Cell migration in Drosophila optic lobe neurons is controlled by eyeless/pax6
|
|
|
- Griffin Davis
- 6 years ago
- Views:
Transcription
1 687 Development 138, (2011) doi: /dev Published by The Company of Biologists Ltd Cell migration in Drosophila optic lobe neurons is controlled by eyeless/pax6 Javier Morante 1,2, Ted Erclik 1 and Claude Desplan 1, * SUMMARY In the developing Drosophila optic lobe, eyeless, apterous and distal-less, three genes that encode transcription factors with important functions during development, are expressed in broad subsets of medulla neurons. Medulla cortex cells follow two patterns of cell movements to acquire their final position: first, neurons are arranged in columns below each neuroblast. Then, during pupation, they migrate laterally, intermingling with each other to reach their retinotopic position in the adult optic lobe. eyeless, which encodes a Pax6 transcription factor, is expressed early in progenitors and controls aspects of this cell migration. Its loss in medulla neurons leads to overgrowth and a failure of lateral migration during pupation. These defects in cell migration among medulla cortex cells can be rescued by removing DE-Cadherin. Thus, eyeless links neurogenesis and neuronal migration. KEY WORDS: Cell adhesion, Cell migration, Neuroblast, Optic lobe INTRODUCTION The adult optic lobe is one of the major structures in the Drosophila brain. It is composed of ~60,000 cells (Hofbauer and Campos- Ortega, 1990) and can be divided into four neuropils: lamina, medulla, lobula and lobula plate (Meinertzhagen and Hanson, 1993). The optic lobe derives from an embryonic optic placode (Green et al., 1993). During larval development, this primordium contains two proliferation centers: the outer and the inner proliferation centers (OPC and IPC) (Fig. 1A,C) (Hofbauer and Campos-Ortega, 1990). Successive cell divisions of a limited number of neuroblasts, which last until early pupation (Hofbauer and Campos-Ortega, 1990; Ito and Hotta, 1992; White and Kankel, 1978), generate the correct number of cells present in the adult optic lobe: the IPC gives rise to neurons of the lobula complex and proximal medulla (presumably cells from the medulla rim), while the OPC generates most medulla neurons (presumably cells from the medulla cortex) as well as cells that will generate the lamina (Meinertzhagen and Hanson, 1993). The medulla represents the largest structure in the adult optic lobe with an estimated 40,000 neurons (Hofbauer and Campos-Ortega, 1990), the cell bodies of which are located either in the medulla cortex, the region between the lamina and the medulla neuropil, or the medulla rim, the region between the medulla and the lobula plate. These two populations have been proposed to have different larval origins (Meinertzhagen and Hanson, 1993). We have identified three genes encoding transcription factors that are expressed in broad subsets of medulla cortex cells: eyeless (ey), apterous (ap) and distalless (dll). These three transcription factors play important functions in the development of a large number of organs in Drosophila, as well as in vertebrates. In the adult medulla, their expression is mostly non-overlapping and covers over 90% of all medulla neurons (Morante and Desplan, 2008). In previous work, we had expanded 1 Center for Developmental Genetics, Department of Biology, New York University, 100 Washington Square East, New York, NY 10003, USA. 2 Instituto de Neurociencias, Consejo Superior de Investigaciones Científicas, Universidad Miguel Hernández, Avenida Santiago Ramón y Cajal s/n, San Juan de Alicante, Spain. *Author for correspondence (cd38@nyu.edu) Accepted 26 November 2010 on the work of Ramón y Cajal, Strausfeld and Fischbach (Ramón y Cajal and Sanchez, 1915; Fischbach and Dittrich, 1989; Strausfeld, 1976), and identified at least 63 distinct neuronal cell types that express these transcription factors in the medulla and might be involved in processing of visual information (Morante and Desplan, 2008). ey-positive cells are present only in the adult medulla cortex, whereas ap- and dll-expressing cells are both present in the adult medulla cortex and medulla rim (Morante and Desplan, 2008). Therefore, at least five different cell populations marked by ey, ap or Dll coexist in the adult medulla. To investigate how each of these medulla cortex cell types is first determined in the OPC and comes to occupy its final position, we followed the early expression patterns of ey, ap and dll during larval and pupal development using reporter constructs or antibodies. We confirm that medulla cortex cells originate from the OPC, the main body of the crescent-shaped OPC. During larval development, the progeny of each medulla cortex neuroblast forms columns where newly born neurons displace older neurons away from their neuroblast. We show that, later in pupation, cells marked with each transcription factor disperse laterally, mixing with other cell types to reach their final position. We also show that one of the genes, ey/pax6, is expressed in OPC neuroblasts and controls migration of a subpopulation of cells in the optic lobe. Loss of ey function causes overproliferation of neuroblasts and prevents dispersion, leading to large clusters of eypositive neurons. Formation of these large clusters can be rescued by removal of DE-Cadherin (Shotgun FlyBase), which regulates cell adhesion and neuroblast proliferation (Dumstrei et al., 2003). Thus, eyeless links neurogenesis and migration in the optic lobe, revealing new mechanisms of brain formation in Drosophila that are similar to cortical development in mammals (Ge et al., 2006). MATERIALS AND METHODS Fly stocks The following lines were used for the study: act-frt-stop-frt-gal4; ap md544 -Gal4, ap rk568 -lacz, dll md23 -Gal4, ey OK107 -Gal4, G-TRACE (Evans et al., 2009), shg-lacz, UAS-p35, UAS-UEE3 and UAS-UEE7 (referred as ey DN ) (Niimi et al., 2002), tub-gal80 ts, UAS-shg-GFP, UAS-CD8-GFP, UAS-H2B-YFP, UAS-nuGFP and FRTG13 shg R69.
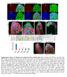
2 688 Development 138 (4) G-TRACE analysis G-TRACE analysis with ap- and dll-gal4 caused larval lethality; thus, we made use of the tub-gal80 ts transgene (McGuire et al., 2004). Larvae containing ap- or dll-gal4, G-TRACE and tub-gal80 ts were raised at 18 C until the second instar stage to allow Gal80 repression of Gal4 activity. They were then incubated at 30 C until third instar larval stages or P40 to relieve repression and allow induction of G-TRACE. G-TRACE analysis was performed using the following stocks (Evans et al., 2009): for ap-gal4, yw hs-flp 122 ; ap-gal4/tub-gal80 ts ; UAS-flp, UAS-RFP, ubi p63 -FRT-stop- FRT-nuGFP/+; for dll-gal4, yw hs-flp 122 ; dll-gal4/tub-gal80 ts ; UAS-flp, UAS-RFP, ubi p63 -FRT-stop-FRT-nuGFP/+; for ey-gal4, yw hs-flp 122 ; tub- Gal80 ts /+; UAS-flp, UAS-RFP, ubi p63 -FRT-stop-FRT-nuGFP/+; ey-gal4. MARCM clonal analysis MARCM clones were induced in larvae 72 hours after egg laying (AEL) with a 20-minute heat-shock at 37 C using the following stocks: for ap- Gal, yw hs-flp 122 UAS-CD8-GFP; ap-gal4/cyo; FRT82B tubp- Gal80/FRT82B; for dll-gal, yw hs-flp 122 UAS-CD8-GFP; FRT40A tubp- Gal80 dll-gal4/frt40a; for ey-gal, yw hs-flp 122 UAS-CD8-GFP; FRT82B tubp-gal80/frt82b; ey-gal4/+, yw hs-flp 122 UAS-CD8-GFP; UAS-UEE3/+; FRT82B tubp-gal80/frt82b; ey-gal4/+, yw hs-flp 122 UAS-CD8-GFP; FRT42D/FRT42D tubp-gal80; UAS-UEE7/TM2; ey- Gal4/+ and yw hs-flp 122 UAS-CD8-GFP; FRTG13 shg R69 /FRTG13 tubp- Gal80; UAS-UEE7/TM2; ey-gal4/+; for tub-gal, yw hs-flp 122 UAS-CD8- GFP;UAS-p35/+; tub-gal4 FRT82B tubp-gal80/frt82b. Brains were dissected at L3, P5, P20, P44, P68 and adulthood. To establish the cell migration pattern of clonally related cells, a 5- minute heat-shock (37 C) was induced in larvae 72 hours AEL using the following stock: yw hs-flp 122 UAS-CD8-GFP;ap-lacZ/+; tub-gal4 FRT82B tubp-gal80/frt82b. Brains were dissected at L3, P10 and around P40. Immunocytochemistry and confocal microscopy Flies were raised on standard medium at 25 C. Larval, pupal and adult brains were dissected in cold PBS and fixed in PFA (4%) for 20 minutes (Morante and Desplan, 2010). Samples were incubated in a cocktail of primary antibodies diluted in PBST (0.3% Triton X-100) overnight at room temperature. Primary antibodies used were as follows: guinea pig anti-dll (dilution 1/3000, from R. Mann, Columbia University, New York, NY, USA), guinea pig anti-dpn (1/3000, from J. Skeath, Washington University, St Louis, MO, USA), mouse anti-24b10 (1/50, DSHB), mouse anti- -Gal (1/500, Promega), mouse anti-elav (Embryonic lethal abnormal vision) (1/50, DSHB), mouse anti-ey (1/10, from P. Callaerts, VIB, Leuven, Belgium), mouse anti-mira (1/50, from F. Matsuzaki, RIKEN, Kobe, Japan), mouse anti-nc82 (1/50, DSHB), rabbit anti-gfp (1/1000, Molecular Probes), rabbit anti- -Gal (1/20000, Cappel), rabbit anti-mira (1/1000, from F. Matsuzaki), rabbit anti-ph3 (1/2000, Upstate), rat anti-de-cad (1/50, DSHB), rat anti-dn-cad (1/50, DSHB) and rat anti-elav (1/50, DSHB). Brains were washed three times for 5 minutes in PBS and then incubated in secondary antibodies diluted in PBST for 3 hours. Secondary antibodies were used as follows: donkey anti-rabbit 405 (1/400, Molecular Probes), donkey anti-guinea pig Alexa488 (1/400, Molecular Probes), donkey anti-mouse Alexa488 (1/1000, Molecular Probes), donkey anti-rabbit Alexa488 (1/1000, Molecular Probes), donkey anti-mouse Alexa555 (1/1000, Molecular Probes), donkey anti-rabbit Alexa555 (1/1000, Molecular Probes), donkey anti-rat Cy5 (1/400, Jackson ImmunoResearch), goat anti-rat Alexa555 (1/500, Molecular Probes), goat anti-guinea pig Alexa555 (1/500, Molecular Probes), goat anti-guinea pig Cy5 (1/500, Jackson ImmunoResearch) and donkey anti-mouse Alexa647 (1/200, Molecular Probes). In Fig. S3 in the supplementary material, samples were incubated in a PBST solution containing DAPI (Sigma) for 30 minutes. After washing overnight, brains were mounted in Vectashield (Vector Labs) keeping their 3D configuration. Samples were imaged using a Leica TCS SP2 confocal using a 20 immersion lens. Images were assembled using Photoshop (Adobe). Fig. S1A-C in the supplementary material was made with Volocity 4 (Improvision) using a 80 m sample acquisition from a late L3 larval brain showing expression of ey (red), Distal-less (green), ap (white) and DE- Cadherin (blue). Number of ph3 cells per clone in pupal optic lobes MARCM clonal analysis of ey-expressing and ey-misexpressing ey DN flies was carried out after a 20-minutes heat-shock (37 C) in larvae 72 hours AEL and dissecting P5 pupal optic lobes. To determine the number of ph3- positive cells per clone, we counted the number of ph3-positive cells in each clone. Number of cells per clone in adult optic lobes MARCM clonal analysis of ey-expressing, ey-misexpressing ey DN and eyexpressing shg ey DN flies was carried out after a 20 minutes heat-shock (37 C) in larvae at 72 hours AEL and in dissecting adults. To determine the number of neurons per clone in adult optic lobes, we fully reconstructed the optic lobes (25 per genotype), collected stacks every 2 m (between m) and counted the number of cells positive for GFP in each cluster. RESULTS Generation of the medulla cortex We analyzed the simultaneous expression patterns of Ey, ap and Dll using an antibody against Eyeless (Clements et al., 2008) (Fig. 1), or ey OK107 -Gal4 (Connolly et al., 1996) crossed with UASnuGFP (Figs S1, S2 in the supplementary material), a lacz enhancer trap in apterous (ap-lacz) (Cohen et al., 1992). The third cell population was labeled with an antibody against Distal-less (Estella et al., 2008). To follow migratory paths, we looked at these three markers in different sections of optic lobes (Betschinger et al., 2006) from larval stages until adulthood. During the late third instar larval stage (L3), we observed three distinct cell populations corresponding to each marker in horizontal views of anterior (Fig. 1B) and middle sections (Fig. 1D,E) of the OPC. In anterior sections (Fig. 1A), Ey, ap- and Dll were expressed in three parallel stripes of cells that represent rows of neurons that emerge from the OPC (Fig. 1B). They correspond to progeny from the youngest to oldest neuroblasts (Fig. 1B). In middle sections (Fig. 1C), Dll-positive cells were generated in the progeny of the oldest neuroblasts, with ap- and Ey-positive cells often placed below Dll-positive cells, i.e. in cells that had emerged earlier from these neuroblasts (Fig. 1D,E). In the progeny of younger neuroblasts, we detected only ap- and Ey-positive cells, and no Dllpositive cells (Fig. 1D,E). Furthermore, Ey and ey-gal4 were coexpressed in neuroblasts with Miranda (Mira) and Deadpan (Dpn) (Fig. 1F,G) (Betschinger et al., 2006; Egger et al., 2007; Ikeshima- Kataoka et al., 1997; Ohshiro et al., 2000; Yasugi et al., 2008). Therefore, three cell populations marked by Ey, ap- and Dll are already spatially and temporally segregated at this early stage (Fig. 1H; see Fig. S1 in the supplementary material). We then followed how the expression patterns of the different cell populations in the OPC evolved during development until adulthood (Fig. 1I-L; see Fig. S2 in the supplementary material). By the beginning of pupation (P0), the number of cells originating from the OPC had increased (Fig. 1I). A major reorganization of the optic lobe structure occurred around P20: cells became intermingled with each other, making the three medial stripes observed earlier no longer distinguishable (compare Fig. 1B with Fig. 1J,K). Thus, the three Ey-, ap- and Dll-positive cell populations lost their spatial segregation and were interspersed within the adult medulla cortex (Fig. 1L).
3 Cell migration in Drosophila optic lobe 689 Fig. 1. Medulla cortex organogenesis. (A,C) Anterior (A) and middle (C) sections of late L3 larval brains. Neuroepithelial cells were visualized with DE-Cadherin (blue) and neuroblasts with Deadpan (red). Solid white line in A separates optic lobe from central brain (CB). IPC, inner proliferation center; OPC, outer proliferation center. (B,D,E) Expression patterns of Eyeless (green), Distal-less (red) and ap-lacz (blue) in cell populations in anterior (B) and middle (D,E) sections of late L3 larvae. Arrowheads in E indicate Ey expression in postmitotic cells and arrows indicate Ey-positive neuroblasts. (F,G) Expression pattern of Ey (F) and ey-gal4 driving UAS-H2B-YFP (G) in OPC-derived neuroblasts (red). Neuroblasts were labeled with Miranda in F and Deadpan in G. Neuroepithelial cells were visualized with DE-Cadherin (blue). Arrows indicate neuroblasts. (H) Neurogenesis in the OPC. Lamina precursor cells (LPC) differentiate into lamina neurons (gray) in the most lateral part of the neuroepithelium (NE) (yellow). Medulla neurons (MeC) derive from eyeless neuroblasts (Nb) on the medial side of the NE. Postmitotic Ey-positive cells (green), appositive cells (blue), Dll-positive cells (red) and other cell types (white) are shown. A, anterior; D, dorsal; M, medial; L, lateral. (I-L) Expression patterns of Ey (green), Dll (red) and ap-lacz (blue) in cell populations in anterior sections at P0 (I), P20 (J,K) and adult (L). Next, we used the G-TRACE system to mark the cell lineages derived from cells expressing a given Gal4 driver (GFP) and, at the same time, to reveal real-time larval expression (RFP) of the given Gal4 driver that serves as a medulla cell marker (Fig. 2) (Evans et al., 2009). In G-trace, Gal4 mediates the expression of FLP recombinase that, in turn, removes an FRT-flanked transcriptional termination cassette inserted between Ubiquitin-p63E promoter fragment and the GFP open reading frame. Thereafter, the Ubip63E promoter maintains GFP expression perpetually in all subsequent daughter cells, independently of Gal4 activity. With ey-gal4, this allowed permanent expression of GFP in all the progeny of cells that expressed ey-gal4 at some point (Evans et al., 2009). In L3, ey-gal4 was expressed in neuroblasts (Fig. 1G) and in the vast majority of neurons derived from ey-positive neuroblasts that still maintained ey-gal4 expression (most cells were both red and green; Fig. 2A-B), although this might be due to perdurance of Gal4 during L3 (see below). Expression of G-TRACE with ap- and dll-gal4 caused larval lethality and precluded the analysis in L3 larval stage. To avoid these early developmental defects, we made use of the tub-gal80 ts construct (McGuire et al., 2004). We raised at 18 C larvae containing ap- or dll-gal4, G-TRACE and tub-gal80 ts until the second instar stage to allow Gal80 repression of Gal4 activity. Then, these larvae were incubated at 30 C for 2 days during third instar larval stages to relieve repression and to allow induction of G-TRACE when neuroblasts actively divide (Fig. 2E,F,I,J). ap-gal4 was observed in neurons derived from ey-positive neuroblasts (Fig. 2E,F) but was not expressed in neuroblasts (see Fig. S3A,B in the supplementary material). dll-gal4 (which precisely mimics Dll expression) showed expression in a small population of neurons located at the most proximal part of the OPC, i.e. in neurons generated by the oldest neuroblasts to have emerged from the neuroepithelium (Fig. 2I,J), but not in the neuroblasts themselves (see Fig. S3C,D in the supplementary material). Thus, ap and dll expression showed a delay before being turned on in maturing neurons. Next, using the G-TRACE we analyzed the lineage followed by those larval cells in pupae (P40) to determine whether the expression of those Gal4 lines persisted during development. Indeed, ap and dll expression persisted during pupal development (Fig. 2G,H,K,L). By contrast, although ey-gal4 expression was detectable in the vast majority of larval cells (Fig. 2A,B), its expression did not persist in pupae and a proportion of those cells were only green (Fig. 2C,D). Therefore, ey-gal4 expression shows perdurance or is downregulated in most OPC-derived cells during pupal stages, with Ey being expressed in a subset of postmitotic cells that are negative for ap and Dll expression (Fig. 1). Therefore, at least three different OPC-derived postmitotic cell populations marked by Ey, ap or Dll are spatially and temporally segregated early in larvae and pupae. These distinct cell populations appear to be pre-patterned early and subsequently become intermingled (Fig. 1, see Fig. S2 in the supplementary material). Patterns of cell movements in optic lobe cells The spatial and temporal segregation displayed by the different medulla cell populations coming from the OPC during larval and early pupal development is in sharp contrast with the extensive intermingling of cell types in the adult medulla cortex (compare Fig. 1B with 1L). This indicates that extensive cell movements take place in OPC-derived cells to achieve the final positioning of cell bodies and projections in the adult optic lobe. Although cell movement has been studied in detail in the Drosophila ovary and embryo (Kunwar et al., 2006; Rorth, 2007), its mechanisms have not been investigated in detail in the central nervous system. The different cell populations that we observe might move similarly to each other, or each might use distinct mechanisms to achieve the final distribution of cells in the medulla cortex. We followed cell movements exhibited by ey-, ap- and dll-positive cells by performing mosaic analysis with a repressible cell marker (MARCM) (Lee and Luo, 1999) to generate neuroblast clones marked by the corresponding Gal4 drivers. The clones were
4 690 Development 138 (4) Fig. 2. G-TRACE analysis of OPC-derived neurons. (A-L) G-TRACE analysis with ey-gal4 (A-D), ap-gal4 (E-H) and dll-gal4 (I-L) reporter lines. Images show OPC-derived cells at late L3 (A,B,E,F,I,J) and P40 (C,D,G,H,K,L). Neuroepithelial cells in A,B,E,F,I,J were labeled with DECadherin (blue). Arrowheads in C,D indicate persisting ey expression in pupal cells (red and green) and arrows indicate cells with previous ey expression that no longer express the reporter (green). given neuroblast sequentially produces different cell types. The same columnar organization was maintained in OPC-derived clones analyzed during early pupal (P10) development (15 clones analyzed in 10 optic lobes) (Fig. 4B). By contrast, cells lost their columnar arrangement during mid-pupation and became dispersed in all axes throughout medulla cortex (70 clones analyzed in 38 optic lobes) (Fig. 4C). Despite this major dispersion, some medulla cells remained in small clusters of three or four cells. Fig. 3. Cell migration pattern of eyeless and apterous cells. (A-F,H-J) Migration patterns of ap- (A-C), ey- (D-F) and eymisexpressing eydn (H-J) MARCM clones (green) generated 72 hours AEL and analyzed in late L3 (A,D,H), in P44 (B,E,I) and in P68 (C,F,J). Brackets in B,C,E,F indicate layers of medulla neuropils. Dotted circles in I,J highlight the accumulation of neurons in large clumps. Neurons were visualized with Elav (red) and the neuropil with nc82 (blue). (G,K) Proliferation analysis in ey-expressing (G) and ey-misexpressing eydn (K) MARCM clones (green) generated 72 hours AEL and analyzed at P5. Mitosis was visualized with anti-phospho Histone 3 (red) and the neuropil with DN-Cad (blue). generated in early L3 [72 hours after egg laying (AEL)] and brains were dissected at different stages throughout late larval and pupal development until adulthood (Fig. 3A-F and data not shown). This allowed us to visualize small subsets of cells. In late L3 larvae, ey- and ap-marked OPC-derived clones presented a striking arrangement as discrete columns of cells (Fig. 3A,D), with the progeny of a neuroblast displacing cells away from the OPC (Egger et al., 2007). In the case of dll-positive cells coming from the OPC, the columnar organization was not evident in single confocal sections or through reconstructions (data not shown), probably owing to the very small number of dll-gal4 neurons produced from each neuroblast. By contrast, at mid-pupation (P44), profound reorganization occurred. Cells lost the columnar arrangement observed in larvae and became dispersed, while dendritic ramifications started to form hints of medulla layers (Fig. 3B,E). By late pupation (P68), medulla cells had reached their final position and were dispersed throughout the medulla cortex (Fig. 3C,F). Their dendritic arbors and axonal projections resembled those of the adult (Fischbach and Dittrich, 1989; Gao et al., 2008; Morante and Desplan, 2008), although we could still observe significant growth of branches during subsequent stages of development. To visualize the patterns of cell movements followed by neurons derived from the OPC, we followed the fate of clonally marked cells derived from a single progenitor. We generated MARCM GFP clones using a tubulin-gal4 driver and we studied the movement displayed by ap-positive and Dll-positive cells using fixed tissues. Clones were generated in early L3 (72 hours AEL) and brains were dissected at different stages throughout late larval and pupal development to follow their migratory paths (Fig. 4). In late L3 larval brains, tub-gal4 MARCM clones (72 clones analyzed in 43 optic lobes) derived from the OPC showed a columnar organization (Fig. 4A). When we analyzed the progeny of the oldest neuroblasts that emerged from the neuroepithelium with ap-lacz and Dll, we observed Dll in the latest born neurons, whereas ap-lacz was expressed in older neurons (Fig. 4A). These cells were interspersed with unlabelled neurons that presumably expressed other markers (Fig. 4A). This confirms the columnar organization observed by eyand ap-marked OPC-derived clones (Fig. 3A,D) and shows that a
5 Cell migration in Drosophila optic lobe 691 acquire their final position in the optic lobe and form the retinotopic map, with the different cell populations intermingling with each other (Fig. 4D). Fig. 4. Cell migration pattern of clonally related cells. (A-C) Migration patterns of tub-marcm clones (green) generated 72 hours AEL and analyzed in late L3 (A), P10 (B) and P44 (C). Dll-positive cells visualized with anti-dll (red) and ap-positive cells with ap-lacz (blue). White arrows in A-C mark ap-positive cells, green arrows mark GFP-positive cells negative for ap and arrowheads label Dll-positive cells. (D) Schematic representation of a larval column in the OPC. During pupation, these OPC-derived cells lose their columnar organization and become dispersed throughout the medulla cortex with non-lineage-related cells (gray cells). In order to address whether cell death plays a role in the dispersion of medulla cortex neurons, we generated MARCM GFP clones using a tub-gal4 promoter mis-expressing UAS-p35, an apoptosis inhibitor. In adult brains (n 30 brains), medulla cortex cells did not show cell accumulation or alteration in the medulla neuropil lamination (data not shown). Thus, optic lobe cells derived from the OPC follow two patterns of cell movement to achieve their final position in the adult optic lobe: first, neurons derived from the same progenitors are arranged in columns of cells, where neuroblasts sequentially produce cells that express different neuronal markers. These cells probably move away from the neuroblast because they are displaced by newly generated neurons, or they could undergo radial migration. Then, neurons lose this configuration in columns and disperse laterally to Altered migration of optic lobe cells lacking eyeless function As previously described, Ey is expressed in OPC-derived neuroblasts (Fig. 1). Thus, we addressed the function of ey in neuroblasts. For this purpose, we expressed a dominant-negative form of the Eyeless protein (hereafter referred as ey DN ) in which the N-terminal domain is replaced by an Engrailed-repressor domain (Niimi et al., 2002), rendering the Ey activator a constitutive transcriptional repressor. We generated MARCM clones expressing ey DN under the control of ey-gal4. We compared the movement of these cells (Fig. 3H-J, Fig. 5B) with those of wild-type cells (Fig. 3D-F, Fig. 5A). Clones were generated in early L3 (72 hours AEL) and brains were dissected at different stages throughout larval and pupal development until adulthood. Wild-type clones forming columns of cells were observed in L3 and early pupation in wild type (Fig. 3D); by mid-pupation, these columns were lost, and dendritic ramifications were observed in the neuropil (Fig. 3E,F). The ey DN clones also formed columns of cells early (Fig. 3H). However, by mid-pupation, these clones formed clumps of cells (compare Fig. 3E,F with 3I,J). Clusters of cells significantly larger than in wild type could still be observed in adult brain sections [number of ey cells per cluster in wild-type adult brains (mean±s.e.m.), 4.08±1.47; in ey DN brains, 19.4±1.56) (Fig. 5E). This accumulation of clustered ey-cells disrupted the normal layering of the medulla as the cells were unable to disperse and reach their final retinotopic position. To understand the genesis of clustering in ey DN MARCM clones, we analyzed the pattern of proliferation with phospho-histone 3 in early pupal brains (P5). In wild-type MARCM clones (63 clones analyzed in 26 optic lobes), ey-positive cells formed columns of cells where some individuals cells started to move laterally (Fig. 3G). By contrast, ey DN MARCM clones (75 clones analyzed in 32 optic lobes) were rounder with many cells proliferating (mean±s.e.m.): 1.01±0.12 ph3-positive cells/clone in ey DN MARCM clones versus 0.2±0.04 ph3-positive cells/clone in control) and expressing neuroblast markers (Fig. 3K and data not shown), although other cells clearly showed signs of differentiation. In addition, these neuronal clusters exhibited thick axonal bundles (data not shown). Fig. 5. Impaired optic lobe cell migration in ey DN MARCM clones rescued by DE-Cadherin. (A-D) Migration patterns of ey-positive cells in wildtype MARCM clones (A), in clones misexpressing ey DN (B), in clones lacking DE-Cadherin (C), and in clones misexpressing ey DN and lacking DE-Cadherin (D) (green) generated 72 hours AEL and analyzed in adult. Neuropil (red) was visualized with DN-Cadherin and photoreceptors with 24B10 (blue). Dotted circles in B highlight the accumulation of neurons in large clumps. (E) Number of cells per clone in ey-positive MARCM clones (mean±s.e.m.: 4.08±0.3), in clones misexpressing ey DN (19.43±1.56), and in clones lacking DE-Cadherin and mis-expressing ey DN (6.04±0.31). (F) Number of large clumps per optic lobe in ey-positive MARCM clones (0), in clones mis-expressing ey DN (5.44±0.28), and in clones lacking DE-Cadherin and mis-expressing ey DN (3.11±0.35). **P<0.001.
6 692 Development 138 (4) In larval ey DN MARCM clones, upregulation of DE-Cadherin was observed (Fig. 6C,D). In wild-type brains, DE-Cadherin is expressed by postembryonic neuroblasts (Fig. 1F,G) (Dumstrei et al., 2003). Dumstrei et al. (Dumstrei et al., 2003) showed that global expression of a dominant-negative form of DE-Cadherin driven by a heat pulse during early second instar results in a severe phenotype that includes deficits in neural proliferation: although neuroblasts appears in approximately normal numbers, they have highly reduced mitotic activity. In order to test whether the clustering of cells in ey DN resulted from increased expression of DE-Cadherin, we removed DE- Cadherin using a shotgun (shg) mutation in MARCM clones that also expressed ey DN. These clones (Fig. 5D) formed clumps of cells (defined by the presence of more than six cells in a cluster and thick axonal bundles) at a much lower frequency [18/63 brains in ey DN shg exhibited clumps of cells (mean±s.e.m.): 3.11±0.35 clumps/optic lobe, when compared with 129/129 in ey DN brains with 5.44±0.28 clumps/optic lobe] (Fig. 5F), and most ey DN -expressing cells were found distributed throughout the medulla cortex (6.0±0.31 cells per cluster in ey DN shg compared with 19.4±1.56 in ey DN and 4.08±1.47 in wild-type clusters in adult brains) (Fig. 5E). Therefore, the loss of DEcadherin appears to rescue the clumping phenotype of ey mutant cells, presumably by reducing the mitotic activity of neuroblasts and allowing neurons to migrate. Thus, the lack of ey causes an increase in neuroblast proliferation and this impedes cells from reaching their final position in the medulla part of the optic lobe. This occurs at least in part by upregulation of DE-Cadherin. Therefore, eyeless links neurogenesis and migration in the optic lobe. DISCUSSION We show here that, as previously reported (Meinertzhagen and Hanson, 1993), medulla cortex cells indeed originate from the OPC (Fig. 1). In adults, there are ~800 columns in the medulla, each formed by more than 60 different cell types that repetitively contact every R7-R8 fascicle (Morante and Desplan, 2008). The spatial segregation of ey-, ap- and dll-positive cells observed early in larval brain lobes is lost during pupal development through two modes of cell movements happening during larval (radial) and pupal life (mostly lateral) (Figs 3, 4). As a result, each adult column in wild-type medulla is composed of the precise complement of neurons necessary to process visual information (Gao et al., 2008; Morante and Desplan, 2008). Each R7/R8 termination pair is surrounded by at least 47 different cell types, while 13 other cell types do not appear to contact PRs (Morante and Desplan, 2008). Further experiments will determine whether the cell movements shown by medulla cortex cells during optic lobe organogenesis involve the dispersion of the entire neuron, or only the cell body. ey-, dll- and ap-positive neurons originating from the OPCderived neuroblasts show a radial organization, while the number of medulla neurons expands (Fig. 1H). This organization resembles the radial units present in the embryonic mammalian neocortex where cells reach their appropriate layer via radial migration along glial processes, expanding the size of the cerebral cortex (Noctor et al., 2001; Rakic, 1988; Rakic, 1992). However, it is unlikely that there is active radial migration in the medulla, as neurons appear to be simply displaced by newly formed neurons above them. The lack of eyeless causes an increase in neuroblast proliferation and impedes cells from reaching their final position in the medulla. It should be noted that some ey DN cells still manage to disperse in Fig. 6. Increased expression of DE-Cadherin in ey DN MARCM clones. (A-D) DE-Cadherin (red) staining in ey-marcm wild-type clones (A,B) and in ey-marcm clones misexpressing ey DN (C,D) generated 72 hours AEL and analyzed in L3. the medulla cortex. They might represent cells that express ey-gal4 but do not express or require ey for their migration, or cells that express lower levels of ey-gal4 in which ey DN is not sufficient to affect function. We also analyzed the effect of ey DN on eye imaginal discs and observed the same phenotype as that reported by Niimi et al. (Niimi et al., 2002); expression of ey DN resulted in underproliferation of eye imaginal disc cells and a partial or complete loss of eye structures (data not shown), which is similar to what is seen in eyeless mutants. Thus, the effects of ey DN in the eye disc are, surprisingly, the opposite of what we observe in medulla neuroblasts. Furthermore, to address a possible role of eyeless in postmitotic cells, we mis-expressed ey DN using an act-frt-stop-frt-gal4 construct both in larvae and in postmitotic cells (after P25). This mis-expression resulted in lethality, even with very short heatshocks, probably owing to the strength of the act-gal4 driver; this precludes analysis in adults. Thus, at this point, we cannot eliminate a possible role of eyeless in postmitotic cells. Interestingly, Sey mice embryos, which are mutant for the mouse ortholog of ey (Pax6), exhibit an increase in the number of Cajal-Retzius cells, which migrate tangentially (Stoykova et al., 2003). Simultaneously, late-born cortical precursors show abnormalities in their patterns of radial migration, forming heterotopic clusters in the path towards their final position in the embryonic cerebral cortex (Caric et al., 1997). Although this defective neuronal radial migration has been proposed to be due to an altered radial glia morphology (Gotz et al., 1998), other studies have shown that these clusters exhibit an increase in adhesion molecules in neurons (Caric et al., 1997). Biochemical studies have demonstrated the presence of binding sites for the Pax6 transcription factor in the NCAM and L1 promoters (Holst
7 Cell migration in Drosophila optic lobe 693 et al., 1997; Meech et al., 1999). Thus, increased proliferation or adhesion among neurons in the clusters could prevent Sey mutant cells from detaching from each other to reach their final position in the cerebral cortex. Therefore, development of medulla neurons of the optic lobe resembles the neurogenesis and migration mechanisms observed during the development of the embryonic mammalian brain. Acknowledgements We are very grateful to P. Callaerts, R. Carthew, C. Estella, T. Hayashi, R. Mann, F. Matsuzaki, J. Skeath, J. Treisman, the Bloomington Stock Center and the Developmental Studies Hybridoma Bank at the University of Iowa for reagents, and to I. Tan for help with the Volocity software. We thank Justin Blau, Ching- Man Choi, Volker Hartenstein, Gord Fishell, Juan Luque, Daniel Vasiliauskas and members of the Desplan laboratory for very useful comments on the manuscript and for support and discussions. This work was supported by Grant NIH R01 EY to C.D. We especially thank Maria Dominguez with whom this study was finished with the support of the Ministerio de Ciencia e Innovación (BFU and MEC-Consolider CSD ), Generalitat Valenciana (PROMETEO 2008/134), Fundacion Marcelino Botin and a European Union research grant UE-HEALTH-F J.M. was supported by a JAE-Doc fellowship funded by the Ministerio de Ciencia e Innovacion. Deposited in PMC for release after 12 months. Competing interests statement The authors declare no competing financial interests. Supplementary material Supplementary material for this article is available at References Betschinger, J., Mechtler, K. and Knoblich, J. A. (2006). Asymmetric segregation of the tumor suppressor brat regulates self-renewal in Drosophila neural stem cells. Cell 124, Caric, D., Gooday, D., Hill, R. E., McConnell, S. K. and Price, D. J. (1997). Determination of the migratory capacity of embryonic cortical cells lacking the transcription factor Pax-6. Development 124, Clements, J., Hens, K., Francis, C., Schellens, A. and Callaerts, P. (2008). Conserved role for the Drosophila Pax6 homolog Eyeless in differentiation and function of insulin-producing neurons. Proc. Natl. Acad. Sci. USA 105, Cohen, B., McGuffin, M. E., Pfeifle, C., Segal, D. and Cohen, S. M. (1992). apterous, a gene required for imaginal disc development in Drosophila encodes a member of the LIM family of developmental regulatory proteins. Genes Dev. 6, Connolly, J. B., Roberts, I. J., Armstrong, J. D., Kaiser, K., Forte, M., Tully, T. and O Kane, C. J. (1996). Associative learning disrupted by impaired Gs signaling in Drosophila mushroom bodies. Science 274, Dumstrei, K., Wang, F. and Hartenstein, V. (2003). Role of DE-cadherin in neuroblast proliferation, neural morphogenesis, and axon tract formation in Drosophila larval brain development. J. Neurosci. 23, Egger, B., Boone, J. Q., Stevens, N. R., Brand, A. H. and Doe, C. Q. (2007). Regulation of spindle orientation and neural stem cell fate in the Drosophila optic lobe. Neural Dev. 2, 1. Estella, C., McKay, D. J. and Mann, R. S. (2008). Molecular integration of Wingless, Decapentaplegic, and autoregulatory inputs into Distalless during Drosophila leg development. Dev. Cell 14, Evans, C. J., Olson, J. M., Ngo, K. T., Kim, E., Lee, N. E., Kuoy, E., Patananan, A. N., Sitz, D., Tran, P., Do, M. T. et al. (2009). G-TRACE: rapid Gal4-based cell lineage analysis in Drosophila. Nat. Methods 6, Fischbach, K. F. and Dittrich, A. P. M. (1989). The optic lobe of Drosophila melanogaster. I. A Golgi analysis of wild-type structure. Cell Tissue Res. 258, Gao, S., Takemura, S. Y., Ting, C. Y., Huang, S., Lu, Z., Luan, H., Rister, J., Thum, A. S., Yang, M., Hong, S. T. et al. (2008). The neural substrate of spectral preference in Drosophila. Neuron 60, Ge, W., He, F., Kim, K. J., Blanchi, B., Coskun, V., Nguyen, L., Wu, X., Zhao, J., Heng, J. I., Martinowich, K. et al. (2006). Coupling of cell migration with neurogenesis by proneural bhlh factors. Proc. Natl. Acad. Sci. USA 103, Gotz, M., Stoykova, A. and Gruss, P. (1998). Pax6 controls radial glia differentiation in the cerebral cortex. Neuron 21, Green, P., Hartenstein, A. Y. and Hartenstein, V. (1993). The embryonic development of the Drosophila visual system. Cell Tissue Res. 273, Hofbauer, A. and Campos-Ortega, J. A. (1990). Proliferation pattern and early differentiation of the optic lobes in Drosophila melanogaster. Roux s Arch. Dev. Biol. 198, Holst, B. D., Wang, Y., Jones, F. S. and Edelman, G. M. (1997). A binding site for Pax proteins regulates expression of the gene for the neural cell adhesion molecule in the embryonic spinal cord. Proc. Natl. Acad. Sci. USA 94, Ikeshima-Kataoka, H., Skeath, J. B., Nabeshima, Y., Doe, C. Q. and Matsuzaki, F. (1997). Miranda directs Prospero to a daughter cell during Drosophila asymmetric divisions. Nature 390, Ito, K. and Hotta, Y. (1992). Proliferation pattern of postembryonic neuroblasts in the brain of Drosophila melanogaster. Dev. Biol. 149, Kunwar, P. S., Siekhaus, D. E. and Lehmann, R. (2006). In vivo migration: a germ cell perspective. Annu. Rev. Cell Dev. Biol. 22, Lee, T. and Luo, L. (1999). Mosaic analysis with a repressible cell marker for studies of gene function in neuronal morphogenesis. Neuron 22, McGuire, S. E., Mao, Z. and Davis, R. L. (2004). Spatiotemporal gene expression targeting with the TARGET and gene-switch systems in Drosophila. Sci STKE 2004, pl6. Meech, R., Kallunki, P., Edelman, G. M. and Jones, F. S. (1999). A binding site for homeodomain and Pax proteins is necessary for L1 cell adhesion molecule gene expression by Pax-6 and bone morphogenetic proteins. Proc. Natl. Acad. Sci. USA 96, Meinertzhagen, I. A. and Hanson, T. E. (1993). The Development of the Optic Lobe. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press. Morante, J. and Desplan, C. (2008). The color-vision circuit in the medulla of Drosophila. Curr. Biol. 18, Morante, J. and Desplan, C. (2010). Dissecting and staining Drosophila optic lobes. In Drosophila Neurobiology: A Laboratory Manual (ed. B. Zhang, M. R. Freeman and S. Waddell), pp Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press. Niimi, T., Clements, J., Gehring, W. J. and Callaerts, P. (2002). Dominantnegative form of the Pax6 homolog eyeless for tissue-specific loss-of-function studies in the developing eye and brain in drosophila. Genesis 34, Noctor, S. C., Flint, A. C., Weissman, T. A., Dammerman, R. S. and Kriegstein, A. R. (2001). Neurons derived from radial glial cells establish radial units in neocortex. Nature 409, Ohshiro, T., Yagami, T., Zhang, C. and Matsuzaki, F. (2000). Role of cortical tumour-suppressor proteins in asymmetric division of Drosophila neuroblast. Nature 408, Rakic, P. (1988). Specification of cerebral cortical areas. Science 241, Rakic, P. (1992). Dividing up the neocortex. Science 258, Ramón y Cajal, S. and Sanchez, D. (1915). Contribucion al conocimiento de los centros nerviosos de los insectos. Trab. Lab. Invest. Biol. XIII, Rorth, P. (2007). Collective guidance of collective cell migration. Trends Cell Biol. 17, Stoykova, A., Hatano, O., Gruss, P. and Gotz, M. (2003). Increase in reelinpositive cells in the marginal zone of Pax6 mutant mouse cortex. Cereb. Cortex 13, Strausfeld, N. J. (1976). Atlas of an Insect Brain. Berlin, Heidelberg, New York: Springer-Verlag. White, K. and Kankel, D. R. (1978). Patterns of cell division and cell movement in the formation of the imaginal nervous system in Drosophila melanogaster. Dev. Biol. 65, Yasugi, T., Umetsu, D., Murakami, S., Sato, M. and Tabata, T. (2008). Drosophila optic lobe neuroblasts triggered by a wave of proneural gene expression that is negatively regulated by JAK/STAT. Development 135,
glial cells missing and gcm2 Cell-autonomously Regulate Both Glial and Neuronal
 glial cells missing and gcm2 Cell-autonomously Regulate Both Glial and Neuronal Development in the Visual System of Drosophila Carole Chotard, Wendy Leung and Iris Salecker Supplemental Data Supplemental
glial cells missing and gcm2 Cell-autonomously Regulate Both Glial and Neuronal Development in the Visual System of Drosophila Carole Chotard, Wendy Leung and Iris Salecker Supplemental Data Supplemental
Article. The Color-Vision Circuit in the Medulla of Drosophila. Javier Morante 1 and Claude Desplan 1, * 1
 Current Biology 18, 1 13, April 22, 2008 ª2008 Elsevier Ltd All rights reserved DOI 10.1016/j.cub.2008.02.075 The Color-Vision Circuit in the Medulla of Drosophila Article Javier Morante 1 and Claude Desplan
Current Biology 18, 1 13, April 22, 2008 ª2008 Elsevier Ltd All rights reserved DOI 10.1016/j.cub.2008.02.075 The Color-Vision Circuit in the Medulla of Drosophila Article Javier Morante 1 and Claude Desplan
Nature Neuroscience: doi: /nn Supplementary Figure 1
 Supplementary Figure 1 Expression of escargot (esg) and genetic approach for achieving IPC-specific knockdown. (a) esg MH766 -Gal4 UAS-cd8GFP (green) and esg-lacz B7-2-22 (red) show similar expression
Supplementary Figure 1 Expression of escargot (esg) and genetic approach for achieving IPC-specific knockdown. (a) esg MH766 -Gal4 UAS-cd8GFP (green) and esg-lacz B7-2-22 (red) show similar expression
Dissecting and Staining Drosophila Optic Lobes PAGE PROOFS
 Ch10_DNB:Drosophila Neurobiology Manual 2/19/10 9:11 AM Page 1 10 Dissecting and Staining Drosophila Optic Lobes ABSTRACT The Drosophila visual system is composed of Optic Lobes at Different Stages of
Ch10_DNB:Drosophila Neurobiology Manual 2/19/10 9:11 AM Page 1 10 Dissecting and Staining Drosophila Optic Lobes ABSTRACT The Drosophila visual system is composed of Optic Lobes at Different Stages of
Supplemental Data SUPPLEMENTAL EXPERIMENTAL PROCEDURES
 Temporal transcription factors and their targets schedule the end of neural proliferation in Drosophila. Cédric Maurange, Louise Cheng and Alex P. Gould Supplemental Data SUPPLEMENTAL EXPERIMENTAL PROCEDURES
Temporal transcription factors and their targets schedule the end of neural proliferation in Drosophila. Cédric Maurange, Louise Cheng and Alex P. Gould Supplemental Data SUPPLEMENTAL EXPERIMENTAL PROCEDURES
G-TRACE: rapid Gal4-based cell lineage analysis in Drosophila
 nature methods G-TRACE: rapid Gal4-based cell lineage analysis in Drosophila Cory J Evans, John M Olson, Kathy T Ngo, Eunha Kim, Noemi E Lee, Edward Kuoy, Alexander N Patananan, Daniel Sitz, PhuongThao
nature methods G-TRACE: rapid Gal4-based cell lineage analysis in Drosophila Cory J Evans, John M Olson, Kathy T Ngo, Eunha Kim, Noemi E Lee, Edward Kuoy, Alexander N Patananan, Daniel Sitz, PhuongThao
Neurogenesis and neuronal circuit formation in the Drosophila visual center
 The Japanese Society of Developmental Biologists Develop. Growth Differ. (2014) 56, 491 498 doi: 10.1111/dgd.12151 Review Article Neurogenesis and neuronal circuit formation in the Drosophila visual center
The Japanese Society of Developmental Biologists Develop. Growth Differ. (2014) 56, 491 498 doi: 10.1111/dgd.12151 Review Article Neurogenesis and neuronal circuit formation in the Drosophila visual center
Cell Birth and Death. Chapter Three
 Cell Birth and Death Chapter Three Neurogenesis All neurons and glial cells begin in the neural tube Differentiated into neurons rather than ectoderm based on factors we have already discussed If these
Cell Birth and Death Chapter Three Neurogenesis All neurons and glial cells begin in the neural tube Differentiated into neurons rather than ectoderm based on factors we have already discussed If these
Neurodevelopment II Structure Formation. Reading: BCP Chapter 23
 Neurodevelopment II Structure Formation Reading: BCP Chapter 23 Phases of Development Ovum + Sperm = Zygote Cell division (multiplication) Neurogenesis Induction of the neural plate Neural proliferation
Neurodevelopment II Structure Formation Reading: BCP Chapter 23 Phases of Development Ovum + Sperm = Zygote Cell division (multiplication) Neurogenesis Induction of the neural plate Neural proliferation
SUPPLEMENTARY INFORMATION
 doi: 10.1038/nature07173 SUPPLEMENTARY INFORMATION Supplementary Figure Legends: Supplementary Figure 1: Model of SSC and CPC divisions a, Somatic stem cells (SSC) reside adjacent to the hub (red), self-renew
doi: 10.1038/nature07173 SUPPLEMENTARY INFORMATION Supplementary Figure Legends: Supplementary Figure 1: Model of SSC and CPC divisions a, Somatic stem cells (SSC) reside adjacent to the hub (red), self-renew
The Tumor Suppressors Brat and Numb Regulate Transit-Amplifying Neuroblast Lineages in Drosophila
 Article The Tumor Suppressors Brat and Numb Regulate Transit-Amplifying Neuroblast Lineages in Drosophila Sarah K. Bowman, 1 Vivien Rolland, 1 Joerg Betschinger, 1,3 Kaolin A. Kinsey, 2 Gregory Emery,
Article The Tumor Suppressors Brat and Numb Regulate Transit-Amplifying Neuroblast Lineages in Drosophila Sarah K. Bowman, 1 Vivien Rolland, 1 Joerg Betschinger, 1,3 Kaolin A. Kinsey, 2 Gregory Emery,
Supplementary Figure 1 Madm is not required in GSCs and hub cells. (a,b) Act-Gal4-UAS-GFP (a), Act-Gal4-UAS- GFP.nls (b,c) is ubiquitously expressed
 Supplementary Figure 1 Madm is not required in GSCs and hub cells. (a,b) Act-Gal4-UAS-GFP (a), Act-Gal4-UAS- GFP.nls (b,c) is ubiquitously expressed in the testes. The testes were immunostained with GFP
Supplementary Figure 1 Madm is not required in GSCs and hub cells. (a,b) Act-Gal4-UAS-GFP (a), Act-Gal4-UAS- GFP.nls (b,c) is ubiquitously expressed in the testes. The testes were immunostained with GFP
Viktorin, G. and Riebli, N. and Popkova, A. and Giangrande, A. and Reichert, H.
 Institutional Repository of the University of Basel University Library Schoenbeinstrasse 18-20 CH-4056 Basel, Switzerland http://edoc.unibas.ch/ Year: 2011 Multipotent neural stem cells generate glial
Institutional Repository of the University of Basel University Library Schoenbeinstrasse 18-20 CH-4056 Basel, Switzerland http://edoc.unibas.ch/ Year: 2011 Multipotent neural stem cells generate glial
Temporal Patterning of Neuroblasts Controls Notch-Mediated Cell Survival through Regulation of Hid or Reaper
 Temporal Patterning of Neuroblasts Controls Notch-Mediated Cell Survival through Regulation of Hid or Reaper Claire Bertet, 1 Xin Li, 1 Ted Erclik, 1 Matthieu Cavey, 1 Brent Wells, 1 and Claude Desplan
Temporal Patterning of Neuroblasts Controls Notch-Mediated Cell Survival through Regulation of Hid or Reaper Claire Bertet, 1 Xin Li, 1 Ted Erclik, 1 Matthieu Cavey, 1 Brent Wells, 1 and Claude Desplan
Concentric zones, cell migration and neuronal circuits in the Drosophila visual center
 983 Development 138, 983-993 (2011) doi:10.1242/dev.058370 2011. Published by The Company of Biologists Ltd Concentric zones, cell migration and neuronal circuits in the Drosophila visual center Eri Hasegawa
983 Development 138, 983-993 (2011) doi:10.1242/dev.058370 2011. Published by The Company of Biologists Ltd Concentric zones, cell migration and neuronal circuits in the Drosophila visual center Eri Hasegawa
SUPPLEMENTARY INFORMATION
 Supplementary Figure 1. Ras V12 expression in the entire eye-antennal disc does not cause invasive tumours. a, Eye-antennal discs expressing Ras V12 in all cells (marked with GFP, green) overgrow moderately
Supplementary Figure 1. Ras V12 expression in the entire eye-antennal disc does not cause invasive tumours. a, Eye-antennal discs expressing Ras V12 in all cells (marked with GFP, green) overgrow moderately
Developmental Biology
 Developmental iology 356 (2011) 553 565 ontents lists available at ScienceDirect Developmental iology journal homepage: www.elsevier.com/developmentalbiology Multipotent neural stem cells generate glial
Developmental iology 356 (2011) 553 565 ontents lists available at ScienceDirect Developmental iology journal homepage: www.elsevier.com/developmentalbiology Multipotent neural stem cells generate glial
Supporting Online Material for
 www.sciencemag.org/cgi/content/full/1171320/dc1 Supporting Online Material for A Frazzled/DCC-Dependent Transcriptional Switch Regulates Midline Axon Guidance Long Yang, David S. Garbe, Greg J. Bashaw*
www.sciencemag.org/cgi/content/full/1171320/dc1 Supporting Online Material for A Frazzled/DCC-Dependent Transcriptional Switch Regulates Midline Axon Guidance Long Yang, David S. Garbe, Greg J. Bashaw*
Nature Neuroscience: doi: /nn Supplementary Figure 1. MADM labeling of thalamic clones.
 Supplementary Figure 1 MADM labeling of thalamic clones. (a) Confocal images of an E12 Nestin-CreERT2;Ai9-tdTomato brain treated with TM at E10 and stained for BLBP (green), a radial glial progenitor-specific
Supplementary Figure 1 MADM labeling of thalamic clones. (a) Confocal images of an E12 Nestin-CreERT2;Ai9-tdTomato brain treated with TM at E10 and stained for BLBP (green), a radial glial progenitor-specific
Development Supplementary information
 Supplemental Materials and Methods Mosaic clonal analysis GSC and SP clones were induced with the FLP/FRT-mediated mitotic recombination technique (Xu and Rubin, 1993) in files with following genotypes:
Supplemental Materials and Methods Mosaic clonal analysis GSC and SP clones were induced with the FLP/FRT-mediated mitotic recombination technique (Xu and Rubin, 1993) in files with following genotypes:
Brat Is a Miranda Cargo Protein that Promotes Neuronal Differentiation and Inhibits Neuroblast Self-Renewal
 Developmental Cell 10, 441 449, April, 2006 ª2006 Elsevier Inc. DOI 10.1016/j.devcel.2006.01.017 Brat Is a Miranda Cargo Protein that Promotes Neuronal Differentiation and Inhibits Neuroblast Self-Renewal
Developmental Cell 10, 441 449, April, 2006 ª2006 Elsevier Inc. DOI 10.1016/j.devcel.2006.01.017 Brat Is a Miranda Cargo Protein that Promotes Neuronal Differentiation and Inhibits Neuroblast Self-Renewal
Mammalian Cerebral Cortex: Embryonic Development and Cytoarchitecture
 Mammalian Cerebral Cortex: Embryonic Development and Cytoarchitecture 2 The prenatal developmental of the mammalian cerebral cortex, including that of humans, is characterized by two sequential and interrelated
Mammalian Cerebral Cortex: Embryonic Development and Cytoarchitecture 2 The prenatal developmental of the mammalian cerebral cortex, including that of humans, is characterized by two sequential and interrelated
Supplementary Figure 1
 Supplementary Figure 1 Kif1a RNAi effect on basal progenitor differentiation Related to Figure 2. Representative confocal images of the VZ and SVZ of rat cortices transfected at E16 with scrambled or Kif1a
Supplementary Figure 1 Kif1a RNAi effect on basal progenitor differentiation Related to Figure 2. Representative confocal images of the VZ and SVZ of rat cortices transfected at E16 with scrambled or Kif1a
Neuroepithelial Cells and Neural Differentiation
 Neuroepithelial Cells and Neural Differentiation Neurulation The cells of the neural tube are NEUROEPITHELIAL CELLS Neural crest cells migrate out of neural tube Neuroepithelial cells are embryonic stem
Neuroepithelial Cells and Neural Differentiation Neurulation The cells of the neural tube are NEUROEPITHELIAL CELLS Neural crest cells migrate out of neural tube Neuroepithelial cells are embryonic stem
ErbB4 migrazione II parte
 ErbB4 migrazione II parte Control SVZ cells prefer to migrate on the NRG1 type III substrate the substrate preference of the neuroblasts migrating out of the SVZ explant was evaluated SVZ cells had a strong
ErbB4 migrazione II parte Control SVZ cells prefer to migrate on the NRG1 type III substrate the substrate preference of the neuroblasts migrating out of the SVZ explant was evaluated SVZ cells had a strong
Cell Migration II: CNS Cell Migration. Steven McLoon Department of Neuroscience University of Minnesota
 Cell Migration II: CNS Cell Migration Steven McLoon Department of Neuroscience University of Minnesota 1 Hey! The major concepts discussed relative to neural crest cell migration apply to cell migration
Cell Migration II: CNS Cell Migration Steven McLoon Department of Neuroscience University of Minnesota 1 Hey! The major concepts discussed relative to neural crest cell migration apply to cell migration
Review Article Neural Stem Cells in Drosophila: Molecular Genetic Mechanisms Underlying Normal Neural Proliferation and Abnormal Brain Tumor Formation
 Stem Cells International Volume 2012, Article ID 486169, 10 pages doi:10.1155/2012/486169 Review Article Neural Stem Cells in Drosophila: Molecular Genetic Mechanisms Underlying Normal Neural Proliferation
Stem Cells International Volume 2012, Article ID 486169, 10 pages doi:10.1155/2012/486169 Review Article Neural Stem Cells in Drosophila: Molecular Genetic Mechanisms Underlying Normal Neural Proliferation
Nature Neuroscience: doi: /nn Supplementary Figure 1. Neuron class-specific arrangements of Khc::nod::lacZ label in dendrites.
 Supplementary Figure 1 Neuron class-specific arrangements of Khc::nod::lacZ label in dendrites. Staining with fluorescence antibodies to detect GFP (Green), β-galactosidase (magenta/white). (a, b) Class
Supplementary Figure 1 Neuron class-specific arrangements of Khc::nod::lacZ label in dendrites. Staining with fluorescence antibodies to detect GFP (Green), β-galactosidase (magenta/white). (a, b) Class
Plasticity of Cerebral Cortex in Development
 Plasticity of Cerebral Cortex in Development Jessica R. Newton and Mriganka Sur Department of Brain & Cognitive Sciences Picower Center for Learning & Memory Massachusetts Institute of Technology Cambridge,
Plasticity of Cerebral Cortex in Development Jessica R. Newton and Mriganka Sur Department of Brain & Cognitive Sciences Picower Center for Learning & Memory Massachusetts Institute of Technology Cambridge,
marker. DAPI labels nuclei. Flies were 20 days old. Scale bar is 5 µm. Ctrl is
 Supplementary Figure 1. (a) Nos is detected in glial cells in both control and GFAP R79H transgenic flies (arrows), but not in deletion mutant Nos Δ15 animals. Repo is a glial cell marker. DAPI labels
Supplementary Figure 1. (a) Nos is detected in glial cells in both control and GFAP R79H transgenic flies (arrows), but not in deletion mutant Nos Δ15 animals. Repo is a glial cell marker. DAPI labels
Tlx3 and Tlx1 are post-mitotic selector genes determining glutamatergic over GABAergic cell fates
 Tlx3 and Tlx1 are post-mitotic selector genes determining glutamatergic over GABAergic cell fates L. Cheng, A. Arata, R. Mizuguchi, Y. Qian, A. Karunaratne, P.A. Gray, S. Arata, S. Shirasawa, M. Bouchard,
Tlx3 and Tlx1 are post-mitotic selector genes determining glutamatergic over GABAergic cell fates L. Cheng, A. Arata, R. Mizuguchi, Y. Qian, A. Karunaratne, P.A. Gray, S. Arata, S. Shirasawa, M. Bouchard,
CONTRACTING ORGANIZATION: Cold Spring Harbor Laboratory Cold Spring Harbor, NY 11724
 AD Award Number: W81XWH-10-1-0450 TITLE: The role of NF1 in memory retrieval PRINCIPAL INVESTIGATOR: Yi Zhong, Ph.D. CONTRACTING ORGANIZATION: Cold Spring Harbor Laboratory Cold Spring Harbor, NY 11724
AD Award Number: W81XWH-10-1-0450 TITLE: The role of NF1 in memory retrieval PRINCIPAL INVESTIGATOR: Yi Zhong, Ph.D. CONTRACTING ORGANIZATION: Cold Spring Harbor Laboratory Cold Spring Harbor, NY 11724
Postembryonic Development of Amplifying Neuroblast Lineages in the Drosophila Brain: Proliferation, Differentiation and Projection Patterns.
 Postembryonic Development of Amplifying Neuroblast Lineages in the Drosophila Brain: Proliferation, Differentiation and Projection Patterns. Inauguraldissertation zur Erlangung der Würde eines Doktors
Postembryonic Development of Amplifying Neuroblast Lineages in the Drosophila Brain: Proliferation, Differentiation and Projection Patterns. Inauguraldissertation zur Erlangung der Würde eines Doktors
Control of proliferation activation in quiescent neuroblasts of the Drosophila central nervous system
 Development 121, 1173-1182 (1995) Printed in Great Britain The Company of Biologists Limited 1995 1173 Control of proliferation activation in quiescent neuroblasts of the Drosophila central nervous system
Development 121, 1173-1182 (1995) Printed in Great Britain The Company of Biologists Limited 1995 1173 Control of proliferation activation in quiescent neuroblasts of the Drosophila central nervous system
Cell Type Nervous System I. Developmental Readout. Foundations. Stem cells. Organ formation. Human issues.
 7.72 10.11.06 Cell Type Nervous System I Human issues Organ formation Stem cells Developmental Readout Axes Cell type Axon guidance 3D structure Analysis Model + + organisms Foundations Principles 1 What
7.72 10.11.06 Cell Type Nervous System I Human issues Organ formation Stem cells Developmental Readout Axes Cell type Axon guidance 3D structure Analysis Model + + organisms Foundations Principles 1 What
SUPPLEMENTARY INFORMATION
 SUPPLEMENTARY INFORMATION doi:10.1038/nature11306 Supplementary Figures Supplementary Figure 1. Basic characterization of GFP+ RGLs in the dentate gyrus of adult nestin-gfp mice. a, Sample confocal images
SUPPLEMENTARY INFORMATION doi:10.1038/nature11306 Supplementary Figures Supplementary Figure 1. Basic characterization of GFP+ RGLs in the dentate gyrus of adult nestin-gfp mice. a, Sample confocal images
A region-specific neurogenesis mode requires migratory progenitors in the Drosophila visual system
 Europe PMC Funders Group Author Manuscript Published in final edited form as: Nat Neurosci. 2015 January ; 18(1): 46 55. doi:10.1038/nn.3896. A region-specific neurogenesis mode requires migratory progenitors
Europe PMC Funders Group Author Manuscript Published in final edited form as: Nat Neurosci. 2015 January ; 18(1): 46 55. doi:10.1038/nn.3896. A region-specific neurogenesis mode requires migratory progenitors
File name: Supplementary Information Description: Supplementary Figures, Supplementary Table and Supplementary References
 File name: Supplementary Information Description: Supplementary Figures, Supplementary Table and Supplementary References File name: Supplementary Data 1 Description: Summary datasheets showing the spatial
File name: Supplementary Information Description: Supplementary Figures, Supplementary Table and Supplementary References File name: Supplementary Data 1 Description: Summary datasheets showing the spatial
doi: /nature09554
 SUPPLEMENTARY INFORMATION doi:10.1038/nature09554 Supplementary Figure 1: Optical Tracing with New Photoactivatable GFP Variants Reveals Enhanced Labeling of Neuronal Processes We qualitatively compare
SUPPLEMENTARY INFORMATION doi:10.1038/nature09554 Supplementary Figure 1: Optical Tracing with New Photoactivatable GFP Variants Reveals Enhanced Labeling of Neuronal Processes We qualitatively compare
Supplementary Figures
 Supplementary Figures Supplementary Figure 1. nrg1 bns101/bns101 embryos develop a functional heart and survive to adulthood (a-b) Cartoon of Talen-induced nrg1 mutation with a 14-base-pair deletion in
Supplementary Figures Supplementary Figure 1. nrg1 bns101/bns101 embryos develop a functional heart and survive to adulthood (a-b) Cartoon of Talen-induced nrg1 mutation with a 14-base-pair deletion in
Head of College Scholars List Scheme. Summer Studentship Report Form
 Head of College Scholars List Scheme Summer Studentship 2019 Report Form This report should be completed by the student with his/her project supervisor. It should summarise the work undertaken during the
Head of College Scholars List Scheme Summer Studentship 2019 Report Form This report should be completed by the student with his/her project supervisor. It should summarise the work undertaken during the
Neural stem cells and the neurobiology of ageing. Chen Siyun 1, Dawe G.S. 2
 ABSTRACT Neural stem cells and the neurobiology of ageing Chen Siyun 1, Dawe G.S. 2 Department of Physics, Faculty of Science, National University of Singapore 10 Kent Ridge Road, Singapore 117546 The
ABSTRACT Neural stem cells and the neurobiology of ageing Chen Siyun 1, Dawe G.S. 2 Department of Physics, Faculty of Science, National University of Singapore 10 Kent Ridge Road, Singapore 117546 The
Supplementary Figure 1
 Supplementary Figure 1 The average sigmoid parametric curves of capillary dilation time courses and average time to 50% peak capillary diameter dilation computed from individual capillary responses averaged
Supplementary Figure 1 The average sigmoid parametric curves of capillary dilation time courses and average time to 50% peak capillary diameter dilation computed from individual capillary responses averaged
Supplemental Information. Tissue Myeloid Progenitors Differentiate. into Pericytes through TGF-b Signaling. in Developing Skin Vasculature
 Cell Reports, Volume 18 Supplemental Information Tissue Myeloid Progenitors Differentiate into Pericytes through TGF-b Signaling in Developing Skin Vasculature Tomoko Yamazaki, Ani Nalbandian, Yutaka Uchida,
Cell Reports, Volume 18 Supplemental Information Tissue Myeloid Progenitors Differentiate into Pericytes through TGF-b Signaling in Developing Skin Vasculature Tomoko Yamazaki, Ani Nalbandian, Yutaka Uchida,
100 mm Sucrose. +Berberine +Quinine
 8 mm Sucrose Probability (%) 7 6 5 4 3 Wild-type Gr32a / 2 +Caffeine +Berberine +Quinine +Denatonium Supplementary Figure 1: Detection of sucrose and bitter compounds is not affected in Gr32a / flies.
8 mm Sucrose Probability (%) 7 6 5 4 3 Wild-type Gr32a / 2 +Caffeine +Berberine +Quinine +Denatonium Supplementary Figure 1: Detection of sucrose and bitter compounds is not affected in Gr32a / flies.
A new subtype of progenitor cell in the mouse embryonic neocortex. Xiaoqun Wang, Jin-Wu Tsai, Bridget LaMonica & Arnold R.
 A new subtype of progenitor cell in the mouse embryonic neocortex Xiaoqun Wang, Jin-Wu Tsai, Bridget LaMonica & Arnold R. Kriegstein Supplementary Figures 1-6: Supplementary Movies 1-9: Supplementary
A new subtype of progenitor cell in the mouse embryonic neocortex Xiaoqun Wang, Jin-Wu Tsai, Bridget LaMonica & Arnold R. Kriegstein Supplementary Figures 1-6: Supplementary Movies 1-9: Supplementary
effects on organ development. a-f, Eye and wing discs with clones of ε j2b10 show no
 Supplementary Figure 1. Loss of function clones of 14-3-3 or 14-3-3 show no significant effects on organ development. a-f, Eye and wing discs with clones of 14-3-3ε j2b10 show no obvious defects in Elav
Supplementary Figure 1. Loss of function clones of 14-3-3 or 14-3-3 show no significant effects on organ development. a-f, Eye and wing discs with clones of 14-3-3ε j2b10 show no obvious defects in Elav
Department of Cell and Developmental Biology, Vanderbilt University, Nashville, TN
 UNC-6/Netrin mediates dendritic self-avoidance Cody J. Smith 1, Joseph D. Watson 1,4,5, Miri K. VanHoven 2, Daniel A. Colón-Ramos 3 and David M. Miller III 1,4,6 1 Department of Cell and Developmental
UNC-6/Netrin mediates dendritic self-avoidance Cody J. Smith 1, Joseph D. Watson 1,4,5, Miri K. VanHoven 2, Daniel A. Colón-Ramos 3 and David M. Miller III 1,4,6 1 Department of Cell and Developmental
EMBO REPORT SUPPLEMENTARY SECTION. Quantitation of mitotic cells after perturbation of Notch signalling.
 EMBO REPORT SUPPLEMENTARY SECTION Quantitation of mitotic cells after perturbation of Notch signalling. Notch activation suppresses the cell cycle indistinguishably both within and outside the neural plate
EMBO REPORT SUPPLEMENTARY SECTION Quantitation of mitotic cells after perturbation of Notch signalling. Notch activation suppresses the cell cycle indistinguishably both within and outside the neural plate
Supplementary Figures
 J. Cell Sci. 128: doi:10.1242/jcs.173807: Supplementary Material Supplementary Figures Fig. S1 Fig. S1. Description and/or validation of reagents used. All panels show Drosophila tissues oriented with
J. Cell Sci. 128: doi:10.1242/jcs.173807: Supplementary Material Supplementary Figures Fig. S1 Fig. S1. Description and/or validation of reagents used. All panels show Drosophila tissues oriented with
Chapter 5. Summary and Future directions
 95 Chapter 5 Summary and Future directions 96 Much of our knowledge about glial development in the vertebrate CNS comes from studies of purified oligodendrocyte precursor cells (Raff 1989; Pfeiffer et
95 Chapter 5 Summary and Future directions 96 Much of our knowledge about glial development in the vertebrate CNS comes from studies of purified oligodendrocyte precursor cells (Raff 1989; Pfeiffer et
SUPPLEMENTARY FIG. S2. Representative counting fields used in quantification of the in vitro neural differentiation of pattern of dnscs.
 Supplementary Data SUPPLEMENTARY FIG. S1. Representative counting fields used in quantification of the in vitro neural differentiation of pattern of anpcs. A panel of lineage-specific markers were used
Supplementary Data SUPPLEMENTARY FIG. S1. Representative counting fields used in quantification of the in vitro neural differentiation of pattern of anpcs. A panel of lineage-specific markers were used
Development of the Nervous System. Leah Militello, class of 2018
 Development of the Nervous System Leah Militello, class of 2018 Learning Objectives 1. Describe the formation and fate of the neural tube and neural crest including timing and germ layer involved. 2. Describe
Development of the Nervous System Leah Militello, class of 2018 Learning Objectives 1. Describe the formation and fate of the neural tube and neural crest including timing and germ layer involved. 2. Describe
Emx2 patterns the neocortex by regulating FGF positional signaling
 Emx2 patterns the neocortex by regulating FGF positional signaling Tomomi Fukuchi-Shimogori and Elizabeth A Grove Presented by Sally Kwok Background Cerebral cortex has anatomically and functionally distinct
Emx2 patterns the neocortex by regulating FGF positional signaling Tomomi Fukuchi-Shimogori and Elizabeth A Grove Presented by Sally Kwok Background Cerebral cortex has anatomically and functionally distinct
Cancer Stem Cells & Glioblastoma
 Cancer Stem Cells & Glioblastoma JP Hugnot «Brain plasticity, Neural stem cells and Glial tumors» INSERM U1051-UM2 Institut des Neurosciences de Montpellier Montpellier 1-Stem cells and Brain Stem Cells
Cancer Stem Cells & Glioblastoma JP Hugnot «Brain plasticity, Neural stem cells and Glial tumors» INSERM U1051-UM2 Institut des Neurosciences de Montpellier Montpellier 1-Stem cells and Brain Stem Cells
Supplementary Figure S1: TIPF reporter validation in the wing disc.
 Supplementary Figure S1: TIPF reporter validation in the wing disc. a,b, Test of put RNAi. a, In wildtype discs the Dpp target gene Sal (red) is expressed in a broad stripe in the centre of the ventral
Supplementary Figure S1: TIPF reporter validation in the wing disc. a,b, Test of put RNAi. a, In wildtype discs the Dpp target gene Sal (red) is expressed in a broad stripe in the centre of the ventral
Zhu et al, page 1. Supplementary Figures
 Zhu et al, page 1 Supplementary Figures Supplementary Figure 1: Visual behavior and avoidance behavioral response in EPM trials. (a) Measures of visual behavior that performed the light avoidance behavior
Zhu et al, page 1 Supplementary Figures Supplementary Figure 1: Visual behavior and avoidance behavioral response in EPM trials. (a) Measures of visual behavior that performed the light avoidance behavior
BIOL2005 WORKSHEET 2008
 BIOL2005 WORKSHEET 2008 Answer all 6 questions in the space provided using additional sheets where necessary. Hand your completed answers in to the Biology office by 3 p.m. Friday 8th February. 1. Your
BIOL2005 WORKSHEET 2008 Answer all 6 questions in the space provided using additional sheets where necessary. Hand your completed answers in to the Biology office by 3 p.m. Friday 8th February. 1. Your
ErbB4 migrazione I parte. 3- ErbB4- NRG1
 ErbB4 migrazione I parte 3- ErbB4- NRG1 1 In rodent brains postnatal neuronal migration is evident in three main areas: the cerebellum (CB), the hippocampus (Hipp) and the rostral migratory stream (RMS).
ErbB4 migrazione I parte 3- ErbB4- NRG1 1 In rodent brains postnatal neuronal migration is evident in three main areas: the cerebellum (CB), the hippocampus (Hipp) and the rostral migratory stream (RMS).
Conserved properties of dentate gyrus neurogenesis across postnatal development revealed by single-cell RNA sequencing
 SUPPLEMENTARY INFORMATION Resource https://doi.org/10.1038/s41593-017-0056-2 In the format provided by the authors and unedited. Conserved properties of dentate gyrus neurogenesis across postnatal development
SUPPLEMENTARY INFORMATION Resource https://doi.org/10.1038/s41593-017-0056-2 In the format provided by the authors and unedited. Conserved properties of dentate gyrus neurogenesis across postnatal development
Overview of the Nervous System (some basic concepts) Steven McLoon Department of Neuroscience University of Minnesota
 Overview of the Nervous System (some basic concepts) Steven McLoon Department of Neuroscience University of Minnesota 1 Coffee Hour Tuesday (Sept 11) 10:00-11:00am Friday (Sept 14) 8:30-9:30am Surdyk s
Overview of the Nervous System (some basic concepts) Steven McLoon Department of Neuroscience University of Minnesota 1 Coffee Hour Tuesday (Sept 11) 10:00-11:00am Friday (Sept 14) 8:30-9:30am Surdyk s
OSVZ progenitors of human and ferret neocortex are epithelial-like and
 OSVZ progenitors of human and ferret neocortex are epithelial-like and expand by integrin signaling Simone A Fietz, Iva Kelava, Johannes Vogt, Michaela Wilsch-Bräuninger, Denise Stenzel, Jennifer L Fish,
OSVZ progenitors of human and ferret neocortex are epithelial-like and expand by integrin signaling Simone A Fietz, Iva Kelava, Johannes Vogt, Michaela Wilsch-Bräuninger, Denise Stenzel, Jennifer L Fish,
Development of the Nervous System 1 st month
 Development of the Nervous System 1 st month day 1 - fertilization of egg day 6 - uterine implantation day 18 - trilaminar (3-layered) disc (blastoderm, embryo) ectoderm (dorsal) - nervous system and skin
Development of the Nervous System 1 st month day 1 - fertilization of egg day 6 - uterine implantation day 18 - trilaminar (3-layered) disc (blastoderm, embryo) ectoderm (dorsal) - nervous system and skin
Department of Cognitive Science UCSD
 Department of Cognitive Science UCSD Verse 1: Neocortex, frontal lobe, Brain stem, brain stem, Hippocampus, neural node, Right hemisphere, Pons and cortex visual, Brain stem, brain stem, Sylvian fissure,
Department of Cognitive Science UCSD Verse 1: Neocortex, frontal lobe, Brain stem, brain stem, Hippocampus, neural node, Right hemisphere, Pons and cortex visual, Brain stem, brain stem, Sylvian fissure,
A Drosophila model for alcohol reward. Supplemental Material
 Kaun et al. Supplemental Material 1 A Drosophila model for alcohol reward Supplemental Material Kaun, K.R. *, Azanchi, R. *, Maung, Z. *, Hirsh, J. and Heberlein, U. * * Department of Anatomy, University
Kaun et al. Supplemental Material 1 A Drosophila model for alcohol reward Supplemental Material Kaun, K.R. *, Azanchi, R. *, Maung, Z. *, Hirsh, J. and Heberlein, U. * * Department of Anatomy, University
A Genetic Program for Embryonic Development
 Concept 18.4: A program of differential gene expression leads to the different cell types in a multicellular organism During embryonic development, a fertilized egg gives rise to many different cell types
Concept 18.4: A program of differential gene expression leads to the different cell types in a multicellular organism During embryonic development, a fertilized egg gives rise to many different cell types
Negative regulation of atonal in proneural cluster formation of Drosophila R8 photoreceptors
 Proc. Natl. Acad. Sci. USA Vol. 96, pp. 5055 5060, April 1999 Developmental Biology Negative regulation of atonal in proneural cluster formation of Drosophila R8 photoreceptors CHIEN-KUO CHEN AND CHENG-TING
Proc. Natl. Acad. Sci. USA Vol. 96, pp. 5055 5060, April 1999 Developmental Biology Negative regulation of atonal in proneural cluster formation of Drosophila R8 photoreceptors CHIEN-KUO CHEN AND CHENG-TING
P. Hitchcock, Ph.D. Department of Cell and Developmental Biology Kellogg Eye Center. Wednesday, 16 March 2009, 1:00p.m. 2:00p.m.
 Normal CNS, Special Senses, Head and Neck TOPIC: CEREBRAL HEMISPHERES FACULTY: LECTURE: READING: P. Hitchcock, Ph.D. Department of Cell and Developmental Biology Kellogg Eye Center Wednesday, 16 March
Normal CNS, Special Senses, Head and Neck TOPIC: CEREBRAL HEMISPHERES FACULTY: LECTURE: READING: P. Hitchcock, Ph.D. Department of Cell and Developmental Biology Kellogg Eye Center Wednesday, 16 March
SUPPLEMENTARY INFORMATION
 SUPPLEMENTARY INFORMATION Human cerebral cortex development from pluripotent stem cells to functional excitatory synapses Yichen Shi 1,2, Peter Kirwan 1,2, James Smith 1,2, Hugh P.C. Robinson 3 and Frederick
SUPPLEMENTARY INFORMATION Human cerebral cortex development from pluripotent stem cells to functional excitatory synapses Yichen Shi 1,2, Peter Kirwan 1,2, James Smith 1,2, Hugh P.C. Robinson 3 and Frederick
T H E J O U R N A L O F C E L L B I O L O G Y
 T H E J O U R N A L O F C E L L B I O L O G Y Supplemental material Lu et al., http://www.jcb.org/cgi/content/full/jcb.201012063/dc1 Figure S1. Kinetics of nuclear envelope assembly, recruitment of Nup133
T H E J O U R N A L O F C E L L B I O L O G Y Supplemental material Lu et al., http://www.jcb.org/cgi/content/full/jcb.201012063/dc1 Figure S1. Kinetics of nuclear envelope assembly, recruitment of Nup133
SUPPLEMENTARY DATA. Supplementary Table 2. Antibodies used for Immunofluoresence. Supplementary Table 3. Real-time PCR primer sequences.
 Supplementary Table 2. Antibodies used for Immunofluoresence. Antibody Dilution Source Goat anti-pdx1 1:100 R&D Systems Rabbit anti-hnf6 1:100 Santa Cruz Biotechnology Mouse anti-nkx6.1 1:200 Developmental
Supplementary Table 2. Antibodies used for Immunofluoresence. Antibody Dilution Source Goat anti-pdx1 1:100 R&D Systems Rabbit anti-hnf6 1:100 Santa Cruz Biotechnology Mouse anti-nkx6.1 1:200 Developmental
SUPPLEMENTARY INFORMATION
 DOI: 10.1038/ncb2419 Figure S1 NiGFP localization in Dl mutant dividing SOPs. a-c) time-lapse analysis of NiGFP (green) in Dl mutant SOPs (H2B-RFP, red; clones were identified by the loss of nlsgfp) showing
DOI: 10.1038/ncb2419 Figure S1 NiGFP localization in Dl mutant dividing SOPs. a-c) time-lapse analysis of NiGFP (green) in Dl mutant SOPs (H2B-RFP, red; clones were identified by the loss of nlsgfp) showing
Olfactory ensheathing glia
 Olfactory ensheathing glia From Wikipedia, the free encyclopedia Neuroglia of the brain shown by Golgi's method. Olfactory ensheathing glia (OEG), also known as olfactory ensheathing cells (OECs) or olfactory
Olfactory ensheathing glia From Wikipedia, the free encyclopedia Neuroglia of the brain shown by Golgi's method. Olfactory ensheathing glia (OEG), also known as olfactory ensheathing cells (OECs) or olfactory
Supplementary Figure 1. Electroporation of a stable form of β-catenin causes masses protruding into the IV ventricle. HH12 chicken embryos were
 Supplementary Figure 1. Electroporation of a stable form of β-catenin causes masses protruding into the IV ventricle. HH12 chicken embryos were electroporated with β- Catenin S33Y in PiggyBac expression
Supplementary Figure 1. Electroporation of a stable form of β-catenin causes masses protruding into the IV ventricle. HH12 chicken embryos were electroporated with β- Catenin S33Y in PiggyBac expression
Dampening the Signals Transduced through Hedgehog via MicroRNA mir-7 Facilitates Notch-Induced Tumourigenesis
 Dampening the Signals Transduced through Hedgehog via MicroRNA mir-7 Facilitates Notch-Induced Tumourigenesis Vanina G. Da Ros, Irene Gutierrez-Perez, Dolors Ferres-Marco, Maria Dominguez* Instituto de
Dampening the Signals Transduced through Hedgehog via MicroRNA mir-7 Facilitates Notch-Induced Tumourigenesis Vanina G. Da Ros, Irene Gutierrez-Perez, Dolors Ferres-Marco, Maria Dominguez* Instituto de
Cell Migration II: CNS Cell Migration. Steven McLoon Department of Neuroscience University of Minnesota
 Cell Migration II: CNS Cell Migration Steven McLoon Department of Neuroscience University of Minnesota 1 Course News Coffee Hour Wednesday (Oct 18) 9:00-10:00am Surdyk s Café in Northrop Auditorium Stop
Cell Migration II: CNS Cell Migration Steven McLoon Department of Neuroscience University of Minnesota 1 Course News Coffee Hour Wednesday (Oct 18) 9:00-10:00am Surdyk s Café in Northrop Auditorium Stop
CNS Developmental. Anke van Eekelen, PhD. Telethon Institute for Child Health Research
 CNS Developmental Anke van Eekelen, PhD Telethon Institute for Child Health Research (Some slides are modified versions of Prof. Alan Harvey s Neuroscience lecture at ANHB and Dr. Joanne Britto s Dev Neuroscience
CNS Developmental Anke van Eekelen, PhD Telethon Institute for Child Health Research (Some slides are modified versions of Prof. Alan Harvey s Neuroscience lecture at ANHB and Dr. Joanne Britto s Dev Neuroscience
The subcortical maternal complex controls symmetric division of mouse zygotes by
 The subcortical maternal complex controls symmetric division of mouse zygotes by regulating F-actin dynamics Xing-Jiang Yu 1,2, Zhaohong Yi 1, Zheng Gao 1,2, Dan-dan Qin 1,2, Yanhua Zhai 1, Xue Chen 1,
The subcortical maternal complex controls symmetric division of mouse zygotes by regulating F-actin dynamics Xing-Jiang Yu 1,2, Zhaohong Yi 1, Zheng Gao 1,2, Dan-dan Qin 1,2, Yanhua Zhai 1, Xue Chen 1,
T H E J O U R N A L O F C E L L B I O L O G Y
 Supplemental material Wang and Page-McCaw, http://www.jcb.org/cgi/content/full/jcb.201403084/dc1 T H E J O U R N A L O F C E L L B I O L O G Y Figure S1. Extracellular anti-wg staining is specific. Note
Supplemental material Wang and Page-McCaw, http://www.jcb.org/cgi/content/full/jcb.201403084/dc1 T H E J O U R N A L O F C E L L B I O L O G Y Figure S1. Extracellular anti-wg staining is specific. Note
Segmentation. Serial Homology. Hox genes
 Segmentation Hox genes Serial Homology William Bateson 1861-1926 Homeosis: a variation in which something has been changed into the likeness of something else Calvin Bridges at Columbia bithorax 1923 1
Segmentation Hox genes Serial Homology William Bateson 1861-1926 Homeosis: a variation in which something has been changed into the likeness of something else Calvin Bridges at Columbia bithorax 1923 1
Supplementary Figure 1. Flies form water-reward memory only in the thirsty state
 1 2 3 4 5 6 7 Supplementary Figure 1. Flies form water-reward memory only in the thirsty state Thirsty but not sated wild-type flies form robust 3 min memory. For the thirsty group, the flies were water-deprived
1 2 3 4 5 6 7 Supplementary Figure 1. Flies form water-reward memory only in the thirsty state Thirsty but not sated wild-type flies form robust 3 min memory. For the thirsty group, the flies were water-deprived
Supplemental Information. Otic Mesenchyme Cells Regulate. Spiral Ganglion Axon Fasciculation. through a Pou3f4/EphA4 Signaling Pathway
 Neuron, Volume 73 Supplemental Information Otic Mesenchyme Cells Regulate Spiral Ganglion Axon Fasciculation through a Pou3f4/EphA4 Signaling Pathway Thomas M. Coate, Steven Raft, Xiumei Zhao, Aimee K.
Neuron, Volume 73 Supplemental Information Otic Mesenchyme Cells Regulate Spiral Ganglion Axon Fasciculation through a Pou3f4/EphA4 Signaling Pathway Thomas M. Coate, Steven Raft, Xiumei Zhao, Aimee K.
Title: Chapter 5 Recorded Lecture. Speaker: Amit Dhingra Created by: (remove if same as speaker) online.wsu.edu
 Title: Chapter 5 Recorded Lecture Speaker: Title: What Anthony is the title Berger/Angela of this lecture? Williams Speaker: Amit Dhingra Created by: (remove if same as speaker) online.wsu.edu Chapter
Title: Chapter 5 Recorded Lecture Speaker: Title: What Anthony is the title Berger/Angela of this lecture? Williams Speaker: Amit Dhingra Created by: (remove if same as speaker) online.wsu.edu Chapter
Genesis of cerebellar interneurons and the prevention of neural DNA damage require XRCC1.
 Genesis of cerebellar interneurons and the prevention of neural DNA damage require XRCC1. Youngsoo Lee, Sachin Katyal, Yang Li, Sherif F. El-Khamisy, Helen R. Russell, Keith W. Caldecott and Peter J. McKinnon.
Genesis of cerebellar interneurons and the prevention of neural DNA damage require XRCC1. Youngsoo Lee, Sachin Katyal, Yang Li, Sherif F. El-Khamisy, Helen R. Russell, Keith W. Caldecott and Peter J. McKinnon.
Ahtiainen et al., http :// /cgi /content /full /jcb /DC1
 Supplemental material JCB Ahtiainen et al., http ://www.jcb.org /cgi /content /full /jcb.201512074 /DC1 THE JOURNAL OF CELL BIOLOGY Figure S1. Distinct distribution of different cell cycle phases in the
Supplemental material JCB Ahtiainen et al., http ://www.jcb.org /cgi /content /full /jcb.201512074 /DC1 THE JOURNAL OF CELL BIOLOGY Figure S1. Distinct distribution of different cell cycle phases in the
Transgenic Expression of the Helicobacter pylori Virulence Factor CagA Promotes Apoptosis or Tumorigenesis through JNK Activation in Drosophila
 Transgenic Expression of the Helicobacter pylori Virulence Factor CagA Promotes Apoptosis or Tumorigenesis through JNK Activation in Drosophila Anica M. Wandler, Karen Guillemin* Institute of Molecular
Transgenic Expression of the Helicobacter pylori Virulence Factor CagA Promotes Apoptosis or Tumorigenesis through JNK Activation in Drosophila Anica M. Wandler, Karen Guillemin* Institute of Molecular
MIDTERM EXAM 1 COGNITIVE SCIENCE 107A
 MIDTERM EXAM 1 COGNITIVE SCIENCE 107A FALL 2011 Name: Points: / 100 PID: I. SHORT ANSWERS (6 points each for a total of 30 points) 1. Describe two contributions made by Ramon y Cajal (1852-1934) in terms
MIDTERM EXAM 1 COGNITIVE SCIENCE 107A FALL 2011 Name: Points: / 100 PID: I. SHORT ANSWERS (6 points each for a total of 30 points) 1. Describe two contributions made by Ramon y Cajal (1852-1934) in terms
Supplementary Figure 1: Signaling centers contain few proliferating cells, express p21, and
 Supplementary Figure 1: Signaling centers contain few proliferating cells, express p21, and exclude YAP from the nucleus. (a) Schematic diagram of an E10.5 mouse embryo. (b,c) Sections at B and C in (a)
Supplementary Figure 1: Signaling centers contain few proliferating cells, express p21, and exclude YAP from the nucleus. (a) Schematic diagram of an E10.5 mouse embryo. (b,c) Sections at B and C in (a)
Option A: Neurobiology & Behavior HL BIOLOGY 2 ND EDITION DAMON, MCGONEGAL, TOSTO, AND
 Option A: Neurobiology & Behavior A1: NEURAL DEVELOPMENT USE THE INFO IN THE PRESENTATION TO COMPLETE A1 NOTES GUIDE INFORMATION TAKEN FROM: HL BIOLOGY 2 ND EDITION DAMON, MCGONEGAL, TOSTO, AND WARD BIOLOGY
Option A: Neurobiology & Behavior A1: NEURAL DEVELOPMENT USE THE INFO IN THE PRESENTATION TO COMPLETE A1 NOTES GUIDE INFORMATION TAKEN FROM: HL BIOLOGY 2 ND EDITION DAMON, MCGONEGAL, TOSTO, AND WARD BIOLOGY
Linkage Mapping in Drosophila Melanogaster
 Linkage Mapping in Drosophila Melanogaster Genetics: Fall 2012 Joshua Hanau Introduction: An experiment was performed in order to determine the presence and degree of gene linkage in Drosophila Melanogaster.
Linkage Mapping in Drosophila Melanogaster Genetics: Fall 2012 Joshua Hanau Introduction: An experiment was performed in order to determine the presence and degree of gene linkage in Drosophila Melanogaster.
9.14 Classes #21-23: Visual systems
 9.14 Classes #21-23: Visual systems Questions based on Schneider chapter 20 and classes: 1) What was in all likelihood the first functional role of the visual sense? Describe the nature of the most primitive
9.14 Classes #21-23: Visual systems Questions based on Schneider chapter 20 and classes: 1) What was in all likelihood the first functional role of the visual sense? Describe the nature of the most primitive
Sheep Brain Dissection
 Sheep Brain Dissection Mammalian brains have many features in common. Human brains may not be available, so sheep brains often are dissected as an aid to understanding the mammalian brain since he general
Sheep Brain Dissection Mammalian brains have many features in common. Human brains may not be available, so sheep brains often are dissected as an aid to understanding the mammalian brain since he general
SUPPLEMENTARY INFORMATION
 Supplementary Figures Supplementary Figure S1. Binding of full-length OGT and deletion mutants to PIP strips (Echelon Biosciences). Supplementary Figure S2. Binding of the OGT (919-1036) fragments with
Supplementary Figures Supplementary Figure S1. Binding of full-length OGT and deletion mutants to PIP strips (Echelon Biosciences). Supplementary Figure S2. Binding of the OGT (919-1036) fragments with
Meigo governs dendrite targeting specificity by modulating Ephrin level and N-glycosylation
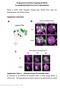 Meigo governs dendrite targeting specificity by modulating Ephrin level and N-glycosylation Sayaka U. Sekine, Shuka Haraguchi, Kinhong Chao, Tomoko Kato, Liqun Luo, Masayuki Miura, and Takahiro Chihara
Meigo governs dendrite targeting specificity by modulating Ephrin level and N-glycosylation Sayaka U. Sekine, Shuka Haraguchi, Kinhong Chao, Tomoko Kato, Liqun Luo, Masayuki Miura, and Takahiro Chihara
Regulation of Self-renewal and Differentiation in the Drosophila Nervous System
 Regulation of Self-renewal and Differentiation in the Drosophila Nervous System T.D. SOUTHALL, B. EGGER, K.S. GOLD, AND A.H. BRAND The Gurdon Institute and Department of hysiology, Development, and Neuroscience,
Regulation of Self-renewal and Differentiation in the Drosophila Nervous System T.D. SOUTHALL, B. EGGER, K.S. GOLD, AND A.H. BRAND The Gurdon Institute and Department of hysiology, Development, and Neuroscience,
Developmental Biology
 Developmental Biology 327 (2009) 288 300 Contents lists available at ScienceDirect Developmental Biology journal homepage: www.elsevier.com/developmentalbiology The HLH protein Extramacrochaetae is required
Developmental Biology 327 (2009) 288 300 Contents lists available at ScienceDirect Developmental Biology journal homepage: www.elsevier.com/developmentalbiology The HLH protein Extramacrochaetae is required
Bio 3411 Midterm Review:
 Midterm Review: Structure/Development/Systems/ Plastics/Talents/Diseases/Genes Structure General Overview Wednesday 1( 2( THE BRAIN ATLAS 3 rd ed, p. 8! THE BRAIN ATLAS 3 rd ed, p. 9! Mid-line (sagittal)
Midterm Review: Structure/Development/Systems/ Plastics/Talents/Diseases/Genes Structure General Overview Wednesday 1( 2( THE BRAIN ATLAS 3 rd ed, p. 8! THE BRAIN ATLAS 3 rd ed, p. 9! Mid-line (sagittal)
A quantitative three-dimensional model of the Drosophila optic lobes Karlheinz Rein*, Malte Zöckler and Martin Heisenberg*
 Brief Communication 93 A quantitative three-dimensional model of the Drosophila optic lobes Karlheinz Rein*, Malte Zöckler and Martin Heisenberg* A big step in the neurobiology of Drosophila would be to
Brief Communication 93 A quantitative three-dimensional model of the Drosophila optic lobes Karlheinz Rein*, Malte Zöckler and Martin Heisenberg* A big step in the neurobiology of Drosophila would be to
Nature Neuroscience: doi: /nn Supplementary Figure 1
 Supplementary Figure 1 Subcellular segregation of VGluT2-IR and TH-IR within the same VGluT2-TH axon (wild type rats). (a-e) Serial sections of a dual VGluT2-TH labeled axon. This axon (blue outline) has
Supplementary Figure 1 Subcellular segregation of VGluT2-IR and TH-IR within the same VGluT2-TH axon (wild type rats). (a-e) Serial sections of a dual VGluT2-TH labeled axon. This axon (blue outline) has
Brain Development III
 Brain Development III Neural Development In the developing nervous system there must be: 1. The formation of different regions of the brain. 2. The ability of a neuron to differentiate. 3. The ability
Brain Development III Neural Development In the developing nervous system there must be: 1. The formation of different regions of the brain. 2. The ability of a neuron to differentiate. 3. The ability
