Oncostatin M overexpression induces skin inflammation but is not required in the mouse model of imiquimod-induced psoriasis-like inflammation
|
|
|
- Marylou Howard
- 5 years ago
- Views:
Transcription
1 Eur. J. Immunol : DOI: /eji Mathilde Pohin et al Research Article Allergy and inflammation Oncostatin M overexpression induces skin inflammation but is not required in the mouse model of imiquimod-induced psoriasis-like inflammation Basic Mathilde Pohin 1, William Guesdon 1, Adela Andrine Tagne Mekouo 1, Hanitriniaina Rabeony 1, Isabelle Paris 1,2, Hristo Atanassov 1,2, Laure Favot 1, Jiad Mcheik 1,2,François-Xavier Bernard 1,3, Carl D. Richards 4,Jérôme Amiaud 5,Frédéric Blanchard 5, Jean-Claude Lecron 1,2, Franck Morel 1 and Jean-François Jégou 1 1 Laboratoire Inflammation, Tissus Epithéliaux et Cytokines (LITEC), EA 4331, Université de Poitiers, Poitiers, France 2 Centre Hospitalier Universitaire de Poitiers, Poitiers, France 3 BioAlternatives, Gençay, France 4 McMaster Immunology Research Centre, McMaster University, Hamilton, Ontario, Canada 5 INSERM UMR 957, Université de Nantes, Nantes, France Oncostatin M (OSM) has been reported to be overexpressed in psoriasis skin lesions and to exert proinflammatory effects in vitro on human keratinocytes. Here, we report the proinflammatory role of OSM in vivo in a mouse model of skin inflammation induced by intradermal injection of murine OSM-encoding adenovirus (AdOSM) and compare with that induced by IL-6 injection. Here, we show that OSM potently regulates the expression of genes involved in skin inflammation and epidermal differentiation in murine primary keratinocytes. In vivo, intradermal injection of AdOSM in mouse ears provoked robust skin inflammation with epidermal thickening and keratinocyte proliferation, while minimal effect was observed after AdIL-6 injection. OSM overexpression in the skin increased the expression of the S100A8/9 antimicrobial peptides, CXCL3, CCL2, CCL5, CCL20, and Th1/Th2 cytokines, in correlation with neutrophil and macrophage infiltration. In contrast, OSM downregulated the expression of epidermal differentiation genes, such as cytokeratin-10 or filaggrin. Collectively, these results support the proinflammatory role of OSM when it is overexpressed in the skin. However, OSM expression was not required in the murine model of psoriasis induced by topical application of imiquimod, as demonstrated by the inflammatory phenotype of OSM-deficient mice or wild-type mice treated with anti-osm antibodies. Keywords: Imiquimod Keratinocyte Oncostatin M Psoriasis Skin inflammation Additional supporting information may be found in the online version of this article at the publisher s web-site Introduction Correspondence: Dr. Jean-François Jégou jean-francois.jegou@univ-poitiers.fr Psoriasis is the most common inflammatory skin disease affecting 2 3% of the adult population, mainly in developed countries,
2 1738 Mathilde Pohin et al. Eur. J. Immunol : in which patients develop skin lesions characterized by erythematous and scaly plaques [1]. The histological features of psoriatic skins are a thickening of the epidermis resulting from an altered keratinocyte differentiation associated with a hyperplasia at the basement membrane, a hyperkeratosis and a parakeratosis (loss of the granular layer and abnormal presence of cell nuclei in the cornified layer) [1, 2]. At the inflammatory site, these alterations of the epidermis are the consequence of a crosstalk between infiltrating immune cells and tissue resident cells (e.g. dermal fibroblasts, epidermal keratinocytes, and resident immune cells) through the release of numerous cytokines. In this complex network of interactions, keratinocytes are now considered to play a key role as bona fide innate immune cells, capable of secreting cytokines, chemokines, and antimicrobial peptides in response to various stimuli [3]. Psoriasis has been reported to be a Th1- and Th17-driven pathology [4]. The IL-23/IL-17 axis plays a crucial role in the pathogenesis of the disease, as demonstrated by the detection of IL-23 producing DCs and the expression of IL-17 and IL-22 by T cells and type 3 innate lymphoid cells in psoriatic lesions [5, 6]. The improved clinical outcomes of psoriatic patients with treatments targeting the Th17 cytokines support the involvement of these cytokines in underlying physiopathology [7]. Other cytokines such as TNF-α, IL-1α/β, or IL-18 have been involved in the pathogenesis of psoriasis as well [8, 9]. The contribution of these cytokines has been mainly studied using the standard mouse model of psoriasis-like skin inflammation induced by topical application of imiquimod (IMQ) [10 13]. Oncostatin M (OSM) has also been reported to be overexpressed in skin lesions of patients with psoriasis and shown to have proinflammatory activities in vitro on human primary keratinocytes and reconstituted epidermis [14]. However, its contribution to skin inflammation remains to be determined in vivo. OSM is a multifunctional cytokine belonging to the gp130 cytokine family, mainly secreted by T cells, monocytes/macrophages, DCs, and neutrophils [15]. Among the pleiotropic activities of OSM, some of them are redundant with other members of the gp130 family that also includes IL-6, IL-11, Leukemia Inhibitory Factor (LIF), cardiotrophin-1, ciliary neutrotrophic factor, neurotrophin-1, IL-27, and IL-31. However, OSM was also shown to possess unique biological effects in homeostasis and inflammatory processes, which were recently reviewed [15, 16]. These cytokines generally bind to heteromeric receptor complexes which share the common gp130 subunit involved in signal transduction, thus explaining some redundant activities among them. In addition to gp130, OSMRβ and LIFRα chains have the capacity to transduce either common or distinct activation signals [17]. In humans, OSM exerts its effects through the LIF receptor (LIFRα/gp130 complex, also named OSMR type I) and the OSMRβ/gp130 complex (OSMR type II), while in mouse, OSM binds only to OSMRβ/gp130 but not to LIFR. The engagement of these receptors leads mainly to the activation of the mitogenactivated protein kinases and the Janus kinase/signal transducer and activator of transcription (JAK/STAT) pathway with STAT3 and STAT1 phosphorylation [17]. Among cytokines of the gp130 family, only OSM and IL-6 have been shown to activate directly STAT3 phosphorylation in epidermal keratinocytes, IL-31 and LIF having minor or no effect [18]. With respect to skin inflammation, we previously described the proinflammatory activity of OSM on human keratinocytes, which were shown to express only a functional OSMR type II. In vitro, OSM induced the expression of antimicrobial peptides of the S100 family, chemokines (IL-8, CXCL5) and downregulated the expression of genes associated with keratinocyte differentiation such as cytokeratins (CKs)-10, involucrin, loricrin, or filaggrin [14]. OSM was also described as one of the five essential cytokines, with IL-1α, TNF-α, IL-17, and IL-22, acting in synergy to reproduce a psoriasislike phenotype on human primary keratinocytes [19], OSM being the most powerful cytokine in altering epidermal differentiation [20]. Among the gp130 cytokines, IL-6 was also reported to be overexpressed in psoriatic skin and to induce the proliferation of keratinocytes in vitro [21]. Surprisingly, transgenic mice with a specific overexpression of IL-6 in the basal layer of the epidermis did not present epidermal hyperplasia, but showed a thickening of the cornified layer in the absence of skin infiltration by leucocytes [22]. The data we previously obtained in vitro on the proinflammatory activities of OSM on human keratinocytes [14] prompted us to investigate the role of OSM in vivo in mouse skin inflammation, in comparison with the prototype gp130 family member IL-6, and its contribution in the model of psoriasislike IMQ-induced skin inflammation. We first compared the proinflammatory effects of OSM and IL-6 in vitro on normal murine epidermal primary keratinocytes (NMEKs). Then, we examined their proinflammatory activity in vivo using a single intradermal injection of recombinant adenoviruses overexpressing murine OSM (AdOSM) or murine IL-6 (AdIL-6). Finally, we addressed the contribution of OSM in the development of IMQ-induced inflammation by comparing WT C57BL/6 and OSM-deficient mice, or through the use of anti-osm blocking antibodies. Results OSM induces a potent inflammatory phenotype on mouse primary keratinocytes We first stimulated NMEKs with increasing doses of recombinant OSM or IL-6 and analyzed the expression of two representative target genes, the antimicrobial peptide S100A9 and CK1, known to be respectively upregulated and downregulated in keratinocytes by proinflammatory cytokines [14]. The data clearly show a major activity of OSM on murine keratinocytes by comparison with IL-6 (Fig. 1A). S100A9 expression was upregulated by OSM at the lowest concentration tested (0.3 pm) and rapidly reached a plateau at the concentration of 80 pm, whereas the maximum effect was obtained at the concentration of 1 nm for IL-6. Moreover, the effect obtained at saturating doses of OSM was tenfold higher than those obtained with saturating doses of IL-6. In addition, OSM decreased the expression of CK1, with a higher efficiency than IL-6 (Fig. 1A),
3 Eur. J. Immunol : Allergy and inflammation 1739 Figure 1. OSM is a potent proinflammatory cytokine on mouse primary keratinocytes. (A) NMEKs were stimulated with increasing concentrations of recombinant OSM or IL-6. S100A9 (left) and CK1 (right) mrna expression was analyzed by qrt-pcr. Data are expressed as mrna fold change above nonstimulated cells and shown as mean ± SEM of data from two independent experiments using primary keratinocyte cultures. (B) NMEKs were infected with adenovirus encoding GFP, OSM, or IL-6. mrna expression of OSM and IL-6 was determined by qrt-pcr. Data are expressed as percentage of housekeeping gene expression of data from three independent experiments. * p < 0.05; ** p < 0.01; *** p < 0.001, compared to AdGFP stimulation, Mann Whitney test. (C). NMEKs were stimulated by adenoviruses (black bars) or recombinant cytokines (10 ng/ml; gray bars). Expression of S100A9 mrna was analyzed by qrt-pcr. Data are expressed as mrna fold change above nonstimulated cells and are shown as mean ± SEM (n = 3 4 for each condition of stimulation) from three independent experiments. ** p < 0.01; *** p < 0.001, compared to AdGFP stimulation for adenoviruses and to unstimulated cells for recombinant protein stimulations, Mann Whitney test. (D) NMEKs were stimulated for 24 h by the different adenoviruses and recombinant proteins. S100A9 concentrations were determined in culture supernatants by ELISA. (E) NMEKs were infected by the adenovirus and the expression of cytokine receptor chains mrna was determined by qrt-pcr. Data are expressed as mrna fold change above nonstimulated cells and are shown as mean ± SEM (n = 3 4 for each condition of infection/experiment) from three independent experiments. * p < 0.05; ** p < 0.01; *** p < 0.001, compared to AdGFP stimulation, Mann Whitney test. (F) NMEKs were infected by the adenoviral vectors encoding OSM or IL-6 or the combination of both adenoviruses for 24 h. Gene expression profiles were determined by RT-qPCR. Data represent mean ± SEM mrna fold change above nonstimulated cells (n = 3 for each condition of infection/experiment) from three independent experiments. * p < 0.05; ** p < 0.01; *** p < 0.001, compared to AdGFP stimulation, Mann Whitney test. even though both cytokines reached the same level of inhibition at high concentrations. In order to validate the adenoviral tools used further in the in vivo study, we analyzed the in vitro expression of each cytokine 24 h after NMEKs infection either with AdOSM, AdIL-6, or AdGFP and compared their effects to recombinant cytokines. Cell infection by the different adenoviruses led to the selective expression of the relevant specific cytokine (encoded by the Ad construct) at the mrna level (Fig. 1B). Their expression was also confirmed by ELISA in culture supernatants of infected NMEKs with concentrations >10 ng/ml, corresponding to the maximum effective doses for each cytokine (not shown). In order to compare the activity of adenovirus-derived cytokines with the recombinant proteins, we analyzed the expression levels of the representative target gene S100A9 (Fig. 1C). The expression of the antimicrobial peptide was upregulated in a similar fashion by AdOSM versus rosm stimulations, and by AdIL-6 versus ril-6 stimulations, respectively. Infection of keratinocytes with control AdGFP did not significantly
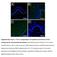
4 1740 Mathilde Pohin et al. Eur. J. Immunol : modulate the expression of S100A9, suggesting that transient exposure of keratinocytes to viral particles had no consequence on the studied parameter. This stronger induction of S100A9 after AdOSM or rosm stimulation was further confirmed at the protein level by Western blot (not shown) and by ELISA (Fig. 1D). We also investigated whether NMEKs stimulation by Ad vectors or recombinant proteins could modulate the expression of cytokine receptors and their capacity to respond to cytokines (data shown only for adenovirus stimulation). No modification was observed in the expression of the common gp130 chain and IL-6R (Fig. 1E). In contrast, OSMRβ chain expression was elevated in cells stimulated with AdOSM (Fig. 1E) or rosm (not shown). Finally, we analyzed more widely the response of NMEKs to adenovirus-derived cytokine stimulations, alone or in combination, to identify synergy or redundancy, by measuring the expression of a panel of target genes which were previously described to be regulated in humans keratinocytes [14]. Both S100A8 and S100A9 antimicrobial peptides were upregulated by AdOSM and, to a lesser extent ( fivefold less), by AdIL-6 (Fig. 1F). When used together, AdIL-6 did not amplify the effect induced by AdOSM alone. Chemokines were also reported to be released by human keratinocytes after OSM stimulation, especially IL-8 and CXCL5 involved in the recruitment of neutrophils [14]. As there is no ortholog of the human IL-8 in mouse, we analyzed CXCL3 which exerts a potent chemoattractant activity on neutrophils through CXCR2 and showed that only AdOSM induced its expression (Fig. 1F). In contrast, AdOSM repressed the expression of CCL2, a chemokine implicated in monocyte and lymphocyte recruitment, while AdIL-6 had no effect alone or in combination with AdOSM. As skin inflammation also results in an alteration of keratinocyte differentiation, we next studied the expression of genes classically associated to this process. When NMEKs were exposed to AdOSM, a strong downregulation of genes encoding CK1, CK10, desmoglein-1 (DSG-1) was observed, as well as gene expression of the terminal differentiation markers filaggrin and involucrin. By comparison, adenovirus expressing IL-6 decreased only the expression of CK1, CK10, and filaggrin in a significant manner, and less effectively than AdOSM (Fig. 1F). Collectively, these results demonstrate a potent proinflammatory activity of OSM in vitro on murine keratinocytes compared to IL-6, similar to the trend observed in human systems. OSM strongly induces skin inflammation with keratinocyte proliferation and epidermal thickening C57BL/6 mice received a single intradermal injection of adenovirus vectors in the ear in order to overexpress locally the cytokine of interest. This experimental approach avoids repeated daily injections of recombinant cytokines that could induce mechanical lesions of the skin interfering with the cytokine-induced inflammation. We first assessed that the corresponding cytokines were expressed during 7 days, with a maximum at 24 h postinfection (data not shown). We checked by real-time PCR the expression of the cytokines and their specific receptors in ears 24 h after injection (Supporting Information Fig. 1). As expected, each cytokine was specifically expressed after injection of its corresponding adenovirus, excepted for AdOSM which also induced the expression of endogenous IL-6. Only AdOSM injection resulted in a significant increase of gp130 and OSMRβ expression levels. All mice presented minor clinical signs of inflammation (skin redness) 24 h after the injection, probably due to the intradermal injection (Fig. 2A). At day 7, only mice injected with AdOSM displayed inflamed ears with a marked erythema, scaly and thickened skins, while signs of inflammation disappeared in the other groups of mice. As negative control, we injected OSMRβ-KO mice with AdOSM and observed no clinical signs of skin inflammation at day 7, thus demonstrating that OSM exerts its proinflammatory activity through its specific interaction with the heterodimeric receptor requiring OSMRβ chain (Fig. 2A). Ears were collected at day 7 and examined histologically after H&E staining. In AdGFP mice, the epidermis thickness was similar to those of control mice, confirming that the single injection of an adenovirus expressing a nonrelevant protein did not modify skin morphology (Fig. 2B). An epidermis thickening with cellular hyperplasia was observed only in ears from WT mice injected with AdOSM (Fig. 2C). This was associated with marked cell proliferation, as demonstrated by the large number of Ki67-positive cells in the epidermis, close to the basement membrane (Fig. 2D). In correlation with the lack of skin inflammation, the epidermal hyperplasia was not observed in OSMRβ-KO mice that received AdOSM injection (Fig. 2B). In addition, OSM-exposed ears from WT mice presented a parakeratosis, as well as a loss or a reduction of the granular layer at the site of injection, thus reproducing some histological features of psoriatic skin lesions (Fig. 2B). Finally, the histology of epidermis from AdIL-6 injected mice presented minor changes when compared to AdOSM-treated mice (Fig. 2 B D). OSM regulates the expression of inflammation markers in the skin We next analyzed the expression of S100A8 and S100A9 as typical markers of cutaneous inflammation. Levels of both antimicrobial peptides were highly increased in WT mice 7 days after AdOSM injection and slightly upregulated by AdIL-6 by comparison with AdGFP (Fig. 3). We verified that S1009 induction was specifically abolished in ears from OSMRβ-KO mice after OSM overexpression by comparison with AdOSM-treated WT mice (Supporting Information Fig. 2). The expression of S100A9 was further confirmed at the protein level by immunohistofluorescence on WT AdOSM-injected ear sections, revealing a strong specific staining in the dermis which was absent in the other conditions (Fig. 4). A weaker S100A9-positive staining could also be observed in the epidermis of AdOSM skins when the laser strength was increased in confocal microscopy (not shown). As the inflamed skin lesions show abundant leukocyte accumulation, we determined by quantitative RT-PCR the mrna expression of chemokines in adenovirus-injected ears. CXCL3 involved
5 Eur. J. Immunol : Allergy and inflammation 1741 Figure 2. Skin inflammation induced by OSM in vivo results in epidermal hyperplasia and keratinocyte proliferation. WT and OSMRβ-KO mice were injected in the ear with adenovirus expressing GFP, OSM, IL-6 or received control PBS. (A) Twenty-four hours and 7 days after adenovirus injection, representative pictures of inflamed ears from WT and OSMRβ- KO mice were taken. (B) H&E-stained section of ears at day 7 postinjection. Scale bar: 20 μm. (C) The thickness of the epidermis on ears from mice subjected to the indicated injections was measured every 25 μm onthewhole length of ear sections. Data shown as the mean ± SEM of five ears pooled from two experiments. ** p < 0.01 compared to AdGFP injection from three independent experiments, Mann Whitney test. (D) Frozen sections of ears were stained with anti-ki67 antibody (in green), cell nuclei stained using TOPRO (in blue) and analyzed by confocal microscopy (magnification x400). The same images are shown with (+) or without ( ) TOPRO staining for each condition. White dashed lines in the lower panel represent the limits of epidermis (basement membrane on the left). No background was observed in control ear sections when incubated with fluorochrome-conjugated secondary antibody only (Ab Ctl). Images shown are representative of five mice analyzed in two experiments. in neutrophil recruitment was found to be induced by AdOSM 24 h after injection (data not shown) and at day 7 (Fig. 3). CCL2 and CCL5, involved in monocytic and lymphocytic recruitment, as well as CCL20 chemotactic for Th17 cells, were upregulated at day 1 and day 7 only by AdOSM (data not shown and Fig. 3). The profile of cytokine expression was also determined showing a strong increase in the expression of IFN-γ and IL-4, and a modest but significant increase in IL-5 and IL-13 expression after AdOSM injection. In addition, IL-1β and TNF-α expressions were enhanced in OSM-exposed skins (Fig. 3). However, no changes in IL-17A, IL-17C, and IL-22 mrna expressions were observed in skin from AdOSM-treated mice (data not shown). In correlation with the strong erythema exhibited by ears injected with AdOSM, suggesting vascular remodeling, we could also detect a significant induction of the proangiogenic factors VEGF-A and D in these inflamed skins (Fig. 3). Consistent with the chemokine expression profile, the analysis of ear sections by specific immunofluorescent labeling clearly revealed the accumulation of Gr1-postive neutrophils in the dermis of AdOSM-treated mice, as well as monocytes/macrophages expressing F4/80, while these cells were absent in AdIL-6- and AdGFP-injected mice (Fig. 4). In healthy skin, both the dermis and the epidermis contained resident
6 1742 Mathilde Pohin et al. Eur. J. Immunol : Figure 3. OSM contributes to the regulation of inflammation markers expression. WT mice were injected in the ears with adenovirus expressing GFP, OSM, and IL-6. Seven days later, the expression of the indicated antimicrobial peptides, chemokines, and cytokines in ears was measured by RT-qPCR. Data are expressed as mrna fold change above noninjected mice and shown as mean ± SEM of nine ears pooled from three independent experiments. * p < 0.05; ** p < 0.01; *** p < 0.001, compared to AdGFP injection, Mann Whitney test. T cells. We here detected T cells at a low frequency in the skin of AdGFP- and AdIL-6 injected mice, but foci of CD3 + T cells could be observed in the hyperplasic epidermis of AdOSM-injected skins (Fig. 4). In summary, OSM induces antimicrobial peptide production, chemokine, and both Th1 and Th2 cytokine release, contributing to the recruitment of numerous immune cells at the inflammatory site. OSM alters epidermal differentiation The inhibition of epidermal differentiation obtained with AdOSM in vitro on primary keratinocytes and the profound histological changes observed in vivo after AdOSM injection led us to investigate the expression of a panel of genes differentially expressed in mouse epidermis. The expression of CK6 and CK16, mainly
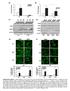
7 Eur. J. Immunol : Allergy and inflammation 1743 Figure 4. OSM induces S100A9 expression and leucocyte infiltration in the skin. WT mice were injected in the ears with adenovirus expressing GFP, OSM, and IL-6. Ears were collected 7 days later, immediately frozen. Cryocut sections were analyzed by confocal microscopy for S100A9 expression and the presence of Gr1 + neutrophils (in green), F4/80 + monocytes/macrophages (in green), and CD3 + T cells (in red). Magnification x200 or x400 as indicated. White dashed lines represent the external limit of epidermis and yellow dashed lines the dermis/epidermis junction. Cell nuclei are stained with TOPRO in the lower panel (in blue). No background was observed in control ear sections when incubated with fluorochrome-conjugated secondary antibody only (Ab Ctl). Images shown are representative of five mice analyzed in two experiments. expressed by basal keratinocytes, was found to be strongly upregulated in AdOSM-injected ears (in WT mice, but not in OSMRβ- KO mice, Supporting Information Fig. 2 and not shown), while AdIL-6 failed to regulate their expression (Fig. 5A). In addition, the expression of CK17, upregulated in skin lesions of psoriatic patients [23], was also induced in ears of AdOSM-treated mice and not modified in all the other groups tested. The expression of CK1, CK10, and DSG-1 decreased discretely but not significantly in AdOSM-injected skin compared to AdGFP (Fig. 5A). On another hand, the expression of genes involved in terminal keratinocyte differentiation, filaggrin, and involucrin was significantly lowered by AdOSM compared to control skin and remained unchanged after AdIL-6 treatment. CK10 immunostainings performed on AdOSM-injected ear sections confirmed the reduced expression of the CK previously observed at the transcriptional level. Indeed, ears injected with AdOSM presented a faint specific staining in the hyperplasic regions of the epidermis, by comparison to AdGFP-injected ears, while it persisted in AdIL-6 injected ears (Fig. 5B). Although we could observe a decrease in filaggrin and involucrin-specific immunostaining, the weaker intensity of the labeling was compensated by the larger thickness of the stained area (data not shown). The expression of loricrin, another late differentiation marker expressed by keratinocytes of the granular layer and corneocytes, was decreased after AdOSM injection, in contrast to AdIL-6 injected skins (Fig. 5B). Altogether, these results highlight an important role of OSM in the alteration of epidermal differentiation and the absence of IL-6 involvement in this process. OSM is expressed but not required for psoriasis-like IMQ-induced skin inflammation To investigate the contribution of endogenous OSM in the development of skin inflammation in vivo, we have used the murine model of psoriasis-like IMQ-induced skin inflammation. Back skins of WT and OSM-KO mice were treated either with Vaseline (VAS) or Aldara cream for six consecutive days and were monitored daily for clinical signs of cutaneous inflammation. We first showed that OSM mrna expression level was effectively higher in skins from IMQ-treated WT mice than in VAS-treated WT mice and, as expected, absent in OSM-KO mice (Fig. 6A). Both IMQ-WT and IMQ-OSM-KO mice presented the phenotypical features of psoriasis-like skin lesions including erythema, thickening, and scales, with a similar severity, while skins from VAS-treated mice were unaffected (Fig. 6B). This clinical observation is supported by the absence of statistical difference between the disease severity scores from IMQ-treated WT and OSM-KO groups of mice (Fig. 6C). The H&E staining of skin sections revealed similar levels of acanthosis and hyperkeratosis in both groups of IMQ-treated mice (Fig. 6B). Alternatively, we also injected i.p. 100 μg of neutralizing goat anti-mouse OSM antibodies in WT mice at day 1 and day 3 of the IMQ treatment in order to block endogenous OSM activity. The cutaneous phenotype and clinical scores of IMQ-treated mice that received injections of anti-osm antibodies were similar to those of control groups injected with PBS or with 100 μg of irrelevant goat IgG (Fig. 6D and E).
8 1744 Mathilde Pohin et al. Eur. J. Immunol : Figure 5. OSM alters the expression of genes associated with keratinocyte proliferation and differentiation in vivo. WT mice were injected in the ears with adenovirus expressing GFP, OSM, and IL-6. (A) Ears were collected 24 h after adenovirus infection for transcriptional analysis by qrt-pcr of genes associated with keratinocyte proliferation or differentiation. Data represent mean ± SEM mrna fold change above noninjected mice from three independent experiments (n = 3 4 mice/group). * p < 0.05; ** p < 0.01; *** p < 0.001, compared to AdGFP injection, Mann Whitney test. (B) Ear sections at day 7 were analyzed by confocal microscopy for CK10 and loricrin expressions (stained in red). Magnification: x400. White dashed lines represent the external limit of epidermis, while the dermis/epidermis junction is indicated with green dashed lines. Cell nuclei are stained with TOPRO (in blue). No background was observed in control ear sections when incubated with fluorochrome-conjugated secondary antibody only (Ab Ctl). Images shown are representative of five mice analyzed in two experiments. Overall, these data support the interpretation that OSM is expressed but not required for the development of skin inflammation in the murine model of psoriasis induced by IMQ. To explore whether the absence of OSM activity in this model could be compensated by other cytokines, we analyzed the skin expression of a panel of cytokines relevant to cutaneous inflammation, with a particular attention to cytokines involving STAT3 signaling. Indeed, by immunohistochemistry using an antiphospho-stat3 antibody, we observed a strong activation of STAT3, in vivo, in the epidermis of both WT and OSM-KO mice after 3 days of IMQ treatment by comparison with nontreated skins (Fig. 7A). Then, we showed that the mrna expressions of STAT-3 activating cytokines such as IL-6, IL-19, IL-20, IL-22, and IL-24 were increased at day 7 in all groups of IMQ-treated skins compared to untreated skins,
9 Eur. J. Immunol : Allergy and inflammation 1745 Figure 6. OSM is expressed but not required for the induction of psoriasis-like IMQ-induced skin inflammation. (A, B, and C) Back skins of WT and OSM-KO mice were treated either with Vaseline (VAS) or IMQ. (A) Skin biopsies were analyzed for OSM expression by RT-qPCR. Data represent mean ± SEM mrna fold increase above nontreated mice from three independent experiments including 5 15 animals per group. * p < 0.05 compared to untreated mice, Mann Whitney test. (B) Phenotypical presentation (upper panel) and histological analyses by H&E staining (lower panels) of mouse back skin from VAS and IMQ-treated WT and OSM-KO mice. Pictures are representative of 5 15 mice per group from three independent experiments. (C) Mean cumulative clinical scores (from 0 to 12) ± SEM of erythema, thickness, and scaling of the back skin during IMQ treatment from three independent experiments including 5 15 animals per group. (D and E) WT mice were treated with IMQ or not treated (NT) and received either an i.p. injection of PBS, control IgG, or neutralizing anti-osm antibodies (anti-osm Ab). (D) Phenotypical presentation (upper panel) and histological analyses (lower panels) of mouse back skins. Pictures are representative of five to eight mice per group from two independent experiments. (E) Mean cumulative clinical scores (from 0 to 12) ± SEM of erythema, thickness, and scaling of the back skin during IMQ treatment from two independent experiments including five to eight animals per group. (B and D) Magnification: x400. Bar = 40 μm.
10 1746 Mathilde Pohin et al. Eur. J. Immunol : Figure 7. Effect of OSM deficiency or anti-osm Ab-mediated blockade on STAT3-activating cytokine mrna levels in IMQ-treated skins. (A) WT and OSM-KO mice were treated with IMQ for 3 days or nontreated (NT). Skins biopsies were collected and formaldehyde-fixed sections were analyzed for STAT3 activation by immunochemistry using an antiphospho-stat3 antibody. Positive nuclei in the epidermis were stained in brown. Magnification: x400. Bar = 50 μm. Pictures are representative of five mice from two independent experiments. (B) WT and OSM-KO mice were treated with VAS or IMQ, and IMQ-treated mice were injected with control IgG or neutralizing anti-osm antibodies. Skins were collected after 7 days of treatment and analyzed for the expression of the antimicrobial peptide S100A8 and cytokines by qrt-pcr. Data are expressed as means ± SEM relative expression of the gene of interest to the housekeeping gene GAPDH, from two to three independent experiments including 5 15 animals per group.
11 Eur. J. Immunol : Allergy and inflammation 1747 although no difference in mrna levels could be observed between WT, OSM-KO mice, and mice that received anti-osm treatment (Fig. 7B). The expression of IL-27, previously reported to exert a proinflammatory role in IMQ model [24], remained unchanged in our hands (Fig. 7B). As IMQ is reported to induce a Th17-driven skin inflammation, we also analyzed the expression of IL-23 and IL-17. As expected, both cytokines were upregulated after IMQ treatment, but no difference could be seen between WT and OSM- KO mice. Both TNF-α and IFN-γ mrna levels were unchanged in the different groups of mice (data not shown). Discussion In previous studies, we described the proinflammatory role of OSM on human primary keratinocytes in vitro and its effects on epidermal differentiation using a 3D model of human reconstituted epidermis [14, 20]. In the present study, we demonstrated that OSM is also a potent inductor of skin inflammation in the mouse system, leading to profound alterations of the epidermis structure. First, we stimulated mouse primary keratinocytes with OSM in vitro, comparing its activity to IL-6. IL-6 was previously shown to be the only cytokine of the gp130 family, other than OSM, which acts on human epidermal keratinocytes through STAT3 and ERK phosphorylation [18]. We thus showed the capacity of OSM to increase the expression of genes classically associated to cutaneous inflammation (antimicrobial peptides, chemokines) and to repress genes involved in epidermal differentiation in mouse. These results obtained with mouse primary keratinocytes were in agreement with studies on OSM-stimulated human keratinocytes, showing an induction of antimicrobial peptides of the S100 family and neutrophil-attracting chemokines (CXCL8, CXCL5) [14]. Moreover, a downregulation of genes involved in epidermal differentiation including CK1, CK10, DSG-1, filaggrin, and involucrin has been reported in human keratinocytes [14]. Such modulation was also observed when murine keratinocytes were stimulated by OSM. Altogether, these results led to the conclusion that OSM exerts comparable activities on keratinocytes in human and mouse systems. Compared to IL-6, murine OSM showed elevated peak activities on NMEKs, as demonstrated for human OSM on NHEK [14]. This finding might be partly explained by an increased sensitivity of keratinocytes after OSM stimulation, as suggested by the upregulation of the OSMRβ chain expression. The weaker activity of IL-6 on murine keratinocytes is in agreement with a previous study reporting minor modifications of the epidermis structure in transgenic mice overexpressing IL-6 under the control of CK14 promoter [22]. In addition, we showed that IL-6 did not contribute to neither additive nor synergistic effects on murine keratinocytes. In order to characterize OSM proinflammatory activity in vivo, mice received an intradermal injection of adenoviral vectors expressing OSM, IL-6 or as a control comparator GFP. Several studies have used AdOSM as a tool to overexpress mouse OSM transiently at the site of injection. For example, intra-articular injection of AdOSM was shown to induce joint inflammation [25, 26]. Overexpression of OSM after intratracheal adenovirus administration elicited persistent lung inflammation with eosinophil and B-cell accumulation in lung parenchyma [27, 28]. Finally, tibial injections of AdOSM in mice promoted osteogenesis uncoupled from bone resorption [29]. In our model of skin inflammation, the sustained expression of cytokine after a single injection of adenovirus has the advantage of minimizing skin damage compared to daily repeated injections of recombinant cytokines. Seven days after adenovirus injection, only mouse ears receiving AdOSM presented a persistent inflammation sharing many features of human psoriatic plaques, such as erythema, thickened and scaly skins. The histological analysis also revealed an epidermal hyperplasia in AdOSM-treated mice, as commonly found in psoriasis [1]. This might be the consequence of basal keratinocyte proliferation, as suggested by CK6 and CK16 upregulation and demonstrated by Ki-67 staining. The epidermal hyperplasia could also be explained partly by an alteration of keratinocyte differentiation leading to a thickening of suprabasal layers of epidermis. This is supported by the analysis of inflamed skins, both at transcriptional and protein levels, showing a lowered expression of CKs (CK1, CK10), DSG-1, and terminal differentiation proteins (involucrin, loricrin, and filaggrin). The altered differentiation is also evidenced by the observation of parakeratosis, hypogranulosis or even the loss of the granular layer at the site of AdOSM injection, which are classically described in skin lesions from psoriatic patients. In contrast, the overexpression of IL-6 in mouse ear did not cause persistent skin inflammation nor significant alteration of inflammation and epidermal differentiation markers. Our results are in agreement with the study using transgenic mice expressing IL-6 by basal keratinocytes, in which no epidermal hyperplasia, hyperkeratosis, or leucocyte infiltration was observed [22]. Although we clearly showed a direct activity of OSM on keratinocytes in vitro, we could hypothesize that, in vivo, it might also act directly or indirectly on other cell types such as dermal fibroblasts or skin resident immune cells, to promote skin inflammation. For example, OSMRβ expression has been reported on fibroblastic cells and infiltrating macrophages [30] and OSM has been shown to induce the expression of CCL1, CCL7, and CCL8 by primary dermal fibroblasts [31]. In addition, other proinflammatory cytokines might be released secondarily at the site of AdOSM injection and could contribute to synergistic effects with OSM. For instance, we could detect a significant induction of IL-1β and TNF-α expression in OSM-induced inflamed skin. These additional cytokines might be released by skin cells or by resident or recruited immune cells at the site of AdOSM injection. Complex synergy associating OSM with IL-1α, IL-17A, IL-22, and TNF-α has been described in the development of a psoriasiform phenotype in mouse [19, 20], and to a larger extent, in other animal models of inflammation, for example between OSM and IL-1β in joint inflammation [32] or in cutaneous manifestations of hypertensive leg ulcer [33]. Within the gp130 family of cytokines, we did not observe either additive or synergistic effects on keratinocytes between OSM and IL-6, even though some distinct signaling pathways have been described for these cytokines [17, 18, 34]. Our results also showed a marked infiltration of neutrophils in the dermis after skin exposure to
12 1748 Mathilde Pohin et al. Eur. J. Immunol : AdOSM, which is consistent with elevated CXCL3 expression. Neutrophil accumulation in skin lesions is a feature of human psoriasis and these cells were described as a potential source of OSM, IL-1β, and IL-17 [30, 35]. In addition, an important monocyte infiltrate was also observed in the skin of AdOSM-treated mice, which could be a source of TNF-α. Resident T cells are commonly found in normal skin [36] and we could also find some T cells along the epidermis of noninjected control mice. When injected with AdOSM, we did not observe any marked recruitment of T cell in the epidermis or in the dermis; nonetheless, some discrete foci of T cells were present in inflamed skins suggesting a relocalization process induced by OSM. Together, these results obtained in vivo in mouse demonstrate that OSM has a potent proinflammatory activity when overexpressed in the skin, thereby mimicking some features of psoriasis plaques in humans. To determine the contribution of OSM in a mouse model of psoriasis, we used the well-described mouse model of psoriasislike IMQ-induced skin inflammation [13]. The inflammatory phenotype of IMQ-treated OSM-KO mice and mice that received neutralizing anti-osm antibodies led to the conclusion that OSM is not required for the development of inflammatory skin in this model. Although we showed that OSM possesses a strong proinflammatory activity on skin when overexpressed, the absence of attenuated phenotype in OSM-deficient mice or after OSM blockade prompted us to identify cytokines that may compensate its effect. We paid a particular attention to STAT3-activating cytokines since similar levels of STAT3 phosphorylation where observed in the skin of IMQ-treated WT and OSM-KO mice. We found increased mrna levels of IL-6, IL-19, IL-20, IL-22, and IL-24, in all groups of IMQ-treated mice. In the context of cytokine synergy, IL-6 could partially compensate OSM activity, as previously demonstrated in vitro on human primary keratinocytes [19]. Within the family of IL-10 cytokines, IL-22 is preferentially secreted by Th17 cells while IL-19, IL-20, and IL-24 are mostly produced by keratinocytes, secondarily to Th17-related cytokine stimulation [37, 38]. All these cytokines were described to involve STAT3 signaling [39] and to play a redundant role in the alteration of epidermis functions [40], which might contribute together, with IL-6, to compensate the absence of OSM activity. The lack of requirement of OSM in the induction of the IMQ model could also be explained by its strong dependence on the IL-23/IL-17 axis [13]. Both cytokines were found upregulated in a similar manner in all groups of IMQ-treated mice. In addition, we showed that OSM overexpression failed to increase the expression of IL-17A/C. Together these results suggest that OSM might act independently and in parallel to the IL-23/IL-17 axis. It can also be hypothesized that a strong stimulation by the TLR7 agonist does not need further stimulation or amplification signal by OSM to induce skin inflammation; this should incite to explore the role of OSM in spontaneous or genetically engineered mouse models of psoriasis, in which chronic inflammation develops in a less strong and acute manner than in IMQ treatment. Interestingly, we could detect higher expression of both IFN-γ and IL4/5/13 in AdOSM-treated skins. This finding suggests that OSM might be involved in Th1- or Th2-driven pathologies. It would be therefore interesting to investigate the role of OSM, for example, in nickel- or dinitrofluorobenzene-mediated contact hypersensitivity, known to induce a Th1-polarized response, or in paraphenylenediamine-mediated allergic contact dermatitis and calcipotriol-induced mouse model of atopic dermatitis, both characterized by a Th2 bias [41, 42]. In conclusion, we provide evidence that OSM exerts proinflammatory effects both in vitro on mouse primary keratinocytes, as in humans, and in vivo, when the cytokine is overexpressed in the skin. However, OSM is not required for the induction of the Th17- driven IMQ-induced inflammation. The absence of OSM requirement may involve compensatory processes by STAT3-dependent cytokines, but this needs further investigation. In addition, the capacity of OSM to modulate Th1 and Th2 cytokines in mouse skin indicates potential value in exploring the role of OSM in other animal models of skin pathologies driven by these cytokines. Materials and methods Animals Male C57BL/6 mice (8 10-week-old) were purchased from Janvier Laboratories (Le Genest-Saint-Isle, France). Mice deficient for OSM (OSM-KO) and OSM receptor β chain (OSMRβ-KO) were previously described [43, 44] and obtained from Pr. A. Miyajima (Laboratory of Cell Growth and Differentiation, Institute of Molecular and Cellular Biosciences, The University of Tokyo, Japan). All transgenic mice and their WT littermates were housed in the same facility, under specific pathogen-free conditions and maintained on a 12 h light/dark cycle, with food and water ad libitum. Mice were acclimatized for at least 1 week before experiments. Protocols and animals were approved by the regional ethics committee for animal experimentation (COMETHEA-CE86) under the agreement number CE Adenoviruses Nonreplicative adenoviruses encoding mouse OSM (AdOSM), mouse IL-6 (AdIL-6), or green fluorescent protein (AdGFP) were previously described [29, 45, 46] and produced in the vector facility of the INSERM U1089 laboratory (Nantes, France). Protocols using adenoviruses have been approved by the French Ministry of Research (Direction Générale pour la Recherche et l Innovation; Agreement n 5637). Keratinocyte culture and stimulation Cultures of normal murine epidermal keratinocytes (NMEKs) were established from pooled newborn mice skins (1 2 days postpartum) and grown in CnT-07 complete medium (CellnTec, Switzerland). Skins were incubated overnight with dispase (Gibco,
13 Eur. J. Immunol : Allergy and inflammation U/mL diluted in CnT-07) to separate the epidermis from the dermis. Keratinocyte isolation was performed by incubating pooled fragments of epidermis in Trypsin LE-express (Gibco) for 10 min at 37 C. Enzymatic digestion was stopped by adding 5 volumes of CnT-07 medium. Cells were counted and grown according to CellnTec s recommendations. At 70% of confluency, cells were starved overnight with basal medium (BM.1 medium, CellnTec) and stimulated for 24 h either with adenoviruses at a multiplicity of infection of 10, or with recombinant murine OSM (rosm; R&D Systems) or IL-6 (ril-6; Peprotech) at a final concentration of 10 ng/ml. In vivo murine skin inflammation For cytokine overexpression in the skin, mice were intradermally injected with pfu of adenovirus diluted in 20 μl of sterile PBS into the ear under anesthesia after injection of a xylazine (10 mg/ml)/ketamine (1 mg/ml) solution (100 μl/10 g of animal weight). After 24 h or 7 days postinjection, mice were euthanized and ears were collected and rapidly frozen in liquid nitrogen. Ears were stored at 80 C until use for RNA extraction or histology. For the induction of psoriasis-like inflammation, mice were shaved and depilated on their back before a daily topical application of 62.5 mg of Aldara cream (5% IMQ; Meda) for six consecutive days, according to the protocol initially described by van der Fits et al. [13]. Control mice were treated with a similar dose of Vaseline (Cooper). Mice were weighed and monitored daily for clinical signs of skin inflammation. Based on the human Psoriasis Area Severity Index, the degree of erythema, thickness, and scaling were scored for mice on a scale from 0 to 4 (0: none; 1: weak; 2: moderate; 3: marked; 4: severe), determining a cumulative clinical score (from 0 to 12) for each mouse and each day of treatment. Dehydrated mice presenting a significant loss of weight received a substitutive i.p. injection of 300 μl of sterile PBS. After 6 days, mice were euthanized and back skin biopsies were collected, rapidly frozen in liquid nitrogen and stored at 80 C until use. In experiments aiming at blocking endogenous OSM, mice were i.p. injected either with 200 μl of sterile PBS, control goat IgG, or goat anti-mouse OSM antibodies (100 μg/mouse diluted in sterile PBS; R&D systems) at day 1 and day 3 of the IMQ treatment, according to the protocol previously described [47]. RNA and real-time quantitative RT-PCR Total RNA was extracted from NMEKs using Nucleospin RNA II kit (Macherey Nagel). For mouse ear and back skin, tissue dissociation was performed using a gentlemacs dissociator (Miltenyi Biotec). RNA was reverse transcribed with Superscript II Reverse Transcriptase (Invitrogen) and transcripts were amplified and quantified using the LightCycler-FastStart DNA Master Plus SYBR Green I kit on LightCycler 480 instrument (Roche Diagnostics, Meylan, France). Oligonucleotides were designed with Primer- Blast software (NCBI) and purchased from Eurogentec (Eurogentec, Angers, France). Samples were normalized to the independent control housekeeping gene, glyceraldehyde 3-phosphate dehydrogenase (GAPDH), and reported according to the CT method as RNA fold increase over untreated samples or expressed in percentage of housekeeping gene expression. Histology and immunohistofluorescence Frozen sections from adenovirus injected ears or back skins (8 μm thick) were fixed in formaldehyde 4% before H&E coloration. Measurements of epidermis thickness were performed with the CellSens software (Olympus) every 50 μm over the entire length of the section. All the values presented the mean thickness of the epidermis ± SEM. For immunohistofluorescence, sections were fixed either in cold acetone/methanol (20/80, v/v) for 10 min or with a 4% paraformaldehyde/pbs solution (for CD3 and S100A9 stainings), before incubation with primary antibodies directed against the following mouse antigens: Ki67 (Dako); Gr1 (BD Biosciences); CD3 (BD Biosciences); F4/80 (Serotec), CK10 (Covance); Loricrin (Covance); S100A9 (R&D systems). RRX- or FITC-conjugated goat or donkey antibodies were used as secondary antibodies (Jackson Immunoresearch). Cell nuclei were detected with TOPRO (Invitrogen). Image acquisition was performed with an Olympus FV1000 confocal microscope using FluoView software (ImageUP platform, University of Poitiers). STAT3 activation was analyzed by immunohistochemistry, using a rabbit antiphospho-stat3 antibody (Cell Signaling Technologies) as described previously [48]. Pictures were obtained using the NDPView software (Hamamatsu). Western blot and ELISA Supernatants from stimulated cells were concentrated 50-fold in Amicon Ultra-4 Centrifugal Filter Units with Ultracel-10 membrane (Millipore). Then, proteins were separated on SDS-PAGE and transferred onto a nitrocellulose membrane (0.2 μm, Biorad) using a Fast semidry blotter (Pierce). Membranes were probed with antibodies against mouse S100A9 (R&D Systems) and revealed with peroxidase-conjugated rabbit anti-goat IgG by chemiluminescence (ECL) using an LAS-3000 imager (Fujifilm). ELISA for mouse OSM, IL-6, and S100A9 detection were provided with R&D Systems and carried out according to the manufacturer s instructions. Statistical analysis Statistical analyses were performed using Mann Whitney U test (two-tailed; GraphPad Prism 5 software). Values of p < 0.05 were considered as significant. All data are expressed as mean ± SEM from three to four independent experiments.
Supplementary Figure 1 IMQ-Induced Mouse Model of Psoriasis. IMQ cream was
 Supplementary Figure 1 IMQ-Induced Mouse Model of Psoriasis. IMQ cream was painted on the shaved back skin of CBL/J and BALB/c mice for consecutive days. (a, b) Phenotypic presentation of mouse back skin
Supplementary Figure 1 IMQ-Induced Mouse Model of Psoriasis. IMQ cream was painted on the shaved back skin of CBL/J and BALB/c mice for consecutive days. (a, b) Phenotypic presentation of mouse back skin
Supplementary Figure 1: Fn14 is upregulated in the epidermis and dermis of mice
 Supplementary Figure 1: Fn14 is upregulated in the epidermis and dermis of mice undergoing AD- and psoriasis-like disease. Immunofluorescence staining for Fn14 (green) and DAPI (blue) in skin of naïve
Supplementary Figure 1: Fn14 is upregulated in the epidermis and dermis of mice undergoing AD- and psoriasis-like disease. Immunofluorescence staining for Fn14 (green) and DAPI (blue) in skin of naïve
SUPPLEMENTARY INFORMATION. Involvement of IL-21 in the epidermal hyperplasia of psoriasis
 SUPPLEMENTARY INFORMATION Involvement of IL-21 in the epidermal hyperplasia of psoriasis Roberta Caruso 1, Elisabetta Botti 2, Massimiliano Sarra 1, Maria Esposito 2, Carmine Stolfi 1, Laura Diluvio 2,
SUPPLEMENTARY INFORMATION Involvement of IL-21 in the epidermal hyperplasia of psoriasis Roberta Caruso 1, Elisabetta Botti 2, Massimiliano Sarra 1, Maria Esposito 2, Carmine Stolfi 1, Laura Diluvio 2,
Supplementary Figure 1 Role of Raf-1 in TLR2-Dectin-1-mediated cytokine expression
 Supplementary Figure 1 Supplementary Figure 1 Role of Raf-1 in TLR2-Dectin-1-mediated cytokine expression. Quantitative real-time PCR of indicated mrnas in DCs stimulated with TLR2-Dectin-1 agonist zymosan
Supplementary Figure 1 Supplementary Figure 1 Role of Raf-1 in TLR2-Dectin-1-mediated cytokine expression. Quantitative real-time PCR of indicated mrnas in DCs stimulated with TLR2-Dectin-1 agonist zymosan
Nature Immunology: doi: /ni Supplementary Figure 1. Production of cytokines and chemokines after vaginal HSV-2 infection.
 Supplementary Figure 1 Production of cytokines and chemokines after vaginal HSV-2 infection. C57BL/6 mice were (a) treated intravaginally with 20 µl of PBS or infected with 6.7x10 4 pfu of HSV-2 in the
Supplementary Figure 1 Production of cytokines and chemokines after vaginal HSV-2 infection. C57BL/6 mice were (a) treated intravaginally with 20 µl of PBS or infected with 6.7x10 4 pfu of HSV-2 in the
IMO-8400, a novel TLR7, TLR8 and TLR9 antagonist, psoriasis
 IMO-8400, a novel TLR7, TLR8 and TLR9 antagonist, inhibits disease development in mouse models of psoriasis Weiwen e Ja Jiang, Fu-Gang Zhu, Dong Yu, Ekambar R. Kandimalla, a a, Nicola La Monica, and Sudhir
IMO-8400, a novel TLR7, TLR8 and TLR9 antagonist, inhibits disease development in mouse models of psoriasis Weiwen e Ja Jiang, Fu-Gang Zhu, Dong Yu, Ekambar R. Kandimalla, a a, Nicola La Monica, and Sudhir
SUPPLEMENTARY INFORMATION
 DOI:.38/ncb3399 a b c d FSP DAPI 5mm mm 5mm 5mm e Correspond to melanoma in-situ Figure a DCT FSP- f MITF mm mm MlanaA melanoma in-situ DCT 5mm FSP- mm mm mm mm mm g melanoma in-situ MITF MlanaA mm mm
DOI:.38/ncb3399 a b c d FSP DAPI 5mm mm 5mm 5mm e Correspond to melanoma in-situ Figure a DCT FSP- f MITF mm mm MlanaA melanoma in-situ DCT 5mm FSP- mm mm mm mm mm g melanoma in-situ MITF MlanaA mm mm
Supplementary Figure 1. H-PGDS deficiency does not affect GI tract functions and anaphylactic reaction. (a) Representative pictures of H&E-stained
 1 2 3 4 5 6 7 8 9 10 11 Supplementary Figure 1. H-PGDS deficiency does not affect GI tract functions and anaphylactic reaction. (a) Representative pictures of H&E-stained jejunum sections ( 200 magnification;
1 2 3 4 5 6 7 8 9 10 11 Supplementary Figure 1. H-PGDS deficiency does not affect GI tract functions and anaphylactic reaction. (a) Representative pictures of H&E-stained jejunum sections ( 200 magnification;
(A) PCR primers (arrows) designed to distinguish wild type (P1+P2), targeted (P1+P2) and excised (P1+P3)14-
 1 Supplemental Figure Legends Figure S1. Mammary tumors of ErbB2 KI mice with 14-3-3σ ablation have elevated ErbB2 transcript levels and cell proliferation (A) PCR primers (arrows) designed to distinguish
1 Supplemental Figure Legends Figure S1. Mammary tumors of ErbB2 KI mice with 14-3-3σ ablation have elevated ErbB2 transcript levels and cell proliferation (A) PCR primers (arrows) designed to distinguish
Role of Tyk-2 in Th9 and Th17 cells in allergic asthma
 Supplementary File Role of Tyk-2 in Th9 and Th17 cells in allergic asthma Caroline Übel 1*, Anna Graser 1*, Sonja Koch 1, Ralf J. Rieker 2, Hans A. Lehr 3, Mathias Müller 4 and Susetta Finotto 1** 1 Laboratory
Supplementary File Role of Tyk-2 in Th9 and Th17 cells in allergic asthma Caroline Übel 1*, Anna Graser 1*, Sonja Koch 1, Ralf J. Rieker 2, Hans A. Lehr 3, Mathias Müller 4 and Susetta Finotto 1** 1 Laboratory
Sestrin2 and BNIP3 (Bcl-2/adenovirus E1B 19kDa-interacting. protein3) regulate autophagy and mitophagy in renal tubular cells in. acute kidney injury
 Sestrin2 and BNIP3 (Bcl-2/adenovirus E1B 19kDa-interacting protein3) regulate autophagy and mitophagy in renal tubular cells in acute kidney injury by Masayuki Ishihara 1, Madoka Urushido 2, Kazu Hamada
Sestrin2 and BNIP3 (Bcl-2/adenovirus E1B 19kDa-interacting protein3) regulate autophagy and mitophagy in renal tubular cells in acute kidney injury by Masayuki Ishihara 1, Madoka Urushido 2, Kazu Hamada
Supplementary Figures
 Supplementary Figures Supplementary Figure 1. NKT ligand-loaded tumour antigen-presenting B cell- and monocyte-based vaccine induces NKT, NK and CD8 T cell responses. (A) The cytokine profiles of liver
Supplementary Figures Supplementary Figure 1. NKT ligand-loaded tumour antigen-presenting B cell- and monocyte-based vaccine induces NKT, NK and CD8 T cell responses. (A) The cytokine profiles of liver
SUPPLEMENTARY METHODS
 SUPPLEMENTARY METHODS Histological analysis. Colonic tissues were collected from 5 parts of the middle colon on day 7 after the start of DSS treatment, and then were cut into segments, fixed with 4% paraformaldehyde,
SUPPLEMENTARY METHODS Histological analysis. Colonic tissues were collected from 5 parts of the middle colon on day 7 after the start of DSS treatment, and then were cut into segments, fixed with 4% paraformaldehyde,
Supplementary data Supplementary Figure 1 Supplementary Figure 2
 Supplementary data Supplementary Figure 1 SPHK1 sirna increases RANKL-induced osteoclastogenesis in RAW264.7 cell culture. (A) RAW264.7 cells were transfected with oligocassettes containing SPHK1 sirna
Supplementary data Supplementary Figure 1 SPHK1 sirna increases RANKL-induced osteoclastogenesis in RAW264.7 cell culture. (A) RAW264.7 cells were transfected with oligocassettes containing SPHK1 sirna
Endocannabinoid-activated Nlrp3 inflammasome in infiltrating macrophages mediates β- cell loss in type 2 diabetes
 Endocannabinoid-activated Nlrp3 inflammasome in infiltrating macrophages mediates β- cell loss in type 2 diabetes T Jourdan, G Godlewski, R Cinar, A Bertola, G Szanda, J Liu, J Tam, T Han, B Mukhopadhyay,
Endocannabinoid-activated Nlrp3 inflammasome in infiltrating macrophages mediates β- cell loss in type 2 diabetes T Jourdan, G Godlewski, R Cinar, A Bertola, G Szanda, J Liu, J Tam, T Han, B Mukhopadhyay,
Supplementary Figure 1: si-craf but not si-braf sensitizes tumor cells to radiation.
 Supplementary Figure 1: si-craf but not si-braf sensitizes tumor cells to radiation. (a) Embryonic fibroblasts isolated from wildtype (WT), BRAF -/-, or CRAF -/- mice were irradiated (6 Gy) and DNA damage
Supplementary Figure 1: si-craf but not si-braf sensitizes tumor cells to radiation. (a) Embryonic fibroblasts isolated from wildtype (WT), BRAF -/-, or CRAF -/- mice were irradiated (6 Gy) and DNA damage
(a) Significant biological processes (upper panel) and disease biomarkers (lower panel)
 Supplementary Figure 1. Functional enrichment analyses of secretomic proteins. (a) Significant biological processes (upper panel) and disease biomarkers (lower panel) 2 involved by hrab37-mediated secretory
Supplementary Figure 1. Functional enrichment analyses of secretomic proteins. (a) Significant biological processes (upper panel) and disease biomarkers (lower panel) 2 involved by hrab37-mediated secretory
Unisooth PN-47 A complete reduction of pro-inflammatory factors for an instant soothing
 Unisooth PN-47 A complete reduction of pro-inflammatory factors for an instant soothing Skin irritation is amplified and controlled by various signaling pathways. By acting directly on the causes of skin
Unisooth PN-47 A complete reduction of pro-inflammatory factors for an instant soothing Skin irritation is amplified and controlled by various signaling pathways. By acting directly on the causes of skin
Supplementary Figure 1. ETBF activate Stat3 in B6 and Min mice colons
 Supplementary Figure 1 ETBF activate Stat3 in B6 and Min mice colons a pstat3 controls Pos Neg ETBF 1 2 3 4 b pstat1 pstat2 pstat3 pstat4 pstat5 pstat6 Actin Figure Legend: (a) ETBF induce predominantly
Supplementary Figure 1 ETBF activate Stat3 in B6 and Min mice colons a pstat3 controls Pos Neg ETBF 1 2 3 4 b pstat1 pstat2 pstat3 pstat4 pstat5 pstat6 Actin Figure Legend: (a) ETBF induce predominantly
Supplementary Figure 1.
 Supplementary Figure 1. Female Pro-ins2 -/- mice at 5-6 weeks of age were either inoculated i.p. with a single dose of CVB4 (1x10 5 PFU/mouse) or PBS and treated with αgalcer or control vehicle. On day
Supplementary Figure 1. Female Pro-ins2 -/- mice at 5-6 weeks of age were either inoculated i.p. with a single dose of CVB4 (1x10 5 PFU/mouse) or PBS and treated with αgalcer or control vehicle. On day
TGF-β Signaling Regulates Neuronal C1q Expression and Developmental Synaptic Refinement
 Supplementary Information Title: TGF-β Signaling Regulates Neuronal C1q Expression and Developmental Synaptic Refinement Authors: Allison R. Bialas and Beth Stevens Supplemental Figure 1. In vitro characterization
Supplementary Information Title: TGF-β Signaling Regulates Neuronal C1q Expression and Developmental Synaptic Refinement Authors: Allison R. Bialas and Beth Stevens Supplemental Figure 1. In vitro characterization
Supplementary Figure 1: STAT3 suppresses Kras-induced lung tumorigenesis
 Supplementary Figure 1: STAT3 suppresses Kras-induced lung tumorigenesis (a) Immunohistochemical (IHC) analysis of tyrosine 705 phosphorylation status of STAT3 (P- STAT3) in tumors and stroma (all-time
Supplementary Figure 1: STAT3 suppresses Kras-induced lung tumorigenesis (a) Immunohistochemical (IHC) analysis of tyrosine 705 phosphorylation status of STAT3 (P- STAT3) in tumors and stroma (all-time
Supporting Information
 Supporting Information Desnues et al. 10.1073/pnas.1314121111 SI Materials and Methods Mice. Toll-like receptor (TLR)8 / and TLR9 / mice were generated as described previously (1, 2). TLR9 / mice were
Supporting Information Desnues et al. 10.1073/pnas.1314121111 SI Materials and Methods Mice. Toll-like receptor (TLR)8 / and TLR9 / mice were generated as described previously (1, 2). TLR9 / mice were
Suppl Video: Tumor cells (green) and monocytes (white) are seeded on a confluent endothelial
 Supplementary Information Häuselmann et al. Monocyte induction of E-selectin-mediated endothelial activation releases VE-cadherin junctions to promote tumor cell extravasation in the metastasis cascade
Supplementary Information Häuselmann et al. Monocyte induction of E-selectin-mediated endothelial activation releases VE-cadherin junctions to promote tumor cell extravasation in the metastasis cascade
University of Ulsan College of Medicine, Seoul 05505, Republic of Korea.
 Nanoparticle-Assisted Transcutaneous Delivery of a Signal Transducer and Activator of Transcription 3-Inhibiting Peptide Ameliorates Psoriasis-like Skin Inflammation Jin Yong Kim 1,5, Jinhyo Ahn 3,4, Jinjoo
Nanoparticle-Assisted Transcutaneous Delivery of a Signal Transducer and Activator of Transcription 3-Inhibiting Peptide Ameliorates Psoriasis-like Skin Inflammation Jin Yong Kim 1,5, Jinhyo Ahn 3,4, Jinjoo
% of live splenocytes. STAT5 deletion. (open shapes) % ROSA + % floxed
 Supp. Figure 1. a 14 1 1 8 6 spleen cells (x1 6 ) 16 % of live splenocytes 5 4 3 1 % of live splenocytes 8 6 4 b 1 1 c % of CD11c + splenocytes (closed shapes) 8 6 4 8 6 4 % ROSA + (open shapes) % floxed
Supp. Figure 1. a 14 1 1 8 6 spleen cells (x1 6 ) 16 % of live splenocytes 5 4 3 1 % of live splenocytes 8 6 4 b 1 1 c % of CD11c + splenocytes (closed shapes) 8 6 4 8 6 4 % ROSA + (open shapes) % floxed
Supplementary Information
 Nature Immunology doi:1.138/ni.2477 Supplementary Information Capillary and arteriolar pericytes attract innate leukocytes exiting through venules and instruct them with pattern recognition and motility
Nature Immunology doi:1.138/ni.2477 Supplementary Information Capillary and arteriolar pericytes attract innate leukocytes exiting through venules and instruct them with pattern recognition and motility
MATERIALS AND METHODS. Neutralizing antibodies specific to mouse Dll1, Dll4, J1 and J2 were prepared as described. 1,2 All
 MATERIALS AND METHODS Antibodies (Abs), flow cytometry analysis and cell lines Neutralizing antibodies specific to mouse Dll1, Dll4, J1 and J2 were prepared as described. 1,2 All other antibodies used
MATERIALS AND METHODS Antibodies (Abs), flow cytometry analysis and cell lines Neutralizing antibodies specific to mouse Dll1, Dll4, J1 and J2 were prepared as described. 1,2 All other antibodies used
well for 2 h at rt. Each dot represents an individual mouse and bar is the mean ±
 Supplementary data: Control DC Blimp-1 ko DC 8 6 4 2-2 IL-1β p=.5 medium 8 6 4 2 IL-2 Medium p=.16 8 6 4 2 IL-6 medium p=.3 5 4 3 2 1-1 medium IL-1 n.s. 25 2 15 1 5 IL-12(p7) p=.15 5 IFNγ p=.65 4 3 2 1
Supplementary data: Control DC Blimp-1 ko DC 8 6 4 2-2 IL-1β p=.5 medium 8 6 4 2 IL-2 Medium p=.16 8 6 4 2 IL-6 medium p=.3 5 4 3 2 1-1 medium IL-1 n.s. 25 2 15 1 5 IL-12(p7) p=.15 5 IFNγ p=.65 4 3 2 1
ECM1 controls T H 2 cell egress from lymph nodes through re-expression of S1P 1
 ZH, Li et al, page 1 ECM1 controls T H 2 cell egress from lymph nodes through re-expression of S1P 1 Zhenhu Li 1,4,Yuan Zhang 1,4, Zhiduo Liu 1, Xiaodong Wu 1, Yuhan Zheng 1, Zhiyun Tao 1, Kairui Mao 1,
ZH, Li et al, page 1 ECM1 controls T H 2 cell egress from lymph nodes through re-expression of S1P 1 Zhenhu Li 1,4,Yuan Zhang 1,4, Zhiduo Liu 1, Xiaodong Wu 1, Yuhan Zheng 1, Zhiyun Tao 1, Kairui Mao 1,
Intracellular MHC class II molecules promote TLR-triggered innate. immune responses by maintaining Btk activation
 Intracellular MHC class II molecules promote TLR-triggered innate immune responses by maintaining Btk activation Xingguang Liu, Zhenzhen Zhan, Dong Li, Li Xu, Feng Ma, Peng Zhang, Hangping Yao and Xuetao
Intracellular MHC class II molecules promote TLR-triggered innate immune responses by maintaining Btk activation Xingguang Liu, Zhenzhen Zhan, Dong Li, Li Xu, Feng Ma, Peng Zhang, Hangping Yao and Xuetao
Figure S1. Generation of inducible PTEN deficient mice and the BMMCs (A) B6.129 Pten loxp/loxp mice were mated with B6.
 Figure S1. Generation of inducible PTEN deficient mice and the BMMCs (A) B6.129 Pten loxp/loxp mice were mated with B6.129-Gt(ROSA)26Sor tm1(cre/ert2)tyj /J mice. To induce deletion of the Pten locus,
Figure S1. Generation of inducible PTEN deficient mice and the BMMCs (A) B6.129 Pten loxp/loxp mice were mated with B6.129-Gt(ROSA)26Sor tm1(cre/ert2)tyj /J mice. To induce deletion of the Pten locus,
Supplementary Figures
 Inhibition of Pulmonary Anti Bacterial Defense by IFN γ During Recovery from Influenza Infection By Keer Sun and Dennis W. Metzger Supplementary Figures d a Ly6G Percentage survival f 1 75 5 1 25 1 5 1
Inhibition of Pulmonary Anti Bacterial Defense by IFN γ During Recovery from Influenza Infection By Keer Sun and Dennis W. Metzger Supplementary Figures d a Ly6G Percentage survival f 1 75 5 1 25 1 5 1
Supplementary Figure 1. Deletion of Smad3 prevents B16F10 melanoma invasion and metastasis in a mouse s.c. tumor model.
 A B16F1 s.c. Lung LN Distant lymph nodes Colon B B16F1 s.c. Supplementary Figure 1. Deletion of Smad3 prevents B16F1 melanoma invasion and metastasis in a mouse s.c. tumor model. Highly invasive growth
A B16F1 s.c. Lung LN Distant lymph nodes Colon B B16F1 s.c. Supplementary Figure 1. Deletion of Smad3 prevents B16F1 melanoma invasion and metastasis in a mouse s.c. tumor model. Highly invasive growth
GFP/Iba1/GFAP. Brain. Liver. Kidney. Lung. Hoechst/Iba1/TLR9!
 Supplementary information a +KA Relative expression d! Tlr9 5!! 5! NSC Neuron Astrocyte Microglia! 5! Tlr7!!!! NSC Neuron Astrocyte! GFP/Sβ/! Iba/Hoechst Microglia e Hoechst/Iba/TLR9! GFP/Iba/GFAP f Brain
Supplementary information a +KA Relative expression d! Tlr9 5!! 5! NSC Neuron Astrocyte Microglia! 5! Tlr7!!!! NSC Neuron Astrocyte! GFP/Sβ/! Iba/Hoechst Microglia e Hoechst/Iba/TLR9! GFP/Iba/GFAP f Brain
Supporting Information Table of Contents
 Supporting Information Table of Contents Supporting Information Figure 1 Page 2 Supporting Information Figure 2 Page 4 Supporting Information Figure 3 Page 5 Supporting Information Figure 4 Page 6 Supporting
Supporting Information Table of Contents Supporting Information Figure 1 Page 2 Supporting Information Figure 2 Page 4 Supporting Information Figure 3 Page 5 Supporting Information Figure 4 Page 6 Supporting
Blocking antibodies and peptides. Rat anti-mouse PD-1 (29F.1A12, rat IgG2a, k), PD-
 Supplementary Methods Blocking antibodies and peptides. Rat anti-mouse PD-1 (29F.1A12, rat IgG2a, k), PD- L1 (10F.9G2, rat IgG2b, k), and PD-L2 (3.2, mouse IgG1) have been described (24). Anti-CTLA-4 (clone
Supplementary Methods Blocking antibodies and peptides. Rat anti-mouse PD-1 (29F.1A12, rat IgG2a, k), PD- L1 (10F.9G2, rat IgG2b, k), and PD-L2 (3.2, mouse IgG1) have been described (24). Anti-CTLA-4 (clone
Abnormally differentiating keratinocytes in the epidermis of systemic sclerosis patients show enhanced secretion of CCN2 and S100A9
 Abnormally differentiating keratinocytes in the epidermis of systemic sclerosis patients show enhanced secretion of CCN2 and S1A9 Joanna Nikitorowicz-Buniak UCL Division of Medicine Systemic sclerosis
Abnormally differentiating keratinocytes in the epidermis of systemic sclerosis patients show enhanced secretion of CCN2 and S1A9 Joanna Nikitorowicz-Buniak UCL Division of Medicine Systemic sclerosis
Supplementary Figure 1. IDH1 and IDH2 mutation site sequences on WHO grade III
 Supplementary Materials: Supplementary Figure 1. IDH1 and IDH2 mutation site sequences on WHO grade III patient samples. Genomic DNA samples extracted from punch biopsies from either FFPE or frozen tumor
Supplementary Materials: Supplementary Figure 1. IDH1 and IDH2 mutation site sequences on WHO grade III patient samples. Genomic DNA samples extracted from punch biopsies from either FFPE or frozen tumor
The toll-like receptor 4 ligands Mrp8 and Mrp14 play a critical role in the development of autoreactive CD8 + T cells
 1 SUPPLEMENTARY INFORMATION The toll-like receptor 4 ligands Mrp8 and Mrp14 play a critical role in the development of autoreactive CD8 + T cells Karin Loser 1,2,6, Thomas Vogl 2,3, Maik Voskort 1, Aloys
1 SUPPLEMENTARY INFORMATION The toll-like receptor 4 ligands Mrp8 and Mrp14 play a critical role in the development of autoreactive CD8 + T cells Karin Loser 1,2,6, Thomas Vogl 2,3, Maik Voskort 1, Aloys
SUPPLEMENTARY INFORMATION
 Supplementary Figures Supplementary Figure S1. Binding of full-length OGT and deletion mutants to PIP strips (Echelon Biosciences). Supplementary Figure S2. Binding of the OGT (919-1036) fragments with
Supplementary Figures Supplementary Figure S1. Binding of full-length OGT and deletion mutants to PIP strips (Echelon Biosciences). Supplementary Figure S2. Binding of the OGT (919-1036) fragments with
Figure S1. A Non-lesional Lesional
 Figure S1 Non-lesional Lesional GEN1 Non-lesional GEN1 Lesional C Normal Negative control Figure S1. Dermal Populations of + cells are found in human skin. Immunohistochemistry for on fixed, frozen skin
Figure S1 Non-lesional Lesional GEN1 Non-lesional GEN1 Lesional C Normal Negative control Figure S1. Dermal Populations of + cells are found in human skin. Immunohistochemistry for on fixed, frozen skin
Étude de la coopération entre les cellules dendritiques et les lymphocytes T dans les allergies aux produits chimiques FPH42
 Étude de la coopération entre les cellules dendritiques et les lymphocytes T dans les allergies aux produits chimiques FPH42 Dr Diane Antonios-Gholam PharmD, PhD Laboratoire de Toxicologie Clinique et
Étude de la coopération entre les cellules dendritiques et les lymphocytes T dans les allergies aux produits chimiques FPH42 Dr Diane Antonios-Gholam PharmD, PhD Laboratoire de Toxicologie Clinique et
Type of file: PDF Title of file for HTML: Supplementary Information Description: Supplementary Figures
 Type of file: PDF Title of file for HTML: Supplementary Information Description: Supplementary Figures Type of file: MOV Title of file for HTML: Supplementary Movie 1 Description: NLRP3 is moving along
Type of file: PDF Title of file for HTML: Supplementary Information Description: Supplementary Figures Type of file: MOV Title of file for HTML: Supplementary Movie 1 Description: NLRP3 is moving along
Supplementary Materials for
 www.sciencesignaling.org/cgi/content/full/8/389/ra79/dc1 Supplementary Materials for HDL-bound sphingosine 1-phosphate acts as a biased agonist for the endothelial cell receptor S1P 1 to limit vascular
www.sciencesignaling.org/cgi/content/full/8/389/ra79/dc1 Supplementary Materials for HDL-bound sphingosine 1-phosphate acts as a biased agonist for the endothelial cell receptor S1P 1 to limit vascular
Cytokines modulate the functional activities of individual cells and tissues both under normal and pathologic conditions Interleukins,
 Cytokines http://highered.mcgraw-hill.com/sites/0072507470/student_view0/chapter22/animation the_immune_response.html Cytokines modulate the functional activities of individual cells and tissues both under
Cytokines http://highered.mcgraw-hill.com/sites/0072507470/student_view0/chapter22/animation the_immune_response.html Cytokines modulate the functional activities of individual cells and tissues both under
Crucial role for human Toll-like receptor 4 in the development of contact allergy to nickel
 Supplementary Figures 1-8 Crucial role for human Toll-like receptor 4 in the development of contact allergy to nickel Marc Schmidt 1,2, Badrinarayanan Raghavan 1,2, Verena Müller 1,2, Thomas Vogl 3, György
Supplementary Figures 1-8 Crucial role for human Toll-like receptor 4 in the development of contact allergy to nickel Marc Schmidt 1,2, Badrinarayanan Raghavan 1,2, Verena Müller 1,2, Thomas Vogl 3, György
Role of cyclooxygenase-2 in H5N1 viral pathogenesis and the potential use of its inhibitors
 Title Role of cyclooxygenase-2 in HN viral pathogenesis and the potential use of its inhibitors Author(s) Lee, MY; Cheung, CY; Peiris, JSM Citation Hong Kong Medical Journal, 2, v. 9 n. Suppl. 4, p. 29-
Title Role of cyclooxygenase-2 in HN viral pathogenesis and the potential use of its inhibitors Author(s) Lee, MY; Cheung, CY; Peiris, JSM Citation Hong Kong Medical Journal, 2, v. 9 n. Suppl. 4, p. 29-
Supplementary Information
 Supplementary Information An orally available, small-molecule interferon inhibits viral replication Hideyuki Konishi 1, Koichi Okamoto 1, Yusuke Ohmori 1, Hitoshi Yoshino 2, Hiroshi Ohmori 1, Motooki Ashihara
Supplementary Information An orally available, small-molecule interferon inhibits viral replication Hideyuki Konishi 1, Koichi Okamoto 1, Yusuke Ohmori 1, Hitoshi Yoshino 2, Hiroshi Ohmori 1, Motooki Ashihara
Supporting Information
 Supporting Information M1 macrophage-derived nanovesicles potentiate the anticancer efficacy of immune checkpoint inhibitors Yeon Woong Choo, 1, Mikyung Kang, 2, Han Young Kim, 1 Jin Han, 1 Seokyung Kang,
Supporting Information M1 macrophage-derived nanovesicles potentiate the anticancer efficacy of immune checkpoint inhibitors Yeon Woong Choo, 1, Mikyung Kang, 2, Han Young Kim, 1 Jin Han, 1 Seokyung Kang,
LPS LPS P6 - + Supplementary Fig. 1.
 P6 LPS - - - + + + - LPS + + - - P6 + Supplementary Fig. 1. Pharmacological inhibition of the JAK/STAT blocks LPS-induced HMGB1 nuclear translocation. RAW 267.4 cells were stimulated with LPS in the absence
P6 LPS - - - + + + - LPS + + - - P6 + Supplementary Fig. 1. Pharmacological inhibition of the JAK/STAT blocks LPS-induced HMGB1 nuclear translocation. RAW 267.4 cells were stimulated with LPS in the absence
Irf1 fold changes (D) 24h 48h. p-p65. t-p65. p-irf3. t-irf3. β-actin SKO TKO 100% 80% 60% 40% 20%
 Irf7 Fold changes 3 1 Irf1 fold changes 3 1 8h h 8h 8h h 8h p-p6 p-p6 t-p6 p-irf3 β-actin p-irf3 t-irf3 β-actin TKO TKO STKO (E) (F) TKO TKO % of p6 nuclear translocation % % 1% 1% % % p6 TKO % of IRF3
Irf7 Fold changes 3 1 Irf1 fold changes 3 1 8h h 8h 8h h 8h p-p6 p-p6 t-p6 p-irf3 β-actin p-irf3 t-irf3 β-actin TKO TKO STKO (E) (F) TKO TKO % of p6 nuclear translocation % % 1% 1% % % p6 TKO % of IRF3
SUPPLEMENTARY INFORMATION
 SUPPLEMENTARY INFORMATION Supplementary Figure 1. Long-term protection studies. 45 minutes of ischemia was induced in wild type (S1pr2 +/+ ) and S1pr2 -/- by MCAO. A) 5 days later brains were harvested
SUPPLEMENTARY INFORMATION Supplementary Figure 1. Long-term protection studies. 45 minutes of ischemia was induced in wild type (S1pr2 +/+ ) and S1pr2 -/- by MCAO. A) 5 days later brains were harvested
CYTOKINE RECEPTORS AND SIGNAL TRANSDUCTION
 CYTOKINE RECEPTORS AND SIGNAL TRANSDUCTION What is Cytokine? Secreted popypeptide (protein) involved in cell-to-cell signaling. Acts in paracrine or autocrine fashion through specific cellular receptors.
CYTOKINE RECEPTORS AND SIGNAL TRANSDUCTION What is Cytokine? Secreted popypeptide (protein) involved in cell-to-cell signaling. Acts in paracrine or autocrine fashion through specific cellular receptors.
Title of file for HTML: Supplementary Information Description: Supplementary Figures and Supplementary Table
 Title of file for HTML: Supplementary Information Description: Supplementary Figures and Supplementary Table Title of file for HTML: Peer Review File Description: Innate Scavenger Receptor-A regulates
Title of file for HTML: Supplementary Information Description: Supplementary Figures and Supplementary Table Title of file for HTML: Peer Review File Description: Innate Scavenger Receptor-A regulates
Expression of acid base transporters in the kidney collecting duct in Slc2a7 -/-
 Supplemental Material Results. Expression of acid base transporters in the kidney collecting duct in Slc2a7 -/- and Slc2a7 -/- mice. The expression of AE1 in the kidney was examined in Slc26a7 KO mice.
Supplemental Material Results. Expression of acid base transporters in the kidney collecting duct in Slc2a7 -/- and Slc2a7 -/- mice. The expression of AE1 in the kidney was examined in Slc26a7 KO mice.
Introduction: 年 Fas signal-mediated apoptosis. PI3K/Akt
 Fas-ligand (CD95-L; Fas-L) Fas (CD95) Fas (apoptosis) 年 了 不 度 Fas Fas-L 力 不 Fas/Fas-L T IL-10Fas/Fas-L 不 年 Fas signal-mediated apoptosis 度降 不 不 力 U-118, HeLa, A549, Huh-7 MCF-7, HepG2. PI3K/Akt FasPI3K/Akt
Fas-ligand (CD95-L; Fas-L) Fas (CD95) Fas (apoptosis) 年 了 不 度 Fas Fas-L 力 不 Fas/Fas-L T IL-10Fas/Fas-L 不 年 Fas signal-mediated apoptosis 度降 不 不 力 U-118, HeLa, A549, Huh-7 MCF-7, HepG2. PI3K/Akt FasPI3K/Akt
SUPPLEMENTARY INFORMATION
 doi:1.138/nature1554 a TNF-α + in CD4 + cells [%] 1 GF SPF 6 b IL-1 + in CD4 + cells [%] 5 4 3 2 1 Supplementary Figure 1. Effect of microbiota on cytokine profiles of T cells in GALT. Frequencies of TNF-α
doi:1.138/nature1554 a TNF-α + in CD4 + cells [%] 1 GF SPF 6 b IL-1 + in CD4 + cells [%] 5 4 3 2 1 Supplementary Figure 1. Effect of microbiota on cytokine profiles of T cells in GALT. Frequencies of TNF-α
Supplemental Table 1. Primer sequences for transcript analysis
 Supplemental Table 1. Primer sequences for transcript analysis Primer Sequence (5 3 ) Primer Sequence (5 3 ) Mmp2 Forward CCCGTGTGGCCCTC Mmp15 Forward CGGGGCTGGCT Reverse GCTCTCCCGGTTTC Reverse CCTGGTGTGCCTGCTC
Supplemental Table 1. Primer sequences for transcript analysis Primer Sequence (5 3 ) Primer Sequence (5 3 ) Mmp2 Forward CCCGTGTGGCCCTC Mmp15 Forward CGGGGCTGGCT Reverse GCTCTCCCGGTTTC Reverse CCTGGTGTGCCTGCTC
T H E J O U R N A L O F C E L L B I O L O G Y
 T H E J O U R N A L O F C E L L B I O L O G Y Supplemental material Amelio et al., http://www.jcb.org/cgi/content/full/jcb.201203134/dc1 Figure S1. mir-24 regulates proliferation and by itself induces
T H E J O U R N A L O F C E L L B I O L O G Y Supplemental material Amelio et al., http://www.jcb.org/cgi/content/full/jcb.201203134/dc1 Figure S1. mir-24 regulates proliferation and by itself induces
PBMC from each patient were suspended in AIM V medium (Invitrogen) with 5% human
 Anti-CD19-CAR transduced T-cell preparation PBMC from each patient were suspended in AIM V medium (Invitrogen) with 5% human AB serum (Gemini) and 300 international units/ml IL-2 (Novartis). T cell proliferation
Anti-CD19-CAR transduced T-cell preparation PBMC from each patient were suspended in AIM V medium (Invitrogen) with 5% human AB serum (Gemini) and 300 international units/ml IL-2 (Novartis). T cell proliferation
SUPPLEMENTARY FIGURES AND TABLE
 SUPPLEMENTARY FIGURES AND TABLE Supplementary Figure S1: Characterization of IRE1α mutants. A. U87-LUC cells were transduced with the lentiviral vector containing the GFP sequence (U87-LUC Tet-ON GFP).
SUPPLEMENTARY FIGURES AND TABLE Supplementary Figure S1: Characterization of IRE1α mutants. A. U87-LUC cells were transduced with the lentiviral vector containing the GFP sequence (U87-LUC Tet-ON GFP).
Supplemental Table 1. Primers used for RT-PCR analysis of inflammatory cytokines Gene Primer Sequence
 Supplemental Table 1. Primers used for RT-PCR analysis of inflammatory cytokines Gene Primer Sequence IL-1α Forward primer 5 -CAAGATGGCCAAAGTTCGTGAC-3' Reverse primer 5 -GTCTCATGAAGTGAGCCATAGC-3 IL-1β
Supplemental Table 1. Primers used for RT-PCR analysis of inflammatory cytokines Gene Primer Sequence IL-1α Forward primer 5 -CAAGATGGCCAAAGTTCGTGAC-3' Reverse primer 5 -GTCTCATGAAGTGAGCCATAGC-3 IL-1β
Nature Immunology: doi: /ni.3866
 Nature Immunology: doi:10.1038/ni.3866 Supplementary Figure 1 The effect of TIPE2 on chemotaxis. a, The expression of TIPE2 in dhl-60c, dhl-60t, TIPE2-expressing and 15/16Q-expressing dhl-60t neutrophils
Nature Immunology: doi:10.1038/ni.3866 Supplementary Figure 1 The effect of TIPE2 on chemotaxis. a, The expression of TIPE2 in dhl-60c, dhl-60t, TIPE2-expressing and 15/16Q-expressing dhl-60t neutrophils
IL-34 is a tissue-restricted ligand of CSF1R required for the development of Langerhans cells and microglia
 Supplementary Figures IL-34 is a tissue-restricted ligand of CSF1R required for the development of Langerhans cells and microglia Yaming Wang, Kristy J. Szretter, William Vermi, Susan Gilfillan, Cristina
Supplementary Figures IL-34 is a tissue-restricted ligand of CSF1R required for the development of Langerhans cells and microglia Yaming Wang, Kristy J. Szretter, William Vermi, Susan Gilfillan, Cristina
Neutrophils contribute to fracture healing by synthesizing fibronectin+ extracellular matrix rapidly after injury
 Neutrophils contribute to fracture healing by synthesizing fibronectin+ extracellular matrix rapidly after injury Bastian OW, Koenderman L, Alblas J, Leenen LPH, Blokhuis TJ. Neutrophils contribute to
Neutrophils contribute to fracture healing by synthesizing fibronectin+ extracellular matrix rapidly after injury Bastian OW, Koenderman L, Alblas J, Leenen LPH, Blokhuis TJ. Neutrophils contribute to
Endogenous TNFα orchestrates the trafficking of neutrophils into and within lymphatic vessels during acute inflammation
 SUPPLEMENTARY INFORMATION Endogenous TNFα orchestrates the trafficking of neutrophils into and within lymphatic vessels during acute inflammation Samantha Arokiasamy 1,2, Christian Zakian 1, Jessica Dilliway
SUPPLEMENTARY INFORMATION Endogenous TNFα orchestrates the trafficking of neutrophils into and within lymphatic vessels during acute inflammation Samantha Arokiasamy 1,2, Christian Zakian 1, Jessica Dilliway
Programmed necrosis, not apoptosis, is a key mediator of cell loss and DAMP-mediated inflammation in dsrna-induced retinal degeneration
 Programmed necrosis, not apoptosis, is a key mediator of cell loss and DAMP-mediated inflammation in dsrna-induced retinal degeneration The Harvard community has made this article openly available. Please
Programmed necrosis, not apoptosis, is a key mediator of cell loss and DAMP-mediated inflammation in dsrna-induced retinal degeneration The Harvard community has made this article openly available. Please
Supplemental Figure 1. Western blot analysis indicated that MIF was detected in the fractions of
 Supplemental Figure Legends Supplemental Figure 1. Western blot analysis indicated that was detected in the fractions of plasma membrane and cytosol but not in nuclear fraction isolated from Pkd1 null
Supplemental Figure Legends Supplemental Figure 1. Western blot analysis indicated that was detected in the fractions of plasma membrane and cytosol but not in nuclear fraction isolated from Pkd1 null
for six pairs of mice. (b) Representative FACS analysis of absolute number of T cells (CD4 + and
 SUPPLEMENTARY DATA Supplementary Figure 1: Peripheral lymphoid organs of SMAR1 -/- mice have an effector memory phenotype. (a) Lymphocytes collected from MLNs and Peyer s patches (PPs) of WT and SMAR1
SUPPLEMENTARY DATA Supplementary Figure 1: Peripheral lymphoid organs of SMAR1 -/- mice have an effector memory phenotype. (a) Lymphocytes collected from MLNs and Peyer s patches (PPs) of WT and SMAR1
Supplementary Figure 1: Hsp60 / IEC mice are embryonically lethal (A) Light microscopic pictures show mouse embryos at developmental stage E12.
 Supplementary Figure 1: Hsp60 / IEC mice are embryonically lethal (A) Light microscopic pictures show mouse embryos at developmental stage E12.5 and E13.5 prepared from uteri of dams and subsequently genotyped.
Supplementary Figure 1: Hsp60 / IEC mice are embryonically lethal (A) Light microscopic pictures show mouse embryos at developmental stage E12.5 and E13.5 prepared from uteri of dams and subsequently genotyped.
Supplementary Materials for
 immunology.sciencemag.org/cgi/content/full/2/16/eaan6049/dc1 Supplementary Materials for Enzymatic synthesis of core 2 O-glycans governs the tissue-trafficking potential of memory CD8 + T cells Jossef
immunology.sciencemag.org/cgi/content/full/2/16/eaan6049/dc1 Supplementary Materials for Enzymatic synthesis of core 2 O-glycans governs the tissue-trafficking potential of memory CD8 + T cells Jossef
Reviewers' comments: Reviewer #1 Expert in EAE and IL-17a (Remarks to the Author):
 Reviewers' comments: Reviewer #1 Expert in EAE and IL-17a (Remarks to the Author): This study shows that the inducible camp early repressor (ICER) is involved in development of Th17 cells that are pathogenic
Reviewers' comments: Reviewer #1 Expert in EAE and IL-17a (Remarks to the Author): This study shows that the inducible camp early repressor (ICER) is involved in development of Th17 cells that are pathogenic
Supplementary Figure 1 IL-27 IL
 Tim-3 Supplementary Figure 1 Tc0 49.5 0.6 Tc1 63.5 0.84 Un 49.8 0.16 35.5 0.16 10 4 61.2 5.53 10 3 64.5 5.66 10 2 10 1 10 0 31 2.22 10 0 10 1 10 2 10 3 10 4 IL-10 28.2 1.69 IL-27 Supplementary Figure 1.
Tim-3 Supplementary Figure 1 Tc0 49.5 0.6 Tc1 63.5 0.84 Un 49.8 0.16 35.5 0.16 10 4 61.2 5.53 10 3 64.5 5.66 10 2 10 1 10 0 31 2.22 10 0 10 1 10 2 10 3 10 4 IL-10 28.2 1.69 IL-27 Supplementary Figure 1.
Supplementary Figure 1
 Supplementary Figure 1 a Percent of body weight! (%) 4! 3! 1! Epididymal fat Subcutaneous fat Liver SD Percent of body weight! (%) ** 3! 1! SD Percent of body weight! (%) 6! 4! SD ** b Blood glucose (mg/dl)!
Supplementary Figure 1 a Percent of body weight! (%) 4! 3! 1! Epididymal fat Subcutaneous fat Liver SD Percent of body weight! (%) ** 3! 1! SD Percent of body weight! (%) 6! 4! SD ** b Blood glucose (mg/dl)!
Islet viability assay and Glucose Stimulated Insulin Secretion assay RT-PCR and Western Blot
 Islet viability assay and Glucose Stimulated Insulin Secretion assay Islet cell viability was determined by colorimetric (3-(4,5-dimethylthiazol-2-yl)-2,5- diphenyltetrazolium bromide assay using CellTiter
Islet viability assay and Glucose Stimulated Insulin Secretion assay Islet cell viability was determined by colorimetric (3-(4,5-dimethylthiazol-2-yl)-2,5- diphenyltetrazolium bromide assay using CellTiter
Procaspase-3. Cleaved caspase-3. actin. Cytochrome C (10 M) Z-VAD-fmk. Procaspase-3. Cleaved caspase-3. actin. Z-VAD-fmk
 A HeLa actin - + + - - + Cytochrome C (1 M) Z-VAD-fmk PMN - + + - - + actin Cytochrome C (1 M) Z-VAD-fmk Figure S1. (A) Pan-caspase inhibitor z-vad-fmk inhibits cytochrome c- mediated procaspase-3 cleavage.
A HeLa actin - + + - - + Cytochrome C (1 M) Z-VAD-fmk PMN - + + - - + actin Cytochrome C (1 M) Z-VAD-fmk Figure S1. (A) Pan-caspase inhibitor z-vad-fmk inhibits cytochrome c- mediated procaspase-3 cleavage.
Supplementary Materials for
 www.sciencesignaling.org/cgi/content/full/4/199/ra75/dc1 Supplementary Materials for Signaling by the Matrix Proteoglycan Decorin Controls Inflammation and Cancer Through PDCD4 and MicroRNA-21 Rosetta
www.sciencesignaling.org/cgi/content/full/4/199/ra75/dc1 Supplementary Materials for Signaling by the Matrix Proteoglycan Decorin Controls Inflammation and Cancer Through PDCD4 and MicroRNA-21 Rosetta
General Laboratory methods Plasma analysis: Gene Expression Analysis: Immunoblot analysis: Immunohistochemistry:
 General Laboratory methods Plasma analysis: Plasma insulin (Mercodia, Sweden), leptin (duoset, R&D Systems Europe, Abingdon, United Kingdom), IL-6, TNFα and adiponectin levels (Quantikine kits, R&D Systems
General Laboratory methods Plasma analysis: Plasma insulin (Mercodia, Sweden), leptin (duoset, R&D Systems Europe, Abingdon, United Kingdom), IL-6, TNFα and adiponectin levels (Quantikine kits, R&D Systems
Supplemental Figure 1. Signature gene expression in in vitro differentiated Th0, Th1, Th2, Th17 and Treg cells. (A) Naïve CD4 + T cells were cultured
 Supplemental Figure 1. Signature gene expression in in vitro differentiated Th0, Th1, Th2, Th17 and Treg cells. (A) Naïve CD4 + T cells were cultured under Th0, Th1, Th2, Th17, and Treg conditions. mrna
Supplemental Figure 1. Signature gene expression in in vitro differentiated Th0, Th1, Th2, Th17 and Treg cells. (A) Naïve CD4 + T cells were cultured under Th0, Th1, Th2, Th17, and Treg conditions. mrna
Electron micrograph of phosphotungstanic acid-stained exosomes derived from murine
 1 SUPPLEMENTARY INFORMATION SUPPLEMENTARY FIGURES Supplementary Figure 1. Physical properties of murine DC-derived exosomes. a, Electron micrograph of phosphotungstanic acid-stained exosomes derived from
1 SUPPLEMENTARY INFORMATION SUPPLEMENTARY FIGURES Supplementary Figure 1. Physical properties of murine DC-derived exosomes. a, Electron micrograph of phosphotungstanic acid-stained exosomes derived from
Stewart et al. CD36 ligands promote sterile inflammation through assembly of a TLR 4 and 6 heterodimer
 NFκB (fold induction) Stewart et al. ligands promote sterile inflammation through assembly of a TLR 4 and 6 heterodimer a. mrna (fold induction) 5 4 3 2 1 LDL oxldl Gro1a MIP-2 RANTES mrna (fold induction)
NFκB (fold induction) Stewart et al. ligands promote sterile inflammation through assembly of a TLR 4 and 6 heterodimer a. mrna (fold induction) 5 4 3 2 1 LDL oxldl Gro1a MIP-2 RANTES mrna (fold induction)
Supplementary Figure S1. Flow cytometric analysis of the expression of Thy1 in NH cells. Flow cytometric analysis of the expression of T1/ST2 and
 Supplementary Figure S1. Flow cytometric analysis of the expression of Thy1 in NH cells. Flow cytometric analysis of the expression of T1/ST2 and Thy1 in NH cells derived from the lungs of naïve mice.
Supplementary Figure S1. Flow cytometric analysis of the expression of Thy1 in NH cells. Flow cytometric analysis of the expression of T1/ST2 and Thy1 in NH cells derived from the lungs of naïve mice.
Supplemental Information. Otic Mesenchyme Cells Regulate. Spiral Ganglion Axon Fasciculation. through a Pou3f4/EphA4 Signaling Pathway
 Neuron, Volume 73 Supplemental Information Otic Mesenchyme Cells Regulate Spiral Ganglion Axon Fasciculation through a Pou3f4/EphA4 Signaling Pathway Thomas M. Coate, Steven Raft, Xiumei Zhao, Aimee K.
Neuron, Volume 73 Supplemental Information Otic Mesenchyme Cells Regulate Spiral Ganglion Axon Fasciculation through a Pou3f4/EphA4 Signaling Pathway Thomas M. Coate, Steven Raft, Xiumei Zhao, Aimee K.
As outlined under External contributions (see appendix 7.1), the group of Prof. Gröne at the
 3 RESULTS As outlined under External contributions (see appendix 7.1), the group of Prof. Gröne at the DKFZ in Heidelberg (Dept. of Cellular and Molecular pathology) contributed to this work by performing
3 RESULTS As outlined under External contributions (see appendix 7.1), the group of Prof. Gröne at the DKFZ in Heidelberg (Dept. of Cellular and Molecular pathology) contributed to this work by performing
NKTR-255: Accessing IL-15 Therapeutic Potential through Robust and Sustained Engagement of Innate and Adaptive Immunity
 NKTR-255: Accessing IL-15 Therapeutic Potential through Robust and Sustained Engagement of Innate and Adaptive Immunity Peiwen Kuo Scientist, Research Biology Nektar Therapeutics August 31 st, 2018 Emerging
NKTR-255: Accessing IL-15 Therapeutic Potential through Robust and Sustained Engagement of Innate and Adaptive Immunity Peiwen Kuo Scientist, Research Biology Nektar Therapeutics August 31 st, 2018 Emerging
Supplementary Materials and Methods
 Supplementary Materials and Methods Hepatocyte toxicity assay. Freshly isolated hepatocytes were incubated for overnight with varying concentrations (-25 µm) of sodium glycochenodeoxycholate (GCDC) or
Supplementary Materials and Methods Hepatocyte toxicity assay. Freshly isolated hepatocytes were incubated for overnight with varying concentrations (-25 µm) of sodium glycochenodeoxycholate (GCDC) or
Nature Medicine: doi: /nm.3922
 Title: Glucocorticoid-induced tumor necrosis factor receptor-related protein co-stimulation facilitates tumor regression by inducing IL-9-producing helper T cells Authors: Il-Kyu Kim, Byung-Seok Kim, Choong-Hyun
Title: Glucocorticoid-induced tumor necrosis factor receptor-related protein co-stimulation facilitates tumor regression by inducing IL-9-producing helper T cells Authors: Il-Kyu Kim, Byung-Seok Kim, Choong-Hyun
Bone marrow-derived mesenchymal stem cells improve diabetes-induced cognitive impairment by
 Nakano et al. Supplementary information 1. Supplementary Figure 2. Methods 3. References Bone marrow-derived mesenchymal stem cells improve diabetes-induced cognitive impairment by exosome transfer into
Nakano et al. Supplementary information 1. Supplementary Figure 2. Methods 3. References Bone marrow-derived mesenchymal stem cells improve diabetes-induced cognitive impairment by exosome transfer into
Supplementary fig. 1. Crystals induce necroptosis does not involve caspases, TNF receptor or NLRP3. A. Mouse tubular epithelial cells were pretreated
 Supplementary fig. 1. Crystals induce necroptosis does not involve caspases, TNF receptor or NLRP3. A. Mouse tubular epithelial cells were pretreated with zvad-fmk (10µM) and exposed to calcium oxalate
Supplementary fig. 1. Crystals induce necroptosis does not involve caspases, TNF receptor or NLRP3. A. Mouse tubular epithelial cells were pretreated with zvad-fmk (10µM) and exposed to calcium oxalate
Supplemental Materials for. Effects of sphingosine-1-phosphate receptor 1 phosphorylation in response to. FTY720 during neuroinflammation
 Supplemental Materials for Effects of sphingosine-1-phosphate receptor 1 phosphorylation in response to FTY7 during neuroinflammation This file includes: Supplemental Table 1. EAE clinical parameters of
Supplemental Materials for Effects of sphingosine-1-phosphate receptor 1 phosphorylation in response to FTY7 during neuroinflammation This file includes: Supplemental Table 1. EAE clinical parameters of
SUPPLEMENTARY INFORMATION
 1. Supplementary Figures and Legends Supplementary Fig. 1. S1P-mediated transcriptional regulation of integrins expressed in OP/monocytoid cells. Real-time quantitative PCR analyses of mrna for two integrins,
1. Supplementary Figures and Legends Supplementary Fig. 1. S1P-mediated transcriptional regulation of integrins expressed in OP/monocytoid cells. Real-time quantitative PCR analyses of mrna for two integrins,
SUPPLEMENTAL MATERIAL. Supplementary Methods
 SUPPLEMENTAL MATERIAL Supplementary Methods Culture of cardiomyocytes, fibroblasts and cardiac microvascular endothelial cells The isolation and culturing of neonatal rat ventricular cardiomyocytes was
SUPPLEMENTAL MATERIAL Supplementary Methods Culture of cardiomyocytes, fibroblasts and cardiac microvascular endothelial cells The isolation and culturing of neonatal rat ventricular cardiomyocytes was
Supplementary Information
 Supplementary Information TABLE S1. SUBJECT CHARACTERISTICS* Normal Control Subjects Subjects with Asthma p Value Number 23 48 Age (years) 35±10 35±10 0.75 Sex, M:F (% F) 9:12 (57) 17:26 (60) 0.76 FEV1
Supplementary Information TABLE S1. SUBJECT CHARACTERISTICS* Normal Control Subjects Subjects with Asthma p Value Number 23 48 Age (years) 35±10 35±10 0.75 Sex, M:F (% F) 9:12 (57) 17:26 (60) 0.76 FEV1
Supplemental Figure 1
 Supplemental Figure 1 1a 1c PD-1 MFI fold change 6 5 4 3 2 1 IL-1α IL-2 IL-4 IL-6 IL-1 IL-12 IL-13 IL-15 IL-17 IL-18 IL-21 IL-23 IFN-α Mut Human PD-1 promoter SBE-D 5 -GTCTG- -1.2kb SBE-P -CAGAC- -1.kb
Supplemental Figure 1 1a 1c PD-1 MFI fold change 6 5 4 3 2 1 IL-1α IL-2 IL-4 IL-6 IL-1 IL-12 IL-13 IL-15 IL-17 IL-18 IL-21 IL-23 IFN-α Mut Human PD-1 promoter SBE-D 5 -GTCTG- -1.2kb SBE-P -CAGAC- -1.kb
Supporting Information
 Supporting Information Pang et al. 10.1073/pnas.1322009111 SI Materials and Methods ELISAs. These assays were performed as previously described (1). ELISA plates (MaxiSorp Nunc; Thermo Fisher Scientific)
Supporting Information Pang et al. 10.1073/pnas.1322009111 SI Materials and Methods ELISAs. These assays were performed as previously described (1). ELISA plates (MaxiSorp Nunc; Thermo Fisher Scientific)
Therapeutic PD L1 and LAG 3 blockade rapidly clears established blood stage Plasmodium infection
 Supplementary Information Therapeutic PD L1 and LAG 3 blockade rapidly clears established blood stage Plasmodium infection Noah S. Butler, Jacqueline Moebius, Lecia L. Pewe, Boubacar Traore, Ogobara K.
Supplementary Information Therapeutic PD L1 and LAG 3 blockade rapidly clears established blood stage Plasmodium infection Noah S. Butler, Jacqueline Moebius, Lecia L. Pewe, Boubacar Traore, Ogobara K.
Supplementary Table 1. The primers used for quantitative RT-PCR. Gene name Forward (5 > 3 ) Reverse (5 > 3 )
 770 771 Supplementary Table 1. The primers used for quantitative RT-PCR. Gene name Forward (5 > 3 ) Reverse (5 > 3 ) Human CXCL1 GCGCCCAAACCGAAGTCATA ATGGGGGATGCAGGATTGAG PF4 CCCCACTGCCCAACTGATAG TTCTTGTACAGCGGGGCTTG
770 771 Supplementary Table 1. The primers used for quantitative RT-PCR. Gene name Forward (5 > 3 ) Reverse (5 > 3 ) Human CXCL1 GCGCCCAAACCGAAGTCATA ATGGGGGATGCAGGATTGAG PF4 CCCCACTGCCCAACTGATAG TTCTTGTACAGCGGGGCTTG
Supplemental Information
 Supplemental Information Tobacco-specific Carcinogen Induces DNA Methyltransferases 1 Accumulation through AKT/GSK3β/βTrCP/hnRNP-U in Mice and Lung Cancer patients Ruo-Kai Lin, 1 Yi-Shuan Hsieh, 2 Pinpin
Supplemental Information Tobacco-specific Carcinogen Induces DNA Methyltransferases 1 Accumulation through AKT/GSK3β/βTrCP/hnRNP-U in Mice and Lung Cancer patients Ruo-Kai Lin, 1 Yi-Shuan Hsieh, 2 Pinpin
Supplemental Figures:
 Supplemental Figures: Figure 1: Intracellular distribution of VWF by electron microscopy in human endothelial cells. a) Immunogold labeling of LC3 demonstrating an LC3-positive autophagosome (white arrow)
Supplemental Figures: Figure 1: Intracellular distribution of VWF by electron microscopy in human endothelial cells. a) Immunogold labeling of LC3 demonstrating an LC3-positive autophagosome (white arrow)
