Viktorin, G. and Riebli, N. and Popkova, A. and Giangrande, A. and Reichert, H.
|
|
|
- Gerald McKinney
- 6 years ago
- Views:
Transcription
1 Institutional Repository of the University of Basel University Library Schoenbeinstrasse CH-4056 Basel, Switzerland Year: 2011 Multipotent neural stem cells generate glial cells of the central complex through transit amplifying intermediate progenitors in Drosophila brain development Viktorin, G. and Riebli, N. and Popkova, A. and Giangrande, A. and Reichert, H. Posted at edoc, University of Basel Official URL: Originally published as: Viktorin, G. and Riebli, N. and Popkova, A. and Giangrande, A. and Reichert, H.. (2011) Multipotent neural stem cells generate glial cells of the central complex through transit amplifying intermediate progenitors in Drosophila brain development. Developmental biology, Vol S
2 Multipotent neural stem cells generate glial cells of the central complex through transit amplifying intermediate progenitors in Drosophila brain development Gudrun Viktorin 1*, Nadia Riebli 1, Anna Popkova 2, Angela Giangrande 2, and Heinrich Reichert 1 1 Biozentrum, University of Basel, Basel, Switzerland 2 Institut de Génétique et de Biologie Moleculaire et Cellulaire, CNRS/INSERM/ULP, C.U. de Strasbourg, France *Corresponding author: Gudrun Viktorin Biozentrum, University of Basel Klingelbergstr. 70 CH-4056 BASEL Switzerland gudrunviktorin@gmail.com Phone (+) Fax (+) Keywords Drosophila; larval brain; neuroblast lineages; intermediate progenitor cells; neuroglioblast; gliogenesis Viktorin et al. 1
3 Abstract The neural stem cells that give rise to the neural lineages of the brain can generate their progeny directly or through transit amplifying intermediate neural progenitor cells (INPs). The INP-producing neural stem cells in Drosophila are called type II neuroblasts, and their neural progeny innervate the central complex, a prominent integrative brain center. Here we use genetic lineage tracing and clonal analysis to show that the INPs of these type II neuroblast lineages give rise to glial cells as well as neurons during postembryonic brain development. Our data indicate that two main types of INP lineages are generated, namely mixed neuronal/glial lineages and neuronal lineages. Genetic loss-of-function and gain-of-function experiments show that the gcm gene is necessary and sufficient for gliogenesis in these lineages. The INP-derived glial cells, like the INP-derived neuronal cells, make major contributions to the central complex. In postembryonic development, these INP-derived glial cells surround the entire developing central complex neuropile, and once the major compartments of the central complex are formed, they also delimit each of these compartments. During this process, the number of these glial cells in the central complex is increased markedly through local proliferation based on glial cell mitosis. Taken together, these findings uncover a novel and complex form of neurogliogenesis in Drosophila involving transit amplifying intermediate progenitors. Moreover, they indicate that type II neuroblasts are remarkably multipotent neural stem cells that can generate both the neuronal and the glial progeny that make major contributions to one and the same complex brain structure. Viktorin et al. 2
4 Introduction Neural stem cells are the primary progenitor cells at different developmental stages that initiate the neural lineages comprising the differentiated neurons and glia of the brain (Kriegstein and Alvarez-Buylla, 2009). Analysis of stem cell-dependent neurogenesis in several model systems indicates that neural cells are not always produced directly from the primary progenitors, but can also be generated by secondary progenitors of more restricted potential (Götz and Huttner, 2005; Kriegstein et al., 2006). In mammals, neural stem cells can generate neural cells either directly through asymmetric division or indirectly through secondary progenitors which either divide only once to produce two neural progeny or divide more than once to amplify the number of neural progeny further (Haubensak et al., 2004; Miyata et al., 2004; Noctor et al., 2004, 2007). Remarkably similar findings have been obtained in studies of neurogenesis in the developing CNS of Drosophila. There, neural stem cell-like primary progenitors called neuroblasts can also generate neurons directly through asymmetric division or, more typically, indirectly through ganglion mother cells (GMC) which divide only once to generate two postmitotic progeny (Skeath and Thor, 2003; Technau et al., 2006; Knoblich, 2008; Doe, 2008). Moreover, recently a third type of neurogenesis has been discovered in the Drosophila central brain where identified neuroblasts generate intermediate neural progenitor cells (INPs) which undergo several rounds of asymmetric divisions, each of which results in INP self-renewal and generation of a GMC that produces two neural progeny (Bello et al., 2008; Boone and Doe, 2008; Bowman et al., 2008). These INPs have also been referred to as IPs, transit amplifying GMCs, or secondary neuroblasts (Weng and Lee, 2010.) Through the Viktorin et al. 3
5 resulting amplification of proliferation these INP producing type II neuroblasts generate large neural lineages that contribute to major neuropile substructures of the brain such as the central complex (Izergina et al., 2009; Bayraktar et al., 2010; Pereanu et al., 2011). In mammalian brain development, neural stem cells can give rise to both neurons and glial cells, however the neurogenic phase and the gliogenic phase of these primary progenitors are generally separate (Miller and Gauthier, 2007; Miyata et al., 2010). Neural stem cells that can generate both neurons and glia are also found in Drosophila (Van De Bor and Giangrande, 2002). These so-called neuroglioblasts have been identified on a single-cell basis in the embryonic ventral nerve cord, and together with glioblasts, which only generate glial cells, they give rise to several subtypes of glial cells in the ventral CNS (Broadus et al., 1995; Bossing et al., 1996; Schmidt et al., 1997; Beckervordersandforth et al., 2008). In the ventral nerve cord, gliogenesis is controlled by the glial cells missing (gcm) gene that acts as a key switch-like regulator in the development of all embryonic glial cells except those generated from mesectodermal precursors (Hosoya et al., 1995; Jones et al., 1995; Vincent et al., 1996). Much less is known about the developmental origins of the glial cells in the Drosophila brain. The glial cells of the early larval brain are thought to be generated by a few neuroglioblasts during embryogenesis (Hartenstein et al., 1998). However, most of the glial cells in the adult central brain are generated postembryonically, and during larval development a marked increase in glial cell number occurs (Pereanu et al., 2005). While some of these glial cells apparently arise by mitosis of other glial Viktorin et al. 4
6 cells, the bulk of the postembryonically added glial cells is thought to be generated through the proliferation of neuroglioblasts, which, however, have not yet been identified (Pereanu et al., 2005; Awasaki et al., 2008). This lack of identification of the postulated postembryonic neuroglioblasts has been a major obstacle for understanding the mechanisms by which glial cells are generated during postembryonic development of the central brain. Here we show that identified type II neuroblasts have neuroglioblast function during postembryonic development of the central brain. We use genetic lineage tracing and clonal analysis to demonstrate that the INPs of type II neuroblast lineages can give rise to glial cells as well as neurons. Our data indicate that two main types of INPderived lineages are generated postembryonically, namely mixed neuronal/glial lineages and neuronal lineages. Moreover, they show that INP-derived glial cells, like INP-derived neuronal cells from the same type II neuroblast lineages, make major contributions to the developing neuropile of the central complex. They surround the entire developing central complex neuropile, and once its major compartments are formed, they also delimit each of these. During this process, the population of INPderived glial cells associated with central complex undergoes clonal expansion through local proliferation. Taken together, these findings uncover novel mechanisms for neurogliogenesis in Drosophila involving the transit amplifying intermediate progenitors of type II lineages. Thus, type II neuroblasts are remarkably multipotent neural stem cells that can generate both the neuronal and the glial progeny of one and the same complex brain structure. Viktorin et al. 5
7 Materials and Methods Fly stocks, MARCM, and G-TRACE analysis Flies were maintained on standard cornmeal medium at 25 C unless noted otherwise. To visualize the potentially glial offspring of neuroblasts, we used insc-gal4 MZ1407, UAS-mCD8GFP LL5 homozygous flies, or mated them to gcm-lacz ra87 /CyO, actgfp JMR1. For erm-gal4 (R09D11; Pfeiffer et al., 2008) G-Trace (flp-out and real-time; Evans et al., 2009) expression patterns we mated UAS-flp, ubi>stop>ngfp, UAS- RFP/CyO, act-gfp JMR1 ; erm-gal4 to gcm-lacz ra87 /CyO, act-gfp JMR1 ; erm-gal4. To generate wild type MARCM (Lee and Luo, 1999) clones, we mated y w hs-flp 1 ; tubp- Gal4, UAS-mCD8GFP LL5 /CyO, act-gfp JMR1 ; FRT82B, tub-gal80 LL3 (Bello et al., 2003) to gcm-lacz ra87 /CyO, act-gfp JMR1 ; FRT82B males. To generate clones misexpressing gcm, we mated elav-gal4 C155, hs-flp 1 ; UAS-mCD8GFP LL5 /CyO, actgfp JMR1 ; FRT82B tub-gal80 LL3 to UAS-gcm m24a /CyO, act-gfp JMR1 ; FRT82B (UASgcm m24a from Bernardoni et al., 1998). To induce gcm loss-of-function clones, we mated y w hs-flp 1 ; FRT40A, tubp-gal80 LL10 /CyO, act-gfp JMR1 ; tubp-gal4 LL7, UASmCD8GFP LL6 (Bello et al., 2006) to FRT40A, gcm N7-4 /CyO, act-gfp JMR1. Eggs were collected for 2-4 hours, grown to first larval instar (22-30 hours after egg laying, AEL), plates immersed in a 37 C waterbath for 5 minutes (sparse wild type clones) or up to 30 minutes (other clones), and grown to the desired stage at 25 C. When recovering clones from wandering L3 larvae, some bottles were raised at 18 C during third instar to delay development until dissection. When recovering clones from earlier stages, larvae were grown at 25 C throughout, and kept at a maximum density of 170 larvae per bottle to avoid developmental delay due to food competition and to ensure exact staging. Viktorin et al. 6
8 Immunohistochemistry and in situ hybridization Brains were dissected in ice-cold PBS and fixed in 2% Paraformaldehyde for minutes at room temperature, washed several times in PBS/0.5% Triton X-100, and preincubated in PBS containing 0.5% Triton X-100 and 10% normal goat serum. Antibodies were incubated overnight at 4 C. We used chicken anti-gfp 1:500 (ab13970, Abcam, Cambridge, UK), rabbit anti-beta-galactosidase 1:500 (55976, MP Biomedicals, Solon, Ohio, USA), mouse anti-neurotactin 1:20 (BP106, DSHB, Iowa City, Iowa, USA), rat anti-elav 1:30 (7E8A10, DSHB), mouse anti-repo 1:30 (8D12, DSHB), rabbit anti-repo 1:400 (kindly provided by Veronica Rodrigues), rabbit antiphospho histone H3 (Ser10, , Millipore, Temecula, CA, USA), rat anti- Deadpan and rabbit anti-asense (both kind gifts from Cheng-Yu Lee), and Alexaconjugated secondary antibodies 1:300 (A11039, A21247, A11036, A11031, A21244, Molecular Probes, Eugene, OR, USA). In situ hybridization was performed as described previously (Soustelle et al., 2007). Identification of dorsomedial type II lineages DM1 was uniquely identified in Nrt/BP106 staining as the dorsoanterior-most large lineage in late third instar brains. DM5 was identified by a unique gcm-lacz and Repo-positive cell cluster within the lineage (Figs 1D, 5E). Other DM lineages were identified by their position relative to DM1 and DM5, their very large cell number (Bello et al., 2008), complex axon branching pattern (Izergina et al., 2009), and location at the posterior/medial brain surface. In younger larvae, DM1-3 lineages were recognized by their similar shapes and positions compared to those in wandering larvae. In addition, they were confirmed to be Type II lineages by weak or absent asense staining in the neuroblast, the presence of numerous asense- or deadpan- Viktorin et al. 7
9 positive cells apart from the neuroblast, or equally numerous prospero- and elavnegative cells throughout the lineage. Microscopy and image processing Fluorescent images were recorded on a Leica TCS SP5 confocal microscope, and processed using Fiji (Schindelin, 2008) or ImageJ (Rasband, ). All adjustments were linear and were performed on whole images. Cells were counted using the CellCounter plugin for Fiji/ImageJ (Kurt De Vos). Viktorin et al. 8
10 Results Postembryonic development of neurons and glial cells in the central brain During postembryonic development, the majority of the neurons (95%) that make up the adult central brain are generated by approximately 100 pairs of neural stem celllike neuroblasts, each of which generate a specific lineage of neural progeny (reviewed in Hartenstein et al, 2008). Figure 1A shows the neuroblast-derived lineages in the central brain of the third larval instar. In addition to neurons, a large number of glial cells are also generated postembryonically in the central brain (Fig. 1B,C). Newly generated glia express both gcm-lacz ra87 and the glial identity marker Repo, whereas older (or gcm-independent) glia express only Repo (Fig. 1B,C). Among the 100 central brain neuroblasts in each brain hemisphere, there are a total of eight identified type II neuroblasts, and six of these occupy most of the posterior medial edge of the hemisphere (Fig. 1D,E). Because they are easier to identify, most studies of type II neuroblasts have focused on the six posterior medially located lineages. These six lineages have been termed DM1-DM6 by Bello et al. (2008), and tentatively correspond to the DPMm1 (DM1), DPMpm1 (DM2), DPMpm2 (DM3), CM4 (DM4), CM3 (DM5), and CM1 (DM6) lineages (Pereanu and Hartenstein, 2006; Pereanu et al., 2011). A previous analysis of the postmitotic cells generated in these six identified type II neuroblast lineages during postembryonic development indicates that the majority of these are neurons, but glial cells also present (Izergina et al., 2009). This is supported by the observation that some neuroblast lineage-specific Gal4 lines label glial cells in DM lineages (Fig. 1F,G). Viktorin et al. 9
11 These findings suggest that type II neuroblasts may in fact function as neuroglioblasts that produce glia in a novel mode involving INPs. To investigate this, we first required a method for specific labeling of all INP-derived cells in type II neuroblast lineages. INP progeny are labeled by erm-gal4-dependent lineage tracing In contrast to other progenitor cells in the brain, all mature and proliferating INPs express the Drosophila Fezf homolog earmuff (erm) and can be reliably labeled by an erm-gal4 line (Weng et al., 2010; Pfeiffer et al., 2008). Although the erm-gal4 line labels all mature INPs of the type II neuroblast lineages, it does not reveal all of their progeny (see below). However, by combining the erm-gal4 line with the Gal4/flpout based cell lineage tracing methodology G-Trace, the INPs and their entire (clonal) set of progeny in the developing brain can be labeled (Fig. 2A,B). In G-Trace, two fluorescent reporters differentially reveal real-time Gal4 expression in precursor cells versus flp-out based expression in all of the cells that are clonally derived from these precursors (Evans et al., 2009). In experiments in which erm-gal4 is coupled with G-Trace for lineage tracing, three populations of labeled cells are seen in the brain hemispheres of the third instar larva (Fig. 2C,D). First, large groups of labeled cells are seen at the dorsomedial midline of each brain hemisphere. These labeled midline cells represent progeny of the six medial type II neuroblasts. Because they are easily identified, and because sparse MARCM clones are recovered frequently enough to allow conclusions about the association with migratory glia derived from them, these dorsomedial cell groups are the focus of this investigation. Second, smaller groups of more laterally located cells Viktorin et al. 10
12 are manifest in each brain hemisphere; they represent the progeny of the two lateral type II neuroblasts. Third, numerous labeled cells are located in the developing optic lobes; their precursors are unknown. Other lineages in the central brain are not labeled by erm-gal4-dependent flp-out, indicating that few, if any, non-type IIderived cells express erm-gal4 in the central brain at the stages investigated. Previous studies have shown that INP cell bodies are clustered in the vicinity of their parent type II neuroblasts near the cortical surface of the brain hemisphere, while the cell bodies of their neural progeny extend in a clustered array away from the INPs (and their neuroblast) towards the developing neuropile (Bello et al., 2008; Weng et al., 2010). Given this spatial array of the erm-positive INPs and their erm-negative neural progeny, spatially distinct expression of the two labels in erm-gal4 G-Trace experiments is expected. This is the case (Fig. 2E,F). Real-time erm-gal4 expression (red/orange label) is concentrated in a small group of cell bodies clustered near the cortical surface (all INPs), while flip-out based clonal expression (green label) reveals a much larger group of labeled cell bodies (most INPs and all of their clonal progeny) that extend towards the developing neuropile. These findings confirm previous observations on the spatial location of INPs versus their differentiated neural progeny in type II lineages and also demonstrate that (realtime) erm-gal4 only labels a subset of the total number of cells that comprise these lineages. INP lineages can give rise to glial as well as neuronal progeny Viktorin et al. 11
13 During postembryonic development, the six type II neuroblasts generate large lineages, and most of the differentiated cells in these lineages are adult-specific neural cells that express the neuronal marker Elav (Bello et al., 2008; Boone and Doe, 2008; Bowman et al., 2008; Izergina et al., 2009). The cell bodies of these neurons remain clustered together with their neuroblast of origin in the cortical cell layer. In addition to numerous ( ) neurons, type II lineages in the late larval brain have also been observed to contain a small number (10-15) of postembryonically generated glial cells that express Repo (Izergina et al., 2009). However, the mode of gliogenesis that gives rise to these glial cells in type II neuroblast lineages as well as the mature phenotype of these glial cells are not known. To determine if these glial cells are generated via INPs, we combined erm-gal4 G-Trace experiments with anti-repo immunolabeling. Figure 3 shows the results of this type of experiment in three optical sections taken at different depths through the late third larval instar brain. In all three sections, two large clusters of erm-gal4 lineage-labeled cells are visible that represent cells of INPderived lineages, and a subset of these cells is Repo-positive indicating that they are glial cells belonging to INP lineages. These INP-derived glial cells are located closer to the midline commissural region and are more spatially dispersed towards the neuropile than the non-glial cell bodies of the INP lineages that remain peripherally clustered in the cortical cell layer. While the INP-derived glial cells are not the only Repo-positive cells in this part of the larval brain, they do represent the majority of the glial cells in this region. These findings indicate that glial cells as well as neurons can be generated via INPs in type II neuroblast lineages. To confirm this, we generated labeled INP clones in the Viktorin et al. 12
14 six medial type II neuroblast lineages using the MARCM technique (Lee and Luo, 1999). In addition to neuroblast clones, single-cell clones and two-cell clones, INP clones represent a fourth type of MARCM clone which consists of more than two cells but lacks a neuroblast and can be generated if the somatic recombination event takes place in the neuroblast (for details, see Bello et al., 2008; Boone and Doe, 2008; Bowman et al., 2008). Mitotic recombination was induced at the first larval instar stage and labeled clones were examined at the mid- and late third larval instar stage. Several different types of INP clones were found. First, we recovered INP clones that contained both neurons and gcm-lacz-expressing glial cells (Fig. 4A, B, C). (In the type II lineages of the third larval instar, virtually all Repo-expressing glial cells also express gcm-lacz, indicating that they are newly specified; Fig. 1B and data not shown.) These mixed neuronal/glial INP clones usually contained 8-9 cells, of which 1-3 glial cells were found in a given clone. Mixed neuronal/glial cell clones were generally located within the DM1, DM2 and DM3 lineages. Second, we recovered INP clones of similar size in which all of the cells were neurons (Fig. 4D,F). Neuronal INP clones were found predominantly in the region occupied by the lineages DM4 (Fig. 4D) and DM6 (Fig. 4F). In addition to these two types of INP clones, a third multicellular clone type consisting entirely of glial cells was sometimes recovered in the area laterally adjacent to the DM5 lineage (Fig. 4E). Together with the results of erm-gal4-based lineage tracing, these clonal MARCM experiments demonstrate that INPs can give rise to both neurons and glial cells. While some of the INPs generate neuronal lineages, many other INPs generate mixed lineages composed of both neuronal and glial cells. This indicates that a novel form Viktorin et al. 13
15 of gliogenesis involving transit amplifying secondary progenitors operates in the type II neuroblast lineages. This, in turn, implies that type II neuroblasts have features of multipotent neural stem cells that can generate both neuronal and glial progeny though transit amplifying intermediate progenitors. Lineage-specific heterogeneity of INP-derived progeny in type II neuroblast lineages The relative frequency with which neuronal INP clones versus mixed neuronal/glial INP clones were recovered varied in different regions of the dorsomedial brain. This suggests that the type II neuroblasts might be heterogeneous in the number and/or type of glial cells generated via their INPs. To investigate this, we recovered MARCM labeled clones of entire type II lineages (neuroblast clones) induced at the first larval instar stage, and analyzed the glial cells comprised in each lineage in wandering third instar brains. The cellular resolution obtained with this type of clonal analysis is documented in single optical sections for the DM1 lineage (and for a control type I lineage) in Figure S1. In these single optical sections, the majority of cells MARCM-labeled with GFP are Elav-expressing neurons whose cell bodies are localized together in a cluster extending from the non-neural progenitor region towards the brain midline. In contrast, only a small set of glial cells is GFP labeled and these are localized at the distal end of the labeled clone near the brain midline. The overall morphology and relative position in the brain of the entire MARCMlabeled clone for each of the six type II neuroblast lineages (DM1-DM6) was highly reproducible, making identification of all six type II lineages in the larval brain possible (Fig. 5A-F, see Materials and Methods). Analysis of the number and relative Viktorin et al. 14
16 position of glial cells within each type II lineage revealed a marked heterogeneity. This is documented in Table 1 which gives the total number of glial cells in each of the six lineages, and in Figure 5A -F in which the position of the gcm-labeled glial cell bodies comprised in each DM lineage is presented semi-schematically. The DM1, DM2 and DM3 lineages each contain glial cells and most of these are clustered together in the part of the lineage that is distal to the neuroblast and close to the brain midline. These glia make up the majority of newly formed glia around the developing commissure that essentially corresponds to the fan-shaped body primordium, as well as the glia around the prospective protocerebral bridge. In contrast, only a small number of glial cells are seen in the DM4 lineage, and the DM6 rarely contains any glial cells at all. The DM5 lineage differs from the others in that it contains a larger number (average of 35) of glial cells, some of which are closely associated with the main cluster of neural cells, while others are more dispersed and positioned more laterally in the brain hemisphere. DM5-derived glia are set apart from the central complex primordia. Taken together, the positions of DM-derived glia cover the entire region of nascent glia in the medial central brain of the third instar larva. These results confirm the specificity of the erm-gal4 line and flp-out experiments, suggesting that most of those glia originate from type II lineages. Progression of glial differentiation in type II neuroblast-derived lineages According to previous reports, glia numbers in the larval central brain remain relatively constant during early instars and increase rapidly during the third larval instar (Pereanu et al., 2005). We assessed the first appearance of glia in the dorsomedial region by gcm in situ hybridization as well as gcm-lacz and Repo immunolabeling in larval brains (Fig. S2). In late L2 larvae, dorso-medial glia were Viktorin et al. 15
17 visible only by in situ hybridization but not by gcm-lacz expression (Figure S2A,B). The earliest gcm-lacz expression appeared in L3 larvae shortly after the L2/L3 transition (Figure S2C). Most of these glia already expressed Repo at that stage (Fig. S2C'). To investigate how DM lineages form these early glia and how their population expands, we induced MARCM clones in newly hatched larvae and recovered them between late L2 and mid-l3 stages. We focused on the lineages DM1, DM2, and DM3 that form most of the glia in the dorsomedial region and are easily distinguished from surrounding lineages in younger brains. L2 larval brains recovered during the L2/L3 transition (65-71 hours after egg laying, AEL), no DM1-3 MARCM clones or INP clones from the DM1-3 region contained glial cells (Table 2, Fig. 6). In brains from hour old L3 larvae recovered during the same experiments, none of the DM clones but 50% of INP clones contained glia. At hours AEL, 20% of DM1-3 clones and 70% of INP clones contained glia. From 85 hours AEL on, all DM1-3 lineages and INP clones from the DM1-3 region contained glia. The number of glia per DM1-3 lineage increased from 6±4 glial cells at 85 hours AEL to 14±5 glial cells at 96 hours AEL, which roughly corresponds to the number of DM1-3 glia in wandering L3 brains (15±5 glial cells, Table 1). Similarly, INP clones increased in size and glial content from 5±1 cells including 0-1 glia to 9±2 cells including 3±1 glial cells (Table 2). Thus the appearance of glia in DM1-3 roughly coincides with the first appearance of glia in the dorso-medial region, indicating that at least some of these glia are postembryonic. Glial numbers increase most rapidly during the first half of the third larval instar. Viktorin et al. 16
18 Next we investigated how gliogenesis is related to the developmental progression of type II lineages. We co-labeled DM and INP MARCM clones with the glial markers gcm-lacz or Repo, and with the neuroblast/inp markers Deadpan (Dpn) or Asense (Ase). We found no overlap between neuroblast and glial marker expression in MARCM clones, indicating that glial markers appear only in differentiating glia and not in their precursor cells (Fig. S1C, Fig. 6B). Furthermore, of 23 INP clones colabeled for glial markers and either Dpn or Ase, only one clone (recovered at 69 hours AEL) contained both a glial cell and a Dpn-expressing cell. The other clones either contained 1-2 cells expressing a neuroblast marker but no glial cells, or contained glial cells, but no cells expressing neuroblast markers. This suggests that within an INP lineage, glia start to be formed around the time when the INP stops expressing neuroblast markers. We were interested in how the formation of type II-derived glia correlated with the expression of cell and neural differentiation markers Prospero (Pros) and Elav in these lineages. Generally, we found that glial markers Repo/gcm-lacZ and Prospero, or Elav, are expressed in a mutually exclusive way (Fig. S1, Fig. 7). However, in younger brains we found several instances of co-expression (Fig. 6C, Fig. 7). During early L3 stage, when glial markers first appear, we recovered 2 of 4 INP clones that contained cells co-expressing gcm-lacz and Elav (Figure 7A), and 5 of 7 DM neuroblast and INP MARCM clones that contained cells co-expressing gcmlacz/repo and Prospero (Figure 7B-D), as well as numerous examples of coexpressing cells outside of clones in the DM region (data not shown). In such coexpressing cells, especially glial markers were expressed more weakly than in neighboring cells (Figs. 6C,7A,C,D ), in accordance with a possible transition to Viktorin et al. 17
19 glial marker gene expression. Notably, not all cells that weakly expressed gcm-lacz also co-expressed Prospero or Elav (Fig. 7A and data not shown), indicating that the transition time is either very short, or that not all glia differentiate from Pros- or Elavpositive precursors. Together these results suggest that at least some type II-derived glial precursors express Elav and/or Prospero when they start to express glial markers, and turn them off rapidly as glial differentiation proceeds. INP-derived glial cells associate with the developing central complex During larval development, the neurons of the six medial type II lineages fasciculate to form several secondary axon tracts and some of these project to the dorsomedial commissure of the larval brain, which is part of the larval primordium of the central complex (Izergina et al., 2009; Young and Armstrong, 2010; Pereanu et al., 2011). During subsequent pupal development, as the central complex primordium grows and differentiates, these commissural projections arborize profusely to form dendrites and axonal arbors that contribute to specific neuropile compartments of the adult central complex such as the fan-shaped body, ellipsoid body, protocerebral bridge, and noduli. Given that many of the neurons in type II neuroblast lineages contribute to the neuropile of the central complex, we wondered if the INP-derived glial cells from these lineages might also be involved in central complex development. By the end of larval development, the neurotactin-positive axons of several lineages including the six dorsomedial type II lineages form a plexus of commissural fibers that represents the central complex primordium. To determine if INP-derived glial cells are associated with this central complex primordium, we combined erm-gal4 G- Trace lineage labeling with anti-neurotactin (BP106) and anti-repo immunolabeling. Viktorin et al. 18
20 Figure 8 shows the results of this type of experiment in the early pupal central brain (1-2 hours APF, After Puparium Formation) in three optical sections taken through the central commissure primordium. In all cases, Repo-positive glial cells that derive lineally from INPs are closely associated with the neuropile primordia of the fanshaped body (Fig. 8A-B) and the protocerebral bridge (Fig. 8C). They are seen aggregated at the two lateral entry zones to the fan-shaped body primordium, surrounding almost the entire midline domain of the primordium, and in some cases are intercalated in its fiber tracts. In addition, INP-derived glial nuclei clearly delineate the protocerebral bridge primordium. Although a few glial cells surrounding the primordia are not INP-derived, the clear majority of the glial cells associated with this central complex structure are INP lineal descendants. The close association of INP-derived glial cells with the developing central complex is also observed during subsequent pupal development in comparable experiments performed at 20 and 40 hours APF. At 20 hours APF, when the central complex has grown significantly in size and shows initial internal compartmentalization, its neuropile is surrounded by numerous INP-derived glial cells, and glial cells are also intercalated within the fiber bundles of the neuropile (Fig. 9A-D; Fig. S3). At 40 hours APF, when the major compartments of the adult central complex, the fanshaped body, ellipsoid body, noduli and protocerebral bridge, have become apparent, INP-derived glial cells are seen surrounding, delimiting and intercalated into all of these compartments (Fig. 9 E-H, Fig. S3). A comparable arrangement of INP-derived glia was seen in the central complex at 75h APF (data not shown). Indeed most of the glial cell bodies associated with in the neuropile compartments of the central complex throughout its development appear to be INP-derived. Based on their appearance and Viktorin et al. 19
21 location within the central complex neuropile, the INP-derived glial cells are likely to be neuropile glia of the ensheathing (non-astrocyte) type (Awasaki et al., 2008). This is confirmed by labeling the INP-derived glial cells in the developing central complex with membrane-targeted mcd8-gfp, which reveals the typical spindle-like morphology of this type of glia (Fig. S4). We have recovered dorso-medially located astrocyte-like glia as well as interhemispheric ring glia (Simon et al., 1998) in our MARCM experiments (both have much larger nuclei than DM-derived glia and express high levels of Prospero and glial markers), but not in association with neuroblast or INP lineages (data not shown). Taken together, these findings indicate that INP-derived glial cells of the type II neuroblast lineages differentiate into ensheathing neuropile glia of the central complex. Thus, the type II neuroblasts via their INPs not only give rise to neuronal progeny that contributes to the central complex neuropile they also generate the glial progeny that ensheaths this neuropile. INP-derived glial cells proliferate locally in the developing central complex Quantification of the number of INP-derived glial cells associated with the developing central complex indicates that a fourfold increase in number occurs during pupal development between 0h (50 glial cells) and 40h APF (200 glial cells). This suggests that the INP-derived glial cells associated with the central complex might be capable of proliferating locally as its neuropile expands and differentiates. To determine if INP-derived glial cells do proliferate in the developing central complex, we combined erm-gal4 G-Trace lineage labeling with anti-ph3 (phospho- Viktorin et al. 20
22 histone H3) and anti-repo immunolabeling. Figure 10 shows results of these experiments in the pupal central brain at 20 hours APF. Several INP-derived glial cells surrounding the central complex are labeled with anti-ph3 indicating that they are mitotically active. At that stage, up to 12 INP-derived glial cells around the developing fan-shaped body were in mitosis (6±5 cells, n=7 brains). Similarly, at 0-3 hours APF, up to 8 mitotically active cells (3±3 cells, n=10 brains) surrounded the fan-shaped body primordium. These findings imply that the population of INP-derived glial cells associated with the central complex expands during pupal development through local proliferation. Thus, gliogenesis in these lineages occurs in two distinct modes both of which amplify proliferation. While glial cells are initially generated by type II neuroblasts via transient amplifying INPs, they subsequently amplify their number through local mitotic activity as the central complex neuropile differentiates and forms the compartments characteristic of its adult structure. The gcm gene controls the development of INP-derived glial cells Given that the glial cells studied here differ developmentally from all other glia in the brain in that they derive from INP generating type II neuroblasts, we investigated if their generation required gcm, a key regulator in the development of many (but not all) glial cell types (Jones et al., 1995; Hosoya et al., 1995; Vincent et al., 1996; Awasaki et al., 2008). For this, we carried out clonal gain-of-function (misexpression) and loss-of-function experiments using MARCM. Clones were induced at first larval instar and recovered in late third larval instar. Viktorin et al. 21
23 For gcm gain-of-function experiments, either elav-gal4 or tub-gal4, both of which drive reporter expression throughout secondary neuroblast lineages (Dumstrei et al., 2003; Bello et al., 2006), was used to misexpress UAS-gcm in MARCM-labeled neuroblast clones. In all cases, misexpression of gcm in type II neuroblast clones resulted in a marked increase of Repo-positive cells (Fig. 11A,B and data not shown) % of the cells in these MARCM-labeled clones expressed Repo (33±5%, n=8 clones), implying that gcm misexpression leads to a marked increase in the number of glial cells in type II neuroblast clones. Similar findings were obtained by driving misexpression of gcm in INPs with erm-gal4 (data not shown). In most type I neuroblast lineages of the central brain that do not give rise to Repopositive cells, misexpression of gcm also leads to formation of few Repo-positive cells (Fig. 11C,D). Thus, gcm is gliogenic in both type I and type II lineages, but the latter appear to be more sensitive to misexpression of gcm. For gcm loss-of-function experiments, we used MARCM to generate mutant DM1, DM2, or DM3 neuroblast lineages that were homozygous for the gcm N7-4 allele. The resulting mutant neuroblast clones consistently showed a loss of glial cells. Repopositive glial cells were completely absent within gcm N7-4 mutant clones (n=7), and all distal clonal progeny expressed the neuronal marker Elav (Fig. 11E-H). Axonal patterns in those clones appeared normal, which indicates that glia were not required for the establishment and proper fasciculation of secondary axon tracts. These finding show that gcm is necessary and sufficient for INP-derived glial cell formation in type II lineages, and that glia from DM1-3 mutant for gcm assume a neural fate. Viktorin et al. 22
24 Discussion In this report we use a combination of genetic lineage tracing, clonal MARCM techniques and molecular labeling to study the developmental mechanisms that give rise to the glial cells of the amplifying type II neuroblast lineages. Our findings uncover a novel mode of neurogliogenesis in these lineages that involves transit amplifying INPs, which can generate glial cells as well as neurons. Our analysis also shows that lineal INP-derived glial and neuronal cells both make major contributions to the central complex. INP-derived neurons project into the neuropile compartments of the central complex and INP-derived glial cells surround and delimit these compartments while undergoing clonal expansion through local proliferation. In the following we discuss the implications of these findings. Gliogenesis in type II neuroblast lineages involves transit amplifying progenitor cells The majority of the glial cells in the adult brain are generated postembryonically. Hitherto unidentified neuroglioblasts have been postulated to give rise to the bulk of these adult-specific glial cells, although some of these arise via proliferative glial cell divisions (Pereanu et al., 2005; Awasaki et al., 2008). Here we report the identification of type II neuroblasts as neural stem cells with neuroglioblast function in postembryonic development of the central brain. Previous work has shown that these amplifying type II neuroblasts augment proliferation through the generation of INPs resulting in the generation of remarkably large neuronal lineages (Bello et al., 2008; Boone and Doe, 2008; Bowman et al., 2008). Our data indicate that type II Viktorin et al. 23
25 neuroblasts also generate glial progeny through INPs and, hence, reveal a novel form of CNS neurogliogenesis that involves transit amplifying cells (Fig. 12). Although type II neuroblasts represent a new type of neuroglioblast in Drosophila brain development, the six identified type II neuroblasts are heterogeneous in terms of their gliogenic activity. While four of these neuroblasts generate comparable numbers of glial cells, a fifth neuroblast (DM4) gives rise to only a few glial cells and the sixth (DM6) rarely generates glia during larval development. This heterogeneity in gliogenic activity is also reflected in the INPs of these type II lineages. Thus, while mixed glial/neuronal INP clones were recovered for most type II lineages, all of the INP clones that contained glia in the DM5 lineage were purely glial, and no glial INP clones were recovered for DM6. Despite this heterogeneity, the generation of INPderived glial cells in all of these type II lineages appears dependent on the gcm gene. Indeed, this seems to be feature common to most glial progenitors in the embryonic and postembryonic CNS (Jones et al., 1995; Hosoya et al., 1995; Vincent et al., 1996; Awasaki et al., 2008). While glia can derive from the very first larval INPs produced by DM neuroblasts, glia differentiate late in the progression of the lineage, at a time when neuroblast markers are no longer present in the INP. At that time, lineages already contain large amounts of differentiating cells, positive for Prospero and/or the neural differentiation marker Elav. Interestingly, both Prospero and Elav are also found in many young glia, some of them verified to be part of Type II-derived lineages. This co-expression is reminiscent of embryonic glia that have been reported to transiently express Elav (Berger et al., 2007), which may thus be a common feature of glial cells also in the Viktorin et al. 24
26 larval brain. In addition, there may be Prospero-positive, gliogenic ganglion mother cells that could divide symmetrically to contribute to the variable number of glia observed in late larval INP lineages. Neural stem cells in the mammalian brain, notably the radial glia of the cortex, also represent mixed progenitors that can give rise to both neuronal and glial cells in different proliferative modes, and one of these proliferative modes involves transit amplifying INPs (Kriegstein and Alvarez-Buylla, 2009; Miyata et al., 2010). Moreover, some of the amplifying INPs involved in mammalian neocortex development are thought to give rise to neuronal progeny while others give rise to glial progeny. The amplification of proliferation through INPs has been postulated to be fundamental for the increase in cortical size during evolution (Kriegstein et al., 2006). The fact that a very comparable mode of INP-dependent proliferation operates in the generation of complex brain architecture in Drosophila suggests that this might represent a general strategy for increased size and complexity in brain development and evolution. Multipotent neural stem cells generate neurons and glial cells in central complex development Previous work has shown that a large subset of the neurons generated by type II neuroblasts contributes to the development of the central complex (Izergina et al., 2009; Bayraktar et al., 2010; Pereanu et al., 2011). The data presented here indicate that the INP-derived glial cells from the type II lineages are also involved in central complex development. INP-derived glial cell bodies associate with the larval primordium and, throughout pupal development; they surround and delimit all of the Viktorin et al. 25
27 compartments of the differentiating central complex neuropile. Thus neural and glial cells from the same neuroblast lineage participate in the development of the same complex brain neuropile. This reveals a remarkable multipotential nature of the type II neuroblasts; they are neural stem cells that have the potential to generate both neural and glial cells of one and the same complex brain structure. Neuroblast lineages have been viewed as units of projection in that the neurons of a given lineage often project their axons along a common trajectory and contribute to the formation of a common neuropile; this is exemplified by the four neuroblast lineages that give rise to the intrinsic cells of the mushroom body neuropile (Hartenstein et al., 2008; Ito and Awasaki, 2008). The neurons of the type II neuroblast lineages do not strictly conform to this notion, since subsets of neurons in the lineage project to different parts of the brain (Izergina et al., 2009). However, for the large subset of neurons in the type II lineages that project to the developing central complex, the notion of a lineal unit of projection is valid. Indeed this concept can be expanded to include the lineally related glial cells that also contribute to the development of the same neuropile compartment. Local proliferation of INP-derived glial cells during postembryonic brain development In type II neuroblast clones, most of the INPs, as well as the cell bodies of the neurons that they produce, remain clustered together in the peripheral cell body layer of the brain (Bello et al., 2008; Boone and Doe, 2008; Bowman et al., 2008; Weng et al., 2010). Although a few of the INP-derived glial cells are also found in these clusters, most are not. During larval and pupal development, the majority of the INP-derived Viktorin et al. 26
28 glial cells are found in or near midline commissural structures such as the central complex precursor (late larva) or the developing central complex neuropile (pupa). One reason for this is that INP-derived glial cells probably migrate away from their site of origin to a different site of final differentiation. Migration of glial cells during CNS development is a common feature in many species and has been studied in detail in the developing ventral nerve cord and optic lobes of Drosophila (Klämbt, 2009). Migration of glial cells has also been reported in postembryonic development of the central brain, and in some cases the migrating cells appear to form clusters suggesting that they might derive from common progenitors (Hartenstein et al., 1998; Awasaki et al, 2008). Another reason for the fact that so many INP-derived glial cells are found in or near the developing central complex is that they proliferate locally. This implies that INPderived glial cells can undergo clonal expansion in the neuropile. Clonal expansion has been described for the perineurial glial cells localized on the surface of the brain and has also been postulated to take place during the postembryonic development of neuropile glial cells (Pereanu et al., 2005; Awasaki et al., 2008). Since INP-derived glial cells undergo substantial (at least fourfold) proliferative clonal expansion, it will be important to determine what controls their mitotic activity. In view of the vulnerability of the amplifying type II neuroblast lineages to overproliferation and brain tumor formation, a tight control of self-renewing glial cell proliferation in these lineages is likely to be essential. Viktorin et al. 27
29 Acknowledgements We thank Utpal Banerjee, Bruno Bello, Cory Evans, Cheng-Yu Lee, Barrett Pfeiffer, Veronica Rodrigues, Jerry Rubin, the Bloomington Stock Center and the DSHB for strains and antibodies, Volker Hartenstein for assistance in matching type II lineages with current nomenclature, and Susanne Flister for excellent technical help. This work was supported by SNSF grant 31003A12488/1 and SNSF/NFP63 Neural stem cells and regenerative medicine grant /1 to HR. Work in the laboratory of AG was supported by INSERM, CNRS, UDS, Hôpital de Strasbourg, ARC, INCA and ANR; AP was supported by FRM. Viktorin et al. 28
30 References Awasaki, T., Lai, S.L., Ito, K., Lee, T., Organization and postembryonic development of glial cells in the adult central brain of Drosophila. J Neurosci 28, Bayraktar, O.A., Boone, J.Q., Drummond, M.L., Doe, C.Q., Drosophila type II neuroblast lineages keep Prospero levels low to generate large clones that contribute to the adult brain central complex. Neural Dev 5, 26. Beckervordersandforth, R.M., Rickert, C., Altenhein, B., Technau, G.M., Subtypes of glial cells in the Drosophila embryonic ventral nerve cord as related to lineage and gene expression. Mech Dev 125, Berger, C., Renner, S., Lüer, K., Technau, G.M., The commonly used marker ELAV is transiently expressed in neuroblasts and glial cells in the Drosophila embryonic CNS. Dev Dyn 236, Bello, B., Reichert, H., Hirth, F., The brain tumor gene negatively regulates neural progenitor cell proliferation in the larval central brain of Drosophila. Development 133, Bello, B.C., Hirth, F., Gould, A.P., A pulse of the Drosophila Hox protein Abdominal-A schedules the end of neural proliferation via neuroblast apoptosis. Neuron 37, Bello, B.C., Izergina, N., Caussinus, E., Reichert, H., Amplification of neural stem cell proliferation by intermediate progenitor cells in Drosophila brain development. Neural Dev 3, 5. Bernardoni, R., Miller, A.A., Giangrande, A., Glial differentiation does not require a neural ground state. Development 125, Boone, J.Q., Doe, C.Q., Identification of Drosophila type II neuroblast lineages containing transit amplifying ganglion mother cells. Dev Neurobiol 68, Bossing, T., Udolph, G., Doe, C.Q., Technau, G.M., The embryonic central nervous system lineages of Drosophila melanogaster. I. Neuroblast lineages derived from the ventral half of the neuroectoderm. Dev Biol 179, Bowman, S.K., Rolland, V., Betschinger, J., Kinsey, K.A., Emery, G., Knoblich, J.A., The tumor suppressors Brat and Numb regulate transit-amplifying neuroblast lineages in Drosophila. Dev Cell 14, Broadus, J., Skeath, J.B., Spana, E.P., Bossing, T., Technau, G., Doe, C.Q., New neuroblast markers and the origin of the acc/pcc neurons in the Drosophila central nervous system. Mech Dev 53, Doe, C.Q., Neural stem cells: balancing self-renewal with differentiation. Development 135, Dumstrei, K., Wang, F., Hartenstein, V., Role of DE-cadherin in neuroblast proliferation, neural morphogenesis, and axon tract formation in Drosophila larval brain development. J Neurosci 23, Evans, C.J., Olson, J.M., Ngo, K.T., Kim, E., Lee, N.E., Kuoy, E., Patananan, A.N., Sitz, D., Tran, P., Do, M.T., Yackle, K., Cespedes, A., Hartenstein, V., Call, G.B., Banerjee, U., G-TRACE: rapid Gal4-based cell lineage analysis in Drosophila. Nat Methods 6, Götz, M., Huttner, W.B., The cell biology of neurogenesis. Nat Rev Mol Cell Biol 6, Hartenstein, V., Nassif, C., Lekven, A., Embryonic development of the Drosophila brain. II. Pattern of glial cells. J Comp Neurol 402, Viktorin et al. 29
31 Hartenstein, V., Spindler, S., Pereanu, W., Fung, S., The development of the Drosophila larval brain. Adv Exp Med Biol 628, Haubensak, W., Attardo, A., Denk, W., Huttner, W.B., Neurons arise in the basal neuroepithelium of the early mammalian telencephalon: a major site of neurogenesis. Proc Natl Acad Sci U S A 101, Hosoya, T., Takizawa, K., Nitta, K., Hotta, Y., glial cells missing: a binary switch between neuronal and glial determination in Drosophila. Cell 82, Ito, K., Awasaki, T., Clonal unit architecture of the adult fly brain. Adv Exp Med Biol 628, Izergina, N., Balmer, J., Bello, B., Reichert, H., Postembryonic development of transit amplifying neuroblast lineages in the Drosophila brain. Neural Dev 4, 44. Jones, B.W., Fetter, R.D., Tear, G., Goodman, C.S., glial cells missing: a genetic switch that controls glial versus neuronal fate. Cell 82, Klämbt, C., Modes and regulation of glial migration in vertebrates and invertebrates. Nat Rev Neurosci 10, Knoblich, J.A., Mechanisms of asymmetric stem cell division. Cell 132, Kriegstein, A., Alvarez-Buylla, A., The glial nature of embryonic and adult neural stem cells. Annu Rev Neurosci 32, Kriegstein, A., Noctor, S., Martinez-Cerdeno, V., Patterns of neural stem and progenitor cell division may underlie evolutionary cortical expansion. Nat Rev Neurosci 7, Lee, T., Luo, L., Mosaic Analysis with a Repressible Cell Marker for Studies of Gene Function in Neuronal Morphogenesis. Neuron 22, Miller, F.D., Gauthier, A.S., Timing is everything: making neurons versus glia in the developing cortex. Neuron 54, Miyata, T., Kawaguchi, A., Saito, K., Kawano, M., Muto, T., Ogawa, M., Asymmetric production of surface-dividing and non-surface-dividing cortical progenitor cells. Development 131, Miyata, T., Kawaguchi, D., Kawaguchi, A., Gotoh, Y., Mechanisms that regulate the number of neurons during mouse neocortical development. Curr Opin Neurobiol 20, Noctor, S.C., Martinez-Cerdeno, V., Ivic, L., Kriegstein, A.R., Cortical neurons arise in symmetric and asymmetric division zones and migrate through specific phases. Nat Neurosci 7, Noctor, S.C., Martinez-Cerdeno, V., Kriegstein, A.R., Contribution of intermediate progenitor cells to cortical histogenesis. Arch Neurol 64, Pereanu, W., Hartenstein, V., Neural lineages of the Drosophila brain: a threedimensional digital atlas of the pattern of lineage location and projection at the late larval stage. J Neurosci 26, Pereanu, W., Shy, D., Hartenstein, V., Morphogenesis and proliferation of the larval brain glia in Drosophila. Dev Biol 283, Pereanu, W., Younossi-Hartenstein, A., Lovick, J., Spindler, S., Hartenstein, V., A lineage-based analysis of the development of the central complex of the Drosophila brain. J Comp Neurol 519: Pfeiffer, B.D., Jenett, A., Hammonds, A.S., Ngo, T.T., Misra, S., Murphy, C., Scully, A., Carlson, J.W., Wan, K.H., Laverty, T.R., Mungall, C., Svirskas, R., Viktorin et al. 30
32 Kadonaga, J.T., Doe, C.Q., Eisen, M.B., Celniker, S.E., Rubin, G.M., Tools for neuroanatomy and neurogenetics in Drosophila. Proc Natl Acad Sci U S A 105, Rasband, W.S., ImageJ, U. S. National Institutes of Health, Bethesda, Maryland, USA, Schmidt, H., Rickert, C., Bossing, T., Vef, O., Urban, J., Technau, G.M., The embryonic central nervous system lineages of Drosophila melanogaster. II. Neuroblast lineages derived from the dorsal part of the neuroectoderm. Dev Biol 189, Schindelin, J., Fiji is just ImageJ -- batteries included. ImageJ User and Developer Conference Skeath, J.B., Thor, S., Genetic control of Drosophila nerve cord development. Curr Opin Neurobiol 13, Simon, A., Boquet, I., Synguélakis, M., Préat, T., The Drosophila putative kinase Linotte (Derailed) prevents central brain axons from converging on a newly described interhemispheric ring. Mech Dev 76, Soustelle, L., Trousse, F., Jacques, C., Ceron, J., Cochard, P., Soula, C., Giangrande, A., Neurogenic role of Gcm transcription factors is conserved in chicken spinal cord. Development 134, Technau, G.M., Berger, C., Urbach, R., Generation of cell diversity and segmental pattern in the embryonic central nervous system of Drosophila. Dev Dyn 235, Van De Bor, V., Heitzler, P., Leger, S., Plessy, C., Giangrande, A., Precocious expression of the Glide/Gcm glial-promoting factor in Drosophila induces neurogenesis. Genetics 160, Vincent, S., Vonesch, J.L., Giangrande, A., Glide directs glial fate commitment and cell fate switch between neurones and glia. Development 122, Weng, M., Golden, K.L., Lee, C.Y., dfezf/earmuff maintains the restricted developmental potential of intermediate neural progenitors in Drosophila. Dev Cell 18, Weng, M., Lee, C.Y., Keeping neural progenitor cells on a short leash during Drosophila neurogenesis. Curr Opin Neurobiol. 21, 1-7. Young, J.M., Armstrong, J.D., Building the central complex in Drosophila: the generation and development of distinct neural subsets. J Comp Neurol 518, Viktorin et al. 31
33 Figure Legends Fig. 1. Central brain neuroblast lineages give rise to glial and neuronal progeny. (A-E) Wandering third instar larval brain (genotype insc-gal4, UAS-mCD8GFP / gcm-lacz ra87, 80 µm maximum intensity projection) labeled with Repo (blue) and gcm-lacz (red). (A-C) Within the region of central brain neuroblast lineages (A; insc>mcd8gfp), many newly formed glia are present (B,C; magenta overlap) expressing both Repo and gcm-lacz. They are positioned in the commissure (arrowheads) as well as medially (short arrows) and laterally (long arrows) in the central brain. Dotted line indicates border between central brain (CB) and optic lobe (OL). (D,E) DM1-DM6 lineages in the same brain; numbers indicate identified DM lineages. (F,G) insc-gal4, UAS-mCD8GFP homozygous larvae have Repo-positive cells (magenta) in GFP-labeled DM lineages (arrows). Numbers indicate tracts of identified DM lineages. Other glia (presumably surface, cortex, or astrocyte-like glia) are not GFP-labeled (asterisks). All figures present anterior to the top. Scale bars, 20 µm. Fig. 2. erm-gal4-dependent lineage tracing labels INP-derived progeny of type II neuroblast lineages. (A) Schematic of the type II neuroblast lineages in the larval central brain. (B) Expression of erm-gal4 in G-Trace flies (UAS-flp, ubi>*>ngfp, UAS-RFP) leads to erm-gal4 independent GFP expression in erm-gal4 expressing cells and their progeny. Real-time expression of erm-gal4 is reported by RFP expression. (C) erm-gal4 driven lineage tracing labels the six dorsomedial type II lineages, the two lateral type II lineages (asterisk) and cells in the optic lobe (OL; delimited by dashed lines). (D) Close-up of the DM lineages. (E,F) Close-up of the Viktorin et al. 32
34 DM1 region. Real-time expression (red) is located near the cortical surface, flip-out based clonal expression (green) is located in cells that extend further from the cortical surface towards the commissure. Scale bars, 25 µm. Fig. 3. erm-gal4 expressing INPs give rise to glial cells. (A-C) Three optical sections at different depths in the third larval instar brain of erm-gal4 flp-out (UASflp, ubi>*>ngfp) driven ngfp expression show the DM1 regions of both hemispheres. Most glia (Repo, magenta) are flp-out progeny of erm-gal4 expressing INPs (green; overlap white). erm-gal4-derived glia are indicated with white dotted outlines, glia not derived from erm-gal4 with orange dotted outlines. Scale bar, 25 µm. Fig. 4. Progeny of single INPs are composed of neurons and/or glia in a lineagespecific manner. tub-gal4 UAS-mCD8GFP / gcm-lacz ra87 - labeled MARCM clones of INPs within identified DM lineages. (A-C) INP clones derived from DM1-3 contain 8-9 cells in total, including one or two glial cells. (D,F) INP clones from DM4 and DM6 contain a similar number of cells but no glia. (E) DM5-derived subclones are positioned in the center of the hemisphere and are composed of only glial cells. Scale bar, 20 µm. Fig. 5. Lineage-specific heterogeneity of INP-derived glia. (A-E; upper panels) Maximum intensity projections of MARCM clones from lineages DM1-DM6, labeled with tubulin-gal4 UAS-mCD8GFP (white), and co-labeled with gcm-lacz (green) identify glial progeny of the DM neuroblasts. Clones were induced at hatching and recovered in wandering third instar. Insets in (A-E) show single confocal slices of Viktorin et al. 33
35 gcm-lacz positive nuclei (green) within the clone. (F) The gcm-lacz positive nuclei (magenta) were associated with DM6 axon tracts but not part of the clone. (A'-F'; lower panels) Semi-schematic representation of glial cell bodies located within the respective clones (green) or outside of the clones (magenta). All gcm-lacz positive nuclei of each particular brain in all confocal sections that are part of the corresponding maximum projection are shown. Brain hemispheres are outlined with dotted lines for orientation. Scale bar, 50 µm. Fig. 6. DM1/2 neuroblast lineages contain cells positive for Dpn, Prospero, and Elav when the first glia start to differentiate. (A-A''') DM1 or DM2 lineage in an L3 brain from the L2/L3 transition at 70 hours AEL, containing numerous Elavpositive cells but no glia. (B-B'') DM1 lineage from an L3 brain at 80 hours AEL containing numerous Deadpan-positive nuclei (example outlined in cyan) and one glial cell (magenta dotted outline). (C-C'') DM1 lineage at from an L3 brain at 85 hours AEL containing several glial cells (magenta dotted outline) and Prosperoexpressing cells (example outlined in cyan), as well as 1-2 cells coexpressing both (white dotted outline). Fig. 7. Glia differentiate from precursors that express Elav or Prospero. (A- A''') A single cell within an INP lineage from DM1 or DM2 coexpresses gcmlacz ra87 and Elav weakly (white dotted line), indicating a transition. Other cells that express gcm-lacz either strongly (magenta dotted line) or weakly (red dotted line) do not express Elav. (B-C'') A single cell within an INP lineage from DM2 or DM3 coexpresses gcm-lacz and Pros (white dotted line), indicating a transition. (D-D ) Another cell from the same MARCM clone that strongly expresses gcm-lacz does not Viktorin et al. 34
36 express Pros (magenta dotted outline). (C) and (D) represent single focal planes of the clone shown in (B) as a maximum intensity projection. Scale bars, 20 µm. Fig. 8. INP-derived glial cells are closely associated with the central complex primordium. (A-B) Two optical sections through the central commissure primordium in 1-2 hour old pupae show that erm-gal4 derived glia (UAS-flp, ubi>*>ngfp, green; Repo, magenta) are located in close association with the fiber tracts (BP106/Neurotactin, cyan) that constitute the fan-shaped body primordium (FB, orange outline in A'). (C) 30 µm posterior to the fan-shaped body, erm-gal4 derived glia outline the primordium of the protocerebral bridge (PB, orange outline in C'). Scale bar, 25 µm. Fig. 9. INP-derived glial cells are associated with the developing central complex throughout pupal development. (A-D) Single optical sections through the central complex primordium in a 20 hour old pupa are labeled with erm-gal4 G-trace (green), Repo (magenta), and Nrt/BP106 (cyan). INP-derived glia make up most of the glia surrounding the fan-shaped body at the level of its anterior surface (A,A'), the noduli (B,B'), the posterior chiasma (C,C'), and surround the protocerebral bridge (D,D'). (E-H) Single optical sections through the central complex primordium in a 40 hour old pupa are labeled with erm-gal4 G-trace (green), Repo (magenta), and nc82 (cyan). INP-derived glia are found on the anterior surface of the fan-shaped body up to the level of the ellipsoid body (E,E'), surrounding and in between fan-shaped body and noduli (F,F'), on the posterior surface of the fan-shaped body (G,G'), and outlining the protocerebral bridge (H,H'). EB, ellipsoid body; FB, fan-shaped body, NO, noduli, PB protocerebral bridge, pch, posterior chiasm. Scale bars, 20 µm. Viktorin et al. 35
37 Fig. 10. INP-derived glia surrounding the pupal fan-shaped body are mitotically active. (A-C) Single confocal section with three Repo-positive erm-gal4-derived central complex glia expressing the mitotic marker ph3 at 20 hours APF; inset and arrows denote dividing cells. (A) INP progeny (white) positive for ph3 (magenta); (B) INP progeny (green) positive for Repo (magenta); (C) Repo (white). Scale bar, 20 µm. Fig. 11. Type II lineages generate glia in a gcm-dependent manner. (A-D) Ectopic glia caused by misexpression of gcm in a type II (DM1) MARCM clone (A,B) and a type I clone (C,D). (A,B) A maximum intensity projection of the complete bilateral DM1 lineages shows numerous additional Repo-expressing cells in an elav-gal4, UAS-mCD8GFP, UAS-gcm m24a MARCM clone (right side, white dotted line) compared to its wild type counterpart (left side, orange dotted line). (C,D) A single confocal slice of a type I tub-gal4, UAS-mCD8GFP, UAS-gcm m24a MARCM clone shows ectopic Repo expression (magenta, single channel in D). (E-H) A gcm N7-4 mutant DM MARCM clone labeled with tub-gal4 UAS-mCD8GFP lacks Repo-positive cells. (E,F) Optical projection of all cells in the clone reveals no Repo positive cell (magenta) within the clone (dotted line). In the opposite hemisphere, glial cells in this region are present (orange dotted outline). (G,H) A single section of the same clone shows that all mutant cells express Elav (blue), indicating that loss of gcm causes a transformation of glial (Repo, magenta) into neuronal fate. Scale bars, 20 µm. Viktorin et al. 36
38 Fig. 12. Simplified summary scheme of glia formation in type II lineages. Type II neuroblasts generate INPs that give rise to either mixed neuronal and glial lineages (A), neuronal lineages (B), or glial lineages (C). gcm is necessary and sufficient for gliogenesis in these lineages and begins to be expressed late in the progression of the lineage. Table legends Table 1. Each type II DM lineage produces a specific number of glial cells. Glial cells were counted from identified MARCM labeled DM neuroblast clones. DM5- derived glia located near the midline and located laterally were counted separately. s.d., standard deviation; n, number of lineages counted. Table 2. Type II derived glia form during early third instar. Glial cells were counted from MARCM labeled DM neuroblast clones identifed as DM1, DM2, or DM3, and INP clones from the DM1-3 region. Rows 1-2: The percentage of lineages with glia identifies the beginning of glial production in lineages around hours AEL in early third instar. Lineages from second instar larvae of the same age did not contain glia. Neuroblast clones differentiate glia later than their INP sister clones induced at the same time. Row 3: The number of glia located within DM1-3 neuroblast clones increases rapidly until mid-l3, when the approximate number counted in wandering larvae is reached (compare with Table 1). Rows 4-5: In INP clones, glial numbers increase concomitantly. Cell counts are given in mean±s.d. Viktorin et al. 37
39 A B C CB OL insc>mcd8gfp gcm-lacz Repo gcm-lacz D 0!m E -23!m F 0!m G -19!m * * * 3 2 gcm-lacz Repo insc>mcd8gfp Repo insc>mcd8gfp 20!m
40 A OL OL B VNC C * * D * E F
41 A Repo 0!m B -10!m C -23!m A' B' C' erm>{ubi>>ngfp} A'' B'' C''
42 Figure 4 AClick here to download Figure: B fig4.pdf C D DM1 DM2 DM3 DM4 tub>gfp clone A' B' C' D' gcm-lacz E DM5 lateral E' F DM6 F' 20 m
43 tub>mcd8gfp MARCM gcm-lacz in clone / outside of clone A B C D DM1 DM2 DM3 DM4 DM5 DM6 A' B' C' D' E' F' 50!m E -23!m 0!m F
44 Figure 6 Click here to download Figure: fig6.pdf A B C 70 h L3 A' A'' A''' tub> mcd8gfp gcm-lacz Elav 80 h L3 B' B'' B''' tub> mcd8gfp Repo Dpn 85 h L3 C' C'' Repo tub> mcd8gfp +gcm-z C''' Pros
45 A Figure 7 A' A'' A''' Click here to download Figure: fig7.pdf tub> mcd8gfp gcm-lacz Elav B C C' C'' Repo+gcmZ Pros D D' D'' tub-mcd8gfp Repo+gcmZ Pros
46 A B anterior 0!m 7!m posterior 32!m C FB Repo BP106/Nrt erm>{ubi>>ngfp} PB A' FB B' C' PB A'' FB B'' C'' PB
47 Figure 9 Click here to download Figure: fig9.pdf anterior 20 h APF erm>{ubi>>ngfp} Repo BP106 A A' 0 m B B' FB EB FB E F 40 h APF erm>{ubi>>ngfp} Repo nc82 E' EB 0 m FB F' FB ant. 8 m NO NO 8 m NO NO C C' G G' posterior D 22 m 44 m D' BP106 FB pch PB H 21 m 32 m H' nc82 FB post. PB
48 Repo A Figure 10 Click here to download Figure: fig10.pdf 20 h APF ph3 erm>{ubi>>ngfp} B Repo erm>{ubi>>ngfp} C
49 A Figure 11 Click here to download Figure: fig11.pdf gcm N7-4 tub>mcd8gfp Repo B gcm N7-4 tub>mcd8gfp Elav C Repo 20 m BP106 elav>gcm, >mcd8gfp E F G H D tub>gcm Repo
50 A B C NB NB NB INP INP INP gcm gcm neurons neurons glia glia
51 Tables 1,2 Viktorin et al. Table 1 Mean ± s.d. (n 9 clones) DM1 DM2 DM3 DM4 DM5 DM5 lateral DM6 10 ± 2 20 ± 2 15 ± 2 2 ± 2 14 ± 4 20 ± ± 0.5 (0-1 glia) Table 2 1 percentage of DM1-3 clones with glia (n 10 clones) 2 percentage of INP1-3 clones with glia (n 11 clones) h L h L h L3 85 h L3 96 h L3 0 % 0 % 20 % 100 % 100 % 0 % 50 % 72 % 100 % 100 % 3 number of glia in DM1-3 clones (n 10 clones) 0 glia 0 glia 0.2±0.4 (0-1 glia) 6±4 glia 14±5 glia 4 number of cells in INP1-3 clones (n 11 clones) 5 number of glia in INP1-3 clones (n 11 clones) 5±1 cells 5±1 cells 5±1 cells 6±2 cells 9±2 cells 0 glia 1±1 glia 1±1 glia 3±1 glia 3±1 glia 1
52 Supplemental Figure legends Fig. S1. INP-derived glia express the glial marker gcm-lacz, but not the neuronal marker Elav. (A-D') Example of a type II lineage MARCM clone (green; white outline, identified as DM1) colabeled with gcm-lacz (magenta) and anti-elav (cyan). (A-D) Clone-derived cells that express gcm-lacz (magenta arrows) do not express Elav and are found far away from the neuroblast (orange outline) and its immediate, Elav-negative progeny (asterisks). (A'-D') The presence of Elavnegative cells (arrows) up to 10 cell diameters away from the neuroblast (orange outline copied from A-D) are indicative of type II lineages and correspond to intermediate progenitor cells and ganglion mother cells. (E-H) In a control type I neuroblast clone, Elav-negative cells (asterisks) are only found in close contact with the neuroblast (orange outline; out of focus, immediately above and touching the cells shown). Scale bar, 20 µm. Fig. S2. Glia in the DM region first appear during early L3. (A-C) Brains of L2 and L3 larvae during the L2-L3 transition around 70 hours AEL. (A) While gcm mrna is already detectable in L2 larvae by in situ hybridization, gcm-lacz ra87 expression in the DM1-3 region is not visible from L2 (B) and appears during L3 (C). Those cells already coexpress Repo (C', maximum projection of the central 71 µm excluding large, Repo-expressing surface glia overlying the glia shown). A brain containing both a DM1 and a DM3 MARCM clone, shown magnified in (C''), was chosen to indicate the DM1-3 region. The area of the clone is outlined in (C-C') to show the position of DM1-3 in relation to the newly formed glia. Note that none of Viktorin et al. Supp 1
53 the glia shown are part of the MARCM clones, as at that developmental time only IPC clones contain glia (see Table 2). Scale bars, 50 µm. Fig. S3. INP-derived glia surround the developing central complex primordium. (A-C) Confocal sections of erm-gal4 G-trace (green) and Repo (magenta) co-labeled brains at the level of the fan-shaped body primordium at 10 hours (A-A"), 20 hours (B-B'''), and 40 hours (C-C''') APF. Single confocal sections in A, B; projection of 5 µm confocal sections in C shows glia around the fan-shaped body primordium. Scale bar, 25 µm. Fig. S4. Membrane labeling of INP-derived glia and neurons at the level of the fan-shaped body primordium at 20 hours APF. (A-D) Single optical sections through the central complex primordium reveals membrane structures of erm-gal4 neuronal and glial progeny (erm-gal4, UAS-flp, act>*>gal4, UAS-mCD8GFP; A,B). All cell bodies (UAS-nLacZ; A,D) at this focal level are glial (Repo; A,C). The membrane structure, while containing axonal processes in addition to glial membranes (white in A,B), resembles that of ensheathing glia, as opposed to astrocyte-like glia, as reported in Awasaki et al. (2008). Scale bar, 20 µm. Viktorin et al. Supp 2
54 A Type II B * * * (DM1) * * * ** C * * * * * D * * * * * * 0 m tub>mcd8gfp clone Elav A' Type II B' C' (DM1) gcm-lacz D' E +20 m Type I F ** * * ** * * ** * * * * * * *** * G H 20 µm
55 A L2 B L2 C L3 C' C" DM1-3 DM1-3 DM1-3 DM5 DM5 DM5 gcm ISH gcm-nlacz gcm-nlacz Repo gcm-nlacz DM1+DM3 tub>mcd8gfp
56 Repo A B C 10h APF 20h APF 40h APF stack D'' 25!m stack A' B' C' D''' erm>{ubi>>ngfp} A'' B'' C''
57 A 20h APF B C 50 µm erm>{act>> mcd8gfp} D Repo erm>{act>> nlacz}
Developmental Biology
 Developmental iology 356 (2011) 553 565 ontents lists available at ScienceDirect Developmental iology journal homepage: www.elsevier.com/developmentalbiology Multipotent neural stem cells generate glial
Developmental iology 356 (2011) 553 565 ontents lists available at ScienceDirect Developmental iology journal homepage: www.elsevier.com/developmentalbiology Multipotent neural stem cells generate glial
glial cells missing and gcm2 Cell-autonomously Regulate Both Glial and Neuronal
 glial cells missing and gcm2 Cell-autonomously Regulate Both Glial and Neuronal Development in the Visual System of Drosophila Carole Chotard, Wendy Leung and Iris Salecker Supplemental Data Supplemental
glial cells missing and gcm2 Cell-autonomously Regulate Both Glial and Neuronal Development in the Visual System of Drosophila Carole Chotard, Wendy Leung and Iris Salecker Supplemental Data Supplemental
Postembryonic Development of Amplifying Neuroblast Lineages in the Drosophila Brain: Proliferation, Differentiation and Projection Patterns.
 Postembryonic Development of Amplifying Neuroblast Lineages in the Drosophila Brain: Proliferation, Differentiation and Projection Patterns. Inauguraldissertation zur Erlangung der Würde eines Doktors
Postembryonic Development of Amplifying Neuroblast Lineages in the Drosophila Brain: Proliferation, Differentiation and Projection Patterns. Inauguraldissertation zur Erlangung der Würde eines Doktors
Cell Birth and Death. Chapter Three
 Cell Birth and Death Chapter Three Neurogenesis All neurons and glial cells begin in the neural tube Differentiated into neurons rather than ectoderm based on factors we have already discussed If these
Cell Birth and Death Chapter Three Neurogenesis All neurons and glial cells begin in the neural tube Differentiated into neurons rather than ectoderm based on factors we have already discussed If these
Nature Neuroscience: doi: /nn Supplementary Figure 1
 Supplementary Figure 1 Expression of escargot (esg) and genetic approach for achieving IPC-specific knockdown. (a) esg MH766 -Gal4 UAS-cd8GFP (green) and esg-lacz B7-2-22 (red) show similar expression
Supplementary Figure 1 Expression of escargot (esg) and genetic approach for achieving IPC-specific knockdown. (a) esg MH766 -Gal4 UAS-cd8GFP (green) and esg-lacz B7-2-22 (red) show similar expression
The Tumor Suppressors Brat and Numb Regulate Transit-Amplifying Neuroblast Lineages in Drosophila
 Article The Tumor Suppressors Brat and Numb Regulate Transit-Amplifying Neuroblast Lineages in Drosophila Sarah K. Bowman, 1 Vivien Rolland, 1 Joerg Betschinger, 1,3 Kaolin A. Kinsey, 2 Gregory Emery,
Article The Tumor Suppressors Brat and Numb Regulate Transit-Amplifying Neuroblast Lineages in Drosophila Sarah K. Bowman, 1 Vivien Rolland, 1 Joerg Betschinger, 1,3 Kaolin A. Kinsey, 2 Gregory Emery,
Neurodevelopment II Structure Formation. Reading: BCP Chapter 23
 Neurodevelopment II Structure Formation Reading: BCP Chapter 23 Phases of Development Ovum + Sperm = Zygote Cell division (multiplication) Neurogenesis Induction of the neural plate Neural proliferation
Neurodevelopment II Structure Formation Reading: BCP Chapter 23 Phases of Development Ovum + Sperm = Zygote Cell division (multiplication) Neurogenesis Induction of the neural plate Neural proliferation
Supplemental Data SUPPLEMENTAL EXPERIMENTAL PROCEDURES
 Temporal transcription factors and their targets schedule the end of neural proliferation in Drosophila. Cédric Maurange, Louise Cheng and Alex P. Gould Supplemental Data SUPPLEMENTAL EXPERIMENTAL PROCEDURES
Temporal transcription factors and their targets schedule the end of neural proliferation in Drosophila. Cédric Maurange, Louise Cheng and Alex P. Gould Supplemental Data SUPPLEMENTAL EXPERIMENTAL PROCEDURES
Role of Notch signaling in establishing the hemilineages of secondary neurons in Drosophila melanogaster
 RESEARCH ARTICLE 53 Development 137, 53-61 (2010) doi:10.1242/dev.041749 Role of Notch signaling in establishing the hemilineages of secondary neurons in Drosophila melanogaster James W. Truman*,, Wanda
RESEARCH ARTICLE 53 Development 137, 53-61 (2010) doi:10.1242/dev.041749 Role of Notch signaling in establishing the hemilineages of secondary neurons in Drosophila melanogaster James W. Truman*,, Wanda
G-TRACE: rapid Gal4-based cell lineage analysis in Drosophila
 nature methods G-TRACE: rapid Gal4-based cell lineage analysis in Drosophila Cory J Evans, John M Olson, Kathy T Ngo, Eunha Kim, Noemi E Lee, Edward Kuoy, Alexander N Patananan, Daniel Sitz, PhuongThao
nature methods G-TRACE: rapid Gal4-based cell lineage analysis in Drosophila Cory J Evans, John M Olson, Kathy T Ngo, Eunha Kim, Noemi E Lee, Edward Kuoy, Alexander N Patananan, Daniel Sitz, PhuongThao
Cell migration in Drosophila optic lobe neurons is controlled by eyeless/pax6
 687 Development 138, 687-693 (2011) doi:10.1242/dev.056069 2011. Published by The Company of Biologists Ltd Cell migration in Drosophila optic lobe neurons is controlled by eyeless/pax6 Javier Morante
687 Development 138, 687-693 (2011) doi:10.1242/dev.056069 2011. Published by The Company of Biologists Ltd Cell migration in Drosophila optic lobe neurons is controlled by eyeless/pax6 Javier Morante
Nature Neuroscience: doi: /nn Supplementary Figure 1. MADM labeling of thalamic clones.
 Supplementary Figure 1 MADM labeling of thalamic clones. (a) Confocal images of an E12 Nestin-CreERT2;Ai9-tdTomato brain treated with TM at E10 and stained for BLBP (green), a radial glial progenitor-specific
Supplementary Figure 1 MADM labeling of thalamic clones. (a) Confocal images of an E12 Nestin-CreERT2;Ai9-tdTomato brain treated with TM at E10 and stained for BLBP (green), a radial glial progenitor-specific
Control of neural stem cell self-renewal and differentiation in Drosophila
 Institutional Repository of the University of Basel University Library Schoenbeinstrasse 18-20 CH-4056 Basel, Switzerland http://edoc.unibas.ch/ Year: 2014 Control of neural stem cell self-renewal and
Institutional Repository of the University of Basel University Library Schoenbeinstrasse 18-20 CH-4056 Basel, Switzerland http://edoc.unibas.ch/ Year: 2014 Control of neural stem cell self-renewal and
SUPPLEMENTARY INFORMATION
 doi: 10.1038/nature07173 SUPPLEMENTARY INFORMATION Supplementary Figure Legends: Supplementary Figure 1: Model of SSC and CPC divisions a, Somatic stem cells (SSC) reside adjacent to the hub (red), self-renew
doi: 10.1038/nature07173 SUPPLEMENTARY INFORMATION Supplementary Figure Legends: Supplementary Figure 1: Model of SSC and CPC divisions a, Somatic stem cells (SSC) reside adjacent to the hub (red), self-renew
marker. DAPI labels nuclei. Flies were 20 days old. Scale bar is 5 µm. Ctrl is
 Supplementary Figure 1. (a) Nos is detected in glial cells in both control and GFAP R79H transgenic flies (arrows), but not in deletion mutant Nos Δ15 animals. Repo is a glial cell marker. DAPI labels
Supplementary Figure 1. (a) Nos is detected in glial cells in both control and GFAP R79H transgenic flies (arrows), but not in deletion mutant Nos Δ15 animals. Repo is a glial cell marker. DAPI labels
Brat Is a Miranda Cargo Protein that Promotes Neuronal Differentiation and Inhibits Neuroblast Self-Renewal
 Developmental Cell 10, 441 449, April, 2006 ª2006 Elsevier Inc. DOI 10.1016/j.devcel.2006.01.017 Brat Is a Miranda Cargo Protein that Promotes Neuronal Differentiation and Inhibits Neuroblast Self-Renewal
Developmental Cell 10, 441 449, April, 2006 ª2006 Elsevier Inc. DOI 10.1016/j.devcel.2006.01.017 Brat Is a Miranda Cargo Protein that Promotes Neuronal Differentiation and Inhibits Neuroblast Self-Renewal
Lineage diversity in the Drosophila nervous system Yohanns Bellaïche* and François Schweisguth
 418 Lineage diversity in the Drosophila nervous system Yohanns Bellaïche* and François Schweisguth The detailed descriptions of cellular lineages in the Drosophila nervous system have provided the foundations
418 Lineage diversity in the Drosophila nervous system Yohanns Bellaïche* and François Schweisguth The detailed descriptions of cellular lineages in the Drosophila nervous system have provided the foundations
A new subtype of progenitor cell in the mouse embryonic neocortex. Xiaoqun Wang, Jin-Wu Tsai, Bridget LaMonica & Arnold R.
 A new subtype of progenitor cell in the mouse embryonic neocortex Xiaoqun Wang, Jin-Wu Tsai, Bridget LaMonica & Arnold R. Kriegstein Supplementary Figures 1-6: Supplementary Movies 1-9: Supplementary
A new subtype of progenitor cell in the mouse embryonic neocortex Xiaoqun Wang, Jin-Wu Tsai, Bridget LaMonica & Arnold R. Kriegstein Supplementary Figures 1-6: Supplementary Movies 1-9: Supplementary
Analysis of neural stem cell self-renewal and differentiation by transgenic RNAi in Drosophila
 Institutional Repository of the University of Basel University Library Schoenbeinstrasse 18-20 CH-4056 Basel, Switzerland http://edoc.unibas.ch/ Year: 2013 Analysis of neural stem cell self-renewal and
Institutional Repository of the University of Basel University Library Schoenbeinstrasse 18-20 CH-4056 Basel, Switzerland http://edoc.unibas.ch/ Year: 2013 Analysis of neural stem cell self-renewal and
Linkage Mapping in Drosophila Melanogaster
 Linkage Mapping in Drosophila Melanogaster Genetics: Fall 2012 Joshua Hanau Introduction: An experiment was performed in order to determine the presence and degree of gene linkage in Drosophila Melanogaster.
Linkage Mapping in Drosophila Melanogaster Genetics: Fall 2012 Joshua Hanau Introduction: An experiment was performed in order to determine the presence and degree of gene linkage in Drosophila Melanogaster.
Review Article Neural Stem Cells in Drosophila: Molecular Genetic Mechanisms Underlying Normal Neural Proliferation and Abnormal Brain Tumor Formation
 Stem Cells International Volume 2012, Article ID 486169, 10 pages doi:10.1155/2012/486169 Review Article Neural Stem Cells in Drosophila: Molecular Genetic Mechanisms Underlying Normal Neural Proliferation
Stem Cells International Volume 2012, Article ID 486169, 10 pages doi:10.1155/2012/486169 Review Article Neural Stem Cells in Drosophila: Molecular Genetic Mechanisms Underlying Normal Neural Proliferation
Supplementary Figure 1 Madm is not required in GSCs and hub cells. (a,b) Act-Gal4-UAS-GFP (a), Act-Gal4-UAS- GFP.nls (b,c) is ubiquitously expressed
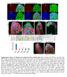 Supplementary Figure 1 Madm is not required in GSCs and hub cells. (a,b) Act-Gal4-UAS-GFP (a), Act-Gal4-UAS- GFP.nls (b,c) is ubiquitously expressed in the testes. The testes were immunostained with GFP
Supplementary Figure 1 Madm is not required in GSCs and hub cells. (a,b) Act-Gal4-UAS-GFP (a), Act-Gal4-UAS- GFP.nls (b,c) is ubiquitously expressed in the testes. The testes were immunostained with GFP
Tramtrack controls glial number and identity in the Drosophila embryonic CNS
 Development 128, 4093-4101 (2001) Printed in Great Britain The Company of Biologists Limited 2001 DEV7891 4093 Tramtrack controls glial number and identity in the Drosophila embryonic CNS Paul Badenhorst
Development 128, 4093-4101 (2001) Printed in Great Britain The Company of Biologists Limited 2001 DEV7891 4093 Tramtrack controls glial number and identity in the Drosophila embryonic CNS Paul Badenhorst
Cancer Stem Cells & Glioblastoma
 Cancer Stem Cells & Glioblastoma JP Hugnot «Brain plasticity, Neural stem cells and Glial tumors» INSERM U1051-UM2 Institut des Neurosciences de Montpellier Montpellier 1-Stem cells and Brain Stem Cells
Cancer Stem Cells & Glioblastoma JP Hugnot «Brain plasticity, Neural stem cells and Glial tumors» INSERM U1051-UM2 Institut des Neurosciences de Montpellier Montpellier 1-Stem cells and Brain Stem Cells
Prss56, a novel marker of adult neurogenesis in the mouse brain. - Supplemental Figures 1 to 5- Brain Structure and Function
 Prss56, a novel marker of adult neurogenesis in the mouse brain - Supplemental Figures 1 to 5- Brain Structure and Function Alexandre Jourdon 1,2, Aurélie Gresset 1, Nathalie Spassky 1, Patrick Charnay
Prss56, a novel marker of adult neurogenesis in the mouse brain - Supplemental Figures 1 to 5- Brain Structure and Function Alexandre Jourdon 1,2, Aurélie Gresset 1, Nathalie Spassky 1, Patrick Charnay
Plasticity of Cerebral Cortex in Development
 Plasticity of Cerebral Cortex in Development Jessica R. Newton and Mriganka Sur Department of Brain & Cognitive Sciences Picower Center for Learning & Memory Massachusetts Institute of Technology Cambridge,
Plasticity of Cerebral Cortex in Development Jessica R. Newton and Mriganka Sur Department of Brain & Cognitive Sciences Picower Center for Learning & Memory Massachusetts Institute of Technology Cambridge,
Control of proliferation activation in quiescent neuroblasts of the Drosophila central nervous system
 Development 121, 1173-1182 (1995) Printed in Great Britain The Company of Biologists Limited 1995 1173 Control of proliferation activation in quiescent neuroblasts of the Drosophila central nervous system
Development 121, 1173-1182 (1995) Printed in Great Britain The Company of Biologists Limited 1995 1173 Control of proliferation activation in quiescent neuroblasts of the Drosophila central nervous system
ErbB4 migrazione II parte
 ErbB4 migrazione II parte Control SVZ cells prefer to migrate on the NRG1 type III substrate the substrate preference of the neuroblasts migrating out of the SVZ explant was evaluated SVZ cells had a strong
ErbB4 migrazione II parte Control SVZ cells prefer to migrate on the NRG1 type III substrate the substrate preference of the neuroblasts migrating out of the SVZ explant was evaluated SVZ cells had a strong
A Cxcl12-Cxcr4 Chemokine Signaling Pathway Defines
 Supplemental Data A Cxcl12-Cxcr4 Chemokine Signaling Pathway Defines the Initial Trajectory of Mammalian Motor Axons Ivo Lieberam, Dritan Agalliu, Takashi Nagasawa, Johan Ericson, and Thomas M. Jessell
Supplemental Data A Cxcl12-Cxcr4 Chemokine Signaling Pathway Defines the Initial Trajectory of Mammalian Motor Axons Ivo Lieberam, Dritan Agalliu, Takashi Nagasawa, Johan Ericson, and Thomas M. Jessell
Supporting Online Material for
 www.sciencemag.org/cgi/content/full/1171320/dc1 Supporting Online Material for A Frazzled/DCC-Dependent Transcriptional Switch Regulates Midline Axon Guidance Long Yang, David S. Garbe, Greg J. Bashaw*
www.sciencemag.org/cgi/content/full/1171320/dc1 Supporting Online Material for A Frazzled/DCC-Dependent Transcriptional Switch Regulates Midline Axon Guidance Long Yang, David S. Garbe, Greg J. Bashaw*
100 mm Sucrose. +Berberine +Quinine
 8 mm Sucrose Probability (%) 7 6 5 4 3 Wild-type Gr32a / 2 +Caffeine +Berberine +Quinine +Denatonium Supplementary Figure 1: Detection of sucrose and bitter compounds is not affected in Gr32a / flies.
8 mm Sucrose Probability (%) 7 6 5 4 3 Wild-type Gr32a / 2 +Caffeine +Berberine +Quinine +Denatonium Supplementary Figure 1: Detection of sucrose and bitter compounds is not affected in Gr32a / flies.
Glia dictate pioneer axon trajectories in the Drosophila embryonic CNS
 Development 127, 393-402 (2000) Printed in Great Britain The Company of Biologists Limited 2000 DEV1479 393 Glia dictate pioneer axon trajectories in the Drosophila embryonic CNS Alicia Hidalgo* and Gwendolen
Development 127, 393-402 (2000) Printed in Great Britain The Company of Biologists Limited 2000 DEV1479 393 Glia dictate pioneer axon trajectories in the Drosophila embryonic CNS Alicia Hidalgo* and Gwendolen
Neuroepithelial Cells and Neural Differentiation
 Neuroepithelial Cells and Neural Differentiation Neurulation The cells of the neural tube are NEUROEPITHELIAL CELLS Neural crest cells migrate out of neural tube Neuroepithelial cells are embryonic stem
Neuroepithelial Cells and Neural Differentiation Neurulation The cells of the neural tube are NEUROEPITHELIAL CELLS Neural crest cells migrate out of neural tube Neuroepithelial cells are embryonic stem
Developmental nicotine exposure affects larval brain size and the adult dopaminergic system of Drosophila melanogaster
 Morris et al. BMC Developmental Biology (2018) 18:13 https://doi.org/10.1186/s12861-018-0172-6 RESEARCH ARTICLE Open Access Developmental nicotine exposure affects larval brain size and the adult dopaminergic
Morris et al. BMC Developmental Biology (2018) 18:13 https://doi.org/10.1186/s12861-018-0172-6 RESEARCH ARTICLE Open Access Developmental nicotine exposure affects larval brain size and the adult dopaminergic
Milestones of neuronal development in the adult hippocampus
 Milestones of neuronal development in the adult hippocampus Gerd Kempermann 1,2, Sebastian Jessberger 2, Barbara Steiner 2 and Golo Kronenberg 1,3 1 Max Delbrück Center for Molecular Medicine (MDC) Berlin-Buch,
Milestones of neuronal development in the adult hippocampus Gerd Kempermann 1,2, Sebastian Jessberger 2, Barbara Steiner 2 and Golo Kronenberg 1,3 1 Max Delbrück Center for Molecular Medicine (MDC) Berlin-Buch,
Article. The Color-Vision Circuit in the Medulla of Drosophila. Javier Morante 1 and Claude Desplan 1, * 1
 Current Biology 18, 1 13, April 22, 2008 ª2008 Elsevier Ltd All rights reserved DOI 10.1016/j.cub.2008.02.075 The Color-Vision Circuit in the Medulla of Drosophila Article Javier Morante 1 and Claude Desplan
Current Biology 18, 1 13, April 22, 2008 ª2008 Elsevier Ltd All rights reserved DOI 10.1016/j.cub.2008.02.075 The Color-Vision Circuit in the Medulla of Drosophila Article Javier Morante 1 and Claude Desplan
A region-specific neurogenesis mode requires migratory progenitors in the Drosophila visual system
 Europe PMC Funders Group Author Manuscript Published in final edited form as: Nat Neurosci. 2015 January ; 18(1): 46 55. doi:10.1038/nn.3896. A region-specific neurogenesis mode requires migratory progenitors
Europe PMC Funders Group Author Manuscript Published in final edited form as: Nat Neurosci. 2015 January ; 18(1): 46 55. doi:10.1038/nn.3896. A region-specific neurogenesis mode requires migratory progenitors
Department of Cell and Developmental Biology, Vanderbilt University, Nashville, TN
 UNC-6/Netrin mediates dendritic self-avoidance Cody J. Smith 1, Joseph D. Watson 1,4,5, Miri K. VanHoven 2, Daniel A. Colón-Ramos 3 and David M. Miller III 1,4,6 1 Department of Cell and Developmental
UNC-6/Netrin mediates dendritic self-avoidance Cody J. Smith 1, Joseph D. Watson 1,4,5, Miri K. VanHoven 2, Daniel A. Colón-Ramos 3 and David M. Miller III 1,4,6 1 Department of Cell and Developmental
Mammalian Cerebral Cortex: Embryonic Development and Cytoarchitecture
 Mammalian Cerebral Cortex: Embryonic Development and Cytoarchitecture 2 The prenatal developmental of the mammalian cerebral cortex, including that of humans, is characterized by two sequential and interrelated
Mammalian Cerebral Cortex: Embryonic Development and Cytoarchitecture 2 The prenatal developmental of the mammalian cerebral cortex, including that of humans, is characterized by two sequential and interrelated
Drosophila Hey is a target of Notch in asymmetric divisions during embryonic and larval neurogenesis
 AND STEM CELLS 191 Development 137, 191-201 (2010) doi:10.1242/dev.043604 Drosophila Hey is a target of Notch in asymmetric divisions during embryonic and larval neurogenesis Maria Monastirioti 1, Nikolaos
AND STEM CELLS 191 Development 137, 191-201 (2010) doi:10.1242/dev.043604 Drosophila Hey is a target of Notch in asymmetric divisions during embryonic and larval neurogenesis Maria Monastirioti 1, Nikolaos
Inheritance of Aldehyde Oxidase in Drosophila melanogaster
 Inheritance of Aldehyde Oxidase in Drosophila melanogaster (adapted from Morgan, J. G. and V. Finnerty. 1991. Inheritance of aldehyde oxidase in Drosophilia melanogaster. Pages 33-47, in Tested studies
Inheritance of Aldehyde Oxidase in Drosophila melanogaster (adapted from Morgan, J. G. and V. Finnerty. 1991. Inheritance of aldehyde oxidase in Drosophilia melanogaster. Pages 33-47, in Tested studies
Neurogenesis in the insect brain: cellular identification and molecular characterization of brain neuroblasts in the grasshopper embryo
 Development 118, 941-955 (1993) Printed in Great Britain The Company of Biologists Limited 1993 941 Neurogenesis in the insect brain: cellular identification and molecular characterization of brain neuroblasts
Development 118, 941-955 (1993) Printed in Great Britain The Company of Biologists Limited 1993 941 Neurogenesis in the insect brain: cellular identification and molecular characterization of brain neuroblasts
Tlx3 and Tlx1 are post-mitotic selector genes determining glutamatergic over GABAergic cell fates
 Tlx3 and Tlx1 are post-mitotic selector genes determining glutamatergic over GABAergic cell fates L. Cheng, A. Arata, R. Mizuguchi, Y. Qian, A. Karunaratne, P.A. Gray, S. Arata, S. Shirasawa, M. Bouchard,
Tlx3 and Tlx1 are post-mitotic selector genes determining glutamatergic over GABAergic cell fates L. Cheng, A. Arata, R. Mizuguchi, Y. Qian, A. Karunaratne, P.A. Gray, S. Arata, S. Shirasawa, M. Bouchard,
Emx2 patterns the neocortex by regulating FGF positional signaling
 Emx2 patterns the neocortex by regulating FGF positional signaling Tomomi Fukuchi-Shimogori and Elizabeth A Grove Presented by Sally Kwok Background Cerebral cortex has anatomically and functionally distinct
Emx2 patterns the neocortex by regulating FGF positional signaling Tomomi Fukuchi-Shimogori and Elizabeth A Grove Presented by Sally Kwok Background Cerebral cortex has anatomically and functionally distinct
Supplementary Figure 1
 Supplementary Figure 1 Kif1a RNAi effect on basal progenitor differentiation Related to Figure 2. Representative confocal images of the VZ and SVZ of rat cortices transfected at E16 with scrambled or Kif1a
Supplementary Figure 1 Kif1a RNAi effect on basal progenitor differentiation Related to Figure 2. Representative confocal images of the VZ and SVZ of rat cortices transfected at E16 with scrambled or Kif1a
Cells of the nervous system
 Neurobiology Cells of the nervous system Anthony Heape 2011 1 Cells of the nervous system Neuroglia : part 2 The non excitable cells of the nervous system that provide support to neuronal survival and
Neurobiology Cells of the nervous system Anthony Heape 2011 1 Cells of the nervous system Neuroglia : part 2 The non excitable cells of the nervous system that provide support to neuronal survival and
Supplemental Information. Tissue Myeloid Progenitors Differentiate. into Pericytes through TGF-b Signaling. in Developing Skin Vasculature
 Cell Reports, Volume 18 Supplemental Information Tissue Myeloid Progenitors Differentiate into Pericytes through TGF-b Signaling in Developing Skin Vasculature Tomoko Yamazaki, Ani Nalbandian, Yutaka Uchida,
Cell Reports, Volume 18 Supplemental Information Tissue Myeloid Progenitors Differentiate into Pericytes through TGF-b Signaling in Developing Skin Vasculature Tomoko Yamazaki, Ani Nalbandian, Yutaka Uchida,
Programmed Cell Death (apoptosis)
 Programmed Cell Death (apoptosis) Stereotypic death process includes: membrane blebbing nuclear fragmentation chromatin condensation and DNA framentation loss of mitochondrial integrity and release of
Programmed Cell Death (apoptosis) Stereotypic death process includes: membrane blebbing nuclear fragmentation chromatin condensation and DNA framentation loss of mitochondrial integrity and release of
READING ASSIGNMENT GENETIC ANALYSIS OF DROSOPHILA POPULATIONS I. HOW DO MITOSIS AND MEIOSIS COMPARE?
 READING ASSIGNMENT GENETIC ANALYSIS OF DROSOPHILA POPULATIONS I. HOW DO MITOSIS AND MEIOSIS COMPARE? II. HOW CAN WE DETERMINE EXPECTED RATIOS OF OFFSPRING? What rules can we learn from Mendel s work with
READING ASSIGNMENT GENETIC ANALYSIS OF DROSOPHILA POPULATIONS I. HOW DO MITOSIS AND MEIOSIS COMPARE? II. HOW CAN WE DETERMINE EXPECTED RATIOS OF OFFSPRING? What rules can we learn from Mendel s work with
Concentric zones, cell migration and neuronal circuits in the Drosophila visual center
 983 Development 138, 983-993 (2011) doi:10.1242/dev.058370 2011. Published by The Company of Biologists Ltd Concentric zones, cell migration and neuronal circuits in the Drosophila visual center Eri Hasegawa
983 Development 138, 983-993 (2011) doi:10.1242/dev.058370 2011. Published by The Company of Biologists Ltd Concentric zones, cell migration and neuronal circuits in the Drosophila visual center Eri Hasegawa
OSVZ progenitors of human and ferret neocortex are epithelial-like and
 OSVZ progenitors of human and ferret neocortex are epithelial-like and expand by integrin signaling Simone A Fietz, Iva Kelava, Johannes Vogt, Michaela Wilsch-Bräuninger, Denise Stenzel, Jennifer L Fish,
OSVZ progenitors of human and ferret neocortex are epithelial-like and expand by integrin signaling Simone A Fietz, Iva Kelava, Johannes Vogt, Michaela Wilsch-Bräuninger, Denise Stenzel, Jennifer L Fish,
R1 Neural Circuitry on Ellipsoid Body Promotes Sleep in Drosophila Melanogaster. Senior Thesis. Presented to
 R1 Neural Circuitry on Ellipsoid Body Promotes Sleep in Drosophila Melanogaster Senior Thesis Presented to The Faculty of the School of Arts and Sciences Brandeis University Undergraduate Program in Neuroscience
R1 Neural Circuitry on Ellipsoid Body Promotes Sleep in Drosophila Melanogaster Senior Thesis Presented to The Faculty of the School of Arts and Sciences Brandeis University Undergraduate Program in Neuroscience
The developmental history of a sensory ganglion cell
 (bozza v1, luglio 2016 per VC) The developmental history of a sensory ganglion cell by Brian Freeman, School of Medical Sciences, UNSW (b.freeman@unsw.edu.au) The sensory ganglion cell (e.g., in spinal
(bozza v1, luglio 2016 per VC) The developmental history of a sensory ganglion cell by Brian Freeman, School of Medical Sciences, UNSW (b.freeman@unsw.edu.au) The sensory ganglion cell (e.g., in spinal
Development of the Nervous System 1 st month
 Development of the Nervous System 1 st month day 1 - fertilization of egg day 6 - uterine implantation day 18 - trilaminar (3-layered) disc (blastoderm, embryo) ectoderm (dorsal) - nervous system and skin
Development of the Nervous System 1 st month day 1 - fertilization of egg day 6 - uterine implantation day 18 - trilaminar (3-layered) disc (blastoderm, embryo) ectoderm (dorsal) - nervous system and skin
Development Supplementary information
 Supplemental Materials and Methods Mosaic clonal analysis GSC and SP clones were induced with the FLP/FRT-mediated mitotic recombination technique (Xu and Rubin, 1993) in files with following genotypes:
Supplemental Materials and Methods Mosaic clonal analysis GSC and SP clones were induced with the FLP/FRT-mediated mitotic recombination technique (Xu and Rubin, 1993) in files with following genotypes:
File name: Supplementary Information Description: Supplementary Figures, Supplementary Table and Supplementary References
 File name: Supplementary Information Description: Supplementary Figures, Supplementary Table and Supplementary References File name: Supplementary Data 1 Description: Summary datasheets showing the spatial
File name: Supplementary Information Description: Supplementary Figures, Supplementary Table and Supplementary References File name: Supplementary Data 1 Description: Summary datasheets showing the spatial
SUPPLEMENTARY INFORMATION
 DOI: 10.1038/ncb2419 Figure S1 NiGFP localization in Dl mutant dividing SOPs. a-c) time-lapse analysis of NiGFP (green) in Dl mutant SOPs (H2B-RFP, red; clones were identified by the loss of nlsgfp) showing
DOI: 10.1038/ncb2419 Figure S1 NiGFP localization in Dl mutant dividing SOPs. a-c) time-lapse analysis of NiGFP (green) in Dl mutant SOPs (H2B-RFP, red; clones were identified by the loss of nlsgfp) showing
Ensheathing Glia Function as Phagocytes in the Adult Drosophila Brain
 4768 The Journal of Neuroscience, April 15, 2009 29(15):4768 4781 Cellular/Molecular Ensheathing Glia Function as Phagocytes in the Adult Drosophila Brain Johnna Doherty,* Mary A. Logan,* Özge E. Taşdemir,
4768 The Journal of Neuroscience, April 15, 2009 29(15):4768 4781 Cellular/Molecular Ensheathing Glia Function as Phagocytes in the Adult Drosophila Brain Johnna Doherty,* Mary A. Logan,* Özge E. Taşdemir,
Citation for published version (APA): Martina-Mamber, C. E. (2014). GFAP as an understudy in adult neurogenesis. 's-hertogenbosch: Boxpress.
 UvA-DARE (Digital Academic Repository) GFAP as an understudy in adult neurogenesis Mamber, C.E. Link to publication Citation for published version (APA): Martina-Mamber, C. E. (2014). GFAP as an understudy
UvA-DARE (Digital Academic Repository) GFAP as an understudy in adult neurogenesis Mamber, C.E. Link to publication Citation for published version (APA): Martina-Mamber, C. E. (2014). GFAP as an understudy
Neurons vs. glia. Traditionally, glia have been viewed as passive cells that help to maintain the function of neurons.
 GLIA Neurons vs. glia The defining characteristic of a neuron is its ability to transmit rapid electrical signals in the form of action potentials. All other neural cells that lack this property are broadly
GLIA Neurons vs. glia The defining characteristic of a neuron is its ability to transmit rapid electrical signals in the form of action potentials. All other neural cells that lack this property are broadly
Title: Chapter 5 Recorded Lecture. Speaker: Amit Dhingra Created by: (remove if same as speaker) online.wsu.edu
 Title: Chapter 5 Recorded Lecture Speaker: Title: What Anthony is the title Berger/Angela of this lecture? Williams Speaker: Amit Dhingra Created by: (remove if same as speaker) online.wsu.edu Chapter
Title: Chapter 5 Recorded Lecture Speaker: Title: What Anthony is the title Berger/Angela of this lecture? Williams Speaker: Amit Dhingra Created by: (remove if same as speaker) online.wsu.edu Chapter
CNS pathology Third year medical students. Dr Heyam Awad 2018 Lecture 12: CNS tumours 2/3
 CNS pathology Third year medical students Dr Heyam Awad 2018 Lecture 12: CNS tumours 2/3 Pilocytic astrocytoma Relatively benign ( WHO grade 1) Occurs in children and young adults Mostly: in the cerebellum
CNS pathology Third year medical students Dr Heyam Awad 2018 Lecture 12: CNS tumours 2/3 Pilocytic astrocytoma Relatively benign ( WHO grade 1) Occurs in children and young adults Mostly: in the cerebellum
Supplemental Information. Octopamine Neurons Mediate Flight-Induced Modulation of Visual Processing in Drosophila. Supplemental Inventory
 1 Current Biology, Volume 22 Supplemental Information Octopamine Neurons Mediate Flight-Induced Modulation of Visual Processing in Drosophila Marie P. Suver, Akira Mamiya, and Michael H. Dickinson Supplemental
1 Current Biology, Volume 22 Supplemental Information Octopamine Neurons Mediate Flight-Induced Modulation of Visual Processing in Drosophila Marie P. Suver, Akira Mamiya, and Michael H. Dickinson Supplemental
Department of Cognitive Science UCSD
 Department of Cognitive Science UCSD Verse 1: Neocortex, frontal lobe, Brain stem, brain stem, Hippocampus, neural node, Right hemisphere, Pons and cortex visual, Brain stem, brain stem, Sylvian fissure,
Department of Cognitive Science UCSD Verse 1: Neocortex, frontal lobe, Brain stem, brain stem, Hippocampus, neural node, Right hemisphere, Pons and cortex visual, Brain stem, brain stem, Sylvian fissure,
A Genetic Program for Embryonic Development
 Concept 18.4: A program of differential gene expression leads to the different cell types in a multicellular organism During embryonic development, a fertilized egg gives rise to many different cell types
Concept 18.4: A program of differential gene expression leads to the different cell types in a multicellular organism During embryonic development, a fertilized egg gives rise to many different cell types
Notch Maintains Drosophila Type II Neuroblasts by Suppressing the. Expression of the Fez Transcription Factor Earmuff
 First posted online on 5 May 2016 as 10.1242/dev.136184 Access the most recent version at http://dev.biologists.org/lookup/doi/10.1242/dev.136184 Notch Maintains Drosophila Type II Neuroblasts by Suppressing
First posted online on 5 May 2016 as 10.1242/dev.136184 Access the most recent version at http://dev.biologists.org/lookup/doi/10.1242/dev.136184 Notch Maintains Drosophila Type II Neuroblasts by Suppressing
Neurogenesis and neuronal circuit formation in the Drosophila visual center
 The Japanese Society of Developmental Biologists Develop. Growth Differ. (2014) 56, 491 498 doi: 10.1111/dgd.12151 Review Article Neurogenesis and neuronal circuit formation in the Drosophila visual center
The Japanese Society of Developmental Biologists Develop. Growth Differ. (2014) 56, 491 498 doi: 10.1111/dgd.12151 Review Article Neurogenesis and neuronal circuit formation in the Drosophila visual center
Chapter 5 A Dose Dependent Screen for Modifiers of Kek5
 Chapter 5 A Dose Dependent Screen for Modifiers of Kek5 "#$ ABSTRACT Modifier screens in Drosophila have proven to be a powerful tool for uncovering gene interaction and elucidating molecular pathways.
Chapter 5 A Dose Dependent Screen for Modifiers of Kek5 "#$ ABSTRACT Modifier screens in Drosophila have proven to be a powerful tool for uncovering gene interaction and elucidating molecular pathways.
Chapter 5. Summary and Future directions
 95 Chapter 5 Summary and Future directions 96 Much of our knowledge about glial development in the vertebrate CNS comes from studies of purified oligodendrocyte precursor cells (Raff 1989; Pfeiffer et
95 Chapter 5 Summary and Future directions 96 Much of our knowledge about glial development in the vertebrate CNS comes from studies of purified oligodendrocyte precursor cells (Raff 1989; Pfeiffer et
Gene co-expression networks in the mouse, monkey, and human brain July 16, Jeremy Miller Scientist I
 Gene co-expression networks in the mouse, monkey, and human brain July 16, 2013 Jeremy Miller Scientist I jeremym@alleninstitute.org Outline 1. Brief introduction to previous WGCNA studies in brain 2.
Gene co-expression networks in the mouse, monkey, and human brain July 16, 2013 Jeremy Miller Scientist I jeremym@alleninstitute.org Outline 1. Brief introduction to previous WGCNA studies in brain 2.
Neural stem cells and the neurobiology of ageing. Chen Siyun 1, Dawe G.S. 2
 ABSTRACT Neural stem cells and the neurobiology of ageing Chen Siyun 1, Dawe G.S. 2 Department of Physics, Faculty of Science, National University of Singapore 10 Kent Ridge Road, Singapore 117546 The
ABSTRACT Neural stem cells and the neurobiology of ageing Chen Siyun 1, Dawe G.S. 2 Department of Physics, Faculty of Science, National University of Singapore 10 Kent Ridge Road, Singapore 117546 The
BRAIN REJUVENATION HOW TO KEEP BRAIN STEM CELLS HAPPY
 BRAIN REJUVENATION HOW TO KEEP BRAIN STEM CELLS HAPPY Natalia Surzenko, PhD Research Assistant Professor UNC Chapel Hill Nutrition Research Institute October 13, 2015 MY JOURNEY Tallinn, Estonia Aiken,
BRAIN REJUVENATION HOW TO KEEP BRAIN STEM CELLS HAPPY Natalia Surzenko, PhD Research Assistant Professor UNC Chapel Hill Nutrition Research Institute October 13, 2015 MY JOURNEY Tallinn, Estonia Aiken,
Supplemental Information. Otic Mesenchyme Cells Regulate. Spiral Ganglion Axon Fasciculation. through a Pou3f4/EphA4 Signaling Pathway
 Neuron, Volume 73 Supplemental Information Otic Mesenchyme Cells Regulate Spiral Ganglion Axon Fasciculation through a Pou3f4/EphA4 Signaling Pathway Thomas M. Coate, Steven Raft, Xiumei Zhao, Aimee K.
Neuron, Volume 73 Supplemental Information Otic Mesenchyme Cells Regulate Spiral Ganglion Axon Fasciculation through a Pou3f4/EphA4 Signaling Pathway Thomas M. Coate, Steven Raft, Xiumei Zhao, Aimee K.
The Nervous System. Functions of the Nervous System input gathering To monitor occurring inside and outside the body Changes =
 The Nervous System Functions of the Nervous System input gathering To monitor occurring inside and outside the body Changes = To process and sensory input and decide if is needed output A response to integrated
The Nervous System Functions of the Nervous System input gathering To monitor occurring inside and outside the body Changes = To process and sensory input and decide if is needed output A response to integrated
Zhu et al, page 1. Supplementary Figures
 Zhu et al, page 1 Supplementary Figures Supplementary Figure 1: Visual behavior and avoidance behavioral response in EPM trials. (a) Measures of visual behavior that performed the light avoidance behavior
Zhu et al, page 1 Supplementary Figures Supplementary Figure 1: Visual behavior and avoidance behavioral response in EPM trials. (a) Measures of visual behavior that performed the light avoidance behavior
4) Modification of Development by Sensory Experience (Nurture)
 Lecture 7 (Jan 29 th ): BRAIN DEVELOPMENT and EVOLUTION Lecture Outline 1) Overview of Neural Development 2) Stages of Neural Development 3) The Nature vs. Nurture Issue 4) Modification of Development
Lecture 7 (Jan 29 th ): BRAIN DEVELOPMENT and EVOLUTION Lecture Outline 1) Overview of Neural Development 2) Stages of Neural Development 3) The Nature vs. Nurture Issue 4) Modification of Development
Olfactory ensheathing glia
 Olfactory ensheathing glia From Wikipedia, the free encyclopedia Neuroglia of the brain shown by Golgi's method. Olfactory ensheathing glia (OEG), also known as olfactory ensheathing cells (OECs) or olfactory
Olfactory ensheathing glia From Wikipedia, the free encyclopedia Neuroglia of the brain shown by Golgi's method. Olfactory ensheathing glia (OEG), also known as olfactory ensheathing cells (OECs) or olfactory
effects on organ development. a-f, Eye and wing discs with clones of ε j2b10 show no
 Supplementary Figure 1. Loss of function clones of 14-3-3 or 14-3-3 show no significant effects on organ development. a-f, Eye and wing discs with clones of 14-3-3ε j2b10 show no obvious defects in Elav
Supplementary Figure 1. Loss of function clones of 14-3-3 or 14-3-3 show no significant effects on organ development. a-f, Eye and wing discs with clones of 14-3-3ε j2b10 show no obvious defects in Elav
Supplemental Information. Induction of Expansion and Folding. in Human Cerebral Organoids
 Cell Stem Cell, Volume 20 Supplemental Information Induction of Expansion and Folding in Human Cerebral Organoids Yun Li, Julien Muffat, Attya Omer, Irene Bosch, Madeline A. Lancaster, Mriganka Sur, Lee
Cell Stem Cell, Volume 20 Supplemental Information Induction of Expansion and Folding in Human Cerebral Organoids Yun Li, Julien Muffat, Attya Omer, Irene Bosch, Madeline A. Lancaster, Mriganka Sur, Lee
Nerve tissue & the Nervous System
 Nerve tissue & the Nervous System The human nervous system, by far the most complex system in the body, is formed by a network of many billion nerve cells (neurons), all assisted by many more supporting
Nerve tissue & the Nervous System The human nervous system, by far the most complex system in the body, is formed by a network of many billion nerve cells (neurons), all assisted by many more supporting
Cell Migration II: CNS Cell Migration. Steven McLoon Department of Neuroscience University of Minnesota
 Cell Migration II: CNS Cell Migration Steven McLoon Department of Neuroscience University of Minnesota 1 Course News Coffee Hour Wednesday (Oct 18) 9:00-10:00am Surdyk s Café in Northrop Auditorium Stop
Cell Migration II: CNS Cell Migration Steven McLoon Department of Neuroscience University of Minnesota 1 Course News Coffee Hour Wednesday (Oct 18) 9:00-10:00am Surdyk s Café in Northrop Auditorium Stop
Cell Type Nervous System I. Developmental Readout. Foundations. Stem cells. Organ formation. Human issues.
 7.72 10.11.06 Cell Type Nervous System I Human issues Organ formation Stem cells Developmental Readout Axes Cell type Axon guidance 3D structure Analysis Model + + organisms Foundations Principles 1 What
7.72 10.11.06 Cell Type Nervous System I Human issues Organ formation Stem cells Developmental Readout Axes Cell type Axon guidance 3D structure Analysis Model + + organisms Foundations Principles 1 What
Nature Neuroscience: doi: /nn Supplementary Figure 1
 Supplementary Figure 1 Subcellular segregation of VGluT2-IR and TH-IR within the same VGluT2-TH axon (wild type rats). (a-e) Serial sections of a dual VGluT2-TH labeled axon. This axon (blue outline) has
Supplementary Figure 1 Subcellular segregation of VGluT2-IR and TH-IR within the same VGluT2-TH axon (wild type rats). (a-e) Serial sections of a dual VGluT2-TH labeled axon. This axon (blue outline) has
Quantification of early stage lesions for loss of p53 should be shown in the main figures.
 Reviewer #1 (Remarks to the Author): Expert in prostate cancer The manuscript "Clonal dynamics following p53 loss of heterozygosity in Kras-driven cancers" uses a number of novel genetically engineered
Reviewer #1 (Remarks to the Author): Expert in prostate cancer The manuscript "Clonal dynamics following p53 loss of heterozygosity in Kras-driven cancers" uses a number of novel genetically engineered
SUPPLEMENTARY INFORMATION
 SUPPLEMENTARY INFORMATION doi:10.1038/nature11306 Supplementary Figures Supplementary Figure 1. Basic characterization of GFP+ RGLs in the dentate gyrus of adult nestin-gfp mice. a, Sample confocal images
SUPPLEMENTARY INFORMATION doi:10.1038/nature11306 Supplementary Figures Supplementary Figure 1. Basic characterization of GFP+ RGLs in the dentate gyrus of adult nestin-gfp mice. a, Sample confocal images
Drosophila model for fetal alcohol syndrome disorders: Role for the insulin pathway
 San Jose State University SJSU ScholarWorks Faculty Publications Biological Sciences 5-1-2011 Drosophila model for fetal alcohol syndrome disorders: Role for the insulin pathway Rachael L. French San Jose
San Jose State University SJSU ScholarWorks Faculty Publications Biological Sciences 5-1-2011 Drosophila model for fetal alcohol syndrome disorders: Role for the insulin pathway Rachael L. French San Jose
Brain Development III
 Brain Development III Neural Development In the developing nervous system there must be: 1. The formation of different regions of the brain. 2. The ability of a neuron to differentiate. 3. The ability
Brain Development III Neural Development In the developing nervous system there must be: 1. The formation of different regions of the brain. 2. The ability of a neuron to differentiate. 3. The ability
Sensory coding and somatosensory system
 Sensory coding and somatosensory system Sensation and perception Perception is the internal construction of sensation. Perception depends on the individual experience. Three common steps in all senses
Sensory coding and somatosensory system Sensation and perception Perception is the internal construction of sensation. Perception depends on the individual experience. Three common steps in all senses
The subcortical maternal complex controls symmetric division of mouse zygotes by
 The subcortical maternal complex controls symmetric division of mouse zygotes by regulating F-actin dynamics Xing-Jiang Yu 1,2, Zhaohong Yi 1, Zheng Gao 1,2, Dan-dan Qin 1,2, Yanhua Zhai 1, Xue Chen 1,
The subcortical maternal complex controls symmetric division of mouse zygotes by regulating F-actin dynamics Xing-Jiang Yu 1,2, Zhaohong Yi 1, Zheng Gao 1,2, Dan-dan Qin 1,2, Yanhua Zhai 1, Xue Chen 1,
Cell Migration II: CNS Cell Migration. Steven McLoon Department of Neuroscience University of Minnesota
 Cell Migration II: CNS Cell Migration Steven McLoon Department of Neuroscience University of Minnesota 1 Hey! The major concepts discussed relative to neural crest cell migration apply to cell migration
Cell Migration II: CNS Cell Migration Steven McLoon Department of Neuroscience University of Minnesota 1 Hey! The major concepts discussed relative to neural crest cell migration apply to cell migration
Chemokine Regulation of Oligodendrocyte Development in the Spinal Cord. Bob Avino Saint Louis University Senior Honors Thesis April 19, 2011
 Chemokine Regulation of Oligodendrocyte Development in the Spinal Cord Bob Avino Saint Louis University Senior Honors Thesis April 19, 2011 Richard J. Miller, PhD Northwestern University Feinberg School
Chemokine Regulation of Oligodendrocyte Development in the Spinal Cord Bob Avino Saint Louis University Senior Honors Thesis April 19, 2011 Richard J. Miller, PhD Northwestern University Feinberg School
9.14 Classes #21-23: Visual systems
 9.14 Classes #21-23: Visual systems Questions based on Schneider chapter 20 and classes: 1) What was in all likelihood the first functional role of the visual sense? Describe the nature of the most primitive
9.14 Classes #21-23: Visual systems Questions based on Schneider chapter 20 and classes: 1) What was in all likelihood the first functional role of the visual sense? Describe the nature of the most primitive
doi: /nature09554
 SUPPLEMENTARY INFORMATION doi:10.1038/nature09554 Supplementary Figure 1: Optical Tracing with New Photoactivatable GFP Variants Reveals Enhanced Labeling of Neuronal Processes We qualitatively compare
SUPPLEMENTARY INFORMATION doi:10.1038/nature09554 Supplementary Figure 1: Optical Tracing with New Photoactivatable GFP Variants Reveals Enhanced Labeling of Neuronal Processes We qualitatively compare
Gross Organization I The Brain. Reading: BCP Chapter 7
 Gross Organization I The Brain Reading: BCP Chapter 7 Layout of the Nervous System Central Nervous System (CNS) Located inside of bone Includes the brain (in the skull) and the spinal cord (in the backbone)
Gross Organization I The Brain Reading: BCP Chapter 7 Layout of the Nervous System Central Nervous System (CNS) Located inside of bone Includes the brain (in the skull) and the spinal cord (in the backbone)
Medical Neuroscience Tutorial
 Pain Pathways Medical Neuroscience Tutorial Pain Pathways MAP TO NEUROSCIENCE CORE CONCEPTS 1 NCC1. The brain is the body's most complex organ. NCC3. Genetically determined circuits are the foundation
Pain Pathways Medical Neuroscience Tutorial Pain Pathways MAP TO NEUROSCIENCE CORE CONCEPTS 1 NCC1. The brain is the body's most complex organ. NCC3. Genetically determined circuits are the foundation
Meigo governs dendrite targeting specificity by modulating Ephrin level and N-glycosylation
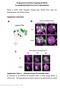 Meigo governs dendrite targeting specificity by modulating Ephrin level and N-glycosylation Sayaka U. Sekine, Shuka Haraguchi, Kinhong Chao, Tomoko Kato, Liqun Luo, Masayuki Miura, and Takahiro Chihara
Meigo governs dendrite targeting specificity by modulating Ephrin level and N-glycosylation Sayaka U. Sekine, Shuka Haraguchi, Kinhong Chao, Tomoko Kato, Liqun Luo, Masayuki Miura, and Takahiro Chihara
Supplementary information. Nkx2.1 regulates the generation of telencephalic astrocytes during embryonic
 Supplementary information Nkx2.1 regulates the generation of telencephalic astrocytes during embryonic development Shilpi Minocha 1*, Delphine Valloton 1*, Yvan Arsenijevic 2, Jean-René Cardinaux 3, Raffaella
Supplementary information Nkx2.1 regulates the generation of telencephalic astrocytes during embryonic development Shilpi Minocha 1*, Delphine Valloton 1*, Yvan Arsenijevic 2, Jean-René Cardinaux 3, Raffaella
Overview of the Nervous System (some basic concepts) Steven McLoon Department of Neuroscience University of Minnesota
 Overview of the Nervous System (some basic concepts) Steven McLoon Department of Neuroscience University of Minnesota 1 Coffee Hour Tuesday (Sept 11) 10:00-11:00am Friday (Sept 14) 8:30-9:30am Surdyk s
Overview of the Nervous System (some basic concepts) Steven McLoon Department of Neuroscience University of Minnesota 1 Coffee Hour Tuesday (Sept 11) 10:00-11:00am Friday (Sept 14) 8:30-9:30am Surdyk s
Faculty of Dental Medicine and Surgery. Sem 4 Peripheral nervous system and nerve plexus Dr. Abbas Garib Alla
 Faculty of Dental Medicine and Surgery Sem 4 Peripheral nervous system and nerve plexus Dr. Abbas Garib Alla PNS Terminology Ganglia neuron cell bodies Peripheral nerves neuronal axons PNS neuroglia Satellite
Faculty of Dental Medicine and Surgery Sem 4 Peripheral nervous system and nerve plexus Dr. Abbas Garib Alla PNS Terminology Ganglia neuron cell bodies Peripheral nerves neuronal axons PNS neuroglia Satellite
THE ORGANIZATION OF AMYGDALOPETAL PROJECTIONS FROM THE LATERAL HYPOTHALAMUS AND PREOPTIC AREA IN THE RAT
 ACTA NEUROBIOL. EXP. 1977, 37: 247-252 THE ORGANIZATION OF AMYGDALOPETAL PROJECTIONS FROM THE LATERAL HYPOTHALAMUS AND PREOPTIC AREA IN THE RAT Liliana NITECKA, Olgierd NARKIEWICZ and Czeslaw JAKIEL Department
ACTA NEUROBIOL. EXP. 1977, 37: 247-252 THE ORGANIZATION OF AMYGDALOPETAL PROJECTIONS FROM THE LATERAL HYPOTHALAMUS AND PREOPTIC AREA IN THE RAT Liliana NITECKA, Olgierd NARKIEWICZ and Czeslaw JAKIEL Department
