Ash1l controls quiescence and self-renewal potential in hematopoietic stem cells
|
|
|
- Nathaniel Turner
- 5 years ago
- Views:
Transcription
1 The Journal of Clinical Investigation Research article Ash1l controls quiescence and self-renewal potential in hematopoietic stem cells Morgan Jones, 1,2,3 Jennifer Chase, 1,2 Michelle Brinkmeier, 4 Jing Xu, 5 Daniel N. Weinberg, 1 Julien Schira, 1 Ann Friedman, 1 Sami Malek, 6 Jolanta Grembecka, 5 Tomasz Cierpicki, 5 Yali Dou, 5 Sally A. Camper, 4 and Ivan Maillard 1,6,7 1 Center for Stem Cell Biology, Life Sciences Institute, 2 Graduate Program in Cellular and Molecular Biology, 3 Medical Scientist Training Program, 4 Department of Human Genetics, 5 Department of Pathology, 6 Division of Hematology-Oncology, Department of Internal Medicine, and 7 Department of Cell and Developmental Biology, University of Michigan, Ann Arbor, Michigan, USA. Rapidly cycling fetal and neonatal hematopoietic stem cells (HSCs) generate a pool of quiescent adult HSCs after establishing hematopoiesis in the bone marrow. We report an essential role for the trithorax group gene absent, small, or homeotic 1-like (Ash1l) at this developmental transition. Emergence and expansion of Ash1l-deficient fetal/neonatal HSCs were preserved; however, in young adult animals, HSCs were profoundly depleted. Ash1l-deficient adult HSCs had markedly decreased quiescence and reduced cyclin-dependent kinase inhibitor 1b/c (Cdkn1b/1c) expression and failed to establish long-term trilineage bone marrow hematopoiesis after transplantation to irradiated recipients. Wild-type HSCs could efficiently engraft when transferred to unirradiated, Ash1l-deficient recipients, indicating increased availability of functional HSC niches in these mice. Ash1l deficiency also decreased expression of multiple Hox genes in hematopoietic progenitors. Ash1l cooperated functionally with mixed-lineage leukemia 1 (Mll1), as combined loss of Ash1l and Mll1, but not isolated Ash1l or Mll1 deficiency, induced overt hematopoietic failure. Our results uncover a trithorax group gene network that controls quiescence, niche occupancy, and self-renewal potential in adult HSCs. Introduction Fetal hematopoietic stem cells (HSCs) proliferate rapidly and contribute to massive expansion of the HSC pool (1, 2). In contrast, adult HSCs divide rarely in steady-state conditions, as a majority of cells reside in the quiescent G 0 phase of the cell cycle and return to quiescence after periods of proliferation (3 8). In mice, transition from the fetal/neonatal to the adult HSC program occurs in the bone marrow (BM) niche within weeks after birth and coincides with establishment of HSC quiescence, functional and phenotypic changes, and downregulated expression of the transcription factor Sox17 and other fetal HSC genes (1, 9 12). Failure to establish or maintain quiescence has been linked to adult HSC depletion, increased sensitivity to myeloablation, and reduced engraftment in transplantation assays (1, 13 16). However, the regulatory mechanisms controlling quiescence and successful initiation of the adult HSC program in the BM remain poorly understood. The trithorax group (TrxG) is a diverse family of epigenetic regulators originally identified to control body patterning in Drosophila (17). In flies, individual TrxG members cooperate to promote expression of homeobox (Hox) genes, and combined heterozygous TrxG gene mutations act as dominant enhancers of one another (18, 19). Mixed-lineage leukemia 1 (Mll1) is the trithorax homolog and founding TrxG member in mammals. In humans, MLL1 was first identified as a recurrent translocation partner in acute leukemias characterized by upregulated HOX gene expression (20 23). Authorship note: Morgan Jones and Jennifer Chase contributed equally to this work. Conflict of interest: The authors have declared that no conflict of interest exists. Submitted: July 21, 2014; Accepted: March 12, Reference information: J Clin Invest. 2015;125(5): doi: /jci Endogenous Mll1 regulates expression of Hox genes and other targets as well as the function of normal HSCs (24 26). Mll1 encodes a large protein containing a C-terminal Su(var)3-9/enhancer-ofzeste/trithorax (SET) domain with H3K4 histone methyltransferase activity (27). MLL1 functions as a part of a multiprotein complex that includes RBBP5, WDR5, and ASH2L (28 31). Limited information is available about cooperative interactions among mammalian TrxG members, in particular with proteins in which an association with the MLL complex has not been as yet identified. The TrxG gene absent, small, or homeotic 1 (ash1) was first discovered in genetic screens seeking regulators of Drosophila imaginal discs (32). ash1 encodes a large protein containing an internal SET domain with putative histone methyltransferase activity (33). Its mammalian homolog Ash1l also encodes a SET domain containing protein that can associate with actively transcribed loci, including at several Hox genes (34 36). However, unlike the much smaller protein ASH2L, ASH1L has not been identified so far as a member of the MLL protein complex. Recently, the ASH1L SET domain was reported to have intrinsic H3K36 dimethyltransferase activity using in vitro biochemical assays (37 39). Neither the physiological significance of Ash1l nor the idea of cooperativity with Mll1 and other TrxG members has been evaluated in vivo. In this report, we describe an essential role for the TrxG member Ash1l in the maintenance and function of adult HSCs but not in the in vivo expansion of fetal or neonatal hematopoietic progenitors. Ash1l was essential for the establishment of quiescence at the fetal to adult HSC transition in the BM within weeks after birth. Ash1l deficiency led to profound depletion of adult HSCs and multipotent progenitors as well as to a lack of functional HSCs capable of long-term BM reconstitution in transplantation assays. Unlike jci.org Volume 125 Number 5 May
2 Research article The Journal of Clinical Investigation Figure 1. Preserved overall fetal and adult hematopoietic output in Ash1l GT/GT mice. (A) Generation of the Ash1l GT allele by insertion of a splice-acceptor gene trap cassette into the first Ash1l intron. Homozygosity led to >90% reduction in wild-type transcripts in fetal (E15.5) and adult LSK progenitors, as shown by quantitative RT-PCR (qrt-pcr) with primers amplifying cdna across the exon 1 2 boundary (mean ± SD). (B) qrt-pcr analysis of Ash1l expression normalized to Hprt1 in selected hematopoietic populations (LT-HSC, LSK CD150 + CD48 LT-HSCs; MPP, LSK MPPs; HPC1, LSK CD150 CD48 + hematopoietic progenitor cells; HPC2, LSK CD150 + CD48 + hematopoietic progenitor cells; myeloid, CD11b + Gr1 + myeloid cells; B cells, B220 + AA4.1 B cells; CD4 T cells, TCRβ + CD4 + cells; CD8 T cells, TCRβ + CD8 + T cells) (mean ± SD). (C) Cellularity and percentage of myeloid, erythroid, and B lineage cells in E14.5 wild-type and Ash1l GT/GT (GT) fetal liver (n 4 per genotype from 2 independent experiments; mean ± SEM). (D) Cellularity and percentage of myeloid, erythroid, and B lineage cells in young adult (6- to 12-week-old) wild-type and Ash1l GT/GT BM (n 6 per genotype from >2 independent experiments; mean ± SEM). (E) Myeloid colony formation by wildtype and Ash1l GT/GT BM in CFU-GM assays (mean ± SEM, representative of 2 experiments). No statistically significant differences by t test. in wild-type recipients, after nonmyeloablative transplantation of normal HSCs, cells could efficiently engraft the BM and establish durable hematopoiesis in Ash1l-deficient mice, suggesting poor competition of residual HSCs for niche space. Despite profound defects in transplantation assays, Ash1l-deficient mice exhibited no overt hematopoietic failure in steady-state conditions, with evidence of increased self-renewal in progenitors downstream of HSCs. However, combining Ash1l deficiency with inactivation of the TrxG gene Mll1 or its cofactor gene Men1 led to rapid BM failure. These data reveal an essential physiological function for the TrxG gene Ash1l in adult HSCs and represent the first genetic demonstration of cooperativity between TrxG members in mammals. Results Ash1l-deficient mice have normal numbers of fetal and neonatal HSCs but profound depletion of adult HSCs. To examine the function of Ash1l in hematopoiesis, we used a gene trap insertion allele con- taining a splice-acceptor cassette in the first Ash1l intron (Figure 1A). This strategy resulted in a >90% reduction of full-length Ash1l transcripts in fetal liver and BM lineage SCA1 + c-kit + (LSK) HSCs and hematopoietic progenitor cells. Ash1l was expressed in all subpopulations of LSK progenitors and in selected mature cell subsets (Figure 1B). Homozygosity for the Ash1l GT allele preserved fetal liver and BM cellularity as well as myeloid, erythroid, and B lineage cells in Ash1l GT/GT mice compared with that in Ashl +/+ littermates (Figure 1, C and D). The capacity to form myeloid colonies was normal in Ash1l-deficient BM, consistent with preserved progenitor numbers and function (Figure 1E). We quantified HSCs as the CD150 + CD48 fraction of LSK progenitors, a definition that identifies both fetal and adult long-term HSCs (LT-HSCs) (40, 41). Ash1l-deficient LT-HSCs were present at normal frequency in the fetal liver, showing that phenotypically defined fetal LT-HSCs emerged and expanded normally in these mice (Figure 2A). HSC numbers were also preserved in the 2008 jci.org Volume 125 Number 5 May 2015
3 The Journal of Clinical Investigation Research article Figure 2. Young adult Ash1l GT/GT mice show profound LT-HSC depletion, as defined phenotypically. (A) Frequency of E14.5 fetal liver LSK and LT-HSC (CD150 + CD48 LSK) cells in wild-type and Ash1l GT/GT fetuses (wild-type n = 4, Ash1l GT/GT n = 8 from 2 experiments; mean ± SEM). (B) Flow cytometric analysis showing >5-fold reduced frequency of LT-HSCs in young adult (6- to 12-week-old) Ash1l GT/GT mice (wild-type n = 6, Ash1l GT/GT n = 8 from 3 experiments; mean ± SEM ). (C) Reduced CD34 FLT3 LSK, but not CD34 + FLT3 LSK or CD34 + FLT3 + LSK, progenitors in young adult Ash1l GT/GT mice (n = 4 mice per genotype from 2 experiments; mean ± SEM). (D) Complete blood counts of 24-week-old mice, showing reduced platelets but preserved lymphocytes, neutrophils, and hemoglobin contents (n = 6 mice per genotype; mean ± SEM). (E) BM cellularity and progenitor contents in 24-week-old mice (n = 6 mice per genotype from 3 experiments; mean ± SEM) and 48-week-old mice (n = 3 per genotype from 2 experiments; mean ± SEM). Flow cytometric analysis demonstrates a reduction in LSK progenitors and persistent 5- to 10-fold reduction in LT-HSCs. *P < 0.05, **P < 0.01, ***P < 0.001, t test. jci.org Volume 125 Number 5 May
4 Research article The Journal of Clinical Investigation neonatal liver (data not shown). However, by 6 to 12 weeks after birth, LT-HSCs had decreased by 5- to 10-fold in Ash1l GT/GT BM as compared with BM of wild-type littermates (Figure 2B). A similar profound reduction was observed using the CD34 FLT3 LSK phenotype as an alternative definition of LT-HSCs, with preserved frequencies of CD34 + FLT3 and CD34 + FLT3 + downstream progenitors (Figure 2C and refs. 42, 43). We next fractionated LSK progenitors using CD150/CD48 expression, as the functional potential of these subpopulations was recently characterized in detail (44). Both LSK CD150 + CD48 LT-HSCs and CD150 CD48 multipotent progenitors (MPPs), but not CD48 + LSK hematopoietic progenitor cells (HPC1/2), were decreased in Ash1l GT/GT mice (Supplemental Figure 1; supplemental material available online with this article; doi: /jci78124ds1). Despite persistent LT-HSC depletion, 24-week-old Ash1l-deficient mice maintained normal blood counts, except for modest thrombocytopenia (Figure 2D). Overall BM cellularity remained preserved at 24 weeks as well as at 48 weeks of life. Flow cytometric analysis showed persistent profound LT-HSC depletion, while numbers of LSK progenitors were reduced only by about 30% at 24 weeks and 50% at 48 weeks (Figure 2E). These data suggest a specific role of Ash1l in the maintenance of adult BM HSCs, with downstream compensatory mechanisms preserving overall hematopoietic function in steady-state conditions. To evaluate whether the hematopoietic phenotype of Ash1ldeficient mice was associated with mobilization of BM HSCs and hematopoietic progenitor cells into the periphery, we assessed LSK and LT-HSC frequency as well as colony-forming activity in the spleen (Supplemental Figure 2). The frequency of spleen LSK/LT- HSCs and granulocyte-macrophage CFU (CFU-GM) colonies was decreased in Ash1l GT/GT mice. These findings indicate the absence of a mobilization phenotype in these mice and suggest that the maintenance of adult Ash1l-deficient hematopoietic progenitors was relatively more impaired outside as compared with that within the BM. Ash1l-deficient mice lack HSCs capable of long-term BM reconstitution after transplantation. As the gold standard definition of LT-HSCs is based on their function (45, 46), we studied the long-term reconstitution potential of Ash1l-deficient progenitors in transplantation assays (Figure 3A). This strategy allowed us to evaluate LT-HSC function irrespective of surface phenotype. We transplanted lethally irradiated CD recipient mice with CD Ash1l GT/GT or Ash1l +/+ BM and an equal number of CD competitor BM cells. Although transient output was observed 2 weeks after transplantation, Ash1l GT/GT BM failed to support long-term trilineage reconstitution of the myeloid, B, and T cell compartments (Figure 3B). Analysis of BM LT-HSCs weeks after transplantation showed a complete absence of Ash1l GT/GT LT-HSCs (Figure 3, C and D). The lack of significant reconstitution beyond a few weeks was consistent with the depletion of both LT-HSCs and multipotent progenitors in Ash1l-deficient mice, as defined using CD150/CD48 expression (43). To examine the relative contribution of CD48 LSK cells (containing LT-HSCs and multipotent progenitors) and CD48 + LSK cells (containing HPC1/2 progenitors) to transient reconstitution after transplant, we competitively transferred sort-purified CD48 LSK and CD48 + LSK progenitors to lethally irradiated recipients (Supplemental Figure 3). Consistent with past data (44), only transient hematopoietic output was detected from wild-type HPC1/2 cells, while LSK MPP/LT-HSC sustained long-term reconstitution. This pattern of activity was preserved with Ash1l-deficient progenitors at early time points after transplantation, with the bulk of short-term reconstitution coming from the CD48 LSK fraction (2 4 weeks after transplant). As observed with transplantation of total BM, however, phenotypically defined MPP/LT-HSCs were functionally unable to sustain long-term reconstitution. These data suggest that Ash1l-deficient CD48 LSK cells can home to the BM and provide initial progeny but lack long-term self-renewal potential. We next examined Ash1l GT/GT LT-HSC function after transplantation without competition. The majority of irradiated Ash1l GT/GT BM recipients died within 10 to 150 days after transplantation, consistent with transient radioprotection but suggestive of LT-HSC dysfunction (Figure 3E). Surviving Ash1l GT/GT BM recipients exhibited host CD reconstitution and no Ash1l GT/GT LT-HSCs (Figure 3F). Thus, Ash1l GT/GT BM did not house transplantable LT-HSCs, even as defined by noncompetitive transplantation. We then performed transplantation using fetal liver as the donor source, thereby normalizing phenotypic LT-HSC frequency and eliminating the possibility that stromal BM defects contributed to LT-HSC dysfunction (Figure 4A). Ash1l GT/GT fetal liver progenitors were incapable of sustaining myeloid, B, and T lineage reconstitution after transplantation (Figure 4B). Analysis of the BM at 17 to 26 weeks after transplantation showed no detectable Ash1l GT/GT LT-HSCs (Figure 4C). Thus, fetal Ash1l GT/GT LT-HSCs were present in normal numbers, as defined phenotypically, but could not establish durable hematopoiesis in the BM after transplantation, indicating that they were functionally defective once transferred into the postnatal environment. To evaluate the homing capacity of Ash1l-deficient progenitors, we analyzed recipient BM 24 hours after transplantation into nonirradiated mice (Supplemental Figure 4A). Equivalent numbers of donor-derived wild-type and Ash1l GT/GT cells were recovered (Supplemental Figure 4B). Similar observations were made in irradiated recipients (data not shown). Altogether, our findings reveal profound LT-HSC dysfunction that becomes apparent in the context of the adult BM niche after transplantation, despite preserved initial homing and even when fetal cells are used as a source of progenitors. The Ash1l-deficient BM supports wild-type HSC engraftment in the absence of myeloablation. Since Ash1l GT/GT BM had reduced LT-HSCs that were nonfunctional in transplantation assays, we reasoned that available niche space and/or defective progenitors could allow engraftment of donor HSCs without myeloablation. We infused 2 boluses of wild-type CD BM (or B6-GFP BM in selected experiments) into CD Ash1l +/+ or Ash1l GT/GT mice without prior irradiation (Figure 5A). Few, if any, donor-derived cells engrafted in wild-type recipients, as expected. In contrast, the majority (7 of 8) of Ash1l GT/GT recipients achieved stable or steadily rising trilineage output from infused BM, as shown by the 20% to 90% donor-derived contribution to blood lineages for 12 weeks (Figure 5B). At completion of the experiment, donor LT-HSCs were present in the Ash1l GT/GT BM (Figure 5C). Thus, the Ash1l GT/GT BM niche supported long-term stable engraftment by wild-type HSCs, while residual Ash1l GT/GT HSCs and hematopoietic progenitor cells competed poorly for niche space. Neonatal Ash1l GT/GT HSCs home to the BM but fail to establish a quiescent adult HSC pool. As LT-HSC loss was first observed in young adult Ash1l GT/GT BM, we studied BM HSCs within 1 to 3 weeks after 2010 jci.org Volume 125 Number 5 May 2015
5 The Journal of Clinical Investigation Research article Figure 3. Competitive and noncompetitive transplantation assays reveal a lack of Ash1l-deficient HSCs capable of long-term hematopoietic reconstitution. (A) Experimental strategy: Ash1l +/+ or Ash1l GT/GT B6-CD45.2 BM was injected into irradiated (9 Gy) B6-CD45.1 recipients, with or without B6-CD45.1 competitor BM ( cells each for competitive transplantation and 10 6 cells for noncompetitive transplantation). (B) Peripheral blood analysis 2 16 weeks after competitive transplantation, showing a profound reduction of Ash1l GT/GT BM contribution to myeloid, B, and T lineage reconstitution (wildtype n = 11, Ash1l GT/GT n = 23; mean ± SD from 2 experiments). BMT, BM transplantation. (C and D) CD45.2/CD45.1 chimerism in BM LT-HSCs weeks after transplantation, showing absence of Ash1l GT/GT LT-HSCs (wild-type n = 11, Ash1l GT/GT n = 22; data from individual mice, with mean shown, pooled from 2 experiments, ***P < 0.001, t test). (E) Survival after noncompetitive BM transplantation, showing only partial radioprotection by Ash1l GT/GT BM (n = 17 mice per genotype from 2 experiments; 8 mice for no BM transplantation control). (F) Flow cytometric analysis of surviving Ash1l GT/GT recipients, showing exclusively host-derived LSK cells. Control Ash1l +/+ recipients were reconstituted with CD donor-derived progenitors (representative of 3 mice per genotype from 2 experiments). birth. At this stage, Ash1l GT/GT mice had a normal frequency of phenotypically defined LT-HSCs in the BM, indicating preserved initial homing from the liver (Figure 6, A and B, and data not shown). However, P19 Ash1l GT/GT HSCs showed increased expression of CD34 (Figure 6B). In mice, CD34 is upregulated in cycling HSCs and marks fetal but not the most quiescent adult mouse LT-HSCs (3, 10). To test whether Ash1l GT/GT BM HSCs aberrantly maintained a fetal program, we used a Sox17-GFP reporter to study expression of Sox17, a master regulator of fetal HSCs (11). Ash1l GT/GT HSCs were Sox17-GFP + in the fetal liver and extinguished Sox17 expression by 2 weeks after birth (Figure 6C). A similar pattern was observed for Lin28b transcripts, which were detected in fetal HSCs, as previously reported (12), but absent in both wild-type and Ash1l GT/GT adult HSCs (Figure 6C). Thus, Ash1l deficiency did not result in the maintenance of a global fetal HSC state. We next assessed cell cycle status in Ash1l GT/GT HSCs. As shown by Ki67/DAPI staining, P19 Ash1l GT/GT HSCs had a markedly reduced G 0 fraction, increased entry into G 1, and a trend for more HSCs in S/G 2 /M phases of the cell cycle (Figure 6D). Since most HSCs exit the cell cycle in the BM between P14 and P21 (1), this suggested that Ash1l-deficient HSCs failed to establish a quiescent stem cell pool at the fetal to adult transition. P19 Ash1l GT/GT LT-HSCs but not total BM or c-kit hi SCA1 hi cells also showed increased BrdU incorporation (Supplemental Figure 5). Recent work showed that BM HSC jci.org Volume 125 Number 5 May
6 Research article The Journal of Clinical Investigation Figure 4. Ash1l-deficient fetal liver cells do not sustain long-term BM reconstitution after competitive transplantation. (A) Experimental strategy: Ash1l +/+ or Ash1l GT/GT B6-CD45.2 E15.5 fetal liver was mixed with wild-type B6-CD45.1 competitor BM (1:1 ratio; cells each) and injected into lethally irradiated (9 Gy) B6-CD45.1 recipients. (B) Analysis of peripheral blood 2 26 weeks after transplantation, showing a profound reduction of Ash1l GT/GT contribution to myeloid, B, and T lineage reconstitution (n = mice per genotype; mean ± SD from 2 experiments). (C) CD45.2/CD45.1 chimerism in CD150 + CD48 LSKs (LT-HSCs) weeks after transplantation, showing markedly decreased fetal liver-derived Ash1l GT/GT LT-HSCs (wildtype n = 11, Ash1l GT/GT n = 10 mice, ***P < 0.001, t test; horizontal bars show the mean). quiescence is dependent on combined effects of the cyclin-dependent kinase inhibitors p27 (encoded by Cdkn1b) and p57 (encoded by Cdkn1c) (15, 47). Ash1l GT/GT BM LSK cells had markedly decreased expression of these two critical regulators (Figure 6E). Expression of p21 (encoded by Cdkn1a) was not altered. These data suggest that Ash1l directly or indirectly regulates p27 and p57 expression during establishment of quiescence in young adult HSCs. We next challenged Ash1l GT/GT and control mice with 5-fluorouracil (5-FU) (Figure 7A). 5-FU treatment is toxic to dividing cells but spares quiescent cells, including many normal LT-HSCs. A single 5-FU treatment resulted in a 2-log reduction of Ash1l GT/GT LT-HSCs compared with controls, thereby worsening LT-HSC loss in Ash1l GT/GT mice by 10- to 20-fold (Figure 7B). This was consistent with increased sensitivity of Ash1l GT/GT HSCs to 5-FU, due, at least in part, to decreased quiescence. Consistent with these findings, 5-FU administration was associated with impaired survival of Ash1l-deficient mice, even after administration of a single dose (Figure 7, C and D). Increased cumulative proliferation of hematopoietic progenitors compensates for HSC loss in Ash1l-deficient mice. HSC loss and profound defects in transplantation assays contrasted with preservation of hematopoietic output under steady-state conditions in Ash1l- deficient mice. To explore this paradox, we used a pulsechase H2B-GFP labeling system to study proliferation history of phenotypically defined HSCs as well as downstream progenitors that normally have limited self-renewal potential (Figure 8A and ref. 8). This strategy allows tetracycline-induced expression of H2B-GFP in all cells (pulse), followed by dilution of GFP fluorescence upon cell division after tetracycline withdrawal (chase). Ash1l GT/GT LT-HSCs had increased proliferation throughout the 6-week chase period, as indicated by enhanced GFP dilution (Figure 8B). The entire Ash1l GT/GT LSK compartment had markedly increased proliferation as compared with control LSK cells. We could not detect a residual population of LSK or Lin cells with high GFP fluorescence in Ash1l-deficient mice, consistent with the absence of highly quiescent progenitors (Figure 8B). In contrast, we observed a markedly increased proportion of Ash1l GT/GT Lin GFP cells that had preserved a primitive LSK phenotype after 6 weeks of chase (Figure 8C). These findings suggest that increased selfrenewal of progenitors downstream of HSCs could compensate for LT-HSC loss and sustain hematopoietic output in Ash1l GT/GT mice jci.org Volume 125 Number 5 May 2015
7 The Journal of Clinical Investigation Research article Figure 5. Ash1l deficiency allows engraftment of wild-type HSCs in the absence of myeloablation. (A) Experimental strategy: B6-CD45.2 Ash1l +/+ or Ash1l GT/GT mice received B6-CD45.1 or B6-GFP BM cells (2 doses 1 week apart i.v.), without prior irradiation. (B) Analysis of peripheral blood, showing myeloid, B, and T cell output from CD donor BM in 7 of 8 Ash1l GT/GT recipients versus 0 of 6 Ash1l +/+ recipients (data from 3 experiments). Lines represent individual recipients. Reconstitution was sustained for 12 weeks. (C) Flow cytometric analysis of LT-HSCs 12 to 21 weeks after infusion, showing donor LT-HSC engraftment in 7 of 8 Ash1l GT/GT recipients versus 0 of 6 wild-type recipients (pooled from 3 experiments, with horizontal bars showing the mean; ***P < 0.001, t test). Ash1l regulates the expression of multiple Hox genes. In flies, TrxG members collaborate to maintain Hox gene expression during development. We reasoned that, if mammalian TrxG members cooperated with one another, they might also share target genes and functional effects. Mll1, the mammalian homolog of fly trithorax, regulates expression of multiple Hoxa genes and Hox-related genes, such as Meis1. Ash1l GT/GT LSK progenitors had reduced expression of Hoxa3, Hoxa4, Hoxa5, Hoxa6, Hoxa9, Hoxa10, and Meis1 (Figure 9). Thus, Ash1l and Mll1 might share multiple target genes, including Hox genes (25, 48 50). Of note, the expression of Mll1 itself and that of other genes previously associated with HSC homing and niche retention (Rac1, Rac2, Dnmt1) was not changed (refs. 51, 52, and data not shown). Ash1l cooperates with Mll1 and Men1 to maintain hematopoiesis. To further explore the functional cooperativity of Ash1l and Mll1 in hematopoiesis, we bred Ash1l GT/+ mice with Mll1 fl/fl Mx1-Cre + mice, using a conditional Mll1 allele reported to induce only mild hematopoietic defects upon inactivation in steady-state conditions (26). This strategy allowed deletion of one or both Mll1 alleles via poly(i:c) injection in the presence of graded Ash1l doses (Figure 10A). Combined Mll1 and Ash1l deficiency resulted in rapid and profound reduction in BM cellularity, suggesting hematopoietic failure (Figure 10B). Flow cytometric analysis of LT-HSCs and hematopoietic progenitors showed that Ash1l GT/GT Mll1 fl/fl Mx1-Cre + mice had profound reductions in both LT-HSCs and LSK progenitors (Figure 10, C and D). In addition, we observed a unique sensitivity of LT-HSCs to the combined Mll1 and Ash1l gene dose, as numbers of LT-HSCs decreased more profoundly when homozygous Ash1l or Mll1 inactivation was combined with heterozygous inactivation of Mll1 or Ash1l, respectively (Figure 10C). CD34 LT-HSCs, a highly quiescent adult HSC subset identified with surface markers in mice, were particularly sensitive to inactivation of an increasing number of Ash1l and Mll1 alleles (Figure 10D), suggesting that Ash1l and Mll1 cooperate to maintain quiescent HSCs. However, multipotent progenitors and overall BM cellularity were only depleted upon targeting of both Mll1 and Ash1l alleles (Figure 10, B D). Finally, we assessed the combined effects of Ash1l deficiency and inactivation of Men1, encoding the MLL1 cofactor menin (Supplemental Figure 6). Menin is essential for recruitment of the MLL1 complex, at least to a subset of target genes, and was previously shown to regulate HSC homeostasis (53 55). Profound thrombocytopenia, BM hypocellularity, and loss of all hematopoietic progenitors were observed upon combined deficiency of both Ash1l and Men1 alleles but not when even one of the four alleles was maintained (Supplemental Figure 6, B D). LT-HSCs were particularly sensitive to graded loss of Ash1l and Men1 alleles. (Supplemental Figure 6D). Although only some Mll1 target genes are sensitive to Men1 loss (50), these genes may be important for the cooperative effects of Ash1l and Mll1 in hematopoiesis. Together, these findings reveal cooperativity between Ash1l and Mll1 or Men1 in HSCs and hematopoietic progenitor cells, uncovering a unique sensitivity of quiescent adult HSCs to regulation by the TrxG family. Discussion Our findings uncover an essential function for the TrxG gene Ash1l in the regulation of adult HSCs and reveal functional cooperativity of TrxG members in hematopoiesis. When Ash1l levels were jci.org Volume 125 Number 5 May
8 Research article The Journal of Clinical Investigation Figure 6. Ash1l GT/GT LT-HSCs home to the BM but fail to establish normal quiescence, despite appropriate extinction of the fetal HSC program. (A) Flow cytometric analysis of P10 BM, showing comparable frequencies of phenotypically defined LT-HSCs (CD150 + CD48 LSK) in Ash1l GT/GT and Ash1l +/+ littermates ( 9 mice per genotype; mean ± SEM). (B) Decreased percentage of quiescent CD34 cells in Ash1l GT/GT LT-HSCs (P19) (4 mice per genotype; mean ± SEM). (C) Sox17-GFP expression in E15.5 fetal liver and P14 BM LT-HSCs (CD150 + CD48 LSK cells). Sox17-GFP was present in fetal LT-HSCs but extinguished in wild-type and Ash1l GT/GT P14 BM (n 3 per genotype, 4 experiments; symbols show individual mice and the bars show the mean). qrt-pcr analysis shows similar Lin28b gene expression in Ash1l GT/GT and Ash1l +/+ fetal LSK progenitors, with loss of expression in adult progenitors of both genotypes (n = 3 5 per group; mean ± SEM) (n.d., not detectable). (D) Flow cytometry plots (Ki67 vs. DAPI) showing decreased G 0 (quiescent fraction) and increased distribution into G 1 and S/G 2 /M phases of the cell cycle in P19 Ash1l GT/GT LT-HSCs (5 mice per genotype; mean ± SEM). (E) Reduced Cdkn1b and Cdkn1c expression relative to Hprt1 in P10 Ash1l GT/GT LSK progenitors (qrt-pcr, n = 3 per group, mean ± SEM). *P < 0.05, **P < 0.01, ***P < 0.001, t test. reduced ability to establish quiescence occurred despite transition from fetal to adult state. Altogether, we believe that Ash1l is a novel key regulator of changes associated with the adaptation of HSCs to the BM niche at the fetal to adult transition or upon transplantation of HSCs into an adult recipient. Ash1l GT/GT BM had profoundly reduced LT-HSC numbers but maintained relatively normal mature hematopoietic cell output. This paradox did not result from the presence of phenotypically abnormal but functional HSCs residing in the Ash1l GT/GT BM, as young adult Ash1l GT/GT BM failed to achieve long-term reconstitution in irradiated recipients after competitive and noncomprofoundly reduced in Ash1l GT/GT mice, the ability of young adult LT-HSCs to establish and maintain quiescence was impaired. Ash1l- deficient HSCs developed and expanded normally in the fetal liver and initially seeded the BM. Once in the BM, these LT-HSCs did not upregulate expression of Cdkn1b/c, which was associated with reduced numbers of quiescent HSCs. Because fetal HSCs are known to divide rapidly, these results could have arisen from the persistence of a fetal program upon reaching the BM. However, expression of Sox17 and Lin28b, two essential regulators of the fetal HSC transcriptional program, was properly extinguished in BM Ash1l GT/GT LT-HSCs, indicating that their 2014 jci.org Volume 125 Number 5 May 2015
9 The Journal of Clinical Investigation Research article Figure 7. Ash1l GT/GT mice are more sensitive to 5-FU challenge than wild-type mice. (A) Experimental strategy: mice of indicated genotypes were injected with 150 mg/kg 5-FU and sacrificed 8 days later. (B) Flow cytometric analysis of LT-HSCs (CD150 + CD48 LSK cells), showing 2-log reduction in frequency and 2.5-log reduction in LT-HSC numbers in Ash1l GT/GT mice as compared with control mice after 5-FU exposure (n 4 mice per genotype; mean ± SEM, *P < 0.05, t test). (C) Experimental strategy: mice were injected with 150 mg/kg 5-FU and monitored for survival. (D) Survival of mice after 5-FU challenge (7 mice per genotype, representative of 2 experiments, *P < 0.05, log-rank Mantel-Cox test). petitive transplantation. Based on this gold standard approach of HSC biology, we could not detect LT-HSC function in the Ash1l GT/GT BM, indicating that the presence of functional HSCs with an alternative phenotype was unlikely. Furthermore, we could also not detect functional LT-HSCs after transplantation of Ash1l GT/GT fetal liver progenitors, which had LT-HSC frequency comparable to that of Ash1l +/+ mice by phenotypic criteria. Thus, despite their normal in vivo expansion in the fetal/neonatal liver, Ash1l GT/GT LT-HSCs could not establish durable hematopoiesis in the BM niche after transplantation, indicating that they were functionally abnormal, even if they expanded normally in the fetal environment. As normal numbers of Ash1l GT/GT HSCs were initially present in the neonatal BM, our data are consistent with preserved HSC homing to the BM in these mice but also with failure to respond to niche factors that are required for the establishment of quiescence and subsequent stable engraftment. Among putative essential niche-derived factors in the BM, thrombopoietin (TPO) and TGF-β have been linked to induction of quiescence through Cdkn1b/c upregulation (14, 56, 57). Furthermore, decreased expression of multiple posterior Hoxa genes was observed in young adult HSCs of TPO-deficient mice (14). Thus, Ash1l may be necessary to establish a normal transcriptional response downstream of TPO or other essential BM niche factors required for adult HSC quiescence, maintenance, and function. As genetic inactivation of Hoxa9 and other Hoxa genes was shown to cause HSC defects, we speculate that decreased expression of multiple Hoxa genes accounts for part of the defects in Ash1l-deficient HSCs, although other factors are likely involved as well (58, 59). Ash1l-deficient mice did not progress to frank hematopoietic failure, despite severe depletion of phenotypically defined LT- HSCs and lack of transplantable HSCs. A possible explanation for these findings is that few residual LT-HSCs could maintain steady-state hematopoiesis through a compensatory increase in proliferation. Using H2B-GFP in a pulse-chase system (8), we indeed observed increased cumulative proliferation of all primitive Ash1l GT/GT hematopoietic progenitors, consistent with the absence of a slowly cycling progenitor pool. A striking feature of Ash1l-deficient hematopoiesis, however, was the large increase in cells that maintained a primitive LSK phenotype, despite the complete loss of GFP at the end of the chase period. Thus, Ash1l-deficient progenitors downstream of LT-HSCs showed evidence of increased self-renewal in vivo in the absence of a normal HSC compartment. This phenomenon is reminiscent of recent observations that early thymocyte progenitors acquire extended self-renewal in vivo when the normal input of blood-derived T lineage progenitors is impaired, suggesting that self-renewal potential can be modulated in individual hematopoietic progenitors as an adaptation to pathological conditions (60, 61). It is interesting to speculate about why compensatory changes in Ash1l-deficient mice were not sustained after transplantation into irradiated recipients. Emerging evidence indicates that steady-state hematopoiesis and stress hematopoiesis after transplantation are regulated by different mechanisms (62). After transplantation, hematopoiesis is driven by a small number of LT-HSCs that outcompete other progenitors, while steadystate hematopoiesis can be maintained for extended periods of time by a broader range of downstream progenitors with more jci.org Volume 125 Number 5 May
10 Research article The Journal of Clinical Investigation Figure 8. In vivo proliferation history shows decreased quiescence of Ash1l-deficient HSCs but increased persistence of proliferating downstream progenitors. (A) Experimental strategy: Ash1l +/+ or Ash1l GT/GT mice with M2rtTA and H2B-GFP transgenes were maintained on doxycycline for 6 weeks to label hematopoietic cells with GFP. GFP dilution was monitored by flow cytometry after a 6-week chase period. (B) Flow cytometric analysis after chase, showing significantly increased GFP dilution in Ash1l GT/GT LSK CD150 + CD48 cells (containing LT-HSCs), LSK CD150 CD48 (MPP), and LSK CD48 + progenitors (HPC1/2) compared with controls, consistent with increased cell division ( 6 mice per genotype; mean ± SEM). (C) Flow cytometric analysis, demonstrating that the H2B-GFP negative fraction of Lin cells was enriched for primitive LSK progenitors in Ash1l GT/GT versus Ash1l +/+ mice ( 4 mice per genotype; mean ± SEM). *P < 0.05, **P < 0.01, ***P < 0.001, t test. restricted potential. Thus, it is possible that Ash1l-deficient mice were competent for this type of hematopoiesis but selectively deficient in LT-HSC function after transplant. Only a few genetic models of HSC dysfunction that support nonablative transplantation have been reported in hematopoietic biology (52, 63). In Ash1l GT/GT mice, successful engraftment in nonablative transplantation experiments suggested that the Ash1ldeficient BM niche was not defective, as it could support maintenance of wild-type LT-HSCs. Stable wild-type LT-HSC engraftment suggested that niche spaces were available due to LT-HSC depletion and/or that remaining Ash1l GT/GT LT-HSCs competed poorly for niche space. Either scenario is consistent with extreme LT-HSC dysfunction when Ash1l levels are reduced. Of note, the full effects of Ash1l could be even more profound than observed in Ash1l GT/GT mice, as these mice had low residual levels of normal Ash1l transcripts, consistent with Ash1l GT being a severely hypomorphic but not a null allele. Cooperative effects of Mll1 and Ash1l in hematopoiesis highlight an evolutionarily conserved feature of TrxG members, a phenomenon identified in flies but not previously described in mammals. Loss of a single Ash1l GT allele amplified LT-HSC depletion in Men1-deficient and Mll1-deficient mice, while complete 2016 jci.org Volume 125 Number 5 May 2015
11 The Journal of Clinical Investigation Research article Figure 9. Ash1l regulates expression of Hox and Hox-related genes in hematopoietic progenitors. Relative abundance of Hoxa and Meis1 normalized to Hprt1 transcripts in sort-purified adult Ash1l GT/GT LSK progenitors, as assessed by qrt-pcr (triplicate analysis of 4 to 8 individual biological samples per genotype, mean ± SEM; *P < 0.05, **P < 0.01, ***P < 0.001, t test). hematopoietic failure was observed only when all 4 alleles were deficient. This dominant phenotypic enhancement is reminiscent of criteria used to identify TrxG members in Drosophila. To date, the biochemical mechanisms underlying this genetic observation remain poorly understood. Given that both Mll1 and Ash1l are required for Hox gene expression, it is possible that MLL1 and ASH1L proteins act nonredundantly to promote transcription at a shared subset of target genes. Indeed, both Ash1l GT/GT and Mll1- or Men1-deficient hematopoietic progenitors displayed a reduced, but not absent, expression of posterior Hoxa genes, suggesting multifactorial regulation at this locus (25, 50, 54). Since MLL1 and ASH1L SET domains have H3K4 and H3K36 methyltransferase activity, two histone marks that are involved in transcription initiation and elongation steps, respectively, it can be envisioned that they target different regulatory steps of transcription. In this scenario, either Mll1 or Ash1l inactivation can lead to compromised Hoxa gene expression. It also remains to be determined whether the cooperative interaction between Mll1 and Ash1l is limited to the regulation of Hoxa genes in LT- HSCs, especially since Mll1 was reported to control multiple other genes in normal hematopoiesis (50). Future work should explore the detailed molecular mechanisms by which TrxG members cooperatively regulate quiescent adult HSCs. Methods Mice. C57BL/6.Ptprca (B6-SJL, CD ) mice were from the National Cancer Institute. B6-GFP transgenic mice expressing GFP under the control of the human ubiquitin C promoter were from The Jackson Laboratory (64). Ash1l GT/+ embryonic stem cells were obtained from Sanger Institute. After blastocyst injection, Ash1l GT/+ mice were generated by the University of Michigan Transgenic Animal Core and intercrossed. In most experiments, Ash1l GT/+ mice were backcrossed to the C57BL/6 background (B6, CD ) for at least 6 generations. In selected experiments, Ash1l GT/+ mice were bred with Sox17 GFP/+ mice, with transgenic mice containing both Rosa26- rtta and TetOP-H2B-GFP, with Men1 fl/fl Mx1-Cre + mice or with Mll1 fl/fl Mx1-Cre + mice (8, 11, 26, 65, 66). To label hematopoietic cells with H2B-GFP, Rosa26-rtTA TetOP-H2B-GFP mice were maintained on doxycycline (2 mg/ml in drinking water) for 6 weeks. Mice were maintained on normal water for a chase period (6 weeks) to allow GFP dilution in dividing cells. Men1 or Mll1 excision was achieved with poly(i:c) (Amersham; 50 μg i.p. every 2 days for 5 doses). Flow cytometry. Single cell suspensions were prepared from fetal liver, BM, or blood, followed by red blood cell lysis (ACK buffer, Cambrex). The following antibodies were from BioLegend: anti-cd3, anti-cd4, anti-cd8, anti-cd11b, anti-cd11c, anti-cd19, anti-cd48, anti-cd150, anti-gr1/ly-6g, anti-b220, anti-nk1.1, anti-tcrβ, anti-tcrγδ, and anti-ckit. Anti-SCA1 was from ebiosciences. We used the following antibody cocktail to exclude lineage + cells: anti-cd11b, anti-gr1, anti-cd11c, anti-b220, anti-cd19, anti- CD3, anti-tcrβ, anti-tcrγδ, anti-cd8, anti-nk1.1, and anti-ter119. In fetal samples, CD11b was omitted. BrdU analysis was performed using a BrdU Labeling Kit (BD Biosciences). Ki67 staining was achieved using the BD Ki67 Set (BD Biosciences). Analysis was performed on a FACSCanto and sorting was performed on a FACSAria II/III (BD Biosciences). Dead cells were excluded with 4 6-diamidino-2-phenylindole (Sigma-Aldrich). Flow cytometry files were analyzed with FlowJo (TreeStar). BM and fetal liver cell transplantation. Six- to eight-week-old B6-SJL (CD ) mice were lethally irradiated (900 Gy, 37 Cs source). Four hours after irradiation, mice were transplanted with 6- to 10-week-old donor BM or E15.5 fetal liver cells via tail vein injection. For competitive transplantation, we mixed equal numbers of competitor B6-SJL BM and tester CD BM or fetal liver cells. For nonablative transplantation, 5- to 8-week-old Ash1l GT/GT mice or wild-type controls were injected twice at 1-week intervals with B6-SJL (CD ) or B6-GFP (CD ) BM cells. For BM homing studies, recipients were analyzed 24 hours after transplantation. Complete blood counts. Blood was obtained through retroorbital bleeding and transferred to EDTA-treated tubes. Complete blood counts were determined using the Advia 120 Hematology System (Siemens). CFU-GM. 20,000 BM cells or 200,000 spleen cells were plated per ml of MethoCult GF M3534 (Stem Cell Technologies). Colonies were scored 7 to 10 days later. Quantitative real-time PCR. For gene expression analyses, at least 5,000 cells from each population of interest were sort purified directly into Trizol (Invitrogen). After RNA extraction, cdna was generated using the SuperScript III First-Strand Synthesis Kit (Invitrogen) or the Nugen jci.org Volume 125 Number 5 May
12 Research article The Journal of Clinical Investigation Figure 10. Combined Ash1l and Mll1 deficiency induces overt hematopoietic failure and profound depletion of LT-HSCs and LSK progenitors. (A) Experimental strategy: mice of indicated genotypes were injected with poly(i:c) (20 μg every 2 days 5 times). (B) Reduced BM cellularity in Ash1l GT/GT Mll1 fl/fl Mx1-Cre + mice ( 2 mice per genotype; mean ± SEM). (C) Flow cytometric analysis showing severe reduction in CD34 LT-HSCs, LT-HSCs, and LSK progenitors in Ash1l GT/GT Mll1 fl/fl Mx1-Cre + mice and reduced CD34 LT-HSC and LT-HSC frequency with cumulative inactivation of Ash1l and Mll1 alleles. The representative plot for Ash1l GT/+ Mll1 fl/fl Mx1-Cre + BM is derived from a separate experiment, thus gating definitions differ from the other samples. (D) LSK and LT-HSC numbers, reflecting hematopoietic failure in Ash1l GT/GT Mll1 fl/fl Mx1-Cre + mice and LT-HSC sensitivity to loss of Ash1l and Mll1 alleles ( 2 mice per genotype; mean ± SEM), and CD34 LT-HSC numbers in 2 hind legs, showing high sensitivity of this population to regulation by TrxG members. *P < 0.05, **P < 0.01, ***P < 0.001, compared with wild type; # P < 0.05, ## P < 0.01, compared with Ash1l GT/GT ; P < 0.05, P < 0.01, P < 0.001, compared with induced Mll fl/fl Mx1-Cre +, t test. Ovation PicoSL WTA System (Nugen Technologies). Relative gene expression was measured using TaqMan primers and probe sets from Applied Biosystems (Hoxa5: Mm _m1; Hoxa7: Mm _ m1; Hoxa9: Mm _m1; Hoxa10: Mm _m1; Meis1: Mm _m1; Cdkn1a: Mm _m1; Cdkn1b: Mm _ m1; Cdkn1c: Mm _m1) or SYBR Green (Fisher) with the fol- lowing primer pairs: Hoxa3, AAGCGACCTACTACGACAG and TGG- CATTGTAAGCGAACC; Hoxa4, CAGAACCGGAGAATGAAGTG and CGAGGCAGTGTTGGAAGA; and Hoxa6, TTACCAACAGTCCAACTC and GGTCTTTATCAGAATAGAAACA. Reactions were carried out on a Mastercycler realplex (Eppendorf). Relative expression was calculated after normalization with Hprt1 expression using the ΔΔCT method jci.org Volume 125 Number 5 May 2015
13 The Journal of Clinical Investigation Research article Statistics. Comparison of two means was performed with 2-tailed unpaired Student s t test. Where indicated, we used a log-rank Mantel- Cox test. Study approval. The University of Michigan Committee on Use and Care of Animals approved all experiments. Acknowledgments We are grateful to Hugh J.M. Brady for Mll1 fl/fl mice, Sean Morrison for Sox17-GFP mice, David Ginsburg for B6-GFP mice, and Hanno Hock for Rosa26-rtTA and TetOP-H2B-GFP mice. This work was supported by the Sidney Kimmel Cancer Research Foundation, the University of Michigan Comprehensive Cancer Center, the D. Dan and Betty Kahn Foundation, and the NIH (RO1-AI to I. Maillard, R37-HD30428 to S.A. Camper). Individual support included T32 training grants from the University of Michigan s Medical Scientist Training Program (GM07863 to M. Jones), Center for Organogenesis (HD to M. Jones and J. Chase), and Cellular and Molecular Biology Program (GM to J. Chase). Flow cytometry was partially supported by a core grant from the NIH to the University of Michigan Cancer Center (P30-CA46592). Address correspondence to: Ivan Maillard, Life Sciences Institute #6382A, 210 Washtenaw Avenue, University of Michigan, Ann Arbor, Michigan 48109, USA. Phone: ; imaillar@umich.edu. 1. Bowie MB, McKnight KD, Kent DG, McCaffrey L, Hoodless PA, Eaves CJ. Hematopoietic stem cells proliferate until after birth and show a reversible phase-specific engraftment defect. J Clin Invest. 2006;116(10): Ema H, Nakauchi H. Expansion of hematopoietic stem cells in the developing liver of a mouse embryo. Blood. 2000;95(7): Wilson A, et al. Hematopoietic stem cells reversibly switch from dormancy to selfrenewal during homeostasis and repair. Cell. 2008;135(6): Takizawa H, Regoes RR, Boddupalli CS, Bonhoeffer S, Manz MG. Dynamic variation in cycling of hematopoietic stem cells in steady state and inflammation. J Exp Med. 2011;208(2): Venezia TA, et al. Molecular signatures of proliferation and quiescence in hematopoietic stem cells. PLoS Biol. 2004;2(10):e Cheshier SH, Morrison SJ, Liao X, Weissman IL. In vivo proliferation and cell cycle kinetics of longterm self-renewing hematopoietic stem cells. Proc Natl Acad Sci U S A. 1999;96(6): Baldridge MT, King KY, Boles NC, Weksberg DC, Goodell MA. Quiescent haematopoietic stem cells are activated by IFN-γ in response to chronic infection. Nature. 2010;465(7299): Foudi A, et al. Analysis of histone 2B-GFP retention reveals slowly cycling hematopoietic stem cells. Nat Biotechnol. 2009;27(1): Bowie MB, et al. Identification of a new intrinsically timed developmental checkpoint that reprograms key hematopoietic stem cell properties. Proc Natl Acad Sci U S A. 2007;104(14): Ito T, Tajima F, Ogawa M. Developmental changes of CD34 expression by murine hematopoietic stem cells. Exp Hematol. 2000;28(11): Kim I, Saunders TL, Morrison SJ. Sox17 dependence distinguishes the transcriptional regulation of fetal from adult hematopoietic stem cells. Cell. 2007;130(3): Yuan J, Nguyen CK, Liu X, Kanellopoulou C, Muljo SA. Lin28b reprograms adult bone marrow hematopoietic progenitors to mediate fetal-like lymphopoiesis. Science. 2012;335(6073): Fleming WH, Alpern EJ, Uchida N, Ikuta K, Spangrude GJ, Weissman IL. Functional heterogeneity is associated with the cell cycle status of murine hematopoietic stem cells. J Cell Biol. 1993;122(4): Qian H, et al. Critical role of thrombopoietin in maintaining adult quiescent hematopoietic stem cells. Cell Stem Cell. 2007;1(6): Zou P, et al. p57(kip2) and p27(kip1) cooperate to maintain hematopoietic stem cell quiescence through interactions with Hsc70. Cell Stem Cell. 2011;9(3): Orford KW, Scadden DT. Deconstructing stem cell self-renewal: genetic insights into cell-cycle regulation. Nat Rev Genet. 2008;9(2): Ringrose L, Paro R. Epigenetic regulation of cellular memory by the Polycomb and Trithorax group proteins. Annu Rev Genet. 2004;38: Shearn A. The ash-1, ash-2 and trithorax genes of Drosophila melanogaster are functionally related. Genetics. 1989;121(3): Tripoulas NA, Hersperger E, La Jeunesse D, Shearn A. Molecular genetic analysis of the Drosophila melanogaster gene absent, small or homeotic discs1 (ash1). Genetics. 1994;137(4): Armstrong SA, et al. MLL translocations specify a distinct gene expression profile that distinguishes a unique leukemia. Nat Genet. 2002;30(1): Gu Y, et al. The t(4;11) chromosome translocation of human acute leukemias fuses the ALL-1 gene, related to Drosophila trithorax, to the AF-4 gene. Cell. 1992;71(4): Tkachuk DC, Kohler S, Cleary ML. Involvement of a homolog of Drosophila trithorax by 11q23 chromosomal translocations in acute leukemias. Cell. 1992;71(4): Ziemin-van der Poel S, et al. Identification of a gene, MLL, that spans the breakpoint in 11q23 translocations associated with human leukemias. Proc Natl Acad Sci U S A. 1991;88(23): Yu BD, Hess JL, Horning SE, Brown GA, Korsmeyer SJ. Altered Hox expression and segmental identity in Mll-mutant mice. Nature. 1995;378(6556): Jude CD, Climer L, Xu D, Artinger E, Fisher JK, Ernst P. Unique and independent roles for MLL in adult hematopoietic stem cells and progenitors. Cell Stem Cell. 2007;1(3): McMahon KA, et al. Mll has a critical role in fetal and adult hematopoietic stem cell self-renewal. Cell Stem Cell. 2007;1(3): Milne TA, et al. MLL targets SET domain methyltransferase activity to Hox gene promoters. Mol Cell. 2002;10(5): Dou Y, et al. Regulation of MLL1 H3K4 methyltransferase activity by its core components. Nat Struct Mol Biol. 2006;13(8): Southall SM, Wong PS, Odho Z, Roe SM, Wilson JR. Structural basis for the requirement of additional factors for MLL1 SET domain activity and recognition of epigenetic marks. Mol Cell. 2009;33(2): Steward MM, Lee JS, O Donovan A, Wyatt M, Bernstein BE, Shilatifard A. Molecular regulation of H3K4 trimethylation by ASH2L, a shared subunit of MLL complexes. Nat Struct Mol Biol. 2006;13(9): Ruthenburg AJ, et al. Histone H3 recognition and presentation by the WDR5 module of the MLL1 complex. Nat Struct Mol Biol. 2006;13(8): Shearn A, Rice T, Garen A, Gehring W. Imaginal disc abnormalities in lethal mutants of Drosophila. Proc Natl Acad Sci U S A. 1971;68(10): Tripoulas N, LaJeunesse D, Gildea J, Shearn A. The Drosophila ash1 gene product, which is localized at specific sites on polytene chromosomes, contains a SET domain and a PHD finger. Genetics. 1996;143(2): Gregory GD, et al. Mammalian ASH1L is a histone methyltransferase that occupies the transcribed region of active genes. Mol Cell Biol. 2007;27(24): Tanaka Y, Kawahashi K, Katagiri Z, Nakayama Y, Mahajan M, Kioussis D. Dual function of histone H3 lysine 36 methyltransferase ASH1 in regulation of Hox gene expression. PLoS One. 2011;6(11):e Tanaka Y, Nakayama Y, Taniguchi M, Kioussis D. Regulation of early T cell development by the PHD finger of histone lysine methyltransferase ASH1. Biochem Biophys Res Commun. 2008;365(3): An S, Yeo KJ, Jeon YH, Song JJ. Crystal structure of the human histone methyltransferase ASH1L catalytic domain and its implications for the regulatory mechanism. J Biol Chem. 2011;286(10): Yuan W, Xu M, Huang C, Liu N, Chen S, Zhu B. H3K36 methylation antagonizes PRC2- mediated H3K27 methylation. J Biol Chem. 2011;286(10): Tanaka Y, Katagiri Z, Kawahashi K, Kioussis D, Kita- jci.org Volume 125 Number 5 May
Hematopoiesis. - Process of generation of mature blood cells. - Daily turnover of blood cells (70 kg human)
 Hematopoiesis - Process of generation of mature blood cells - Daily turnover of blood cells (70 kg human) 1,000,000,000,000 total cells 200,000,000,000 red blood cells 70,000,000,000 neutrophils Hematopoiesis
Hematopoiesis - Process of generation of mature blood cells - Daily turnover of blood cells (70 kg human) 1,000,000,000,000 total cells 200,000,000,000 red blood cells 70,000,000,000 neutrophils Hematopoiesis
Nature Immunology: doi: /ni Supplementary Figure 1. Huwe1 has high expression in HSCs and is necessary for quiescence.
 Supplementary Figure 1 Huwe1 has high expression in HSCs and is necessary for quiescence. (a) Heat map visualizing expression of genes with a known function in ubiquitin-mediated proteolysis (KEGG: Ubiquitin
Supplementary Figure 1 Huwe1 has high expression in HSCs and is necessary for quiescence. (a) Heat map visualizing expression of genes with a known function in ubiquitin-mediated proteolysis (KEGG: Ubiquitin
MATERIALS AND METHODS. Neutralizing antibodies specific to mouse Dll1, Dll4, J1 and J2 were prepared as described. 1,2 All
 MATERIALS AND METHODS Antibodies (Abs), flow cytometry analysis and cell lines Neutralizing antibodies specific to mouse Dll1, Dll4, J1 and J2 were prepared as described. 1,2 All other antibodies used
MATERIALS AND METHODS Antibodies (Abs), flow cytometry analysis and cell lines Neutralizing antibodies specific to mouse Dll1, Dll4, J1 and J2 were prepared as described. 1,2 All other antibodies used
CD34+ Cells: A Comparison of Stem and Progenitor Cells in Cord Blood, Peripheral Blood, and the Bone Marrow
 White Paper September 2016 CD34+ Cells: A Comparison of Stem and Progenitor Cells in Cord Blood, Peripheral Blood, and the Bone Marrow Lily C. Trajman, PhD Introduction: Hematopoietic Stem Cells (HSCs)
White Paper September 2016 CD34+ Cells: A Comparison of Stem and Progenitor Cells in Cord Blood, Peripheral Blood, and the Bone Marrow Lily C. Trajman, PhD Introduction: Hematopoietic Stem Cells (HSCs)
New Insights into the Regulation of Hematopoietic Stem Cell Self-Renewal
 New Insights into the Regulation of Hematopoietic Stem Cell Self-Renewal By Morgan Jones A dissertation submitted in partial fulfillment of the requirements for the degree of Doctor of Philosophy (Cellular
New Insights into the Regulation of Hematopoietic Stem Cell Self-Renewal By Morgan Jones A dissertation submitted in partial fulfillment of the requirements for the degree of Doctor of Philosophy (Cellular
Haematopoietic stem cells
 Haematopoietic stem cells Neil P. Rodrigues, DPhil NIH Centre for Biomedical Research Excellence in Stem Cell Biology Boston University School of Medicine neil.rodrigues@imm.ox.ac.uk Haematopoiesis: An
Haematopoietic stem cells Neil P. Rodrigues, DPhil NIH Centre for Biomedical Research Excellence in Stem Cell Biology Boston University School of Medicine neil.rodrigues@imm.ox.ac.uk Haematopoiesis: An
Meeting Report. From December 8 to 11, 2012 at Atlanta, GA, U.S.A
 Meeting Report Affiliation Department of Transfusion Medicine and Cell Therapy Name Hisayuki Yao Name of the meeting Period and venue Type of your presentation Title of your presentation The 54 th Annual
Meeting Report Affiliation Department of Transfusion Medicine and Cell Therapy Name Hisayuki Yao Name of the meeting Period and venue Type of your presentation Title of your presentation The 54 th Annual
sequences of a styx mutant reveals a T to A transversion in the donor splice site of intron 5
 sfigure 1 Styx mutant mice recapitulate the phenotype of SHIP -/- mice. (A) Analysis of the genomic sequences of a styx mutant reveals a T to A transversion in the donor splice site of intron 5 (GTAAC
sfigure 1 Styx mutant mice recapitulate the phenotype of SHIP -/- mice. (A) Analysis of the genomic sequences of a styx mutant reveals a T to A transversion in the donor splice site of intron 5 (GTAAC
Nature Immunology: doi: /ni Supplementary Figure 1. Cellularity of leukocytes and their progenitors in naive wild-type and Spp1 / mice.
 Supplementary Figure 1 Cellularity of leukocytes and their progenitors in naive wild-type and Spp1 / mice. (a, b) Gating strategies for differentiated cells including PMN (CD11b + Ly6G hi and CD11b + Ly6G
Supplementary Figure 1 Cellularity of leukocytes and their progenitors in naive wild-type and Spp1 / mice. (a, b) Gating strategies for differentiated cells including PMN (CD11b + Ly6G hi and CD11b + Ly6G
Stem cells: units of development and regeneration. Fernando D. Camargo Ph.D. Whitehead Fellow Whitehead Institute for Biomedical Research.
 Stem cells: units of development and regeneration Fernando D. Camargo Ph.D. Whitehead Fellow Whitehead Institute for Biomedical Research Concepts 1. Embryonic vs. adult stem cells 2. Hematopoietic stem
Stem cells: units of development and regeneration Fernando D. Camargo Ph.D. Whitehead Fellow Whitehead Institute for Biomedical Research Concepts 1. Embryonic vs. adult stem cells 2. Hematopoietic stem
SUPPLEMENTARY INFORMATION
 a. Smo+/+ b. Smo+/+ 5.63 5.48 c. Lin- d. e. 6 5 4 3 Ter119 Mac B T Sca1 Smo+/+ 25 15 2 o BMT 2 1 5 * Supplementary Figure 1: Deletion of Smoothened does not alter the frequency of hematopoietic lineages
a. Smo+/+ b. Smo+/+ 5.63 5.48 c. Lin- d. e. 6 5 4 3 Ter119 Mac B T Sca1 Smo+/+ 25 15 2 o BMT 2 1 5 * Supplementary Figure 1: Deletion of Smoothened does not alter the frequency of hematopoietic lineages
Chronic variable stress activates hematopoietic stem cells
 SUPPLEMENTARY INFORMATION Chronic variable stress activates hematopoietic stem cells Timo Heidt *, Hendrik B. Sager *, Gabriel Courties, Partha Dutta, Yoshiko Iwamoto, Alex Zaltsman, Constantin von zur
SUPPLEMENTARY INFORMATION Chronic variable stress activates hematopoietic stem cells Timo Heidt *, Hendrik B. Sager *, Gabriel Courties, Partha Dutta, Yoshiko Iwamoto, Alex Zaltsman, Constantin von zur
Highly Efficient CRISPR/Cas9 Gene Editing and Long-Term Engraftment of Human Hematopoietic Stem and Progenitor Cells
 Highly Efficient CRISPR/Cas9 Gene Editing and Long-Term Engraftment of Human Hematopoietic Stem and Progenitor Cells J. M. Heath, A. Chalishazar, C.S. Lee, W. Selleck, C. Cotta-Ramusino, D. Bumcrot, J.L.
Highly Efficient CRISPR/Cas9 Gene Editing and Long-Term Engraftment of Human Hematopoietic Stem and Progenitor Cells J. M. Heath, A. Chalishazar, C.S. Lee, W. Selleck, C. Cotta-Ramusino, D. Bumcrot, J.L.
Canberra, Australia). CD11c-DTR-OVA-GFP (B6.CD11c-OVA), B6.luc + and. Cancer Research Center, Germany). B6 or BALB/c.FoxP3-DTR-GFP mice were
 Supplemental Materials and Methods Mice Female C57BL/6 (B6, I-E null, H-2 b ), BALB/c (H-2 d ) + ), FVB/N (H-2 q, I-E null, CD45.1 + ), and B6D2F1 (H-2 b/d ) mice were purchased from the Animal Resources
Supplemental Materials and Methods Mice Female C57BL/6 (B6, I-E null, H-2 b ), BALB/c (H-2 d ) + ), FVB/N (H-2 q, I-E null, CD45.1 + ), and B6D2F1 (H-2 b/d ) mice were purchased from the Animal Resources
SUPPLEMENTARY INFORMATION
 doi:10.1038/nature12215 Supplementary Figure 1. The effects of full and dissociated GR agonists in supporting BFU-E self-renewal divisions. BFU-Es were cultured in self-renewal medium with indicated GR
doi:10.1038/nature12215 Supplementary Figure 1. The effects of full and dissociated GR agonists in supporting BFU-E self-renewal divisions. BFU-Es were cultured in self-renewal medium with indicated GR
CRISPR-mediated Editing of Hematopoietic Stem Cells for the Treatment of β-hemoglobinopathies
 CRISPR-mediated Editing of Hematopoietic Stem Cells for the Treatment of β-hemoglobinopathies Jennifer Gori American Society of Gene & Cell Therapy May 11, 2017 editasmedicine.com 1 Highlights Developed
CRISPR-mediated Editing of Hematopoietic Stem Cells for the Treatment of β-hemoglobinopathies Jennifer Gori American Society of Gene & Cell Therapy May 11, 2017 editasmedicine.com 1 Highlights Developed
SUPPLEMENTARY INFORMATION
 doi:10.1038/nature11095 Supplementary Table 1. Summary of the binding between Angptls and various Igdomain containing receptors as determined by flow cytometry analysis. The results were summarized from
doi:10.1038/nature11095 Supplementary Table 1. Summary of the binding between Angptls and various Igdomain containing receptors as determined by flow cytometry analysis. The results were summarized from
Nature Genetics: doi: /ng Supplementary Figure 1
 Supplementary Figure 1 MSI2 interactors are associated with the riboproteome and are functionally relevant. (a) Coomassie blue staining of FLAG-MSI2 immunoprecipitated complexes. (b) GO analysis of MSI2-interacting
Supplementary Figure 1 MSI2 interactors are associated with the riboproteome and are functionally relevant. (a) Coomassie blue staining of FLAG-MSI2 immunoprecipitated complexes. (b) GO analysis of MSI2-interacting
Supplement Material. Spleen weight (mg) LN cells (X106) Acat1-/- Acat1-/- Mouse weight (g)
 Supplement Material A Spleen weight (mg) C Mouse weight (g) 1 5 1 2 9 6 3 2 5 2 1 5 Male LN cells (X16) 4 ** ** Female B 3 2 1 Supplemental Figure I. Spleen weight (A), Inguinal lymph node (LN) cell number
Supplement Material A Spleen weight (mg) C Mouse weight (g) 1 5 1 2 9 6 3 2 5 2 1 5 Male LN cells (X16) 4 ** ** Female B 3 2 1 Supplemental Figure I. Spleen weight (A), Inguinal lymph node (LN) cell number
Molecular Characterization of Leukemia Stem Cell Development. Scott A. Armstrong MD, Ph.D.
 Molecular Characterization of Leukemia Stem Cell Development Scott A. Armstrong MD, Ph.D. Normal and Leukemic Hierarchies NORMAL HSC (SRC) Myeloid progenitor LTC-IC CFU AML LSC (SL-IC) Leukemic LTC-IC
Molecular Characterization of Leukemia Stem Cell Development Scott A. Armstrong MD, Ph.D. Normal and Leukemic Hierarchies NORMAL HSC (SRC) Myeloid progenitor LTC-IC CFU AML LSC (SL-IC) Leukemic LTC-IC
Scientific report: Delineating cellular stages and regulation of human NK cell development to improve NK cell-based therapy for cancer (Dnr )
 Scientific report: Delineating cellular stages and regulation of human NK cell development to improve NK cell-based therapy for cancer (Dnr 130259) The main goal of this project focuses on establishing
Scientific report: Delineating cellular stages and regulation of human NK cell development to improve NK cell-based therapy for cancer (Dnr 130259) The main goal of this project focuses on establishing
The autoimmune disease-associated PTPN22 variant promotes calpain-mediated Lyp/Pep
 SUPPLEMENTARY INFORMATION The autoimmune disease-associated PTPN22 variant promotes calpain-mediated Lyp/Pep degradation associated with lymphocyte and dendritic cell hyperresponsiveness Jinyi Zhang, Naima
SUPPLEMENTARY INFORMATION The autoimmune disease-associated PTPN22 variant promotes calpain-mediated Lyp/Pep degradation associated with lymphocyte and dendritic cell hyperresponsiveness Jinyi Zhang, Naima
SUPPLEMENTARY INFORMATION doi: /nature12026
 doi:1.138/nature1226 a 4 35 3 MCSF level (pg/ml) 25 2 15 1 5 1h3 3h 5h 7h 15h 24h b MPP (CD135 KSL) HSC (CD34 CD15 KSLF) c % 4 ** LPS 3 GFP pos cells 2 PU.1 GFP LPS 1 FSCA Ctl NI 24h LPS Sup.Fig.1 Effect
doi:1.138/nature1226 a 4 35 3 MCSF level (pg/ml) 25 2 15 1 5 1h3 3h 5h 7h 15h 24h b MPP (CD135 KSL) HSC (CD34 CD15 KSLF) c % 4 ** LPS 3 GFP pos cells 2 PU.1 GFP LPS 1 FSCA Ctl NI 24h LPS Sup.Fig.1 Effect
SUPPLEMENTARY INFORMATION
 SUPPLEMENTARY INFORMATION Pleiotrophin Regulates the Expansion and Regeneration of Hematopoietic Stem Cells Heather A Himburg 1, Garrett G Muramoto 1 *, Pamela Daher 1*, Sarah K Meadows 1, J. Lauren Russell
SUPPLEMENTARY INFORMATION Pleiotrophin Regulates the Expansion and Regeneration of Hematopoietic Stem Cells Heather A Himburg 1, Garrett G Muramoto 1 *, Pamela Daher 1*, Sarah K Meadows 1, J. Lauren Russell
X P. Supplementary Figure 1. Nature Medicine: doi: /nm Nilotinib LSK LT-HSC. Cytoplasm. Cytoplasm. Nucleus. Nucleus
 a b c Supplementary Figure 1 c-kit-apc-eflu780 Lin-FITC Flt3-Linc-Kit-APC-eflu780 LSK Sca-1-PE-Cy7 d e f CD48-APC LT-HSC CD150-PerCP-cy5.5 g h i j Cytoplasm RCC1 X Exp 5 mir 126 SPRED1 SPRED1 RAN P SPRED1
a b c Supplementary Figure 1 c-kit-apc-eflu780 Lin-FITC Flt3-Linc-Kit-APC-eflu780 LSK Sca-1-PE-Cy7 d e f CD48-APC LT-HSC CD150-PerCP-cy5.5 g h i j Cytoplasm RCC1 X Exp 5 mir 126 SPRED1 SPRED1 RAN P SPRED1
c-myb binds MLL through menin in human leukemia cells and is an important driver of MLL-associated leukemogenesis
 Research article c-myb binds MLL through menin in human leukemia cells and is an important driver of MLL-associated leukemogenesis Shenghao Jin, Huiwu Zhao, Yan Yi, Yuji Nakata, Anna Kalota, and Alan M.
Research article c-myb binds MLL through menin in human leukemia cells and is an important driver of MLL-associated leukemogenesis Shenghao Jin, Huiwu Zhao, Yan Yi, Yuji Nakata, Anna Kalota, and Alan M.
Nature Immunology: doi: /ni.3412
 Supplementary Figure 1 Gata1 expression in heamatopoietic stem and progenitor populations. (a) Unsupervised clustering according to 100 top variable genes across single pre-gm cells. The two main cell
Supplementary Figure 1 Gata1 expression in heamatopoietic stem and progenitor populations. (a) Unsupervised clustering according to 100 top variable genes across single pre-gm cells. The two main cell
The Role of Rac Signaling in The Perivascular Niche
 The Role of Rac Signaling in The Perivascular Niche Felicia Ciuculescu Diaspora and Higher Education and Research Perspectives in Personalized Medicine- from Concept to Clinical Application Center for
The Role of Rac Signaling in The Perivascular Niche Felicia Ciuculescu Diaspora and Higher Education and Research Perspectives in Personalized Medicine- from Concept to Clinical Application Center for
Supplemental Information. Gut Microbiota Promotes Hematopoiesis to Control Bacterial Infection. Cell Host & Microbe, Volume 15
 Cell Host & Microbe, Volume 15 Supplemental Information Gut Microbiota Promotes Hematopoiesis to Control Bacterial Infection Arya Khosravi, Alberto Yáñez, Jeremy G. Price, Andrew Chow, Miriam Merad, Helen
Cell Host & Microbe, Volume 15 Supplemental Information Gut Microbiota Promotes Hematopoiesis to Control Bacterial Infection Arya Khosravi, Alberto Yáñez, Jeremy G. Price, Andrew Chow, Miriam Merad, Helen
B6.SJL (Ly5.2) mice were obtained from Taconic Farms. Rag1-deficient mice were
 Supplementary Methods Mice. B6.SJL (Ly5.2) mice were obtained from Taconic Farms. Rag1-deficient mice were purchased from The Jackson Laboratory. Real-time PCR Total cellular RNA was extracted from the
Supplementary Methods Mice. B6.SJL (Ly5.2) mice were obtained from Taconic Farms. Rag1-deficient mice were purchased from The Jackson Laboratory. Real-time PCR Total cellular RNA was extracted from the
Supporting Information
 Supporting Information Franco et al. 10.1073/pnas.1015557108 SI Materials and Methods Drug Administration. PD352901 was dissolved in 0.5% (wt/vol) hydroxyl-propyl-methylcellulose, 0.2% (vol/vol) Tween
Supporting Information Franco et al. 10.1073/pnas.1015557108 SI Materials and Methods Drug Administration. PD352901 was dissolved in 0.5% (wt/vol) hydroxyl-propyl-methylcellulose, 0.2% (vol/vol) Tween
BCR-ABL - LSK BCR-ABL + LKS - (%)
 Marker Clone BCR-ABL + LSK (%) BCR-ABL + LKS - (%) BCR-ABL - LSK (%) P value vs. BCR-ABL + LKS - P value vs. BCR-ABL - LSK CD2 RM2-5 12.9 ± 3.6 36.7 ± 6.5 19.3 ± 2.4 0.01 0.10 CD5 53-7.3 13.9 ± 3.2 20.8
Marker Clone BCR-ABL + LSK (%) BCR-ABL + LKS - (%) BCR-ABL - LSK (%) P value vs. BCR-ABL + LKS - P value vs. BCR-ABL - LSK CD2 RM2-5 12.9 ± 3.6 36.7 ± 6.5 19.3 ± 2.4 0.01 0.10 CD5 53-7.3 13.9 ± 3.2 20.8
Supplementary Figure 1. Successful excision of genes from WBM lysates and
 Supplementary Information: Supplementary Figure 1. Successful excision of genes from WBM lysates and survival of mice with different genotypes. (a) The proper excision of Pten, p110α, p110α and p110δ was
Supplementary Information: Supplementary Figure 1. Successful excision of genes from WBM lysates and survival of mice with different genotypes. (a) The proper excision of Pten, p110α, p110α and p110δ was
% of live splenocytes. STAT5 deletion. (open shapes) % ROSA + % floxed
 Supp. Figure 1. a 14 1 1 8 6 spleen cells (x1 6 ) 16 % of live splenocytes 5 4 3 1 % of live splenocytes 8 6 4 b 1 1 c % of CD11c + splenocytes (closed shapes) 8 6 4 8 6 4 % ROSA + (open shapes) % floxed
Supp. Figure 1. a 14 1 1 8 6 spleen cells (x1 6 ) 16 % of live splenocytes 5 4 3 1 % of live splenocytes 8 6 4 b 1 1 c % of CD11c + splenocytes (closed shapes) 8 6 4 8 6 4 % ROSA + (open shapes) % floxed
Normal & Leukaemic haematopoiesis. Dr. Liu Te Chih Dept of Haematology / Oncology National University Health Services Singapore
 Normal & Leukaemic haematopoiesis 2010 Dr. Liu Te Chih Dept of Haematology / Oncology National University Health Services Singapore Use of Immunophenotyping today Lineage assignment Differentiation of
Normal & Leukaemic haematopoiesis 2010 Dr. Liu Te Chih Dept of Haematology / Oncology National University Health Services Singapore Use of Immunophenotyping today Lineage assignment Differentiation of
Repressive Transcription
 Repressive Transcription The MIT Faculty has made this article openly available. Please share how this access benefits you. Your story matters. Citation As Published Publisher Guenther, M. G., and R. A.
Repressive Transcription The MIT Faculty has made this article openly available. Please share how this access benefits you. Your story matters. Citation As Published Publisher Guenther, M. G., and R. A.
Cell isolation. Spleen and lymph nodes (axillary, inguinal) were removed from mice
 Supplementary Methods: Cell isolation. Spleen and lymph nodes (axillary, inguinal) were removed from mice and gently meshed in DMEM containing 10% FBS to prepare for single cell suspensions. CD4 + CD25
Supplementary Methods: Cell isolation. Spleen and lymph nodes (axillary, inguinal) were removed from mice and gently meshed in DMEM containing 10% FBS to prepare for single cell suspensions. CD4 + CD25
Supplemental Information. Granulocyte-Monocyte Progenitors and. Monocyte-Dendritic Cell Progenitors Independently
 Immunity, Volume 47 Supplemental Information Granulocyte-Monocyte Progenitors and Monocyte-endritic ell Progenitors Independently Produce Functionally istinct Monocytes lberto Yáñez, Simon G. oetzee, ndre
Immunity, Volume 47 Supplemental Information Granulocyte-Monocyte Progenitors and Monocyte-endritic ell Progenitors Independently Produce Functionally istinct Monocytes lberto Yáñez, Simon G. oetzee, ndre
Getting to the root of Cancer
 Cancer Stem Cells: Getting to the root of Cancer Dominique Bonnet, Ph.D Senior Group Leader, Haematopoietic Stem Cell Laboratory Cancer Research UK, London Research Institute Venice, Sept 2009 Overview
Cancer Stem Cells: Getting to the root of Cancer Dominique Bonnet, Ph.D Senior Group Leader, Haematopoietic Stem Cell Laboratory Cancer Research UK, London Research Institute Venice, Sept 2009 Overview
Supplementary Figure 1. Characterization of basophils after reconstitution of SCID mice
 Supplementary figure legends Supplementary Figure 1. Characterization of after reconstitution of SCID mice with CD4 + CD62L + T cells. (A-C) SCID mice (n = 6 / group) were reconstituted with 2 x 1 6 CD4
Supplementary figure legends Supplementary Figure 1. Characterization of after reconstitution of SCID mice with CD4 + CD62L + T cells. (A-C) SCID mice (n = 6 / group) were reconstituted with 2 x 1 6 CD4
The Central Role of Menin and Wild-Type MLL in MLL-AF9-Induced Leukemia
 University of Pennsylvania ScholarlyCommons Publicly Accessible Penn Dissertations 1-1-2012 The Central Role of Menin and Wild-Type MLL in MLL-AF9-Induced Leukemia Austin Thiel University of Pennsylvania,
University of Pennsylvania ScholarlyCommons Publicly Accessible Penn Dissertations 1-1-2012 The Central Role of Menin and Wild-Type MLL in MLL-AF9-Induced Leukemia Austin Thiel University of Pennsylvania,
T cell development October 28, Dan Stetson
 T cell development October 28, 2016 Dan Stetson stetson@uw.edu 441 Lecture #13 Slide 1 of 29 Three lectures on T cells (Chapters 8, 9) Part 1 (Today): T cell development in the thymus Chapter 8, pages
T cell development October 28, 2016 Dan Stetson stetson@uw.edu 441 Lecture #13 Slide 1 of 29 Three lectures on T cells (Chapters 8, 9) Part 1 (Today): T cell development in the thymus Chapter 8, pages
Supporting Online Material for
 www.sciencemag.org/cgi/content/full/1171320/dc1 Supporting Online Material for A Frazzled/DCC-Dependent Transcriptional Switch Regulates Midline Axon Guidance Long Yang, David S. Garbe, Greg J. Bashaw*
www.sciencemag.org/cgi/content/full/1171320/dc1 Supporting Online Material for A Frazzled/DCC-Dependent Transcriptional Switch Regulates Midline Axon Guidance Long Yang, David S. Garbe, Greg J. Bashaw*
Hosoya et al. TRIM28 is essential for erythroblast differentiation in the mouse (supplemental information)
 TaqMan assay Gene TRIM28 GATA-1 Applied Biosystems TaqMan assay Mm00495594_m1 Mm01352636_m1 SYBR Green assay Gene Forward primer Reverse primer Reference adult α-globin CCCGGTGCCTTGTCTGCT GTGAAATCGGCAGGGTGG
TaqMan assay Gene TRIM28 GATA-1 Applied Biosystems TaqMan assay Mm00495594_m1 Mm01352636_m1 SYBR Green assay Gene Forward primer Reverse primer Reference adult α-globin CCCGGTGCCTTGTCTGCT GTGAAATCGGCAGGGTGG
Stem cells are undifferentiated cells which are maintained within a specific niche. A stem cell
 Abstract Stem cells are undifferentiated cells which are maintained within a specific niche. A stem cell niche is a microenvironment of cells that maintain stem cell functionality, and one example is the
Abstract Stem cells are undifferentiated cells which are maintained within a specific niche. A stem cell niche is a microenvironment of cells that maintain stem cell functionality, and one example is the
Alternative splicing. Biosciences 741: Genomics Fall, 2013 Week 6
 Alternative splicing Biosciences 741: Genomics Fall, 2013 Week 6 Function(s) of RNA splicing Splicing of introns must be completed before nuclear RNAs can be exported to the cytoplasm. This led to early
Alternative splicing Biosciences 741: Genomics Fall, 2013 Week 6 Function(s) of RNA splicing Splicing of introns must be completed before nuclear RNAs can be exported to the cytoplasm. This led to early
SUPPLEMENTARY INFORMATION
 Supplementary Discussion The cell cycle machinery and the DNA damage response network are highly interconnected and co-regulated in assuring faithful duplication and partition of genetic materials into
Supplementary Discussion The cell cycle machinery and the DNA damage response network are highly interconnected and co-regulated in assuring faithful duplication and partition of genetic materials into
SUPPLEMENTARY FIGURES
 SUPPLEMENTARY FIGURES Supplementary Figure 1: Chemokine receptor expression profiles of CCR6 + and CCR6 - CD4 + IL-17A +/ex and Treg cells. Quantitative PCR analysis of chemokine receptor transcript abundance
SUPPLEMENTARY FIGURES Supplementary Figure 1: Chemokine receptor expression profiles of CCR6 + and CCR6 - CD4 + IL-17A +/ex and Treg cells. Quantitative PCR analysis of chemokine receptor transcript abundance
The role of DNMT3A and HOXA9 hypomethylation in acute myeloid leukemia (AML)
 Campbell Drohan BIOL 463 December 2016 The role of DNMT3A and HOXA9 hypomethylation in acute myeloid leukemia (AML) Introduction Epigenetic modifications of DNA and histones are key to gene regulation
Campbell Drohan BIOL 463 December 2016 The role of DNMT3A and HOXA9 hypomethylation in acute myeloid leukemia (AML) Introduction Epigenetic modifications of DNA and histones are key to gene regulation
A pediatric patient with acute leukemia of ambiguous lineage with a NUP98-NSD1 rearrangement SH
 A pediatric patient with acute leukemia of ambiguous lineage with a NUP98NSD1 rearrangement SH20170203 Rebecca LeemanNeill, Ronald Rice, Anita Malek, Patricia Raciti, Susan Hsiao, Mahesh Mansukhani, Bachir
A pediatric patient with acute leukemia of ambiguous lineage with a NUP98NSD1 rearrangement SH20170203 Rebecca LeemanNeill, Ronald Rice, Anita Malek, Patricia Raciti, Susan Hsiao, Mahesh Mansukhani, Bachir
September 20, Submitted electronically to: Cc: To Whom It May Concern:
 History Study (NOT-HL-12-147), p. 1 September 20, 2012 Re: Request for Information (RFI): Building a National Resource to Study Myelodysplastic Syndromes (MDS) The MDS Cohort Natural History Study (NOT-HL-12-147).
History Study (NOT-HL-12-147), p. 1 September 20, 2012 Re: Request for Information (RFI): Building a National Resource to Study Myelodysplastic Syndromes (MDS) The MDS Cohort Natural History Study (NOT-HL-12-147).
SUPPLEMENTARY METHODS
 SUPPLEMENTARY METHODS Histological analysis. Colonic tissues were collected from 5 parts of the middle colon on day 7 after the start of DSS treatment, and then were cut into segments, fixed with 4% paraformaldehyde,
SUPPLEMENTARY METHODS Histological analysis. Colonic tissues were collected from 5 parts of the middle colon on day 7 after the start of DSS treatment, and then were cut into segments, fixed with 4% paraformaldehyde,
Supplementary Information
 Supplementary Information Distinct bone marrow-derived and tissue resident macrophage lineages proliferate at key stages during inflammation. 1 Luke C. Davies, 1 Marcela Rosas, 2 Stephen J. Jenkins, 1
Supplementary Information Distinct bone marrow-derived and tissue resident macrophage lineages proliferate at key stages during inflammation. 1 Luke C. Davies, 1 Marcela Rosas, 2 Stephen J. Jenkins, 1
Hematopoiesis. BHS Liège 27/1/2012. Dr Sonet Anne UCL Mont-Godinne
 Hematopoiesis BHS Liège 27/1/2012 Dr Sonet Anne UCL Mont-Godinne Hematopoiesis: definition = all the phenomenons to produce blood cells Leukocytes = White Blood Cells Polynuclear = Granulocytes Platelet
Hematopoiesis BHS Liège 27/1/2012 Dr Sonet Anne UCL Mont-Godinne Hematopoiesis: definition = all the phenomenons to produce blood cells Leukocytes = White Blood Cells Polynuclear = Granulocytes Platelet
Effective Targeting of Quiescent Chronic Myelogenous
 Cancer Cell, Volume 7 Supplemental Information Effective Targeting of Quiescent Chronic Myelogenous Leukemia Stem Cells by Histone Deacetylase Inhibitors in Combination with Imatinib Mesylate Bin Zhang,
Cancer Cell, Volume 7 Supplemental Information Effective Targeting of Quiescent Chronic Myelogenous Leukemia Stem Cells by Histone Deacetylase Inhibitors in Combination with Imatinib Mesylate Bin Zhang,
Molecular Hematopathology Leukemias I. January 14, 2005
 Molecular Hematopathology Leukemias I January 14, 2005 Chronic Myelogenous Leukemia Diagnosis requires presence of Philadelphia chromosome t(9;22)(q34;q11) translocation BCR-ABL is the result BCR on chr
Molecular Hematopathology Leukemias I January 14, 2005 Chronic Myelogenous Leukemia Diagnosis requires presence of Philadelphia chromosome t(9;22)(q34;q11) translocation BCR-ABL is the result BCR on chr
Structure and Function of Fusion Gene Products in. Childhood Acute Leukemia
 Structure and Function of Fusion Gene Products in Childhood Acute Leukemia Chromosomal Translocations Chr. 12 Chr. 21 der(12) der(21) A.T. Look, Science 278 (1997) Distribution Childhood ALL TEL-AML1 t(12;21)
Structure and Function of Fusion Gene Products in Childhood Acute Leukemia Chromosomal Translocations Chr. 12 Chr. 21 der(12) der(21) A.T. Look, Science 278 (1997) Distribution Childhood ALL TEL-AML1 t(12;21)
TISSUE-SPECIFIC STEM CELLS
 TISSUE-SPECIFIC STEM CELLS Inhibition of Aldehyde Dehydrogenase Expands Hematopoietic Stem Cells with Radioprotective Capacity GARRETT G. MURAMOTO, a J. LAUREN RUSSELL, a RACHID SAFI, b ALICE B. SALTER,
TISSUE-SPECIFIC STEM CELLS Inhibition of Aldehyde Dehydrogenase Expands Hematopoietic Stem Cells with Radioprotective Capacity GARRETT G. MURAMOTO, a J. LAUREN RUSSELL, a RACHID SAFI, b ALICE B. SALTER,
of TERT, MLL4, CCNE1, SENP5, and ROCK1 on tumor development were discussed.
 Supplementary Note The potential association and implications of HBV integration at known and putative cancer genes of TERT, MLL4, CCNE1, SENP5, and ROCK1 on tumor development were discussed. Human telomerase
Supplementary Note The potential association and implications of HBV integration at known and putative cancer genes of TERT, MLL4, CCNE1, SENP5, and ROCK1 on tumor development were discussed. Human telomerase
One Day BMT Course by Thai Society of Hematology. Management of Graft Failure and Relapsed Diseases
 One Day BMT Course by Thai Society of Hematology Management of Graft Failure and Relapsed Diseases Piya Rujkijyanont, MD Division of Hematology-Oncology Department of Pediatrics Phramongkutklao Hospital
One Day BMT Course by Thai Society of Hematology Management of Graft Failure and Relapsed Diseases Piya Rujkijyanont, MD Division of Hematology-Oncology Department of Pediatrics Phramongkutklao Hospital
TITLE: Effects of Hematopoietic Stem Cell Age on CML Disease Progression
 AD Award Number: W81XWH-04-1-0795 TITLE: Effects of Hematopoietic Stem Cell Age on CML Disease Progression PRINCIPAL INVESTIGATOR: Kenneth Dorshkind, Ph.D. CONTRACTING ORGANIZATION: University of California,
AD Award Number: W81XWH-04-1-0795 TITLE: Effects of Hematopoietic Stem Cell Age on CML Disease Progression PRINCIPAL INVESTIGATOR: Kenneth Dorshkind, Ph.D. CONTRACTING ORGANIZATION: University of California,
UMBILICAL CORD BLOOD STEM CELLS EXPANDED IN THE PRESENCE OF NICOTINAMIDE (NICORD) PROVIDE LONG TERM MULITI-LINEAGE ENGRAFTMENT
 UMBILICAL CORD BLOOD STEM CELLS EXPANDED IN THE PRESENCE OF NICOTINAMIDE (NICORD) PROVIDE LONG TERM MULITI-LINEAGE ENGRAFTMENT Mitchell E. Horwitz, MD Duke University Medical Center Duke Cancer Institute
UMBILICAL CORD BLOOD STEM CELLS EXPANDED IN THE PRESENCE OF NICOTINAMIDE (NICORD) PROVIDE LONG TERM MULITI-LINEAGE ENGRAFTMENT Mitchell E. Horwitz, MD Duke University Medical Center Duke Cancer Institute
RUNX1 and FPD/AML Translational Research. The Leukemia and Lymphoma Society / Babich Family Foundation Partnership. September 2016
 www.lls.org www.runx1.com RUNX1 and FPD/AML Translational Research The Leukemia and Lymphoma Society / Babich Family Foundation Partnership September 2016 Prepared by L. Greenberger, PhD Chief Scientific
www.lls.org www.runx1.com RUNX1 and FPD/AML Translational Research The Leukemia and Lymphoma Society / Babich Family Foundation Partnership September 2016 Prepared by L. Greenberger, PhD Chief Scientific
Supplementary Figure 1. Generation of knockin mice expressing L-selectinN138G. (a) Schematics of the Sellg allele (top), the targeting vector, the
 Supplementary Figure 1. Generation of knockin mice expressing L-selectinN138G. (a) Schematics of the Sellg allele (top), the targeting vector, the targeted allele in ES cells, and the mutant allele in
Supplementary Figure 1. Generation of knockin mice expressing L-selectinN138G. (a) Schematics of the Sellg allele (top), the targeting vector, the targeted allele in ES cells, and the mutant allele in
Ablation of Fbxw7 Eliminates Leukemia-Initiating Cells by Preventing Quiescence
 Article Ablation of Fbxw7 Eliminates Leukemia-Initiating Cells by Preventing Quiescence Shoichiro Takeishi, 1,2 Akinobu Matsumoto, 1,2 Ichiro Onoyama, 1,2 Kazuhito Naka, 3 Atsushi Hirao, 2,3 and Keiichi
Article Ablation of Fbxw7 Eliminates Leukemia-Initiating Cells by Preventing Quiescence Shoichiro Takeishi, 1,2 Akinobu Matsumoto, 1,2 Ichiro Onoyama, 1,2 Kazuhito Naka, 3 Atsushi Hirao, 2,3 and Keiichi
TITLE: Properties of Leukemia Stem Cells in a Novel Model of CML Progression to Lymphoid Blast Crisis
 AD Award Number: W81XWH-05-1-0608 TITLE: Properties of Leukemia Stem Cells in a Novel Model of CML Progression to Lymphoid Blast Crisis PRINCIPAL INVESTIGATOR: Craig T. Jordan, Ph.D. CONTRACTING ORGANIZATION:
AD Award Number: W81XWH-05-1-0608 TITLE: Properties of Leukemia Stem Cells in a Novel Model of CML Progression to Lymphoid Blast Crisis PRINCIPAL INVESTIGATOR: Craig T. Jordan, Ph.D. CONTRACTING ORGANIZATION:
Peli1 negatively regulates T-cell activation and prevents autoimmunity
 Peli1 negatively regulates T-cell activation and prevents autoimmunity Mikyoung Chang 1,*, Wei Jin 1,5,*, Jae-Hoon Chang 1, Yi-chuan Xiao 1, George Brittain 1, Jiayi Yu 1, Xiaofei Zhou 1, Yi-Hong Wang
Peli1 negatively regulates T-cell activation and prevents autoimmunity Mikyoung Chang 1,*, Wei Jin 1,5,*, Jae-Hoon Chang 1, Yi-chuan Xiao 1, George Brittain 1, Jiayi Yu 1, Xiaofei Zhou 1, Yi-Hong Wang
DISCOVERING ATCC IMMUNOLOGICAL CELLS - MODEL SYSTEMS TO STUDY THE IMMUNE AND CARDIOVASCULAR SYSTEMS
 DISCOVERING ATCC IMMUNOLOGICAL CELLS - MODEL SYSTEMS TO STUDY THE IMMUNE AND CARDIOVASCULAR SYSTEMS James Clinton, Ph.D. Scientist, ATCC February 19, 2015 About ATCC Founded in 1925, ATCC is a non-profit
DISCOVERING ATCC IMMUNOLOGICAL CELLS - MODEL SYSTEMS TO STUDY THE IMMUNE AND CARDIOVASCULAR SYSTEMS James Clinton, Ph.D. Scientist, ATCC February 19, 2015 About ATCC Founded in 1925, ATCC is a non-profit
The genetics of heterochromatin. in metazoa. mutations by means of X-ray irradiation" "for the discovery of the production of
 The genetics of heterochromatin in metazoa 1 Hermann Joseph Muller 1946 Nobel Prize in Medicine: "for the discovery of the production of mutations by means of X-ray irradiation" 3 4 The true meaning of
The genetics of heterochromatin in metazoa 1 Hermann Joseph Muller 1946 Nobel Prize in Medicine: "for the discovery of the production of mutations by means of X-ray irradiation" 3 4 The true meaning of
Figure S1. Generation of inducible PTEN deficient mice and the BMMCs (A) B6.129 Pten loxp/loxp mice were mated with B6.
 Figure S1. Generation of inducible PTEN deficient mice and the BMMCs (A) B6.129 Pten loxp/loxp mice were mated with B6.129-Gt(ROSA)26Sor tm1(cre/ert2)tyj /J mice. To induce deletion of the Pten locus,
Figure S1. Generation of inducible PTEN deficient mice and the BMMCs (A) B6.129 Pten loxp/loxp mice were mated with B6.129-Gt(ROSA)26Sor tm1(cre/ert2)tyj /J mice. To induce deletion of the Pten locus,
Transfer protocol of human HSC into NOG mice
 Transfer protocol of human HSC into NOG mice Mice: Adult NOG mice are aged 8-12 weeks. Newborn mice are 1 2 days old. 8-12 week old NOG mice irradiated with 2.5 Gy Intravenous transfer of 1-0.5 x 10 5
Transfer protocol of human HSC into NOG mice Mice: Adult NOG mice are aged 8-12 weeks. Newborn mice are 1 2 days old. 8-12 week old NOG mice irradiated with 2.5 Gy Intravenous transfer of 1-0.5 x 10 5
SUPPLEMENTARY INFORMATION
 Haematopoietic stem cell release is regulated by circadian oscillations Simón Méndez-Ferrer *, Daniel Lucas *, Michela Battista * and Paul S. Frenette * Mount Sinai School of Medicine, *Departments of
Haematopoietic stem cell release is regulated by circadian oscillations Simón Méndez-Ferrer *, Daniel Lucas *, Michela Battista * and Paul S. Frenette * Mount Sinai School of Medicine, *Departments of
Blocking antibodies and peptides. Rat anti-mouse PD-1 (29F.1A12, rat IgG2a, k), PD-
 Supplementary Methods Blocking antibodies and peptides. Rat anti-mouse PD-1 (29F.1A12, rat IgG2a, k), PD- L1 (10F.9G2, rat IgG2b, k), and PD-L2 (3.2, mouse IgG1) have been described (24). Anti-CTLA-4 (clone
Supplementary Methods Blocking antibodies and peptides. Rat anti-mouse PD-1 (29F.1A12, rat IgG2a, k), PD- L1 (10F.9G2, rat IgG2b, k), and PD-L2 (3.2, mouse IgG1) have been described (24). Anti-CTLA-4 (clone
Nature Genetics: doi: /ng Supplementary Figure 1. HOX fusions enhance self-renewal capacity.
 Supplementary Figure 1 HOX fusions enhance self-renewal capacity. Mouse bone marrow was transduced with a retrovirus carrying one of three HOX fusion genes or the empty mcherry reporter construct as described
Supplementary Figure 1 HOX fusions enhance self-renewal capacity. Mouse bone marrow was transduced with a retrovirus carrying one of three HOX fusion genes or the empty mcherry reporter construct as described
Nature Immunology: doi: /ni Supplementary Figure 1. Characteristics of SEs in T reg and T conv cells.
 Supplementary Figure 1 Characteristics of SEs in T reg and T conv cells. (a) Patterns of indicated transcription factor-binding at SEs and surrounding regions in T reg and T conv cells. Average normalized
Supplementary Figure 1 Characteristics of SEs in T reg and T conv cells. (a) Patterns of indicated transcription factor-binding at SEs and surrounding regions in T reg and T conv cells. Average normalized
Nature Genetics: doi: /ng.3812
 Nature Genetics: doi:10.1038/ng.3812 Supplementary Figure 1 Smarcd2-knockout mice die perinatally with impaired energy homeostasis. (a) Generation of the Smarcd2 conditional knockout allele. Deletion of
Nature Genetics: doi:10.1038/ng.3812 Supplementary Figure 1 Smarcd2-knockout mice die perinatally with impaired energy homeostasis. (a) Generation of the Smarcd2 conditional knockout allele. Deletion of
SUPPLEMENTARY INFORMATION
 doi:10.1038/nature10134 Supplementary Figure 1. Anti-inflammatory activity of sfc. a, Autoantibody immune complexes crosslink activating Fc receptors, promoting activation of macrophages, and WWW.NATURE.COM/NATURE
doi:10.1038/nature10134 Supplementary Figure 1. Anti-inflammatory activity of sfc. a, Autoantibody immune complexes crosslink activating Fc receptors, promoting activation of macrophages, and WWW.NATURE.COM/NATURE
Expanded View Figures
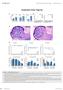 The EMO Journal Dnmt regulates translational fidelity Francesca Tuorto et al Expanded View Figures x E3 cells/µl W g/l HG x e3 cells/µl PLT % of cell type 8 day 8 month % of cell type 8 day 8 month 8 day
The EMO Journal Dnmt regulates translational fidelity Francesca Tuorto et al Expanded View Figures x E3 cells/µl W g/l HG x e3 cells/µl PLT % of cell type 8 day 8 month % of cell type 8 day 8 month 8 day
The nucleotide sugar UDP-glucose mobilizes long-term repopulating primitive hematopoietic cells
 Research article The nucleotide sugar UDP-glucose mobilizes long-term repopulating primitive hematopoietic cells Sungho Kook, 1 Joonseok Cho, 1 Sean Bong Lee, 2 and Byeong-Chel Lee 1 1 University of Pittsburgh
Research article The nucleotide sugar UDP-glucose mobilizes long-term repopulating primitive hematopoietic cells Sungho Kook, 1 Joonseok Cho, 1 Sean Bong Lee, 2 and Byeong-Chel Lee 1 1 University of Pittsburgh
Supplementary Information Titles Journal: Nature Medicine
 Supplementary Information Titles Journal: Nature Medicine Article Title: Corresponding Author: Supplementary Item & Number Supplementary Fig.1 Fig.2 Fig.3 Fig.4 Fig.5 Fig.6 Fig.7 Fig.8 Fig.9 Fig. Fig.11
Supplementary Information Titles Journal: Nature Medicine Article Title: Corresponding Author: Supplementary Item & Number Supplementary Fig.1 Fig.2 Fig.3 Fig.4 Fig.5 Fig.6 Fig.7 Fig.8 Fig.9 Fig. Fig.11
PREPARED FOR: U.S. Army Medical Research and Materiel Command Fort Detrick, Maryland
 AD Award Number: W81XWH-13-1-0057 TITLE: Role of TIRAP in Myelodysplastic Syndromes PRINCIPAL INVESTIGATOR: Linda Ya-ting Chang CONTRACTING ORGANIZATION: British Columbia Cancer Agency Branch Vancouver,
AD Award Number: W81XWH-13-1-0057 TITLE: Role of TIRAP in Myelodysplastic Syndromes PRINCIPAL INVESTIGATOR: Linda Ya-ting Chang CONTRACTING ORGANIZATION: British Columbia Cancer Agency Branch Vancouver,
m 6 A mrna methylation regulates AKT activity to promote the proliferation and tumorigenicity of endometrial cancer
 SUPPLEMENTARY INFORMATION Articles https://doi.org/10.1038/s41556-018-0174-4 In the format provided by the authors and unedited. m 6 A mrna methylation regulates AKT activity to promote the proliferation
SUPPLEMENTARY INFORMATION Articles https://doi.org/10.1038/s41556-018-0174-4 In the format provided by the authors and unedited. m 6 A mrna methylation regulates AKT activity to promote the proliferation
Supplementary Figure 1. Efficiency of Mll4 deletion and its effect on T cell populations in the periphery. Nature Immunology: doi: /ni.
 Supplementary Figure 1 Efficiency of Mll4 deletion and its effect on T cell populations in the periphery. Expression of Mll4 floxed alleles (16-19) in naive CD4 + T cells isolated from lymph nodes and
Supplementary Figure 1 Efficiency of Mll4 deletion and its effect on T cell populations in the periphery. Expression of Mll4 floxed alleles (16-19) in naive CD4 + T cells isolated from lymph nodes and
Early cell death (FGF) B No RunX transcription factor produced Yes No differentiation
 Solution Key - Practice Questions Question 1 a) A recent publication has shown that the fat stem cells (FSC) can act as bone stem cells to repair cavities in the skull, when transplanted into immuno-compromised
Solution Key - Practice Questions Question 1 a) A recent publication has shown that the fat stem cells (FSC) can act as bone stem cells to repair cavities in the skull, when transplanted into immuno-compromised
SUPPLEMENTARY INFORMATION
 Complete but curtailed T-cell response to very-low-affinity antigen Dietmar Zehn, Sarah Y. Lee & Michael J. Bevan Supp. Fig. 1: TCR chain usage among endogenous K b /Ova reactive T cells. C57BL/6 mice
Complete but curtailed T-cell response to very-low-affinity antigen Dietmar Zehn, Sarah Y. Lee & Michael J. Bevan Supp. Fig. 1: TCR chain usage among endogenous K b /Ova reactive T cells. C57BL/6 mice
DISCLOSURE. I have the following financial relationships:
 DISCLOSURE I have the following financial relationships: Consultant for: Fate Therapeutics, GlaxoSmithKline, Bone Therapeutics, G1 Therapeutics Contracted Research for: GlaxoSmithKline Royalties from:
DISCLOSURE I have the following financial relationships: Consultant for: Fate Therapeutics, GlaxoSmithKline, Bone Therapeutics, G1 Therapeutics Contracted Research for: GlaxoSmithKline Royalties from:
Supplementary Figures
 Supplementary Figures Supplementary Figure 1. Confirmation of Dnmt1 conditional knockout out mice. a, Representative images of sorted stem (Lin - CD49f high CD24 + ), luminal (Lin - CD49f low CD24 + )
Supplementary Figures Supplementary Figure 1. Confirmation of Dnmt1 conditional knockout out mice. a, Representative images of sorted stem (Lin - CD49f high CD24 + ), luminal (Lin - CD49f low CD24 + )
Nature Medicine doi: /nm.3150
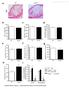 Nature Medicine doi:10.1038/nm.3150 Supplementary Table 1 Primer sequences used for q-pcr analysis Gene Forward primer Reverse primer Abca1 CAGCTTCCATCCTCCTTGTC CCACATCCACAACTGTCTGG Abcg1 GTACCATGACATCGCTGGTG
Nature Medicine doi:10.1038/nm.3150 Supplementary Table 1 Primer sequences used for q-pcr analysis Gene Forward primer Reverse primer Abca1 CAGCTTCCATCCTCCTTGTC CCACATCCACAACTGTCTGG Abcg1 GTACCATGACATCGCTGGTG
Soft Agar Assay. For each cell pool, 100,000 cells were resuspended in 0.35% (w/v)
 SUPPLEMENTARY MATERIAL AND METHODS Soft Agar Assay. For each cell pool, 100,000 cells were resuspended in 0.35% (w/v) top agar (LONZA, SeaKem LE Agarose cat.5004) and plated onto 0.5% (w/v) basal agar.
SUPPLEMENTARY MATERIAL AND METHODS Soft Agar Assay. For each cell pool, 100,000 cells were resuspended in 0.35% (w/v) top agar (LONZA, SeaKem LE Agarose cat.5004) and plated onto 0.5% (w/v) basal agar.
Suppression of Cytochrome P450 Reductase Enhances Long-Term Hematopoietic Stem Cell Repopulation Efficiency in Mice
 Suppression of Cytochrome P450 Reductase Enhances Long-Term Hematopoietic Stem Cell Repopulation Efficiency in Mice Yan Zhang 1,2., Fang Dong 1,2., Na Zhang 1,2, Hui Cheng 1,2, Yakun Pang 1,2, Xiaomin
Suppression of Cytochrome P450 Reductase Enhances Long-Term Hematopoietic Stem Cell Repopulation Efficiency in Mice Yan Zhang 1,2., Fang Dong 1,2., Na Zhang 1,2, Hui Cheng 1,2, Yakun Pang 1,2, Xiaomin
Impaired DNA replication within progenitor cell pools promotes leukemogenesis
 Impaired DNA replication within progenitor cell pools promotes leukemogenesis Ganna Bilousova, University of Colorado Andriy Marusyk, University of Colorado Christopher Porter, Emory University Robert
Impaired DNA replication within progenitor cell pools promotes leukemogenesis Ganna Bilousova, University of Colorado Andriy Marusyk, University of Colorado Christopher Porter, Emory University Robert
Therapeutic implications of cancer stem cells. Cédric Blanpain, MD, PhD Laboratory of stem cells and cancer WELBIO, Université Libre de Bruxelles
 Therapeutic implications of cancer stem cells Cédric Blanpain, MD, PhD Laboratory of stem cells and cancer WELBIO, Université Libre de Bruxelles Stem cell properties Differentiation Self-renewal Tumor
Therapeutic implications of cancer stem cells Cédric Blanpain, MD, PhD Laboratory of stem cells and cancer WELBIO, Université Libre de Bruxelles Stem cell properties Differentiation Self-renewal Tumor
Interferon γ regulates idiopathic pneumonia syndrome, a. Th17 + CD4 + T-cell-mediated GvH disease
 Interferon γ regulates idiopathic pneumonia syndrome, a Th17 + CD4 + T-cell-mediated GvH disease Nora Mauermann, Julia Burian, Christophe von Garnier, Stefan Dirnhofer, Davide Germano, Christine Schuett,
Interferon γ regulates idiopathic pneumonia syndrome, a Th17 + CD4 + T-cell-mediated GvH disease Nora Mauermann, Julia Burian, Christophe von Garnier, Stefan Dirnhofer, Davide Germano, Christine Schuett,
Supplemental Materials
 Supplemental Materials Programmed death one homolog maintains the pool size of regulatory T cells by promoting their differentiation and stability Qi Wang 1, Jianwei He 1, Dallas B. Flies 2, Liqun Luo
Supplemental Materials Programmed death one homolog maintains the pool size of regulatory T cells by promoting their differentiation and stability Qi Wang 1, Jianwei He 1, Dallas B. Flies 2, Liqun Luo
Supplementary Figure S1. Flow cytometric analysis of the expression of Thy1 in NH cells. Flow cytometric analysis of the expression of T1/ST2 and
 Supplementary Figure S1. Flow cytometric analysis of the expression of Thy1 in NH cells. Flow cytometric analysis of the expression of T1/ST2 and Thy1 in NH cells derived from the lungs of naïve mice.
Supplementary Figure S1. Flow cytometric analysis of the expression of Thy1 in NH cells. Flow cytometric analysis of the expression of T1/ST2 and Thy1 in NH cells derived from the lungs of naïve mice.
0.0 All T-lymph B-lymph Sarcomas Carcinomas Germ cell. Tumor type
 Fig S1 A B Tumors per mouse 1.2 1.0 0.8 0.6 0.4 0.2 0.0 All T-lymph B-lymph Sarcomas Carcinomas Germ cell Tumor type -/- (n = 46) Q/- (n = 76) Q/Q (n = 31) Tumors per mouse 1.2 1.0 0.8 0.6 0.4 0.2 0.0
Fig S1 A B Tumors per mouse 1.2 1.0 0.8 0.6 0.4 0.2 0.0 All T-lymph B-lymph Sarcomas Carcinomas Germ cell Tumor type -/- (n = 46) Q/- (n = 76) Q/Q (n = 31) Tumors per mouse 1.2 1.0 0.8 0.6 0.4 0.2 0.0
Nature Immunology: doi: /ni.3631
 Supplementary Figure 1 SPT analyses of Zap70 at the T cell plasma membrane. (a) Total internal reflection fluorescent (TIRF) excitation at 64-68 degrees limits single molecule detection to 100-150 nm above
Supplementary Figure 1 SPT analyses of Zap70 at the T cell plasma membrane. (a) Total internal reflection fluorescent (TIRF) excitation at 64-68 degrees limits single molecule detection to 100-150 nm above
Supplementary Figures. T Cell Factor-1 initiates T helper 2 fate by inducing GATA-3 and repressing Interferon-γ
 Supplementary Figures T Cell Factor-1 initiates T helper 2 fate by inducing GATA-3 and repressing Interferon-γ Qing Yu, Archna Sharma, Sun Young Oh, Hyung-Geun Moon, M. Zulfiquer Hossain, Theresa M. Salay,
Supplementary Figures T Cell Factor-1 initiates T helper 2 fate by inducing GATA-3 and repressing Interferon-γ Qing Yu, Archna Sharma, Sun Young Oh, Hyung-Geun Moon, M. Zulfiquer Hossain, Theresa M. Salay,
PRC2 crystal clear. Matthieu Schapira
 PRC2 crystal clear Matthieu Schapira Epigenetic mechanisms control the combination of genes that are switched on and off in any given cell. In turn, this combination, called the transcriptional program,
PRC2 crystal clear Matthieu Schapira Epigenetic mechanisms control the combination of genes that are switched on and off in any given cell. In turn, this combination, called the transcriptional program,
Supplemental Figure S1. RANK expression on human lung cancer cells.
 Supplemental Figure S1. RANK expression on human lung cancer cells. (A) Incidence and H-Scores of RANK expression determined from IHC in the indicated primary lung cancer subgroups. The overall expression
Supplemental Figure S1. RANK expression on human lung cancer cells. (A) Incidence and H-Scores of RANK expression determined from IHC in the indicated primary lung cancer subgroups. The overall expression
