Hepatic oleate regulates adipose tissue lipogenesis and fatty acid oxidation
|
|
|
- Simon Cameron
- 5 years ago
- Views:
Transcription
1 Hepatic oleate regulates adipose tissue lipogenesis and fatty acid oxidation Maggie S. Burhans 1, Matthew T. Flowers 2, Kristin R. Harrington 2, Laura M. Bond 2, Chang-An Guo 2, Rozalyn M. Anderson 3,4, and James M. Ntambi 1,2, * 1 Department of Nutritional Sciences University of Wisconsin-Madison, 433 Babcock Drive, Madison, WI USA 2 Department of Biochemistry, University of Wisconsin-Madison, 433 Babcock Drive, Madison, WI USA 3 Department of Medicine, University of Wisconsin-Madison, Madison, WI USA 4 Geriatric Research, Education, and Clinical Center, VA Medical Center, Madison, Wisconsin USA *Corresponding author: James M. Ntambi, jmntambi@wisc.edu Running title: Hepatic oleate regulates WAT metabolism Abbreviations: cholesteryl ester (CE), de novo lipogenesis (DNL), SCD1 global knockout (GKO), GKO liver-specific SCD 5 transgenic (GLS5), GKO liver-specific SCD 3 transgenic (GLS3), lipogenic diet (LD), SCD1 liver knockout (LKO), monounsaturated fatty acids (MUFA), phospholipid (PL), stearoyl-coa desaturase (SCD), triglycerides (TG), white adipose tissue (WAT) 1
2 Abstract Hepatic steatosis is associated with detrimental metabolic phenotypes including enhanced risk for diabetes. Stearoyl-CoA desaturases (SCD) catalyze the synthesis of monounsaturated fatty acids (MUFA). In mice, genetic ablation of SCD reduces hepatic de novo lipogenesis (DNL) and protects against diet-induced hepatic steatosis and adiposity. To understand the mechanism by which hepatic MUFA production influences adipose tissue stores we created two liver-specific transgenic mouse models in the SCD1 knockout that express either human SCD5 or mouse SCD3, that synthesize oleate and palmitoleate respectively. We demonstrate that hepatic de novo synthesized oleate, but not palmitoleate, stimulated hepatic lipid accumulation and adiposity, reversing the protective effect of the global SCD1 knockout under lipogenic conditions. Unexpectedly, the accumulation of hepatic lipid occurred without induction of the hepatic DNL program. Changes in hepatic lipid composition were reflected in plasma and in adipose tissue. Importantly, endogenously synthesized hepatic oleate was associated with suppressed DNL and fatty acid oxidation in white adipose tissue. Regression analysis revealed a strong correlation between adipose tissue lipid fuel utilization and hepatic and adipose tissue lipid storage. These data suggest an extrahepatic mechanism where endogenous hepatic oleate regulates lipid homeostasis in adipose tissues. Supplementary keywords: adipose tissue, beta-oxidation, de novo lipogenesis, fatty acid/desaturases, fatty acid/metabolism, lipids, liver, triglycerides 2
3 Introduction Recently, the prevalence of overweight among all adults in the U.S. was estimated to be nearly 70% (1), and has increased more than two-fold since 1980 (2). With increased adiposity, lipids accumulate ectopically and are associated with increased risk for detrimental metabolic conditions such as nonalcoholic fatty liver disease, insulin resistance and hypertriglyceridemia increases (3 5). The balance of lipid storage between liver and adipose depots may play an important role in disease vulnerability. Fat may be synthesized from dietary carbohydrates via de novo lipogenesis (DNL), or sequestered from circulating lipids. The contribution of hepatic and adipose tissue DNL-derived fat to whole body adiposity is controversial. While it is considered to be quantitatively minimal by some (6, 7), others have demonstrated that DNLderived lipids can negatively impact metabolic health and contribute to hepatic steatosis and visceral adiposity in humans (8 10). Of particular importance in the context of whole body metabolic homeostasis, recent evidence suggests that de novo synthesized fatty acids and lipids serve important signaling and regulatory roles in cellular and systemic metabolism (7, 11). Monounsaturated fatty acids (MUFA) are major components of tissue lipids such as TG, cholesteryl esters (CE) and phospholipids (PL), and high levels of MUFA are inversely associated with metabolic health. The stearoyl-coa desaturase (SCD) family of enzymes catalyzes the synthesis of MUFA by insertion of a cis double bond at the Δ9 position of saturated fatty acids. The preferred substrates for SCD-catalyzed reactions are palmitate (16:0) and stearate (18:0) and the major products of SCD activity are oleate (18:1n-9) and palmitoleate (16:1n-7). In humans, skeletal muscle SCD1 mrna abundance is positively correlated with TG synthesis and negatively correlated with TG oxidation and there is also a strong positive association between muscle SCD1 activity and BMI (12). Serum TG are largely hepatic in origin and the desaturation index (18:1n-9/18:0) is used to estimate liver SCD activity. In humans, the serum lipid desaturation index positively correlates with overall TG levels, explaining more than 50% of the variation in serum TG levels among individuals (13). Another study revealed that 3
4 four single nucleotide polymorphisms in human SCD1 are associated with improved insulin sensitivity, lower BMI and reduced abdominal adiposity (14). There are two models of diet-induced obesity, high-carbohydrate diets that drive DNL and high-fat diets that provide excess exogenous lipid. We previously demonstrated that wholebody or global SCD1 knockout mice (GKO) have increased energy expenditure and are protected from obesity and hepatic steatosis induced by both high-carbohydrate and high-fat diets (15, 16). Mice with deletion of SCD1 in liver only (LKO) exhibit the adiposity and hepatic steatosis phenotypes observed in GKO mice when fed a high-carbohydrate diet. In contrast, high-fat diet fed LKO mice are not protected from weight gain and hepatic steatosis (16). These data suggest that the phenotypes induced by high-carbohydrate diets are driven by liver metabolic events. Although the hepatic lipogenic program is impaired in LKO mice fed a highcarbohydrate diet, dietary supplementation with oleate restores the molecular changes and related lipid accumulation phenotypes (16). Taken together, this body of past work reveals that hepatic SCD1 mediates the lipogenic effects of a high-carbohydrate diet. However, the question of whether local hepatic synthesis of MUFA is sufficient to restore the response to a highcarbohydrate diet in GKO mice remains unknown. Furthermore, whether oleate and palmitoleate, the major products of SCD-catalyzed reactions, differentially regulate these metabolic processes has not been directly assessed. Two SCD isoforms have been identified in humans (SCD1 and SCD5) and four in mice (SCD1 4). While mouse SCD1 is expressed at high levels in adipose tissue and is induced in liver under high-carbohydrate conditions, SCD3 expression is restricted to skin, Harderian gland and the preputial gland (17 19). We took advantage of the substrate preferences of distinct SCD isoforms to restore oleate (human SCD5) or palmitoleate (mouse SCD3) synthesis in liver only in GKO mice. We show that in the context of a high-sucrose lipogenic diet, hepatic oleate, but not palmitoleate, synthesis increases hepatic lipid accumulation and adiposity in GKO mice. In addition, hepatic oleate is associated with suppression of DNL and fatty acid oxidation in 4
5 white adipose tissue (WAT). These data suggest that the unfavorable metabolic effects of lipogenic diets are mediated by hepatic oleate, the major product of human SCD5. Methods Animals and diets Two liver transgenic mouse lines were generated by cloning either the human SCD5 cdna sequence or mouse SCD3 cdna sequence into the pliv.le6 vector construct (a kind gift from John Taylor, Gladstone Institute) (20). Mice were backcrossed at least seven generations with C57BL/6 mice to generate SCD5Tg+ and SCD3Tg+ mice. SCD5Tg+ and SCD3Tg+ mice were then crossed with SCD1 GKO mice (in C57BL/6 background) to generate compound heterozygous mice, SCD1+/- carrying one copy of the SCD5 or SCD3 transgene. These compound heterozygous mice were then bred with female SCD1+/- or male SCD1-/- mice to generate SCD5Tg+;SCD1-/- (GLS5) and SCD3Tg+;SCD1-/- (GLS3) mice. Mice were housed in the University of Wisconsin-Madison Department of Biochemistry animal care facility and maintained on a 12 hour light-dark cycle (6PM to 6AM) and had free access to food and water unless specified otherwise. All mice were male and were fed a standard rodent chow diet (Purina 5008) at weaning and then either maintained on the chow diet or fed a lipogenic diet (LD) that is high in sucrose and very low in fat (Harlan Teklad TD.03045; 2.5% kcal from fat (corn oil), 76.7% kcal from carbohydrate) for a period of 10 days. The age at LD feeding varied among experiments, from weeks of age, and is described specifically for each figure. Unless noted otherwise, all mice were fasted 4 hours and killed at the same time of day by isoflurane overdose. Blood was collected via cardiac puncture and tissues were collected. All in vivo experimental animal procedures were approved by the Institutional Animal Care and Use Committee at the University of Wisconsin-Madison. Lipid extraction and gas chromatography 5
6 Liver, adipose tissue and plasma fatty acid analyses were carried out as previously described (21). Lipids were extracted following a modified Folch method (22). Pentadecanoic acid was added as an internal control of transmethylation efficiency. Neutral lipid species were separated on silica gel-60 TLC plates (EMD Millipore) using a heptane/isopropyl ether/acetic acid (60/40/3) solvent system. TG, CE, FFA and PL bands were scraped from plates, lipids were extracted and transmethylated using boron trifluoride in 14% methanol (Sigma). Fatty acid methyl esters were suspended in hexane and analyzed by gas liquid chromatography (GLC). Chromatograms were analyzed using HP ChemStation software. Results were calculated to express fatty acid composition as a percent of total and as concentration of µg/mg tissue or µg/100 µl plasma. Total hepatic oleate and total hepatic palmitoleate levels were calculated based on the sum of the respective fatty acid in TG, CE, FFA and PL fractions for each animal and expressed as a percentage of the level in WT mice. Total fatty acids in each lipid fraction were calculated based on the sum of the total fatty acid mass (µg/mg tissue) detected by GLC analysis in each fraction. Heptadecanoic acid, triheptadecanoin, cholesteryl heptadecanoate or diheptadecanoyl phosphocholine internal standards were used to quantify fatty acids in FFA, TG, CE and PL fractions, respectively. Liver histology Fresh liver tissue was fixed in 10% neutral buffered formalin for 2 days at 4 C and then stored in 70% ethanol at 4 C until sectioning and histological analysis. Oil red O staining was performed on cryosections, as previously described (23). Slides were imaged with a 40x objective in a Leica DM4000B microscope. Plasma and liver biochemical analyses Plasma TG were measured in 5 µl plasma using a colorimetric assay with the Infinity Triglycerides Reagent (Thermo Scientific). Liver TG were measured in lipid extract from 10 mg liver tissue using a colorimetric enzymatic assay (Wako Chemicals USA). Blood glucose values were measured using a spectrophotometric glucose oxidase and peroxidase assay, as 6
7 described previously (16). Plasma insulin (Crystal Chem, #90080), plasma leptin (Millipore, #EZML-82K) and plasma FGF21 (R & D Systems, #MF2100) were all measured by ELISA. RNA isolation and real-time quantitative PCR Liver total RNA was isolated with Tri Reagent (Molecular Research Center) and WAT total RNA was isolated with RNeasy Lipid Tissue Mini Kit (Qiagen) and treated with Turbo DNase (Ambion). RNA was reverse transcribed with High Capacity cdna Reverse Transcription Kit (Applied Biosystems). Quantitative real-time PCR was performed using SYBR Green PCR Master Mix (Applied Biosystems) and ABI 7500 instrument (Applied Biosystems). Relative mrna expression levels of SCD5 and Scd3 were calculated using the comparative C T method, and normalized to 18S rrna. Primer sequences used to detect SCD5 expression were: Fwd 5 GCTGTTTGTTCGCAAGCATCGAGA and Rev 5 AAAGCACATGAGCACCACGGAGAT. For Scd3 expression, the primer sequences were Fwd 5 CATTGGAGCCGGAGTCCATC and Rev 5 GCCATGGTGTTGGCAATGAT. Other primer sequences available upon request. Microsomal protein and stearoyl-coa desaturase activity assays Hepatic microsomes were prepared by sequential centrifugation as previously described (21). SCD activity assays were performed using 100 µg liver microsomal protein. Microsomes were incubated in potassium phosphate buffer in the presence of 30 µm stearoyl-coa and 1 µci 3 H-9,10-Stearoyl-CoA (or palmitoyl-coa and 3 H-9,10-Palmitoyl-CoA) (American Radiolabeled Chemicals) and NADH for 15 minutes at 24 C. The reaction was quenched by 6% perchloric acid and unused substrate was adsorbed by activated charcoal (Sigma). Samples were counted in a scintillation counter and results were calculated as 3 H DPM/100 µg protein and are presented as percent of mean WT activity level. Immunoblotting Microsomal protein was used for immunoblot analysis of SCD1 (Santa Cruz Biotechnology, #sc-14720), HA tag for SCD5 and SCD3 transgenic protein (Roche Applied Science, # ), human SCD5, GAPDH (Millipore) and transferrin receptor-1 (TfR1; 7
8 Invitrogen, # ). Proteins were separated by SDS-PAGE in 8-10% gels. Proteins were transferred to PVDF membrane, incubated with primary antibody overnight, followed by secondary antibody and visualized using Pierce ECL western blotting substrate. In vivo de novo lipogenesis Mice were fasted for 2 hours and then injected intraperitoneally with 50 mci 3 H-H 2 O. Mice were killed by isoflurane overdose 1 hour post-injection. Blood was collected by cardiac puncture to determine specific activity. Approximately 100 mg liver tissue or 10 mg gonadal WAT was saponified in 2.5 M KOH/EtOH for 2.5 hr at 75 C. The tissues were neutralized with formic acid and lipids extracted with hexane. Fatty acids and sterols were separated on TLC with heptane/isopropyl ether/acetic acid (60/40/3) solvent system. Fatty acid and sterol bands were visualized with iodine vapors, scraped and counted in a liquid scintillation counter. Results were calculated as µmol 3 H-H 2 O per g tissue per hour incorporated into fatty acids or cholesterol (24). Ex vivo fatty acid oxidation Mice were fasted for 4 hours and killed by isoflurane overdose. Liver and gonadal WAT were collected and processed as previously described (25). Tissue homogenates were incubated for 1 h in the presence of 300 µm palmitic acid with 0.4 µci 1-14 C-palmitic acid. Fatty acid oxidation inhibitor rotenone (0.75 µm, Sigma Aldrich) was used as a control. 14 C-containing acid soluble metabolites and trapped CO 2 were measured in a liquid scintillation counter. Fatty acid oxidation of the two fractions was calculated by [(DPM-blank)/reaction mixture specific activity]/g tissue and the total fatty acid oxidation values reported reflect the sum of the CO 2 and acid soluble metabolite fractions. Ex vivo lipolysis Gonadal fat explants isolated from mice (8 mice per group) were washed with ice-cold PBS and cut into small pieces (75~150mg). Fat pads were preincubated for 1 hour in 140 µl of DMEM (Life Technologies) containing 2% fatty acid-free serum albumin (Sigma-Aldrich). 8
9 Subsequently, fat pads were incubated in 250 µl of KRH buffer (125 mm NaCl, 5 mm KCl, 1.8 mm CaCl2, 2.6 mm MgSO4, 5 mm HEPES, ph 7.2) plus 2% BSA (fatty acid free) for 2 hours under agitation at 37 C. Free glycerol content was quantified from supernatants using the Free Glycerol Determination Kit (Sigma-Aldrich). Glycerol release from each sample was normalized to the weight of each fat pad. Glucose tolerance tests Mice were fasted for 4 hours prior to the start of glucose tolerance tests. Intraperitoneal injections of 20% dextrose solution at 2 g/kg body weight were administered. Tail vein blood was used to determine blood glucose concentrations at 0, 30, 60, 90 and 120 minutes following the glucose dose using a blood glucose meter and test strips (One Touch Ultra, Diabetic Express). Statistical analyses Results are expressed as mean ± SEM. Variables that did not have a Gaussian distribution were log transformed prior to ANOVA analyses. Data were analyzed using linear regression analyses and one-way ANOVA with Tukey s post-hoc test. Results with P-value < 0.05 were considered statistically significant. All statistical analyses were performed using GraphPad Prism 6 (GraphPad Software). Results Liver SCD5 and SCD3 transgenic mice in the SCD1 GKO background produce hepatic oleate and palmitoleate, respectively To determine if hepatic MUFA exert differential metabolic effects, we generated mice with constitutive expression of either human SCD5 or mouse Scd3 in liver tissue only in a GKO background. These SCD isoforms synthesize oleate (SCD5) and palmitoleate (SCD3) (Figure 1A). To generate the liver transgenic mice, the cdna sequences of the transgenes were cloned into the pliv.le6 vector construct in which liver-specific expression is achieved by using 9
10 elements of the human APOE gene, including the promoter, exons 1 and 2, and the liver control element (20, Figure 1B). As expected, expression of the transgenes was robust in liver tissue with minimal to no protein expression detected in a panel of extrahepatic tissues (Figure 1C). SCD5 and SCD3 transgenic mice in the C57Bl/6 genetic background were then crossed into the GKO background that lacks Scd1 expression in all tissues producing GKO Liver-specific SCD5 (GLS5) and GKO Liver-specific SCD3 (GLS3) transgenic mice. Quantitative PCR and western blot analysis revealed that transgenic mice retained high levels of hepatic transgenic gene (Figure 1D) and protein (Figure 1E) expression but lacked detectable levels of SCD1 protein in liver microsomes (Figure 1E). Like GKO and SCD1 skin-specific knockout mice (26), GLS5 and GLS3 mice exhibit alopecia, closed eye fissures and dry skin (Supplemental Figure 1) and were visibly indistinguishable from GKO mice at weaning and throughout adulthood, indicating that these phenotypes are not rescued by restoration of liver MUFA synthesis. We used a modified high-carbohydrate diet (high in sucrose) that supplies 2.5% kcal from fat, hereafter referred to as the lipogenic diet (LD), to potently induce DNL (16). WT, GKO, GLS5 and GLS3 mice were fed the LD for 10 days. First, changes in SCD activity and hepatic MUFA levels conferred by the expression of SCD5 or Scd3 were assessed. Desaturation of 3 H- stearoyl-coa (18:0) was 7-fold higher in GLS5 mice as compared to GKO mice but not changed in GLS3 mice (Figure 1F). In contrast, desaturation of 3 H-palmitoyl-CoA (16:0) was 5-fold higher in GLS3 mice compared to GKO but not changed in GLS5 mice (Figure 1F). These activity assay results confirmed substrate specificities in vivo of the human SCD5 and mouse SCD3 isoforms, in which SCD5 selectively desaturates 18:0 to 18:1n-9 and SCD3 selectively desaturates 16:0 to 16:1n-7. As expected, and in accordance with the SCD activity assays, gas chromatographic analysis of fatty acids in hepatic lipids (summed values in TG, CE, FFA and PL fractions, reported in Tables S1-S4) revealed 18:1n-9 was significantly increased only in GLS5 mice and 16:1n-7 was increased only in GLS3 mice, relative to GKO mice (Figure 1G). Body weight and adiposity phenotypes are restored in GLS5 mice fed a lipogenic diet 10
11 When fed a chow diet, body weight of male mice did not differ with the exception of GLS5, which was lower than WT mice (Supplemental Figure 2A). Adipose and liver weights and liver and plasma TG did not differ among GLS5, GLS3 and GKO mice (Supplemental Figure 2B 2E). To assess potential changes in the lipogenic capacity we measured body weight and adipose tissue weights of WT, GKO, GLS5 and GLS3 male mice fed the LD for 10 days. There were no significant differences in body weight at the start of the study (Supplemental Figure 3). Consistent with our previous results (27), LD-fed GKO mice lost a significant amount of body weight over the 10-day period, with an average loss of nearly 20% of their initial body weight. However, male GLS5 mice lost only 8.6% of the initial body weight. In contrast, GLS3 mice lost nearly double that with an average loss of 16.5%, which was not significantly different from GKO. At the end of the feeding period, the body weight of GLS5 mice was significantly greater than that of GKO mice (Figure 2A) and was not different from WT mice. Body weight of GLS3 and GKO mice did not differ and both were significantly lighter than WT mice (Figures 2A). Subcutaneous and gonadal WAT depot weights were significantly reduced in GKO mice relative to WT mice. Both adipose tissue depots were significantly increased in weight in GLS5 and GLS3 mice relative to GKO mice (Figure 2B). However, while the weight of the gonadal adipose depot was partially restored in both GLS5 and GLS3, the subcutaneous depot was fully restored to WT level only in GLS5 mice (Figure 2B). GLS5 mice ate significantly more food over the course of the 10-day feeding study as compared to GKO and WT mice (Figure 2C). Total food intake was increased by 32% in GLS5 relative to the WT counterparts. However, when expressed relative to final body weight, GKO, GLS5 and GLS3 groups ate significantly more food than WT mice (data not shown). Although GLS5 mice consumed more food than GKO mice, plasma leptin was not different between these groups and was significantly lower in GKO, GLS5 and GLS3 mice relative to the WT level (Figure 2D). 11
12 To determine whether circulating FGF21 is influenced by hepatic MUFA levels and could perhaps explain the differential body weight and adiposity phenotypes among the LD-fed mice, we measured plasma FGF21 levels by ELISA. FGF21 was significantly increased in GKO relative to WT and was also increased but only trended towards significance in GLS5 (P=0.07) and GLS3 (P<0.06) mice (Figure 2E). These results suggest that although plasma FGF21 is generally increased by whole-body SCD1 deficiency, it is not likely to be the factor regulating the differential body weight and adiposity responses among the GKO, GLS5 and GLS3 mice. The differences in GLS5 and GLS3 are consistent with a model where the phenotypes of GKO mice are driven by attenuated oleate production. Overall, these results suggest a role for de novo synthesized hepatic oleate in the regulation of adiposity and body weight under LD conditions but little effect of palmitoleate. Blood glucose is increased in GLS5 mice fed a lipogenic diet In the absence of SCD1, LD-fed mice are unable to maintain blood glucose levels and become severely hypoglycemic (27). In addition, pyruvate tolerance tests suggest that LD-fed SCD1 LKO mice are unable to synthesize glucose, but this defect in gluconeogenesis is corrected by dietary oleate provided at a level of 20% (w/w) (16). These data suggest that MUFA availability plays a role in the regulation of blood glucose levels. To investigate this further, we measured blood glucose levels in 4 hour fasted male mice fed the LD for 10 days. Blood glucose was significantly increased in GLS5 mice compared to GKO mice (Figure 3A). In contrast, hepatic palmitoleate synthesis in GLS3 mice did not alter blood glucose relative to GKO mice (Figure 3A). Although fasted blood glucose was significantly elevated in GLS5, the relative hepatic mrna levels of the gluconeogenic genes Pck1 and G6pc were not significantly different among GKO, GLS5 and GLS3 mice (Figure 3B). Plasma insulin levels were significantly lower in GKO, GLS5 and GLS3 mice compared to WT (Figure 3C). GKO mice are highly insulin sensitive and glucose tolerant, as previously reported (15, 28). Despite greater adiposity and increased fasted blood glucose levels in GLS5 mice, glucose clearance was not 12
13 significantly different from GKO or GLS3 mice at any time point during a glucose tolerance test (Figure 3D) and area under the curve above baseline levels was significantly smaller for all three genotypes as compared to WT mice. Overall, these data suggest that hepatic oleate, at the level produced in GLS5 mice, modestly influences blood glucose regulation. Hepatic lipids accumulate in GLS5 mice during lipogenic dietary conditions Since GKO and LKO mice display protection against high-carbohydrate diet-induced hepatic steatosis, we questioned whether this protection would be lost with an increase in MUFA availability through the local hepatic synthesis of either oleate or palmitoleate. Biochemical measurement of hepatic TG revealed that GKO mice were protected from lipid accumulation but GLS5 mice were not (Figure 4A) and accumulated TG to the same level as WT mice. Hepatic TG level in GLS3 mice was not significantly different from that of GKO (Figure 4A). We also assessed hepatic lipid burden in males by staining neutral lipids in liver tissue sections with oil red O. This histological assessment confirmed the biochemical assay results and demonstrated that while lipid droplets accumulated in GLS5 liver, there was minimal to no accumulation in GKO and GLS3 mice (Figure 4B). These results reveal that hepatic oleate synthesis is sufficient to rescue the lipid accumulation phenotype of GKO mice during lipogenic dietary conditions but palmitoleate does not have an effect. Using gas liquid chromatography, qualitative and quantitative fatty acid analyses of hepatic TG, CE, PL lipid fractions and nonesterified fatty acids were performed. The mean sum of the fatty acids in each fraction is reported for each genotype (Figures 4C 4F). The sum of fatty acids in the TG fraction further confirmed high levels of hepatic TG in GLS5 mice and lower levels in GKO and GLS3 mice (Figure 4C). CE did not accumulate in liver of GLS5 mice (Figure 4D). This suggests that neutral lipid accumulation observed by oil red O in GLS5 mice was due to TG and not CE. There were no significant changes in total liver PL levels among the four genotypes (Figure 4E). FFA were significantly elevated in GLS5 mice compared to GKO and 13
14 significantly lower in GLS3 compared to WT mice (Figure 4F). Despite increased hepatic FFA in GLS5, plasma total FFA levels did not differ between groups (data not shown). Hepatic oleate, but not palmitoleate, positively correlates with adiposity and liver triglycerides The results of the hepatic TG measurements suggested that hepatic oleate, but not palmitoleate, may partially explain variation in hepatic TG accumulation during lipogenic dietary conditions. We measured total hepatic oleate and palmitoleate in WT, GKO, GLS5 and GLS3 mice using gas liquid chromatography. Linear regression analyses of all animals revealed that total hepatic oleate levels significantly and directly correlated with hepatic TG (R 2 =0.60, Figure 5A) while there was no significant relationship between total hepatic palmitoleate and hepatic TG (R 2 =0.15, Figure 5B). Hepatic oleate (R 2 =0.70, Figure 5C) and hepatic palmitoleate (R 2 =0.53, Figure 5D) positively correlated with gonadal WAT weight. However, the correlation with hepatic palmitoleate was less robust and the WT group explained the relationship, as the removal of this group from the analysis resulted in complete loss of the correlation (R 2 =0.007) while the correlation between oleate and gonadal WAT remained significant even without the WT group. Hepatic oleate significantly correlated with the subcutaneous WAT depot (R 2 =0.50, Figure 5E) but palmitoleate did not (R 2 =0.19, Figure 5F). Taken together, these data suggest that the de novo synthesis of oleate, as compared to that of palmitoleate, is more potent in the positive regulation of lipid accumulation in liver and WAT in mice on a LD. Furthermore, data from the GLS5 group suggest that when hepatic MUFA are limited, even a modest increase in hepatic oleate availability is sufficient to induce a shift towards susceptibility to hepatic steatosis and increased adiposity. Hepatic de novo fatty acid synthesis is not restored in GLS5 or GLS3 mice fed a lipogenic diet Next we asked whether hepatic TG accumulation in response to LD in GLS5 mice was due to restoration of hepatic DNL. To address this question, we fed week old male mice 14
15 the LD diet for 10 days, performed intraperitoneal injections of 3 H-H 2 O and collected tissues 1 hour later to measure 3 H incorporation into de novo synthesized fatty acids and sterols. As would be predicted from the liver lipid analysis, GKO and GLS3 mice exhibited significantly reduced de novo fatty acid synthesis as compared to the WT rate (Figure 6A). Surprisingly, fatty acid synthesis in GLS5 mice was also low and did not differ from that of the GKO group (Figure 6A). Hepatic sterol synthesis was not significantly different among the four genotypes (data not shown). Consistent with the fatty acid synthesis results, linear regression analysis revealed that hepatic fatty acid synthesis was not correlated with liver TG (Figure 6B). We also measured relative expression levels of genes in the de novo fatty acid synthesis pathway (Acaca, Fasn, Elovl6) and consistent with the in vivo experiment, the relative mrna levels of these genes remained low in GKO, GLS5 and GLS3 mice relative to WT, although partially restored in GLS5 (Figure 6C). Taken together, these results indicate that hepatic DNL was not responsible for the restored hepatic lipid accumulation observed in GLS5 mice. Ex vivo hepatic fatty acid oxidation is not significantly changed in GLS5 or GLS3 mice In absence of an increase in hepatic DNL, another explanation for hepatic lipid accumulation is a defect in fatty acid oxidation. To probe whether differences in hepatic β- oxidation could in part explain the differential susceptibility to hepatic TG accumulation between the GLS5 and GLS3 transgenic models, we directly assessed hepatic mitochondrial fatty acid oxidation. We performed an ex vivo fatty acid oxidation experiment and incubated liver homogenates with 14 C-palmitate and measured 14 C-labeled CO 2 and acid soluble metabolites (ASM) 1 hour later. Total hepatic fatty acid oxidation was not different and there were no differences in CO 2 or ASM (data not shown), even though relative mrna levels of hepatic fatty acid oxidation-related genes (Cpt1α, Acadm, Acadl) revealed increased expression of all genes measured in GKO and GLS3 mice while only Cpt1α was increased in GLS5 mice relative to WT (Figure 6D). Regression analysis indicated that differences in liver TG accumulation are not 15
16 explained by hepatic fatty acid oxidation (R 2 =0.07, Figure 6E). Taken together these results show that TG accumulation is not associated with a defect in fatty acid oxidation. Liver MUFA synthesis influences plasma and WAT MUFA composition GKO mice are protected from LD-induced hypertriglyceridemia and we questioned whether liver-restored SCD transgenic mice would lose this protection. Plasma TG were partially restored in GLS5 and GLS3 mice, with an intermediate level between that of GKO and WT mice (Figure 6F). These data may suggest that altered hepatic MUFA production leads to changes in circulating lipids. Next we tested whether hepatic de novo synthesized oleate and palmitoleate influence plasma and WAT lipid composition. The secretion of TG-rich VLDL particles from the liver and subsequent lipolysis and fatty acid uptake by peripheral tissues is the primary mechanism by which hepatic derived lipids can influence lipid metabolism and fatty acid composition of extrahepatic tissues. We first analyzed the fatty acid composition of TG, CE, FFA and PL lipid fractions in liver tissue. Hepatic oleate was significantly increased in TG and PL species in GLS5 relative to GKO (Figure 7A) while palmitoleate was significantly increased in TG, CE and PL fractions in GLS3 mice compared to GKO (Figure 7A). The absolute mass of oleate in GLS5 mice was greater than the absolute mass of palmitoleate in GLS3 in every lipid fraction analyzed. We predicted that the hepatic MUFA composition would be reflected in circulating lipid species in plasma. Indeed, oleate was significantly increased by 2.5 fold in plasma TG in GLS5 mice relative to GKO (Figure 7B). Palmitoleate in the TG fraction was more dramatically changed, as it was increased in the GLS3 by more than 17 times the level measured in GKO (Figure 7B). Oleate and palmitoleate were also significantly increased as percent of total fatty acids in plasma TG in GLS5 and GLS3 mice, respectively (Supplemental Table 6). Oleate was not significantly changed in any other plasma lipid class in GLS5 mice although it was significantly reduced in PL of GLS3 mice (Figure 7B). Palmitoleate was significantly increased in plasma PL of GLS3 mice as compared to GKO but not altered in any fraction in GLS5 mice 16
17 (Figure 7B). These results show that changes in hepatic MUFA synthesis influence the composition of circulating lipids. The fatty acids contained in circulating VLDL particles that are taken up by adipose tissue are re-esterified into TG molecules, which are the major lipid species of adipose tissue. In the TG fraction of gonadal adipose tissue we detected significantly elevated oleate in GLS5 mice and significantly elevated palmitoleate in GLS3 mice (Figure 7C). These data show that hepatic MUFA production influences lipid composition of adipose tissue stores. See Tables S1- S9 in supplemental information for detailed fatty acid composition of liver, plasma and gonadal WAT lipids. White adipose tissue de novo lipogenesis is reduced in GLS5 mice Differences in adiposity among the SCD models could be explained by differences in WAT DNL and next we assessed in vivo lipid synthesis in the gonadal WAT depot. In this experiment the weight of the gonadal WAT depot was partially restored in both GLS5 and GLS3 to a level between that of the WT and GKO groups and the difference between GLS5 and GLS3 adiposity was also significant (Figure 8A). Unexpectedly, the rate of de novo fatty acid synthesis was dramatically and significantly increased in GKO and GLS3 mice despite lower adiposity (Figure 8B). De novo sterol synthesis followed the same pattern (Figure 8C). In contrast, GLS5 mice exhibited repressed adipose DNL that was significantly lower than GKO and was not different from WT (Figure 8B and 8C). Expression of major lipid metabolism regulators, including ChREBP (Mlxipl), SREBP-1c (Srebf1c), LXRα (Nr1h3) and PPARγ were not significantly changed among the genotypes (Figure 8D). Relative expression of genes encoding enzymes involved in de novo fatty acid (Fasn and Elovl6) and TG synthesis (Gpam, Dgat1, and Dgat2) largely were also not different among the models, with the exception of Gpam, which was elevated in both GLS5 and GLS3 mice (Figure 8D). There was a trend towards significance of Dgat2, with reduced expression in GKO. Expression of SREBP2 (Srebf2), the major transcriptional regulator of cholesterol synthesis, was not significantly changed but its target 17
18 Hmgcr was significantly increased in GKO relative to WT mice (Figure 8D), consistent with increased in vivo sterol synthesis. Taken together, the gene expression data suggest that differences in WAT DNL are unlikely to be transcriptionally mediated. Linear regression analyses revealed an inverse relationship between gonadal WAT fatty acid synthesis and liver TG (R 2 =0.26, Figure 8E), WAT weight (R 2 =0.31, Figure 8F) and body weight (R 2 =0.39, Figure 8G). The decrease in WAT DNL in GLS5 mice and increase in WAT DNL in GKO and GLS3 mice suggests that adipose DNL may be sensitive to changes in hepatic MUFA production. White adipose tissue fatty acid oxidation is reduced in GLS5 mice Given the differences in adiposity and unexpected DNL results, we next tested if WAT fatty acid oxidation was different among the models. We performed an ex vivo mitochondrial fatty acid oxidation experiment using gonadal WAT homogenates. Total fatty acid oxidation was significantly increased in GKO and GLS3 mice relative to WT mice (Figure 9A). The enhanced fatty acid oxidation of GKO was reversed in the GLS5 mice (Figure 9A). There was a trend towards increased CO 2 production in GKO and GLS3 mice, but this was not statistically significant (Figure 9B). Levels of 14 C-ASM were significantly different among the genotypes and followed the same pattern as total fatty acid oxidation (Figure 9C). We also performed an ex vivo experiment to assess WAT lipolysis. Glycerol release from gonadal WAT was not different among GKO, GLS5 and GLS3 and was elevated in all groups compared to WT mice (Figure 9D), indicating lipolysis was increased in all three groups. We next measured genes involved in fatty acid oxidation. There were no significant differences in gonadal WAT gene expression although the expression patterns of Acadm and Acadl largely mirrored the trend of the ex vivo fatty acid oxidation results (Figure 9E). The expression of Ucp1 was significantly induced GLS5 while Ucp2 was significantly induced in GKO mice (Figure 9E). Regression analysis revealed that adipose tissue fatty acid oxidation and liver TG accumulation are not correlated (Figure 9F). However, gonadal WAT fatty acid oxidation strongly and negatively correlated with gonadal WAT weight (R 2 =0.70, Figure 9G). The negative relationship between WAT fatty acid oxidation 18
19 and final body weight was also significant, although less robust (R 2 =0.20, Figure 9H). These results, taken together with the gonadal WAT DNL results and hepatic lipid data, suggest that hepatic de novo synthesized oleate influences hepatic TG accumulation, adiposity and is associated with adipose DNL and tissue fatty acid oxidation (Figure 10). Discussion This study follows-up on past work describing the role of hepatic SCD1 in mediating the metabolic effects of a LD (16, 27). Some phenotypes of GKO mice are recapitulated in LKO mice, such as protection from LD-induced liver steatosis and adipose tissue lipid accumulation (16). The focus of this study was to determine which product of hepatic SCD activity drives the phenotypes of increased lipid accumulation in liver and adipose tissue. We developed two novel mouse models where either oleate or palmitoleate production was restored in liver in the GKO background. We show that restoring liver synthesis of oleate, but not palmitoleate, rescues adaptation to LD feeding in GKO mice, blocking the prevention of ectopic lipid accumulation. Furthermore total hepatic oleate strongly correlated with total hepatic TG accumulation and with adipose tissue mass whereas total hepatic palmitoleate did not. These experiments show that a modest change in hepatic oleate synthesis can have dramatic whole-body metabolic effects. At the outset of the study we hypothesized that reduced hepatic de novo fatty acid synthesis accounted for the protection against lipid accumulation in LKO mice on the LD. However, data shown here demonstrate that despite increased hepatic oleate availability at the level produced in the liver of GLS5 mice, hepatic de novo fatty acid synthesis is not enhanced, indicating that the lipid accumulation is not driven by increased lipid synthesis. Our data also eliminate deficient hepatic fatty acid oxidation as the underlying cause of the steatosis phenotype, as there were no significant differences in hepatic β-oxidation among the GKO, GLS5 and GLS3 models. An alternate possible explanation for increased lipid stores in liver of GLS5 mice is based on studies of substrate preference of DGAT2. DGAT2 synthesizes TG from 19
20 diacylglycerol and prefers de novo synthesized fatty acids to fatty acids supplied in the diet (29, 30), and competition assays suggest that DGAT2 prefers oleate (31, 32). In this case, restoration of oleate could enhance flux through DGAT2. The proximity of SCD and DGAT2 in the ER membrane ensures channeling of de novo synthesized MUFA for esterification due to local enrichment at the ER membrane. This could explain how the modest restoration of stearate desaturation in the GLS5 mice is sufficient to drive a 40% enrichment in oleate in liver fatty acid composition and an increase in total TG accumulation. Relative to GKO mice, GLS5 restored liver steatosis and expanded the adipose tissue mass. This suggests that liver MUFA synthesis may be involved in the regulation of lipid homeostasis outside of the liver. Although changes in liver MUFA composition of the transgenic models were reflected in the plasma TG fatty acid composition, plasma lipid load may drive the adiposity phenotypes in the GKO and transgenic models, in which plasma TG and adipose tissue weights were low in GKO and intermediate in GLS5 and GLS3 mice. An alternate explanation is that lipid fatty acid composition influences the metabolic response in adipose tissue. In analyzing lipid metabolism in expanded and non-responsive adipose tissue, we unexpectedly determined that the two models with the lowest adipose tissue mass had the highest rates of DNL, with fatty acid synthesis 25- and 10-fold higher in WAT of GKO and GLS3, respectively, as compared to WT mice. These results demonstrate that increased WAT mass is not predictive of increased DNL and that increases in DNL do not necessarily direct increased adiposity. Regression analyses showed that adipose tissue fatty acid synthesis was negatively correlated with liver TG, gonadal WAT weight and total body weight. These findings are consistent with the concept that the ability to upregulate WAT DNL is metabolically beneficial in the context of a LD. In clinical studies, decreased lipogenic gene expression has been reported in obese human subjects, which correlated inversely with markers of insulin resistance and hepatic steatosis (33). Eissing et al. also demonstrated that in subjects who underwent bariatric 20
21 surgery, increased lipogenic gene expression in WAT was associated with weight loss and improved glucose homeostasis post-surgery (33). It is not unprecedented for WAT DNL to be increased when liver DNL is low. Independent models of altered lipid metabolism have demonstrated a relationship between liver DNL and WAT DNL, with reduced hepatic DNL accompanied by upregulated adipose DNL, likely as a compensatory response (34, 35). The apparent incongruity of reduced adiposity with increased DNL in GKO and GLS3 mice is explained in part by increased rate of fatty acid oxidation. Regression analysis showed a strong negative correlation between fatty acid oxidation in WAT and gonadal adipose tissue weight, explaining up to 70% of the weight variation. This suggests that the oxidation of fatty acids in adipose tissue exerts control over total adiposity. In contrast, regression analysis showed adipose tissue DNL was negatively associated with hepatic lipid accumulation. Taken together these results reveal that lipid storage homeostasis is associated with the metabolic status of adipose tissue. Potential mechanisms by which hepatic de novo synthesized oleate exerts systemic effects to regulate specific metabolic pathways in WAT remain unknown. One possibility is that oleate itself or a more complex lipid that it is incorporated into exerts transcriptional regulation over genes in the β-oxidation or DNL pathways via regulation of transcription factors. Indeed, fatty acid-mediated regulation of transcription factors has been well described (36). However, we previously demonstrated that in SCD1 GKO the protection from hepatic steatosis and increased adiposity is not meditated through peroxisome proliferator-activated receptor-α (PPARα), a transcription factor that exerts significant control over fatty acid oxidation (37) and the results of the current study do not support a transcriptional mechanism. Another possibility is that oleate or a more complex lipid directly inhibits enzymes in the β-oxidation or DNL pathways. MUFA have been shown to be required for a number of specific protein interactions that elicit a broad range of biological effects. For example, Liu et al. recently demonstrated that 21
22 endogenous, but not exogenous, oleate and palmitoleate inhibit the activity of fatty acid amide hydrolase, an enzyme that degrades endocannanbinoids, and explains the mechanism through which endocannanbinoids decrease insulin sensitivity (38). Activation of Wnt proteins requires them to first become acylated with palmitoleate (39), while oleate is a required component of specific PL species that activate PPARα in liver (40) and muscle (41). Perhaps enzymes in the fatty acid oxidation or synthesis pathways distinguish between oleate and palmitoleate and are regulated by the former but not the latter. However, it should also be emphasized that the dramatic metabolic changes in WAT in this study were in SCD1-deficient tissue, as GKO, GLS5, and GLS3 mice were all SCD1 global knockouts. Therefore, the effects of oleate on metabolic regulation should be further tested in adipocytes with WT SCD1expression. Recent reports support a role for palmitoleate in maintenance of metabolic health. In mice, Cao et al. showed that circulating palmitoleate synthesized in WAT acts as a lipokine in peripheral tissues, suppressing hepatic lipogenesis and increasing skeletal muscle insulin sensitivity (42). Parallel independent studies showed that palmitoleate supplementation increases insulin sensitivity (43, 44), although consensus on its effect on hepatic lipid accumulation is lacking. In humans, the role of plasma palmitoleate is less clear. Palmitoleate positively correlates with insulin sensitivity in overweight subjects (45), but the association is not observed in obese adults or children (46, 47). Although hepatic palmitoleate synthesis is restored in GLS3 mice, GKO mice are already dramatically protected against diet-induced metabolic derangements, and further benefits of increased palmitoleate were not observed. A striking result of the current study was the significant increase in total food intake in LD-fed GLS5 mice as compared to WT mice. With increased adiposity one could expect plasma leptin levels to be increased in GLS5 mice relative to GKO mice, and that in turn would be predicted to reduce food intake. In this study, plasma leptin in GLS5 was reduced compared to WT and not different from GKO while food intake was enhanced for GLS5 only, separating adiposity and food intake from absolute plasma leptin levels. A potential explanation for the 22
23 similar body weights in GLS5 and WT mice despite the increased food intake in GLS5 is an induction of uncoupling. Indeed, we observed significantly induced Ucp1 expression in WAT of GLS5 mice, which may suggest energy dissipation is increased in these mice. The possibility that the absolute mass of oleate is driving the phenotypes in this study cannot be excluded based on the current study. However, it is unlikely that palmitoleate levels would reach oleate levels as the metabolic fates of these fatty acids are not equivalent and conversion to elongated species is not equivalent. In general the elongation of the product of SCD5, 18:1n-9, to 20:1n-9 is minimal and 20:1n-9 accumulates to approximately 2% that of 18:1n-9. In contrast, the elongation of the product of SCD3, 16:1n-7, to 18:1n-7 readily occurs and approximately equal levels of these two n-7 fatty acids are present in the liver. In GLS5 mice, levels of 18:1n-9 were restored to 50% of WT levels, whereas in GLS3 mice, levels of 16:1n-7 reached only 20% of WT levels. Given the differences in metabolic fate, the 20% increase in 16:1n-7 detecting in the GLS3 liver likely underestimates the increase in its rate of de novo synthesis. We have identified a model of lipid metabolism homeostasis involving communication between liver and adipose tissue stores. Importantly, this work reveals that lipid metabolism and lipid storage homeostasis are both sensitive to hepatic oleate production, where one of the major products of SCD activity can influence the balance of storage and utilization in adipose tissue. However, additional work will be required to understand whether these are direct or indirect effects of hepatic oleate. An understanding of factors that regulate the balance of lipid storage among adipose depots and in hepatic tissue is relevant to human health. The degree of risk for metabolic impairment is dependent on the location of ectopic lipid stores, where fatty liver is associated with poorer outcomes than expanded adipose depots. Our studies suggest that an imbalance in lipid stores may be corrected through interventions targeting SCD activities in liver. 23
24 Acknowledgements We thank Dr. Makoto Miyazaki for design and development of the transgenic mouse lines. We also thank Laura Vanderploeg in the UW-Madison Biochemistry Department Media Center and Mary Cantu for assistance with design and production of figures. We thank Dr. Tom Pugh for assistance with liver histology. This work was supported by NIH R01 DK062388, ADA 7-13-BS-118 and USDA Hatch W2005 (to J.M.N.) and NIH RO1 AG (to R.M.A.). M.S.B was supported by NIH predoctoral training grant (T32 DK ), M.T.F was supported by NIH postdoctoral training grant (T32 DK ) and an American Heart Association postdoctoral fellowship, L.M.B. was supported by NIH National Research Service Award (T32 GM07215). References 1. Flegal KM, Carroll MD, Kit BK, Ogden CL. Prevalence of obesity and trends in the distribution of body mass index among US adults, JAMA 2012;307: Nguyen D, El-Serag HB. The epidemiology of obesity. Gastroenterol. Clin. North Am. 2010;39: Moore JB. Non-alcoholic fatty liver disease: the hepatic consequence of obesity and the metabolic syndrome. Proc. Nutr. Soc. 2010;69: Cohen JC, Horton JD, Hobbs HH. Human fatty liver disease: old questions and new insights. Science. 2011;332: Birkenfeld AL, Shulman GI. Nonalcoholic fatty liver disease, hepatic insulin resistance and type 2 diabetes. Hepatology. 2014;59: Aarsland A, Chinkes D, Wolfe R. Hepatic and whole-body fat synthesis in humans during carbohydrate overfeeding. Am. J. Clin. Nutr. 1997;65:
25 7. Hellerstein MK, Schwarz JM, Neese RA. Regulation of hepatic de novo lipogenesis in humans. Annu. Rev. Nutr. 1996;16: Stanhope KL, Schwarts JM, Keim NL, Griffen SC, Bremer AA, Graham JL, Hatcher B, Cox CL, Dyachenko A, Zhang W, McGahan JP, Seibert A, Karuss RM, Chiu S, Schaefer EJ, Ai M, Otokozawa S, Nakajima K, Nakano T, Beysen C, Hellerstein MK, Berglund L, Havel PJ. Consuming fructose-sweetened, not glucose-sweetened, beverages increases visceral adiposity and lipids and decreases insulin sensitivity in overweight/obese humans. J. Clin. Invest. 2009;119: Vos MB, Lavine JE. Dietary fructose in nonalcoholic fatty liver disease. Hepatology. 2013;57: Donnelly KL, Smith CI, Schwarzenberg SJ, Jessurun J, Boldt MD, Parks EJ. Sources of fatty acids stored in liver and secreted via lipoproteins in patients with nonalcoholic fatty liver disease. J. Clin. Invest. 2005;115: Lodhi IJ, Wei X, Semenkovich CF. Lipoexpediency: de novo lipogenesis as a metabolic signal transmitter. Trends Endocrinol. Metab. 2011;22: Hulver MW, Berggren JR, Carper MJ, Miyazaki M, Ntambi JM, Hoffman EP, Thyfault JP, Stevens R, Dohm GL, Houmard JA, Muoio DM. Elevated stearoyl-coa desaturase-1 expression in skeletal muscle contributes to abnormal fatty acid partitioning in obese humans. Cell Metab. 2005;4: Attie AD, Krauss RM, Gray-Keller MP, Brownlie A, Miyazaki M, Kastelein JJ, Lusis AJ, Stalenhoef 25
26 AF, Stoehr JP, Hayden MR, Ntambi JM. Relationship between stearoyl-coa desaturase activity and plasma triglycerides in human and mouse hypertriglyceridemia. J. Lipid Res. 2002;43: Warensjö E, Ingelsson E, Lundmark P, Lannfelt L, Syvanen AC, Vessby B, Riserus U. Polymorphisms in the SCD1 gene: associations with body fat distribution and insulin sensitivity. Obesity. 2007;15: Ntambi JM, Miyazaki M, Stoehr JP, Lan H, Kendziorski CM, Yandell BS, Song Y, Cohen P, Friedman JM, Attie AD. Loss of stearoyl CoA desaturase-1 function protects mice against adiposity. Proc. Natl. Acad. Sci. USA. 2002;99: Miyazaki M, Flowers MT, Sampath H, Chu K, Otzelberger C, Liu X, Ntambi JM. Hepatic stearoyl- CoA desaturase-1 deficiency protects mice from carbohydrate-induced adiposity and hepatic steatosis. Cell Metab. 2007;6: Zheng Y, Prouty SM, Harmon A, Sundberg JP, Stenn KS, Parimoo S. Scd3 a novel gene of the stearoyl-coa desaturase family with restricted expression in skin. Genomics. 71; Miyazaki, M., H. J. Kim, W. C. Man, and J. M. Ntambi Oleoyl-CoA is the major de novo product of stearoyl-coa desaturase 1 gene isoform and substrate for the biosynthesis of the Harderian gland 1-alkyl-2,3-diacylglycerol. J. Biol. Chem. 276: Miyazaki, M., F. E. Gomez, and J. M. Ntambi Lack of stearoyl-coa desaturase-1 function induces a palmitoyl-coa Delta6 desaturase and represses the stearoyl-coa desaturase-3 gene in the preputial glands of the mouse. J. Lipid Res. 43: Simonet WS, Bucay N, Lauer SJ, Taylor JM. A far-downstream hepatocyte-specific control 26
27 region directs expression of the linked human apolipoprotein E and C-I genes in transgenic mice. J. Biol. Chem. 1993;268: Flowers MT, Ade L, Strable MS, Ntambi JM. Combined deletion of SCD1 from adipose tissue and liver does not protect mice from obesity. J. Lipid Res. 2012;53: Folch J, Lees M, Stanley GHS. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957;226: Koopman R, Schaart G, Hesselink M. Optimization of oil red O staining permits combination with immunofluorescence and automated quantification of lipids. Histochem. Cell Biol. 2001;116: Jeske DJ, Dietschy JM. Regulation of rates of cholesterol synthesis in vivo in the liver and carcass of the rat measured using [3H]water. J. Lipid Res. 1980;21: Hirschey MD, Shimazu T, Goetzman E, Jing E, Schwer B, Lombard DB, Grueter CA, Harris C, Biddinger S, Ilkayeva OR, Stevens RD, Li Y, Saha AK, Ruderman NB, Bain JR, Newgard CB, Farese RV Jr, Alt FW, Kahn CR, Verdin E. SIRT3 regulates mitochondrial fatty-acid oxidation by reversible enzyme deacetylation. Nature. 2010;464: Sampath H, Flowers MT, Liu X, Paton CM, Sullivan R, Chu K, Zhao M, Ntambi JM. Skin-specific deletion of stearoyl-coa desaturase-1 alters skin lipid composition and protects mice from high fat diet-induced obesity. J. Biol. Chem. 2009;284: Flowers MT, Groen AK, Oler TA, Keller MP, Choi Y, Schueler KL, Richards OC, Lan H, Miyazaki M, Kuipers F, Kendziorski CM, Ntambi JM, Attie AD. Cholestasis and hypercholesterolemia in SCD1-deficient mice fed a low-fat, high-carbohydrate diet. J. Lipid Res. 2006;47:
28 28. Rahman SM, Dobrzyn A, Dobrzyn P, Lee SH, Miyazaki M, Ntambi JM Stearoyl-CoA desaturase 1 deficiency elevates insulin-signaling components and down-regulates protein-tyrosine phosphatase 1B in muscle. Proc. Natl. Acad. Sci. U.S.A. 2003;100: Yen C-LE, Stone SJ, Koliwad S, Harris C, Farese RV. Thematic review series: glycerolipids. DGAT enzymes and triacylglycerol biosynthesis. J. Lipid Res. 2008;49: Wurie HR, Buckett L, Zammit VA. Diacylglycerol acyltransferase 2 acts upstream of diacylglycerol acyltransferase 1 and utilizes nascent diglycerides and de novo synthesized fatty acids in HepG2 cells. FEBS J. 2012;279: Man WC, Miyazaki M, Chu K, Ntambi J. Colocalization of SCD1 and DGAT2: implying preference for endogenous monounsaturated fatty acids in triglyceride synthesis. J. Lipid Res. 2006;47: Cases S, Stone SJ, Zhou P, Yen E, Tow B, Lardizabel KD, Voelker T, Farese RV Jr. Cloning of DGAT2, a second mammalian diacylglycerol acyltransferase, and related family members. J. Biol. Chem. 2001;276: Eissing L, Scherer T, Todter K, Knippschild U, Greve JW, Buurman WA, Pinnschmidt HO, Rensen SS, Wolf AM, Bartelt A, Heeren J, Buettner C, Scheja L. De novo lipogenesis in human fat and liver is linked to ChREBP-β and metabolic health. Nat. Commun. 2013;4: Beaven SW, Matveyenko A, Wroblewski K, Chao L, Wilpitz D, Hsu TW, Lentz J, Drew B, Hevener AL, Tontonoz P. Reciprocal regulation of hepatic and adipose lipogenesis by liver x receptors in obesity and insulin resistance. Cell Metab. 2013;18:
29 35. Kuriyama H, Liang G, Engelking LJ, Horton JD, Goldstein JL, Brown MS. Compensatory increase in fatty acid synthesis in adipose tissue of mice with conditional deficiency of SCAP in liver. Cell Metab. 2005;1: Jump DB, Tripathy S, Depner CM. Fatty acid-regulated transcription factors in the liver. Annu. Rev. Nutr. 2013;33: Miyazaki M, Dobrzyn A, Sampath H, Lee SH, Man WC, Chu K, Peters JM, Gonzales FJ, Ntambi JM. Reduced adiposity and liver steatosis by stearoyl-coa desaturase deficiency are independent of peroxisome proliferator-activated receptor-alpha. J. Biol. Chem. 2004;279: Liu J, Cinar R, Xiong K,Godlewski G, Jourdan T, Lin Y, Ntambi JM, Kunos G. Monounsaturated fatty acids generated via stearoyl CoA desaturase-1 are endogenous inhibitors of fatty acid amide hydrolase. Proc. Natl. Acad. Sci. U.S.A. 2013;110: Rios-Esteves J, Resh MD. Stearoyl CoA desaturase is required to produce active, lipid-modified Wnt proteins. Cell Rep. 2013;4: Chakravarthy MV, Lodhi IJ, Yin L, Malapaka RR, Xu HE, Turk J, Semenkovich CF. Identification of a physiologically relevant endogenous ligand for PPARalpha in liver. Cell. 2009;138: Liu S, brown JD, Stanya KJ, Homan E, Leidl M, Inouye K, Bhargava P, Gangl MR, Dai L, Hatano B, Hotamisligil GS, Saghatelian A, Plutzky J, Lee CH. A diurnal serum lipid integrates hepatic lipogenesis and peripheral fatty acid use. Nature. 2013;502: Cao H, Gerhold K, Mayers JR, Wiest MM, Watkins SM, Hotamisligil GS. Identification of a lipokine, a lipid hormone linking adipose tissue to systemic metabolism. Cell. 2008;134:
30 43. Yang Z-H, Miyahara H, Hatanaka A. Chronic administration of palmitoleic acid reduces insulin resistance and hepatic lipid accumulation in KK-Ay Mice with genetic type 2 diabetes. Lipids Health Dis. 2011;10: Guo X, Li H, Xu H, Halim V, Zhang W, Wang H, Ong KT, Woo SL, Walzem RL, Mashek DG, Dong H, Lu F, Wei L, Huo Y, Wu C. Palmitoleate induces hepatic steatosis but suppresses liver inflammatory response in mice. PLoS One. 2012;7:e Arregui M. Buijsse B, Stefan N, Corella D, Fisher E, di Giuseppe R, Coltell O, Knuppel S, Aleksandrova K, Joost HG, Boeing H, Weikert C. Heterogeneity of the stearoyl-coa desaturase-1 (SCD1) gene and metabolic risk factors in the EPIC-Potsdam study. PLoS One. 2012;7:e Fabbrini E, Magkos F, Su X, Abumrad NA, Nejedly N, Coughlin CC, Okunade AL, Patterson BW, Klein S. Insulin sensitivity is not associated with palmitoleate availability in obese humans. J. Lipid Res. 2011;52: Okada T, Furuhashi N, Kuromori Y, Miyashita M, Iwata F, Harada K. Plasma palmitoleic acid content and obesity in children. Am. J. Clin. Nutr. 2005;82:
31 Figure legends Figure 1. Generation and characterization of SCD5 and SCD3 liver-specific transgenic mice. (A) Monounsaturated fatty acids oleate (18:1n-9) and palmitoleate (16:1n-7) are synthesized by human SCD5 and mouse SCD3, respectively, from saturated fatty acid precursors. (B) Construct design for the generation of human SCD5 and mouse Scd3 transgenes (Tg) driven by the human APOE gene promoter. (C) Immunoblot analysis of HA-tagged SCD5 and SCD3 microsomal protein in liver, kidney, white adipose tissue (WAT), brown adipose tissue (BAT), heart and skin tissue in chow-fed SCD5Tg-, SCD5Tg+, SCD3Tg- and SCD3Tg+ mice in C57Bl/6 background. Transferrin receptor 1 (TfR1) used as loading control. (D G) 10 week old male mice were fed a lipogenic diet for 10 days (n=5 6/group) and fasted for 4 hours prior to tissue collection. (D) Relative mrna levels of SCD5 and Scd3 in liver tissue. The mean cycle threshold value for the transgenic group for each gene is indicated. (E) Immunoblot analysis of mouse SCD1 and transgenic protein expression in liver microsomes of WT, GKO and GLS5 and GLS3 mice. TfR1 and GAPDH used as loading controls. (F) SCD activity in liver microsomes assessed by desaturation of 3 H-(9,10)-stearoyl-CoA (18:0) and 3 H-(9,10)-palmitoyl-CoA (16:0). Data are presented as mean percentage of WT activity level for each substrate. (G) Relative total (sum of levels in TG, CE, FFA and PL fractions as reported in Tables S1-S4) hepatic 18:1n-9 and 16:1n-7 levels measured by gas liquid chromatography. Data are presented as percentage of WT fatty acid level. GKO, SCD1 global knockout; GLS5, GKO Liver-specific SCD5 transgenic; GLS3, GKO Liver-specific SCD3 transgenic. Values are mean ± SEM, *P < 0.05 vs. GKO; one-way ANOVA analysis with Tukey s post-hoc test. Figure 2. Body weight and adiposity are rescued in GLS5 mice fed a lipogenic diet. 31
32 10 week old male mice (n=4 6/group) were fed a lipogenic diet for 10 days. Food was measured at the start and end of the feeding study. (A) Body weight and (B) gonadal and subcutaneous WAT weight were measured at the end of the 10 day feeding study. (C) Total food intake was determined. (D) 4 hour fasted plasma leptin values were measured using ELISA (n=6 7/group). (E) Plasma FGF21 values were measured using ELISA from 4 hour fasted mice. Values are mean ± SEM, *P < 0.05 vs. WT, # P < 0.05 vs. GKO; one-way ANOVA analysis with Tukey s post-hoc test. Figure 3. Blood glucose is increased in GLS5 mice fed a lipogenic diet. 10 week old male mice were fed a lipogenic diet for 10 days. (A and C) Blood was collected retro-orbitally from 4 hour fasted mice (n=4 6/group). (A) Glucose was determined using an enzymatic colorimetric assay. (B) Relative hepatic mrna levels of gluconeogenic genes Pck1 and G6pc were measured. (C) Plasma insulin levels were measured using ELISA in samples collected as described for (A). (D) Glucose tolerance tests were performed in 4 hour fasted male mice. Mice were intraperitoneally injected with 2 g/kg body weight glucose and blood glucose was measured using a glucometer at 0, 30, 60, 90 and 120 minutes post-injection. Values are mean ± SEM, *P < 0.05 vs. WT, # P < 0.05 vs. GKO; one-way ANOVA analysis with Tukey s post-hoc test. Figure 4. Hepatic lipid accumulation is restored in male GLS5 mice. 10 week old male mice were fed a lipogenic diet for 10 days and 4 hour fasted prior to tissue collection (n=4 6/group). (A) Hepatic TG were extracted from liver tissue and mass was determined by biochemical assay. (B) Liver sections from WT, GKO, GLS5 and GLS3 mice were formalin fixed, cryosectioned and neutral lipids were stained with oil red O and imaged with a 40x objective. (C F) Gas liquid chromatography was used to measure total fatty acids in hepatic lipid fractions of (C) Triglycerides, (D) Cholesteryl Esters (CE), (E) Phospholipids, and (F) Free Fatty Acids. Values 32
33 are mean ± SEM, *P < 0.05 vs. WT, # P < 0.05 vs. GKO; ^P < 0.05 vs. GLS5; one-way ANOVA analysis with Tukey s post-hoc test. Figure 5. Hepatic oleate mass correlates with liver TG and adiposity. Total hepatic oleate and palmitoleate concentrations in WT, GKO, GLS5 and GLS3 male mice fed a lipogenic diet for 10 days were measured by gas liquid chromatography. (A) Hepatic oleate significantly correlates with liver TG accumulation while (B) total hepatic palmitoleate mass does not correlate with liver TG. (C) Total hepatic oleate levels strongly correlated with gonadal WAT and (D) total hepatic palmitoleate correlated with gonadal WAT. (E) Hepatic oleate correlated with subcutaneous WAT weights but (F) total hepatic palmitoleate did not correlate with subcutaneous WAT. Data were analyzed using linear regression analysis, n=19. Figure 6. Hepatic DNL and fatty acid oxidation are not changed in GLS5 and GLS3 mice fed a lipogenic diet. (A) Hepatic de novo lipogenesis in adult week old male mice fed a LD for 10 days. Mice were fasted for 2 hours and injected intraperitoneally with 50 mci 3 H-H 2 0 and tissues were collected 1 hour post-injection. Lipids were saponified and separated by TLC (n=4 6/group). De novo fatty acid synthesis was determined by measuring 3 H incorporation into fatty acids by liquid scintillation. (B) Hepatic de novo fatty acid synthesis was not significantly correlated with liver TG mass (n=19). (C) Relative expression levels of genes encoding for enzymes involved in de novo fatty acid synthesis were measured in livers of 4 hour fasted 10- day LD-fed male mice. (D) Fatty acid oxidation genes were measured in livers of 4 hour fasted 10-day LD-fed male mice (n=4 6/group). (E) Hepatic fatty acid oxidation was not correlated with liver TG (n=19). (F) Plasma TG were measured in 4 hour fasted mice fed the LD for 10 days (n=4 6/group). Values are mean ± SEM, *P < 0.05 vs. WT, ^P < 0.05 vs. GLS5; one-way ANOVA analysis with Tukey s post-hoc test (A, C, D, F), linear regression analysis (B and E). 33
34 Figure 7. Hepatic monounsaturated fatty acid synthesis influences plasma and gonadal WAT fatty acid composition. 10 week old male WT, GKO, GLS5 and GLS3 mice were fed a lipogenic diet for 10 days (n=4 6/group) and fasted 4 hours prior to tissue collection. Gas liquid chromatography was used to analyze oleate (18:1n-9) and palmitoleate (16:1n-7) fatty acid composition of (A) liver, (B) plasma and (C) gonadal WAT lipids. Total lipids were extracted from tissues, lipid classes were separated by TLC and triglyceride (TG), cholesteryl ester (CE), free fatty acid (FFA) and phospholipid (PL) fractions were analyzed in liver and plasma and TG was analyzed in gonadal WAT. All data are presented on a fatty acid concentration basis as µg/mg tissue or µg/100 µl plasma with the exception of plasma PL data, which are expressed as percent of total fatty acids. See supplementary information for complete fatty acid analysis results of these tissues. Values are mean ± SEM, *P < 0.05 vs. WT; # P < 0.05 vs. GKO; ^P < 0.05 vs. GLS5; one-way ANOVA analysis with Tukey s post-hoc test. Figure 8. De novo lipogenesis is repressed in gonadal WAT of GLS5 mice and negatively correlates with lipid accumulation and adiposity. De novo lipogenesis of gonadal WAT (n=4 6/group) in week old mice. (A) Mean gonadal WAT weights. Lipid synthesis was determined by measuring the incorporation of 3 H-H 2 O into (B) fatty acids and (C) sterols. The experimental procedure and mice are as described in the caption of Figure 6. (D) Relative expression levels of genes encoding for enzymes involved in de novo fatty acid and TG synthesis were measured in gonadal WAT of 4 hour fasted male mice fed a LD for 10 days (n=5 6/group). (E G) Gonadal WAT fatty acid synthesis was significantly and negatively correlated with (E) liver TG mass (R 2 =0.26), (F) gonadal WAT weight (R 2 =0.31) and (G) body weight (R 2 =0.39), (n=19). Values are mean ± SEM, *P < 0.05 vs. WT, # P < 0.05 vs. GKO; ^P < 0.05 vs. GLS5; one-way ANOVA analysis with Tukey s post-hoc test (A D), and linear regression analysis (E G). 34
35 Figure 9. Fatty acid oxidation in gonadal WAT is repressed in GLS5 mice week old male mice were fed a lipogenic diet for 10 days. Mice were fasted 4 hours prior to tissue collection (n=5 6/group). 300 µm palmitate with 0.4 µci 1-14 C-palmitate was incubated with WAT homogenates for 1 hour. (A) Total fatty acid oxidation in gonadal WAT represents the sum of nmol of palmitate oxidized to CO 2 and ASM. Fatty acid oxidation was assessed and 14 C- enriched (B) CO 2 and (C) acid-soluble metabolites (ASM) levels were measured using liquid scintillation. (D) Free glycerol was measured in gonadal WAT of male mice (n=6 9/group). (E) Relative mrna levels of fatty acid oxidation-related and uncoupling genes were measured in gonadal WAT of 4 h fasted 10-day LD fed male mice (n=5 6/group). (F) WAT fatty acid oxidation was not correlated with liver TG accumulation (n=22). (G) WAT fatty acid oxidation was highly and negatively correlated (R 2 =0.70) with gonadal WAT weight and (H) WAT fatty acid oxidation was negatively correlated with body weight (R 2 =0.20) (n=22). Values are mean ± SEM, *P < 0.05 vs. WT, ^P < 0.05 vs. GLS5; one-way ANOVA analysis with Tukey s post-hoc test (A E) and linear regression analysis (F H). Figure 10. Proposed model of the metabolic effects of de novo synthesized hepatic 18:1n-9 within the context of a lipogenic diet. (A) A lipogenic, high-carbohydrate diet stimulates hepatic SCD activity, causing an increase in the de novo synthesis of 18:1n-9 and subsequent hepatic TG accumulation. Hepatic 18:1n-9 becomes available to peripheral tissues through circulating 18:1n-9-containing TG-rich VLDL particles. In gonadal WAT there is a net effect of increased adiposity and the hepatic-derived 18:1n-9 is associated with repression of the de novo lipogenesis (DNL) and fatty acid oxidation (FAO) pathways. (B) However, when hepatic SCD activity is reduced, there is a reduction in de novo synthesized hepatic 18:1n-9 and a lipogenic diet fails to induce hepatic lipid accumulation. Plasma TG 18:1n-9 content and peripheral tissue 18:1n-9 uptake are both reduced with a net effect of reduced adiposity. With decreased 18:1n-9, fatty acid synthesis and oxidation pathways are activated. 35
36 Figure 1 36
37 Figure 2 37
38 Figure 3 38
39 Figure 4 39
Gene Polymorphisms and Carbohydrate Diets. James M. Ntambi Ph.D
 Gene Polymorphisms and Carbohydrate Diets James M. Ntambi Ph.D Fatty Acids that Flux into Tissue Lipids are from Dietary Sources or are Made De novo from Glucose or Fructose Glucose Fructose Acetyl-CoA
Gene Polymorphisms and Carbohydrate Diets James M. Ntambi Ph.D Fatty Acids that Flux into Tissue Lipids are from Dietary Sources or are Made De novo from Glucose or Fructose Glucose Fructose Acetyl-CoA
SUPPLEMENTARY INFORMATION
 SUPPLEMENTARY INFORMATION FOR Liver X Receptor α mediates hepatic triglyceride accumulation through upregulation of G0/G1 Switch Gene 2 (G0S2) expression I: SUPPLEMENTARY METHODS II: SUPPLEMENTARY FIGURES
SUPPLEMENTARY INFORMATION FOR Liver X Receptor α mediates hepatic triglyceride accumulation through upregulation of G0/G1 Switch Gene 2 (G0S2) expression I: SUPPLEMENTARY METHODS II: SUPPLEMENTARY FIGURES
General Laboratory methods Plasma analysis: Gene Expression Analysis: Immunoblot analysis: Immunohistochemistry:
 General Laboratory methods Plasma analysis: Plasma insulin (Mercodia, Sweden), leptin (duoset, R&D Systems Europe, Abingdon, United Kingdom), IL-6, TNFα and adiponectin levels (Quantikine kits, R&D Systems
General Laboratory methods Plasma analysis: Plasma insulin (Mercodia, Sweden), leptin (duoset, R&D Systems Europe, Abingdon, United Kingdom), IL-6, TNFα and adiponectin levels (Quantikine kits, R&D Systems
ANSC/NUTR 601 GENERAL ANIMAL NUTRITION Stearoyl-CoA desaturase, VLDL metabolism, and obesity
 ANSC/NUTR 601 GENERAL ANIMAL NUTRITION Stearoyl-CoA desaturase, VLDL metabolism, and obesity I. Stearoyl coenzyme A desaturase and obesity in rodents A. Stearoyl coenzyme A desaturase (SCD) is the 9 desaturase.
ANSC/NUTR 601 GENERAL ANIMAL NUTRITION Stearoyl-CoA desaturase, VLDL metabolism, and obesity I. Stearoyl coenzyme A desaturase and obesity in rodents A. Stearoyl coenzyme A desaturase (SCD) is the 9 desaturase.
control kda ATGL ATGLi HSL 82 GAPDH * ** *** WT/cTg WT/cTg ATGLi AKO/cTg AKO/cTg ATGLi WT/cTg WT/cTg ATGLi AKO/cTg AKO/cTg ATGLi iwat gwat ibat
 body weight (g) tissue weights (mg) ATGL protein expression (relative to GAPDH) HSL protein expression (relative to GAPDH) ### # # kda ATGL 55 HSL 82 GAPDH 37 2.5 2. 1.5 1..5 2. 1.5 1..5.. Supplementary
body weight (g) tissue weights (mg) ATGL protein expression (relative to GAPDH) HSL protein expression (relative to GAPDH) ### # # kda ATGL 55 HSL 82 GAPDH 37 2.5 2. 1.5 1..5 2. 1.5 1..5.. Supplementary
GPR120 *** * * Liver BAT iwat ewat mwat Ileum Colon. UCP1 mrna ***
 a GPR120 GPR120 mrna/ppia mrna Arbitrary Units 150 100 50 Liver BAT iwat ewat mwat Ileum Colon b UCP1 mrna Fold induction 20 15 10 5 - camp camp SB202190 - - - H89 - - - - - GW7647 Supplementary Figure
a GPR120 GPR120 mrna/ppia mrna Arbitrary Units 150 100 50 Liver BAT iwat ewat mwat Ileum Colon b UCP1 mrna Fold induction 20 15 10 5 - camp camp SB202190 - - - H89 - - - - - GW7647 Supplementary Figure
AAV-TBGp-Cre treatment resulted in hepatocyte-specific GH receptor gene recombination
 AAV-TBGp-Cre treatment resulted in hepatocyte-specific GH receptor gene recombination Supplementary Figure 1. Generation of the adult-onset, liver-specific GH receptor knock-down (alivghrkd, Kd) mouse
AAV-TBGp-Cre treatment resulted in hepatocyte-specific GH receptor gene recombination Supplementary Figure 1. Generation of the adult-onset, liver-specific GH receptor knock-down (alivghrkd, Kd) mouse
The art of tracing dietary fat in humans. Leanne Hodson
 The art of tracing dietary fat in humans Leanne Hodson Dietary fat Other lipoproteins: IDL, LDL, HDL Hodson and Fielding linical Lipidology (2010) Relationship between blood & dietary fatty acids Typically:
The art of tracing dietary fat in humans Leanne Hodson Dietary fat Other lipoproteins: IDL, LDL, HDL Hodson and Fielding linical Lipidology (2010) Relationship between blood & dietary fatty acids Typically:
SUPPLEMENTARY INFORMATION
 doi:10.1038/nature12652 Supplementary Figure 1. PRDM16 interacts with endogenous EHMT1 in brown adipocytes. Immunoprecipitation of PRDM16 complex by flag antibody (M2) followed by Western blot analysis
doi:10.1038/nature12652 Supplementary Figure 1. PRDM16 interacts with endogenous EHMT1 in brown adipocytes. Immunoprecipitation of PRDM16 complex by flag antibody (M2) followed by Western blot analysis
Hmgcoar AGCTTGCCCGAATTGTATGTG TCTGTTGTAACCATGTGACTTC. Cyp7α GGGATTGCTGTGGTAGTGAGC GGTATGGAATCAACCCGTTGTC
 Supplement Table I: primers for Real Time RT-PCR Gene Foward Reverse Hmgcoar AGCTTGCCCGAATTGTATGTG TCTGTTGTAACCATGTGACTTC Cyp7α GGGATTGCTGTGGTAGTGAGC GGTATGGAATCAACCCGTTGTC Cyp27a1 GTGGTCTTATTGGGTACTTGC
Supplement Table I: primers for Real Time RT-PCR Gene Foward Reverse Hmgcoar AGCTTGCCCGAATTGTATGTG TCTGTTGTAACCATGTGACTTC Cyp7α GGGATTGCTGTGGTAGTGAGC GGTATGGAATCAACCCGTTGTC Cyp27a1 GTGGTCTTATTGGGTACTTGC
SUPPLEMENTARY DATA. Supplementary Table 1. Primers used in qpcr
 Supplementary Table 1. Primers used in qpcr Gene forward primer (5'-3') reverse primer (5'-3') β-actin AGAGGGAAATCGTGCGTGAC CAATAGTGATGACCTGGCCGT Hif-p4h-2 CTGGGCAACTACAGGATAAAC GCGTCCCAGTCTTTATTTAGATA
Supplementary Table 1. Primers used in qpcr Gene forward primer (5'-3') reverse primer (5'-3') β-actin AGAGGGAAATCGTGCGTGAC CAATAGTGATGACCTGGCCGT Hif-p4h-2 CTGGGCAACTACAGGATAAAC GCGTCCCAGTCTTTATTTAGATA
Fig. S1. Dose-response effects of acute administration of the β3 adrenoceptor agonists CL316243, BRL37344, ICI215,001, ZD7114, ZD2079 and CGP12177 at
 Fig. S1. Dose-response effects of acute administration of the β3 adrenoceptor agonists CL316243, BRL37344, ICI215,001, ZD7114, ZD2079 and CGP12177 at doses of 0.1, 0.5 and 1 mg/kg on cumulative food intake
Fig. S1. Dose-response effects of acute administration of the β3 adrenoceptor agonists CL316243, BRL37344, ICI215,001, ZD7114, ZD2079 and CGP12177 at doses of 0.1, 0.5 and 1 mg/kg on cumulative food intake
Free Fatty Acid Assay Kit (Fluorometric)
 Product Manual Free Fatty Acid Assay Kit (Fluorometric) Catalog Number STA-619 100 assays FOR RESEARCH USE ONLY Not for use in diagnostic procedures Introduction Triglycerides (TAG) are a type of lipid
Product Manual Free Fatty Acid Assay Kit (Fluorometric) Catalog Number STA-619 100 assays FOR RESEARCH USE ONLY Not for use in diagnostic procedures Introduction Triglycerides (TAG) are a type of lipid
For pair feeding, mice were fed 2.7g of HFD containing tofogliflozin
 Materials and Methods Pair Feeding Experiment For pair feeding, mice were fed 2.7g of HFD containing tofogliflozin (0.005%), which is average daily food intake of mice fed control HFD ad libitum at week
Materials and Methods Pair Feeding Experiment For pair feeding, mice were fed 2.7g of HFD containing tofogliflozin (0.005%), which is average daily food intake of mice fed control HFD ad libitum at week
Supplementary Table 2. Plasma lipid profiles in wild type and mutant female mice submitted to a HFD for 12 weeks wt ERα -/- AF-1 0 AF-2 0
 Supplementary Table 1. List of specific primers used for gene expression analysis. Genes Primer forward Primer reverse Hprt GCAGTACAGCCCCAAAATGG AACAAAGTCTGGCCTGTATCCA Srebp-1c GGAAGCTGTCGGGGTAGCGTC CATGTCTTCAAATGTGCAATCCAT
Supplementary Table 1. List of specific primers used for gene expression analysis. Genes Primer forward Primer reverse Hprt GCAGTACAGCCCCAAAATGG AACAAAGTCTGGCCTGTATCCA Srebp-1c GGAAGCTGTCGGGGTAGCGTC CATGTCTTCAAATGTGCAATCCAT
LIPID METABOLISM. Sri Widia A Jusman Department of Biochemistry & Molecular Biology FMUI
 LIPID METABOLISM Sri Widia A Jusman Department of Biochemistry & Molecular Biology FMUI Lipid metabolism is concerned mainly with fatty acids cholesterol Source of fatty acids from dietary fat de novo
LIPID METABOLISM Sri Widia A Jusman Department of Biochemistry & Molecular Biology FMUI Lipid metabolism is concerned mainly with fatty acids cholesterol Source of fatty acids from dietary fat de novo
Males- Western Diet WT KO Age (wks) Females- Western Diet WT KO Age (wks)
 Relative Arv1 mrna Adrenal 33.48 +/- 6.2 Skeletal Muscle 22.4 +/- 4.93 Liver 6.41 +/- 1.48 Heart 5.1 +/- 2.3 Brain 4.98 +/- 2.11 Ovary 4.68 +/- 2.21 Kidney 3.98 +/-.39 Lung 2.15 +/-.6 Inguinal Subcutaneous
Relative Arv1 mrna Adrenal 33.48 +/- 6.2 Skeletal Muscle 22.4 +/- 4.93 Liver 6.41 +/- 1.48 Heart 5.1 +/- 2.3 Brain 4.98 +/- 2.11 Ovary 4.68 +/- 2.21 Kidney 3.98 +/-.39 Lung 2.15 +/-.6 Inguinal Subcutaneous
298 Biomed Environ Sci, 2015; 28(4):
 298 Biomed Environ Sci, 2015; 28(4): 298-302 Letter to the Editor Effects of Maternal Linseed Oil Supplementation on Metabolic Parameters in Cafeteria Diet-induced Obese Rats * BENAISSA Nawel 1, MERZOUK
298 Biomed Environ Sci, 2015; 28(4): 298-302 Letter to the Editor Effects of Maternal Linseed Oil Supplementation on Metabolic Parameters in Cafeteria Diet-induced Obese Rats * BENAISSA Nawel 1, MERZOUK
Requires Signaling though Akt2 Independent of the. Transcription Factors FoxA2, FoxO1, and SREBP1c
 Cell Metabolism, Volume 14 Supplemental Information Postprandial Hepatic Lipid Metabolism Requires Signaling though Akt2 Independent of the Transcription Factors FoxA2, FoxO1, and SREBP1c Min Wan, Karla
Cell Metabolism, Volume 14 Supplemental Information Postprandial Hepatic Lipid Metabolism Requires Signaling though Akt2 Independent of the Transcription Factors FoxA2, FoxO1, and SREBP1c Min Wan, Karla
Supplementary Figure 1. DJ-1 modulates ROS concentration in mouse skeletal muscle.
 Supplementary Figure 1. DJ-1 modulates ROS concentration in mouse skeletal muscle. (a) mrna levels of Dj1 measured by quantitative RT-PCR in soleus, gastrocnemius (Gastroc.) and extensor digitorum longus
Supplementary Figure 1. DJ-1 modulates ROS concentration in mouse skeletal muscle. (a) mrna levels of Dj1 measured by quantitative RT-PCR in soleus, gastrocnemius (Gastroc.) and extensor digitorum longus
SUPPLEMENTARY INFORMATION
 SUPPLEMENTARY INFORMATION doi:10.1038/nature11464 Supplemental Figure S1. The expression of Vegfb is increased in obese and diabetic mice as compared to lean mice. a-b, Body weight and postprandial blood
SUPPLEMENTARY INFORMATION doi:10.1038/nature11464 Supplemental Figure S1. The expression of Vegfb is increased in obese and diabetic mice as compared to lean mice. a-b, Body weight and postprandial blood
Metabolic defects underlying dyslipidemia in abdominal obesity
 Metabolic defects underlying dyslipidemia in abdominal obesity Professor Marja-Riitta Taskinen Department of Medicine, Division of Cardiology Helsinki University Hospital, Finland Disclosures: Honorariums/
Metabolic defects underlying dyslipidemia in abdominal obesity Professor Marja-Riitta Taskinen Department of Medicine, Division of Cardiology Helsinki University Hospital, Finland Disclosures: Honorariums/
Supplementary Information. Glycogen shortage during fasting triggers liver-brain-adipose. neurocircuitry to facilitate fat utilization
 Supplementary Information Glycogen shortage during fasting triggers liver-brain-adipose neurocircuitry to facilitate fat utilization Supplementary Figure S1. Liver-Brain-Adipose neurocircuitry Starvation
Supplementary Information Glycogen shortage during fasting triggers liver-brain-adipose neurocircuitry to facilitate fat utilization Supplementary Figure S1. Liver-Brain-Adipose neurocircuitry Starvation
Taylor Yohe. Project Advisor: Dr. Martha A. Belury. Department of Human Nutrition at the Ohio State University
 Atherosclerosis Development and the Inflammatory Response of Hepatocytes to Sesame Oil Supplementation Taylor Yohe Project Advisor: Dr. Martha A. Belury Department of Human Nutrition at the Ohio State
Atherosclerosis Development and the Inflammatory Response of Hepatocytes to Sesame Oil Supplementation Taylor Yohe Project Advisor: Dr. Martha A. Belury Department of Human Nutrition at the Ohio State
Supplementary Figure S1. Effect of Glucose on Energy Balance in WT and KHK A/C KO
 Supplementary Figure S1. Effect of Glucose on Energy Balance in WT and KHK A/C KO Mice. WT mice and KHK-A/C KO mice were provided drinking water containing 10% glucose or tap water with normal chow ad
Supplementary Figure S1. Effect of Glucose on Energy Balance in WT and KHK A/C KO Mice. WT mice and KHK-A/C KO mice were provided drinking water containing 10% glucose or tap water with normal chow ad
Supplementary Fig. 1 eif6 +/- mice show a reduction in white adipose tissue, blood lipids and normal glycogen synthesis. The cohort of the original
 Supplementary Fig. 1 eif6 +/- mice show a reduction in white adipose tissue, blood lipids and normal glycogen synthesis. The cohort of the original phenotypic screening was n=40. For specific tests, the
Supplementary Fig. 1 eif6 +/- mice show a reduction in white adipose tissue, blood lipids and normal glycogen synthesis. The cohort of the original phenotypic screening was n=40. For specific tests, the
18s AAACGGCTACCACATCCAAG CCTCCAATGGATCCTCGTTA. 36b4 GTTCTTGCCCATCAGCACC AGATGCAGCAGATCCGCAT. Acc1 AGCAGATCCGCAGCTTG ACCTCTGCTCGCTGAGTGC
 Supplementary Table 1. Quantitative PCR primer sequences Gene symbol Sequences (5 to 3 ) Forward Reverse 18s AAACGGCTACCACATCCAAG CCTCCAATGGATCCTCGTTA 36b4 GTTCTTGCCCATCAGCACC AGATGCAGCAGATCCGCAT Acc1
Supplementary Table 1. Quantitative PCR primer sequences Gene symbol Sequences (5 to 3 ) Forward Reverse 18s AAACGGCTACCACATCCAAG CCTCCAATGGATCCTCGTTA 36b4 GTTCTTGCCCATCAGCACC AGATGCAGCAGATCCGCAT Acc1
3-Thia Fatty Acids A New Generation of Functional Lipids?
 Conference on Food Structure and Food Quality 3-Thia Fatty Acids A New Generation of Functional Lipids? Rolf K. Berge rolf.berge@med.uib.no Fatty acids- Essential cellular metabolites Concentrations must
Conference on Food Structure and Food Quality 3-Thia Fatty Acids A New Generation of Functional Lipids? Rolf K. Berge rolf.berge@med.uib.no Fatty acids- Essential cellular metabolites Concentrations must
Increasing Marbling Gene Expression in Beef Cattle with Dietary Lipids
 Increasing Marbling Gene Expression in Beef Cattle with Dietary Lipids Stephen B. Smith and Seong Ho Choi Texas A&M University Bradley J. Johnson Texas Tech University, Lubbock RMC 2012 June 18, 2012 Researchers
Increasing Marbling Gene Expression in Beef Cattle with Dietary Lipids Stephen B. Smith and Seong Ho Choi Texas A&M University Bradley J. Johnson Texas Tech University, Lubbock RMC 2012 June 18, 2012 Researchers
SUPPLEMENTARY INFORMATION
 DOI: 10.1038/ncb3461 In the format provided by the authors and unedited. Supplementary Figure 1 (associated to Figure 1). Cpeb4 gene-targeted mice develop liver steatosis. a, Immunoblot displaying CPEB4
DOI: 10.1038/ncb3461 In the format provided by the authors and unedited. Supplementary Figure 1 (associated to Figure 1). Cpeb4 gene-targeted mice develop liver steatosis. a, Immunoblot displaying CPEB4
BMP6 treatment compensates for the molecular defect and ameliorates hemochromatosis in Hfe knockout mice
 SUPPLEMENTARY MATERIALS BMP6 treatment compensates for the molecular defect and ameliorates hemochromatosis in Hfe knockout mice Elena Corradini, Paul J. Schmidt, Delphine Meynard, Cinzia Garuti, Giuliana
SUPPLEMENTARY MATERIALS BMP6 treatment compensates for the molecular defect and ameliorates hemochromatosis in Hfe knockout mice Elena Corradini, Paul J. Schmidt, Delphine Meynard, Cinzia Garuti, Giuliana
LIPID METABOLISM
 LIPID METABOLISM LIPOGENESIS LIPOGENESIS LIPOGENESIS FATTY ACID SYNTHESIS DE NOVO FFA in the blood come from :- (a) Dietary fat (b) Dietary carbohydrate/protein in excess of need FA TAG Site of synthesis:-
LIPID METABOLISM LIPOGENESIS LIPOGENESIS LIPOGENESIS FATTY ACID SYNTHESIS DE NOVO FFA in the blood come from :- (a) Dietary fat (b) Dietary carbohydrate/protein in excess of need FA TAG Site of synthesis:-
ANSC/NUTR 618 Lipids & Lipid Metabolism
 I. Overall concepts A. Definitions ANC/NUTR 618 Lipids & Lipid Metabolism 1. De novo synthesis = synthesis from non-fatty acid precursors a. Carbohydrate precursors (glucose, lactate, and pyruvate) b.
I. Overall concepts A. Definitions ANC/NUTR 618 Lipids & Lipid Metabolism 1. De novo synthesis = synthesis from non-fatty acid precursors a. Carbohydrate precursors (glucose, lactate, and pyruvate) b.
Supplementary Figure 1. DNA methylation of the adiponectin promoter R1, Pparg2, and Tnfa promoter in adipocytes is not affected by obesity.
 Supplementary Figure 1. DNA methylation of the adiponectin promoter R1, Pparg2, and Tnfa promoter in adipocytes is not affected by obesity. (a) Relative amounts of adiponectin, Ppar 2, C/ebp, and Tnf mrna
Supplementary Figure 1. DNA methylation of the adiponectin promoter R1, Pparg2, and Tnfa promoter in adipocytes is not affected by obesity. (a) Relative amounts of adiponectin, Ppar 2, C/ebp, and Tnf mrna
CHY2026: General Biochemistry. Lipid Metabolism
 CHY2026: General Biochemistry Lipid Metabolism Lipid Digestion Lipid Metabolism Fats (triglycerides) are high metabolic energy molecules Fats yield 9.3 kcal of energy (carbohydrates and proteins 4.1 kcal)
CHY2026: General Biochemistry Lipid Metabolism Lipid Digestion Lipid Metabolism Fats (triglycerides) are high metabolic energy molecules Fats yield 9.3 kcal of energy (carbohydrates and proteins 4.1 kcal)
SUPPLEMENTARY INFORMATION
 Supplementary Figures Supplementary Figure S1. Binding of full-length OGT and deletion mutants to PIP strips (Echelon Biosciences). Supplementary Figure S2. Binding of the OGT (919-1036) fragments with
Supplementary Figures Supplementary Figure S1. Binding of full-length OGT and deletion mutants to PIP strips (Echelon Biosciences). Supplementary Figure S2. Binding of the OGT (919-1036) fragments with
Skeletal muscle metabolism was studied by measuring arterio-venous concentration differences
 Supplemental Data Dual stable-isotope experiment Skeletal muscle metabolism was studied by measuring arterio-venous concentration differences across the forearm, adjusted for forearm blood flow (FBF) (1).
Supplemental Data Dual stable-isotope experiment Skeletal muscle metabolism was studied by measuring arterio-venous concentration differences across the forearm, adjusted for forearm blood flow (FBF) (1).
Supporting Information Table of content
 Supporting Information Table of content Supporting Information Fig. S1 Supporting Information Fig. S2 Supporting Information Fig. S3 Supporting Information Fig. S4 Supporting Information Fig. S5 Supporting
Supporting Information Table of content Supporting Information Fig. S1 Supporting Information Fig. S2 Supporting Information Fig. S3 Supporting Information Fig. S4 Supporting Information Fig. S5 Supporting
PROMINENT ROLE OF LIVER IN ELEVATED PLASMA PALMITOLEATE LEVELS IN RESPONSE TO ROSIGLITAZONE IN MICE FED HIGH-FAT DIET
 JOURNAL OF PHYSIOLOGY AND PHARMACOLOGY 2009, 60, 4, 135-140 www.jpp.krakow.pl O. KUDA 1, B. STANKOVA 2, E. TVRZICKA 2, M. HENSLER 1, T. JELENIK 1, M. ROSSMEISL 1, P. FLACHS 1, J. KOPECKY 1 PROMINENT ROLE
JOURNAL OF PHYSIOLOGY AND PHARMACOLOGY 2009, 60, 4, 135-140 www.jpp.krakow.pl O. KUDA 1, B. STANKOVA 2, E. TVRZICKA 2, M. HENSLER 1, T. JELENIK 1, M. ROSSMEISL 1, P. FLACHS 1, J. KOPECKY 1 PROMINENT ROLE
Supplementary Materials for
 www.sciencesignaling.org/cgi/content/full/8/407/ra127/dc1 Supplementary Materials for Loss of FTO in adipose tissue decreases Angptl4 translation and alters triglyceride metabolism Chao-Yung Wang,* Shian-Sen
www.sciencesignaling.org/cgi/content/full/8/407/ra127/dc1 Supplementary Materials for Loss of FTO in adipose tissue decreases Angptl4 translation and alters triglyceride metabolism Chao-Yung Wang,* Shian-Sen
Effects of growth hormone secretagogue receptor agonist and antagonist in nonobese type 2 diabetic MKR mice
 Effects of growth hormone secretagogue receptor agonist and antagonist in nonobese type 2 diabetic MKR mice Rasha Mosa (MBCHC, M.D, PhD candidate) School of Biomedical Sciences University of Queensland
Effects of growth hormone secretagogue receptor agonist and antagonist in nonobese type 2 diabetic MKR mice Rasha Mosa (MBCHC, M.D, PhD candidate) School of Biomedical Sciences University of Queensland
Supplementary Table 1. Metabolic parameters in GFP and OGT-treated mice
 Supplementary Table 1. Metabolic parameters in GFP and OGT-treated mice Fasted Refed GFP OGT GFP OGT Liver G6P (mmol/g) 0.03±0.01 0.04±0.02 0.60±0.04 0.42±0.10 A TGs (mg/g of liver) 20.08±5.17 16.29±0.8
Supplementary Table 1. Metabolic parameters in GFP and OGT-treated mice Fasted Refed GFP OGT GFP OGT Liver G6P (mmol/g) 0.03±0.01 0.04±0.02 0.60±0.04 0.42±0.10 A TGs (mg/g of liver) 20.08±5.17 16.29±0.8
Lecithin Cholesterol Acyltransferase (LCAT) ELISA Kit
 Product Manual Lecithin Cholesterol Acyltransferase (LCAT) ELISA Kit Catalog Number STA-616 96 assays FOR RESEARCH USE ONLY Not for use in diagnostic procedures Introduction Cholesterol is a lipid sterol
Product Manual Lecithin Cholesterol Acyltransferase (LCAT) ELISA Kit Catalog Number STA-616 96 assays FOR RESEARCH USE ONLY Not for use in diagnostic procedures Introduction Cholesterol is a lipid sterol
SUPPLEMENTARY INFORMATION
 doi: 1.138/nature7221 Brown fat selective genes 12 1 Control Q-RT-PCR (% of Control) 8 6 4 2 Ntrk3 Cox7a1 Cox8b Cox5b ATPase b2 ATPase f1a1 Sirt3 ERRα Elovl3/Cig3 PPARα Zic1 Supplementary Figure S1. stimulates
doi: 1.138/nature7221 Brown fat selective genes 12 1 Control Q-RT-PCR (% of Control) 8 6 4 2 Ntrk3 Cox7a1 Cox8b Cox5b ATPase b2 ATPase f1a1 Sirt3 ERRα Elovl3/Cig3 PPARα Zic1 Supplementary Figure S1. stimulates
Niacin Metabolism: Effects on Cholesterol
 Niacin Metabolism: Effects on Cholesterol By Julianne R. Edwards For Dr. William R. Proulx, PhD, RD Associate Professor of Nutrition and Dietetics In partial fulfillments for the requirements of NUTR342
Niacin Metabolism: Effects on Cholesterol By Julianne R. Edwards For Dr. William R. Proulx, PhD, RD Associate Professor of Nutrition and Dietetics In partial fulfillments for the requirements of NUTR342
Supplementary Figure 1.
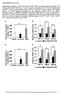 Supplementary Figure 1. FGF21 does not exert direct effects on hepatic glucose production. The liver explants from C57BL/6J mice (A, B) or primary rat hepatocytes (C, D) were incubated with rmfgf21 (2
Supplementary Figure 1. FGF21 does not exert direct effects on hepatic glucose production. The liver explants from C57BL/6J mice (A, B) or primary rat hepatocytes (C, D) were incubated with rmfgf21 (2
Supplementary Information
 Supplementary Information Akt regulates hepatic metabolism by suppressing a Foxo1 dependent global inhibition of adaptation to nutrient intake Mingjian Lu 1, Min Wan 1, Karla F. Leavens 1, Qingwei Chu
Supplementary Information Akt regulates hepatic metabolism by suppressing a Foxo1 dependent global inhibition of adaptation to nutrient intake Mingjian Lu 1, Min Wan 1, Karla F. Leavens 1, Qingwei Chu
Investigations on the mechanism of hypercholesterolemia observed in copper deficiency in rats
 J. Biosci., Vol. 12, Number 2, June 1987, pp. 137 142. Printed in India. Investigations on the mechanism of hypercholesterolemia observed in copper deficiency in rats P. VALSALA and P. A. KURUP Department
J. Biosci., Vol. 12, Number 2, June 1987, pp. 137 142. Printed in India. Investigations on the mechanism of hypercholesterolemia observed in copper deficiency in rats P. VALSALA and P. A. KURUP Department
Defective Hepatic Autophagy in Obesity Promotes ER Stress and Causes Insulin Resistance
 Cell Metabolism, Volume 11 Supplemental Information Defective Hepatic Autophagy in Obesity Promotes ER Stress and Causes Insulin Resistance Ling Yang, Ping Li, Suneng Fu, Ediz S. Calay, and Gökhan S. Hotamisligil
Cell Metabolism, Volume 11 Supplemental Information Defective Hepatic Autophagy in Obesity Promotes ER Stress and Causes Insulin Resistance Ling Yang, Ping Li, Suneng Fu, Ediz S. Calay, and Gökhan S. Hotamisligil
The Role of LCPUFA in Obesity. M.Tom Clandinin. The Alberta Institute for Human Nutrition The University of Alberta Edmonton, Alberta, Canada
 The Role of LCPUFA in Obesity by M.Tom Clandinin The Alberta Institute for Human Nutrition The University of Alberta Edmonton, Alberta, Canada How big is the Conceptual Problem? Some assumptions: 150lb
The Role of LCPUFA in Obesity by M.Tom Clandinin The Alberta Institute for Human Nutrition The University of Alberta Edmonton, Alberta, Canada How big is the Conceptual Problem? Some assumptions: 150lb
ANSC/NUTR 618 LIPIDS & LIPID METABOLISM. Fatty Acid Elongation and Desaturation
 ANSC/NUTR 618 LIPIDS & LIPID METABOLISM I. Fatty acid elongation A. General 1. At least 60% of fatty acids in triacylglycerols are C18. 2. Free palmitic acid (16:0) synthesized in cytoplasm is elongated
ANSC/NUTR 618 LIPIDS & LIPID METABOLISM I. Fatty acid elongation A. General 1. At least 60% of fatty acids in triacylglycerols are C18. 2. Free palmitic acid (16:0) synthesized in cytoplasm is elongated
Supporting Information. Supporting Tables. S-Table 1 Primer pairs for RT-PCR. Product size. Gene Primer pairs
 Supporting Information Supporting Tables S-Table 1 Primer pairs for RT-PCR. Gene Primer pairs Product size (bp) FAS F: 5 TCTTGGAAGCGATGGGTA 3 429 R: 5 GGGATGTATCATTCTTGGAC 3 SREBP-1c F: 5 CGCTACCGTTCCTCTATCA
Supporting Information Supporting Tables S-Table 1 Primer pairs for RT-PCR. Gene Primer pairs Product size (bp) FAS F: 5 TCTTGGAAGCGATGGGTA 3 429 R: 5 GGGATGTATCATTCTTGGAC 3 SREBP-1c F: 5 CGCTACCGTTCCTCTATCA
The SCAP/SREBP Pathway: A Mediator of Hepatic Steatosis
 Review Article Endocrinol Metab 2017;32:6-10 https://doi.org/10.3803/enm.2017.32.1.6 pissn 2093-596X eissn 2093-5978 The SCAP/SREBP Pathway: A Mediator of Hepatic Steatosis Young-Ah Moon Department of
Review Article Endocrinol Metab 2017;32:6-10 https://doi.org/10.3803/enm.2017.32.1.6 pissn 2093-596X eissn 2093-5978 The SCAP/SREBP Pathway: A Mediator of Hepatic Steatosis Young-Ah Moon Department of
In The Name Of God. In The Name Of. EMRI Modeling Group
 In The Name Of God In The Name Of God EMRI Modeling Group Cells work together in functionally related groups called tissues Types of tissues: Epithelial lining and covering Connective support Muscle movement
In The Name Of God In The Name Of God EMRI Modeling Group Cells work together in functionally related groups called tissues Types of tissues: Epithelial lining and covering Connective support Muscle movement
BCM 221 LECTURES OJEMEKELE O.
 BCM 221 LECTURES BY OJEMEKELE O. OUTLINE INTRODUCTION TO LIPID CHEMISTRY STORAGE OF ENERGY IN ADIPOCYTES MOBILIZATION OF ENERGY STORES IN ADIPOCYTES KETONE BODIES AND KETOSIS PYRUVATE DEHYDROGENASE COMPLEX
BCM 221 LECTURES BY OJEMEKELE O. OUTLINE INTRODUCTION TO LIPID CHEMISTRY STORAGE OF ENERGY IN ADIPOCYTES MOBILIZATION OF ENERGY STORES IN ADIPOCYTES KETONE BODIES AND KETOSIS PYRUVATE DEHYDROGENASE COMPLEX
Dietary α-linolenic acid-rich flaxseed oil prevents against alcoholic hepatic steatosis
 Dietary α-linolenic acid-rich flaxseed oil prevents against alcoholic hepatic steatosis via ameliorating lipid homeostasis at adipose tissue-liver axis in mice Meng Wang a, Xiao-Jing Zhang a, Kun Feng
Dietary α-linolenic acid-rich flaxseed oil prevents against alcoholic hepatic steatosis via ameliorating lipid homeostasis at adipose tissue-liver axis in mice Meng Wang a, Xiao-Jing Zhang a, Kun Feng
HIV VPR alters fat metabolism. Dorothy E Lewis PhD/Ashok Balasubramanyam MD
 HIV VPR alters fat metabolism Dorothy E Lewis PhD/Ashok Balasubramanyam MD Old Dogma for HIV associated lipodystrophy Differentiation Block (PI) Lipoatrophy Apoptosis (NRTI) Stem cell Preadipocyte Adipocyte
HIV VPR alters fat metabolism Dorothy E Lewis PhD/Ashok Balasubramanyam MD Old Dogma for HIV associated lipodystrophy Differentiation Block (PI) Lipoatrophy Apoptosis (NRTI) Stem cell Preadipocyte Adipocyte
Supplemental Table 1 Primer sequences (mouse) used for real-time qrt-pcr studies
 Supplemental Table 1 Primer sequences (mouse) used for real-time qrt-pcr studies Gene symbol Forward primer Reverse primer ACC1 5'-TGAGGAGGACCGCATTTATC 5'-GCATGGAATGGCAGTAAGGT ACLY 5'-GACACCATCTGTGATCTTG
Supplemental Table 1 Primer sequences (mouse) used for real-time qrt-pcr studies Gene symbol Forward primer Reverse primer ACC1 5'-TGAGGAGGACCGCATTTATC 5'-GCATGGAATGGCAGTAAGGT ACLY 5'-GACACCATCTGTGATCTTG
Energy metabolism - the overview
 Energy metabolism - the overview Josef Fontana EC - 40 Overview of the lecture Important terms of the energy metabolism The overview of the energy metabolism The main pathways of the energy metabolism
Energy metabolism - the overview Josef Fontana EC - 40 Overview of the lecture Important terms of the energy metabolism The overview of the energy metabolism The main pathways of the energy metabolism
Islet viability assay and Glucose Stimulated Insulin Secretion assay RT-PCR and Western Blot
 Islet viability assay and Glucose Stimulated Insulin Secretion assay Islet cell viability was determined by colorimetric (3-(4,5-dimethylthiazol-2-yl)-2,5- diphenyltetrazolium bromide assay using CellTiter
Islet viability assay and Glucose Stimulated Insulin Secretion assay Islet cell viability was determined by colorimetric (3-(4,5-dimethylthiazol-2-yl)-2,5- diphenyltetrazolium bromide assay using CellTiter
(A) PCR primers (arrows) designed to distinguish wild type (P1+P2), targeted (P1+P2) and excised (P1+P3)14-
 1 Supplemental Figure Legends Figure S1. Mammary tumors of ErbB2 KI mice with 14-3-3σ ablation have elevated ErbB2 transcript levels and cell proliferation (A) PCR primers (arrows) designed to distinguish
1 Supplemental Figure Legends Figure S1. Mammary tumors of ErbB2 KI mice with 14-3-3σ ablation have elevated ErbB2 transcript levels and cell proliferation (A) PCR primers (arrows) designed to distinguish
: Overview of EFA metabolism
 Figure 1 gives an overview of the metabolic fate of the EFAs when consumed in the diet. The n-6 and n-3 PUFAs when consumed in the form of dietary triglyceride from various food sources undergoes digestion
Figure 1 gives an overview of the metabolic fate of the EFAs when consumed in the diet. The n-6 and n-3 PUFAs when consumed in the form of dietary triglyceride from various food sources undergoes digestion
The role of apolipoprotein D in lipid metabolism and metabolic syndrome
 UQÀM: Department of Biological Sciences The role of apolipoprotein D in lipid metabolism and metabolic syndrome Catherine Mounier PhD Apolipoprotein D Apolipoprotéine D (apod) Secreted protein Associated
UQÀM: Department of Biological Sciences The role of apolipoprotein D in lipid metabolism and metabolic syndrome Catherine Mounier PhD Apolipoprotein D Apolipoprotéine D (apod) Secreted protein Associated
Quantitative Real-Time PCR was performed as same as Materials and Methods.
 Supplemental Material Quantitative Real-Time PCR Quantitative Real-Time PCR was performed as same as Materials and Methods. Expression levels in the aorta were normalized to peptidylprolyl isomerase B
Supplemental Material Quantitative Real-Time PCR Quantitative Real-Time PCR was performed as same as Materials and Methods. Expression levels in the aorta were normalized to peptidylprolyl isomerase B
Supplementary Table 1. Primer Sequences Used for Quantitative Real-Time PCR
 Supplementary Table 1. Primer Sequences Used for Quantitative Real-Time PCR Gene Forward Primer (5-3 ) Reverse Primer (5-3 ) cadl CTTGGGGGCGCGTCT CTGTTCTTTTGTGCCGTTTCG cyl-coenzyme Dehydrogenase, very
Supplementary Table 1. Primer Sequences Used for Quantitative Real-Time PCR Gene Forward Primer (5-3 ) Reverse Primer (5-3 ) cadl CTTGGGGGCGCGTCT CTGTTCTTTTGTGCCGTTTCG cyl-coenzyme Dehydrogenase, very
ALT (U/L) (Relative expression) HDL (mm) (Relative expression) ALT (U/L) (Relative expression)
 a DMT mrna () 8 6 r =.96 P =. DMT mrna () 8 6 r =. P =.6 DMT mrna () 8 6 r =.99 P =.6 DMT mrna () 8 6 r =. P =.9 DMT mrna () BMI (kg/m ) 8 6 r =.7 P =.966 DMT mrna () 8 ALT (U/L) 8 6 r = -.66 P =.76 DMT
a DMT mrna () 8 6 r =.96 P =. DMT mrna () 8 6 r =. P =.6 DMT mrna () 8 6 r =.99 P =.6 DMT mrna () 8 6 r =. P =.9 DMT mrna () BMI (kg/m ) 8 6 r =.7 P =.966 DMT mrna () 8 ALT (U/L) 8 6 r = -.66 P =.76 DMT
Unit IV Problem 3 Biochemistry: Cholesterol Metabolism and Lipoproteins
 Unit IV Problem 3 Biochemistry: Cholesterol Metabolism and Lipoproteins - Cholesterol: It is a sterol which is found in all eukaryotic cells and contains an oxygen (as a hydroxyl group OH) on Carbon number
Unit IV Problem 3 Biochemistry: Cholesterol Metabolism and Lipoproteins - Cholesterol: It is a sterol which is found in all eukaryotic cells and contains an oxygen (as a hydroxyl group OH) on Carbon number
The lipogenic transcription factor ChREBP dissociates hepatic steatosis from insulin resistance in mice and humans
 Research article The lipogenic transcription factor ChREBP dissociates hepatic steatosis from insulin resistance in mice and humans Fadila Benhamed, 1,2,3 Pierre-Damien Denechaud, 1,2,3 Maud Lemoine, 4,5,6
Research article The lipogenic transcription factor ChREBP dissociates hepatic steatosis from insulin resistance in mice and humans Fadila Benhamed, 1,2,3 Pierre-Damien Denechaud, 1,2,3 Maud Lemoine, 4,5,6
Lipids digestion and absorption, Biochemistry II
 Lipids digestion and absorption, blood plasma lipids, lipoproteins Biochemistry II Lecture 1 2008 (J.S.) Triacylglycerols (as well as free fatty acids and both free and esterified cholesterol) are very
Lipids digestion and absorption, blood plasma lipids, lipoproteins Biochemistry II Lecture 1 2008 (J.S.) Triacylglycerols (as well as free fatty acids and both free and esterified cholesterol) are very
Plasma lipoproteins & atherosclerosis by. Prof.Dr. Maha M. Sallam
 Biochemistry Department Plasma lipoproteins & atherosclerosis by Prof.Dr. Maha M. Sallam 1 1. Recognize structures,types and role of lipoproteins in blood (Chylomicrons, VLDL, LDL and HDL). 2. Explain
Biochemistry Department Plasma lipoproteins & atherosclerosis by Prof.Dr. Maha M. Sallam 1 1. Recognize structures,types and role of lipoproteins in blood (Chylomicrons, VLDL, LDL and HDL). 2. Explain
2-Deoxyglucose Assay Kit (Colorimetric)
 2-Deoxyglucose Assay Kit (Colorimetric) Catalog Number KA3753 100 assays Version: 01 Intended for research use only www.abnova.com Table of Contents Introduction... 3 Background... 3 General Information...
2-Deoxyglucose Assay Kit (Colorimetric) Catalog Number KA3753 100 assays Version: 01 Intended for research use only www.abnova.com Table of Contents Introduction... 3 Background... 3 General Information...
Chapter (5) Etiology of Low HDL- Cholesterol
 Chapter (5) Etiology of Low HDL- Cholesterol The aim of this chapter is to summarize the different etiological factors mainly the role of life-style and different disease conditions contributing to the
Chapter (5) Etiology of Low HDL- Cholesterol The aim of this chapter is to summarize the different etiological factors mainly the role of life-style and different disease conditions contributing to the
Figure S1. Body composition, energy homeostasis and substrate utilization in LRH-1 hep+/+ (white bars) and LRH-1 hep-/- (black bars) mice.
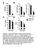 Figure S1. Body composition, energy homeostasis and substrate utilization in LRH-1 hep+/+ (white bars) and LRH-1 hep-/- (black bars) mice. (A) Lean and fat masses, determined by EchoMRI. (B) Food and water
Figure S1. Body composition, energy homeostasis and substrate utilization in LRH-1 hep+/+ (white bars) and LRH-1 hep-/- (black bars) mice. (A) Lean and fat masses, determined by EchoMRI. (B) Food and water
Supplementary Information Titles Journal: Nature Medicine
 Supplementary Information Titles Journal: Nature Medicine Article Title: Corresponding Author: Supplementary Item & Number Supplementary Fig.1 Fig.2 Fig.3 Fig.4 Fig.5 Fig.6 Fig.7 Fig.8 Fig.9 Fig. Fig.11
Supplementary Information Titles Journal: Nature Medicine Article Title: Corresponding Author: Supplementary Item & Number Supplementary Fig.1 Fig.2 Fig.3 Fig.4 Fig.5 Fig.6 Fig.7 Fig.8 Fig.9 Fig. Fig.11
number Done by Corrected by Doctor Faisal Al-Khatibe
 number 24 Done by Mohammed tarabieh Corrected by Doctor Faisal Al-Khatibe 1 P a g e *Please look over the previous sheet about fatty acid synthesis **Oxidation(degradation) of fatty acids, occurs in the
number 24 Done by Mohammed tarabieh Corrected by Doctor Faisal Al-Khatibe 1 P a g e *Please look over the previous sheet about fatty acid synthesis **Oxidation(degradation) of fatty acids, occurs in the
TFEB-mediated increase in peripheral lysosomes regulates. Store Operated Calcium Entry
 TFEB-mediated increase in peripheral lysosomes regulates Store Operated Calcium Entry Luigi Sbano, Massimo Bonora, Saverio Marchi, Federica Baldassari, Diego L. Medina, Andrea Ballabio, Carlotta Giorgi
TFEB-mediated increase in peripheral lysosomes regulates Store Operated Calcium Entry Luigi Sbano, Massimo Bonora, Saverio Marchi, Federica Baldassari, Diego L. Medina, Andrea Ballabio, Carlotta Giorgi
Fig. S1. REGN1500 reduces plasma levels of cholesterol, TG and NEFA in WT and Ldlr -/- mice. (A) WT
 Figure Legends for Supplementary Figures. Fig. S1. REGN15 reduces plasma levels of cholesterol, TG and NEF in WT and Ldlr -/- mice. () WT and Ldlr -/- mice were injected with control IgG or REGN15 (1 mg/kg)
Figure Legends for Supplementary Figures. Fig. S1. REGN15 reduces plasma levels of cholesterol, TG and NEF in WT and Ldlr -/- mice. () WT and Ldlr -/- mice were injected with control IgG or REGN15 (1 mg/kg)
2.5. AMPK activity
 Supplement Fig. A 3 B phos-ampk 2.5 * Control AICAR AMPK AMPK activity (Absorbance at 45 nm) 2.5.5 Control AICAR Supplement Fig. Effects of AICAR on AMPK activation in macrophages. J774. macrophages were
Supplement Fig. A 3 B phos-ampk 2.5 * Control AICAR AMPK AMPK activity (Absorbance at 45 nm) 2.5.5 Control AICAR Supplement Fig. Effects of AICAR on AMPK activation in macrophages. J774. macrophages were
Role of fatty acids in the development of insulin resistance and type 2 diabetes mellitus
 Emerging Science Role of fatty acids in the development of insulin resistance and type 2 diabetes mellitus George Wolf Insulin resistance is defined as the reduced responsiveness to normal circulating
Emerging Science Role of fatty acids in the development of insulin resistance and type 2 diabetes mellitus George Wolf Insulin resistance is defined as the reduced responsiveness to normal circulating
Application Note. Introduction
 Simultaneously Measuring Oxidation of Exogenous and Endogenous Fatty Acids Using the XF Palmitate-BSA FAO Substrate with the Agilent Seahorse XF Cell Mito Stress Test Application Note Introduction The
Simultaneously Measuring Oxidation of Exogenous and Endogenous Fatty Acids Using the XF Palmitate-BSA FAO Substrate with the Agilent Seahorse XF Cell Mito Stress Test Application Note Introduction The
CHAPTER FORTY FIVE ENDOGENOUS LIPID TRANSPORT PATHWAY: VLDL AND IDL
 CHAPTER FORTY FIVE ENDOGENOUS LIPID TRANSPORT PATHWAY: VLDL AND IDL You will notice that the endogenous pathway is very similar to the exogenous pathway What is the average daily amount of triacylglycerol
CHAPTER FORTY FIVE ENDOGENOUS LIPID TRANSPORT PATHWAY: VLDL AND IDL You will notice that the endogenous pathway is very similar to the exogenous pathway What is the average daily amount of triacylglycerol
Supplementary Figure 1
 VO (ml kg - min - ) VCO (ml kg - min - ) Respiratory exchange ratio Energy expenditure (cal kg - min - ) Locomotor activity (x count) Body temperature ( C) Relative mrna expression TA Sol EDL PT Heart
VO (ml kg - min - ) VCO (ml kg - min - ) Respiratory exchange ratio Energy expenditure (cal kg - min - ) Locomotor activity (x count) Body temperature ( C) Relative mrna expression TA Sol EDL PT Heart
ZL ZDF ZDF + E2 *** Visceral (g) ZDF
 Body Weight (g) 4 3 2 1 ** * ZL ZDF 6 8 1 12 14 16 Age (weeks) B * Sub-cutaneous (g) 16 12 8 4 ZL ZDF Visceral (g) 25 2 15 1 5 ZL ZDF Total fat pad weight (g) 4 3 2 1 ZDF ZL Supplemental Figure 1: Effect
Body Weight (g) 4 3 2 1 ** * ZL ZDF 6 8 1 12 14 16 Age (weeks) B * Sub-cutaneous (g) 16 12 8 4 ZL ZDF Visceral (g) 25 2 15 1 5 ZL ZDF Total fat pad weight (g) 4 3 2 1 ZDF ZL Supplemental Figure 1: Effect
Supplementary Materials
 Supplementary Materials 1 Supplementary Table 1. List of primers used for quantitative PCR analysis. Gene name Gene symbol Accession IDs Sequence range Product Primer sequences size (bp) β-actin Actb gi
Supplementary Materials 1 Supplementary Table 1. List of primers used for quantitative PCR analysis. Gene name Gene symbol Accession IDs Sequence range Product Primer sequences size (bp) β-actin Actb gi
Reviewers' comments: Reviewer #1 (Remarks to the Author):
 Reviewers' comments: Reviewer #1 (Remarks to the Author): Bempedoic acid (ETC-1002) is an ATP-citrate lyase (ACL) inhibitor that in clinical studies has been shown to decrease plasma LDL cholesterol apparently
Reviewers' comments: Reviewer #1 (Remarks to the Author): Bempedoic acid (ETC-1002) is an ATP-citrate lyase (ACL) inhibitor that in clinical studies has been shown to decrease plasma LDL cholesterol apparently
ANSC/NUTR 618 LIPIDS & LIPID METABOLISM. Triacylglycerol and Fatty Acid Metabolism
 ANSC/NUTR 618 LIPIDS & LIPID METABOLISM II. Triacylglycerol synthesis A. Overall pathway Glycerol-3-phosphate + 3 Fatty acyl-coa à Triacylglycerol + 3 CoASH B. Enzymes 1. Acyl-CoA synthase 2. Glycerol-phosphate
ANSC/NUTR 618 LIPIDS & LIPID METABOLISM II. Triacylglycerol synthesis A. Overall pathway Glycerol-3-phosphate + 3 Fatty acyl-coa à Triacylglycerol + 3 CoASH B. Enzymes 1. Acyl-CoA synthase 2. Glycerol-phosphate
1Why lipids cannot be transported in blood alone? 2How we transport Fatty acids and steroid hormones?
 1Why lipids cannot be transported in blood alone? 2How we transport Fatty acids and steroid hormones? 3How are dietary lipids transported? 4How lipids synthesized in the liver are transported? 5 Lipoprotien
1Why lipids cannot be transported in blood alone? 2How we transport Fatty acids and steroid hormones? 3How are dietary lipids transported? 4How lipids synthesized in the liver are transported? 5 Lipoprotien
Hepatic De Novo Lipogenesis and Regulation of Metabolism
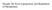 Hepatic De Novo Lipogenesis and Regulation of Metabolism James M. Ntambi Editor Hepatic De Novo Lipogenesis and Regulation of Metabolism Editor James M. Ntambi Department of Biochemistry University of
Hepatic De Novo Lipogenesis and Regulation of Metabolism James M. Ntambi Editor Hepatic De Novo Lipogenesis and Regulation of Metabolism Editor James M. Ntambi Department of Biochemistry University of
Biosynthesis of Triacylglycerides (TG) in liver. Mobilization of stored fat and oxidation of fatty acids
 Biosynthesis of Triacylglycerides (TG) in liver Mobilization of stored fat and oxidation of fatty acids Activation of hormone sensitive lipase This enzyme is activated when phosphorylated (3,5 cyclic AMPdependent
Biosynthesis of Triacylglycerides (TG) in liver Mobilization of stored fat and oxidation of fatty acids Activation of hormone sensitive lipase This enzyme is activated when phosphorylated (3,5 cyclic AMPdependent
Supplementary Information. MicroRNA-33b knock-in mice for an intron of sterol regulatory
 Supplementary Information MicroRNA-33b knock-in mice for an intron of sterol regulatory element-binding factor 1 (Srebf1) exhibit reduced HDL-C in vivo Takahiro Horie, Tomohiro Nishino, Osamu Baba, Yasuhide
Supplementary Information MicroRNA-33b knock-in mice for an intron of sterol regulatory element-binding factor 1 (Srebf1) exhibit reduced HDL-C in vivo Takahiro Horie, Tomohiro Nishino, Osamu Baba, Yasuhide
THE GLUCOSE-FATTY ACID-KETONE BODY CYCLE Role of ketone bodies as respiratory substrates and metabolic signals
 Br. J. Anaesth. (1981), 53, 131 THE GLUCOSE-FATTY ACID-KETONE BODY CYCLE Role of ketone bodies as respiratory substrates and metabolic signals J. C. STANLEY In this paper, the glucose-fatty acid cycle
Br. J. Anaesth. (1981), 53, 131 THE GLUCOSE-FATTY ACID-KETONE BODY CYCLE Role of ketone bodies as respiratory substrates and metabolic signals J. C. STANLEY In this paper, the glucose-fatty acid cycle
Supplementary Figure 1: Additional metabolic parameters of obesity mouse models and controls. (a) Body weight, (b) blood glucose and (c) insulin
 Supplementary Figure 1: Additional metabolic parameters of obesity mouse models and controls. (a) Body weight, (b) blood glucose and (c) insulin resistance index of homeostatic model assessment (HOMA IR)
Supplementary Figure 1: Additional metabolic parameters of obesity mouse models and controls. (a) Body weight, (b) blood glucose and (c) insulin resistance index of homeostatic model assessment (HOMA IR)
Supplementary Fig. 1. Identification of acetylation of K68 of SOD2
 Supplementary Fig. 1. Identification of acetylation of K68 of SOD2 A B H. sapiens 54 KHHAAYVNNLNVTEEKYQEALAK 75 M. musculus 54 KHHAAYVNNLNATEEKYHEALAK 75 X. laevis 55 KHHATYVNNLNITEEKYAEALAK 77 D. rerio
Supplementary Fig. 1. Identification of acetylation of K68 of SOD2 A B H. sapiens 54 KHHAAYVNNLNVTEEKYQEALAK 75 M. musculus 54 KHHAAYVNNLNATEEKYHEALAK 75 X. laevis 55 KHHATYVNNLNITEEKYAEALAK 77 D. rerio
IL METABOLISMO EPATICO DEI CARBOIDRATI IN FISIOLOGIA E PATOLOGIA
 UNIGASTRO Il fegato come centrale metabolica e i fattori di danno oltre ai virus epatitici IL METABOLISMO EPATICO DEI CARBOIDRATI IN FISIOLOGIA E PATOLOGIA Dr Elisabetta Bugianesi Divisione di Gastro-Epatologia
UNIGASTRO Il fegato come centrale metabolica e i fattori di danno oltre ai virus epatitici IL METABOLISMO EPATICO DEI CARBOIDRATI IN FISIOLOGIA E PATOLOGIA Dr Elisabetta Bugianesi Divisione di Gastro-Epatologia
Fatty Acid and Triacylglycerol Metabolism 1
 Fatty Acid and Triacylglycerol Metabolism 1 Mobilization of stored fats and oxidation of fatty acids Lippincott s Chapter 16 What is the first lecture about What is triacylglycerol Fatty acids structure
Fatty Acid and Triacylglycerol Metabolism 1 Mobilization of stored fats and oxidation of fatty acids Lippincott s Chapter 16 What is the first lecture about What is triacylglycerol Fatty acids structure
Supplemental Information. Increased 4E-BP1 Expression Protects. against Diet-Induced Obesity and Insulin. Resistance in Male Mice
 Cell Reports, Volume 16 Supplemental Information Increased 4E-BP1 Expression Protects against Diet-Induced Obesity and Insulin Resistance in Male Mice Shih-Yin Tsai, Ariana A. Rodriguez, Somasish G. Dastidar,
Cell Reports, Volume 16 Supplemental Information Increased 4E-BP1 Expression Protects against Diet-Induced Obesity and Insulin Resistance in Male Mice Shih-Yin Tsai, Ariana A. Rodriguez, Somasish G. Dastidar,
Integration Of Metabolism
 Integration Of Metabolism Metabolism Consist of Highly Interconnected Pathways The basic strategy of catabolic metabolism is to form ATP, NADPH, and building blocks for biosyntheses. 1. ATP is the universal
Integration Of Metabolism Metabolism Consist of Highly Interconnected Pathways The basic strategy of catabolic metabolism is to form ATP, NADPH, and building blocks for biosyntheses. 1. ATP is the universal
Interplay between FGF21 and insulin action in the liver regulates metabolism
 Research article Interplay between FGF21 and insulin action in the liver regulates metabolism Brice Emanuelli, 1 Sara G. Vienberg, 1 Graham Smyth, 1 Christine Cheng, 2 Kristin I. Stanford, 1 Manimozhiyan
Research article Interplay between FGF21 and insulin action in the liver regulates metabolism Brice Emanuelli, 1 Sara G. Vienberg, 1 Graham Smyth, 1 Christine Cheng, 2 Kristin I. Stanford, 1 Manimozhiyan
Metabolic integration and Regulation
 Metabolic integration and Regulation 109700: Graduate Biochemistry Trimester 2/2016 Assistant Prof. Dr. Panida Khunkaewla kpanida@sut.ac.th School of Chemistry Suranaree University of Technology 1 Overview
Metabolic integration and Regulation 109700: Graduate Biochemistry Trimester 2/2016 Assistant Prof. Dr. Panida Khunkaewla kpanida@sut.ac.th School of Chemistry Suranaree University of Technology 1 Overview
Biosynthesis of Fatty Acids. By Dr.QUTAIBA A. QASIM
 Biosynthesis of Fatty Acids By Dr.QUTAIBA A. QASIM Fatty Acids Definition Fatty acids are comprised of hydrocarbon chains terminating with carboxylic acid groups. Fatty acids and their associated derivatives
Biosynthesis of Fatty Acids By Dr.QUTAIBA A. QASIM Fatty Acids Definition Fatty acids are comprised of hydrocarbon chains terminating with carboxylic acid groups. Fatty acids and their associated derivatives
