Arf family GTP loading is activated by, and generates, positive membrane curvature
|
|
|
- Kory Washington
- 5 years ago
- Views:
Transcription
1 Biochem. J. (2008) 414, (Printed in Great Britain) doi: /bj ACCELERATED PUBLICATION Arf family GTP loading is activated by, and generates, positive membrane curvature Richard LUNDMARK 1,2, Gary J. DOHERTY, Yvonne VALLIS, Brian J. PETER and Harvey T. MCMAHON 2 MRC Laboratory of Molecular Biology, Hills Road, Cambridge, CB2 0QH, U.K. Small G-proteins belonging to the Arf (ADP-ribosylation factor) family serve as regulatory proteins for numerous cellular processes through GTP-dependent recruitment of effector molecules. In the present study we demonstrate that proteins in this family regulate, and are regulated by, membrane curvature. Arf1 and Arf6 were shown to load GTP in a membrane-curvaturedependent manner and stabilize, or further facilitate, changes in membrane curvature through the insertion of an amphipathic helix. Key words: ADP-ribosylation factor family (Arf family), amphipathic helix, clathrin-mediated endocytosis, coatamer protein (COP), membrane curvature sensing and generation, small G-protein. INTRODUCTION Small G-proteins cycle between a GTP-bound active state and a GDP-bound inactive state [1,2]. In the active state, these proteins are able to recruit a wide variety of effector molecules that participate in various cellular processes. The GTP-loaded status is regulated positively by GEFs (guanine-nucleotide-exchange factors) and negatively by GAPs (GTPase-activating proteins). A common theme for small G-proteins is that they localize to membranes, and it is here that they execute their functions. Most members of the Ras superfamily of small G-proteins acquire a C- terminal lipid modification (geranylgeranylation or farnesylation) that anchors these proteins to the membrane. Arf (ADPribosylation factor) and Arl (Arf-like) family proteins have a myristoylated (C 14:0 ) N-terminal helix that is thought to facilitate membrane binding. The myristoyl moiety is attached to the second residue (a glycine) following removal of the first residue (a methionine). The six mammalian Arfs can be categorized into three subclasses based on sequence similarities. Class I Arfs (Arf1 Arf3) are involved in the recruitment of membrane-budding machineries, whereas the roles of Class II Arfs (Arf4 and Arf5) remain unclear. Arf6 alone constitutes the third class and this protein has been shown to regulate trafficking in the endocytic system, actin polymerization and cell migration. Structural details of Arf1 and Arf6 have been described through crystallographic interrogation of the GDP- and GTP-bound proteins [3 5]. Surprisingly, the rearrangements induced by GTP loading are relatively minor, but must be sufficient to provide information for the specific recruitment of effectors. In addition, the precise localization of the Arfs has been suggested to allow for specific effector recruitment [6]. The exposure of the N-terminal helix is thought to be closely linked to the GDP/GTP cycle of Arfs. Here binding of GTP causes a shift in the intermolecular switch region, which suggestively displaces the helix that is normally buried in a hydrophobic pocket in the GDP-bound state. Arf1 and Arf6 are each vital for particular membrane-trafficking events, although it seems that they act via slightly different mechanisms. Arf1 is absolutely required for the recruitment of COPI (coatamer protein I), AP-1 (adaptor protein-1) and GGA (Golgi-localizing γ -adaptin ear homology domain Arf-binding proteins) coat proteins to the Golgi. Furthermore, Arf1 has been suggested to recruit AP-3 and AP-4 [7]. However, the precise mechanism of action of Arf1 at the budding vesicle remains elusive. It has been shown that, in addition to recruiting coat proteins, Arf1 couples the generation of vesicles to the incorporation of cargo molecules [8,9]. It has previously been demonstrated that, when cargo is present, the association between Arf1 and AP-1 is stabilized and ArfGAP1 activity is inhibited [10]. Arf6 localizes to the plasma membrane and endosomal system, where it is known to regulate both clathrin-dependent and -independent endocytic pathways, as well as endomembrane recycling mechanisms. This is not thought to be facilitated via direct recruitment of coat proteins, but rather through the generation of distinct membrane domains [11]. Arf6 activates phospholipase D and PIP5K (phosphatidylinositol-4-phosphate 5-kinase). Arf6 and PIP5K act synergistically to generate membrane domains enriched in PtdIns(4,5)P 2, thereby helping to recruit the proteinbudding machineries that rely on this phospholipid. Generation of membrane curvature is a prerequisite for the budding of vesicles from a flat membrane. Recent evidence suggests that Arf effectors and Arfs themselves might be directly involved in membrane remodelling. The recruitment of the COPII (coatamer protein II) budding machinery to the endoplasmic reticulum is dependent on Sar1, a small G-protein that is closely related to the Arfs. Sar1 was fairly recently shown to induce membrane curvature [12]. In addition, overexpression of regulatory GAPs (centaurins) or GEFs [EFA6 (exchange factor for Arf6)] for Arfs have been found to result in the tubulation of cellular membranes [13,14]. This, coupled with our previous observations of amphipathic helix-mediated membrane curvature generation [15], led us to study the role of Arfs as membrane curvature regulators. In the present study we have used purified proteins to study the role of Arfs in generating membrane curvature and how this ability is influenced by, and influences, GTP loading. Abbreviations used: AP, adaptor protein; Arf, ADP-ribosylation factor; Arl, Arf-like; BAR, Bin/Amphiphysin/Rvs; COP, coatamer protein; GAP, GTPaseactivating protein; GEF, guanine-nucleotide-exchange factor; PIP5K, phosphatidylinositol-4-phosphate 5-kinase; Sf9, Spodoptera frugiperda 9. 1 Present address: Department of Medical Biochemistry and Biophysics, Umeå University, Umeå, Sweden. 2 Correspondence may be addressed to either of these authors ( hmm@mrc-lmb.cam.ac.uk or richard.lundmark@medchem.umu.se).
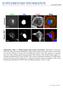
2 190 R. Lundmark and others EXPERIMENTAL cdna construct preparation cdna constructs encoding human Arf1 and Arf6 were obtained from the cdna Resource Center, Guthrie Research Institute, Sayre, PA, U.S.A. Full-length Arf1 (residues 1 181), Arf1 N-terminus (residues ) and full-length Arf6 (residues 1 175) were subsequently cloned into the pbac4 1 vector for baculovirus expression or the pet15b vector for bacterial expression. The Arf6 K3E mutation was generated by sitedirected mutagenesis. The plasmid encoding N-myristoyltransferase was kindly provided by Dr Sean Munro of this laboratory. Protein expression and protein purification Histidine-tagged myristoylated Arf1, Arf6 and the Arf6 K3E mutant were purified from baculovirus-infected Sf9 (Spodoptera frugiperda 9) insect cells using nickel agarose beads and gel filtration. Arf1 was expressed in Escherichia coli BL21 strain with or without the N-myristoyltransferase-encoding plasmid mentioned above and purified as described in [16]. Liposome binding and tubulation Generation of liposomes from total brain lipids (Folch fraction I) (Sigma Aldrich) or synthetic lipids (Avanti Polar Lipids), and specification of liposome diameter (made by sequential filtration) were performed as previously described [13]. The sequential filtration results in slightly lower concentrations of smaller-diameter versus larger-diameter liposomes being present in assays. Liposome-binding assays were performed as previously described [13]. Briefly, proteins were incubated together with 1 mg/ml liposomes, followed by centrifugation and analysis of the pellet and supernatant by SDS/PAGE and Coomassie Blue staining. In vitro liposome-tubulation assays were performed and the results analysed as previously described [13]. Real-time fluorescence GTP-loading assay GTP loading of Arfs was measured as described in [17]. Briefly, purified GDP-loaded Arfs (0.5 μm) were incubated at 37 Cin the presence or absence of liposomes (final concn. 0.1 mg/ml Folch lipids) and HKM buffer (50 mm Hepes, ph 7.2, 120 mm potassium acetate and 1 mm MgCl 2 ) and loading was induced by sequential addition of 1 mm GTP and 2 mm EDTA. Tryptophan fluorescence was measured at 340 nm after excitation at nm in a FluoroMax-2 fluorimeter (Horiba Jobin Yvon Ltd.). Data was normalized by setting the fluorescence value before induction of GTP loading to zero and the value obtained after 1000 s for the 50 nm liposomes and Arf1/Arf6 respectively as the maximum value in each experimental set. All data were processed identically and are presented as a percentage of the maximum value to demonstrate differences in GTP loading. RESULTS In the Arf GDP crystal structure, amino acids 2 10 form a helical structure buried in a hydrophobic pocket in the core of the protein (PDB code: 1HUR). However, upon the structural rearrangements induced by GTP binding, this helix is thought to be displaced from its groove. It was previously shown that myristoylation of the N- terminal helix is absolutely required for membrane binding in the GDP-bound state and that point mutations in this helix decreased binding to membranes [17,18]. Helical-wheel presentations of the N-terminal amino acids of Arf1 and Arf6 show that, when the myristoylation of the first glycine residue is taken into account, the helices have an amphipathic nature (Figure 1A). Sequence comparison of the N-termini of Arfs and Arls shows that the amphipathic properties of the first 13 amino acids are conserved in many family members (Figure 1B). We analysed the binding of purified Arf1 to artificially generated liposomes in the presence of GTP or GDP. When Arf1 was purified from insect cells or bacteria co-expressing N-myristoyltransferase, which enables myristoylation, robust membrane binding was observed. This was true for both GTP- and GDP-loaded Arf1, although more efficient binding was observed when GTP was included in the assay. However, if Arf1 was purified without myristoylation or without the N-terminus, no significant binding could be detected (Figure 1C). These results are in agreement with the findings of previous studies and demonstrate that our proteins are functional and behave as would be expected [16,17]. Similar results were obtained for Arf6, but Arf6 bound even more strongly to membranes in the absence of GTP, probably due to binding sites on the protein core ([19,20] and results not shown). The association of Arfs with membranes even in the GDPbound state suggested that membrane binding of the myristoylated protein does not necessarily involve the exposure of the amphipathic helix. Thus the helix itself might not be absolutely required for binding, but may instead have a more elaborate role. We reasoned that, following GTP-dependent release of the amphipathic helix, it might insert into the lipid bilayer and subsequently impose positive membrane curvature by acting as a wedge [21]. To study this, liposomes were incubated together with myristoylated Arf1 or Arf6 and GTP or GDP and analysed using electron microscopy (Figure 1D). We found that the normal spherical morphology of the liposomes always persisted after incubation with either GDP-loaded Arf1 or Arf6. However, after incubation with either GTP-loaded Arf1 or Arf6, we consistently observed a drastic change in the shape of many of the liposomes. Frequently, straight tubular structures with an apparent diameter of nm were observed (Figure 1D; arrowheads). In addition, smaller nm-diameter tubular structures were detected (Figure 1D; asterisks). We have observed similar structures on rare occasions with the N-BAR domain of amphiphysin (results not shown). [N- BAR domains are BAR (Bin/Amphiphysin/Rvs) domains with an N-terminal amphipathic helix.] The nature of such small structures is uncertain, but might result from single layers of lipids being forced into tubular micelles. These results suggest that Arfs generate high membrane curvature in a GTP-dependent manner through the insertion of a helix in the lipid bilayer. In theory, the insertion of a helix into the membrane would be favoured by positive membrane curvature, since the lipid headgroups apposing the helix would be more separate from one another. This would be reflected by increased membrane binding as the liposome diameter decreases. To test this, we generated liposomes with low curvature (800 nm in diameter) and high curvature (50 nm in diameter) and assayed binding of Arf1 GTP and Arf6 GTP to these liposomes (see the insets to Figures 2A and 2B and see the Discussion). The results indicate that both Arf1 and Arf6 show a small increase in binding in the presence of higher-curvature liposomes (insets to Figures 2A and 2B). Curvature-sensitivity in this assay may reflect an increased amount of GTP loading, since amphipathic helix insertion occurs in response to GTP binding (Figure 1D), which would decrease protein off-rates from membranes. We therefore studied GTP loading in the presence of liposomes of different diameters. The structural rearrangements in Arfs upon GTP binding can be detected by an increase in tryptophan fluorescence [22]. This can be used to analyse GTP loading in real time [17]. No
3 Curvature-sensitive activation of Arf family small G-proteins 191 Figure 1 Arfs induce membrane curvature in a GTP-dependent manner (A) Helical-wheel representation of amino acids (a.a.) 2 13 in Arf1 (top wheel) and 2 11 in Arf6 (bottom wheel). Hydrophobic and charged amino acids are highlighted by dark grey circles or light grey circles respectively. Myristoylation of the second residue (glycine) is depicted by the long oval containing the word myristoyl. (B) Sequence alignment of the N-terminal amino acids of Arf/Arl family members. Hydrophobic amino acids are highlighted in dark grey. (C) Liposome co-sedimentation assays. Liposomes generated from brain-derived lipids (Folch fraction I) were incubated with the indicated proteins and GTP or GDP before centrifugation and analysis of supernatants (S) and pellets (P) by SDS/PAGE and Coomassie Blue staining. A control experiment where protein was incubated in the absence of liposomes is shown. The proteins used were myristoylated Arf1 purified from Sf9 insect cells [myr-arf1 (Sf9)], Arf1 purified from bacteria [Arf1 (bacteria)], myristoylated Arf1 purified from bacteria co-expressing N-myristoyltransferase [myr-arf1 (bacteria)] and N-terminally truncated Arf1 purified from bacteria [ N-term Arf1 (bacteria)]. (D) Electron micrographs of negatively stained 800 nm liposomes incubated in the presence of equal concentrations of proteins (10 μm) and nucleotides (100 μm) as indicated. Arrowheads indicate tubules that are approx. 45 nm in diameter and asterisks indicate tubules that are nm wide. Scale bars represent 100 nm. Abbreviation: myr, myristoylated. increase in tryptophan fluorescence was observed on the binding of GDP-loaded Arf1/Arf6 to membranes (results not shown). This strongly suggests that the amphipathic helix does not change conformation upon lipid binding per se, a finding consistent with the observation that this helix is buried in the core of the GDPbound Arf [15]. By incubating Arf1 with liposomes of different diameters in the presence of GTP, we were able to observe that Arf1 was loaded more quickly with GTP, and to a greater extent, in the context of higher liposome curvature (Figure 2A). As was previously observed, GTP loading was very slow in the absence of membranes [17,23]. Similar results were obtained for the loading of Arf6 (Figure 2B). Thus curvature-sensitive GTP loading may be
4 192 R. Lundmark and others Figure 2 Arf-family proteins are activated by high positive membrane curvature (A) (C) Indicated proteins were incubated together with or without liposomes ( lip) of the indicated diameters and GTP. GTP loading was analysed in real time by measuring tryptophan fluorescence (see the Experimental section). Fluorescence is presented as a percentage of the value at 1000 s for 50 nm liposomes and Arf1 and Arf6 respectively. Insets in (A) and(b) shows liposome binding assays of Arf1 (A)andArf6(B) in the absence or presence of liposomes of different diameters. S, supernatant; P, pellet. Results shown in (A) (C) are representative of three independent experiments with the same set of proteins and lipids. (D) Electron micrographs of negatively stained liposomes incubated together with GTP-loaded Arf6 (left) and Arf6 K3E (right). The scale bar represents 100 nm. The asterisks and arrowheads have the same meaning as in Figure 1. myr, myristoylated. (E) Epifluorescent micrograph of a HeLa cell expressing a myc-tagged constitutively active Arf6 mutant (Arf6 Q67L-myc). Arrowheads indicate tubular localization. The scale bar represents 5 μm. (F) Schematic model of the GTP-dependent role of Arfs as curvature sensors at the rim of a budding vesicle. Arfs loaded with GDP (ARF GDP) display a transient membrane binding while Arfs bound to GTP (ARF GTP) stay membrane-associated within the diffusion barrier generated by positive membrane curvature. a general property of Arf-family proteins. Liposomes of different diameters did not on their own yield any significant change in fluorescence (Figure 2C and results not shown). The increase in the extent of GTP loading in the context of higher membrane curvature probably reflects a change in the steady-state distribution of Arfs, with more being found bound to the morehighly-curved membranes. Our present results suggest that the N-terminus of Arfs is responsible for both curvature sensing and generation. We therefore created a point mutant (Arf6 K3E) in the helix to disrupt a positively charged residue that would be expected to align with the negatively charged lipid headgroups once the helix is inserted in the membrane. We used Arf6 as a model, since this protein showed greater curvature-sensitivity than did Arf1. We chose to mutate this particular residue since, according to the structure, this mutation should not affect myristoylation or localization of the helix in the GDP-bound state (having no interaction with other residues in the crystal structure), while being sufficiently N- terminal to be readily accessible for membrane insertion. When the Arf6 K3E mutant was assayed for its ability to load GTP,
5 Curvature-sensitive activation of Arf family small G-proteins 193 we found that this protein loads GTP at the same rate as wildtype Arf6 in the absence of liposomes (Figure 2C). However, when liposomes of differing diameters were added to the reaction mixture, we found that Arf6 K3E showed a slight increase in the GTP-loading rate, but this increase was independent of liposome diameter. This strongly suggests that the introduced mutation renders the helix unable to properly insert in the membrane and therefore Arf6 K3E cannot respond to membrane curvature. We next examined whether the K3E mutation affected membrane curvature generation. Liposomes that had been incubated together with wild-type Arf6 or Arf6 K3E were analysed by electron microscopy. Indeed we found that, in contrast with wild-type Arf6, Arf6 K3E was unable to tubulate liposomes (Figure 2D). To resolve whether GTP loading might result in a tubular localization of Arf6 in vivo, we expressed constitutively GTP-associated Arf6 (Arf6 Q67L-myc). This protein was indeed found on tubular structures in cells, which is consistent with curvature-sensitive membrane binding of this protein. Taken together, our data are consistent with a model in which Arf family proteins function as membrane curvature sensors and further facilitate membrane budding through direct GTPdependent membrane remodelling (Figure 2E). In the GDP-bound state, myristoyl-enabled membrane binding of Arfs is insufficient, owing to a relatively high off-rate. This can be overcome by the local high positive membrane curvature seen, for example, at a budding vesicle. In addition, GEFs function to induce local GTP loading of Arfs that subsequently generate curvature via insertion of the helix, thereby creating a positive-feedback loop. Once the helix is inserted, a diffusion barrier is probably created, since Arfs are probably unable move into the negative curvature surrounding the positive curvature of the budding membrane (tubule or vesicle). A high local concentration of Arf GTP with lowered off-rate is obtained that can recruit effector molecules to this precise location where budding is in progress. Subsequently, coat proteins are recruited that help to stabilize the budding vesicle. DISCUSSION Cellular processes such as morphogenesis and signalling are complex, and regulatory proteins are required to orchestrate and drive the membrane remodelling, cargo sorting and cytoskeletal rearrangements that must occur during such events. Small G- proteins of the Arf and Arl families function as such regulatory units, owing to their ability to define processes in a spatial and temporal manner and transmit this information to effector molecules. In the present study we have shown that Arf family proteins generate and sense membrane curvature through an amphipathic helix that can be inserted into the lipid bilayer in a GTP-driven manner. Given that amphipathic helix insertion leads to the induction of local positive membrane curvature, this can result in curvature-sensitivity in the rate of Arf insertion. Furthermore, once a helix has inserted, this will help to drive the curvature preferred by the next Arf molecules. Through this mechanism, GTP-loaded Arfs are organized in high concentrations at local sites of positive membrane curvature. In this context, membrane curvature functions as a base for protein recruitment. This ability makes Arfs unique among small G-proteins and might explain why they are used in membrane budding. Previous studies on Arf1 and Sar1p have shown that mutations in the helix that renders it less hydrophobic results in an increased off-rate from the membrane [12,17]. These findings are consistent with our results and show that mutations in the helix affect both membrane binding and curvature-sensitivity. Also, the binding of Arf GTP to a membrane feeds back to generate or stabilize even higher curvature, which will drive the progression of membrane budding both directly and indirectly through increased recruitment of curvature effector proteins. Since insertion of the helix is dependent upon the GDP/GTP status of Arf, the procedure can be arrested or promoted by GAPs and GEFs respectively. Interestingly several Arf-specific GAPs and GEFs are predicted to contain BAR domains that are known to sense, stabilize and generate membrane curvature [13]. This is in agreement with our model for the function and regulation of Arfs. Our results could also suggest that the need for GEFs in membrane budding might be to initially recruit Arfs and that, once the curvature is generated, Arfs can drive the process via their curvature-sensitivity. It has been proposed that ArfGAP1, which is curvature-sensitive owing to helix insertion, promotes hydrolysis of Arf GTP at the positive curvature on the vesicle, but that Arf GTP at the vesicular neck, with negative curvature, is unaffected [24]. However, from our results it is difficult to envisage how Arf-GTP can be located at sites of negative curvature while ArfGAP cannot, since they both localize better to increasing positive membrane curvature. Arf6 has been ascribed several roles, which include the regulation of membrane trafficking, actin rearrangements and membrane cytoskeleton interactions [2,25]. We found that the amphipathic helix of Arf6 is shorter than the helix in other Arf-family members. In addition, Arf6 is thought to be continuously associated with membranes, perhaps since core residues in Arf6 bind more efficiently to membranes than those of others such as Arf1. This suggests that the role of Arf6 may have evolved to function more as a sensor than as a generator of membrane curvature. Arf6 has been shown to function in clathrinindependent endocytic and recycling pathways for MHC class I proteins and CD59 [26,27]. Morphologically, these pathways are characterized by tubular rather than vesicular structures. Our results suggest that Arf6 is adapted to localize and function at such membrane tubules. It is possible that the shorter helix in Arf6 is more suitable in this system. The role of Arf6 has been suggested to be largely dependent upon effects on PtdIns(4,5)P 2 levels [14]. Our in vitro data, however, show that Arf6 also has a direct effect on lipid packing, resulting in, and depending upon, membrane shape modulation. In conclusion, we propose that proteins in the Arf and Arl families of small G-proteins serve as GTP-dependent sensors and drivers of membrane shape. This ability will help to define local areas of activity that are fine-tuned by the characteristics of effector molecules. The unresolved functions of the less-studied Arfs will probably also require the ability to sense membrane curvature. Whether this includes direct roles in vesicular budding, or indirect regulation of membrane remodelling, remains to be seen. We thank Dr Sven Carlsson (Department of Medical Biochemistry and Biophysics, Umeå University, Umeå, Sweden) for advice. R. L. was supported by the Swedish Research Council, the Medical Faculty of Umeå University and the Magn. Bergvalls Foundation. G. J. D. was supported by a Trinity College Cambridge Internal Graduate Studentship. We declare no competing interests. REFERENCES 1 Gillingham, A. K. and Munro, S. (2007) The small G proteins of the Arf family and their regulators. Annu. Rev. Cell Dev. Biol. 23, D Souza-Schorey, C. and Chavrier, P. (2006) ARF proteins: roles in membrane traffic and beyond. Nat. Rev. Mol. Cell Biol. 7, Pasqualato, S., Menetrey, J., Franco, M. and Cherfils, J. (2001) The structural GDP/GTP cycle of human Arf6. EMBO Rep. 2, Amor, J. C., Harrison, D. H., Kahn, R. A. and Ringe, D. (1994) Structure of the human ADP-ribosylation factor 1 complexed with GDP. Nature 372,
6 194 R. Lundmark and others 5 Menetrey, J., Macia, E., Pasqualato, S., Franco, M. and Cherfils, J. (2000) Structure of Arf6 GDP suggests a basis for guanine nucleotide exchange factors specificity. Nat. Struct. Biol. 7, Peters, P. J., Hsu, V. W., Ooi, C. E., Finazzi, D., Teal, S. B., Oorschot, V., Donaldson, J. G. and Klausner, R. D. (1995) Overexpression of wild-type and mutant ARF1 and ARF6: distinct perturbations of nonoverlapping membrane compartments. J. Cell Biol. 128, Robinson, M. S. (2004) Adaptable adaptors for coated vesicles. Trends Cell Biol. 14, Bremser, M., Nickel, W., Schweikert, M., Ravazzola, M., Amherdt, M., Hughes, C. A., Sollner, T. H., Rothman, J. E. and Wieland, F. T. (1999) Coupling of coat assembly and vesicle budding to packaging of putative cargo receptors. Cell 96, Lanoix, J., Ouwendijk, J., Lin, C. C., Stark, A., Love, H. D., Ostermann, J. and Nilsson, T. (1999) GTP hydrolysis by Arf-1 mediates sorting and concentration of Golgi resident enzymes into functional COPI vesicles. EMBO J. 18, Meyer, D. M., Crottet, P., Maco, B., Degtyar, E., Cassel, D. and Spiess, M. (2005) Oligomerization and dissociation of AP-1 adaptors are regulated by cargo signals and by ArfGAP1-induced GTP hydrolysis. Mol. Biol. Cell 16, Donaldson, J. G. (2005) Arfs, phosphoinositides and membrane traffic. Biochem. Soc. Trans. 33, Lee, M. C., Orci, L., Hamamoto, S., Futai, E., Ravazzola, M. and Schekman, R. (2005) Sar1p N-terminal helix initiates membrane curvature and completes the fission of a COPII vesicle. Cell 122, Peter, B. J., Kent, H. M., Mills, I. G., Vallis, Y., Butler, P. J., Evans, P. R. and McMahon, H. T. (2004) BAR domains as sensors of membrane curvature: the amphiphysin BAR structure. Science 303, Brown, F. D., Rozelle, A. L., Yin, H. L., Balla, T. and Donaldson, J. G. (2001) Phosphatidylinositol 4,5-bisphosphate and Arf6-regulated membrane traffic. J. Cell Biol. 154, Ford, M. G., Mills, I. G., Peter, B. J., Vallis, Y., Praefcke, G. J., Evans, P. R. and McMahon, H. T. (2002) Curvature of clathrin-coated pits driven by epsin. Nature 419, Franco, M., Chardin, P., Chabre, M. and Paris, S. (1995) Myristoylation of ADP-ribosylation factor 1 facilitates nucleotide exchange at physiological Mg 2+ levels. J. Biol. Chem. 270, Antonny, B., Beraud-Dufour, S., Chardin, P. and Chabre, M. (1997) N-terminal hydrophobic residues of the G-protein ADP-ribosylation factor-1 insert into membrane phospholipids upon GDP to GTP exchange. Biochemistry 36, Randazzo, P. A., Terui, T., Sturch, S., Fales, H. M., Ferrige, A. G. and Kahn, R. A. (1995) The myristoylated amino terminus of ADP-ribosylation factor 1 is a phospholipid- and GTP-sensitive switch. J. Biol. Chem. 270, Paleotti, O., Macia, E., Luton, F., Klein, S., Partisani, M., Chardin, P., Kirchhausen, T. and Franco, M. (2005) The small G-protein Arf6GTP recruits the AP-2 adaptor complex to membranes. J. Biol. Chem. 280, Amor, J. C., Horton, J. R., Zhu, X., Wang, Y., Sullards, C., Ringe, D., Cheng, X. and Kahn, R. A. (2001) Structures of yeast ARF2 and ARL1: distinct roles for the N terminus in the structure and function of ARF family GTPases. J. Biol. Chem. 276, Campelo, F., McMahon, H. T. and Kozlov, M. M. (2008) The hydrophobic insertion mechanism of membrane curvature generation by proteins. Biophys. J., doi: /biophys.j Kahn, R. A. and Gilman, A. G. (1986) The protein cofactor necessary for ADP-ribosylation of Gs by cholera toxin is itself a GTP binding protein. J. Biol. Chem. 261, Weiss, O., Holden, J., Rulka, C. and Kahn, R. A. (1989) Nucleotide binding and cofactor activities of purified bovine brain and bacterially expressed ADP-ribosylation factor. J. Biol. Chem. 264, Antonny, B., Bigay, J., Casella, J. F., Drin, G., Mesmin, B. and Gounon, P. (2005) Membrane curvature and the control of GTP hydrolysis in Arf1 during COPI vesicle formation. Biochem. Soc. Trans. 33, Doherty, G. J. and McMahon, H. T. (2008) Mediation, modulation, and consequences of membrane-cytoskeleton interactions. Annu. Rev. Biophys. 37, Radhakrishna, H. and Donaldson, J. G. (1997) ADP-ribosylation factor 6 regulates a novel plasma membrane recycling pathway. J. Cell Biol. 139, Naslavsky, N., Weigert, R. and Donaldson, J. G. (2004) Characterization of a nonclathrin endocytic pathway: membrane cargo and lipid requirements. Mol. Biol. Cell 15, Received 17 June 2008/2 July 2008; accepted 3 July 2008 Published as BJ Immediate Publication 3 July 2008, doi: /bj
Signal Transduction Cascades
 Signal Transduction Cascades Contents of this page: Kinases & phosphatases Protein Kinase A (camp-dependent protein kinase) G-protein signal cascade Structure of G-proteins Small GTP-binding proteins,
Signal Transduction Cascades Contents of this page: Kinases & phosphatases Protein Kinase A (camp-dependent protein kinase) G-protein signal cascade Structure of G-proteins Small GTP-binding proteins,
Mechanism of Vesicular Transport
 Mechanism of Vesicular Transport Transport vesicles play a central role in the traffic of molecules between different membrane-enclosed enclosed compartments. The selectivity of such transport is therefore
Mechanism of Vesicular Transport Transport vesicles play a central role in the traffic of molecules between different membrane-enclosed enclosed compartments. The selectivity of such transport is therefore
T H E J O U R N A L O F C E L L B I O L O G Y
 Supplemental material Beck et al., http://www.jcb.org/cgi/content/full/jcb.201011027/dc1 T H E J O U R N A L O F C E L L B I O L O G Y Figure S1. Membrane binding of His-tagged proteins to Ni-liposomes.
Supplemental material Beck et al., http://www.jcb.org/cgi/content/full/jcb.201011027/dc1 T H E J O U R N A L O F C E L L B I O L O G Y Figure S1. Membrane binding of His-tagged proteins to Ni-liposomes.
Overview of clathrin-mediated endocytosis
 Overview of clathrin-mediated endocytosis Accessory and adaptor proteins promote clathrin nucleation on the plasma membrane and some help deform membrane. Clathrin assembly into lattices stabilize the
Overview of clathrin-mediated endocytosis Accessory and adaptor proteins promote clathrin nucleation on the plasma membrane and some help deform membrane. Clathrin assembly into lattices stabilize the
Supplementary Materials. High affinity binding of phosphatidylinositol-4-phosphate. by Legionella pneumophila DrrA
 Supplementary Materials High affinity binding of phosphatidylinositol-4-phosphate by Legionella pneumophila DrrA Running title: Molecular basis of PtdIns(4)P-binding by DrrA Stefan Schoebel, Wulf Blankenfeldt,
Supplementary Materials High affinity binding of phosphatidylinositol-4-phosphate by Legionella pneumophila DrrA Running title: Molecular basis of PtdIns(4)P-binding by DrrA Stefan Schoebel, Wulf Blankenfeldt,
Homework Hanson section MCB Course, Fall 2014
 Homework Hanson section MCB Course, Fall 2014 (1) Antitrypsin, which inhibits certain proteases, is normally secreted into the bloodstream by liver cells. Antitrypsin is absent from the bloodstream of
Homework Hanson section MCB Course, Fall 2014 (1) Antitrypsin, which inhibits certain proteases, is normally secreted into the bloodstream by liver cells. Antitrypsin is absent from the bloodstream of
Molecular Cell Biology - Problem Drill 17: Intracellular Vesicular Traffic
 Molecular Cell Biology - Problem Drill 17: Intracellular Vesicular Traffic Question No. 1 of 10 1. Which of the following statements about clathrin-coated vesicles is correct? Question #1 (A) There are
Molecular Cell Biology - Problem Drill 17: Intracellular Vesicular Traffic Question No. 1 of 10 1. Which of the following statements about clathrin-coated vesicles is correct? Question #1 (A) There are
Lysosomes and endocytic pathways 9/27/2012 Phyllis Hanson
 Lysosomes and endocytic pathways 9/27/2012 Phyllis Hanson General principles Properties of lysosomes Delivery of enzymes to lysosomes Endocytic uptake clathrin, others Endocytic pathways recycling vs.
Lysosomes and endocytic pathways 9/27/2012 Phyllis Hanson General principles Properties of lysosomes Delivery of enzymes to lysosomes Endocytic uptake clathrin, others Endocytic pathways recycling vs.
Chapter 1: Vesicular traffic. Biochimica cellulare parte B 2017/18
 Chapter 1: Vesicular traffic Biochimica cellulare parte B 2017/18 Major Protein-sorting pathways in eukaryotic cells Secretory and endocytic pathways Unifying principle governs all protein trafficking
Chapter 1: Vesicular traffic Biochimica cellulare parte B 2017/18 Major Protein-sorting pathways in eukaryotic cells Secretory and endocytic pathways Unifying principle governs all protein trafficking
Selective protection of an ARF1-GTP signaling axis by a bacterial scaffold induces bidirectional trafficking arrest.
 Selective protection of an ARF1-GTP signaling axis by a bacterial scaffold induces bidirectional trafficking arrest. Andrey S. Selyunin, L. Evan Reddick, Bethany A. Weigele, and Neal M. Alto Supplemental
Selective protection of an ARF1-GTP signaling axis by a bacterial scaffold induces bidirectional trafficking arrest. Andrey S. Selyunin, L. Evan Reddick, Bethany A. Weigele, and Neal M. Alto Supplemental
Vesicle Transport. Vesicle pathway: many compartments, interconnected by trafficking routes 3/17/14
 Vesicle Transport Vesicle Formation Curvature (Self Assembly of Coat complex) Sorting (Sorting Complex formation) Regulation (Sar1/Arf1 GTPases) Fission () Membrane Fusion SNARE combinations Tethers Regulation
Vesicle Transport Vesicle Formation Curvature (Self Assembly of Coat complex) Sorting (Sorting Complex formation) Regulation (Sar1/Arf1 GTPases) Fission () Membrane Fusion SNARE combinations Tethers Regulation
In the previous chapter we explored how proteins are targeted
 17 VESICULAR TRAFFIC, SECRETION, AND ENDOCYTOSIS Electron micrograph of clathrin cages, like those that surround clathrin-coated transport vesicles, formed by the in vitro polymerization of clathrin heavy
17 VESICULAR TRAFFIC, SECRETION, AND ENDOCYTOSIS Electron micrograph of clathrin cages, like those that surround clathrin-coated transport vesicles, formed by the in vitro polymerization of clathrin heavy
Lecture Readings. Vesicular Trafficking, Secretory Pathway, HIV Assembly and Exit from Cell
 October 26, 2006 1 Vesicular Trafficking, Secretory Pathway, HIV Assembly and Exit from Cell 1. Secretory pathway a. Formation of coated vesicles b. SNAREs and vesicle targeting 2. Membrane fusion a. SNAREs
October 26, 2006 1 Vesicular Trafficking, Secretory Pathway, HIV Assembly and Exit from Cell 1. Secretory pathway a. Formation of coated vesicles b. SNAREs and vesicle targeting 2. Membrane fusion a. SNAREs
Protein Trafficking in the Secretory and Endocytic Pathways
 Protein Trafficking in the Secretory and Endocytic Pathways The compartmentalization of eukaryotic cells has considerable functional advantages for the cell, but requires elaborate mechanisms to ensure
Protein Trafficking in the Secretory and Endocytic Pathways The compartmentalization of eukaryotic cells has considerable functional advantages for the cell, but requires elaborate mechanisms to ensure
Cellular Biochemistry
 Cellular Biochemistry Fall Semester 2013 Sept. 23 Benoit Kornmann Institute of Biochemistry Introduction to biological membranes General functions and properties Membrane lipids Physical properties Distribution/asymmetry
Cellular Biochemistry Fall Semester 2013 Sept. 23 Benoit Kornmann Institute of Biochemistry Introduction to biological membranes General functions and properties Membrane lipids Physical properties Distribution/asymmetry
Ras and Cell Signaling Exercise
 Ras and Cell Signaling Exercise Learning Objectives In this exercise, you will use, a protein 3D- viewer, to explore: the structure of the Ras protein the active and inactive state of Ras and the amino
Ras and Cell Signaling Exercise Learning Objectives In this exercise, you will use, a protein 3D- viewer, to explore: the structure of the Ras protein the active and inactive state of Ras and the amino
Signal Transduction: G-Protein Coupled Receptors
 Signal Transduction: G-Protein Coupled Receptors Federle, M. (2017). Lectures 4-5: Signal Transduction parts 1&2: nuclear receptors and GPCRs. Lecture presented at PHAR 423 Lecture in UIC College of Pharmacy,
Signal Transduction: G-Protein Coupled Receptors Federle, M. (2017). Lectures 4-5: Signal Transduction parts 1&2: nuclear receptors and GPCRs. Lecture presented at PHAR 423 Lecture in UIC College of Pharmacy,
Intracellular Vesicular Traffic Chapter 13, Alberts et al.
 Intracellular Vesicular Traffic Chapter 13, Alberts et al. The endocytic and biosynthetic-secretory pathways The intracellular compartments of the eucaryotic ell involved in the biosynthetic-secretory
Intracellular Vesicular Traffic Chapter 13, Alberts et al. The endocytic and biosynthetic-secretory pathways The intracellular compartments of the eucaryotic ell involved in the biosynthetic-secretory
PHSI3009 Frontiers in Cellular Physiology 2017
 Overview of PHSI3009 L2 Cell membrane and Principles of cell communication L3 Signalling via G protein-coupled receptor L4 Calcium Signalling L5 Signalling via Growth Factors L6 Signalling via small G-protein
Overview of PHSI3009 L2 Cell membrane and Principles of cell communication L3 Signalling via G protein-coupled receptor L4 Calcium Signalling L5 Signalling via Growth Factors L6 Signalling via small G-protein
Chapt. 10 Cell Biology and Biochemistry. The cell: Student Learning Outcomes: Describe basic features of typical human cell
 Chapt. 10 Cell Biology and Biochemistry Cell Chapt. 10 Cell Biology and Biochemistry The cell: Lipid bilayer membrane Student Learning Outcomes: Describe basic features of typical human cell Integral transport
Chapt. 10 Cell Biology and Biochemistry Cell Chapt. 10 Cell Biology and Biochemistry The cell: Lipid bilayer membrane Student Learning Outcomes: Describe basic features of typical human cell Integral transport
Conformational changes of coat proteins during vesicle formation
 FEBS Letters 581 (2007) 2083 2088 Minireview Conformational changes of coat proteins during vesicle formation Julian D. Langer, Emily H. Stoops, Julien Béthune, Felix T. Wieland * Biochemie Zentrum der
FEBS Letters 581 (2007) 2083 2088 Minireview Conformational changes of coat proteins during vesicle formation Julian D. Langer, Emily H. Stoops, Julien Béthune, Felix T. Wieland * Biochemie Zentrum der
BAR domains and membrane curvature: bringing your curves to the BAR
 Biochem. Soc. Symp. 72, 223 231 (Printed in Great Britain) 21 BAR domains and membrane curvature: bringing your curves to the BAR Jennifer L. Gallop 1 and Harvey T. McMahon 1 MRC Laboratory of Molecular
Biochem. Soc. Symp. 72, 223 231 (Printed in Great Britain) 21 BAR domains and membrane curvature: bringing your curves to the BAR Jennifer L. Gallop 1 and Harvey T. McMahon 1 MRC Laboratory of Molecular
Practice Exam 2 MCBII
 1. Which feature is true for signal sequences and for stop transfer transmembrane domains (4 pts)? A. They are both 20 hydrophobic amino acids long. B. They are both found at the N-terminus of the protein.
1. Which feature is true for signal sequences and for stop transfer transmembrane domains (4 pts)? A. They are both 20 hydrophobic amino acids long. B. They are both found at the N-terminus of the protein.
The formation of coat protein complex I (COPI)
 Published Online: 17 November, 2008 Supp Info: http://doi.org/10.1083/jcb.200806140 Downloaded from jcb.rupress.org on October 31, 2018 JCB: ARTICLE Differential roles of ArfGAP1, ArfGAP2, and ArfGAP3
Published Online: 17 November, 2008 Supp Info: http://doi.org/10.1083/jcb.200806140 Downloaded from jcb.rupress.org on October 31, 2018 JCB: ARTICLE Differential roles of ArfGAP1, ArfGAP2, and ArfGAP3
Name: Multiple choice questions. Pick the BEST answer (2 pts ea)
 Exam 1 202 Oct. 5, 1999 Multiple choice questions. Pick the BEST answer (2 pts ea) 1. The lipids of a red blood cell membrane are all a. phospholipids b. amphipathic c. glycolipids d. unsaturated 2. The
Exam 1 202 Oct. 5, 1999 Multiple choice questions. Pick the BEST answer (2 pts ea) 1. The lipids of a red blood cell membrane are all a. phospholipids b. amphipathic c. glycolipids d. unsaturated 2. The
MOLECULAR CELL BIOLOGY
 1 Lodish Berk Kaiser Krieger scott Bretscher Ploegh Matsudaira MOLECULAR CELL BIOLOGY SEVENTH EDITION CHAPTER 13 Moving Proteins into Membranes and Organelles Copyright 2013 by W. H. Freeman and Company
1 Lodish Berk Kaiser Krieger scott Bretscher Ploegh Matsudaira MOLECULAR CELL BIOLOGY SEVENTH EDITION CHAPTER 13 Moving Proteins into Membranes and Organelles Copyright 2013 by W. H. Freeman and Company
Lecture Series 4 Cellular Membranes. Reading Assignments. Selective and Semi-permeable Barriers
 Lecture Series 4 Cellular Membranes Reading Assignments Read Chapter 11 Membrane Structure Review Chapter 12 Membrane Transport Review Chapter 15 regarding Endocytosis and Exocytosis Read Chapter 20 (Cell
Lecture Series 4 Cellular Membranes Reading Assignments Read Chapter 11 Membrane Structure Review Chapter 12 Membrane Transport Review Chapter 15 regarding Endocytosis and Exocytosis Read Chapter 20 (Cell
Cell Membranes. Dr. Diala Abu-Hassan School of Medicine Cell and Molecular Biology
 Cell Membranes Dr. Diala Abu-Hassan School of Medicine Dr.abuhassand@gmail.com Cell and Molecular Biology Organelles 2Dr. Diala Abu-Hassan Membrane proteins Major components of cells Nucleic acids DNA
Cell Membranes Dr. Diala Abu-Hassan School of Medicine Dr.abuhassand@gmail.com Cell and Molecular Biology Organelles 2Dr. Diala Abu-Hassan Membrane proteins Major components of cells Nucleic acids DNA
Legionella pneumophila: an intracellular pathogen of phagocytes Prof. Craig Roy
 an intracellular pathogen of phagocytes Section of Microbial Pathogenesis, Yale University School of Medicine 1 Legionella pneumophila Gram-negative bacterium Facultative intracellular pathogen Protozoa
an intracellular pathogen of phagocytes Section of Microbial Pathogenesis, Yale University School of Medicine 1 Legionella pneumophila Gram-negative bacterium Facultative intracellular pathogen Protozoa
The contribution of proteins and lipids to COPI vesicle formation and consumption. Fredrik Kartberg
 The contribution of proteins and lipids to COPI vesicle formation and consumption Fredrik Kartberg Institute of Biomedicine Department of Medical Genetics 2008 A doctoral thesis at a Swedish University
The contribution of proteins and lipids to COPI vesicle formation and consumption Fredrik Kartberg Institute of Biomedicine Department of Medical Genetics 2008 A doctoral thesis at a Swedish University
Studying dynamic protein:protein interactions in vesicle trafficking. David Owen CIMR University of Cambridge
 Studying dynamic protein:protein interactions in vesicle trafficking David Owen CIMR University of Cambridge The importance of vesicle trafficking in mammalian cells Transducing signals small molecules/nutrients
Studying dynamic protein:protein interactions in vesicle trafficking David Owen CIMR University of Cambridge The importance of vesicle trafficking in mammalian cells Transducing signals small molecules/nutrients
Lipids and Membranes
 Lipids and Membranes Presented by Dr. Mohammad Saadeh The requirements for the Pharmaceutical Biochemistry I Philadelphia University Faculty of pharmacy Membrane transport D. Endocytosis and Exocytosis
Lipids and Membranes Presented by Dr. Mohammad Saadeh The requirements for the Pharmaceutical Biochemistry I Philadelphia University Faculty of pharmacy Membrane transport D. Endocytosis and Exocytosis
Subcellular biochemistry
 Department of Medical Biochemistry Semmelweis University Subcellular biochemistry February-March 2017 Subcellular biochemistry (biochemical aspects of cell biology) Miklós Csala Semmelweis University Dept.
Department of Medical Biochemistry Semmelweis University Subcellular biochemistry February-March 2017 Subcellular biochemistry (biochemical aspects of cell biology) Miklós Csala Semmelweis University Dept.
Lecture Series 4 Cellular Membranes
 Lecture Series 4 Cellular Membranes Reading Assignments Read Chapter 11 Membrane Structure Review Chapter 12 Membrane Transport Review Chapter 15 regarding Endocytosis and Exocytosis Read Chapter 20 (Cell
Lecture Series 4 Cellular Membranes Reading Assignments Read Chapter 11 Membrane Structure Review Chapter 12 Membrane Transport Review Chapter 15 regarding Endocytosis and Exocytosis Read Chapter 20 (Cell
ARTICLE IN PRESS. Review. Dorothee Lay, Karin Gorgas, Wilhelm W. Just
 BBAMCR-15485; No. of pages: 10; 4C: 6 + model Biochimica et Biophysica Acta xx (2006) xxx xxx www.elsevier.com/locate/bbamcr Review Peroxisome biogenesis: Where Arf and coatomer might be involved Dorothee
BBAMCR-15485; No. of pages: 10; 4C: 6 + model Biochimica et Biophysica Acta xx (2006) xxx xxx www.elsevier.com/locate/bbamcr Review Peroxisome biogenesis: Where Arf and coatomer might be involved Dorothee
The Molecular Mechanism of Intracellular Membrane Fusion. Richard H. Scheller
 The Molecular Mechanism of Intracellular Membrane Fusion Richard H. Scheller The human brain contains approximately 10 15 connections between nerve cells (Figure 2). The specific formation and modulation
The Molecular Mechanism of Intracellular Membrane Fusion Richard H. Scheller The human brain contains approximately 10 15 connections between nerve cells (Figure 2). The specific formation and modulation
Molecular Cell Biology 5068 In Class Exam 1 September 29, Please print your name:
 Molecular Cell Biology 5068 In Class Exam 1 September 29, 2015 Exam Number: Please print your name: Instructions: Please write only on these pages, in the spaces allotted and not on the back. Write your
Molecular Cell Biology 5068 In Class Exam 1 September 29, 2015 Exam Number: Please print your name: Instructions: Please write only on these pages, in the spaces allotted and not on the back. Write your
Chapter 20. Cell - Cell Signaling: Hormones and Receptors. Three general types of extracellular signaling. endocrine signaling. paracrine signaling
 Chapter 20 Cell - Cell Signaling: Hormones and Receptors Three general types of extracellular signaling endocrine signaling paracrine signaling autocrine signaling Endocrine Signaling - signaling molecules
Chapter 20 Cell - Cell Signaling: Hormones and Receptors Three general types of extracellular signaling endocrine signaling paracrine signaling autocrine signaling Endocrine Signaling - signaling molecules
Membrane curvature and mechanisms of dynamic cell membrane remodelling Harvey T. McMahon 1 & Jennifer L. Gallop 1
 INSIGHT REVIEW NATURE Vol 438 1 December 2005 doi:10.1038/nature04396 Membrane curvature and mechanisms of dynamic cell membrane remodelling Harvey T. McMahon 1 & Jennifer L. Gallop 1 Membrane curvature
INSIGHT REVIEW NATURE Vol 438 1 December 2005 doi:10.1038/nature04396 Membrane curvature and mechanisms of dynamic cell membrane remodelling Harvey T. McMahon 1 & Jennifer L. Gallop 1 Membrane curvature
endomembrane system internal membranes origins transport of proteins chapter 15 endomembrane system
 endo system chapter 15 internal s endo system functions as a coordinated unit divide cytoplasm into distinct compartments controls exocytosis and endocytosis movement of molecules which cannot pass through
endo system chapter 15 internal s endo system functions as a coordinated unit divide cytoplasm into distinct compartments controls exocytosis and endocytosis movement of molecules which cannot pass through
Eukaryotic cells use internal membrane-bound compartments
 Exo1: A new chemical inhibitor of the exocytic pathway Yan Feng*, Sidney Yu, Troy K. R. Lasell, Ashutosh P. Jadhav*, Eric Macia, Pierre Chardin, Paul Melancon, Michael Roth, Timothy Mitchison*, and Tomas
Exo1: A new chemical inhibitor of the exocytic pathway Yan Feng*, Sidney Yu, Troy K. R. Lasell, Ashutosh P. Jadhav*, Eric Macia, Pierre Chardin, Paul Melancon, Michael Roth, Timothy Mitchison*, and Tomas
Lecture Series 4 Cellular Membranes
 Lecture Series 4 Cellular Membranes Reading Assignments Read Chapter 11 Membrane Structure Review Chapter 21 pages 709-717 717 (Animal( Cell Adhesion) Review Chapter 12 Membrane Transport Review Chapter
Lecture Series 4 Cellular Membranes Reading Assignments Read Chapter 11 Membrane Structure Review Chapter 21 pages 709-717 717 (Animal( Cell Adhesion) Review Chapter 12 Membrane Transport Review Chapter
1. endoplasmic reticulum This is the location where N-linked oligosaccharide is initially synthesized and attached to glycoproteins.
 Biology 4410 Name Spring 2006 Exam 2 A. Multiple Choice, 2 pt each Pick the best choice from the list of choices, and write it in the space provided. Some choices may be used more than once, and other
Biology 4410 Name Spring 2006 Exam 2 A. Multiple Choice, 2 pt each Pick the best choice from the list of choices, and write it in the space provided. Some choices may be used more than once, and other
Nature Biotechnology: doi: /nbt.3828
 Supplementary Figure 1 Development of a FRET-based MCS. (a) Linker and MA2 modification are indicated by single letter amino acid code. indicates deletion of amino acids and N or C indicate the terminus
Supplementary Figure 1 Development of a FRET-based MCS. (a) Linker and MA2 modification are indicated by single letter amino acid code. indicates deletion of amino acids and N or C indicate the terminus
Membrane Fission Is Promoted by Insertion of Amphipathic Helices and Is Restricted by Crescent BAR Domains
 Membrane Fission Is Promoted by Insertion of Amphipathic Helices and Is Restricted by Crescent BAR Domains Emmanuel Boucrot, 1,3 Adi Pick, 2,3 Gamze Çamdere, 1,3 Nicole Liska, 1 Emma Evergren, 1 Harvey
Membrane Fission Is Promoted by Insertion of Amphipathic Helices and Is Restricted by Crescent BAR Domains Emmanuel Boucrot, 1,3 Adi Pick, 2,3 Gamze Çamdere, 1,3 Nicole Liska, 1 Emma Evergren, 1 Harvey
Zool 3200: Cell Biology Exam 4 Part II 2/3/15
 Name:Key Trask Zool 3200: Cell Biology Exam 4 Part II 2/3/15 Answer each of the following questions in the space provided, explaining your answers when asked to do so; circle the correct answer or answers
Name:Key Trask Zool 3200: Cell Biology Exam 4 Part II 2/3/15 Answer each of the following questions in the space provided, explaining your answers when asked to do so; circle the correct answer or answers
Molecular Cell Biology 5068 In class Exam 1 October 2, Please print your name: Instructions:
 Molecular Cell Biology 5068 In class Exam 1 October 2, 2012 Exam Number: Please print your name: Instructions: Please write only on these pages, in the spaces allotted and not on the back. Write your number
Molecular Cell Biology 5068 In class Exam 1 October 2, 2012 Exam Number: Please print your name: Instructions: Please write only on these pages, in the spaces allotted and not on the back. Write your number
Lecture 6 - Intracellular compartments and transport I
 01.25.10 Lecture 6 - Intracellular compartments and transport I Intracellular transport and compartments 1. Protein sorting: How proteins get to their appropriate destinations within the cell 2. Vesicular
01.25.10 Lecture 6 - Intracellular compartments and transport I Intracellular transport and compartments 1. Protein sorting: How proteins get to their appropriate destinations within the cell 2. Vesicular
SUPPLEMENTARY INFORMATION
 doi:10.1038/nature12004 Supplementary Figure 1 Shigella disrupts Golgi structure and function. Representative fluorescence microscopy images showing the redistribution of Golgi resident proteins upon Shigella
doi:10.1038/nature12004 Supplementary Figure 1 Shigella disrupts Golgi structure and function. Representative fluorescence microscopy images showing the redistribution of Golgi resident proteins upon Shigella
Supplementary Information
 Gureaso et al., Supplementary Information Supplementary Information Membrane-dependent Signal Integration by the Ras ctivator Son of Sevenless Jodi Gureaso, William J. Galush, Sean Boyevisch, Holger Sondermann,
Gureaso et al., Supplementary Information Supplementary Information Membrane-dependent Signal Integration by the Ras ctivator Son of Sevenless Jodi Gureaso, William J. Galush, Sean Boyevisch, Holger Sondermann,
7.06 Cell Biology EXAM #3 April 24, 2003
 7.06 Spring 2003 Exam 3 Name 1 of 8 7.06 Cell Biology EXAM #3 April 24, 2003 This is an open book exam, and you are allowed access to books and notes. Please write your answers to the questions in the
7.06 Spring 2003 Exam 3 Name 1 of 8 7.06 Cell Biology EXAM #3 April 24, 2003 This is an open book exam, and you are allowed access to books and notes. Please write your answers to the questions in the
Tala Saleh. Ahmad Attari. Mamoun Ahram
 23 Tala Saleh Ahmad Attari Minna Mushtaha Mamoun Ahram In the previous lecture, we discussed the mechanisms of regulating enzymes through inhibitors. Now, we will start this lecture by discussing regulation
23 Tala Saleh Ahmad Attari Minna Mushtaha Mamoun Ahram In the previous lecture, we discussed the mechanisms of regulating enzymes through inhibitors. Now, we will start this lecture by discussing regulation
Protein regulation Protein motion
 Lecture 13 Protein regulation Protein motion Antoine van Oijen BCMP201 Spring 2008 04/02 Section IV 04/09 Hands-on methods session / PS 4 due 1 Today s lecture 1) Mechanisms of protein regulation 2) Molecular
Lecture 13 Protein regulation Protein motion Antoine van Oijen BCMP201 Spring 2008 04/02 Section IV 04/09 Hands-on methods session / PS 4 due 1 Today s lecture 1) Mechanisms of protein regulation 2) Molecular
Zool 3200: Cell Biology Exam 4 Part I 2/3/15
 Name: Key Trask Zool 3200: Cell Biology Exam 4 Part I 2/3/15 Answer each of the following questions in the space provided, explaining your answers when asked to do so; circle the correct answer or answers
Name: Key Trask Zool 3200: Cell Biology Exam 4 Part I 2/3/15 Answer each of the following questions in the space provided, explaining your answers when asked to do so; circle the correct answer or answers
Supplementary information. Membrane curvature enables N-Ras lipid anchor sorting to. liquid-ordered membrane phases
 Supplementary information Membrane curvature enables N-Ras lipid anchor sorting to liquid-ordered membrane phases Jannik Bruun Larsen 1,2,3, Martin Borch Jensen 1,2,3,6, Vikram K. Bhatia 1,2,3,7, Søren
Supplementary information Membrane curvature enables N-Ras lipid anchor sorting to liquid-ordered membrane phases Jannik Bruun Larsen 1,2,3, Martin Borch Jensen 1,2,3,6, Vikram K. Bhatia 1,2,3,7, Søren
Cell Biology Lecture 9 Notes Basic Principles of cell signaling and GPCR system
 Cell Biology Lecture 9 Notes Basic Principles of cell signaling and GPCR system Basic Elements of cell signaling: Signal or signaling molecule (ligand, first messenger) o Small molecules (epinephrine,
Cell Biology Lecture 9 Notes Basic Principles of cell signaling and GPCR system Basic Elements of cell signaling: Signal or signaling molecule (ligand, first messenger) o Small molecules (epinephrine,
Signal Transduction Pathways. Part 2
 Signal Transduction Pathways Part 2 GPCRs G-protein coupled receptors > 700 GPCRs in humans Mediate responses to senses taste, smell, sight ~ 1000 GPCRs mediate sense of smell in mouse Half of all known
Signal Transduction Pathways Part 2 GPCRs G-protein coupled receptors > 700 GPCRs in humans Mediate responses to senses taste, smell, sight ~ 1000 GPCRs mediate sense of smell in mouse Half of all known
CMB621: Cytoskeleton. Also known as How the cell plays with LEGOs to ensure order, not chaos, is temporally and spatially achieved
 CMB621: Cytoskeleton Also known as How the cell plays with LEGOs to ensure order, not chaos, is temporally and spatially achieved Lecture(s) Overview Lecture 1: What is the cytoskeleton? Membrane interaction
CMB621: Cytoskeleton Also known as How the cell plays with LEGOs to ensure order, not chaos, is temporally and spatially achieved Lecture(s) Overview Lecture 1: What is the cytoskeleton? Membrane interaction
The clathrin adaptor Numb regulates intestinal cholesterol. absorption through dynamic interaction with NPC1L1
 The clathrin adaptor Numb regulates intestinal cholesterol absorption through dynamic interaction with NPC1L1 Pei-Shan Li 1, Zhen-Yan Fu 1,2, Ying-Yu Zhang 1, Jin-Hui Zhang 1, Chen-Qi Xu 1, Yi-Tong Ma
The clathrin adaptor Numb regulates intestinal cholesterol absorption through dynamic interaction with NPC1L1 Pei-Shan Li 1, Zhen-Yan Fu 1,2, Ying-Yu Zhang 1, Jin-Hui Zhang 1, Chen-Qi Xu 1, Yi-Tong Ma
MAE 545: Lecture 14 (11/10) Mechanics of cell membranes
 MAE 545: ecture 14 (11/10) Mechanics of cell membranes Cell membranes Eukaryotic cells E. Coli FIBROBAST 10 mm E. COI nuclear pore complex 1 mm inner membrane plasma membrane secretory complex ribosome
MAE 545: ecture 14 (11/10) Mechanics of cell membranes Cell membranes Eukaryotic cells E. Coli FIBROBAST 10 mm E. COI nuclear pore complex 1 mm inner membrane plasma membrane secretory complex ribosome
October 26, Lecture Readings. Vesicular Trafficking, Secretory Pathway, HIV Assembly and Exit from Cell
 October 26, 2006 Vesicular Trafficking, Secretory Pathway, HIV Assembly and Exit from Cell 1. Secretory pathway a. Formation of coated vesicles b. SNAREs and vesicle targeting 2. Membrane fusion a. SNAREs
October 26, 2006 Vesicular Trafficking, Secretory Pathway, HIV Assembly and Exit from Cell 1. Secretory pathway a. Formation of coated vesicles b. SNAREs and vesicle targeting 2. Membrane fusion a. SNAREs
Fig. S1. Subcellular localization of overexpressed LPP3wt-GFP in COS-7 and HeLa cells. Cos7 (top) and HeLa (bottom) cells expressing for 24 h human
 Fig. S1. Subcellular localization of overexpressed LPP3wt-GFP in COS-7 and HeLa cells. Cos7 (top) and HeLa (bottom) cells expressing for 24 h human LPP3wt-GFP, fixed and stained for GM130 (A) or Golgi97
Fig. S1. Subcellular localization of overexpressed LPP3wt-GFP in COS-7 and HeLa cells. Cos7 (top) and HeLa (bottom) cells expressing for 24 h human LPP3wt-GFP, fixed and stained for GM130 (A) or Golgi97
BIOLOGICAL CHEMISTRY Prof. J.H.P. Bayley, Dr. R.M. Adlington and Dr. L. Smith Trinity Term First Year. Lecture 2 Hagan Bayley
 BIOLOGICAL CHEMISTRY Prof. J.H.P. Bayley, Dr. R.M. Adlington and Dr. L. Smith Trinity Term 2007 - First Year Lecture 2 Hagan Bayley Introduction to the macromolecules of life and cell structures. Introduction
BIOLOGICAL CHEMISTRY Prof. J.H.P. Bayley, Dr. R.M. Adlington and Dr. L. Smith Trinity Term 2007 - First Year Lecture 2 Hagan Bayley Introduction to the macromolecules of life and cell structures. Introduction
Module 3 Lecture 7 Endocytosis and Exocytosis
 Module 3 Lecture 7 Endocytosis and Exocytosis Endocytosis: Endocytosis is the process by which cells absorb larger molecules and particles from the surrounding by engulfing them. It is used by most of
Module 3 Lecture 7 Endocytosis and Exocytosis Endocytosis: Endocytosis is the process by which cells absorb larger molecules and particles from the surrounding by engulfing them. It is used by most of
should be addressed. 2 The abbreviations used are: ER, endoplasmic reticulum; TGN, trans-golgi
 THE JOURNAL OF BIOLOGICAL CHEMISTRY VOL. 286, NO. 41, pp. 35634 35642, October 14, 2011 2011 by The American Society for Biochemistry and Molecular Biology, Inc. Printed in the U.S.A. Several ADP-ribosylation
THE JOURNAL OF BIOLOGICAL CHEMISTRY VOL. 286, NO. 41, pp. 35634 35642, October 14, 2011 2011 by The American Society for Biochemistry and Molecular Biology, Inc. Printed in the U.S.A. Several ADP-ribosylation
Chapter 1 Membrane Structure and Function
 Chapter 1 Membrane Structure and Function Architecture of Membranes Subcellular fractionation techniques can partially separate and purify several important biological membranes, including the plasma and
Chapter 1 Membrane Structure and Function Architecture of Membranes Subcellular fractionation techniques can partially separate and purify several important biological membranes, including the plasma and
Lecture 15. Signal Transduction Pathways - Introduction
 Lecture 15 Signal Transduction Pathways - Introduction So far.. Regulation of mrna synthesis Regulation of rrna synthesis Regulation of trna & 5S rrna synthesis Regulation of gene expression by signals
Lecture 15 Signal Transduction Pathways - Introduction So far.. Regulation of mrna synthesis Regulation of rrna synthesis Regulation of trna & 5S rrna synthesis Regulation of gene expression by signals
Intracellular vesicular traffic. B. Balen
 Intracellular vesicular traffic B. Balen Three types of transport in eukaryotic cells Figure 12-6 Molecular Biology of the Cell ( Garland Science 2008) Endoplasmic reticulum in all eucaryotic cells Endoplasmic
Intracellular vesicular traffic B. Balen Three types of transport in eukaryotic cells Figure 12-6 Molecular Biology of the Cell ( Garland Science 2008) Endoplasmic reticulum in all eucaryotic cells Endoplasmic
Rama Abbady. Odai Bani-Monia. Diala Abu-Hassan
 5 Rama Abbady Odai Bani-Monia Diala Abu-Hassan Lipid Rafts Lipid rafts are aggregates (accumulations) of sphingolipids. They re semisolid clusters (10-200 nm) of cholesterol and sphingolipids (sphingomyelin
5 Rama Abbady Odai Bani-Monia Diala Abu-Hassan Lipid Rafts Lipid rafts are aggregates (accumulations) of sphingolipids. They re semisolid clusters (10-200 nm) of cholesterol and sphingolipids (sphingomyelin
Lecture Series 5 Cellular Membranes
 Lecture Series 5 Cellular Membranes Cellular Membranes A. Membrane Composition and Structure B. Animal Cell Adhesion C. Passive Processes of Membrane Transport D. Active Transport E. Endocytosis and Exocytosis
Lecture Series 5 Cellular Membranes Cellular Membranes A. Membrane Composition and Structure B. Animal Cell Adhesion C. Passive Processes of Membrane Transport D. Active Transport E. Endocytosis and Exocytosis
A. Membrane Composition and Structure. B. Animal Cell Adhesion. C. Passive Processes of Membrane Transport. D. Active Transport
 Cellular Membranes A. Membrane Composition and Structure Lecture Series 5 Cellular Membranes B. Animal Cell Adhesion E. Endocytosis and Exocytosis A. Membrane Composition and Structure The Fluid Mosaic
Cellular Membranes A. Membrane Composition and Structure Lecture Series 5 Cellular Membranes B. Animal Cell Adhesion E. Endocytosis and Exocytosis A. Membrane Composition and Structure The Fluid Mosaic
Summary and Discussion antigen presentation
 Summary and Discussion antigen presentation 247 248 Summary & Discussion Summary and discussion: antigen presentation For a cell to communicate information about its internal health and status to the immune
Summary and Discussion antigen presentation 247 248 Summary & Discussion Summary and discussion: antigen presentation For a cell to communicate information about its internal health and status to the immune
Enzymes Part III: regulation II. Dr. Mamoun Ahram Summer, 2017
 Enzymes Part III: regulation II Dr. Mamoun Ahram Summer, 2017 Advantage This is a major mechanism for rapid and transient regulation of enzyme activity. A most common mechanism is enzyme phosphorylation
Enzymes Part III: regulation II Dr. Mamoun Ahram Summer, 2017 Advantage This is a major mechanism for rapid and transient regulation of enzyme activity. A most common mechanism is enzyme phosphorylation
Effects of Second Messengers
 Effects of Second Messengers Inositol trisphosphate Diacylglycerol Opens Calcium Channels Binding to IP 3 -gated Channel Cooperative binding Activates Protein Kinase C is required Phosphorylation of many
Effects of Second Messengers Inositol trisphosphate Diacylglycerol Opens Calcium Channels Binding to IP 3 -gated Channel Cooperative binding Activates Protein Kinase C is required Phosphorylation of many
Chapter 13: Vesicular Traffic
 Chapter 13: Vesicular Traffic Know the terminology: ER, Golgi, vesicle, clathrin, COP-I, COP-II, BiP, glycosylation, KDEL, microtubule, SNAREs, dynamin, mannose-6-phosphate, M6P receptor, endocytosis,
Chapter 13: Vesicular Traffic Know the terminology: ER, Golgi, vesicle, clathrin, COP-I, COP-II, BiP, glycosylation, KDEL, microtubule, SNAREs, dynamin, mannose-6-phosphate, M6P receptor, endocytosis,
Molecular Cell Biology 5068 In Class Exam 1 October 3, 2013
 Molecular Cell Biology 5068 In Class Exam 1 October 3, 2013 Exam Number: Please print your name: Instructions: Please write only on these pages, in the spaces allotted and not on the back. Write your number
Molecular Cell Biology 5068 In Class Exam 1 October 3, 2013 Exam Number: Please print your name: Instructions: Please write only on these pages, in the spaces allotted and not on the back. Write your number
AFM In Liquid: A High Sensitivity Study On Biological Membranes
 University of Wollongong Research Online Faculty of Science - Papers (Archive) Faculty of Science, Medicine and Health 2006 AFM In Liquid: A High Sensitivity Study On Biological Membranes Michael J. Higgins
University of Wollongong Research Online Faculty of Science - Papers (Archive) Faculty of Science, Medicine and Health 2006 AFM In Liquid: A High Sensitivity Study On Biological Membranes Michael J. Higgins
Biosignals, Chapter 8, rearranged, Part I
 Biosignals, Chapter 8, rearranged, Part I Nicotinic Acetylcholine Receptor: A Ligand-Binding Ion Channel Classes of Receptor Proteins in Eukaryotes, Heterotrimeric G Proteins Signaling View the Heterotrimeric
Biosignals, Chapter 8, rearranged, Part I Nicotinic Acetylcholine Receptor: A Ligand-Binding Ion Channel Classes of Receptor Proteins in Eukaryotes, Heterotrimeric G Proteins Signaling View the Heterotrimeric
The elements of G protein-coupled receptor systems
 The elements of G protein-coupled receptor systems Prostaglandines Sphingosine 1-phosphate a receptor that contains 7 membrane-spanning domains a coupled trimeric G protein which functions as a switch
The elements of G protein-coupled receptor systems Prostaglandines Sphingosine 1-phosphate a receptor that contains 7 membrane-spanning domains a coupled trimeric G protein which functions as a switch
Appendix: Subcellular localization of MIG-14::GFP in C. elegans body wall muscle cells. Pei-Tzu Yang and Hendrik C. Korswagen
 Appendix: Subcellular localization of MIG-14::GFP in C. elegans body wall muscle cells Pei-Tzu Yang and Hendrik C. Korswagen 129 SUBCELLULAR LOCALIZATION OF MIG-14 Abstract MIG-14/Wls is a Wnt binding
Appendix: Subcellular localization of MIG-14::GFP in C. elegans body wall muscle cells Pei-Tzu Yang and Hendrik C. Korswagen 129 SUBCELLULAR LOCALIZATION OF MIG-14 Abstract MIG-14/Wls is a Wnt binding
Chapter 3. Expression of α5-megfp in Mouse Cortical Neurons. on the β subunit. Signal sequences in the M3-M4 loop of β nachrs bind protein factors to
 22 Chapter 3 Expression of α5-megfp in Mouse Cortical Neurons Subcellular localization of the neuronal nachr subtypes α4β2 and α4β4 depends on the β subunit. Signal sequences in the M3-M4 loop of β nachrs
22 Chapter 3 Expression of α5-megfp in Mouse Cortical Neurons Subcellular localization of the neuronal nachr subtypes α4β2 and α4β4 depends on the β subunit. Signal sequences in the M3-M4 loop of β nachrs
Chapter 5 Ground Rules of Metabolism Sections 6-10
 Chapter 5 Ground Rules of Metabolism Sections 6-10 5.6 Cofactors in Metabolic Pathways Most enzymes require cofactors Energy in ATP drives many endergonic reactions Table 5-1 p86 Cofactors and Coenzymes
Chapter 5 Ground Rules of Metabolism Sections 6-10 5.6 Cofactors in Metabolic Pathways Most enzymes require cofactors Energy in ATP drives many endergonic reactions Table 5-1 p86 Cofactors and Coenzymes
Enzyme-coupled Receptors. Cell-surface receptors 1. Ion-channel-coupled receptors 2. G-protein-coupled receptors 3. Enzyme-coupled receptors
 Enzyme-coupled Receptors Cell-surface receptors 1. Ion-channel-coupled receptors 2. G-protein-coupled receptors 3. Enzyme-coupled receptors Cell-surface receptors allow a flow of ions across the plasma
Enzyme-coupled Receptors Cell-surface receptors 1. Ion-channel-coupled receptors 2. G-protein-coupled receptors 3. Enzyme-coupled receptors Cell-surface receptors allow a flow of ions across the plasma
Biol403 MAP kinase signalling
 Biol403 MAP kinase signalling The mitogen activated protein kinase (MAPK) pathway is a signalling cascade activated by a diverse range of effectors. The cascade regulates many cellular activities including
Biol403 MAP kinase signalling The mitogen activated protein kinase (MAPK) pathway is a signalling cascade activated by a diverse range of effectors. The cascade regulates many cellular activities including
Signaling. Dr. Sujata Persad Katz Group Centre for Pharmacy & Health research
 Signaling Dr. Sujata Persad 3-020 Katz Group Centre for Pharmacy & Health research E-mail:sujata.persad@ualberta.ca 1 Growth Factor Receptors and Other Signaling Pathways What we will cover today: How
Signaling Dr. Sujata Persad 3-020 Katz Group Centre for Pharmacy & Health research E-mail:sujata.persad@ualberta.ca 1 Growth Factor Receptors and Other Signaling Pathways What we will cover today: How
Biophys J BioFAST, published on May 30, 2008 as doi: /biophysj
 Biophys J BioFAST, published on May 30, 008 as doi:10.159/biophysj.108.133173 This un-edited manuscript has been accepted for publication in Biophysical Journal and is freely available on BioFast at http://www.biophysj.org.
Biophys J BioFAST, published on May 30, 008 as doi:10.159/biophysj.108.133173 This un-edited manuscript has been accepted for publication in Biophysical Journal and is freely available on BioFast at http://www.biophysj.org.
Localization and Retention of Glycosyltransferases And the Role of Vesicle Trafficking in Glycosylation
 Localization and Retention of Glycosyltransferases And the Role of Vesicle Trafficking in Glycosylation Richard Steet, Ph.D. 3/8/2011 glycosylation is a non-template derived phenomenon - the presence of
Localization and Retention of Glycosyltransferases And the Role of Vesicle Trafficking in Glycosylation Richard Steet, Ph.D. 3/8/2011 glycosylation is a non-template derived phenomenon - the presence of
ADP-ribosylation factor and phosphatidic acid levels in Golgi membranes during budding of coatomer-coated vesicles
 Proc. Natl. Acad. Sci. USA Vol. 95, pp. 13676 13680, November 1998 Cell Biology ADP-ribosylation factor and phosphatidic acid levels in Golgi membranes during budding of coatomer-coated vesicles MARK STAMNES*,
Proc. Natl. Acad. Sci. USA Vol. 95, pp. 13676 13680, November 1998 Cell Biology ADP-ribosylation factor and phosphatidic acid levels in Golgi membranes during budding of coatomer-coated vesicles MARK STAMNES*,
I. Fluid Mosaic Model A. Biological membranes are lipid bilayers with associated proteins
 Lecture 6: Membranes and Cell Transport Biological Membranes I. Fluid Mosaic Model A. Biological membranes are lipid bilayers with associated proteins 1. Characteristics a. Phospholipids form bilayers
Lecture 6: Membranes and Cell Transport Biological Membranes I. Fluid Mosaic Model A. Biological membranes are lipid bilayers with associated proteins 1. Characteristics a. Phospholipids form bilayers
04_polarity. The formation of synaptic vesicles
 Brefeldin prevents assembly of the coats required for budding Nocodazole disrupts microtubules Constitutive: coatomer-coated Selected: clathrin-coated The formation of synaptic vesicles Nerve cells (and
Brefeldin prevents assembly of the coats required for budding Nocodazole disrupts microtubules Constitutive: coatomer-coated Selected: clathrin-coated The formation of synaptic vesicles Nerve cells (and
Regulation of Golgi structure and function by ARF-like protein 1 (Arl1)
 RESEARCH ARTICLE 4543 Regulation of Golgi structure and function by ARF-like protein 1 (Arl1) Lei Lu, Heinz Horstmann, Cheepeng Ng and Wanjin Hong Membrane Biology Laboratory, Institute of Molecular and
RESEARCH ARTICLE 4543 Regulation of Golgi structure and function by ARF-like protein 1 (Arl1) Lei Lu, Heinz Horstmann, Cheepeng Ng and Wanjin Hong Membrane Biology Laboratory, Institute of Molecular and
Localization and Retention of Glycosyltransferases And the Role of Vesicle Trafficking in Glycosylation
 Localization and Retention of Glycosyltransferases And the Role of Vesicle Trafficking in Glycosylation Richard Steet, Ph.D. 2/21/17 glycosylation is a non-template derived phenomenon - the presence of
Localization and Retention of Glycosyltransferases And the Role of Vesicle Trafficking in Glycosylation Richard Steet, Ph.D. 2/21/17 glycosylation is a non-template derived phenomenon - the presence of
Dynamic Interaction of HIV-1 Nef with the Clathrin-Mediated Endocytic Pathway at the Plasma Membrane
 Traffic 2007; 8: 61 76 Blackwell Munksgaard Dynamic Interaction of HIV-1 Nef with the Clathrin-Mediated Endocytic Pathway at the Plasma Membrane # 2006 The Authors Journal compilation # 2006 Blackwell
Traffic 2007; 8: 61 76 Blackwell Munksgaard Dynamic Interaction of HIV-1 Nef with the Clathrin-Mediated Endocytic Pathway at the Plasma Membrane # 2006 The Authors Journal compilation # 2006 Blackwell
Organization of ATPases
 The Primary Active Transporter II: The ATPase Objectives: Organization P type with NPA domains Proton pumps of the rotary V type ATPase 1 Organization of P type, solute transport, found in plasma membranes
The Primary Active Transporter II: The ATPase Objectives: Organization P type with NPA domains Proton pumps of the rotary V type ATPase 1 Organization of P type, solute transport, found in plasma membranes
Lecture: CHAPTER 13 Signal Transduction Pathways
 Lecture: 10 17 2016 CHAPTER 13 Signal Transduction Pathways Chapter 13 Outline Signal transduction cascades have many components in common: 1. Release of a primary message as a response to a physiological
Lecture: 10 17 2016 CHAPTER 13 Signal Transduction Pathways Chapter 13 Outline Signal transduction cascades have many components in common: 1. Release of a primary message as a response to a physiological
Summary of Endomembrane-system
 Summary of Endomembrane-system 1. Endomembrane System: The structural and functional relationship organelles including ER,Golgi complex, lysosome, endosomes, secretory vesicles. 2. Membrane-bound structures
Summary of Endomembrane-system 1. Endomembrane System: The structural and functional relationship organelles including ER,Golgi complex, lysosome, endosomes, secretory vesicles. 2. Membrane-bound structures
Section 6. Junaid Malek, M.D.
 Section 6 Junaid Malek, M.D. The Golgi and gp160 gp160 transported from ER to the Golgi in coated vesicles These coated vesicles fuse to the cis portion of the Golgi and deposit their cargo in the cisternae
Section 6 Junaid Malek, M.D. The Golgi and gp160 gp160 transported from ER to the Golgi in coated vesicles These coated vesicles fuse to the cis portion of the Golgi and deposit their cargo in the cisternae
MCB130 Midterm. GSI s Name:
 1. Peroxisomes are small, membrane-enclosed organelles that function in the degradation of fatty acids and in the degradation of H 2 O 2. Peroxisomes are not part of the secretory pathway and peroxisomal
1. Peroxisomes are small, membrane-enclosed organelles that function in the degradation of fatty acids and in the degradation of H 2 O 2. Peroxisomes are not part of the secretory pathway and peroxisomal
Cell Membrane Structure (1.3) IB Diploma Biology
 Cell Membrane Structure (1.3) IB Diploma Biology Essential idea: The structure of biological membranes makes them fluid and dynamic http://www.flickr.com/photos/edsweeney/6346198056/ 1.3.1 Phospholipids
Cell Membrane Structure (1.3) IB Diploma Biology Essential idea: The structure of biological membranes makes them fluid and dynamic http://www.flickr.com/photos/edsweeney/6346198056/ 1.3.1 Phospholipids
Structure and Activation of the Visual Pigment Rhodopsin
 Seminar 02.12.2011 Structure and Activation of the Visual Pigment Rhodopsin by Steven O.Smith, 2010 Constantin Schneider FB Physik, FU Berlin GPCR: Overview Rhodopsin is a G protein-coupled receptor (GPCR)
Seminar 02.12.2011 Structure and Activation of the Visual Pigment Rhodopsin by Steven O.Smith, 2010 Constantin Schneider FB Physik, FU Berlin GPCR: Overview Rhodopsin is a G protein-coupled receptor (GPCR)
