The Building Blocks of Mammalian Lung Development
|
|
|
- Ralph Watts
- 5 years ago
- Views:
Transcription
1 a DEVELOPMENTAL DYNAMICS 240: , 2011 SPECIAL ISSUE REVIEWS-A PEER REVIEWED FORUM The Building Blocks of Mammalian Lung Development Emma L. Rawlins* Progress has recently been made in identifying progenitor cell populations in the embryonic lung. Some progenitor cell types have been definitively identified by lineage-tracing studies. However, others are not as well characterized and their existence is inferred on the basis of lung morphology, or mutant phenotypes. Here, I focus on lung development after the specification of the initial lung primordium. The evidence for various lung embryonic progenitor cell types is discussed and future experiments are suggested. The regulation of progenitor proliferation in the embryonic lung, and its coordinate control with morphogenesis, is also discussed. In addition, the relationship between embryonic and adult lung progenitors is considered. 240: , VC 2010 Wiley-Liss, Inc. Key words: progenitor cell; stem cell; lung; lineage; mouse Accepted 1 October 2010 INTRODUCTION Our lungs are highly complex organs that are exquisitely specialized for gas exchange and host defense. They have a large surface area exposed to the external environment, which makes them vulnerable to both infection and environmental damage. The adult lung has a tremendous capacity to regenerate following an acute insult. An example of an acute lung injury is partial pneumonectomy. If the entire left lobe of a mouse lung is removed, total lung volume is restored within 3 weeks by compensatory growth of the right lung. Detailed stereological analysis has shown that this compensatory growth involves both expansion of remaining alveoli and the formation of new alveolar units (neoalveolarization; Voswinckel et al., 2004; Fehrenbach et al., 2008). In contrast, many chronic injuries are not repairable and can lead to lung conditions, such as emphysema in which alveolar units are lost.numerouslabsarecharacterizing adult lung stem cells with the long-term aim of manipulating their behavior for therapeutic purposes. One successful clinical example of stem cell therapy for the lung has been reported: transplantation of a decellularized trachea reseeded with the patient s own bronchial epithelial cells (Macchiarini et al., 2008). Similar techniques have been used to obtain bioengineered rat lungs, which were able to function for up to 6 hours after transplantation (Ott et al., 2010; Petersen et al., 2010). However, the successful bioengineering of an entire human lung is vastly more complex, and these advances remain far from routine clinical use. One area of current and future research of great clinical relevance is the normal developmental biology of the mammalian lung. Variation in adult lung function, and lung conditions, can result from changes in our lungs during embryogenesis or infancy. It has become apparent that many developmental abnormalities of the lung cannot be corrected postnatally. These can result from premature birth, genetic variation, or antenatal conditions such as intrauterine infection (Baraldi and Filippone, 2007; Shi et al., 2007). Longitudinal studies of individuals born prematurely, with or without acute lung disease at birth, show that these people generally have decreased lung function and/or more respiratory problems throughout adolescence (Vrijlandt et al., 2006; Narang et al., 2008; Wong et al., 2008). Furthermore, it is often suggested that these individuals will be at increased risk of developing diseases associated with the normal age-related decline in lung function, Gurdon Institute and Department of Pathology, University of Cambridge, Cambridge, United Kingdom Grant sponsor: MRC; Grant number: GO *Correspondence to: Emma L. Rawlins, Gurdon Institute and Department of Pathology, University of Cambridge, Cambridge, CB2 1QN, UK. e.rawlins@gurdon.cam.ac.uk DOI /dvdy Published online 18 November 2010 in Wiley Online Library (wileyonlinelibrary.com). VC 2010 Wiley-Liss, Inc.
2 464 RAWLINS such as chronic obstructive pulmonary disease (COPD; Maciewicz et al., 2009). Even in normal healthy individuals there is variation in lung function. Meta-analysis of genome-wide studies (16 studies totaling >40,000 individuals) identified loci associated with natural variations in lung function of healthy volunteers (Hancock et al., 2010; Repapi et al., 2010). These loci included HHIP and PTCH1 which are both required for lung development in mice (Chuang et al., 2003). A better understanding of embryonic lung development will improve our knowledge of these developmental conditions and may eventually lead to additional therapeutic options. LUNG STRUCTURE The adult lung consists of a pair of primary bronchi that branch from the trachea and give rise to progressively finer branches of the conducting airways (the bronchioles) which open to the ductal alveolar region where gas exchange occurs (Fig. 1A). The different regions of the lung contain specific specialized epithelial and mesenchymal cells. In mice, the primary bronchi are lined with a columnar epithelium consisting of basal, Clara (secretory), ciliated, and a small number of neuroendocrine (NE) cells. The mesenchyme of these airways has evenly spaced C- ring cartilage on the ventral side and smooth muscle (parabronchial smooth muscle) on the dorsal side (Fig. 1B). The bronchioles are lined by a similar columnar epithelium consisting of Clara and ciliated cells and clusters of NE cells known as neuroendocrine bodies, or NEBs (Fig. 1C,D). The epithelial composition of the bronchioles, particularly the ratio of Clara:ciliated cells, changes stereotypically along the proximal distal axis; the smaller airways having an increased proportion of Clara cells (Toskala et al., 2005). The bronchioles are completely circled by parabronchial smooth muscle and do not have cartilage (Fig. 1F). Running parallel with the branched network of conducting airways are similar networks of blood vessels (arteries and veins, composed of endothelium, vascular smooth muscle, and pericytes), nerves (sympathetic and parasymphathetic neurons innervating both the airways and vasculature), and lymphatic tissue. The terminal branch of the bronchioles opens directly into the alveolar ducts where gas exchange occurs. The alveolar epithelium is squamous and consists of surfactant-secreting type 2 cells and highly elongated type 1 cells (Fig. 1E G). The type 1 cells line the entire alveolar epithelial surface and run adjacent to capillaries in the underlying mesenchyme, providing a minimal barrier for gas exchange. The mesenchyme of the alveoli also contains alveolar smooth muscle cells and stromal fibroblasts, including lipofibroblasts and myofibroblasts, although these cell types may be interconvertible (Kapanci et al., 1974; Lindahl et al., 1997; McGowan et al., 2008; Perl and Gale, 2009). In all regions of the lung, it is difficult to distinguish the different mesenchymal cells, either morphologically or with molecular markers, and there may be additional undescribed subtypes. The entire lung is surrounded by a layer of squamous epithelium, the mesothelium or pleura. This provides a lubricated surface for movement of the organ. An Overview of Lung Development When and where do all these different cell types originate? The lung arises from the ventral foregut at embryonic day (E) 9.5 in the mouse as two primary buds. The lung buds consist of three cell layers: the inner epithelium (endodermal origin), which is surrounded by loosely packed mesenchyme and a thin outer mesothelial (squamous epithelial) layer. Lung morphogenesis has been the subject of several excellent recent reviews (Cardoso and Lu, 2006; Morrisey and Hogan, 2010) and is described briefly here. During embryonic development the epithelium of the lung bud undergoes branching morphogenesis to elaborate the structure of the bronchioles and alveoli (Fig. 2A D). The inner, endodermally derived, epithelium of the lung buds consists of a blind-ended hollow tube. Initial branches are mostly side-branches, which form by budding along the length of the tube, this type of budding has been termed domain branching (Metzger et al., 2008). The branches elongate and subsequently bud again, this time by bifurcation of the distal-most epithelial tip, termed planar, or orthogonal, bifurcation depending on the orientation of the two new branches (Metzger et al., 2008). Metzger and Krasnow have proposed a conceptual framework for lung branching that includes genetic subroutines that control timing, orientation, and birfurcation of the branches. In this model, the branch timing subroutine would be the program that regulates overall growth of the lung and also controls progenitor cell proliferation. However, these ideas have yet to be specifically tested. The branching epithelium initially generates the future bronchioles (E9.5 E16.5, embryonic and pseudoglandular stages of lung development). Between E16.5 and E17.5 (canalicular stage), the distal epithelium continues to branch, although budding morphology and frequency changes, and it now gives rise to a framework for future alveolar development, the terminal sacs (Fig. 2, compare A,B, with C,D). These sacs increase in number from E17.5 to postnatal day (P) 5 (terminal sac stage). During the alveolar phases (P5 P30), the terminal sacs are remodeled into mature alveoli and alveolar ducts. Alveolarization involves the generation of septae, which initially form as ridges along the terminal sac walls, and microvascular remodeling. The trachea forms from the ventral side of the foregut just caudal to the lung buds (Que et al., 2006). The trachea and lung both arise from an Nkx2-1 þ region of the foregut and presumably from a common progenitor population (reviewed in Cardoso and Lu, 2006). However, the trachea does not undergo branching morphogenesis, rather it separates from the future esophagus by means of a process of septation (Ioannides et al., 2010). Indeed after the initial specification of the Nkx2-1 þ anterior foregut domain, the lung and trachea have somewhat different patterns of gene expression. Specific tracheal progenitor cell populations are not discussed in this article. Mammalian lung development has largely been studied by analysis of gene-targeted mice. These studies have identified genes and cellular mechanisms that regulate progenitor cell fate and overall lung morphogenesis. More recently, various methods of transient and long-term lineage-
3 PROGENITOR CELLS IN THE DEVELOPING LUNG 465 Fig. 1. Fig. 1. Cellular organization of the adult mouse lung. A E: Hematoxylin and eosin stained sections of the adult mouse lung. A: Overview showing the branching bronchioles (Br) opening up into the alveoli (Al). A large blood vessel (Bv) is running parallel with the bronchioles. Dashed lines indicate the position of the images in (C,D). B: Distal region of a primary bronchus, dorsal side. Part of the bronchiolar smooth muscle layer is visible (arrows). The epithelium is largely composed of ciliated and basal cells. C: Proximal bronchiole branch (level corresponds to the upper dashed line in A). The epithelium is largely composed of ciliated cells, but greater numbers of Clara cells (arrowheads) are present than in the more proximal airways. The bronchiolar smooth muscle is less extensive (arrow). D: Distal bronchiole branch (level corresponds to the lower dashed line in A). The epithelium has many more Clara cells; ciliated cells (arrowheads) are located between the Clara cells, but do not extend as far apically and are now difficult to see without a specific stain. Similarly, the bronchiolar smooth muscle (arrow) is less extensive and cannot readily be distinguished. E: Alveolar region. The cell types cannot be distinguished without specific antibody labeling. F: Section through a terminal bronchiole (Br) and adjacent alveolar region (A) stained to show muscle cells (antiasmooth muscle actin [SMA], red) and nuclei (DAPI [4 0,6-diamidine-2-phenylidole-dihydrochloride], blue). The most extensive SMA staining is the vascular smooth muscle around a blood vessel (Bv). Bronchiolar (arrows) and alveolar (arrowheads) smooth muscle can also be seen. G: Alveolar section stained to show type 2 cells (anti-pro surfactant protein C, green), type 1 cells (anti-t1a, red), and endothelial cells (anti-pecam, blue). Note the proximity of the type 1 and endothelial cells throughout the alveolar network. Scale bars ¼ 0.25 mm in A, 25 mm in B E, 200 mm inf,50mm ing. Fig. 2. Fig. 2. Lung development. A: Whole-mount image of an embryonic day (E) 11.5 left lung lobe. The epithelium (E) appears translucent and can be seen branching within the surrounding mesenchyme (M). The distal tips of the epithelium (arrowheads) are in different stages of the budding process. B: Confocal image of a whole-mount E12.5 lung lobe stained with anti E-cadherin (green) to show the epithelium. The budding tip is in the early stages of dichotomous branching. C: E-cadherin stained (green) confocal image of a 100 mm Vibratome section of an E16.5 lung. Note that branching is much more frequent at this stage of lung development and that the buds themselves are more rounded. D: Confocal image of a 100 mm Vibratome section of an E16.5 Sox2-GFP lung accessory lobe (Taranova et al., 2006) stained to show the developing bronchioles (Sox2-GFP, green), the distal epithelial progenitors (anti-sox9, red) and the nuclei (DAPI, blue). Note the increased number of distal epithelial buds compared to E11.5. Scale bars ¼ 0.25 mm in A, 50 mm in B,C, 100 mm ind.
4 466 RAWLINS labeling have been used to identify specific embryonic lung progenitor cell populations. These studies have successfully identified a multipotent epithelial progenitor cell, parabronchial smooth muscle progenitors, and a subset of vascular smooth muscle progenitors. They provide a framework for analyzing the genetic regulation of individual populations of lung progenitor cells. Other lung embryonic progenitor cell populations are less well characterized and lineage-tracing studies have either not been performed, or are just beginning. Here I consider the evidence for the existence of various embryonic lung progenitor cell populations in the branching lung and their molecular regulation. This synthesis of the current literature highlights hypotheses to be tested and additional areas where further research is urgently needed. I also discuss the relationship between lung progenitor cell proliferation and branching morphogenesis, and the embryonic and adult lung progenitor populations. EPITHELIAL PROGENITOR CELL POPULATIONS Multipotent Epithelial Progenitors Embryonic progenitor cells can selfrenew during development and give rise to one or more cell types. They do not necessarily persist in the postnatal animal. The best characterized progenitor population in the embryonic lung is an epithelial progenitor cell located at the distal tip of the endodermally derived branching epithelium. The work of many laboratories has suggested that these cells are a multipotent epithelial progenitor population (reviewed in Cardoso and Lu, 2006). Compared with the epithelial stalk cells, the distal tip cells have a unique pattern of gene expression including high levels of Bmp4, Shh, Nmyc, Sox9, Id2, and Etv4/5 (reviewed in Rawlins, 2008), faster cell cycle kinetics (Okubo et al., 2005), and a subtly different morphology (Liu et al., 2004). Moreover, lung epithelial-specific deletion, or overexpression, of Nmyc results in phenotypes consistent with a premature loss, or overproliferation, of epithelial progenitor cells (Okubo et al., 2005). To directly test the hypothesis that the distal epithelial tip cells are a multipotent epithelial progenitor population, an Id2-CreER 2T knock-in mouse line was generated and used to lineage-label the distal lung epithelium at different stages of development. When lineage-labeled during the pseudoglandular stage, the distal Id2 þ epithelial cells both self-renewed during development and produced descendents which differentiated into all of the identifiable bronchiolar and alveolar epithelial cell types: Clara, ciliated, neuroendocrine, type 2, type 1, including the adult progenitor cells (Figs. 3A,C, 5; Rawlins et al., 2009a). These data definitively show that the Id2 þ distal epithelial cells are a multipotent epithelial progenitor population. To test the hypothesis that individual Id2 þ distal tip cells are multipotent, single epithelial cells were lineage-labeled at E11.5 by exposing pregnant dams to a lower dose of tamoxifen. In >80% of the clones examined, the descendents of these single labeled Id2 þ cells differentiated into both bronchiolar and alveolar cell types, strongly supporting the hypothesis that they are multipotent (Rawlins et al., 2009a). (However, NE cell differentiation was not specifically tested in the single cell lineage-tracing experiments.) The remaining 20% of single labeled cells gave rise to small (up to 5 cells) bronchiolar, or alveolar-restricted clones. Further work is needed to determine if this 20% of more lineage-restricted clones actually represent lineage-restricted Id2 þ progenitor cells. Or, alternatively, if the Id2 þ distal epithelial cells are a homogenous population, but by chance some of the labeled cells did not divide many times in the single cell lineage-tracing experiments. Id2 expression is maintained at the lung distal epithelial tip throughout embryogenesis. When the Id2 þ distal tip cells were lineage-labeled during the canalicular stage, their descendents differentiated as type 1 or type 2 alveolar cells (Fig. 3B). Taken together, these data suggest a model in which the Id2 þ distal epithelial cells are multipotent progenitors for lung embryonic development. They self-renew throughout embryogenesis, initially generate bronchiolar daughters, and then alveolar daughters (Figs. 3C, 5). A similar distal epithelial multipotent progenitor population has been identified in the endodermally derived pancreas (Zhou et al., 2007). Many questions remain to be addressed. For example, how do the multipotent lung progenitor cells switch from making bronchiolar to alveolar descendents? The mechanism must be lung-intrinsic because isolated E11.5 lungs will branch in culture and generate both bronchiolar and alveolar cell types. Transplantation experiments will be required to determine if the epithelial progenitors themselves undergo an intrinsic change in competence, or if signaling from the surrounding mesenchyme determines daughter fate. These studies may benefit from comparison with the developing cortex and retina where transplant experiments have shown that progenitors undergo cell intrinsic changes in competence to produce a sequence of different daughters (Livesey and Cepko, 2001). One hypothesis to explain how this is achieved in the nervous system is a link between the time of cell cycle exit and fate determination, perhaps regulated by cyclin kinase inhibitors (Dyer and Cepko, 2000, 2001). Lineage-restricted Epithelial Progenitors As cells leave the Id2 þ pool they continue to proliferate. One model is that as cells exit the distal tip they become more-restricted bronchiolar, or alveolar, progenitors (Fig. 3D). Directly testing this hypothesis will require a better understanding of the molecular mechanisms which are responsible for cell fate specification in the developing lung. Differentiation of the airway epithelium has been observed to move down the airways from the proximal to the more distal regions (McDowell et al., 1994; Toskala et al., 2005). Molecular markers for NE cell fate specification (for example, the transcription factor Ascl1) and Clara and ciliated cell differentiation (Secretoglobin1a1 and the transcription factor Foxj1) are first observed at E12.5 (NE cells) and E14.5, or 15.5 (ciliated and Clara cells), always at least one airway generation from the multipotent progenitors in the distal tip (Rawlins et al., 2007; McGovern et al., 2010).
5 PROGENITOR CELLS IN THE DEVELOPING LUNG 467 These results suggest that cells are not being assigned to specific fates as soon as they leave the multipotent progenitor pool, consistent with the presence of more restricted progenitors. The reported effects of loss or gain of Notch signaling in the developing lung also support the restricted progenitor hypothesis. Ectopic expression of the active Notch intracellular domain throughout the developing airways, resulted in increased numbers of secretory (mucous-producing) cells and a decrease in ciliated cell specification (Guseh et al., 2009). Conversely, when Notch signaling was ablated throughout the entire lung epithelium by conditional deletion of Pofut1 (which attaches O-fucose to the EGF repeats of Notch), or Rbpjk (the Notch transcriptional effector), the bronchiolar epithelium largely differentiated as ciliated cells at the expense of the secretory (Clara) cells (Tsao et al., 2009; Morimoto et al., 2010). In the loss-of-function experiments, the multipotent progenitors were unaffected, alveolar development continued normally, and the mice were born with normal-sized lungs. These data are consistent with Notch signaling occurring between undifferentiated bronchiolar progenitor cells to restrict ciliated cell fate. The existence of these bronchiolar-restricted epithelial progenitors, and a similar putative alveolar progenitor, needs to be tested directly. Lineage-labeling the Id2 þ distal epithelial cells showed that their descendents can differentiate as NE cells. However, no completely labeled NE cell clusters (NEBs) were identified (Rawlins et al., 2009a). By contrast, when a human SftpC (Surfactant Protein C) promoter fragment was used for doxycycline-dependent, Cre-mediated lineage-labeling throughout the developing lung epithelium, no labeled NE cells were identified (Perl et al., 2002). These results could be partially reconciled if a subset of the NE cells, possibly the founding cells of each cluster, are neural crest-derived. This hypothesis needs to be formally tested. MESENCHYMAL PROGENITOR CELL POPULATIONS The mesenchymal cells of the lung are not as well characterized as the epithelial cells. There is still no general consensus around the number of different differentiated mesenchymal cell types in the adult lung, or the molecular markers which can be used to distinguish between them. Indeed, a recent study of adult human lung mesenchymal cells suggests that no single molecular marker yet identified is specific for an individual mesenchymal cell type (Singh et al., 2010). This makes identification of the mesenchymal progenitor cells difficult, although progress has been made. The initial lung buds are composed of endodermal epithelium surrounded by mesenchyme derived from the splanchnic mesoderm, which itself comes from the lateral plate. Two different populations of mesenchymal cells can be identified morphologically at E12.5 (White et al., 2006). The subepithelial mesenchyme is tightly packed and oriented around the epithelial tubes, whereas the submesothelial mesenchyme is more loosely packed and not oriented. These morphological differences are subtle (Fig. 4A). However, they do seem to coincide with some gene expression differences. For example, Fgf10 is specifically expressed in the submesothelial mesenchyme (Bellusci et al., 1997b), whereas ptch1 (patched1) is expressed throughout the distal lung mesenchyme (Weaver et al., 2003). Bronchiolar Smooth Muscle Progenitors Fibroblast growth factor-10 (FGF10) is highly expressed in the submesothelial mesenchyme, several cell diameters from the branching tip of the lung epithelium (Bellusci et al., 1997b) and is required for branching morphogenesis (for example, Sekine et al., 1999; Weaver et al., 2000). The distal FGF10 þ mesenchymal cells were followed for a short time by taking advantage of the perdurance of the b-galactosidase protein in an Fgf10-LacZ strain. In this strain, the b-galactosidase protein remains in the cells after Fgf10 transcription has been turned off. Although formal lineage-tracing experiments remain to be done, the descendents of the distal FGF10 þ mesenchymal cells have been partially characterized. In these experiments, the FGF10 þ,sma (a-smooth muscle actin) distal mesenchyme cells gave rise to FGF10,SMA þ parabronchial smooth muscle (Mailleux et al., 2005). The authors confirmed the result by grafting Fgf10-LacZ mesenchyme onto cultured wild-type lungs and vice versa. These data conclusively show that the distal tip mesenchyme includes progenitors for the parabronchial smooth muscle. It is not yet clear if all of the FGF10 þ distal mesenchymal cells become parabronchial smooth muscle or, if they can also acquire other mesenchymal fates. Similarly, is all of the bronchiolar smooth muscle derived from this cell population? And how do the cells exit the submesothelial mesenchyme and move to a subepithelial position? Do similar submesothelial mesenchymal progenitor cells give rise to the alveolar smooth muscle at later stages of lung development? Using retrovirus-mediated lineagetracing in E11.5 lung/tracheal explants, it was shown that labeled proximal mesenchymal cells, just adjacent to the trachea and mainstem bronchi, gave rise to descendents which were dispersed throughout the lung mesenchyme after 11 days culture. These included cells with the morphological appearance of parabronchiolar smooth muscle (Shan et al., 2008). Laser capture microdissection and sequencing of the retroviral genome showed clonal origins for some of these widespread cells. This result suggests that there might be a second, more proximal, progenitor for the bronchiolar smooth muscle (and possibly other mesenchymal cell types). The existence of two populations of parabronchial smooth muscle progenitors (proximal bronchi region, and distal tip region) is also supported by lineage-tracing using the Id2-CreER mouse strain (E.L.R., unpublished data, Rawlins et al., 2009a). Id2-CreER drives recombination in small numbers of both proximal and distal mesenchymal cells at E11.5, these subsequently differentiate as smooth muscle (Fig. 4B,C). Id2 is not expressed at high levels in the developing lung mesenchyme and the Id2- CreER strain is not useful for further characterization of mesenchymal progenitor populations. It will be important to better-characterize the molecular profile of different mesenchymal cell types throughout lung development to allow for an improved analysis of the cellular and molecular regulation of the different mesenchymal progenitors.
6 468 RAWLINS Fig. 3. Epithelial progenitor cell populations in the developing lung. A,B: Eosin-stained sections of Id2-CreER; Rosa26R-LacZ lungs labeled with X-gal (blue) to show a subset of the multipotent progenitor cells. A: Embryonic day (E) 12.5, exposed to tamoxifen at E11.5. The lineage-labeled cells will contribute descendents to all of the lung epithelium. B: E17.5, exposed to tamoxifen at E16.5. The lineage-labeled cells will contribute descendents to the alveoli only. C: Model of the Id2 þ multipotent progenitor cells. The cells self-renew throughout lung development. Initially they contribute descendents to the bronchioles. After E16.5, they contribute descendents to the alveoli. D: Diagram of the progenitors in the pseudoglandular stage epithelial buds. The distal tip epithelium contains Id2 þ multipotent progenitors, which were exposed to high levels of fibroblast growth factor (FGF), bone morphogenetic protein (BMP), and Wnt signaling. As cells are left behind in the stalk they are gradually exposed to lower levels of the signaling molecules and become a putative bronchiolar progenitor population. These cells continue to proliferate and also signal to each other by means of the Notch pathway to determine specific cell identities. Fig. 4. Mesenchymal progenitor cell populations in the developing lung. A: Hematoxylin and eosin stained section of an embryonic day (E) 12.5 left lung lobe. The endodermally derived epithelium (E) grows into the mesenchyme (M). The mesenchyme surrounding the distal budding epithelium has been categorized as subepithelial mesenchyme (sem) and submesothelial mesenchyme (smm). However, the morphological differences between the cells are subtle. The whole lung is surrounded by the mesothelium (Mt) which is an epithelial layer. B: Whole-mount image of E12.5 Id2-CreER; Rosa26R-LacZ lung. Lineage-labeled cells are blue (X-gal). At E11.5, the lung was exposed to tamoxifen to label a subset of the Id2 þ cells and then was dissected and X-gal stained at E12.5. Some of the distal epithelial multipotent progenitor cells are X-gal þ, as are a small number of distal and proximal mesenchymal cells (arrows). The mesothelium was never observed to express the reporter. C: X-gal-stained section of E13.5 Id2-CreER; Rosa26R- LacZ lung exposed to tamoxifen at E11.5. Lineage-labeled mesenchymal cells (arrows) are already beginning to differentiate into bronchiolar smooth muscle. Scale bars ¼ 200 mm in A,C, 1mminB. Vascular Smooth Muscle Progenitors In the heart and gut, lineage-tracing of the outer mesothelial layer, using mesothelial-specific Wt1-Cre mouse strains, has shown that the mesothelium is the source of 80% of the vascular smooth muscle cells during embryonic development (Wilm et al., 2005; Zhou et al., 2008). Similar Fig. 5. Summary of the lineage-tracing experiments performed in the embryonic lung. Cartoon representing lineage-tracing of the distal epithelial and mesothelial populations have during embryonic lung development. During the pseudoglandular stage the outer mesothelial epithelial layer (Wt1 þ, represented in green) gives rise to vascular smooth muscle and unidentified population(s) of alveolar cells. The mesothelial layer has not been specifically lineage-traced at later developmental stages. During the pseudoglandular stage the distal epithelial tip cells (Id2 þ, represented in blue) give rise to the bronchiolar and alveolar epithelium. During the canalicular stage of development, the descendents of the Id2 þ cells generate alveolar daughters only. Br, Bronchiole; Bv, Blood Vessel; Av, Alveoli.
7 PROGENITOR CELLS IN THE DEVELOPING LUNG 469 lineage-tracing experiments in the lung demonstrated that the mesothelium contributes 30% of the vascular smooth muscle in both arteries and veins, but that it makes no contribution to the parabronchial smooth muscle(queetal.,2008;morimotoetal., 2010; Fig. 5). Where do the remaining vascular smooth muscle cells originate? Lineage-tracing of the endothelium using Tie1-Cre; Rosa26R-YFP showed that overall 30% of the vascular smooth muscle at E16.5 was derived from the endothelium. The proportion of endothelial-derived vascular smooth muscle cells varied along the proximal distal axis of the lung. Most of the vascular smooth muscle in the proximal vessels, but almost none in the distal vessels, was of endothelial origin (Morimoto et al., 2010). It would be desirable to repeat this experiment with an inducible Cre, and also to confirm that the Tie1-Cre transgene was never expressed in the vascular smooth muscle cells. Nevertheless, there is also indirect evidence for an endothelial origin of some lung vascular smooth muscle cells (reviewed in Arciniegas et al., 2007). Together the mesothelial and endothelial progenitor populations account for only approximately 60% of the lung vascular smooth muscle. Where does the remaining 40% originate? Does it come from another progenitor located within the mesenchyme? In support of a separate mesenchymal origin, morphological studies of human embryonic lungs have concluded that both the endothelium and mesenchyme contain vascular smooth muscle progenitors (Hall et al., 2000). However, this needs to be confirmed in an animal model using a lineagelabeling technique. Other important questions include, does the origin of the vessel (angiogenesis vs. vasculogenesis), as well as its location, affect the origin of its smooth muscle? Do the different vascular smooth muscle progenitor populations share aspects of their molecular regulation? How much flexibility is there between the contributions made by the different vascular smooth muscle progenitor types? Are these multiple vascular smooth muscle progenitor populations relevant to lung vascular maintenance or repair during postnatal life, particularly to the development of lung vascular conditions such as hypertension? Cellular Origin of the Vasculature Two opposing models of lung vasculature development exist: angiogenesis (sprouting from existing vessels) and vasculogenesis (de novo formation of blood vessels within the lung mesenchyme from angioblasts). Recent descriptive studies, in which mouse embryos were processed for histology with an intact blood circulation, support the hypothesis that at least some of the blood vessels form by angiogenesis. At the earliest stages of branching morphogenesis (E9.5 E10) simple vessels composed of Tie2-LacZ þ and Pecam þ endothelial cells, but no vascular smooth muscle, were observed to contain primitive erythrocytes, suggesting that they were already connected to the overall circulation and had likely arisen by angiogenesis (Parera et al., 2005). Moreover, the budding endodermal epithelial tip of the developing lung was surrounded by a capillary network (Parera et al., 2005). Subsequent studies have confirmed and extended these results. A fluorescent lectin-tracer was used to demonstrate that the embryonic lung vasculature is connected to the circulation during the pseudoglandular stage, and mitotic figures were observed at the tips of the endothelium only, indicative of angiogenesis (Schwarz et al., 2009). Multiple reports have provided evidence for the occurrence of vasculogenesis within the pseudoglandular stage lung mesenchyme. For example, using a Flk1-LacZ reporter mouse, vascular progenitor cells were detected from E10.5 onward, arranged in vessels without a lumen (Schachtner et al., 2000; Yamamoto et al., 2007). It is likely that both vasculogenesis and angiogenesis occur during lung development. Indeed, this has been suggested by careful anatomical studies of both mouse and human fetal lungs (demello et al., 1997; demello and Reid, 2000). Further molecular genetic studies will be needed to clarify the relative contributions of the two processes at different stages of development and in different regions of the lung. Vascular development continues as the lung grows and the vasculature is extensively remodeled during alveolarization, although no new vessels are formed at this stage (reviewed in Stenmark and Abman, 2005). Other Mesenchymal Progenitor Populations The Wt1-Cre lineage-labeling experiments showed that other lung mesenchymal cells (in addition to a subset of the vascular smooth muscle) were also derived from the mesothelium. These were not specifically identified and could have been alveolar myofibroblasts, microvascular pericytes, or even endothelial cells (Que et al., 2008). This finding warrants further investigation, preferably with an inducible Cre line, to identify all of the mesothelial-derived cells and the developmental stage at which they are produced; moreover, to determine how many different progenitor populations the embryonic lung mesothelium contains and whether any of these also function in the adult. Other mesenchymal cell types (various fibroblasts, pericytes, alveolar smooth muscle) are thought to be produced by progenitors within the splanchnic mesoderm which surrounds the lung buds. It is not known how homogenous the cells within the mesoderm are at the early stages of lung development. For example, are there multiple populations of lineagerestricted mesenchymal progenitor cells as the lung buds initiate? Or, is there a multipotent mesenchymal progenitor at this stage? Answering these questions will require new genetic tools and an improved knowledge of gene expression profiles within the lung mesenchyme. It is now incontrovertible that epithelial mesothelial cells delaminate and undergo EMT (epithelial mesenchymal transitions) during lung development (Que et al., 2008; Morimoto et al., 2010). By contrast, all of the available evidence suggests that the endodermally derived epithelium does not undergo EMT during normal development. For example, in lineagetracing experiments in which the entire lung bud endoderm was labeled, using a human Surfactant protein C (SftpC) promoter fragment to drive Cre expression, the lineage
8 470 RAWLINS label was never observed in the mesenchyme (Perl et al., 2002). GENETIC MECHANISMS OF SELF-RENEWAL IN EMBRYONIC LUNG PROGENITOR POPULATIONS Lung morphogenesis is controlled by reciprocal signaling between all cell layers: the epithelium, mesenchyme (including developing blood vessels), and the mesothelium (reviewed in, Cardoso and Lu, 2006; Morrisey and Hogan, 2010). Multiple interacting signaling pathways and transcription factors regulate the proliferation of lung progenitor cells (Fig. 6). The direct targets of most of the genes implicated in embryonic lung progenitor proliferation have not been identified. This makes it hard to determine if the effect observed, for example proliferation, is directly downstream of the gene/pathway under investigation. Epithelial Progenitor Regulation The distal lung epithelial progenitors express a repertoire of transcription factors. Nmyc is both necessary and sufficient for the division of multipotent lung epithelial progenitors and likely promotes self-renewal (Okubo et al., 2005). Similarly, the forkhead genes Foxp1 and Foxp2 are enriched in the multipotent epithelial progenitors and Foxp2 / ; Foxp1 þ/ mutants have smaller lungs with decreased levels of proliferation and Nmyc expression, but normal proximal distal patterning (Shu et al., 2007). Sox9 is a useful marker for the multipotent epithelial progenitors, but lung epithelial specific deletion did not result in a phenotype (Perl et al., 2005). Fgfr signaling mediates branching morphogenesis of the lung. In vitro FGF10 is both a chemoattractant and a mitogen for the lung epithelium during branching (Bellusci et al., 1997b; Park et al., 1998). Fgf10 hypomorphs have decreased levels of epithelial proliferation during the pseudoglandular stage and this is associated with decreased levels of distal epithelial phosphorylated-erk MAPK and decreased expression of a Fig. 6. Schematic diagram of the factors regulating progenitor cell proliferation in the pseudoglandular stage developing lung. A: Multipotent epithelial progenitors. B: Mesenchymal progenitors. It is not known if any of these molecules directly control cell proliferation, for example by regulation of cyclins. Many of the arrows represent genetic links only. Not all of the putative interactions between different molecules and pathways have been illustrated. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.] canonical Wnt pathway reporter (Ramasamy et al., 2007). By contrast, overexpression of FGF10 in the developing lung apparently arrests epithelial progenitors in an undifferentiated state (Nyeng et al., 2008). These results are consistent with a role for mesenchymal FGF10 in promoting self-renewal, and inhibiting differentiation, of the multipotent lung epithelial progenitors. The transcription factors Etv4/5 and Elf5 mediate some of the effects of Fgfr signaling in these cells (Liu et al., 2003; Metzger et al., 2007). Wnt7b is expressed in the distal tips of the developing lung epithelium and acts by means of a canonical
9 PROGENITOR CELLS IN THE DEVELOPING LUNG 471 signaling pathway to promote both epithelial and mesenchymal proliferation (Rajagopal et al., 2008). Wnt7b null embryos have a normal overall body size, but severely hypoplastic lungs. Lung progenitor proliferation is significantly decreased, particularly in the distal tip epithelium and subepithelial mesenchyme, but the number of Sox9 þ multipotent progenitor cells per tip is normal and differentiation proceeds at the normal rate (Rajagopal et al., 2008). The effects of Wnt7b in the epithelium are possibly mediated through loss of distal epithelial Bmp4 and Id2 expression (Rajagopal et al., 2008). However, Id2 null lungs have normal rates of BrdU incorporation during the pseudoglandular stage (E.L.R., unpublished data). Wnt2 is expressed in the mesenchyme surrounding the epithelial buds and also promotes lung progenitor proliferation. Similar to Wnt7b null animals, Wnt2 nullmicehavesmaller lungs with both epithelial and mesenchymal proliferation defects, but normal overall patterning (Goss et al., 2009). Wnt5a is highly expressed in the distal lung epithelium and surrounding mesenchyme. The null phenotype suggeststhatitmayactantagonisticallyto Wnt7b and Wnt2 and repress epithelial and mesenchymal progenitor proliferation (Li et al., 2002). Wnt signaling has also been implicated in proximal distal epithelial patterning and differentiation during lung development and the roles of other Wnt ligands remain obscure (Shu et al., 2005; Zhang et al., 2008). Interestingly, Wnt signaling is also absolutely required for the initial specification of the respiratory epithelial lineage from the foregut endoderm (Goss et al., 2009; Harris-Johnson et al., 2009; Chen et al., 2010). The role of Bmp signaling in lung epithelial progenitor regulation has been difficult to define. In vitro and overexpression studies gave conflicting results, suggesting that the responses of the different progenitor populations to Bmp signaling are highly dose-specific (for example, Bellusci et al., 1996; Weaver et al., 2000). Bmp4 is expressed in the distal epithelial tip cells, as well as the mesenchyme surrounding the developing bronchioles, whereas Bmpr1a is found throughout the epithelium and mesenchyme (Weaver et al., 2000, 2003). Lung epithelial-specific deletion of either Bmpr1a or Bmp4 results in hypoplastic lungs with significantly reduced rates of epithelial proliferation and a decrease in the levels of Shh and Nmyc (Eblaghie et al., 2006). Analysis of the phenotype suggested that Bmp signaling may be important for the self-renewal of the distal epithelial progenitor cells, but that it also affects cell morphology and survival. The specific roles of other Bmp signaling components have not been determined. Tgfb signaling has been implicated in lung epithelial progenitor proliferation in vitro, perhaps acting antagonistically to FGF10 in the multipotent progenitors through effects on Pten transcription (Xing et al., 2008). The lung epithelial-specific disruption of individual transforming growth factor-beta (TGFb) superfamily ligands, receptors and signaling components is in process, but specific effects on progenitor populations have yet to be identified (for example, Chen et al., 2005). Deletion of Pten throughout the developing lung epithelium led to epithelial hyperplasia and changes in cell fate allocation (Tiozzo et al., 2009). However, Pten expression is widespread in the lung and it is not clear in which specific embryonic progenitor populations its function is required. The phenotypes of transgenic overexpression or targeted deletion of the mir17-92 microrna cluster are consistent with a normal role in promoting self-renewal, and inhibiting differentiation, of the multipotent lung epithelial progenitors (Lu et al., 2007; Ventura et al., 2008). Direct targets of the mir17 family in the lung include Rbl2, Mapk14, and Stat3 mrnas (Lu et al., 2007; Carraro et al., 2009). Mesenchymal Progenitor Regulation Specific transcription factors expressed in the different mesenchymal progenitor cell populations have not been identified, even for the well-established parabronchial smooth muscle progenitors. Nevertheless, tremendous progress has been made in identifying molecules which regulate overall lung embryonic mesenchymal proliferation, rather than specific progenitor populations. FGF9 is highly expressed in both the mesothelium and the distal endoderm during the pseudoglandular stage of lung development. Fgf9 null lungs have a smaller mesenchymal compartment than wild-type and, as a consequence, branching morphogenesis is reduced (Colvin et al., 2001). This initial observation has lead to the elucidation of a complex signaling loop, involving the FGF, Shh, and Wnt pathways, which regulates the proliferation and differentiation of mesenchymal progenitor cell populations in the pseudoglandular stage lung. The cross-talk between these pathways is in the process of being extensively characterized. However, the detailed consequences of signaling for specific mesenchymal progenitor populations continues to lag behind. It is clear that the different progenitor populations respond differently to the same signaling molecules, even though they are developmentally related and in close proximity. For example, an increase in the levels of FGF9 protein inhibits the development of bronchiolar smooth muscle, but not vascular smooth muscle (Weaver et al., 2003; White et al., 2006). The cell biology underlying these varying responses is likely to include, first, the different developmental histories of the progenitor populations (different transcription factors, signaling pathway components, epigenetic marks). Second, slight differences in progenitor location within the organ resulting in varying levels of signaling, or a different combinatorial pattern of signals. Genetic evidence suggests that FGF9 is itself a mitogen for the subepithelial mesenchyme where it signals through Fgfr1 and Fgfr2. The submesothelial mesenchyme was absent in the Fgf9 null lungs and expanded when Fgf9 was overexpressed. Moreover, adding FGF9 to cultured lungs caused a preferential increase in submesothelial mesenchymal proliferation (White et al., 2006). By contrast, the effects on proliferation of the subepithelial mesenchyme in these conditions were much weaker and most likely mediated by Shh. Shh expression was decreased in the Fgf9 null lungs. Moreover, simultaneously adding FGF9 and the Shh pathway inhibitor cyclopamine to cultured lungs blocked the effects of FGF9 on
10 472 RAWLINS the subepithelial mesenchyme (White et al., 2006). Consistent with a role for Shh in subepithelial mesenchymal proliferation, it is highly expressed in the distal epithelial endoderm budding tips and its receptor, Patched1 (Ptch1), is highly expressed throughout the distal mesenchyme (Weaver et al., 2003). In vitro and in vivo overexpression experiments also support the idea that Shh functions as a mitogen for the mesenchymal progenitors (Bellusci et al., 1997a; Weaver et al., 2003). There is evidence that TGFb signaling negatively regulates the expression of Shh signaling components in the lung mesenchyme (Li et al., 2008). Subsequent studies identified an FGF9 Wnt regulatory loop that also promotes mesenchymal proliferation. FGF9 in the mesothelium induces Wnt2a expression in the submesothelial mesenchyme. Blocking Wnt signaling throughout the mesenchyme and mesothelium by deleting a floxed b-catenin allele with Dermo1-Cre resulted in lower levels of proliferation, decreased mesenchymal levels of Fgfr1 and 2, and was sufficient to prevent the mesenchyme from responding to exogenous FGF9 (De Langhe et al., 2008; Yin et al., 2008). The model suggested by Yin et al. is that Wnt2 (downstream of FGF9 and previously known as Wnt2a) functions to mediate submesothelial mesenchyme proliferation. Whereas, Wnt7b (which is expressed independently of FGF9) in the distal epithelium acts on the subepithelial mesenchyme (Shu et al., 2002; Rajagopal et al., 2008; Yin et al., 2008). Loss of function studies have also shown that Wnt7b signaling from the epithelium by means of the canonical b-catenin mediated pathway is required for smooth muscle precursor cell proliferation (Cohen et al., 2009). FGF10 þ cells in the distal mesenchyme at the pseudoglandular stage are parabronchial smooth muscle progenitors and moreover, Fgf10 hypomorphic lungs have a smaller number of smooth muscle cells (Mailleux et al., 2005). However, the hypomorphs do not display changes in proliferation or apoptosis of the mesenchymal progenitors and FGF10 elicited no direct response (neither activation of Erk or Akt) on primary lung mesenchymal cells (Mailleux et al., 2005). These data suggest that, unlike the epithelial progenitors, the function of FGF10 is not to promote progenitor self-renewal, rather it is required for smooth muscle specification. Similarly, no requirement for canonical Notch signaling in proliferation of the mesenchymal progenitor populations has been identified (Morimoto et al., 2010). The roles of other pathways, including Egfr, Bmp, cytokine, in the different populations of lung mesenchymal progenitors remain to be tested. Vascular Progenitor Regulation In addition to their roles in mesenchymal proliferation and differentiation, FGF9 and Shh also affect vascular development in the lung. Fgf9 null lungs have a smaller capillary network around the budding epithelial tips at E11.5. This could be partially phenocopied by deletion of Fgfr1 and Fgfr2 throughout the developing mesenchyme and mesothelium, but not by endothelial-specific Fgfr deletion (White et al., 2007). The effects of FGF9 on the developing vasculature are therefore indirect and the authors show that it most likely regulates mesenchymal Vegfa expression. Loss of Shh in the lung epithelium resulted in a simplification of the lung vascular network (Miller et al., 2004). Similarly, lung-specific deletion of the Shh receptor, Smoothened, also resulted in a decreased vascular network (White et al., 2007). However, Shh and FGF9 were unable to compensate for one another in vitro, suggesting that they act independently in the embryonic lung mesenchyme to regulate vascular development (White et al., 2007). LUNG PROGENITORS AND BRANCHING MORPHOGENESIS Lung development involves the coordinated proliferation, migration, and differentiation of epithelial, mesenchymal, and endothelial cell types during branching morphogenesis. The multipotent epithelial progenitor cells are located in the distal budding tip and these cells also mediate epithelial branching. The detailed cell biology of lung branching is poorly characterized. However, it is clear that during branching the distal epithelial cells migrate, alter their shape, make changes in cell substratum and cell cell adhesion, and possibly also undergo oriented cell division and local cell rearrangements (Andrew and Ewald, 2010). Many of the signals that regulate lung embryonic progenitor proliferation also mediate branching. It is tempting to speculate that a master-regulator controls both processes coordinately. One candidate for a master-regulator is FGF10. In Fgf10, or its receptor Fgfr2-IIIb, null animals the lungs do not branch (Sekine et al., 1999; De Moerlooze et al., 2000). Moreover, as we have seen, the Fgf10 hypomorphic lungs have defects in epithelial progenitor proliferation and mesenchymal, including vascular, differentiation (Ramasamy et al., 2007). Testing the hypothesis that FGF10 is a master-regulator for lung development will require both the targeted deletion of its receptor in specific progenitor cell populations and a full analysis of the gene regulatory network controlling progenitor proliferation and morphogenesis. A lung epithelial-specific deletion of Fgfr2 has been performed and the results are consistent with roles in branching, survival, and proliferation (Abler et al., 2009). As yet, there has been no attempt to build a gene regulatory network for lung morphogenesis. Gene Regulatory Networks are systems-level models of the molecular circuitry governing cell fate decisions. When complete, these include all of the transcription factors, their cis-binding sites and the intercellular signaling molecules required for these decisions (Stathopoulos and Levine, 2005; Oliveri et al., 2008). The establishment of a gene regulatory network for lung progenitor proliferation and branching morphogenesis will be the work of many labs over the course of many years. Nevertheless, the tools to build it are now available and the process of building the network will generate testable predictions and provide new insights into these highly complex cellular events (Materna and Oliveri, 2008). An alternative hypothesis is that a signaling network centered on the distal budding endoderm and
Development of Respiratory System. Dr. Sanaa Alshaarawy& Dr. Saeed Vohra
 Development of Respiratory System Dr. Sanaa Alshaarawy& Dr. Saeed Vohra OBJECTIVES At the end of the lecture the students should be able to: Identify the development of the laryngeotracheal (respiratory)
Development of Respiratory System Dr. Sanaa Alshaarawy& Dr. Saeed Vohra OBJECTIVES At the end of the lecture the students should be able to: Identify the development of the laryngeotracheal (respiratory)
Lung Physiology. Jamie Havrilak, PhD Postdoctoral Research Associate Layden Lab October 26th, 2018
 Lung Physiology Jamie Havrilak, PhD Postdoctoral Research Associate Layden Lab October 26th, 2018 Nkx2.1- Lung epithelium Endomucin- Vasculature Alveoli/Capillaries: Site of Gas Exchange in the Lung
Lung Physiology Jamie Havrilak, PhD Postdoctoral Research Associate Layden Lab October 26th, 2018 Nkx2.1- Lung epithelium Endomucin- Vasculature Alveoli/Capillaries: Site of Gas Exchange in the Lung
Histology and development of the respiratory system
 Histology and development of the respiratory system Árpád Dobolyi Semmelweis University, Department of Anatomy, Histology and Embryology Outline of the lecture 1. Structure of the trachea 2. Histology
Histology and development of the respiratory system Árpád Dobolyi Semmelweis University, Department of Anatomy, Histology and Embryology Outline of the lecture 1. Structure of the trachea 2. Histology
Bronchioles. Alveoli. Type I alveolar cells are very thin simple squamous epithelial cells and form most of the lining of an alveolus.
 276 Bronchioles Bronchioles continue on to form bronchi. The primary identifying feature is the loss of hyaline cartilage. The epithelium has become simple ciliated columnar, and there is a complete ring
276 Bronchioles Bronchioles continue on to form bronchi. The primary identifying feature is the loss of hyaline cartilage. The epithelium has become simple ciliated columnar, and there is a complete ring
Function of Breathing. Jeanine D Armiento, M.D., Ph.D. Respiratory Portion. Conducting Portion. Critical to the Development of the Lung
 Function of Breathing Jeanine D Armiento, M.D., Ph.D. Associate Professor Department of Medicine P&S 9-449 5-3745 jmd12@columbia.edu Air Sacs (alveoli) Ventilation-air conduction Moving gas in and out
Function of Breathing Jeanine D Armiento, M.D., Ph.D. Associate Professor Department of Medicine P&S 9-449 5-3745 jmd12@columbia.edu Air Sacs (alveoli) Ventilation-air conduction Moving gas in and out
WNT2 and WNT7B Cooperative Signaling in Lung Development
 University of Pennsylvania ScholarlyCommons Publicly Accessible Penn Dissertations 1-1-2012 WNT2 and WNT7B Cooperative Signaling in Lung Development Mayumi Miller University of Pennsylvania, mayumimiller@gmail.com
University of Pennsylvania ScholarlyCommons Publicly Accessible Penn Dissertations 1-1-2012 WNT2 and WNT7B Cooperative Signaling in Lung Development Mayumi Miller University of Pennsylvania, mayumimiller@gmail.com
Branching morphogenesis of the lung: new molecular insights into an old problem
 86 Review TRENDS in Cell Biology Vol.13 No.2 February 2003 Branching morphogenesis of the lung: new molecular insights into an old problem Pao-Tien Chuang 1 and Andrew P. McMahon 2 1 Cardiovascular Research
86 Review TRENDS in Cell Biology Vol.13 No.2 February 2003 Branching morphogenesis of the lung: new molecular insights into an old problem Pao-Tien Chuang 1 and Andrew P. McMahon 2 1 Cardiovascular Research
Organs Histology D. Sahar AL-Sharqi. Respiratory system
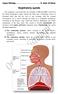 Respiratory system The respiratory system provides for exchange of O2 and CO2 to and from the blood. Respiratory organs include the lungs and a branching system of bronchial tubes that link the sites of
Respiratory system The respiratory system provides for exchange of O2 and CO2 to and from the blood. Respiratory organs include the lungs and a branching system of bronchial tubes that link the sites of
SUPPLEMENTARY INFORMATION
 b 350 300 250 200 150 100 50 0 E0 E10 E50 E0 E10 E50 E0 E10 E50 E0 E10 E50 Number of organoids per well 350 300 250 200 150 100 50 0 R0 R50 R100 R500 1st 2nd 3rd Noggin 100 ng/ml Noggin 10 ng/ml Noggin
b 350 300 250 200 150 100 50 0 E0 E10 E50 E0 E10 E50 E0 E10 E50 E0 E10 E50 Number of organoids per well 350 300 250 200 150 100 50 0 R0 R50 R100 R500 1st 2nd 3rd Noggin 100 ng/ml Noggin 10 ng/ml Noggin
Signaling Vascular Morphogenesis and Maintenance
 Signaling Vascular Morphogenesis and Maintenance Douglas Hanahan Science 277: 48-50, in Perspectives (1997) Blood vessels are constructed by two processes: vasculogenesis, whereby a primitive vascular
Signaling Vascular Morphogenesis and Maintenance Douglas Hanahan Science 277: 48-50, in Perspectives (1997) Blood vessels are constructed by two processes: vasculogenesis, whereby a primitive vascular
Cell Birth and Death. Chapter Three
 Cell Birth and Death Chapter Three Neurogenesis All neurons and glial cells begin in the neural tube Differentiated into neurons rather than ectoderm based on factors we have already discussed If these
Cell Birth and Death Chapter Three Neurogenesis All neurons and glial cells begin in the neural tube Differentiated into neurons rather than ectoderm based on factors we have already discussed If these
Lecture 21Development of respiratory system Dr. Rehan Asad At the end of session students should able to Describe formation of lung buds Describe
 Lecture 21Development of respiratory system Dr. Rehan Asad At the end of session students should able to Describe formation of lung buds Describe development of larynx, trachea and bronchi. Describe the
Lecture 21Development of respiratory system Dr. Rehan Asad At the end of session students should able to Describe formation of lung buds Describe development of larynx, trachea and bronchi. Describe the
Lower Respiratory Tract (Trachea, Bronchi, Bronchioles) & the Lung
 Lower Respiratory Tract (Trachea, Bronchi, Bronchioles) & the Lung Color code: Important Extra & Doctor notes Editing file Objectives: By the end of this lecture, the student should be able to describe:
Lower Respiratory Tract (Trachea, Bronchi, Bronchioles) & the Lung Color code: Important Extra & Doctor notes Editing file Objectives: By the end of this lecture, the student should be able to describe:
HISTOLOGY OF THE RESPIRATORY SYSTEM I. Introduction A. The respiratory system provides for gas exchange between the environment and the blood. B.
 HISTOLOGY OF THE RESPIRATORY SYSTEM I. Introduction A. The respiratory system provides for gas exchange between the environment and the blood. B. The human respiratory system may be subdivided into two
HISTOLOGY OF THE RESPIRATORY SYSTEM I. Introduction A. The respiratory system provides for gas exchange between the environment and the blood. B. The human respiratory system may be subdivided into two
Chapter 6. Villous Growth
 Core Curriculum in Perinatal Pathology Chapter 6 Villous Growth Overview of vasculogenesis and angiogenesis Vasculogenesis Extraembryonic Vasculogenesis Angiogenesis Branching angiogenesis Sprouting angiogenesis
Core Curriculum in Perinatal Pathology Chapter 6 Villous Growth Overview of vasculogenesis and angiogenesis Vasculogenesis Extraembryonic Vasculogenesis Angiogenesis Branching angiogenesis Sprouting angiogenesis
Supplementary Figure 1. EC-specific Deletion of Snail1 Does Not Affect EC Apoptosis. (a,b) Cryo-sections of WT (a) and Snail1 LOF (b) embryos at
 Supplementary Figure 1. EC-specific Deletion of Snail1 Does Not Affect EC Apoptosis. (a,b) Cryo-sections of WT (a) and Snail1 LOF (b) embryos at E10.5 were double-stained for TUNEL (red) and PECAM-1 (green).
Supplementary Figure 1. EC-specific Deletion of Snail1 Does Not Affect EC Apoptosis. (a,b) Cryo-sections of WT (a) and Snail1 LOF (b) embryos at E10.5 were double-stained for TUNEL (red) and PECAM-1 (green).
Lec #2 histology. Bronchioles:
 Lec #2 histology. Last lecture we talked about the upper respiratory tract histology, this one is about the lower part histology. We will discuss the histology of: -bronchioles -respiratory bronchioles
Lec #2 histology. Last lecture we talked about the upper respiratory tract histology, this one is about the lower part histology. We will discuss the histology of: -bronchioles -respiratory bronchioles
SUPPLEMENTARY INFORMATION
 DOI: 10.1038/ncb2610 Figure S1 FSMCs derived from MSLN CLN transgenic mice express smooth muscle-specific proteins. Beta-galactosidase is ubiquitously expressed within cultured FSMCs derived from MSLN
DOI: 10.1038/ncb2610 Figure S1 FSMCs derived from MSLN CLN transgenic mice express smooth muscle-specific proteins. Beta-galactosidase is ubiquitously expressed within cultured FSMCs derived from MSLN
The Respiratory System. Dr. Ali Ebneshahidi
 The Respiratory System Dr. Ali Ebneshahidi Functions of The Respiratory System To allow gases from the environment to enter the bronchial tree through inspiration by expanding the thoracic volume. To allow
The Respiratory System Dr. Ali Ebneshahidi Functions of The Respiratory System To allow gases from the environment to enter the bronchial tree through inspiration by expanding the thoracic volume. To allow
Supporting Information
 Supporting Information Rock et al. 10.1073/pnas.1117988108 Fig. S1. Heterogeneity of stromal cells in normal and fibrotic mouse lungs. Sections of normal mouse lungs (A and D) and fibrotic lungs collected
Supporting Information Rock et al. 10.1073/pnas.1117988108 Fig. S1. Heterogeneity of stromal cells in normal and fibrotic mouse lungs. Sections of normal mouse lungs (A and D) and fibrotic lungs collected
Hepatogenesis I Liver development
 Hepatogenesis I Liver development HB 308 George Yeoh Room 2.59 MCS Building yeoh@cyllene.uwa.edu.au Topics Early liver development Tissue interaction - role of morphogens and cytokines Liver enriched transcription
Hepatogenesis I Liver development HB 308 George Yeoh Room 2.59 MCS Building yeoh@cyllene.uwa.edu.au Topics Early liver development Tissue interaction - role of morphogens and cytokines Liver enriched transcription
When you see this diagram, remember that you are looking at the embryo from above, through the amniotic cavity, where the epiblast appears as an oval
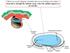 When you see this diagram, remember that you are looking at the embryo from above, through the amniotic cavity, where the epiblast appears as an oval disc 2 Why the embryo needs the vascular system? When
When you see this diagram, remember that you are looking at the embryo from above, through the amniotic cavity, where the epiblast appears as an oval disc 2 Why the embryo needs the vascular system? When
SCPA602 Respiratory System
 SCPA602 Respiratory System Associate Professor Dr. Wannee Jiraungkoorskul Department of Pathobiology, Faculty of Science, Mahidol University Tel: 02-201-5563, E-mail: wannee.jir@mahidol.ac.th 1 Objectives
SCPA602 Respiratory System Associate Professor Dr. Wannee Jiraungkoorskul Department of Pathobiology, Faculty of Science, Mahidol University Tel: 02-201-5563, E-mail: wannee.jir@mahidol.ac.th 1 Objectives
CHAPTER 6 SUMMARIZING DISCUSSION
 CHAPTER 6 SUMMARIZING DISCUSSION More than 20 years ago the founding member of the Wnt gene family, Wnt-1/Int1, was discovered as a proto-oncogene activated in mammary gland tumors by the mouse mammary
CHAPTER 6 SUMMARIZING DISCUSSION More than 20 years ago the founding member of the Wnt gene family, Wnt-1/Int1, was discovered as a proto-oncogene activated in mammary gland tumors by the mouse mammary
Mesodermal Pten inactivation leads to alveolar capillary dysplasia-like phenotype
 Research article Mesodermal Pten inactivation leads to alveolar capillary dysplasia-like phenotype Caterina Tiozzo, 1,2 Gianni Carraro, 2,3 Denise Al Alam, 2 Sheryl Baptista, 2 Soula Danopoulos, 2 Aimin
Research article Mesodermal Pten inactivation leads to alveolar capillary dysplasia-like phenotype Caterina Tiozzo, 1,2 Gianni Carraro, 2,3 Denise Al Alam, 2 Sheryl Baptista, 2 Soula Danopoulos, 2 Aimin
Supplementary Materials for
 www.sciencetranslationalmedicine.org/cgi/content/full/4/117/117ra8/dc1 Supplementary Materials for Notch4 Normalization Reduces Blood Vessel Size in Arteriovenous Malformations Patrick A. Murphy, Tyson
www.sciencetranslationalmedicine.org/cgi/content/full/4/117/117ra8/dc1 Supplementary Materials for Notch4 Normalization Reduces Blood Vessel Size in Arteriovenous Malformations Patrick A. Murphy, Tyson
Development of the nasal cavity :
 Development of the nasal cavity : several processes contribute to the development of the nose, the nose consists of 2 cavities separated by a septum, and the nasal cavity is separated from the oral cavity
Development of the nasal cavity : several processes contribute to the development of the nose, the nose consists of 2 cavities separated by a septum, and the nasal cavity is separated from the oral cavity
Study of different tissues Abnormal cells and tissues can be compared to normal tissues to identify disease, such as cancer Being able to know and
 CHAPTER 4 Study of different tissues Abnormal cells and tissues can be compared to normal tissues to identify disease, such as cancer Being able to know and recognize normal tissues under the microscope
CHAPTER 4 Study of different tissues Abnormal cells and tissues can be compared to normal tissues to identify disease, such as cancer Being able to know and recognize normal tissues under the microscope
-Tamara Wahbeh. -Razan Abu Rumman. Dr. Mohammed Al-Muhtaseb
 -2 -Tamara Wahbeh -Razan Abu Rumman Dr. Mohammed Al-Muhtaseb I tried to include everything the doctor mentioned in both the lecture and his slides in the simplest way possible, so hopefully there would
-2 -Tamara Wahbeh -Razan Abu Rumman Dr. Mohammed Al-Muhtaseb I tried to include everything the doctor mentioned in both the lecture and his slides in the simplest way possible, so hopefully there would
Organogenesis Part 2. V. Lateral Plate Mesoderm VI. Endoderm VII. Development of the Tetrapod Limb VIII. Sex Determination. V. Lateral Plate Mesoderm
 Organogenesis Part 2 V. Lateral Plate Mesoderm VI. Endoderm VII. Development of the Tetrapod Limb VIII. Sex Determination V. Lateral Plate Mesoderm chordamesoderm paraxial mesoderm intermediate mesoderm
Organogenesis Part 2 V. Lateral Plate Mesoderm VI. Endoderm VII. Development of the Tetrapod Limb VIII. Sex Determination V. Lateral Plate Mesoderm chordamesoderm paraxial mesoderm intermediate mesoderm
Tissues. tissue = many cells w/ same structure and function. cell shape aids its function tissue shape aids its function
 Tissues tissue = many cells w/ same structure and function cell shape aids its function tissue shape aids its function Histology = study of tissues 4 types of tissues Epithelial coverings contact openings
Tissues tissue = many cells w/ same structure and function cell shape aids its function tissue shape aids its function Histology = study of tissues 4 types of tissues Epithelial coverings contact openings
SUPPLEMENTARY INFORMATION
 DOI: 10.1038/ncb2697 Figure S1 Cytokeratin 5 is a specific marker for basal and intermediate cells in all mouse prostate lobes. (a) Immunofluorescence staining showing co-localization of YFP with p63 in
DOI: 10.1038/ncb2697 Figure S1 Cytokeratin 5 is a specific marker for basal and intermediate cells in all mouse prostate lobes. (a) Immunofluorescence staining showing co-localization of YFP with p63 in
Heart Development. Robert G. Kelly Developmental Biology Institute of Marseilles - Luminy
 ESC CBCS Summer School on Cardiovascular Sciences 15th June 2011 Heart Development Robert G. Kelly Developmental Biology Institute of Marseilles - Luminy Animal models of heart development Tinman/Nkx2.5
ESC CBCS Summer School on Cardiovascular Sciences 15th June 2011 Heart Development Robert G. Kelly Developmental Biology Institute of Marseilles - Luminy Animal models of heart development Tinman/Nkx2.5
Supplementary Figure 1: Signaling centers contain few proliferating cells, express p21, and
 Supplementary Figure 1: Signaling centers contain few proliferating cells, express p21, and exclude YAP from the nucleus. (a) Schematic diagram of an E10.5 mouse embryo. (b,c) Sections at B and C in (a)
Supplementary Figure 1: Signaling centers contain few proliferating cells, express p21, and exclude YAP from the nucleus. (a) Schematic diagram of an E10.5 mouse embryo. (b,c) Sections at B and C in (a)
The Evolution and Development of the Gut. Dr Mike Wride School of Natural Sciences Zoology Department
 The Evolution and Development of the Gut Dr Mike Wride School of Natural Sciences Zoology Department email: wridem@tcd.ie The gut? Gut Function and Regulation (Dr. Alan Tuffery) Absorption of nutrients
The Evolution and Development of the Gut Dr Mike Wride School of Natural Sciences Zoology Department email: wridem@tcd.ie The gut? Gut Function and Regulation (Dr. Alan Tuffery) Absorption of nutrients
AP VP DLP H&E. p-akt DLP
 A B AP VP DLP H&E AP AP VP DLP p-akt wild-type prostate PTEN-null prostate Supplementary Fig. 1. Targeted deletion of PTEN in prostate epithelium resulted in HG-PIN in all three lobes. (A) The anatomy
A B AP VP DLP H&E AP AP VP DLP p-akt wild-type prostate PTEN-null prostate Supplementary Fig. 1. Targeted deletion of PTEN in prostate epithelium resulted in HG-PIN in all three lobes. (A) The anatomy
Early cell death (FGF) B No RunX transcription factor produced Yes No differentiation
 Solution Key - Practice Questions Question 1 a) A recent publication has shown that the fat stem cells (FSC) can act as bone stem cells to repair cavities in the skull, when transplanted into immuno-compromised
Solution Key - Practice Questions Question 1 a) A recent publication has shown that the fat stem cells (FSC) can act as bone stem cells to repair cavities in the skull, when transplanted into immuno-compromised
PBS Class #2 Introduction to the Immune System part II Suggested reading: Abbas, pgs , 27-30
 PBS 803 - Class #2 Introduction to the Immune System part II Suggested reading: Abbas, pgs. 15-25, 27-30 Learning Objectives Compare and contrast the maturation of B and T lymphocytes Compare and contrast
PBS 803 - Class #2 Introduction to the Immune System part II Suggested reading: Abbas, pgs. 15-25, 27-30 Learning Objectives Compare and contrast the maturation of B and T lymphocytes Compare and contrast
Prelab #4 BLOOD; BONE MARROW; RESPIRATORY; INTEGUEMENT Page 1
 Prelab #4 BLOOD; BONE MARROW; RESPIRATORY; INTEGUEMENT Page 1 Blood Slide 101 This a classic slide of blood cells using a Wright stain. Inspect red blood cells and their appearance. Note the approximate
Prelab #4 BLOOD; BONE MARROW; RESPIRATORY; INTEGUEMENT Page 1 Blood Slide 101 This a classic slide of blood cells using a Wright stain. Inspect red blood cells and their appearance. Note the approximate
THE RESPIRATORY SYSTEM
 THE RESPIRATORY SYSTEM Functions of the Respiratory System Provides extensive gas exchange surface area between air and circulating blood Moves air to and from exchange surfaces of lungs Protects respiratory
THE RESPIRATORY SYSTEM Functions of the Respiratory System Provides extensive gas exchange surface area between air and circulating blood Moves air to and from exchange surfaces of lungs Protects respiratory
Heart Development. Origins of congenital heart defects Properties of cardiac progenitor cells. Robert G. Kelly
 ESC CBCS Summer School on Cardiovascular Sciences Heart Development 19th June 2013 Origins of congenital heart defects Properties of cardiac progenitor cells Robert G. Kelly Animal models of heart development
ESC CBCS Summer School on Cardiovascular Sciences Heart Development 19th June 2013 Origins of congenital heart defects Properties of cardiac progenitor cells Robert G. Kelly Animal models of heart development
The Respiratory System
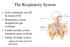 The Respiratory System Cells continually use O2 & release CO2 Respiratory system designed for gas exchange Cardiovascular system transports gases in blood Failure of either system rapid cell death from
The Respiratory System Cells continually use O2 & release CO2 Respiratory system designed for gas exchange Cardiovascular system transports gases in blood Failure of either system rapid cell death from
The Anatomy and Physiology of the Respiratory System
 CHAPTER 1 The Anatomy and Physiology of the Respiratory System Sagittal Section of Upper Airway Fig. 1-1. Sagittal section of upper airway. Structure of the Nose Fig. 1-2. Structure of the nose. Sagittal
CHAPTER 1 The Anatomy and Physiology of the Respiratory System Sagittal Section of Upper Airway Fig. 1-1. Sagittal section of upper airway. Structure of the Nose Fig. 1-2. Structure of the nose. Sagittal
Neurodevelopment II Structure Formation. Reading: BCP Chapter 23
 Neurodevelopment II Structure Formation Reading: BCP Chapter 23 Phases of Development Ovum + Sperm = Zygote Cell division (multiplication) Neurogenesis Induction of the neural plate Neural proliferation
Neurodevelopment II Structure Formation Reading: BCP Chapter 23 Phases of Development Ovum + Sperm = Zygote Cell division (multiplication) Neurogenesis Induction of the neural plate Neural proliferation
Tissues. Tissues - Overview. Bio 101 Laboratory 3. Epithelial Tissues and Integument
 Bio 101 Laboratory 3 Epithelial Tissues and Integument 1 Tissues Tissues to be examined under the microscope Epithelial Tissue Integument Connective Tissue **We will be doing muscle and nervous tissues
Bio 101 Laboratory 3 Epithelial Tissues and Integument 1 Tissues Tissues to be examined under the microscope Epithelial Tissue Integument Connective Tissue **We will be doing muscle and nervous tissues
Prepared By Student. Dania Abed Al-majeed. Rahma Raad Hanna. Balqees Mohammed Aasim. Dania Hisham. Rasha Rafiee
 Prepared By Student Rahma Raad Hanna Balqees Mohammed Aasim Dania Hisham Dania Abed Al-majeed Rasha Rafiee Epithelia Epithelia can be derived from ectoderm, mesoderm or endoderm -ectoderm gives rise to
Prepared By Student Rahma Raad Hanna Balqees Mohammed Aasim Dania Hisham Dania Abed Al-majeed Rasha Rafiee Epithelia Epithelia can be derived from ectoderm, mesoderm or endoderm -ectoderm gives rise to
Epithelial Lecture Test Questions
 Epithelial Lecture Test Questions 1. Which of the following free surfaces lack(s) epithelia: a. lung alveoli (air sacs) b. hard palate c. joint cavities d. abdominal cavity e. salivary gland ducts 2. Which
Epithelial Lecture Test Questions 1. Which of the following free surfaces lack(s) epithelia: a. lung alveoli (air sacs) b. hard palate c. joint cavities d. abdominal cavity e. salivary gland ducts 2. Which
Supplemental Figure 1. Intracranial transduction of a modified ptomo lentiviral vector in the mouse
 Supplemental figure legends Supplemental Figure 1. Intracranial transduction of a modified ptomo lentiviral vector in the mouse hippocampus targets GFAP-positive but not NeuN-positive cells. (A) Stereotaxic
Supplemental figure legends Supplemental Figure 1. Intracranial transduction of a modified ptomo lentiviral vector in the mouse hippocampus targets GFAP-positive but not NeuN-positive cells. (A) Stereotaxic
SUPPLEMENTARY INFORMATION
 doi:10.1038/nature10188 Supplementary Figure 1. Embryonic epicardial genes are down-regulated from midgestation stages and barely detectable post-natally. Real time qrt-pcr revealed a significant down-regulation
doi:10.1038/nature10188 Supplementary Figure 1. Embryonic epicardial genes are down-regulated from midgestation stages and barely detectable post-natally. Real time qrt-pcr revealed a significant down-regulation
Basic Histology. By Mrs. Bailey
 Basic Histology By Mrs. Bailey Primary Tissues 1. Epithelial Tissue 2. Connective Tissue 3. Muscle Tissue 4. Nervous Tissue Very cellular Supported by underlying connective tissue Epithelial & connective
Basic Histology By Mrs. Bailey Primary Tissues 1. Epithelial Tissue 2. Connective Tissue 3. Muscle Tissue 4. Nervous Tissue Very cellular Supported by underlying connective tissue Epithelial & connective
A Cxcl12-Cxcr4 Chemokine Signaling Pathway Defines
 Supplemental Data A Cxcl12-Cxcr4 Chemokine Signaling Pathway Defines the Initial Trajectory of Mammalian Motor Axons Ivo Lieberam, Dritan Agalliu, Takashi Nagasawa, Johan Ericson, and Thomas M. Jessell
Supplemental Data A Cxcl12-Cxcr4 Chemokine Signaling Pathway Defines the Initial Trajectory of Mammalian Motor Axons Ivo Lieberam, Dritan Agalliu, Takashi Nagasawa, Johan Ericson, and Thomas M. Jessell
7/12/2012. Respiratory system. Respiratory Response to Toxic Injury (Lung) Ninth Industrial Toxicology and Pathology Short Course.
 Ninth Industrial Toxicology and Pathology Short Course 23 27 July, 2012 Contemporary Concepts in Target Organ Toxicologic Pathology Respiratory system Respiratory Response to Toxic Injury (Lung) Eric Wheeldon
Ninth Industrial Toxicology and Pathology Short Course 23 27 July, 2012 Contemporary Concepts in Target Organ Toxicologic Pathology Respiratory system Respiratory Response to Toxic Injury (Lung) Eric Wheeldon
Lab Animal Tissue. LEARNING OBJECTIVES: To understand the relationship between the structure and function of different animal tissues
 Name: Bio A.P. PURPOSE: HYPOTHESIS: NONE Lab Animal Tissue BACKGROUND: In animals, groups of closely related cells specialized to perform the same function are called tissues. There are four general classes
Name: Bio A.P. PURPOSE: HYPOTHESIS: NONE Lab Animal Tissue BACKGROUND: In animals, groups of closely related cells specialized to perform the same function are called tissues. There are four general classes
Neuroepithelial Cells and Neural Differentiation
 Neuroepithelial Cells and Neural Differentiation Neurulation The cells of the neural tube are NEUROEPITHELIAL CELLS Neural crest cells migrate out of neural tube Neuroepithelial cells are embryonic stem
Neuroepithelial Cells and Neural Differentiation Neurulation The cells of the neural tube are NEUROEPITHELIAL CELLS Neural crest cells migrate out of neural tube Neuroepithelial cells are embryonic stem
Cell and Tissue Types. Epithelial, Connective, Muscle, Nerve
 Cell and Tissue Types Epithelial, Connective, Muscle, Nerve Objectives Explain the major stages of the cell cycle and cellular division (mitosis). Describe specific events occurring in each of the phases
Cell and Tissue Types Epithelial, Connective, Muscle, Nerve Objectives Explain the major stages of the cell cycle and cellular division (mitosis). Describe specific events occurring in each of the phases
Respiratory System. Functional Anatomy of the Respiratory System
 Respiratory System Overview of the Respiratory System s Job Major Duty Respiration Other important aspects ph control Vocalization Processing incoming air Protection Metabolism (ACE) What structures allow
Respiratory System Overview of the Respiratory System s Job Major Duty Respiration Other important aspects ph control Vocalization Processing incoming air Protection Metabolism (ACE) What structures allow
Lung hypoplasia and neonatal death in Fgf9-null mice identify this gene as an essential regulator of lung mesenchyme
 Development 128, 2095-2106 (2001) Printed in Great Britain The Company of Biologists Limited 2001 DEV4537 2095 Lung hypoplasia and neonatal death in Fgf9-null mice identify this gene as an essential regulator
Development 128, 2095-2106 (2001) Printed in Great Britain The Company of Biologists Limited 2001 DEV4537 2095 Lung hypoplasia and neonatal death in Fgf9-null mice identify this gene as an essential regulator
The normal lung: histology, embryology, development, aging and function
 Chapter1 The normal lung: histology, embryology, development, aging and function Neil Sahasrabudhe, John R. Gosney and Philip Hasleton Introduction Knowledge of normal lung anatomy and function is important
Chapter1 The normal lung: histology, embryology, development, aging and function Neil Sahasrabudhe, John R. Gosney and Philip Hasleton Introduction Knowledge of normal lung anatomy and function is important
Respiratory System. Organization of the Respiratory System
 Respiratory System In addition to the provision of oxygen and elimination of carbon dioxide, the respiratory system serves other functions, as listed in (Table 15 1). Respiration has two quite different
Respiratory System In addition to the provision of oxygen and elimination of carbon dioxide, the respiratory system serves other functions, as listed in (Table 15 1). Respiration has two quite different
glial cells missing and gcm2 Cell-autonomously Regulate Both Glial and Neuronal
 glial cells missing and gcm2 Cell-autonomously Regulate Both Glial and Neuronal Development in the Visual System of Drosophila Carole Chotard, Wendy Leung and Iris Salecker Supplemental Data Supplemental
glial cells missing and gcm2 Cell-autonomously Regulate Both Glial and Neuronal Development in the Visual System of Drosophila Carole Chotard, Wendy Leung and Iris Salecker Supplemental Data Supplemental
Tissue: The Living Fabric: Part A
 PowerPoint Lecture Slides prepared by Janice Meeking, Mount Royal College C H A P T E R 4 Tissue: The Living Fabric: Part A Tissues Groups of cells similar in structure and function Types of tissues Epithelial
PowerPoint Lecture Slides prepared by Janice Meeking, Mount Royal College C H A P T E R 4 Tissue: The Living Fabric: Part A Tissues Groups of cells similar in structure and function Types of tissues Epithelial
Angiogenesis in Human Development. Vascular Development
 Angiogenesis in Human Development Jan Kitajewski ICRC 217B, ph 851-4688, email: jkk9 BACKGROUND READING: Vascular Development Signaling Vascular Morphogenesis and Maintenance Douglas Hanahan. Science 277:
Angiogenesis in Human Development Jan Kitajewski ICRC 217B, ph 851-4688, email: jkk9 BACKGROUND READING: Vascular Development Signaling Vascular Morphogenesis and Maintenance Douglas Hanahan. Science 277:
2/2/2011. Primitive Gut Tube Proctodeum and Stomodeum Stomach Duodenum Pancreas Liver and Biliary Apparatus Spleen Midgut
 DEVELOPMENT OF THE DIGESTIVE SYSTEM Development of Endodermal Organs Primitive Gut Tube Proctodeum and Stomodeum Stomach Duodenum Pancreas Liver and Biliary Apparatus Spleen Midgut Wednesday, February
DEVELOPMENT OF THE DIGESTIVE SYSTEM Development of Endodermal Organs Primitive Gut Tube Proctodeum and Stomodeum Stomach Duodenum Pancreas Liver and Biliary Apparatus Spleen Midgut Wednesday, February
Meeting Report. From December 8 to 11, 2012 at Atlanta, GA, U.S.A
 Meeting Report Affiliation Department of Transfusion Medicine and Cell Therapy Name Hisayuki Yao Name of the meeting Period and venue Type of your presentation Title of your presentation The 54 th Annual
Meeting Report Affiliation Department of Transfusion Medicine and Cell Therapy Name Hisayuki Yao Name of the meeting Period and venue Type of your presentation Title of your presentation The 54 th Annual
Ahtiainen et al., http :// /cgi /content /full /jcb /DC1
 Supplemental material JCB Ahtiainen et al., http ://www.jcb.org /cgi /content /full /jcb.201512074 /DC1 THE JOURNAL OF CELL BIOLOGY Figure S1. Distinct distribution of different cell cycle phases in the
Supplemental material JCB Ahtiainen et al., http ://www.jcb.org /cgi /content /full /jcb.201512074 /DC1 THE JOURNAL OF CELL BIOLOGY Figure S1. Distinct distribution of different cell cycle phases in the
The embryonic endoderm initially is widely connected with the yolk sac. As a consequence of cephalocaudal and lateral folding, a portion of the
 DIGESTIVE SYSTEM The embryonic endoderm initially is widely connected with the yolk sac. As a consequence of cephalocaudal and lateral folding, a portion of the endoderm-lined yolk sac cavity is incorporated
DIGESTIVE SYSTEM The embryonic endoderm initially is widely connected with the yolk sac. As a consequence of cephalocaudal and lateral folding, a portion of the endoderm-lined yolk sac cavity is incorporated
Histology = the study of tissues. Tissue = a complex of cells that have a common function
 { EPITHELIAL TISSUE Histology = the study of tissues Tissue = a complex of cells that have a common function The Four Primary Tissue Types: Epithelium (epithelial tissue) covers body surfaces, lines body
{ EPITHELIAL TISSUE Histology = the study of tissues Tissue = a complex of cells that have a common function The Four Primary Tissue Types: Epithelium (epithelial tissue) covers body surfaces, lines body
SOPten flox/flox (KO) Pten flox/flox (WT) flox allele 6.0 kb. Pten. Actin. ! allele 2.3 kb. Supplementary Figure S1. Yanagi, et al.
 s1 A Pten flox/flox () SOPten flox/flox () flox allele 6. kb B Pten flox/flox () SOPten flox/flox () Pten Actin! allele 2.3 kb Supplementary Figure S1. Yanagi, et al. A B BrdU BrdU positive cells ( ) 3
s1 A Pten flox/flox () SOPten flox/flox () flox allele 6. kb B Pten flox/flox () SOPten flox/flox () Pten Actin! allele 2.3 kb Supplementary Figure S1. Yanagi, et al. A B BrdU BrdU positive cells ( ) 3
CHAPTER VII CONCLUDING REMARKS AND FUTURE DIRECTION. Androgen deprivation therapy is the most used treatment of de novo or recurrent
 CHAPTER VII CONCLUDING REMARKS AND FUTURE DIRECTION Stathmin in Prostate Cancer Development and Progression Androgen deprivation therapy is the most used treatment of de novo or recurrent metastatic PCa.
CHAPTER VII CONCLUDING REMARKS AND FUTURE DIRECTION Stathmin in Prostate Cancer Development and Progression Androgen deprivation therapy is the most used treatment of de novo or recurrent metastatic PCa.
يراهظلا( يئلاطلا جيسنلا
 Epithelium النسيج الطالئي )الظهاري( Features of Epithelium Epithelium occurs in the body as a sheet of cells that covers a body surface, lines a cavity, or forms a gland. Coverings, linings, glands. Derived
Epithelium النسيج الطالئي )الظهاري( Features of Epithelium Epithelium occurs in the body as a sheet of cells that covers a body surface, lines a cavity, or forms a gland. Coverings, linings, glands. Derived
Biology 325 Fall 2003
 Name: MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1) Which of the following is not one of the primary tissue types? A) germinative tissue B) muscle
Name: MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1) Which of the following is not one of the primary tissue types? A) germinative tissue B) muscle
Organs of the Respiratory System Laboratory Exercise 52
 Organs of the Respiratory System Laboratory Exercise 52 Background The organs of the respiratory system include the nose, nasal cavity, sinuses, pharynx, larynx, trachea, bronchial tree, and lungs. They
Organs of the Respiratory System Laboratory Exercise 52 Background The organs of the respiratory system include the nose, nasal cavity, sinuses, pharynx, larynx, trachea, bronchial tree, and lungs. They
Urinary System Laboratory
 Urinary System Laboratory 1 Adrenal gland Organs of The Urinary System Renal artery and vein Kidney Ureter Urinary bladder Figure 26.1 2 Urethra Functions of the urinary system organs: Urethra expels urine
Urinary System Laboratory 1 Adrenal gland Organs of The Urinary System Renal artery and vein Kidney Ureter Urinary bladder Figure 26.1 2 Urethra Functions of the urinary system organs: Urethra expels urine
Urogenital Development
 2-5-03 Urogenital Development Greg Dressler Assoc. Professor Dept. of Pathology x46490 Dressler@umich.edu The Origin of the Kidney In the vertebrate embryo, the first stage of kidney development occurs
2-5-03 Urogenital Development Greg Dressler Assoc. Professor Dept. of Pathology x46490 Dressler@umich.edu The Origin of the Kidney In the vertebrate embryo, the first stage of kidney development occurs
Regulation of retinoic acid signaling during lung morphogenesis
 Development 127, 3057-3067 (2000) Printed in Great Britain The Company of Biologists Limited 2000 DEV4324 3057 Regulation of retinoic acid signaling during lung morphogenesis Sarah Malpel 1, Cathy Mendelsohn
Development 127, 3057-3067 (2000) Printed in Great Britain The Company of Biologists Limited 2000 DEV4324 3057 Regulation of retinoic acid signaling during lung morphogenesis Sarah Malpel 1, Cathy Mendelsohn
Histology Notes -Part 1: Epithelial Tissues
 Introduction Group of cells w/ similar structure & function = TISSUE Four Basic Tissue Types 1. Epithelial-covers 2. Connective-supports 3. Muscular*-produces movement (will discuss in the muscular system
Introduction Group of cells w/ similar structure & function = TISSUE Four Basic Tissue Types 1. Epithelial-covers 2. Connective-supports 3. Muscular*-produces movement (will discuss in the muscular system
A. cells that perform related functions and are similar in structure. B. extracellular material - made by cells and secreted into interstitial space
 I. tissue components A. cells that perform related functions and are similar in structure B. extracellular material - made by cells and secreted into interstitial space II. tissue types A. epithelium (e.)
I. tissue components A. cells that perform related functions and are similar in structure B. extracellular material - made by cells and secreted into interstitial space II. tissue types A. epithelium (e.)
Emx2 patterns the neocortex by regulating FGF positional signaling
 Emx2 patterns the neocortex by regulating FGF positional signaling Tomomi Fukuchi-Shimogori and Elizabeth A Grove Presented by Sally Kwok Background Cerebral cortex has anatomically and functionally distinct
Emx2 patterns the neocortex by regulating FGF positional signaling Tomomi Fukuchi-Shimogori and Elizabeth A Grove Presented by Sally Kwok Background Cerebral cortex has anatomically and functionally distinct
T Cell Development. Xuefang Cao, MD, PhD. November 3, 2015
 T Cell Development Xuefang Cao, MD, PhD November 3, 2015 Thymocytes in the cortex of the thymus Early thymocytes development Positive and negative selection Lineage commitment Exit from the thymus and
T Cell Development Xuefang Cao, MD, PhD November 3, 2015 Thymocytes in the cortex of the thymus Early thymocytes development Positive and negative selection Lineage commitment Exit from the thymus and
Basic Tissue Types and Functions
 Tissues Histology Basic Tissue Types and Functions 1) Epithelial tissue covering 2) Connective tissue support 3) Muscle tissue movement 4) Nervous tissue control Epithelial Tissue 1) Covers a body surface
Tissues Histology Basic Tissue Types and Functions 1) Epithelial tissue covering 2) Connective tissue support 3) Muscle tissue movement 4) Nervous tissue control Epithelial Tissue 1) Covers a body surface
Tissues. tissue = many cells w/ same structure and function. cell shape aids function tissue shape aids function. Histology = study of tissues
 Tissues tissue = many cells w/ same structure and function cell shape aids function tissue shape aids function Histology = study of tissues 4 types of tissues Epithelial coverings contact openings Connective
Tissues tissue = many cells w/ same structure and function cell shape aids function tissue shape aids function Histology = study of tissues 4 types of tissues Epithelial coverings contact openings Connective
a) They are the most common cause of pediatric kidney failure. b) They are always symptomatic. c) They can be asymmetric.
 Practice questions: 1. The paraxial mesoderm gives rise to somites. The structure of the somite a) is a loose mesenchymal sheet that will migrate toward the notochord. b) is an epithelial rosette with
Practice questions: 1. The paraxial mesoderm gives rise to somites. The structure of the somite a) is a loose mesenchymal sheet that will migrate toward the notochord. b) is an epithelial rosette with
Outline. Bio 105: Tissues Laboratory. Organization of the Human Body. Tissue - Epithelium. Tissues 3/2/ Copyright 2009 Pearson Education, Inc
 Outline Bio 105: Tissues Laboratory Laboratory 5 Reading: Chapter 4 I. Cell to cell contact II. Body Cavities III. Membranes IV. Homeostasis V. Integumentary System I. Includes skin, hair and nails 1 2
Outline Bio 105: Tissues Laboratory Laboratory 5 Reading: Chapter 4 I. Cell to cell contact II. Body Cavities III. Membranes IV. Homeostasis V. Integumentary System I. Includes skin, hair and nails 1 2
Endocrine Pancreas Development and Regeneration: Noncanonical Ideas From Neural Stem Cell Biology
 314 Diabetes Volume 65, February 2016 Jimmy Masjkur, 1 Steven W. Poser, 1 Polyxeni Nikolakopoulou, 1 George Chrousos, 2 Ronald D. McKay, 3 Stefan R. Bornstein, 1 Peter M. Jones, 4 and Andreas Androutsellis-Theotokis
314 Diabetes Volume 65, February 2016 Jimmy Masjkur, 1 Steven W. Poser, 1 Polyxeni Nikolakopoulou, 1 George Chrousos, 2 Ronald D. McKay, 3 Stefan R. Bornstein, 1 Peter M. Jones, 4 and Andreas Androutsellis-Theotokis
Lecture Overview. Marieb s Human Anatomy and Physiology. Chapter 4 Tissues: The Living Fabric Epithelial Tissues Lecture 9. Introduction to Tissues
 Marieb s Human Anatomy and Physiology Marieb Hoehn Chapter 4 Tissues: The Living Fabric Epithelial Tissues Lecture 9 Lecture Overview Introduction to Tissues Epithelial Tissues Location General characteristics
Marieb s Human Anatomy and Physiology Marieb Hoehn Chapter 4 Tissues: The Living Fabric Epithelial Tissues Lecture 9 Lecture Overview Introduction to Tissues Epithelial Tissues Location General characteristics
Brain Development III
 Brain Development III Neural Development In the developing nervous system there must be: 1. The formation of different regions of the brain. 2. The ability of a neuron to differentiate. 3. The ability
Brain Development III Neural Development In the developing nervous system there must be: 1. The formation of different regions of the brain. 2. The ability of a neuron to differentiate. 3. The ability
Tissue repair. (3&4 of 4)
 Tissue repair (3&4 of 4) What will we discuss today: Regeneration in tissue repair Scar formation Cutaneous wound healing Pathologic aspects of repair Regeneration in tissue repair Labile tissues rapid
Tissue repair (3&4 of 4) What will we discuss today: Regeneration in tissue repair Scar formation Cutaneous wound healing Pathologic aspects of repair Regeneration in tissue repair Labile tissues rapid
Cooperative and independent functions of FGF and Wnt signaling during early inner ear development
 Wright et al. BMC Developmental Biology (2015) 15:33 DOI 10.1186/s12861-015-0083-8 RESEARCH ARTICLE Open Access Cooperative and independent functions of FGF and Wnt signaling during early inner ear development
Wright et al. BMC Developmental Biology (2015) 15:33 DOI 10.1186/s12861-015-0083-8 RESEARCH ARTICLE Open Access Cooperative and independent functions of FGF and Wnt signaling during early inner ear development
5 Dr. Heba Kalbouneh
 5 Dr. Heba Kalbouneh Glandular epithelium Gland: Is a collection of epithelial cells the secrets a certain product, like: proteins, lipids and carbohydrates. Secretion : A certain material that is produced
5 Dr. Heba Kalbouneh Glandular epithelium Gland: Is a collection of epithelial cells the secrets a certain product, like: proteins, lipids and carbohydrates. Secretion : A certain material that is produced
CHAPTER 05 Histology: EPITHELIUM
 BIO 211: ANATOMY & PHYSIOLOGY I 1 CHAPTER 05 Histology: EPITHELIUM Part 01: Brief Introduction Part 02: Survey of Types Dr. Lawrence G. G. Altman www.lawrencegaltman.com Some illustrations are courtesy
BIO 211: ANATOMY & PHYSIOLOGY I 1 CHAPTER 05 Histology: EPITHELIUM Part 01: Brief Introduction Part 02: Survey of Types Dr. Lawrence G. G. Altman www.lawrencegaltman.com Some illustrations are courtesy
TISSUES TYPES. CHAPTER 05 Histology: EPITHELIUM BIO 211: ANATOMY & PHYSIOLOGY I. HISTOLOGY = the study of tissues
 BIO 211: ANATOMY & PHYSIOLOGY I 1 CHAPTER 05 Histology: EPITHELIUM Part 01: Brief Introduction Part 02: Survey of Types Dr. Lawrence G. G. Altman www.lawrencegaltman.com Some illustrations are courtesy
BIO 211: ANATOMY & PHYSIOLOGY I 1 CHAPTER 05 Histology: EPITHELIUM Part 01: Brief Introduction Part 02: Survey of Types Dr. Lawrence G. G. Altman www.lawrencegaltman.com Some illustrations are courtesy
NOTES: CH 40 Introduction to Human Anatomy & Physiology
 NOTES: CH 40 Introduction to Human Anatomy & Physiology THE HUMAN BODY Anatomy Physiology (= structures) (= functions or processes) Characteristics of LIFE: 1) Made up of 1 or more CELLS. 2) Obtain and
NOTES: CH 40 Introduction to Human Anatomy & Physiology THE HUMAN BODY Anatomy Physiology (= structures) (= functions or processes) Characteristics of LIFE: 1) Made up of 1 or more CELLS. 2) Obtain and
(b) Stomach s function 1. Dilution of food materials 2. Acidification of food (absorption of dietary Fe in small intestine) 3. Partial chemical digest
 (1) General features a) Stomach is widened portion of gut-tube: between tubular and spherical; Note arranged of smooth muscle tissue in muscularis externa. 1 (b) Stomach s function 1. Dilution of food
(1) General features a) Stomach is widened portion of gut-tube: between tubular and spherical; Note arranged of smooth muscle tissue in muscularis externa. 1 (b) Stomach s function 1. Dilution of food
Chapter 4 - Epithelial Tissues
 Chapter 4 - Epithelial Tissues Tissues Definition A group of closely associated cells that work together to perform a specific function Types Epithelial - covering Connective - support Muscle - movement
Chapter 4 - Epithelial Tissues Tissues Definition A group of closely associated cells that work together to perform a specific function Types Epithelial - covering Connective - support Muscle - movement
Supplemental Information. Otic Mesenchyme Cells Regulate. Spiral Ganglion Axon Fasciculation. through a Pou3f4/EphA4 Signaling Pathway
 Neuron, Volume 73 Supplemental Information Otic Mesenchyme Cells Regulate Spiral Ganglion Axon Fasciculation through a Pou3f4/EphA4 Signaling Pathway Thomas M. Coate, Steven Raft, Xiumei Zhao, Aimee K.
Neuron, Volume 73 Supplemental Information Otic Mesenchyme Cells Regulate Spiral Ganglion Axon Fasciculation through a Pou3f4/EphA4 Signaling Pathway Thomas M. Coate, Steven Raft, Xiumei Zhao, Aimee K.
THE GOOFY ANATOMIST QUIZZES
 THE GOOFY ANATOMIST QUIZZES 7. LUNGS Q1. Fill in the blanks: the lung has lobes and fissures. A. Right, three, two. B. Right, two, one. C. Left, three, two. D. Left, two, three. Q2. The base of the lung
THE GOOFY ANATOMIST QUIZZES 7. LUNGS Q1. Fill in the blanks: the lung has lobes and fissures. A. Right, three, two. B. Right, two, one. C. Left, three, two. D. Left, two, three. Q2. The base of the lung
A adipose cells. B capillary. C epithelium
 EPITHELIA Objective The objective of this class is to observe how different epithelia vary in terms of cell shape, size and number of cell layers enabling them to be well adapted for functions in different
EPITHELIA Objective The objective of this class is to observe how different epithelia vary in terms of cell shape, size and number of cell layers enabling them to be well adapted for functions in different
Includes : - the lung - a system of tube
 FYH - ERDS 1 Includes : - the lung - a system of tube Divided into 2 principal regions : - conducting portion : nasal cavity, nasopharynx, larynx, trachea, bronchi, bronchioles & terminal bronchioles -
FYH - ERDS 1 Includes : - the lung - a system of tube Divided into 2 principal regions : - conducting portion : nasal cavity, nasopharynx, larynx, trachea, bronchi, bronchioles & terminal bronchioles -
Tissues. Definition. A group of similar cells and their intercellular substances specialized to perform a specific function.
 Chapter 4 - Tissues Tissues Definition A group of similar cells and their intercellular substances specialized to perform a specific function. Tissues Epithelial covers exposed surfaces, lines internal
Chapter 4 - Tissues Tissues Definition A group of similar cells and their intercellular substances specialized to perform a specific function. Tissues Epithelial covers exposed surfaces, lines internal
Normal Development, Anatomy, Histology and Aging of the Lung
 Chapter 1 Normal Development, Anatomy, Histology and Aging of the Lung Xuchen Zhang*, and Robert J. Homer 1. Introduction The primary function of the lungs is to exchange gas between air in the external
Chapter 1 Normal Development, Anatomy, Histology and Aging of the Lung Xuchen Zhang*, and Robert J. Homer 1. Introduction The primary function of the lungs is to exchange gas between air in the external
