Alterations in growth and apoptosis of IRS-1 deficient beta-cells
|
|
|
- Martin Elliott
- 5 years ago
- Views:
Transcription
1 Articles in PresS. Am J Physiol Endocrinol Metab (April 12, 2005). doi: /ajpendo Alterations in growth and apoptosis of IRS-1 deficient beta-cells Anita M. Hennige* 1,4, Umut Ozcan* 1, Terumasa Okada 1, Ulupi S. Jhala 2, Markus Schubert 1, Morris F. White 3 and Rohit N. Kulkarni 1 1 Research Division, Joslin Diabetes Center & Department of Medicine, Harvard Medical School, Boston, MA 02215; 2 Islet Research Labs, The Whittier Institute-UCSD, La Jolla CA 92037; 3 Children s Hospital, Howard Hughes Medical Institute, Harvard Medical School, Boston, MA *These authors contributed equally Address correspondence to: Rohit N. Kulkarni MD PhD Rm 602, Joslin Diabetes Center, One Joslin Place, Boston MA rohit.kulkarni@joslin.harvard.edu 4 Current address of A.M. Hennige: University of Tuebingen, Internal Medicine 4, Tuebingen, Germany. Copyright 2005 by the American Physiological Society. 1
2 Abstract Insulin and insulin-like growth factor-i (IGF-I) activate anti-apoptotic pathways via insulin receptor substrate (IRS) proteins in most mammalian cells including β-cells. IRS-1 knockout (IRS-1KO) mice show growth retardation, hyperinsulinemia and hyperplastic but dysfunctional islets without developing overt diabetes, while IRS-2KOs develop insulin resistance and islet hypoplasia leading to diabetes. Since both models display insulin resistance it is difficult to differentiate islet response to insulin resistance from islet defects due to loss of proteins in the islets itself. We used a transplantation approach, as a means of separating host insulin resistance from islet function, to examine alterations in proteins in insulin/igf-i signaling pathways that may contribute to β-cell proliferation and/or apoptosis in IRS-1KO islets. Islets isolated from wildtype (WT) or IRS-1KO mice were transplanted into WT or insulin resistant IRS-1KO males under the kidney capsule. The β-cell mitotic rate in transplanted islets in IRS-1KO recipients was increased 1.5-fold compared to WT recipients, and was similar to that in endogenous pancreases of IRS-1KOs, while β-cell apoptosis was reduced by ~80% in IRS-1KO grafts in IRS-1KO recipients compared to WT recipients. Immunohistochemistry showed a substantial increase in IRS-2 expression in IRS-1KO islets transplanted into IRS-1KO mice as well as in endogenous islets from IRS-1KOs. Furthermore, enhanced cytosolic Forkhead transcription factor (FoxO1) staining in IRS-1KO grafts suggests intact Akt/PKB activity. Together, these data indicate that even in the absence of insulin resistance, β-cells deficient in IRS-1 exhibit a compensatory increase in IRS-2, which is associated with islet growth and is characterized by both proliferative and anti-apoptotic effects that likely occur via an insulin/igf-1/irs-2 pathway. Key words: IRS proteins, islets, transplantation, insulin resistance, beta-cell growth Running Title: beta-cell growth in IRS-1 deficient mice 2
3 Introduction Type 2 diabetes is a metabolic disease characterized by insulin resistance in peripheral organs including liver, skeletal muscle and adipose tissue coupled with a failure of the β-cells to compensate for the increasing demand (15; 32; 33; 48). Naturally occurring rodent models of diabetes and obesity, such as the ob/ob or db/db mouse, and genetically engineered models of insulin resistance such as mice heterozygous for deletion of the insulin receptor and insulin receptor-substrate-1 (IR/IRS-1) or homozygous for deletion of IRS-1 (IRS-1KO) all show islet hyperplasia (18; 25; 34). Although the precise pathway(s) that underlie the islet hyperplasia in each of the different models are not fully defined, several factors including glucose, insulin, growth hormone, prolactin, placental lactogen, glucagon-like peptide-1, hepatocyte growth factor/scatter factor, and the insulin-like growth factors (IGFs) have all been implicated in the β- cell proliferation response (25; 46; 47)(11; 39). In addition to the effects on classic target tissues, including the skeletal muscle, liver and adipose, it is now well established that the insulin/igf-i signaling pathway plays an important role in non classical tissues such as pancreatic islets (21) and the central nervous system (4). For example, mice lacking functional receptors for insulin (ßIRKO) or IGF-I (ßIGFKO) in β-cells manifest a type 2 diabetes phenotype characterized by loss of first phase insulin response to glucose stimulation and progressive glucose intolerance (22; 23; 51). Although, neither βirko nor βigfko mice exhibit developmental defects in islets, the βirko mice do show failure to increase β-cell mass in an age-dependent manner suggesting a role for insulin in growth and/or survival of β-cells. Furthermore, we have previously shown that islets from normal WT mice transplanted under the renal capsule of insulin-resistant normoglycemic insulin receptor/insulin receptor substrate-1 double heterozygous (DH) or ob/ob mice, proliferate at a higher rate 3
4 compared to WT recipients (10), suggesting that either insulin itself and/or a glucose-independent factor promotes islet growth in these models. In many different types of cells, insulin exerts anti-apoptotic and proliferative effects by activating IRS proteins (35; 44). All four IRS proteins, including IRS-1, -2, -3 and 4, are expressed in mouse islets (27). Each IRS-protein contains a highly conserved N-terminal pleckstrin homology (PH) domain followed by a phosphotyrosine-binding domain (PTB), which together couple IRS proteins to the activated insulin or IGF-1 receptors. Upon phosphorylation, IRS proteins bind to SH2 domains of effector proteins, including the regulatory subunit of the lipid kinase phosphatidylinositol 3-kinase (PI 3-kinase) (37). Products of PI 3-kinase activate a network of serine/threonine kinases implicated in the pleiotropic effects of insulin including glucose transport, glycogen and protein synthesis, hepatic gluconeogenesis, anti-apoptosis and proliferation (6). Although the precise pathways that mediate growth and anti-apoptotic effects of insulin in pancreatic β-cells have not been fully explored, the forkhead transcription factor, FKHR, has been recently implicated in β-cell growth (17), while other studies suggest a role for signals from endothelial cells including vascular endothelial growth factor A (VEGFA) (29; 31). IRS-1 is an important mediator of insulin/igf-1 receptor signaling in most tissues, including islets. IRS-1KO mice develop hyperplastic islets that is likely due to the insulin resistance and/or ambient hyperinsulinemia (1; 27; 41). In contrast, IRS-2KOs manifest hepatic insulin resistance and islet hypoplasia of varying severity depending on the genetic background, leading to a phenotype ranging from mild to severe diabetes, and suggesting that IRS-2 is involved in β-cell growth and/or survival (20; 49; 50). The potential role of IRS-2 in β-cell growth and/or anti-apoptosis prompted us to examine whether this substrate protein is also involved in the islet hyperplastic response in mice lacking IRS-1. Using a transplantation 4
5 approach, as a means of separating host insulin resistance from islet function, we show that enhanced β-cell proliferation and survival in islets deficient in IRS-1 is associated with a compensatory increase in expression of IRS-2. Materials and Methods: Animals IRS-1 knockout mice (IRS-1KO) were backcrossed onto the C57Bl/6 background at Taconic Farms and were essentially syngeneic. All mice were maintained on a 12-hour light/dark cycle and housed at the Joslin Diabetes Center in accordance with IACUC protocols. Mice were fed standard mouse chow ad libitum and were housed individually after transplantation. Islet Isolation and Transplantation Islets were isolated from 8-week-old male mice as described previously (27) using the intraductal liberase technique and hand-picked under a stereomicroscope (Stereozoom GZ7, Leica, Deerfield, IL). Following isolation, 150 islets were hand-picked and kept on ice until transplantation. We chose 150 size-matched islets based on pilot studies wherein 150 islets did not alter metabolic variables including serum insulin and blood glucose levels in the recipients. In the event some of the isolated islets were larger we transplanted similar islet equivalents (IEs) so that total amount of islet tissue was comparable between all groups. Surgery was performed under anesthesia that was induced by an intra-peritoneal injection of a 1:1 mixture of 2,2,2- tribromoethanol and tert-amyl alcohol and diluted 1:50 in PBS (ph 7.4) at a dose of 15 µl/g body weight. Using a retroperitoneal approach, the capsule of one of the kidneys was incised, and islets were implanted near the upper pole in 8-week-old male recipient mice. The capsule was cauterized, and mice were allowed to recover on a heating pad. 5
6 Metabolic analysis. Body weight, blood glucose and serum insulin levels were followed weekly after transplantation. Blood was obtained from the tail vein in the fed state and glucose levels were measured using Glucometer Elite (Bayer). For insulin measurement, blood was collected in chilled heparinized capillaries and immediately centrifuged to obtain plasma that was stored at 20 C for subsequent insulin ELISA (Crystal Chem, Chicago, IL) or for serum IGF-I by RIA (ALPCO). DNA Laddering To examine whether islet cells lacking IRS-1 are more resistant to cell death we treated WT and IRS-1KO islets with TNFα and subjected the extracted DNA to laddering using standard protocols (Biosource Kit) (12). Briefly, islets are plated on 6 cm dishes and cultured overnight. Following treatment with TNFα (50 ng/ml), the supernatant is discarded and attached cells are removed. Lysis buffer and enzyme are added following manufacturers instructions; the solution is vortexed and placed at 37 C for 10 min. Ammonium acetate and cold methanol (-20 C) are added and tubes placed in -20C for 15 min. The tubes are centrifuged at 12,000 rpm for 5 min and pellet is washed with cold 70% ethanol. The supernatant is discarded and pellet is allowed to dry at RT for 5 min. The DNA pellet is resuspended in 30 µl of DNAse-free water and allowed to dissolve for 24 h. DNA content is measured using a spectrophotometer and 1µg is run on a 1.5% agarose gel. Three mice from each group were evaluated. Sectioning of Endogenous Pancreas and Islet Grafts. 6
7 Five weeks after transplantation, the random fed recipient mice were injected with 5-bromo-2- deoxyuridine (BrdU, 100 µg/g b.wt; Roche, IN) and 6 hours later were anesthetized and sacrificed by perfusion using 4% PFA solution. The endogenous pancreas and kidney tissue including islet graft was rapidly harvested from recipient mice and fixed in 4% PFA for 16 hours. Pancreas were embedded in paraffin and sectioned (7-10 µm thickness). Further, kidney tissue was embedded in Araldite 502 resin and cured at 60º C for 48 hours. One micron sections were cut, adhered to glass slides with heat and stained with 1% methylene blue containing 1% sodium borate. Capillaries were counted as mean ± SEM of capillaries over number of ß-cell nuclei per section, 3 sections per animal and at least 3 mice per group (at least 900 capillaries per animal). Semi-quantitative fluorescence immunocytochemistry. Following re-hydration and permeabilization (1 % Triton X-100), sections were incubated with guinea pig anti-insulin (Zymed Laboratories, Inc.), rabbit anti-irs-2, or rabbit anti-fkhr (Santa Cruz) antibodies overnight at 4 C. To minimize variability between sections, the staining procedures were performed in parallel with the same batch of antisera and same incubation times for fixation, permeabilization, blocking, and exposure to antisera were employed for all sections. Islet proliferation was estimated by following double-label insulin and BrdU immunohistochemistry. Detection was performed with rhodamine and fluorescein-conjugated secondary antibodies (Jackson Immunoresearch). We identified apoptotic cells in de-paraffinized sections using a Rhodamine DNA fragmentation detection assay (TUNEL, Intergen Company). BrdU and TUNEL positive cells were calculated as the mean ± SEM of at least 500 insulin positive ß-cells over total ß-cells per section, 3 sections per animal and at least 3 mice per group. 7
8 To determine the cell distribution of IRS-2 in islets we immunostained pancreas sections from WT mice with anti-irs-2 antibody (Santa Cruz) using standard protocols (22). Western blotting: Islets were lyzed with buffer A containing 25 mm Tris HCl (ph 7.4), 2 mm Na 3 VO 4, 10 mm NaF, 10 mm Na 4 P 2 O 7, 1 mm EGTA, 1 mm EDTA, 5 µg/ml of leupeptin, 5 µg/ml of aprotinin, 1 mm phenylmethylsulfonyl fluoride, and 1 % Nonidet P-40. The lysates were subjected to immunoblotting and visualized by an enhanced chemiluminescence system (Roche Applied Science, Indianapolis, IN). Antibodies recognizing Akt and phospho-akt (Ser 473) were purchased from Cell Signaling Technology (Beverly, MA) and used at dilution 1:1000 for immunoblotting (45). Statistics. Results are expressed as mean ± SEM. For comparison between groups the unpaired Student s t- test (two tailed) was used. p-values less than 0.05 were considered significant. Results: Four groups of 8-week-old WT or IRS-1KO male mice on a pure C57Bl/6 background were transplanted with either 150 WT or IRS-1KO islets under the kidney capsule and followed weekly with measurements of body weight, serum glucose and insulin levels. Before transplantation, IRS-1KO mice were ~35% smaller than WT mice as previously described (20; 27) (Fig. 1a). The transplanted islet mass did not alter blood glucose or body weight over the 5- week transplantation period (Fig. 1a,b). Eight-week-old IRS-1KO mice were hyperinsulinemic with ~3-fold elevated serum insulin levels compared to WT mice as previously observed (1; 41), and these did not alter significantly over the transplantation period (Fig. 1c). Serum IGF-I 8
9 concentrations were comparable in IRS-1KO and WT mice (284 ± 29 vs 310 ± 37 ng/ml; n=6; p = NS). Compensation for insulin resistance can occur either by enhanced insulin secretion, an increase in β-cell mass or both (9; 16; 24). An enhanced β-cell mass can be secondary to replication of preexisting β-cells or an increase in β-cell survival (2; 3). To measure β-cell proliferation and apoptosis, we performed in situ assays using 5-bromodeoxyuridine (BrdU) and terminal deoxyribotransferase (TdT)-UTP nick end labeling (TUNEL) staining on endogenous pancreas and islet graft sections harvested 5 weeks after transplantation. As expected, the percentage of BrdU positive β-cells was increased in pancreas sections of IRS-1KO mice, likely due to peripheral insulin resistance (Fig 2a). In grafts of IRS-1KO islets into IRS-1KO hosts (IRS-1-IRS-1), a profound increase in BrdU incorporation was observed while replication in all other groups was unchanged (Fig. 2b). The increase in BrdU positive cells in IRS-1KO transplanted islets into IRS-1KO mice was similar to that present in endogenous islets of the IRS- 1KO host animal and represented a ~41% increase compared to WT. Interestingly, IRS-1KO islets transplanted into WT animals (IRS-1-wt) exhibited a slight, but non-significant, decrease in BrdU incorporation compared to WT islets transplanted into WT mice (wt-wt). Conversely, BrdU incorporation was increased in WT islets transplanted into IRS-1KO mice (wt-irs-1), suggesting that insulin resistance in IRS-1KO mice is one potential factor promoting β-cell mitosis in WT grafts. In fact, examination of serum insulin levels of host animals in the WT to IRS-1KO group revealed a positive and linear correlation with BrdU incorporation (Fig. 2c), suggesting that insulin itself is a potential islet growth factor (25; 26). To assess apoptosis, we stained pancreas and graft sections using the terminal deoxyribotransferase (TdT)-UTP nick end labeling (TUNEL) assay. By contrast to proliferation, 9
10 TUNEL staining showed a 50% decrease in apoptosis in endogenous IRS-1KO islets as compared to WT islets (Fig. 3a). Next, TUNEL staining in graft tissue showed a ~3-fold increase in apoptosis in the IRS-1KO into WT group, while the number of apoptotic cells in the IRS-1KO into IRS-1KO group was lower but did not reach significant levels (Fig. 3b, c). To confirm the alterations in cell death in IRS-1 knockout islet cells we used a different approach by treating freshly isolated islets with TNF-α for 24 h and subjecting the isolated DNA to laddering. As expected, significant laddering was evident in WT islets and significantly minimal effects were observed in the IRS-1KO group (Fig 3d). These data clearly indicate that cell death is lower in the absence of IRS-1 and it is likely that there are compensatory effects that contribute to protection against cell death in the mutants. Maximal cell death in a transplant setting has been reported to occur two weeks after surgery (8). Therefore, to assess whether a significant reduction in cell death in IRS-1KO beta cells in the presence of hyperinsulinemia can be unmasked at an earlier time after transplantation, we examined apoptosis in the grafts 2 to 3 weeks after transplanting IRS-1KO islets in IRS-1KO recipients. Again, we observed a trend towards a decrease in apoptosis, but no significant differences were evident (data not shown). It is possible that several factors including a greater level of circulating insulin is necessary to significantly impact on apoptosis in β-cells (25). To determine potential alterations in proteins in the insulin/igf-i signaling pathway that promote β-cell proliferation and anti-apoptosis we performed immunohistochemistry on endogenous pancreas and islet graft sections. IRS-2 is one of the intermediates suggested to promote β-cell development, proliferation and survival (50). Although we could barely detect IRS-2 expression in WT islets of 13-week old mice, we were able to easily detect IRS-2 in islets from IRS-1KO mice by immunohistochemistry (Fig. 4a). Furthermore, immunostaining for IRS-2 10
11 in islet grafts also showed an increase in IRS-2 expression in the IRS-1KO into IRS-1KO group (Fig. 4b), suggesting that the compensatory increase in IRS-2 promotes both proliferation and anti-apoptotic signals in these islets. IRS-2 staining in IRS-1KO islets transplanted into WT mice, on the other hand, was comparable to WT islets, suggesting that elevated serum insulin levels in IRS-1KO host animals likely promote expression of IRS-2 in the absence of IRS-1 (Fig. 4b). Interestingly, we observed expression of IRS-2 protein in α-cells in WT islets using immunohistochemistry (Fig 4c). Further work is necessary to evaluate whether IRS-2 indeed has a potential role in regulation of islet α-cell growth and/or function. The IRS-2-PI 3-kinase cascade controls many downstream elements, including Akt/PKB, BAD/Bcl2, and the FoxO family of transcription factors (AFX, FKHR, and FKHRL1) (17; 36). FKHR is located in the nucleus under basal conditions where it is transcriptionally active. Insulin and IGF-I, acting via Akt, stimulate phosphorylation of FKHR, which causes it to bind to proteins and accumulates in the cytosol where it is unable to regulate gene expression. Compared to WT islets, IRS-1KO islets transplanted into IRS-1KO mice exhibited increased phosphorylation of FKHR as determined by nuclear exclusion using immunohistochemistry (Fig. 5). Moreover, IRS-1KO islets in WT mice show lower level of cytoplasmic FKHR, suggesting that these cells are undergoing apoptosis. This is consistent with nuclear restriction of FoxO1 (FKHR) in β-cells lacking insulin receptors (T. Okada and R. N. Kulkarni, unpublished observations). Together, these findings are in agreement with an increased number of apoptotic cells in IRS-1KO into WT group compared to transplanted IRS-1KO islets into IRS-1KO mice. We did not detect significant differences in the expression of total or phospho-akt protein levels between WT and IRS-1 KO groups by Western blotting of freshly isolated islets in the basal state (data not shown). It is possible that exposing the isolated islets to the ambient environment of 11
12 hyperinsulinemia found in vivo in the IRS-1KO mice is necessary to unmask differences in Akt levels between groups. Thus, increased expression of IRS-2 is associated with alterations in gene transcription and anti-apoptosis that likely occurs via a PKB/forkhead-transcription-factormediated pathway. PDX-1 is critical for the development of the pancreas in mice and humans and complete disruption of PDX-1 results in pancreatic agenesis (28; 40). Expression of PDX-1 has been shown to be diminished or unchanged in islets from IRS-2KO mice depending on the genetic background of the mutants (28; 42). Immunostaining for insulin and PDX-1 revealed no significant changes among the groups in our study suggesting that proliferation in cells lacking IRS-1 is independent of changes in PDX-1 in this model (Fig. 6). Microvascularization of islets is essential for successful engraftment and long-term function of islet grafts. To investigate whether islets from IRS-1KO mice show a revascularization pattern similar to those from WT donors, we measured capillary density in plastic sections of islet grafts (Fig. 7a). A significant increase in capillary density was detected in IRS-1KO islets transplanted into IRS-1KO mice compared with other groups (Fig. 7b). Preliminary data suggest that expression of VEGF is up-regulated in grafts that exhibit more capillaries (data not shown) and other reports indicate a role for morphogens derived from endothelial cells as potential signals for islet cell growth (19; 30; 31). Whether the increase in capillary density is a consequence of increased β-cell proliferation in IRS-1KO islets transplanted into IRS-1KO mice or is secondary to a specific process involving IRS-signaling in endothelial cells (19; 30; 31) requires further investigation. 12
13 Discussion Islet hyperplasia is a feature of many metabolic disorders including obesity, type 2 diabetes, glucocorticoid excess and elevated levels of growth hormone. Although insulin and IGF-I promote growth and anti-apoptosis in most mammalian tissues, a role for these hormones in mediating the islet proliferative response in hyperinsulinemic states has not been fully explored. In the present study we used a transplantation approach to demonstrate that enhanced IRS-2 expression is associated with enhanced growth and anti-apoptosis of β-cells in IRS-1KO islet grafts. We have previously shown that islets deficient in IRS-1 show defects in nutrientstimulated insulin secretion and reduced insulin content (22). However, IRS-1KOs and obese IRS-1 heterozygotes are able to compensate and maintain glucose homeostasis and do not become overtly diabetic even as they age (38). The factors that promote the islet compensatory response in the absence of one or both alleles for IRS-1 are not fully understood, but show a strong correlation with insulin resistance. The basal proliferation of β-cells in the endogenous pancreas of hyperinsulinemic IRS-1KO recipients is significantly increased while basal apoptosis levels are low. When WT islets were transplanted into hyperinsulinemic but normoglycemic IRS- 1KO recipients, we detected an increase in β-cell proliferation and this showed a significant correlation with circulating insulin levels. Interestingly, the IRS-1 islets transplanted into IRS- 1KO recipients responded with the highest BrdU incorporation and lowest levels of apoptosis while IRS-1KO islets transplanted into WT recipients revealed enhanced apoptosis of β-cells and reduced proliferation. Together these data indicate that after a period of adaptation to hyperinsulinemic conditions in the donor animal when β-cell proliferation is high, exposure of the islets to a relatively hypoinsulinemic environment in the recipient leads to β-cell apoptosis 13
14 suggesting that insulin either plays an important anti-apoptotic role or promotes β-cell growth in the hyperinsulinemic state. In the present study we observed that IRS-2 is up-regulated in IRS-1KO islets and protein levels of IRS-2 were also increased in WT grafts exposed to the ambient hyperinsulinemia in IRS-1KO recipients. Although IGF-I has been suggested to be an important ligand in the IGF- 1/IRS-2 axis (49), our data indicate that insulin likely recruits IRS-2 via insulin and/or an insulin/igf-1 hybrid receptor since serum IGF-I levels in IRS-1KO mice are normal. Whether local IGF-I is altered in the mutant islets and potentially plays a role in this scenario is not clear (7). Growth factors including insulin and IGF-I signal via the IRS-2, PI3-K/Akt pathway to promote cell survival (5; 14). Activation of Akt has been demonstrated to inhibit apoptosis by inhibiting the release of cythochrome c from mitochondria (13) and to phosphorylate the forkhead transcription factor, FKHR, thereby blocking its pro-apoptotic effects. In the absence of IRS-2, IGF-I-stimulated Akt phosphorylation has been shown to be reduced with consequent high levels of cleaved/activated caspase-3. Furthermore, FKHR is also phosphorylated to a lesser degree in IRS-2KO islets suggesting a partial disruption in the Akt pathway. Thus, it is possible that upregulation of IRS-2 levels in IRS-1 mutant islets activates the major survival pathway mediated by Akt activation and FKHR phosphorylation in response to the elevated circulating insulin levels. Another factor that may participate in the islet growth response is the enhanced capillary density in the grafts in IRS-1KO recipients. Indeed, continuous infusion of VEFG has been shown to improve vascularization in rats (43) and VEGF-A has been recently shown to play a role in vascularization of islets (31). Whether increased formation of capillaries in IRS-1KO islets is the consequence of increased β-cell proliferation or a specific process involving VEGF (31) 14
15 due to the deletion of IRS-1 needs further investigation. In summary, we provide evidence that IRS-2 plays a role in the islet compensatory response in IRS-1 null mice likely via an IRS- 2/Akt/FoxO1 (FKHR) pathway in IRS-1 deficient islets. Acknowldgements The authors thank C. Ronald Kahn for critical comments on the manuscript and IRS-1 KO mice, Jane Hu for excellent assistance with immunohistochemistry and Julie Marr for excellent secretarial assistance. R.N. Kulkarni is supported by a K08 Clinician Scientist Development Award (DK 02885). A. M. Hennige was supported by a grant from Deutsche Forschungsgemeinschaft (HE-3321/1-1). This study was supported by NIH grants R03 DK66207, R01 DK67536, R01 DK66207 to (R.N.K.) and the Joslin DERC Grant P30 DK (Specialized Assay and Advanced Microscopy Cores). Figure Legends: Fig 1 Metabolic effects of islet transplantation A) Body weight of male islet recipients followed up for 5 weeks after transplantation. B) Blood glucose levels were measured in samples obtained through tail bleeds of fed male recipients at the indicated ages (weeks after transplantation). Values are means ± SEM (n=5 mice per genotype). C) Serum insulin levels were measured using ELISA in fed recipient mice. Data are means ± SEM (n=5 mice per genotype). In all labels, the donor is represented followed by the recipient. 15
16 Fig 2 β-cell proliferation. A) BrdU incorporation analysis was performed in anti-insulin and anti-brdu double-labeled pancreas sections of 13-week old recipient mice and expressed as % of insulin-positive β-cells. Results are means ± SEM of five mice per genotype, *p< B) BrdU incorporation analysis in anti-insulin and anti-brdu double-labeled islet-graft sections. Results are means ± SEM (n=5 mice per genotype); *p< C) BrdU incorporation analysis in WT into IRS-1KO islet graft sections expressed as % BrdU-positive cells 5 weeks after transplantation. Each data point represents one animal. A significant correlation was observed with an r value of 0.99 (P<0.002). In all labels, the donor is represented followed by the recipient. Fig 3 β-cell apoptosis. A) Apoptotic cells were detected in de-paraffinized pancreas sections using a Flourescein DNA fragmentation detection assay (TUNEL). Number of apoptotic nuclei is shown for 13-week-old mice. Values are expressed as means ± SEM of four mice per genotype. B) Number of apoptotic nuclei per β-cells is shown in grafts, 5 weeks after transplantation. Values are expressed as means ± SEM of five mice per genotype, *p< In all labels, the donor is represented followed by the recipient. C) A representative magnified image showing coimmunostaining of insulin and TUNEL in pancreas sections from a wild type mouse. Arrows indicate cells positive for both TUNEL and insulin. D) DNA laddering in islets isolated from WT and IRS-1KO mice. Three individual mice from Controls (C1, C2, C3) and IRS-1 knockouts (IRS-1KO1, IRS-1KO2, IRS- 1KO3) are shown. 16
17 Fig 4 Immunostaining for IRS-2 A) Immunohistochemistry was performed on pancreas sections of WT and IRS-1KO mice fixed in 4% PFA and embedded in paraffin. Following re-hydration sections were incubated with antiinsulin, and anti-irs-2 antibodies. Sections were processed identically and simultaneously as described. Representative sections of 3 mice per genotype 5 weeks after transplantation are shown. B) Anti-insulin and anti-irs-2 co-stained sections of transplanted islets under the kidney capsule. Representative sections of 13-week-old mice are shown. In all labels, the donor is represented followed by the recipient. C) Co-immunostaining for IRS-2 and glucagon and DAPI in pancreas section from a 10-week-old WT adult male mouse. Red, IRS-2; Green, glucagon; Blue, DAPI. Magnification 40X, bar = 50 micron. Fig 5 Immunostaining for FKHR Immunostaining was performed on transplanted islets under the kidney capsule fixed in 4% PFA and embedded in paraffin. Following re-hydration sections were incubated with anti-insulin and anti-fkhr antibodies. Sections were processed identically and simultaneously as described in Methods. Representative sections of 3 mice per genotype 5 weeks after transplantation are shown. In all labels, the donor is represented followed by the recipient. Fig 6 Immunostaining for PDX-1 A) Immunohistochemistry was performed on transplanted islets under the kidney capsule fixed in 4% PFA and embedded in paraffin. Following re-hydration sections were incubated with anti- PDX-1 antibody. Sections were processed identically and simultaneously as described in methods. Representative sections of 2 mice per genotype 5 weeks after transplantation are shown. 17
18 Fig 7 Capillary density A) Methylene blue stained plastic sections (1µm) are shown at a magnification of 63x. In stained graft-sections, islet tissue was easily identified and capillaries counted. B) Values are expressed as number of capillaries per β-cell nucleus ± SEM of at least three mice per genotype, *p< In all labels, the donor is represented followed by the recipient. Reference List 1. Araki E, Lipes MA, Patti ME, Brüning JC, Haag BL, III, Johnson RS and Kahn CR. Alternative pathway of insulin signaling in mice with targeted disruption of the IRS-1 gene. Nature 372: , Bonner-Weir S, Baxter L, Schuppin GT and Smith FE. A second pathway for regeneration of adult exocrine and enocringe pancrease. A possible recapitulation of embryonic development. Diabetes 42: , Bonner-Weir S, Deery D, Leahy JL and Weir GC. Compensatory growth of pancreatic β-cells in adult rats after short-term glucose infusion. Diabetes 38: 49-53, Bruning JC, Gautam D, Burks DJ, Gillette J, Schubert M, Orban PC, Klein R, Krone W, Muller-Wieland D and Kahn CR. Role of brain insulin receptor in control of body weight and reproduction. Science 289: ,
19 5. Budihardjo I, Oliver H, Lutter M, Luo X and Wang X. Biochemical pathways of caspase activation during apoptosis. Annu Rev Cell Dev Biol 15: , Cheatham B and Kahn CR. Insulin action and the insulin signaling network. Endocr Rev 16: , D'Ercole AJ and Calikoglu AS. Editorial review: the case of local versus endocrine IGF-I actions: the jury is still out. Growth Horm IGF Res 11: , Davalli AM, Scaglia L, Zangen DH, Hollister J, Bonner-Weir S and Weir GC. Vulnerability of islets in the immediate posttransplantation period. Dynamic changes in structure and function. Diabetes 45: , Dor Y, Brown J, Martinez OI and Melton DA. Adult pancreatic beta-cells are formed by self-duplication rather than stem-cell differentiation. Nature 429: 41-46, Flier SN, Kulkarni RN and Kahn CR. Evidence for a circulating islet cell growth factor in insulin- resistant states. Proc Natl Acad Sci U S A 98: , Garcia-Ocana A, Takane KK, Syed MA, Philbrick WM, Vasavada RC and Stewart AF. Hepatocyte growth factor overexpression in the islet of transgenic mice increases beta cell proliferation, enhances islet mass, and induces mild hypoglycemia. J Biol Chem 275: ,
20 12. Han X, Sun Y, Scott S and Bleich D. Tissue inhibitor of metalloproteinase-1 prevents cytokine-mediated dysfunction and cytotoxicity in pancreatic islets and beta-cells. Diabetes 50: , Hennige AM, Burks DJ, Ozcan U, Kulkarni RN, Ye J, Park S, Schubert M, Fisher TL, Dow MA, Leshan R, Zakaria M, Mossa-Basha M and White MF. Upregulation of insulin receptor substrate-2 in pancreatic beta cells prevents diabetes. J Clin Invest 112: , Hugl SR, White MF and Rhodes CJ. Insulin-like growth factor I (IGF-I)-stimulated pancreatic beta-cell growth is glucose-dependent. Synergistic activation of insulin receptor substrate-mediated signal transduction pathways by glucose and IGF-I in INS-1 cells. J Biol Chem 273: , Kahn CR. Insulin action, diabetogenes, and the cause of type II diabetes (Banting Lecture). Diabetes 43: , Kahn CR, Bruning JC, Michael MD and Kulkarni RN. Knockout mice challenge our concepts of glucose homeostasis and the pathogenesis of diabetes mellitus. J Pediatr Endocrinol Metab 13 Suppl 6: , Kitamura T, Nakae J, Kitamura Y, Kido Y, Biggs WH3, Wright CV, White MF, Arden KC and Accili D. The forkhead transcription factor Foxo1 links insulin signaling to Pdx1 regulation of pancreatic beta cell growth. J Clin Invest 110: ,
21 18. Kloppel G, Lohr M, Habich K, Oberholzer M and Heitz PU. Islet pathology and the pathogenesis of type 1 and type 2 diabetes mellitus revisited. Surv Synth Pathol Res 4: , Kondo T, Vicent D, Suzuma K, Yanagisawa M, King GL, Holzenberger M and Kahn CR. Knockout of insulin and IGF-1 receptors on vascular endothelial cells protects against retinal neovascularization. J Clin Invest 111: , Kubota N, Tobe K, Terauchi Y, Eto K, Yamauchi T, Suzuki R, Tsubamoto Y, Komeda K, Nakano R, Miki H, Satoh S, Sekihara H, Sciacchitano S, Lesniak M, Aizawa S, Nagai R, Kimura S, Akanuma Y, Taylor SI and Kadowaki T. Disruption of insulin receptor substrate 2 causes type 2 diabetes because of liver insulin resistance and lack of compensatory β-cell hyperplasia. Diabetes 49: , Kulkarni RN. Receptors for insulin and insulin-like growth factor-1 and insulin receptor substrate-1 mediate pathways that regulate islet function. Biochem Soc Trans 30: , Kulkarni RN, Bruning JC, Winnay JN, Postic C, Magnuson MA and Kahn CR. Tissue-specific knockout of the insulin receptor in pancreatic β cells creates an insulin secretory defect similar to that in Type 2 diabetes. Cell 96: ,
22 23. Kulkarni RN, Holzenberger M, Shih DQ, Ozcan U, Stoffel M, Magnuson MA and Kahn CR. beta-cell-specific deletion of the Igf1 receptor leads to hyperinsulinemia and glucose intolerance but does not alter beta-cell mass. Nat Genet 31: , Kulkarni RN, Jhala US, Winnay JN, Krajewski S, Montminy M and Kahn CR. PDX-1 haploinsufficiency limits the compensatory islet hyperplasia that occurs in response to insulin resistance. J Clin Invest 114: , Kulkarni RN and Kahn CR. Genetic models of insulin resistance: alterations in ß-cell biology. In: Molecular basis of pancreas development and function, edited by Habener JF and Hussain M. New York City: Kluwer Academic Publishers, 2001, p Kulkarni RN and Kahn CR. Molecular biology. HNFs--linking the liver and pancreatic islets in diabetes. Science 303: , Kulkarni RN, Winnay JN, Daniels M, Bruning JC, Flier SN, Hanahan D and Kahn CR. Altered function of insulin receptor substrate-1-deficient mouse islets and cultured beta-cell lines. J Clin Invest 104: R69-R75, Kushner JA, Ye J, Schubert M, Burks DJ, Dow MA, Flint CL, Dutta S, Wright CV, Montminy MR and White MF. Pdx1 restores beta cell function in Irs2 knockout mice. J Clin Invest 109: ,
23 29. Lammert E, Cleaver O and Melton D. Induction of pancreatic differentiation by siganls from blood vessels. Science 1-10, Lammert E, Cleaver O and Melton D. Role of endothelial cells in early pancreas and liver development. Mech Dev 120: 59-64, Lammert E, Gu G, McLaughlin M, Brown D, Brekken R, Murtaugh LC, Gerber HP, Ferrara N and Melton DA. Role of VEGF-A in Vascularization of Pancreatic Islets. Curr Biol 13: , Lillioja S, Mott DM, Spraul M, Ferraro R, Foley JE, Ravussin E, Knowler WC, Bennett PH and Bogardus C. Insulin resistance and insulin secretory dysfunction as precursors of non-insulin-dependent diabetes mellitus: prospective studies of Pima Indians. N Engl J Med 329: 1992, Martin BC, Warram J, Krolewski AS, Bergman RN, Soeldner JS and Kahn CR. Role of glucose and insulin resistance in development of type 2 diabetes mellitus: results of a 25- year follow-up study+. The Lancet 340: , Michael MD, Kulkarni RN, Postic C, Previs SF, Shulman GI, Magnuson MA and Kahn CR. Loss of insulin signaling in hepatocytes leads to severe insulin resistance and progressive hepatic dysfunction. Mol Cell 6: 87-97,
24 35. Myers MG, Jr. and White MF. Insulin signal transduction and the IRS proteins. Annu Rev Pharmacol Toxicol 36: , Nakae J, Kido Y and Accili D. Distinct and overlapping functions of insulin and IGF-1 receptors. Endo Reviews 22: , Shepherd PR, Withers DJ and Siddle K. Phosphoinositide 3-kinase: the key switch mechanism in insulin signalling. Biochem J 333: , Shirakami A, Toyonaga T, Tsuruzoe K, Shirotani T, Matsumoto K, Yoshizato K, Kawashima J, Hirashima Y, Miyamura N, Kahn CR and Araki E. Heterozygous knockout of the IRS-1 gene in mice enhances obesity-linked insulin resistance: a possible model for the development of type 2 diabetes. J Endocrinol 174: , Smith FE, Rosen KM, Villa-Komaroff L, Weir GC and Bonner-Weir S. Enhanced insulin-like growth factor I gene expression in regenerating rat pancreas. Proc Natl Acad Sci U S A 88: , Stoffers DA, Heller RS, Miller CP and Habener JF. Developmental expression of the homeodomain protein IDX-1 in mice transgenic for an IDX-1 promoter/lacz transcriptional reporter. Endocrinology 140: , Tamemoto H, Kadowaki T, Tobe K, Yagi T, Sakura H, Hayakawa T, Terauchi Y, Ueki K, Kaburagi Y, Satoh S, Sekihara H, Yoshioka S, Horikoshi H, Furuta Y, Ikawa 24
25 Y, Kasuga M, Yazaki Y and Aizawa S. Insulin resistance and growth retardation in mice lacking insulin receptor substrate-1. Nature 372: , Terauchi Y, Matsui J, Suzuki R, Kubota N, Komeda K, Aizawa S, Eto K, Kimura S, Nagai R, Tobe K, Lienhard GE and Kadowaki T. Impact of genetic background and ablation of insulin receptor substrate (IRS)-3 on IRS-2 knock-out mice. J Biol Chem 278: , Trivedi N, Steil GM, Colton CK, Bonner-Weir S and Weir GC. Improved vascularization of planar membrane diffusion devices following continuous infusion of vascular endothelial growth factor. Cell Transplant 9: , Tseng YH, Ueki K, Kriauciunas KM and Kahn CR. Differential roles of insulin receptor substrates in the anti-apoptotic function of insulin-like growth factor-1 and insulin. J Biol Chem 277: , Ueki K, Fruman DA, Brachmann SM, Tseng YH, Cantley LC and Kahn CR. Molecular balance between the regulatory and catalytic subunits of phosphoinositide 3- kinase regulates cell signaling and survival. Mol Cell Biol 22: , Vasavada RC, Cavaliere C, D'Ercole AJ, Dann P, Burtis WJ, Madlener AL, Zawalich K, Zawalich W, Philbrick W and Stewart AF. Overexpression of parathyroid hormonerelated protein in the pancreatic islets of transgenic mice causes islet hyperplasia, hyperinsulinemia, and hypoglycemia. J Biol Chem 271: ,
26 47. Vasavada RC, Garcia-Ocana A, Zawalich WS, Sorenson RL, Dann P, Syed M, Ogren L, Talamantes F and Stewart AF. Targeted expression of placental lactogen in the beta cells of transgenic mice results in beta cell proliferation, islet mass augmentation, and hypoglycemia. J Biol Chem 275: , Warram JH, Martin BC, Krolewski AS, Soeldner JS and Kahn CR. Slow glucose removal rate and hyperinsulinemia are predictors of the risk of Type II diabetes in offspring of diabetic parents. Ann Intern Med 113: , Withers DJ, Burks DJ, Towery HH, Altamuro SL, Flint CL and White MF. Irs-2 coordinates Igf-1 receptor-mediated beta-cell development and peripheral insulin signalling. Nat Genet 23: 32-40, Withers DJ, Gutierrez JS, Towery H, Burks DJ, Ren JM, Previs S, Zhang Y, Bernal D, Pons S, Shulman GI, Bonner-Weir S and White MF. Disruption of IRS-2 causes type 2 diabetes in mice. Nature 391: , Xuan S, Kitamura T, Nakae J, Politi K, Kido Y, Fisher PE, Morroni M, Cinti S, White MF, Herrera PL, Accili D and Efstratiadis A. Defective insulin secretion in pancreatic beta cells lacking type 1 IGF receptor. J Clin Invest 110: ,
27
28
29 IRS1KO2 IRS1KO3 C3 C2 c) d) Insulin TUNEL Merge IRS1KO1 C1
30
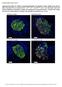
31 Fig 4c) IRS-2 Glucagon DAPI IRS-2/ Glucagon IRS-2/ Glucagon/ DAPI
32
33 Fig 6
34
Perspective: The Insulin Signaling System A Common Link in the Pathogenesis of Type 2 Diabetes
 Perspective: The Insulin Signaling System A Common Link in the Pathogenesis of Type 2 Diabetes Dominic J. Withers and Morris White Endocrinology 2000 141: 1917-1921, doi: 10.1210/en.141.6.1917 To subscribe
Perspective: The Insulin Signaling System A Common Link in the Pathogenesis of Type 2 Diabetes Dominic J. Withers and Morris White Endocrinology 2000 141: 1917-1921, doi: 10.1210/en.141.6.1917 To subscribe
(A) PCR primers (arrows) designed to distinguish wild type (P1+P2), targeted (P1+P2) and excised (P1+P3)14-
 1 Supplemental Figure Legends Figure S1. Mammary tumors of ErbB2 KI mice with 14-3-3σ ablation have elevated ErbB2 transcript levels and cell proliferation (A) PCR primers (arrows) designed to distinguish
1 Supplemental Figure Legends Figure S1. Mammary tumors of ErbB2 KI mice with 14-3-3σ ablation have elevated ErbB2 transcript levels and cell proliferation (A) PCR primers (arrows) designed to distinguish
Journal Club WS 2012/13 Stefanie Nickl
 Journal Club WS 2012/13 Stefanie Nickl Background Mesenchymal Stem Cells First isolation from bone marrow 30 ys ago Isolation from: spleen, heart, skeletal muscle, synovium, amniotic fluid, dental pulp,
Journal Club WS 2012/13 Stefanie Nickl Background Mesenchymal Stem Cells First isolation from bone marrow 30 ys ago Isolation from: spleen, heart, skeletal muscle, synovium, amniotic fluid, dental pulp,
Insights into insulin resistance and type 2 diabetes from knockout mouse models
 Insights into insulin resistance and type 2 diabetes from knockout mouse models Takashi Kadowaki Department of Metabolic Diseases, Graduate School of Medicine, University of Tokyo, Tokyo 113-8655, Japan.
Insights into insulin resistance and type 2 diabetes from knockout mouse models Takashi Kadowaki Department of Metabolic Diseases, Graduate School of Medicine, University of Tokyo, Tokyo 113-8655, Japan.
SUPPLEMENTARY INFORMATION
 Supplementary Figures Supplementary Figure S1. Binding of full-length OGT and deletion mutants to PIP strips (Echelon Biosciences). Supplementary Figure S2. Binding of the OGT (919-1036) fragments with
Supplementary Figures Supplementary Figure S1. Binding of full-length OGT and deletion mutants to PIP strips (Echelon Biosciences). Supplementary Figure S2. Binding of the OGT (919-1036) fragments with
Role of fatty acids in the development of insulin resistance and type 2 diabetes mellitus
 Emerging Science Role of fatty acids in the development of insulin resistance and type 2 diabetes mellitus George Wolf Insulin resistance is defined as the reduced responsiveness to normal circulating
Emerging Science Role of fatty acids in the development of insulin resistance and type 2 diabetes mellitus George Wolf Insulin resistance is defined as the reduced responsiveness to normal circulating
Upregulation of insulin receptor substrate-2 in pancreatic β cells prevents diabetes
 Upregulation of insulin receptor substrate-2 in pancreatic β cells prevents diabetes Anita M. Hennige, 1 Deborah J. Burks, 1 Umut Ozcan, 2 Rohit N. Kulkarni, 2 Jing Ye, 1 Sunmin Park, 1 Markus Schubert,
Upregulation of insulin receptor substrate-2 in pancreatic β cells prevents diabetes Anita M. Hennige, 1 Deborah J. Burks, 1 Umut Ozcan, 2 Rohit N. Kulkarni, 2 Jing Ye, 1 Sunmin Park, 1 Markus Schubert,
Supporting Information
 Supporting Information Pang et al. 10.1073/pnas.1322009111 SI Materials and Methods ELISAs. These assays were performed as previously described (1). ELISA plates (MaxiSorp Nunc; Thermo Fisher Scientific)
Supporting Information Pang et al. 10.1073/pnas.1322009111 SI Materials and Methods ELISAs. These assays were performed as previously described (1). ELISA plates (MaxiSorp Nunc; Thermo Fisher Scientific)
VEGFR2-Mediated Vascular Dilation as a Mechanism of VEGF-Induced Anemia and Bone Marrow Cell Mobilization
 Cell Reports, Volume 9 Supplemental Information VEGFR2-Mediated Vascular Dilation as a Mechanism of VEGF-Induced Anemia and Bone Marrow Cell Mobilization Sharon Lim, Yin Zhang, Danfang Zhang, Fang Chen,
Cell Reports, Volume 9 Supplemental Information VEGFR2-Mediated Vascular Dilation as a Mechanism of VEGF-Induced Anemia and Bone Marrow Cell Mobilization Sharon Lim, Yin Zhang, Danfang Zhang, Fang Chen,
Reduced Insulin Signaling and Endoplasmic Reticulum Stress Act Synergistically to Deteriorate Pancreatic β Cell Function
 Kobe J. Med. Sci., Vol. 54, No. 2, pp. E114-E121, 2008 Reduced Insulin Signaling and Endoplasmic Reticulum Stress Act Synergistically to Deteriorate Pancreatic β Cell Function TOMOKAZU MATSUDA, YOSHIAKI
Kobe J. Med. Sci., Vol. 54, No. 2, pp. E114-E121, 2008 Reduced Insulin Signaling and Endoplasmic Reticulum Stress Act Synergistically to Deteriorate Pancreatic β Cell Function TOMOKAZU MATSUDA, YOSHIAKI
Genetic manipulation of IRS proteins: animal models for understanding the molecular basis of diabetes
 avances en Diabetología Av Diabetol. 2009;25:21-6 Revisión Genetic manipulation of IRS proteins: animal models for understanding the molecular basis of diabetes S.M. Sanz González, D.J. Burks Centro de
avances en Diabetología Av Diabetol. 2009;25:21-6 Revisión Genetic manipulation of IRS proteins: animal models for understanding the molecular basis of diabetes S.M. Sanz González, D.J. Burks Centro de
Supplementary Information
 Supplementary Information Akt regulates hepatic metabolism by suppressing a Foxo1 dependent global inhibition of adaptation to nutrient intake Mingjian Lu 1, Min Wan 1, Karla F. Leavens 1, Qingwei Chu
Supplementary Information Akt regulates hepatic metabolism by suppressing a Foxo1 dependent global inhibition of adaptation to nutrient intake Mingjian Lu 1, Min Wan 1, Karla F. Leavens 1, Qingwei Chu
Inhibition of DYRK1A stimulates human beta-cell proliferation
 Inhibition of DYRK1A stimulates human beta-cell proliferation Ercument Dirice 1,, Deepika Walpita 2,, Amedeo Vetere 2, Bennett C. Meier 2,5, Sevim Kahraman 1, Jiang Hu 1, Vlado Dančík 2, Sean M. Burns
Inhibition of DYRK1A stimulates human beta-cell proliferation Ercument Dirice 1,, Deepika Walpita 2,, Amedeo Vetere 2, Bennett C. Meier 2,5, Sevim Kahraman 1, Jiang Hu 1, Vlado Dančík 2, Sean M. Burns
Secondary Negative Effects of Isolation Enzyme (s) On Human Islets. A.N.Balamurugan
 Secondary Negative Effects of Isolation Enzyme (s) On Human Islets A.N.Balamurugan Human Islets Functional Mass Preservation 18-25 min 10 min 8-12 min 10-15 min 45-60 min pancreas in chamber 37º Sub-Optimal
Secondary Negative Effects of Isolation Enzyme (s) On Human Islets A.N.Balamurugan Human Islets Functional Mass Preservation 18-25 min 10 min 8-12 min 10-15 min 45-60 min pancreas in chamber 37º Sub-Optimal
RayBio KinaseSTAR TM Akt Activity Assay Kit
 Activity Assay Kit User Manual Version 1.0 March 13, 2015 RayBio KinaseSTAR TM Akt Activity Kit Protocol (Cat#: 68AT-Akt-S40) RayBiotech, Inc. We Provide You With Excellent Support And Service Tel:(Toll
Activity Assay Kit User Manual Version 1.0 March 13, 2015 RayBio KinaseSTAR TM Akt Activity Kit Protocol (Cat#: 68AT-Akt-S40) RayBiotech, Inc. We Provide You With Excellent Support And Service Tel:(Toll
Disruption of Insulin Receptor Substrate 2 Causes Type 2 Diabetes Because of Liver Insulin Resistance and Lack of Compensatory
 Disruption of Insulin Receptor Substrate 2 Causes Type 2 Diabetes Because of Liver Insulin Resistance and Lack of Compensatory -Cell Hyperplasia Naoto Kubota, Kazuyuki Tobe, Yasuo Terauchi, Kazuhiro Eto,
Disruption of Insulin Receptor Substrate 2 Causes Type 2 Diabetes Because of Liver Insulin Resistance and Lack of Compensatory -Cell Hyperplasia Naoto Kubota, Kazuyuki Tobe, Yasuo Terauchi, Kazuhiro Eto,
Insulin Resistance. Biol 405 Molecular Medicine
 Insulin Resistance Biol 405 Molecular Medicine Insulin resistance: a subnormal biological response to insulin. Defects of either insulin secretion or insulin action can cause diabetes mellitus. Insulin-dependent
Insulin Resistance Biol 405 Molecular Medicine Insulin resistance: a subnormal biological response to insulin. Defects of either insulin secretion or insulin action can cause diabetes mellitus. Insulin-dependent
Supplementary Information. Induction of human pancreatic beta cell replication by inhibitors of dual specificity tyrosine regulated kinase
 Journal: Nature Medicine Supplementary Information Induction of human pancreatic beta cell replication by inhibitors of dual specificity tyrosine regulated kinase 1,2 Peng Wang PhD, 1,2 Juan-Carlos Alvarez-Perez
Journal: Nature Medicine Supplementary Information Induction of human pancreatic beta cell replication by inhibitors of dual specificity tyrosine regulated kinase 1,2 Peng Wang PhD, 1,2 Juan-Carlos Alvarez-Perez
Phospho-AKT Sampler Kit
 Phospho-AKT Sampler Kit E 0 5 1 0 0 3 Kits Includes Cat. Quantity Application Reactivity Source Akt (Ab-473) Antibody E021054-1 50μg/50μl IHC, WB Human, Mouse, Rat Rabbit Akt (Phospho-Ser473) Antibody
Phospho-AKT Sampler Kit E 0 5 1 0 0 3 Kits Includes Cat. Quantity Application Reactivity Source Akt (Ab-473) Antibody E021054-1 50μg/50μl IHC, WB Human, Mouse, Rat Rabbit Akt (Phospho-Ser473) Antibody
Protocol for Gene Transfection & Western Blotting
 The schedule and the manual of basic techniques for cell culture Advanced Protocol for Gene Transfection & Western Blotting Schedule Day 1 26/07/2008 Transfection Day 3 28/07/2008 Cell lysis Immunoprecipitation
The schedule and the manual of basic techniques for cell culture Advanced Protocol for Gene Transfection & Western Blotting Schedule Day 1 26/07/2008 Transfection Day 3 28/07/2008 Cell lysis Immunoprecipitation
SUPPLEMENTARY INFORMATION
 SUPPLEMENTARY INFORMATION FOR Liver X Receptor α mediates hepatic triglyceride accumulation through upregulation of G0/G1 Switch Gene 2 (G0S2) expression I: SUPPLEMENTARY METHODS II: SUPPLEMENTARY FIGURES
SUPPLEMENTARY INFORMATION FOR Liver X Receptor α mediates hepatic triglyceride accumulation through upregulation of G0/G1 Switch Gene 2 (G0S2) expression I: SUPPLEMENTARY METHODS II: SUPPLEMENTARY FIGURES
A Hepatocyte Growth Factor Receptor (Met) Insulin Receptor hybrid governs hepatic glucose metabolism SUPPLEMENTARY FIGURES, LEGENDS AND METHODS
 A Hepatocyte Growth Factor Receptor (Met) Insulin Receptor hybrid governs hepatic glucose metabolism Arlee Fafalios, Jihong Ma, Xinping Tan, John Stoops, Jianhua Luo, Marie C. DeFrances and Reza Zarnegar
A Hepatocyte Growth Factor Receptor (Met) Insulin Receptor hybrid governs hepatic glucose metabolism Arlee Fafalios, Jihong Ma, Xinping Tan, John Stoops, Jianhua Luo, Marie C. DeFrances and Reza Zarnegar
General Laboratory methods Plasma analysis: Gene Expression Analysis: Immunoblot analysis: Immunohistochemistry:
 General Laboratory methods Plasma analysis: Plasma insulin (Mercodia, Sweden), leptin (duoset, R&D Systems Europe, Abingdon, United Kingdom), IL-6, TNFα and adiponectin levels (Quantikine kits, R&D Systems
General Laboratory methods Plasma analysis: Plasma insulin (Mercodia, Sweden), leptin (duoset, R&D Systems Europe, Abingdon, United Kingdom), IL-6, TNFα and adiponectin levels (Quantikine kits, R&D Systems
Perspectives Home > Literature > Perspectives > Holz et al., pp. pe2
 AAAS Science's STKE Holz et al., pp. pe2 http://stke.sciencemag.org/cgi/content/abstract/sigtrans;2005/268/pe2... Institution: NEW YORK UNIV MED CENTER Sign In as Individual Access Rights Subscription
AAAS Science's STKE Holz et al., pp. pe2 http://stke.sciencemag.org/cgi/content/abstract/sigtrans;2005/268/pe2... Institution: NEW YORK UNIV MED CENTER Sign In as Individual Access Rights Subscription
Section 4: -Cell Mass and Function in Type 2 Diabetes IRS Proteins and -Cell Function Deborah J. Burks and Morris F. White
 Section 4: -Cell Mass and Function in Type 2 Diabetes IRS Proteins and -Cell Function Deborah J. Burks and Morris F. White Insulin receptor substrate (IRS) proteins mediate a variety of the metabolic and
Section 4: -Cell Mass and Function in Type 2 Diabetes IRS Proteins and -Cell Function Deborah J. Burks and Morris F. White Insulin receptor substrate (IRS) proteins mediate a variety of the metabolic and
NIH Public Access Author Manuscript Diabetologia. Author manuscript; available in PMC 2014 February 01.
 NIH Public Access Author Manuscript Published in final edited form as: Diabetologia. 2013 February ; 56(2): 231 233. doi:10.1007/s00125-012-2788-6. Lipotoxicity impairs incretin signalling V. Poitout 1,2
NIH Public Access Author Manuscript Published in final edited form as: Diabetologia. 2013 February ; 56(2): 231 233. doi:10.1007/s00125-012-2788-6. Lipotoxicity impairs incretin signalling V. Poitout 1,2
SUPPLEMENTARY INFORMATION
 Figure S1 Treatment with both Sema6D and Plexin-A1 sirnas induces the phenotype essentially identical to that induced by treatment with Sema6D sirna alone or Plexin-A1 sirna alone. (a,b) The cardiac tube
Figure S1 Treatment with both Sema6D and Plexin-A1 sirnas induces the phenotype essentially identical to that induced by treatment with Sema6D sirna alone or Plexin-A1 sirna alone. (a,b) The cardiac tube
SUPPLEMENTARY DATA. Supplementary Table 2. Antibodies used for Immunofluoresence. Supplementary Table 3. Real-time PCR primer sequences.
 Supplementary Table 2. Antibodies used for Immunofluoresence. Antibody Dilution Source Goat anti-pdx1 1:100 R&D Systems Rabbit anti-hnf6 1:100 Santa Cruz Biotechnology Mouse anti-nkx6.1 1:200 Developmental
Supplementary Table 2. Antibodies used for Immunofluoresence. Antibody Dilution Source Goat anti-pdx1 1:100 R&D Systems Rabbit anti-hnf6 1:100 Santa Cruz Biotechnology Mouse anti-nkx6.1 1:200 Developmental
Requires Signaling though Akt2 Independent of the. Transcription Factors FoxA2, FoxO1, and SREBP1c
 Cell Metabolism, Volume 14 Supplemental Information Postprandial Hepatic Lipid Metabolism Requires Signaling though Akt2 Independent of the Transcription Factors FoxA2, FoxO1, and SREBP1c Min Wan, Karla
Cell Metabolism, Volume 14 Supplemental Information Postprandial Hepatic Lipid Metabolism Requires Signaling though Akt2 Independent of the Transcription Factors FoxA2, FoxO1, and SREBP1c Min Wan, Karla
GPR120 *** * * Liver BAT iwat ewat mwat Ileum Colon. UCP1 mrna ***
 a GPR120 GPR120 mrna/ppia mrna Arbitrary Units 150 100 50 Liver BAT iwat ewat mwat Ileum Colon b UCP1 mrna Fold induction 20 15 10 5 - camp camp SB202190 - - - H89 - - - - - GW7647 Supplementary Figure
a GPR120 GPR120 mrna/ppia mrna Arbitrary Units 150 100 50 Liver BAT iwat ewat mwat Ileum Colon b UCP1 mrna Fold induction 20 15 10 5 - camp camp SB202190 - - - H89 - - - - - GW7647 Supplementary Figure
AMPK Phosphorylation Assay Kit
 AMPK Phosphorylation Assay Kit Catalog Number KA3789 100 assays Version: 02 Intended for research use only www.abnova.com Table of Contents Introduction... 3 Intended Use... 3 Background... 3 Principle
AMPK Phosphorylation Assay Kit Catalog Number KA3789 100 assays Version: 02 Intended for research use only www.abnova.com Table of Contents Introduction... 3 Intended Use... 3 Background... 3 Principle
PRODUCT INFORMATION & MANUAL
 PRODUCT INFORMATION & MANUAL Mitochondrial Extraction Kit NBP2-29448 Research use only. Not for diagnostic or therapeutic procedures www.novusbio.com P: 303.760.1950 P: 888.506.6887 F: 303.730.1966 technical@novusbio.com
PRODUCT INFORMATION & MANUAL Mitochondrial Extraction Kit NBP2-29448 Research use only. Not for diagnostic or therapeutic procedures www.novusbio.com P: 303.760.1950 P: 888.506.6887 F: 303.730.1966 technical@novusbio.com
Primer sequences Target Sequence F Sequence R TNF-α (Tnfa) TCAGCCGATTTGCTATCTCAT A
 Supplementary Table 1. Q- and RT-PR primers used in this study. Primer sequences Target Sequence F Sequence R TNF-α (Tnfa) TGGTTTGTTTT GTTTGGGGTTG T hemokine (- motif) ligand 5 (cl5) GTGTTTGTTT TGGTGGTG
Supplementary Table 1. Q- and RT-PR primers used in this study. Primer sequences Target Sequence F Sequence R TNF-α (Tnfa) TGGTTTGTTTT GTTTGGGGTTG T hemokine (- motif) ligand 5 (cl5) GTGTTTGTTT TGGTGGTG
Programmed necrosis, not apoptosis, is a key mediator of cell loss and DAMP-mediated inflammation in dsrna-induced retinal degeneration
 Programmed necrosis, not apoptosis, is a key mediator of cell loss and DAMP-mediated inflammation in dsrna-induced retinal degeneration The Harvard community has made this article openly available. Please
Programmed necrosis, not apoptosis, is a key mediator of cell loss and DAMP-mediated inflammation in dsrna-induced retinal degeneration The Harvard community has made this article openly available. Please
Procaspase-3. Cleaved caspase-3. actin. Cytochrome C (10 M) Z-VAD-fmk. Procaspase-3. Cleaved caspase-3. actin. Z-VAD-fmk
 A HeLa actin - + + - - + Cytochrome C (1 M) Z-VAD-fmk PMN - + + - - + actin Cytochrome C (1 M) Z-VAD-fmk Figure S1. (A) Pan-caspase inhibitor z-vad-fmk inhibits cytochrome c- mediated procaspase-3 cleavage.
A HeLa actin - + + - - + Cytochrome C (1 M) Z-VAD-fmk PMN - + + - - + actin Cytochrome C (1 M) Z-VAD-fmk Figure S1. (A) Pan-caspase inhibitor z-vad-fmk inhibits cytochrome c- mediated procaspase-3 cleavage.
(a) Significant biological processes (upper panel) and disease biomarkers (lower panel)
 Supplementary Figure 1. Functional enrichment analyses of secretomic proteins. (a) Significant biological processes (upper panel) and disease biomarkers (lower panel) 2 involved by hrab37-mediated secretory
Supplementary Figure 1. Functional enrichment analyses of secretomic proteins. (a) Significant biological processes (upper panel) and disease biomarkers (lower panel) 2 involved by hrab37-mediated secretory
Supplementary Figure 1. Normal T lymphocyte populations in Dapk -/- mice. (a) Normal thymic development in Dapk -/- mice. Thymocytes from WT and Dapk
 Supplementary Figure 1. Normal T lymphocyte populations in Dapk -/- mice. (a) Normal thymic development in Dapk -/- mice. Thymocytes from WT and Dapk -/- mice were stained for expression of CD4 and CD8.
Supplementary Figure 1. Normal T lymphocyte populations in Dapk -/- mice. (a) Normal thymic development in Dapk -/- mice. Thymocytes from WT and Dapk -/- mice were stained for expression of CD4 and CD8.
Signal transduction of insulin
 Signal transduction of insulin Diabetes mellitus is a severe chronic disease, affecting 6-11 percent of the populations aged 30-64 and about 20 percent of those older than age 65 throughout the world.
Signal transduction of insulin Diabetes mellitus is a severe chronic disease, affecting 6-11 percent of the populations aged 30-64 and about 20 percent of those older than age 65 throughout the world.
Pathogenesis of Diabetes Mellitus
 Pathogenesis of Diabetes Mellitus Young-Bum Kim, Ph.D. Associate Professor of Medicine Harvard Medical School Definition of Diabetes Mellitus a group of metabolic diseases characterized by hyperglycemia
Pathogenesis of Diabetes Mellitus Young-Bum Kim, Ph.D. Associate Professor of Medicine Harvard Medical School Definition of Diabetes Mellitus a group of metabolic diseases characterized by hyperglycemia
Expression of acid base transporters in the kidney collecting duct in Slc2a7 -/-
 Supplemental Material Results. Expression of acid base transporters in the kidney collecting duct in Slc2a7 -/- and Slc2a7 -/- mice. The expression of AE1 in the kidney was examined in Slc26a7 KO mice.
Supplemental Material Results. Expression of acid base transporters in the kidney collecting duct in Slc2a7 -/- and Slc2a7 -/- mice. The expression of AE1 in the kidney was examined in Slc26a7 KO mice.
Generating Mouse Models of Pancreatic Cancer
 Generating Mouse Models of Pancreatic Cancer Aom Isbell http://www2.massgeneral.org/cancerresourceroom/types/gi/index.asp Spring/Summer 1, 2012 Alexandros Tzatsos, MD PhD Bardeesy Lab: Goals and Objectives
Generating Mouse Models of Pancreatic Cancer Aom Isbell http://www2.massgeneral.org/cancerresourceroom/types/gi/index.asp Spring/Summer 1, 2012 Alexandros Tzatsos, MD PhD Bardeesy Lab: Goals and Objectives
G-Protein Signaling. Introduction to intracellular signaling. Dr. SARRAY Sameh, Ph.D
 G-Protein Signaling Introduction to intracellular signaling Dr. SARRAY Sameh, Ph.D Cell signaling Cells communicate via extracellular signaling molecules (Hormones, growth factors and neurotransmitters
G-Protein Signaling Introduction to intracellular signaling Dr. SARRAY Sameh, Ph.D Cell signaling Cells communicate via extracellular signaling molecules (Hormones, growth factors and neurotransmitters
Islet viability assay and Glucose Stimulated Insulin Secretion assay RT-PCR and Western Blot
 Islet viability assay and Glucose Stimulated Insulin Secretion assay Islet cell viability was determined by colorimetric (3-(4,5-dimethylthiazol-2-yl)-2,5- diphenyltetrazolium bromide assay using CellTiter
Islet viability assay and Glucose Stimulated Insulin Secretion assay Islet cell viability was determined by colorimetric (3-(4,5-dimethylthiazol-2-yl)-2,5- diphenyltetrazolium bromide assay using CellTiter
Western Immunoblotting Preparation of Samples:
 Western Immunoblotting Preparation of Samples: Total Protein Extraction from Culture Cells: Take off the medium Wash culture with 1 x PBS 1 ml hot Cell-lysis Solution into T75 flask Scrap out the cells
Western Immunoblotting Preparation of Samples: Total Protein Extraction from Culture Cells: Take off the medium Wash culture with 1 x PBS 1 ml hot Cell-lysis Solution into T75 flask Scrap out the cells
Week 3, Lecture 5a. Pathophysiology of Diabetes. Simin Liu, MD, ScD
 Week 3, Lecture 5a Pathophysiology of Diabetes Simin Liu, MD, ScD General Model of Peptide Hormone Action Hormone Plasma Membrane Activated Nucleus Cellular Trafficking Enzymes Inhibited Receptor Effector
Week 3, Lecture 5a Pathophysiology of Diabetes Simin Liu, MD, ScD General Model of Peptide Hormone Action Hormone Plasma Membrane Activated Nucleus Cellular Trafficking Enzymes Inhibited Receptor Effector
Anti-ceramide Antibody Prevents the Radiation GI Syndrome in Mice
 Anti-ceramide Antibody Prevents the Radiation GI Syndrome in Mice Jimmy A. Rotolo 1, Branka Stancevic 1, Jianjun Zhang 1, Guoqiang Hua 1, John Fuller 1, Xianglei Yin 1, Adriana Haimovitz-Friedman 2, Kisu
Anti-ceramide Antibody Prevents the Radiation GI Syndrome in Mice Jimmy A. Rotolo 1, Branka Stancevic 1, Jianjun Zhang 1, Guoqiang Hua 1, John Fuller 1, Xianglei Yin 1, Adriana Haimovitz-Friedman 2, Kisu
KEY CONCEPT QUESTIONS IN SIGNAL TRANSDUCTION
 Signal Transduction - Part 2 Key Concepts - Receptor tyrosine kinases control cell metabolism and proliferation Growth factor signaling through Ras Mutated cell signaling genes in cancer cells are called
Signal Transduction - Part 2 Key Concepts - Receptor tyrosine kinases control cell metabolism and proliferation Growth factor signaling through Ras Mutated cell signaling genes in cancer cells are called
Supplementary data Supplementary Figure 1 Supplementary Figure 2
 Supplementary data Supplementary Figure 1 SPHK1 sirna increases RANKL-induced osteoclastogenesis in RAW264.7 cell culture. (A) RAW264.7 cells were transfected with oligocassettes containing SPHK1 sirna
Supplementary data Supplementary Figure 1 SPHK1 sirna increases RANKL-induced osteoclastogenesis in RAW264.7 cell culture. (A) RAW264.7 cells were transfected with oligocassettes containing SPHK1 sirna
β-cell Preservation and Regeneration After Islet Transplantation
 β-cell Preservation and Regeneration After Islet Transplantation Jyuhn-Huarng Juang, MD Division of Endocrinology and Metabolism, Department of Internal Medicine, Chang Gung University and Memorial Hospital,
β-cell Preservation and Regeneration After Islet Transplantation Jyuhn-Huarng Juang, MD Division of Endocrinology and Metabolism, Department of Internal Medicine, Chang Gung University and Memorial Hospital,
β cell replication is the primary mechanism for maintaining postnatal β cell mass
 Research article β cell replication is the primary mechanism for maintaining postnatal β cell mass Senta Georgia and Anil Bhushan Department of Biochemistry and Molecular Biology, University of Southern
Research article β cell replication is the primary mechanism for maintaining postnatal β cell mass Senta Georgia and Anil Bhushan Department of Biochemistry and Molecular Biology, University of Southern
(Stratagene, La Jolla, CA) (Supplemental Fig. 1A). A 5.4-kb EcoRI fragment
 SUPPLEMENTAL INFORMATION Supplemental Methods Generation of RyR2-S2808D Mice Murine genomic RyR2 clones were isolated from a 129/SvEvTacfBR λ-phage library (Stratagene, La Jolla, CA) (Supplemental Fig.
SUPPLEMENTAL INFORMATION Supplemental Methods Generation of RyR2-S2808D Mice Murine genomic RyR2 clones were isolated from a 129/SvEvTacfBR λ-phage library (Stratagene, La Jolla, CA) (Supplemental Fig.
Figure legends Supplemental Fig.1. Glucose-induced insulin secretion and insulin content of islets. Supplemental Fig. 2.
 Figure legends Supplemental Fig.. Glucose-induced insulin secretion and insulin content of islets. Insulin secretory responses to.,., and. mm glucose (A) (n = 7-), and the insulin content in the islets
Figure legends Supplemental Fig.. Glucose-induced insulin secretion and insulin content of islets. Insulin secretory responses to.,., and. mm glucose (A) (n = 7-), and the insulin content in the islets
The Schedule and the Manual of Basic Techniques for Cell Culture
 The Schedule and the Manual of Basic Techniques for Cell Culture 1 Materials Calcium Phosphate Transfection Kit: Invitrogen Cat.No.K2780-01 Falcon tube (Cat No.35-2054:12 x 75 mm, 5 ml tube) Cell: 293
The Schedule and the Manual of Basic Techniques for Cell Culture 1 Materials Calcium Phosphate Transfection Kit: Invitrogen Cat.No.K2780-01 Falcon tube (Cat No.35-2054:12 x 75 mm, 5 ml tube) Cell: 293
Supplementary Figure S1. Distinct compartmentalization of proinsulin in obese db/db mouse islet ß- cells.
 Supplementary Figure S1. Distinct compartmentalization of proinsulin in obese db/db mouse islet ß- cells. Pancreata from 16-weeks-old 6J +/+, 6J db/db, KS +/+ and KS db/db mice were harvested, fixed with
Supplementary Figure S1. Distinct compartmentalization of proinsulin in obese db/db mouse islet ß- cells. Pancreata from 16-weeks-old 6J +/+, 6J db/db, KS +/+ and KS db/db mice were harvested, fixed with
hexahistidine tagged GRP78 devoid of the KDEL motif (GRP78-His) on SDS-PAGE. This
 SUPPLEMENTAL FIGURE LEGEND Fig. S1. Generation and characterization of. (A) Coomassie staining of soluble hexahistidine tagged GRP78 devoid of the KDEL motif (GRP78-His) on SDS-PAGE. This protein was expressed
SUPPLEMENTAL FIGURE LEGEND Fig. S1. Generation and characterization of. (A) Coomassie staining of soluble hexahistidine tagged GRP78 devoid of the KDEL motif (GRP78-His) on SDS-PAGE. This protein was expressed
Type of file: PDF Title of file for HTML: Supplementary Information Description: Supplementary Figures
 Type of file: PDF Title of file for HTML: Supplementary Information Description: Supplementary Figures Type of file: MOV Title of file for HTML: Supplementary Movie 1 Description: NLRP3 is moving along
Type of file: PDF Title of file for HTML: Supplementary Information Description: Supplementary Figures Type of file: MOV Title of file for HTML: Supplementary Movie 1 Description: NLRP3 is moving along
Supplemental Figure 1. Western blot analysis indicated that MIF was detected in the fractions of
 Supplemental Figure Legends Supplemental Figure 1. Western blot analysis indicated that was detected in the fractions of plasma membrane and cytosol but not in nuclear fraction isolated from Pkd1 null
Supplemental Figure Legends Supplemental Figure 1. Western blot analysis indicated that was detected in the fractions of plasma membrane and cytosol but not in nuclear fraction isolated from Pkd1 null
Crosstalk between Adiponectin and IGF-IR in breast cancer. Prof. Young Jin Suh Department of Surgery The Catholic University of Korea
 Crosstalk between Adiponectin and IGF-IR in breast cancer Prof. Young Jin Suh Department of Surgery The Catholic University of Korea Obesity Chronic, multifactorial disorder Hypertrophy and hyperplasia
Crosstalk between Adiponectin and IGF-IR in breast cancer Prof. Young Jin Suh Department of Surgery The Catholic University of Korea Obesity Chronic, multifactorial disorder Hypertrophy and hyperplasia
A high-fructose diet induces changes in pp185 phosphorylation in muscle and liver of rats
 Fructose Brazilian diet Journal induces of Medical changes and in Biological rat pp185 Research () 33: 1421-1427 ISSN -879X Short Communication 1421 A high-fructose diet induces changes in pp185 phosphorylation
Fructose Brazilian diet Journal induces of Medical changes and in Biological rat pp185 Research () 33: 1421-1427 ISSN -879X Short Communication 1421 A high-fructose diet induces changes in pp185 phosphorylation
Supplemental Information. Increased 4E-BP1 Expression Protects. against Diet-Induced Obesity and Insulin. Resistance in Male Mice
 Cell Reports, Volume 16 Supplemental Information Increased 4E-BP1 Expression Protects against Diet-Induced Obesity and Insulin Resistance in Male Mice Shih-Yin Tsai, Ariana A. Rodriguez, Somasish G. Dastidar,
Cell Reports, Volume 16 Supplemental Information Increased 4E-BP1 Expression Protects against Diet-Induced Obesity and Insulin Resistance in Male Mice Shih-Yin Tsai, Ariana A. Rodriguez, Somasish G. Dastidar,
Supplementary Information POLO-LIKE KINASE 1 FACILITATES LOSS OF PTEN-INDUCED PROSTATE CANCER FORMATION
 Supplementary Information POLO-LIKE KINASE 1 FACILITATES LOSS OF PTEN-INDUCED PROSTATE CANCER FORMATION X. Shawn Liu 1, 3, Bing Song 2, 3, Bennett D. Elzey 3, 4, Timothy L. Ratliff 3, 4, Stephen F. Konieczny
Supplementary Information POLO-LIKE KINASE 1 FACILITATES LOSS OF PTEN-INDUCED PROSTATE CANCER FORMATION X. Shawn Liu 1, 3, Bing Song 2, 3, Bennett D. Elzey 3, 4, Timothy L. Ratliff 3, 4, Stephen F. Konieczny
Supplementary Figures
 Supplementary Figures Supplementary Figure 1. NKT ligand-loaded tumour antigen-presenting B cell- and monocyte-based vaccine induces NKT, NK and CD8 T cell responses. (A) The cytokine profiles of liver
Supplementary Figures Supplementary Figure 1. NKT ligand-loaded tumour antigen-presenting B cell- and monocyte-based vaccine induces NKT, NK and CD8 T cell responses. (A) The cytokine profiles of liver
Over-expression of MKP-3 and knockdown of MKP-3 and FOXO1 in primary rat. day, cells were transduced with adenoviruses expressing GFP, MKP-3 or shgfp,
 SUPPLEMENTAL METHODS Over-expression of MKP-3 and knockdown of MKP-3 and FOXO1 in primary rat hepatocytes Primary rat hepatocytes were seeded as described in experimental procedures. The next day, cells
SUPPLEMENTAL METHODS Over-expression of MKP-3 and knockdown of MKP-3 and FOXO1 in primary rat hepatocytes Primary rat hepatocytes were seeded as described in experimental procedures. The next day, cells
Lipoprotein Lipase Activity Assay Kit (Fluorometric)
 Lipoprotein Lipase Activity Assay Kit (Fluorometric) Catalog Number KA4538 100 assays Version: 02 Intended for research use only www.abnova.com Table of Contents Introduction... 3 Background... 3 General
Lipoprotein Lipase Activity Assay Kit (Fluorometric) Catalog Number KA4538 100 assays Version: 02 Intended for research use only www.abnova.com Table of Contents Introduction... 3 Background... 3 General
Part-4. Cell cycle regulatory protein 5 (Cdk5) A novel target of ERK in Carb induced cell death
 Part-4 Cell cycle regulatory protein 5 (Cdk5) A novel target of ERK in Carb induced cell death 95 1. Introduction The process of replicating DNA and dividing cells can be described as a series of coordinated
Part-4 Cell cycle regulatory protein 5 (Cdk5) A novel target of ERK in Carb induced cell death 95 1. Introduction The process of replicating DNA and dividing cells can be described as a series of coordinated
(A) RT-PCR for components of the Shh/Gli pathway in normal fetus cell (MRC-5) and a
 Supplementary figure legends Supplementary Figure 1. Expression of Shh signaling components in a panel of gastric cancer. (A) RT-PCR for components of the Shh/Gli pathway in normal fetus cell (MRC-5) and
Supplementary figure legends Supplementary Figure 1. Expression of Shh signaling components in a panel of gastric cancer. (A) RT-PCR for components of the Shh/Gli pathway in normal fetus cell (MRC-5) and
Improving Diabetes Research: Moving Beyond Animal Models. Charu Chandrasekera, Ph.D. Anne Bunner, Ph.D.
 Improving Diabetes Research: Moving Beyond Animal Models Charu Chandrasekera, Ph.D. Anne Bunner, Ph.D. July 19, 2014 From Bench-to-Bedside Sulfonylurea Biguanide Dipeptidyl peptidase-4 inhibitor Glucagon-like
Improving Diabetes Research: Moving Beyond Animal Models Charu Chandrasekera, Ph.D. Anne Bunner, Ph.D. July 19, 2014 From Bench-to-Bedside Sulfonylurea Biguanide Dipeptidyl peptidase-4 inhibitor Glucagon-like
Insulin Glulisine Insulin Receptor Signaling Characteristics In Vivo
 Insulin Glulisine Insulin Receptor Signaling Characteristics In Vivo Anita M. Hennige, Rainer Lehmann, Cora Weigert, Klaus Moeschel, Myriam Schäuble, Elisabeth Metzinger, Reiner Lammers, and Hans-Ulrich
Insulin Glulisine Insulin Receptor Signaling Characteristics In Vivo Anita M. Hennige, Rainer Lehmann, Cora Weigert, Klaus Moeschel, Myriam Schäuble, Elisabeth Metzinger, Reiner Lammers, and Hans-Ulrich
SUPPLEMENTARY INFORMATION
 DOI: 10.1038/ncb3461 In the format provided by the authors and unedited. Supplementary Figure 1 (associated to Figure 1). Cpeb4 gene-targeted mice develop liver steatosis. a, Immunoblot displaying CPEB4
DOI: 10.1038/ncb3461 In the format provided by the authors and unedited. Supplementary Figure 1 (associated to Figure 1). Cpeb4 gene-targeted mice develop liver steatosis. a, Immunoblot displaying CPEB4
FOR REVIEW. BMB Reports - Manuscript Submission. Manuscript Draft. Manuscript Number: BMB
 BMB Reports - Manuscript Submission Manuscript Draft Manuscript Number: BMB-18-095 Title: Insulin Receptor Substrate 2:A Bridge between Hippo and AKT Pathways Article Type: Perspective (Invited Only) Keywords:
BMB Reports - Manuscript Submission Manuscript Draft Manuscript Number: BMB-18-095 Title: Insulin Receptor Substrate 2:A Bridge between Hippo and AKT Pathways Article Type: Perspective (Invited Only) Keywords:
Problem Set 8 Key 1 of 8
 7.06 2003 Problem Set 8 Key 1 of 8 7.06 2003 Problem Set 8 Key 1. As a bright MD/PhD, you are interested in questions about the control of cell number in the body. Recently, you've seen three patients
7.06 2003 Problem Set 8 Key 1 of 8 7.06 2003 Problem Set 8 Key 1. As a bright MD/PhD, you are interested in questions about the control of cell number in the body. Recently, you've seen three patients
Early cell death (FGF) B No RunX transcription factor produced Yes No differentiation
 Solution Key - Practice Questions Question 1 a) A recent publication has shown that the fat stem cells (FSC) can act as bone stem cells to repair cavities in the skull, when transplanted into immuno-compromised
Solution Key - Practice Questions Question 1 a) A recent publication has shown that the fat stem cells (FSC) can act as bone stem cells to repair cavities in the skull, when transplanted into immuno-compromised
Gamma-aminobutyric acid (GABA) treatment blocks inflammatory pathways and promotes survival and proliferation of pancreatic beta cells
 Gamma-aminobutyric acid (GABA) treatment blocks inflammatory pathways and promotes survival and proliferation of pancreatic beta cells Gérald J. Prud homme, MD, FRCPC Keenan Research Centre for Biomedical
Gamma-aminobutyric acid (GABA) treatment blocks inflammatory pathways and promotes survival and proliferation of pancreatic beta cells Gérald J. Prud homme, MD, FRCPC Keenan Research Centre for Biomedical
Insulin Signaling After Exercise in Insulin Receptor Substrate-2 Deficient Mice
 Insulin Signaling After Exercise in Insulin Receptor Substrate-2 Deficient Mice Kirsten F. Howlett, Kei Sakamoto, Michael F. Hirshman, William G. Aschenbach, Matthew Dow, Morris F. White, and Laurie J.
Insulin Signaling After Exercise in Insulin Receptor Substrate-2 Deficient Mice Kirsten F. Howlett, Kei Sakamoto, Michael F. Hirshman, William G. Aschenbach, Matthew Dow, Morris F. White, and Laurie J.
islets scored 1 week month months
 Supplementary Table 1. Sampling parameters for the morphometrical analyses Time (post- DT) Control mice (age-matched) α-cells mice pancreatic surface (mm 2 ) scored DT-treated mice islets scored mice pancreatic
Supplementary Table 1. Sampling parameters for the morphometrical analyses Time (post- DT) Control mice (age-matched) α-cells mice pancreatic surface (mm 2 ) scored DT-treated mice islets scored mice pancreatic
Supplementary Figure 1.
 Supplementary Figure 1. Increased β cell mass and islet diameter in βtsc2 -/- mice up to 35 weeks A: Reconstruction of multiple anti-insulin immunofluorescence images showing differences in β cell mass
Supplementary Figure 1. Increased β cell mass and islet diameter in βtsc2 -/- mice up to 35 weeks A: Reconstruction of multiple anti-insulin immunofluorescence images showing differences in β cell mass
Digestive system L 4. Lecturer Dr. Firdous M. Jaafar Department of Anatomy/Histology section
 Digestive system L 4 Lecturer Dr. Firdous M. Jaafar Department of Anatomy/Histology section objectives 1-Describe the structure of liver. 2-Define liver lobule, and identify its zones. 3-Define portal
Digestive system L 4 Lecturer Dr. Firdous M. Jaafar Department of Anatomy/Histology section objectives 1-Describe the structure of liver. 2-Define liver lobule, and identify its zones. 3-Define portal
Making Mature Human Islet Cells from Stem Cells to Model Disease and Treat Diabetes
 University of British Columbia Departments of Surgery and Cellular & Physiological Sciences Making Mature Human Islet Cells from Stem Cells to Model Disease and Treat Diabetes 2016 International Conference
University of British Columbia Departments of Surgery and Cellular & Physiological Sciences Making Mature Human Islet Cells from Stem Cells to Model Disease and Treat Diabetes 2016 International Conference
Supplementary Table 1. Primer sequences for conventional RT-PCR on mouse islets
 Supplementary Table 1. Primer sequences for conventional RT-PCR on mouse islets Gene 5 Forward 3 5 Reverse 3.T. Product (bp) ( C) mnox1 GTTCTTGGGCTGCCTTGG GCTGGGGCGGCGG 60 300 mnoxa1 GCTTTGCCGCGTGC GGTTCGGGTCCTTTGTGC
Supplementary Table 1. Primer sequences for conventional RT-PCR on mouse islets Gene 5 Forward 3 5 Reverse 3.T. Product (bp) ( C) mnox1 GTTCTTGGGCTGCCTTGG GCTGGGGCGGCGG 60 300 mnoxa1 GCTTTGCCGCGTGC GGTTCGGGTCCTTTGTGC
Supplemental Table 1. Biochemical and Cellular Potency and Selectivity of PF
 Supplemental Table 1. Biochemical and Cellular Potency and Selectivity of PF- 02341066 Assay IC 50 nm Selectivity Ratio d Biochemical Activity In Vitro c-met/hgfr enzyme (Ki, nm) a 4 NA Cellular Activity
Supplemental Table 1. Biochemical and Cellular Potency and Selectivity of PF- 02341066 Assay IC 50 nm Selectivity Ratio d Biochemical Activity In Vitro c-met/hgfr enzyme (Ki, nm) a 4 NA Cellular Activity
Kit for assay of thioredoxin
 FkTRX-02-V2 Kit for assay of thioredoxin The thioredoxin system is the major protein disulfide reductase in cells and comprises thioredoxin, thioredoxin reductase and NADPH (1). Thioredoxin systems are
FkTRX-02-V2 Kit for assay of thioredoxin The thioredoxin system is the major protein disulfide reductase in cells and comprises thioredoxin, thioredoxin reductase and NADPH (1). Thioredoxin systems are
Supplemental Data. Wnt/β-Catenin Signaling in Mesenchymal Progenitors. Controls Osteoblast and Chondrocyte
 Supplemental Data Wnt/β-Catenin Signaling in Mesenchymal Progenitors Controls Osteoblast and Chondrocyte Differentiation during Vertebrate Skeletogenesis Timothy F. Day, Xizhi Guo, Lisa Garrett-Beal, and
Supplemental Data Wnt/β-Catenin Signaling in Mesenchymal Progenitors Controls Osteoblast and Chondrocyte Differentiation during Vertebrate Skeletogenesis Timothy F. Day, Xizhi Guo, Lisa Garrett-Beal, and
PKC-θ knockout mice are protected from fat-induced insulin resistance
 Research article PKC-θ knockout mice are protected from fat-induced insulin resistance Jason K. Kim, 1 Jonathan J. Fillmore, 1 Mary Jean Sunshine, 2 Bjoern Albrecht, 2 Takamasa Higashimori, 1 Dong-Wook
Research article PKC-θ knockout mice are protected from fat-induced insulin resistance Jason K. Kim, 1 Jonathan J. Fillmore, 1 Mary Jean Sunshine, 2 Bjoern Albrecht, 2 Takamasa Higashimori, 1 Dong-Wook
Insulin resistance and pancreatic b cell failure
 Review series introduction Insulin resistance and pancreatic b cell failure Masato Kasuga Department of Clinical Molecular Medicine, Kobe University Graduate School of Medicine, Kobe, Japan. It is now
Review series introduction Insulin resistance and pancreatic b cell failure Masato Kasuga Department of Clinical Molecular Medicine, Kobe University Graduate School of Medicine, Kobe, Japan. It is now
UNIVERSITY OF PNG SCHOOL OF MEDICINE AND HEALTH SCIENCES DIVISION OF BASIC MEDICAL SCIENCES DISCIPLINE OF BIOCHEMISTRY AND MOLECULAR BIOLOGY
 1 UNIVERSITY OF PNG SCHOOL OF MEDICINE AND HEALTH SCIENCES DIVISION OF BASIC MEDICAL SCIENCES DISCIPLINE OF BIOCHEMISTRY AND MOLECULAR BIOLOGY GLUCOSE HOMEOSTASIS An Overview WHAT IS HOMEOSTASIS? Homeostasis
1 UNIVERSITY OF PNG SCHOOL OF MEDICINE AND HEALTH SCIENCES DIVISION OF BASIC MEDICAL SCIENCES DISCIPLINE OF BIOCHEMISTRY AND MOLECULAR BIOLOGY GLUCOSE HOMEOSTASIS An Overview WHAT IS HOMEOSTASIS? Homeostasis
Evaluation of directed and random motility in microslides Assessment of leukocyte adhesion in flow chambers
 Evaluation of directed and random motility in microslides Motility experiments in IBIDI microslides, image acquisition and processing were performed as described. PMN, which ended up in an angle < 180
Evaluation of directed and random motility in microslides Motility experiments in IBIDI microslides, image acquisition and processing were performed as described. PMN, which ended up in an angle < 180
Protocol for purification of recombinant protein from 300 ml yeast culture
 Protocol for purification of recombinant protein from 300 ml yeast culture Equipment and reagents needed: Zirconia beads (0.5 mm diameter from BSP, Germany) Paint Shaker (at 4 C) Tube rotator for 15 ml
Protocol for purification of recombinant protein from 300 ml yeast culture Equipment and reagents needed: Zirconia beads (0.5 mm diameter from BSP, Germany) Paint Shaker (at 4 C) Tube rotator for 15 ml
EFFECTS OF VANADATE ON OLEIC ACID INDUCED INSULIN RESISTANCE IN CULTURED RAT HEPATOCYTES
 1 Department of Physiology, School of Dentistry, University of Zagreb, Gunduliæeva 5, HR-1 Zagreb, Croatia Department of Physiology, School of Medicine, University of Zagreb, Šalata 3, HR-1 Zagreb, Croatia
1 Department of Physiology, School of Dentistry, University of Zagreb, Gunduliæeva 5, HR-1 Zagreb, Croatia Department of Physiology, School of Medicine, University of Zagreb, Šalata 3, HR-1 Zagreb, Croatia
SUPPLEMENTARY INFORMATION
 doi:10.1038/nature12652 Supplementary Figure 1. PRDM16 interacts with endogenous EHMT1 in brown adipocytes. Immunoprecipitation of PRDM16 complex by flag antibody (M2) followed by Western blot analysis
doi:10.1038/nature12652 Supplementary Figure 1. PRDM16 interacts with endogenous EHMT1 in brown adipocytes. Immunoprecipitation of PRDM16 complex by flag antibody (M2) followed by Western blot analysis
PRODUCT INFORMATION & MANUAL
 PRODUCT INFORMATION & MANUAL Nuclear Extraction Kit NBP2-29447 Research use only. Not for diagnostic or therapeutic procedures. www.novusbio.com - P: 888.506.6887 - technical@novusbio.com Novus kits are
PRODUCT INFORMATION & MANUAL Nuclear Extraction Kit NBP2-29447 Research use only. Not for diagnostic or therapeutic procedures. www.novusbio.com - P: 888.506.6887 - technical@novusbio.com Novus kits are
PREPARATION OF IF- ENRICHED CYTOSKELETAL PROTEINS
 TMM,5-2011 PREPARATION OF IF- ENRICHED CYTOSKELETAL PROTEINS Ice-cold means cooled in ice water. In order to prevent proteolysis, make sure to perform all steps on ice. Pre-cool glass homogenizers, buffers
TMM,5-2011 PREPARATION OF IF- ENRICHED CYTOSKELETAL PROTEINS Ice-cold means cooled in ice water. In order to prevent proteolysis, make sure to perform all steps on ice. Pre-cool glass homogenizers, buffers
Insulin resistance and insulin secretory dysfunction as precursors of non- insulin-dependent diabetes mellitus: Prospective studies of Pima Indians
 University of Wollongong Research Online Graduate School of Medicine - Papers (Archive) Faculty of Science, Medicine and Health 1993 Insulin resistance and insulin secretory dysfunction as precursors of
University of Wollongong Research Online Graduate School of Medicine - Papers (Archive) Faculty of Science, Medicine and Health 1993 Insulin resistance and insulin secretory dysfunction as precursors of
Supplementary material: Materials and suppliers
 Supplementary material: Materials and suppliers Electrophoresis consumables including tris-glycine, acrylamide, SDS buffer and Coomassie Brilliant Blue G-2 dye (CBB) were purchased from Ameresco (Solon,
Supplementary material: Materials and suppliers Electrophoresis consumables including tris-glycine, acrylamide, SDS buffer and Coomassie Brilliant Blue G-2 dye (CBB) were purchased from Ameresco (Solon,
p47 negatively regulates IKK activation by inducing the lysosomal degradation of polyubiquitinated NEMO
 Supplementary Information p47 negatively regulates IKK activation by inducing the lysosomal degradation of polyubiquitinated NEMO Yuri Shibata, Masaaki Oyama, Hiroko Kozuka-Hata, Xiao Han, Yuetsu Tanaka,
Supplementary Information p47 negatively regulates IKK activation by inducing the lysosomal degradation of polyubiquitinated NEMO Yuri Shibata, Masaaki Oyama, Hiroko Kozuka-Hata, Xiao Han, Yuetsu Tanaka,
Supplementary Figure 1.TRIM33 binds β-catenin in the nucleus. a & b, Co-IP of endogenous TRIM33 with β-catenin in HT-29 cells (a) and HEK 293T cells
 Supplementary Figure 1.TRIM33 binds β-catenin in the nucleus. a & b, Co-IP of endogenous TRIM33 with β-catenin in HT-29 cells (a) and HEK 293T cells (b). TRIM33 was immunoprecipitated, and the amount of
Supplementary Figure 1.TRIM33 binds β-catenin in the nucleus. a & b, Co-IP of endogenous TRIM33 with β-catenin in HT-29 cells (a) and HEK 293T cells (b). TRIM33 was immunoprecipitated, and the amount of
Insulin Receptor Substrate 3 (IRS-3) and IRS-4 Impair IRS-1- and IRS-2-Mediated Signaling
 MOLECULAR AND CELLULAR BIOLOGY, Jan. 2001, p. 26 38 Vol. 21, No. 1 0270-7306/01/$04.00 0 DOI: 10.1128/MCB.21.1.26 38.2001 Copyright 2001, American Society for Microbiology. All Rights Reserved. Insulin
MOLECULAR AND CELLULAR BIOLOGY, Jan. 2001, p. 26 38 Vol. 21, No. 1 0270-7306/01/$04.00 0 DOI: 10.1128/MCB.21.1.26 38.2001 Copyright 2001, American Society for Microbiology. All Rights Reserved. Insulin
Supplementary Information
 Supplementary Information Supplementary Figure 1. CD4 + T cell activation and lack of apoptosis after crosslinking with anti-cd3 + anti-cd28 + anti-cd160. (a) Flow cytometry of anti-cd160 (5D.10A11) binding
Supplementary Information Supplementary Figure 1. CD4 + T cell activation and lack of apoptosis after crosslinking with anti-cd3 + anti-cd28 + anti-cd160. (a) Flow cytometry of anti-cd160 (5D.10A11) binding
Reduced PDX-1 expression impairs islet response to insulin resistance and worsens glucose homeostasis
 Am J Physiol Endocrinol Metab 288: E707 E714, 2005. First published November 23, 2004; doi:10.1152/ajpendo.00252.2004. Reduced PDX-1 expression impairs islet response to insulin resistance and worsens
Am J Physiol Endocrinol Metab 288: E707 E714, 2005. First published November 23, 2004; doi:10.1152/ajpendo.00252.2004. Reduced PDX-1 expression impairs islet response to insulin resistance and worsens
Growth and Differentiation Phosphorylation Sampler Kit
 Growth and Differentiation Phosphorylation Sampler Kit E 0 5 1 0 1 4 Kits Includes Cat. Quantity Application Reactivity Source Akt (Phospho-Ser473) E011054-1 50μg/50μl IHC, WB Human, Mouse, Rat Rabbit
Growth and Differentiation Phosphorylation Sampler Kit E 0 5 1 0 1 4 Kits Includes Cat. Quantity Application Reactivity Source Akt (Phospho-Ser473) E011054-1 50μg/50μl IHC, WB Human, Mouse, Rat Rabbit
Figure S1. Generation of inducible PTEN deficient mice and the BMMCs (A) B6.129 Pten loxp/loxp mice were mated with B6.
 Figure S1. Generation of inducible PTEN deficient mice and the BMMCs (A) B6.129 Pten loxp/loxp mice were mated with B6.129-Gt(ROSA)26Sor tm1(cre/ert2)tyj /J mice. To induce deletion of the Pten locus,
Figure S1. Generation of inducible PTEN deficient mice and the BMMCs (A) B6.129 Pten loxp/loxp mice were mated with B6.129-Gt(ROSA)26Sor tm1(cre/ert2)tyj /J mice. To induce deletion of the Pten locus,
