Angiotensin type 1a receptor-deficient mice develop diabetes-induced cardiac dysfunction, which is prevented by renin-angiotensin system inhibitors
|
|
|
- Maximilian Porter
- 5 years ago
- Views:
Transcription
1 Yong et al. Cardiovascular Diabetology 2013, 12:169 CARDIO VASCULAR DIABETOLOGY ORIGINAL INVESTIGATION Open Access Angiotensin type 1a receptor-deficient mice develop diabetes-induced cardiac dysfunction, which is prevented by renin-angiotensin system inhibitors Qian Chen Yong 1, Candice M Thomas 1, Rachid Seqqat 1,2, Niketa Chandel 1, Kenneth M Baker 1 and Rajesh Kumar 1* Abstract Background: Diabetes-induced organ damage is significantly associated with the activation of the renin-angiotensin system (RAS). Recently, several studies have demonstrated a change in the RAS from an extracellular to an intracellular system, in several cell types, in response to high ambient glucose levels. In cardiac myocytes, intracellular angiotensin (ANG) II synthesis and actions are ACE and AT 1 independent, respectively. However, a role of this system in diabetes-induced organ damage is not clear. Methods: To determine a role of the intracellular ANG II in diabetic cardiomyopathy, we induced diabetes using streptozotocin in AT 1a receptor deficient (AT 1a -KO) mice to exclude any effects of extracellular ANG II. Further, diabetic animals were treated with a renin inhibitor aliskiren, an ACE inhibitor benazeprilat, and an AT 1 receptor blocker valsartan. Results: AT 1a -KO mice developed significant diastolic and systolic dysfunction following 10 wks of diabetes, as determined by echocardiography. All three drugs prevented the development of cardiac dysfunction in these animals, without affecting blood pressure or glucose levels. A significant down regulation of components of the kallikrein-kinin system (KKS) was observed in diabetic animals, which was largely prevented by benazeprilat and valsartan, while aliskiren normalized kininogen expression. Conclusions: These data indicated that the AT 1a receptor, thus extracellular ANG II, are not required for the development of diabetic cardiomyopathy. The KKS might contribute to the beneficial effects of benazeprilat and valsartan in diabetic cardiomyopathy. A role of intracellular ANG II is suggested by the inhibitory effects of aliskiren, which needs confirmation in future studies. Keywords: Renin-angiotensin system, Intracrine, Renin inhibitor, Diabetic cardiomyopathy kallikrein, Kininogen, Kinin B2 receptor * Correspondence: kumar@medicine.tamhsc.edu Equal contributors 1 Division of Molecular Cardiology, Department of Medicine, Texas A&M Health Science Center, College of Medicine; Scott & White; Central Texas Veterans Health Care System, 1901 South First Street, Building 205, Temple, Texas 76504, USA Full list of author information is available at the end of the article 2013 Yong et al.; licensee BioMed Central Ltd. This is an Open Access article distributed under the terms of the Creative Commons Attribution License ( which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. The Creative Commons Public Domain Dedication waiver ( applies to the data made available in this article, unless otherwise stated.
2 Yong et al. Cardiovascular Diabetology 2013, 12:169 Page 2 of 13 Background Diabetic cardiomyopathy, defined as ventricular dysfunction that occurs independent of vascular or valvular pathology, is a major morbidity factor in diabetic patients [1,2]. Several mechanisms have been implicated in the development of diabetic cardiomyopathy, including upregulated oxidative stress, impaired calcium homeostasis, and activation of the renin-angiotensin system (RAS) [3]. Clinical and experimental studies have shown beneficial effects of RAS inhibitors in diabetes-induced organ damage [4]. Currently, two classes of RAS inhibitors, angiotensin type 1 receptor (AT 1 ) blockers (ARBs) and ACE inhibitors, are used in the clinic. However, cardiovascular morbidity and mortality remain higher in diabetic patients on RAS inhibitors compared to non-diabetics [5]. The latter observations have suggested the existence of residual risk in patients, which might be related to insufficient RAS blockade, among other factors. With regard to insufficient RAS blockade by ACE inhibitors and ARBs, we described an intracellular cardiac RAS that is significantly upregulated in diabetes [6-9]. An intracrine role of ANG II has also been described by other investigators [10,11]. The intracellular RAS in cardiac myocytes is not inhibited by ACE inhibitors and ARBs, due to chymase-mediated synthesis and AT 1 independent actions of intracellular ANG II. However, a renin inhibitor prevents both intra- and extracellular ANG II synthesis. We reported that a renin inhibitor was more effective than an ACE inhibitor or ARB in preventing diabetes-induced cardiac superoxide production, apoptosis and fibrosis [8], suggesting the intracellular RAS as a possible residual risk factor in diabetes. To determine whether the intracellular cardiac RAS has a role in diabetes-induced cardiac dysfunction, we induced diabetes in AT 1a receptor-deficient (AT 1a -KO) mice by streptozotocin (STZ). Our hypothesis was that in the absence of extracellular ANG II-mediated effects in these animals, development of cardiac dysfunction and prevention of the latter by a renin inhibitor would indicate involvement of intracellular ANG II in diabetic cardiomyopathy. We report that AT 1a -KO mice develop diabetes-induced cardiac dysfunction, which is prevented by a renin inhibitor, as well as by an ACE inhibitor and ARB. Materials and methods Animals All animal protocols were approved by the Institutional Animal Care and Use Committee and conformed to the National Institutes of Health guidelines. Male AT 1a -KO mice were purchased from the Jackson Laboratory (stock number , Bar Harbor, Maine) and fed ad libitum. At 12 wks of age, animals were randomized into control and diabetic groups (Figure 1). Animals in the diabetic group received STZ (50 mg kg -1 day -1 ; zanosar) intraperitoneally (i.p.) for 5 consecutive days, while those in the control group received 0.1 M sodium citrate buffer (ph 4.5). After 2 wks, mice with a blood glucose value of 250 mg/dl were considered diabetic. The diabetic group was divided into 5 subgroups (n = 10) that were treated with either vehicle or RAS inhibitors, as follows: 1) STZ + saline (STZ + Veh), 2) STZ + Aliskiren (20 mg/kg, STZ + Alsk), 3) STZ + Benazeprilat (10 mg/kg, STZ + Benz), 4) STZ + Valsartan (2 mg/kg, STZ + Vals), and STZ + PD (3 mg/kg) + Valsartan (2 mg/kg, STZ + PD + Vals). The drug dosages were based on our previously published study [12]. Drugs were delivered by subcutaneous osmotic minipumps (ALZET 1004, 0.11 μl/hr), for 10 wks, with minipump replacement every 4 wks. Echocardiography was performed at the beginning of diabetes (0 wk) and every 2 wks thereafter. At the end of the study (10 wks of diabetes), the right carotid artery was cannulated and arterial pressure measured under isoflurane/oxygen (1.5/2%) anesthesia with a blood pressure Figure 1 Study design. Twelve wk old male AT 1a receptor knockout (AT 1a -KO) mice were injected with either 0.1 M citrate buffer (ph 4.5) or streptozotocin (50 mg/kg) for five days. After 2 wks, animals with blood glucose levels of 250 mg/dl were considered diabetic. Echocardiographic measurements were taken in all groups at the beginning (Before STZ), onset of diabetes (0 wk), and at two wk intervals thereafter. Osmotic minipumps containing one of the drugs were implanted subcutaneously at 0 wk and replaced after 4 and 8 wks. At the conclusion of the study (10 wks), blood pressure was measured by carotid artery cannulation and tissues were collected.
3 Yong et al. Cardiovascular Diabetology 2013, 12:169 Page 3 of 13 analyzer (BPA 400, Micro-Med, Louisville, KY). Studies have shown a close correlation between systolic blood pressure measured under the conscious state and steady isoflurane anesthesia and that the latter can be reliably used to monitor changes in blood pressure in mice [13]. Hearts were immediately collected and processed for either cardiac myocytes isolation or fixed with paraformaldehyde for staining. In addition to the 10 wks study, a short study of one wk was performed to measure cardiac RAS activation. Echocardiographic measurements Transthoracic echocardiography was performed using a VisualSonics Vevo 2100 with a 35-MHz probe. Briefly, mice were anesthetized with 3-5% isoflurane (with 2% oxygen) that was reduced to 1.5% to maintain the heart rate at about 450 beats per minute. The heart was imaged in the 2-dimensional, short-axis, and 4-chamber view. Left ventricular (LV) fractional shortening (FS), ejection fraction (EF), stroke volume (SV), cardiac output (CO), LV internal dimension at end-diastole (LVI Dd), LV internal dimension at end-systole (LVIDs), LV posterior wall thickness at end-diastole (LVPWd), LV posterior wall thickness at end-systole (LVPWs), interventricular septum thickness at end-diastole (IVSd), intra-ventricular septum thickness at end-systole (IVSs), isovolumic relaxation time (IVRT), isovolumic contraction time (IVCT), early diastolic (E ) and late diastolic (A ) mitral annulus tissue Doppler velocities, and peak velocity of early (E) and late (A) LV filling waves were measured. Isolation of adult mouse cardiomyocytes Adult mouse cardiomyocytes were isolated using a temperature-controlled (37 C) Langendorff s perfusion system, as previously described with some modifications [14]. Briefly, hearts were perfused with perfusion buffer for 5 min, followed by digestion with collagenase II (0.17% W/V, 330 U/mg, Worthington Biochemical Corp), for 10 min, at a rate of 3 ml/min. After stopping digestion with a serum- and calcium-containing buffer, cells were washed with PBS and collected by centrifugation (180 rcf, 1 min). One portion of cardiac myocytes was snap frozen in liquid nitrogen for protein analysis or ANG II ELISA measurement, and the other was stored in RNAlater solution for RNA analysis. Real-time PCR Gene expression of angiotensinogen (AGT), renin, AT 1a, AT 1b, tissue kallikrein, kininogen 2, prorenin receptor (PRR), ACE2, and Mas was determined using TaqMan assays (Applied Biosystems). RNA was extracted using an RNeasy Fibrous Tissue Kit (Qiagen). cdna was made using a High Capacity cdna Reverse Transcription Kit (Applied Biosystems). Real-time PCR was performed in 20 μl reaction containing cdna, TaqMan Universal PCR master mix, and 20X specific gene expression assay mix (Applied Biosystems). Data were normalized to 18S mrna. Each sample was run in duplicate, and the threshold cycle, ΔCt, was calculated as Ct (target gene) - Ct (18S). The relative changes in target gene in different treatment groups were determined by the formula 2 -ΔΔ Ct, where ΔΔ Ct = ΔCt (control) - ΔCt (treatment group). The efficiency of AT 1a and AT 1b PCR was determined using a 10-fold dilution series of mouse adrenal gland cdna. The slope of the standard curve was determined and the efficiency calculated using the formula E = 10 ( 1/slope) -1. Western blot analysis Protein expression of renin, ANG II Type 2 receptor (AT 2 ), and bradykinin Type 2 (B2) receptor was assessed with the standard Western immunoblotting technique. Briefly, cardiac myocytes were sonicated in ice-cold lysis buffer (Cell Signaling) supplemented with protease and phosphatase inhibitor cocktails (Roche Applied Science). Homogenates were centrifuged at 16,000 g and protein concentration in the supernatant was determined using DC protein assay kit (BioRad). Equal amounts of cell lysate (60 μg) or plasma (0.05 μl) were separated on 4-20% SDS-polyacrylamide gels (120 V, 1 hr) and transferred to nitrocellulose membranes (27 V, 2 hrs). Blots were probed with anti-renin (Sigma-Aldrich), anti-at 2, and anti-b2 receptor (Santa Cruz Biotechnology) antibodies. Equal protein loading was confirmed by Ponceau staining, because GAPDH and beta-actin levels in the hearts of AT 1a -KO mice were significantly affected by diabetes or the treatments (data not shown). Protein bands were detected using secondary antibodies labeled with infrared dye 680/800 and the enhanced Odyssey Infrared Imaging System (LI-COR, Biosciences). ANG II measurement ANG II levels in isolated cardiac myocytes were measured by a competitive quantitative ELISA, following extraction with 1 M acetic acid and purification over a reverse phase C18 column, as described previously [15]. Since the cardiac myocyte isolation procedure involves perfusion of the heart, enzymatic digestion, and washing of the dispersed myocytes with PBS, the steps would wash-off extracellular ANG II; ANG II measured this way represents intracellular ANG II. The above intracellular ANG II method had previously been validated by mass spectrometry and confocal microscopy [9]. ANG II data have been presented in terms of per unitheartweighttobeconsistentwiththeliterature. Reactive oxygen species staining Hearts were fixed in 4% paraformaldehyde and frozen in O.C.T. compound (Tissue-Tek). Frozen sections (20 μm)
4 Yong et al. Cardiovascular Diabetology 2013, 12:169 Page 4 of 13 were incubated with 10 μm dihydroethidium (DHE, Sigma-Aldrich), at 37 C, for 30 min in a humidified chamber protected from light. Fluorescent images (60X) were obtained with a Leica TCS SP5X confocal microscope and analyzed using ImageJ. Mean DHE fluorescence was calculated by subtracting integrated density of the background signal from the integrated density of the fluorescent staining for 10 fields/heart, 5 hearts/ group, and normalized to control. Apoptosis Hearts were fixed in 10% paraformaldehyde and paraffin embedded. Paraffin embedded sections (5 μm) were incubated at 60 C for 15 minutes, de-waxed, and rehydrated. Apoptosis was detected using terminal deoxynucleotide transferase-mediated dutp nick-end labeling (TUNEL) assay, according to the manufacturer s instructions (In situ Cell Death Detection Kit, Roche). Actin filaments and nuclei of cardiac myocytes were counterstained using Alexa Fluor 546 phalloidin (Invitrogen) and DAPI, respectively. Positively stained nuclei were counted from 6 12 sections per heart and 3 hearts per treatment group. Statistical analysis All data were expressed as the mean ± SEM. One-way ANOVA with Tukey s post hoc test or multiple comparisons using two-way ANOVA with the Bonferroni post hoc test, where appropriate, were used for statistical analysis (GraphPad). P <0.05 was considered statistically significant. Results Diabetes activates the cardiac intracellular RAS We had previously demonstrated intracellular ANG II synthesis in neonatal and adult cardiac myocytes following stimulation with high glucose in vitro and in rat hearts after one wk of diabetes [8,9]. In these studies, an ARB, candesartan, was used to prevent uptake of extracellular ANG II, to distinguish intracellularly synthesized ANG II from extracellular ANG II internalized by cells. To further confirm intracellular synthesis of ANG II and activation of the RAS, we produced diabetes in AT 1a -KO and WT mice for one wk and measured ANG II levels in cardiac myocytes isolated from control and diabetic animals. As shown in Figure 2, a several fold increase in intracellular ANG II levels was observed in both AT 1a - KO and WT diabetic mice, compared to non-diabetic controls. Additionally, AGT and renin gene expression was increased in cardiac myocytes of diabetic animals compared to controls. These results demonstrated activation of the cardiac intracellular RAS by hyperglycemia in AT 1a -KO mice. Figure 2 Activation of the renin-angiotensin system. Activity of the RAS was measured in cardiac myocytes isolated from wild type (A-C) and AT 1a -KO (A, D-E) mice after 1 wk of diabetes. ANG II (A) was extracted from cardiac myocytes and measured using a competitive ELISA. Angiotensinogen (AGT, B and D) and renin (C and E) expression was measured in control and diabetic (STZ) samples, by real-time RT-PCR and normalized to 18S mrna. Values are expressed as the fold change compared to control ± SEM. *P < 0.05 vs. control. Diabetes induces diastolic dysfunction in AT 1a -KO mice, which is prevented by aliskiren, benazeprilat, and valsartan Others and we have shown prevention of cardiac dysfunction by ARBs and ACE inhibitors in animal models of diabetes [12]. To determine whether AT 1a -KO mice would develop diastolic dysfunction, we used echocardiography to monitor ventricular function of control and diabetic mice for up to 10 wks following the onset of
5 Yong et al. Cardiovascular Diabetology 2013, 12:169 Page 5 of 13 diabetes. Further, we treated diabetic mice with a renin inhibitor (aliskiren), ACE inhibitor (benazeprilat), and ARB (valsartan) to determine whether these agents would have a protective effect in the absence of AT 1a receptor. Diastolic function was evaluated by measuring the ratio of mitral valve flow velocities (E/A ratio) and isovolumic relaxation time (IVRT). As shown in Figure 3C and D, the E/A ratio was significantly lower at 8 and 10 wks of diabetes, compared to before STZ. Further, IVRT increased significantly after 10 wks of diabetes (Figure 3E, only 10 wk data shown). Intriguingly, treatment with all three classes of RAS inhibitors, including an ARB, completely prevented the progression of diastolic dysfunction. Blocking AT 2 receptor with PD in the presence of valsartan reduced the protective effects of the latter on E/A (Figure 3D), suggesting a beneficial role of AT 2. Diabetes induces systolic dysfunction in AT 1a -KO mice, which is prevented by aliskiren, benazeprilat, and valsartan In addition to diastolic dysfunction, we observed impairment of systolic function in diabetic AT 1a -KO mice, as determined by measurement of EF and FS (Figure 4). Similar to WT animals [12], systolic dysfunction in the diabetic AT 1a -KO animals manifested at 10 wks after the establishment of diabetes. All three RAS inhibitors protected the diabetic hearts from systolic impairment. Combined treatment with AT 1 and AT 2 blockers reduced protection provided by the AT 1 blocker alone. Additional parameters of cardiac structure and function Several other indices of cardiac structure and function, as measured by echocardiography, are presented in Table 1. There was no statistical difference in the heart rate among groups at the time of echocardiography. Cardiac output and stroke volume were reduced in diabetic mice compared to controls, which were significantly improved in all single treatment groups. The isovolumic contraction time was increased in the diabetic group, indicative of systolic dysfunction, as described above. Diastolic dysfunction was further confirmed by a decrease in the mitral annulus tissue Doppler velocities (E /A ) in diabetic animals, which was prevented in the treatment groups. Significantly, left ventricular posterior wall thickness, the interventricular septum thickness (IVS), and the left ventricular internal dimension, measured in diastole, did not change over the course of diabetes, indicating no change in cardiac structure. However, the IVS measured in systole decreased, the significance of which is not known. The latter was prevented by all three RAS inhibitors. Figure 3 Measurement of diastolic function by echocardiography. Representative pulsed-wave Doppler images of Mitral valve flow of Control and STZ + Veh mice at 10 wks of diabetes (A and B, respectively). Temporal recordings of Mitral valve flow velocity (E/A) in control and diabetic mice implanted with saline minipumps (STZ + Veh), demonstrating development of diastolic dysfunction (C). Mitral valve flow velocity (D) and isovolumic relaxation time (E) of Control, STZ + Veh, and those treated with aliskiren (STZ + Alsk), benazeprilat (STZ + Benz), valsartan (STZ + Vals), and valsartan + PD (STZ + Vals + PD); before STZ treatment and at 10 wks after becoming diabetic. 2 wk values for STZ + Vals + PD are shown, as we could not collect reliable (before STZ) data in this group. Values are expressed as the mean ± SEM. *P < 0.05 vs. Control (C) or respective Before STZ (D and E).
6 Yong et al. Cardiovascular Diabetology 2013, 12:169 Page 6 of 13 Figure 4 Measurement of systolic function by echocardiography. Representative M-mode short axis views of Control and STZ + Veh mice at 10 wks of diabetes (A and B, respectively). Ejection fraction (C) and fractional shortening (D) of Control, STZ + Veh, STZ + Alsk, STZ + Benz, STZ + Vals, and STZ + Vals + PD, before STZ treatment and at 10 wks after becoming diabetic. 2 wk values for STZ + Vals + PD are shown, as we could not obtain reliable (before STZ) data in this group. Values are expressed as the mean ± SEM. *P < 0.05 vs. respective Before STZ. # P < 0.05 vs. Control (10wk). Blood glucose levels and mean arterial pressure Blood glucose levels were monitored biweekly and were consistently elevated. After 10 wks, diabetic animals exhibited elevated blood glucose levels (>500 mg/dl), which were not lowered by aliskiren, benazeprilat, and valsartan (Figure 5A). Mean arterial pressure (MAP) in AT 1a -KO mice did not change as a result of hyperglycemia (Figure 5B), which is consistent with other reports in the STZ-induced model of diabetes [16]. Treatment with any of the RAS inhibitors did not reduce blood pressure, which corroborated previous reports of lack of hypotensive effects of these agents at comparable therapeutic doses in the absence of hypertension [17,18]. These observations suggested that the therapeutic effect of the RAS blockers on cardiac function were not secondary to the changes in hyperglycemia or blood pressure. Aliskiren and valsartan prevent diabetes-induced oxidative stress and apoptosis in the heart Diabetic AT 1a -KO mice showed a significant increase in DHE staining, compared to control mice. As shown in Table 1 Echocardiography measurements in control, diabetic (10 wks), and treatment groups Control STZ + Veh STZ + Alsk STZ + Benz STZ + Vals STZ + PD + Vals HR 477 ± ± ± ± ± ± 26 CO 24 ± ± 0.62* 22.5 ± ± ± ± 0.84* SV 52.8 ± ± 1.60* 45.3 ± 2.40* ± ± ± 1.70* IVCT 15.5 ± ± 0.60* 15.2 ± ± ± ± 1.40 LVPWd 0.93 ± ± ± ± ± ± 0.08 LVPWs 1.29 ± ± ± ± ± ± 0.06 IVSd 0.93 ± ± ± ± ± ± 0.04 IVSs 1.36 ± ± 0.05* 1.38 ± ± ± ± 0.06 LVIDd 3.91 ± ± ± 0.09* 3.47 ± 0.14* 3.68 ± ± 0.12 LVIDs 2.83 ± ± ± 0.10* 2.42 ± ± ± 0.13 E'/A' 0.90 ± ± 0.06* 0.84 ± ± ± ± 0.11 For control group, n = 8; STZ + Veh, n = 9; STZ + Alsk, n = 8; STZ + Benz, n = 9; STZ + Vals, n = 7; STZ + Vals + PD, n = 7. CO = cardiac output (mls/min), SV = stroke volume (μl), IVCT = isovolumic contraction time (ms), LVPWd = left ventricular posterior wall thickness in diastole (mm), LVPWs = left ventricular posterior wall thickness in systole, IVSd = interventricular septum thickness in diastole (mm), IVSs = interventricular septum thickness in systole, LVIDd = left ventricular internal dimension in diastole, LVIDs = left ventricular internal dimension in systole (mm), E A = mitral annulus tissue Doppler velocities. * = p < 0.05 vs. Control; = p < 0.05 vs. STZ + Veh.
7 Yong et al. Cardiovascular Diabetology 2013, 12:169 Page 7 of 13 Figure 5 Measurement of blood glucose, mean arterial pressure, oxidative stress, and cardiac myocyte apoptosis. Blood glucose values shown are from animals before injecting STZ and after 10 wks of diabetes in different groups (A). Mean arterial pressure (MAP) was calculated from blood pressure values measured through carotid artery cannulation following 10 wks of diabetes (B). Apoptosis was measured by TUNEL staining of heart sections and is represented as TUNEL positive nuclei as a percentage of total nuclei (C). Oxidative stress was measured by DHE staining (D and E). The staining intensity was calculated from five images per heart, three hearts per group and normalized to the section area (D). Representative images of DHE staining are shown (E). Values are expressed as the mean ± SEM. *P < 0.05 vs. before STZ or Control, # P <0.05vs.STZ+Veh. Figure 5D and E, the increased staining was completely abolished by aliskiren and valsartan; but, not by benazeprilat treatment. The number of apoptotic cells, detected by TUNEL staining, increased significantly in the diabetic heart (Figure 5C). The changes in apoptosis in diabetic and treated hearts were consistent with the changes in the oxidative stress. However, we did not observe an increase in cardiac fibrosis or a change in cardiac structure, as measured by LV posterior wall and interventricular septum thickness using echocardiography (data not shown). These results are consistent with our previous observations in WT mice [12]. Cardiac AT 1b expression in AT 1a -KO mice To determine any potential functional compensation by AT 1b in AT 1a -KO mice, we measured mrna levels of AT 1b in cardiac myocytes from WT mice, which were barely detectable with Ct values around 40. To compare the expression of AT 1a and AT 1b, we determined the efficiency of the two PCRs, which were 0.91 and 0.94, respectively (Figure 6A and B). Comparatively, AT 1b expression was ~1/1316th of AT 1a. In AT 1a -KO mice, AT 1b was upregulated slightly but was still ~1/543th of AT 1a in WT mice (Figure 6C). Importantly, AT 1b expression was reduced in diabetic AT 1a -KO mice, compared to non-diabetics. Renal mrna levels of AT 1b and AT 1a were about 3-fold higher than the hearts; however, AT 1b expression remained scarce in comparison to AT 1a in WT mice. There was a small increase in renal AT 1b expression in AT 1a -KO mice, but it was ~700 fold lower than renal AT 1a in WT mice (Figure 6D). Plasma renin levels are not changed following treatment with RAS inhibitors RAS inhibitors cause a reactive rise in plasma renin by interfering with the negative feedback effects of ANG II on kidneys via AT 1 receptor [19]. Similarly, we had previously reported an increase in plasma renin following RAS inhibition in WT mice [12]. To examine whether levels of AT 1b in AT 1a -KO mice, though extremely low, are sufficient to cause a physiological response; we performed Western blot analysis of plasma samples, using an antibody which recognizes both prorenin and renin. As shown in Figure 7A, only prorenin was detected, which was reduced in diabetic animals compared to controls. None of the RAS inhibitors used in this study upregulated plasma prorenin. These results indicated that levels of renal AT 1b were not sufficient to respond to RAS inhibition in AT 1a -KO mice; and by derivation, cardiac AT 1b receptor were also functionally irrelevant.
8 Yong et al. Cardiovascular Diabetology 2013, 12:169 Page 8 of 13 Figure 6 Relative expression of AT 1a and AT 1b in WT and AT 1a -KO mice. Efficiency of the PCR for AT 1a and AT 1b was determined using serial dilutions of mouse adrenal gland cdna (A and B). Parallel lines of the amplification reactions demonstrate similar efficiency of both PCRs. Expression of AT 1a and AT 1b was measured by real-time RT-PCR in cardiac myocytes (C) and kidneys (D) of wild type and AT 1a -KO (control and diabetic (STZ, 10 wk)) mice. Values are expressed as the fold change compared to control ± SEM. *P < 0.05 vs. control. Prorenin receptor and AT 2 expression does not change in AT 1a -KO mice following hyperglycemia or treatment with RAS inhibitors Upregulation of the PRR in kidneys and hearts of diabetic animals has been reported, which may contribute to the pathological mechanism of tissue damage [20,21]. We determined mrna and protein levels of the PRR in cardiac myocytes isolated from control, diabetic, and diabetic animals treated with RAS inhibitors. No significant change in PRR expression was observed in any of these groups, suggesting that the PRR may not have a role in diabetic cardiomyopathy in AT 1a -KO mice (Figure 7B, protein data Figure 7 Plasma renin levels and prorenin receptor and AT 2 expression in cardiac myocytes. Plasma renin (A) and AT 2 (C) levels were determined by Western blot analysis and PRR (B) expression was measured by real-time RT-PCR, in control, diabetic (STZ + Veh), and diabetic mice treated with different RAS inhibitors, after 10 wks of diabetes (C). Representative Western blot images are shown. Values are expressed as the fold change compared to control ± SEM. *P < 0.05 vs. control.
9 Yong et al. Cardiovascular Diabetology 2013, 12:169 Page 9 of 13 not shown). We also observed that AT 2 receptor expression did not change in diabetes or following treatment with RAS inhibitors (Figure 7C). Aliskiren, benazeprilat, and valsartan normalize the kallikrein-kinin system in diabetic hearts The kallikrein-kinin system (KKS) has been implicated in the development of diabetic cardiomyopathy [22,23]; and interaction between the KKS and the RAS at the level of ACE is well established [24]. In the present study, mrna levels of tissue kallikrein and kininogen were reduced in isolated cardiac myocytes from diabetic hearts. Treatment with benazeprilat and valsartan reversed the effect of diabetes on these two genes, whereas aliskiren treatment only normalized kininogen mrna expression (Figure 8A and B). Protein expression data is not presented, as available antibodies did not recognize tissue isoforms of mouse kallikrein and kininogen with certainty. Further, the protein expression of B2 receptor, which is responsible for the cardioprotective actions of bradykinin, was significantly diminished in diabetic hearts (Figure 8C). Benazeprilat significantly normalized the protein expression of B2 receptor, whereas aliskiren and valsartan did not have any effect. Together, these results suggest that part of the beneficial effects of RAS inhibitors was mediated by the KKS. Effect on ACE2/Ang 1-7/Mas pathway ACE2/Ang 1-7/Mas pathway represents a protective mechanism in the heart, which is downregulated in diabetes (Figure 8D and E). We observed that the expression of ACE2 was enhanced in all treatment groups except in the STZ + Vals + PD group. A reduction in Mas expression in diabetes was not reversed by any treatment. Discussion In this study, we determined a role of the intracellular cardiac RAS in the development of diabetes-induced cardiac dysfunction. We report three significant findings: 1) activation of the intracellular RAS, indicated by increased intracellular levels of ANG II in the absence of AT 1a - mediated uptake; 2) development of cardiac dysfunction in AT 1a -KO mice, which is prevented by a renin inhibitor and; 3) prevention of cardiac dysfunction by an ACE inhibitor and ARB in AT 1a -KO mice, possibly through activation of the kallikrein-kinin system. The basic premise of this study was the activation of the intracellular cardiac RAS in diabetes. In our previous Figure 8 Expression of tissue kallikrein, kininogen, kinin B2 receptor, ACE2, and Mas. mrna expression of tissue kallikrein (A), kininogen (B), ACE2 (D), and Mas (E) in cardiac myocytes of control, STZ + Veh, STZ + Alsk, STZ + Benz, STZ + Vals, and STZ + Vals + PD after 10 wks of diabetes was measured by real-time RT-PCR and normalized to 18S mrna. B2 receptor expression was measured by Western blot analysis (C). A representative Western blot image is shown. Values are expressed as the fold change compared to control ± SEM. *P < 0.05 vs. control, # P < 0.05 vs. STZ + Veh.
10 Yong et al. Cardiovascular Diabetology 2013, 12:169 Page 10 of 13 studies, demonstration of intracellular ANG II synthesis was based on an increase in cellular ANG II levels, after pharmacological blockade of ANG II uptake by cells using an ARB [8,9]. Here we demonstrated activation of the intracellular RAS in a genetic model of AT 1a -deficiency. We measured AGT and renin expression and ANG II concentration in cardiac myocytes isolated from AT 1a -KO mice after one wk of diabetes. A significant upregulation of AGT and renin expression was observed, which was accompanied by a several fold increase in ANG II levels in AT 1a -KO mice. The increase in ANG II levels was similar to that in WT mice (Figure 2). Intracellular ANG II synthesis in cardiac myocytes of AT 1a - KO mice was likely due to retention of AGT and renin inside cells in response to high ambient glucose levels, as we had reported in neonatal rat ventricular myocytes [9]. However, the intracellular site of synthesis and mechanism of renin activation in cardiac myocytes are not known. Since intracellular ANG II in AT 1a -KO mice could not have been due to cellular uptake, these observations clearly demonstrated activation of the intracellular RAS in the diabetic heart. Rodents express two subtypes of AT 1 receptor, AT 1a and AT 1b. It may be argued that some of the observations in the current study could be related to AT 1b receptor. Several studies have examined tissue-specific expression of AT 1a and AT 1b in mice and rats. In rats, one study reported solely AT 1a expression in the heart, another study detected very low AT 1b expression, about 1/10th of AT 1a expression [25,26]. However, in mice, AT 1b expression was detected only in the adrenal gland, brain, and testis; not in the heart [27]. In another mouse study, in which the AT 1b coding exon was replaced with the reporter gene lacz, there was no detectable lacz expression in kidneys and heart [28]. Consistent with these studies, we observed extremely low levels of cardiac myocyte AT 1b expression, i.e., 1/1316th and 1/543th in WT and AT 1a -KO mice, respectively, as compared to AT 1a expression in WT mice. AT 1b expression was detectable because we performed PCR up to 40 cycles, while routine protocols use only 30 cycles. Kidneys had higher expression of AT 1b compared to the heart; however, were not responsive to RAS inhibitors, as shown by the lack of a reactive rise in renin expression. These observations suggested that the levels of AT 1b in the heart were not sufficient to be physiologically relevant. Functional studies in AT 1a -KO or AT 1a -AT 1b double knockout mice by other investigators also did not find any role for AT 1b in the heart [29,30]. Further, AT 1b expression was reduced in the diabetic group compared to controls, indicating that AT 1b did not contribute to diabetesinduced cardiac dysfunction. To determine the role of the AT 2 receptor, we treated diabetic animals with the AT 2 antagonist PD123319, along with valsartan. Treatment with the AT 2 blocker reduced the protective effects of valsartan, suggesting that the AT 2 receptor had a beneficial role and was not involved in diabetesinduced cardiac dysfunction. Nuclear AT 1 and AT 2 receptors have been described in cardiac myocytes, which respond to ANG II stimulation by initiating nuclear signaling events [31]. Genetic removal of AT 1a and the lack of functional levels of AT 1b expression would indicate that AT 1a -KO mice will not have cardiac nuclear AT 1 receptors. AT 2 has a protective role, as discussed above. Thus, a role of the known nuclear ANG II receptors in intracellular ANG II-mediated diabetic cardiomyopathy is unlikely. Further studies are required to understand the mechanism of intracellular ANG II-induced diabetic cardiomyopathy. Given that RAS inhibitors are cardioprotective in diabetes, the development of cardiac dysfunction in diabetic AT 1a -KO mice represents an important finding. A decrease in systolic and diastolic function and increase in oxidative stress and apoptosis in the heart of diabetic animals was observed. The systolic and diastolic dysfunction in AT 1a -KO mice appeared similar to that we described in WT animals [12]. The onset of cardiac dysfunction was slightly delayed (E/A: 6 wks vs. 8 wks in WT vs. AT 1a -KO mice, respectively) and the degree of severity was less after 10 wks of diabetes in AT 1a -KO mice (E/A: 1.11 vs. 1.24; IVRT: 19.8 vs ms; EF: 47 vs. 52%; FS: 23 vs. 26%; in WT vs. AT 1a -KO mice, respectively). However, these differences could be due to inter-experimental variation. These observations suggested that the development of diabetic cardiomyopathy was independent of AT 1a. A similar observation was made regarding STZ-induced diabetic nephropathy in AT 1a -KO mice [20]. It was reported that the AT 1a deficiency delayed the onset of diabetes-induced glomerulosclerosis and proteinuria; but, did not prevent full development of these pathological endpoints [20]. Treatment with the ACE inhibitor imidapril partially prevented diabetic nephropathy; these investigators did not study the effect of an ARB. However, a handle region peptide, that blocked binding of prorenin to the prorenin receptor (PRR) and inhibited downstream signaling, completely prevented glomerulosclerosis and proteinuria. Based on these observations, the investigators concluded that an ANG II-independent mechanism contributed to the development of diabetic nephropathy [20]. We observed no change in PRR expression in cardiac myocytes of diabetic AT 1a -KO animals or following treatment with RAS inhibitors, compared to non-diabetic controls, suggesting that the PRR was not involved in cardiac dysfunction in AT 1a -KO mice. We had previously observed increased cardiac PRR in diabetes in WT mice [12]. Given that PRR expression is increased in conditions that activate the RAS (low salt and hyperglycemia) and is decreased by RAS inhibition, we believe that AT 1 receptor
11 Yong et al. Cardiovascular Diabetology 2013, 12:169 Page 11 of 13 deficiency likely explains the lack of PRR upregulation in this study. Further, development of diabetic cardiomyopathy in AT 1a -KO mice supported our hypothesis and previous reports of AT 1 -independent actions of intracellular ANG II in the heart [8,15,32]. However, to confirm the involvement of ANG II, we studied whether blocking ANG II synthesis with a renin inhibitor would prevent diabetes-induced cardiac dysfunction in AT 1a -KO mice. We also investigated whether an ACE inhibitor or ARB would provide protection via non-ras mechanisms, as was suggested by our previous study in WT mice [12]. We observed that the renin inhibitor aliskiren completely protected animals from developing cardiac dysfunction up to 10 wks of diabetes. We have previously reported that high glucose-induced intracellular ANG II synthesis is both renin and chymase-dependent in cardiac myocytes and renin and ACE-dependent in cardiac fibroblasts [9,33]. Aliskiren accumulates in tissues, including cardiac myocytes and therefore inhibits renin-dependent intracellular ANG II production [8,34,35]. We previously reported that aliskiren was better than benazeprilat and candesartan in reducing cardiac ANG II levels and preventing diabetes-induced cardiac myocyte apoptosis, oxidative stress, and cardiac fibrosis after one wk of diabetes in rats [8]. In the current study, preservation of heart function by aliskiren in AT 1a -KO animals, suggested the involvement of intracellular ANG II in diabetic cardiomyopathy. Significantly, benazeprilat and valsartan also showed protective effects, similar to aliskiren, in AT 1a -KO mice. Regarding protection by the ACE inhibitor, it may be argued that ACE-mediated ANG II synthesis in the circulation or by cardiac fibroblasts had a role in cardiac dysfunction. However, in the absence of AT 1a receptor, extracellular ANG II would be ineffective in the heart. The latter argument also applies to any change in the circulating RAS, as a result of AT 1a deficiency or the RAS inhibitor treatment. It has been reported that ACE inhibitors also have cardioprotective effects through inhibition of kinin degradation [24,36]. Additionally, these drugs enhance kinin B1 and B2 receptor function via allosteric mechanisms that involve ACE and B2 receptor heterodimerization and direct binding of ACE inhibitors to B1 receptor [37]. Beneficial effects of kallikrein overexpression and B2 receptor signaling, and detrimental effects of B1 receptor-induced inflammation, have been described in diabetic cardiomyopathy [23,38,39]. In this study, benazeprilat normalized the expression of tissue kallikrein, kininogen, and B2 receptor, which were impaired in diabetic animals, suggesting a role of the KKS in cardioprotection by benazeprilat in AT 1a -KO mice. The blockade of the B2 receptor reduced the renal protective effects of the ACE inhibitor ramipril in db/db mice, further supporting our conclusion [40]. Particularly intriguing was the observation that the AT 1 receptor blocker valsartan was as effective as the other two drugs, despite the lack of AT 1a receptor in these animals. As discussed above, the mouse heart does not express physiologically relevant levels of AT 1b ; thus, the protective effects of valsartan were likely mediated via non-ras mechanisms. Valsartan was recently shown to markedly inhibit lipopolysaccharide-induced cytokine production in macrophages and improve insulin resistance in co-cultured adipocytes, via an unidentified AT 1a receptor-independent pathway [41]. Another ARB, losartan, protected WT mice against myocardial ischemiareperfusion injury, which was not observed in kallikrein gene-deficient mice, suggesting that the KKS was a major determinant of the cardioprotective effects of losartan [42]. Similarly, the inhibitory effects of valsartan on ANG II-induced MAP and urinary sodium excretion in healthy humans were shown to be mediated via the B2 receptor [43]. We observed that valsartan significantly increased mrna expression of tissue kallikrein and kininogen, which might have contributed to the effects of valsartan in this study. To determine whether these actions of valsartan on the KKS were mediated through AT 2 receptor, as suggested by some studies [42,44], we measured AT 2 expression and treated diabetic mice with the AT 2 antagonist PD in combination with valsartan. No change in AT 2 expression was observed following diabetes or treatment with valsartan; however, we did observe reduced protection by valsartan when used in combination with PD Further, activation of the KKS, with the exception of B2 receptor, was blunted with the co-treatment, suggesting a contribution of AT 2 in cardiac protection. It is noteworthy that functional AT 2 receptors on cardiac myocyte nuclei have been demonstrated, which are accessible to intracellular ANG II [31]. However, how valsartan would modulate AT 2 function in AT 1a -KO mice is not clear. Aliskiren treatment also increased kininogen levels; thus, a partial contribution of the KKS in aliskiren-mediated cardioprotection cannot be excluded [45]. ACE2 expression was increased in animals treated with all three RAS inhibitors, suggesting increased Ang 1 7 generation; however, Mas expression was not rescued by these treatments. Therefore, a role of the Ang 1-7/Mas axis, independently or in concert with the KKS, in cardioprotection is not clear. Conclusions In conclusion, we demonstrated an elevation of cardiac intracellular ANG II and the development of diabetesinduced cardiac dysfunction in AT 1a -KO mice. All three classes of RAS inhibitors protected the heart against diabetic cardiomyopathy. Whereas protection provided by a renin inhibitor suggested the involvement of intracellular
12 Yong et al. Cardiovascular Diabetology 2013, 12:169 Page 12 of 13 ANG II in the pathological process, the beneficial effects of the ACE inhibitor and ARB confounded the interpretation. Two possibilities emerged from this study; either the RAS did not have a significant role in the development of diabetic cardiomyopathy, or if it did, then it was likely via the intracellular RAS. Interestingly, our knowledge of the role of the RAS in diabetes-induced organ damage is based entirely on pharmacological benefits of RAS inhibitors. More studies utilizing genetic animal models are required to determine precisely the role of ANG II in diabetic tissue damage. If a role of intracellular ANG II in diabetic cardiomyopathy is established, delineation of AT 1 -independent mechanisms of ANG II would require substantial future research. In summary, this study provides new insights into the role of the RAS and RAS inhibitors in diabetic cardiomyopathy. Limitations This study focused on the role of cardiac intracellular RAS in diabetic cardiomyopathy. Thus, isolated cardiac myocytes, rather than whole hearts, were used to measure various molecular parameters, which severely limited the amount of sample available for analysis. For the latter reason, ANG II levels were not measured in the treated groups. However, we had reported in wild type mice and rats that AT 1 receptor blockers and ACE inhibitors did not significantly reduce intracellular ANG II levels in cardiac myocytes from diabetic hearts. Only a renin inhibitor prevented diabetes-induced ANG II formation [8,12]. We did not expect different results in AT 1a -KO animals. Any change in the circulating ANG II levels, as a result of the treatments with RAS inhibitors, would not be of any consequence in the heart of AT 1a - KO animals. Another limitation of this study is that gene expression data for some parameters described in Figure 8 could not be confirmed by protein analysis due to non-specific bands on Western blots that precluded identification of protein-specific bands with certainty. Regarding the mechanism of action of benazeprilat and valsartan via the KKS, our data are consistent with the literature; however, more studies will be required. Finally, whereas our hypothesis of the role of intracellular ANG II in diabetic cardiomyopathy is strengthened by the data presented, extended studies will be required to unequivocally prove it. Abbreviations ACE: Angiotensin-converting enzyme; ACE2: Angiotensin-converting enzyme; ACEi: ACE inhibitor; AGT: Angiotensinogen; Alsk: Aliskiren; Ang-(1 7): Angiotensin- (1 7); ANG I: Angiotensin I; ANG II: Angiotensin II; ARB: Angiotensin receptor blocker; ANP: Atrial natriuretic peptide; AT1 receptor: AngII type 1 receptor; AT1a-KO: AT1a receptor-deficient; AT2 receptor: AngII type 2 receptor; B2R: B2 receptor; Benz: Benazeprilat; BNP: Brain natriuretic peptide; CO: Cardiac output; DHE: Dihydroethidium; E/A ratio: Ratio of mitral valve flow velocities; E /A ratio: Ratio of early to late mitral annulus tissue Doppler velocities; ERK: Extracellular-signal-regulated kinase; EF: Ejection fraction; FS: Fractional shortening; IVCT: Isovolumic contraction time; IVRT: Isovolumic relaxation time; IVSd: Interventricular septum thickness at end-diastole; IVSs: Interventricular septum thickness at end-systole; KKS: Kallikrein-kinin system; KLK: Kallikrein; KNG: Kininogen; LV: Left ventricular; LVIDd: LV internal dimension at end-diastole; LVIDs: LV internal dimension at end-systole; LVPWd: LV posterior wall thickness at end-diastole; LVPWs: LV posterior wall thickness at end-systole; Mas: Mas receptor; MHC: Myosin heavy chain; MAP: Mean arterial pressure; PRR: (Pro)renin receptor;ras:renin angiotensin system; ROS: Reactive oxygen species; SV: Stroke volume; TUNEL: Terminal deoxynucleotide transferase-mediated dutp nick-end labeling; Vals: Valsartan; Veh: Vehicle; WT: Wild type. Competing interests RK and KMB had previously received research funding from Novartis Pharmaceuticals Corporation. In addition, Novartis provided aliskiren, valsartan, and benazeprilat for use in this study. CMT, QCY, RS and NC do not have any conflicts of interest. This work was published in the abstract form in Hypertension 58(5):e128, Authors contributions QCY researched data and wrote the manuscript, CMT researched data and reviewed the manuscript, RS researched data, NC researched data, KMB reviewed/edited the manuscript and contributed to discussion, RK designed and supervised research and wrote the manuscript. KMB and RK are guarantors of this work and, as such, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. All authors read and approved the final manuscript. Acknowledgments This material is the result of work supported with resources and the use of facilities at the Central Texas Veterans Health Care System, Temple, Texas. This work was supported by funding from NIH grant 5R01HL Author details 1 Division of Molecular Cardiology, Department of Medicine, Texas A&M Health Science Center, College of Medicine; Scott & White; Central Texas Veterans Health Care System, 1901 South First Street, Building 205, Temple, Texas 76504, USA. 2 Current address: SENESCYT/Proyecto Prometeo, Laboratorio de Biotecnología Humana, Escuela Politécnica del Ejército, Sangolquí, Ecuador. Received: 25 September 2013 Accepted: 9 November 2013 Published: 12 November 2013 References 1. From AM, Scott CG, Chen HH: The development of heart failure in patients with diabetes mellitus and pre-clinical diastolic dysfunction a population-based study. J Am Coll Cardiol 2010, 55(4): Carnethon MR, De Chavez PJ, Biggs ML, Lewis CE, Pankow JS, Bertoni AG, Golden SH, Liu K, Mukamal KJ, Campbell-Jenkins B, et al: Association of weight status with mortality in adults with incident diabetes. JAMA 2012, 308(6): Boudina S, Abel ED: Diabetic cardiomyopathy revisited. Circulation 2007, 115(25): Lim HS, MacFadyen RJ, Lip GY: Diabetes mellitus, the renin-angiotensinaldosterone system, and the heart. Arch Intern Med 2004, 164(16): Azizi M, Menard J: Renin inhibitors and cardiovascular and renal protection: an endless quest? Cardiovasc Drugs Ther 2013, 27(2): Kumar R, Thomas CM, Yong QC, Chen W, Baker KM: The intracrine renin-angiotensin system. Clin Sci 2012, 123(5): Kumar R, Yong QC, Thomas CM, Baker KM: Review: Intracardiac intracellular angiotensin system in diabetes. Am J Physiol Regul Integr Comp Physiol 2012, 302:R510 R Singh VP, Le B, Khode R, Baker KM, Kumar R: Intracellular angiotensin II production in diabetic rats is correlated with cardiomyocyte apoptosis, oxidative stress, and cardiac fibrosis. Diabetes 2008, 57(12): Singh VP, Le B, Bhat VB, Baker KM, Kumar R: High glucose induced regulation of intracellular angiotensin II synthesis and nuclear redistribution in cardiac myocytes. Am J Physiol Heart Circ Physiol 2007, 293:H939 H948.
c Ischemia (30 min) Reperfusion (8 w) Supplementary Figure bp 300 bp Ischemia (30 min) Reperfusion (4 h) Dox 20 mg/kg i.p.
 a Marker Ripk3 +/ 5 bp 3 bp b Ischemia (3 min) Reperfusion (4 h) d 2 mg/kg i.p. 1 w 5 w Sacrifice for IF size A subset for echocardiography and morphological analysis c Ischemia (3 min) Reperfusion (8
a Marker Ripk3 +/ 5 bp 3 bp b Ischemia (3 min) Reperfusion (4 h) d 2 mg/kg i.p. 1 w 5 w Sacrifice for IF size A subset for echocardiography and morphological analysis c Ischemia (3 min) Reperfusion (8
Supplementary Figure 1. Confocal immunofluorescence showing mitochondrial translocation of Drp1. Cardiomyocytes treated with H 2 O 2 were prestained
 Supplementary Figure 1. Confocal immunofluorescence showing mitochondrial translocation of Drp1. Cardiomyocytes treated with H 2 O 2 were prestained with MitoTracker (red), then were immunostained with
Supplementary Figure 1. Confocal immunofluorescence showing mitochondrial translocation of Drp1. Cardiomyocytes treated with H 2 O 2 were prestained with MitoTracker (red), then were immunostained with
hemodynamic stress. A. Echocardiographic quantification of cardiac dimensions and function in
 SUPPLEMENTAL FIGURE LEGENDS Supplemental Figure 1. Fbn1 C1039G/+ hearts display normal cardiac function in the absence of hemodynamic stress. A. Echocardiographic quantification of cardiac dimensions and
SUPPLEMENTAL FIGURE LEGENDS Supplemental Figure 1. Fbn1 C1039G/+ hearts display normal cardiac function in the absence of hemodynamic stress. A. Echocardiographic quantification of cardiac dimensions and
Fetal gene upregulation by 1-wk TAC is significantly increased in mice lacking RGS2.
 3562-RG-1 Supplementary Figure 1 Fetal gene upregulation by 1-wk is significantly increased in mice lacking RGS2. ANP(Nppa) /BNP(Nppb) A-type and B-type natriuretic peptide; β-mhc (Myh7) beta myosin heavy
3562-RG-1 Supplementary Figure 1 Fetal gene upregulation by 1-wk is significantly increased in mice lacking RGS2. ANP(Nppa) /BNP(Nppb) A-type and B-type natriuretic peptide; β-mhc (Myh7) beta myosin heavy
SUPPLEMENTAL MATERIAL. Supplementary Methods
 SUPPLEMENTAL MATERIAL Supplementary Methods Culture of cardiomyocytes, fibroblasts and cardiac microvascular endothelial cells The isolation and culturing of neonatal rat ventricular cardiomyocytes was
SUPPLEMENTAL MATERIAL Supplementary Methods Culture of cardiomyocytes, fibroblasts and cardiac microvascular endothelial cells The isolation and culturing of neonatal rat ventricular cardiomyocytes was
Control. csarnt -/- Cre, f/f
 ody weight (g) A re,f/f re x f/f f/+ re, f/+ re,f/+ f/f x f/f f/+ cs -/- re, f/f re f/f re, f/f Normal chow Tamoxifen Tamoxifen Tamoxifen W 4W re f/f re, re/ff f/f re f/f re, re/ff f/f Normal chow Tamoxifen
ody weight (g) A re,f/f re x f/f f/+ re, f/+ re,f/+ f/f x f/f f/+ cs -/- re, f/f re f/f re, f/f Normal chow Tamoxifen Tamoxifen Tamoxifen W 4W re f/f re, re/ff f/f re f/f re, re/ff f/f Normal chow Tamoxifen
SUPPLEMENTARY INFORMATION
 SUPPLEMENTARY INFORMATION FOR Liver X Receptor α mediates hepatic triglyceride accumulation through upregulation of G0/G1 Switch Gene 2 (G0S2) expression I: SUPPLEMENTARY METHODS II: SUPPLEMENTARY FIGURES
SUPPLEMENTARY INFORMATION FOR Liver X Receptor α mediates hepatic triglyceride accumulation through upregulation of G0/G1 Switch Gene 2 (G0S2) expression I: SUPPLEMENTARY METHODS II: SUPPLEMENTARY FIGURES
Tcf21 MCM ; R26 mtmg Sham GFP Col 1/3 TAC 8W TAC 2W. Postn MCM ; R26 mtmg Sham GFP Col 1/3 TAC 8W TAC 2W
 A Tcf21 MCM ; R26 mtmg Sham GFP Col 1/3 Tcf21 MCM ; R26 mtmg TAC 2W Tcf21 MCM ; R26 mtmg TAC 8W B Postn MCM ; R26 mtmg Sham GFP Col 1/3 Postn MCM ; R26 mtmg TAC 2W Postn MCM ; R26 mtmg TAC 8W Supplementary
A Tcf21 MCM ; R26 mtmg Sham GFP Col 1/3 Tcf21 MCM ; R26 mtmg TAC 2W Tcf21 MCM ; R26 mtmg TAC 8W B Postn MCM ; R26 mtmg Sham GFP Col 1/3 Postn MCM ; R26 mtmg TAC 2W Postn MCM ; R26 mtmg TAC 8W Supplementary
Echocardiographic and Doppler Assessment of Cardiac Functions in Patients of Non-Insulin Dependent Diabetes Mellitus
 ORIGINAL ARTICLE JIACM 2002; 3(2): 164-8 Echocardiographic and Doppler Assessment of Cardiac Functions in Patients of Non-Insulin Dependent Diabetes Mellitus Rajesh Rajput*, Jagdish**, SB Siwach***, A
ORIGINAL ARTICLE JIACM 2002; 3(2): 164-8 Echocardiographic and Doppler Assessment of Cardiac Functions in Patients of Non-Insulin Dependent Diabetes Mellitus Rajesh Rajput*, Jagdish**, SB Siwach***, A
Postn MCM Smad2 fl/fl Postn MCM Smad3 fl/fl Postn MCM Smad2/3 fl/fl. Postn MCM. Tgfbr1/2 fl/fl TAC
 A Smad2 fl/fl Smad3 fl/fl Smad2/3 fl/fl Tgfbr1/2 fl/fl 1. mm B Tcf21 MCM Tcf21 MCM Smad3 fl/fl Tcf21 MCM Smad2/3 fl/fl Tcf21 MCM Tgfbr1/2 fl/fl αmhc MCM C 1. mm 1. mm D Smad2 fl/fl Smad3 fl/fl Smad2/3
A Smad2 fl/fl Smad3 fl/fl Smad2/3 fl/fl Tgfbr1/2 fl/fl 1. mm B Tcf21 MCM Tcf21 MCM Smad3 fl/fl Tcf21 MCM Smad2/3 fl/fl Tcf21 MCM Tgfbr1/2 fl/fl αmhc MCM C 1. mm 1. mm D Smad2 fl/fl Smad3 fl/fl Smad2/3
Serum cytokine levels in control and tumor-bearing male and female mice at day 15.
 Supplementary Table 1. Serum cytokine levels in control and tumor-bearing male and female mice at day 15. Male Female Cytokine Control C-26 Control C-26 IL-1β 2.0 ± 0.8 9.6 ± 1.5* 1.8 ± 0.2 6.8 ± 1.4*
Supplementary Table 1. Serum cytokine levels in control and tumor-bearing male and female mice at day 15. Male Female Cytokine Control C-26 Control C-26 IL-1β 2.0 ± 0.8 9.6 ± 1.5* 1.8 ± 0.2 6.8 ± 1.4*
Supplementary Figure 1: si-craf but not si-braf sensitizes tumor cells to radiation.
 Supplementary Figure 1: si-craf but not si-braf sensitizes tumor cells to radiation. (a) Embryonic fibroblasts isolated from wildtype (WT), BRAF -/-, or CRAF -/- mice were irradiated (6 Gy) and DNA damage
Supplementary Figure 1: si-craf but not si-braf sensitizes tumor cells to radiation. (a) Embryonic fibroblasts isolated from wildtype (WT), BRAF -/-, or CRAF -/- mice were irradiated (6 Gy) and DNA damage
In Vivo Animal Models of Heart Disease. Why Animal Models of Disease? Timothy A Hacker, PhD Department of Medicine University of Wisconsin-Madison
 In Vivo Animal Models of Heart Disease Timothy A Hacker, PhD Department of Medicine University of Wisconsin-Madison Why Animal Models of Disease? Heart Failure (HF) Leading cause of morbidity and mortality
In Vivo Animal Models of Heart Disease Timothy A Hacker, PhD Department of Medicine University of Wisconsin-Madison Why Animal Models of Disease? Heart Failure (HF) Leading cause of morbidity and mortality
SUPPLEMENTARY INFORMATION
 a c e doi:10.1038/nature10407 b d f Supplementary Figure 1. SERCA2a complex analysis. (a) Two-dimensional SDS-PAGE gels of SERCA2a complexes. A silver-stained SDSPAGE gel is shown, which reveals a 12 kda
a c e doi:10.1038/nature10407 b d f Supplementary Figure 1. SERCA2a complex analysis. (a) Two-dimensional SDS-PAGE gels of SERCA2a complexes. A silver-stained SDSPAGE gel is shown, which reveals a 12 kda
Prospect Cardiac Packages. S-Sharp
 Prospect Cardiac Packages S-Sharp B mode: Teichholz: Teichholz formula LV Volume 2D: modified Simpson's rule method ALM: area length method LV Volume (Intg.): integral method M mode: Long axis: Teichholz
Prospect Cardiac Packages S-Sharp B mode: Teichholz: Teichholz formula LV Volume 2D: modified Simpson's rule method ALM: area length method LV Volume (Intg.): integral method M mode: Long axis: Teichholz
Gallic acid prevents isoproterenol-induced cardiac hypertrophy and fibrosis through regulation of JNK2 signaling and Smad3 binding activity
 Gallic acid prevents isoproterenol-induced cardiac hypertrophy and fibrosis through regulation of JNK2 signaling and Smad3 binding activity Yuhee Ryu 1,+, Li Jin 1,2+, Hae Jin Kee 1,, Zhe Hao Piao 3, Jae
Gallic acid prevents isoproterenol-induced cardiac hypertrophy and fibrosis through regulation of JNK2 signaling and Smad3 binding activity Yuhee Ryu 1,+, Li Jin 1,2+, Hae Jin Kee 1,, Zhe Hao Piao 3, Jae
Protection against doxorubicin-induced myocardial dysfunction in mice by cardiac-specific expression of carboxyl terminus of hsp70-interacting protein
 Protection against doxorubicin-induced myocardial dysfunction in mice by cardiac-specific expression of carboxyl terminus of hsp70-interacting protein Lei Wang 1, Tian-Peng Zhang 1, Yuan Zhang 2, Hai-Lian
Protection against doxorubicin-induced myocardial dysfunction in mice by cardiac-specific expression of carboxyl terminus of hsp70-interacting protein Lei Wang 1, Tian-Peng Zhang 1, Yuan Zhang 2, Hai-Lian
(A) PCR primers (arrows) designed to distinguish wild type (P1+P2), targeted (P1+P2) and excised (P1+P3)14-
 1 Supplemental Figure Legends Figure S1. Mammary tumors of ErbB2 KI mice with 14-3-3σ ablation have elevated ErbB2 transcript levels and cell proliferation (A) PCR primers (arrows) designed to distinguish
1 Supplemental Figure Legends Figure S1. Mammary tumors of ErbB2 KI mice with 14-3-3σ ablation have elevated ErbB2 transcript levels and cell proliferation (A) PCR primers (arrows) designed to distinguish
References. Plasma renin activity (PRA) PRA was measured by a radioimmunoassay kit (Wallac, Tokyo, Japan).
 Detailed Methods Experiment I enos / mice were purchased from Jackson Laboratory (Bar Harbor, USA). C57BL/6J mice on the same genetic background were purchased from KBT Oriental (Hamamatsu, Japan). Eleven-week-old
Detailed Methods Experiment I enos / mice were purchased from Jackson Laboratory (Bar Harbor, USA). C57BL/6J mice on the same genetic background were purchased from KBT Oriental (Hamamatsu, Japan). Eleven-week-old
General Laboratory methods Plasma analysis: Gene Expression Analysis: Immunoblot analysis: Immunohistochemistry:
 General Laboratory methods Plasma analysis: Plasma insulin (Mercodia, Sweden), leptin (duoset, R&D Systems Europe, Abingdon, United Kingdom), IL-6, TNFα and adiponectin levels (Quantikine kits, R&D Systems
General Laboratory methods Plasma analysis: Plasma insulin (Mercodia, Sweden), leptin (duoset, R&D Systems Europe, Abingdon, United Kingdom), IL-6, TNFα and adiponectin levels (Quantikine kits, R&D Systems
E10.5 E18.5 P2 10w 83w NF1 HF1. Sham ISO. Bmi1. H3K9me3. Lung weight (g)
 Myociyte cross-sectional Relative mrna levels Relative levels Relative mrna levels Supplementary Figures and Legends a 8 6 4 2 Ezh2 E1.5 E18.5 P2 1w 83w b Ezh2 p16 amhc b-actin P2 43w kd 37 86 16 wt mouse
Myociyte cross-sectional Relative mrna levels Relative levels Relative mrna levels Supplementary Figures and Legends a 8 6 4 2 Ezh2 E1.5 E18.5 P2 1w 83w b Ezh2 p16 amhc b-actin P2 43w kd 37 86 16 wt mouse
Vevo 2100 System Cardio Measurements. Dieter Fuchs, PhD FUJIFILM VisualSonics, Inc.
 Vevo 2100 System Cardio Measurements Dieter Fuchs, PhD FUJIFILM VisualSonics, Inc. dfuchs@visualsonics.com Instructions This document is a guideline on how to assess cardiac function in rodents imaged
Vevo 2100 System Cardio Measurements Dieter Fuchs, PhD FUJIFILM VisualSonics, Inc. dfuchs@visualsonics.com Instructions This document is a guideline on how to assess cardiac function in rodents imaged
Impact factor: Reporter:4A1H0019 Chen Zi Hao 4A1H0023 Huang Wan ting 4A1H0039 Sue Yi Zhu 4A1H0070 Lin Guan cheng 4A1H0077 Chen Bo xuan
 Curcumin Protects Neonatal Rat Cardiomyocytes against High Glucose-Induced Apoptosis via PI3K/Akt Signalling Pathway Wei Yu,1,2 Wenliang Zha,1 Zhiqiang Ke,1 Qing Min,2 Cairong Li,1 Huirong Sun,3 and Chao
Curcumin Protects Neonatal Rat Cardiomyocytes against High Glucose-Induced Apoptosis via PI3K/Akt Signalling Pathway Wei Yu,1,2 Wenliang Zha,1 Zhiqiang Ke,1 Qing Min,2 Cairong Li,1 Huirong Sun,3 and Chao
Drugs acting on the reninangiotensin-aldosterone
 Drugs acting on the reninangiotensin-aldosterone system John McMurray Eugene Braunwald Scholar in Cardiovascular Diseases, Brigham and Women s Hospital, Boston & Visiting Professor, Harvard Medical School
Drugs acting on the reninangiotensin-aldosterone system John McMurray Eugene Braunwald Scholar in Cardiovascular Diseases, Brigham and Women s Hospital, Boston & Visiting Professor, Harvard Medical School
LCZ696 A First-in-Class Angiotensin Receptor Neprilysin Inhibitor
 The Angiotensin Receptor Neprilysin Inhibitor LCZ696 in Heart Failure with Preserved Ejection Fraction The Prospective comparison of ARNI with ARB on Management Of heart failure with preserved ejection
The Angiotensin Receptor Neprilysin Inhibitor LCZ696 in Heart Failure with Preserved Ejection Fraction The Prospective comparison of ARNI with ARB on Management Of heart failure with preserved ejection
Entresto Development of sacubitril/valsartan (LCZ696) for the treatment of heart failure with reduced ejection fraction
 Cardio-Metabolic Franchise Entresto Development of sacubitril/valsartan (LCZ696) for the treatment of heart failure with reduced ejection fraction Randy L Webb, PhD Rutgers Workshop October 21, 2016 Heart
Cardio-Metabolic Franchise Entresto Development of sacubitril/valsartan (LCZ696) for the treatment of heart failure with reduced ejection fraction Randy L Webb, PhD Rutgers Workshop October 21, 2016 Heart
A Long-Term and Slow-Releasing Hydrogen Sulfide Donor Protects against Myocardial. Ischemia/Reperfusion Injury
 Supporting Information A Long-Term and Slow-Releasing Hydrogen Sulfide Donor Protects against Myocardial Ischemia/Reperfusion Injury Xiaotian Sun 1 *, Wenshuo Wang 2, Jing Dai 3, Sheng Jin 4, Jiechun Huang
Supporting Information A Long-Term and Slow-Releasing Hydrogen Sulfide Donor Protects against Myocardial Ischemia/Reperfusion Injury Xiaotian Sun 1 *, Wenshuo Wang 2, Jing Dai 3, Sheng Jin 4, Jiechun Huang
Kidney. Heart. Lung. Sirt1. Gapdh. Mouse IgG DAPI. Rabbit IgG DAPI
 a e Na V 1.5 Ad-LacZ Ad- 110KD b Scn5a/ (relative to Ad-LacZ) f 150 100 50 0 p = 0.65 Ad-LacZ Ad- c Heart Lung Kidney Spleen 110KD d fl/fl c -/- DAPI 20 µm Na v 1.5 250KD fl/fl Rabbit IgG DAPI fl/fl Mouse
a e Na V 1.5 Ad-LacZ Ad- 110KD b Scn5a/ (relative to Ad-LacZ) f 150 100 50 0 p = 0.65 Ad-LacZ Ad- c Heart Lung Kidney Spleen 110KD d fl/fl c -/- DAPI 20 µm Na v 1.5 250KD fl/fl Rabbit IgG DAPI fl/fl Mouse
renoprotection therapy goals 208, 209
 Subject Index Aldosterone, plasminogen activator inhibitor-1 induction 163, 164, 168 Aminopeptidases angiotensin II processing 64 66, 214 diabetic expression 214, 215 Angiotensin I intrarenal compartmentalization
Subject Index Aldosterone, plasminogen activator inhibitor-1 induction 163, 164, 168 Aminopeptidases angiotensin II processing 64 66, 214 diabetic expression 214, 215 Angiotensin I intrarenal compartmentalization
Appendix II: ECHOCARDIOGRAPHY ANALYSIS
 Appendix II: ECHOCARDIOGRAPHY ANALYSIS Two-Dimensional (2D) imaging was performed using the Vivid 7 Advantage cardiovascular ultrasound system (GE Medical Systems, Milwaukee) with a frame rate of 400 frames
Appendix II: ECHOCARDIOGRAPHY ANALYSIS Two-Dimensional (2D) imaging was performed using the Vivid 7 Advantage cardiovascular ultrasound system (GE Medical Systems, Milwaukee) with a frame rate of 400 frames
Introduction. Acute sodium overload produces renal tubulointerstitial inflammation in normal rats
 Acute sodium overload produces renal tubulointerstitial inflammation in normal rats MI Roson, et al. Kidney International (2006) Introduction Present by Kanya Bunnan and Wiraporn paebua Tubular sodium
Acute sodium overload produces renal tubulointerstitial inflammation in normal rats MI Roson, et al. Kidney International (2006) Introduction Present by Kanya Bunnan and Wiraporn paebua Tubular sodium
Left atrial function. Aliakbar Arvandi MD
 In the clinic Left atrial function Abstract The left atrium (LA) is a left posterior cardiac chamber which is located adjacent to the esophagus. It is separated from the right atrium by the inter-atrial
In the clinic Left atrial function Abstract The left atrium (LA) is a left posterior cardiac chamber which is located adjacent to the esophagus. It is separated from the right atrium by the inter-atrial
Optimal blockade of the Renin- Angiotensin-Aldosterone. in chronic heart failure
 Optimal blockade of the Renin- Angiotensin-Aldosterone Aldosterone- (RAA)-System in chronic heart failure Jan Östergren Department of Medicine Karolinska University Hospital Stockholm, Sweden Key Issues
Optimal blockade of the Renin- Angiotensin-Aldosterone Aldosterone- (RAA)-System in chronic heart failure Jan Östergren Department of Medicine Karolinska University Hospital Stockholm, Sweden Key Issues
INTRODUCTION. Induction of Monocyte Chemoattractant Protein-1 (MCP-1) Expression by Angiotensin II (AngII) in the Pancreatic Islets and Beta Cells
 Induction of Monocyte Chemoattractant Protein-1 (MCP-1) Expression by Angiotensin II (AngII) in the Pancreatic Islets and Beta Cells Galina Chipitsyna, Qiaoke Gong, Chance F. Gray et al. Endocrinology,
Induction of Monocyte Chemoattractant Protein-1 (MCP-1) Expression by Angiotensin II (AngII) in the Pancreatic Islets and Beta Cells Galina Chipitsyna, Qiaoke Gong, Chance F. Gray et al. Endocrinology,
A ccumulating evidence supports the proposal that
 1080 BASIC RESEARCH Angiotensin converting enzyme inhibitor prevents left ventricular remodelling after myocardial infarction in angiotensin II type 1 receptor knockout mice M Yoshiyama, Y Nakamura, T
1080 BASIC RESEARCH Angiotensin converting enzyme inhibitor prevents left ventricular remodelling after myocardial infarction in angiotensin II type 1 receptor knockout mice M Yoshiyama, Y Nakamura, T
International Graduate Research Programme in Cardiovascular Science
 1 International Graduate Research Programme in Cardiovascular Science This work has been supported by the European Community s Sixth Framework Programme under grant agreement n LSHM-CT-2005-01883 EUGeneHeart.
1 International Graduate Research Programme in Cardiovascular Science This work has been supported by the European Community s Sixth Framework Programme under grant agreement n LSHM-CT-2005-01883 EUGeneHeart.
Supplemental Table 1. Echocardiography Control (n=4)
 Supplemental Table 1. Echocardiography (n=4) Mlc2v cre/+ ; DNMAML (n=4) LVIDd, mm 3.9±0.3 4.3±0.3 LVIDs, mm 2.6±0.4 2.9±0.2 d, mm 0.72±0.06 0.75±0.1 LVPWd, mm 0.72±0.06 0.77±0.11 FS, % 33±6 33±1 EF, %
Supplemental Table 1. Echocardiography (n=4) Mlc2v cre/+ ; DNMAML (n=4) LVIDd, mm 3.9±0.3 4.3±0.3 LVIDs, mm 2.6±0.4 2.9±0.2 d, mm 0.72±0.06 0.75±0.1 LVPWd, mm 0.72±0.06 0.77±0.11 FS, % 33±6 33±1 EF, %
Copyright 2011, 2007 by Mosby, Inc., an affiliate of Elsevier Inc. Normal Cardiac Anatomy
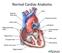 Mosby,, an affiliate of Elsevier Normal Cardiac Anatomy Impaired cardiac pumping Results in vasoconstriction & fluid retention Characterized by ventricular dysfunction, reduced exercise tolerance, diminished
Mosby,, an affiliate of Elsevier Normal Cardiac Anatomy Impaired cardiac pumping Results in vasoconstriction & fluid retention Characterized by ventricular dysfunction, reduced exercise tolerance, diminished
Therapeutic Targets and Interventions
 Therapeutic Targets and Interventions Ali Valika, MD, FACC Advanced Heart Failure and Pulmonary Hypertension Advocate Medical Group Midwest Heart Foundation Disclosures: 1. Novartis: Speaker Honorarium
Therapeutic Targets and Interventions Ali Valika, MD, FACC Advanced Heart Failure and Pulmonary Hypertension Advocate Medical Group Midwest Heart Foundation Disclosures: 1. Novartis: Speaker Honorarium
The toll-like receptor 4 ligands Mrp8 and Mrp14 play a critical role in the development of autoreactive CD8 + T cells
 1 SUPPLEMENTARY INFORMATION The toll-like receptor 4 ligands Mrp8 and Mrp14 play a critical role in the development of autoreactive CD8 + T cells Karin Loser 1,2,6, Thomas Vogl 2,3, Maik Voskort 1, Aloys
1 SUPPLEMENTARY INFORMATION The toll-like receptor 4 ligands Mrp8 and Mrp14 play a critical role in the development of autoreactive CD8 + T cells Karin Loser 1,2,6, Thomas Vogl 2,3, Maik Voskort 1, Aloys
Pathophysiology and Diagnosis of Heart Failure
 Pathophysiology and Diagnosis of Heart Failure Francesco Paneni, MD, PhD, FESC Cardiology Unit Karolinska University Hospital Stockholm, Sweden Cardiology University Hospital Zurich Switzerland francesco.paneni@gmail.com
Pathophysiology and Diagnosis of Heart Failure Francesco Paneni, MD, PhD, FESC Cardiology Unit Karolinska University Hospital Stockholm, Sweden Cardiology University Hospital Zurich Switzerland francesco.paneni@gmail.com
C57BL/6 Mice are More Appropriate. than BALB/C Mice in Inducing Dilated Cardiomyopathy with Short-Term Doxorubicin Treatment
 Original Article C57BL/6 Mice are More Appropriate Acta Cardiol Sin 2012;28:236 240 Heart Failure & Cardiomyopathy C57BL/6 Mice are More Appropriate than BALB/C Mice in Inducing Dilated Cardiomyopathy
Original Article C57BL/6 Mice are More Appropriate Acta Cardiol Sin 2012;28:236 240 Heart Failure & Cardiomyopathy C57BL/6 Mice are More Appropriate than BALB/C Mice in Inducing Dilated Cardiomyopathy
Chemical Chaperones Mitigate Experimental Asthma By Attenuating Endoplasmic
 Chemical Chaperones Mitigate Experimental Asthma By Attenuating Endoplasmic Reticulum Stress Lokesh Makhija, BE, Veda Krishnan, MSc, Rakhshinda Rehman, MTech, Samarpana Chakraborty, MSc, Shuvadeep Maity,
Chemical Chaperones Mitigate Experimental Asthma By Attenuating Endoplasmic Reticulum Stress Lokesh Makhija, BE, Veda Krishnan, MSc, Rakhshinda Rehman, MTech, Samarpana Chakraborty, MSc, Shuvadeep Maity,
HCC1937 is the HCC1937-pcDNA3 cell line, which was derived from a breast cancer with a mutation
 SUPPLEMENTARY INFORMATION Materials and Methods Human cell lines and culture conditions HCC1937 is the HCC1937-pcDNA3 cell line, which was derived from a breast cancer with a mutation in exon 20 of BRCA1
SUPPLEMENTARY INFORMATION Materials and Methods Human cell lines and culture conditions HCC1937 is the HCC1937-pcDNA3 cell line, which was derived from a breast cancer with a mutation in exon 20 of BRCA1
Supporting Information
 Supporting Information Muraski et al. 10.1073/pnas.0709135105 SI Text Generation of Transgenic Animals. Pim-WT and Pim-DN cdnas were subcloned NheI/SmaI from pegfp-c1 Pim-1 and pegfp-c1 Pim-DN plasmids
Supporting Information Muraski et al. 10.1073/pnas.0709135105 SI Text Generation of Transgenic Animals. Pim-WT and Pim-DN cdnas were subcloned NheI/SmaI from pegfp-c1 Pim-1 and pegfp-c1 Pim-DN plasmids
Supplementary Figures and Figure Legends
 1 Supplementary Figures and Figure Legends 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 Supplementary Figure 1: camp levels in myocytes confirm studies in perfused hearts. (a) Time-resolved camp dynamics (presented
1 Supplementary Figures and Figure Legends 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 Supplementary Figure 1: camp levels in myocytes confirm studies in perfused hearts. (a) Time-resolved camp dynamics (presented
MTC-TT and TPC-1 cell lines were cultured in RPMI medium (Gibco, Breda, The Netherlands)
 Supplemental data Materials and Methods Cell culture MTC-TT and TPC-1 cell lines were cultured in RPMI medium (Gibco, Breda, The Netherlands) supplemented with 15% or 10% (for TPC-1) fetal bovine serum
Supplemental data Materials and Methods Cell culture MTC-TT and TPC-1 cell lines were cultured in RPMI medium (Gibco, Breda, The Netherlands) supplemented with 15% or 10% (for TPC-1) fetal bovine serum
Supplementary Figure 1. Spatial distribution of LRP5 and β-catenin in intact cardiomyocytes. (a) and (b) Immunofluorescence staining of endogenous
 Supplementary Figure 1. Spatial distribution of LRP5 and β-catenin in intact cardiomyocytes. (a) and (b) Immunofluorescence staining of endogenous LRP5 in intact adult mouse ventricular myocytes (AMVMs)
Supplementary Figure 1. Spatial distribution of LRP5 and β-catenin in intact cardiomyocytes. (a) and (b) Immunofluorescence staining of endogenous LRP5 in intact adult mouse ventricular myocytes (AMVMs)
Effects of sitagliptin on cardiac metabolism in mice
 Effects of sitagliptin on cardiac metabolism in mice M. Lenski, J.-C. Reil, M. Böhm, U. Laufs Saarland University Hospital Department of Internal Medicine III, Cardiology Homburg - Germany Disclosures
Effects of sitagliptin on cardiac metabolism in mice M. Lenski, J.-C. Reil, M. Böhm, U. Laufs Saarland University Hospital Department of Internal Medicine III, Cardiology Homburg - Germany Disclosures
Disclosure of Relationships
 Disclosure of Relationships Over the past 12 months Dr Ruilope has served as Consultant and Speakers Bureau member of Astra-Zeneca, Bayer, Daiichi-Sankyo, Menarini, Novartis, Otsuka, Pfizer, Relypsa, Servier
Disclosure of Relationships Over the past 12 months Dr Ruilope has served as Consultant and Speakers Bureau member of Astra-Zeneca, Bayer, Daiichi-Sankyo, Menarini, Novartis, Otsuka, Pfizer, Relypsa, Servier
SUPPLEMENTARY INFORMATION
 Figure S1 Treatment with both Sema6D and Plexin-A1 sirnas induces the phenotype essentially identical to that induced by treatment with Sema6D sirna alone or Plexin-A1 sirna alone. (a,b) The cardiac tube
Figure S1 Treatment with both Sema6D and Plexin-A1 sirnas induces the phenotype essentially identical to that induced by treatment with Sema6D sirna alone or Plexin-A1 sirna alone. (a,b) The cardiac tube
Supplementary Figure 1:
 Supplementary Figure 1: (A) Whole aortic cross-sections stained with Hematoxylin and Eosin (H&E), 7 days after porcine-pancreatic-elastase (PPE)-induced AAA compared to untreated, healthy control aortas
Supplementary Figure 1: (A) Whole aortic cross-sections stained with Hematoxylin and Eosin (H&E), 7 days after porcine-pancreatic-elastase (PPE)-induced AAA compared to untreated, healthy control aortas
Heart Failure in Women: Dr Goh Ping Ping Cardiologist Asian Heart & Vascular Centre
 Heart Failure in Women: More than EF? Dr Goh Ping Ping Cardiologist Asian Heart & Vascular Centre Overview Review pathophysiology as it relates to diagnosis and management Rational approach to workup:
Heart Failure in Women: More than EF? Dr Goh Ping Ping Cardiologist Asian Heart & Vascular Centre Overview Review pathophysiology as it relates to diagnosis and management Rational approach to workup:
Imaging Guide Echocardiography
 Imaging Guide Guide to Small Animal Echocardiography using the Vevo Imaging Systems System Compatibility: This guide contains instructions and suggestions for work on the Vevo2100, VevoLAZR, Vevo 3100
Imaging Guide Guide to Small Animal Echocardiography using the Vevo Imaging Systems System Compatibility: This guide contains instructions and suggestions for work on the Vevo2100, VevoLAZR, Vevo 3100
Individual Study Table Referring to Part of Dossier: Volume: Page:
 Synopsis Abbott Laboratories Name of Study Drug: Paricalcitol Capsules (ABT-358) (Zemplar ) Name of Active Ingredient: Paricalcitol Individual Study Table Referring to Part of Dossier: Volume: Page: (For
Synopsis Abbott Laboratories Name of Study Drug: Paricalcitol Capsules (ABT-358) (Zemplar ) Name of Active Ingredient: Paricalcitol Individual Study Table Referring to Part of Dossier: Volume: Page: (For
TFEB-mediated increase in peripheral lysosomes regulates. Store Operated Calcium Entry
 TFEB-mediated increase in peripheral lysosomes regulates Store Operated Calcium Entry Luigi Sbano, Massimo Bonora, Saverio Marchi, Federica Baldassari, Diego L. Medina, Andrea Ballabio, Carlotta Giorgi
TFEB-mediated increase in peripheral lysosomes regulates Store Operated Calcium Entry Luigi Sbano, Massimo Bonora, Saverio Marchi, Federica Baldassari, Diego L. Medina, Andrea Ballabio, Carlotta Giorgi
J Jpn Coll Angiol, 2009, 49:
 Online publication October 6, 2009 48 2 20 J Jpn Coll Angiol, 2009, 49: 293 297 atrial natriuretic peptide, brain natriuretic peptide, guanylyl cyclase-a receptor, cardiac remodeling, cardiac hypertrophy
Online publication October 6, 2009 48 2 20 J Jpn Coll Angiol, 2009, 49: 293 297 atrial natriuretic peptide, brain natriuretic peptide, guanylyl cyclase-a receptor, cardiac remodeling, cardiac hypertrophy
GPR120 *** * * Liver BAT iwat ewat mwat Ileum Colon. UCP1 mrna ***
 a GPR120 GPR120 mrna/ppia mrna Arbitrary Units 150 100 50 Liver BAT iwat ewat mwat Ileum Colon b UCP1 mrna Fold induction 20 15 10 5 - camp camp SB202190 - - - H89 - - - - - GW7647 Supplementary Figure
a GPR120 GPR120 mrna/ppia mrna Arbitrary Units 150 100 50 Liver BAT iwat ewat mwat Ileum Colon b UCP1 mrna Fold induction 20 15 10 5 - camp camp SB202190 - - - H89 - - - - - GW7647 Supplementary Figure
For pair feeding, mice were fed 2.7g of HFD containing tofogliflozin
 Materials and Methods Pair Feeding Experiment For pair feeding, mice were fed 2.7g of HFD containing tofogliflozin (0.005%), which is average daily food intake of mice fed control HFD ad libitum at week
Materials and Methods Pair Feeding Experiment For pair feeding, mice were fed 2.7g of HFD containing tofogliflozin (0.005%), which is average daily food intake of mice fed control HFD ad libitum at week
Supplementary Material
 Supplementary Material Induction of myocardial infarction Mice were anesthetized by intraperitoneal injection of pentobarbital (7 mg/kg). In the supine position, endotracheal intubation was performed.
Supplementary Material Induction of myocardial infarction Mice were anesthetized by intraperitoneal injection of pentobarbital (7 mg/kg). In the supine position, endotracheal intubation was performed.
Index. Note: Page numbers of article titles are in boldface type.
 Heart Failure Clin 2 (2006) 101 105 Index Note: Page numbers of article titles are in boldface type. A ACE inhibitors, in diabetic hypertension, 30 31 Adipokines, cardiovascular events related to, 6 Advanced
Heart Failure Clin 2 (2006) 101 105 Index Note: Page numbers of article titles are in boldface type. A ACE inhibitors, in diabetic hypertension, 30 31 Adipokines, cardiovascular events related to, 6 Advanced
Segmental Tissue Doppler Image-Derived Tei Index in Patients With Regional Wall Motion Abnormalities
 ORIGINAL ARTICLE DOI 10.4070 / kcj.2010.40.3.114 Print ISSN 1738-5520 / On-line ISSN 1738-5555 Copyright c 2010 The Korean Society of Cardiology Open Access Segmental Tissue Doppler Image-Derived Tei Index
ORIGINAL ARTICLE DOI 10.4070 / kcj.2010.40.3.114 Print ISSN 1738-5520 / On-line ISSN 1738-5555 Copyright c 2010 The Korean Society of Cardiology Open Access Segmental Tissue Doppler Image-Derived Tei Index
Pretargeting and Bioorthogonal Click Chemistry-Mediated Endogenous Stem Cell Homing for Heart Repair
 Pretargeting and Bioorthogonal Click Chemistry-Mediated Endogenous Stem Cell Homing for Heart Repair Mouse Model of Myocardial Infarction (MI) All animal work was compliant with the Institutional Animal
Pretargeting and Bioorthogonal Click Chemistry-Mediated Endogenous Stem Cell Homing for Heart Repair Mouse Model of Myocardial Infarction (MI) All animal work was compliant with the Institutional Animal
HFPEF Echo with Strain vs. MRI T1 Mapping
 HFPEF Echo with Strain vs. MRI T1 Mapping Erik Schelbert, MD MS Director, Cardiovascular Magnetic Resonance Assistant Professor of Medicine Heart & Vascular Institute University of Pittsburgh Disclosures
HFPEF Echo with Strain vs. MRI T1 Mapping Erik Schelbert, MD MS Director, Cardiovascular Magnetic Resonance Assistant Professor of Medicine Heart & Vascular Institute University of Pittsburgh Disclosures
Supporting Information
 Supporting Information Kuroda et al. 10.1073/pnas.1002178107 SI Methods Monoclonal Antibodies Against Nox4. Generation of the anti-nox4 mouse monoclonal antibody (3D2), which detects Nox4 and does not
Supporting Information Kuroda et al. 10.1073/pnas.1002178107 SI Methods Monoclonal Antibodies Against Nox4. Generation of the anti-nox4 mouse monoclonal antibody (3D2), which detects Nox4 and does not
Angiotensin-Converting Enzyme-2 Overexpression Improves Left Ventricular Remodeling and Function in a Rat Model of Diabetic Cardiomyopathy
 Journal of the American College of Cardiology Vol. 59, No. 8, 2012 2012 by the American College of Cardiology Foundation ISSN 0735-1097/$36.00 Published by Elsevier Inc. doi:10.1016/j.jacc.2011.09.071
Journal of the American College of Cardiology Vol. 59, No. 8, 2012 2012 by the American College of Cardiology Foundation ISSN 0735-1097/$36.00 Published by Elsevier Inc. doi:10.1016/j.jacc.2011.09.071
Supplementary Information. Glycogen shortage during fasting triggers liver-brain-adipose. neurocircuitry to facilitate fat utilization
 Supplementary Information Glycogen shortage during fasting triggers liver-brain-adipose neurocircuitry to facilitate fat utilization Supplementary Figure S1. Liver-Brain-Adipose neurocircuitry Starvation
Supplementary Information Glycogen shortage during fasting triggers liver-brain-adipose neurocircuitry to facilitate fat utilization Supplementary Figure S1. Liver-Brain-Adipose neurocircuitry Starvation
Definition of Congestive Heart Failure
 Heart Failure Definition of Congestive Heart Failure A clinical syndrome of signs & symptoms resulting from the heart s inability to supply adequate tissue perfusion. CHF Epidemiology Affects 4.7 million
Heart Failure Definition of Congestive Heart Failure A clinical syndrome of signs & symptoms resulting from the heart s inability to supply adequate tissue perfusion. CHF Epidemiology Affects 4.7 million
Circulation. Blood Pressure and Antihypertensive Medications. Venous Return. Arterial flow. Regulation of Cardiac Output.
 Circulation Blood Pressure and Antihypertensive Medications Two systems Pulmonary (low pressure) Systemic (high pressure) Aorta 120 mmhg Large arteries 110 mmhg Arterioles 40 mmhg Arteriolar capillaries
Circulation Blood Pressure and Antihypertensive Medications Two systems Pulmonary (low pressure) Systemic (high pressure) Aorta 120 mmhg Large arteries 110 mmhg Arterioles 40 mmhg Arteriolar capillaries
Bone marrow-derived mesenchymal stem cells improve diabetes-induced cognitive impairment by
 Nakano et al. Supplementary information 1. Supplementary Figure 2. Methods 3. References Bone marrow-derived mesenchymal stem cells improve diabetes-induced cognitive impairment by exosome transfer into
Nakano et al. Supplementary information 1. Supplementary Figure 2. Methods 3. References Bone marrow-derived mesenchymal stem cells improve diabetes-induced cognitive impairment by exosome transfer into
Cells and reagents. Synaptopodin knockdown (1) and dynamin knockdown (2)
 Supplemental Methods Cells and reagents. Synaptopodin knockdown (1) and dynamin knockdown (2) podocytes were cultured as described previously. Staurosporine, angiotensin II and actinomycin D were all obtained
Supplemental Methods Cells and reagents. Synaptopodin knockdown (1) and dynamin knockdown (2) podocytes were cultured as described previously. Staurosporine, angiotensin II and actinomycin D were all obtained
ONLINE DATA SUPPLEMENT. Impact of Obstructive Sleep Apnea on Left Ventricular Mass and. Diastolic Function
 ONLINE DATA SUPPLEMENT Impact of Obstructive Sleep Apnea on Left Ventricular Mass and Diastolic Function Mitra Niroumand Raffael Kuperstein Zion Sasson Patrick J. Hanly St. Michael s Hospital University
ONLINE DATA SUPPLEMENT Impact of Obstructive Sleep Apnea on Left Ventricular Mass and Diastolic Function Mitra Niroumand Raffael Kuperstein Zion Sasson Patrick J. Hanly St. Michael s Hospital University
Islet viability assay and Glucose Stimulated Insulin Secretion assay RT-PCR and Western Blot
 Islet viability assay and Glucose Stimulated Insulin Secretion assay Islet cell viability was determined by colorimetric (3-(4,5-dimethylthiazol-2-yl)-2,5- diphenyltetrazolium bromide assay using CellTiter
Islet viability assay and Glucose Stimulated Insulin Secretion assay Islet cell viability was determined by colorimetric (3-(4,5-dimethylthiazol-2-yl)-2,5- diphenyltetrazolium bromide assay using CellTiter
Combination of renin-angiotensinaldosterone. how to choose?
 Combination of renin-angiotensinaldosterone system inhibitors how to choose? Karl Swedberg Professor of Medicine Sahlgrenska Academy University of Gothenburg karl.swedberg@gu.se Disclosures Research grants
Combination of renin-angiotensinaldosterone system inhibitors how to choose? Karl Swedberg Professor of Medicine Sahlgrenska Academy University of Gothenburg karl.swedberg@gu.se Disclosures Research grants
Direct blood pressure monitoring was done using radiotelemetry (DataSciences
 Supplemental Methods: Blood Pressure Monitoring Direct blood pressure monitoring was done using radiotelemetry (DataSciences International; DSI). Surgical implantation of TA11PA-C1 transmitters was performed
Supplemental Methods: Blood Pressure Monitoring Direct blood pressure monitoring was done using radiotelemetry (DataSciences International; DSI). Surgical implantation of TA11PA-C1 transmitters was performed
Genetic ablation of Acp1 (Lmptp) in mice prevents heart failure
 Genetic ablation of Acp1 (Lmptp) in mice prevents heart failure Coralie Poizat, Ph.D. Director, Cardiovascular Research Program KFSHRC-Riyadh Saudi Heart Failure Working Group Jeddah, 5 December 2015 Cardiovascular
Genetic ablation of Acp1 (Lmptp) in mice prevents heart failure Coralie Poizat, Ph.D. Director, Cardiovascular Research Program KFSHRC-Riyadh Saudi Heart Failure Working Group Jeddah, 5 December 2015 Cardiovascular
Supplementary Figure 1. Characterization of human carotid plaques. (a) Flash-frozen human plaques were separated into vulnerable (V) and stable (S),
 Supplementary Figure 1. Characterization of human carotid plaques. (a) Flash-frozen human plaques were separated into vulnerable (V) and stable (S), regions which were then quantified for mean fluorescence
Supplementary Figure 1. Characterization of human carotid plaques. (a) Flash-frozen human plaques were separated into vulnerable (V) and stable (S), regions which were then quantified for mean fluorescence
LV geometric and functional changes in VHD: How to assess? Mi-Seung Shin M.D., Ph.D. Gachon University Gil Hospital
 LV geometric and functional changes in VHD: How to assess? Mi-Seung Shin M.D., Ph.D. Gachon University Gil Hospital LV inflow across MV LV LV outflow across AV LV LV geometric changes Pressure overload
LV geometric and functional changes in VHD: How to assess? Mi-Seung Shin M.D., Ph.D. Gachon University Gil Hospital LV inflow across MV LV LV outflow across AV LV LV geometric changes Pressure overload
ANGIOTENSIN II RECEPTOR BLOCKERS: MORE THAN THE ALTERNATIVE PRESENTATION BY: PATRICK HO, USC PHARM D. CANDIDATE OF 2017 MENTOR: DR.
 ANGIOTENSIN II RECEPTOR BLOCKERS: MORE THAN THE ALTERNATIVE PRESENTATION BY: PATRICK HO, USC PHARM D. CANDIDATE OF 2017 MENTOR: DR. CRAIG STERN, PHARMD, MBA, RPH, FASCP, FASHP, FICA, FLMI, FAMCP RENIN-ANGIOTENSIN
ANGIOTENSIN II RECEPTOR BLOCKERS: MORE THAN THE ALTERNATIVE PRESENTATION BY: PATRICK HO, USC PHARM D. CANDIDATE OF 2017 MENTOR: DR. CRAIG STERN, PHARMD, MBA, RPH, FASCP, FASHP, FICA, FLMI, FAMCP RENIN-ANGIOTENSIN
Minute TM Plasma Membrane Protein Isolation and Cell Fractionation Kit User Manual (v5)
 Minute TM Plasma Membrane Protein Isolation and Cell Fractionation Kit Catalog number: SM-005 Description Minute TM plasma membrane (PM) protein isolation kit is a novel and patented native PM protein
Minute TM Plasma Membrane Protein Isolation and Cell Fractionation Kit Catalog number: SM-005 Description Minute TM plasma membrane (PM) protein isolation kit is a novel and patented native PM protein
There is no conflict of interests for the following presentation
 There is no conflict of interests for the following presentation Inflammation and Left Ventricular Diastolic Dysfunction Cho-Kai Wu MD National Taiwan University Hospital, Taipei, Taiwan Diastolic heart
There is no conflict of interests for the following presentation Inflammation and Left Ventricular Diastolic Dysfunction Cho-Kai Wu MD National Taiwan University Hospital, Taipei, Taiwan Diastolic heart
Blood Pressure Fox Chapter 14 part 2
 Vert Phys PCB3743 Blood Pressure Fox Chapter 14 part 2 T. Houpt, Ph.D. 1 Cardiac Output and Blood Pressure How to Measure Blood Pressure Contribution of vascular resistance to blood pressure Cardiovascular
Vert Phys PCB3743 Blood Pressure Fox Chapter 14 part 2 T. Houpt, Ph.D. 1 Cardiac Output and Blood Pressure How to Measure Blood Pressure Contribution of vascular resistance to blood pressure Cardiovascular
CNS Effects of Aldosterone :
 CNS Effects of Aldosterone : Critical Roles in salt-sensitive sensitive hypertension and CHF. Frans HH Leenen MD, PhD, FRCPC, FAHA 2 Renin Angiotensin Aldosterone System Circulatory RAAS Tissue RAAS: -
CNS Effects of Aldosterone : Critical Roles in salt-sensitive sensitive hypertension and CHF. Frans HH Leenen MD, PhD, FRCPC, FAHA 2 Renin Angiotensin Aldosterone System Circulatory RAAS Tissue RAAS: -
A novel role for vitamin D: modulation of expression and function of the local renin angiotensin system in mouse pancreatic islets
 Diabetologia () 5:77 DOI.7/s5--- SHORT COMMUNICATION A novel role for vitamin D: modulation of expression and function of the local renin angiotensin system in mouse pancreatic islets Q. Cheng & Y. C.
Diabetologia () 5:77 DOI.7/s5--- SHORT COMMUNICATION A novel role for vitamin D: modulation of expression and function of the local renin angiotensin system in mouse pancreatic islets Q. Cheng & Y. C.
Supplementary Online Content
 Supplementary Online Content Inohara T, Manandhar P, Kosinski A, et al. Association of renin-angiotensin inhibitor treatment with mortality and heart failure readmission in patients with transcatheter
Supplementary Online Content Inohara T, Manandhar P, Kosinski A, et al. Association of renin-angiotensin inhibitor treatment with mortality and heart failure readmission in patients with transcatheter
Review of Cardiac Imaging Modalities in the Renal Patient. George Youssef
 Review of Cardiac Imaging Modalities in the Renal Patient George Youssef ECHO Left ventricular hypertrophy (LVH) assessment Diastolic dysfunction Stress ECHO Cardiac CT angiography Echocardiography - positives
Review of Cardiac Imaging Modalities in the Renal Patient George Youssef ECHO Left ventricular hypertrophy (LVH) assessment Diastolic dysfunction Stress ECHO Cardiac CT angiography Echocardiography - positives
Mechanisms of heart failure with normal EF Arterial stiffness and ventricular-arterial coupling. What is the pathophysiology at presentation?
 Mechanisms of heart failure with normal EF Arterial stiffness and ventricular-arterial coupling What is the pathophysiology at presentation? Ventricular-arterial coupling elastance Central arterial pressure
Mechanisms of heart failure with normal EF Arterial stiffness and ventricular-arterial coupling What is the pathophysiology at presentation? Ventricular-arterial coupling elastance Central arterial pressure
Supplementary Materials and Methods
 Supplementary Materials and Methods Immunoblotting Immunoblot analysis was performed as described previously (1). Due to high-molecular weight of MUC4 (~ 950 kda) and MUC1 (~ 250 kda) proteins, electrophoresis
Supplementary Materials and Methods Immunoblotting Immunoblot analysis was performed as described previously (1). Due to high-molecular weight of MUC4 (~ 950 kda) and MUC1 (~ 250 kda) proteins, electrophoresis
Influence of RAAS inhibition on outflow tract obstruction in hypertrophic cardiomyopathy
 ORIGINAL ARTICLE 5 RAAS inhibitors should be avoided if possible in patients with obstructive HCM Influence of RAAS inhibition on outflow tract obstruction in hypertrophic cardiomyopathy Katrin Witzel,
ORIGINAL ARTICLE 5 RAAS inhibitors should be avoided if possible in patients with obstructive HCM Influence of RAAS inhibition on outflow tract obstruction in hypertrophic cardiomyopathy Katrin Witzel,
ACE inhibitors: still the gold standard?
 ACE inhibitors: still the gold standard? Session: Twenty-five years after CONSENSUS What have we learnt about the RAAS in heart failure? Lars Køber, MD, D.Sci Department of Cardiology Rigshospitalet University
ACE inhibitors: still the gold standard? Session: Twenty-five years after CONSENSUS What have we learnt about the RAAS in heart failure? Lars Køber, MD, D.Sci Department of Cardiology Rigshospitalet University
Left ventricular hypertrophy: why does it happen?
 Nephrol Dial Transplant (2003) 18 [Suppl 8]: viii2 viii6 DOI: 10.1093/ndt/gfg1083 Left ventricular hypertrophy: why does it happen? Gerard M. London Department of Nephrology and Dialysis, Manhes Hospital,
Nephrol Dial Transplant (2003) 18 [Suppl 8]: viii2 viii6 DOI: 10.1093/ndt/gfg1083 Left ventricular hypertrophy: why does it happen? Gerard M. London Department of Nephrology and Dialysis, Manhes Hospital,
MCD-microRNA. WU Hong-quan, LIU Di-chuan *, TONG Xin, CAI Min, HUANG Jing. (LVIDd) A [ ] (2012)
![MCD-microRNA. WU Hong-quan, LIU Di-chuan *, TONG Xin, CAI Min, HUANG Jing. (LVIDd) A [ ] (2012) MCD-microRNA. WU Hong-quan, LIU Di-chuan *, TONG Xin, CAI Min, HUANG Jing. (LVIDd) A [ ] (2012)](/thumbs/81/83672149.jpg) MCD-microRNA [ ] A (MCD)-microRNA MCD MCD 4 MCD-microRNA HEK293 PCR MCD mrna 28 + 3 ( + + + + + + ) 7 7 ( + ) MCD-microRNA 4 4d 1 28d (LVEF) (FS) (LVIDd)A PCR 1 MCD mrna82% + + + LVEF FS (P 0.05) (P 0.05)
MCD-microRNA [ ] A (MCD)-microRNA MCD MCD 4 MCD-microRNA HEK293 PCR MCD mrna 28 + 3 ( + + + + + + ) 7 7 ( + ) MCD-microRNA 4 4d 1 28d (LVEF) (FS) (LVIDd)A PCR 1 MCD mrna82% + + + LVEF FS (P 0.05) (P 0.05)
Supplementary Figure 1: Hsp60 / IEC mice are embryonically lethal (A) Light microscopic pictures show mouse embryos at developmental stage E12.
 Supplementary Figure 1: Hsp60 / IEC mice are embryonically lethal (A) Light microscopic pictures show mouse embryos at developmental stage E12.5 and E13.5 prepared from uteri of dams and subsequently genotyped.
Supplementary Figure 1: Hsp60 / IEC mice are embryonically lethal (A) Light microscopic pictures show mouse embryos at developmental stage E12.5 and E13.5 prepared from uteri of dams and subsequently genotyped.
SUPPLEMENTAL DATA. Lumen area ( m 2 )
 Elastin Lumen area ( m 2 ) Media to lumen ratio (x1) H.E. Medium thickness ( m) Medium area ( m 2 ) SUPPLEMENTAL DATA A (Bmal1 flox/flox ) (SM-Bmal1 -/- ) B 1 8 8 6 6 4 4 2 2 1µm 5 8 4 6 3 2 4 1 2 Supplemental
Elastin Lumen area ( m 2 ) Media to lumen ratio (x1) H.E. Medium thickness ( m) Medium area ( m 2 ) SUPPLEMENTAL DATA A (Bmal1 flox/flox ) (SM-Bmal1 -/- ) B 1 8 8 6 6 4 4 2 2 1µm 5 8 4 6 3 2 4 1 2 Supplemental
(Stratagene, La Jolla, CA) (Supplemental Fig. 1A). A 5.4-kb EcoRI fragment
 SUPPLEMENTAL INFORMATION Supplemental Methods Generation of RyR2-S2808D Mice Murine genomic RyR2 clones were isolated from a 129/SvEvTacfBR λ-phage library (Stratagene, La Jolla, CA) (Supplemental Fig.
SUPPLEMENTAL INFORMATION Supplemental Methods Generation of RyR2-S2808D Mice Murine genomic RyR2 clones were isolated from a 129/SvEvTacfBR λ-phage library (Stratagene, La Jolla, CA) (Supplemental Fig.
The Patient with Atrial Fibrilation
 Assessment of Diastolic Function The Patient with Atrial Fibrilation Assoc. Prof. Adriana Ilieşiu, FESC University of Medicine Carol Davila Bucharest, Romania Associated Conditions with Atrial Fibrillation
Assessment of Diastolic Function The Patient with Atrial Fibrilation Assoc. Prof. Adriana Ilieşiu, FESC University of Medicine Carol Davila Bucharest, Romania Associated Conditions with Atrial Fibrillation
SUPPLEMENTARY INFORMATION
 doi:10.1038/nature12652 Supplementary Figure 1. PRDM16 interacts with endogenous EHMT1 in brown adipocytes. Immunoprecipitation of PRDM16 complex by flag antibody (M2) followed by Western blot analysis
doi:10.1038/nature12652 Supplementary Figure 1. PRDM16 interacts with endogenous EHMT1 in brown adipocytes. Immunoprecipitation of PRDM16 complex by flag antibody (M2) followed by Western blot analysis
PHARMACEUTICAL INFORMATION AZILSARTAN
 AZEARLY Tablets Each Tablet Contains Azilsartan 20/40/80 mg PHARMACEUTICAL INFORMATION AZILSARTAN Generic name: Azilsartan Chemical name: 2-Ethoxy-1-{[2'-(5-oxo-2,5-dihydro-1,2,4-oxadiazol-3-yl)-4-biphenylyl]methyl}-
AZEARLY Tablets Each Tablet Contains Azilsartan 20/40/80 mg PHARMACEUTICAL INFORMATION AZILSARTAN Generic name: Azilsartan Chemical name: 2-Ethoxy-1-{[2'-(5-oxo-2,5-dihydro-1,2,4-oxadiazol-3-yl)-4-biphenylyl]methyl}-
FOCUS ON CARDIOVASCULAR DISEASE
 The Consequences of Vitamin D Deficiency: FOCUS ON CARDIOVASCULAR DISEASE Vitamin D deficiency is a global health problem. With all the medical advances of the century, vitamin D deficiency is still epidemic.
The Consequences of Vitamin D Deficiency: FOCUS ON CARDIOVASCULAR DISEASE Vitamin D deficiency is a global health problem. With all the medical advances of the century, vitamin D deficiency is still epidemic.
PUBLIC SUMMARY OF RISK MANAGEMENT PLAN (RMP) CANDESARTAN/HYDROCHLOROTHIAZIDE ORION 8 MG/12.5 MG, 16 MG/12.5 MG, 32 MG/12.5 MG, 32 MG/25 MG ORION
 PUBLIC SUMMARY OF RISK MANAGEMENT PLAN (RMP) CANDESARTAN/HYDROCHLOROTHIAZIDE ORION 8 MG/12.5 MG, 16 MG/12.5 MG, 32 MG/12.5 MG, 32 MG/25 MG ORION CORPORATION DATE: 17-04-2015, VERSION 2 VI.2 Elements for
PUBLIC SUMMARY OF RISK MANAGEMENT PLAN (RMP) CANDESARTAN/HYDROCHLOROTHIAZIDE ORION 8 MG/12.5 MG, 16 MG/12.5 MG, 32 MG/12.5 MG, 32 MG/25 MG ORION CORPORATION DATE: 17-04-2015, VERSION 2 VI.2 Elements for
