Transforming Growth Factor b/activin Signaling Functions as a Sugar-Sensing Feedback Loop to Regulate Digestive Enzyme Expression
|
|
|
- Esmond Owens
- 6 years ago
- Views:
Transcription
1 Article Transforming Growth Factor b/activin Signaling Functions as a Sugar-Sensing Feedback Loop to Regulate Digestive Enzyme Expression Graphical Abstract Authors Wen-bin Alfred Chng, Maroun S. Bou Sleiman, Fanny Schüpfer, Bruno Lemaitre Correspondence alfred.chng@epfl.ch (W.A.C.), bruno.lemaitre@epfl.ch (B.L.) Highlights Glucose represses midgut-expressed carbohydrases and lipases In Brief Organisms modulate their digestive processes to reflect their nutritional state. In this study, Chng et al. demonstrate that the TGF-b/Activin pathway functions as a carbohydrate-sensing mechanism in the adult Drosophila midgut to regulate digestive enzyme expression. They show that the TGFb ligand, Dawdle, and the canonical TGF-b/ Activin signaling are essential to couple carbohydrate sensing with digestive enzyme expression. Thus, their study highlights an unexpected function of TGF-b/ Activin signaling that is beyond their established roles in development and immunity. Accession Numbers GSE54755 Nutritional value of the sugars and the nutritional state of host affects repression TGF-b ligand, Dawdle (Daw), derived from the fat body mediates glucose repression Activation of the TGF-b/Activin signaling in the midgut underlies glucose repression Chng et al., 2014, Cell Reports 9, October 9, 2014 ª2014 The Authors
2 Cell Reports Article Transforming Growth Factor b/activin Signaling Functions as a Sugar-Sensing Feedback Loop to Regulate Digestive Enzyme Expression Wen-bin Alfred Chng, 1, * Maroun S. Bou Sleiman, 1 Fanny Schüpfer, 1 and Bruno Lemaitre 1, * 1 Global Health Institute, School of Life Sciences, EPFL, Station 19, 1015 Lausanne, Switzerland *Correspondence: alfred.chng@epfl.ch (W.A.C.), bruno.lemaitre@epfl.ch (B.L.) This is an open access article under the CC BY-NC-ND license ( SUMMARY Organisms need to assess their nutritional state and adapt their digestive capacity to the demands for various nutrients. Modulation of digestive enzyme production represents a rational step to regulate nutriment uptake. However, the role of digestion in nutrient homeostasis has been largely neglected. In this study, we analyzed the mechanism underlying glucose repression of digestive enzymes in the adult Drosophila midgut. We demonstrate that glucose represses the expression of many carbohydrases and lipases. Our data reveal that the consumption of nutritious sugars stimulates the secretion of the transforming growth factor b (TGF-b) ligand, Dawdle, from the fat body. Dawdle then acts via circulation to activate TGF-b/Activin signaling in the midgut, culminating in the repression of digestive enzymes that are highly expressed during starvation. Thus, our study not only identifies a mechanism that couples sugar sensing with digestive enzyme expression but points to an important role of TGF-b/Activin signaling in sugar metabolism. INTRODUCTION Digestion and absorption are two principal functions of the digestive tract. To efficiently extract nutrients from the ingested food, a repertoire of digestive enzymes is expressed to breakdown the macronutrients in the diet into a form that can be readily absorbed. Given that dietary intake in the environment is inherently variable, organisms need to readily assess their nutritional state and adapt their digestive capacity to the demands for various nutrients. To date, most metabolic studies are focused on the postabsorption events for nutrient homeostasis (Grönke et al., 2007; Havula et al., 2013; Quesada et al., 2008; Saltiel and Kahn, 2001), and our understanding of digestion and its regulation by nutrient-sensing pathways remains limited. In Drosophila, the gut is traditionally divided into three distinct regions: the foregut, the midgut, and the hindgut (Hakim et al., 2010), with digestion and absorption of food being accomplished predominantly in the midgut (Lehane et al., 1995; Terra and Ferreira, 1994). For many dietary components, hydrolysis by endogenous or microbial-derived digestive enzymes precedes absorption through the gut lumen. On the principle of economy, release of digestive enzymes often reflects the quantity of nutrients, with more enzymes generally released in fed than in unfed insects (Karasov et al., 2011). However, several studies in Drosophila have reported that the activity of amylase, an enzyme required for the hydrolysis of glucosidic linkages in polysaccharides, is repressed in flies fed with a high-sugar diet. This phenomenon whereby glucose reduces amylase expression was termed glucose repression (Benkel and Hickey, 1986; Hickey and Benkel, 1982; Figure 1A). Whereas the effect of dietary sugar on amylase activity has long been recognized, the mechanistic underpinnings of such regulation and its effects on other digestive enzymes remain to be established. In this study, we have analyzed the mechanism underlying glucose repression in the adult Drosophila midgut and show that glucose repression affects many other carbohydrases and lipases. We also show that Dawdle (Daw), a transforming growth factor b (TGF-b) ligand, is induced in the fat body by nutritious carbohydrates in the diet and is indispensable for glucose repression. Thus, our study not only identifies a mechanism regulating digestive enzyme expression but points to an important role of the TGF-b/Activin signaling in sugar sensing and metabolism, which is independent of the insulin-signaling pathway. RESULTS Amylase Is Repressed in Flies Fed Ad Libitum Previous studies have shown that intestinal amylase activity is repressed when flies were fed on a glucose-rich diet, an effect described as glucose repression (Benkel and Hickey, 1986; Hickey and Benkel, 1982; Figure 1A). To confirm and further characterize this response, we compared the expression level of all three Drosophila amylase genes (Amy-p, Amy-d, and Amyrel) in the gut of adult flies fed ad libitum on a standard laboratory medium, starved on agar, or refed for 8 hr after starvation on agar. The transcript of all three genes was significantly reduced when flies were fed ad libitum or when flies were refed, as illustrated by the ratio (<1) of amylase expression relative to starved flies (Figure 1B). Consistent with this, we also found 336 Cell Reports 9, , October 9, 2014 ª2014 The Authors
3 Figure 1. Glucose Repression Affects Carbohydrate and Lipid-Acting Digestive Enzymes (A) A schematic representation of carbohydrate digestion from polysaccharides to monosaccharaides. Amylases expression is repressed by glucose in the diet (known as glucose repression). (B) qrt-pcr on whole gut of ad-libitum-fed, starved, or 8 hr refed flies. Transcript levels of all three amylases were reduced (ratio <1) in flies fed ad libitum or after refeeding on a standard medium. Data are expressed relative to starved as mean ± SEM. For western blot analysis of amylase, see also Figures S1A and S1B. (C) Graphic representation of amylase expression along the anterior/posterior axis of the midgut based on previous microarray data (Buchon et al., 2013; top panel). Amylase expression, as revealed by immunostaining (bottom panel), was mainly observed in region R1, R2, and R4 (red). Amylase expression in the R2 and R4 regions were low when flies are fed. Note that the amylase signal (red) from the crop is due to autofluorescence. The scale bar represents 1,000 mm. (D) Venn diagram for the RNA-seq results. Sixty-two genes were differentially regulated (at least 2-fold) in both comparison (top panel). Gene Ontology (GO) analysis was done with GOrilla (bottom panel). Thirty-four genes were associated with GO term, including hydrolase activity on glycosyl bonds, oxidoreductases activity, glucose transmembrane transporter activity, and lipase activity. All the 34 genes were repressed. Red shades represent the extent of repression, whereas numeric values represent transcript level relative to starved. The asterisk denotes genes with putative digestive enzyme function (Lemaitre and Miguel-Aliaga, 2013). See also Figures S2 and S3, supplemental text S1, and Table S3. less amylase protein in ad-libitum-fed flies in western blot analysis using an anti-amylase antibody that we generated (Figures S1A and S1B). Our laboratory and others (Buchon et al., 2013; Marianes and Spradling, 2013) had previously demonstrated that the adult midgut is compartmentalized into discrete regions, with amylase expression enriched in the anterior (R1 and R2) and posterior (R4) regions of the midgut (Figure 1C, upper panel; Thompson et al., 1992). As such, we immunostained the adult gut with our amylase antibody to visualize amylase expression in the gut of ad-libitum-fed flies and starved flies. Interestingly, amylase repression in the fed state was associated with very low Cell Reports 9, , October 9, 2014 ª2014 The Authors 337
4 expression of amylase in the R2 and R4 regions (Figure 1C, lower panel). All these indicate that amylase expression is reduced in the fed state and that the high level of amylase expression during starvation is reversible upon refeeding. Glucose Repression Extends beyond Amylases To date, only amylase has been shown to be subjected to glucose repression. In order to extend our results beyond amylases, we performed a RNA sequencing (RNA-seq) study on adult gut samples to identify genes that are differentially regulated by glucose in the diet. To ensure that the gene set derived from our comparison between agar versus glucose-only diet remains relevant for a nutritionally complete diet regime, we identified differentially regulated genes that were common between agar versus glucose and agar versus standard medium conditions. Sixty-two genes were differentially expressed in both comparisons (Figure 1D). A closer examination of these genes revealed that many possess hydrolase activity acting on glycosyl bonds, including several alpha-mannosidases and maltases, enzymes that are involved in the later steps of carbohydrate hydrolysis to produce glucose and/or mannose (Figures 1A and 1D). In addition, our analysis also revealed that several genes with lipase activity, glucose transmembrane transporter activity, as well as genes with oxidoreductase activity were repressed (Figure 1D; see also Figures S2 and S3, Supplemental Text S1, and Table S3). As many of the genes were more repressed by the standard medium, it is possible that other macro- and micronutrients may affect the repression of these genes, either through direct modulation of signaling pathways governing their expression or indirect mechanisms affecting food intake and intestinal transit. Independent quantitative RT-PCR (qrt-pcr) analysis of carbohydrase and lipase genes identified by our RNA-seq, as well as other members within those gene cluster, showed that seven out of eight maltase genes in the Mal-A cluster (all except Mal-A5), all four alpha-mannosidases of the cluster (CG9463, CG9465, CG9466, and CG9468), and lipases were downregulated in wild-type (MyoIA-gal4 ts >w 1118 ) glucose-fed flies relative to starved flies (Figure 5A). Because our qrt- PCR results revealed additional genes subjected to glucose repression (i.e., CG9466, CG9468, and CG6283), the actual pool of affected carbohydrases and lipases could be potentially larger. In agreement with our transcriptome analysis, guts derived from flies fed a glucose-only diet also had reduced amylase activity relative to starved flies (Figure S1C). Collectively, our study demonstrates the ability of the gut to modulate digestive enzymes expression in response to their nutritional states and that the previously characterized glucose repression encompasses a broader digestion response, affecting many carbohydrases and lipases. Glucose Repression Is Induced by Nutritious Carbohydrate Previous studies have shown that Drosophila can also utilize a variety of sugars (Hassett, 1948) hereafter referred to as nutritious sugars but not arabinose and L-glucose, two sweet-tasting sugars with no nutritional value (Fujita and Tanimura, 2011; Hassett, 1948). To determine if the nutritional value of sugars is essential for repression, we examined the effect of a glucose, fructose, trehalose, arabinose, or L-glucoseonly diet on repression. For this and subsequent analysis, we quantified Mal-A1 and Amy-p transcript in flies, as they provide appropriate readout for glucose repression. Both Mal-A1 and Amy-p are strongly enriched in the midgut (Chintapalli et al., 2007), repressed by glucose in the diet (Figure 1B), and encompass the early and later steps of polysaccharide digestion. Amy-p was also demonstrated to be one of the two dietary amylases expressed in adults (Haj-Ahmad and Hickey, 1982; Hickey et al., 1988). Whereas nutritious sugars (glucose, fructose, and trehalose) efficiently repressed Mal-A1 and Amyp, arabinose and L-glucose did not (Figure 2A). In addition, we also observed a statistically significant repression of Mal-A1 and a weak repression of Amy-p (not statistically significant) by a starch-only diet (Figure S1D). Amylases and maltases are suited for their respective substrates: polysaccharides for amylases and lower-molecular-weight dextrin/disaccharides for maltases. Hence, enzymes involved in different stages of polysaccharide digestion may have different sensitivity toward glucose repression. In contrast, both Mal-A1 and Amy-p transcripts were not repressed by a casein (protein) or palmitic acid (fatty-acid)-only diet (Figure 2A), asserting that the repression of Mal-A1 and Amy-p was not determined solely by the caloric content of the nutrients. Of note, the level of Mal-A1 and Amy-p expression in flies fed with our standard laboratory medium (a nutritionally complete diet) was already low and was not further repressed by supplementing the same medium with 10% glucose (Figure S1E). Together, these results indicate that Mal-A1 and Amy-p repression depends upon the carbohydrate contents of the diet, which activates a negative feedback loop that reduces digestive enzymes expression when flies are in the starved state, but not further when flies are in the fed state. Glucose Repression Is Independent of Insulin and AKH Signaling In Drosophila, the insulin and adipokinetic hormone (AKH)- signaling pathways are two critical mediators of sugar homeostasis (Brogiolo et al., 2001; Kim and Rulifson, 2004; Lee and Park, 2004; Rulifson et al., 2002). As such, a simple mechanistic explanation for glucose repression would be through the insulin and/or AKH-signaling pathway. To determine if insulin signaling is required for repression of amylase and maltase, we examined glucose repression in chico 1 homozygous mutant flies lacking the insulin receptor substrate (Böhni et al., 1999) and flies whereby dilp3 producing cells were ablated through the overexpression of a proapoptotic gene, reaper (rpr). The dilp3-gal4 driver was used for targeted ablation of the insulin-producing cells in the brain and in the midgut (Veenstra et al., 2008). Interestingly, in chico 1 homozygous flies and dilp3-gal4 > rpr cellablated flies, glucose repression was unaffected (Figures 2B). In addition, glucose repression was unaffected in AKHR mutants or when we knocked down AKH using two independent RNAi lines with an AKH-gal4 driver (Figures 2C and 2D). Thus, our data indicate that glucose repression of Mal-A1 and Amy-p involves a carbohydrate-sensing mechanism independent of the insulin- and AKH-signaling pathway. 338 Cell Reports 9, , October 9, 2014 ª2014 The Authors
5 Figure 2. Nutritious Sugars Repress Mal-A1 and Amy-p Independent of the AKH- and Insulin-Signaling Pathway (A) Repression analysis on flies fed ad libitum with various sugars (glucose, fructose, trehalose arabinose, or L-glucose), protein (casein), fatty acid (palmitic acid), or standard medium for 24 hr. Only nutritious sugars (glucose, trehalose, and fructose) reduced Mal-A1 and Amy-p expression. Data are expressed relative to starved as mean ± SEM. For glucose repression of amylase enzymatic activity, see Figure S1C. For effects of starch and the effects of glucose under nonstarving conditions, see also Figures S1D and S1E. (B) Ablation of dilp3-expressing cells by overexpression of reaper (dilp3-gal4>rpr) and compromised insulin signaling (chico 1 an insulin receptor substrate mutant) did not abolish repression in flies fed on a glucose-only diet. Data are expressed relative to starved as mean ± SEM. (C) Glucose repression in AKH-receptor mutants (AKHR 1 ) and a genotype-matched control (AKHR rev ). Glucose repression was not affected in AKHR 1 in flies fed on a glucose-only diet. Data are expressed relative to starved as mean ± SEM. (D) Reducing AKH expression with two independent RNAi lines (UAS-AKH-IR) using AKH-gal4 did not affect glucose repression in flies fed on a glucose-only diet. Data are expressed relative to starved as mean ± SEM. Loss of Smad2 Abolished Glucose Repression To obtain insights into how glucose repression is achieved, we performed an in vivo RNAi screen on 60 candidate transcription factors, selected based on their expression profile (see Supplemental Experimental Procedures). For each RNAi line driven by an inducible enterocyte driver in the adult midgut (MyoIA-gal4 ts ), the expression of both Mal-A1 and Amy-p were monitored in glucose-fed and starved flies to assess for glucose repression. Among the transcription factors screened, knocking down Smad2 abolished glucose repression of both Mal-A1 and Amyp (Table S1). Three independent RNAi lines against Smad2 all abrogated glucose repression (Figure 3A). A Smad2-null mutant (Smad2 F4 ), whereby the entire coding region is deleted, has previously been described (Peterson et al., 2012). Because Smad2 F4 mutants are larval lethal, we used second instar larvae (L2) maintained on a standard medium without glucose or on the same medium but supplemented with 10% glucose to confirm if Smad2 is required for glucose repression. Whereas Mal-A1 and Amy-p were strongly repressed by glucose supplementation in wild-type larvae, this was lost in Smad2 F4 larvae (Figure 3B). Because the repression of amylase in the R2b and R2c subregions of the midgut was most prominent in the fed state (Figure 1C), we monitored amylase expression in the R2b subregion in flies whereby midgut Smad2 was knocked down (MyoIAgal4 ts > Smad2-IR (1)). Immunostaining for amylase showed similar levels of amylase protein in the R2b region between agar- and glucose-fed flies (Figure 3C). Knockdown of Smad2 also affected amylase repression in other regions, such that amylase expression level along the midgut between glucosefed and starved guts were indistinguishable (Figure S4A). To confirm if the high-amylase signal in the R2b region was indeed due to changes in gene expression, we examined the expression of Amy-p along the midgut using a newly generated Amy-p-EGFP reporter. The nuclear enhanced GFP (EGFP) reporter expression was under the control of a 2 kb region upstream of the Amy-p coding sequence. Whereas Amy-p- EGFP was repressed in the R2b region in glucose-fed control flies, Amy-p expression remained high in the same region when Smad2 was knocked down in the midgut (Figure 3D). Because many of our manipulations were conducted such that Smad2 was only compromised in differentiated adult enterocytes, we expect little, if any, effects on enterocyte cell fate and differentiation. Consistent with this, enterocytes expressed the gut-specific brush border myosin IA (Morgan et al., 1994); the marker of differentiated enterocyte, Pdm1 (Lee et al., 2009; data not shown); and amylases, even when Smad2 was silenced. Next, to determine if the effects of Smad2 knockdown on glucose repression is cell autonomous, we made positively marked clones of Smad2 knockdown using the esg ts F/O system (Jiang et al., 2009) and examined the expression of amylase by immunostaining whole guts derived from glucosefed flies. In both the R1 and R2b regions, amylase expression was consistently higher within the Smad2 knockdown clones (Figure 3E). Hence, increased amylase expression when Smad2 was knocked down in the midgut (MyoIA-gal4 ts > Smad2-IR (1)) was not due to changes in feeding behavior. Instead, Smad2 is required cell autonomously along the midgut for repression of Mal-A1 and Amy-p in response to dietary glucose. Cell Reports 9, , October 9, 2014 ª2014 The Authors 339
6 Figure 3. Smad2 Is Cell Autonomously Required for Glucose Repression (A) Glucose repression was determined by qrt- PCR on whole flies. Three independent RNAi lines against Smad2, all abolished Mal-A1 and Amy-p repression by glucose. Data are expressed relative to starved as mean ± SEM. For RNAi screen, see also Table S1. (B) Mal-A1 and Amy-p repression in L2 larvae. Feeding L2 larvae were maintained on standard medium without glucose (glucose [ ]) or standard medium supplemented with 10% glucose (glucose [+]). Repression of Mal-A1 and Amy-p by glucose was lost in Smad2 F4 larvae. Data are expressed relative to glucose ( )-fed larvae as mean ± SEM. (C) Glucose repression of amylase expression in the R2b region as revealed by immunostaining. When Smad2 was knocked down in the midgut, amylase signal remained high in the R2b region in glucose-fed flies. The scale bar represents 100 mm. For immunostaining images of whole gut, see also Figure S4A. (D) Amy-p expression was monitored using a nuclear localized EGFP reporter under the control of a 2 kb region upstream of the Amy-p coding sequence (Amy-p-EGFP). In the R2b region of Smad2 knockdown, Amy-p expression remained high in glucose-fed flies. The scale bar represents 100 mm. (E) Clonal analysis of Smad2 knockdown using the esg ts F/O system. In the midgut, Smad2-IR-activated clones (green) expressed higher level of amylase (red) compared to surrounding enterocytes in glucose-fed gut, both in the R1 (top panel) and R2b (bottom panel) region. The scale bar represents 50 mm. Glucose Repression Is Dependent on the Canonical TGF-b/Activin Pathway The transcription factor Smad2 is an R-Smad of the TGF-b/Activin pathway. The conserved TGF-b/Activin pathway consists of a tetrameric complex of two type 1 and two type 2 receptor serine/ threonine kinases activated by ligand dimers. In Drosophila, there is one type 1 receptor, named Baboon (Babo), which is specific to the TGF-b/Activin pathway, and two type 2 receptors, Wishful thinking (Wit) and Punt (Put), common to both the TGF-b/ Activin and the BMP/Dpp pathway. To confirm and extend our results with our Smad2 RNAi, we examined if the TGF-b/ Activin pathway is required for glucose repression by targeting Babo, Wit, and Put. Whereas glucose repression was lost by knockdown of Babo and Put, Mal-A1 and Amy-p expression was still repressed by glucose in Wit knockdown flies (Figure 4A). Previous studies have demonstrated that the activated Babo receptor can also phosphorylate Mad when endogenous Smad2 protein is depleted (Gesualdi and Haerry, 2007; Hevia and de Celis, 2013; Peterson et al., 2012). To exclude ectopic Mad activation as an explanation for the loss of glucose repression, we also knocked down Dad, a negative regulator of Mad, to hyperactivate Mad (Ogiso et al., 2011) and showed that Dad knockdown did not affect Mal-A1 and Amy-p repression by glucose (Figure 4A). In agreement with this, Babo knockdown 340 Cell Reports 9, , October 9, 2014 ª2014 The Authors
7 (see above), which reduces Smad2 activation without any corresponding Mad phosphorylation, also effectively abolished Mal-A1 and Amy-p repression by glucose. Given that Babo and Smad2 were both required for glucose repression, we reasoned that the hyperactivation of the same pathway would be associated with the repression of Mal-A1 and Amy-p expression, even in absence of glucose. Indeed, when we overexpressed the constitutive active form of Babo (Babo*) or Smad2 (Smad2*) in the adult midgut, both Mal-A1 and Amy-p expression was reduced (Figure 4B). Taken together, glucose repression of Mal-A1 and Amy-p in the adult midgut is mediated through the canonical TGF-b/Activin pathway involving Babo, Punt, and Smad2. Babo C Receptor Isoform Mediates Glucose Repression of Carbohydrases and Lipases The Drosophila genome encodes a single Babo gene that is alternatively spliced to produce three different receptor isoforms, each with distinct extracellular domains. Specifically, the Babo C receptor isoform was previously found to be enriched in the larval gut (Jensen et al., 2009). Consistent with those findings, we also found Babo C receptor to be the major isoform expressed in the adult gut (Figure 4C). Importantly, knocking down Babo C (but not Babo A and Babo B ) with an isoform-specific RNAi (Awasaki et al., 2011) also abolished glucose repression of Mal-A1 and Amy-p (Figure 4D). Similarly, knocking down Babo C or Put in specific subset of clones using the esg ts F/O system (Jiang et al., 2009) also renders those cells insensitive to glucose repression (Figures 4E and S4B). To verify if the TGF-b/Activin pathway may act to repress other putative digestive enzymes identified in our RNA-seq analysis, we examined the effects of Babo C knockdown on amylases, maltases, alpha-mannosidases, and lipases identified from our RNA-seq analysis. Strikingly, glucose repression of all amylase, maltase, alpha-mannosidase, and lipase genes tested was abolished when Babo C was knocked down in the adult midgut (Figure 5A). Consistent with this observation, glucose repression of amylase activity was also abrogated when Smad2 and Babo were silenced in the adult midgut (Figure 5B). We then analyzed the metabolic consequence of disrupting the TGF-b pathway in the midgut. Glucose is the principal sugar absorbed through the gut lumen into the hemolymph. To determine if increased digestive enzyme expression may affect postprandial level of circulating glucose, we quantified the level of glucose in the hemolymph before refeeding and 1 hr post refeeding. As expected, the increase in postprandial level of glucose was consistently higher in the flies whereby the TGFb/Activin signaling was compromised in the midgut (Figure 5C). However, there were no consistent changes in the level of trehalose 1 hr postrefeeding (data not shown). Thus, we examined the glucose, trehalose, glycogen, and triglyceride (TAG) levels in flies fed on a high-sugar diet for 2 weeks. Unexpectedly, we did not observe any significant differences between Babo C or Smad2 knockdown flies and control (Figures 5D and S5A). Also, Smad2 knockdown flies fed on a nutrient-poor medium, deprived of sugar, had similar survival kinetics as control (Figure S5B). Hence, there are probably compensating mechanisms to counteract any postprandial increase in circulating sugars when TGF-b/Activin pathway is disrupted in the midgut. Taken together, the TGF-b/Activin signaling through Babo C is required in the adult midgut to repress both carbohydrate and lipid-acting digestive enzymes in response to glucose in the diet; however, the metabolic significance of this process remains unclear. Nutritious Sugar Induces Daw Expression, which Represses Amylase and Maltase The transcript levels of all three Babo receptor isoforms and Smad2 were similar in the guts derived from flies fed ad libitum with agar, glucose, or standard medium (Figure 4C). Thus, the modulation of the TGF-b/Activin pathway after feeding does not rely on transcriptional changes involving Babo receptor or Smad2. In Drosophila, the TGF-b/Activin pathway can potentially be activated by four different ligands: Dawdle (Daw), Myoglianin (Myo), Maverick (Mav), and Activin-b (Actb)(Lo and Frasch, 1999; Nguyen et al., 2000; Parker et al., 2006; Zhu et al., 2008). To test which of the ligands are sensitive to glucose, we compared their expression in glucose-fed flies relative to starved flies. Among the four ligands, only Daw level was induced by glucose (Figure 6A). More importantly, Daw transcript was also increased in flies fed the various nutritious sugars (Figure S6A), which repressed both Mal-A1 and Amy-p (Figure 2A). Casein, palmitic acid, and the nonnutritious sugar arabinose did not stimulate Daw expression. These results suggest that Daw may act as the nutritional cue to repress digestive enzyme expression in the midgut in response to sugar in the diet. To confirm whether these ligands have a repressive function, we examined the level of Mal-A1 in flies individually overexpressing the various ligands of the TGF-b/Activin pathway using a ubiquitous driver in adults. Among the four ligands tested, only Daw significantly reduced Mal-A1 level (Figures 6B and S6B). Because Daw was also the only TGF-b/Activin ligand that was induced by glucose, we focused our subsequent analysis only on Daw. As expected, overexpression of Daw also reduced amylase protein expression in western blot and immunostaining analysis on gut samples (Figures 6C and S6C). We then confirmed if Daw is essential for glucose repression by using a previously described null mutant of Daw (Gesualdi and Haerry, 2007). Given that Daw mutants (Daw Acct1 ) do not survive to adulthood, we studied glucose repression in feeding L2 larvae. Concurring with our results whereby overexpression of Daw repressed Mal-A1, glucose repression was abolished in Daw Acct1 larvae (Figure 6D). Altogether, our data demonstrate that Daw expression is induced by nutritious sugar and one of its functions is to reduce digestive enzyme expression in the adult midgut. Systemic Release of Daw Can Mediate Glucose Repression in the Gut In adults, Daw is highly expressed in the fat body and muscle; whereas in the brain, gut, and ovary tissues, Daw expression is low (Bai et al., 2013; Chintapalli et al., 2007). To determine the source of adult Daw induction in glucose-fed flies, we examined in which of those tissues Daw might be induced. After 24 hr on a glucose diet, transcript levels of Daw were elevated only in the abdomen carcass (without gut and ovaries; containing mainly fat body) samples. Expression of Daw in the head (brain), thorax Cell Reports 9, , October 9, 2014 ª2014 The Authors 341
8 342 Cell Reports 9, , October 9, 2014 ª2014 The Authors (legend on next page)
9 (consisting mainly muscle), and whole-gut samples was not significantly altered (Figure 6E). All these results suggest that glucose repression is unlikely mediated by a local release of Daw from the gut. Next, to confirm that Daw can function in an endocrine manner, we overexpressed Daw in the fat body (using yolk-gal4 and ppl-gal4), visceral muscle (using how-gal4 ts ), or gut (using MyoIA-gal4 ts ). Overexpression with the different gal4 drivers all repressed Mal-A1 and Amy-p expression (Figures 6F and S6D), showing that Daw can function in an endocrine manner via the circulation to regulate midgut expression of Mal-A1 and Amy-p. To better define the source of Daw for glucose repression, we used various gal4 drivers to knock down Daw in a tissue-specific manner (Figures 6G and S6E). Whereas glucose repression remained when Daw was knocked down in the adult midgut (MyoIA-gal4 ts ), the muscle (MHC-gal4), and the brain (elav-gal4), glucose repression was abolished when Daw expression was compromised in the fat body (ppl-gal4 and C564-gal4 ts ). Fat body knocked down of Daw also increased the expression of Mal-A1 and Amy-p (Figure S6F). In conclusion, our results are consistent with a model whereby Daw expression is induced and secreted by the fat body in response to nutritious carbohydrate in the diet and, via the circulation, represses digestive enzyme expression in the midgut through the TGF-b/Activinsignaling pathway (Figure 7). DISCUSSION Digestive enzymes expression is subjected to complex regulation. However, apart from the regulation of magro (lipase) by the nutrient-sensitive DHR96 and dfoxo (Karpac et al., 2013; Sieber and Thummel, 2012), regulatory mechanisms controlling digestive enzymes expression have not been carefully studied in Drosophila. A rare but poorly characterized example of digestive enzyme regulation concerns amylase repression by the end product of carbohydrate digestion, glucose (Benkel and Hickey, 1986; Hickey and Benkel, 1982). Although glucose repression was initially described in the context of amylase, our transcriptome analyses have showed that glucose repression encompasses a broader spectrum response. Glucose also repressed genes with predicted carbohydrase, glucose transport, and lipase function. Many of the genes affected by glucose repression are expressed almost exclusively in the midgut (Chintapalli et al., 2007) and are organized in clusters in the genome (e.g., lipase cluster: CG6283, CG6271, and CG6277), a feature pertaining to many digestive-enzyme-encoding genes (Lemaitre and Miguel-Aliaga, 2013). It is noteworthy that our arbitrary threshold for RNA-seq analysis has rejected several genes whose repression was more subtle (Figure S2). For this, we have independently verified Amy-p, Amy-d, CG9466, CG9468, and CG6283 to be repressed by glucose through qrt-pcr. Thus, the actual repertoire of carbohydrases and lipases affected by glucose could be potentially larger. To date, little is known about the contribution of digestion on sugar homeostasis. Although a detailed profiling for the metabolic effects of digestion is beyond the scope of this paper, it seemed likely that glucose repression of carbohydrases and lipases is aimed at reducing the amount of sugars and lipids that are available for absorption. Consistent with this view, we also found glucose transmembrane transporters among genes that were downregulated by dietary glucose. A high-sugar diet in Drosophila is associated with dire consequences such as hyperglycemia, insulin resistance, and increased fat accumulation (Havula et al., 2013; Musselman et al., 2011). Thus, reducing both carbohydrases and lipases expression may restrict the nutritional load available for absorption into the circulation when carbohydrate stores in the organism are sufficient and fats are accumulating. In accordance with this, early postprandial glucose level was elevated in the hemolymph when TGF-b/ Activin pathway function was compromised in the midgut, a condition associated with elevated digestive enzymes expression. However, when we monitored the levels of TAG, glycogen, glucose, and trehalose after 2 weeks on a high-sugar diet, we did not observe any significant differences between flies whereby Smad2 or Babo were knocked down in the midgut and control. Sugar homeostasis is a tightly regulated process involving multiple tissues. One possibility would be that the postprandial increase in glucose was counteracted by early acting satiety response when hemolymph glucose level passed a certain threshold, thus limiting the net amount of glucose entering the circulation. Clearly, the role of glucose repression in sugar homeostasis and metabolism warrants additional research. An understanding of how the repertoire of digestive enzymes respond to other nutriments in the diet will provide insights into how an organism may rebalance its diet after ingestion and improve our understanding of nutrients homeostasis. In this study, we also showed that digestive enzyme repression is induced only by nutritious carbohydrates in the diet. Arabinose, a sweet-tasting sugar with no nutritional value, and L-glucose, another nonutilizable sugar (Fujita and Tanimura, Figure 4. The Canonical TGF-b/Activin Pathway Is Required for Glucose Repression in the Adult Midgut (A) Mal-A1 and Amy-p repression was determined by qrt-pcr on midgut-activated Babo-IR (does not distinguish between isoforms), Dad-IR, Put-IR, and Wit-IR flies. Glucose repression of Mal-A1 and Amy-p was lost in Babo-IR and Put-IR. Data are expressed relative to starved as mean ± SEM. (B) Overexpression of the constitutive active form of Babo (Babo*) and Smad2 (Smad2*) in the adult midgut reduced Mal-A1 and Amy-p expression in starved flies. Data are relative to control flies (MyoIA-gal4 ts >ywfor Smad2*; MyoIA-gal4 ts >w 1118 for Babo*) as mean ± SEM. (C) The transcript level of Smad2 and the three Babo isoforms were monitored by qrt-pcr on adult gut from flies fed ad libitum on various medium for 24 hr. Babo C and to a lesser extent Babo B receptor isoform were expressed in the adult gut. Expression of Babo receptors and Smad2 was not sensitive to the different diet. Data are expressed relative to rp49 as mean ± SEM. (D) Effects of knocking down specific Babo receptor isoform on glucose repression. Loss of Babo C receptor isoform, but not Babo A and Babo B, abolished glucose repression. Data are expressed relative to starved as mean ± SEM. (E) Clonal analysis of Babo C or Put knockdown using the esg ts F/O system. In the R2b region of the gut, knockdown of Babo C or Put (green) increased amylase (red) expression relative to surrounding enterocytes in glucose-fed flies. This effect was also observed in the R1 region of the midgut (Figure S4B). The scale bar represents 50 mm. Cell Reports 9, , October 9, 2014 ª2014 The Authors 343
10 Figure 5. TGF-b/Activin Pathway Affects Repression of Many Digestive Enzymes and Postprandial Glucose Level (A) Glucose repression analysis for all other maltases, amylases, alpha-mannosidases, and lipases identified by the RNA-seq analysis as well as other members within the same cluster, but not identified in analysis. Knockdown of Babo C by RNAi abolished repression of many of the genes in response to glucose in the diet. Data are expressed relative to starved as mean ± SEM. (B) Glucose repression of amylase activity. Midgut knockdown of Smad2 or Babo-IR, but not control, abolished amylase repression. Amylase activities were normalized to protein level (mu activity/mg protein) and expressed relative to starved as mean ± SEM. (C) Circulating glucose after 1 hr refeeding. Flies were refed with standard medium supplemented with 5% maltose, 5% sucrose, and 2.5% starch. The increase in hemolymph glucose postfeeding was higher in MyoIA-gal4 ts > Smad2-IR (1) and MyoIA-gal4 ts > Babo-IR compared to control. Values are hemolymph glucose level relative to level before refeeding. (D) Amounts of glucose, trehalose, glycogen, and TAG derived from whole flies fed on a high-sugar diet (standard medium supplemented with 20% sucrose, 5% maltose, and 5% starch) for 2 weeks. There were no significant differences between Babo knockdown and control. Amounts were normalized to the level of protein and expressed as mg/mg protein. Values are mean ± SEM. For metabolic measurements and sensitivity to sugar deprivation of Smad2 knockdown, see Figures S5A and S5B. 2011; Hassett, 1948), did not suppress amylase and maltase expression. Hence, we consider postprandial activation of gustatory receptors (Park and Kwon, 2011) in the gut to be an unlikely mechanism for glucose repression of digestive enzymes. Instead, all these are suggestive of an underlying sugar-sensing mechanism to ensure that carbohydrate digestive capacity toward utilizable carbohydrate sources are not comprised until nutritional sufficiency is attained. In Drosophila, sugar homeostasis is often associated with the AKH and insulin signaling, whereas insulin signaling is also modulated by proteins and amino acids in the diet (Brogiolo et al., 2001; Buch et al., 2008; Kim and Rulifson, 2004; Lee and Park, 2004; Rulifson et al., 2002). Recently, Bai and colleagues have showed that Daw expression is modulated by insulin signaling and identified Daw as a target of dfoxo (Bai et al., 2013), raising the possibility that glucose repression may be similarly affected by insulin signaling. Surprisingly, disrupting both AKH and insulin signaling did not compromise glucose repression. Instead, we identified a key role for TGF-b/Activin signaling in this process. Whereas Daw expression may be modulated by insulin signaling, our results clearly showed that glucose repression is mediated through an insulin-independent mechanism. More recently, Ghosh and O Connor have demonstrated that Daw is required for insulin secretion, suggesting 344 Cell Reports 9, , October 9, 2014 ª2014 The Authors
11 that the TGF-b/Activin pathway may function upstream of the insulin signaling (Ghosh and O Connor, 2014). It is also noteworthy that, whereas compromising insulin signaling is known to raise circulating sugar levels, this did not affect the ability of flies to repress digestive enzymes in response to dietary glucose. One possible explanation is that Daw expression in response to glucose is dependent on the nutritional state perceived cell autonomously by the fat body cells. Thus, if nutrient sensing in these cells is not compromised, Daw induction and glucose repression can be achieved. Future research should clarify the mechanism underlying Daw induction by nutritious sugar and define the possible interactions between TGF-b/Activin and other sugar-sensing mechanisms. The TGF-b/Activin pathway in Drosophila has been previously studied in the context of larval brain development, neuronal remodeling, wing disc development, and, more recently, aging and ph homeostasis (Bai et al., 2013; Ghosh and O Connor, 2014; Gibbens et al., 2011; Hevia and de Celis, 2013; Peterson et al., 2012; Zheng et al., 2003; Zhu et al., 2008). This study addresses the physiological function of the TGF-b/Activin pathway in the adult midgut. When we disrupted the TGF-b/Activin signaling in the adult midgut, glucose repression was abolished. Conversely, increasing TGF-b/Activin signaling in the midgut, through the overexpression of the constitutive active form of Babo or Smad2, was sufficient to repress both amylase and maltase expression. Furthermore, we showed that glucose repression is mediated by the TGF-b ligand Daw, produced and secreted from the fat body, a metabolic tissue functionally analogous to the mammalian liver and adipose tissue. Thus, our study uncovers a physiological role for the TGF-b/Activin pathway in adapting carbohydrate and lipase digestion in response to the nutritional state of the organism. Because many features of digestion and absorption are conserved between flies and mammals, it will be of interest to investigate the role of TGF-b/Activin pathway in mammalian digestion. Figure 6. Daw Is Induced in the Fat Body by Glucose in the Diet and Is Necessary for Repression of Mal-A1 and Amy-p (A) qrt-pcr of the four possible TGF-b/Activin pathway ligand genes on flies fed with glucose. Only Daw expression was induced upon glucose feeding. Data are expressed relative to starved as mean ± SEM. For the effects of other sugars on Daw, see also Figure S6A. (B) Mal-A1 level in starved flies ubiquitously overexpressing each of the four TGF-b/Activin ligand genes. Overexpression of Daw (Da-gal4 ts > Daw) reduced Mal-A1 expression relative to control flies (Da-gal4 ts >yw). Data are expressed relative to rp49 as mean ± SEM. See also Figure S6B for quantification with gut lysate. (C) Western blot analysis of amylase protein on gut lysate derived from flies overexpressing Daw. Overexpression of Daw reduced amylase protein expression in the gut. Tubulin is shown as loading control. For immunostaining of the gut when Daw is overexpressed, see Figure S6C. (D) Mal-A1 and Amy-p gene repression in Daw Acct1 was monitored in feeding L2 larvae maintained on standard medium without glucose or standard medium supplemented with 10% glucose. Repression of Mal-A1 and Amy-p by glucose was compromised in Daw Acct1 larvae. Data are expressed relative to glucose ( )-fed larvae as mean ± SEM. (E) Expression of Daw in different tissues after 24 hr on glucose. Daw was induced in the abdomen carcass (mainly fat body tissue) of glucose-fed flies. Data are expressed relative to starved as mean ± SEM. (F) Mal-A1 transcript level in starved flies overexpressing Daw through different tissue-specific gal4 drivers. Overexpression of Daw by different drivers all reduced Mal-A1 expression. Data are expressed relative to control (gal4>yw) as mean ± SEM. For Amy-p expression, see also Figure S6D. (G) Mal-A1 repression when Daw was knocked down in specific tissue. Fat body knockdown (ppl-gal4 and C564-gal4 ts ) of Daw abolished glucose repression of Mal-A1. Data are expressed relative to starved as mean ± SEM. For Amy-p repression, see also Figure S6E. Cell Reports 9, , October 9, 2014 ª2014 The Authors 345
12 rons for satiety (You et al., 2008). There were also several observations that hyperglycemia is linked to increased TGF-b activity in mammals (Iglesias-de la Cruz et al., 2002; Kolm-Litty et al., 1998). Hence, the role of TGF-b/Activin signaling in sugar homeostasis requires further investigation in Drosophila and other organisms. In conclusion, our study revealed a remarkable resilience in the regulation of carbohydrate and lipid-acting enzymes expression to ensure that digestive capacity in the midgut is not compromised before certain metabolic criteria in the fat body is attained. The study also unraveled a role of the TGF-b/Activin-signaling pathway in the adult Drosophila midgut, which has not been appreciated. It reinforced the notion that the gut is not a passive tube for nutriment flow. Rather, it dynamically modulates digestive enzyme expression in response to the organism s nutritional state through endocrine signals derived from other metabolic tissues. EXPERIMENTAL PROCEDURES Fly Stocks and Maintenance For stocks and diets, see Supplemental Experimental Procedures. For inducible GAL4 activation, F1 flies were raised at 18 Cor22 C for larval and pupal development and switched to 29 C 3 days after eclosion for at least 5 days. Validation for the various RNAi, overexpression, and cell ablation is provided in Table S2. All crosses and stocks were kept at 25 C on a 12 hr light dark cycle unless otherwise stated. Figure 7. Model for Sugar Sensing and Repression of Carbohydrase and Lipase Nutritious sugars, when consumed sufficiently, induce Daw expression in the fat body. Secreted Daw then activates the canonical TGF-b/Activin signaling in the midgut through Babo C and Punt receptors, leading to the activation of Smad2 and reduction of carbohydrase and lipase expression. Recent studies have attributed a role for Daw in aging (Bai et al., 2013) and ph homeostasis (Ghosh and O Connor, 2014), two processes tightly linked to metabolism. Thus, it is likely that Daw induced from the fat body in response to carbohydrate in the diet will induce a more global response instead of a local response, affecting only digestive enzyme expression. As such, Daw may act as a central mediator for glucose homeostasis by regulating sugar level in the circulation. When there are sufficient carbohydrates in the diet, Daw expression restricts the expression of carbohydrase and glucose transporters. Concurrently, at the postabsorption level, Daw in the circulation may act directly or indirectly (via insulin signaling) to maintain circulating sugar level. A broader role for Daw in sugar homeostasis is reinforced by the findings that Daw mutant larvae were more sensitive to a high-sugar diet (Ghosh and O Connor 2014). Similarly, we found overexpression of Daw, but not Myo, Mav, or Actb, renders flies sensitive to sugar starvation (W.A.C., unpublished data). Along this line, in C. elegans, the TGF-b signaling is reported to be elevated and required in neu- Statistical Analysis All analyses were performed in R. For qrt-pcr data, relative ratios of target gene to rp49 were Log2 transformed. Before performing any parametric test, we used Levene s test to test for equality of variances and the Shapiro- Wilk test to test for normality. For pairwise comparisons, we used Student s t test on the transformed data. ANOVA followed by Dunnett s test was used when comparing more than two groups. Pooled data (n R 3) were expressed as means ± SEM. ***p < ; **p < 0.005; *p < ACCESSION NUMBERS The National Center for Biotechnology Information Gene Expression Omnibus accession number for the RNA-seq raw data reported in this paper is GSE SUPPLEMENTAL INFORMATION Supplemental Information includes Supplemental Experimental Procedures, Supplemental Text, six figures, and three tables and can be found with this article online at AUTHOR CONTRIBUTION W.A.C. designed the project, assembled the data, analyzed and interpreted the results, and wrote the manuscript. M.S.B.S. analyzed the RNA-seq data and statistics. F.S. collected data for metabolic analysis. B.L. designed the project and wrote the manuscript. ACKNOWLEDGMENTS We acknowledge the VDCR in Vienna and Bloomington Stock Center in Indiana University for the fly stocks and the Lausanne Genomic Technologies Facility, Switzerland for RNA sequencing. We thank Michael O Connor (University of Minnesota), Jean-Luc Da Lage (LEGS, CNRS), Hugo Stocker (ETH, Zurich), and Ronald R. Kühnlein (Max Planck Institute for Biophysical 346 Cell Reports 9, , October 9, 2014 ª2014 The Authors
13 Chemistry) for providing the necessary materials for this work. We are grateful to Aidan Petterson, Arpan Ghosh (University of Minnesota), Dani Osman, and Zhongzhao Zhai for their helpful discussions and advice and Sveta Chakrabarti for critical reading of the manuscript. This work was supported by the Swiss National Science Foundation (3100A /1). Received: February 20, 2014 Revised: July 24, 2014 Accepted: August 25, 2014 Published: October 2, 2014 REFERENCES Awasaki, T., Huang, Y., O Connor, M.B., and Lee, T. (2011). Glia instruct developmental neuronal remodeling through TGF-b signaling. Nat. Neurosci. 14, Bai, H., Kang, P., Hernandez, A.M., and Tatar, M. (2013). Activin signaling targeted by insulin/dfoxo regulates aging and muscle proteostasis in Drosophila. PLoS Genet. 9, e Benkel, B.F., and Hickey, D.A. (1986). Glucose repression of amylase gene expression in Drosophila melanogaster. Genetics 114, Böhni, R., Riesgo-Escovar, J., Oldham, S., Brogiolo, W., Stocker, H., Andruss, B.F., Beckingham, K., and Hafen, E. (1999). Autonomous control of cell and organ size by CHICO, a Drosophila homolog of vertebrate IRS1-4. Cell 97, Brogiolo, W., Stocker, H., Ikeya, T., Rintelen, F., Fernandez, R., and Hafen, E. (2001). An evolutionarily conserved function of the Drosophila insulin receptor and insulin-like peptides in growth control. Curr. Biol. 11, Buch, S., Melcher, C., Bauer, M., Katzenberger, J., and Pankratz, M.J. (2008). Opposing effects of dietary protein and sugar regulate a transcriptional target of Drosophila insulin-like peptide signaling. Cell Metab. 7, Buchon, N., Osman, D., David, F.P., Fang, H.Y., Boquete, J.P., Deplancke, B., and Lemaitre, B. (2013). Morphological and molecular characterization of adult midgut compartmentalization in Drosophila. Cell Reports 3, Chintapalli, V.R., Wang, J., and Dow, J.A.T. (2007). Using FlyAtlas to identify better Drosophila melanogaster models of human disease. Nat. Genet. 39, Fujita, M., and Tanimura, T. (2011). Drosophila evaluates and learns the nutritional value of sugars. Curr. Biol. 21, Gesualdi, S.C., and Haerry, T.E. (2007). Distinct signaling of Drosophila Activin/ TGF-beta family members. Fly (Austin) 1, Ghosh, A.C., and O Connor, M.B. (2014). Systemic Activin signaling independently regulates sugar homeostasis, cellular metabolism, and ph balance in Drosophila melanogaster. Proc. Natl. Acad. Sci. USA 111, Gibbens, Y.Y., Warren, J.T., Gilbert, L.I., and O Connor, M.B. (2011). Neuroendocrine regulation of Drosophila metamorphosis requires TGFbeta/Activin signaling. Development 138, Grönke, S., Müller, G., Hirsch, J., Fellert, S., Andreou, A., Haase, T., Jäckle, H., and Kühnlein, R.P. (2007). Dual lipolytic control of body fat storage and mobilization in Drosophila. PLoS Biol. 5, e137. Haj-Ahmad, Y., and Hickey, D.A. (1982). A molecular explanation of frequencydependent selection in Drosophila. Nature 299, Hakim, R.S., Baldwin, K., and Smagghe, G. (2010). Regulation of midgut growth, development, and metamorphosis. Annu. Rev. Entomol. 55, Hassett, C.C. (1948). The utilization of sugars and other substances by Drosophila. Biol. Bull. 95, Havula, E., Teesalu, M., Hyötyläinen, T., Seppälä, H., Hasygar, K., Auvinen, P., Oresic, M., Sandmann, T., and Hietakangas, V. (2013). Mondo/ChREBP-Mlxregulated transcriptional network is essential for dietary sugar tolerance in Drosophila. PLoS Genet. 9, e Hevia, C.F., and de Celis, J.F. (2013). Activation and function of TGFb signalling during Drosophila wing development and its interactions with the BMP pathway. Dev. Biol. 377, Hickey, D.A., and Benkel, B. (1982). Regulation of amylase activity in drosophila melanogaster: effects of dietary carbohydrate. Biochem. Genet. 20, Hickey, D.A., Benkel, B.F., Abukashawa, S., and Haus, S. (1988). DNA rearrangement causes multiple changes in gene expression at the amylase locus in Drosophila melanogaster. Biochem. Genet. 26, Iglesias-de la Cruz, M.C., Ziyadeh, F.N., Isono, M., Kouahou, M., Han, D.C., Kalluri, R., Mundel, P., and Chen, S. (2002). Effects of high glucose and TGF-beta1 on the expression of collagen IV and vascular endothelial growth factor in mouse podocytes. Kidney Int. 62, Jensen, P.A., Zheng, X., Lee, T., and O Connor, M.B. (2009). The Drosophila Activin-like ligand Dawdle signals preferentially through one isoform of the Type-I receptor Baboon. Mech. Dev. 126, Jiang, H., Patel, P.H., Kohlmaier, A., Grenley, M.O., McEwen, D.G., and Edgar, B.A. (2009). Cytokine/Jak/Stat signaling mediates regeneration and homeostasis in the Drosophila midgut. Cell 137, Karasov, W.H., Martínez del Rio, C., and Caviedes-Vidal, E. (2011). Ecological physiology of diet and digestive systems. Annu. Rev. Physiol. 73, Karpac, J., Biteau, B., and Jasper, H. (2013). Misregulation of an adaptive metabolic response contributes to the age-related disruption of lipid homeostasis in Drosophila. Cell Reports 4, Kim, S.K., and Rulifson, E.J. (2004). Conserved mechanisms of glucose sensing and regulation by Drosophila corpora cardiaca cells. Nature 431, Kolm-Litty, V., Sauer, U., Nerlich, A., Lehmann, R., and Schleicher, E.D. (1998). High glucose-induced transforming growth factor beta1 production is mediated by the hexosamine pathway in porcine glomerular mesangial cells. J. Clin. Invest. 101, Lee, G., and Park, J.H. (2004). Hemolymph sugar homeostasis and starvation-induced hyperactivity affected by genetic manipulations of the adipokinetic hormone-encoding gene in Drosophila melanogaster. Genetics 167, Lee, W.-C., Beebe, K., Sudmeier, L., and Micchelli, C.A. (2009). Adenomatous polyposis coli regulates Drosophila intestinal stem cell proliferation. Development 136, Lehane, M.J., Blakemore, D., Williams, S., and Moffatt, M.R. (1995). Regulation of digestive enzyme levels in insects. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 110, Lemaitre, B., and Miguel-Aliaga, I. (2013). The digestive tract of Drosophila melanogaster. Annu. Rev. Genet. 47, Lo, P.C.H., and Frasch, M. (1999). Sequence and expression of myoglianin, a novel Drosophila gene of the TGF-b superfamily. Mech. Dev. 86, Marianes, A., and Spradling, A.C. (2013). Physiological and stem cell compartmentalization within the Drosophila midgut. elife 2, e Morgan, N.S., Skovronsky, D.M., Artavanis-Tsakonas, S., and Mooseker, M.S. (1994). The molecular cloning and characterization of Drosophila melanogaster myosin-ia and myosin-ib. J. Mol. Biol. 239, Musselman, L.P., Fink, J.L., Narzinski, K., Ramachandran, P.V., Hathiramani, S.S., Cagan, R.L., and Baranski, T.J. (2011). A high-sugar diet produces obesity and insulin resistance in wild-type Drosophila. Dis. Model. Mech. 4, Nguyen, M., Parker, L., and Arora, K. (2000). Identification of maverick, a novel member of the TGF-b superfamily in Drosophila. Mech. Dev. 95, Ogiso, Y., Tsuneizumi, K., Masuda, N., Sato, M., and Tabata, T. (2011). Robustness of the Dpp morphogen activity gradient depends on negative feedback regulation by the inhibitory Smad, Dad. Dev. Growth Differ. 53, Park, J.-H., and Kwon, J.Y. (2011). Heterogeneous expression of Drosophila gustatory receptors in enteroendocrine cells. PLoS ONE 6, e Parker, L., Ellis, J.E., Nguyen, M.Q., and Arora, K. (2006). The divergent TGF-b ligand Dawdle utilizes an activin pathway to influence axon guidance in Drosophila. Development 133, Cell Reports 9, , October 9, 2014 ª2014 The Authors 347
14 Peterson, A.J., Jensen, P.A., Shimell, M., Stefancsik, R., Wijayatonge, R., Herder, R., Raftery, L.A., and O Connor, M.B. (2012). R-Smad competition controls activin receptor output in Drosophila. PLoS ONE 7, e Quesada, I., Tudurí, E., Ripoll, C., and Nadal, A. (2008). Physiology of the pancreatic a-cell and glucagon secretion: role in glucose homeostasis and diabetes. J. Endocrinol. 199, Rulifson, E.J., Kim, S.K., and Nusse, R. (2002). Ablation of insulin-producing neurons in flies: growth and diabetic phenotypes. Science 296, Saltiel, A.R., and Kahn, C.R. (2001). Insulin signalling and the regulation of glucose and lipid metabolism. Nature 414, Sieber, M.H., and Thummel, C.S. (2012). Coordination of triacylglycerol and cholesterol homeostasis by DHR96 and the Drosophila LipA homolog magro. Cell Metab. 15, Terra, W.R., and Ferreira, C. (1994). Insect digestive enzymes: properties, compartmentalization and function. Comp. Biochem. Physiol. B Comp. Biochem. 109, Thompson, D.B., Treat-Clemons, L.G., and Doane, W.W. (1992). Tissue-specific and dietary control of alpha-amylase gene expression in the adult midgut of Drosophila melanogaster. J. Exp. Zool. 262, Veenstra, J.A., Agricola, H.-J., and Sellami, A. (2008). Regulatory peptides in fruit fly midgut. Cell Tissue Res. 334, You, Y.J., Kim, J., Raizen, D.M., and Avery, L. (2008). Insulin, cgmp, and TGFb signals regulate food intake and quiescence in C. elegans: a model for satiety. Cell Metab. 7, Zheng, X., Wang, J., Haerry, T.E., Wu, A.Y.H., Martin, J., O Connor, M.B., Lee, C.-H.J., and Lee, T. (2003). TGF-b signaling activates steroid hormone receptor expression during neuronal remodeling in the Drosophila brain. Cell 112, Zhu, C.C., Boone, J.Q., Jensen, P.A., Hanna, S., Podemski, L., Locke, J., Doe, C.Q., and O Connor, M.B. (2008). Drosophila Activin- and the Activin-like product Dawdle function redundantly to regulate proliferation in the larval brain. Development 135, Cell Reports 9, , October 9, 2014 ª2014 The Authors
15 SUPPLEMENTAL RESULTS Text S1. Related to Figures 1D, S2 and S3. Genes encoding proteases are regulated differently upon feeding Dietary glucose has been shown to repress amylase activity, in a response known as glucose repression (Benkel and Hickey, 1986; Hickey and Benkel, 1982). To expand upon this work, we analyzed the transcriptomic changes in response to glucose in the diet by performing RNA-seq with adult guts (consisting of the crop, the midgut and the hindgut; minus malpighian tubules), to exclude confounding results when analysing whole flies or the developing larvae. Our RNA-seq data showed that the transcriptome of guts derived from flies that were fed on an agar diet for 24h was very similar to flies that were fed on 10% glucose; with only 73 genes differentially expressed. Although this list is small, 62 (or 85%) of the genes changed in a similar manner between agar-fed and standard medium fed (Fig S2), indicating that glucose in the standard medium is responsible for the differential expression. In general, our results showed that more genes are repressed than induced by glucose feeding. Notably, the expression of all genes with carbohydrase (GO: hydrolases acting on glycosyl bonds) and lipase function were found to be reduced, relative to starved flies (Figure S2). To know if a similar regulatory loop is applicable to midgut expressed protease, we compared the transcriptome of agar and standard medium fed guts. Intriguingly, we found the expression of many genes encoding proteases to be enhanced (21 out of 47, or 44%) in standard medium fed guts relative to starved guts (Figure S3 and Table S3). These results suggest that the strategy underlying the regulation of protease expression in the adult midgut for the purpose of digestion may be fundamentally different from that of carbohydrases and lipases.
16 SUPPLEMENTAL FIGURES AND LEGENDS Figure S1. Related to Figures 1 and 2. Carbohydrate in the diet represses amylase protein expression and activity in the midgut. (A) Anti-amylase antibody generated in this study recognizes a ~55KDa band in wild-type (OR) flies which is absent in amy null flies. The size of this band is close to the predicted size of amylase encoded by both Amy-p and Amy-d (predicted to be 53.8KDa). Due to the high amino acid sequence identity between Amy-p and Amy-d, our antibody does not distinguish between the two amylases. (B) Western Blot analysis for Drosophila amylase protein on whole flies derived protein lysate. Amylase protein was reduced in flies fed ad libitum on a standard medium. Tubulin was used as a loading control. Band intensity from 3 independent experiments were quantified and expressed in arbitrary units (AU) on the right. (C) Amylase activity of starved and glucose-fed flies. Amylase activity, expressed as mu activity per unit protein, was consistently higher when flies were starved. Values are mean ±SEM. (D) Repression analysis on flies fed ad libitum with starch for 24h. Starch reduced Mal-A1 expression. Transcript levels are expressed relative to starved as mean ±SEM. (E) Repression analysis on flies fed ad libitum with standard laboratory medium without glucose and the same medium supplemented with 10% glucose. There was no significant reduction in the level of Mal-A1 and Amy-p between flies fed either diet. Data is expressed relative to rp49 as mean ±SEM. Figure S2. Related to Figure 1D. Gene expression changes between agar and glucose fed adult guts. Gene ontology (GO) analysis was performed with GOrilla. GO enrichment analysis for the complete set of 73 differentially regulated genes (at least 2- fold) is provided. Genes with no enriched GO, and genes which were not differentially expressed (i.e. <2 fold and/or p>0.05) in standard medium fed guts are also included. The FDR q-value is the correction for multiple testing using the Benjamini and Hochberg method. Enrichment is calculated using formula: (b/n) / (B/N), where N, is the total number of genes; B, is the total number of genes associated with a specific GO term; n, is the number of genes in the top of the input list or in the target set and b, is the number of genes in the intersection. Glucose effect refers to the transcript level in glucose-fed guts relative to agar-fed guts. Standard medium effect refers to the transcript level in
17 standard medium-fed guts relative to agar-fed guts. Red bar represents decreased expression, while green bar represents increased expression relative to agar fed flies. Figure S3. Related to Figure 1D. Gene expression changes between agar and standard medium fed adult guts. A heat map of triplicate experiments (left panel). GO enrichment for the 741 differentially regulated genes (at least 2-fold) is provided on the right panel. GO term is provided on the top. Each horizontal line (red or green) represent one gene. Red horizontal lines represent genes with decreased expression in standard medium fed relative to starved guts. Green horizontal lines represent genes with increased expression in standard medium fed relative to starved guts. The number of genes from our RNA-seq in each GO is provided next to the GO term. See also Table S3 for details. Figure S4. Related to Figures 3 and 4. Amylase expression when TGFβ/Activin signalling was compromised in the midgut. (A) Immunostaining of whole gut samples from two independent Smad2 RNAi knockdown. Knockdown of Smad2 abolished amylase repression by glucose. In wild-type flies (MyoIA-gal4 ts >mcherry-ir), amylase expression was reduced predominantly in the R2 and R4 region of the midgut after 24h on a glucose-only diet. This repression was lost in flies whereby Smad2-IR was induced in the adult midgut enterocytes (MyoIA-gal4 ts >Smad2-IR (1) and MyoIA-gal4 ts >Smad2- IR (2)). Scale bar 1000µM. (B) Clonal analysis was performed using the esg ts F/O system. Clones in the R1 region of the guts with Babo C or Put knocked down (green), expressed higher level of amylase (red) compared to the surrounding enterocytes in glucose fed flies. The difference in amylase expression between knockdown clones and the surrounding region were less obvious compared to the R2b region as amylase is highly expressed in the R1 region (see with Figure 4E). Scale bar 50µM. Figure S5. Related to Figure 5. Metabolic effects of compromising TGFβ/Activin signalling in the adult midgut. (A) Amounts of glucose, trehalose, glycogen and TAG derived from whole flies fed on a high sugar diet (standard medium supplemented with 20% sucrose, 5% maltose and 5% starch) for 2 weeks. Amounts were normalized to the
18 level of protein and expressed as µg/µg protein. There were no significant differences between MyoIA-gal4 ts >Smad2-IR(1) and MyoIA-gal4 ts >mcherry-ir. Values are mean ±SEM. (B) Survival analysis on a nutrient poor medium deprived of sugar. There was no significant differences in survival between MyoIA-gal4 ts >Smad2-IR(1) and MyoIAgal4 ts >mcherry-ir. Figure S6. Related to Figure 6. Nutritious sugar induction of Daw is required for glucose repression. (A) Expression of Daw in flies fed ad libitum with various sugars (glucose, trehalose, fructose or arabinose), protein (casein), fatty acid (palmitic acid) or standard medium for 24h. Daw expression was induced when flies were fed with nutritious sugars, but not arabinose, casein or palmitic acid. Data is expressed relative to starved flies as mean ±SEM. (B) Mal-A1 and Amy-p expression in whole-gut samples as quantified by RT-qPCR. Both Mal-A1 and Amy-p were reduced when Daw was overexpressed ubiquitously. Data is expressed relative to rp49 as mean ±SEM. (C) Immunostaining of whole-gut samples derived from flies overexpressing Daw. Overexpression of Daw (MyoIA-gal4 ts > Daw) reduced amylase expression (red) relative to control (MyoIA-gal4 ts > yw). Scale bar 1000µM. (D) Amy-p expression in flies overexpressing Daw through different tissue-specific drivers. Overexpression of Daw with various gal4 drivers all reduced Amy-p expression. Data is expressed relative to control (driver-gal4>yw) as mean ±SEM. (E) Amy-p repression was analysed when Daw is knocked down in specific tissue. Fat body knockdown (ppl-gal4 and C564-gal4 ts ) of Daw abolished glucose repression of Amy-p. Data is expressed relative to starved flies as mean ±SEM. (F) Mal-A1 and Amy-p expression as quantified by RT-qPCR when Daw was knocked down in the fat body. Both Mal-A1 and Amy-p level were elevated when Daw was knocked down in the adult fat body tissue. Data is expressed relative to rp49 as mean ±SEM.
19 Figure S1 A amy null OR B α-amy OR (Whole flies) Agar Standard medium Band intensity (AU) C * 15 * Amylase activity (Whole guts) 10 5 Tubulin ~55KDa 0 Agar Standard medium 0 Agar 10% Glucose D Transcript relative to starved (Whole flies) 1.5 Standard medium Glucose Starch *** *** Mal-A1 * ** Amy-p *** E Transcript relative to rp49 (Whole flies) 0.10 Standard medium Standard medium % Glucose Mal-A1 Amy-p
20 Figure S2 GO term Description FDR q- value GO: GO: no GO enrichment hydrolase 3.82E activity, acting on glycosyl bonds oxidoreductase activity differentially expressed only in glucose 1.14E GO: glucose 2.05E transmembrane transporter activity GO: lipase activity 1.06E Enrichment Genes Molecular Function Glucose effect Standard medium effect Amyrel Mal-A1 Mal-A2 Mal-A3 Mal-A4 Mal-A7 Mal-A8 tobi Gal Mal-A6 CG31148 CG9463 CG9465 CG12224 CG5009 Cyp309a1 Men-b CG12766 CG9512 Cyp28d1 Cyp4s3 Cyp6g1 Cyp12d1-p CG8665 CG9331 CG3902 CG31075 CG10960 CG1208 CG6484 CG6271 CG6277 CG31089 CG8093 CenG1A CG13160 CG32152 CG44098 CG32751 CG9396 CG12512 Reg-2 CG1139 GstE6 GstD10 gb CG31741 smp-30 CG31104 CG11891 CG11889 CG11878 CG10559 CG CG CG CG CG CG CG CG CG CG15093 CG7433 CG3348 CG10725 GstE9 CG6283 CG9466 CG9468 Jon99Ci net ect - alpha-amylase alpha-glucosidase alpha-glucosidase alpha-glucosidase alpha-glucosidase alpha-glucosidase alpha-glucosidase alpha-glucosidase beta-galactosidase catalytic activity glucosylceramidase alpha-mannosidase alpha-mannosidase oxidoreductase activity acyl-coa dehydrogenase electron carrier activity malate dehydrogenase alditol:nadp+ 1-oxidoreductase choline dehydrogenase electron carrier activity electron carrier activity electron carrier activity electron carrier activity formyltetrahydrofolate dehydrogenase oxidoreductase activity acyl-coa dehydrogenase aldehyde dehydrogenase (NAD) activity glucose transmembrane transporter glucose transmembrane transporter glucose transmembrane transporter phosphatidylcholine 1-acylhydrolase phosphatidylcholine 1-acylhydrolase triglyceride lipase lipase activity ARF GTPase activator peptidase activity protein-arginine N-methyltransferase organic cation transmembrane transporter pantetheine hydrolase activity pyruvate transmembrane transporter long-chain fatty acid-coa ligase hydrolase amino acid transmembrane transporter glutathione transferase activity glutathione transferase activity amino acid transmembrane transporter scavenger receptor activity calcium ion binding phosphorus-containing groups transferase phosphorus-containing groups transferase phosphorus-containing groups transferase phosphorus-containing groups transferase phosphorus-containing groups transferase 3-hydroxyisobutyrate dehydrogenase 4-aminobutyrate transaminase chitin binding 4-aminobutyrate transaminase glutathione transferase activity phosphatidylcholine 1-acylhydrolase alpha-mannosidase alpha-mannosidase serine-type endopeptidase transcription factor
21 Figure S3 oxidation-reduction process (66: 6;60) lipid metabolic process (47: 7;40) lipid catabolic process (17: 4;13) nucleosome assembly (10:10;0) carbohydrate metabolic process (35: 1;34) organic acid catabolic process (13: 2;11) carboxylic acid metabolic process (35: 6;29) sphingolipid catabolic process (5: 0;5) regulation of hormone levels (9: 0;9) proteolysis (47: 21;26) RPKM value Standard medium (3 repeats) Agar (3 repeats) not in a GO group (545: 201;344)
22 esg F/O>Put-IR (R1 region) ts esg F/O>BaboC-IR (R1 region) ts MyoIA-gal4ts >Smox-IR (2) (10% Glucose) MyoIA-gal4ts >Smox-IR (2) (Agar) MyoIA-gal4ts >Smox-IR (1) (10% Glucose) MyoIA-gal4ts >Smox-IR (1) (Agar) MyoIA-gal4ts >mcherry-ir (10% Glucose) MyoIA-gal4ts >mcherry-ir (Agar) Figure S4 A AMY DAPI AMY DAPI AMY DAPI AMY DAPI AMY DAPI AMY DAPI B AMY DAPI BaboC-IR DAPI BaboC- IR AMY AMY DAPI Put-IR DAPI Put-IR AMY
23 Figure S5 A MyoIA-gal4 ts >Smad2-IR(1) MyoIA-gal4 ts >mcherry-ir B 150 MyoIA-gal4 ts >Smad2-IR(1) MyoIA-gal4 ts >mcherry-ir 2.0 Glucose 15 Trehalose 8 Glycogen 2.0 TAG µg/µg protein Percent survival Days
24 Figure S6 A 4 Daw transcipt relative to starved (Whole flies) * *** *** ** B Transcipt relative to rp49 (Whole guts) *** Da-gal4 ts >Daw Da-gal4 ts >yw ** C MyoIA-gal4 ts >yw (Agar) MyoIA-gal4 ts >Daw (Agar) 0 Standard medium Glucose Fructose Trehalose Arabinose Casein Palmitic acid AMY DAPI AMY DAPI D 0.0 Amy-p transcipt relative to gal4>yw (Whole flies) Mal-A1 ** how-gal4 ts >Daw ** yolk-gal4> Daw Amy-p * ppl-gal4> Daw ** MyoIA-gal4 ts >Daw E Amy-p transcipt relative to starved (Whole flies) Da-gal4 ts ** ** MyoIA-gal4 ts MHC-gal4 elav-gal4 ppl-gal4 gal4>daw-ir gal4>w Ryu ** C564-gal4 ts F Trascript relative to rp49 (Whole flies) ** Mal-A1 C564-gal4 ts >Daw-IR C564-gal4 ts >w Ryu * Amy-p
Midgut-Derived Activin Regulates Glucagon-like Action in the Fat Body and Glycemic Control
 Article Midgut-Derived Activin Regulates Glucagon-like Action in the Fat Body and Glycemic Control Graphical Abstract Authors Wei Song, Daojun Cheng, Shangyu Hong,..., Michael B. O Connor, Pavlos Pissios,
Article Midgut-Derived Activin Regulates Glucagon-like Action in the Fat Body and Glycemic Control Graphical Abstract Authors Wei Song, Daojun Cheng, Shangyu Hong,..., Michael B. O Connor, Pavlos Pissios,
50mM D-Glucose. Percentage PI. L-Glucose
 Monica Dus, Minrong Ai, Greg S.B Suh. Taste-independent nutrient selection is mediated by a brain-specific Na+/solute cotransporter in Drosophila. Control + Phlorizin mm D-Glucose 1 2mM 1 L-Glucose Gr5a;Gr64a
Monica Dus, Minrong Ai, Greg S.B Suh. Taste-independent nutrient selection is mediated by a brain-specific Na+/solute cotransporter in Drosophila. Control + Phlorizin mm D-Glucose 1 2mM 1 L-Glucose Gr5a;Gr64a
Supplementary Information
 Supplementary Information Supplementary s Supplementary 1 All three types of foods suppress subsequent feeding in both sexes when the same food is used in the pre-feeding test feeding. (a) Adjusted pre-feeding
Supplementary Information Supplementary s Supplementary 1 All three types of foods suppress subsequent feeding in both sexes when the same food is used in the pre-feeding test feeding. (a) Adjusted pre-feeding
SUPPLEMENTARY INFORMATION
 doi:10.1038/nature12652 Supplementary Figure 1. PRDM16 interacts with endogenous EHMT1 in brown adipocytes. Immunoprecipitation of PRDM16 complex by flag antibody (M2) followed by Western blot analysis
doi:10.1038/nature12652 Supplementary Figure 1. PRDM16 interacts with endogenous EHMT1 in brown adipocytes. Immunoprecipitation of PRDM16 complex by flag antibody (M2) followed by Western blot analysis
effects on organ development. a-f, Eye and wing discs with clones of ε j2b10 show no
 Supplementary Figure 1. Loss of function clones of 14-3-3 or 14-3-3 show no significant effects on organ development. a-f, Eye and wing discs with clones of 14-3-3ε j2b10 show no obvious defects in Elav
Supplementary Figure 1. Loss of function clones of 14-3-3 or 14-3-3 show no significant effects on organ development. a-f, Eye and wing discs with clones of 14-3-3ε j2b10 show no obvious defects in Elav
UNIVERSITY OF PNG SCHOOL OF MEDICINE AND HEALTH SCIENCES DIVISION OF BASIC MEDICAL SCIENCES DISCIPLINE OF BIOCHEMISTRY AND MOLECULAR BIOLOGY
 1 UNIVERSITY OF PNG SCHOOL OF MEDICINE AND HEALTH SCIENCES DIVISION OF BASIC MEDICAL SCIENCES DISCIPLINE OF BIOCHEMISTRY AND MOLECULAR BIOLOGY GLUCOSE HOMEOSTASIS An Overview WHAT IS HOMEOSTASIS? Homeostasis
1 UNIVERSITY OF PNG SCHOOL OF MEDICINE AND HEALTH SCIENCES DIVISION OF BASIC MEDICAL SCIENCES DISCIPLINE OF BIOCHEMISTRY AND MOLECULAR BIOLOGY GLUCOSE HOMEOSTASIS An Overview WHAT IS HOMEOSTASIS? Homeostasis
Supplementary Information
 Supplementary Information Akt regulates hepatic metabolism by suppressing a Foxo1 dependent global inhibition of adaptation to nutrient intake Mingjian Lu 1, Min Wan 1, Karla F. Leavens 1, Qingwei Chu
Supplementary Information Akt regulates hepatic metabolism by suppressing a Foxo1 dependent global inhibition of adaptation to nutrient intake Mingjian Lu 1, Min Wan 1, Karla F. Leavens 1, Qingwei Chu
Functional studies of Drosophila zinc transporters reveal the mechanism for dietary zinc absorption and regulation
 Qin et al. BMC Biology 2013, 11:101 RESEARCH ARTICLE Open Access Functional studies of Drosophila zinc transporters reveal the mechanism for dietary zinc absorption and regulation Qiuhong Qin, Xiaoxi Wang
Qin et al. BMC Biology 2013, 11:101 RESEARCH ARTICLE Open Access Functional studies of Drosophila zinc transporters reveal the mechanism for dietary zinc absorption and regulation Qiuhong Qin, Xiaoxi Wang
Summary and conclusions
 Summary and conclusions 133 Part 1: Toxicity effects of Cry toxins upon hemocoelic delivery to A. janata larvae The first investigation was aimed to find out whether other modes of delivery of Cry toxins
Summary and conclusions 133 Part 1: Toxicity effects of Cry toxins upon hemocoelic delivery to A. janata larvae The first investigation was aimed to find out whether other modes of delivery of Cry toxins
SUPPLEMENTARY INFORMATION Glucosylceramide synthase (GlcT-1) in the fat body controls energy metabolism in Drosophila
 SUPPLEMENTARY INFORMATION Glucosylceramide synthase (GlcT-1) in the fat body controls energy metabolism in Drosophila Ayako Kohyama-Koganeya, 1,2 Takuji Nabetani, 1 Masayuki Miura, 2,3 Yoshio Hirabayashi
SUPPLEMENTARY INFORMATION Glucosylceramide synthase (GlcT-1) in the fat body controls energy metabolism in Drosophila Ayako Kohyama-Koganeya, 1,2 Takuji Nabetani, 1 Masayuki Miura, 2,3 Yoshio Hirabayashi
Identification of Sphingolipid Metabolites That Induce Obesity via Misregulation of Appetite, Caloric Intake and Fat Storage in Drosophila
 Identification of Sphingolipid Metabolites That Induce Obesity via Misregulation of Appetite, Caloric Intake and Fat Storage in Drosophila Stanley M. Walls, Jr 1, Steve J. Attle 1, Gregory B. Brulte 1,
Identification of Sphingolipid Metabolites That Induce Obesity via Misregulation of Appetite, Caloric Intake and Fat Storage in Drosophila Stanley M. Walls, Jr 1, Steve J. Attle 1, Gregory B. Brulte 1,
Supplementary Figures
 J. Cell Sci. 128: doi:10.1242/jcs.173807: Supplementary Material Supplementary Figures Fig. S1 Fig. S1. Description and/or validation of reagents used. All panels show Drosophila tissues oriented with
J. Cell Sci. 128: doi:10.1242/jcs.173807: Supplementary Material Supplementary Figures Fig. S1 Fig. S1. Description and/or validation of reagents used. All panels show Drosophila tissues oriented with
marker. DAPI labels nuclei. Flies were 20 days old. Scale bar is 5 µm. Ctrl is
 Supplementary Figure 1. (a) Nos is detected in glial cells in both control and GFAP R79H transgenic flies (arrows), but not in deletion mutant Nos Δ15 animals. Repo is a glial cell marker. DAPI labels
Supplementary Figure 1. (a) Nos is detected in glial cells in both control and GFAP R79H transgenic flies (arrows), but not in deletion mutant Nos Δ15 animals. Repo is a glial cell marker. DAPI labels
A Neuronal Relay Mediates a Nutrient Responsive Gut/Fat Body Axis Regulating Energy Homeostasis in Adult Drosophila
 Article A Neuronal Relay Mediates a Nutrient Responsive Gut/Fat Body Axis Regulating Energy Homeostasis in Adult Drosophila Graphical Abstract Authors Alessandro Scopelliti, Christin Bauer, Yachuan Yu,...,
Article A Neuronal Relay Mediates a Nutrient Responsive Gut/Fat Body Axis Regulating Energy Homeostasis in Adult Drosophila Graphical Abstract Authors Alessandro Scopelliti, Christin Bauer, Yachuan Yu,...,
Mir-184 affects lifespan via regulating the Activin pathway in Drosophila
 1 Mir-184 affects lifespan via regulating the Activin pathway in Drosophila Senior Thesis Presented to The Faculty of the School of Arts and Sciences Brandeis University Undergraduate Program in the Department
1 Mir-184 affects lifespan via regulating the Activin pathway in Drosophila Senior Thesis Presented to The Faculty of the School of Arts and Sciences Brandeis University Undergraduate Program in the Department
Fat body glycogen serves as a metabolic safeguard for the maintenance of sugar levels in Drosophila
 First posted online on 21 February 2018 as 10.1242/dev.158865 Access the most recent version at http://dev.biologists.org/lookup/doi/10.1242/dev.158865 Fat body glycogen serves as a metabolic safeguard
First posted online on 21 February 2018 as 10.1242/dev.158865 Access the most recent version at http://dev.biologists.org/lookup/doi/10.1242/dev.158865 Fat body glycogen serves as a metabolic safeguard
Supplementary Materials for
 www.sciencesignaling.org/cgi/content/full/8/364/ra18/dc1 Supplementary Materials for The tyrosine phosphatase (Pez) inhibits metastasis by altering protein trafficking Leila Belle, Naveid Ali, Ana Lonic,
www.sciencesignaling.org/cgi/content/full/8/364/ra18/dc1 Supplementary Materials for The tyrosine phosphatase (Pez) inhibits metastasis by altering protein trafficking Leila Belle, Naveid Ali, Ana Lonic,
Week 3 The Pancreas: Pancreatic ph buffering:
 Week 3 The Pancreas: A gland with both endocrine (secretion of substances into the bloodstream) & exocrine (secretion of substances to the outside of the body or another surface within the body) functions
Week 3 The Pancreas: A gland with both endocrine (secretion of substances into the bloodstream) & exocrine (secretion of substances to the outside of the body or another surface within the body) functions
What systems are involved in homeostatic regulation (give an example)?
 1 UNIVERSITY OF PNG SCHOOL OF MEDICINE AND HEALTH SCIENCES DIVISION OF BASIC MEDICAL SCIENCES DISCIPLINE OF BIOCHEMISTRY AND MOLECULAR BIOLOGY GLUCOSE HOMEOSTASIS (Diabetes Mellitus Part 1): An Overview
1 UNIVERSITY OF PNG SCHOOL OF MEDICINE AND HEALTH SCIENCES DIVISION OF BASIC MEDICAL SCIENCES DISCIPLINE OF BIOCHEMISTRY AND MOLECULAR BIOLOGY GLUCOSE HOMEOSTASIS (Diabetes Mellitus Part 1): An Overview
Supplementary Figure 1. Procedures to independently control fly hunger and thirst states.
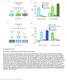 Supplementary Figure 1 Procedures to independently control fly hunger and thirst states. (a) Protocol to produce exclusively hungry or thirsty flies for 6 h water memory retrieval. (b) Consumption assays
Supplementary Figure 1 Procedures to independently control fly hunger and thirst states. (a) Protocol to produce exclusively hungry or thirsty flies for 6 h water memory retrieval. (b) Consumption assays
RASA: Robust Alternative Splicing Analysis for Human Transcriptome Arrays
 Supplementary Materials RASA: Robust Alternative Splicing Analysis for Human Transcriptome Arrays Junhee Seok 1*, Weihong Xu 2, Ronald W. Davis 2, Wenzhong Xiao 2,3* 1 School of Electrical Engineering,
Supplementary Materials RASA: Robust Alternative Splicing Analysis for Human Transcriptome Arrays Junhee Seok 1*, Weihong Xu 2, Ronald W. Davis 2, Wenzhong Xiao 2,3* 1 School of Electrical Engineering,
Supplementary Fig. 1 V-ATPase depletion induces unique and robust phenotype in Drosophila fat body cells.
 Supplementary Fig. 1 V-ATPase depletion induces unique and robust phenotype in Drosophila fat body cells. a. Schematic of the V-ATPase proton pump macro-complex structure. The V1 complex is composed of
Supplementary Fig. 1 V-ATPase depletion induces unique and robust phenotype in Drosophila fat body cells. a. Schematic of the V-ATPase proton pump macro-complex structure. The V1 complex is composed of
Digestion of Carbohydrates. BCH 340 Lecture 2
 Digestion of Carbohydrates BCH 340 Lecture 2 Carbohydrates are called carbohydrates because they are essentially hydrates of carbon (i.e. they are composed of carbon and water and have a composition of
Digestion of Carbohydrates BCH 340 Lecture 2 Carbohydrates are called carbohydrates because they are essentially hydrates of carbon (i.e. they are composed of carbon and water and have a composition of
Drosophila HNF4 Directs a Switch in Lipid Metabolism that Supports the Transition to Adulthood
 Article Drosophila HNF4 Directs a Switch in Lipid Metabolism that Supports the Transition to Adulthood Graphical Abstract Authors Gilles Storelli, Hyuck-Jin Nam, Judith Simcox, Claudio J. Villanueva, Carl
Article Drosophila HNF4 Directs a Switch in Lipid Metabolism that Supports the Transition to Adulthood Graphical Abstract Authors Gilles Storelli, Hyuck-Jin Nam, Judith Simcox, Claudio J. Villanueva, Carl
The effect of lactose and fructose based- diets on Drosophila melanogaster maturation time
 The effect of lactose and fructose based- diets on Drosophila melanogaster maturation time Nicole D. Gehring, Mandy L. Huang, Brian B. Kahnamelli, Rebecca H. Liu Abstract Using the model organism, Drosophila
The effect of lactose and fructose based- diets on Drosophila melanogaster maturation time Nicole D. Gehring, Mandy L. Huang, Brian B. Kahnamelli, Rebecca H. Liu Abstract Using the model organism, Drosophila
Supporting Information
 Supporting Information Fig. S1. Overexpression of Rpr causes progenitor cell death. (A) TUNEL assay of control intestines. No progenitor cell death could be observed, except that some ECs are undergoing
Supporting Information Fig. S1. Overexpression of Rpr causes progenitor cell death. (A) TUNEL assay of control intestines. No progenitor cell death could be observed, except that some ECs are undergoing
Chapter 4 Reading Guide
 Chapter 4 Reading Guide 1. How many covalent bonds does carbon (C) form? 2. What is the chemical formula for glucose? 3. List the major dietary monosaccharides and disaccharides. What are the components
Chapter 4 Reading Guide 1. How many covalent bonds does carbon (C) form? 2. What is the chemical formula for glucose? 3. List the major dietary monosaccharides and disaccharides. What are the components
Metabolic integration and Regulation
 Metabolic integration and Regulation 109700: Graduate Biochemistry Trimester 2/2016 Assistant Prof. Dr. Panida Khunkaewla kpanida@sut.ac.th School of Chemistry Suranaree University of Technology 1 Overview
Metabolic integration and Regulation 109700: Graduate Biochemistry Trimester 2/2016 Assistant Prof. Dr. Panida Khunkaewla kpanida@sut.ac.th School of Chemistry Suranaree University of Technology 1 Overview
Supplementary Figure 1 hlrrk2 promotes CAP dependent protein translation.
 ` Supplementary Figure 1 hlrrk2 promotes CAP dependent protein translation. (a) Overexpression of hlrrk2 in HeLa cells enhances total protein synthesis in [35S] methionine/cysteine incorporation assays.
` Supplementary Figure 1 hlrrk2 promotes CAP dependent protein translation. (a) Overexpression of hlrrk2 in HeLa cells enhances total protein synthesis in [35S] methionine/cysteine incorporation assays.
ENERGY FROM INGESTED NUTREINTS MAY BE USED IMMEDIATELY OR STORED
 QUIZ/TEST REVIEW NOTES SECTION 1 SHORT TERM METABOLISM [METABOLISM] Learning Objectives: Identify primary energy stores of the body Differentiate the metabolic processes of the fed and fasted states Explain
QUIZ/TEST REVIEW NOTES SECTION 1 SHORT TERM METABOLISM [METABOLISM] Learning Objectives: Identify primary energy stores of the body Differentiate the metabolic processes of the fed and fasted states Explain
Carbohydrates Dr. Ameerah M. Zarzoor
 Carbohydrates Dr. Ameerah M. Zarzoor What Are Carbohydrates? Carbohydrates are the most abundant biomolecules on Earth Produced by plants during photosynthesis Carbohydrates are polyhydroxyl aldehydes
Carbohydrates Dr. Ameerah M. Zarzoor What Are Carbohydrates? Carbohydrates are the most abundant biomolecules on Earth Produced by plants during photosynthesis Carbohydrates are polyhydroxyl aldehydes
The Physiology of Weight Regulation: Implications for Effective Clinical Care
 Roundtable on Obesity Solutions The Physiology of Weight Regulation: Implications for Effective Clinical Care Lee M. Kaplan, MD, PhD Obesity, Metabolism & Nutrition Institute Massachusetts General Hospital
Roundtable on Obesity Solutions The Physiology of Weight Regulation: Implications for Effective Clinical Care Lee M. Kaplan, MD, PhD Obesity, Metabolism & Nutrition Institute Massachusetts General Hospital
Molecular Structure and Function Polysaccharides as Energy Storage. Biochemistry
 1 1.Objectives Dr. Vijaya Khader Dr. MC Varadaraj To understand how polysaccharides act as energy source To understand the structure and energy generation process from glycogen To understand the structure
1 1.Objectives Dr. Vijaya Khader Dr. MC Varadaraj To understand how polysaccharides act as energy source To understand the structure and energy generation process from glycogen To understand the structure
Diet-induced plasticity in the adult Drosophila melanogaster midgut
 Diet-induced plasticity in the adult Drosophila melanogaster midgut Honors Thesis Presented to the College of Arts and Sciences, Cornell University in Partial Fulfillment of the Requirements for the Biological
Diet-induced plasticity in the adult Drosophila melanogaster midgut Honors Thesis Presented to the College of Arts and Sciences, Cornell University in Partial Fulfillment of the Requirements for the Biological
We are IntechOpen, the world s leading publisher of Open Access books Built by scientists, for scientists. International authors and editors
 We are IntechOpen, the world s leading publisher of Open Access books Built by scientists, for scientists 4,000 116,000 120M Open access books available International authors and editors Downloads Our
We are IntechOpen, the world s leading publisher of Open Access books Built by scientists, for scientists 4,000 116,000 120M Open access books available International authors and editors Downloads Our
Comparison of open chromatin regions between dentate granule cells and other tissues and neural cell types.
 Supplementary Figure 1 Comparison of open chromatin regions between dentate granule cells and other tissues and neural cell types. (a) Pearson correlation heatmap among open chromatin profiles of different
Supplementary Figure 1 Comparison of open chromatin regions between dentate granule cells and other tissues and neural cell types. (a) Pearson correlation heatmap among open chromatin profiles of different
a. What is the stimulus? Consuming a large pumpkin spice muffin and caramel macchiato.
 : Homeostasis and Macromolecules Unit Study Guide Homeostasis 1. Define homeostasis and give an example. Homeostasis is the ability of the body to maintain relatively constant internal physical and chemical
: Homeostasis and Macromolecules Unit Study Guide Homeostasis 1. Define homeostasis and give an example. Homeostasis is the ability of the body to maintain relatively constant internal physical and chemical
Problem Set 5 KEY
 2006 7.012 Problem Set 5 KEY ** Due before 5 PM on THURSDAY, November 9, 2006. ** Turn answers in to the box outside of 68-120. PLEASE WRITE YOUR ANSWERS ON THIS PRINTOUT. 1. You are studying the development
2006 7.012 Problem Set 5 KEY ** Due before 5 PM on THURSDAY, November 9, 2006. ** Turn answers in to the box outside of 68-120. PLEASE WRITE YOUR ANSWERS ON THIS PRINTOUT. 1. You are studying the development
Name # Class Regents Review: Characteristics of Life and Biochemistry
 Name # Class Regents Review: Characteristics of Life and Biochemistry 6. Some processes that occur in a cell are listed below. A. utilize energy B. detect changes in the environment C. rearrange and synthesize
Name # Class Regents Review: Characteristics of Life and Biochemistry 6. Some processes that occur in a cell are listed below. A. utilize energy B. detect changes in the environment C. rearrange and synthesize
Organic Molecules. 8/27/2004 Mr. Davenport 1
 Organic Molecules 8/27/2004 Mr. Davenport 1 Carbohydrates Commonly called sugars and starches Consist of C, H, O with H:O ration 2:1 Usually classified as to sugar units Monosaccharide are single sugar
Organic Molecules 8/27/2004 Mr. Davenport 1 Carbohydrates Commonly called sugars and starches Consist of C, H, O with H:O ration 2:1 Usually classified as to sugar units Monosaccharide are single sugar
CHEMISTRY OF LIFE 05 FEBRUARY 2014
 CHEMISTRY OF LIFE 05 FEBRUARY 2014 In this lesson we will: Lesson Description Discuss inorganic compounds and their importance Discuss organic compounds and their biological importance. Summary Inorganic
CHEMISTRY OF LIFE 05 FEBRUARY 2014 In this lesson we will: Lesson Description Discuss inorganic compounds and their importance Discuss organic compounds and their biological importance. Summary Inorganic
Topic 3.1 Nutrients. - Lipids are an essential part of the and are a part of cell in the body.
 Name: Topic 3.1 Nutrients Date: IB SEHS 3.1.1. List the macronutrients and micronutrients Macronutrients: - lipid (fat) - carbohydrate - protein - water (says the book) Micronutrients: - vitamins - minerals
Name: Topic 3.1 Nutrients Date: IB SEHS 3.1.1. List the macronutrients and micronutrients Macronutrients: - lipid (fat) - carbohydrate - protein - water (says the book) Micronutrients: - vitamins - minerals
2. In terms of appearance, what is the main difference between a monomer, dimer and a polymer?
 Biology Ms. Ye Name Date Block Monomers vs. Polymers 1. The prefix mono- means one. The prefix di- means two. The prefix poly- means many. Based on the given definitions, label the pictures of paperclips
Biology Ms. Ye Name Date Block Monomers vs. Polymers 1. The prefix mono- means one. The prefix di- means two. The prefix poly- means many. Based on the given definitions, label the pictures of paperclips
SUPPLEMENTARY INFORMATION
 doi: 10.1038/nature07173 SUPPLEMENTARY INFORMATION Supplementary Figure Legends: Supplementary Figure 1: Model of SSC and CPC divisions a, Somatic stem cells (SSC) reside adjacent to the hub (red), self-renew
doi: 10.1038/nature07173 SUPPLEMENTARY INFORMATION Supplementary Figure Legends: Supplementary Figure 1: Model of SSC and CPC divisions a, Somatic stem cells (SSC) reside adjacent to the hub (red), self-renew
BIOLOGICAL MOLECULES REVIEW-UNIT 1 1. The factor being tested in an experiment is the A. data. B. variable. C. conclusion. D. observation. 2.
 BIOLOGICAL MOLECULES REVIEW-UNIT 1 1. The factor being tested in an experiment is the A. data. B. variable. C. conclusion. D. observation. 2. A possible explanation for an event that occurs in nature is
BIOLOGICAL MOLECULES REVIEW-UNIT 1 1. The factor being tested in an experiment is the A. data. B. variable. C. conclusion. D. observation. 2. A possible explanation for an event that occurs in nature is
Digestion and Absorption
 Digestion and Absorption General Considerations - No absorption in esophagus, little in the stomach and vast majority of absorption occurs in small intestine. - The small intestine has specialized structures
Digestion and Absorption General Considerations - No absorption in esophagus, little in the stomach and vast majority of absorption occurs in small intestine. - The small intestine has specialized structures
Ch 7 Nutrition in humans
 Ch 7 Nutrition in humans Think about (Ch 7, p.2) 1. The stomach churns food into smaller pieces physically. The stomach wall secretes proteases to chemically digest proteins. It also releases hydrochloric
Ch 7 Nutrition in humans Think about (Ch 7, p.2) 1. The stomach churns food into smaller pieces physically. The stomach wall secretes proteases to chemically digest proteins. It also releases hydrochloric
College of Science Department of Biochemistry
 Dr. Abir Alghanouchi College of Science Department of Biochemistry Carbohydrates Carbohydrates are called carbohydrates because they are essentially hydrates of carbon(i.e. they are composed of carbon
Dr. Abir Alghanouchi College of Science Department of Biochemistry Carbohydrates Carbohydrates are called carbohydrates because they are essentially hydrates of carbon(i.e. they are composed of carbon
Supplementary Materials for
 www.sciencesignaling.org/cgi/content/full/8/407/ra127/dc1 Supplementary Materials for Loss of FTO in adipose tissue decreases Angptl4 translation and alters triglyceride metabolism Chao-Yung Wang,* Shian-Sen
www.sciencesignaling.org/cgi/content/full/8/407/ra127/dc1 Supplementary Materials for Loss of FTO in adipose tissue decreases Angptl4 translation and alters triglyceride metabolism Chao-Yung Wang,* Shian-Sen
Proteins. Biomolecules. Nucleic Acids. The Building Blocks of Life
 Proteins Biomolecules Nucleic Acids The Building Blocks of Life Carbohydrates Lipids Biomolecules are 1. Organic molecules that are (at least 1 Carbon molecule and often chains of Carbon) They all contain.
Proteins Biomolecules Nucleic Acids The Building Blocks of Life Carbohydrates Lipids Biomolecules are 1. Organic molecules that are (at least 1 Carbon molecule and often chains of Carbon) They all contain.
5.0 HORMONAL CONTROL OF CARBOHYDRATE METABOLISM
 5.0 HORMONAL CONTROL OF CARBOHYDRATE METABOLISM Introduction: Variety of hormones and other molecules regulate the carbohydrates metabolism. Some of these have already been cited in previous sections.
5.0 HORMONAL CONTROL OF CARBOHYDRATE METABOLISM Introduction: Variety of hormones and other molecules regulate the carbohydrates metabolism. Some of these have already been cited in previous sections.
Biochemistry Name: Practice Questions
 Name: Practice Questions 1. Carbohydrate molecules A and B come in contact with the cell membrane of the same cell. Molecule A passes through the membrane readily, but molecule B does not. It is most likely
Name: Practice Questions 1. Carbohydrate molecules A and B come in contact with the cell membrane of the same cell. Molecule A passes through the membrane readily, but molecule B does not. It is most likely
Head of College Scholars List Scheme. Summer Studentship Report Form
 Head of College Scholars List Scheme Summer Studentship 2019 Report Form This report should be completed by the student with his/her project supervisor. It should summarise the work undertaken during the
Head of College Scholars List Scheme Summer Studentship 2019 Report Form This report should be completed by the student with his/her project supervisor. It should summarise the work undertaken during the
Figure Nutrition: omnivore, herbivore, carnivore
 Figure 41.1 Nutrition: omnivore, herbivore, carnivore Essential Nutrients: Amino acids Fatty acids Vitamins Minerals Figure 41.2 Complete vs incomplete Omnivore vs herbivore (vegetarian) Table 41.1 Table
Figure 41.1 Nutrition: omnivore, herbivore, carnivore Essential Nutrients: Amino acids Fatty acids Vitamins Minerals Figure 41.2 Complete vs incomplete Omnivore vs herbivore (vegetarian) Table 41.1 Table
The addition of sugar moiety determines the blood group
 The addition of sugar moiety determines the blood group Sugars attached to glycoproteins and glycolipids on the surfaces of red blood cells determine the blood group termed A, B, and O. The A and B antigens
The addition of sugar moiety determines the blood group Sugars attached to glycoproteins and glycolipids on the surfaces of red blood cells determine the blood group termed A, B, and O. The A and B antigens
SUPPLEMENTARY INFORMATION
 doi:10.1038/nature10643 Supplementary Table 1. Identification of hecw-1 coding polymorphisms at amino acid positions 322 and 325 in 162 strains of C. elegans. WWW.NATURE.COM/NATURE 1 Supplementary Figure
doi:10.1038/nature10643 Supplementary Table 1. Identification of hecw-1 coding polymorphisms at amino acid positions 322 and 325 in 162 strains of C. elegans. WWW.NATURE.COM/NATURE 1 Supplementary Figure
Chapter 12. Ingestive Behavior
 Chapter 12 Ingestive Behavior Drinking a. fluid compartments b. osmometric thirst c. volumetric thirst Eating a. energy sources b. starting a meal c. stopping a meal d. eating disordersd Drinking a. fluid
Chapter 12 Ingestive Behavior Drinking a. fluid compartments b. osmometric thirst c. volumetric thirst Eating a. energy sources b. starting a meal c. stopping a meal d. eating disordersd Drinking a. fluid
Expanded View Figures
 Expanded View Figures A B C D E F G H I J K L Figure EV1. The dysregulated lipid metabolic phenotype of mouse models of metabolic dysfunction is most pronounced in the fasted state. A L Male 12-weeks-old
Expanded View Figures A B C D E F G H I J K L Figure EV1. The dysregulated lipid metabolic phenotype of mouse models of metabolic dysfunction is most pronounced in the fasted state. A L Male 12-weeks-old
Digestive and Excretory Systems
 Digestive and Excretory Systems Homeostasis Q: How are the materials that enter and leave your body related to the processes that maintain homeostasis? 30.1 How is the human body organized and regulated?
Digestive and Excretory Systems Homeostasis Q: How are the materials that enter and leave your body related to the processes that maintain homeostasis? 30.1 How is the human body organized and regulated?
Bio& 242 Unit 1 / Lecture 4
 Bio& 242 Unit 1 / Lecture 4 system: Gastric hormones GASTRIN: Secretion: By enteroendocrine (G) in gastric pits of the mucosa. Stimulus: Stomach distention and acid ph of chyme causes Gastrin. Action:
Bio& 242 Unit 1 / Lecture 4 system: Gastric hormones GASTRIN: Secretion: By enteroendocrine (G) in gastric pits of the mucosa. Stimulus: Stomach distention and acid ph of chyme causes Gastrin. Action:
Macromolecules. Polymer Overview: The 4 major classes of macromolecules also called are: 1) 2) 3) 4)
 Macromolecules Polymer Overview: The 4 major classes of macromolecules also called are: 1) 2) 3) 4) Q: Which of the above are polymers? (put a star by them). Polymer literally means. Polymers are long
Macromolecules Polymer Overview: The 4 major classes of macromolecules also called are: 1) 2) 3) 4) Q: Which of the above are polymers? (put a star by them). Polymer literally means. Polymers are long
6. The diagram below represents an interaction between parts of an organism.
 Endocrine Review 1. Base your answer to the following question on the diagram below and on your knowledge of biology. Each arrow in the diagram represents a different hormone released by the pituitary
Endocrine Review 1. Base your answer to the following question on the diagram below and on your knowledge of biology. Each arrow in the diagram represents a different hormone released by the pituitary
Do Now Makeups. 4. In which organelle would water and dissolved materials be stored? A. 1 B. 2 C. 3 D. 5. A. mitochondria B.
 Do Now Makeups Name: Date: 1. Which organelle is primarily concerned with the conversion of potential energy of organic compounds into suitable form for immediate use by the cell? A. mitochondria B. centrosomes
Do Now Makeups Name: Date: 1. Which organelle is primarily concerned with the conversion of potential energy of organic compounds into suitable form for immediate use by the cell? A. mitochondria B. centrosomes
Connections of Carbohydrate, Protein, and Lipid Metabolic Pathways
 OpenStax-CNX module: m44441 1 Connections of Carbohydrate, Protein, and Lipid Metabolic Pathways OpenStax College This work is produced by OpenStax-CNX and licensed under the Creative Commons Attribution
OpenStax-CNX module: m44441 1 Connections of Carbohydrate, Protein, and Lipid Metabolic Pathways OpenStax College This work is produced by OpenStax-CNX and licensed under the Creative Commons Attribution
Supplemental Figure S1. Expression of Cirbp mrna in mouse tissues and NIH3T3 cells.
 SUPPLEMENTAL FIGURE AND TABLE LEGENDS Supplemental Figure S1. Expression of Cirbp mrna in mouse tissues and NIH3T3 cells. A) Cirbp mrna expression levels in various mouse tissues collected around the clock
SUPPLEMENTAL FIGURE AND TABLE LEGENDS Supplemental Figure S1. Expression of Cirbp mrna in mouse tissues and NIH3T3 cells. A) Cirbp mrna expression levels in various mouse tissues collected around the clock
Nature Genetics: doi: /ng Supplementary Figure 1. Assessment of sample purity and quality.
 Supplementary Figure 1 Assessment of sample purity and quality. (a) Hematoxylin and eosin staining of formaldehyde-fixed, paraffin-embedded sections from a human testis biopsy collected concurrently with
Supplementary Figure 1 Assessment of sample purity and quality. (a) Hematoxylin and eosin staining of formaldehyde-fixed, paraffin-embedded sections from a human testis biopsy collected concurrently with
Why No Calorie makes No Sense
 Why No Calorie makes No Sense Nancy E. Rawson, M.Sc., Ph.D. Associate Director Monell Chemical Senses Center 3500 Market St. Philadelphia PA 19104-3308 nrawson@monell.org Outline Defining the problem How
Why No Calorie makes No Sense Nancy E. Rawson, M.Sc., Ph.D. Associate Director Monell Chemical Senses Center 3500 Market St. Philadelphia PA 19104-3308 nrawson@monell.org Outline Defining the problem How
18. PANCREATIC FUNCTION AND METABOLISM. Pancreatic secretions ISLETS OF LANGERHANS. Insulin
 18. PANCREATIC FUNCTION AND METABOLISM ISLETS OF LANGERHANS Some pancreatic functions have already been discussed in the digestion section. In this one, the emphasis will be placed on the endocrine function
18. PANCREATIC FUNCTION AND METABOLISM ISLETS OF LANGERHANS Some pancreatic functions have already been discussed in the digestion section. In this one, the emphasis will be placed on the endocrine function
Minireview Shrinkage control: regulation of insulin-mediated growth by FOXO transcription factors Thomas P Neufeld
 Journal of Biology BioMed Central Minireview Shrinkage control: regulation of insulin-mediated growth by FOXO transcription factors Thomas P Neufeld Address: Department of Genetics, Cell Biology, and Development,
Journal of Biology BioMed Central Minireview Shrinkage control: regulation of insulin-mediated growth by FOXO transcription factors Thomas P Neufeld Address: Department of Genetics, Cell Biology, and Development,
Digestive System Processes *
 OpenStax-CNX module: m44742 1 Digestive System Processes * OpenStax This work is produced by OpenStax-CNX and licensed under the Creative Commons Attribution License 3.0 By the end of this section, you
OpenStax-CNX module: m44742 1 Digestive System Processes * OpenStax This work is produced by OpenStax-CNX and licensed under the Creative Commons Attribution License 3.0 By the end of this section, you
The functional investigation of the interaction between TATA-associated factor 3 (TAF3) and p53 protein
 THESIS BOOK The functional investigation of the interaction between TATA-associated factor 3 (TAF3) and p53 protein Orsolya Buzás-Bereczki Supervisors: Dr. Éva Bálint Dr. Imre Miklós Boros University of
THESIS BOOK The functional investigation of the interaction between TATA-associated factor 3 (TAF3) and p53 protein Orsolya Buzás-Bereczki Supervisors: Dr. Éva Bálint Dr. Imre Miklós Boros University of
Soft Agar Assay. For each cell pool, 100,000 cells were resuspended in 0.35% (w/v)
 SUPPLEMENTARY MATERIAL AND METHODS Soft Agar Assay. For each cell pool, 100,000 cells were resuspended in 0.35% (w/v) top agar (LONZA, SeaKem LE Agarose cat.5004) and plated onto 0.5% (w/v) basal agar.
SUPPLEMENTARY MATERIAL AND METHODS Soft Agar Assay. For each cell pool, 100,000 cells were resuspended in 0.35% (w/v) top agar (LONZA, SeaKem LE Agarose cat.5004) and plated onto 0.5% (w/v) basal agar.
Physiology 12. Overview. The Gastrointestinal Tract. Germann Ch 19
 Physiology 12 The Gastrointestinal Tract Germann Ch 19 Overview 1 Basic functions of the GI tract Digestion Secretion Absorption Motility Basic functions of the GI tract Digestion: : Dissolving and breaking
Physiology 12 The Gastrointestinal Tract Germann Ch 19 Overview 1 Basic functions of the GI tract Digestion Secretion Absorption Motility Basic functions of the GI tract Digestion: : Dissolving and breaking
Supporting Online Material for
 www.sciencemag.org/cgi/content/full/1171320/dc1 Supporting Online Material for A Frazzled/DCC-Dependent Transcriptional Switch Regulates Midline Axon Guidance Long Yang, David S. Garbe, Greg J. Bashaw*
www.sciencemag.org/cgi/content/full/1171320/dc1 Supporting Online Material for A Frazzled/DCC-Dependent Transcriptional Switch Regulates Midline Axon Guidance Long Yang, David S. Garbe, Greg J. Bashaw*
BIOL212 Biochemistry of Disease. Metabolic Disorders - Obesity
 BIOL212 Biochemistry of Disease Metabolic Disorders - Obesity Obesity Approx. 23% of adults are obese in the U.K. The number of obese children has tripled in 20 years. 10% of six year olds are obese, rising
BIOL212 Biochemistry of Disease Metabolic Disorders - Obesity Obesity Approx. 23% of adults are obese in the U.K. The number of obese children has tripled in 20 years. 10% of six year olds are obese, rising
REVISION: CHEMISTRY OF LIFE 19 MARCH 2014
 REVISION: CHEMISTRY OF LIFE 19 MARCH 2014 Lesson Description In this lesson we revise: The Chemistry of Life Food tests Summary Inorganic Nutrients Water Solvent Medium in which chemical reactions occur
REVISION: CHEMISTRY OF LIFE 19 MARCH 2014 Lesson Description In this lesson we revise: The Chemistry of Life Food tests Summary Inorganic Nutrients Water Solvent Medium in which chemical reactions occur
Control of Metabolic Homeostasis by Stress Signaling Is Mediated by the Lipocalin NLaz
 Control of Metabolic Homeostasis by Stress Signaling Is Mediated by the Lipocalin NLaz Julie Hull-Thompson 1., Julien Muffat 2., Diego Sanchez 3, David W. Walker 2, Seymour Benzer 2, Maria D. Ganfornina
Control of Metabolic Homeostasis by Stress Signaling Is Mediated by the Lipocalin NLaz Julie Hull-Thompson 1., Julien Muffat 2., Diego Sanchez 3, David W. Walker 2, Seymour Benzer 2, Maria D. Ganfornina
Rumination or cud chewing consists of regurgitation, remastication, reinsalvation, and reswallowing.
 Nutrition 115 Midterm Exam 2 February 25, 2000 Name Please be sure to put your name at the top of each page. Any page without a name in the appropriate place will not be graded. Read each question carefully,
Nutrition 115 Midterm Exam 2 February 25, 2000 Name Please be sure to put your name at the top of each page. Any page without a name in the appropriate place will not be graded. Read each question carefully,
VI. SUMMARY. One hundred and eighty day old commercial Cobb broiler chicks. randomly divided into five groups, each comprising of 36 chicks, were
 VI. SUMMARY One hundred and eighty day old commercial Cobb broiler chicks randomly divided into five groups, each comprising of 36 chicks, were used in the present study to evaluate the physiological effects
VI. SUMMARY One hundred and eighty day old commercial Cobb broiler chicks randomly divided into five groups, each comprising of 36 chicks, were used in the present study to evaluate the physiological effects
Topic 10: Nutrition & Digestion Ch. 41. Nutritional Requirements pp Essential Amino Acids p.939. Essential Fatty Acids p.
 Topic 10: Nutrition & Digestion Ch. 41 Nutritional Requirements pp.939-941 Diets for animals must satisfy three needs : 1. Metabolic fuel Energy needed to do cellular work. E.g. glucose 2. Materials for
Topic 10: Nutrition & Digestion Ch. 41 Nutritional Requirements pp.939-941 Diets for animals must satisfy three needs : 1. Metabolic fuel Energy needed to do cellular work. E.g. glucose 2. Materials for
Chemistry of Carbon. All living things rely on one particular type of molecule: carbon
 Ach Chemistry of Carbon All living things rely on one particular type of molecule: carbon Carbon atom with an outer shell of four electrons can form covalent bonds with four atoms. In organic molecules,
Ach Chemistry of Carbon All living things rely on one particular type of molecule: carbon Carbon atom with an outer shell of four electrons can form covalent bonds with four atoms. In organic molecules,
Assignment #1: Biological Molecules & the Chemistry of Life
 Assignment #1: Biological Molecules & the Chemistry of Life A. Important Inorganic Molecules Water 1. Explain why water is considered a polar molecule. The partial negative charge of the oxygen and the
Assignment #1: Biological Molecules & the Chemistry of Life A. Important Inorganic Molecules Water 1. Explain why water is considered a polar molecule. The partial negative charge of the oxygen and the
7.06 Cell Biology EXAM #3 April 24, 2003
 7.06 Spring 2003 Exam 3 Name 1 of 8 7.06 Cell Biology EXAM #3 April 24, 2003 This is an open book exam, and you are allowed access to books and notes. Please write your answers to the questions in the
7.06 Spring 2003 Exam 3 Name 1 of 8 7.06 Cell Biology EXAM #3 April 24, 2003 This is an open book exam, and you are allowed access to books and notes. Please write your answers to the questions in the
Digestion and Human Health
 Digestion and Human Health The Molecules of Living Systems There are three main fluid components in your body Cytoplasm in your cells Fluid between your cells Fluid in your blood The also contain many
Digestion and Human Health The Molecules of Living Systems There are three main fluid components in your body Cytoplasm in your cells Fluid between your cells Fluid in your blood The also contain many
SCHOOL OF HEALTH SCIENCES DIVISION OF DIETETICS, NUTRITION AND BIOLOGICAL SCIENCES, PHYSIOTHERAPY, PODIATRY, RADIOGRAPHY LEVEL 2 / DIET 1
 SCHOOL OF HEALTH SCIENCES DIVISION OF DIETETICS, NUTRITION AND BIOLOGICAL SCIENCES, PHYSIOTHERAPY, PODIATRY, RADIOGRAPHY LEVEL 2 / DIET 1 D2143/ Nutrition DATE: 28/04/2014 WRITING TIME: 120 minutes TIME:
SCHOOL OF HEALTH SCIENCES DIVISION OF DIETETICS, NUTRITION AND BIOLOGICAL SCIENCES, PHYSIOTHERAPY, PODIATRY, RADIOGRAPHY LEVEL 2 / DIET 1 D2143/ Nutrition DATE: 28/04/2014 WRITING TIME: 120 minutes TIME:
Males- Western Diet WT KO Age (wks) Females- Western Diet WT KO Age (wks)
 Relative Arv1 mrna Adrenal 33.48 +/- 6.2 Skeletal Muscle 22.4 +/- 4.93 Liver 6.41 +/- 1.48 Heart 5.1 +/- 2.3 Brain 4.98 +/- 2.11 Ovary 4.68 +/- 2.21 Kidney 3.98 +/-.39 Lung 2.15 +/-.6 Inguinal Subcutaneous
Relative Arv1 mrna Adrenal 33.48 +/- 6.2 Skeletal Muscle 22.4 +/- 4.93 Liver 6.41 +/- 1.48 Heart 5.1 +/- 2.3 Brain 4.98 +/- 2.11 Ovary 4.68 +/- 2.21 Kidney 3.98 +/-.39 Lung 2.15 +/-.6 Inguinal Subcutaneous
10/27/2016. Processing in the Large Intestine. The colon of the large intestine is connected to the small intestine
 The hepatic portal vein carries nutrient-rich blood from the capillaries of the villi to the liver, then to the heart The liver regulates nutrient distribution, interconverts many organic molecules, and
The hepatic portal vein carries nutrient-rich blood from the capillaries of the villi to the liver, then to the heart The liver regulates nutrient distribution, interconverts many organic molecules, and
NIH Public Access Author Manuscript Kidney Int. Author manuscript; available in PMC 2013 November 01.
 NIH Public Access Author Manuscript Published in final edited form as: Kidney Int. 2013 May ; 83(5): 779 782. doi:10.1038/ki.2012.468. Need to quickly excrete K +? Turn off NCC Alicia A. McDonough 1 and
NIH Public Access Author Manuscript Published in final edited form as: Kidney Int. 2013 May ; 83(5): 779 782. doi:10.1038/ki.2012.468. Need to quickly excrete K +? Turn off NCC Alicia A. McDonough 1 and
Dietary Fibres Soluble Fibres: can be.. Insoluble Fibres : can be..
 Dietary Fibres The fraction of edible parts of plants or analogous carbohydrates that are: Resistant to digestion and absorption in the human small intestine with.. Complete or partial fermentation in
Dietary Fibres The fraction of edible parts of plants or analogous carbohydrates that are: Resistant to digestion and absorption in the human small intestine with.. Complete or partial fermentation in
Src-INACTIVE / Src-INACTIVE
 Biology 169 -- Exam 1 February 2003 Answer each question, noting carefully the instructions for each. Repeat- Read the instructions for each question before answering!!! Be as specific as possible in each
Biology 169 -- Exam 1 February 2003 Answer each question, noting carefully the instructions for each. Repeat- Read the instructions for each question before answering!!! Be as specific as possible in each
Supplementary Figure 1. HGL-DTG levels in uninduced and herbivory induced leaves. Total HGL-
 Supplementary Figure 1. HGL-DTG levels in uninduced and herbivory induced leaves. Total HGL- DTG concentrations [F 1,4 = 36.88, P 0.0037; significant differences (threshold: P 0.05) between means (± SE)
Supplementary Figure 1. HGL-DTG levels in uninduced and herbivory induced leaves. Total HGL- DTG concentrations [F 1,4 = 36.88, P 0.0037; significant differences (threshold: P 0.05) between means (± SE)
Unit 2 - Characteristics of Living Things
 Living Environment Answer Key to Practice Exam- Parts A and B-1 1. A fully functioning enzyme molecule is arranged in a complex three-dimensional shape. This shape determines the A) specific type of molecule
Living Environment Answer Key to Practice Exam- Parts A and B-1 1. A fully functioning enzyme molecule is arranged in a complex three-dimensional shape. This shape determines the A) specific type of molecule
Topic 4 - #2 Carbohydrates Topic 2
 Topic 4 - #2 Carbohydrates Topic 2 Biologically Important Monosaccharide Derivatives There are a large number of monosaccharide derivatives. A variety of chemical and enzymatic reactions produce these
Topic 4 - #2 Carbohydrates Topic 2 Biologically Important Monosaccharide Derivatives There are a large number of monosaccharide derivatives. A variety of chemical and enzymatic reactions produce these
In The Name Of God. In The Name Of. EMRI Modeling Group
 In The Name Of God In The Name Of God EMRI Modeling Group Cells work together in functionally related groups called tissues Types of tissues: Epithelial lining and covering Connective support Muscle movement
In The Name Of God In The Name Of God EMRI Modeling Group Cells work together in functionally related groups called tissues Types of tissues: Epithelial lining and covering Connective support Muscle movement
Hormonal Regulations Of Glucose Metabolism & DM
 Hormonal Regulations Of Glucose Metabolism & DM What Hormones Regulate Metabolism? What Hormones Regulate Metabolism? Insulin Glucagon Thyroid hormones Cortisol Epinephrine Most regulation occurs in order
Hormonal Regulations Of Glucose Metabolism & DM What Hormones Regulate Metabolism? What Hormones Regulate Metabolism? Insulin Glucagon Thyroid hormones Cortisol Epinephrine Most regulation occurs in order
Station One: Nutrition
 Station One: Nutrition Name that thing! 1. Chemical substances, found in foods, which are used in the human body. 2. Nutrient in human diet where foods are the only possible source of the nutrient. 3.
Station One: Nutrition Name that thing! 1. Chemical substances, found in foods, which are used in the human body. 2. Nutrient in human diet where foods are the only possible source of the nutrient. 3.
Nature Structural & Molecular Biology: doi: /nsmb Supplementary Figure 1
 Supplementary Figure 1 Effect of HSP90 inhibition on expression of endogenous retroviruses. (a) Inducible shrna-mediated Hsp90 silencing in mouse ESCs. Immunoblots of total cell extract expressing the
Supplementary Figure 1 Effect of HSP90 inhibition on expression of endogenous retroviruses. (a) Inducible shrna-mediated Hsp90 silencing in mouse ESCs. Immunoblots of total cell extract expressing the
1 (a) The control of blood glucose is a very important aspect of homeostasis [2]
![1 (a) The control of blood glucose is a very important aspect of homeostasis [2] 1 (a) The control of blood glucose is a very important aspect of homeostasis [2]](/thumbs/80/81464401.jpg) 1 (a) The control of blood glucose is a very important aspect of homeostasis. (i) Explain what is meant by the term homeostasis.... [2] Describe how negative feedback is used to control blood glucose concentration.
1 (a) The control of blood glucose is a very important aspect of homeostasis. (i) Explain what is meant by the term homeostasis.... [2] Describe how negative feedback is used to control blood glucose concentration.
Myelin suppresses axon regeneration by PIR-B/SHPmediated inhibition of Trk activity
 Manuscript EMBO-2010-76298 Myelin suppresses axon regeneration by PIR-B/SHPmediated inhibition of Trk activity Yuki Fujita, Shota Endo, Toshiyuki Takai and Toshihide Yamashita Corresponding author: Toshihide
Manuscript EMBO-2010-76298 Myelin suppresses axon regeneration by PIR-B/SHPmediated inhibition of Trk activity Yuki Fujita, Shota Endo, Toshiyuki Takai and Toshihide Yamashita Corresponding author: Toshihide
Comprehensive and Easy Course Notes for BIOL1040 Exams and Assessment
 Comprehensive and Easy Course Notes for BIOL1040 Exams and Assessment MODULE 1: PRINCIPLES OF CELL FUNCTION Membrane Structure & Function Cellular membranes are fluid mosaics of lipids and proteins Phospholipids
Comprehensive and Easy Course Notes for BIOL1040 Exams and Assessment MODULE 1: PRINCIPLES OF CELL FUNCTION Membrane Structure & Function Cellular membranes are fluid mosaics of lipids and proteins Phospholipids
