Cilia-driven fluid flow in the zebrafish pronephros, brain and Kupffer s vesicle is required for normal organogenesis
|
|
|
- Kerrie Hunter
- 6 years ago
- Views:
Transcription
1 Research article 1907 Cilia-driven fluid flow in the zebrafish pronephros, brain and Kupffer s vesicle is required for normal organogenesis Albrecht G. Kramer-Zucker 1, Felix Olale 2, Courtney J. Haycraft 3, Bradley K. Yoder 3, Alexander F. Schier 2 and Iain A. Drummond 1, * 1 Renal Unit, Massachusetts General Hospital, th Street, Charlestown, MA 02129, USA 2 al Genetics Program, Skirball Institute of Biomolecular Medicine, New York University School of Medicine, New York, NY 10016, USA 3 Department of Cell Biology, University of Alabama at Birmingham Medical Center, Birmingham, AL 35294, USA *Author for correspondence ( idrummon@receptor.mgh.harvard.edu) Accepted 4 February , Published by The Company of Biologists 2005 doi: /dev Summary Cilia, as motile and sensory organelles, have been implicated in normal development, as well as diseases including cystic kidney disease, hydrocephalus and situs inversus. In kidney epithelia, cilia are proposed to be nonmotile sensory organelles, while in the mouse node, two cilia populations, motile and non-motile have been proposed to regulate situs. We show that cilia in the zebrafish larval kidney, the spinal cord and Kupffer s vesicle are motile, suggesting that fluid flow is a common feature of each of these organs. Disruption of cilia structure or motility resulted in pronephric cyst formation, Introduction Cilia and flagella have well-established roles as motile and sensory organelles in various species; however, their function in vertebrate organogenesis is only now coming to light. The identification of mutations in ciliogenic genes as the underlying cause of several mutant phenotypes and human pathologies, including polycystic kidney disease, left-right asymmetry defects and retinal degeneration, has implicated cilia function in the normal development of several organs (Ibanez-Tallon et al., 2003). The establishment of left-right asymmetry is the earliest embryonic process associated with cilia function. To generate proper organ laterality, mechanisms must exist that translate existing anterior-posterior polarity into signals that break the bilateral symmetry of the gastrulating embryo. The asymmetric expression of genes such as nodal and southpaw (Bisgrove et al., 2003; Hamada et al., 2002) and the position of organs such as the heart on the left side of the trunk reflect the outcome of early left-right signaling. In humans, abnormalities in organ situs in the primary ciliary dyskinesia (PCD) syndrome (Afzelius, 1985) are due to mutations in several different axonemal dyneins affecting ciliary motility (Ibanez-Tallon et al., 2003). The inversus viscerum (iv) mouse, which is mutant in the left-right dynein gene (lrd; Dnahc11 Mouse Genome Informatics) encoding an axonemal dynein heavy chain present in node cilia (Supp et al., 1997), displays randomization of early gene expression and later organ laterality (Layton, 1976; hydrocephalus and left-right asymmetry defects. The data show that loss of fluid flow leads to fluid accumulation, which can account for organ distension pathologies in the kidney and brain. In Kupffer s vesicle, loss of flow is associated with loss of left-right patterning, indicating that the nodal flow mechanism of generating situs is conserved in non-mammalian vertebrates. Key words: Cilia, Pronephros, Kupffer s vesicle, Ependymal cell, Spinal canal, Kidney cyst, Hydrocephalus, Left-right asymmetry Lowe et al., 1996). Cilia paralysis and loss of fluid flow in this and other mutants (Brody et al., 2000; Chen et al., 1998; Marszalek et al., 1999; Nonaka et al., 1998) suggested that nodal fluid flow was the key factor in establishing organ situs. Artificial reversal of nodal flow has been shown to randomize the left-right axis, providing further support for the nodal flow hypothesis (Nonaka et al., 2002). McGrath and coworkers have proposed that two types of cilia exist in the node: motile lrd-expressing cilia and non-motile polycystin2- expressing sensory cilia (McGrath et al., 2003). Nodal flow could generate a morphogen gradient regulating situs, or, alternatively, mechanosensory ion fluxes mediated by polycystin2 may be the signal that initiates left-sided gene expression. Whatever the final signal may be, the demonstration that dynein-expressing node monocilia exist in a range of vertebrate embryos (Brummett and Dumont, 1978; Essner et al., 2002) indicates that cilia-driven fluid flow may be part of a general mechanism for establishing left-right asymmetry. Currently, however, nodal flow has been directly demonstrated only in mouse embryos, and it is unclear whether or not ciliary motion and fluid flow is relevant to other vertebrates. Cilia dysfunction has been implicated in polycystic kidney disease, based on the findings that disruption of ciliogenic and cilia-associated genes leads to cyst formation in the kidney (Igarashi and Somlo, 2002; Nauli and Zhou, 2004). The two proteins associated with human autosomal dominant PKD, Polycystin-1 and Polycystin-2, have been detected in the renal
2 (8) cilium (Pazour et al., 2002; Yoder et al., 2002) as has the product of the inversin gene, which is mutant in the human kidney cystic condition nephronophthisis type 2 (Otto et al., 2003). Cystin, the protein encoded by the mutant gene in the cpk cystic mouse, is also localized to apical cilia (Hou et al., 2002; Yoder et al., 2002). In zebrafish, the results of a largescale retroviral insertional mutagenesis screen revealed that several genes associated with cilia assembly were mutated in fish that developed pronephric cysts (Sun et al., 2004). In mammalian epithelial cultured cells, cilia are proposed to be non-motile mechanosensors that initiate signals controlling tubular epithelial cell proliferation or homeostasis (Nauli et al., 2003; Pazour and Witman, 2003). Non-motile cilia with sensory functions have been described in Caenorhabditis elegans neurons and several mutant strains with altered sensory behavior have been identified (Perkins et al., 1986). However, whether kidney cilia are always immotile, or whether they might play an additional role in kidney tubule fluid movement, remains an unresolved question. Our insight into cilia assembly has been significantly advanced by the discovery of the cellular machinery responsible for moving particles along the microtubule scaffold of cilia or flagella, called intraflagellar transport (IFT) (Rosenbaum and Witman, 2002). The pleiotropic phenotype observed in animals carrying mutations in IFT genes has confirmed the importance of cilia in organogenesis and tissue physiology. A hypomorphic mutation in the mouse polaris gene (Tg737/IFT88) results in cystic kidney disease, pancreatic and bile duct hyperplasia, hydrocephalus, and skeletal patterning defects (Cano et al., 2004; Moyer et al., 1994; Richards et al., 1996; Yoder et al., 1995). In zebrafish, the oval mutant is a stop codon in the polaris/ift88 homolog; these fish show widespread neurosensory cell death (Tsujikawa and Malicki, 2004). Three different zebrafish IFT proteins associated with cystic kidneys were also identified in a large-scale insertional mutagenesis screen (Sun et al., 2004). Despite the implication that cilia defects are associated with mutant phenotypes, the mechanism by which ciliary malfunction may lead to the various organ pathologies remains unclear. To better understand the developmental roles of cilia in organogenesis, we examined cilia in the pronephric kidney, the spinal cord and Kupffer s vesicle (KV, the equivalent of the mouse embryonic node) of zebrafish larvae and assessed the consequence that loss of cilia has on the formation and function of these organs. We found that cilia in all three of these structures are motile, suggesting that cilia function to drive fluid flow. Indeed, we show by using injected fluorescent tracers that this is the case. Disruption of cilia function in IFT morphant embryos resulted in loss of fluid flow and subsequent development of kidney cysts, hydrocephalus and laterality defects. The association between defects in fluid flow and organ pathology when cilia biogenesis was perturbed suggests that a common mechanism, namely loss of fluid flow, leads to fluid backup and subsequent organ distension, with formation of cysts in the kidney and hydrocephalus in the brain. Our data also demonstrate that fluid flow is a conserved feature of gastrulation-stage midline structures that regulate left-right asymmetry and, further, that disruption of this flow in zebrafish also causes abnormalities in situs. Materials and methods Research article Zebrafish lines Wild-type TL or TÜAB zebrafish were maintained and raised as described (Westerfield, 1995). Dechorionated embryos were kept at 28.5 C in E3 solution with or without 0.003% 1-Phenyl-2-thiourea (PTU, Sigma) to suppress pigmentation and staged according to somite number (som) or hours post-fertilization (hpf) (Westerfield, 1995). Cloning of polaris, hippi and a fragment of pronephric axonemal dynein heavy chain 9 Zebrafish polaris was cloned by RT-PCR based on sequence predicted from Sanger Center zebrafish genomic DNA sequence (Sanger Institute). RT-PCR products were subcloned in pcrii TOPO (Invitrogen) and sequenced. Zebrafish hippi sequence was derived partially by tblastn searches (Sanger Institute) and used for 5 and 3 RACE reactions (Invitrogen). Finally, the coding sequence was obtained by reverse transcription and nested PCR of wild-type total RNA (outer primer-set: forward CCCTTTGCGAGTAAAGAGTGT- TAAATGTGA, reverse CATCATCTGCTGCAAACTAGCCCTCT, nested primer-set forward CGGGATCCGCCACCATGGCGGAG- GAGGAAGAG reverse CGGAATTCCGGCGGTGAGTGTGT- GTTTCAATA) and subcloned into the expression vector pcs2+. The hippi gene maps to linkage group 2. The nested PCR for murine hippi was performed based on known sequence in GenBank (NM_028680) using total RNA from mouse brain (kind gift from Dr Ruth Luthi- Carter, Neurology, MGH) and the following primer (outer primer-set: forward GGCGCTGGGGGTCTGAGCA, reverse AAATTGT- GTTTGGAAATCAATGCAACA, nested primer-set forward CG- GAATTCGCCACCATGGCCGCCGCGGCCGCG, reverse GCTC- TAGAGAAGCATGGAAGCCCACGTGTTTA). The amplicon was subcloned into the expression vector pcs2+. For isolation of pronephros specific axonemal dynein heavy chains, 72 hpf. zebrafish embryos were incubated in 10 mmol/l DTT in egg water for 1 hour at room temperature and then washed three times with egg water. They were then incubated at 28.5 C in 5 mg/ml collagenase II in Hank s saline with calcium (Worthington) for 4 hours. The larvae were then put in Hank s saline and triturated gently five times with a 1000 µl pipette tip, so that the disintegrated, approximately 20 pronephric duct fragments were collected by visual identification. Total RNA from the collected tissue was used for reverse transcription and nested PCR. The following degenerate primers were used (I=inosine): GTIAT(AC)A- CICCICTIACIGA (forward primer), GCIGGIACIGGIAA(AG)A- CIGA (nested forward primer), C(GT)ICCIGC(AG)TAICCIG- G(AG)TT (reverse primer for reverse transcription and first and second PCR). A 323 bp fragment was subcloned and 15 clones were sequenced. Accession numbers ift57/hppi, AY956331; dynein heavy chain 9, AY956332; ift88/polaris, AY In situ hybridization Whole-mount in situ hybridization was performed as previously described (Thisse and Thisse, 1998) with some minor modifications. For the polaris antisense probe the template (pcrii-topo-polaris 1200 bp, flanking primers forward AGCAGGCTGTCAGGA- CAAGTC and reverse GTTTGAAGTCTCTCTGTCTTAGGT was linearized with Not1 and the antisense riboprobes were transcribed using SP6 RNA polymerase. The hippi antisense riboprobes were generated using a BamHI linearized template and T7 RNA polymerase. In situ hybridization experiments with southpaw (spaw) (Long et al., 2003) and pitx2 (Yan et al., 1999) were performed using standard techniques. Embryos were then mounted in Permount (Fisher Scientific) and photographed on a Zeiss Axioplan microscope equipped with a Zeiss AxioCam digital camera.
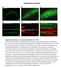
3 Cilia and zebrafish organogenesis 1909 Morpholino antisense oligonucleotides Morpholino antisense oligonucleotides were designed either to target the translation of the mrna (abbreviation AUG) leading to a protein knockdown phenotype or to target an exon splice donor site causing splicing defects of the mrna (abbreviation SP). For the design of the antisense oligonucleotides the translation start site and the splice donor site of the second coding exon were chosen and the morpholino oligonucleotides obtained from GENE TOOLS, LLC, Philomath, OR. The following morpholinos were used: polarisaug CTGGGACAAGATGCACATTCTCCAT, polarissp AGCAGATG- CAAAATGACTCACTGGG, hippiaug CCTCCGCCATCCCT- CTCTCTTTCT, hippiaugmis CCTgCGgCATCCgTCTCTgTTaCT (5 mismatches in lower case), hippisp AGTGTTATCGCCT- CACCAGGGTTCG, dhc9p1sp GATTTACACACCTTGTAGTC- CATTT. The morpholinos were diluted in 100 mmol/l KCl, 10 mmol/l HEPES, 0.1% Phenol Red (Sigma). The injections were done using a microinjector PLI-90 (Harvard Apparatus, Cambridge, MA). The effect of the splice-morpholinos was verified by RT-PCR from single embryo total RNA with nested primers in flanking exons yielding a bp amplicon. Rescue experiments were done by co-injection of capped mrna together with a morpholino. Capped mrna was made using mmessage mmachine (Ambion). For the injection of the capped mrna or capped mrna together with morpholino, a microprocessor controlled nanoliter injector Nanoliter 2000 (World Precision Instruments, Inc.) was used. Histochemistry and immunohistochemistry Embryos were fixed in 2% glutaraldehyde/1.5% paraformaldehyde/70 mmol/l Na 2 HPO 4 ph 7.2/3% sucrose at 4 C overnight. After being washed in PBS and taken through an ethanol dehydration series they were embedded in JB-4 resin (Polysciences Inc.) and sectioned at 3-5 µm. Slides were stained in Methylene Blue/Azure II (Humphrey and Pittman, 1974), mounted and examined using a Nikon immunofluorescence microscope. For acetylated tubulin staining, the embryos were fixed in Dent s Fix (80% methanol/20% DMSO) at 4 C overnight. After gradual rehydration they were washed several times in 1 PBS with 0.5% Tween20 and blocked in 1 PBS-DBT (1% DMSO/1% BSA/0.5% Tween20) with 10% normal goat serum (NGS) (Sigma) at room temperature for 2 hours. Primary antibody incubation in 1 PBS- DBT 10% NGS [1:500 monoclonal anti-acetylated tubulin 6-11B-1 (Piperno and Fuller, 1985) (Sigma)] was at 4 C overnight. The embryos were washed in 1 PBS with 0.5% Tween20 and blocked in 1 PBS-DBT 10% NGS at RT for 1 hour and then incubated in 1:1000 goat anti-mouse Alexa 546 (Molecular Probes) in 1 PBS- DBT 10% NGS at 4 C overnight. After rinsing in 1 PBS, the embryos were washed with methanol and equilibrated in clearing solution (1/3 benzoyl-alcohol and 2/3 benzoyl-benzoate) and examined using a Bio-Rad Radiance 2000 confocal microscope. Z- stacks were acquired and used for creation of projections with extended focus. Cilia length measurements were performed using Image J 1.32j (National Institute of Health) in two to four different embryos per group. Confocal images where individual cilia base and ends could be discerned (>60 individual measurements) were outlined and the calculated length recorded. Our measurements may underestimate cilia length owing to a foreshortening effect caused by viewing some cilia at an angle. For double labeling with two monoclonal antibodies, the embryos were stained as above and the procedure was repeated with 1:20 monoclonal antibody alpha 6F, raised against the chicken alpha1 subunit of the Na+/K+ ATPase (Takeyasu et al., 1988), obtained from the al Studies Hybridoma Bank, as primary and 1:1000 goat anti-mouse Alexa 633 (Molecular Probes) as secondary antibody after incubation with goat anti-mouse Fab fragments 1:20 in 1 PBS- DBT at 4 C overnight. Electron microscopy Embryos were prepared for electron microscopy by previously published protocols (Drummond et al., 1998). High speed videomicroscopy PTU-treated embryos were put in E3 egg water containing 40 mmol/l BDM (2,3-butanedione monoxime, Sigma), for 5 minutes to stop the heartbeat and then changed to 20 mmol/l BDM containing egg water for observation. The embryos were then analyzed using a 40 /0.55 water immersion lens on a Zeiss Axioplan microscope (Zeiss, Germany) equipped with a high-speed Photron FastCAM-PCI 500 videocamera (Photron LTD). Image acquisition of beating cilia was 250 frames per second and 1088 frames total per take by Photron FastCAM version (Photron LTD). Image processing was done using Photoshop 7.0 (Adobe) and movies compiled in Graphic Converter v (Lemke Software, Germany). Three-dimensional illustrations were drawn using Strata3D Software (Strata). Fluorescent dye/bead injection and fluorescence videomicroscopy For urine excretion assays a 5% solution of Tetramethylrhodaminconjugated 70 k MW dextran (Molecular Probes) was injected into the common cardinal vein (CCV) of dpf. embryos anesthetized with 0.2 mg/ml tricaine (3-aminobenzoic acid ethylester, Sigma) in egg water, these were then examined using a 40 /0.55 water immersion lens on a Zeiss Axioplan microscope equipped with a MTI SIT68 fluorescence camera the video was recorded at real time with a Panasonic PV-8400 tape recorder. Digitalization was done using SonicMyDVD Version software (Adaptec), still frames were captured using QuickTime v and movies were recompiled by Graphic Converter (Lemke Software, Germany). For the dye transport in the central canal, the dye was injected into the brain ventricle of 60 hpf. embryos anesthetized with tricaine. Sequential images were taken with a Nikon fluorescence microscope. Fluorescent beads of 0.02 µm diameter (Fluospheres (580/605), Molecular Probes) were dispersed 1:50 in 0.1 mol/l saline with 0.1% Phenol Red (Sigma) and used for injection into KV of embryos at 8-10 somite stage. Statistics The two-tailed Student s t-test was applied to the quantitative results. Results Apical cilia are present in KV, in the central canal of the spinal cord, and in the pronephric ducts To survey zebrafish larvae for the presence of ciliated cells during organogenesis, we performed immunocytochemistry using an anti-acetylated tubulin antibody. Immunostaining confirmed an earlier report that apical cilia are present in cells lining KV at the 8-somite stage (Fig. 1A,D) (Essner et al., 2002). Also, ependymal cells along the central canal bore apical cilia at 24 hpf (Fig. 1B,E), as did pronephric duct cells at 48 hpf (Fig. 1C,F). KV cilia and pronephric duct cilia showed an ultrastructure consisting of nine peripheral microtubules and a central pair (9+2 pattern; Fig. 1G,I), whereas a 9+0 formation was present in ependymal cell cilia along the central canal of the spinal cord (Fig. 1H). Outer dynein arms were present in cilia of all three organs (arrows). All kidney cilia examined were 9+2; no 9+0 cilia were found in the zebrafish pronephros. Cilia in pronephros, central canal and KV are motile The presence of dynein outer arms in the cilia of all three tissues suggested that these cilia are motile (Smith and Yang,
4 (8) Research article 2004). We therefore examined cilia for motility using highspeed videomicroscopy. To examine pronephric cilia, embryos at 2.5 days post-fertilization (dpf) were treated with butanedione monoxime (BDM) to stop the heartbeat and Fig. 1. Apical cilia are present in Kupffer s vesicle, the central canal of the spinal cord and pronephric ducts. Immunostaining of acetylated tubulin. (A) Apical cilia are present in cells lining Kupffer s vesicle at the 8-somite stage (arrowhead) in midline longitudinal sections. (D) Kupffer s vesicle; higher magnification (DAPI nuclear staining in blue). (B) Ependymal cells along the central canal bear cilia at 24 hpf (arrowheads). (E) Cross section at 44 hpf; cilia arise from all cells of the spinal central canal. (C) Cilia can also be seen in the pronephric duct at 48 hpf (arrowheads). (F) Cells double stained for acetylated tubulin and the alpha1 subunit of the NaK-ATPase confirmed the apical position of the pronephric cilia. (G-I) EM cross sections of cilia in Kupffer s vesicle (G) show a 9+2 structure; ependymal cell cilia (H) are 9+0 in structure; pronephric cilia (I) are 9+2 with clear dynein outer arms (arrows). Cilia beat pattern: (J) The cilia in the of the spinal central canal and Kupffer s vesicle rotate in an counterclockwise orientation. (K) In the pronephric duct monociliated and multiciliated cells can be observed. Their cilia beat in rotation like a corkscrew with an undulating appearance along their longitudinal axis. Mean values for cilia length and beat frequency are given for comparison. Scale bars: 100 µm in A-C; 10 µm in D-F; 250 nm in G-I. KV, Kupffer s vesicle; PND, pronephric duct; SCC, spinal central canal. circulation in order to eliminate glomerular filtration pressure. Images were acquired at 250 frames per second and then replayed in slow motion at 15 frames per second to count the beat frequency. Under these conditions, cilia beating at a frequency of 20.0±3.2 Hz (n=34) were observed in all parts of the pronephros, including the tubules and ducts (see Movies 1, 2 in the supplementary material). As a similar cilia beat frequency was observed in embryos not treated with BDM, we conclude that BDM does not influence cilia motility. In BDM-treated embryos there was no luminal fluid flow generated by glomerular filtration, so the observed cilia movements must represent active beating and not passive deflection. Rotational cilia movement generated a corkscrewlike wave pattern in the lumen of the duct directed toward the cloaca (Fig. 1K). While the majority of pronephric epithelial cells displayed a single apical motile cilium, a subset of cells with up to 16 apical cilia could be observed in the midpart of the pronephric duct. The cilia in the central canal of the spinal cord were filmed under the same BDM conditions as above to avoid disturbances by circulating blood cells. The ependymal cilia were approximately 2 µm in length and also showed a rotary pattern of motility (Fig. 1J). The frequency of rotation was approximately 12 Hz (see Movie 4 in the supplementary material). The cilia in KV were similar in length (3 µm) to the ependymal cilia and rotated in a counterclockwise orientation (Fig. 1J; see Movie 8 in the supplementary material). In addition to images of moving KV cilia themselves, cilia motility could be detected by the movement of small pieces of debris suspended in the fluid of KV. Debris was observed to travel in a counterclockwise orbit, interrupted by small counterclockwise spins corresponding to the radii of circular cilia beat patterns (see Movie 9 in the supplementary material). This type of particle and fluid movement was subsequently confirmed with fluorescent bead injections (see below). Cilia length is shortened in IFT morphants and oval mutant embryos In order to manipulate cilia structure and assess their function in vivo, we cloned and disrupted the expression of zebrafish homologs of the IFT proteins of polaris/ift88/osm-5 and hippi/ift57/che-13. The sequence homology and identity between human, mouse and zebrafish IFT genes are shown in Fig. S1 in the supplementary material. In addition, we analyzed the oval mutant (ovl tz288b ), which carries a point mutation in the zebrafish homolog of polaris/ift88/osm-5 leading to a protein truncation (L260X) (Tsujikawa and Malicki, 2004). The in situ expression of both polaris(ift88) and hippi(ift57) in hpf embryos was ubiquitous with some enrichment along the pronephric ducts (see Fig. S2 in the supplementary material) and the brain ventricles, and also around KV (data not shown). Using morpholino antisense oligonucleotides (MO), we disrupted protein function of the hippi and polaris genes. AUG and SP-morpholinos were designed for both genes. The effectiveness of SP-morpholinos was verified by RT-PCR using RNA from single embryos. The results show that morpholino suppression of mrna splicing persists for at least 72 hours (Fig. 2O,P; see Fig. S5 in the supplementary material). Wholemount immunostains for acetylated tubulin of 44 hpf. embryos
5 Cilia and zebrafish organogenesis 1911 were performed and the specimens examined by confocal microscopy with extended focus. Wild-type pronephric tubules and ducts are ciliated along the entire length of the nephron. Individual cilia were visible in the posterior segment of the pronephric duct (Fig. 2A). In the pronephros of IFT morphant embryos and oval homozygous mutants, severe shortening or absence of cilia was observed along the entire length of the pronephric nephron, from the cloaca (Fig. 2B- D) to the anterior region of the pronephric tubules (data not shown). Cilia length was reduced from wildtype control 8.8±2.0 µm (n=107) to polaris MO 2.5±1.9 µm (n=271, P<0.001) and hippi MO 3.5±2.0 µm (n=141, P<0.001). In oval heterozygotes cilia were 10.0±2.5 µm (n=68) in length, while in oval homozygotes they were 3.7±2.1 µm (n=104, P<0.001). Ependymal cilia of the spinal central canal were similarly shortened or reduced in number in the morphant and mutant embryos (Fig. 2F-H) compared with wild-type controls (Fig. 2E). Length measurements of the cilia showed 2.1±0.7 µm (n=63) in wild type versus 0.9±0.5 µm (n=21, P<0.001) in polaris MO and 1.2±0.8 µm (n=46, P<0.001) in hippi MO. At the 8-10 somite stage KV cilia were also shortened or missing in IFT morphants compared with controls (Fig. 2I-K). When present, cilia length was 3.3±1.1 µm (n=119) in wild type versus 2.0±0.8 µm (n=25, P<0.001) in polaris MO and 1.4±0.6 µm (n=15, P<0.001) in hippi MO. In several instances cilia appeared largely absent in hippi MO embryos (Fig. 2K). Although reduced in length, pronephric cilia in IFT morphant or mutant embryos were comparable in structure and maintained a relatively normal 9+2 microtubule doublet ultrastructure (Fig. 2L-N). Fig. 2. Cilia structure is altered in IFT morphant embryos. A whole-mount confocal immunostaining of acetylated tubulin in 44 hpf embryos shows apical cilia in the pronephric ducts (A-D), the spinal canal (E-H) and Kupffer s vesicle (I-K). At 44 hpf polaris and hippi morphants (B,C), as well as oval homozygous mutant embryos (D), exhibit severely shortened cilia (arrowheads) compared with wild type (A). For reference, arrows in A-D indicate the point were the pronephric ducts merge at the cloaca. (E-H) Cilia (arrowheads) in the central canal (lumen indicated by arrow in E) of the spinal cord are also shortened or absent in polaris (F) and hippi (G) morphants and in oval / embryos (H) compared with wild type (E). Immunostaining of pronephros/central canal: anterior is to the left, dorsal to the top. Kupffer s vesicle cilia in polaris (J) and hippi (K) morphant embryos are greatly reduced compared with control (I). (L,M) Ultrastructure of the pronephric cilia in wild-type (L), polaris (M) and hippi (N) morphants show a typical 9+2 microtubule doublet pattern. (O) Molecular analysis of the effectiveness of SPmorpholinos inducing splice defects: RT-PCR of single embryos generates a 354 bp polaris fragment in control embryos, bridging part of exon 1 to part of exon 5 at 24, 48, 72 hpf (lane M, 1 Kb Plus DNA Ladder). polarissp-injected embryos analyzed with the same primer sets at the same timepoints show a larger amplicon of 457 bp caused by a non-splicing of intron 2, which encodes a premature stop codon; the lower wild-type band recovers over time. Lower panel: RT- PCR of β-actin of the same RNA samples. (P) RT-PCR of hippi mrna results in a 553 bp fragment in control embryos, whereas the amplicon is reduced in size in the hippisp-treated embryos, indicating an in-frame deletion of exon 2 and 3 (lower band, 260 bp) or an out-of-frame deletion of exon 2 only (middle band, 378 bp); there is recovery of the wild-type band over time. Cyst formation and hydrocephalus in IFT morphants and oval / embryos To determine whether the previously described phenotypes associated with IFT88/polaris loss of function were also observed when expression of other IFT proteins was disrupted, we compared the phenotype of embryos injected with morpholinos targeting both IFT88/polaris and IFT57/hippi. Morpholinos targeting the translation start site (AUG) or the splice donor site (SP) of the second coding exon of IFT88/polaris or IFT57/hippi all led to pronephric cyst formation, hydrocephalus, ventrally curved body axis and pericardial edema in 72 hpf embryos (Fig. 3). Edema became generalized by day 5/6, when most of the embryos died. The kidney cyst and hydrocephalus were easily recognizable in the living fish, in which pigmentation was inhibited by PTU and also by histology (Fig. 3K). Body axis curvature, kidney cysts
6 (8) Research article and hydrocephalus were also observed in oval (IFT88/polaris) homozygous mutant embryos, confirming that the morpholino phenotypes we observed are due to IFT88/polaris loss of function (Fig. 3H) (Tsujikawa and Malicki, 2004). The specificity of the morpholino was tested for the hippiaug morpholino, for which an introduction of five mismatches completely abolished its effects (Fig. 3D). To further establish the specificity of the morpholino action, we performed rescue experiments for the hippisp morpholino by co-injection of capped RNA made in vitro from zebrafish hippi cdna. Zebrafish hippi mrna injection alone did not cause an obvious phenotype; injected embryos appeared wild type. Co-injection of zebrafish hippi mrna with morpholino completely rescued 36 out of 44 injected larvae to a wild-typelike phenotype; histological cross sections confirmed the absence of hydrocephalus and cyst formation in the co-injected larvae (see Fig. S3 in the supplementary material). Co-injected capped murine hippi RNA with hippisp morpholino showed a complete rescue in 6 out of 26 embryos. Partial rescue was observed in 14 out of 26 embryos, with embryos showing a straight body axis and substantially reduced cyst formation. In 1 out of 26 doubly injected embryos, axis deformity and hydrocephalus were observed, but no cysts formed and the remaining five embryos did not show rescue (data not shown). The data demonstrate that the observed phenotypes are specific to loss of IFT57/hippi function and further suggest that IFT57/hippi protein function is in large part conserved in vertebrates. Pronephric fluid flow is impaired in the IFT morphant/mutant embryos and can lead to cyst formation The reduction in cilia length in IFT morphant embryos suggested that cilia motility might also be affected and contribute to the observed organ phenotypes. Indeed, in the IFT morphant embryos and oval homozygotes, moving cilia were rarely detected. The remaining motile cilia in these embryos appeared to be stumpy and had a faster, uncoordinated flickering movement (PolAUG 32.2±2.3 Hz, n=8, HippiSP 30.6±4.2 Hz, n=7, significantly different from control 20.0±3.2 Hz, n=34, P<0.001) (see Movie 6, Movie 5 in the supplementary material). The cloaca-directed, helical wave pattern of cilia beat observed in wild-type embryos was never seen in IFT morphant tubules or ducts. To test if disturbed ciliary motility had an impact on fluid output from the pronephros, we performed dye excretion experiments. Tetramethylrhodamine-conjugated dextran (70 kd) injected into the common cardinal vein of living 3.5-dayold embryos was filtered in the glomerulus and excreted via the pronephric ducts at the cloaca (Fig. 4C). The time span after injection until the first visible urine excretion at the cloaca was 4.5±2.9 minutes (n=12) in wild-type Fig. 3. IFT morphant phenotype: kidney cysts and hydrocephalus. Disruption of polaris function by injection of polarisaug (B) and polarissp (C,I) results in hydrocephalus (black arrowhead), pronephric cyst formation (arrow), pericardial edema (white arrowhead), and ventrally bent body axis at 72 hpf compared with non-injected control embryos (A). This phenotype imitates the oval homozygous mutant (H), in which the polaris gene is mutated. Heterozygous embryos are indistinguishable from wild-type controls (G). Embryos injected with the control hippiaug mismatch morpholino have a normal morphology (D), whereas hippiaug (E) and hippisp (F) cause a phenotype similar to the polaris morphants and the oval homozygous mutant. (J) Wild-type embryo in longitudinal section. (K) hippisp morphant embryo showing severe hydrocephalus (**) and kidney cyst (*). (L) hippisp morphant embryo showing axis curvature, cysts (arrow) and hydrocephalus (arrowhead). (M) Histological cross sections of a 72 hpf control embryo show the midline fused glomerulus, pronephric tubules and pronephric ducts on either side. (N,O) hippisp morphant embryos at 72 hpf show a cystic dilatation (*) of the pronephric tubules with a stretched glomerulus in the midline. (O) polarisaug morphants show kidney cysts (*) and distension of the pronephric ducts. gl, glomerulus; pd, pronephric ducts; pt, pronephric tubules.
7 Cilia and zebrafish organogenesis 1913 control embryos. A movie available in the supplemental data shows in fast motion how the fluorescent urine output is observed from 3-8 minutes postinjection (see Movie 3 in the supplementary material). In the morphant embryos, dye excretion fell to levels below our detection limits; no jet of fluorescence at the cloaca was observed in 9 out of 9 polarisaug and 9 out of 9 hippisp morphants, even at timepoints more than 30 minutes post-injection (Fig. 4G,K), compared with a visible excretion in 22 out of 27 wild types. To demonstrate that the failure to detect fluorescent output was not because of blocked glomerular filtration, the embryos were sectioned and examined for dye passage and uptake by pronephric epithelial cells: all embryos showed endocytic uptake of the filtered dye by proximal duct cells, indicating that the fluorescent dextran was efficiently filtered in IFT morphant embryos (Fig. 4D,H,L). Similar to control wild-type embryos, oval heterozygotes showed dye excretion starting at 5.3±0.4 minutes (n=2) after injection. By contrast, two out of five oval homozygotes did not show dye excretion, and in the remaining three embryos, dye excretion was delayed, being first detectable at 13.7±5.5 minutes (n=3) (P=0.1, not significant) after injection, and the flow of excreted dye was markedly reduced. In these embryos, only the lumen of the common pronephric duct was visibly fluorescent (arrowhead), and there was no jet of fluorescence in the medium outside the cloaca (arrow) (Fig. 4R). The data indicate that cilia function is required to maintain normal rates of fluid flow in the pronephros. To demonstrate that impaired fluid flow in the zebrafish pronephros could lead to cyst formation, we mechanically obstructed the pronephric ducts close to the cloaca (Fig. 4S). Number 5 Inox tweezers were used to pinch and physically obstruct the cloaca. This obstruction of fluid flow resulted in rapid pronephric cyst formation, within 30 minutes. Cystic Fig. 4. Fluid flow is impaired by lack of normal cilia movement in the pronephros. Living embryos were injected with 5% tetramethylrhodamine-conjugated 70 k MW dextran into the circulation. After passage of the pronephric kidney, the dye was excreted at the cloaca (C, arrow). The images of the first column (A,E,I,M,P) are transmitted light images. The images in the second column (B,F,J,N,Q) were taken at 2-3 minutes post-injection, while the images in the third column (C,G,K,O,R) were captured when maximum excretion was reached. The time after injection that the image was captured is indicated in the bottom left of each panel. No fluorescent dye excretion via the cloaca was observed at timepoints >30 minutes in polarisaug morphants (n=9) or hippisp morphants (n=9), whereas in the control embryos excretion was observed in 22 individuals (n=27). On histology of the same embryos, all showed endocytic uptake of the dye in anterior duct cells (arrowhead) (D,H,L), indicating that the dye had been filtered via the glomerulus (double arrows) into the cyst lumens (*). In oval heterozygous embryos, excretion started at 5.3±0.4 seconds (n=2) (O), and 3 out of 5 oval homozygous embryos showed weak dye excretion at 13.7±5.5 seconds (n=3), whereas 2 did not show a visible output. In A-R anterior is to the left and dorsal is to the top. Mechanical obstruction of the pronephric ducts close to the cloaca (S, arrow) causes cystic distension of the anterior pronephric tubules within 30 minutes (T, arrow). The dilated tubules/glomerulus can be seen in cross sections (U, arrows). Scale bar: 100 µm. distension of the pronephric tubules and the anterior segment of the pronephric ducts occurred well anterior to the point of occlusion. Cyst structure was verified in histological cross sections (Fig. 4T,U). Taken together with our data on disruption of cilia function, the results suggest that a reduction in flow rate in the pronephros may lead to back pressure at the site of fluid input to the pronephros and result in tubule luminal expansion and cyst formation.
8 (8) Research article Distension of the brain ventricles is associated with impaired fluid flow along the central canal of the spinal cord To determine whether a similar loss of fluid flow could account for the distension of the brain ventricles seen in IFT morphant embryos, we injected 70 kd rhodamine-dextran into the fourth ventricle at the level of the hindbrain and labeled the cerebrospinal fluid in order to monitor its transport along the central canal of the spinal cord by fluorescence microscopy. The embryos were pretreated with BDM in order to prevent dye movement by an active circulation. The leading front of the dye traveling along the central canal was imaged at various timepoints and transport rate was quantified. In wild-type controls, the mean velocity of fluid movement in the spinal central canal was 27.0±1.9 µm/minute (n=4) (Fig. 5A,B), whereas it was reduced in the polarisaug morphants to 11.3±3.3 µm/minute (n=5, P<0.001) (Fig. 5D) and in the hippisp morphants to 12.0±1.7 µm/minute (n=3, P<0.001) (Fig. 5F). Identical results were obtained for the oval mutant (Fig. 5J). Impaired fluid flow probably results in fluid backup and distension of the brain ventricles and the development of hydrocephalus. Disruption of dynein heavy chain 9 expression partially phenocopies the IFT-morphants Previous studies of the role of cilia in kidney cystic disorders have suggested that kidney cilia are non-motile and act to sense fluid flow. Our results in zebrafish indicate that cilia motility is a primary factor in maintaining proper lumen size and kidney function. To distinguish between sensory versus motile cilia function, we sought to decrease cilia beat rate without causing gross changes in cilia length or structure. The outer doublet microtubules and associated dynein arms are critical for the initiation and propagation of ciliary bending, while the central pair/radial spokes system serves to regulate beat frequency and wave form (Smith and Yang, 2004). In order to disrupt cilia beat rate and pattern, we isolated an axonemal dynein heavy chain by RT-PCR using degenerate primers and RNA from isolated pronephric ducts of 72 hpf zebrafish larvae. A cdna encoding the P1 domain, the primary ATP binding site that is essential for dynein motor function, of a dynein heavy chain homologous to human dynein heavy chain 9 (DYH9, AAF69004) was obtained. Injection of a morpholino (dhc9p1sp) targeting the splice donor site of the P1-domain coding exon resulted in mis-splicing of dhc9 mrna, producing either an out-of-frame deletion of the P1 ATPbinding domain or truncation after the P1 domain (see Fig. S5 in the supplementary material). The reduction of dhc9 function partially phenocopied the IFT morphants/mutants (Fig. 6A) with injected embryos showing hydrocephalus and pericardial edema. Histological sectioning revealed distension of the pronephric tubules (Fig. 6G) and dilated pronephric ducts (Fig. 6H,I). The movement of the cilia in dhc9p1sp larvae was significantly slower than in wild-type control embryos: wild type 20.0±3.2 Hz (n=34) versus dhc9p1sp 14.7±3.9 Hz (n=15, P<0.001) (see Movie 7 in the supplementary material). The cilia beat frequency was also reduced in the central canal of the spinal cord with wild type 12.3±3.4 Hz (n=18) versus dhc9p1sp 7.3±1.9 Hz (n=6, P<0.001). The rate of dye transport in the spinal canal was also reduced in dhc9p1sp morphants (Fig. 6C) compared with wild type (Fig. 6B): wild Fig. 5. Fluid flow is impaired by lack of normal cilia movement in the central canal of the spinal cord. BDM-pretreated embryos were injected with 5% tetramethylrhodamine-conjugated 70 k MW dextran into the fourth brain ventricle and dye distribution along the central canal of the spinal cord was recorded at various timepoints; shown are 2 and 40 minutes post-injection. Control (A,B) and oval heterozygous (G,H) show a distribution of the dye up to an anteriorposterior level of the tip of the yolk extension at 40 minutes (arrowheads) (B,H), whereas polarisaug morphant (C,D), hippisp morphant (E,F) and oval homozygotes (I,J) show reduced dye migration. Anterior to the left. Scale bar: 100 µm. type 27.0±1.9 µm/minute (n=7) versus dhc9p1sp 20.6±2.6 µm/minute (n=7, P<0.001). Excretion of circulating 70 kd rhodamine-dextran by the pronephros could be detected in only 5 out of 19 morphant embryos (Fig. 6L,M), and these embryos showed a delayed onset of 12.8±5.9 minutes (n=5) versus 4.5±2.9 minutes (n=12) in wild-type controls (P<0.01) (Fig. 6J,K). The embryos used for this experiment had sufficient circulation (circulating blood cells) despite pericardial edema. The data indicate that a reduction in beat frequency without changes in cilia structure is sufficient to cause the phenotypes associated with IFT protein loss of function.
9 Cilia and zebrafish organogenesis 1915 Fig. 6. Dynein heavy chain 9 knockdown morphants show abnormal cilia movements and phenotypic changes similar to the IFT morphants. A morpholino targeting the splice-donor site of the exon coding for the P1-domain of dhc9 causes kidney cysts, hydrocephalus (arrow) and axis curvature (A). Sequencing RT-PCR of aberrant splice products (A, inset) revealed non-splicing of the adjacent intron with a premature stop codon (upper band) and an out-of-frame deletion of the P1-domain coding exon (lower band). The transport of injected fluorescent dye along the central canal of the spinal cord (B,C) is impaired in dhc9sp morphants (C) versus control (B). Histologically, Dhc9P1SP morphant embryos show distension of the tubules near the glomerulus (G) and dilated ducts (H) compared with wild-type control (D,E). The dilatation of the duct can also be seen in frames taken from Movie 7 (see supplementary material) (F, wild type; I, Dhc9P1SP morphant). (J-M) Dye excretion via the urine was not detected in dhc9sp morphants (arrows in L,M) versus control (J,K). Impaired fluid flow in KV is associated with laterality defects The presence of motile cilia in KV suggested that the fluid enclosed by the KV epithelium is stirred and that, similar to the role of nodal flow in the mouse, fluid movement might also play a role in establishing proper organ laterality in zebrafish. We visualized fluid movement in KV by injecting small fluorescent beads into the lumen of KV of 8-10 somite embryos. When viewed dorsally by videomicroscopy, injected beads moved in a counterclockwise direction (Fig. 7A-D, see Movie 10 in the supplementary material). Larger bead aggregates tended to collect in the center of KV, where they rotated in place in a counterclockwise direction. As noted above, cilia beating and the movement of small pieces of debris in KV was also in a counterclockwise direction (see Movie 9 in the supplementary material). To test whether fluid motion in KV required intact cilia, we examined injected bead movements in IFT88/polaris and IFT57hippi morphant embryos. The absence of normal cilia in KV of IFT morphant embryos completely abolished bead movement (Fig. 7E-H). The data indicate that counterclockwise cilia beating drives fluid in a counterclockwise flow pattern inside the confined space of KV. It has been suggested that in order to achieve right-to-left fluid flow in the mouse node, only a portion of the circular cilia beat must be involved in fluid propulsion (Cartwright et al., 2004). If cilia were, for instance, tipped toward the posterior, then a cilium would extend into node fluid only on the rightto-left portion of the cycle. On the return left-to-right portion of stroke, the cilium would move close to the cell surface and produce only a small drag on node fluid. We therefore examined KV cilia by electron microscopy for signs that the cilia might be polarized in an anterior-posterior orientation. As shown in figure 7P, cilia and their associated basal bodies on the dorsal aspect ( roof ) of KV were observed to be tipped to the posterior approximately 45 relative to the surface of the roof. We also observed that the cell membrane and cytoplasm adjacent to the cilium insertion appeared to be a site of active vesicle fusion (Fig. 7P). To test whether loss of cilia-dependent fluid flow in KV resulted in laterality defects, we assayed expression of two conserved left-right genes, the nodal-related protein southpaw (spaw) and the bicoid-related transcription factor pitx2, which is downstream of nodal. At mid-somite stages (15-23) southpaw is expressed in the left lateral plate mesoderm (LPM) (Long et al., 2003). pitx2 is also expressed in the same location at similar stages (Campione et al., 1999; Yan et al., 1999). Observed expression patterns for the IFT morphant embryos are shown in Fig. 7I-O. In IFT morphant embryos, right-sided or bilateral expression of southpaw was significantly increased, and in 33% of polarisaug embryos southpaw expression was missing (Fig. 7R). Right-sided pitx2 expression was increased in polarisaug-injected embryos compared with wild type, and the frequency of absent signal was also increased. Significantly, hippispinjected embryos showed a complete absence of pitx2
10 (8) Research article Fig. 7. Impaired fluid flow in Kupffer s vesicle is associated with defects in laterality. Embryos at the 8-10 somite stage were dechorionated and fluorescent beads were injected into Kupffer s vesicle. Control embryos showed a rotating movement of bead aggregates in a counterclockwise orientation (arrowheads in A-C) when viewed dorsally. Relative timepoints in seconds are indicated in the bottom right of each panel. The wall of Kupffer s vesicle is indicated by dotted lines. (D) Superposition and enlargement of frames (A-C) with an additional transmitted light frame showing the counterclockwise direction of movement. None of the injected morphant embryos (polarisaug and hippisp) showed this phenomenon (E-H). Abnormal expression of the laterality markers pitx2 and spaw in polarisaug and hippisp embryos (I-O,R). In situ experiments were performed on 14-somite (spaw) and 20-somite (pitx2) embryos. Dorsal views of the lateral plate mesoderm are shown with the different expression patterns seen in polarisaug embryos. southpaw was expressed on the left (I), right (J) or bilaterally (K), or in many cases absent (L). pitx2 shows the same patterns (M, left-sided; N, right-sided; O, absent expression), with the exception of bilateral expression. Sagittal section electron micrograph (P) of the roof of Kupffer s vesicle showing, a single cilium and associated basal body. (Q) Diagram of the micrograph in P, detailing how the angle of the basal body [approximately 45 to the posterior (P)] would result in the cilium projecting into Kupffer s vesicle on the right-to-left portion of the counterclockwise rotary beat. (R) Frequency of laterality defects in polaris and hippi morphant embryos. Expression of pitx2 and southpaw was randomized in polarisaug embryos, while hippisp embryos showed significantly higher numbers of embryos with no expression of southpaw and pitx2 (control, n=25; polarisaug/pitx2, n=29; polarisaug/southpaw, n=36; hippisp/pitx2, n=40; hippisp/southpaw, n=57). In embryos lacking laterality signals in the lateral plate mesoderm, gene expression was nevertheless maintained in the tailbud (spaw) and Rohon-Beard neurons (pitx2). Scale bar: 10 µm. a, anterior; d, dorsal. expression and a near-complete absence of southpaw expression (Fig. 7R). Discussion The recent convergence of studies of kidney cystic disease, left-right asymmetry defects, retinal degeneration and flagella formation has led to the idea that defects in the formation or function of cilia may underlie pathologies observed in all these conditions. However, despite the focus on cilia as a central organelle in these phenotypes, it has been unclear what exactly cilia do to support normal organ development and function and how loss of this organelle can lead to such pathology. Our analysis of cilia during zebrafish organogenesis has demonstrated that cilia in the zebrafish pronephros, in the central canal of the spinal cord and in KV are motile. Beating cilia were found to induce fluid flow in all three organs. Lack of proper cilia formation due to inhibition of IFT88/osm- 5/polaris or IFT57/che-13/hippi expression was associated with loss of fluid movement and resulted in pronephric cyst formation, left-right asymmetry defects and hydrocephalus. We conclude that back pressure from blocked flow and subsequent fluid accumulation may account for organ distension pathologies in the brain and kidney, while the loss of fluid flow in KV may result in absence of a mechanosensory signal regulating organ laterality.
SUPPLEMENTARY INFORMATION
 Supplementary Information included with Nature MS 2008-02-01484B by Colantonio et al., entitled The dynein regulatory complex is required for ciliary motility and otolith biogenesis in the inner ear. This
Supplementary Information included with Nature MS 2008-02-01484B by Colantonio et al., entitled The dynein regulatory complex is required for ciliary motility and otolith biogenesis in the inner ear. This
Polaris and Polycystin-2 in dorsal forerunner cells and Kupffer s vesicle are required for specification of the zebrafish left right axis
 Developmental Biology 287 (2005) 274 288 www.elsevier.com/locate/ydbio Polaris and Polycystin-2 in dorsal forerunner cells and Kupffer s vesicle are required for specification of the zebrafish left right
Developmental Biology 287 (2005) 274 288 www.elsevier.com/locate/ydbio Polaris and Polycystin-2 in dorsal forerunner cells and Kupffer s vesicle are required for specification of the zebrafish left right
Supplementary Figures
 Supplementary Figures Supplementary Figure 1. nrg1 bns101/bns101 embryos develop a functional heart and survive to adulthood (a-b) Cartoon of Talen-induced nrg1 mutation with a 14-base-pair deletion in
Supplementary Figures Supplementary Figure 1. nrg1 bns101/bns101 embryos develop a functional heart and survive to adulthood (a-b) Cartoon of Talen-induced nrg1 mutation with a 14-base-pair deletion in
Supplemental Information. Ciliary Beating Compartmentalizes. Cerebrospinal Fluid Flow in the Brain. and Regulates Ventricular Development
 Current Biology, Volume Supplemental Information Ciliary Beating Compartmentalizes Cerebrospinal Fluid Flow in the Brain and Regulates Ventricular Development Emilie W. Olstad, Christa Ringers, Jan N.
Current Biology, Volume Supplemental Information Ciliary Beating Compartmentalizes Cerebrospinal Fluid Flow in the Brain and Regulates Ventricular Development Emilie W. Olstad, Christa Ringers, Jan N.
SUPPLEMENTARY INFORMATION
 Supplementary Figure 1. Formation of the AA5x. a, Camera lucida drawing of embryo at 48 hours post fertilization (hpf, modified from Kimmel et al. Dev Dyn. 1995 203:253-310). b, Confocal microangiogram
Supplementary Figure 1. Formation of the AA5x. a, Camera lucida drawing of embryo at 48 hours post fertilization (hpf, modified from Kimmel et al. Dev Dyn. 1995 203:253-310). b, Confocal microangiogram
Axis Formation and Mesoderm Induction
 Developmental Biology Biology 4361 Axis Formation and Mesoderm Induction October 27, 2005 Amphibian anteroposterior specification polarized eggs animal/vegetal pigment yolk v. clear cytoplasm mitochondrial
Developmental Biology Biology 4361 Axis Formation and Mesoderm Induction October 27, 2005 Amphibian anteroposterior specification polarized eggs animal/vegetal pigment yolk v. clear cytoplasm mitochondrial
The coiled-coil domain containing protein CCDC40 is essential for motile cilia function and left-right axis formation
 The coiled-coil domain containing protein CCDC40 is essential for motile cilia function and left-right axis formation Anita Becker-Heck#, Irene Zohn#, Noriko Okabe#, Andrew Pollock#, Kari Baker Lenhart,
The coiled-coil domain containing protein CCDC40 is essential for motile cilia function and left-right axis formation Anita Becker-Heck#, Irene Zohn#, Noriko Okabe#, Andrew Pollock#, Kari Baker Lenhart,
Supplementary Information
 Supplementary Information CEP41 is mutated in Joubert syndrome and is required for tubulin glutamylation at the cilium Ji Eun Lee, Jennifer L. Silhavy, Maha S. Zaki, Jana Schroth, Stephanie L. Bielas,
Supplementary Information CEP41 is mutated in Joubert syndrome and is required for tubulin glutamylation at the cilium Ji Eun Lee, Jennifer L. Silhavy, Maha S. Zaki, Jana Schroth, Stephanie L. Bielas,
Lack of cadherins Celsr2 and Celsr3 impairs ependymal ciliogenesis, leading to fatal
 Lack of cadherins Celsr2 and Celsr3 impairs ependymal ciliogenesis, leading to fatal hydrocephalus Fadel TISSIR, Yibo QU, Mireille MONTCOUQUIOL, Libing ZHOU, Kouji KOMATSU, Dongbo SHI, Toshihiko FUJIMORI,
Lack of cadherins Celsr2 and Celsr3 impairs ependymal ciliogenesis, leading to fatal hydrocephalus Fadel TISSIR, Yibo QU, Mireille MONTCOUQUIOL, Libing ZHOU, Kouji KOMATSU, Dongbo SHI, Toshihiko FUJIMORI,
Kupffer s vesicle is a ciliated organ of asymmetry in the zebrafish embryo that initiates left-right development of the brain, heart and gut
 First posted online on 16 February 2005 as 10.1242/dev.01663 Access the most recent epress version at online http://dev.biologists.org/lookup/doi/10.1242/dev.01663 publication date 16 February 2005 1247
First posted online on 16 February 2005 as 10.1242/dev.01663 Access the most recent epress version at online http://dev.biologists.org/lookup/doi/10.1242/dev.01663 publication date 16 February 2005 1247
SUPPLEMENTARY INFORMATION
 DOI: 10.1038/ncb2988 Supplementary Figure 1 Kif7 L130P encodes a stable protein that does not localize to cilia tips. (a) Immunoblot with KIF7 antibody in cell lysates of wild-type, Kif7 L130P and Kif7
DOI: 10.1038/ncb2988 Supplementary Figure 1 Kif7 L130P encodes a stable protein that does not localize to cilia tips. (a) Immunoblot with KIF7 antibody in cell lysates of wild-type, Kif7 L130P and Kif7
RECONSTITUTION OF METACHRONAL WAVES IN CILIATED CORTICAL SHEETS OF PARAMECIUM
 J. exp. Biol. 192, 73 81 (1994) Printed in Great Britain The Company of Biologists Limited 1994 73 RECONSTITUTION OF METACHRONAL WAVES IN CILIATED CORTICAL SHEETS OF PARAMECIUM II. ASYMMETRY OF THE CILIARY
J. exp. Biol. 192, 73 81 (1994) Printed in Great Britain The Company of Biologists Limited 1994 73 RECONSTITUTION OF METACHRONAL WAVES IN CILIATED CORTICAL SHEETS OF PARAMECIUM II. ASYMMETRY OF THE CILIARY
Collective Cell Migration Drives Morphogenesis of the Kidney Nephron
 Collective Cell Migration Drives Morphogenesis of the Kidney Nephron PLoS BIOLOGY Aleksandr Vasilyev 1, Yan Liu 1, Sudha Mudumana 1, Steve Mangos 1, Pui-Ying Lam 1, Arindam Majumdar 1 a, Jinhua Zhao 1
Collective Cell Migration Drives Morphogenesis of the Kidney Nephron PLoS BIOLOGY Aleksandr Vasilyev 1, Yan Liu 1, Sudha Mudumana 1, Steve Mangos 1, Pui-Ying Lam 1, Arindam Majumdar 1 a, Jinhua Zhao 1
(a) Significant biological processes (upper panel) and disease biomarkers (lower panel)
 Supplementary Figure 1. Functional enrichment analyses of secretomic proteins. (a) Significant biological processes (upper panel) and disease biomarkers (lower panel) 2 involved by hrab37-mediated secretory
Supplementary Figure 1. Functional enrichment analyses of secretomic proteins. (a) Significant biological processes (upper panel) and disease biomarkers (lower panel) 2 involved by hrab37-mediated secretory
tom tom 24hpf tom tom 48hpf tom 60hpf tom tom 72hpf tom
 a 24hpf c 48hpf d e 60hpf f g 72hpf h i j k ISV ISV Figure 1. Vascular integrity defects and endothelial regression in mutant emryos. (a,c,e,g,i) Bright-field and (,d,f,h,j) corresponding fluorescent micrographs
a 24hpf c 48hpf d e 60hpf f g 72hpf h i j k ISV ISV Figure 1. Vascular integrity defects and endothelial regression in mutant emryos. (a,c,e,g,i) Bright-field and (,d,f,h,j) corresponding fluorescent micrographs
SUPPLEMENTARY INFORMATION
 DOI: 1.138/ncb222 / b. WB anti- WB anti- ulin Mitotic index (%) 14 1 6 2 T (h) 32 48-1 1 2 3 4 6-1 4 16 22 28 3 33 e. 6 4 2 Time (min) 1-6- 11-1 > 1 % cells Figure S1 depletion leads to mitotic defects
DOI: 1.138/ncb222 / b. WB anti- WB anti- ulin Mitotic index (%) 14 1 6 2 T (h) 32 48-1 1 2 3 4 6-1 4 16 22 28 3 33 e. 6 4 2 Time (min) 1-6- 11-1 > 1 % cells Figure S1 depletion leads to mitotic defects
Supplemental Information. Otic Mesenchyme Cells Regulate. Spiral Ganglion Axon Fasciculation. through a Pou3f4/EphA4 Signaling Pathway
 Neuron, Volume 73 Supplemental Information Otic Mesenchyme Cells Regulate Spiral Ganglion Axon Fasciculation through a Pou3f4/EphA4 Signaling Pathway Thomas M. Coate, Steven Raft, Xiumei Zhao, Aimee K.
Neuron, Volume 73 Supplemental Information Otic Mesenchyme Cells Regulate Spiral Ganglion Axon Fasciculation through a Pou3f4/EphA4 Signaling Pathway Thomas M. Coate, Steven Raft, Xiumei Zhao, Aimee K.
Supporting Online Material for
 www.sciencemag.org/cgi/content/full/1171320/dc1 Supporting Online Material for A Frazzled/DCC-Dependent Transcriptional Switch Regulates Midline Axon Guidance Long Yang, David S. Garbe, Greg J. Bashaw*
www.sciencemag.org/cgi/content/full/1171320/dc1 Supporting Online Material for A Frazzled/DCC-Dependent Transcriptional Switch Regulates Midline Axon Guidance Long Yang, David S. Garbe, Greg J. Bashaw*
Expression of acid base transporters in the kidney collecting duct in Slc2a7 -/-
 Supplemental Material Results. Expression of acid base transporters in the kidney collecting duct in Slc2a7 -/- and Slc2a7 -/- mice. The expression of AE1 in the kidney was examined in Slc26a7 KO mice.
Supplemental Material Results. Expression of acid base transporters in the kidney collecting duct in Slc2a7 -/- and Slc2a7 -/- mice. The expression of AE1 in the kidney was examined in Slc26a7 KO mice.
Available online at
 Available online at www.sciencedirect.com Developmental Biology 314 (2008) 261 275 www.elsevier.com/developmentalbiology Zebrafish mutations affecting cilia motility share similar cystic phenotypes and
Available online at www.sciencedirect.com Developmental Biology 314 (2008) 261 275 www.elsevier.com/developmentalbiology Zebrafish mutations affecting cilia motility share similar cystic phenotypes and
BIOL2005 WORKSHEET 2008
 BIOL2005 WORKSHEET 2008 Answer all 6 questions in the space provided using additional sheets where necessary. Hand your completed answers in to the Biology office by 3 p.m. Friday 8th February. 1. Your
BIOL2005 WORKSHEET 2008 Answer all 6 questions in the space provided using additional sheets where necessary. Hand your completed answers in to the Biology office by 3 p.m. Friday 8th February. 1. Your
Supplementary Figure 1
 Supplementary Figure 1 Global TeNT expression effectively impairs synaptic transmission. Injection of 100 pg tent mrna leads to a reduction of vesicle mediated synaptic transmission in the spinal cord
Supplementary Figure 1 Global TeNT expression effectively impairs synaptic transmission. Injection of 100 pg tent mrna leads to a reduction of vesicle mediated synaptic transmission in the spinal cord
Problem Set 5 KEY
 2006 7.012 Problem Set 5 KEY ** Due before 5 PM on THURSDAY, November 9, 2006. ** Turn answers in to the box outside of 68-120. PLEASE WRITE YOUR ANSWERS ON THIS PRINTOUT. 1. You are studying the development
2006 7.012 Problem Set 5 KEY ** Due before 5 PM on THURSDAY, November 9, 2006. ** Turn answers in to the box outside of 68-120. PLEASE WRITE YOUR ANSWERS ON THIS PRINTOUT. 1. You are studying the development
klp-18 (RNAi) Control. supplementary information. starting strain: AV335 [emb-27(g48); GFP::histone; GFP::tubulin] bleach
![klp-18 (RNAi) Control. supplementary information. starting strain: AV335 [emb-27(g48); GFP::histone; GFP::tubulin] bleach klp-18 (RNAi) Control. supplementary information. starting strain: AV335 [emb-27(g48); GFP::histone; GFP::tubulin] bleach](/thumbs/91/104639484.jpg) DOI: 10.1038/ncb1891 A. starting strain: AV335 [emb-27(g48); GFP::histone; GFP::tubulin] bleach embryos let hatch overnight transfer to RNAi plates; incubate 5 days at 15 C RNAi food L1 worms adult worms
DOI: 10.1038/ncb1891 A. starting strain: AV335 [emb-27(g48); GFP::histone; GFP::tubulin] bleach embryos let hatch overnight transfer to RNAi plates; incubate 5 days at 15 C RNAi food L1 worms adult worms
A study of cilia density in sea urchin embryos during gastrulation
 A study of cilia density in sea urchin embryos during gastrulation Steve Das Independent Research Project Report Bio 254 Developmental Biology May 3, 2012 Introduction Embryo growth and development is
A study of cilia density in sea urchin embryos during gastrulation Steve Das Independent Research Project Report Bio 254 Developmental Biology May 3, 2012 Introduction Embryo growth and development is
Pathophysiology of inherited kidney disorders: lessons from the zebrafish
 Pathophysiology of inherited kidney disorders: lessons from the zebrafish Francisco J. Arjona Radboud Institute for Molecular Life Sciences Radboud university medical center Nijmegen, The Netherlands www.physiomics.eu
Pathophysiology of inherited kidney disorders: lessons from the zebrafish Francisco J. Arjona Radboud Institute for Molecular Life Sciences Radboud university medical center Nijmegen, The Netherlands www.physiomics.eu
Supplemental Information. Supernumerary Centrosomes. Nucleate Extra Cilia and Compromise. Primary Cilium Signaling. Current Biology, Volume 22
 Current Biology, Volume 22 Supplemental Information Supernumerary Centrosomes Nucleate Extra Cilia and Compromise Primary Cilium Signaling Moe R. Mahjoub and Tim Stearns Supplemental Inventory 1. Supplemental
Current Biology, Volume 22 Supplemental Information Supernumerary Centrosomes Nucleate Extra Cilia and Compromise Primary Cilium Signaling Moe R. Mahjoub and Tim Stearns Supplemental Inventory 1. Supplemental
T H E J O U R N A L O F C E L L B I O L O G Y
 Supplemental material Brooks and Wallingford, http://www.jcb.org/cgi/content/full/jcb.201204072/dc1 T H E J O U R N A L O F C E L L B I O L O G Y Figure S1. Quantification of ciliary compartments in control
Supplemental material Brooks and Wallingford, http://www.jcb.org/cgi/content/full/jcb.201204072/dc1 T H E J O U R N A L O F C E L L B I O L O G Y Figure S1. Quantification of ciliary compartments in control
Supplementary Figure 1. AdipoR1 silencing and overexpression controls. (a) Representative blots (upper and lower panels) showing the AdipoR1 protein
 Supplementary Figure 1. AdipoR1 silencing and overexpression controls. (a) Representative blots (upper and lower panels) showing the AdipoR1 protein content relative to GAPDH in two independent experiments.
Supplementary Figure 1. AdipoR1 silencing and overexpression controls. (a) Representative blots (upper and lower panels) showing the AdipoR1 protein content relative to GAPDH in two independent experiments.
Nature Biotechnology: doi: /nbt Supplementary Figure 1. Analysis of hair bundle morphology in Ush1c c.216g>a mice at P18 by SEM.
 Supplementary Figure 1 Analysis of hair bundle morphology in Ush1c c.216g>a mice at P18 by SEM. (a-c) Heterozygous c.216ga mice displayed normal hair bundle morphology at P18. (d-i) Disorganized hair bundles
Supplementary Figure 1 Analysis of hair bundle morphology in Ush1c c.216g>a mice at P18 by SEM. (a-c) Heterozygous c.216ga mice displayed normal hair bundle morphology at P18. (d-i) Disorganized hair bundles
When you see this diagram, remember that you are looking at the embryo from above, through the amniotic cavity, where the epiblast appears as an oval
 When you see this diagram, remember that you are looking at the embryo from above, through the amniotic cavity, where the epiblast appears as an oval disc 2 Why the embryo needs the vascular system? When
When you see this diagram, remember that you are looking at the embryo from above, through the amniotic cavity, where the epiblast appears as an oval disc 2 Why the embryo needs the vascular system? When
Studying The Role Of DNA Mismatch Repair In Brain Cancer Malignancy
 Kavya Puchhalapalli CALS Honors Project Report Spring 2017 Studying The Role Of DNA Mismatch Repair In Brain Cancer Malignancy Abstract Malignant brain tumors including medulloblastomas and primitive neuroectodermal
Kavya Puchhalapalli CALS Honors Project Report Spring 2017 Studying The Role Of DNA Mismatch Repair In Brain Cancer Malignancy Abstract Malignant brain tumors including medulloblastomas and primitive neuroectodermal
Supplementary Figure 1. EC-specific Deletion of Snail1 Does Not Affect EC Apoptosis. (a,b) Cryo-sections of WT (a) and Snail1 LOF (b) embryos at
 Supplementary Figure 1. EC-specific Deletion of Snail1 Does Not Affect EC Apoptosis. (a,b) Cryo-sections of WT (a) and Snail1 LOF (b) embryos at E10.5 were double-stained for TUNEL (red) and PECAM-1 (green).
Supplementary Figure 1. EC-specific Deletion of Snail1 Does Not Affect EC Apoptosis. (a,b) Cryo-sections of WT (a) and Snail1 LOF (b) embryos at E10.5 were double-stained for TUNEL (red) and PECAM-1 (green).
The Rho kinase Rock2b establishes anteroposterior asymmetry of the ciliated Kupffer s vesicle in zebrafish
 RESEARCH ARTICLE 45 Development 138, 45-54 (2011) doi:10.1242/dev.052985 2011. Published by The Company of Biologists Ltd The Rho kinase Rock2b establishes anteroposterior asymmetry of the ciliated Kupffer
RESEARCH ARTICLE 45 Development 138, 45-54 (2011) doi:10.1242/dev.052985 2011. Published by The Company of Biologists Ltd The Rho kinase Rock2b establishes anteroposterior asymmetry of the ciliated Kupffer
Tanimoto et al., http ://www.jcb.org /cgi /content /full /jcb /DC1
 Supplemental material JCB Tanimoto et al., http ://www.jcb.org /cgi /content /full /jcb.201510064 /DC1 THE JOURNAL OF CELL BIOLOGY Figure S1. Method for aster 3D tracking, extended characterization of
Supplemental material JCB Tanimoto et al., http ://www.jcb.org /cgi /content /full /jcb.201510064 /DC1 THE JOURNAL OF CELL BIOLOGY Figure S1. Method for aster 3D tracking, extended characterization of
Qilin Is Essential for Cilia Assembly and Normal Kidney Development in Zebrafish
 and Normal Kidney Development in Zebrafish Jade Li, Zhaoxia Sun* Department of Genetics, Yale University School of Medicine, New Haven, Connecticut, United States of America Abstract Defects in the cilium,
and Normal Kidney Development in Zebrafish Jade Li, Zhaoxia Sun* Department of Genetics, Yale University School of Medicine, New Haven, Connecticut, United States of America Abstract Defects in the cilium,
Supplemental Figure 1. Western blot analysis indicated that MIF was detected in the fractions of
 Supplemental Figure Legends Supplemental Figure 1. Western blot analysis indicated that was detected in the fractions of plasma membrane and cytosol but not in nuclear fraction isolated from Pkd1 null
Supplemental Figure Legends Supplemental Figure 1. Western blot analysis indicated that was detected in the fractions of plasma membrane and cytosol but not in nuclear fraction isolated from Pkd1 null
SUPPLEMENTARY INFORMATION
 SUPPLEMENTARY INFORMATION Advances in pancreatic islet monolayer culture on glass surfaces enable superresolution microscopy and insights into beta cell ciliogenesis and proliferation Edward A. Phelps,
SUPPLEMENTARY INFORMATION Advances in pancreatic islet monolayer culture on glass surfaces enable superresolution microscopy and insights into beta cell ciliogenesis and proliferation Edward A. Phelps,
Developmental Biology
 Developmental Biology 362 (2012) 141 153 Contents lists available at SciVerse ScienceDirect Developmental Biology journal homepage: www.elsevier.com/developmentalbiology Dual function of suppressor of
Developmental Biology 362 (2012) 141 153 Contents lists available at SciVerse ScienceDirect Developmental Biology journal homepage: www.elsevier.com/developmentalbiology Dual function of suppressor of
* * A3027. A4623 e A3507 A3507 A3507
 a c L A327 d e A37 A37 A37 Supplementary Figure 1. Clinical manifestations of individuals with mutations. (a) Renal ultrasound of right kidney in A327 reveals small renal cysts, loss of corticomedullary
a c L A327 d e A37 A37 A37 Supplementary Figure 1. Clinical manifestations of individuals with mutations. (a) Renal ultrasound of right kidney in A327 reveals small renal cysts, loss of corticomedullary
Intravital Microscopic Interrogation of Peripheral Taste Sensation
 Supplementary Information Intravital Microscopic Interrogation of Peripheral Taste Sensation Myunghwan Choi 1, Woei Ming Lee 1,2, and Seok-Hyun Yun 1 * 1 Harvard Medical School and Wellman Center for Photomedicine,
Supplementary Information Intravital Microscopic Interrogation of Peripheral Taste Sensation Myunghwan Choi 1, Woei Ming Lee 1,2, and Seok-Hyun Yun 1 * 1 Harvard Medical School and Wellman Center for Photomedicine,
MII. Supplement Figure 1. CapZ β2. Merge. 250ng. 500ng DIC. Merge. Journal of Cell Science Supplementary Material. GFP-CapZ β2 DNA
 A GV GVBD MI DNA CapZ β2 CapZ β2 Merge B DIC GFP-CapZ β2 Merge CapZ β2-gfp 250ng 500ng Supplement Figure 1. MII A early MI late MI Control RNAi CapZαβ DNA Actin Tubulin B Phalloidin Intensity(A.U.) n=10
A GV GVBD MI DNA CapZ β2 CapZ β2 Merge B DIC GFP-CapZ β2 Merge CapZ β2-gfp 250ng 500ng Supplement Figure 1. MII A early MI late MI Control RNAi CapZαβ DNA Actin Tubulin B Phalloidin Intensity(A.U.) n=10
A Precise Bicoid Gradient is Nonessential During Cycles for Precise Patterning in the Drosophila Blastoderm
 Supporting Information for A Precise Bicoid Gradient is Nonessential During Cycles 11-13 for Precise Patterning in the Drosophila Blastoderm Elena M. Lucchetta, Meghan E. Vincent and Rustem F. Ismagilov*
Supporting Information for A Precise Bicoid Gradient is Nonessential During Cycles 11-13 for Precise Patterning in the Drosophila Blastoderm Elena M. Lucchetta, Meghan E. Vincent and Rustem F. Ismagilov*
Supplemental Information. Myocardial Polyploidization Creates a Barrier. to Heart Regeneration in Zebrafish
 Developmental Cell, Volume 44 Supplemental Information Myocardial Polyploidization Creates a Barrier to Heart Regeneration in Zebrafish Juan Manuel González-Rosa, Michka Sharpe, Dorothy Field, Mark H.
Developmental Cell, Volume 44 Supplemental Information Myocardial Polyploidization Creates a Barrier to Heart Regeneration in Zebrafish Juan Manuel González-Rosa, Michka Sharpe, Dorothy Field, Mark H.
Correspondence: mirna regulation of Sdf1 chemokine signaling provides genetic robustness to germ cell migration
 Correspondence: mirna regulation of Sdf1 chemokine signaling provides genetic robustness to germ cell migration Alison A. Staton, Holger Knaut, and Antonio J. Giraldez Supplementary Note Materials and
Correspondence: mirna regulation of Sdf1 chemokine signaling provides genetic robustness to germ cell migration Alison A. Staton, Holger Knaut, and Antonio J. Giraldez Supplementary Note Materials and
Supplemental Information. Autophagy in Oncogenic K-Ras. Promotes Basal Extrusion. of Epithelial Cells by Degrading S1P. Current Biology, Volume 24
 Current Biology, Volume 24 Supplemental Information Autophagy in Oncogenic K-Ras Promotes Basal Extrusion of Epithelial Cells by Degrading S1P Gloria Slattum, Yapeng Gu, Roger Sabbadini, and Jody Rosenblatt
Current Biology, Volume 24 Supplemental Information Autophagy in Oncogenic K-Ras Promotes Basal Extrusion of Epithelial Cells by Degrading S1P Gloria Slattum, Yapeng Gu, Roger Sabbadini, and Jody Rosenblatt
Nature Neuroscience: doi: /nn Supplementary Figure 1
 Supplementary Figure 1 Subcellular segregation of VGluT2-IR and TH-IR within the same VGluT2-TH axon (wild type rats). (a-e) Serial sections of a dual VGluT2-TH labeled axon. This axon (blue outline) has
Supplementary Figure 1 Subcellular segregation of VGluT2-IR and TH-IR within the same VGluT2-TH axon (wild type rats). (a-e) Serial sections of a dual VGluT2-TH labeled axon. This axon (blue outline) has
Inhibitory Effects of Torin2 on Proximal Tubular Development of the Xenopus laevis Pronephric Kidney
 John Carroll University Carroll Collected Senior Honors Projects Theses, Essays, and Senior Honors Projects Spring 2013 Inhibitory Effects of Torin2 on Proximal Tubular Development of the Xenopus laevis
John Carroll University Carroll Collected Senior Honors Projects Theses, Essays, and Senior Honors Projects Spring 2013 Inhibitory Effects of Torin2 on Proximal Tubular Development of the Xenopus laevis
ASSET Student Laboratory
 ASSET Student Laboratory The Effect of Cigarette Smoke and Alcohol on Tetrahymena Background Information While the general effects of smoking and alcohol use on humans are well documented, it is useful
ASSET Student Laboratory The Effect of Cigarette Smoke and Alcohol on Tetrahymena Background Information While the general effects of smoking and alcohol use on humans are well documented, it is useful
This article appeared in a journal published by Elsevier. The attached copy is furnished to the author for internal non-commercial research and
 This article appeared in a journal published by Elsevier. The attached copy is furnished to the author for internal non-commercial research and education use, including for instruction at the authors institution
This article appeared in a journal published by Elsevier. The attached copy is furnished to the author for internal non-commercial research and education use, including for instruction at the authors institution
McWilliams et al., http :// /cgi /content /full /jcb /DC1
 Supplemental material JCB McWilliams et al., http ://www.jcb.org /cgi /content /full /jcb.201603039 /DC1 THE JOURNAL OF CELL BIOLOGY Figure S1. In vitro characterization of mito-qc. (A and B) Analysis
Supplemental material JCB McWilliams et al., http ://www.jcb.org /cgi /content /full /jcb.201603039 /DC1 THE JOURNAL OF CELL BIOLOGY Figure S1. In vitro characterization of mito-qc. (A and B) Analysis
Supplementary Information
 Supplementary Information Supplementary Figure 1: Luminal localization of CCM-3. (a) The CCM-3::GFP fusion protein localizes along the apical (luminal) surface of the pharynx (b) as well as the lumen of
Supplementary Information Supplementary Figure 1: Luminal localization of CCM-3. (a) The CCM-3::GFP fusion protein localizes along the apical (luminal) surface of the pharynx (b) as well as the lumen of
(A) PCR primers (arrows) designed to distinguish wild type (P1+P2), targeted (P1+P2) and excised (P1+P3)14-
 1 Supplemental Figure Legends Figure S1. Mammary tumors of ErbB2 KI mice with 14-3-3σ ablation have elevated ErbB2 transcript levels and cell proliferation (A) PCR primers (arrows) designed to distinguish
1 Supplemental Figure Legends Figure S1. Mammary tumors of ErbB2 KI mice with 14-3-3σ ablation have elevated ErbB2 transcript levels and cell proliferation (A) PCR primers (arrows) designed to distinguish
Programmed Cell Death (apoptosis)
 Programmed Cell Death (apoptosis) Stereotypic death process includes: membrane blebbing nuclear fragmentation chromatin condensation and DNA framentation loss of mitochondrial integrity and release of
Programmed Cell Death (apoptosis) Stereotypic death process includes: membrane blebbing nuclear fragmentation chromatin condensation and DNA framentation loss of mitochondrial integrity and release of
Supplementary Figure 1. Gene schematics of hyls-1, gasr-8 and k10g6.4, and TEM analysis of TFs in WT and hyls-1 cilia. (a) Gene structure of hyls-1,
 Supplementary Figure 1. Gene schematics of hyls-1, gasr-8 and k10g6.4, and TEM analysis of TFs in WT and hyls-1 cilia. (a) Gene structure of hyls-1, gasr-8 and k10g6.4 based on WormBase (http://wormbase.org),
Supplementary Figure 1. Gene schematics of hyls-1, gasr-8 and k10g6.4, and TEM analysis of TFs in WT and hyls-1 cilia. (a) Gene structure of hyls-1, gasr-8 and k10g6.4 based on WormBase (http://wormbase.org),
Supplementary Figure S1. Venn diagram analysis of mrna microarray data and mirna target analysis. (a) Western blot analysis of T lymphoblasts (CLS)
 Supplementary Figure S1. Venn diagram analysis of mrna microarray data and mirna target analysis. (a) Western blot analysis of T lymphoblasts (CLS) and their exosomes (EXO) in resting (REST) and activated
Supplementary Figure S1. Venn diagram analysis of mrna microarray data and mirna target analysis. (a) Western blot analysis of T lymphoblasts (CLS) and their exosomes (EXO) in resting (REST) and activated
Epithelium-1. Hanan Jafar BDS.MSc.PhD
 Epithelium-1 Hanan Jafar BDS.MSc.PhD General features Epithelium is an avascular tissue composed of cells that cover the exterior body surfaces and line internal closed cavities and tubes. It also forms
Epithelium-1 Hanan Jafar BDS.MSc.PhD General features Epithelium is an avascular tissue composed of cells that cover the exterior body surfaces and line internal closed cavities and tubes. It also forms
Supplementary Materials and Methods
 Supplementary Materials and Methods Whole Mount X-Gal Staining Whole tissues were collected, rinsed with PBS and fixed with 4% PFA. Tissues were then rinsed in rinse buffer (100 mm Sodium Phosphate ph
Supplementary Materials and Methods Whole Mount X-Gal Staining Whole tissues were collected, rinsed with PBS and fixed with 4% PFA. Tissues were then rinsed in rinse buffer (100 mm Sodium Phosphate ph
Review from Biology A
 Chapter 4 Review from Biology A The Cell Theory All organisms are made of cells Cells come from pre-existing cells The cell is the simplest collection of matter that can live Scientists whose work you
Chapter 4 Review from Biology A The Cell Theory All organisms are made of cells Cells come from pre-existing cells The cell is the simplest collection of matter that can live Scientists whose work you
SUPPLEMENTAL MATERIAL. Supplementary Methods
 SUPPLEMENTAL MATERIAL Supplementary Methods Culture of cardiomyocytes, fibroblasts and cardiac microvascular endothelial cells The isolation and culturing of neonatal rat ventricular cardiomyocytes was
SUPPLEMENTAL MATERIAL Supplementary Methods Culture of cardiomyocytes, fibroblasts and cardiac microvascular endothelial cells The isolation and culturing of neonatal rat ventricular cardiomyocytes was
100 mm Sucrose. +Berberine +Quinine
 8 mm Sucrose Probability (%) 7 6 5 4 3 Wild-type Gr32a / 2 +Caffeine +Berberine +Quinine +Denatonium Supplementary Figure 1: Detection of sucrose and bitter compounds is not affected in Gr32a / flies.
8 mm Sucrose Probability (%) 7 6 5 4 3 Wild-type Gr32a / 2 +Caffeine +Berberine +Quinine +Denatonium Supplementary Figure 1: Detection of sucrose and bitter compounds is not affected in Gr32a / flies.
Vertebrate Limb Patterning
 Vertebrate Limb Patterning What makes limb patterning an interesting/useful developmental system How limbs develop Key events in limb development positioning and specification initiation of outgrowth establishment
Vertebrate Limb Patterning What makes limb patterning an interesting/useful developmental system How limbs develop Key events in limb development positioning and specification initiation of outgrowth establishment
Supplementary Figures
 Supplementary Figures Supplementary Figure 1. Confirmation of Dnmt1 conditional knockout out mice. a, Representative images of sorted stem (Lin - CD49f high CD24 + ), luminal (Lin - CD49f low CD24 + )
Supplementary Figures Supplementary Figure 1. Confirmation of Dnmt1 conditional knockout out mice. a, Representative images of sorted stem (Lin - CD49f high CD24 + ), luminal (Lin - CD49f low CD24 + )
The subcortical maternal complex controls symmetric division of mouse zygotes by
 The subcortical maternal complex controls symmetric division of mouse zygotes by regulating F-actin dynamics Xing-Jiang Yu 1,2, Zhaohong Yi 1, Zheng Gao 1,2, Dan-dan Qin 1,2, Yanhua Zhai 1, Xue Chen 1,
The subcortical maternal complex controls symmetric division of mouse zygotes by regulating F-actin dynamics Xing-Jiang Yu 1,2, Zhaohong Yi 1, Zheng Gao 1,2, Dan-dan Qin 1,2, Yanhua Zhai 1, Xue Chen 1,
Supplementary Figure 1: Signaling centers contain few proliferating cells, express p21, and
 Supplementary Figure 1: Signaling centers contain few proliferating cells, express p21, and exclude YAP from the nucleus. (a) Schematic diagram of an E10.5 mouse embryo. (b,c) Sections at B and C in (a)
Supplementary Figure 1: Signaling centers contain few proliferating cells, express p21, and exclude YAP from the nucleus. (a) Schematic diagram of an E10.5 mouse embryo. (b,c) Sections at B and C in (a)
The T Box Transcription Factor No Tail in Ciliated Cells Controls Zebrafish Left-Right Asymmetry
 Current Biology, Vol. 14, 685 690, April 20, 2004, 2004 Elsevier Ltd. All rights reserved. DOI 10.1016/j.cub.2004.04.002 The T Box Transcription Factor No Tail in Ciliated Cells Controls Zebrafish Left-Right
Current Biology, Vol. 14, 685 690, April 20, 2004, 2004 Elsevier Ltd. All rights reserved. DOI 10.1016/j.cub.2004.04.002 The T Box Transcription Factor No Tail in Ciliated Cells Controls Zebrafish Left-Right
XBX-1 Encodes a Dynein Light Intermediate Chain Required for Retrograde Intraflagellar Transport and Cilia Assembly in Caenorhabditis elegans V
 Molecular Biology of the Cell Vol. 14, 2057 2070, May 2003 XBX-1 Encodes a Dynein Light Intermediate Chain Required for Retrograde Intraflagellar Transport and Cilia Assembly in Caenorhabditis elegans
Molecular Biology of the Cell Vol. 14, 2057 2070, May 2003 XBX-1 Encodes a Dynein Light Intermediate Chain Required for Retrograde Intraflagellar Transport and Cilia Assembly in Caenorhabditis elegans
SUPPLEMENTARY INFORMATION. Supplementary Figures S1-S9. Supplementary Methods
 SUPPLEMENTARY INFORMATION SUMO1 modification of PTEN regulates tumorigenesis by controlling its association with the plasma membrane Jian Huang 1,2#, Jie Yan 1,2#, Jian Zhang 3#, Shiguo Zhu 1, Yanli Wang
SUPPLEMENTARY INFORMATION SUMO1 modification of PTEN regulates tumorigenesis by controlling its association with the plasma membrane Jian Huang 1,2#, Jie Yan 1,2#, Jian Zhang 3#, Shiguo Zhu 1, Yanli Wang
JCB. Supplemental material. Gu et al.,
 Supplemental material Gu et al., http://www.jcb.org/cgi/content/full/jcb.201010075/dc1 T H E J O U R N A L O F C E L L B I O L O G Y Figure S1. S1P directly induces actin assembly. Actin assembly at the
Supplemental material Gu et al., http://www.jcb.org/cgi/content/full/jcb.201010075/dc1 T H E J O U R N A L O F C E L L B I O L O G Y Figure S1. S1P directly induces actin assembly. Actin assembly at the
Tay Sachs, Cystic Fibrosis, Sickle Cell Anemia and PKU. Tay Sachs Disease (also called Hexosaminidase deficiency)
 Tay Sachs, Cystic Fibrosis, Sickle Cell Anemia and PKU Tay Sachs Disease (also called Hexosaminidase deficiency) Introduction 1. Tay Sachs is a rare condition named after 2 physicians, Tay and Sachs, who
Tay Sachs, Cystic Fibrosis, Sickle Cell Anemia and PKU Tay Sachs Disease (also called Hexosaminidase deficiency) Introduction 1. Tay Sachs is a rare condition named after 2 physicians, Tay and Sachs, who
Human height. Length of some nerve and muscle cells. Chicken egg. Frog egg. Most plant and animal cells Nucleus Most bacteria Mitochondrion
 10 m 1 m 0.1 m 1 cm Human height Length of some nerve and muscle cells Chicken egg Unaided eye 1 mm Frog egg 100 µm 10 µm 1 µm 100 nm 10 nm Most plant and animal cells Nucleus Most bacteria Mitochondrion
10 m 1 m 0.1 m 1 cm Human height Length of some nerve and muscle cells Chicken egg Unaided eye 1 mm Frog egg 100 µm 10 µm 1 µm 100 nm 10 nm Most plant and animal cells Nucleus Most bacteria Mitochondrion
Influenza virus exploits tunneling nanotubes for cell-to-cell spread
 Supplementary Information Influenza virus exploits tunneling nanotubes for cell-to-cell spread Amrita Kumar 1, Jin Hyang Kim 1, Priya Ranjan 1, Maureen G. Metcalfe 2, Weiping Cao 1, Margarita Mishina 1,
Supplementary Information Influenza virus exploits tunneling nanotubes for cell-to-cell spread Amrita Kumar 1, Jin Hyang Kim 1, Priya Ranjan 1, Maureen G. Metcalfe 2, Weiping Cao 1, Margarita Mishina 1,
CELLS. Cells. Basic unit of life (except virus)
 Basic unit of life (except virus) CELLS Prokaryotic, w/o nucleus, bacteria Eukaryotic, w/ nucleus Various cell types specialized for particular function. Differentiation. Over 200 human cell types 56%
Basic unit of life (except virus) CELLS Prokaryotic, w/o nucleus, bacteria Eukaryotic, w/ nucleus Various cell types specialized for particular function. Differentiation. Over 200 human cell types 56%
Cytosolic Carboxypeptidase 5 and Cilia Development in Zebrafish
 Andrews University Digital Commons @ Andrews University Honors Theses Undergraduate Research 2014 Cytosolic Carboxypeptidase 5 and Cilia Development in Zebrafish Philip E. Giddings IV This research is
Andrews University Digital Commons @ Andrews University Honors Theses Undergraduate Research 2014 Cytosolic Carboxypeptidase 5 and Cilia Development in Zebrafish Philip E. Giddings IV This research is
Life Sciences 1A Midterm Exam 2. November 13, 2006
 Name: TF: Section Time Life Sciences 1A Midterm Exam 2 November 13, 2006 Please write legibly in the space provided below each question. You may not use calculators on this exam. We prefer that you use
Name: TF: Section Time Life Sciences 1A Midterm Exam 2 November 13, 2006 Please write legibly in the space provided below each question. You may not use calculators on this exam. We prefer that you use
Two Populations of Node Monocilia Initiate Left-Right Asymmetry in the Mouse
 Cell, Vol. 114, 61 73, July 11, 2003, Copyright 2003 by Cell Press Two Populations of Node Monocilia Initiate Left-Right Asymmetry in the Mouse comes restricted to the left side of the node (Collignon
Cell, Vol. 114, 61 73, July 11, 2003, Copyright 2003 by Cell Press Two Populations of Node Monocilia Initiate Left-Right Asymmetry in the Mouse comes restricted to the left side of the node (Collignon
Nature Genetics: doi: /ng Supplementary Figure 1. Brain magnetic resonance imaging in patient 9 at age 3.6 years.
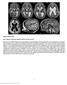 Supplementary Figure 1 Brain magnetic resonance imaging in patient 9 at age 3.6 years. (a d) Axial T2-weighted images show a marked degree of ventriculomegaly. Note the diencephalic mesencephalic junction
Supplementary Figure 1 Brain magnetic resonance imaging in patient 9 at age 3.6 years. (a d) Axial T2-weighted images show a marked degree of ventriculomegaly. Note the diencephalic mesencephalic junction
Supplemental Information. Tissue Myeloid Progenitors Differentiate. into Pericytes through TGF-b Signaling. in Developing Skin Vasculature
 Cell Reports, Volume 18 Supplemental Information Tissue Myeloid Progenitors Differentiate into Pericytes through TGF-b Signaling in Developing Skin Vasculature Tomoko Yamazaki, Ani Nalbandian, Yutaka Uchida,
Cell Reports, Volume 18 Supplemental Information Tissue Myeloid Progenitors Differentiate into Pericytes through TGF-b Signaling in Developing Skin Vasculature Tomoko Yamazaki, Ani Nalbandian, Yutaka Uchida,
Ciliary Movement of Sea-urchin Embryos
 Ciliary Movement of Sea-urchin Embryos Kogiku Shiba*, Yoshihiro Mogami** and Shoji A. Baba*** *Division of Life Sciences, Graduate School of Humanities and Sciences g0040425@edu.cc.ocha.ac.jp **Department
Ciliary Movement of Sea-urchin Embryos Kogiku Shiba*, Yoshihiro Mogami** and Shoji A. Baba*** *Division of Life Sciences, Graduate School of Humanities and Sciences g0040425@edu.cc.ocha.ac.jp **Department
Sestrin2 and BNIP3 (Bcl-2/adenovirus E1B 19kDa-interacting. protein3) regulate autophagy and mitophagy in renal tubular cells in. acute kidney injury
 Sestrin2 and BNIP3 (Bcl-2/adenovirus E1B 19kDa-interacting protein3) regulate autophagy and mitophagy in renal tubular cells in acute kidney injury by Masayuki Ishihara 1, Madoka Urushido 2, Kazu Hamada
Sestrin2 and BNIP3 (Bcl-2/adenovirus E1B 19kDa-interacting protein3) regulate autophagy and mitophagy in renal tubular cells in acute kidney injury by Masayuki Ishihara 1, Madoka Urushido 2, Kazu Hamada
Supplementary Figure 1. Electroporation of a stable form of β-catenin causes masses protruding into the IV ventricle. HH12 chicken embryos were
 Supplementary Figure 1. Electroporation of a stable form of β-catenin causes masses protruding into the IV ventricle. HH12 chicken embryos were electroporated with β- Catenin S33Y in PiggyBac expression
Supplementary Figure 1. Electroporation of a stable form of β-catenin causes masses protruding into the IV ventricle. HH12 chicken embryos were electroporated with β- Catenin S33Y in PiggyBac expression
Supplementary Figure 1 Expression of Crb3 in mouse sciatic nerve: biochemical analysis (a) Schematic of Crb3 isoforms, ERLI and CLPI, indicating the
 Supplementary Figure 1 Expression of Crb3 in mouse sciatic nerve: biochemical analysis (a) Schematic of Crb3 isoforms, ERLI and CLPI, indicating the location of the transmembrane (TM), FRM binding (FB)
Supplementary Figure 1 Expression of Crb3 in mouse sciatic nerve: biochemical analysis (a) Schematic of Crb3 isoforms, ERLI and CLPI, indicating the location of the transmembrane (TM), FRM binding (FB)
(a) TEM of a plasma. Fimbriae. Nucleoid. Ribosomes. Plasma membrane. Cell wall Capsule. Bacterial chromosome
 0 m m 0. m cm mm 00 µm 0 µm 00 nm 0 nm Human height Length of some nerve and muscle cells Chicken egg Frog egg Most plant and animal cells Most bacteria Smallest bacteria Viruses Proteins Unaided eye Light
0 m m 0. m cm mm 00 µm 0 µm 00 nm 0 nm Human height Length of some nerve and muscle cells Chicken egg Frog egg Most plant and animal cells Most bacteria Smallest bacteria Viruses Proteins Unaided eye Light
Figure S1 Time-dependent down-modulation of HER3 by EZN No Treatment. EZN-3920, 2 μm. Time, h
 Figure S1 Time-dependent down-modulation of HER3 by EZN-392 HE ER3 mrna A, %Contr rol 12 No Treatment EZN-392, 2 μm 1 8 6 4 2 2 8 24 Time, h Figure S2. Specific target down-modulation by HER3 (EZN-392)
Figure S1 Time-dependent down-modulation of HER3 by EZN-392 HE ER3 mrna A, %Contr rol 12 No Treatment EZN-392, 2 μm 1 8 6 4 2 2 8 24 Time, h Figure S2. Specific target down-modulation by HER3 (EZN-392)
Supplementary material Legends to Supplementary Figures Figure S1. Figure S2. Figure S3.
 Supplementary material Legends to Supplementary Figures. Figure S1. Expression of BICD-N-MTS fusion does not affect the distribution of the Golgi and endosomes. HeLa cells were transfected with GFP-BICD-N-MTS
Supplementary material Legends to Supplementary Figures. Figure S1. Expression of BICD-N-MTS fusion does not affect the distribution of the Golgi and endosomes. HeLa cells were transfected with GFP-BICD-N-MTS
A Genetic Program for Embryonic Development
 Concept 18.4: A program of differential gene expression leads to the different cell types in a multicellular organism During embryonic development, a fertilized egg gives rise to many different cell types
Concept 18.4: A program of differential gene expression leads to the different cell types in a multicellular organism During embryonic development, a fertilized egg gives rise to many different cell types
41B. Metabolism produces wastes that must be eliminated from the body. This. Renal System Physiology: Computer Simulation
 41B E X E R C I S E Renal System Physiology: Computer Simulation O B J E C T I V E S 1. To define the following terms: glomerulus, glomerular capsule, renal corpuscle, renal tubule, nephron, proximal convoluted
41B E X E R C I S E Renal System Physiology: Computer Simulation O B J E C T I V E S 1. To define the following terms: glomerulus, glomerular capsule, renal corpuscle, renal tubule, nephron, proximal convoluted
Supporting Information
 Supporting Information Franco et al. 10.1073/pnas.1015557108 SI Materials and Methods Drug Administration. PD352901 was dissolved in 0.5% (wt/vol) hydroxyl-propyl-methylcellulose, 0.2% (vol/vol) Tween
Supporting Information Franco et al. 10.1073/pnas.1015557108 SI Materials and Methods Drug Administration. PD352901 was dissolved in 0.5% (wt/vol) hydroxyl-propyl-methylcellulose, 0.2% (vol/vol) Tween
Supplementary table and figures
 3D single molecule tracking with multifocal plane microscopy reveals rapid intercellular transferrin transport at epithelial cell barriers Sripad Ram, Dongyoung Kim, Raimund J. Ober and E. Sally Ward Supplementary
3D single molecule tracking with multifocal plane microscopy reveals rapid intercellular transferrin transport at epithelial cell barriers Sripad Ram, Dongyoung Kim, Raimund J. Ober and E. Sally Ward Supplementary
Nature Medicine: doi: /nm.4322
 1 2 3 4 5 6 7 8 9 10 11 Supplementary Figure 1. Predicted RNA structure of 3 UTR and sequence alignment of deleted nucleotides. (a) Predicted RNA secondary structure of ZIKV 3 UTR. The stem-loop structure
1 2 3 4 5 6 7 8 9 10 11 Supplementary Figure 1. Predicted RNA structure of 3 UTR and sequence alignment of deleted nucleotides. (a) Predicted RNA secondary structure of ZIKV 3 UTR. The stem-loop structure
STRUCTURES LINKING THE TIPS OF CILIARY AND FLAGELLAR MICROTUBULES TO THE MEMBRANE
 J. Cell Sci. 42, 207-220 (1980) 207 Printed in Great Britain Company of Biologists Limited ig8o STRUCTURES LINKING THE TIPS OF CILIARY AND FLAGELLAR MICROTUBULES TO THE MEMBRANE WILLIAM L. DENTLER Department
J. Cell Sci. 42, 207-220 (1980) 207 Printed in Great Britain Company of Biologists Limited ig8o STRUCTURES LINKING THE TIPS OF CILIARY AND FLAGELLAR MICROTUBULES TO THE MEMBRANE WILLIAM L. DENTLER Department
Physiology of Parasites (512) Zoo 3(2+1) Ultrastructure of protozoa and its adaption for host cell invasion
 Physiology of Parasites (512) Zoo 3(2+1) Ultrastructure of protozoa and its adaption for host cell invasion 1 Introduction protozoa Many are important nutrient cyclers. Many are photoautotrophic & make
Physiology of Parasites (512) Zoo 3(2+1) Ultrastructure of protozoa and its adaption for host cell invasion 1 Introduction protozoa Many are important nutrient cyclers. Many are photoautotrophic & make
BIO 5099: Molecular Biology for Computer Scientists (et al)
 BIO 5099: Molecular Biology for Computer Scientists (et al) Lecture 15: Being a Eukaryote: From DNA to Protein, A Tour of the Eukaryotic Cell. Christiaan van Woudenberg Being A Eukaryote Basic eukaryotes
BIO 5099: Molecular Biology for Computer Scientists (et al) Lecture 15: Being a Eukaryote: From DNA to Protein, A Tour of the Eukaryotic Cell. Christiaan van Woudenberg Being A Eukaryote Basic eukaryotes
Supplemental Materials and Methods Plasmids and viruses Quantitative Reverse Transcription PCR Generation of molecular standard for quantitative PCR
 Supplemental Materials and Methods Plasmids and viruses To generate pseudotyped viruses, the previously described recombinant plasmids pnl4-3-δnef-gfp or pnl4-3-δ6-drgfp and a vector expressing HIV-1 X4
Supplemental Materials and Methods Plasmids and viruses To generate pseudotyped viruses, the previously described recombinant plasmids pnl4-3-δnef-gfp or pnl4-3-δ6-drgfp and a vector expressing HIV-1 X4
SUPPLEMENTARY INFORMATION
 Supplementary Figures Supplementary Figure S1. Binding of full-length OGT and deletion mutants to PIP strips (Echelon Biosciences). Supplementary Figure S2. Binding of the OGT (919-1036) fragments with
Supplementary Figures Supplementary Figure S1. Binding of full-length OGT and deletion mutants to PIP strips (Echelon Biosciences). Supplementary Figure S2. Binding of the OGT (919-1036) fragments with
Supplementary Appendix
 Supplementary Appendix This appendix has been provided by the authors to give readers additional information about their work. Supplement to: Yatsenko AN, Georgiadis AP, Röpke A, et al. X-linked TEX11
Supplementary Appendix This appendix has been provided by the authors to give readers additional information about their work. Supplement to: Yatsenko AN, Georgiadis AP, Röpke A, et al. X-linked TEX11
Supplementary Information
 Supplementary Information Supplementary Figure 1. EBV-gB 23-431 mainly exists as trimer in HEK 293FT cells. (a) Western blotting analysis for DSS crosslinked FLAG-gB 23-431. HEK 293FT cells transfected
Supplementary Information Supplementary Figure 1. EBV-gB 23-431 mainly exists as trimer in HEK 293FT cells. (a) Western blotting analysis for DSS crosslinked FLAG-gB 23-431. HEK 293FT cells transfected
SUPPLEMENTARY INFORMATION
 doi:10.1038/nature12652 Supplementary Figure 1. PRDM16 interacts with endogenous EHMT1 in brown adipocytes. Immunoprecipitation of PRDM16 complex by flag antibody (M2) followed by Western blot analysis
doi:10.1038/nature12652 Supplementary Figure 1. PRDM16 interacts with endogenous EHMT1 in brown adipocytes. Immunoprecipitation of PRDM16 complex by flag antibody (M2) followed by Western blot analysis
Primary Cilia are Expressed on a Small Fraction of Cells in Trabecular Bone and Marrow
 Primary Cilia are Expressed on a Small Fraction of Cells in Trabecular Bone and Marrow Thomas R. Coughlin 1, Muriel Voisin, Ph.D. 2, Glen L. Niebur, PhD 1, Laoise M. McNamara 2. 1 University of Notre Dame,
Primary Cilia are Expressed on a Small Fraction of Cells in Trabecular Bone and Marrow Thomas R. Coughlin 1, Muriel Voisin, Ph.D. 2, Glen L. Niebur, PhD 1, Laoise M. McNamara 2. 1 University of Notre Dame,
Supplementary Figure 1. Baf60c and baf180 are induced during cardiac regeneration in zebrafish. RNA in situ hybridization was performed on paraffin
 Supplementary Figure 1. Baf60c and baf180 are induced during cardiac regeneration in zebrafish. RNA in situ hybridization was performed on paraffin sections from sham-operated adult hearts (a and i) and
Supplementary Figure 1. Baf60c and baf180 are induced during cardiac regeneration in zebrafish. RNA in situ hybridization was performed on paraffin sections from sham-operated adult hearts (a and i) and
