Cellular Mechanisms for the Release of Exosomes Containing the Amyloid Precursor Protein
|
|
|
- Giles Carter
- 6 years ago
- Views:
Transcription
1 Cellular Mechanisms for the Release of Exosomes Containing the Amyloid Precursor Protein Master s Thesis Presented to The Faculty of the Graduate School of Arts and Sciences Brandeis University Interdepartmental Program in Neuroscience Avital Rodal, Advisor In Partial Fulfillment of the Requirements for Master s Degree by Matthew J. Zunitch May 2013
2 Cellular Mechanisms for the Release of Exosomes Containing the Amyloid Precursor Protein A thesis presented to the Interdepartment Program in Neuroscience Graduate School of Arts and Sciences Brandeis University Waltham, Massachusetts by Matthew J. Zunitch Abstract The Amyloid Precursor Protein (APP) is a transmembrane protein whose cleavage results in the toxic Aβ fragment identified as the major constituent in the amyloid plaques of Alzheimer s Disease. Recent evidence has shown that fragments of APP including its intracellular domain are secreted into the extracellular space, possibly via the formation and release of extracellular vesicles called exosomes, which are derived from endosomes. In the Drosophila model, we have manipulated specific components of the membrane trafficking machinery predicted to be involved in APP traffic, and measured their effects on in vivo APPeGFP exosome secretion using fluorescence quantification. We targeted the Vps35, Snx1, and Snx3 components of the endosomal sorting complex called Retromer (which is implicated in Alzheimer s Disease), and the GTPase Rab11 which has been implicated in exocytosis. Our results indicate that Rab11 and the Snx1 are required for exosome release while Snx3 and Vps35, perhaps working together through the Retromer complex, inhibit the formation of APPeGFP containing exosomes. These findings may provide important distinctions for how specific endo/exosomal trafficking pathways are misregulated and lead to APP defects in Alzheimer s disease. ii
3 Copyright by Matthew J. Zunitch 2013 iii
4 Table of Contents Abstract List of Tables List of Figures ii v v 1. Introduction Alzheimer s Disease and the Amyloid Precursor Protein The role of Retromer in membrane trafficking and AD Sorting Nexins and their contribution to Retromer function Exosome biogenesis and implications for AD Materials and Methods Molecular genetics and cross schemes Immunohistochemistry Confocal imaging and fluorescence quantification Results Rab11 is required for the release of APPeGFP-containing exosomes Vps35-mediated trafficking opposes APPeGFP exosome formation Snx1 and Snx3 exert opposite control over APPeGFP exosome secretion Transgenic human APP and Drosophila homolog APPL localize to similar sites at the neuromuscular junction Discussion and Conclusions Functions of Rab11 and Hrs in APP exosome formation Role of Retromer, Snx1, and Snx3 in APP exosome Formation Relationship of APP exosomes to Alzheimer s Disease and the role of Retromer Appendix Supporting observations from previous experiments Flaws in previous methodology and their effects on results References 37 iv
5 List of Tables 1. Antibodies used for immunohistochemistry Drosophila strains generated for confocal fluorescence microscopy. 17 List of Figures 1a. The structure and processing of APP. 4 1b. Generation of the Aβ fragment Endosomal trafficking of APP and the function of Retromer Molecular modeling of the Retromer core Tubular endosomal structures Intracellularly-tagged APPeGFP localizes to extracellular puncta The effects of Rab11 LOF on APPeGFP exosome secretion The effects of Retromer trafficking mutants on APPeGFP exosome secretion Quantification of extracellular APPeGFP fluorescence The Drosophila APP homolog, APPL, is secreted at the neuromuscular junction Model Overstained neuromuscular junctions display inaccurate phenotypes Quantification of extracellular fluorescence using an alternative method. 36 v
6 1 Introduction 1.1 Alzheimer s Disease and the Amyloid Precursor Protein Alzheimer s Disease (AD) is a neurodegenerative disease characterized by the progressive and pervasive death of cortical neurons in addition to the deposition of two stereotyped protein accumulations: amyloid plaques in the extracellular space and neurofibrillary tangles in the cytosol. A amino acid peptide, now referred to as the Aβ fragment, was identified as the main constituent of the extracellular plaques 1 and is a cleavage product of the Amyloid Precursor Protein (APP). 2 The cleavage of APP depends on numerous proteases and can occur at many places along the full-length protein (Figure 1a). The ectodomain of full-length APP (secreted APP, sapp) can be cleaved by distinct protease activities termed the αsecretase or β-secretase. The cleavage sites of these enzymes differ from each other by only a few amino acids, with the α cutsite being closer to the plasma membrane. Studies using protease inhibitors have demonstrated that at least three Type-I integral membrane zinc metalloproteases (ADAM9, ADAM10, and ADAM17) exhibit α-secretase activity, 3 while the β-secretase BACE (β-site APP Cleavage Enzyme) is a membrane-bound aspartyl protease. 4,5 Aside from α- and β-cleavage, the transmembrane region of APP can be cleaved within the plasma membrane by the multimeric γ-secretase complex, 6 releasing the APP Intracellular Domain (AICD) into the cytosol. Although it was assumed 1
7 that α- or β-secretase activity necessarily preceded γ-secretase activity, recent evidence suggests that γ-cleavage can occur directly from full-length APP as well. 7 The processing of APP by both BACE and the γ-secretase results in the Aβ fragment (Figure 1b). On the other hand, α-secretase processing prevents the formation of Aβ by cleaving the Aβ region in two. APP is a Type-I, single-pass transmembrane glycoprotein. As a transmembrane protein, APP function depends on the processes and mechanisms that control vesicular formation, maturation, and trafficking. The trafficking of APP may be instrumental in Alzheimer s pathogenesis because evidence indicates that β-cleavage is favored within more acidic environments. Endosomes have a characteristically lower ph compared to the plasma membrane, which continues to drop as they mature. Therefore, the membrane trafficking of APP into endosomes is believed to modulate the kinetics of Aβ formation. Furthermore, experiments have shown that the different metabolites of APP are differentially packaged, independently transported, and localized to different subcellular compartments of cultured neurons. 8 The intracellular domain contains at least two different internalization sequences, 9,10 and endocytosis of APP been shown to be the ratelimiting step in Aβ generation. 11 Since its discovery, APP has been implicated in a large array of cellular processes, but a clear picture of its normal molecular functionality in healthy individuals has remained elusive. APP is conserved from C. elegans to Drosophila to mammals, 12 and the Drosophila homolog APPL has ~50% sequence similarity to human APP. APP has been shown to be involved in cell and synaptic adhesion via its extracellular domain, and 2
8 putatively trophic signaling and transcriptional regulation functions via its intracellular domain. 13 In Drosophila, APPL overexpression results in neuromuscular junction (NMJ) synaptic overgrowth, 14 however APPL-null flies display only a slightly reduced bouton number. 15 This indicates that APPL function contributes to but is not required for synapse formation. Studies examining the endosomal trafficking of APP in the fly are lacking. 3
9 1a. 1b. γ α or β Figure 1a. The structure and processing of APP. Full-length APP can be cleaved within the transmembrane (TM) region by the γ-secretase complex, releasing the intracellular domain (blue arrow). Additionally it can be cleaved by either the α- or β-secretase, releasing a form of its extracellular domain (red arrow). Figure 1b. Generation of the Aβ fragment. The combined actions of the β- and γ-secretase produce the Aβ fragment (black), which is the major component of the amyloid plaques found in AD patient brains. Proteolysis resulting in the Aβ fragment depends on the membrane trafficking of APP due to the enhancement of β-secretase activity in acidic endosomal environments. 4
10 1.2 The role of Retromer in membrane trafficking and AD In the Alzheimer s literature, one of the most extensively characterized membrane trafficking machines is the highly conserved Retromer complex, which mediates membrane trafficking and cargo sorting away from endosomes (Figure 2). Homologs of the human Retromer complex have been identified in Saccharomyces cerevisiae, Schizosaccharomyces pombe, Entamoeba histolytica, Caenorhabditis elegans, Drosophila melanogaster, Xenopus tropicalis, Xenopus laevis, and Mus musculus 16 indicating a highly conserved complex. The core of Retromer consists of three proteins named Vps35, Vps29, and Vps25. Molecular modeling data indicates that these three core proteins exist in a dynamic equilibrium between a 1:1:1 trimer and a dimer of trimers that appears roughly horseshoe-shaped (Figure 3). 17 The core of Retromer is held together by the Vps35 protein, which binds Vps29 and Vps25 on its distal ends. Vps29 and Vps25 are not believed to physically interact. Often referred to as the cargo-selective subcomplex, the core unit has been shown to bind numerous cargos including a Vps10 family protein named SorLA. SorLA has been shown to directly bind APP and control its Retromer-dependent trafficking. 18 Retromer has also been shown to control the retrograde trafficking of BACE in mice, 19 putatively mediated by another Vps10 family protein named Sortilin, 20 suggesting that Retromer may have direct and indirect effects on the processing of APP. Both Retromer and SorLA have been genetically linked to Late Onset Alzheimer s Disease (LOAD), 21,22,23 demonstrating that Retromer-dependent APP trafficking is a key aspect of LOAD pathogenesis. In accordance with this, a heterozygous deficiency of 5
11 Retromer leads to elevated levels of endogenous Aβ in mouse brains as well as elevated levels of human Aβ in transgenic fly brains. 24 In actual Alzheimer s Disease patients, Retromer protein levels are markedly decreased in postmortem brain tissue compared to controls. 25 What remains unknown is how the trafficking function of Retromer affects APP and other cargoes relevant to Alzheimer s Disease. 6
12 Figure 2. Endosomal trafficking of APP and the function of Retromer. APP is especially dependent on membrane trafficking due to its transmembrane character. Early endosomes (1) acidify as they mature into late endosomes (2), becoming more permissive to BACE function as an aspartyl protease. Retromer acts as a retrograde trafficking machine which functions off of early and late endosomes, targeting proteins to the trans-golgi network. Late endosomes can develop intralumenal vesicles and become multivesicular bodies (3), which fuse with the membrane releasing their contents as exosomes (4). These exosomes contain fragments of APP (including the Aβ and GFP-tagged AICD) which have been generated during its trafficking. Figure 3. Molecular modeling of the Retromer core. SAXS-based reconstructions (blue and gray) of the Retromer core superimposed with partial crystal structures and rigid body modeling of its constituents, reveals that the dimer of trimers is roughly horseshoe-shaped. Vps26 is shown in purple, Vps35 in red, and Vps29 in green. Figure adapted from reference 17. 7
13 1.3 Sorting Nexins and their contribution to Retromer function The cargo-selective core of Retromer can form an even larger complex with a Sorting Nexin (Snx) dimer of varying composition. The Sorting Nexin family is defined by the presence of a PX domain, which binds 3-phosphoinositide-phosphates in the plasma membrane. 26,27 The association of the Retromer core with Snx dimers is thought to aid in anchoring the core to the endosomal membrane, as the core itself has no known direct membrane-binding motifs. Two Snx family members of special interest are members of the Snx1 group (homologous members Snx1, 2, 5, and 6) and Snx3, which differ from each other by virtue of the Snx1 group possessing two additional features: a BAR domain which mediates membrane tubulation 28 and a coiled-coil region. The BAR domain of Snx1 has the ability to both sense and induce membrane curvature, which enables Snx1 to localize to tubular structures that extend off of early and late endosomes and mediate cargo sorting (Figure 4). Snx1-Retromer tubulation appears to be regulated by the Rab5 and Rab7 GTPases, as Snx1-Retromer decorated tubules have been observed to occur at the highest frequency at the transition between early and late endosomes. 29 In general, it is unclear whether Snx1- and Snx3-Retromer possess different cellular functions or whether the Retromer core has a greater affinity for one type of Snx over another. Both Snx-Retromer combinations have been implicated in the endosomal sorting of specific cargos. Snx1 has been primarily implicated in the lysosomal targeting of a number of proteins 30,31,32,33 as well as the endosome-to-golgi trafficking of Sortilin. 34 On the other hand, Snx3 is essential for the Retromer-dependent trafficking of the Wnt signaling proteins in Drosophila via their sorting factor 8
14 Wntless. 3536,37 It was also demonstrated that active Wnt morphogens are secreted via extracellular membrane-bound compartments called exosomes, and in light of these tandem observations we postulate that the Retromer complex and associated Snx dimers may have regulatory roles in the release of exosome-associated cargo relevant to Alzheimer s Disease. Whether APP is sorted exclusively by Snx1- or Snx3-Retromer, or whether the different forms of Retromer target the same cargo for different destinations remains to be seen. 9
15 Figure 4. Tubular endosomal structures. With the help of membrane deformation machinery, endosomal domains can adopt a tubular morphology which may aid in the sorting of cargo. Snx1 contains a BAR domain capable of membrane deformation and is thought to decorate these tubular structures. Red, green, and yellow figures represent different transmembrane receptors that can be trafficked throughout the different endosomal domains. Figure adapted from Cullen (2008) 10
16 1.4 Exosome biogenesis and implications for Alzheimer s Disease Exosomes and microvesicles are two classes of extracellular vesicles. Exosomes have come under intense investigation in the field of Alzheimer s Disease pathophysiology because proteolytic variants of APP as well as the full-length protein have been localized to brain-derived exosomes that have been purified from human AD patients as well as transgenic mouse models of AD. In addition to APP and its proteolytic fragments, murine exosomes contain ADAM10, BACE1, and Nicastrin (a component of the γ-secretase complex). 38 In order to study exosomes, I enlisted the Drosophila model and focused on the larval neuromuscular junction as a model synapse. The NMJ is a perfect model for studying synaptic exosome release because it is easily accessed via dissection, contains exclusively presynaptic neural connections, and retains stereotyped patterning between animals. Previous work by Dr. Avital Rodal has found that transgenic flies expressing human APP tagged with GFP at its C-terminus (APPeGFP) is secreted from the Drosophila NMJ into postsynaptic, membrane-encapsulated compartments (Figure 5) which also contain the exosome marker Sunglasses, further supporting the hypothesis that APPeGFP is secreted on exosomes. Clinically, the staining of postmortem brain tissue sampled from people who were diagnosed with Alzheimer s Disease reveals that amyloid plaques are enriched in the exosome marker Alix. 39 These data taken together suggest that exosomes may play a role in Alzheimer s Disease as a way for APP and its fragments to reach the extracellular space where amyloid plaques occur, or to spread through the brain. 11
17 One widely accepted model of exosome release depends on the fusion of multivesicular bodies (MVBs) with the plasma membrane (Figure 2), a process which has been shown to depend on the small GTPase Rab Multivesicular bodies contain intralumenal vesicles (ILVs) which appear strikingly similar to purified exosomes under electron microscopy, 41 lending credit to the theory that exosomes originate as ILVs within MVBs. Intralumenal vesicles can be formed from at least two distinct cellular processes. The first involves a complex cascade of intracellular events dependent on the ESCRT (Endosomal Sorting Complex Required for Transport) complex. ESCRT-0, otherwise known as Hrs, is the ESCRT complex nucleating factor which gathers ubiquitinylated proteins as well as lipid bilayer-associated cholesterol molecules. 42 The ESCRT complex is an effector cascade that invaginates and subsequently pinches an endosome s limiting membrane away from the cytosolic face Exosome budding can also occur independent of Hrs and the ESCRT complex, as demonstrated when a dominant negative form of the ESCRT component Vps4 (an AAA-type protein) failed to suppress proteolipid protein-containing exosome secretion in cultured oligodendocytes. Mass spectrometry analysis of purified exosomes revealed that they were enriched in ceramide, which is produced from sphingomyelin by sphingomyelinase enzymes. The addition of a bacterial sphingomyelinase to mixed-phase liposomes resulted in inward curvature and budding from lipid raft domains, leading researchers to conclude that domain-induced exosome generation can depend on ceramide interacting with the membrane independently of the ESCRT complex. 45,46 12
18 In summary, Retromer is a highly conserved molecular machine whose trafficking function relies on the concerted actions of Rab GTPases and Snx dimers. The Retromer core acts on the same subcellular site as a cargo-sequestering protein named Hrs that accounts for some fraction of ILV biogenesis. 47 ILV s can be released as exosomes when the larger vesicle that contains them fuses with the plasma membrane in a Rab11-dependent manner, and exosomes have recently been implicated in the proteomics and histology of Alzheimer s Disease. We seek to explore the molecular activities of Retromer and its associated Snx dimer(s) in the context of exosome biogenesis and release, how this affects the distribution of APP proteolysis within neurons and their exosomes, and how exosome-altering mutations affect Alzheimer s-like neurodegeneration within the Drosophila nervous system. 13
19 Figure 5. Intracellularly-tagged APPeGFP localizes to extracellular puncta. Novel observations by Avital Rodal localize transgenic human APP tagged with an intracellular GFP to extracellular puncta. The presynaptic neuron is visualized by staining against Complexin (cpx), a cytosolic neuronal protein involved in synaptic vesicle release. Due to the presence of an intracellular protein exterior to the presynaptic cytosol (as seen in the merge channel, inset), we must conclude that the GFP is enclosed in a membranous compartment such as an exosome. 14
20 2 Materials and Methods 2.1 Molecular genetics and genome manipulation In an effort to ascertain the roles of Snx1, Snx3, Vps35, Hrs, and Rab11 on the formation of APPeGFP-containing exosomes, I utilized an in vivo approach in which I generated multiple Drosophila lines. UAS-APPeGFP transgenic fly lines were generated by inserting pbi-uasc-appegfp (vector described previously 48 ) into φ-c381 integration sites on the second chromosome (Attp40) or third chromosome (Attp2) (described previously 49 ). vps35 e42 (reference 35) snx1 Δ2 (reference 37), snx3 Δ1 (reference 37), hrs (reference 43) and rab11 mutants have all been previously described. 50,51 Df7068, obtained from the Bloomington Drosophila stock center, is a small genomic deficiency covering the vps35 locus. UAS-APPeGFP was crossed into these mutants backgrounds, which were then crossed to the pan-neuronal Gal4 driver elav-gal4 C155 in each mutant background, to generate the strains used for imaging (shown in Table 2). All lines and crosses were kept at 25ºC. UAS-APPL + and UAS-APPL sd were previously described (reference 15). 15
21 2.2 Immunohistochemistry Wandering male third instar larvae were dissected in ice cold HL3.1 buffer, 52 fixed in 4% formaldehyde in HL3.1 for 30 minutes at RT, then washed 3x 10 minutes in 0.1% Triton- X in phosphate buffered saline (hereafter referred to as 0.1% PBX). Dissections were stained for one hour at room temperature in primary antibody, washed 3x 10 minutes in 0.1% PBX, then stained in secondary antibody for one hour at room temperature. Finally 3x 5 minute washes in 0.1% PBX were performed before mounting larvae in 70% glycerol. Table 1. Antibodies used for immunohistochemistry. Target (source) Presynaptic cytosol (Huntwork 2008) Neuronal membrane (Jackson Immunoresearch) APPL (Kalpana White lab) Primary [1º] Secondary [2º] α-cpx 1:10,000 Goat α-rabbit, 633nm 1:1000 α-hrp-647 1:1000 N/A N/A α-appl 1:1000 Goat α-rabbit, 488nm 1: Confocal imaging and fluorescence quantification Spinning disk confocal imaging was carried out on the motor neurons innervating between muscles 6 and 7 in bilateral hemisegments A2-A4. Samples were imaged with 488 nm, 561 nm and 640 nm lasers and appropriate emission filters on 60X (n.a. 1.4 Plan Apo) objective on a Nikon Ni-E upright microscope, equipped with a Yokogawa CSU- W1 spinning disk head, and an Andor ixon 897U EMCCD camera. Images were collected using Nikon Elements AR software. Some samples were imaged with a 63X n.a. 1.3 objective on a Marianas spinning disk confocal system (3I, Inc.), consisting of a 16
22 Zeiss Observer Z1 microscope equipped with a Yokagawa CSU-X1 spinning disk confocal head and a QuantEM 512SC EMCCD camera. Fluorescence quantification was obtained by exporting maximum projection Z-stack images into a 16-bit.tiff format, and measuring in ImageJ. Measurements of the intracellular fluorescence Integrated Density (ID) were carried out by applying a default threshold to identify the boundary of the neuronal membrane, and the total postsynaptic region was defined by computationally expanding the intracellular region in a radial direction by 20 pixel units. After obtaining the ID values for intracellular and total fluorescence (abbreviated FI and FT, respectively) the extracellular fraction (FE) was calculated using the following formula: FE = (FT - FI ) / FT Table 2. Lines generated for confocal fluorescence microscopy. Genotype imaged elav-gal4 C155 /Y ; UAS-hAPPeGFP[27.1]/+ elav-gal4 C155 /Y ; snx1 2, UAS-hAPPeGFP[27.1]/snx1 2 elav-gal4 C155 /Y ; UAS-hAPPeGFP[27.1]/+ ; snx3 1 elav-gal4 C155 /Y ; vps35 e42, UAS-hAPPeGFP[27.1]/Df6078 elav-gal4 C155 /Y ; hrs D28 /Df6277 ; UAS-hAPPeGFP[27.1]/+ elav-gal4 C155 /Y ; UAS-hAPPeGFP[27.1]/+ ; rab11 ex2 /rab11 93bi Abbreviation wt snx1 snx3 vps35 hrs rab11 elav-gal4 C155 /Y ; UAS-APPL + /+ appl + elav-gal4 C155 /Y ; UAS-APPL sd /+ appl sd 17
23 3 Results 3.1 Rab11 is required for the release of APPeGFP-containing exosomes In order to explore the hypothesis that APPeGFP exosomes are derived from multivesicular body intralumenal vesicles, I generated a mutant line containing two mutant alleles of Rab11. The rab11 ex2 allele contains an excision of the second intron of the Rab11 gene and is amorphic, while the other, Rab11 93bi, is a partial loss-of-function allele due to a R104W point mutation. By confocal microscopy these NMJs secreted the least amount of APPeGFP exosomes out of any genotype imaged-- a mere 28% of the APPeGFP fluorescence localized exterior to the presynaptic membrane in comparison to 50% extracellular in wild-type (Figure 8). The remaining APPeGFP signal appeared bound in vesicles that were both numerous appeared relatively large by eye (Figure 6) compared to wild-type controls. It is unknown whether these vesicles actually represent stalled MVBs, however this interpretation would be consistent with the canonical function of Rab11 in the exosome pathway. 3.2 Vps35-mediated trafficking opposes APPeGFP exosome formation To test the role of Retromer in APP exosome formation, I examined the localization of human APP (tagged with GFP at its C-terminus) at the NMJ of Retromer mutant larvae. I 18
24 used the vps35 e42 allele, which contains a 642-base pair deletion in the transcription unit due to an imprecise P-element excision (reference 35). This allele was combined with a Bloomington deficiency (Df6078) that encapsulates the entire vps35 gene and complements a second-site embryonic lethal mutation on the vps35 e42 chromosome. While I was not able to obtain viable vps35 e42 /Df6078 third instar larvae using standard issue Drosophila media, I was able to partially rescue the third instar lethality by rearing the cross on media plates containing grape juice agar and yeast paste. These larvae, which presumably have a slight maternal contribution of Vps35, survived long enough for dissection and imaging. These animals had a significant increase in the amount of APPeGFP exosomes compared to wild-type controls (Figure 7). Quantification of the extracellular fraction of APPeGFP indicated that the percentage of exosomal APPeGFP increased from 50% in wild-type to 74% in Vps35-deficient animals (Figure 8). Thus, the core of Retromer is required to restrict APP traffic from the exosomal pathway. This could potentially be due to Vps35-mediated retrograde trafficking of APP away from endosomes, preventing APP from being packaged into exosomes. 3.3 Snx1 and Snx3 exert opposite control over APPeGFP exosome secretion I then wanted to examine how the Snx1- or Snx3-associated variants of Retromer contribute to the formation of APPeGFP-containing extracellular vesicles, utilizing the snx1 2 and snx3 1 deletion alleles (reference 37). In animals that are homozygous for the snx3 deletion, we observed an increase in the extracellular APPeGFP fraction from 50% to 65% in comparison with wild-type controls (Figures 7 and 8), though this difference 19
25 did not reach statistical significance for the number of animals we examined. Animals lacking Snx1 on the other hand, displayed a dramatic NMJ phenotype which is opposite to that of a Snx3 deletion (Figure 7). Extracellular APPeGFP levels were reduced from 50% to 36% in Snx1 deletion animals, though this difference also did not reach statistical significance for the number of animals we examined (Figure 8). It is interesting to note that snx3 and vps35 present similar phenotypes, suggesting that they may work in a similar pathway. This is consistent with previous results that indicate Snx3 works with Retromer core to sort cargo. Snx1, on the other hand, appears to be necessary for an unknown step along the exosome biogenesis pathway and either possesses a cellular function distinct from its association with the core of Retromer, or has a function with Retromer downstream of the Snx3-Retromer function. 3.4 Transgenic human APP and Drosophila homolog APPL localize to similar sites at the neuromuscular junction To test the physiological relevance of our findings with overexpressed human APPeGFP, I examined the localization of endogenous APPL, the Drosophila homolog of human APP, as well as UAS-APPL transgenes (Figure 9). Importantly, APPL is predicted to be a predominantly neuronally expressed protein with no expression in the postsynaptic muscle cell. 53 In wild-type (elav C155 ) animals that did not contain any UAS transgenes, we observed a dim but partially postsynaptic localization of endogenous APPL using a polyclonal APPL antibody (obtained from K. White s laboratory). In order to test the specificity of the antibody I performed the same set of experiments on flies 20
26 overexpressing wild-type (appl + ) and secretion defective (appl sd ) variants of APPL. This secretion defective mutant cannot be cleaved in its extracellular domain (reference 15), and thus any extracellular localization must come from APPL that is trafficked out of the neuron on exosomes. appl + animals displayed a stronger and more punctate postsynaptic signal when normalized to wild-type fluorescence ranges, and this phenotype was augmented even further by the secretion defective variant. This indicates that our antibody is likely to be specific for Drosophila APPL and that APPL, when overexpressed, is trafficked and secreted similarly to transgenic APP. It is unknown what effect the genetic manipulations of Retromer and Rab11 we described for human APPeGFP will have on APPL exosome phenotypes, but this should be explored in order to validate previous results. 21
27 Figure 6. The effects of Rab11 LOF on APPeGFP exosome secretion. When imaged using confocal microscopy, rab11 mutants display remarkably less extracellular APPeGFP compared to wild-type controls. Pictured here are merge channels displaying our overexpressed human APPeGFP construct in green and the presynaptic neuron stained in red. Although the identities of the vesicular structures indicated in the lower right panel (white arrows) are unknown, widely-accepted models for Rab11 function suggest that they are stalled multivesicular bodies that are unable to fuse with the plasma membrane. Neuronal cytosol is stained with α-cpx and far red secondary for wt, and neuronal membrane is stained using a far red Hrp for rab11. 22
28 Membrane APPeGFP Merge snx1 snx3 vps35 wt Figure 7. The effects of Retromer trafficking mutants on APPeGFP exosome secretion. Retromer mutants have observable effects on the secretion of exosomal APPeGFP in comparison to wild-type animals (top row). When the core of Retromer is disrupted (second row), APPeGFP exosomes increase to a statistically significant degree suggesting that the Retromer complex functions to remove APP from the exosome secretory pathway. Mutating the Snx3 subunit of Retromer mimics this phenotype (third row) but to a degree that was not statistically significant for the number of animals measured. Mutating Snx1, another Retromer subunit, decreases the amount of APPeGFP exosomes (bottom row), but again this effect was not significant for the number of measurements taken. 23
29 Extracellular APPeGFP Percent Figure 8. Quantification of percent extracellular APPeGFP fluorescence. Percent extracellular APPeGFP was calculated as described in Section 5.3. Bar heights represent mean percentage with error bars indicating the standard error of the mean. N-values are listed as white typeface at the base of the black bars and quantify the number of neuromuscular junctions that were included in the fluorescence quantification. A statistically significant (p < 0.05) decrease was observed in APPeFP exosome release in rab11 mutants, while a significant increase was observed in vps35 mutants. snx3 and snx1 mutants had respectively facilitative and preventative effects on APPeGFP exosome release, although these differences were not statistically significant. 24
30 appl sd appl + C155 APPeGFP Figure 9. The Drosophila APP homolog, APPL, is also secreted at the neuromuscular junction. Similar to the localization of our transgenic human APP (top row), the Drosophila APP homolog APPL is localized to the postsynaptic region using a polyclonal antibody in both wild-type (second row) and APPL overexpressing (third row) animals. The secretion defective allele of APPL (appl sd ) is resistant to the proteolytic processing that APPL usually undergoes causing it to accumulate both within and around the NMJ to much higher relative levels when overexpressed. Since the extracellular domain cannot be shed in appl sd animals, the presence of extracellular APPL indicates that it is secreted on exosomes like its human homolog. Neuronal identification was accomplished using α-cpx primary/far red secondary in the top row and far red Hrp for the last three rows. 25
31 4 Discussion and Conclusions Here, I have examined the roles of Retromer and other membrane trafficking proteins in the biogenesis of APP-containing exosomes. My results suggest that APP is secreted from the Drosophila NMJ in bonafide exosomes, and that this localization is conserved for the Drosophila APP homologue APPL. These findings suggest a novel cell biological explanation for the role of Retromer in APP trafficking to exosomes, which may be relevant to Alzheimer s Disease. 4.1 Functions of Rab11 and Hrs in APP exosome formation Rab11, a key component of the exocytosis pathway, appears to be required for APPeGFP exosome release in a manner which suggests that APPeGFP exosomes are in fact derived from multivesicular bodies. Examining the colocalization in Rab11 mutants between APPeGFP and a known MVB marker such as a fluorescently-tagged Hrs would lend credit to the MVB origin of APPeGFP exosomes. Furthermore we are in the process of generating APPeGFP-expressing Hrs mutants for confocal imaging and extracellular APPeGFP fluorescence quantification. Preliminary images have been acquired however they have not yielded any conclusive results. This is due to weak expression of our APPeGFP construct inserted into the Attp2 site, however this may be circumvented in the future by staining with an anti-gfp antibody to enhancing signal. 26
32 4.2 Role of Retromer, Snx1, and Snx3 in APP exosome Formation Vps35, the essential unit of the Retromer core, is necessary for the formation of the cargo-selective subunit that is responsible for the retrograde (endosome-to-golgi) trafficking of vesicles and membrane-bound cargo. The Amyloid Precursor Protein, a transmembrane protein, is particularly susceptible to this type of trafficking and has been shown to rely on a Vps10 family protein named SorLA for its Retromer-dependent trafficking. Here, I have shown evidence suggesting that mutating vps35 interrupts APP trafficking at the Drosophila neuromuscular junction, and leads to a marked and statistically significant increase of extracellular APPeGFP which is likely contained within exosomes. While a reduction in either Vps35 or Snx3 appears to result in an increase of APPeGFP-containing exosomes, snx1 deletion appears to suppress APPeGFP exosome secretion below wild-type levels. Based on this, I propose a model in which Snx3-Retromer and ILV-forming machinery compete for cargo on a common, limited endosomal surface. Our phenotypic results suggest that Snx3 and Vps35 work together to traffic APP away from endosomes and the molecular factors that generate ILVs. Snx1 presents with an opposite phenotype featuring reduced exosomes, suggesting that Snx1 either has Retromer-independent functions (hypothesis 1), or that the Snx3-Retromer function in exosome restriction is epistatic to a subsequent Snx1-Retromer function in exosome biogenesis (hypothesis 2). One interpretation of these results, consistent with hypothesis 2 and with the wellestablished relationship between both sorting nexins and Retromer, is that Snx1 and Snx3 27
33 have different functions and compete for a limited number of Retromer core units. One direct experimental test of this titration model would examine whether loss of Snx1 would phenocopy a Snx3-Retromer overexpression and vice versa. This possibility is in the process of being explored with the construction of UAS-Snx1 and UAS-Snx3 fly lines. Furthermore, this titration model leaves open the possibility that the actions of Snx3 and Snx1 through Retromer may occur at differing stages of endosomal maturation, allowing for differing effects on exosome formation and release (Figure 10). A possible Snx1- Retromer function that would be supported by hypothesis two is that Snx1-Retromer could act to transport ILV formation-inhibiting proteins away from the late endosomal surface. By examining the colocalization between Vps35, Snx1, and Snx3 with EEA1 (a marker for early endosomes) and LAMP1 (a marker for late endosomes) we may be able to elucidate whether there is a temporal sequence to Snx1- and Snx3-Retromer activity. In addition to colocalization experiments we would be able to analyze the epistasis of Snx1 and Snx3 function using snx1,3 double mutants. Our model would predict that Snx3, acting on a more immature phase of endosomal development, would present with the dominant phenotype in a snx1,3 double mutant animal. An alternative explanation is that Snx1 has Retromer-independent functions, but we favor this hypothesis less since in other systems Snx1 and Retromer functions are so intimately linked. However, if the outcomes of our epistasis experiments do not support a direct relationship between Vps35 and Snx1, we will have to consider this independent function hypothesis as an alternative. 28
34 4.3 Relationship of APP exosomes to Alzheimer s Disease and the role of Retromer Our experiments suggest a cell biological interpretation for the neurotoxicity of APP, and the role of Retromer in Alzheimer s Disease. Since disruption of Retromer leads to increased production of toxic fragments of APP, and in our experiments also leads to increased exosome formation, our results suggest that APP secretion into exosomes may increase its processing to toxic fragments. Further, exosomal cell-to-cell transfer of these toxic fragments may play a role in the spread of Alzheimer s Disease in the brain. We will test these models in several ways. Using our APPeGFP overexpression lines we can test if exosome secretion affects APP processing, by immunoblotting against APP fragments or GFP itself in conditions where we have manipulated membrane traffic and exosome release. These experiments will tell us if any APP proteolytes are under- or overrepresented when exosome formation is altered. Next, human APP overexpression in the Drosophila eye (using the GMR-gal4 driver) leads to neurodegeneration. 54 We can assess how Snx1, Snx3, Vps35, Rab11, or Hrs affect the progression of this neurodegenerative phenotype, to test if APP exosome formation is protecting against or enhancing the disease. Finally, we can express human APP panneuronally in Snx1, Snx3, Vps35, Rab11, and Hrs mutant backgrounds to examine the effects those mutations have on fly lifespan. These tests will help us come to a clearer understanding of how proteins involved in APP trafficking and exosome formation influence Alzheimer s-like neurodegeneration and the processing of APP. 29
35 In conclusion, we have identified four genes implicated in the exosome secretory pathway of the Amyloid Precursor Protein (Figure 10). We have shown that rab11 exerts control over the release of exosomes from the Drosophila nervous system in a manner which suggests the exosomes are derived from multivesicular bodies. In addition, we have shown that vps35, the core of the Retromer complex, is responsible for inhibiting APP exosome formation. Preliminary observations suggest that snx1 encourages APP exosome release while snx3 inhibits it. According to phenotypic data both Vps35 and Snx3 seem to play a role in preventing the exosomal secretion of APPeGFP, while Snx1 seems to promote APP exosome release through a function that is either downstream of Snx3 and Retromer, or independent of the Retromer complex in its entirety. We have also shown that the Drosophila homolog APPL localizes to similar extracellular regions, suggesting that trafficking routes are conserved between the human and Drosophila APP homologs. We seek to further elucidate this pathway and examine its effects on neurodegenerative phenotypes in the future. 30
36 Figure 10. Model. Based on my observations I propose a model in which Rab11, Retromer, Snx1, and Snx3 coordinate the release, formation, and cargo loading of exosomes within the Drosophila nervous system. Loss of Rab11 is associated with a significant decrease in exosomal APPeGFP suggesting that APPeGFP-containing exosomes are derived from multivesicular bodies that fuse with the plasma membrane. Snx3 and Vps35 mutants both display an increase in exosomal APPeGFP, however the Snx3 phenotype is not statistically significant presumably due to a low quantify of measurements. This indicates that Snx3 may be working through Vps35 and the Retromer complex in order to retrogradely traffic APP away from endosomes, preventing it from being packaged into exosomes. Snx1 mutants, on the other hand, display a dramatic retention of APPeGFP and reduction to the amount of APPegFP exosomes. This difference also did not reach statistical significance due to insufficient measurements. Nevertheless, it seems that Snx1 promotes APPeGFP exosomes biogenesis through an unknown mechanism. Two hypotheses exist. The first possibility is that Snx1 acts indpendent of Retromer to promote ILV formation via a novel function (1). The other possibility is that Snx1 workes through the Retromer complex, which may encourage ILV formation if Snx1-Retromer removes factors that inhibit the inward budding of the membrane (2). 31
37 5 Appendix 5.1 Supporting observations from previous experiments In order to address the validity of our proposed model it is important to note that the n-values (representing the number of NMJs imaged and quantified) for percent extracellular APPeGFP were too small to yield a statistically significant effects for our snx1 and snx3 conditions in comparison to wt. Nevertheless it is possible to draw support for my conclusions and model based on previous versions of the NMJ imaging experiments. The prior method for examining APPeGFP exosomes at the Drosophila NMJ involved staining against Complexin overnight at 4ºC and 1:10,000 concentration, then using a Rhodamine Red secondary antibody at room temperature and 1:1000 concentration for four hours. After numerous trials and experiments it was discovered that there was a noticible amount of 488nm excitation/ 561nm emission from the Rhodamine Red secondary, most likely due to overstaining. This was determined by staining non-gfp-expressing larvae with Complexin primary/ Rhodamine Red secondary and observing a clear green channel fluorescence that perfectly superimposed with the neuronal processes illuminated by the Complexin stain. This phenomenon was observed on both confocal microscopy systems described in the methods section. 32
38 What was observed bye the eye in these samples parallels what is described in our results section; snx3 lead to an increase in extracellular APPeGFP puncta while snx1 deletion led to a decrease (Figure 11). These visual results were recapitulated with our new staining protocol, although statistical differences exist between the two sets of data. It is important to note that the percentage of extracellular APPeGFP would have been reduced for all conditions due to the staining protocol and the quantification methods available at the time. 5.2 Flaws in previous methodology and their effects on results Quantification of the NMJ images was performed in a manner which would have the overall effect of reducing the extracellular percentage across all conditions by increasing the intracellular green channel signal due to bleedthrough from the Rhodamine Red secondary antibody originally used to visualize Complexin. In order to more accurately measure APPeGFP fluorescence the staining protocol was changed to what is described in the methods section, and the quantification redone with new samples. In addition to the staining protocol, the process of quantifying my fluorescence data involved a method which resulted in an overall decrease in the calculated percentage of extracellular APPeGFP (FE). This method, also enlisting the ImageJ program, involved measuring only mean fluorescence values for the intracellular (µi) and extracellular regions (µe) after subtracting mean background (µb) levels. These mean fluorescence per unit area values were then converted into raw fluorescence data by 33
39 multiplying by the area over which the measurements were taken (A). Equation shown below: FE = (µe - µb)(ae) / [(µe - µb)(ae) + (µi - µb)(ai)] The measurement of a mean fluorescence value over a large extracellular area where puncta are sparse is extremely sensitive to the size of that area. Because of the large quantity of dim background pixels factored into the mean extracellular fluorescence value, the mean would be skewed towards a dimmer value. In this way, performing calculations with mean fluorescence values would result in lower calculated percentages for extracellular APPeGFP. These differences can be observed by comparing the percent extracellular APPeGFP values for identical genotypes between different staining and quantification methods (Figures 8 and 12). 34
40 Membrane APPeGFP Merge snx1 snx3 wt Figure 11. Overstained neuromuscular junctions display inaccurate phenotypes. The above images, taken before the discovery of the Rhodamine Red bleedthrough signal, display a dramatic APPeGFP retention phenotype for snx1 mutant animals in comparison to wild-type and snx3 mutant animals. The presynaptic neuron is stained as described in Section 8.1, generating a green signal that perfectly overlaps with neuronal processes when APPeGFP expression is not high enough (such as in this particular example of a snx1 mutant NMJ, third row). Due to the unknown relative contributions of APPeGFP and Rhodamine Red bleedthrough, these data were all discarded once the bleedthrough was discovered and the experiments was repeated with a far red secondary. 35
41 Extracellular APPeGFP Percent Figure 12. Quantification of extracellular fluorescence using an alternative method. Scatter plot above displaying the fraction of extracellular APPeGFP as calculated using mean fluorescence values instead of integrated density measurements. Wide bars indicate group means with brackets denoting the standard error of the mean. Due to inaccuracies inherent in the staining and quantification methodology, propagation of error resulted in a global decrease in percent extracellular APPeGFP across all genotypes when compared to the percentages reported utilizing the new method (described in Section 5.2, Section 5.3, and Figure 8). Neuromuscular junctions that were sprawling or had weak APPeGFP expression were especially susceptible to measurement error for reasons described in Section 8.2. According to this methodology, the differences between snx3 and wt animals are less pronounced and the differences between snx1 and wt reach a statistically significant magnitude. 36
42 6 References 1 Masters, C. L., et al. (1985). "Amyloid plaque core protein in Alzheimer disease and Down syndrome." Proceedings of the National Academy of Sciences 82(12): Kang, J., et al. (1987). "The precursor of Alzheimer's disease amyloid A4 protein resembles a cell-surface receptor." 3 Allinson, T. M. J., et al. (2003). "ADAMs family members as amyloid precursor protein α secretases." Journal of neuroscience research 74(3): Yan, R., et al. (1999). "Membrane-anchored aspartyl protease with Alzheimer's disease β-secretase activity." Nature 402(6761): Vassar, R., et al. (1999). "β-secretase cleavage of Alzheimer's amyloid precursor protein by the transmembrane aspartic protease BACE." Science 286(5440): Wolfe, M. S. (2010). "Structure, mechanism and inhibition of γ-secretase and presenilinlike proteases." Biological chemistry 391(8): Sala Frigerio, C., et al. (2010). "β Secretase cleavage is not required for generation of the intracellular C terminal domain of the amyloid precursor family of proteins." FEBS Journal 277(6): Muresan, V., et al. (2009). "The cleavage products of amyloid-β precursor protein are sorted to distinct carrier vesicles that are independently transported within neurites." The Journal of Neuroscience 29(11): Chen, W. J., et al. (1990). "NPXY, a sequence often found in cytoplasmic tails, is required for coated pit-mediated internalization of the low density lipoprotein receptor." Journal of Biological Chemistry 265(6): Lai, A., et al. (1995). "Characterization of sorting signals in the-amyloid precursor protein cytoplasmic domain." Journal of Biological Chemistry 270(8): Cirrito, J. R., et al. (2008). "Endocytosis is required for synaptic activity-dependent release of amyloid-β in vivo." Neuron 58(1): Jacobsen, K. T. and K. Iverfeldt (2009). "Amyloid precursor protein and its homologues: a family of proteolysis-dependent receptors." Cellular and molecular life sciences 66(14):
43 13 Müller, U. C. and H. Zheng (2012). "Physiological Functions of APP Family Proteins." Cold Spring Harb Perspect Med 2(2). 14 Ashley, J., et al. (2005). "Fasciclin II signals new synapse formation through amyloid precursor protein and the scaffolding protein dx11/mint." The Journal of Neuroscience 25(25): Torroja, L., et al. (1999). "The Drosophila β-amyloid precursor protein homolog promotes synapse differentiation at the neuromuscular junction." The Journal of Neuroscience 19(18): Attar, N. and P. J. Cullen (2010). "The Retromer complex." Advances in enzyme regulation 50(1): Norwood, S. J., et al. (2011). "Assembly and solution structure of the core Retromer protein complex." Traffic 12(1): Fjorback, A. W., et al. (2012). "Retromer binds the FANSHY sorting motif in SorLA to regulate amyloid precursor protein sorting and processing." J Neurosci 32(4): Wang, C. L., et al. (2012). "VPS35 regulates developing mouse hippocampal neuronal morphogenesis by promoting retrograde trafficking of BACE1." Biology Open 1(12): Lane, R. F., et al. (2012). "Vps10 Family Proteins and the Retromer Complex in Aging- Related Neurodegeneration and Diabetes." The Journal of Neuroscience 32(41): Tanzi, R. E. (2012). "The genetics of Alzheimer disease." Cold Spring Harb Perspect Med 2(10). 22 Vardarajan, B. N., et al. (2012). "Identification of Alzheimer disease-associated variants in genes that regulate retromer function." Neurobiology of Aging. 23 Rogaeva, E., et al. (2007). "The neuronal sortilin-related receptor SORL1 is genetically associated with Alzheimer disease." Nature genetics 39(2): Muhammad, A., et al. (2008). "Retromer deficiency observed in Alzheimer's disease causes hippocampal dysfunction, neurodegeneration, and Aβ accumulation." Proceedings of the National Academy of Sciences 105(20): Small, S. A., et al. (2005). "Model guided microarray implicates the Retromer complex in Alzheimer's disease." Annals of neurology 58(6): Cozier, G. E., et al. (2002). "The phox homology (PX) domain-dependent, 3- phosphoinositide-mediated association of sorting nexin-1 with an early sorting endosomal compartment is required for its ability to regulate epidermal growth factor receptor degradation." Journal of Biological Chemistry 277(50):
Chapter 3. Expression of α5-megfp in Mouse Cortical Neurons. on the β subunit. Signal sequences in the M3-M4 loop of β nachrs bind protein factors to
 22 Chapter 3 Expression of α5-megfp in Mouse Cortical Neurons Subcellular localization of the neuronal nachr subtypes α4β2 and α4β4 depends on the β subunit. Signal sequences in the M3-M4 loop of β nachrs
22 Chapter 3 Expression of α5-megfp in Mouse Cortical Neurons Subcellular localization of the neuronal nachr subtypes α4β2 and α4β4 depends on the β subunit. Signal sequences in the M3-M4 loop of β nachrs
LDLR-related protein 10 (LRP10) regulates amyloid precursor protein (APP) trafficking and processing: evidence for a role in Alzheimer s disease
 Brodeur et al. Molecular Neurodegeneration 2012, 7:31 RESEARCH ARTICLE Open Access LDLR-related protein 10 (LRP10) regulates amyloid precursor protein (APP) trafficking and processing: evidence for a role
Brodeur et al. Molecular Neurodegeneration 2012, 7:31 RESEARCH ARTICLE Open Access LDLR-related protein 10 (LRP10) regulates amyloid precursor protein (APP) trafficking and processing: evidence for a role
Homework Hanson section MCB Course, Fall 2014
 Homework Hanson section MCB Course, Fall 2014 (1) Antitrypsin, which inhibits certain proteases, is normally secreted into the bloodstream by liver cells. Antitrypsin is absent from the bloodstream of
Homework Hanson section MCB Course, Fall 2014 (1) Antitrypsin, which inhibits certain proteases, is normally secreted into the bloodstream by liver cells. Antitrypsin is absent from the bloodstream of
The Role of Sorting Nexins in Antigen Presentation
 The Role of Sorting Nexins in Antigen Presentation Chng X.R.J 1 and Wong S.H. 2 Department of Microbiology Yong Loo Lin School of Medicine, National University of Singapore Block MD4, 5 Science Drive 2,
The Role of Sorting Nexins in Antigen Presentation Chng X.R.J 1 and Wong S.H. 2 Department of Microbiology Yong Loo Lin School of Medicine, National University of Singapore Block MD4, 5 Science Drive 2,
Lecture Readings. Vesicular Trafficking, Secretory Pathway, HIV Assembly and Exit from Cell
 October 26, 2006 1 Vesicular Trafficking, Secretory Pathway, HIV Assembly and Exit from Cell 1. Secretory pathway a. Formation of coated vesicles b. SNAREs and vesicle targeting 2. Membrane fusion a. SNAREs
October 26, 2006 1 Vesicular Trafficking, Secretory Pathway, HIV Assembly and Exit from Cell 1. Secretory pathway a. Formation of coated vesicles b. SNAREs and vesicle targeting 2. Membrane fusion a. SNAREs
Endocytosis and Intracellular Trafficking of Notch and Its Ligands
 CHA P T E R F IVE Endocytosis and Intracellular Trafficking of Notch and Its Ligands Shinya Yamamoto, *,1 Wu-Lin Charng, *,1 and Hugo J. Bellen *,,, Contents 1. Notch Signaling and its Regulation by Endocytosis
CHA P T E R F IVE Endocytosis and Intracellular Trafficking of Notch and Its Ligands Shinya Yamamoto, *,1 Wu-Lin Charng, *,1 and Hugo J. Bellen *,,, Contents 1. Notch Signaling and its Regulation by Endocytosis
04_polarity. The formation of synaptic vesicles
 Brefeldin prevents assembly of the coats required for budding Nocodazole disrupts microtubules Constitutive: coatomer-coated Selected: clathrin-coated The formation of synaptic vesicles Nerve cells (and
Brefeldin prevents assembly of the coats required for budding Nocodazole disrupts microtubules Constitutive: coatomer-coated Selected: clathrin-coated The formation of synaptic vesicles Nerve cells (and
Lysosomes and endocytic pathways 9/27/2012 Phyllis Hanson
 Lysosomes and endocytic pathways 9/27/2012 Phyllis Hanson General principles Properties of lysosomes Delivery of enzymes to lysosomes Endocytic uptake clathrin, others Endocytic pathways recycling vs.
Lysosomes and endocytic pathways 9/27/2012 Phyllis Hanson General principles Properties of lysosomes Delivery of enzymes to lysosomes Endocytic uptake clathrin, others Endocytic pathways recycling vs.
Institute of Molecular and Cellular Biology FACULTY OF BIOLOGICAL SCIENCES. Lipid rafts in neurodegenerative diseases. Nigel M.
 Institute of Molecular and Cellular Biology FACULTY OF BIOLOGICAL SCIENCES Lipid rafts in neurodegenerative diseases Nigel M. Hooper Institute of Molecular and Cellular Biology FACULTY OF BIOLOGICAL SCIENCES
Institute of Molecular and Cellular Biology FACULTY OF BIOLOGICAL SCIENCES Lipid rafts in neurodegenerative diseases Nigel M. Hooper Institute of Molecular and Cellular Biology FACULTY OF BIOLOGICAL SCIENCES
Vesicle Transport. Vesicle pathway: many compartments, interconnected by trafficking routes 3/17/14
 Vesicle Transport Vesicle Formation Curvature (Self Assembly of Coat complex) Sorting (Sorting Complex formation) Regulation (Sar1/Arf1 GTPases) Fission () Membrane Fusion SNARE combinations Tethers Regulation
Vesicle Transport Vesicle Formation Curvature (Self Assembly of Coat complex) Sorting (Sorting Complex formation) Regulation (Sar1/Arf1 GTPases) Fission () Membrane Fusion SNARE combinations Tethers Regulation
Project report October 2012 March 2013 CRF fellow: Principal Investigator: Project title:
 Project report October 2012 March 2013 CRF fellow: Gennaro Napolitano Principal Investigator: Sergio Daniel Catz Project title: Small molecule regulators of vesicular trafficking to enhance lysosomal exocytosis
Project report October 2012 March 2013 CRF fellow: Gennaro Napolitano Principal Investigator: Sergio Daniel Catz Project title: Small molecule regulators of vesicular trafficking to enhance lysosomal exocytosis
Supplementary Figure S1. Venn diagram analysis of mrna microarray data and mirna target analysis. (a) Western blot analysis of T lymphoblasts (CLS)
 Supplementary Figure S1. Venn diagram analysis of mrna microarray data and mirna target analysis. (a) Western blot analysis of T lymphoblasts (CLS) and their exosomes (EXO) in resting (REST) and activated
Supplementary Figure S1. Venn diagram analysis of mrna microarray data and mirna target analysis. (a) Western blot analysis of T lymphoblasts (CLS) and their exosomes (EXO) in resting (REST) and activated
Protein Trafficking in the Secretory and Endocytic Pathways
 Protein Trafficking in the Secretory and Endocytic Pathways The compartmentalization of eukaryotic cells has considerable functional advantages for the cell, but requires elaborate mechanisms to ensure
Protein Trafficking in the Secretory and Endocytic Pathways The compartmentalization of eukaryotic cells has considerable functional advantages for the cell, but requires elaborate mechanisms to ensure
Intracellular Vesicular Traffic Chapter 13, Alberts et al.
 Intracellular Vesicular Traffic Chapter 13, Alberts et al. The endocytic and biosynthetic-secretory pathways The intracellular compartments of the eucaryotic ell involved in the biosynthetic-secretory
Intracellular Vesicular Traffic Chapter 13, Alberts et al. The endocytic and biosynthetic-secretory pathways The intracellular compartments of the eucaryotic ell involved in the biosynthetic-secretory
Localization and Retention of Glycosyltransferases And the Role of Vesicle Trafficking in Glycosylation
 Localization and Retention of Glycosyltransferases And the Role of Vesicle Trafficking in Glycosylation Richard Steet, Ph.D. 3/8/2011 glycosylation is a non-template derived phenomenon - the presence of
Localization and Retention of Glycosyltransferases And the Role of Vesicle Trafficking in Glycosylation Richard Steet, Ph.D. 3/8/2011 glycosylation is a non-template derived phenomenon - the presence of
Mechanism of Vesicular Transport
 Mechanism of Vesicular Transport Transport vesicles play a central role in the traffic of molecules between different membrane-enclosed enclosed compartments. The selectivity of such transport is therefore
Mechanism of Vesicular Transport Transport vesicles play a central role in the traffic of molecules between different membrane-enclosed enclosed compartments. The selectivity of such transport is therefore
SUPPLEMENTARY FIGURES
 SUPPLEMENTARY FIGURES Supplementary Figure 1. (A) Left, western blot analysis of ISGylated proteins in Jurkat T cells treated with 1000U ml -1 IFN for 16h (IFN) or left untreated (CONT); right, western
SUPPLEMENTARY FIGURES Supplementary Figure 1. (A) Left, western blot analysis of ISGylated proteins in Jurkat T cells treated with 1000U ml -1 IFN for 16h (IFN) or left untreated (CONT); right, western
1. endoplasmic reticulum This is the location where N-linked oligosaccharide is initially synthesized and attached to glycoproteins.
 Biology 4410 Name Spring 2006 Exam 2 A. Multiple Choice, 2 pt each Pick the best choice from the list of choices, and write it in the space provided. Some choices may be used more than once, and other
Biology 4410 Name Spring 2006 Exam 2 A. Multiple Choice, 2 pt each Pick the best choice from the list of choices, and write it in the space provided. Some choices may be used more than once, and other
Molecular Cell Biology - Problem Drill 17: Intracellular Vesicular Traffic
 Molecular Cell Biology - Problem Drill 17: Intracellular Vesicular Traffic Question No. 1 of 10 1. Which of the following statements about clathrin-coated vesicles is correct? Question #1 (A) There are
Molecular Cell Biology - Problem Drill 17: Intracellular Vesicular Traffic Question No. 1 of 10 1. Which of the following statements about clathrin-coated vesicles is correct? Question #1 (A) There are
ALZHEIMER S DISEASE FACTOIDS & STATISTICS
 ALZHEIMER S DISEASE FACTOIDS & STATISTICS ~ 4 million affected in US alone 6-8% if 65+ years old, 30-50% if 80+ By 2030, in US >65 million people >65+ (---> ~14 million with AD) AD is one of the top 10
ALZHEIMER S DISEASE FACTOIDS & STATISTICS ~ 4 million affected in US alone 6-8% if 65+ years old, 30-50% if 80+ By 2030, in US >65 million people >65+ (---> ~14 million with AD) AD is one of the top 10
Localization and Retention of Glycosyltransferases And the Role of Vesicle Trafficking in Glycosylation
 Localization and Retention of Glycosyltransferases And the Role of Vesicle Trafficking in Glycosylation Richard Steet, Ph.D. 2/21/17 glycosylation is a non-template derived phenomenon - the presence of
Localization and Retention of Glycosyltransferases And the Role of Vesicle Trafficking in Glycosylation Richard Steet, Ph.D. 2/21/17 glycosylation is a non-template derived phenomenon - the presence of
Lipids and Membranes
 Lipids and Membranes Presented by Dr. Mohammad Saadeh The requirements for the Pharmaceutical Biochemistry I Philadelphia University Faculty of pharmacy Membrane transport D. Endocytosis and Exocytosis
Lipids and Membranes Presented by Dr. Mohammad Saadeh The requirements for the Pharmaceutical Biochemistry I Philadelphia University Faculty of pharmacy Membrane transport D. Endocytosis and Exocytosis
MCB130 Midterm. GSI s Name:
 1. Peroxisomes are small, membrane-enclosed organelles that function in the degradation of fatty acids and in the degradation of H 2 O 2. Peroxisomes are not part of the secretory pathway and peroxisomal
1. Peroxisomes are small, membrane-enclosed organelles that function in the degradation of fatty acids and in the degradation of H 2 O 2. Peroxisomes are not part of the secretory pathway and peroxisomal
Cell Quality Control. Peter Takizawa Department of Cell Biology
 Cell Quality Control Peter Takizawa Department of Cell Biology Cellular quality control reduces production of defective proteins. Cells have many quality control systems to ensure that cell does not build
Cell Quality Control Peter Takizawa Department of Cell Biology Cellular quality control reduces production of defective proteins. Cells have many quality control systems to ensure that cell does not build
Section 6. Junaid Malek, M.D.
 Section 6 Junaid Malek, M.D. The Golgi and gp160 gp160 transported from ER to the Golgi in coated vesicles These coated vesicles fuse to the cis portion of the Golgi and deposit their cargo in the cisternae
Section 6 Junaid Malek, M.D. The Golgi and gp160 gp160 transported from ER to the Golgi in coated vesicles These coated vesicles fuse to the cis portion of the Golgi and deposit their cargo in the cisternae
Head of College Scholars List Scheme. Summer Studentship Report Form
 Head of College Scholars List Scheme Summer Studentship 2019 Report Form This report should be completed by the student with his/her project supervisor. It should summarise the work undertaken during the
Head of College Scholars List Scheme Summer Studentship 2019 Report Form This report should be completed by the student with his/her project supervisor. It should summarise the work undertaken during the
SUPPLEMENTARY FIGURE LEGENDS
 SUPPLEMENTARY FIGURE LEGENDS Supplemental FIG. 1. Localization of myosin Vb in cultured neurons varies with maturation stage. A and B, localization of myosin Vb in cultured hippocampal neurons. A, in DIV
SUPPLEMENTARY FIGURE LEGENDS Supplemental FIG. 1. Localization of myosin Vb in cultured neurons varies with maturation stage. A and B, localization of myosin Vb in cultured hippocampal neurons. A, in DIV
Module 3 Lecture 7 Endocytosis and Exocytosis
 Module 3 Lecture 7 Endocytosis and Exocytosis Endocytosis: Endocytosis is the process by which cells absorb larger molecules and particles from the surrounding by engulfing them. It is used by most of
Module 3 Lecture 7 Endocytosis and Exocytosis Endocytosis: Endocytosis is the process by which cells absorb larger molecules and particles from the surrounding by engulfing them. It is used by most of
7.06 Cell Biology EXAM #3 April 24, 2003
 7.06 Spring 2003 Exam 3 Name 1 of 8 7.06 Cell Biology EXAM #3 April 24, 2003 This is an open book exam, and you are allowed access to books and notes. Please write your answers to the questions in the
7.06 Spring 2003 Exam 3 Name 1 of 8 7.06 Cell Biology EXAM #3 April 24, 2003 This is an open book exam, and you are allowed access to books and notes. Please write your answers to the questions in the
Chapter 13: Vesicular Traffic
 Chapter 13: Vesicular Traffic Know the terminology: ER, Golgi, vesicle, clathrin, COP-I, COP-II, BiP, glycosylation, KDEL, microtubule, SNAREs, dynamin, mannose-6-phosphate, M6P receptor, endocytosis,
Chapter 13: Vesicular Traffic Know the terminology: ER, Golgi, vesicle, clathrin, COP-I, COP-II, BiP, glycosylation, KDEL, microtubule, SNAREs, dynamin, mannose-6-phosphate, M6P receptor, endocytosis,
SUPPLEMENTARY INFORMATION
 DOI: 10.1038/ncb2988 Supplementary Figure 1 Kif7 L130P encodes a stable protein that does not localize to cilia tips. (a) Immunoblot with KIF7 antibody in cell lysates of wild-type, Kif7 L130P and Kif7
DOI: 10.1038/ncb2988 Supplementary Figure 1 Kif7 L130P encodes a stable protein that does not localize to cilia tips. (a) Immunoblot with KIF7 antibody in cell lysates of wild-type, Kif7 L130P and Kif7
Programmed Cell Death (apoptosis)
 Programmed Cell Death (apoptosis) Stereotypic death process includes: membrane blebbing nuclear fragmentation chromatin condensation and DNA framentation loss of mitochondrial integrity and release of
Programmed Cell Death (apoptosis) Stereotypic death process includes: membrane blebbing nuclear fragmentation chromatin condensation and DNA framentation loss of mitochondrial integrity and release of
7.06 Spring of PROBLEM SET #6
 7.6 Spring 23 1 of 6 7.6 PROBLEM SET #6 1. You are studying a mouse model of hypercholesterolemia, a disease characterized by high levels of cholesterol in the blood. In normal cells, LDL particles in
7.6 Spring 23 1 of 6 7.6 PROBLEM SET #6 1. You are studying a mouse model of hypercholesterolemia, a disease characterized by high levels of cholesterol in the blood. In normal cells, LDL particles in
MBios 401/501: Lecture 12.1 Signaling IV. Slide 1
 MBios 401/501: Lecture 12.1 Signaling IV Slide 1 Pathways that require regulated proteolysis 1. Notch and Delta 2. Wnt/ b-catenin 3. Hedgehog 4. NFk-B Our last topic on cell signaling are pathways that
MBios 401/501: Lecture 12.1 Signaling IV Slide 1 Pathways that require regulated proteolysis 1. Notch and Delta 2. Wnt/ b-catenin 3. Hedgehog 4. NFk-B Our last topic on cell signaling are pathways that
ab SREBP-2 Translocation Assay Kit (Cell-Based)
 ab133114 SREBP-2 Translocation Assay Kit (Cell-Based) Instructions for Use For analysis of translocation of SREBP-2 into nuclei. This product is for research use only and is not intended for diagnostic
ab133114 SREBP-2 Translocation Assay Kit (Cell-Based) Instructions for Use For analysis of translocation of SREBP-2 into nuclei. This product is for research use only and is not intended for diagnostic
Practice Exam 2 MCBII
 1. Which feature is true for signal sequences and for stop transfer transmembrane domains (4 pts)? A. They are both 20 hydrophobic amino acids long. B. They are both found at the N-terminus of the protein.
1. Which feature is true for signal sequences and for stop transfer transmembrane domains (4 pts)? A. They are both 20 hydrophobic amino acids long. B. They are both found at the N-terminus of the protein.
marker. DAPI labels nuclei. Flies were 20 days old. Scale bar is 5 µm. Ctrl is
 Supplementary Figure 1. (a) Nos is detected in glial cells in both control and GFAP R79H transgenic flies (arrows), but not in deletion mutant Nos Δ15 animals. Repo is a glial cell marker. DAPI labels
Supplementary Figure 1. (a) Nos is detected in glial cells in both control and GFAP R79H transgenic flies (arrows), but not in deletion mutant Nos Δ15 animals. Repo is a glial cell marker. DAPI labels
PHSI3009 Frontiers in Cellular Physiology 2017
 Overview of PHSI3009 L2 Cell membrane and Principles of cell communication L3 Signalling via G protein-coupled receptor L4 Calcium Signalling L5 Signalling via Growth Factors L6 Signalling via small G-protein
Overview of PHSI3009 L2 Cell membrane and Principles of cell communication L3 Signalling via G protein-coupled receptor L4 Calcium Signalling L5 Signalling via Growth Factors L6 Signalling via small G-protein
2 Answer all the questions.
 2 Answer all the questions. 1 Fig. 1.1 is a flow diagram showing the main stages involved in making cheese. The starting material is milk, which contains the protein, casein. milk is heated to 72 C for
2 Answer all the questions. 1 Fig. 1.1 is a flow diagram showing the main stages involved in making cheese. The starting material is milk, which contains the protein, casein. milk is heated to 72 C for
Supplementary Fig. 1 V-ATPase depletion induces unique and robust phenotype in Drosophila fat body cells.
 Supplementary Fig. 1 V-ATPase depletion induces unique and robust phenotype in Drosophila fat body cells. a. Schematic of the V-ATPase proton pump macro-complex structure. The V1 complex is composed of
Supplementary Fig. 1 V-ATPase depletion induces unique and robust phenotype in Drosophila fat body cells. a. Schematic of the V-ATPase proton pump macro-complex structure. The V1 complex is composed of
Chapter 2. Investigation into mir-346 Regulation of the nachr α5 Subunit
 15 Chapter 2 Investigation into mir-346 Regulation of the nachr α5 Subunit MicroRNA s (mirnas) are small (< 25 base pairs), single stranded, non-coding RNAs that regulate gene expression at the post transcriptional
15 Chapter 2 Investigation into mir-346 Regulation of the nachr α5 Subunit MicroRNA s (mirnas) are small (< 25 base pairs), single stranded, non-coding RNAs that regulate gene expression at the post transcriptional
Supplementary Figure S1: TIPF reporter validation in the wing disc.
 Supplementary Figure S1: TIPF reporter validation in the wing disc. a,b, Test of put RNAi. a, In wildtype discs the Dpp target gene Sal (red) is expressed in a broad stripe in the centre of the ventral
Supplementary Figure S1: TIPF reporter validation in the wing disc. a,b, Test of put RNAi. a, In wildtype discs the Dpp target gene Sal (red) is expressed in a broad stripe in the centre of the ventral
Organization of ATPases
 The Primary Active Transporter II: The ATPase Objectives: Organization P type with NPA domains Proton pumps of the rotary V type ATPase 1 Organization of P type, solute transport, found in plasma membranes
The Primary Active Transporter II: The ATPase Objectives: Organization P type with NPA domains Proton pumps of the rotary V type ATPase 1 Organization of P type, solute transport, found in plasma membranes
Extracellular vesicle budding is inhibited by redundant regulators of TAT-5 flippase localization and phospholipid asymmetry
 Extracellular vesicle budding is inhibited by redundant regulators of TAT-5 flippase localization and phospholipid asymmetry Katharina. eer a, Jennifer Rivas-Castillo a, Kenneth Kuhn a, Gholamreza Fazeli
Extracellular vesicle budding is inhibited by redundant regulators of TAT-5 flippase localization and phospholipid asymmetry Katharina. eer a, Jennifer Rivas-Castillo a, Kenneth Kuhn a, Gholamreza Fazeli
Supporting Online Material for
 www.sciencemag.org/cgi/content/full/1171320/dc1 Supporting Online Material for A Frazzled/DCC-Dependent Transcriptional Switch Regulates Midline Axon Guidance Long Yang, David S. Garbe, Greg J. Bashaw*
www.sciencemag.org/cgi/content/full/1171320/dc1 Supporting Online Material for A Frazzled/DCC-Dependent Transcriptional Switch Regulates Midline Axon Guidance Long Yang, David S. Garbe, Greg J. Bashaw*
Appendix: Subcellular localization of MIG-14::GFP in C. elegans body wall muscle cells. Pei-Tzu Yang and Hendrik C. Korswagen
 Appendix: Subcellular localization of MIG-14::GFP in C. elegans body wall muscle cells Pei-Tzu Yang and Hendrik C. Korswagen 129 SUBCELLULAR LOCALIZATION OF MIG-14 Abstract MIG-14/Wls is a Wnt binding
Appendix: Subcellular localization of MIG-14::GFP in C. elegans body wall muscle cells Pei-Tzu Yang and Hendrik C. Korswagen 129 SUBCELLULAR LOCALIZATION OF MIG-14 Abstract MIG-14/Wls is a Wnt binding
I. Fluid Mosaic Model A. Biological membranes are lipid bilayers with associated proteins
 Lecture 6: Membranes and Cell Transport Biological Membranes I. Fluid Mosaic Model A. Biological membranes are lipid bilayers with associated proteins 1. Characteristics a. Phospholipids form bilayers
Lecture 6: Membranes and Cell Transport Biological Membranes I. Fluid Mosaic Model A. Biological membranes are lipid bilayers with associated proteins 1. Characteristics a. Phospholipids form bilayers
Intracellular vesicular traffic. B. Balen
 Intracellular vesicular traffic B. Balen Three types of transport in eukaryotic cells Figure 12-6 Molecular Biology of the Cell ( Garland Science 2008) Endoplasmic reticulum in all eucaryotic cells Endoplasmic
Intracellular vesicular traffic B. Balen Three types of transport in eukaryotic cells Figure 12-6 Molecular Biology of the Cell ( Garland Science 2008) Endoplasmic reticulum in all eucaryotic cells Endoplasmic
General information. Cell mediated immunity. 455 LSA, Tuesday 11 to noon. Anytime after class.
 General information Cell mediated immunity 455 LSA, Tuesday 11 to noon Anytime after class T-cell precursors Thymus Naive T-cells (CD8 or CD4) email: lcoscoy@berkeley.edu edu Use MCB150 as subject line
General information Cell mediated immunity 455 LSA, Tuesday 11 to noon Anytime after class T-cell precursors Thymus Naive T-cells (CD8 or CD4) email: lcoscoy@berkeley.edu edu Use MCB150 as subject line
Zool 3200: Cell Biology Exam 4 Part I 2/3/15
 Name: Key Trask Zool 3200: Cell Biology Exam 4 Part I 2/3/15 Answer each of the following questions in the space provided, explaining your answers when asked to do so; circle the correct answer or answers
Name: Key Trask Zool 3200: Cell Biology Exam 4 Part I 2/3/15 Answer each of the following questions in the space provided, explaining your answers when asked to do so; circle the correct answer or answers
(a) Significant biological processes (upper panel) and disease biomarkers (lower panel)
 Supplementary Figure 1. Functional enrichment analyses of secretomic proteins. (a) Significant biological processes (upper panel) and disease biomarkers (lower panel) 2 involved by hrab37-mediated secretory
Supplementary Figure 1. Functional enrichment analyses of secretomic proteins. (a) Significant biological processes (upper panel) and disease biomarkers (lower panel) 2 involved by hrab37-mediated secretory
The Molecular Mechanism of Intracellular Membrane Fusion. Richard H. Scheller
 The Molecular Mechanism of Intracellular Membrane Fusion Richard H. Scheller The human brain contains approximately 10 15 connections between nerve cells (Figure 2). The specific formation and modulation
The Molecular Mechanism of Intracellular Membrane Fusion Richard H. Scheller The human brain contains approximately 10 15 connections between nerve cells (Figure 2). The specific formation and modulation
Nature Neuroscience: doi: /nn Supplementary Figure 1. Neuron class-specific arrangements of Khc::nod::lacZ label in dendrites.
 Supplementary Figure 1 Neuron class-specific arrangements of Khc::nod::lacZ label in dendrites. Staining with fluorescence antibodies to detect GFP (Green), β-galactosidase (magenta/white). (a, b) Class
Supplementary Figure 1 Neuron class-specific arrangements of Khc::nod::lacZ label in dendrites. Staining with fluorescence antibodies to detect GFP (Green), β-galactosidase (magenta/white). (a, b) Class
Zool 3200: Cell Biology Exam 4 Part II 2/3/15
 Name:Key Trask Zool 3200: Cell Biology Exam 4 Part II 2/3/15 Answer each of the following questions in the space provided, explaining your answers when asked to do so; circle the correct answer or answers
Name:Key Trask Zool 3200: Cell Biology Exam 4 Part II 2/3/15 Answer each of the following questions in the space provided, explaining your answers when asked to do so; circle the correct answer or answers
Supplementary Figures
 J. Cell Sci. 128: doi:10.1242/jcs.173807: Supplementary Material Supplementary Figures Fig. S1 Fig. S1. Description and/or validation of reagents used. All panels show Drosophila tissues oriented with
J. Cell Sci. 128: doi:10.1242/jcs.173807: Supplementary Material Supplementary Figures Fig. S1 Fig. S1. Description and/or validation of reagents used. All panels show Drosophila tissues oriented with
Advanced Cell Biology. Lecture 33
 Advanced Cell Biology. Lecture 33 Alexey Shipunov Minot State University April 22, 2013 Shipunov (MSU) Advanced Cell Biology. Lecture 33 April 22, 2013 1 / 38 Outline Questions and answers Intracellular
Advanced Cell Biology. Lecture 33 Alexey Shipunov Minot State University April 22, 2013 Shipunov (MSU) Advanced Cell Biology. Lecture 33 April 22, 2013 1 / 38 Outline Questions and answers Intracellular
1. This is the location where N-linked oligosaccharide is initially synthesized and attached to glycoproteins.
 Biology 4410 Name Spring 2006 Exam 2 A. Multiple Choice, 2 pt each Pick the best choice from the list of choices, and write it in the space provided. Some choices may be used more than once, and other
Biology 4410 Name Spring 2006 Exam 2 A. Multiple Choice, 2 pt each Pick the best choice from the list of choices, and write it in the space provided. Some choices may be used more than once, and other
Supplementary Materials for VAMP4 directs synaptic vesicles to a pool that selectively maintains asynchronous neurotransmission
 Supplementary Materials for VAMP4 directs synaptic vesicles to a pool that selectively maintains asynchronous neurotransmission Jesica Raingo, Mikhail Khvotchev, Pei Liu, Frederic Darios, Ying C. Li, Denise
Supplementary Materials for VAMP4 directs synaptic vesicles to a pool that selectively maintains asynchronous neurotransmission Jesica Raingo, Mikhail Khvotchev, Pei Liu, Frederic Darios, Ying C. Li, Denise
Supplementary Materials for
 www.sciencesignaling.org/cgi/content/full/6/283/ra57/dc1 Supplementary Materials for JNK3 Couples the Neuronal Stress Response to Inhibition of Secretory Trafficking Guang Yang,* Xun Zhou, Jingyan Zhu,
www.sciencesignaling.org/cgi/content/full/6/283/ra57/dc1 Supplementary Materials for JNK3 Couples the Neuronal Stress Response to Inhibition of Secretory Trafficking Guang Yang,* Xun Zhou, Jingyan Zhu,
THE SYNAPTIC VESICLE CYCLE
 Annu. Rev. Neurosci. 2004. 27:509 47 doi: 10.1146/annurev.neuro.26.041002.131412 Copyright c 2004 by Annual Reviews. All rights reserved First published online as a Review in Advance on March 12, 2004
Annu. Rev. Neurosci. 2004. 27:509 47 doi: 10.1146/annurev.neuro.26.041002.131412 Copyright c 2004 by Annual Reviews. All rights reserved First published online as a Review in Advance on March 12, 2004
BIOL2005 WORKSHEET 2008
 BIOL2005 WORKSHEET 2008 Answer all 6 questions in the space provided using additional sheets where necessary. Hand your completed answers in to the Biology office by 3 p.m. Friday 8th February. 1. Your
BIOL2005 WORKSHEET 2008 Answer all 6 questions in the space provided using additional sheets where necessary. Hand your completed answers in to the Biology office by 3 p.m. Friday 8th February. 1. Your
HIV-1 genome organization
 HIV-1 genome organization Gag encodes for a polyprotein that is cleaved into 4 proteins by HIV-1 protease. Gag is present in 2 transcripts: Gag polyprotein precursor and GagPol polyprotein precursor (5%).
HIV-1 genome organization Gag encodes for a polyprotein that is cleaved into 4 proteins by HIV-1 protease. Gag is present in 2 transcripts: Gag polyprotein precursor and GagPol polyprotein precursor (5%).
Intracellular Vesicle Trafficking
 Intracellular Vesicle Trafficking Chi-Kuang Yao (IBC, Academia Sinica) 11-6-2017 ckyao@gate.sinica.edu.tw 1 Compartmentalization makes difference between bacteria and yeast 1. More compartments with specific
Intracellular Vesicle Trafficking Chi-Kuang Yao (IBC, Academia Sinica) 11-6-2017 ckyao@gate.sinica.edu.tw 1 Compartmentalization makes difference between bacteria and yeast 1. More compartments with specific
endomembrane system internal membranes origins transport of proteins chapter 15 endomembrane system
 endo system chapter 15 internal s endo system functions as a coordinated unit divide cytoplasm into distinct compartments controls exocytosis and endocytosis movement of molecules which cannot pass through
endo system chapter 15 internal s endo system functions as a coordinated unit divide cytoplasm into distinct compartments controls exocytosis and endocytosis movement of molecules which cannot pass through
Cellular control of cholesterol. Peter Takizawa Department of Cell Biology
 Cellular control of cholesterol Peter Takizawa Department of Cell Biology Brief overview of cholesterol s biological role Regulation of cholesterol synthesis Dietary and cellular uptake of cholesterol
Cellular control of cholesterol Peter Takizawa Department of Cell Biology Brief overview of cholesterol s biological role Regulation of cholesterol synthesis Dietary and cellular uptake of cholesterol
El Azzouzi et al., http ://www.jcb.org /cgi /content /full /jcb /DC1
 Supplemental material JCB El Azzouzi et al., http ://www.jcb.org /cgi /content /full /jcb.201510043 /DC1 THE JOURNAL OF CELL BIOLOGY Figure S1. Acquisition of -phluorin correlates negatively with podosome
Supplemental material JCB El Azzouzi et al., http ://www.jcb.org /cgi /content /full /jcb.201510043 /DC1 THE JOURNAL OF CELL BIOLOGY Figure S1. Acquisition of -phluorin correlates negatively with podosome
Bulk Transport * OpenStax. 1 Endocytosis
 OpenStax-CNX module: m44419 1 Bulk Transport * OpenStax This work is produced by OpenStax-CNX and licensed under the Creative Commons Attribution License 3.0 By the end of this section, you will be able
OpenStax-CNX module: m44419 1 Bulk Transport * OpenStax This work is produced by OpenStax-CNX and licensed under the Creative Commons Attribution License 3.0 By the end of this section, you will be able
THE ROLE OF ALTERED CALCIUM AND mtor SIGNALING IN THE PATHOGENESIS OF CYSTINOSIS
 Research Foundation, 18 month progress report THE ROLE OF ALTERED CALCIUM AND mtor SIGNALING IN THE PATHOGENESIS OF CYSTINOSIS Ekaterina Ivanova, doctoral student Elena Levtchenko, MD, PhD, PI Antonella
Research Foundation, 18 month progress report THE ROLE OF ALTERED CALCIUM AND mtor SIGNALING IN THE PATHOGENESIS OF CYSTINOSIS Ekaterina Ivanova, doctoral student Elena Levtchenko, MD, PhD, PI Antonella
Chapter 2: Exocytosis and endocytosis. Biochimica cellulare parte B 2016/17
 Chapter 2: Exocytosis and endocytosis Biochimica cellulare parte B 2016/17 Exocytosis and endocytosis Transport from the trans-golgi network to the cell exterior: exocytosis. All eukaryotic cells continuously
Chapter 2: Exocytosis and endocytosis Biochimica cellulare parte B 2016/17 Exocytosis and endocytosis Transport from the trans-golgi network to the cell exterior: exocytosis. All eukaryotic cells continuously
MISSION: understanding the mechanisms of therapeutic strategies
 Telethon Institute of Genetics and Medicine MISSION: understanding the mechanisms of genetic diseases to develop preventive and therapeutic strategies G E N O T Y P E G E Researcher N 1 3 5 O T Y P E 8
Telethon Institute of Genetics and Medicine MISSION: understanding the mechanisms of genetic diseases to develop preventive and therapeutic strategies G E N O T Y P E G E Researcher N 1 3 5 O T Y P E 8
CONTRACTING ORGANIZATION: Cold Spring Harbor Laboratory Cold Spring Harbor, NY 11724
 AD Award Number: W81XWH-10-1-0450 TITLE: The role of NF1 in memory retrieval PRINCIPAL INVESTIGATOR: Yi Zhong, Ph.D. CONTRACTING ORGANIZATION: Cold Spring Harbor Laboratory Cold Spring Harbor, NY 11724
AD Award Number: W81XWH-10-1-0450 TITLE: The role of NF1 in memory retrieval PRINCIPAL INVESTIGATOR: Yi Zhong, Ph.D. CONTRACTING ORGANIZATION: Cold Spring Harbor Laboratory Cold Spring Harbor, NY 11724
Summary and Discussion antigen presentation
 Summary and Discussion antigen presentation 247 248 Summary & Discussion Summary and discussion: antigen presentation For a cell to communicate information about its internal health and status to the immune
Summary and Discussion antigen presentation 247 248 Summary & Discussion Summary and discussion: antigen presentation For a cell to communicate information about its internal health and status to the immune
/07/$15.00/0 Molecular Endocrinology 21(12): Printed in U.S.A.
 0888-8809/07/$15.00/0 Molecular Endocrinology 21(12):3087 3099 Printed in U.S.A. Copyright 2007 by The Endocrine Society doi: 10.1210/me.2006-0476 The Glucose Transporter 4 FQQI Motif Is Necessary for
0888-8809/07/$15.00/0 Molecular Endocrinology 21(12):3087 3099 Printed in U.S.A. Copyright 2007 by The Endocrine Society doi: 10.1210/me.2006-0476 The Glucose Transporter 4 FQQI Motif Is Necessary for
Life Sciences 1A Midterm Exam 2. November 13, 2006
 Name: TF: Section Time Life Sciences 1A Midterm Exam 2 November 13, 2006 Please write legibly in the space provided below each question. You may not use calculators on this exam. We prefer that you use
Name: TF: Section Time Life Sciences 1A Midterm Exam 2 November 13, 2006 Please write legibly in the space provided below each question. You may not use calculators on this exam. We prefer that you use
Intracellular Compartments and Protein Sorting
 Intracellular Compartments and Protein Sorting Intracellular Compartments A eukaryotic cell is elaborately subdivided into functionally distinct, membrane-enclosed compartments. Each compartment, or organelle,
Intracellular Compartments and Protein Sorting Intracellular Compartments A eukaryotic cell is elaborately subdivided into functionally distinct, membrane-enclosed compartments. Each compartment, or organelle,
Why am I working in a Drosophila lab? Dr. Stefan Pulver, Brandeis University
 Why am I working in a Drosophila lab? Dr. Stefan Pulver, Brandeis University !Short life span The advantages of Drosophila as a model organism in neurocience!genetic manipulations are relatively easy!forward
Why am I working in a Drosophila lab? Dr. Stefan Pulver, Brandeis University !Short life span The advantages of Drosophila as a model organism in neurocience!genetic manipulations are relatively easy!forward
Overview of clathrin-mediated endocytosis
 Overview of clathrin-mediated endocytosis Accessory and adaptor proteins promote clathrin nucleation on the plasma membrane and some help deform membrane. Clathrin assembly into lattices stabilize the
Overview of clathrin-mediated endocytosis Accessory and adaptor proteins promote clathrin nucleation on the plasma membrane and some help deform membrane. Clathrin assembly into lattices stabilize the
WDR62 is associated with the spindle pole and mutated in human microcephaly
 WDR62 is associated with the spindle pole and mutated in human microcephaly Adeline K. Nicholas, Maryam Khurshid, Julie Désir, Ofélia P. Carvalho, James J. Cox, Gemma Thornton, Rizwana Kausar, Muhammad
WDR62 is associated with the spindle pole and mutated in human microcephaly Adeline K. Nicholas, Maryam Khurshid, Julie Désir, Ofélia P. Carvalho, James J. Cox, Gemma Thornton, Rizwana Kausar, Muhammad
Molecular Trafficking
 SCBM 251 Molecular Trafficking Assoc. Prof. Rutaiwan Tohtong Department of Biochemistry Faculty of Science rutaiwan.toh@mahidol.ac.th Lecture outline 1. What is molecular trafficking? Why is it important?
SCBM 251 Molecular Trafficking Assoc. Prof. Rutaiwan Tohtong Department of Biochemistry Faculty of Science rutaiwan.toh@mahidol.ac.th Lecture outline 1. What is molecular trafficking? Why is it important?
Supplementary Information
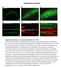 Supplementary Information Supplementary Figure 1: Luminal localization of CCM-3. (a) The CCM-3::GFP fusion protein localizes along the apical (luminal) surface of the pharynx (b) as well as the lumen of
Supplementary Information Supplementary Figure 1: Luminal localization of CCM-3. (a) The CCM-3::GFP fusion protein localizes along the apical (luminal) surface of the pharynx (b) as well as the lumen of
General aspects of this review - specific examples were addressed in class.
 General aspects of this review - specific examples were addressed in class. 1 Exam 1 Lecture 2: Discussed intracellular killing mechanisms Important maturation steps Rapid development into a microbicidal
General aspects of this review - specific examples were addressed in class. 1 Exam 1 Lecture 2: Discussed intracellular killing mechanisms Important maturation steps Rapid development into a microbicidal
Summary of Endomembrane-system
 Summary of Endomembrane-system 1. Endomembrane System: The structural and functional relationship organelles including ER,Golgi complex, lysosome, endosomes, secretory vesicles. 2. Membrane-bound structures
Summary of Endomembrane-system 1. Endomembrane System: The structural and functional relationship organelles including ER,Golgi complex, lysosome, endosomes, secretory vesicles. 2. Membrane-bound structures
Nature Neuroscience: doi: /nn Supplementary Figure 1
 Supplementary Figure 1 Quantification of myelin fragments in the aging brain (a) Electron microscopy on corpus callosum is shown for a 18-month-old wild type mice. Myelin fragments (arrows) were detected
Supplementary Figure 1 Quantification of myelin fragments in the aging brain (a) Electron microscopy on corpus callosum is shown for a 18-month-old wild type mice. Myelin fragments (arrows) were detected
Biol212 Biochemistry of Disease Neurological Disorders: Prions
 Biol212 Biochemistry of Disease Neurological Disorders: Prions Prions Transmissible spongiform encephalopathies (TSEs) are diseases of the central nervous system caused by unconventional infectious agents,
Biol212 Biochemistry of Disease Neurological Disorders: Prions Prions Transmissible spongiform encephalopathies (TSEs) are diseases of the central nervous system caused by unconventional infectious agents,
genome edited transient transfection, CMV promoter
 Supplementary Figure 1. In the absence of new protein translation, overexpressed caveolin-1-gfp is degraded faster than caveolin-1-gfp expressed from the endogenous caveolin 1 locus % loss of total caveolin-1-gfp
Supplementary Figure 1. In the absence of new protein translation, overexpressed caveolin-1-gfp is degraded faster than caveolin-1-gfp expressed from the endogenous caveolin 1 locus % loss of total caveolin-1-gfp
Cellular Physiology (PHSI3009) Contents:
 Cellular Physiology (PHSI3009) Contents: Cell membranes and communication 2 nd messenger systems G-coupled protein signalling Calcium signalling Small G-protein signalling o RAS o MAPK o PI3K RHO GTPases
Cellular Physiology (PHSI3009) Contents: Cell membranes and communication 2 nd messenger systems G-coupled protein signalling Calcium signalling Small G-protein signalling o RAS o MAPK o PI3K RHO GTPases
Chapter 1: Vesicular traffic. Biochimica cellulare parte B 2017/18
 Chapter 1: Vesicular traffic Biochimica cellulare parte B 2017/18 Major Protein-sorting pathways in eukaryotic cells Secretory and endocytic pathways Unifying principle governs all protein trafficking
Chapter 1: Vesicular traffic Biochimica cellulare parte B 2017/18 Major Protein-sorting pathways in eukaryotic cells Secretory and endocytic pathways Unifying principle governs all protein trafficking
USE OF EXOSOMES IN TRANSLATIONAL AND CLINICAL RESEARCH. Eva Colás Ortega Postdoc researcher IRBLleida
 USE OF EXOSOMES IN TRANSLATIONAL AND CLINICAL RESEARCH Eva Colás Ortega Postdoc researcher IRBLleida ecolas@irblleida.cat THERE ARE DIFFERENT TYPE OF VESICLES RELEASED BY CELLS MICROVESICLES Size: 100-1000nm
USE OF EXOSOMES IN TRANSLATIONAL AND CLINICAL RESEARCH Eva Colás Ortega Postdoc researcher IRBLleida ecolas@irblleida.cat THERE ARE DIFFERENT TYPE OF VESICLES RELEASED BY CELLS MICROVESICLES Size: 100-1000nm
HLA and antigen presentation. Department of Immunology Charles University, 2nd Medical School University Hospital Motol
 HLA and antigen presentation Department of Immunology Charles University, 2nd Medical School University Hospital Motol MHC in adaptive immunity Characteristics Specificity Innate For structures shared
HLA and antigen presentation Department of Immunology Charles University, 2nd Medical School University Hospital Motol MHC in adaptive immunity Characteristics Specificity Innate For structures shared
Legionella pneumophila: an intracellular pathogen of phagocytes Prof. Craig Roy
 an intracellular pathogen of phagocytes Section of Microbial Pathogenesis, Yale University School of Medicine 1 Legionella pneumophila Gram-negative bacterium Facultative intracellular pathogen Protozoa
an intracellular pathogen of phagocytes Section of Microbial Pathogenesis, Yale University School of Medicine 1 Legionella pneumophila Gram-negative bacterium Facultative intracellular pathogen Protozoa
Supplemental information contains 7 movies and 4 supplemental Figures
 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 Supplemental information contains 7 movies and 4 supplemental Figures Movies: Movie 1. Single virus tracking of A4-mCherry-WR MV
1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 Supplemental information contains 7 movies and 4 supplemental Figures Movies: Movie 1. Single virus tracking of A4-mCherry-WR MV
Selective filtering defect at the axon initial segment in Alzheimer s disease mouse models. Yu Wu
 Selective filtering defect at the axon initial segment in Alzheimer s disease mouse models Yu Wu Alzheimer s Disease (AD) Mouse models: APP/PS1, PS1δE9, APPswe, hps1 Wirths, O. et al, Acta neuropathologica
Selective filtering defect at the axon initial segment in Alzheimer s disease mouse models Yu Wu Alzheimer s Disease (AD) Mouse models: APP/PS1, PS1δE9, APPswe, hps1 Wirths, O. et al, Acta neuropathologica
Supplementary Figure 1. Spatial distribution of LRP5 and β-catenin in intact cardiomyocytes. (a) and (b) Immunofluorescence staining of endogenous
 Supplementary Figure 1. Spatial distribution of LRP5 and β-catenin in intact cardiomyocytes. (a) and (b) Immunofluorescence staining of endogenous LRP5 in intact adult mouse ventricular myocytes (AMVMs)
Supplementary Figure 1. Spatial distribution of LRP5 and β-catenin in intact cardiomyocytes. (a) and (b) Immunofluorescence staining of endogenous LRP5 in intact adult mouse ventricular myocytes (AMVMs)
COURSE: Medical Microbiology, MBIM 650/720 - Fall TOPIC: Antigen Processing, MHC Restriction, & Role of Thymus Lecture 12
 COURSE: Medical Microbiology, MBIM 650/720 - Fall 2008 TOPIC: Antigen Processing, MHC Restriction, & Role of Thymus Lecture 12 FACULTY: Dr. Mayer Office: Bldg. #1, Rm B32 Phone: 733-3281 Email: MAYER@MED.SC.EDU
COURSE: Medical Microbiology, MBIM 650/720 - Fall 2008 TOPIC: Antigen Processing, MHC Restriction, & Role of Thymus Lecture 12 FACULTY: Dr. Mayer Office: Bldg. #1, Rm B32 Phone: 733-3281 Email: MAYER@MED.SC.EDU
The Cell Organelles. Eukaryotic cell. The plasma membrane separates the cell from the environment. Plasma membrane: a cell s boundary
 Eukaryotic cell The Cell Organelles Enclosed by plasma membrane Subdivided into membrane bound compartments - organelles One of the organelles is membrane bound nucleus Cytoplasm contains supporting matrix
Eukaryotic cell The Cell Organelles Enclosed by plasma membrane Subdivided into membrane bound compartments - organelles One of the organelles is membrane bound nucleus Cytoplasm contains supporting matrix
Lecture 6 - Intracellular compartments and transport I
 01.25.10 Lecture 6 - Intracellular compartments and transport I Intracellular transport and compartments 1. Protein sorting: How proteins get to their appropriate destinations within the cell 2. Vesicular
01.25.10 Lecture 6 - Intracellular compartments and transport I Intracellular transport and compartments 1. Protein sorting: How proteins get to their appropriate destinations within the cell 2. Vesicular
Nature Immunology: doi: /ni.3631
 Supplementary Figure 1 SPT analyses of Zap70 at the T cell plasma membrane. (a) Total internal reflection fluorescent (TIRF) excitation at 64-68 degrees limits single molecule detection to 100-150 nm above
Supplementary Figure 1 SPT analyses of Zap70 at the T cell plasma membrane. (a) Total internal reflection fluorescent (TIRF) excitation at 64-68 degrees limits single molecule detection to 100-150 nm above
Virus Entry/Uncoating
 Virus Entry/Uncoating Delivery of genome to inside of a cell Genome must be available for first step of replication The Problem--barriers to infection Virion Barriers: Non-enveloped viruses capsid Enveloped
Virus Entry/Uncoating Delivery of genome to inside of a cell Genome must be available for first step of replication The Problem--barriers to infection Virion Barriers: Non-enveloped viruses capsid Enveloped
17/01/2017. Protein trafficking between cell compartments. Lecture 3: The cytosol. The mitochondrion - the power plant of the cell
 ell biology 2017 version 13/1 2017 ote endosome vs lysosome handout Lecture 3: Text book Alberts et al.: hapter 12-14 (Topics covered by the lecture) A lot of reading! Focus on principles ell Biology interactive
ell biology 2017 version 13/1 2017 ote endosome vs lysosome handout Lecture 3: Text book Alberts et al.: hapter 12-14 (Topics covered by the lecture) A lot of reading! Focus on principles ell Biology interactive
IP: anti-gfp VPS29-GFP. IP: anti-vps26. IP: anti-gfp - + +
 FAM21 Strump. WASH1 IP: anti- 1 2 3 4 5 6 FAM21 Strump. FKBP IP: anti-gfp VPS29- GFP GFP-FAM21 tail H H/P P H H/P P c FAM21 FKBP Strump. VPS29-GFP IP: anti-gfp 1 2 3 FKBP VPS VPS VPS VPS29 1 = VPS29-GFP
FAM21 Strump. WASH1 IP: anti- 1 2 3 4 5 6 FAM21 Strump. FKBP IP: anti-gfp VPS29- GFP GFP-FAM21 tail H H/P P H H/P P c FAM21 FKBP Strump. VPS29-GFP IP: anti-gfp 1 2 3 FKBP VPS VPS VPS VPS29 1 = VPS29-GFP
