is the result of changes in Lmo7 activity caused by increased or decreased expression of emerin. For this model to be correct, the concentrations of Lmo7 and emerin used for the EMSA studies need to be physiologically relevant. For example, the concentration of Lmo7 and emerin in the nucleus needs to be high enough to ensure that sufficient numbers of emerin Lmo7 complexes would form and compete with Lmo7 for binding to the MyoD promoter. The concentration of emerin (62 M) is approximately twice as high as Lmo7 (36 M) in the nucleus of undifferentiated myoblasts, as determined by western blotting (J.M.H., unpublished). Given that emerin is exclusively nuclear-envelope-associated, this creates a local concentration of 5.6 mm. The affinity (K d ) of Lmo7 C-term for emerin is 125 nm (Holaska et al., 2006). On the basis of the concentrations of Lmo7 and emerin in the nucleus, >99% of Lmo7 is predicted to be emerin-bound. However, only 10% of Lmo7 localizes to the nuclear envelope in myoblasts, suggesting that Lmo7 binding to emerin is regulated by post-translational modification or emerin occupancy by other binding partners [e.g. -catenin, GCL, Btf and the nuclear hormone receptor corepressor (NCoR) complex]. Lmo7 localization at the nuclear envelope also increased during differentiation. We predict that post-translational modification of Lmo7 or emerin, or both, increases their affinity for one another. Emerin contains four serine, one threonine and 13 tyrosine residues that are phosphorylated in vitro and in vivo (Amanchy et al., 2005; Brill et al., 2004; Hirano et al., 2005; Roberts et al., 2006; Schlosser et al., 2006), many of which regulate binding to its partners (Hirano et al., 2005; Roberts et al., 2006; Tifft et al., 2009). However, it is not well documented how emerin phosphorylation changes during myogenic differentiation. Lmo7 is also phosphorylated on multiple residues (Beausoleil et al., 2006; Dephoure et al., 2008). It will be interesting to analyze Lmo7 and emerin phosphorylation during myogenic differentiation and determine how these post-translational modifications affect their interaction. Of course, it cannot be ruled out that the localization of Lmo7 is regulated by binding to other nuclear envelope proteins. There are multiple proposed mechanisms for how nuclear envelope proteins regulate gene expression. MAN1, a LEM domain inner nuclear envelope protein, antagonizes the TGF- pathway (Lin et al., 2005; Osada et al., 2003; Pan et al., 2005; Raju et al., 2003). It was initially proposed that MAN1 inhibits the TGF- pathway by altering the phosphorylation status of the SMAD2 SMAD3 heterodimer (Pan et al., 2005), but this hypothesis remains to be rigorously tested. Nuclear envelope proteins have also been shown to regulate the nuclear localization of transcription factors. Hutchison and colleagues have demonstrated that loss of emerin increases nuclear -catenin localization, resulting in increased - catenin-dependent transcription (Markiewicz et al., 2006) and defective adipogenic differentiation (Tilgner et al., 2009). Another hypothesis is that emerin binding to transcription factors localizes the transcription-factor-bound chromatin to the repressed environment of the nuclear envelope. This localization is predicted to cause the chromatin to adopt a repressed structure, resulting in reduced expression of genes within this region of chromatin.
10 (10) Although there is no direct evidence supporting this model, emerin and LAP2 were found to interact with histone deacetylase complexes (Holaska and Wilson, 2007; Somech et al., 2005). Furthermore, chromatin in cells lacking emerin adopts a more open conformation, (Boyle et al., 2001; Fidzianska and Hausmanowa-Petrusewicz, 2003). The final model, which the results presented here support, is that emerin binds to transcription factors (e.g. Lmo7) at the nuclear envelope and competes with transcription factor binding to regulatory elements (e.g. the MyoD promoter). We propose that emerin utilizes all of these mechanisms to regulate transcription factor activity, although it remains to be determined whether the mechanisms used by emerin to inhibit gene expression are pathway-dependent. This is the first study to provide a mechanism for how loss of emerin might result in increased MyoD expression (i.e. by failing to inhibit Lmo7 activity). Supporting the competition model for emerin inhibition of Lmo7 activity, we found that emerin binding to Lmo7 inhibits Lmo7 binding to the MyoD and Pax3 promoters, resulting in decreased expression of MyoD and Pax3. We predict that emerin binding to Lmo7 will also inhibit binding to, and activation of, the Pax7 and Myf5 promoters, in the same manner as expression of exogenous emerin reduces Myf5 expression in myoblasts. On the basis of the results presented here, we hypothesize that Lmo7 regulation of Pax3, Pax7 and Myf5 expression is important to ensure proper myogenic differentiation during development and regeneration of damaged muscle. We further predict that loss of emerin expression or function, as is seen in X-EDMD patients, results in sustained activation of myogenic progenitor proliferation genes by failing to inhibit Lmo7 activity. Failure to inhibit Lmo7 activity would then be predicted to result in the failure of myogenic progenitors to temporally regulate the expression of crucial myogenic differentiation genes, including Pax3. In support of this model, aberrant expression of Pax3 in satellite cells delays myogenic differentiation (Boutet et al., 2007). This model predicts that emerin-null myoblasts will exhibit greater Lmo7 binding to other satellite cell activation and myoblast proliferation promoters, enhanced myogenic progenitor cell proliferation and exhaustion of the satellite cell niche by increased proliferation of myogenic progenitors that fail to fuse (Boutet et al., 2007). Future experiments will be needed to test whether satellite cells or myogenic precursors derived from X-EDMD patients and emerin-null mice have increased Pax7, Myf5 or Pax3 expression and exhaustion of the satellite cell niche, which would be predicted by these studies. Materials and Methods Expression and localization of myogenic proteins during myoblast differentiation C2C12 myoblasts were seeded onto coverslips and maintained in growth medium [Dulbecco s modified Eagle s medium (DMEM) plus 10% fetal bovine serum (FBS)]. To induce differentiation, C2C12 myoblasts were incubated with differentiation medium (DM; DMEM + 2% horse serum) for 0 7 days. Cells were recovered every day and resuspended in SDS-PAGE sample buffer at a concentration of 10 6 nuclei per ml. A total of 10,000 50,000 nuclear equivalents were separated by SDS-PAGE, transferred onto nitrocellulose and subjected to western blotting with the indicated antibodies. Densitometry was performed for each protein and the results were normalized to -tubulin (loading control). For immunofluorescence microscopy, a coverslip containing differentiating C2C12 myoblasts was removed every day, washed three times with PBS and fixed with 3.7% formaldehyde for 15 minutes. The samples were then permeabilized for 25 minutes by treatment with 0.2% Triton X-100 in PBS and blocked with 3% BSA in PBS containing 0.1% Triton X-100 for 1 hour. Primary antibodies were incubated with the cells overnight at 4 C, which were washed three times with PBS and incubated with either Alexa-Fluor-488-conjugated goat anti-rabbit-igg or Alexa- Fluor-594-conjugated goat anti-mouse-igg secondary antibodies (1:500 dilution) for 1 hour at 22 C. The cells were rinsed in PBS three times, incubated with 250 nm DAPI for 5 minutes and mounted onto slides containing one drop of ProLong Gold antifade reagent (Invitrogen). All antibodies were diluted into 3% BSA in PBS containing 0.1% Triton X-100. Antibodies used for these studies were affinity-purified clone 5688 (20 g/ml) against Lmo7 (J.M.H. and K. Wilson, unpublished), rabbit polyclonal serum against the LIM domain of Lmo7 (serum 5690, 1:10,000; J.M.H. and K. Wilson, unpublished), rabbit polyclonal serum generated against Lmo7 (serum 4001, 1:10,000), rabbit polyclonal antibody against Myf5 (1:200; Santa Cruz Biotechnologyc), rabbit polyclonal antibody against myosin heavy chain (1:100,000; Santa Cruz Biotechnology), rabbit polyclonal antibody against emerin (1:10,000; Santa Cruz Biotechnology), rabbit polyclonal antibody against myogenin (1:5,000; BD Pharmingen), rabbit polyclonal against antibody MyoD (1:500; Santa Cruz Biotechnology) and mouse monoclonal antibody against -tubulin (1:50,000; Santa Cruz Biotechnology). Slides were viewed on an Olympus IX81 DSU spinning disk confocal microscope and images were acquired using a Photometrics Evolve-512 camera controlled by Slide Book 3I software. Subcellular Fractionation A total of 10 6 C2C12 myoblasts or myotube nuclear equivalents were suspended in 500 l ice-cold PBS containing 10 g/ml of pepstatin A, leupeptin and aprotonin and 1 mm PMSF. A total of 4.5 ml ice-cold double-distilled H 2 O was then added to each sample, and the samples were incubated for 10 minutes on ice with gentle agitation every 2 minutes. Myoblasts were dounced 5 10 times and myotubes were dounced times on ice. Lysis was monitored by treating 10 l of each sample with Trypan Blue. The samples were each centrifuged at 400 g to pellet the nuclei. SDS- PAGE sample buffer was added to a sample of the cells prior to fractionation (load) and the nuclear and cytosolic fractions were separated by SDS-PAGE, transferred onto nitrocellulose and blotted with rabbit polyclonal antibodies against -tubulin (1:5000; Santa Cruz Biotechnology), emerin and Lmo7 (sera 5690 and 4001). C2C12 myoblast transfection and quantitative real-time PCR C2C12 myoblasts were transfected using Lipofectamine according to the manufacturer s instructions. Growth medium was replaced with DM after 48 hours. DM was added to C2C12 myoblasts and RNA was isolated every day for 8 days using the RNeasy mini kit (Qiagen) according to the manufacturer s instructions. cdna was generated from RNA using MMLV reverse transcriptase (Invitrogen) according to the manufacturer s instructions. RNA levels were measured by qpcr using the SYBR-GreenER Supermix (Invitrogen) and detecting transcript levels using the iq5 real-time PCR detection system (Bio-Rad). Relative mrna levels were normalized to that of GAPDH. Primers used for all experiments are listed in supplementary material Table S1. GFP emerin and GFP emerin P183H constructs used for these studies were described previously (Lee et al., 2001). Plasmids containing Lmo7 shrna and control shrna (KM33653G, clones 1, 3, 4, NC; SA Biosciences) were used for all Lmo7 downregulation studies. Expression of GFP, GFP emerin or GFP emerin P183H in emerin-null primary myoblasts was performed using the Neon electroporation system (Invitrogen) using two pulses of 1225 V for 30 msecond as per the manufacturer s instructions. Chromatin immunoprecipitation ChIP assays were performed according to the manufacturer s instructions (Millipore) using 10 6 C2C12 myoblasts per reaction and shearing the DNA to bp by sonicating with 10 pulses of 20 seconds each on setting 3 (Fisher 550 sonic dismembranator). A total of 2 l of serum 5688 against Lmo7 or pre-immune serum was used for each reaction. Pax3, Myf5, MyoD and myogenin promoters were detected by amplification of input and immunoprecipitated DNA using primers specific to Pax3, Myf5, MyoD and myogenin regulatory elements (supplementary material Table S1). Electrophoretic mobility-shift assays EMSAs were performed according to the manufacturer s instructions (Invitrogen). Promoter regions of MyoD (nucleotides 478 to 288), Pax3 (nucleotides 1135 to 722) and the myogenin promoter (nucleotides 289 to +7) were generated by PCR using the primers listed in supplementary material Table S1. MyoD, Pax3 and myogenin promoter fragments were then purified using the Qiagen Gel Extraction kit (Qiagen). 5 M His 6 -tagged Lmo7 C-term (residues ) or 5 M GSTtagged Lmo7C1 (residues ) was incubated with 200 ng of each promoter fragment in EMSA binding buffer (10 mm Tris-HCl ph 7.4, 150 mm KCl, 0.1 mm EDTA and 0.1 mm DTT) for 30 minutes and separated by native PAGE. For experiments including emerin (residues 1 222), 1.5 M His 6 -Lmo7 C-term and 4.2 M emerin (residues 1 222) were incubated together with 200 ng of the MyoD or Pax3 promoter fragment for 30 minutes. The recombinant emerin protein used in these assays contains the entire nucleoplasmic domain of emerin, which is sufficient to bind Lmo7 with high affinity (Holaska et al., 2006). Recombinant emerin and Lmo7 C-term were purified as previously described (Holaska et al., 2003; Holaska et al., 2006). DNA was visualized by staining with SYBR Green and protein was visualized using SYPRO-RUBY according to the manufacturer s instructions (Invitrogen).
SUPPLEMENTARY INFORMATION
 Supplementary Figures Supplementary Figure S1. Binding of full-length OGT and deletion mutants to PIP strips (Echelon Biosciences). Supplementary Figure S2. Binding of the OGT (919-1036) fragments with
Supplementary Figures Supplementary Figure S1. Binding of full-length OGT and deletion mutants to PIP strips (Echelon Biosciences). Supplementary Figure S2. Binding of the OGT (919-1036) fragments with
SUPPLEMENTARY INFORMATION
 doi:10.1038/nature12652 Supplementary Figure 1. PRDM16 interacts with endogenous EHMT1 in brown adipocytes. Immunoprecipitation of PRDM16 complex by flag antibody (M2) followed by Western blot analysis
doi:10.1038/nature12652 Supplementary Figure 1. PRDM16 interacts with endogenous EHMT1 in brown adipocytes. Immunoprecipitation of PRDM16 complex by flag antibody (M2) followed by Western blot analysis
Supplemental Experimental Procedures
 Cell Stem Cell, Volume 2 Supplemental Data A Temporal Switch from Notch to Wnt Signaling in Muscle Stem Cells Is Necessary for Normal Adult Myogenesis Andrew S. Brack, Irina M. Conboy, Michael J. Conboy,
Cell Stem Cell, Volume 2 Supplemental Data A Temporal Switch from Notch to Wnt Signaling in Muscle Stem Cells Is Necessary for Normal Adult Myogenesis Andrew S. Brack, Irina M. Conboy, Michael J. Conboy,
Supplementary Figure S1. Venn diagram analysis of mrna microarray data and mirna target analysis. (a) Western blot analysis of T lymphoblasts (CLS)
 Supplementary Figure S1. Venn diagram analysis of mrna microarray data and mirna target analysis. (a) Western blot analysis of T lymphoblasts (CLS) and their exosomes (EXO) in resting (REST) and activated
Supplementary Figure S1. Venn diagram analysis of mrna microarray data and mirna target analysis. (a) Western blot analysis of T lymphoblasts (CLS) and their exosomes (EXO) in resting (REST) and activated
Bio 401 Sp2014. Multiple Choice (Circle the letter corresponding to the correct answer)
 MIDTERM EXAM KEY (60 pts) You should have 5 pages. Please put your name on every page. You will have 70 minutes to complete the exam. You may begin working as soon as you receive the exam. NOTE: the RED
MIDTERM EXAM KEY (60 pts) You should have 5 pages. Please put your name on every page. You will have 70 minutes to complete the exam. You may begin working as soon as you receive the exam. NOTE: the RED
Supplementary Figure 1.TRIM33 binds β-catenin in the nucleus. a & b, Co-IP of endogenous TRIM33 with β-catenin in HT-29 cells (a) and HEK 293T cells
 Supplementary Figure 1.TRIM33 binds β-catenin in the nucleus. a & b, Co-IP of endogenous TRIM33 with β-catenin in HT-29 cells (a) and HEK 293T cells (b). TRIM33 was immunoprecipitated, and the amount of
Supplementary Figure 1.TRIM33 binds β-catenin in the nucleus. a & b, Co-IP of endogenous TRIM33 with β-catenin in HT-29 cells (a) and HEK 293T cells (b). TRIM33 was immunoprecipitated, and the amount of
The functional investigation of the interaction between TATA-associated factor 3 (TAF3) and p53 protein
 THESIS BOOK The functional investigation of the interaction between TATA-associated factor 3 (TAF3) and p53 protein Orsolya Buzás-Bereczki Supervisors: Dr. Éva Bálint Dr. Imre Miklós Boros University of
THESIS BOOK The functional investigation of the interaction between TATA-associated factor 3 (TAF3) and p53 protein Orsolya Buzás-Bereczki Supervisors: Dr. Éva Bálint Dr. Imre Miklós Boros University of
Supplementary Figure 1. AdipoR1 silencing and overexpression controls. (a) Representative blots (upper and lower panels) showing the AdipoR1 protein
 Supplementary Figure 1. AdipoR1 silencing and overexpression controls. (a) Representative blots (upper and lower panels) showing the AdipoR1 protein content relative to GAPDH in two independent experiments.
Supplementary Figure 1. AdipoR1 silencing and overexpression controls. (a) Representative blots (upper and lower panels) showing the AdipoR1 protein content relative to GAPDH in two independent experiments.
Supplementary Figure 1 Role of Raf-1 in TLR2-Dectin-1-mediated cytokine expression
 Supplementary Figure 1 Supplementary Figure 1 Role of Raf-1 in TLR2-Dectin-1-mediated cytokine expression. Quantitative real-time PCR of indicated mrnas in DCs stimulated with TLR2-Dectin-1 agonist zymosan
Supplementary Figure 1 Supplementary Figure 1 Role of Raf-1 in TLR2-Dectin-1-mediated cytokine expression. Quantitative real-time PCR of indicated mrnas in DCs stimulated with TLR2-Dectin-1 agonist zymosan
Cells and reagents. Synaptopodin knockdown (1) and dynamin knockdown (2)
 Supplemental Methods Cells and reagents. Synaptopodin knockdown (1) and dynamin knockdown (2) podocytes were cultured as described previously. Staurosporine, angiotensin II and actinomycin D were all obtained
Supplemental Methods Cells and reagents. Synaptopodin knockdown (1) and dynamin knockdown (2) podocytes were cultured as described previously. Staurosporine, angiotensin II and actinomycin D were all obtained
s u p p l e m e n ta ry i n f o r m at i o n
 Figure S1 Characterization of tet-off inducible cell lines expressing GFPprogerin and GFP-wt lamin A. a, Western blot analysis of GFP-progerin- or GFP-wt lamin A- expressing cells before induction (0d)
Figure S1 Characterization of tet-off inducible cell lines expressing GFPprogerin and GFP-wt lamin A. a, Western blot analysis of GFP-progerin- or GFP-wt lamin A- expressing cells before induction (0d)
Supplements. Figure S1. B Phalloidin Alexa488
 Supplements A, DMSO, PP2, PP3 Crk-myc Figure S1. (A) Src kinase activity is necessary for recruitment of Crk to Nephrin cytoplasmic domain. Human podocytes expressing /7-NephrinCD () were treated with
Supplements A, DMSO, PP2, PP3 Crk-myc Figure S1. (A) Src kinase activity is necessary for recruitment of Crk to Nephrin cytoplasmic domain. Human podocytes expressing /7-NephrinCD () were treated with
MOLECULAR CELL BIOLOGY
 1 Lodish Berk Kaiser Krieger scott Bretscher Ploegh Matsudaira MOLECULAR CELL BIOLOGY SEVENTH EDITION CHAPTER 13 Moving Proteins into Membranes and Organelles Copyright 2013 by W. H. Freeman and Company
1 Lodish Berk Kaiser Krieger scott Bretscher Ploegh Matsudaira MOLECULAR CELL BIOLOGY SEVENTH EDITION CHAPTER 13 Moving Proteins into Membranes and Organelles Copyright 2013 by W. H. Freeman and Company
TFEB-mediated increase in peripheral lysosomes regulates. Store Operated Calcium Entry
 TFEB-mediated increase in peripheral lysosomes regulates Store Operated Calcium Entry Luigi Sbano, Massimo Bonora, Saverio Marchi, Federica Baldassari, Diego L. Medina, Andrea Ballabio, Carlotta Giorgi
TFEB-mediated increase in peripheral lysosomes regulates Store Operated Calcium Entry Luigi Sbano, Massimo Bonora, Saverio Marchi, Federica Baldassari, Diego L. Medina, Andrea Ballabio, Carlotta Giorgi
T H E J O U R N A L O F C E L L B I O L O G Y
 Supplemental material Edens and Levy, http://www.jcb.org/cgi/content/full/jcb.201406004/dc1 T H E J O U R N A L O F C E L L B I O L O G Y Figure S1. Nuclear shrinking does not depend on the cytoskeleton
Supplemental material Edens and Levy, http://www.jcb.org/cgi/content/full/jcb.201406004/dc1 T H E J O U R N A L O F C E L L B I O L O G Y Figure S1. Nuclear shrinking does not depend on the cytoskeleton
Soft Agar Assay. For each cell pool, 100,000 cells were resuspended in 0.35% (w/v)
 SUPPLEMENTARY MATERIAL AND METHODS Soft Agar Assay. For each cell pool, 100,000 cells were resuspended in 0.35% (w/v) top agar (LONZA, SeaKem LE Agarose cat.5004) and plated onto 0.5% (w/v) basal agar.
SUPPLEMENTARY MATERIAL AND METHODS Soft Agar Assay. For each cell pool, 100,000 cells were resuspended in 0.35% (w/v) top agar (LONZA, SeaKem LE Agarose cat.5004) and plated onto 0.5% (w/v) basal agar.
Muscles, muscle fibres and myofibrils
 Muscles, muscle fibres and myofibrils Properties of Muscle Fiber Types Fast fibers Slow fibers Characteristic IIb IIx IIa Type I V max (speed of shortening) Highest Intermediate Low Resistance to fatigue
Muscles, muscle fibres and myofibrils Properties of Muscle Fiber Types Fast fibers Slow fibers Characteristic IIb IIx IIa Type I V max (speed of shortening) Highest Intermediate Low Resistance to fatigue
Supplemental Information
 Supplemental Information Tobacco-specific Carcinogen Induces DNA Methyltransferases 1 Accumulation through AKT/GSK3β/βTrCP/hnRNP-U in Mice and Lung Cancer patients Ruo-Kai Lin, 1 Yi-Shuan Hsieh, 2 Pinpin
Supplemental Information Tobacco-specific Carcinogen Induces DNA Methyltransferases 1 Accumulation through AKT/GSK3β/βTrCP/hnRNP-U in Mice and Lung Cancer patients Ruo-Kai Lin, 1 Yi-Shuan Hsieh, 2 Pinpin
Figure S1, Beyer et al.
 Figure S1, eyer et al. Pax7 Myogenin si sitrl Hoechst T = 72h 14 1.8.6.4.2 12 1 8 6 4 2 24h 48h 96h diff. sitrl siset1 212 72h diff. b1 td r t Se km MyH Vinculin Myogenin β-ctin Vinculin MW b1 ka td r
Figure S1, eyer et al. Pax7 Myogenin si sitrl Hoechst T = 72h 14 1.8.6.4.2 12 1 8 6 4 2 24h 48h 96h diff. sitrl siset1 212 72h diff. b1 td r t Se km MyH Vinculin Myogenin β-ctin Vinculin MW b1 ka td r
Supplementary Figure 1 Expression of Crb3 in mouse sciatic nerve: biochemical analysis (a) Schematic of Crb3 isoforms, ERLI and CLPI, indicating the
 Supplementary Figure 1 Expression of Crb3 in mouse sciatic nerve: biochemical analysis (a) Schematic of Crb3 isoforms, ERLI and CLPI, indicating the location of the transmembrane (TM), FRM binding (FB)
Supplementary Figure 1 Expression of Crb3 in mouse sciatic nerve: biochemical analysis (a) Schematic of Crb3 isoforms, ERLI and CLPI, indicating the location of the transmembrane (TM), FRM binding (FB)
Inner Nuclear Membrane Protein MAN1 and Regulation of R-Smad Signaling
 4th International Melorheostosis Association Conference Inner Nuclear Membrane Protein MAN1 and Regulation of R-Smad Signaling Howard J. Worman Columbia University New York, NY The Nuclear Envelope By
4th International Melorheostosis Association Conference Inner Nuclear Membrane Protein MAN1 and Regulation of R-Smad Signaling Howard J. Worman Columbia University New York, NY The Nuclear Envelope By
TSH Receptor Monoclonal Antibody (49) Catalog Number MA3-218 Product data sheet
 Website: thermofisher.com Customer Service (US): 1 800 955 6288 ext. 1 Technical Support (US): 1 800 955 6288 ext. 441 TSH Receptor Monoclonal Antibody (49) Catalog Number MA3-218 Product data sheet Details
Website: thermofisher.com Customer Service (US): 1 800 955 6288 ext. 1 Technical Support (US): 1 800 955 6288 ext. 441 TSH Receptor Monoclonal Antibody (49) Catalog Number MA3-218 Product data sheet Details
An HMGA2-IGF2BP2 Axis Regulates Myoblast Proliferation and Myogenesis
 Please cite this article in press as: Li et al., An HMGA2-IGF2BP2 Axis Regulates Myoblast Proliferation and Myogenesis, Developmental Cell (2012), http://dx.doi.org/10.1016/j.devcel.2012.10.019 Developmental
Please cite this article in press as: Li et al., An HMGA2-IGF2BP2 Axis Regulates Myoblast Proliferation and Myogenesis, Developmental Cell (2012), http://dx.doi.org/10.1016/j.devcel.2012.10.019 Developmental
Rabbit Polyclonal antibody to NFkB p65 (v-rel reticuloendotheliosis viral oncogene homolog A (avian))
 Datasheet GeneTex, Inc : Toll Free 1-877-GeneTex (1-877-436-3839) Fax:1-949-309-2888 info@genetex.com GeneTex International Corporation : Tel:886-3-6208988 Fax:886-3-6208989 infoasia@genetex.com Date :
Datasheet GeneTex, Inc : Toll Free 1-877-GeneTex (1-877-436-3839) Fax:1-949-309-2888 info@genetex.com GeneTex International Corporation : Tel:886-3-6208988 Fax:886-3-6208989 infoasia@genetex.com Date :
Mesenchymal progenitor cells in intramuscular connective tissue development
 Mesenchymal progenitor cells in intramuscular connective tissue development Min Du, Ph.D. Professor and Endowed Chair Department of Animal Sciences Washington State University Pullman, WA Beef Quality:
Mesenchymal progenitor cells in intramuscular connective tissue development Min Du, Ph.D. Professor and Endowed Chair Department of Animal Sciences Washington State University Pullman, WA Beef Quality:
Supplementary Figure S1: Defective heterochromatin repair in HGPS progeroid cells
 Supplementary Figure S1: Defective heterochromatin repair in HGPS progeroid cells Immunofluorescence staining of H3K9me3 and 53BP1 in PH and HGADFN003 (HG003) cells at 24 h after γ-irradiation. Scale bar,
Supplementary Figure S1: Defective heterochromatin repair in HGPS progeroid cells Immunofluorescence staining of H3K9me3 and 53BP1 in PH and HGADFN003 (HG003) cells at 24 h after γ-irradiation. Scale bar,
Regulation of Gene Expression in Eukaryotes
 Ch. 19 Regulation of Gene Expression in Eukaryotes BIOL 222 Differential Gene Expression in Eukaryotes Signal Cells in a multicellular eukaryotic organism genetically identical differential gene expression
Ch. 19 Regulation of Gene Expression in Eukaryotes BIOL 222 Differential Gene Expression in Eukaryotes Signal Cells in a multicellular eukaryotic organism genetically identical differential gene expression
SUPPLEMENTARY INFORMATION. Supplementary Figures S1-S9. Supplementary Methods
 SUPPLEMENTARY INFORMATION SUMO1 modification of PTEN regulates tumorigenesis by controlling its association with the plasma membrane Jian Huang 1,2#, Jie Yan 1,2#, Jian Zhang 3#, Shiguo Zhu 1, Yanli Wang
SUPPLEMENTARY INFORMATION SUMO1 modification of PTEN regulates tumorigenesis by controlling its association with the plasma membrane Jian Huang 1,2#, Jie Yan 1,2#, Jian Zhang 3#, Shiguo Zhu 1, Yanli Wang
SUPPLEMENTARY LEGENDS...
 TABLE OF CONTENTS SUPPLEMENTARY LEGENDS... 2 11 MOVIE S1... 2 FIGURE S1 LEGEND... 3 FIGURE S2 LEGEND... 4 FIGURE S3 LEGEND... 5 FIGURE S4 LEGEND... 6 FIGURE S5 LEGEND... 7 FIGURE S6 LEGEND... 8 FIGURE
TABLE OF CONTENTS SUPPLEMENTARY LEGENDS... 2 11 MOVIE S1... 2 FIGURE S1 LEGEND... 3 FIGURE S2 LEGEND... 4 FIGURE S3 LEGEND... 5 FIGURE S4 LEGEND... 6 FIGURE S5 LEGEND... 7 FIGURE S6 LEGEND... 8 FIGURE
Supplementary Figure 1: si-craf but not si-braf sensitizes tumor cells to radiation.
 Supplementary Figure 1: si-craf but not si-braf sensitizes tumor cells to radiation. (a) Embryonic fibroblasts isolated from wildtype (WT), BRAF -/-, or CRAF -/- mice were irradiated (6 Gy) and DNA damage
Supplementary Figure 1: si-craf but not si-braf sensitizes tumor cells to radiation. (a) Embryonic fibroblasts isolated from wildtype (WT), BRAF -/-, or CRAF -/- mice were irradiated (6 Gy) and DNA damage
(a) Significant biological processes (upper panel) and disease biomarkers (lower panel)
 Supplementary Figure 1. Functional enrichment analyses of secretomic proteins. (a) Significant biological processes (upper panel) and disease biomarkers (lower panel) 2 involved by hrab37-mediated secretory
Supplementary Figure 1. Functional enrichment analyses of secretomic proteins. (a) Significant biological processes (upper panel) and disease biomarkers (lower panel) 2 involved by hrab37-mediated secretory
9/16/2009. Fast and slow twitch fibres. Properties of Muscle Fiber Types Fast fibers Slow fibers
 Muscles, muscle fibres and myofibrils Fast and slow twitch fibres Rat hindlimb muscle ATPase staining at different ph and NADH Muscle fibre shortening velocity lengths/second Properties of Muscle Fiber
Muscles, muscle fibres and myofibrils Fast and slow twitch fibres Rat hindlimb muscle ATPase staining at different ph and NADH Muscle fibre shortening velocity lengths/second Properties of Muscle Fiber
TRAF6 ubiquitinates TGFβ type I receptor to promote its cleavage and nuclear translocation in cancer
 Supplementary Information TRAF6 ubiquitinates TGFβ type I receptor to promote its cleavage and nuclear translocation in cancer Yabing Mu, Reshma Sundar, Noopur Thakur, Maria Ekman, Shyam Kumar Gudey, Mariya
Supplementary Information TRAF6 ubiquitinates TGFβ type I receptor to promote its cleavage and nuclear translocation in cancer Yabing Mu, Reshma Sundar, Noopur Thakur, Maria Ekman, Shyam Kumar Gudey, Mariya
SUPPLEMENTARY INFORMATION
 -. -. SUPPLEMENTARY INFORMATION DOI: 1.1/ncb86 a WAT-1 WAT- BAT-1 BAT- sk-muscle-1 sk-muscle- mir-133b mir-133a mir-6 mir-378 mir-1 mir-85 mir-378 mir-6a mir-18 mir-133a mir- mir- mir-341 mir-196a mir-17
-. -. SUPPLEMENTARY INFORMATION DOI: 1.1/ncb86 a WAT-1 WAT- BAT-1 BAT- sk-muscle-1 sk-muscle- mir-133b mir-133a mir-6 mir-378 mir-1 mir-85 mir-378 mir-6a mir-18 mir-133a mir- mir- mir-341 mir-196a mir-17
SUPPLEMENTARY INFORMATION
 doi: 10.1038/nature05473 SUPPLEMENTARY FIGURES SUPPLEMENTARY INFORMATION Supplementary Figure 1: Association of Runx2 with mitotic chromosomes Mitotic chromosome spreads were prepared for a. Human Saos-2
doi: 10.1038/nature05473 SUPPLEMENTARY FIGURES SUPPLEMENTARY INFORMATION Supplementary Figure 1: Association of Runx2 with mitotic chromosomes Mitotic chromosome spreads were prepared for a. Human Saos-2
Chapter 4. Estrogen receptor expression in human macrophages
 Chapter 4 Estrogen receptor expression in human macrophages 4.1. Introduction Macrophages respond to estrogen present in their microenvironment and hence should express functional estrogen receptors unless
Chapter 4 Estrogen receptor expression in human macrophages 4.1. Introduction Macrophages respond to estrogen present in their microenvironment and hence should express functional estrogen receptors unless
E3 ligase TRIM72 negatively regulates myogenesis by IRS-1 ubiquitination
 E3 ligase negatively regulates myogenesis by IRS-1 ubiquitination Young-Gyu Ko College of Life Science and Biotechnology Korea University MyHC immunofluorescence Lipid rafts are plasma membrane compartments
E3 ligase negatively regulates myogenesis by IRS-1 ubiquitination Young-Gyu Ko College of Life Science and Biotechnology Korea University MyHC immunofluorescence Lipid rafts are plasma membrane compartments
SUPPLEMENTARY INFORMATION
 SUPPLEMENTARY INFORMATION doi:1.138/nature9814 a A SHARPIN FL B SHARPIN ΔNZF C SHARPIN T38L, F39V b His-SHARPIN FL -1xUb -2xUb -4xUb α-his c Linear 4xUb -SHARPIN FL -SHARPIN TF_LV -SHARPINΔNZF -SHARPIN
SUPPLEMENTARY INFORMATION doi:1.138/nature9814 a A SHARPIN FL B SHARPIN ΔNZF C SHARPIN T38L, F39V b His-SHARPIN FL -1xUb -2xUb -4xUb α-his c Linear 4xUb -SHARPIN FL -SHARPIN TF_LV -SHARPINΔNZF -SHARPIN
MicroRNA sponges: competitive inhibitors of small RNAs in mammalian cells
 MicroRNA sponges: competitive inhibitors of small RNAs in mammalian cells Margaret S Ebert, Joel R Neilson & Phillip A Sharp Supplementary figures and text: Supplementary Figure 1. Effect of sponges on
MicroRNA sponges: competitive inhibitors of small RNAs in mammalian cells Margaret S Ebert, Joel R Neilson & Phillip A Sharp Supplementary figures and text: Supplementary Figure 1. Effect of sponges on
Supplementary Figure 1 Induction of cellular senescence and isolation of exosome. a to c, Pre-senescent primary normal human diploid fibroblasts
 Supplementary Figure 1 Induction of cellular senescence and isolation of exosome. a to c, Pre-senescent primary normal human diploid fibroblasts (TIG-3 cells) were rendered senescent by either serial passage
Supplementary Figure 1 Induction of cellular senescence and isolation of exosome. a to c, Pre-senescent primary normal human diploid fibroblasts (TIG-3 cells) were rendered senescent by either serial passage
SUPPLEMENTARY FIGURES
 SUPPLEMENTARY FIGURES 1 Supplementary Figure 1, Adult hippocampal QNPs and TAPs uniformly express REST a-b) Confocal images of adult hippocampal mouse sections showing GFAP (green), Sox2 (red), and REST
SUPPLEMENTARY FIGURES 1 Supplementary Figure 1, Adult hippocampal QNPs and TAPs uniformly express REST a-b) Confocal images of adult hippocampal mouse sections showing GFAP (green), Sox2 (red), and REST
SUPPLEMENTARY INFORMATION
 Supplementary Discussion The cell cycle machinery and the DNA damage response network are highly interconnected and co-regulated in assuring faithful duplication and partition of genetic materials into
Supplementary Discussion The cell cycle machinery and the DNA damage response network are highly interconnected and co-regulated in assuring faithful duplication and partition of genetic materials into
Supplementary Figure 1. Normal T lymphocyte populations in Dapk -/- mice. (a) Normal thymic development in Dapk -/- mice. Thymocytes from WT and Dapk
 Supplementary Figure 1. Normal T lymphocyte populations in Dapk -/- mice. (a) Normal thymic development in Dapk -/- mice. Thymocytes from WT and Dapk -/- mice were stained for expression of CD4 and CD8.
Supplementary Figure 1. Normal T lymphocyte populations in Dapk -/- mice. (a) Normal thymic development in Dapk -/- mice. Thymocytes from WT and Dapk -/- mice were stained for expression of CD4 and CD8.
BIO360 Fall 2013 Quiz 1
 BIO360 Fall 2013 Quiz 1 1. Examine the diagram below. There are two homologous copies of chromosome one and the allele of YFG carried on the light gray chromosome has undergone a loss-of-function mutation.
BIO360 Fall 2013 Quiz 1 1. Examine the diagram below. There are two homologous copies of chromosome one and the allele of YFG carried on the light gray chromosome has undergone a loss-of-function mutation.
7.06 Cell Biology EXAM #3 April 24, 2003
 7.06 Spring 2003 Exam 3 Name 1 of 8 7.06 Cell Biology EXAM #3 April 24, 2003 This is an open book exam, and you are allowed access to books and notes. Please write your answers to the questions in the
7.06 Spring 2003 Exam 3 Name 1 of 8 7.06 Cell Biology EXAM #3 April 24, 2003 This is an open book exam, and you are allowed access to books and notes. Please write your answers to the questions in the
T H E J O U R N A L O F C E L L B I O L O G Y
 T H E J O U R N A L O F C E L L B I O L O G Y Supplemental material Krenn et al., http://www.jcb.org/cgi/content/full/jcb.201110013/dc1 Figure S1. Levels of expressed proteins and demonstration that C-terminal
T H E J O U R N A L O F C E L L B I O L O G Y Supplemental material Krenn et al., http://www.jcb.org/cgi/content/full/jcb.201110013/dc1 Figure S1. Levels of expressed proteins and demonstration that C-terminal
p = formed with HCI-001 p = Relative # of blood vessels that formed with HCI-002 Control Bevacizumab + 17AAG Bevacizumab 17AAG
 A.. Relative # of ECs associated with HCI-001 1.4 1.2 1.0 0.8 0.6 0.4 0.2 0.0 ol b p < 0.001 Relative # of blood vessels that formed with HCI-001 1.4 1.2 1.0 0.8 0.6 0.4 0.2 0.0 l b p = 0.002 Control IHC:
A.. Relative # of ECs associated with HCI-001 1.4 1.2 1.0 0.8 0.6 0.4 0.2 0.0 ol b p < 0.001 Relative # of blood vessels that formed with HCI-001 1.4 1.2 1.0 0.8 0.6 0.4 0.2 0.0 l b p = 0.002 Control IHC:
Supplementary data Supplementary Figure 1 Supplementary Figure 2
 Supplementary data Supplementary Figure 1 SPHK1 sirna increases RANKL-induced osteoclastogenesis in RAW264.7 cell culture. (A) RAW264.7 cells were transfected with oligocassettes containing SPHK1 sirna
Supplementary data Supplementary Figure 1 SPHK1 sirna increases RANKL-induced osteoclastogenesis in RAW264.7 cell culture. (A) RAW264.7 cells were transfected with oligocassettes containing SPHK1 sirna
Supplementary Figure 1. HOPX is hypermethylated in NPC. (a) Methylation levels of HOPX in Normal (n = 24) and NPC (n = 24) tissues from the
 Supplementary Figure 1. HOPX is hypermethylated in NPC. (a) Methylation levels of HOPX in Normal (n = 24) and NPC (n = 24) tissues from the genome-wide methylation microarray data. Mean ± s.d.; Student
Supplementary Figure 1. HOPX is hypermethylated in NPC. (a) Methylation levels of HOPX in Normal (n = 24) and NPC (n = 24) tissues from the genome-wide methylation microarray data. Mean ± s.d.; Student
Analysis of small RNAs from Drosophila Schneider cells using the Small RNA assay on the Agilent 2100 bioanalyzer. Application Note
 Analysis of small RNAs from Drosophila Schneider cells using the Small RNA assay on the Agilent 2100 bioanalyzer Application Note Odile Sismeiro, Jean-Yves Coppée, Christophe Antoniewski, and Hélène Thomassin
Analysis of small RNAs from Drosophila Schneider cells using the Small RNA assay on the Agilent 2100 bioanalyzer Application Note Odile Sismeiro, Jean-Yves Coppée, Christophe Antoniewski, and Hélène Thomassin
GSK3β mediates muscle pathology in myotonic dystrophy
 Research article GSK3β mediates muscle pathology in myotonic dystrophy Karlie Jones, 1 Christina Wei, 1 Polina Iakova, 2 Enrico Bugiardini, 3 Christiane Schneider-Gold, 4 Giovanni Meola, 3 James Woodgett,
Research article GSK3β mediates muscle pathology in myotonic dystrophy Karlie Jones, 1 Christina Wei, 1 Polina Iakova, 2 Enrico Bugiardini, 3 Christiane Schneider-Gold, 4 Giovanni Meola, 3 James Woodgett,
RAW264.7 cells stably expressing control shrna (Con) or GSK3b-specific shrna (sh-
 1 a b Supplementary Figure 1. Effects of GSK3b knockdown on poly I:C-induced cytokine production. RAW264.7 cells stably expressing control shrna (Con) or GSK3b-specific shrna (sh- GSK3b) were stimulated
1 a b Supplementary Figure 1. Effects of GSK3b knockdown on poly I:C-induced cytokine production. RAW264.7 cells stably expressing control shrna (Con) or GSK3b-specific shrna (sh- GSK3b) were stimulated
Supplementary Figure 1 CD4 + T cells from PKC-θ null mice are defective in NF-κB activation during T cell receptor signaling. CD4 + T cells were
 Supplementary Figure 1 CD4 + T cells from PKC-θ null mice are defective in NF-κB activation during T cell receptor signaling. CD4 + T cells were isolated from wild type (PKC-θ- WT) or PKC-θ null (PKC-θ-KO)
Supplementary Figure 1 CD4 + T cells from PKC-θ null mice are defective in NF-κB activation during T cell receptor signaling. CD4 + T cells were isolated from wild type (PKC-θ- WT) or PKC-θ null (PKC-θ-KO)
Supplementary Figure 1 Transcription assay of nine ABA-responsive PP2C. Transcription assay of nine ABA-responsive PP2C genes. Total RNA was isolated
 Supplementary Figure 1 Transcription assay of nine ABA-responsive PP2C genes. Transcription assay of nine ABA-responsive PP2C genes. Total RNA was isolated from 7 day-old seedlings treated with or without
Supplementary Figure 1 Transcription assay of nine ABA-responsive PP2C genes. Transcription assay of nine ABA-responsive PP2C genes. Total RNA was isolated from 7 day-old seedlings treated with or without
Muscular Dystrophy. Biol 405 Molecular Medicine
 Muscular Dystrophy Biol 405 Molecular Medicine Duchenne muscular dystrophy Duchenne muscular dystrophy is a neuromuscular disease that occurs in ~ 1/3,500 male births. The disease causes developmental
Muscular Dystrophy Biol 405 Molecular Medicine Duchenne muscular dystrophy Duchenne muscular dystrophy is a neuromuscular disease that occurs in ~ 1/3,500 male births. The disease causes developmental
Supplementary Figure 1. Spatial distribution of LRP5 and β-catenin in intact cardiomyocytes. (a) and (b) Immunofluorescence staining of endogenous
 Supplementary Figure 1. Spatial distribution of LRP5 and β-catenin in intact cardiomyocytes. (a) and (b) Immunofluorescence staining of endogenous LRP5 in intact adult mouse ventricular myocytes (AMVMs)
Supplementary Figure 1. Spatial distribution of LRP5 and β-catenin in intact cardiomyocytes. (a) and (b) Immunofluorescence staining of endogenous LRP5 in intact adult mouse ventricular myocytes (AMVMs)
Medical Biology. Dr. Khalida Ibrahim
 Dr. Khalida Ibrahim Medical Biology MUSCLE TISSUE 1. Muscle tissue is characterized by its well-developed properties of contraction. 2. Muscle is responsible for the movements of the body and the various
Dr. Khalida Ibrahim Medical Biology MUSCLE TISSUE 1. Muscle tissue is characterized by its well-developed properties of contraction. 2. Muscle is responsible for the movements of the body and the various
TRAF6 regulates satellite stem cell self-renewal and function during regenerative myogenesis
 The Journal of Clinical Investigation TRAF6 regulates satellite stem cell self-renewal and function during regenerative myogenesis Sajedah M. Hindi and Ashok Kumar Department of Anatomical Sciences and
The Journal of Clinical Investigation TRAF6 regulates satellite stem cell self-renewal and function during regenerative myogenesis Sajedah M. Hindi and Ashok Kumar Department of Anatomical Sciences and
Syntaxin 4 regulates the surface localization of a promyogenic receptor Cdo thereby promoting myogenic differentiation
 Yoo et al. Skeletal Muscle (2015) 5:28 DOI 10.1186/s13395-015-0052-8 RESEARCH Open Access Syntaxin 4 regulates the surface localization of a promyogenic receptor Cdo thereby promoting myogenic differentiation
Yoo et al. Skeletal Muscle (2015) 5:28 DOI 10.1186/s13395-015-0052-8 RESEARCH Open Access Syntaxin 4 regulates the surface localization of a promyogenic receptor Cdo thereby promoting myogenic differentiation
RayBio Human PPAR-alpha Transcription Factor Activity Assay Kit
 RayBio Human PPAR-alpha Transcription Factor Activity Assay Kit Catalog #: TFEH-PPARa User Manual Jan 5, 2018 3607 Parkway Lane, Suite 200 Norcross, GA 30092 Tel: 1-888-494-8555 (Toll Free) or 770-729-2992,
RayBio Human PPAR-alpha Transcription Factor Activity Assay Kit Catalog #: TFEH-PPARa User Manual Jan 5, 2018 3607 Parkway Lane, Suite 200 Norcross, GA 30092 Tel: 1-888-494-8555 (Toll Free) or 770-729-2992,
The clathrin adaptor Numb regulates intestinal cholesterol. absorption through dynamic interaction with NPC1L1
 The clathrin adaptor Numb regulates intestinal cholesterol absorption through dynamic interaction with NPC1L1 Pei-Shan Li 1, Zhen-Yan Fu 1,2, Ying-Yu Zhang 1, Jin-Hui Zhang 1, Chen-Qi Xu 1, Yi-Tong Ma
The clathrin adaptor Numb regulates intestinal cholesterol absorption through dynamic interaction with NPC1L1 Pei-Shan Li 1, Zhen-Yan Fu 1,2, Ying-Yu Zhang 1, Jin-Hui Zhang 1, Chen-Qi Xu 1, Yi-Tong Ma
REGENERATIVE MEDICINE
 REGENERATIVE MEDICINE Ex Vivo Gene Editing of the Dystrophin Gene in Muscle Stem Cells Mediated by Peptide Nucleic Acid Single Stranded Oligodeoxynucleotides Induces Stable Expression of Dystrophin in
REGENERATIVE MEDICINE Ex Vivo Gene Editing of the Dystrophin Gene in Muscle Stem Cells Mediated by Peptide Nucleic Acid Single Stranded Oligodeoxynucleotides Induces Stable Expression of Dystrophin in
(A) PCR primers (arrows) designed to distinguish wild type (P1+P2), targeted (P1+P2) and excised (P1+P3)14-
 1 Supplemental Figure Legends Figure S1. Mammary tumors of ErbB2 KI mice with 14-3-3σ ablation have elevated ErbB2 transcript levels and cell proliferation (A) PCR primers (arrows) designed to distinguish
1 Supplemental Figure Legends Figure S1. Mammary tumors of ErbB2 KI mice with 14-3-3σ ablation have elevated ErbB2 transcript levels and cell proliferation (A) PCR primers (arrows) designed to distinguish
Ratchakrit Srikuea 1 and Muthita Hirunsai 2 1
 J Appl Physiol 120: 1381 1393, 2016. First published March 31, 2016; doi:10.1152/japplphysiol.01018.2015. Effects of intramuscular administration of 1,25(OH) 2 D 3 during skeletal muscle regeneration on
J Appl Physiol 120: 1381 1393, 2016. First published March 31, 2016; doi:10.1152/japplphysiol.01018.2015. Effects of intramuscular administration of 1,25(OH) 2 D 3 during skeletal muscle regeneration on
SUPPLEMENTARY INFORMATION
 sirna pool: Control Tetherin -HA-GFP HA-Tetherin -Tubulin Supplementary Figure S1. Knockdown of HA-tagged tetherin expression by tetherin specific sirnas. HeLa cells were cotransfected with plasmids expressing
sirna pool: Control Tetherin -HA-GFP HA-Tetherin -Tubulin Supplementary Figure S1. Knockdown of HA-tagged tetherin expression by tetherin specific sirnas. HeLa cells were cotransfected with plasmids expressing
Nature Medicine: doi: /nm.4322
 1 2 3 4 5 6 7 8 9 10 11 Supplementary Figure 1. Predicted RNA structure of 3 UTR and sequence alignment of deleted nucleotides. (a) Predicted RNA secondary structure of ZIKV 3 UTR. The stem-loop structure
1 2 3 4 5 6 7 8 9 10 11 Supplementary Figure 1. Predicted RNA structure of 3 UTR and sequence alignment of deleted nucleotides. (a) Predicted RNA secondary structure of ZIKV 3 UTR. The stem-loop structure
Supplementary information. MARCH8 inhibits HIV-1 infection by reducing virion incorporation of envelope glycoproteins
 Supplementary information inhibits HIV-1 infection by reducing virion incorporation of envelope glycoproteins Takuya Tada, Yanzhao Zhang, Takayoshi Koyama, Minoru Tobiume, Yasuko Tsunetsugu-Yokota, Shoji
Supplementary information inhibits HIV-1 infection by reducing virion incorporation of envelope glycoproteins Takuya Tada, Yanzhao Zhang, Takayoshi Koyama, Minoru Tobiume, Yasuko Tsunetsugu-Yokota, Shoji
Transcriptional Regulation of Skeletal Muscle Atrophy-Induced Gene Expression by Muscle Ring Finger-1 and Myogenic Regulatory Factors
 UNF Digital Commons UNF Theses and Dissertations Student Scholarship 2017 Transcriptional Regulation of Skeletal Muscle Atrophy-Induced Gene Expression by Muscle Ring Finger-1 and Myogenic Regulatory Factors
UNF Digital Commons UNF Theses and Dissertations Student Scholarship 2017 Transcriptional Regulation of Skeletal Muscle Atrophy-Induced Gene Expression by Muscle Ring Finger-1 and Myogenic Regulatory Factors
Recruitment of HBO1 Histone Acetylase and Blocks
 Molecular Cell, Volume 44 Supplemental Information JNK1 Phosphorylation of Cdt1 Inhibits Recruitment of HO1 Histone cetylase and locks Replication Licensing in Response to Stress enoit Miotto and Kevin
Molecular Cell, Volume 44 Supplemental Information JNK1 Phosphorylation of Cdt1 Inhibits Recruitment of HO1 Histone cetylase and locks Replication Licensing in Response to Stress enoit Miotto and Kevin
Supplementary Figure 1 IL-27 IL
 Tim-3 Supplementary Figure 1 Tc0 49.5 0.6 Tc1 63.5 0.84 Un 49.8 0.16 35.5 0.16 10 4 61.2 5.53 10 3 64.5 5.66 10 2 10 1 10 0 31 2.22 10 0 10 1 10 2 10 3 10 4 IL-10 28.2 1.69 IL-27 Supplementary Figure 1.
Tim-3 Supplementary Figure 1 Tc0 49.5 0.6 Tc1 63.5 0.84 Un 49.8 0.16 35.5 0.16 10 4 61.2 5.53 10 3 64.5 5.66 10 2 10 1 10 0 31 2.22 10 0 10 1 10 2 10 3 10 4 IL-10 28.2 1.69 IL-27 Supplementary Figure 1.
SUPPLEMENTARY INFORMATION
 DOI: 1.138/ncb222 / b. WB anti- WB anti- ulin Mitotic index (%) 14 1 6 2 T (h) 32 48-1 1 2 3 4 6-1 4 16 22 28 3 33 e. 6 4 2 Time (min) 1-6- 11-1 > 1 % cells Figure S1 depletion leads to mitotic defects
DOI: 1.138/ncb222 / b. WB anti- WB anti- ulin Mitotic index (%) 14 1 6 2 T (h) 32 48-1 1 2 3 4 6-1 4 16 22 28 3 33 e. 6 4 2 Time (min) 1-6- 11-1 > 1 % cells Figure S1 depletion leads to mitotic defects
Recombinant Protein Expression Retroviral system
 Recombinant Protein Expression Retroviral system Viruses Contains genome DNA or RNA Genome encased in a protein coat or capsid. Some viruses have membrane covering protein coat enveloped virus Ø Essential
Recombinant Protein Expression Retroviral system Viruses Contains genome DNA or RNA Genome encased in a protein coat or capsid. Some viruses have membrane covering protein coat enveloped virus Ø Essential
Supplementary Figure 1
 Supplementary Figure 1 Constitutive EGFR signaling does not activate canonical EGFR signals (a) U251EGFRInd cells with or without tetracycline exposure (24h, 1µg/ml) were treated with EGF for 15 minutes
Supplementary Figure 1 Constitutive EGFR signaling does not activate canonical EGFR signals (a) U251EGFRInd cells with or without tetracycline exposure (24h, 1µg/ml) were treated with EGF for 15 minutes
Supplemental Information. Metabolic Maturation during Muscle Stem Cell. Differentiation Is Achieved by mir-1/133a-mediated
 Cell Metabolism, Volume 27 Supplemental Information Metabolic Maturation during Muscle Stem Cell Differentiation Is Achieved by mir-1/133a-mediated Inhibition of the Dlk1-Dio3 Mega Gene Cluster Stas Wüst,
Cell Metabolism, Volume 27 Supplemental Information Metabolic Maturation during Muscle Stem Cell Differentiation Is Achieved by mir-1/133a-mediated Inhibition of the Dlk1-Dio3 Mega Gene Cluster Stas Wüst,
supplementary information
 DOI: 1.138/ncb1 Control Atg7 / NAC 1 1 1 1 (mm) Control Atg7 / NAC 1 1 1 1 (mm) Lamin B Gstm1 Figure S1 Neither the translocation of into the nucleus nor the induction of antioxidant proteins in autophagydeficient
DOI: 1.138/ncb1 Control Atg7 / NAC 1 1 1 1 (mm) Control Atg7 / NAC 1 1 1 1 (mm) Lamin B Gstm1 Figure S1 Neither the translocation of into the nucleus nor the induction of antioxidant proteins in autophagydeficient
SUPPLEMENTARY INFORMATION
 doi:10.1038/nature12215 Supplementary Figure 1. The effects of full and dissociated GR agonists in supporting BFU-E self-renewal divisions. BFU-Es were cultured in self-renewal medium with indicated GR
doi:10.1038/nature12215 Supplementary Figure 1. The effects of full and dissociated GR agonists in supporting BFU-E self-renewal divisions. BFU-Es were cultured in self-renewal medium with indicated GR
Supplementary Information. Preferential associations between co-regulated genes reveal a. transcriptional interactome in erythroid cells
 Supplementary Information Preferential associations between co-regulated genes reveal a transcriptional interactome in erythroid cells Stefan Schoenfelder, * Tom Sexton, * Lyubomira Chakalova, * Nathan
Supplementary Information Preferential associations between co-regulated genes reveal a transcriptional interactome in erythroid cells Stefan Schoenfelder, * Tom Sexton, * Lyubomira Chakalova, * Nathan
The subcortical maternal complex controls symmetric division of mouse zygotes by
 The subcortical maternal complex controls symmetric division of mouse zygotes by regulating F-actin dynamics Xing-Jiang Yu 1,2, Zhaohong Yi 1, Zheng Gao 1,2, Dan-dan Qin 1,2, Yanhua Zhai 1, Xue Chen 1,
The subcortical maternal complex controls symmetric division of mouse zygotes by regulating F-actin dynamics Xing-Jiang Yu 1,2, Zhaohong Yi 1, Zheng Gao 1,2, Dan-dan Qin 1,2, Yanhua Zhai 1, Xue Chen 1,
SUPPORTING MATREALS. Methods and Materials
 SUPPORTING MATREALS Methods and Materials Cell Culture MC3T3-E1 (subclone 4) cells were maintained in -MEM with 10% FBS, 1% Pen/Strep at 37ºC in a humidified incubator with 5% CO2. MC3T3 cell differentiation
SUPPORTING MATREALS Methods and Materials Cell Culture MC3T3-E1 (subclone 4) cells were maintained in -MEM with 10% FBS, 1% Pen/Strep at 37ºC in a humidified incubator with 5% CO2. MC3T3 cell differentiation
Supplementary Figure 1. SC35M polymerase activity in the presence of Bat or SC35M NP encoded from the phw2000 rescue plasmid.
 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 Supplementary Figure 1. SC35M polymerase activity in the presence of Bat or SC35M NP encoded from the phw2000 rescue plasmid. HEK293T
1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 Supplementary Figure 1. SC35M polymerase activity in the presence of Bat or SC35M NP encoded from the phw2000 rescue plasmid. HEK293T
Supplementary Materials and Methods
 Supplementary Materials and Methods Reagents and antibodies was purchased from iaffin GmbH & Co KG. Cisplatin (ristol-myers Squibb Co.) and etoposide (Sandoz Pharma Ltd.) were used. Antibodies recognizing
Supplementary Materials and Methods Reagents and antibodies was purchased from iaffin GmbH & Co KG. Cisplatin (ristol-myers Squibb Co.) and etoposide (Sandoz Pharma Ltd.) were used. Antibodies recognizing
HEK293FT cells were transiently transfected with reporters, N3-ICD construct and
 Supplementary Information Luciferase reporter assay HEK293FT cells were transiently transfected with reporters, N3-ICD construct and increased amounts of wild type or kinase inactive EGFR. Transfections
Supplementary Information Luciferase reporter assay HEK293FT cells were transiently transfected with reporters, N3-ICD construct and increased amounts of wild type or kinase inactive EGFR. Transfections
Studying The Role Of DNA Mismatch Repair In Brain Cancer Malignancy
 Kavya Puchhalapalli CALS Honors Project Report Spring 2017 Studying The Role Of DNA Mismatch Repair In Brain Cancer Malignancy Abstract Malignant brain tumors including medulloblastomas and primitive neuroectodermal
Kavya Puchhalapalli CALS Honors Project Report Spring 2017 Studying The Role Of DNA Mismatch Repair In Brain Cancer Malignancy Abstract Malignant brain tumors including medulloblastomas and primitive neuroectodermal
(a) Schematic diagram of the FS mutation of UVRAG in exon 8 containing the highly instable
 Supplementary Figure 1. Frameshift (FS) mutation in UVRAG. (a) Schematic diagram of the FS mutation of UVRAG in exon 8 containing the highly instable A 10 DNA repeat, generating a premature stop codon
Supplementary Figure 1. Frameshift (FS) mutation in UVRAG. (a) Schematic diagram of the FS mutation of UVRAG in exon 8 containing the highly instable A 10 DNA repeat, generating a premature stop codon
Proteomic profiling of small-molecule inhibitors reveals dispensability of MTH1 for cancer cell survival
 Supplementary Information for Proteomic profiling of small-molecule inhibitors reveals dispensability of MTH1 for cancer cell survival Tatsuro Kawamura 1, Makoto Kawatani 1, Makoto Muroi, Yasumitsu Kondoh,
Supplementary Information for Proteomic profiling of small-molecule inhibitors reveals dispensability of MTH1 for cancer cell survival Tatsuro Kawamura 1, Makoto Kawatani 1, Makoto Muroi, Yasumitsu Kondoh,
Problem Set #5 4/3/ Spring 02
 Question 1 Chloroplasts contain six compartments outer membrane, intermembrane space, inner membrane, stroma, thylakoid membrane, and thylakoid lumen each of which is populated by specific sets of proteins.
Question 1 Chloroplasts contain six compartments outer membrane, intermembrane space, inner membrane, stroma, thylakoid membrane, and thylakoid lumen each of which is populated by specific sets of proteins.
RAS Genes. The ras superfamily of genes encodes small GTP binding proteins that are responsible for the regulation of many cellular processes.
 ۱ RAS Genes The ras superfamily of genes encodes small GTP binding proteins that are responsible for the regulation of many cellular processes. Oncogenic ras genes in human cells include H ras, N ras,
۱ RAS Genes The ras superfamily of genes encodes small GTP binding proteins that are responsible for the regulation of many cellular processes. Oncogenic ras genes in human cells include H ras, N ras,
MTC-TT and TPC-1 cell lines were cultured in RPMI medium (Gibco, Breda, The Netherlands)
 Supplemental data Materials and Methods Cell culture MTC-TT and TPC-1 cell lines were cultured in RPMI medium (Gibco, Breda, The Netherlands) supplemented with 15% or 10% (for TPC-1) fetal bovine serum
Supplemental data Materials and Methods Cell culture MTC-TT and TPC-1 cell lines were cultured in RPMI medium (Gibco, Breda, The Netherlands) supplemented with 15% or 10% (for TPC-1) fetal bovine serum
Supplemental Figure S1. Expression of Cirbp mrna in mouse tissues and NIH3T3 cells.
 SUPPLEMENTAL FIGURE AND TABLE LEGENDS Supplemental Figure S1. Expression of Cirbp mrna in mouse tissues and NIH3T3 cells. A) Cirbp mrna expression levels in various mouse tissues collected around the clock
SUPPLEMENTAL FIGURE AND TABLE LEGENDS Supplemental Figure S1. Expression of Cirbp mrna in mouse tissues and NIH3T3 cells. A) Cirbp mrna expression levels in various mouse tissues collected around the clock
Supplementary Information
 Supplementary Information Supplementary Figure 1. CD4 + T cell activation and lack of apoptosis after crosslinking with anti-cd3 + anti-cd28 + anti-cd160. (a) Flow cytometry of anti-cd160 (5D.10A11) binding
Supplementary Information Supplementary Figure 1. CD4 + T cell activation and lack of apoptosis after crosslinking with anti-cd3 + anti-cd28 + anti-cd160. (a) Flow cytometry of anti-cd160 (5D.10A11) binding
SUPPLEMENTARY FIGURES
 SUPPLEMENTARY FIGURES Supplementary Figure 1. (A) Left, western blot analysis of ISGylated proteins in Jurkat T cells treated with 1000U ml -1 IFN for 16h (IFN) or left untreated (CONT); right, western
SUPPLEMENTARY FIGURES Supplementary Figure 1. (A) Left, western blot analysis of ISGylated proteins in Jurkat T cells treated with 1000U ml -1 IFN for 16h (IFN) or left untreated (CONT); right, western
Muscle Stem Cells in Regeneration
 Muscle Stem Cells in Regeneration Dr. F Jeffrey Dilworth BIM6028/SMC6052 Lecture (February 11, 2016) Duchenne Muscular Dystrophy is an X-linked genetic disorder that affects 1 in 3500 males Wells et al,
Muscle Stem Cells in Regeneration Dr. F Jeffrey Dilworth BIM6028/SMC6052 Lecture (February 11, 2016) Duchenne Muscular Dystrophy is an X-linked genetic disorder that affects 1 in 3500 males Wells et al,
Supplementary Figure 1
 Supplementary Figure 1 Kif1a RNAi effect on basal progenitor differentiation Related to Figure 2. Representative confocal images of the VZ and SVZ of rat cortices transfected at E16 with scrambled or Kif1a
Supplementary Figure 1 Kif1a RNAi effect on basal progenitor differentiation Related to Figure 2. Representative confocal images of the VZ and SVZ of rat cortices transfected at E16 with scrambled or Kif1a
Supplementary Table; Supplementary Figures and legends S1-S21; Supplementary Materials and Methods
 Silva et al. PTEN posttranslational inactivation and hyperactivation of the PI3K/Akt pathway sustain primary T cell leukemia viability Supplementary Table; Supplementary Figures and legends S1-S21; Supplementary
Silva et al. PTEN posttranslational inactivation and hyperactivation of the PI3K/Akt pathway sustain primary T cell leukemia viability Supplementary Table; Supplementary Figures and legends S1-S21; Supplementary
Expression of acid base transporters in the kidney collecting duct in Slc2a7 -/-
 Supplemental Material Results. Expression of acid base transporters in the kidney collecting duct in Slc2a7 -/- and Slc2a7 -/- mice. The expression of AE1 in the kidney was examined in Slc26a7 KO mice.
Supplemental Material Results. Expression of acid base transporters in the kidney collecting duct in Slc2a7 -/- and Slc2a7 -/- mice. The expression of AE1 in the kidney was examined in Slc26a7 KO mice.
Effects of UBL5 knockdown on cell cycle distribution and sister chromatid cohesion
 Supplementary Figure S1. Effects of UBL5 knockdown on cell cycle distribution and sister chromatid cohesion A. Representative examples of flow cytometry profiles of HeLa cells transfected with indicated
Supplementary Figure S1. Effects of UBL5 knockdown on cell cycle distribution and sister chromatid cohesion A. Representative examples of flow cytometry profiles of HeLa cells transfected with indicated
SUPPLEMENTARY INFORMATION
 doi: 1.138/nature89 IFN- (ng ml ) 5 4 3 1 Splenocytes NS IFN- (ng ml ) 6 4 Lymph node cells NS Nfkbiz / Nfkbiz / Nfkbiz / Nfkbiz / IL- (ng ml ) 3 1 Splenocytes IL- (ng ml ) 1 8 6 4 *** ** Lymph node cells
doi: 1.138/nature89 IFN- (ng ml ) 5 4 3 1 Splenocytes NS IFN- (ng ml ) 6 4 Lymph node cells NS Nfkbiz / Nfkbiz / Nfkbiz / Nfkbiz / IL- (ng ml ) 3 1 Splenocytes IL- (ng ml ) 1 8 6 4 *** ** Lymph node cells
7.06 Cell Biology Exam #3 April 23, 2002
 RECITATION TA: NAME: 7.06 Cell Biology Exam #3 April 23, 2002 This is an open book exam and you are allowed access to books, notes, and calculators. Please limit your answers to the spaces allotted after
RECITATION TA: NAME: 7.06 Cell Biology Exam #3 April 23, 2002 This is an open book exam and you are allowed access to books, notes, and calculators. Please limit your answers to the spaces allotted after
SUPPLEMENTARY INFORMATION
 doi:10.1038/nature11095 Supplementary Table 1. Summary of the binding between Angptls and various Igdomain containing receptors as determined by flow cytometry analysis. The results were summarized from
doi:10.1038/nature11095 Supplementary Table 1. Summary of the binding between Angptls and various Igdomain containing receptors as determined by flow cytometry analysis. The results were summarized from
Supplementary Figures
 Supplementary Figures Supplementary Figure 1. Confirmation of Dnmt1 conditional knockout out mice. a, Representative images of sorted stem (Lin - CD49f high CD24 + ), luminal (Lin - CD49f low CD24 + )
Supplementary Figures Supplementary Figure 1. Confirmation of Dnmt1 conditional knockout out mice. a, Representative images of sorted stem (Lin - CD49f high CD24 + ), luminal (Lin - CD49f low CD24 + )
