Rac function in epithelial tube morphogenesis
|
|
|
- Polly Gardner
- 5 years ago
- Views:
Transcription
1 Developmental Biology 290 (2006) Rac function in epithelial tube morphogenesis Carolyn Pirraglia, Rakhi Jattani, Monn Monn Myat Department of Cell and Developmental Biology, Weill Medical College of Cornell University, 1300 York Avenue, New York, NY 10021, USA Received for publication 5 August 2005, revised 18 November 2005, accepted 1 December 2005 Available online 10 January 2006 Abstract Epithelial cell migration and morphogenesis require dynamic remodeling of the actin cytoskeleton and cell cell adhesion complexes. Numerous studies in cell culture and in model organisms have demonstrated the small GTPase Rac to be a critical regulator of these processes; however, little is known about Rac function in the morphogenic movements that drive epithelial tube formation. Here, we use the embryonic salivary glands of Drosophila to understand the role of Rac in epithelial tube morphogenesis. We show that inhibition of Rac function, either through loss of function mutations or dominant-negative mutations, disrupts salivary gland invagination and posterior migration. In contrast, constitutive activation of Rac induces motile behavior and subsequent cell death. We further show that Rac regulation of salivary gland morphogenesis occurs through modulation of cell cell adhesion mediated by the E-cadherin/β-catenin complex and that shibire, the Drosophila homolog of dynamin, functions downstream of Rac in regulating β-catenin localization during gland morphogenesis. Our results demonstrate that regulation of cadherin-based adherens junctions by Rac is critical for salivary gland morphogenesis and that this regulation occurs through dynamin-mediated endocytosis Elsevier Inc. All rights reserved. Keywords: Drosophila; Salivary gland; Epithelial morphogenesis; Tube; Rac; E-cadherin; Dynamin; Endocytosis Introduction Epithelial cells undergo dynamic morphogenic movements to build complex three-dimensional structures from simply organized epithelial sheets. Changes in the actin cytoskeleton and cell cell adhesion are thought to be essential for such morphogenic movements although the molecular mechanisms that control the dynamics and precision of such changes are not well understood. The Rho family of small GTPases, which includes Rac, Rho and Cdc42, regulates a number of cellular events, including remodeling of cadherin-based adhesion junctions (Etienne-Manneville and Hall, 2002). Cadherins mediate cell cell adhesion through homotypic binding of their extracellular domains and are indirectly linked to the actin cytoskeleton through the binding of β-catenin and α- catenin to their cytoplasmic tails (Tepass et al., 2001). Rho GTPases were shown to alter both assembly and disassembly of cadherin-based adherens junctions. Studies Corresponding author. address: mmm2005@med.cornell.edu (M.M. Myat). performed on mammalian Madin Darby canine kidney (MDCK) cells grown in culture and in the Drosophila embryonic epithelia showed that activated Rac1 can increase E-cadherin-mediated cell adhesion (Eaton et al., 1995; Harden et al., 1995; Ridley et al., 1995; Takaishi et al., 1997), whereas dominant-negative Rac1 can inhibit hepatocyte growth factor (HGF)-mediated disruption of junctions in MDCK cells (Potempa and Ridley, 1998). Similarly, Rac has been implicated in both the assembly and disassembly of junctions in cultured human epidermal keratinocytes (Akhtar et al., 2000; Braga et al., 1997, 1999). It is believed that the role of a particular GTPase in either the assembly or disassembly of adherens junctions depends on the cell type, the extracellular matrix and junctional maturity. Rac function has been implicated in the development of numerous tissues in the Drosophila embryo. Rac is essential for axonal migration in the developing nervous system, epithelial sheet movement during dorsal closure, myoblast fusion and establishment of planar cell polarity (Eaton et al., 1996; Fanto et al., 2000; Hakeda-Suzuki et al., 2002; Kaufmann et al., 1998; Luo et al., 1994; Ng et al., 2002; Woolner et al., 2005) /$ - see front matter 2005 Elsevier Inc. All rights reserved. doi: /j.ydbio
2 436 C. Pirraglia et al. / Developmental Biology 290 (2006) However, little is known of Rac's role in epithelial tube morphogenesis, an essential step in the formation of most major organs where several different types of movements, such as invagination and migration, occur coordinately to form a three dimensional tube from a relatively simple epithelial anlage (Hogan and Kolodziej, 2002; Myat, 2005). A recent study of Rac in the Drosophila embryonic trachea showed that Rac negatively regulates cadherin-mediated adhesion (Chihara et al., 2003). Reduction of Rac activity increased the amount of E-cadherin/catenin complexes in the embryonic epidermis and tracheal epithelia and prevented tracheal cell rearrangements (Chihara et al., 2003). Furthermore, activation of Rac inhibited incorporation of E-cadherin into cell cell junctions and led to loss of adhesion between tracheal cells and subsequent detachment from the epithelium. Although Rac might alter cadherin-based cell cell junctions by several molecular mechanisms as suggested from studies on cultured mammalian cells (Briggs and Sacks, 2003; Lozano et al., 2003), it is still unknown how Rac regulates cadherin-mediated adhesion in the context of a developing organ or tissue. Here, we analyze Rac function in the developing salivary gland, a pair of elongated tubes with a single layer of epithelial cells surrounding a central lumen (Myat, 2005). The Drosophila embryonic salivary gland is an ideal system to investigate the role of Rac in tube morphogenesis because of its relatively simple, unbranched structure and the genetic tools available in Drosophila that allow dissection of Rac function. The glands are formed from two placodes, or plates, of ectodermal epithelial cells that invaginate through a series of cell shape changes to form tubes (Myat and Andrew, 2000b). The transcription factor Fork Head (Fkh) is required for the cell shape changes that occur during salivary gland invagination and also for cell survival; in fkh mutant embryos, salivary gland cells remain columnar instead of becoming pyramidal and eventually die by apoptosis (Myat and Andrew, 2000a). Additionally, these cell shape changes need to be polarized in order to form an elongated tube, a process controlled by the transcription factors, Hairy and Huckebein, and their downstream targets, Crumbs, an apical membrane determinant, and Klarsicht, a mediator of microtubule-dependent organelle transport (Myat and Andrew, 2002). After invagination is complete, the salivary gland migrates posteriorly as an intact organ until it reaches its final position along the lateral body wall. Here, we show that the Rac GTPases play an important role in regulating cadherin-mediated cell cell adhesion during salivary gland morphogenesis. Our results further suggest that Rac regulates cell cell adhesion in salivary gland cells by modulating dynamin-mediated endocytosis of E-cadherin. Materials and methods Drosophila strains and genetics Canton-S flies were used as wild-type controls. The following fly lines were obtained from the Bloomington Stock Center and are described in FlyBase ( Rac1 J11, Rac1 J11 Rac2 Δ, Rac1 J11 Rac2 Δ Mtl Δ, Rac1 J10 Rac2 Δ Mtl Δ, Mtl Δ, UAS-Rac1 L89, UAS-Rac1 N17, UAS-Rac1 V12, shi ts2 and UAS-shi K44A. UAS-DEcadherin-GFP was a gift of H. Oda. UAS-p35 was a gift from H. Stellar. fkh-gal4 was used to drive salivary gland specific expression (Henderson and Andrew, 2000). Antibody staining of embryos Embryos were fixed and processed for antibody staining as previously described (Reuter et al., 1990). For staining of β-catenin and E-cadherin, embryos were fixed in 4% paraformaldehyde (Electron Microscopy Sciences, Hatfield, PA) instead of 3.7% formaldehyde. The following antisera were used at the indicated dilutions: rat dcreb-a antiserum at 1:10,000 for whole mount and 1:5000 for fluorescence; rat PH4αSG1 antiserum (a gift from E. Abrams and D. Andrew) at 1:10,000 for whole mount and 1:5000 for fluorescence; mouse monoclonal Crumbs antiserum (Developmental Studies Hybridoma Bank; Iowa City, IA) at 1:100; mouse monoclonal β-catenin (Armadillo) antiserum (Developmental Studies Hybridoma Bank) at 1:100; rat monoclonal DEcadherin antiserum (gift from H. Oda) at 1:20; mouse β-galactosidase (β-gal) antiserum (Promega; Madison, WI) at 1:10,000, rabbit polyclonal apkc antiserum (Santa Cruz Biotechnology, Inc., Santa Cruz, CA) at 1:1000 and rabbit polyclonal Croquemort antiserum at 1:500 (a gift from N. Franc). Appropriate biotinylated- (Jackson Immunoresearch Laboratories, Westgrove, PA), FITC- or Rhodamine- (Molecular Probes, Eugene, OR) conjugated secondary antibodies were used at a dilution of 1:500. Whole-mount-stained embryos were mounted in methyl salicylate (Sigma, St. Louis, MO) before visualization on a Zeiss Axioplan 2 microscope with Axiovision Rel 4.2 software (Carl Zeiss, Thornwood, NY). Fluorescently labeled embryos were mounted in 85% glycerol with 2.5% N- propylgalate. Thick (1 μm) fluorescent images were acquired on a Zeiss Axioplan microscope (Carl Zeiss) equipped with LSM 510 for laser scanning confocal microscopy at the Rockefeller University Bio-imaging Resources Center (New York, NY). Analysis of recombinant lines Recombinant lines carrying different UAS-transgene insertions were identified by PCR analysis of genomic DNA isolated from adult recombinant flies using one primer specific to the UAS sequence and another to the transgene of interest. Expression of the transgene was then confirmed by in situ hybridization with antisense digoxigenin-labeled RNA probes for the respective transgenes prepared as previously described (Lehmann and Tautz, 1994). shotgun (E-cadherin; Research Genetics), Rac1 (Open Biosystems, Huntsville, AL) and shibire (Open Biosystems) cdnas were used as templates for generating antisense digoxygenin-labeled RNA probes. Embryos were mounted in 70% glycerol before visualization as described above for antibody staining. Results Inhibition of Rac function disrupts salivary gland morphogenesis The Drosophila genome encodes three Rac genes, Rac1, Rac2 and Mig 2-like (Mtl). Rac1 and Rac2 share 92% amino acid sequence identity and Mtl is highly related. All three genes are expressed ubiquitously during development (Harden et al., 1995, 2002; Hariharan et al., 1995; Luo et al., 1994). To investigate the role of the Rac GTPases in salivary gland morphogenesis, we analyzed embryos carrying single, double or triple mutations in these Rac genes. In wild-type embryos, salivary gland cells form two placodes on the ventral surface of the embryo and begin their invagination at embryonic stage 11 (Fig. 1A). After invagination is complete, a dorsallyoriented tube has formed which then migrates posteriorly to form an elongated tube along the lateral body wall (Figs. 1B
3 C. Pirraglia et al. / Developmental Biology 290 (2006) Fig. 1. Rac GTPases regulate salivary gland morphogenesis. In wild-type (WT, A C) embryos, salivary gland cells invaginate from the ventral surface at stage 11 (A, arrow), and migrate posteriorly between stages 12 (B, arrow) and 14 (C, arrow). In Rac1 J11 mutants, the salivary gland does not complete its posterior migration (D, arrow). In Rac1 J11 Rac2 Δ (E), Mtl Δ (F) and Rac1 J11 Rac2 Δ Mtl Δ mutants (G), a cluster of cells remains at the ventral surface (E G, arrowheads) and the gland fails to complete its posterior migration (E G, arrows). In embryos expressing the dominant-negative Rac1 mutations (Rac1 N17 or Rac1 L89 ) specifically in the salivary glands with fkh-gal4, the salivary gland also fails to migrate posteriorly (H and I, arrows) and some cells remain at the ventral surface (H and I, arrowheads). The salivary glands of Rac1 L89 mutants are severely narrowed at their mid-points (I, small arrow). All embryos shown were stained for the transcription factor, dcreb-a to mark salivary gland nuclei. and C). In contrast, in embryos homozygous for different combinations of the three Rac genes, salivary gland invagination and migration were perturbed. In Rac1 J11 mutant embryos, all cells invaginated but the gland did not complete its posterior migration and instead was abnormally shaped and appeared attached to the ventral surface (Fig. 1D). In Rac1 J11 Rac2 Δ mutants, the salivary gland phenotype was enhanced, such that more cells remained at the ventral surface and the gland failed to migrate posteriorly (Fig. 1E). Thus, the functions of both Rac1 and Rac2 are required for proper invagination and migration of the salivary gland. In Mtl Δ homozygous embryos (Fig. 1F), salivary gland invagination and migration were disrupted in a similar manner to Rac1 J11 Rac2 Δ mutants (Fig. 1E). Embryos mutant for all three Rac genes, Rac1 J11 Rac2 Δ Mtl Δ, also showed defects in invagination and migration (Fig. 1G). These data suggest that all three Rac genes, Rac1, Rac2 and Mtl, are individually required for salivary gland morphogenesis. To confirm a function for Rac1 in salivary gland morphogenesis and to determine cell autonomy, we expressed two types of Rac1 dominant-negative mutations, Rac1 L89 and Rac1 N17, specifically in the salivary glands of otherwise wild-type embryos using the UAS-GAL4 system (herein referred to as Rac1 L89 and Rac1 N17 ; Figs. 1H and I; Brand and Perrimon, 1993; Harden et al., 1995; Luo et al., 1994). In Rac1 L89 and Rac1 N17 embryos invagination and posterior migration of the gland were similarly affected to that of loss of Rac1 function (Figs. 1H and I). These data demonstrate that the Rac genes are required in salivary gland cells during invagination and migration. Expression of the Rac1 L89 dominant-negative mutation caused the most severe salivary gland phenotype; the gland appeared to be stretched or severed at its approximate midpoint (Fig. 1I). To better understand the Rac1 L89 salivary gland phenotype, we visualized the lumen of the glands by staining for the apical membrane protein Crumbs and the transcription factor dcreb-a. In wild-type embryos, invagination of salivary gland primordial cells formed a tube with a contiguous, central lumen (Fig. 2A). In subsequent stages as the gland elongated and migrated posteriorly, the gland and its lumen remained intact (Figs. 2B and C). After migration was complete, the salivary gland lay along the lateral body wall with its longest axis in the anterior posterior direction (Fig. 2D). In contrast, in Rac1 L89 embryos, the salivary glands were broken apart. Most salivary gland cells invaginated in Rac1 L89 embryos and formed a tube with a slightly expanded lumen (Fig. 2E). At the stage when wildtype salivary gland cells turned to migrate posteriorly (Fig. 2B), the Rac1 L89 salivary gland lumen was thinner at its midpoint (Fig. 2F) or broke completely (Fig. 2G) while the gland proper remained intact (white outline Figs. 2F and G). At later stages, the Rac1 L89 salivary gland proper was also separated into pieces with one segment at the surface and another internal (Figs. 2H and I). One hundred percent of glands scored at embryonic stage 14 displayed the broken gland phenotype (Fig. 5A). By the end of embryogenesis, the salivary glands of Rac1 L89 embryos were globular and consisted of several separated lumena (Fig. 2J). The salivary gland lumen was similarly disrupted in embryos homozygous for Rac1 J11 Rac2 Δ. During posterior migration of the salivary gland in Rac1 J11 Rac2 Δ embryos, breaks in the lumen were detected (Fig. 2K). In the mature gland of Rac1 J11 Rac2 Δ embryos, cyst-like lumena were found (Fig. 2L) similar to those of Rac1 L89 embryos (Fig. 2J). Therefore, these data demonstrate a crucial role for Rac in keeping the lumen and the gland intact during gland morphogenesis.
4 438 C. Pirraglia et al. / Developmental Biology 290 (2006) Fig. 2. Salivary gland lumen integrity is lost in Rac1 L89 mutants. In wild-type embryos (A D), invagination forms a gland with a distinct lumen (A, arrow). Posterior migration of the WT gland is initiated at stage 12 (B, arrow), continues during stage 14 (C, arrow) and is completed by stage 15 (D, arrow). In Rac1 L89 embryos, a wider salivary gland lumen is formed (E, arrow). The lumen of Rac1 L89 glands severs (F and G, arrows) but the gland remains intact (G, arrowhead). Rac1 L89 mutant gland breaks apart into two pieces (panels H and I are different focal planes of the same embryo, arrows). The mature gland of Rac1 L89 mutants contains several lumena (J, arrows). In Rac1 J11 Rac2 Δ embryos, the lumen of the invaginated salivary gland breaks apart (K, arrow) and several lumena are detected in the mature gland (L, arrows). All embryos were stained for the transcription factor, dcreb-a and for Crumbs to mark the lumen. Constitutive activation of Rac causes loss of salivary gland cells To better understand the function of Rac in salivary gland morphogenesis, we expressed the constitutively active form of Rac1 (Rac1 V12 ) specifically in the salivary gland (herein referred to as Rac1 V12 embryos). In wild-type embryos, expression of the transcription factor dcreb-a is maintained throughout gland morphogenesis (Figs. 3A C; Andrew et al., 1997). In contrast, in Rac1 V12 embryos, we observed a gradual disappearance of dcreb-a beginning with cells in the distal tip of the gland (Figs. 3E, F and G). By late embryogenesis (stages 15 16), dcreb-a was detected in only a few cells or was completely lost (Fig. 3G and data not shown). Apical membrane proteins, such as Crumbs and atypical Protein kinase C (apkc), also gradually disappeared from salivary gland cells of Rac1 V12 embryos (Fig. 3H and data not shown). In wild-type embryos, Crumbs is localized to the apical domain and marks the lumen (Fig. 3D), whereas in Rac1 V12 embryos, Crumbs was found either in clumps or was absent from most salivary gland cells (Fig. 3H). An identical expression pattern was observed for apkc (data not shown). These data demonstrate that Rac1 activation altered expression of dcreb-a and apical polarity markers. To determine whether the expression of salivary gland marker proteins other than dcreb-a was similarly affected, we stained wild-type and Rac1 V12 embryos for Prolyl 4-hydroxylase alpha salivary gland 1 (PH4αSG1), which is expressed specifically in salivary gland cells beginning during embryonic stage 12 and continuing throughout embryogenesis (Figs. 3I and J; Abrams and Andrew, 2002). PH4αSG1 was expressed at reduced levels in Rac1 V12 embryos at stage 14 (Fig. 3K). Furthermore, PH4αSG1-expressing Rac1 V12 cells were dispersed and did not form an intact gland. By the end of embryogenesis, PH4αSG1-positive cells were detected in the head region and scattered in more posterior regions (Fig. 3L). The number of PH4αSG1-positive cells in Rac1 V12 mutant glands at stage 16 was consistently less than the normal number of salivary gland cells in wild-type embryos (data not shown). These data suggest that Rac activation induces migratory behavior and alters salivary gland specific gene expression. The reduced number of salivary gland cells in late Rac1 V12 embryos further suggests that a population of cells either dies or completely loses the salivary gland fate when Rac is activated. Rac1 regulates cadherin-mediated adhesion during salivary gland morphogenesis Previous reports have shown that in a number of different contexts Rac GTPases exert their cellular functions through regulation of E-cadherin-mediated adhesion. Therefore, we tested whether the salivary gland defects observed in Rac1 mutants could be due to changes in cadherin-mediated cell cell adhesion by analyzing the localization of the adherens junction proteins, β-catenin and E-cadherin. In salivary gland cells of wild-type embryos, β-catenin and E-cadherin were localized in the apical domain during gland morphogenesis (Figs. 4A D). In invaginating Rac1 L89 mutant glands, β-catenin and E- cadherin expression was increased in the apical domain and β- catenin was also found along the basal lateral membrane (Figs. 4E and F). At the point when Rac1 L89 salivary gland cells began to migrate posteriorly, β-catenin and E-cadherin remained
5 C. Pirraglia et al. / Developmental Biology 290 (2006) Fig. 3. Activation of Rac1 alters salivary gland gene expression. In wild-type embryos, dcreb-a is expressed in all salivary gland cells throughout gland morphogenesis (A C). In Rac1 V12 salivary glands, dcreb-a staining reveals abnormal salivary gland morphology (E, arrow). dcreb-a expression begins to disappear at the time of posterior migration in salivary glands expressing Rac1 V12 (F, arrow). Few dcreb-a-stained cells are detected in late salivary glands expressing Rac1 V12 (G, arrow). In wild-type glands (D), the apical membrane protein, Crumbs (red), marks the lumen which is a contiguous structure (arrow). In Rac1 V12 glands, Crumbs staining is found in clumps (H, arrow) or is absent (H, arrowhead). Wild-type (WT) salivary glands express PH4αSG1 (I and J, arrows). In Rac1 V12 salivary glands, PH4αSG1-stained cells do not form a coherent tube (K, arrow) and become fewer and dispersed at late stages of embryogenesis (L, arrows). Embryos in panels A C and E G were stained for dcreb-a; embryos in panels D and H were stained for Crumbs (red) to mark the lumen and dcreb-a (green) to mark the salivary gland nuclei; embryos in panels I L were stained for PH4αSG1 to mark the endoplasmic reticulum of salivary gland cells. elevated in the distal and proximal portions of the gland, but were absent from the middle of the mutant glands coinciding with the thin or broken regions (Figs. 4G and H). We observed a similar increase in β-catenin expression and distribution in Rac1 J10 Rac2 Δ Mtl Δ homozygous embryos and in wild-type embryos expressing the Rac1 N17 dominant-negative mutation in the salivary gland (data not shown). In contrast to Rac1 L89, the constitutively active Rac1 V12 mutation caused loss of β-catenin and E-cadherin expression in salivary gland cells (compare Figs. 4I and J with E and F). At the invagination stage, only few Rac1 V12 salivary gland cells had apical localization of β-catenin and E-cadherin while most had cytoplasmic accumulation (Figs. 4I and J). By stage 12 when dcreb-a staining began to disappear in Rac1 V12 salivary glands, β-catenin and E-cadherin localization was cytoplasmic in all gland cells (data not shown). Therefore, expression of dominant-negative and constitutively active Rac mutations caused opposite effects on expression levels and localization of β-catenin and E-cadherin in salivary gland cells. To determine whether the changes in localization of β- catenin and E-cadherin reflect changes in adhesiveness of the cadherin-based junctions, we attempted to rescue the Rac1 mutant salivary gland phenotypes by increasing or decreasing the expression of shotgun (shg), which encodes Drosophila E- cadherin. We hypothesized that if the increased E-cadherin staining observed in Rac1 L89 mutant glands increases cell cell adhesion and causes the glands to sever during migration, then reduction of E-cadherin levels by removing one copy of the shg gene should alleviate the Rac1 L89 salivary gland phenotype. Similarly, if loss of cadherin expression in Rac1 V12 mutant glands reduces cell cell adhesion and leads to dispersal and cell death of salivary gland cells, then exogenous expression of wild-type E-cadherin may rescue the Rac1 V12 mutant salivary gland phenotype. We expressed Rac1 L89 in salivary glands of embryos heterozygous for the shg 2 mutation and scored embryos at stage 14 when the gland had completely severed in Rac1 L89 embryos. We observed a dramatic decrease in the Rac1 L89 salivary gland phenotype in Rac1 L89 shg/+ embryos from 100% to 16% (n = 88 glands; Fig. 5A), suggesting that increased cadherin-mediated adhesion is the cause for the break up of the gland in Rac1 L89 embryos. We also expressed wild-type fulllength E-cadherin (shg) fused to GFP (EcadGFP) concomitantly with the Rac1 V12 mutation in salivary gland cells and scored dcreb-a-stained embryos at stage 14 when most dcreb-a expression is lost in Rac1 V12 embryos. Wild-type
6 440 C. Pirraglia et al. / Developmental Biology 290 (2006) Fig. 4. β-catenin and E-cadherin localization is altered in Rac1 mutant salivary glands. In wild-type embryos (A D), β-catenin (A and C, arrows) and E-cadherin (B and D, arrows) are found exclusively in the apical domain during invagination (A and B) and during posterior migration (C and D). In Rac1 L89 salivary glands, β- catenin (E) and E-cadherin (F) are increased in the apical domain (E and F, arrows) and are also present along the basal lateral membrane (E and F, arrowhead) of invaginating salivary gland cells. During posterior migration of Rac1 L89 glands, β-catenin (G) and E-cadherin (H) are present in the distal and proximal regions (G and H, arrows) but absent from the mid-region of the gland (G and H, arrowheads). In Rac1 V12 glands, β-catenin (I) and E-cadherin (J) are present in some cells (I and J, arrows) but lost from others (I and J, arrowheads). Embryos in panels A, C, E, G and I were stained for β-catenin (red) and dcreb-a (green); embryos in panels B, D, F, H and J were stained for E-cadherin. The right panels of panels A, C, E, G and I emphasize the β-catenin staining of the glands in the left panels. embryos expressing EcadGFP in the salivary gland formed normal glands like wild-type embryos (Figs. 5B and C). Expression of EcadGFP in Rac1 V12 mutant salivary gland cells restored dcreb-a expression to normal levels and gland integrity in 40% of embryos (n = 150 glands; Figs. 5A and D). In some Rac1 V12 EcadGFP embryos, the salivary glands were slightly more elongated compared to wild-type (compare Figs. 5D with B). Altogether, these data demonstrate that Rac regulates epithelial integrity during salivary gland morphogenesis through modulation of cadherin function; loss of Rac function increases cadherin function whereas gain of Rac function decreases cadherin function. Rac regulates cadherin activity through endocytosis Mammalian cell culture studies have shown that one mechanism by which Rac controls cell cell adhesion is through selective endocytosis of cell surface cadherins (Akhtar and Hotchin, 2001; Izumi et al., 2004). Therefore, we investigated the possibility that in Rac1 V12 salivary gland
7 C. Pirraglia et al. / Developmental Biology 290 (2006) Fig. 5. Modulation of cadherin function rescues the Rac1 mutant salivary gland phenotypes. Graphical representation of embryos scored for salivary gland phenotypes at stage 14 (A). Loss of one copy of the shg gene ameliorates the Rac1 L89 salivary gland phenotype (A, fkh-gal4; UAS-Rac1 L89 ; shg 2 /+) whereas expression of wild-type full-length EcadGFP partially rescues the Rac1 V12 phenotype (A, fkh-gal4; UAS-Rac1 V12 -UAS-EcadGFP). Wild-type (WT) embryos (B) and WT embryos expressing EcadGFP (C) form normal glands (arrows). Concomitant expression of the Rac1 V12 mutation and EcadGFP (D, arrow) prevents the loss of dcreb-a expression and loss of gland integrity induced by expression of the Rac1 V12 mutation alone (E, arrow). Embryos in panels B through E were stained for dcreb-a. cells changes in E-cadherin endocytosis may lead to the loss of E-cadherin from the apical lateral membrane and subsequent dispersal and/or death of salivary gland cells. We first tested whether inhibition of endocytosis affected salivary gland morphogenesis in a similar manner to loss of Rac function. Different types of endocytosis have been shown to be regulated by the GTPase dynamin (Conner and Schmid, 2003), whose Drosophila homolog is encoded by the shibire (shi) gene (Chen et al., 1991; Obar et al., 1990). Therefore, we analyzed salivary gland development in embryos carrying a temperature-sensitive mutation of shibire, shi ts2. At the permissive temperature (25 C), we observed normal salivary gland development; however, when the embryos were shifted to the non-permissive temperature (30 C) for 15 min and then allowed to recover at 25 C for an hour before being processed for immunocytochemistry, we observed embryos in which the salivary gland either failed to turn posteriorly or did not complete its posterior migration (Fig. 6B and data not shown). To determine whether the salivary gland migration defect observed in shi ts2 mutants was due to alteration of adherens junction proteins, we stained shi ts2 mutant embryos for β-catenin. To keep the experimental conditions and imaging parameters identical, we compared β-catenin expression of the same pool of shi ts2 embryos. We observed embryos with varying levels of β-catenin staining; some embryos had a level and pattern of β-catenin expression identical to that of wild-type salivary glands where β-catenin was concentrated in puncta at the apical lateral membrane (Fig. 6C). In contrast, in other embryos, the level of β-catenin staining was dramatically elevated in the salivary gland where it was found as a continuous band throughout the apical domain (Fig. 6D), similar to the pattern observed in Rac loss of function mutants and wild-type embryos expressing dominant-negative Rac1 mutations in the salivary gland (Fig. 4 and data not shown). Thus, compromised endocytosis leads to an increase in β- catenin levels which may account for the defects in salivary gland migration. We hypothesized that if loss of E-cadherin in Rac1 V12 salivary gland cells is due to increased endocytosis of β-catenin/ E-cadherin, then decreasing endocytosis of E-cadherin in Rac1 V12 mutant salivary gland cells may ameliorate the Rac1 V12 salivary gland phenotype. To disrupt dynaminmediated endocytosis, we expressed a dominant-negative form of shi, shi K44A, specifically in salivary gland cells of wild-type embryos and Rac1 V12 mutant embryos. In a few of the wild-type embryos expressing shi K44A in salivary glands of wild-type embryos, the gland did not complete its posterior migration and resembled Rac mutants (Fig. 6E). Coexpression of Rac1 V12 and shi K44A partially restored dcreb-a expression and gland integrity; however, the glands appeared narrower with fewer cells compared to their wild-type counterparts (Fig. 6F).
8 442 C. Pirraglia et al. / Developmental Biology 290 (2006) We tested for a genetic interaction between shibire and the Rac genes during salivary gland morphogenesis. In transheterozygous embryos of shi ts2 and Rac1 J11 Rac2 Δ Mtl Δ at the permissive temperature, all embryos formed normal salivary glands (Fig. 6G). However, in shi ts2 and Rac1 J11 Rac2 Δ Mtl Δ trans-heterozygous embryos at the non-permissive temperature, posterior migration of the salivary gland was disrupted (Fig. 6H), indicating that shi and Rac genes interact genetically to regulate salivary gland migration. We confirmed that one copy of shi ts2 at the non-permissive temperature did not cause aberrant migration of the salivary gland (Fig. 6I). We also confirmed that Rac1 J11 Rac2 Δ Mtl Δ homozygous embryos at the non-permissive temperature showed the salivary gland migration defect (Fig. 6J) and that heterozygous embryos formed normal glands (data not shown). In conclusion, loss of dynamin function resulted in increased β- catenin at the membrane and interrupted salivary gland migration. Furthermore, inhibition of dynamin function was sufficient to partially restore dcreb-a expression and gland integrity in Rac1 V12 mutant embryos and dynamin interacts genetically with Rac during salivary gland morphogenesis. Thus, Rac-regulated dynamin-mediated endocytosis is critical for salivary gland formation. Rac activation leads to cell death Fig. 6. Regulated endocytosis is required for salivary gland migration. In shi ts2 embryos at the permissive temperature (A), salivary gland development is normal (A, arrow) whereas in shi ts2 embryos at the non-permissive temperature (B), salivary glands fail to migrate posteriorly (B, arrow). In shi ts2 embryos at the non-permissive temperature (C and D), some embryos have a normal pattern of β-catenin expression (C, arrows) whereas others have elevated levels of β- catenin expression in the apical lateral membrane (D, arrow). In embryos that express shi K44A in just the salivary gland of otherwise WT embryos, the salivary gland fails to migrate properly (E, arrow). Coexpression of the shi K44A and Rac1 V12 mutations partially restores dcreb-a expression (F, arrow), but the glands are smaller and appear to have less cells (F, arrow). In embryos heterozygous for shi ts2 and Rac1 J11 Rac2 Δ Mtl Δ, normal glands form at the permissive temperature (G, arrow) whereas glands fail to migrate at the nonpermissive temperature (H, arrow). Embryos carrying one copy of shi ts2 at the non-permissive temperature form normal glands (I, arrow). In Rac1 J11 Rac2 Δ Mtl homozygous embryos at the non-permissive temperature, salivary glands fail to migrate (J, arrow). All embryos were stained for dcreb-a except those in panels C and D which were stained for β-catenin. In Rac1 V12 mutant embryos at late stages of embryogenesis, we observed salivary gland cells dispersed throughout the head region (Fig. 3L). This observation raises the possibility that Rac1 activation induced cell migration. Alternatively, the perceived migrating cells could be apoptotic bodies of salivary gland cells being carried by migrating macrophages. To determine if either or both of these possibilities were true, we stained Rac1 V12 mutant embryos for PH4αSG1 and Croquemort (CRQ), a macrophage marker protein (Franc et al., 1999). In wild-type embryos, PH4αSG1-staining in the salivary gland was distinct from that of the nearby CRQ-positive macrophages (Figs. 7A, A and A ). However, in Rac1 V12 embryos, the residual PH4αSG1-staining colocalized with CRQ-positive macrophages. Indeed, some macrophages showed punctate PH4αSG1 staining which is likely to be ingested apoptotic salivary gland cells (Figs. 7B, B and B ). We were able to detect few PH4αSG1-stained salivary gland cells that did not stain for CRQ. These data suggest that, in Rac1 V12 mutant embryos at late stages, many scattered salivary gland cells die by apoptosis and are engulfed by macrophages. However, a subpopulation of salivary gland cells survives and continues to express salivary gland specific markers such as, PH4αSG1. Thus, activation of Rac1 leads both to cell migration and cell death. The cell death observed in Rac1 V12 embryos raised the possibility that the dispersal of Rac1 V12 salivary gland cells is solely due to cell death and not to interference of cadherinmediated cell adhesion. To determine whether the Rac1 V12 salivary gland phenotype was due to apoptosis or to loss of cell adhesion, we attempted to block apoptosis by coexpressing Rac1 V12 and the antiapoptotic p35 gene from baculovirus (Grether et al., 1995) in salivary glands of wild-type embryos. Expression of p35 in wild-type salivary gland cells did not affect gland morphogenesis (Figs. 7C, C and C ). In contrast, the glands of Rac1 V12 p35 embryos were abnormally shaped and appeared to consist of a disorganized cluster of cells. Moreover, we did not detect a lumen in the glands of Rac1 V12 p35 embryos (data not shown). Although expression of PH4αSG1 was markedly reduced in the Rac1 V12 p35 embryos compared to that in wild-type embryos expressing p35 alone (Figs. 7D, D and D ), it did not overlap with CRQ staining. Thus, inhibition of apoptosis by p35 was able to keep all salivary gland cells from engulfment by macrophages. Although more PH4αSG1-expressing salivary gland cells survive in Rac1 V12 p35 embryos due to inhibition of apoptosis, the abnormal development of the gland
9 C. Pirraglia et al. / Developmental Biology 290 (2006) Fig. 7. Rac1 V12 salivary gland cells die by apoptosis. In wild-type salivary glands (A and A, arrows), and in salivary gland cells expressing p35 (C and C, arrows), PH4αSG1 is found in salivary gland cells and is distinct from surrounding macrophages labeled with CRQ (A and C, arrowheads). In Rac1 V12 mutant salivary glands (B and B ), faint PH4αSG1 staining (B arrow) and bright and punctate PH4αSG1 staining (B, arrowheads) colocalize with CRQ-stained macrophages (B, arrowheads). A PH4αSG1-stained salivary gland cell (B and B, small arrow) that does not stain for CRQ. In embryos coexpressing Rac1 V12 and p35 in the salivary glands (D, D and D ), faint PH4αSG1 staining is detected in the abnormally shaped glands (D and D, arrows) and is distinct from CRQ-staining in the surrounding macrophages (D and D, arrowheads). Salivary glands are detected with PH4αSG1 (green) and macrophages with CRQ (red). All embryos shown are at stage 16. indicates that Rac activation interferes with proper gland morphogenesis. Discussion In this study, we show that the Rac GTPases regulate salivary gland morphogenesis through modulation of cadherin/cateninbased cell cell adhesion, likely by dynamin-mediated endocytosis. Our characterization of the Rac mutant phenotypes suggests a model where Rac normally regulates cadherinmediated cell cell adhesion in salivary gland cells to allow enough plasticity for its invagination and migration yet keep the cells of the tube adhered to one another so that the gland can migrate as a cohesive tube. One mechanism by which cell surface cadherin levels are regulated is through selective endocytosis of E-cadherin from the apical lateral membrane in a dynamin-mediated process (Akhtar and Hotchin, 2001; Paterson et al., 2003). When Rac function is compromised through loss-of-function mutations or expression of dominantnegative mutations, the balance between E-cadherin at the plasma membrane and internalized E-cadherin appears to be abrogated so that more E-cadherin remains at the plasma membrane resulting in increased cell cell adhesion and causing the gland to sever. Our studies reveal the importance of precise regulation of adherens junction remodeling during cell migration in the context of a developing organ. In all stage 14 Rac1 L89 mutant embryos examined, the salivary gland broke apart close to its approximate mid-point. Reduction in cadherin levels rescues the mutant Rac severing phenotype, suggesting that severing occurs because loss of
10 444 C. Pirraglia et al. / Developmental Biology 290 (2006) Rac leads to an increase in cadherin-mediated cell cell adhesion. We envision two possible explanations for the midpoint severing phenotype. In the first scenario, levels of cadherin remodeling may differ throughout the gland such that in Rac1 L89 embryos the cells in the distal tip are least affected and cells in the mid-region of the gland are most affected by the increase in cadherin function. In this situation, when the distal cells begin to migrate posteriorly, the increased adhesivity of the mid-region cells prevents their migration and causes the gland to sever in the middle. In the second scenario, movement of the mid-region and the distal region of the gland may occur through different mechanisms. It is possible that while the distal most cells migrate by undergoing cell shape change and extending prominent protrusions in the direction of migration, as was previously observed (Bradley et al., 2003), cells in the middle of the gland may follow the distal cells by rearranging their positions along the gland, such as occurs during the convergence extension movements observed in epithelial morphogenesis (Keller, 2002). Dynamic remodeling of E- cadherin may be particularly important for proper rearrangement of the mid-region cells and an inability to rearrange when E-cadherin adhesion is increased may cause severing of the gland and subsequent separation of the migrating distal portion from the rest of the gland. Alternatively, it is possible that both of these scenarios are at play during normal salivary gland migration. We currently cannot distinguish between these possibilities. In the developing tracheal tubes, Rac1 was recently shown to be required for cell rearrangements; in tracheal cells expressing a dominant-negative Rac1 mutation, the dorsal branch was shorter than that of wild-type embryos (Chihara et al., 2003). Therefore, it will be important to determine whether cell rearrangement plays a role during salivary gland migration and to further elucidate the role of the Rac genes in this process. When Rac1 function is over-activate, dynamin-mediated endocytosis of E-cadherin may be increased, resulting in decreased cadherin at the plasma membrane, and decreased cell cell adhesion. The loss of adhesion leads to the dispersal of salivary gland cells and ultimately cell death. Preventing Rac1 V12 -induced cell death led to the formation of abnormally shaped glands demonstrating that the Rac1 V12 salivary gland phenotype is primarily due to abrogation of gland morphogenesis and not to activation of the apoptotic pathway. Moreover, since wild-type full-length E-cadherin was sufficient to rescue the Rac1 V12 salivary gland phenotype, loss of cadherin function appears to be the primary cause for salivary gland defects. Thus, the Rac genes function in salivary gland cells to regulate E-cadherin-mediated cell cell adhesion during tube morphogenesis. Endocytosis allows temporal regulation of cell cell adhesion During salivary gland morphogenesis, gland integrity is kept intact while cells perform extensive cell shape changes and movements. Rac-regulated endocytosis of E-cadherin is one mechanism by which cell cell adhesion is likely to be downregulated temporarily. After E-cadherin is endocytosed, it can be recycled back to the cell surface, sequestered transiently inside the cell or routed to late endosomes and lysosomes for degradation (Bryant and Stow, 2004). Once salivary gland migration is complete and the gland has reached its final position, cell cell adhesion may then need to be strengthened again in the mature gland and Rac activity may be downregulated to promote increase in surface cadherins. In addition to endocytosis, studies in mammalian cultured cells have shown that Rac can regulate levels of cell surface E- cadherin by other mechanisms, such as cleavage by presinilins and metalloproteinases (Egeblad and Werb, 2002; Marambaud et al., 2002), or tyrosine phosphorylation of the cadherin adhesion complex in a process involving reactive oxygen species (van Wetering et al., 2002). Thus, it will be interesting to determine whether additional mechanisms of E-cadherin regulation exist in salivary gland cells during gland morphogenesis. Numerous studies in cell culture have demonstrated that recycling of E-cadherin occurs in both a clathrin-dependent and caveolin-dependent manner (Akhtar and Hotchin, 2001; Ivanov et al., 2004; Izumi et al., 2004; Le et al., 1999; Paterson et al., 2003). Since dynamin mediates both clathrin- and caveolindependent endocytosis, our studies do not allow us to distinguish which type is involved in cadherin endocytosis during salivary gland migration. Alternatively, both types of endocytosis may mediate Rac1 regulation of E-cadherin in salivary gland cells. Rac activation and cell death Expression of the Rac1 V12 mutation in salivary gland cells leads to loss of expression of salivary gland specific proteins, apical basal polarity proteins and E-cadherin/β-catenin. Concomitant with changes in gene expression, Rac1 V12 mutant salivary gland cells lose adhesion to each other and subsequently migrate away or die by apoptosis. Our data suggest that overactivation of Rac1 primarily affects E- cadherin/β-catenin-mediated adhesion and salivary gland cell fate and that the observed cell death is a secondary consequence of these earlier changes. When cell death was prevented in Rac1 V12 embryos by expressing p35, more cells expressed the salivary gland specific protein PH4αSG1 than in Rac1 V12 embryos; however, the expression level was drastically reduced compared to wild-type, suggesting that even in the Rac1 V12 p35 cells, cell differentiation was still mostly altered. Moreover, Rac1 V12 p35 salivary gland cells did not form a normal gland, demonstrating a role for Rac1 in gland morphogenesis. It is possible that apoptosis of Rac1 V12 cells is brought about by the loss or reduction of Fkh function. Fkh is expressed early in the salivary gland placode and its expression is maintained throughout embryogenesis (Myat and Andrew, 2000a). In the absence of fkh function, salivary gland cells die by apoptosis during the invagination stage. Since expression of dcreb-a and PH4αSG1 was reduced in Rac1 V12 mutant salivary gland cells, it is possible that Fkh expression was also similarly reduced, thereby, causing the cells to undergo apoptotic cell death.
11 C. Pirraglia et al. / Developmental Biology 290 (2006) Rac activation and cancer Many human cancers are due to epithelial-derived tumors. When epithelial cells metastasize, they first undergo an epithelial to mesenchymal transition (EMT) before migrating away from the primary tumor to invade surrounding tissues. EMT is characterized by the loss of epithelial polarity and cell cell adhesion (Thiery, 2003). When Rac1 V12 was expressed in salivary gland cells, expression of apical membrane proteins, Crumbs and apkc and adherens junction proteins E-cadherin and β-catenin, was either lost or mislocalized. Based on these criteria, activation of Rac1 function induced features characteristic of early changes in EMT and metastasis. Interestingly, the expression levels of Rho GTPases are found to be elevated in a number of human cancers. For example, increased Rac protein levels and fast-cycling Rac mutations have been correlated with colorectal and breast tumors (Fritz et al., 2002). Expression of constitutively active Rac1 caused some salivary gland cells to lose polarity and adhesion to neighboring cells and migrate away in a manner similar to EMT. Our findings suggest that Rac1-regulated endocytosis of E-cadherin in the Drosophila salivary glands may be critical in maintaining epithelial character and preventing the loss of cell cell adhesion and cell polarity. The Drosophila salivary gland might thus be powerful as a simple system to identify and characterize mechanisms that regulate cadherin-based cell cell adhesion and certain aspects of EMT. Acknowledgments We thank the Bloomington Stock Center, the Developmental Biology Hybridoma Bank and our colleagues, Deborah Andrew, Natalie Franc, Hiroki Oda and Herman Steller for fly lines and antisera. We are also grateful to our colleagues, Deborah Andrew, Pamela Bradley, Elizabeth Grevengoed, Markus Schober, Melissa Vining and members of our lab for advice, critical discussions and review of the manuscript. This work was partly supported by a K22 Faculty Transition Grant from the NIDCR to M.M.M. References Abrams, E.W., Andrew, D.J., Prolyl-4-hydroxylase alpha-related proteins in Drosophila melanogaster: tissue-specific embryonic expression of the 99F8-9 cluster. Mech. Dev. 112, Akhtar, N., Hotchin, N.A., Rac1 regulates adherens junctions through endocytosis and E-cadherin. Mol. Biol. Cell 12, Akhtar, N., Hudson, K.R., Hotchin, N.A., Co-localization of Rac1 and E- cadherin in human epidermal keatinocytes. Cell Adhes. Commun. 7, Andrew, D.J., Baig, A., Bhanot, P., Smolik, S.M., Henderson, K.D., The Drosophila dcreb-a gene is required for dorsal/ventral patterning of the larval cuticle. Development 124, Bradley, P.L., Myat, M.M., Comeaux, C.A., Andrew, D.J., Posterior migration of the salivary gland requires an intact visceral mesoderm and integrin function. Dev. Biol. 257, Braga, V.M., Machesky, L.M., Hall, A., Hotchin, N.A., The small GTPase Rho and Rac are required for the establishment of cadherin-dependent cell cell contacts. J. Cell Biol. 137, Braga, V.M., Del Maschio, A., Machesky, L., Dejana, E., Regulation of cadherin function by Rho and Rac: modulation by junction maturation and cellular context. Mol. Biol. Cell 10, Brand, A.H., Perrimon, N., Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development 118, Briggs, M.W., Sacks, D.B., IQGAP proteins are integral components of cytoskeletal regulation. EMBO J. 4, Bryant, D.M., Stow, J.L., The ins and outs of E-cadherin trafficking. Trends Cell Biol. 14, Chen, M.S., Obar, R.A., Schroeder, C.C., Austin, T.W., Poodry, C.A., Wadsworth, S.C., Vallee, R.B., Multiple forms of dynamin are encoded by shibire, a Drosophila gene involved in endocytosis. Nature 351, Chihara, T., Kato, K., Taniguchi, M., NG, J., Hayashi, S., Rac promotes epithelial cell rearrangement during tracheal tubulogenesis in Drosophila. Development 130, Conner, S.D., Schmid, S.L., Regulated portals of entry into the cell. Nature 422, Eaton, S., Auvinen, P., Luo, L., Jan, Y.N., Simons, K., CDC42 and Rac1 control different actin-dependent processes in the Drosophila wing disc epithelium. J. Cell Biol. 131, Eaton, S., Wepf, R., Simons, K., Roles for Rac1 and Cdc42 in planar polarization and hair outgrowth in the wing of Drosophila. J. Cell Biol Egeblad, M., Werb, Z., New functions for the matrix metalloproteinases in cancer progression. Nat. Rev., Cancer 2. Etienne-Manneville, S., Hall, A., Rho GTPases in cell biology. Nature 420, Fanto, M., Weber, U., Strutt, D.I., Mlodzik, M., Nuclear signaling by Rac and Rho GTPases is required in the establishment of epithelial planar polarity in the Drosophila eye. Curr. Biol. 10, Franc, N.C., Heitzler, P., Ezekowitz, R.A., White, K., Requirement for croquemort in phagocytosis of apoptotic cells in Drosophila. Science 284, Fritz, G., Brachetti, C., Bahlmann, F., Schmidt, M., Kaina, B., Rho GTPases in human breast tumours: expression and mutation analyses and correlation with clinical parameters. Br. J. Cancer 87, Grether, M.E., Abrams, J.M., Agapite, J., White, K., Steller, H., The head involution defective gene of Drosophila melanogaster functions in programmed cell death. Genes Dev. 9, Hakeda-Suzuki, S., Ng, J., Tzu, J., Dietzl, G., Sun, Y., Harms, M., Nardine, T., Luo, L., Dickson, B.J., Rac function and regulation during Drosophila development. Nature 416, Harden, N., Loh, H.Y., Chia, W., Lim, L., A dominant inhibitory version of the small GTP-binding protein Rac disrupts cytoskeletal structures and inhibits developmental cell shape changes in Drosophila. Development 121, Harden, N., Ricos, M., Yee, K., Sanny, J., Langmann, C., Yu, H., Chia, W., Lim, L., Drac1 and Crumbs participate in amnioserosa morphogenesis during dorsal closure in Drosophila. J. Cell Sci. 115, Hariharan, I.K., Hu, K.-Q., Asha, H., Quintanilla, A., Ezzell, R.M., Settleman, J., Characterization of rho GTPase family homologues in Drosophila melanogaster: overexpressing Rho1 in retinal cells causes a late developmental defect. EMBO J. 14, Henderson, K.D., Andrew, D.J., Regulation and function of Scr, exd, and hth in the Drosophila salivary gland. Dev. Biol. 217, Hogan, B.L., Kolodziej, P.A., Organogenesis: molecular mechanisms of tubulogenesis. Nat. Rev., Genet. 3, Ivanov, A.I., Nusrat, A., Parkos, C.A., Endocytosis of epithelial apical junction proteins by a clathrin-mediated pathway into a unique storage compartment. Mol. Biol. Cell 15, Izumi, G., Sakisaka, T., Baba, T., Tanaka, S., Morimoto, K., Takai, Y., Endocytosis of E-cadherin regulated by Rac and Cdc42 small G proteins through IQGAP1 and actin filaments. J. Cell Biol. 166, Kaufmann, N., Wills, Z.P., Van Vector, D., Drosophila Rac1 controls motor axon guidance. Development 125,
Processes of Tubulogenesis. Processes of Tubulogenesis. Tube Formation is critical to forming: There are three types of tubes:
 Biology 624 - Developmental Genetics Tubular Organs Lecture #8 - Tube Formation I Tube Formation is critical to forming: 1. Lung* 2. Kidney* 3. Mammary gland 4. Blood vessels* 5. Fly trachea 6. C. elegans
Biology 624 - Developmental Genetics Tubular Organs Lecture #8 - Tube Formation I Tube Formation is critical to forming: 1. Lung* 2. Kidney* 3. Mammary gland 4. Blood vessels* 5. Fly trachea 6. C. elegans
Supplementary Figure S1: TIPF reporter validation in the wing disc.
 Supplementary Figure S1: TIPF reporter validation in the wing disc. a,b, Test of put RNAi. a, In wildtype discs the Dpp target gene Sal (red) is expressed in a broad stripe in the centre of the ventral
Supplementary Figure S1: TIPF reporter validation in the wing disc. a,b, Test of put RNAi. a, In wildtype discs the Dpp target gene Sal (red) is expressed in a broad stripe in the centre of the ventral
Supporting Online Material for
 www.sciencemag.org/cgi/content/full/1171320/dc1 Supporting Online Material for A Frazzled/DCC-Dependent Transcriptional Switch Regulates Midline Axon Guidance Long Yang, David S. Garbe, Greg J. Bashaw*
www.sciencemag.org/cgi/content/full/1171320/dc1 Supporting Online Material for A Frazzled/DCC-Dependent Transcriptional Switch Regulates Midline Axon Guidance Long Yang, David S. Garbe, Greg J. Bashaw*
A Genetic Program for Embryonic Development
 Concept 18.4: A program of differential gene expression leads to the different cell types in a multicellular organism During embryonic development, a fertilized egg gives rise to many different cell types
Concept 18.4: A program of differential gene expression leads to the different cell types in a multicellular organism During embryonic development, a fertilized egg gives rise to many different cell types
Vesicle Transport. Vesicle pathway: many compartments, interconnected by trafficking routes 3/17/14
 Vesicle Transport Vesicle Formation Curvature (Self Assembly of Coat complex) Sorting (Sorting Complex formation) Regulation (Sar1/Arf1 GTPases) Fission () Membrane Fusion SNARE combinations Tethers Regulation
Vesicle Transport Vesicle Formation Curvature (Self Assembly of Coat complex) Sorting (Sorting Complex formation) Regulation (Sar1/Arf1 GTPases) Fission () Membrane Fusion SNARE combinations Tethers Regulation
Appendix: Subcellular localization of MIG-14::GFP in C. elegans body wall muscle cells. Pei-Tzu Yang and Hendrik C. Korswagen
 Appendix: Subcellular localization of MIG-14::GFP in C. elegans body wall muscle cells Pei-Tzu Yang and Hendrik C. Korswagen 129 SUBCELLULAR LOCALIZATION OF MIG-14 Abstract MIG-14/Wls is a Wnt binding
Appendix: Subcellular localization of MIG-14::GFP in C. elegans body wall muscle cells Pei-Tzu Yang and Hendrik C. Korswagen 129 SUBCELLULAR LOCALIZATION OF MIG-14 Abstract MIG-14/Wls is a Wnt binding
04_polarity. The formation of synaptic vesicles
 Brefeldin prevents assembly of the coats required for budding Nocodazole disrupts microtubules Constitutive: coatomer-coated Selected: clathrin-coated The formation of synaptic vesicles Nerve cells (and
Brefeldin prevents assembly of the coats required for budding Nocodazole disrupts microtubules Constitutive: coatomer-coated Selected: clathrin-coated The formation of synaptic vesicles Nerve cells (and
Supplemental Information. Otic Mesenchyme Cells Regulate. Spiral Ganglion Axon Fasciculation. through a Pou3f4/EphA4 Signaling Pathway
 Neuron, Volume 73 Supplemental Information Otic Mesenchyme Cells Regulate Spiral Ganglion Axon Fasciculation through a Pou3f4/EphA4 Signaling Pathway Thomas M. Coate, Steven Raft, Xiumei Zhao, Aimee K.
Neuron, Volume 73 Supplemental Information Otic Mesenchyme Cells Regulate Spiral Ganglion Axon Fasciculation through a Pou3f4/EphA4 Signaling Pathway Thomas M. Coate, Steven Raft, Xiumei Zhao, Aimee K.
Problem Set 5 KEY
 2006 7.012 Problem Set 5 KEY ** Due before 5 PM on THURSDAY, November 9, 2006. ** Turn answers in to the box outside of 68-120. PLEASE WRITE YOUR ANSWERS ON THIS PRINTOUT. 1. You are studying the development
2006 7.012 Problem Set 5 KEY ** Due before 5 PM on THURSDAY, November 9, 2006. ** Turn answers in to the box outside of 68-120. PLEASE WRITE YOUR ANSWERS ON THIS PRINTOUT. 1. You are studying the development
Problem Set 8 Key 1 of 8
 7.06 2003 Problem Set 8 Key 1 of 8 7.06 2003 Problem Set 8 Key 1. As a bright MD/PhD, you are interested in questions about the control of cell number in the body. Recently, you've seen three patients
7.06 2003 Problem Set 8 Key 1 of 8 7.06 2003 Problem Set 8 Key 1. As a bright MD/PhD, you are interested in questions about the control of cell number in the body. Recently, you've seen three patients
MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question.
 Exam Name MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1) All of the following are synthesized along various sites of the endoplasmic reticulum
Exam Name MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1) All of the following are synthesized along various sites of the endoplasmic reticulum
CHAPTER 6 SUMMARIZING DISCUSSION
 CHAPTER 6 SUMMARIZING DISCUSSION More than 20 years ago the founding member of the Wnt gene family, Wnt-1/Int1, was discovered as a proto-oncogene activated in mammary gland tumors by the mouse mammary
CHAPTER 6 SUMMARIZING DISCUSSION More than 20 years ago the founding member of the Wnt gene family, Wnt-1/Int1, was discovered as a proto-oncogene activated in mammary gland tumors by the mouse mammary
In vitro scratch assay: method for analysis of cell migration in vitro labeled fluorodeoxyglucose (FDG)
 In vitro scratch assay: method for analysis of cell migration in vitro labeled fluorodeoxyglucose (FDG) 1 Dr Saeb Aliwaini 13/11/2015 Migration in vivo Primary tumors are responsible for only about 10%
In vitro scratch assay: method for analysis of cell migration in vitro labeled fluorodeoxyglucose (FDG) 1 Dr Saeb Aliwaini 13/11/2015 Migration in vivo Primary tumors are responsible for only about 10%
Src-INACTIVE / Src-INACTIVE
 Biology 169 -- Exam 1 February 2003 Answer each question, noting carefully the instructions for each. Repeat- Read the instructions for each question before answering!!! Be as specific as possible in each
Biology 169 -- Exam 1 February 2003 Answer each question, noting carefully the instructions for each. Repeat- Read the instructions for each question before answering!!! Be as specific as possible in each
BIOL2005 WORKSHEET 2008
 BIOL2005 WORKSHEET 2008 Answer all 6 questions in the space provided using additional sheets where necessary. Hand your completed answers in to the Biology office by 3 p.m. Friday 8th February. 1. Your
BIOL2005 WORKSHEET 2008 Answer all 6 questions in the space provided using additional sheets where necessary. Hand your completed answers in to the Biology office by 3 p.m. Friday 8th February. 1. Your
CHAPTER VII CONCLUDING REMARKS AND FUTURE DIRECTION. Androgen deprivation therapy is the most used treatment of de novo or recurrent
 CHAPTER VII CONCLUDING REMARKS AND FUTURE DIRECTION Stathmin in Prostate Cancer Development and Progression Androgen deprivation therapy is the most used treatment of de novo or recurrent metastatic PCa.
CHAPTER VII CONCLUDING REMARKS AND FUTURE DIRECTION Stathmin in Prostate Cancer Development and Progression Androgen deprivation therapy is the most used treatment of de novo or recurrent metastatic PCa.
Cellular compartments
 Cellular compartments 1. Cellular compartments and their function 2. Evolution of cellular compartments 3. How to make a 3D model of cellular compartment 4. Cell organelles in the fluorescent microscope
Cellular compartments 1. Cellular compartments and their function 2. Evolution of cellular compartments 3. How to make a 3D model of cellular compartment 4. Cell organelles in the fluorescent microscope
Department of Cell and Developmental Biology, Vanderbilt University, Nashville, TN
 UNC-6/Netrin mediates dendritic self-avoidance Cody J. Smith 1, Joseph D. Watson 1,4,5, Miri K. VanHoven 2, Daniel A. Colón-Ramos 3 and David M. Miller III 1,4,6 1 Department of Cell and Developmental
UNC-6/Netrin mediates dendritic self-avoidance Cody J. Smith 1, Joseph D. Watson 1,4,5, Miri K. VanHoven 2, Daniel A. Colón-Ramos 3 and David M. Miller III 1,4,6 1 Department of Cell and Developmental
7.06 Cell Biology EXAM #3 April 24, 2003
 7.06 Spring 2003 Exam 3 Name 1 of 8 7.06 Cell Biology EXAM #3 April 24, 2003 This is an open book exam, and you are allowed access to books and notes. Please write your answers to the questions in the
7.06 Spring 2003 Exam 3 Name 1 of 8 7.06 Cell Biology EXAM #3 April 24, 2003 This is an open book exam, and you are allowed access to books and notes. Please write your answers to the questions in the
Early Embryonic Development
 Early Embryonic Development Maternal effect gene products set the stage by controlling the expression of the first embryonic genes. 1. Transcription factors 2. Receptors 3. Regulatory proteins Maternal
Early Embryonic Development Maternal effect gene products set the stage by controlling the expression of the first embryonic genes. 1. Transcription factors 2. Receptors 3. Regulatory proteins Maternal
Medical Biology. Dr. Khalida Ibrahim
 Dr. Khalida Ibrahim Medical Biology MUSCLE TISSUE 1. Muscle tissue is characterized by its well-developed properties of contraction. 2. Muscle is responsible for the movements of the body and the various
Dr. Khalida Ibrahim Medical Biology MUSCLE TISSUE 1. Muscle tissue is characterized by its well-developed properties of contraction. 2. Muscle is responsible for the movements of the body and the various
Inner ear development Nervous system development
 Upcoming Sessions April 22: Nervous System Development Lecture April 24: Reviews of Axonal Pathfinding in Sensory Systems April 29: Inner Ear Development Lecture May 1: May 6: May 8: Auditory System Pathfinding
Upcoming Sessions April 22: Nervous System Development Lecture April 24: Reviews of Axonal Pathfinding in Sensory Systems April 29: Inner Ear Development Lecture May 1: May 6: May 8: Auditory System Pathfinding
Supplementary Figure 1: Signaling centers contain few proliferating cells, express p21, and
 Supplementary Figure 1: Signaling centers contain few proliferating cells, express p21, and exclude YAP from the nucleus. (a) Schematic diagram of an E10.5 mouse embryo. (b,c) Sections at B and C in (a)
Supplementary Figure 1: Signaling centers contain few proliferating cells, express p21, and exclude YAP from the nucleus. (a) Schematic diagram of an E10.5 mouse embryo. (b,c) Sections at B and C in (a)
Linkage Mapping in Drosophila Melanogaster
 Linkage Mapping in Drosophila Melanogaster Genetics: Fall 2012 Joshua Hanau Introduction: An experiment was performed in order to determine the presence and degree of gene linkage in Drosophila Melanogaster.
Linkage Mapping in Drosophila Melanogaster Genetics: Fall 2012 Joshua Hanau Introduction: An experiment was performed in order to determine the presence and degree of gene linkage in Drosophila Melanogaster.
Supplementary Figure 1 Madm is not required in GSCs and hub cells. (a,b) Act-Gal4-UAS-GFP (a), Act-Gal4-UAS- GFP.nls (b,c) is ubiquitously expressed
 Supplementary Figure 1 Madm is not required in GSCs and hub cells. (a,b) Act-Gal4-UAS-GFP (a), Act-Gal4-UAS- GFP.nls (b,c) is ubiquitously expressed in the testes. The testes were immunostained with GFP
Supplementary Figure 1 Madm is not required in GSCs and hub cells. (a,b) Act-Gal4-UAS-GFP (a), Act-Gal4-UAS- GFP.nls (b,c) is ubiquitously expressed in the testes. The testes were immunostained with GFP
MCB Topic 19 Regulation of Actin Assembly- Prof. David Rivier
 MCB 252 -Topic 19 Regulation of Actin Assembly- Prof. David Rivier MCB 252 Spring 2017 MCB 252 Cell Biology Topic 19 Regulation of Actin Assembly Reading: Lodish 17.2-17.3, 17.7 MCB 252 Actin Cytoskeleton
MCB 252 -Topic 19 Regulation of Actin Assembly- Prof. David Rivier MCB 252 Spring 2017 MCB 252 Cell Biology Topic 19 Regulation of Actin Assembly Reading: Lodish 17.2-17.3, 17.7 MCB 252 Actin Cytoskeleton
Development: is the growth of an individual organism from a simple to a more complex or mature level. A slow process of progressive change
 1. Define the following terms (use your own words): development, growth, differentiation, histogenesis, organogenesis, morphogenesis, reproduction, tissue, organ, organ system, and organism. Development:
1. Define the following terms (use your own words): development, growth, differentiation, histogenesis, organogenesis, morphogenesis, reproduction, tissue, organ, organ system, and organism. Development:
Dr. Heba Kalbouneh. Dr. Heba Kalbouneh. Dr. Heba Kalbouneh
 Dr. Heba Kalbouneh Dr. Heba Kalbouneh Dr. Heba Kalbouneh Tissue: is a group of cells that serve the same function, they are surrounded by extra cellular matrix. The 4 basic types of tissue: 1. epithelial
Dr. Heba Kalbouneh Dr. Heba Kalbouneh Dr. Heba Kalbouneh Tissue: is a group of cells that serve the same function, they are surrounded by extra cellular matrix. The 4 basic types of tissue: 1. epithelial
Determination of the temporal pattern and importance of BALF1 expression in Epstein-Barr viral infection
 Determination of the temporal pattern and importance of BALF1 expression in Epstein-Barr viral infection Melissa Mihelidakis May 6, 2004 7.340 Research Proposal Introduction Apoptosis, or programmed cell
Determination of the temporal pattern and importance of BALF1 expression in Epstein-Barr viral infection Melissa Mihelidakis May 6, 2004 7.340 Research Proposal Introduction Apoptosis, or programmed cell
Homework Hanson section MCB Course, Fall 2014
 Homework Hanson section MCB Course, Fall 2014 (1) Antitrypsin, which inhibits certain proteases, is normally secreted into the bloodstream by liver cells. Antitrypsin is absent from the bloodstream of
Homework Hanson section MCB Course, Fall 2014 (1) Antitrypsin, which inhibits certain proteases, is normally secreted into the bloodstream by liver cells. Antitrypsin is absent from the bloodstream of
Transgenic Expression of the Helicobacter pylori Virulence Factor CagA Promotes Apoptosis or Tumorigenesis through JNK Activation in Drosophila
 Transgenic Expression of the Helicobacter pylori Virulence Factor CagA Promotes Apoptosis or Tumorigenesis through JNK Activation in Drosophila Anica M. Wandler, Karen Guillemin* Institute of Molecular
Transgenic Expression of the Helicobacter pylori Virulence Factor CagA Promotes Apoptosis or Tumorigenesis through JNK Activation in Drosophila Anica M. Wandler, Karen Guillemin* Institute of Molecular
Lysosomes and endocytic pathways 9/27/2012 Phyllis Hanson
 Lysosomes and endocytic pathways 9/27/2012 Phyllis Hanson General principles Properties of lysosomes Delivery of enzymes to lysosomes Endocytic uptake clathrin, others Endocytic pathways recycling vs.
Lysosomes and endocytic pathways 9/27/2012 Phyllis Hanson General principles Properties of lysosomes Delivery of enzymes to lysosomes Endocytic uptake clathrin, others Endocytic pathways recycling vs.
Epithelial Tissue. Functions include: 1. Protection 4. Absorption 2. Secretion 5. Filtration 3. Sensory reception
 Tissues There are 4 primary tissue types in the human body: 1. Epithelial (covering/lining) 2. Connective (support) 3. Muscle (movement) 4. Nervous (control) Epithelium Epithelial Tissue Covers the surface
Tissues There are 4 primary tissue types in the human body: 1. Epithelial (covering/lining) 2. Connective (support) 3. Muscle (movement) 4. Nervous (control) Epithelium Epithelial Tissue Covers the surface
Cell Polarity and Cancer
 Cell Polarity and Cancer Pr Jean-Paul Borg Email: jean-paul.borg@inserm.fr Features of malignant cells Steps in Malignant Progression Cell polarity, cell adhesion, morphogenesis and tumorigenesis pathways
Cell Polarity and Cancer Pr Jean-Paul Borg Email: jean-paul.borg@inserm.fr Features of malignant cells Steps in Malignant Progression Cell polarity, cell adhesion, morphogenesis and tumorigenesis pathways
Vertebrate Limb Patterning
 Vertebrate Limb Patterning What makes limb patterning an interesting/useful developmental system How limbs develop Key events in limb development positioning and specification initiation of outgrowth establishment
Vertebrate Limb Patterning What makes limb patterning an interesting/useful developmental system How limbs develop Key events in limb development positioning and specification initiation of outgrowth establishment
HOMEWORK RUBRICS MECHANOTRANSDUCTION UNIT: HOMEWORK #1 (20 pts towards your grade)
 HOMEWORK RUBRICS MECHANOTRANSDUCTION UNIT: HOMEWORK #1 (20 pts towards your grade) 1. Mesenchymal stem cells (MSC) cultured on extracellular matrices with different stiffness exhibit diverse lineage commitment
HOMEWORK RUBRICS MECHANOTRANSDUCTION UNIT: HOMEWORK #1 (20 pts towards your grade) 1. Mesenchymal stem cells (MSC) cultured on extracellular matrices with different stiffness exhibit diverse lineage commitment
Overview: Conducting the Genetic Orchestra Prokaryotes and eukaryotes alter gene expression in response to their changing environment
 Overview: Conducting the Genetic Orchestra Prokaryotes and eukaryotes alter gene expression in response to their changing environment In multicellular eukaryotes, gene expression regulates development
Overview: Conducting the Genetic Orchestra Prokaryotes and eukaryotes alter gene expression in response to their changing environment In multicellular eukaryotes, gene expression regulates development
Chapter 13: Vesicular Traffic
 Chapter 13: Vesicular Traffic Know the terminology: ER, Golgi, vesicle, clathrin, COP-I, COP-II, BiP, glycosylation, KDEL, microtubule, SNAREs, dynamin, mannose-6-phosphate, M6P receptor, endocytosis,
Chapter 13: Vesicular Traffic Know the terminology: ER, Golgi, vesicle, clathrin, COP-I, COP-II, BiP, glycosylation, KDEL, microtubule, SNAREs, dynamin, mannose-6-phosphate, M6P receptor, endocytosis,
Signaling Vascular Morphogenesis and Maintenance
 Signaling Vascular Morphogenesis and Maintenance Douglas Hanahan Science 277: 48-50, in Perspectives (1997) Blood vessels are constructed by two processes: vasculogenesis, whereby a primitive vascular
Signaling Vascular Morphogenesis and Maintenance Douglas Hanahan Science 277: 48-50, in Perspectives (1997) Blood vessels are constructed by two processes: vasculogenesis, whereby a primitive vascular
Head of College Scholars List Scheme. Summer Studentship Report Form
 Head of College Scholars List Scheme Summer Studentship 2019 Report Form This report should be completed by the student with his/her project supervisor. It should summarise the work undertaken during the
Head of College Scholars List Scheme Summer Studentship 2019 Report Form This report should be completed by the student with his/her project supervisor. It should summarise the work undertaken during the
MBios 401/501: Lecture 12.1 Signaling IV. Slide 1
 MBios 401/501: Lecture 12.1 Signaling IV Slide 1 Pathways that require regulated proteolysis 1. Notch and Delta 2. Wnt/ b-catenin 3. Hedgehog 4. NFk-B Our last topic on cell signaling are pathways that
MBios 401/501: Lecture 12.1 Signaling IV Slide 1 Pathways that require regulated proteolysis 1. Notch and Delta 2. Wnt/ b-catenin 3. Hedgehog 4. NFk-B Our last topic on cell signaling are pathways that
marker. DAPI labels nuclei. Flies were 20 days old. Scale bar is 5 µm. Ctrl is
 Supplementary Figure 1. (a) Nos is detected in glial cells in both control and GFAP R79H transgenic flies (arrows), but not in deletion mutant Nos Δ15 animals. Repo is a glial cell marker. DAPI labels
Supplementary Figure 1. (a) Nos is detected in glial cells in both control and GFAP R79H transgenic flies (arrows), but not in deletion mutant Nos Δ15 animals. Repo is a glial cell marker. DAPI labels
Rearrangements of the Actin Cytoskeleton and E-Cadherin Based Adherens Junctions Caused by Neoplasic Transformation Change Cell Cell Interactions
 Rearrangements of the Actin Cytoskeleton and E-Cadherin Based Adherens Junctions Caused by Neoplasic Transformation Change Cell Cell Interactions Dmitry V. Ayollo., Irina Y. Zhitnyak., Jury M. Vasiliev,
Rearrangements of the Actin Cytoskeleton and E-Cadherin Based Adherens Junctions Caused by Neoplasic Transformation Change Cell Cell Interactions Dmitry V. Ayollo., Irina Y. Zhitnyak., Jury M. Vasiliev,
Biochemistry of Carcinogenesis. Lecture # 35 Alexander N. Koval
 Biochemistry of Carcinogenesis Lecture # 35 Alexander N. Koval What is Cancer? The term "cancer" refers to a group of diseases in which cells grow and spread unrestrained throughout the body. It is difficult
Biochemistry of Carcinogenesis Lecture # 35 Alexander N. Koval What is Cancer? The term "cancer" refers to a group of diseases in which cells grow and spread unrestrained throughout the body. It is difficult
Lecture 13 - Intermediate filaments
 02.12.10 Lecture 13 - Intermediate filaments Intermediate filaments Present in nearly all animals, but absent from plants and fungi Rope-like network of filaments in the cell Principle function is maintenance
02.12.10 Lecture 13 - Intermediate filaments Intermediate filaments Present in nearly all animals, but absent from plants and fungi Rope-like network of filaments in the cell Principle function is maintenance
Intracellular Vesicular Traffic Chapter 13, Alberts et al.
 Intracellular Vesicular Traffic Chapter 13, Alberts et al. The endocytic and biosynthetic-secretory pathways The intracellular compartments of the eucaryotic ell involved in the biosynthetic-secretory
Intracellular Vesicular Traffic Chapter 13, Alberts et al. The endocytic and biosynthetic-secretory pathways The intracellular compartments of the eucaryotic ell involved in the biosynthetic-secretory
Serafino et al. Thymosin α1 activates complement receptor-mediated phagocytosis in human monocyte-derived macrophages. SUPPLEMENTARY FIGURES
 Supplementary Fig. S1. Evaluation of the purity and maturation of macrophage cultures tested by flow cytometry. The lymphocytic/monocytic cellular fraction was isolated from buffy coats of healthy donors
Supplementary Fig. S1. Evaluation of the purity and maturation of macrophage cultures tested by flow cytometry. The lymphocytic/monocytic cellular fraction was isolated from buffy coats of healthy donors
General Biology. Overview: The Key Roles of Cell Division The continuity of life is based upon the reproduction of cells, or cell division
 General Biology Course No: BNG2003" Credits: 3.00 " " " 8. The Cell Cycle Prof. Dr. Klaus Heese Overview: The Key Roles of Cell Division The continuity of life is based upon the reproduction of cells,
General Biology Course No: BNG2003" Credits: 3.00 " " " 8. The Cell Cycle Prof. Dr. Klaus Heese Overview: The Key Roles of Cell Division The continuity of life is based upon the reproduction of cells,
The Alimentary Canal of the Aphid Prociphilus Tesselata Fitch
 The Ohio State University Knowledge Bank kb.osu.edu Ohio Journal of Science (Ohio Academy of Science) Ohio Journal of Science: Volume 38, Issue 3 (May, 1938) 1938-05 The Alimentary Canal of the Aphid Prociphilus
The Ohio State University Knowledge Bank kb.osu.edu Ohio Journal of Science (Ohio Academy of Science) Ohio Journal of Science: Volume 38, Issue 3 (May, 1938) 1938-05 The Alimentary Canal of the Aphid Prociphilus
Ahtiainen et al., http :// /cgi /content /full /jcb /DC1
 Supplemental material JCB Ahtiainen et al., http ://www.jcb.org /cgi /content /full /jcb.201512074 /DC1 THE JOURNAL OF CELL BIOLOGY Figure S1. Distinct distribution of different cell cycle phases in the
Supplemental material JCB Ahtiainen et al., http ://www.jcb.org /cgi /content /full /jcb.201512074 /DC1 THE JOURNAL OF CELL BIOLOGY Figure S1. Distinct distribution of different cell cycle phases in the
VIII Curso Internacional del PIRRECV. Some molecular mechanisms of cancer
 VIII Curso Internacional del PIRRECV Some molecular mechanisms of cancer Laboratorio de Comunicaciones Celulares, Centro FONDAP Estudios Moleculares de la Celula (CEMC), ICBM, Facultad de Medicina, Universidad
VIII Curso Internacional del PIRRECV Some molecular mechanisms of cancer Laboratorio de Comunicaciones Celulares, Centro FONDAP Estudios Moleculares de la Celula (CEMC), ICBM, Facultad de Medicina, Universidad
Biology Developmental Biology Spring Quarter Midterm 1 Version A
 Biology 411 - Developmental Biology Spring Quarter 2013 Midterm 1 Version A 75 Total Points Open Book Choose 15 out the 20 questions to answer (5 pts each). Only the first 15 questions that are answered
Biology 411 - Developmental Biology Spring Quarter 2013 Midterm 1 Version A 75 Total Points Open Book Choose 15 out the 20 questions to answer (5 pts each). Only the first 15 questions that are answered
Endocytosis and Intracellular Trafficking of Notch and Its Ligands
 CHA P T E R F IVE Endocytosis and Intracellular Trafficking of Notch and Its Ligands Shinya Yamamoto, *,1 Wu-Lin Charng, *,1 and Hugo J. Bellen *,,, Contents 1. Notch Signaling and its Regulation by Endocytosis
CHA P T E R F IVE Endocytosis and Intracellular Trafficking of Notch and Its Ligands Shinya Yamamoto, *,1 Wu-Lin Charng, *,1 and Hugo J. Bellen *,,, Contents 1. Notch Signaling and its Regulation by Endocytosis
Supplementary Figure 1. Electroporation of a stable form of β-catenin causes masses protruding into the IV ventricle. HH12 chicken embryos were
 Supplementary Figure 1. Electroporation of a stable form of β-catenin causes masses protruding into the IV ventricle. HH12 chicken embryos were electroporated with β- Catenin S33Y in PiggyBac expression
Supplementary Figure 1. Electroporation of a stable form of β-catenin causes masses protruding into the IV ventricle. HH12 chicken embryos were electroporated with β- Catenin S33Y in PiggyBac expression
Supplemental Figure 1. Quantification of proliferation in thyroid of WT, Ctns -/- and grafted
 Supplemental Figure 1. Quantification of proliferation in thyroid of WT, Ctns -/- and grafted Ctns -/- mice. Cells immunolabeled for the proliferation marker (Ki-67) were counted in sections (n=3 WT, n=4
Supplemental Figure 1. Quantification of proliferation in thyroid of WT, Ctns -/- and grafted Ctns -/- mice. Cells immunolabeled for the proliferation marker (Ki-67) were counted in sections (n=3 WT, n=4
Supplementary Figures
 J. Cell Sci. 128: doi:10.1242/jcs.173807: Supplementary Material Supplementary Figures Fig. S1 Fig. S1. Description and/or validation of reagents used. All panels show Drosophila tissues oriented with
J. Cell Sci. 128: doi:10.1242/jcs.173807: Supplementary Material Supplementary Figures Fig. S1 Fig. S1. Description and/or validation of reagents used. All panels show Drosophila tissues oriented with
10/13/11. Cell Theory. Cell Structure
 Cell Structure Grade 12 Biology Cell Theory All organisms are composed of one or more cells. Cells are the smallest living units of all living organisms. Cells arise only by division of a previously existing
Cell Structure Grade 12 Biology Cell Theory All organisms are composed of one or more cells. Cells are the smallest living units of all living organisms. Cells arise only by division of a previously existing
Supplementary Figure 1. Rab27a-KD inhibits speed and persistence of HEp3 cells migrating in the chick CAM. (a) Western blot analysis of Rab27a
 Supplementary Figure 1. Rab27a-KD inhibits speed and persistence of HEp3 cells migrating in the chick CAM. (a) Western blot analysis of Rab27a expression in GFP-expressing HEp3 cells. (b) Representative
Supplementary Figure 1. Rab27a-KD inhibits speed and persistence of HEp3 cells migrating in the chick CAM. (a) Western blot analysis of Rab27a expression in GFP-expressing HEp3 cells. (b) Representative
Investigating the role of EphAl ephrin-a signalling during trigeminal ganglion axon guidance
 Investigating the role of EphAl ephrin-a signalling during trigeminal ganglion axon guidance A thesis submitted for the degree of Doctor of Philosophy Molecular and Biomedical Science (Discipline of Genetics),
Investigating the role of EphAl ephrin-a signalling during trigeminal ganglion axon guidance A thesis submitted for the degree of Doctor of Philosophy Molecular and Biomedical Science (Discipline of Genetics),
SUPPLEMENTARY FIGURE LEGENDS
 SUPPLEMENTARY FIGURE LEGENDS Supplemental FIG. 1. Localization of myosin Vb in cultured neurons varies with maturation stage. A and B, localization of myosin Vb in cultured hippocampal neurons. A, in DIV
SUPPLEMENTARY FIGURE LEGENDS Supplemental FIG. 1. Localization of myosin Vb in cultured neurons varies with maturation stage. A and B, localization of myosin Vb in cultured hippocampal neurons. A, in DIV
Cancer Biology Course. Invasion and Metastasis
 Cancer Biology Course Invasion and Metastasis 2016 Lu-Hai Wang NHRI Cancer metastasis Major problem: main reason for killing cancer patients, without it cancer can be cured or controlled. Challenging questions:
Cancer Biology Course Invasion and Metastasis 2016 Lu-Hai Wang NHRI Cancer metastasis Major problem: main reason for killing cancer patients, without it cancer can be cured or controlled. Challenging questions:
General Biology. Overview: The Key Roles of Cell Division. Unicellular organisms
 General Biology Course No: BNG2003 Credits: 3.00 8. The Cell Cycle Prof. Dr. Klaus Heese Overview: The Key Roles of Cell Division The continuity of life is based upon the reproduction of cells, or cell
General Biology Course No: BNG2003 Credits: 3.00 8. The Cell Cycle Prof. Dr. Klaus Heese Overview: The Key Roles of Cell Division The continuity of life is based upon the reproduction of cells, or cell
Supplementary Information
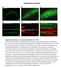 Supplementary Information Supplementary Figure 1: Luminal localization of CCM-3. (a) The CCM-3::GFP fusion protein localizes along the apical (luminal) surface of the pharynx (b) as well as the lumen of
Supplementary Information Supplementary Figure 1: Luminal localization of CCM-3. (a) The CCM-3::GFP fusion protein localizes along the apical (luminal) surface of the pharynx (b) as well as the lumen of
SOS1 and Ras regulate epithelial tight junction formation in the human airway through EMP1
 Manuscript EMBOR-2014-39218 SOS1 and Ras regulate epithelial tight junction formation in the human airway through EMP1 Joanne Durgan, Guangbo Tao, Matthew S. Walters, Oliver Florey, Anja Schmidt, Vanessa
Manuscript EMBOR-2014-39218 SOS1 and Ras regulate epithelial tight junction formation in the human airway through EMP1 Joanne Durgan, Guangbo Tao, Matthew S. Walters, Oliver Florey, Anja Schmidt, Vanessa
Breaking Up is Hard to Do (At Least in Eukaryotes) Mitosis
 Breaking Up is Hard to Do (At Least in Eukaryotes) Mitosis Chromosomes Chromosomes were first observed by the German embryologist Walther Fleming in 1882. Chromosome number varies among organisms most
Breaking Up is Hard to Do (At Least in Eukaryotes) Mitosis Chromosomes Chromosomes were first observed by the German embryologist Walther Fleming in 1882. Chromosome number varies among organisms most
Molecular Cell Biology - Problem Drill 20: Cytoskeleton and Cellular Mobility
 Molecular Cell Biology - Problem Drill 20: Cytoskeleton and Cellular Mobility Question No. 1 of 10 1. Which of the following statements about cytoskeletal filaments is correct? Question #1 (A) The Cytoskeleton
Molecular Cell Biology - Problem Drill 20: Cytoskeleton and Cellular Mobility Question No. 1 of 10 1. Which of the following statements about cytoskeletal filaments is correct? Question #1 (A) The Cytoskeleton
7.012 Quiz 3 Answers
 MIT Biology Department 7.012: Introductory Biology - Fall 2004 Instructors: Professor Eric Lander, Professor Robert A. Weinberg, Dr. Claudette Gardel Friday 11/12/04 7.012 Quiz 3 Answers A > 85 B 72-84
MIT Biology Department 7.012: Introductory Biology - Fall 2004 Instructors: Professor Eric Lander, Professor Robert A. Weinberg, Dr. Claudette Gardel Friday 11/12/04 7.012 Quiz 3 Answers A > 85 B 72-84
Molecular biology :- Cancer genetics lecture 11
 Molecular biology :- Cancer genetics lecture 11 -We have talked about 2 group of genes that is involved in cellular transformation : proto-oncogenes and tumour suppressor genes, and it isn t enough to
Molecular biology :- Cancer genetics lecture 11 -We have talked about 2 group of genes that is involved in cellular transformation : proto-oncogenes and tumour suppressor genes, and it isn t enough to
SUPPLEMENTARY INFORMATION
 Supplementary Figure 1. Ras V12 expression in the entire eye-antennal disc does not cause invasive tumours. a, Eye-antennal discs expressing Ras V12 in all cells (marked with GFP, green) overgrow moderately
Supplementary Figure 1. Ras V12 expression in the entire eye-antennal disc does not cause invasive tumours. a, Eye-antennal discs expressing Ras V12 in all cells (marked with GFP, green) overgrow moderately
Cell Birth and Death. Chapter Three
 Cell Birth and Death Chapter Three Neurogenesis All neurons and glial cells begin in the neural tube Differentiated into neurons rather than ectoderm based on factors we have already discussed If these
Cell Birth and Death Chapter Three Neurogenesis All neurons and glial cells begin in the neural tube Differentiated into neurons rather than ectoderm based on factors we have already discussed If these
Module 3 Lecture 7 Endocytosis and Exocytosis
 Module 3 Lecture 7 Endocytosis and Exocytosis Endocytosis: Endocytosis is the process by which cells absorb larger molecules and particles from the surrounding by engulfing them. It is used by most of
Module 3 Lecture 7 Endocytosis and Exocytosis Endocytosis: Endocytosis is the process by which cells absorb larger molecules and particles from the surrounding by engulfing them. It is used by most of
Fig. S1. RT-PCR analyses of the expression and distribution of Xdscr6 transcripts during early development.
 Fig. S1. RT-PCR analyses of the expression and distribution of Xdscr6 transcripts during early development. (A) Temporal expression of Xdscr6 at various stages (numbers on the top) and its distribution
Fig. S1. RT-PCR analyses of the expression and distribution of Xdscr6 transcripts during early development. (A) Temporal expression of Xdscr6 at various stages (numbers on the top) and its distribution
Animal Tissue Culture SQG 3242 Biology of Cultured Cells. Dr. Siti Pauliena Mohd Bohari
 Animal Tissue Culture SQG 3242 Biology of Cultured Cells Dr. Siti Pauliena Mohd Bohari The Culture Environment Changes of Cell s microenvironment needed that favor the spreading, migration, and proliferation
Animal Tissue Culture SQG 3242 Biology of Cultured Cells Dr. Siti Pauliena Mohd Bohari The Culture Environment Changes of Cell s microenvironment needed that favor the spreading, migration, and proliferation
klp-18 (RNAi) Control. supplementary information. starting strain: AV335 [emb-27(g48); GFP::histone; GFP::tubulin] bleach
![klp-18 (RNAi) Control. supplementary information. starting strain: AV335 [emb-27(g48); GFP::histone; GFP::tubulin] bleach klp-18 (RNAi) Control. supplementary information. starting strain: AV335 [emb-27(g48); GFP::histone; GFP::tubulin] bleach](/thumbs/91/104639484.jpg) DOI: 10.1038/ncb1891 A. starting strain: AV335 [emb-27(g48); GFP::histone; GFP::tubulin] bleach embryos let hatch overnight transfer to RNAi plates; incubate 5 days at 15 C RNAi food L1 worms adult worms
DOI: 10.1038/ncb1891 A. starting strain: AV335 [emb-27(g48); GFP::histone; GFP::tubulin] bleach embryos let hatch overnight transfer to RNAi plates; incubate 5 days at 15 C RNAi food L1 worms adult worms
Expression of acid base transporters in the kidney collecting duct in Slc2a7 -/-
 Supplemental Material Results. Expression of acid base transporters in the kidney collecting duct in Slc2a7 -/- and Slc2a7 -/- mice. The expression of AE1 in the kidney was examined in Slc26a7 KO mice.
Supplemental Material Results. Expression of acid base transporters in the kidney collecting duct in Slc2a7 -/- and Slc2a7 -/- mice. The expression of AE1 in the kidney was examined in Slc26a7 KO mice.
SUPPLEMENTARY INFORMATION
 DOI: 10.1038/ncb2607 Figure S1 Elf5 loss promotes EMT in mammary epithelium while Elf5 overexpression inhibits TGFβ induced EMT. (a, c) Different confocal slices through the Z stack image. (b, d) 3D rendering
DOI: 10.1038/ncb2607 Figure S1 Elf5 loss promotes EMT in mammary epithelium while Elf5 overexpression inhibits TGFβ induced EMT. (a, c) Different confocal slices through the Z stack image. (b, d) 3D rendering
Tumor microenvironment Interactions and Lung Cancer Invasiveness. Pulmonary Grand Rounds Philippe Montgrain, M.D.
 Tumor microenvironment Interactions and Lung Cancer Invasiveness Pulmonary Grand Rounds Philippe Montgrain, M.D. February 26, 2009 Objectives Review epithelial mesenchymal transition (EMT), and its implications
Tumor microenvironment Interactions and Lung Cancer Invasiveness Pulmonary Grand Rounds Philippe Montgrain, M.D. February 26, 2009 Objectives Review epithelial mesenchymal transition (EMT), and its implications
Developmental Biology
 Developmental Biology 362 (2012) 141 153 Contents lists available at SciVerse ScienceDirect Developmental Biology journal homepage: www.elsevier.com/developmentalbiology Dual function of suppressor of
Developmental Biology 362 (2012) 141 153 Contents lists available at SciVerse ScienceDirect Developmental Biology journal homepage: www.elsevier.com/developmentalbiology Dual function of suppressor of
Chapter 11 Intercellular Communication and Tissue Architecture
 Part III Organization of Cell Populations Chapter 11 Multicellular organisms such as the human body consist of various tissues such as epithelial tissues, bones and nerves, and organs such as heart and
Part III Organization of Cell Populations Chapter 11 Multicellular organisms such as the human body consist of various tissues such as epithelial tissues, bones and nerves, and organs such as heart and
C) The graph should look exactly like the graph on the left (Mut1 cells + Mating Pheromone for 3 hours at 25 degrees). The cells arrest in G1.
 706-2000-Exam 4 Answer Key 1) The question asks you to explain peaks A and B in the top graph. The other two graphs were there to give you hints. The question did not ask for these other two graphs to
706-2000-Exam 4 Answer Key 1) The question asks you to explain peaks A and B in the top graph. The other two graphs were there to give you hints. The question did not ask for these other two graphs to
Urogenital Development
 2-5-03 Urogenital Development Greg Dressler Assoc. Professor Dept. of Pathology x46490 Dressler@umich.edu The Origin of the Kidney In the vertebrate embryo, the first stage of kidney development occurs
2-5-03 Urogenital Development Greg Dressler Assoc. Professor Dept. of Pathology x46490 Dressler@umich.edu The Origin of the Kidney In the vertebrate embryo, the first stage of kidney development occurs
Cells and reagents. Synaptopodin knockdown (1) and dynamin knockdown (2)
 Supplemental Methods Cells and reagents. Synaptopodin knockdown (1) and dynamin knockdown (2) podocytes were cultured as described previously. Staurosporine, angiotensin II and actinomycin D were all obtained
Supplemental Methods Cells and reagents. Synaptopodin knockdown (1) and dynamin knockdown (2) podocytes were cultured as described previously. Staurosporine, angiotensin II and actinomycin D were all obtained
Cell Cell Communication
 IBS 8102 Cell, Molecular, and Developmental Biology Cell Cell Communication January 29, 2008 Communicate What? Why do cells communicate? To govern or modify each other for the benefit of the organism differentiate
IBS 8102 Cell, Molecular, and Developmental Biology Cell Cell Communication January 29, 2008 Communicate What? Why do cells communicate? To govern or modify each other for the benefit of the organism differentiate
Biology is the only subject in which multiplication is the same thing as division
 Biology is the only subject in which multiplication is the same thing as division 2007-2008 The Cell Cycle: Cell Growth, Cell Division 2007-2008 Where it all began You started as a cell smaller than a
Biology is the only subject in which multiplication is the same thing as division 2007-2008 The Cell Cycle: Cell Growth, Cell Division 2007-2008 Where it all began You started as a cell smaller than a
Supplementary Figure 1: Hsp60 / IEC mice are embryonically lethal (A) Light microscopic pictures show mouse embryos at developmental stage E12.
 Supplementary Figure 1: Hsp60 / IEC mice are embryonically lethal (A) Light microscopic pictures show mouse embryos at developmental stage E12.5 and E13.5 prepared from uteri of dams and subsequently genotyped.
Supplementary Figure 1: Hsp60 / IEC mice are embryonically lethal (A) Light microscopic pictures show mouse embryos at developmental stage E12.5 and E13.5 prepared from uteri of dams and subsequently genotyped.
Wnt signaling. Ramray Bhat.
 Wnt signaling Ramray Bhat ramray@mrdg.iisc.ernet.in Starting with animal biology and viral infections The discovery of certain laboratory murine strains that were highly susceptible to mammary gland cancer.
Wnt signaling Ramray Bhat ramray@mrdg.iisc.ernet.in Starting with animal biology and viral infections The discovery of certain laboratory murine strains that were highly susceptible to mammary gland cancer.
Breaking Up is Hard to Do (At Least in Eukaryotes) Mitosis
 Breaking Up is Hard to Do (At Least in Eukaryotes) Mitosis Prokaryotes Have a Simpler Cell Cycle Cell division in prokaryotes takes place in two stages, which together make up a simple cell cycle 1. Copy
Breaking Up is Hard to Do (At Least in Eukaryotes) Mitosis Prokaryotes Have a Simpler Cell Cycle Cell division in prokaryotes takes place in two stages, which together make up a simple cell cycle 1. Copy
SUPPLEMENTARY INFORMATION
 DOI: 10.1038/ncb2988 Supplementary Figure 1 Kif7 L130P encodes a stable protein that does not localize to cilia tips. (a) Immunoblot with KIF7 antibody in cell lysates of wild-type, Kif7 L130P and Kif7
DOI: 10.1038/ncb2988 Supplementary Figure 1 Kif7 L130P encodes a stable protein that does not localize to cilia tips. (a) Immunoblot with KIF7 antibody in cell lysates of wild-type, Kif7 L130P and Kif7
Cooperative and independent functions of FGF and Wnt signaling during early inner ear development
 Wright et al. BMC Developmental Biology (2015) 15:33 DOI 10.1186/s12861-015-0083-8 RESEARCH ARTICLE Open Access Cooperative and independent functions of FGF and Wnt signaling during early inner ear development
Wright et al. BMC Developmental Biology (2015) 15:33 DOI 10.1186/s12861-015-0083-8 RESEARCH ARTICLE Open Access Cooperative and independent functions of FGF and Wnt signaling during early inner ear development
Cell Cell Communication
 IBS 8102 Cell, Molecular, and Developmental Biology Cell Cell Communication January 29, 2008 Communicate What? Why do cells communicate? To govern or modify each other for the benefit of the organism differentiate
IBS 8102 Cell, Molecular, and Developmental Biology Cell Cell Communication January 29, 2008 Communicate What? Why do cells communicate? To govern or modify each other for the benefit of the organism differentiate
THE ROLE OF ALTERED CALCIUM AND mtor SIGNALING IN THE PATHOGENESIS OF CYSTINOSIS
 Research Foundation, 18 month progress report THE ROLE OF ALTERED CALCIUM AND mtor SIGNALING IN THE PATHOGENESIS OF CYSTINOSIS Ekaterina Ivanova, doctoral student Elena Levtchenko, MD, PhD, PI Antonella
Research Foundation, 18 month progress report THE ROLE OF ALTERED CALCIUM AND mtor SIGNALING IN THE PATHOGENESIS OF CYSTINOSIS Ekaterina Ivanova, doctoral student Elena Levtchenko, MD, PhD, PI Antonella
Karyotype analysis reveals transloction of chromosome 22 to 9 in CML chronic myelogenous leukemia has fusion protein Bcr-Abl
 Chapt. 18 Cancer Molecular Biology of Cancer Student Learning Outcomes: Describe cancer diseases in which cells no longer respond Describe how cancers come from genomic mutations (inherited or somatic)
Chapt. 18 Cancer Molecular Biology of Cancer Student Learning Outcomes: Describe cancer diseases in which cells no longer respond Describe how cancers come from genomic mutations (inherited or somatic)
SUPPLEMENTARY/SECOND OPPORTUNITY EXAMINATION QUESTION PAPER
 I nnmibin UNIVERSITY OF SCIENCE nno TECHNOLOGY FACULTY OF HEALTH AND APPLIED SCIENCES DEPARTMENT OF NATURAL AND APPLIED SCIENCES QUALIFICATION: BACHELOR OF SCIENCE QUALIFICATION CODE: O7BOSC LEVEL: 6 COURSE
I nnmibin UNIVERSITY OF SCIENCE nno TECHNOLOGY FACULTY OF HEALTH AND APPLIED SCIENCES DEPARTMENT OF NATURAL AND APPLIED SCIENCES QUALIFICATION: BACHELOR OF SCIENCE QUALIFICATION CODE: O7BOSC LEVEL: 6 COURSE
Nature Neuroscience: doi: /nn Supplementary Figure 1. Neuron class-specific arrangements of Khc::nod::lacZ label in dendrites.
 Supplementary Figure 1 Neuron class-specific arrangements of Khc::nod::lacZ label in dendrites. Staining with fluorescence antibodies to detect GFP (Green), β-galactosidase (magenta/white). (a, b) Class
Supplementary Figure 1 Neuron class-specific arrangements of Khc::nod::lacZ label in dendrites. Staining with fluorescence antibodies to detect GFP (Green), β-galactosidase (magenta/white). (a, b) Class
Soft Agar Assay. For each cell pool, 100,000 cells were resuspended in 0.35% (w/v)
 SUPPLEMENTARY MATERIAL AND METHODS Soft Agar Assay. For each cell pool, 100,000 cells were resuspended in 0.35% (w/v) top agar (LONZA, SeaKem LE Agarose cat.5004) and plated onto 0.5% (w/v) basal agar.
SUPPLEMENTARY MATERIAL AND METHODS Soft Agar Assay. For each cell pool, 100,000 cells were resuspended in 0.35% (w/v) top agar (LONZA, SeaKem LE Agarose cat.5004) and plated onto 0.5% (w/v) basal agar.
Campbell Biology in Focus (Urry) Chapter 9 The Cell Cycle. 9.1 Multiple-Choice Questions
 Campbell Biology in Focus (Urry) Chapter 9 The Cell Cycle 9.1 Multiple-Choice Questions 1) Starting with a fertilized egg (zygote), a series of five cell divisions would produce an early embryo with how
Campbell Biology in Focus (Urry) Chapter 9 The Cell Cycle 9.1 Multiple-Choice Questions 1) Starting with a fertilized egg (zygote), a series of five cell divisions would produce an early embryo with how
Biology is the only subject in which multiplication is the same thing as division
 Biology is the only subject in which multiplication is the same thing as division The Cell Cycle: Cell Growth, Cell Division 2007-2008 2007-2008 Getting from there to here Going from egg to baby. the original
Biology is the only subject in which multiplication is the same thing as division The Cell Cycle: Cell Growth, Cell Division 2007-2008 2007-2008 Getting from there to here Going from egg to baby. the original
Histology = the study of tissues. Tissue = a complex of cells that have a common function
 { EPITHELIAL TISSUE Histology = the study of tissues Tissue = a complex of cells that have a common function The Four Primary Tissue Types: Epithelium (epithelial tissue) covers body surfaces, lines body
{ EPITHELIAL TISSUE Histology = the study of tissues Tissue = a complex of cells that have a common function The Four Primary Tissue Types: Epithelium (epithelial tissue) covers body surfaces, lines body
TITLE: Effect of MUC1 Expression on EGFR Endocytosis and Degradation in Human Breast Cancer Cell Lines
 AD AWARD NUMBER: W81XWH-06-1-0464 TITLE: Effect of MUC1 Expression on EGFR Endocytosis and Degradation in Human Breast Cancer Cell Lines PRINCIPAL INVESTIGATOR: Rachid M El Bejjani CONTRACTING ORGANIZATION:
AD AWARD NUMBER: W81XWH-06-1-0464 TITLE: Effect of MUC1 Expression on EGFR Endocytosis and Degradation in Human Breast Cancer Cell Lines PRINCIPAL INVESTIGATOR: Rachid M El Bejjani CONTRACTING ORGANIZATION:
endomembrane system internal membranes origins transport of proteins chapter 15 endomembrane system
 endo system chapter 15 internal s endo system functions as a coordinated unit divide cytoplasm into distinct compartments controls exocytosis and endocytosis movement of molecules which cannot pass through
endo system chapter 15 internal s endo system functions as a coordinated unit divide cytoplasm into distinct compartments controls exocytosis and endocytosis movement of molecules which cannot pass through
