Cisplatin Protects against Acute Liver Failure by Inhibiting Nuclear HMGB1 Release
|
|
|
- Julia Hodge
- 5 years ago
- Views:
Transcription
1 Int. J. Mol. Sci. 2013, 14, ; doi: /ijms Article OPEN ACCESS International Journal of Molecular Sciences ISSN Cisplatin Protects against Acute Liver Failure by Inhibiting Nuclear HMGB1 Release Xun Li, Li-Kun Wang, Lu-Wen Wang, Xiao-Qun Han, Fan Yang and Zuo-Jiong Gong * Department of Infectious Diseases, Renmin Hospital of Wuhan University, Wuhan University, Wuhan , China; s: xunlee2011@gmail.com (X.L.); lkwang999@163.com (L.-K.W.); wangluw8253@163.com (L.-W.W.); hxqqqqq520@126.com (X.-Q.H.); yangfanwhu2012@163.com (F.Y.) * Author to whom correspondence should be addressed; zjgong@163.com; Tel.: (ext ); Fax: Received: 4 April 2013; in revised form: 15 May 2013 / Accepted: 21 May 2013 / Published: 27 May 2013 Abstract: Cisplatin is one of the most widely used chemical drugs for anticancer treatment. Recent studies have focused on the ability of cisplatin to retain the high mobility group box 1 (HMGB1) protein in cisplatin-dna adducts, thereby preventing its release from the nucleus. Because HMGB1 is a powerful inflammatory mediator in many diseases, the aim of this study is to evaluate the therapeutic effect of cisplatin acute liver failure. In this study, low-dose cisplatin was administered to treat PMA-induced macrophage-like cells induced by PMA and rats with acute liver failure. We found that cell viability and liver injury were greatly improved by cisplatin treatment. The extracellular levels of HMGB1, TNF-α and IFN-γ were also significantly decreased by the administration of cisplatin. During inflammation, nuclear HMGB1 translocates from the nucleus to the cytoplasm. The administration of cisplatin reduced the cytoplasmic levels of HMGB1 and increased nuclear HMGB1 levels in vitro and in vivo. In conclusion, cisplatin can protect against acute liver failure by retaining HMGB1 in the nucleus and preventing its release into the extracellular milieu. Keywords: cisplatin; acute liver failure; high mobility group box-1; HMGB1 translocation; inflammation
2 Int. J. Mol. Sci. 2013, Introduction High mobility group box 1 (HMGB1) is a non-histone DNA-binding protein that has been identified as a chromatin architectural factor that facilitates gene transcription, DNA replication and repair by mediating DNA bending [1 3]. In addition to its intranuclear function, the extracellular function of HMGB1 has also been studied in detail. Many studies have shown that extracellular HMGB1 functions as a cytokine. As an inflammatory mediator, HMGB1 plays a crucial role in many diseases including acute pancreatitis [4], acute lung injury [5], rheumatoid arthritis [6], hemorrhagic shock [7] and ischemia-reperfusion injury [8]. Treatment with anti-hmgb1 agents, either chemical or biological, could alleviate inflammatory responses in these diseases. Our previous study (in press) demonstrated that treatment with an anti-hmgb1 antibody protects against acute-on-chronic liver failure in rats. Cisplatin is one of the most widely used anticancer chemical drugs. It has been used to treat a range of tumors including head and neck, lung, breast, ovarian and cervical cancers [9]. As an anticancer agent, cisplatin exerts its cytotoxic role in tumor cells by damaging DNA through the formation of covalent bonds with purine bases [10]. Fundamental cellular processes, including replication, transcription, translation and DNA repair are inhibited by the covalent DNA platinum adducts [11]. It has been reported that cisplatin treatment at relatively high doses results in the production of significant amounts of nitric oxide (NO) and pro-inflammatory cytokines in macrophages and exhibits increased tumoricidal activity [12]. Moreover, low-dose cisplatin administration in murine cecal ligation and puncture (CLP) has been shown to prevent the systemic release of HMGB1 and attenuate lethality [13]. HMGB1 binds to platinized lesions on DNA with a specificity for 1, 2-intrastrand cross-links, which account for the cisplatin DNA adducts formed mostly in vivo [14]. Considering the ability of cisplatin to sequester HMGB1 and the cytokine activity of HMGB1, it is reasonable to hypothesize that cisplatin could be used for the treatment of inflammation. Nontoxic low-dose cisplatin treatment studies have also shown that cisplatin can confer protection against sepsis and ischemia/reperfusion injury in mice [13,15]. To explore whether cisplatin can be used as a new drug for acute liver failure (ALF) treatment, we evaluated the effects of cisplatin on U937 cells and in the ALF model in rats induced by D-Gal combined with LPS. 2. Results and Discussion 2.1. Effect of Cisplatin on the Viability of Macrophage-Like Cells The viability of macrophage-like cells was measured by CCK-8 assay. Cell viability was not decreased by cisplatin at concentrations below 10 μm (Figure 1A). However, viability of the cells decreased remarkably when LPS (1 μg/ml) was added to the culture medium (p < 0.05), and improved when 10 μm cisplatin was added (Figure 1B).
3 Int. J. Mol. Sci. 2013, Figure 1. Viability of macrophage-like cells measured by CCK-8 assay. (A) Toxic effect of cisplatin (0 50 μm), relative to medium without cisplatin, * p < 0.05; (B) Protective effect of low-dose cisplatin. Cell viability is presented as the densitometry value. A higher densitometryvalue indicates higher viability of macrophage-like cells. Compared to the control group, * p < 0.05; compared to the LPS-treated group, ** p < Results are expressed as the mean ± SD (n = 4) Effect of Cisplatin on Cytokine Production in Macrophage-Like Cells Previous data has led us to believe that a low dose of cisplatin can modulate immunoresponses. To test this hypothesis, we measured TNF-α and IFN-γ in cell supernatants after LPS exposure with and without low dose Cisplatin. The levels of TNF-α and IFN-γ in cell supernatants were measured by ELISA.TNF-α and IFN-γ levels were significantly increased in LPS-treated group ( ± pg/ml and ± pg/ml, respectively) relative to the control group (131.5 ± 7.72 pg/ml and ± pg/ml, respectively) (p < 0.05). However, TNF-α and IFN-γ levels were markedly reduced by 46.54% and 43.47% in the LPS + cisplatin-treated group ( ± pg/ml and ± pg/ml, respectively) (p < 0.05) (Figure 2). These data show that cisplatin somehow reduce the sera expression of the inflammatory mediators. Figure 2. Effect of cisplatin on TNF-α and IFN-γ in macrophage-like cells. (A) TNF-α level; (B) IFN-γ level. Compared with the control group, * p < 0.05; compared with the LPS-treated group, ** p < Results are expressed as the mean ± SD (n = 4).
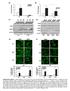
4 Int. J. Mol. Sci. 2013, Effect of Cisplatin on HMGB1 in Macrophage-Like Cells To observe the changes of HMGB1 in location, immunofluorescence experiments were performed. The results showed that HMGB1 exists mainly in the nucleus of macrophage-like cells. LPS treatment induces translocation of HMGB1 from the nucleus to the cytoplasm and extracellular matrix. In the LPS + cisplatin-treated group of macrophage-like cells, HMGB1 was retained in the nucleus (Figure 3A). To confirm the changes of HMGB1 observed in immunofluorescence, HMGB1 levels in macrophage-like cells and cell supernatants were determined by Western blot analysis and ELISA, respectively. The ELISA results showed that supernatanthmgb1 levels increased greatly in the LPS-treated group ( ± ng/ml) relative to the control group (82.94 ± ng/ml) (p < 0.05). However, in the LPS + cisplatin-treated group, supernatant HMGB1 levels ( ± ng/ml) decreased by 51.58% relative to the LPS-treated group (p < 0.05) (Figure 3F). Western blot analysis of macrophage-like cells showed that the cytoplasmic levels of HMGB1 increased evidently in the LPS-treated group (0.946 ± 0.060) relative to the control group (0.227 ± 0.014) (p < 0.05), whereas the relative levels of HMGB1 were reduced by 51.27% in the LPS+cisplatin-treated group (0.461 ± 0.046) relative to the LPS-treated group (p < 0.05) (Figure 3B,C). In addition, nuclear HMGB1 levels decreased in the LPS-treated group (0.238 ± 0.057) relative to the control group (0.822 ± 0.030) (p < 0.05), whereas relative levels of HMGB1 increased by % in LPS + cisplatin-treated group (0.951 ± 0.051) relative to the LPS-treated group(p < 0.05) (Figure 3D,E). These data indicate that cisplatin can prevent the translocation of HMGB1 from the nucleus to the cytoplasm and decrease extracellular HMGB1 level Effect of Cisplatin on ALT Level and Apoptosis of Hepatic Tissue Because cisplatin exerts its anti-tumor effect through the activation of apoptosis, the apoptosis of hepatic tissue was measured to evaluate the apoptosis of hepatocytes when ALF occurred. TUNEL assay showed that apoptosis was rarely observed in the control group, and was evidently increased after ALF induction in the model group. However, compared with the model group, less apoptosis was observed in the cisplatin treatment group (Figure 4A). To determine whether the model was induced successfully and the protective effect of cisplatin on ALF, ALT levels in sera were detected. The results (Figure 4B) showed that ALT levels increased markedly in the model group ( ± IU/L) compared to the control group ( ± IU/L) (p < 0.05), and cisplatin administration attenuated ALT levels by 45.82% in the cisplatin treatment group ( ± IU/L) (p < 0.05). These results suggest that the ALF model is induced successfully with D-Gal and LPS, and treatment with a low dose of cisplatin reduces apoptosis and necrosis of liver cells in ALF.
5 Int. J. Mol. Sci. 2013, Figure 3. Effect of cisplatin on HMGB1in macrophage-like cells. (A) Immunofluorescence analysis of HMGB1. HMGB1 was stained green, and nuclei were stained blue, (original magnification 400). a: Control group, HMGB1 (green fluorescence) was localized mainly in the nucleus; b: LPS-treated group, HMGB1 was localized mainly in the cytoplasm and extracellular matrix; c: LPS+cisplatin-treated group, although HMGB1 present both in nucleus and extracellular matrix, HMGB1 mainly existed in the nucleus; (B) Cytoplasmic levels of HMGB1 detected by Western blot analysis. β-actin was probed as an additional loading control. Control: control group, LPS: LPS-treated group, CISPLATIN: LPS + cisplatin-treated group; (C) Semi-quantification of cytoplasmic levels of HMGB1. The relative protein expression levels from three independent experiments are represented as the density of HMGB1 bands normalized to that of β-actin bands. Compared with the control group, * p < 0.05; compared with the LPS-treated group, ** p < Results are expressed as the mean ± SD (n = 3); (D) Nuclear levels of HMGB1 detected by Western blot analysis. Histone3 was probed as an additional loading control. Control: control group, LPS: LPS-treated group, CISPLATIN: LPS + cisplatin-treated group; (E) Semi-quantification of nuclear levels of HMGB1. The relative protein expression levels from three independent experiments are represented as the density of HMGB1 bands normalized to that of Histone3 bands. Compared with the control group, * p< 0.05; compared with the LPS-treated group, ** p< Results are expressed as the mean ± SD (n = 3); (F) The supernatant levels of HMGB1. Compared to the control group, * p< 0.05; compared with the LPS-treated group, ** p< Results are expressed as the mean ± SD (n = 4).
6 Int. J. Mol. Sci. 2013, Figure 4. Effect of cisplatin on serum ALT levels and apoptosis in hepatic tissue. (A) Apoptosis in hepatic tissue was detected by TUNEL assay, where apoptosis cells were stained green. a: Control group, apoptosis cells were rarely observed; b: Model group, many apoptosis cells were observed; c: Cisplatin treatment group, relatively fewer apoptosis cells were observed relative to the model group; (B) ALT levels in serum, control: Control group, model: Model group, cisplatin: Cisplatin treatment group. Compared to the control group, * p < 0.05; compared to the model group, ** p < Results are expressed as the mean ± SD (n = 5) Effect of Cisplatin on Survival Time To assess the outcome of cisplatin in treating ALF, the survival time of rats were observed. All of the ALF rats died within four days without cisplatin treatment, and their median survival time was 60 h. However, cisplatin treatment prolonged the median survival time of the ALF rats to 90 h. As determined by log-rank test, there was a significant difference between the survival curves for the two groups (χ 2 = 22.21, p < ) (Figure 5). The result indicates that low-dose cisplatin administration increases survival rate of rats in ALF. Figure 5. Effect of cisplatin on the survival time of rats.
7 Int. J. Mol. Sci. 2013, Effect of Cisplatin on Cytokine Production in ALF To evaluate whether the effect of cisplatin on cytokine production was consistent with that in inflammatory cells, TNF-α and IFN-γ levels were also detected in rats with ALF. As shown in Figure 6, TNF-α and IFN-γ levels increased remarkably in the model group ( ± 7.35 pg/ml and ± 6.99 pg/ml, respectively) relative to the control group (38.65 ± 3.37 pg/ml and ± 3.09 pg/ml, respectively). The levels of these cytokines decreased by 23.72% and 25.41% after administration of cisplatin in the model group ( ± pg/ml and ± 4.69 pg/ml, respectively) (p < 0.05). The data suggest that as demonstrated in vitro, a low dose of cisplatin is also able to reduce cytokine production in vivo. Figure 6. Effect of cisplatin on TNF-α and IFN-γ in ALF. (A) Serum levels of TNF-α; (B) Serum levels of IFN-γ. Control: Control group, model: Model group, cisplatin: Cisplatin treatment group. Compared to the control group, * p < 0.05; compared to the model group, ** p < Results are expressed as the mean ± SD (n = 5) Effect of Cisplatin on HMGB1 in ALF Similar to the results seen in macrophage-like cells, the results of immunofluorescence of hepatic tissue showed that HMGB1 existed mainly in the nucleus of liver cells in the control group. However, upon induced ALF, HMGB1 translocated from the nucleus to cytoplasm, whereas HMGB1 was retained in the nucleus in the cisplatin treatment group (Figure 7A). HMGB1 levels in rat sera and hepatic tissue lysates were determined by ELISA and Western blot analysis, respectively. The ELISA results showed that the serum levels of HMGB1 increased greatly in the model group (55.77 ± 8.46 ng/ml) relative to the control group (11.57 ± 3.53 ng/ml) (p < 0.05) but decreased by 40.90% in the cisplatin treatment group (32.96 ± 3.81 ng/ml) relative to the model group (p < 0.05) (Figure 7F). Western blot analysis of HMGB1 levels in hepatic tissue showed that cytoplasmic levels of HMGB1 increased in the model group (0.652 ± 0.049) relative to the control group (0.257 ± 0.041), but relative nuclear HMGB1 levels in the model group (0.458 ± 0.039) were markedly lower than those of the control group (0.629 ± 0.061) (p < 0.05). Treatment with cisplatin reduced the cytoplasmic HMGB1 protein levels by 25% in hepatic tissue (0.470 ± 0.041), and increased the nuclear HMGB1 levels by % (0.918 ± 0.028) (p < 0.05) (Figure 7B E). The data demonstrate that a low dose of cisplatin is able to inhibit HMGB1 translocation in ALF.
8 Int. J. Mol. Sci. 2013, Figure 7. Effect of cisplatin on HMGB1inALF. (A) Immunofluorescence staining of HMGB1 in hepatic tissue. HMGB1 was stained green, and nuclei were stained blue, (original magnification 400). a: Control, HMGB1 (green fluorescence) was observed mainly in nucleus; b: Model group, HMGB1 was observed mainly in the cytoplasm; c: Cisplatin treatment group, HMGB1 was found in the nucleus and cytoplasm; (B) Cytoplasmic levels of HMGB1 detected by Western blot analysis. β-actin was probed as an additional loading control. Control: Control group, model: Model group, Cisplatin: Cisplatin treatment group; (C) Semi-quantification of cytoplasmic HMGB1 levels. The values for expression are from three independent experiments and are represented as the density of HMGB1 bands normalized to that of β-actin bands. Compared to the control group, * p < 0.05; compared to the model group, ** p < Results are expressed as the mean ± SD (n = 3); (D) Nuclear levels of HMGB1 detected by Western blot analysis. Histone3 was probed as an additional loading control. Control: Control group, Model: Model group, Cisplatin: Cisplatin treatment group; (E) Semi-quantification of nuclear HMGB1 levels. The values of relative protein expression levels are from three independent experiments and are represented as the density of HMGB1bands normalized to Histone3 bands. Compared to the control group, * p < 0.05; compared to the model group, ** p < Results are expressed as the mean ± SD (n = 3); (F) Serum levels of HMGB1. Control: Control group, model: Model group, cisplatin: Cisplatin treatment group. Results are expressed as the mean ± SD (n = 5).
9 Int. J. Mol. Sci. 2013, ALF is a severe syndrome with high lethality, and its pathological mechanisms are not yet fully understood. Many factors have been implicated in the pathogenesis of the disease including endotoxin and inflammatory cytokines [16 18]. Intraperitoneal injection with D-Gal and LPS to induce acute liver injury is a classic model for ALF research. The monocyte-like cell line U-937 is widely used in inflammation research, when differentiated by phorbol myristate acetate (PMA) and activated by lipopolysaccharide (LPS) [19]. Because inflammatory cells play an important role in the pathogenesis of ALF, we utilized differentiated U937 cells and an acute liver failure model using D-Gal and LPS to evaluate the therapeutic effects of cisplatin on ALF. Theoretically, cisplatin exerts its biological effects by interacting with DNA to form DNA adducts, which activate several signal transduction pathways, including those involving ATR, p53, p73 and MAPK, thereby culminating in the activation of apoptosis [20]. Many proteins such as mismatch repair (MMR) complex, high-mobility groups 1 and 2 (HMG1 and HMG2) proteins, upstream binding factor (hubf), and TATA binding protein (TBP) contribute to these pathway by binding to the cisplatin DNA adducts [20]. Since its first demonstrated in ischemia/reperfusion injury [15], the anti-inflammation role of cisplatin has sequentially been confirmed in CLP-induced sepsis [13] and post-transplantation pancreatitis [21]. In this study, we demonstrate that a nontoxic dose of cisplatin can improve the viability of macrophage-like cells and significantly attenuated liver cells injury and apoptosis. The survival time of the animals was also prolonged by cisplatin treatment. These results indicate that a nontoxic dose of cisplatin is beneficial for ALF.HMGB1 has been identified as a secreted extracellular mediator of inflammation and repair responses in LPS-induced endotoxemia and sepsis [22]. It is actively secreted by immunostimulated macrophages [23 26] and enterocytes [27] and is also released by necrotic, but not apoptotic cells [28]. Once released into the extracellular milieu, HMGB1 binds to many extracellular receptors, such as RAGE, TLR2, TLR4 and TLR9 [29 31]. By interacting with different receptors, HMGB1 mediates a variety of responses including epithelial barrier dysfunction, the release of pro-inflammatory cytokines and acute inflammation [32]. In addition, the administration of HMGB1 to normal animals leads to systemic inflammatory responses [29], which suggest a role for extracellular HMGB1 in the induction of inflammatory responses. In this study, we also measured the levels of TNF-α and IFN-γ in the supernatant of macrophage-like cells and serum from rats, our results show that TNF-α and IFN-γ levels were also decreased by the administration of cisplatin in vitro and in vivo. In this study, we showed that the production of inflammatory cytokines is attenuated by cisplatin. To determine whether this effect was associated with extracellular HMGB1, the levels of HMGB1 in the serum and cell supernatant were measured. Extracellular levels of HMGB1 increased significantly both in vitro and in vivo, and this increase was attenuated by cisplatin treatment. Cisplatin can bind to DNA and cause a dramatic distortion in the duplex structure. HMGB1 is able to recognize the distorted DNA structures and binds to them stably, especially via the formation of 1, 2-intrastrand cross-links [33]. The main effect of cisplatin on HMGB1 known so far is that it sequesters HMGB1 to the cisplatin-dna adducts and prevents its release from the nucleus to the cytoplasm and extracellular milieu, this effect of cisplatin on HMGB1 in macrophage-like cells and hepatic tissue was evaluated by immunofluorescence. As demonstrated in this study, HMGB1 exists mainly in the nucleus of macrophage-like cells and liver cells, and the translocation of HMGB1 occurs when cells are stimulated by LPS or after ALF induction. To determine whether cisplatin treatment could suppress the release of HMGB1 from the
10 Int. J. Mol. Sci. 2013, nucleus to the extracellular milieu, HMGB1 levels in macrophage-like cells and hepatic tissue were also determined. There were higher levels of HMGB1 in the nucleus and lower levels were observed in the cytoplasm in the cisplatin-treated group relative to the model group. Although the cause and mechanism of ALF pathogenesis are still unclear, liver cell necrosis and inflammatory cell infiltration can be observed in disease-affected tissue. In this study, the effects of cisplatin were evaluated both in vitro and in vivo, and hepato-protective effect of cisplatin was demonstrated in rats with ALF. Our results show that extracellular HMGB1 plays a key role in the inflammatory responses during ALF pathogenesis and that cisplatin reduces extracellular HMGB1 levels by inhibiting the translocation of HMGB1 from the nucleus to the cytoplasm and extracellular milieu. 3. Experimental Section 3.1. Cell Culture and Treatment U-937 cells (ATCC, Manassas, VA, USA) were cultured and maintained at a cell density between and cells/ml in RPMI 1640 (Jinuo, Hangzhou, China) supplemented with 10% fetal bovine serum (Sijiqing, Huzhou, China), 100 U/mL penicillin, and 100 μg/ml streptomycin. For macrophage-like cell differentiation, serum starved U-937 cells were plated in a 35-mm cell culture dish ( cells/ml) and treated with 60 ng/ml PMA for 24 h. The non-adherent cells were removed by two PBS washes. Complete RPMI 1640 medium containing 1 μg/ml LPS or 1 μg/ml LPS combined with cisplatin (different doses) was applied in subsequent experiments. Cultural supernatants were collected for cytokine detection Animals Male-specific pathogen-free (SPF) Wistar rats, weighing 180 to 220 g, were purchased from the Experimental Animal Center of Wuhan University. All animals were housed in a light-controlled room (12 h light/dark cycle) at an ambient temperature of 25 C with free access to water and standard chow. All animals were given humane care in accordance with institutional guidelines Model Production and Sample Collection The animals were randomly divided into three groups: normal group, model group and cisplatin treatment group. The rat ALF model was induced by D-Gal and LPS. The rats in the model group (n = 15) received 400 mg/kg D-Gal 100 μg/kg LPS by intraperitoneal injection. In addition to D-Gal and LPS, the rats in the cisplatin treatment group (n = 15) received 100 μg/kg cisplatin [14] by intraperitoneal injection 2 h after ALF induction. The rats in the control group (n = 15) were injected intraperitoneally with normal saline solution (NSS) as a control. The time of administration of D-Gal combined with LPS was assigned as the baseline (time point 0). Five animals from each group were sacrificed for blood and hepatic tissue collection at the 24 h time point. The remaining 10 rats were observed for survival time every 12 h for 7 days after induction of the ALF.
11 Int. J. Mol. Sci. 2013, Cell Viability Test Cell viability was assessed using Cell Counting Kit-8 (Dojindo, Shanghai, China) assay. Cells ( cells/well) were plated in 96-well plates for viability measurement. Experimental procedures were performed according to the manufacturer s protocol TUNEL Assay Portions of liver samples were fixed in 10% buffered formalin for 24 h, embedded in paraffin and sliced into sections of 5-μm thickness. The sections were incubated in 0.1% Triton X-100 for 8 min and washed twice in PBS for 5 min each. In situ labeling of apoptosis-induced DNA strand breaks (TUNEL assay) was performed using a commercially available kit (in situ Cell Death Detection Kit, Roche, Switzerland) Analysis of Cell Supernatants and Blood Samples The levels of HMGB1, TNF-α and IFN-γ in cell supernatantsand sera were measured using ELISA kits (HMGB1 from Westang, Shanghai, China, TNF-α and IFN-γ from Ebioscience, San Diego, CA, USA) according to the manufacturer s recommendations. The alanine transaminase (ALT) level was determined by routine biochemical methods using a Hitachi Automatic Analyzer (Hitachi, Inc., Tokyo, Japan) Extraction of Nuclear and Cytoplasmic Protein from Cells and Hepatic Tissue Nuclear and cytoplasmic proteins were isolated by using a nuclear and cytoplasmic protein extraction kit (Beyotime, Nantong, China). Experimental procedures were conducted according to the manufacturer s instructions Western Blot Analysis of HMGB1 Levels from U937 Cells and Hepatic Tissue Briefly, 50 μg of different protein extracts was subjected to 10% SDS-PAGE and transferred onto a PVDF membrane (Millipore, Billerica, MA, USA). The membrane was incubated with anti-hmgb1 antibody (Epitomics, Burlingame, CA, USA), followed by incubation with a secondary antibody (LICOR, Lincoln, NE, USA), and then it was analyzed by ODESSEY infrared imaging system (LICOR, Lincoln, NE, USA). Membranes were also probed for beta-actin and histone 3 (Santa Cruz, CA, USA) as additional loading controls Immunohistological Analysis of HMGB1 Tissue sections were dewaxed in xylene and rehydrated in a series of dilutions of alcohol. The sections and cover glasses with fixed U937 cells were incubated in 0.1% Triton X-100 for 10 min, and washed twice in PBS for 5 min. The tissue sections were then placed in 3% H 2 O 2 for 20 min to block endogenous peroxidase activity and boiled in sodium citrate buffer (ph = 6.0) for 15 min. The sections and fixed cells were blocked with 10% normal goat serum in a humid chamber at 37 C for 20 min to reduce non-specific binding, and then incubated with a primary rabbit anti-hmgb1 antibody at a
12 Int. J. Mol. Sci. 2013, :500 dilution at 4 C overnight. The sections were then incubated with fluorescein isothiocyanate (FITC)-conjugated goat anti-rabbit IgG (Boster Biotechnology, Wuhan, China) for 60 min at 37 C in the dark, counterstained with 2 μg/ml 2-(4-Amidinophenyl)-6-indolecarbamidine dihydrochloride (DAPI) (Beyotime, Nantong, China) for 2 min and observed under animmunofluorescence microscope Statistical Analysis Statistical analysis was performed using SPSS software (version 13.0 for Windows). Data are presented as the mean ± SD, and comparisons were made using Student s t test and one-way ANOVA. Animal survival data were evaluated using the Kaplan Meier method and compared using the log-rank test. A probability value of 0.05 or less was considered statistically significant. 4. Conclusions Cisplatin confers protection against acute liver failure. The mechanism of protection potentially involves cisplatin-mediated sequestration of HMGB1 in the nucleus and inhibition of its translocation from the nucleus into the extracellular milieu. Acknowledgments This study was supported by a grant from the National Natural Science Foundation of China (No ). Conflict of Interest The authors declare no conflict of interest. References 1. Lotze, M.T.; Tracey, K.J. High-mobility group box 1 protein (HMGB1): Nuclear weapon in the immune arsenal. Nat. Rev. Immunol. 2005, 5, Zhang, C.C.; Krieg, S.; Shapiro, D.J. HMG-1 stimulates estrogen response element binding by estrogen receptor from stably transfected HeLa cells. Mol. Endocrinol. 1999, 13, Bustin, M. Regulation of DNA-dependent activities by the functional motifs of the high-mobility-group chromosomal proteins. Mol. Cell. Biol. 1999, 19, Yasuda, T.; Ueda, T.; Shinzeki, M.; Sawa, H.; Nakajima, T.; Takeyama, Y.; Kuroda, Y. Increase of high-mobility group box chromosomal protein 1 in blood and injured organs in experimental severe acute pancreatitis. Pancreas 2007, 34, Ueno, H.; Matsuda, T.; Hashimoto, S.; Amaya, F.; Kitamura, Y.; Tanaka, M.; Kobayashi, A.; Maruyama, I.; Yamada, S.; Hasegawa, N.; et al. Contributions of high mobility group box protein in experimental and clinical acute lung injury. Am. J. Respir. Crit. Care Med. 2004, 170, Hamada, T.; Torikai, M.; Kuwazuru, A.; Tanaka, M.; Horai, N.; Fukuda, T.; Yamada, S.; Nagayama, S.; Hashiguchi, K.; Sunahara, N.; et al. Extracellular high mobility group box chromosomal protein 1 is a coupling factor for hypoxia and inflammation in arthritis. Arthritis Rheum. 2008, 58,
13 Int. J. Mol. Sci. 2013, Fan, J.; Li, Y.; Levy, R.M.; Hackam, D.J.; Vodovotz, Y.; Yang, H.; Tracey, K.J.; Billiar, T.R.; Wilson, M.A. Hemorrhagic shock induces NAD(P)H oxidase activation in neutrophils: Role of HMGB1-TLR4 signaling. J. Immunol. 2007, 178, Tsung, A.; Sahai, R.; Tanaka, H.; Nakao, A.; Fink, M.P.; Lotze, M.T.; Yang, H.; Li, J.; Tracey, K.J.; Geller, D.A.; et al. The nuclear factor HMGB1 mediates hepatic injury after murine liver ischemia-reperfusion. J. Exp. Med. 2005, 201, Rabik, C.A.; Dolan, M.E. Molecular mechanisms of resistance and toxicity associated with platinating agents. Cancer Treat Rev. 2007, 33, Jamieson, E.R.; Lippard, S.J. Structure, recognition, and processing of cisplatin-dna adducts. Chem. Rev. 1999, 99, Woźniak, K.; Błasiak, J. Recognition and repair of DNA-cisplatin adducts. Acta Biochim. Pol. 2002, 49, Chauhan, P.; Sodhi, A.; Shrivastava, A. Cisplatin primes murine peritoneal macrophages for enhanced expression of nitric oxide, proinflammatory cytokines, TLRs, transcription factors and activation of MAP kinases upon co-incubation with L929 cells. Immunobiology 2009, 214, Pan, P.; Cardinal, J.; Dhupar, R.; Rosengart, M.R.; Lotze, M.T.; Geller, D.A.; Billiar, T.R.; Tsung, A. Low-dose cisplatin administration in murine cecal ligation and puncture prevents the systemic release of HMGB1 and attenuates lethality. J. Leukoc. Biol. 2009, 86, Park, S.; Lippard, S.J. Redox state-dependent interaction of HMGB1 and cisplatin-modified DNA. Biochemistry 2011, 50, Cardinal, J.; Pan, P.; Dhupar, R.; Ross, M.; Nakao, A.; Lotze, M.; Billiar, T.; Geller, D.; Tsung, A. Cisplatin prevents high mobility group box 1 release and is protective in a murine model of hepatic ischemia/reperfusion injury. Hepatology 2009, 50, Sozinov, A.S. Systemic endotoxemia during chronic viral hepatitis. Bull. Exp. Biol. Med. 2002, 133, Beutler, B.; Rietschel, E.T. Innate immune sensing and its roots: The story of endotoxin. Nat. Rev. Immunol. 2003, 3, Akira, S.; Uematsu, S.; Takeuchi, O. Pathogen recognition and innate immunity. Cell 2006, 124, Del Bufalo, A.; Bernad, J.; Dardenne, C.; Verda, D.; Meunier, J.R.; Rousset, F.; Martinozzi-Teissier, S.; Pipy, B. Contact sensitizers modulate the arachidonic acid metabolism of PMA-differentiated U-937 monocytic cells activated by LPS. Toxicol. Appl. Pharmacol. 2011, 256, Siddik, Z.H. Cisplatin: Mode of cytotoxic action and molecular basis of resistance. Oncogene 2003, 22, Yan, S.; Ding, Y.; Sun, F.; Lu, Z.; Xue, L.; Liu, X.; Shuai, M.; Fang, C.; Wang, Y.; Cheng, H.; et al. Pretreatment of cisplatin in recipients attenuates post-transplantation pancreatitis in murine model. Int. J. Biol. Sci. 2012, 8, Yang, H.; Ochani, M.; Li, J.; Qiang, X.; Tanovic, M.; Harris, H.E.; Susarla, S.M.; Ulloa, L.; Wang, H.; DiRaimo, R.; et al. Reversing established sepsis with antagonists of endogenous high-mobility group box 1. Proc. Natl. Acad. Sci. USA 2004, 101,
14 Int. J. Mol. Sci. 2013, Wang, H.; Bloom, O.; Zhang, M.; Vishnubhakat, J.M.; Ombrellino, M.; Che, J.; Frazier, A.; Yang, H.; Ivanova, S.; Borovikova, L.; et al. HMG-1 as a late mediator of endotoxin lethality in mice. Science 1999, 285, Rendon-Mitchell, B.; Ochani, M.; Li, J.; Han, J.; Wang, H.; Yang, H.; Susarla, S.; Czura, C.; Mitchell, R.A.; Chen, G.; et al. IFN-gamma induces high mobility group box 1 protein release partly through a TNF-dependent mechanism. J. Immunol. 2003, 170, Gardella, S.; Andrei, C.; Ferrera, D.; Lotti, L.V.; Torrisi, M.R.; Bianchi, M.E.; Rubartelli, A. The nuclear protein HMGB1 is secreted by monocytes via a non-classical, vesicle-mediated secretory pathway. EMBO Rep. 2002, 3, Bonaldi, T.; Talamo, F.; Scaffidi, P.; Ferrera, D.; Porto, A.; Bachi, A.; Rubartelli, A.; Agresti, A.; Bianchi, M.E. Monocytic cells hyperacetylate chromatin protein HMGB1 to redirect it towards secretion. EMBO J. 2003, 22, Liu, S.; Stolz, D.B.; Sappington, P.L.; Macias, C.A.; Killeen, M.E.; Tenhunen, J.J.; Delude, R.L.; Fink, M.P. HMGB1 is secreted by immunostimulated enterocytes and contributes to cytomix-induced hyperpermeability of Caco-2 monolayers. Am. J. Physiol. Cell Physiol. 2006, 290, C990 C Scaffidi, P.; Misteli, T.; Bianchi, M.E. Release of chromatin protein HMGB1 by necrotic cells triggers inflammation. Nature 2002, 418, Yang, H.; Wang, H.; Czura, C.J.; Tracey, K.J. The cytokine activity of HMGB1. J. Leukoc. Biol. 2005, 78, Wang, H.; Yang, H.; Czura, C.J.; Sama, A.E.;Tracey, K.J. HMGB1 as a late mediator of lethal systemic inflammation. Am. J. Respir. Crit. Care Med. 2001, 164, Lu, Y.C.; Yeh, W.C.; Ohashi, P.S. LPS/TLR4 signal transduction pathway. Cytokine 2008, 42, Yang, H.; Tracey, K.J. Targeting HMGB1 in inflammation. Biochim. Biophys. Acta 2010, 1799, Zhang, C.X.; Chang, P.V.; Lippard, S.J. Identification of nuclear proteins that interact with platinum-modified DNA by photoaffinity labeling. J. Am. Chem. Soc. 2004, 126, by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (
Part-I: Screening. 3.1 Assessment of non-toxic concentration of test compounds in RAW cells. Results
 Part-I: Screening Our strategy was to look for compounds which can inhibit HMGB1 (high mobility group box 1) release in activated macrophages and check effect of screened compound on endotoxemic mice.
Part-I: Screening Our strategy was to look for compounds which can inhibit HMGB1 (high mobility group box 1) release in activated macrophages and check effect of screened compound on endotoxemic mice.
Programmed necrosis, not apoptosis, is a key mediator of cell loss and DAMP-mediated inflammation in dsrna-induced retinal degeneration
 Programmed necrosis, not apoptosis, is a key mediator of cell loss and DAMP-mediated inflammation in dsrna-induced retinal degeneration The Harvard community has made this article openly available. Please
Programmed necrosis, not apoptosis, is a key mediator of cell loss and DAMP-mediated inflammation in dsrna-induced retinal degeneration The Harvard community has made this article openly available. Please
Impact factor: Reporter:4A1H0019 Chen Zi Hao 4A1H0023 Huang Wan ting 4A1H0039 Sue Yi Zhu 4A1H0070 Lin Guan cheng 4A1H0077 Chen Bo xuan
 Curcumin Protects Neonatal Rat Cardiomyocytes against High Glucose-Induced Apoptosis via PI3K/Akt Signalling Pathway Wei Yu,1,2 Wenliang Zha,1 Zhiqiang Ke,1 Qing Min,2 Cairong Li,1 Huirong Sun,3 and Chao
Curcumin Protects Neonatal Rat Cardiomyocytes against High Glucose-Induced Apoptosis via PI3K/Akt Signalling Pathway Wei Yu,1,2 Wenliang Zha,1 Zhiqiang Ke,1 Qing Min,2 Cairong Li,1 Huirong Sun,3 and Chao
SUPPLEMENTARY INFORMATION. Involvement of IL-21 in the epidermal hyperplasia of psoriasis
 SUPPLEMENTARY INFORMATION Involvement of IL-21 in the epidermal hyperplasia of psoriasis Roberta Caruso 1, Elisabetta Botti 2, Massimiliano Sarra 1, Maria Esposito 2, Carmine Stolfi 1, Laura Diluvio 2,
SUPPLEMENTARY INFORMATION Involvement of IL-21 in the epidermal hyperplasia of psoriasis Roberta Caruso 1, Elisabetta Botti 2, Massimiliano Sarra 1, Maria Esposito 2, Carmine Stolfi 1, Laura Diluvio 2,
HMGB1 release by human liver L02 and HepG2 cells induced by lipopolysaccharide
 MOLECULAR MEDICINE REPORTS 8: 103-112, 2013 HMGB1 release by human liver L02 and HepG2 cells induced by lipopolysaccharide ZE-BING HUANG 1,2, XIA-HONG DAI 3, MEI-FANG XIAO 4, RONG-RONG ZHOU 1,2, SHU-SHAN
MOLECULAR MEDICINE REPORTS 8: 103-112, 2013 HMGB1 release by human liver L02 and HepG2 cells induced by lipopolysaccharide ZE-BING HUANG 1,2, XIA-HONG DAI 3, MEI-FANG XIAO 4, RONG-RONG ZHOU 1,2, SHU-SHAN
LPS LPS P6 - + Supplementary Fig. 1.
 P6 LPS - - - + + + - LPS + + - - P6 + Supplementary Fig. 1. Pharmacological inhibition of the JAK/STAT blocks LPS-induced HMGB1 nuclear translocation. RAW 267.4 cells were stimulated with LPS in the absence
P6 LPS - - - + + + - LPS + + - - P6 + Supplementary Fig. 1. Pharmacological inhibition of the JAK/STAT blocks LPS-induced HMGB1 nuclear translocation. RAW 267.4 cells were stimulated with LPS in the absence
(A) PCR primers (arrows) designed to distinguish wild type (P1+P2), targeted (P1+P2) and excised (P1+P3)14-
 1 Supplemental Figure Legends Figure S1. Mammary tumors of ErbB2 KI mice with 14-3-3σ ablation have elevated ErbB2 transcript levels and cell proliferation (A) PCR primers (arrows) designed to distinguish
1 Supplemental Figure Legends Figure S1. Mammary tumors of ErbB2 KI mice with 14-3-3σ ablation have elevated ErbB2 transcript levels and cell proliferation (A) PCR primers (arrows) designed to distinguish
Supplementary Information POLO-LIKE KINASE 1 FACILITATES LOSS OF PTEN-INDUCED PROSTATE CANCER FORMATION
 Supplementary Information POLO-LIKE KINASE 1 FACILITATES LOSS OF PTEN-INDUCED PROSTATE CANCER FORMATION X. Shawn Liu 1, 3, Bing Song 2, 3, Bennett D. Elzey 3, 4, Timothy L. Ratliff 3, 4, Stephen F. Konieczny
Supplementary Information POLO-LIKE KINASE 1 FACILITATES LOSS OF PTEN-INDUCED PROSTATE CANCER FORMATION X. Shawn Liu 1, 3, Bing Song 2, 3, Bennett D. Elzey 3, 4, Timothy L. Ratliff 3, 4, Stephen F. Konieczny
Intracellular MHC class II molecules promote TLR-triggered innate. immune responses by maintaining Btk activation
 Intracellular MHC class II molecules promote TLR-triggered innate immune responses by maintaining Btk activation Xingguang Liu, Zhenzhen Zhan, Dong Li, Li Xu, Feng Ma, Peng Zhang, Hangping Yao and Xuetao
Intracellular MHC class II molecules promote TLR-triggered innate immune responses by maintaining Btk activation Xingguang Liu, Zhenzhen Zhan, Dong Li, Li Xu, Feng Ma, Peng Zhang, Hangping Yao and Xuetao
Supplementary data Supplementary Figure 1 Supplementary Figure 2
 Supplementary data Supplementary Figure 1 SPHK1 sirna increases RANKL-induced osteoclastogenesis in RAW264.7 cell culture. (A) RAW264.7 cells were transfected with oligocassettes containing SPHK1 sirna
Supplementary data Supplementary Figure 1 SPHK1 sirna increases RANKL-induced osteoclastogenesis in RAW264.7 cell culture. (A) RAW264.7 cells were transfected with oligocassettes containing SPHK1 sirna
Integrin CD11b negatively regulates TLR-triggered inflammatory responses by. activating Syk and promoting MyD88 and TRIF degradation via cbl-b
 Integrin CD11b negatively regulates TLR-triggered inflammatory responses by activating Syk and promoting MyD88 and TRIF degradation via cbl-b Chaofeng Han, Jing Jin, Sheng Xu, Haibo Liu, Nan Li, and Xuetao
Integrin CD11b negatively regulates TLR-triggered inflammatory responses by activating Syk and promoting MyD88 and TRIF degradation via cbl-b Chaofeng Han, Jing Jin, Sheng Xu, Haibo Liu, Nan Li, and Xuetao
Chemical Chaperones Mitigate Experimental Asthma By Attenuating Endoplasmic
 Chemical Chaperones Mitigate Experimental Asthma By Attenuating Endoplasmic Reticulum Stress Lokesh Makhija, BE, Veda Krishnan, MSc, Rakhshinda Rehman, MTech, Samarpana Chakraborty, MSc, Shuvadeep Maity,
Chemical Chaperones Mitigate Experimental Asthma By Attenuating Endoplasmic Reticulum Stress Lokesh Makhija, BE, Veda Krishnan, MSc, Rakhshinda Rehman, MTech, Samarpana Chakraborty, MSc, Shuvadeep Maity,
Page 39 of 44. 8h LTA & AT h PepG & AT h LTA
 Page 39 of 44 Fig. S1 A: B: C: D: 8h LTA 8h LTA & AT7519 E: F: 8h PepG G: 8h PepG & AT7519 Fig. S1. AT7519 overrides the survival effects of lipoteichoic acid (LTA) and peptidoglycan (PepG). (A) Human
Page 39 of 44 Fig. S1 A: B: C: D: 8h LTA 8h LTA & AT7519 E: F: 8h PepG G: 8h PepG & AT7519 Fig. S1. AT7519 overrides the survival effects of lipoteichoic acid (LTA) and peptidoglycan (PepG). (A) Human
A549 and A549-fLuc cells were maintained in high glucose Dulbecco modified
 Cell culture and animal model A549 and A549-fLuc cells were maintained in high glucose Dulbecco modified Eagle medium supplemented with 10% fetal bovine serum at 37 C in humidified atmosphere containing
Cell culture and animal model A549 and A549-fLuc cells were maintained in high glucose Dulbecco modified Eagle medium supplemented with 10% fetal bovine serum at 37 C in humidified atmosphere containing
Correlation between estrogen receptor β expression and the curative effect of endocrine therapy in breast cancer patients
 1568 Correlation between estrogen receptor β expression and the curative effect of endocrine therapy in breast cancer patients LIYING GUO 1, YU ZHANG 2, WEI ZHANG 3 and DILIMINA YILAMU 1 1 Department of
1568 Correlation between estrogen receptor β expression and the curative effect of endocrine therapy in breast cancer patients LIYING GUO 1, YU ZHANG 2, WEI ZHANG 3 and DILIMINA YILAMU 1 1 Department of
Doctoral Degree Program in Marine Biotechnology, College of Marine Sciences, Doctoral Degree Program in Marine Biotechnology, Academia Sinica, Taipei,
 Cyclooxygenase 2 facilitates dengue virus replication and serves as a potential target for developing antiviral agents Chun-Kuang Lin 1,2, Chin-Kai Tseng 3,4, Yu-Hsuan Wu 3,4, Chih-Chuang Liaw 1,5, Chun-
Cyclooxygenase 2 facilitates dengue virus replication and serves as a potential target for developing antiviral agents Chun-Kuang Lin 1,2, Chin-Kai Tseng 3,4, Yu-Hsuan Wu 3,4, Chih-Chuang Liaw 1,5, Chun-
HIGH MOBILITY GROUP BOX 1 PROTEIN AS A LATE-ACTING MEDIATOR OF ACUTE LUNG INFLAMMATION
 International Journal of Occupational Medicine and Environmental Health, 2004; 17(2): 245 254 HIGH MOBILITY GROUP BOX 1 PROTEIN AS A LATE-ACTING MEDIATOR OF ACUTE LUNG INFLAMMATION WALDEMAR LUTZ 1 AND
International Journal of Occupational Medicine and Environmental Health, 2004; 17(2): 245 254 HIGH MOBILITY GROUP BOX 1 PROTEIN AS A LATE-ACTING MEDIATOR OF ACUTE LUNG INFLAMMATION WALDEMAR LUTZ 1 AND
Proteomic profiling of small-molecule inhibitors reveals dispensability of MTH1 for cancer cell survival
 Supplementary Information for Proteomic profiling of small-molecule inhibitors reveals dispensability of MTH1 for cancer cell survival Tatsuro Kawamura 1, Makoto Kawatani 1, Makoto Muroi, Yasumitsu Kondoh,
Supplementary Information for Proteomic profiling of small-molecule inhibitors reveals dispensability of MTH1 for cancer cell survival Tatsuro Kawamura 1, Makoto Kawatani 1, Makoto Muroi, Yasumitsu Kondoh,
Rabbit Polyclonal antibody to NFkB p65 (v-rel reticuloendotheliosis viral oncogene homolog A (avian))
 Datasheet GeneTex, Inc : Toll Free 1-877-GeneTex (1-877-436-3839) Fax:1-949-309-2888 info@genetex.com GeneTex International Corporation : Tel:886-3-6208988 Fax:886-3-6208989 infoasia@genetex.com Date :
Datasheet GeneTex, Inc : Toll Free 1-877-GeneTex (1-877-436-3839) Fax:1-949-309-2888 info@genetex.com GeneTex International Corporation : Tel:886-3-6208988 Fax:886-3-6208989 infoasia@genetex.com Date :
Antioxidant Inhibits HMGB1 Expression and Reduces Pancreas Injury in Rats with Severe Acute Pancreatitis
 DOI 10.1007/s10620-009-1073-0 ORIGINAL ARTICLE Antioxidant Inhibits HMGB1 Expression and Reduces Pancreas Injury in Rats with Severe Acute Pancreatitis Zhong Wei Zhang Qi Yu Zhang Meng Tao Zhou Na Xin
DOI 10.1007/s10620-009-1073-0 ORIGINAL ARTICLE Antioxidant Inhibits HMGB1 Expression and Reduces Pancreas Injury in Rats with Severe Acute Pancreatitis Zhong Wei Zhang Qi Yu Zhang Meng Tao Zhou Na Xin
Original Article TLR4 contributes to mechanical ventilation induced lung injury in the rabbits
 Int J Clin Exp Pathol 2017;10(4):4700-4704 www.ijcep.com /ISSN:1936-2625/IJCEP0040416 Original Article TLR4 contributes to mechanical ventilation induced lung injury in the rabbits Hongmei Zhang 1, Pei
Int J Clin Exp Pathol 2017;10(4):4700-4704 www.ijcep.com /ISSN:1936-2625/IJCEP0040416 Original Article TLR4 contributes to mechanical ventilation induced lung injury in the rabbits Hongmei Zhang 1, Pei
The effects of glycyrrhizin on acute pancreatitis in mice
 European Review for Medical and Pharmacological Sciences The effects of glycyrrhizin on acute pancreatitis in mice 2014; 18: 3943-3947 Y.-L. PAN Department of Emergency Medicine, People s Hospital of Zhengzhou,
European Review for Medical and Pharmacological Sciences The effects of glycyrrhizin on acute pancreatitis in mice 2014; 18: 3943-3947 Y.-L. PAN Department of Emergency Medicine, People s Hospital of Zhengzhou,
- 1 - Cell types Monocytes THP-1 cells Macrophages. LPS Treatment time (Hour) IL-6 level (pg/ml)
 Supplementary Table ST1: The dynamic effect of LPS on IL-6 production in monocytes and THP-1 cells after GdA treatment. Monocytes, THP-1 cells and macrophages (5x10 5 ) were incubated with 10 μg/ml of
Supplementary Table ST1: The dynamic effect of LPS on IL-6 production in monocytes and THP-1 cells after GdA treatment. Monocytes, THP-1 cells and macrophages (5x10 5 ) were incubated with 10 μg/ml of
Supplementary Materials for
 www.sciencesignaling.org/cgi/content/full/4/199/ra75/dc1 Supplementary Materials for Signaling by the Matrix Proteoglycan Decorin Controls Inflammation and Cancer Through PDCD4 and MicroRNA-21 Rosetta
www.sciencesignaling.org/cgi/content/full/4/199/ra75/dc1 Supplementary Materials for Signaling by the Matrix Proteoglycan Decorin Controls Inflammation and Cancer Through PDCD4 and MicroRNA-21 Rosetta
PUBLICATIONS. cells. J. Physiol. (London) 517P:91P (Manchester, England, UK).
 277 PUBLICATIONS Abstracts Haddad JJ, Land SC (1999). Differential activation of oxygen-responsive transcription factors over fetal-to-neonatal alveolar oxygen tensions in rat fetal distal lung epithelial
277 PUBLICATIONS Abstracts Haddad JJ, Land SC (1999). Differential activation of oxygen-responsive transcription factors over fetal-to-neonatal alveolar oxygen tensions in rat fetal distal lung epithelial
Essential Medium, containing 10% fetal bovine serum, 100 U/ml penicillin and 100 µg/ml streptomycin. Huvec were cultured in
 Supplemental data Methods Cell culture media formulations A-431 and U-87 MG cells were maintained in Dulbecco s Modified Eagle s Medium. FaDu cells were cultured in Eagle's Minimum Essential Medium, containing
Supplemental data Methods Cell culture media formulations A-431 and U-87 MG cells were maintained in Dulbecco s Modified Eagle s Medium. FaDu cells were cultured in Eagle's Minimum Essential Medium, containing
General Laboratory methods Plasma analysis: Gene Expression Analysis: Immunoblot analysis: Immunohistochemistry:
 General Laboratory methods Plasma analysis: Plasma insulin (Mercodia, Sweden), leptin (duoset, R&D Systems Europe, Abingdon, United Kingdom), IL-6, TNFα and adiponectin levels (Quantikine kits, R&D Systems
General Laboratory methods Plasma analysis: Plasma insulin (Mercodia, Sweden), leptin (duoset, R&D Systems Europe, Abingdon, United Kingdom), IL-6, TNFα and adiponectin levels (Quantikine kits, R&D Systems
The liver in poisoning: what can we learn from animal models?
 The liver in poisoning: what can we learn from animal models? Stephan Krähenbühl Clinical Pharmacology & Toxicology University Hospital 4031 Basel/Switzerland Kraehenbuehl@uhbs.ch Outcome and causes of
The liver in poisoning: what can we learn from animal models? Stephan Krähenbühl Clinical Pharmacology & Toxicology University Hospital 4031 Basel/Switzerland Kraehenbuehl@uhbs.ch Outcome and causes of
HMGB1 is a potent trigger of arthritis
 Journal of Internal Medicine 2004; 255: 344 350 MINISYMPOSIUM HMGB1 is a potent trigger of arthritis U. ANDERSSON 1,2 & H. ERLANDSSON-HARRIS 1 From the 1 Department of Medicine, Rheumatology Research Unit,
Journal of Internal Medicine 2004; 255: 344 350 MINISYMPOSIUM HMGB1 is a potent trigger of arthritis U. ANDERSSON 1,2 & H. ERLANDSSON-HARRIS 1 From the 1 Department of Medicine, Rheumatology Research Unit,
Role of high mobility group box 1 in myocardial ischemia/reperfusion injury and the effect of ethyl pyruvate
 EXPERIMENTAL AND THERAPEUTIC MEDICINE 9: 1537-1541, 2015 Role of high mobility group box 1 in myocardial ischemia/reperfusion injury and the effect of ethyl pyruvate YUNLING LIN, LIANGLONG CHEN, WEIWEI
EXPERIMENTAL AND THERAPEUTIC MEDICINE 9: 1537-1541, 2015 Role of high mobility group box 1 in myocardial ischemia/reperfusion injury and the effect of ethyl pyruvate YUNLING LIN, LIANGLONG CHEN, WEIWEI
CD14 + S100A9 + Monocytic Myeloid-Derived Suppressor Cells and Their Clinical Relevance in Non-Small Cell Lung Cancer
 CD14 + S1A9 + Monocytic Myeloid-Derived Suppressor Cells and Their Clinical Relevance in Non-Small Cell Lung Cancer Po-Hao, Feng M.D., Kang-Yun, Lee, M.D. Ph.D., Ya-Ling Chang, Yao-Fei Chan, Lu- Wei, Kuo,Ting-Yu
CD14 + S1A9 + Monocytic Myeloid-Derived Suppressor Cells and Their Clinical Relevance in Non-Small Cell Lung Cancer Po-Hao, Feng M.D., Kang-Yun, Lee, M.D. Ph.D., Ya-Ling Chang, Yao-Fei Chan, Lu- Wei, Kuo,Ting-Yu
Sestrin2 and BNIP3 (Bcl-2/adenovirus E1B 19kDa-interacting. protein3) regulate autophagy and mitophagy in renal tubular cells in. acute kidney injury
 Sestrin2 and BNIP3 (Bcl-2/adenovirus E1B 19kDa-interacting protein3) regulate autophagy and mitophagy in renal tubular cells in acute kidney injury by Masayuki Ishihara 1, Madoka Urushido 2, Kazu Hamada
Sestrin2 and BNIP3 (Bcl-2/adenovirus E1B 19kDa-interacting protein3) regulate autophagy and mitophagy in renal tubular cells in acute kidney injury by Masayuki Ishihara 1, Madoka Urushido 2, Kazu Hamada
Supplemental Information
 Electronic Supplementary Material (ESI) for Food & Function. This journal is The Royal Society of Chemistry 2016 Supplemental Information Supplementary Materials and Methods Materials Assay kits of total
Electronic Supplementary Material (ESI) for Food & Function. This journal is The Royal Society of Chemistry 2016 Supplemental Information Supplementary Materials and Methods Materials Assay kits of total
Characterization and significance of MUC1 and c-myc expression in elderly patients with papillary thyroid carcinoma
 Characterization and significance of MUC1 and c-myc expression in elderly patients with papillary thyroid carcinoma Y.-J. Hu 1, X.-Y. Luo 2, Y. Yang 3, C.-Y. Chen 1, Z.-Y. Zhang 4 and X. Guo 1 1 Department
Characterization and significance of MUC1 and c-myc expression in elderly patients with papillary thyroid carcinoma Y.-J. Hu 1, X.-Y. Luo 2, Y. Yang 3, C.-Y. Chen 1, Z.-Y. Zhang 4 and X. Guo 1 1 Department
Role of Tyk-2 in Th9 and Th17 cells in allergic asthma
 Supplementary File Role of Tyk-2 in Th9 and Th17 cells in allergic asthma Caroline Übel 1*, Anna Graser 1*, Sonja Koch 1, Ralf J. Rieker 2, Hans A. Lehr 3, Mathias Müller 4 and Susetta Finotto 1** 1 Laboratory
Supplementary File Role of Tyk-2 in Th9 and Th17 cells in allergic asthma Caroline Übel 1*, Anna Graser 1*, Sonja Koch 1, Ralf J. Rieker 2, Hans A. Lehr 3, Mathias Müller 4 and Susetta Finotto 1** 1 Laboratory
(a) Significant biological processes (upper panel) and disease biomarkers (lower panel)
 Supplementary Figure 1. Functional enrichment analyses of secretomic proteins. (a) Significant biological processes (upper panel) and disease biomarkers (lower panel) 2 involved by hrab37-mediated secretory
Supplementary Figure 1. Functional enrichment analyses of secretomic proteins. (a) Significant biological processes (upper panel) and disease biomarkers (lower panel) 2 involved by hrab37-mediated secretory
p47 negatively regulates IKK activation by inducing the lysosomal degradation of polyubiquitinated NEMO
 Supplementary Information p47 negatively regulates IKK activation by inducing the lysosomal degradation of polyubiquitinated NEMO Yuri Shibata, Masaaki Oyama, Hiroko Kozuka-Hata, Xiao Han, Yuetsu Tanaka,
Supplementary Information p47 negatively regulates IKK activation by inducing the lysosomal degradation of polyubiquitinated NEMO Yuri Shibata, Masaaki Oyama, Hiroko Kozuka-Hata, Xiao Han, Yuetsu Tanaka,
Effects of Gelsolin on Macrophage Inflammatory Responses to Implant Wear Debris
 Effects of Gelsolin on Macrophage Inflammatory Responses to Implant Wear Debris William Michael Mihalko, MD PhD, Lev Djenderedjian, Paramjeet S. Cheema, Richard A. Smith, PhD. University of Tennessee,
Effects of Gelsolin on Macrophage Inflammatory Responses to Implant Wear Debris William Michael Mihalko, MD PhD, Lev Djenderedjian, Paramjeet S. Cheema, Richard A. Smith, PhD. University of Tennessee,
colorimetric sandwich ELISA kit datasheet
 colorimetric sandwich ELISA kit datasheet For the quantitative detection of human TNF-alpha in serum, plasma and cell culture supernatants. general information Catalogue Number Product Name Species cross-reactivity
colorimetric sandwich ELISA kit datasheet For the quantitative detection of human TNF-alpha in serum, plasma and cell culture supernatants. general information Catalogue Number Product Name Species cross-reactivity
Supplementary Figure 1. Expression of CUGBP1 in non-parenchymal liver cells treated with TGF-β
 Supplementary Figures Supplementary Figure 1. Expression of CUGBP1 in non-parenchymal liver cells treated with TGF-β and LPS. Non-parenchymal liver cells were isolated and treated with or without TGF-β
Supplementary Figures Supplementary Figure 1. Expression of CUGBP1 in non-parenchymal liver cells treated with TGF-β and LPS. Non-parenchymal liver cells were isolated and treated with or without TGF-β
TD-BF01: Innate immunity to microorganisms
 TD-BF01: Innate immunity to microorganisms I. Toll receptors (adapted from Takeuchi, O. et al. (1999) Immunity 11:443; Kawai, T. et al. (1999) Immunity 11:115; Hemmi, H. et al. (2000) Nature 408:740; Muzio,
TD-BF01: Innate immunity to microorganisms I. Toll receptors (adapted from Takeuchi, O. et al. (1999) Immunity 11:443; Kawai, T. et al. (1999) Immunity 11:115; Hemmi, H. et al. (2000) Nature 408:740; Muzio,
INTRODUCTION. Induction of Monocyte Chemoattractant Protein-1 (MCP-1) Expression by Angiotensin II (AngII) in the Pancreatic Islets and Beta Cells
 Induction of Monocyte Chemoattractant Protein-1 (MCP-1) Expression by Angiotensin II (AngII) in the Pancreatic Islets and Beta Cells Galina Chipitsyna, Qiaoke Gong, Chance F. Gray et al. Endocrinology,
Induction of Monocyte Chemoattractant Protein-1 (MCP-1) Expression by Angiotensin II (AngII) in the Pancreatic Islets and Beta Cells Galina Chipitsyna, Qiaoke Gong, Chance F. Gray et al. Endocrinology,
Evaluation of directed and random motility in microslides Assessment of leukocyte adhesion in flow chambers
 Evaluation of directed and random motility in microslides Motility experiments in IBIDI microslides, image acquisition and processing were performed as described. PMN, which ended up in an angle < 180
Evaluation of directed and random motility in microslides Motility experiments in IBIDI microslides, image acquisition and processing were performed as described. PMN, which ended up in an angle < 180
Alcoholic hepatitis is a drug-induced disorder
 Alcoholic hepatitis is a drug-induced disorder Gyongyi Szabo, MD, PhD Professor of Medicine University of Massachusetts Medical School Source: 2 Sobernation.com Clinical Progression of ALD Mortality Acute
Alcoholic hepatitis is a drug-induced disorder Gyongyi Szabo, MD, PhD Professor of Medicine University of Massachusetts Medical School Source: 2 Sobernation.com Clinical Progression of ALD Mortality Acute
Li et al. Journal of Experimental & Clinical Cancer Research (2018) 37:108
 Li et al. Journal of Experimental & Clinical Cancer Research (2018) 37:108 https://doi.org/10.1186/s13046-018-0774-7 CORRECTION Correction to: Novel smac mimetic APG- 1387 elicits ovarian cancer cell killing
Li et al. Journal of Experimental & Clinical Cancer Research (2018) 37:108 https://doi.org/10.1186/s13046-018-0774-7 CORRECTION Correction to: Novel smac mimetic APG- 1387 elicits ovarian cancer cell killing
1. TLR. TLR Toll-like receptors. Toll Toll-like receptor, TLR TLR TLR TLR. type I TLR TLR. Toll
 54pp.145 152 2004 1. TLR T B TLR Toll-like receptors TLR TLR I IFN TLR T B B T Toll NF- B 1996 565-0871 3-1 TEL 06-6879-8303 FAX 06-6879-8305 E-mail uemattsu@biken.osaka-u.ac.jp Toll Toll-like receptor,
54pp.145 152 2004 1. TLR T B TLR Toll-like receptors TLR TLR I IFN TLR T B B T Toll NF- B 1996 565-0871 3-1 TEL 06-6879-8303 FAX 06-6879-8305 E-mail uemattsu@biken.osaka-u.ac.jp Toll Toll-like receptor,
FOR OPTIMAL GUT HEALTH KEMIN.COM/GUTHEALTH
 FOR OPTIMAL GUT HEALTH KEMIN.COM/GUTHEALTH ALETA A SOURCE OF 1,3-BETA GLUCANS Aleta is highly bioavailable, offering a concentration greater than 5% of 1,3-beta glucans. Aleta provides a consistent response
FOR OPTIMAL GUT HEALTH KEMIN.COM/GUTHEALTH ALETA A SOURCE OF 1,3-BETA GLUCANS Aleta is highly bioavailable, offering a concentration greater than 5% of 1,3-beta glucans. Aleta provides a consistent response
Supplementary Table 1. Characterization of HNSCC PDX models established at MSKCC
 Supplementary Table 1. Characterization of HNSCC PDX models established at MSKCC Supplementary Table 2. Drug content and loading efficiency estimated with F-NMR and UV- Vis Supplementary Table 3. Complete
Supplementary Table 1. Characterization of HNSCC PDX models established at MSKCC Supplementary Table 2. Drug content and loading efficiency estimated with F-NMR and UV- Vis Supplementary Table 3. Complete
Supplemental Materials. STK16 regulates actin dynamics to control Golgi organization and cell cycle
 Supplemental Materials STK16 regulates actin dynamics to control Golgi organization and cell cycle Juanjuan Liu 1,2,3, Xingxing Yang 1,3, Binhua Li 1, Junjun Wang 1,2, Wenchao Wang 1, Jing Liu 1, Qingsong
Supplemental Materials STK16 regulates actin dynamics to control Golgi organization and cell cycle Juanjuan Liu 1,2,3, Xingxing Yang 1,3, Binhua Li 1, Junjun Wang 1,2, Wenchao Wang 1, Jing Liu 1, Qingsong
SUPPLEMENTAL MATERIAL. Supplementary Methods
 SUPPLEMENTAL MATERIAL Supplementary Methods Culture of cardiomyocytes, fibroblasts and cardiac microvascular endothelial cells The isolation and culturing of neonatal rat ventricular cardiomyocytes was
SUPPLEMENTAL MATERIAL Supplementary Methods Culture of cardiomyocytes, fibroblasts and cardiac microvascular endothelial cells The isolation and culturing of neonatal rat ventricular cardiomyocytes was
Supplementary Material
 Supplementary Material accompanying the manuscript Interleukin 37 is a fundamental inhibitor of innate immunity Marcel F Nold, Claudia A Nold-Petry, Jarod A Zepp, Brent E Palmer, Philip Bufler & Charles
Supplementary Material accompanying the manuscript Interleukin 37 is a fundamental inhibitor of innate immunity Marcel F Nold, Claudia A Nold-Petry, Jarod A Zepp, Brent E Palmer, Philip Bufler & Charles
Sipper BK Experimental Animal Co. (Shanghai, China) and bred in a specific. pathogen-free environment. The animal study protocol was approved by the
 Supplementary information, Data S1 Materials and Methods Mice, Ad vectors and reagents Female C57BL/6 mice, 8-10 weeks of age, were purchased from Joint Ventures Sipper BK Experimental Animal Co. (Shanghai,
Supplementary information, Data S1 Materials and Methods Mice, Ad vectors and reagents Female C57BL/6 mice, 8-10 weeks of age, were purchased from Joint Ventures Sipper BK Experimental Animal Co. (Shanghai,
Irf1 fold changes (D) 24h 48h. p-p65. t-p65. p-irf3. t-irf3. β-actin SKO TKO 100% 80% 60% 40% 20%
 Irf7 Fold changes 3 1 Irf1 fold changes 3 1 8h h 8h 8h h 8h p-p6 p-p6 t-p6 p-irf3 β-actin p-irf3 t-irf3 β-actin TKO TKO STKO (E) (F) TKO TKO % of p6 nuclear translocation % % 1% 1% % % p6 TKO % of IRF3
Irf7 Fold changes 3 1 Irf1 fold changes 3 1 8h h 8h 8h h 8h p-p6 p-p6 t-p6 p-irf3 β-actin p-irf3 t-irf3 β-actin TKO TKO STKO (E) (F) TKO TKO % of p6 nuclear translocation % % 1% 1% % % p6 TKO % of IRF3
International Conference on Biomedical and Biological Engineering (BBE 2016)
 International Conference on Biomedical and Biological Engineering (BBE 2016) Injury Mechanism of Sub-Micron Calcium Oxalate Monohydrate and Dihydrate Crystals on Renal Epithelial Cells Poonam BHADJA, Kai
International Conference on Biomedical and Biological Engineering (BBE 2016) Injury Mechanism of Sub-Micron Calcium Oxalate Monohydrate and Dihydrate Crystals on Renal Epithelial Cells Poonam BHADJA, Kai
ab LDL Uptake Assay Kit (Cell-Based)
 ab133127 LDL Uptake Assay Kit (Cell-Based) Instructions for Use For the detection of LDL uptake into cultured cells. This product is for research use only and is not intended for diagnostic use. Version
ab133127 LDL Uptake Assay Kit (Cell-Based) Instructions for Use For the detection of LDL uptake into cultured cells. This product is for research use only and is not intended for diagnostic use. Version
TSH Receptor Monoclonal Antibody (49) Catalog Number MA3-218 Product data sheet
 Website: thermofisher.com Customer Service (US): 1 800 955 6288 ext. 1 Technical Support (US): 1 800 955 6288 ext. 441 TSH Receptor Monoclonal Antibody (49) Catalog Number MA3-218 Product data sheet Details
Website: thermofisher.com Customer Service (US): 1 800 955 6288 ext. 1 Technical Support (US): 1 800 955 6288 ext. 441 TSH Receptor Monoclonal Antibody (49) Catalog Number MA3-218 Product data sheet Details
Supporting Information
 Supporting Information Desnues et al. 10.1073/pnas.1314121111 SI Materials and Methods Mice. Toll-like receptor (TLR)8 / and TLR9 / mice were generated as described previously (1, 2). TLR9 / mice were
Supporting Information Desnues et al. 10.1073/pnas.1314121111 SI Materials and Methods Mice. Toll-like receptor (TLR)8 / and TLR9 / mice were generated as described previously (1, 2). TLR9 / mice were
Kun Jiang 1, He-Bin Chen 1, Ying Wang 1, Jia-Hui Lin 2, Yan Hu 1, Yu-Rong Fang 1
 Original Article Changes in interleukin-17 and transforming growth factor beta 1 levels in serum and bronchoalveolar lavage fluid and their clinical significance among children with asthma Kun Jiang 1,
Original Article Changes in interleukin-17 and transforming growth factor beta 1 levels in serum and bronchoalveolar lavage fluid and their clinical significance among children with asthma Kun Jiang 1,
Supplementary Figures for TSC1 controls macrophage polarization to prevent inflammatory disorder by Linnan Zhu et al
 Supplementary Figures for TSC1 controls macrophage polarization to prevent inflammatory disorder by Linnan Zhu et al Suppl. Fig. 1 Tissue DN C Proteins kd TSC1-17 TSC 1 loxp bp -48-285 ctin PEMs Neutrophils
Supplementary Figures for TSC1 controls macrophage polarization to prevent inflammatory disorder by Linnan Zhu et al Suppl. Fig. 1 Tissue DN C Proteins kd TSC1-17 TSC 1 loxp bp -48-285 ctin PEMs Neutrophils
Department of Pharmacology, Institute of Health Sciences, College of Medicine, Gyeongsang National University, Jinju, Korea
 Degradation of histone deacetylase 4 via the TLR4/JAK/ STAT1 signaling pathway promotes the acetylation of high mobility group box 1 (HMGB1) in lipopolysaccharideactivated macrophages Eun J. Park, Young
Degradation of histone deacetylase 4 via the TLR4/JAK/ STAT1 signaling pathway promotes the acetylation of high mobility group box 1 (HMGB1) in lipopolysaccharideactivated macrophages Eun J. Park, Young
IKKα Causes Chromatin Modification on Pro-Inflammatory Genes by Cigarette Smoke in Mouse Lung
 IKKα Causes Chromatin Modification on Pro-Inflammatory Genes by Cigarette Smoke in Mouse Lung Se-Ran Yang, Samantha Valvo, Hongwei Yao, Aruna Kode, Saravanan Rajendrasozhan, Indika Edirisinghe, Samuel
IKKα Causes Chromatin Modification on Pro-Inflammatory Genes by Cigarette Smoke in Mouse Lung Se-Ran Yang, Samantha Valvo, Hongwei Yao, Aruna Kode, Saravanan Rajendrasozhan, Indika Edirisinghe, Samuel
DC were seeded into tissue culture dishes in IMDM 2% FCS, and added with PMN. (1:1; PMN: DC) for 16h also in the presence of DNAse (100 U/ml); DC were
 Supplementary methods Flow cytometric analysis of DCs. DC were seeded into tissue culture dishes in IMDM 2% FCS, and added with PMN (1:1; PMN: DC) for 16h also in the presence of DNAse (100 U/ml); DC were
Supplementary methods Flow cytometric analysis of DCs. DC were seeded into tissue culture dishes in IMDM 2% FCS, and added with PMN (1:1; PMN: DC) for 16h also in the presence of DNAse (100 U/ml); DC were
Title of file for HTML: Supplementary Information Description: Supplementary Figures and Supplementary Table
 Title of file for HTML: Supplementary Information Description: Supplementary Figures and Supplementary Table Title of file for HTML: Peer Review File Description: Innate Scavenger Receptor-A regulates
Title of file for HTML: Supplementary Information Description: Supplementary Figures and Supplementary Table Title of file for HTML: Peer Review File Description: Innate Scavenger Receptor-A regulates
1. Materials and Methods 1.1 Animals experiments process The experiments were approved by the Institution Animal Ethics Committee of Jilin University
 1. Materials and Methods 1.1 Animals experiments process The experiments were approved by the Institution Animal Ethics Committee of Jilin University (Reference NO. 2015-003). 96 Kunming (KM) mice (8 weeks;
1. Materials and Methods 1.1 Animals experiments process The experiments were approved by the Institution Animal Ethics Committee of Jilin University (Reference NO. 2015-003). 96 Kunming (KM) mice (8 weeks;
Study on the expression of MMP-9 and NF-κB proteins in epithelial ovarian cancer tissue and their clinical value
 Study on the expression of MMP-9 and NF-κB proteins in epithelial ovarian cancer tissue and their clinical value Shen Wei 1,a, Chen Juan 2, Li Xiurong 1 and Yin Jie 1 1 Department of Obstetrics and Gynecology,
Study on the expression of MMP-9 and NF-κB proteins in epithelial ovarian cancer tissue and their clinical value Shen Wei 1,a, Chen Juan 2, Li Xiurong 1 and Yin Jie 1 1 Department of Obstetrics and Gynecology,
Supplementary Figure S I: Effects of D4F on body weight and serum lipids in apoe -/- mice.
 Supplementary Figures: Supplementary Figure S I: Effects of D4F on body weight and serum lipids in apoe -/- mice. Male apoe -/- mice were fed a high-fat diet for 8 weeks, and given PBS (model group) or
Supplementary Figures: Supplementary Figure S I: Effects of D4F on body weight and serum lipids in apoe -/- mice. Male apoe -/- mice were fed a high-fat diet for 8 weeks, and given PBS (model group) or
B-cell. Astrocyte SCI SCI. T-cell
 RF #2015 P-01 PI: Azizul Haque, PhD Grant Title: Targeting Enolase in Spinal Cord Injury 12-month Technical Progress Report Progress Report (First Six Months): Enolase is one of the most abundantly expressed
RF #2015 P-01 PI: Azizul Haque, PhD Grant Title: Targeting Enolase in Spinal Cord Injury 12-month Technical Progress Report Progress Report (First Six Months): Enolase is one of the most abundantly expressed
Supplementary information
 Supplementary information Supplementary Figure S1: Ex[Ca 2+ ]-induced IL-1ß production of monocytes primed with different TLR ligands IL-1ß release of CD14+ monocytes in response to stimulation for 16
Supplementary information Supplementary Figure S1: Ex[Ca 2+ ]-induced IL-1ß production of monocytes primed with different TLR ligands IL-1ß release of CD14+ monocytes in response to stimulation for 16
NLRX1: 5 -GCTCCATGGCTTAGAGCATC-3 (forward) 5 -AACTCCTCCTCCGTCCTGAT-3 (reverse) β-actin
 NLRX1 β-actin 1 2 3 4 5 6 1 2 3 4 5 6 NLRX1 (667 bp) β-actin (523 bp) Supplementary Figure 1: Expression of NLRX1 in human cell lines. 1: HeLa, 2: HEK293T, 3: MCF-7, 4:Ramos, 5:Jurkat, 6: THP1. The following
NLRX1 β-actin 1 2 3 4 5 6 1 2 3 4 5 6 NLRX1 (667 bp) β-actin (523 bp) Supplementary Figure 1: Expression of NLRX1 in human cell lines. 1: HeLa, 2: HEK293T, 3: MCF-7, 4:Ramos, 5:Jurkat, 6: THP1. The following
Original Article Endotoxin tolerance alleviates experimental acute liver failure via inhibition of high mobility group box 1
 Int J Clin Exp Pathol 2015;8(8):9062-9071 www.ijcep.com /ISSN:1936-2625/IJCEP0011307 Original Article Endotoxin tolerance alleviates experimental acute liver failure via inhibition of high mobility group
Int J Clin Exp Pathol 2015;8(8):9062-9071 www.ijcep.com /ISSN:1936-2625/IJCEP0011307 Original Article Endotoxin tolerance alleviates experimental acute liver failure via inhibition of high mobility group
Biodegradable Zwitterionic Nanogels with Long. Circulation for Antitumor Drug Delivery
 Supporting Information Biodegradable Zwitterionic Nanogels with Long Circulation for Antitumor Drug Delivery Yongzhi Men, Shaojun Peng, Peng Yang, Qin Jiang, Yanhui Zhang, Bin Shen, Pin Dong, *, Zhiqing
Supporting Information Biodegradable Zwitterionic Nanogels with Long Circulation for Antitumor Drug Delivery Yongzhi Men, Shaojun Peng, Peng Yang, Qin Jiang, Yanhui Zhang, Bin Shen, Pin Dong, *, Zhiqing
Supplementary Figures
 Inhibition of Pulmonary Anti Bacterial Defense by IFN γ During Recovery from Influenza Infection By Keer Sun and Dennis W. Metzger Supplementary Figures d a Ly6G Percentage survival f 1 75 5 1 25 1 5 1
Inhibition of Pulmonary Anti Bacterial Defense by IFN γ During Recovery from Influenza Infection By Keer Sun and Dennis W. Metzger Supplementary Figures d a Ly6G Percentage survival f 1 75 5 1 25 1 5 1
Supplementary Figure (OH) 22 nanoparticles did not affect cell viability and apoposis. MDA-MB-231, MCF-7, MCF-10A and BT549 cells were
 Supplementary Figure 1. Gd@C 82 (OH) 22 nanoparticles did not affect cell viability and apoposis. MDA-MB-231, MCF-7, MCF-10A and BT549 cells were treated with PBS, Gd@C 82 (OH) 22, C 60 (OH) 22 or GdCl
Supplementary Figure 1. Gd@C 82 (OH) 22 nanoparticles did not affect cell viability and apoposis. MDA-MB-231, MCF-7, MCF-10A and BT549 cells were treated with PBS, Gd@C 82 (OH) 22, C 60 (OH) 22 or GdCl
Supplemental Figure 1. (A) Western blot for the expression of RIPK1 in HK-2 cells treated with or without LPS (1 µg/ml) for indicated times.
 Supplemental Figure 1. (A) Western blot for the expression of RIPK1 in HK-2 cells treated with or without LPS (1 µg/ml) for indicated times. Western blots shown are representative results from 3 independent
Supplemental Figure 1. (A) Western blot for the expression of RIPK1 in HK-2 cells treated with or without LPS (1 µg/ml) for indicated times. Western blots shown are representative results from 3 independent
Effects of COX-2 Inhibitor on the Proliferation of MCF-7 and LTED MCF-7 Cells
 Mahidol University Journal of Pharmaceutical Sciences 2008; 35(1-4): 47-51. Original Article Effects of COX-2 Inhibitor on the Proliferation of MCF-7 and LTED MCF-7 Cells K. Poemsantitham, N. Sookvanichsilp
Mahidol University Journal of Pharmaceutical Sciences 2008; 35(1-4): 47-51. Original Article Effects of COX-2 Inhibitor on the Proliferation of MCF-7 and LTED MCF-7 Cells K. Poemsantitham, N. Sookvanichsilp
ROMANIAN ACADEMY INSTITUTE OF CELLULAR BIOLOGY AND PATHOLOGY NICOLAE SIMIONESCU. PhD THESIS Summary
 ROMANIAN ACADEMY INSTITUTE OF CELLULAR BIOLOGY AND PATHOLOGY NICOLAE SIMIONESCU PhD THESIS Summary INVOLVEMENT OF ALARMIN HMGB1 IN INFLAMMATORY PROCESSES ASSOCIATED WITH VASCULAR DYSFUNCTION IN HYPERLIPIDEMIA
ROMANIAN ACADEMY INSTITUTE OF CELLULAR BIOLOGY AND PATHOLOGY NICOLAE SIMIONESCU PhD THESIS Summary INVOLVEMENT OF ALARMIN HMGB1 IN INFLAMMATORY PROCESSES ASSOCIATED WITH VASCULAR DYSFUNCTION IN HYPERLIPIDEMIA
The Annexin V Apoptosis Assay
 The Annexin V Apoptosis Assay Development of the Annexin V Apoptosis Assay: 1990 Andree at al. found that a protein, Vascular Anticoagulant α, bound to phospholipid bilayers in a calcium dependent manner.
The Annexin V Apoptosis Assay Development of the Annexin V Apoptosis Assay: 1990 Andree at al. found that a protein, Vascular Anticoagulant α, bound to phospholipid bilayers in a calcium dependent manner.
Innate Immunity & Inflammation
 Innate Immunity & Inflammation The innate immune system is an evolutionally conserved mechanism that provides an early and effective response against invading microbial pathogens. It relies on a limited
Innate Immunity & Inflammation The innate immune system is an evolutionally conserved mechanism that provides an early and effective response against invading microbial pathogens. It relies on a limited
Extracellular roles of high - mobility - group B1
 Chinese Journal of Pathophysiology 2004,20 (4) :673-678 673 [ ] 1000-4718 (2004) 04-0673 - 06 B1 3,, ( 304, 100037) Extracellular roles of high - mobility - group B1 WANG Zhong - tang, YAO Yong - ming,
Chinese Journal of Pathophysiology 2004,20 (4) :673-678 673 [ ] 1000-4718 (2004) 04-0673 - 06 B1 3,, ( 304, 100037) Extracellular roles of high - mobility - group B1 WANG Zhong - tang, YAO Yong - ming,
Writing Effective Grant Proposals
 WritingEffectiveGrantProposals SUPPLEMENTALHANDOUT EXERCISES (toaccompanypowerpointslidepresentation) PamelaDerish ScientificPublicationsManager DepartmentofSurgery,UCSF tel415.885 7686 Pamela.Derish@ucsfmedctr.org
WritingEffectiveGrantProposals SUPPLEMENTALHANDOUT EXERCISES (toaccompanypowerpointslidepresentation) PamelaDerish ScientificPublicationsManager DepartmentofSurgery,UCSF tel415.885 7686 Pamela.Derish@ucsfmedctr.org
Role of cyclooxygenase-2 in H5N1 viral pathogenesis and the potential use of its inhibitors
 Title Role of cyclooxygenase-2 in HN viral pathogenesis and the potential use of its inhibitors Author(s) Lee, MY; Cheung, CY; Peiris, JSM Citation Hong Kong Medical Journal, 2, v. 9 n. Suppl. 4, p. 29-
Title Role of cyclooxygenase-2 in HN viral pathogenesis and the potential use of its inhibitors Author(s) Lee, MY; Cheung, CY; Peiris, JSM Citation Hong Kong Medical Journal, 2, v. 9 n. Suppl. 4, p. 29-
Supporting Information
 Supporting Information Pang et al. 10.1073/pnas.1322009111 SI Materials and Methods ELISAs. These assays were performed as previously described (1). ELISA plates (MaxiSorp Nunc; Thermo Fisher Scientific)
Supporting Information Pang et al. 10.1073/pnas.1322009111 SI Materials and Methods ELISAs. These assays were performed as previously described (1). ELISA plates (MaxiSorp Nunc; Thermo Fisher Scientific)
LDL Uptake Cell-Based Assay Kit
 LDL Uptake Cell-Based Assay Kit Catalog Number KA1327 100 assays Version: 07 Intended for research use only www.abnova.com Table of Contents Introduction... 3 Background... 3 Principle of the Assay...
LDL Uptake Cell-Based Assay Kit Catalog Number KA1327 100 assays Version: 07 Intended for research use only www.abnova.com Table of Contents Introduction... 3 Background... 3 Principle of the Assay...
Type of file: PDF Title of file for HTML: Supplementary Information Description: Supplementary Figures
 Type of file: PDF Title of file for HTML: Supplementary Information Description: Supplementary Figures Type of file: MOV Title of file for HTML: Supplementary Movie 1 Description: NLRP3 is moving along
Type of file: PDF Title of file for HTML: Supplementary Information Description: Supplementary Figures Type of file: MOV Title of file for HTML: Supplementary Movie 1 Description: NLRP3 is moving along
Ligand-mediated cytoplasmic retention of the Ah receptor inhibits. Gulsum E. Muku, Tejas S. Lahoti, Iain A. Murray, Michael A.
 Ligand-mediated cytoplasmic retention of the Ah receptor inhibits macrophage mediated acute inflammatory responses Gulsum E. Muku, Tejas S. Lahoti, Iain A. Murray, Michael A. Podolsky, Kayla Smith, Troy
Ligand-mediated cytoplasmic retention of the Ah receptor inhibits macrophage mediated acute inflammatory responses Gulsum E. Muku, Tejas S. Lahoti, Iain A. Murray, Michael A. Podolsky, Kayla Smith, Troy
Supplementary Information
 Supplementary Information Supplementary Figure 1. CD4 + T cell activation and lack of apoptosis after crosslinking with anti-cd3 + anti-cd28 + anti-cd160. (a) Flow cytometry of anti-cd160 (5D.10A11) binding
Supplementary Information Supplementary Figure 1. CD4 + T cell activation and lack of apoptosis after crosslinking with anti-cd3 + anti-cd28 + anti-cd160. (a) Flow cytometry of anti-cd160 (5D.10A11) binding
Supplementary Figure 1 Induction of cellular senescence and isolation of exosome. a to c, Pre-senescent primary normal human diploid fibroblasts
 Supplementary Figure 1 Induction of cellular senescence and isolation of exosome. a to c, Pre-senescent primary normal human diploid fibroblasts (TIG-3 cells) were rendered senescent by either serial passage
Supplementary Figure 1 Induction of cellular senescence and isolation of exosome. a to c, Pre-senescent primary normal human diploid fibroblasts (TIG-3 cells) were rendered senescent by either serial passage
The Need for a PARP in vivo Pharmacodynamic Assay
 The Need for a PARP in vivo Pharmacodynamic Assay Jay George, Ph.D. Chief Scientific Officer Trevigen, Inc. Gaithersburg, MD Poly(ADP-ribose) polymerases are promising therapeutic targets. In response
The Need for a PARP in vivo Pharmacodynamic Assay Jay George, Ph.D. Chief Scientific Officer Trevigen, Inc. Gaithersburg, MD Poly(ADP-ribose) polymerases are promising therapeutic targets. In response
HCC1937 is the HCC1937-pcDNA3 cell line, which was derived from a breast cancer with a mutation
 SUPPLEMENTARY INFORMATION Materials and Methods Human cell lines and culture conditions HCC1937 is the HCC1937-pcDNA3 cell line, which was derived from a breast cancer with a mutation in exon 20 of BRCA1
SUPPLEMENTARY INFORMATION Materials and Methods Human cell lines and culture conditions HCC1937 is the HCC1937-pcDNA3 cell line, which was derived from a breast cancer with a mutation in exon 20 of BRCA1
HMGB1-TLR4 Interactions: Mediators of Kidney Lung Crosstalk?
 HMGB1-TLR4 Interactions: Mediators of Kidney Lung Crosstalk? Dept of Emergency and Critical Care Medicine The University of Tokyo Kent Doi, MD. PhD Organ crosstalk in AKI Grams ME, Rabb H. Kidney Int.
HMGB1-TLR4 Interactions: Mediators of Kidney Lung Crosstalk? Dept of Emergency and Critical Care Medicine The University of Tokyo Kent Doi, MD. PhD Organ crosstalk in AKI Grams ME, Rabb H. Kidney Int.
LDL Uptake Cell-Based Assay Kit
 LDL Uptake Cell-Based Assay Kit Item No. 10011125 www.caymanchem.com Customer Service 800.364.9897 Technical Support 888.526.5351 1180 E. Ellsworth Rd Ann Arbor, MI USA TABLE OF CONTENTS GENERAL INFORMATION
LDL Uptake Cell-Based Assay Kit Item No. 10011125 www.caymanchem.com Customer Service 800.364.9897 Technical Support 888.526.5351 1180 E. Ellsworth Rd Ann Arbor, MI USA TABLE OF CONTENTS GENERAL INFORMATION
Effect of ST2825 on the proliferation and apoptosis of human hepatocellular carcinoma cells
 Effect of ST2825 on the proliferation and apoptosis of human hepatocellular carcinoma cells Y. Deng, J. Sun and L.D. Zhang Department of Hepatobiliary Surgery Institute, Southwest Hospital, Third Military
Effect of ST2825 on the proliferation and apoptosis of human hepatocellular carcinoma cells Y. Deng, J. Sun and L.D. Zhang Department of Hepatobiliary Surgery Institute, Southwest Hospital, Third Military
ab SREBP-2 Translocation Assay Kit (Cell-Based)
 ab133114 SREBP-2 Translocation Assay Kit (Cell-Based) Instructions for Use For analysis of translocation of SREBP-2 into nuclei. This product is for research use only and is not intended for diagnostic
ab133114 SREBP-2 Translocation Assay Kit (Cell-Based) Instructions for Use For analysis of translocation of SREBP-2 into nuclei. This product is for research use only and is not intended for diagnostic
Pathogenesis of lupus apoptosis clearance and regulation of self tolerance. Durga Prasanna Misra
 Pathogenesis of lupus apoptosis clearance and regulation of self tolerance Durga Prasanna Misra Format Apoptosis Apoptosis in SLE Decreased apoptosis of autoreactive cells Increased apoptosis Defective
Pathogenesis of lupus apoptosis clearance and regulation of self tolerance Durga Prasanna Misra Format Apoptosis Apoptosis in SLE Decreased apoptosis of autoreactive cells Increased apoptosis Defective
Epithelial interleukin-25 is a key mediator in Th2-high, corticosteroid-responsive
 Online Data Supplement: Epithelial interleukin-25 is a key mediator in Th2-high, corticosteroid-responsive asthma Dan Cheng, Zheng Xue, Lingling Yi, Huimin Shi, Kan Zhang, Xiaorong Huo, Luke R. Bonser,
Online Data Supplement: Epithelial interleukin-25 is a key mediator in Th2-high, corticosteroid-responsive asthma Dan Cheng, Zheng Xue, Lingling Yi, Huimin Shi, Kan Zhang, Xiaorong Huo, Luke R. Bonser,
Ines Bohlinger, Mareel Leist, Johannes Barsig, Stefan Uhlig, Gisa Tiegs*, Albreeht Wendel
 a Ines Bohlinger, Mareel Leist, Johannes Barsig, Stefan Uhlig, Gisa Tiegs*, Albreeht Wendel o-galactosamine-sensitized mice challenged with tumor necrosis factor Q' (TNF) developed severe apoptotic and
a Ines Bohlinger, Mareel Leist, Johannes Barsig, Stefan Uhlig, Gisa Tiegs*, Albreeht Wendel o-galactosamine-sensitized mice challenged with tumor necrosis factor Q' (TNF) developed severe apoptotic and
Supplementary Materials and Methods
 Supplementary Materials and Methods Immunoblotting Immunoblot analysis was performed as described previously (1). Due to high-molecular weight of MUC4 (~ 950 kda) and MUC1 (~ 250 kda) proteins, electrophoresis
Supplementary Materials and Methods Immunoblotting Immunoblot analysis was performed as described previously (1). Due to high-molecular weight of MUC4 (~ 950 kda) and MUC1 (~ 250 kda) proteins, electrophoresis
T H E J O U R N A L O F C E L L B I O L O G Y
 Supplemental material Edens and Levy, http://www.jcb.org/cgi/content/full/jcb.201406004/dc1 T H E J O U R N A L O F C E L L B I O L O G Y Figure S1. Nuclear shrinking does not depend on the cytoskeleton
Supplemental material Edens and Levy, http://www.jcb.org/cgi/content/full/jcb.201406004/dc1 T H E J O U R N A L O F C E L L B I O L O G Y Figure S1. Nuclear shrinking does not depend on the cytoskeleton
SUPPLEMENTARY INFORMATION
 Supplemental Figure 1. Furin is efficiently deleted in CD4 + and CD8 + T cells. a, Western blot for furin and actin proteins in CD4cre-fur f/f and fur f/f Th1 cells. Wild-type and furin-deficient CD4 +
Supplemental Figure 1. Furin is efficiently deleted in CD4 + and CD8 + T cells. a, Western blot for furin and actin proteins in CD4cre-fur f/f and fur f/f Th1 cells. Wild-type and furin-deficient CD4 +
A protocol for enhancement of the AAV-mediated expression of transgenes
 A protocol for enhancement of the AAV-mediated expression of transgenes Hiroaki Mizukami, Takeharu Kanazawa, Takashi Okada, and Keiya Ozawa Division of Genetic Therapeutics, Center for Molecular Medicine,
A protocol for enhancement of the AAV-mediated expression of transgenes Hiroaki Mizukami, Takeharu Kanazawa, Takashi Okada, and Keiya Ozawa Division of Genetic Therapeutics, Center for Molecular Medicine,
