Research Article TNF-α and IFN-s-Dependent Muscle Decay Is Linked to NF-κB- and STAT-1α-Stimulated Atrogin1 and MuRF1 Genes in C2C12 Myotubes
|
|
|
- Laurence Stafford
- 5 years ago
- Views:
Transcription
1 Hindwi Pulishing Corportion Meditors of Inflmmtion Volume 213, Artile ID , 18 pges Reserh Artile nd IFN-s-Dependent Musle Dey Is Linked to NF-κB- nd STAT-1α-Stimulted Atrogin1 nd MuRF1 Genes in C2C12 Myotues Brr Pijet, 1 Mj Pijet, 1 Ann Litwiniuk, 1 MBgorzt Gjewsk, 1 Bet Pjdk, 2 nd Arkdiusz Orzehowski 1,2 1 Deprtment of Physiologil Sienes, Fulty of Veterinry Mediine, Wrsw University of Life Sienes SGGW, Nowoursynowsk 159, Wrsw, Polnd 2 Eletron Mirosopy Pltform, Mosskowski Medil Reserh Centre, Polish Ademy of Sienes, Pwińskiego 5, 2-16 Wrsw, Polnd Correspondene should e ddressed to Arkdiusz Orzehowski; orzehowski rkdiusz@wp.pl Reeived 4 July 213; Revised 9 Septemer 213; Aepted 4 Otoer 213 Ademi Editor: Eline Htnk Copyright 213 Brr Pijet et l. This is n open ess rtile distriuted under the Cretive Commons Attriution Liense, whih permits unrestrited use, distriution, nd reprodution in ny medium, provided the originl work is properly ited. ws shown tostimulte mitogeniity inc2c12myolsts. Seleted ytokines,ifnα,or redued the expression of myosin hevy hin (MyHC II) when given together. Moleulr mehnisms of ytokine tivities were ontrolled y NF-κB nd JAK/STAT signling pthwys, s metoli inhiitors, urumin nd AG49, inhiited some of nd IFNα/ effets. Insulin ws hrdly ntgonisti to - ndifnα/-dependent derese in MyHC II protein expression. Cytokines used individully or together lso repressed myogenesis of C2C12 ells. Moreover, -nd IFNα/-dependent effets on C2C12 myotues were ssoited with inresed tivity of Atrogin1 nd MuRF1 genes, whih ode uiquitin ligses. MyHC II gene tivity ws unltered y ytokines. Inhiition of NF-κB or JAK/STAT with speifi metoli inhiitors deresed tivity of Atrogin1 nd MuRF1 ut not MyHC II gene. Overll, these results suggest oopertion etween ytokines in the redution of MyHC II protein expression level vi NF-κB/JAK/STAT signling pthwys nd tivtion of Atrogin1 nd MuRF1 genes s their moleulr trgets. Insulin otretment or pretretment does not protet ginst musle dey indued y exmined proinflmmtory ytokines. 1. Introdution Chexi, n unintentionl loss of len ody weight despite dequte nutrition, is ftl omplition of mny diseses (ner, ongestive hert filure, dietes, kidney filure, hroni ostrutive pulmonry disese, rheumtoid rthritis, nd HIV/AIDS), while the primry usl moleulr mehnisms still remin unknown [1, 2]. Atully, it seems ovious tht ll these omplitions hve something in ommon despite distint etiology. Among severl hypotheses, the insuffiient noli tion of insulin in response to rise in some ytokines during systemi inflmmtion ttrted ourttention.thelossofmusletissueoservedinelevted levels of proinflmmtory ytokines seems to e linked to elerted proteolysis rther thn impired protein synthesis [3]. Some uthors [4] oserved synergism etween nd IFN-γ effets through NF-κB tivtion.moreover,guttridge et l. [5] demonstrted tht inhiition of myogenesis is NF-κB dependent, s ytokine tivtion is responsile for the redution of MyoD protein tht ontrols musle development [6]. Furthermore, s reported y Wheeler et l. [7], MyoD inding to myosin hevy hin II promoter region is neessry for myosin expression in fst twith musles. Interestingly, IGF-1 ould not stop the effet of proinflmmtory ytokines in myotue trophy, even though it ws shown to repress Atrogin1 gene [8]. Nowdys, efforts to fight hexi re sed on trgeting genes prior to their effets evoked in trget orgns [9]. It is elieved tht elerted loss of skeletl musle fiers nd proteins whih our in musle trophy, musle hexi, nd sropeni re driven y intrinsi mehnisms of utophgy [1], poptosis [11], nd deresed stellite ell tivtion [12]. Furthermore, the
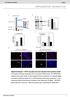
2 2 Meditors of Inflmmtion imlne in regultion of skeletl musle protein retion leds to exessive tivity of protesome, thepsins, lpins, nd/or spse proteolyti systems [3]. A gret del of ppers referring to musle hexi points to erroneous tivity of signling pthwys triggered y ertin ytokines, suh s IL-6, IL-1,,,ndmyosttin[13 17], respetively. ting through TNFR1 is known to trigger two funtionlly opposite nd sequentil signls: (i) first to supportell viilitythroughnf-κb-dependent gene regultion; (ii) seond to initite intrinsi poptosis with prospse 8tivtion[18]. Interestingly, NF-κB trnsription ftor lso plys ritil role in musle hexi [19 21]. Thus, deiphering the moleulr mehnism of musle hexi would provide theoretil kground for therpeuti use of some of the metoli inhiitors. In this study murine C2C12 myotues were sujeted to short- nd long term inutions with, IFNα,, nd/or insulin s potent funtionl ntgonists. The set-up of experiments ws designed to verify the hypothesis tht individully some ytokines ould ffet ertin genes nd proteins ssoited with musle wsting, wheres together they dd or synergize in their respetive tions. It ws ssumed tht they ompete for the ommon trnsdution trget (STAT-1α) thtloks internl signl essentil for elerted protein loss (NF-κB). In this pper the results of oth short (minutes) nd long term (hours) studies performed on C2C12 myolsts nd myotues re presented. We demonstrted tht NF-κB nd JAK/STAT signling pthwys re interseted nd tht they ontrol musle growth nd dey. IFNα redued the level of STAT-1α protein linked to TRADD protein to relese NF- κb from STAT-1α-dependent inhiition. In differentiting C2C12 myotues, dministrtion ugmented ell growth, wheres it inhiited MyHC II protein expression in differentited myotues. To our surprise, -indued effet ws ssoited with oth tivtion of Myh II, Atrogin1 (lso known s MAFx), nd MuRF1 genes, ut proteolysis took over protein retion. 2. Mterils nd Methods 2.1. Mterils. Medi (Duleo s modified Egles medium (DMEM) with Glutmx), PBS (inluding C 2 nd Mg 2 ), ntiiotis, nd het intivted ser (fetl ovine serum FBS nd horse serum HS) were purhsed from Gio Life Tehnologies (Grnd Islnd, NY, USA). Mouse tumor nerosis ftor lph (), interferon lph (IFNα), interferon gmm (), nd insulin porine (Sigm-Aldrih Chemil Co., St. Louis, MO, USA) were dissolved ording to mnufturer s reommendtions nd kept frozen t 2 C. Metoli inhiitors: urumin (NF-κB ndprotesome inhiitor), LY2942 nd PD9859 (PI3-K nd MEK inhiitor, resp., Sigm-Aldrih Chemil Co., St. Louis, MO, USA), nd tyrphostin (AG49, JAK inhiitor, Merk, Drmstdt, Germny) were dissolved in DMSO nd depending on the type were kept frozen t 2 C or refrigerted t 4 8 C. All other regents were ell ulture tested, of high purity, nd unless otherwise stted they were purhsed from Sigm- Aldrih Chemil Co. (St. Louis, MO, USA). Plstis were from Beton Dikinson (BD Biosienes, Frnklin Lkes, NJ, USA) nd tues for deep freezing were from Nunlon (Nun, Roskilde, Denmrk), while syringe filters were purhsed from Corning-Costr In. (Cmridge, MA, USA) Musle nd Myotue Cell Cultures nd Tretments. The mouse C2C12 ell line [22] ws otined from the Europen Colletion of Animl Cell Cultures (ECAAC). Cells were initilly suspended in growth medi (GM) ontining DMEM with Glutmx supplemented with 1% (v/v) fetl ovine serum (FBS), pen:strep (Peniillin:Streptomyin solution, 5 IU/mL/5 μg/ml), Gentmiin sulfte 2 μg/ml, Fungizone-Amphoteriin B 1 μg/ml, nd plted onto plsti nonoted ulture flsks or petri dishes. They were ultured t 37 Cinhumidified5%CO 2 nd 95% ir in inutor. After rehing 7 8% onfluene, myolsts were suultured y trypsyniztion nd the sme volume of ell suspension ws seeded onto 1 mm petri dishes, 96-fltwell pltes or multiwell 4 Chmer Culture Slides (Beton Dikinson, BD Biosienes, Frnklin Lkes, NJ, USA) depending on the experimentl protool. For differentiting nd differentition sttes when the myolsts rehed 8% onfluene, growth medi were swithed to differentition medi (DM) ontining DMEM with Glutmx supplemented with 2% (v/v) horse serum (HS) nd the sme ntiioti:ntimyoti mixture. During ute nd hroni study of myogeni differentition, DM ws repled y freshly prepred medi ontining 2% BSA (w/v) with or without experimentl ftors.intheseofmitogeniitystudy,gminnononfluent ells ws diretly repled y 2% BSA (w/v)/dmem with or without experimentl ftors. When the experimentl ftors were dissolved in DMSO, the equivlent volume of vehile (.1% v/v) ws dded to the ontrol ells. DMSOdissolved regents were dded extly 3 min prior to the pplition of wter-solule regents. Preliminry experiments were rried out with inresing onentrtions of ytokines for different time points in order to hoose the est time/onentrtion omintion to dopt in our shortnd long term studies. Differenes in phosphoryltion sttus nd tivities of prtiulr proteins (NF-κB, STAT-1α) eing fst nd trnsient phenomen were rried out s short term studies. Viility, mitogeniity, or hnges in the protein expression were investigted in the long term study. There were dose- nd time-dependent ell responses (Supplementry dt 1, see Supplementry Mterils ville online t suggesting tht the est onentrtion setting in our experimentl model (determined y the omplete sene of ellulr toxiity in MTT ssy) ws otined using 1 ng/ml of, IFNα,, nd 1 nm of insulin. Cells were removed from the ulture pltes using trypsin (hrvesting), entrifuged in GM t 1 rpm for 5 min, medi were spirted off, nd ell pellets were resuspended in GM. For the differentiting stte, fter 24hinGM,ellswerewshedwithPBSndtheninuted in DM for 3 to 5 dys. Medi were hnged every dy. For differentition stte, the ells were llowed to inute in DM for 6 dys prior to tretment with ytokines or inhiitors rried out for nother 2 dys (8 dys ltogether). Medi were
3 Meditors of Inflmmtion 3 hnged every other dy. Myotues were hrvested on Dy 8 of differentition (i.e., 6 dys without tretment 2 dys with tretment). Floting ded ells were removed during medi hnge or wshing with PBS nd were not inluded in these experiments Determintion of Cell Viility nd Mitogeniity. Cell viility ws sed on the ility of ells grown on 96-well pltes to onvert solule MTT [3-(4,5-dimethylthizol-2-yl)- 2-5-diphenyltetrzolium romide] into n insolule purple formzn retion produt with minor modifitions to protool desried y Joson et l. [23]. Briefly, ells were uniformly seeded in 96-well flt ottomed pltes nd grown in GM for 24 h. Confluent ultures were wshed with PBS nd then exposed to DM inluding (or not) experimentl ftor(s) for 5 suessive dys. Medi were hnged every dy nd reltive viility (perent of ontrol) ws evluted t eh dy (1, 2, 3, 4, 5). Medi were removed, ells were wshed three times with PBS, nd were further inuted with MTT for 1 h t 37 Cinhumidified5%CO 2 nd 95% ir in inutor. Next, MTT solution ws removed nd wter insolule formzn ws immeditely dissolved in DMSO. Cell mitogeniity ws determined y rystl violet (CV) ssy on identil 96-well flt ottomed multiwell pltes. Cells were uniformly seeded nd grown in GM for 24 h. Mitogeniity ws mesured on dy 3 of ulture. Initilly, ells were kept in GM for 24 h, followed y 24 h inution with the experimentl ftor(s) dissolved in serum-free 2% BSA/DMEM, nd finlly reovered in GM for nother 24 h. Cells were exposed (or not) to experimentl ftors during 24 h. Upon ompleting the experiment, ells were wshed with PBS nd fixed with two-step th in ie-old methnol(7%followedy1%,v/v,2min,4 C). Cells were immersed in.2%, w/v rystl violet solution in dd.h 2 O with ethnol 2% (v/v) for 1 min. Susequently, they were gently wshed with dd.h 2 O, ir dried, nd inuted with SDS solution (1%, w/v in dd.h 2 O). The sornes for MTT ndcvweremesuredt49nd57nm,respetively, with ELISA reder type Infinite 2 (TECAN, Austri). Reltive perentges (versus nontreted ontrol) of vile or proliferting ells were mesured y MTT onversion into purple formzn nd quntity of CV ound to ellulr DNA, respetively Antiodies, Immunolotting, Immunopreipittion, Trnsriptionl Ativity of STAT-1α, Immunofluoresene, nd Mirosopi Imging. For whole-ell lystes the ells were ultured with or without experimentl ftors indited in the legend of Figure 7(), hrvested, wshed, nd lysed with RIPA lysis uffer (1x PBS, 1 ml/l Igepl CA-63, 5 g/l sodium deoxyholte, 1 g/l SDS) supplemented with.4 mm PMSF; 1 μg/ml of protinin nd 1 μg/ml of sodium orthovndte were dded. To lyse the ell pellets, ells were roken up y repetitive triturting with the syringe with tthed needle (21 G,.8 mm dimeter). Cell suspension ws then left on ie (4 C) for 3 min nd entrifuged for nother 5 min (4 C, 1 g). Next, visous solution ws divided into smller volumes nd trnsferred to fresh Eppendorf tues nd stored t 8 Cuntilused.Forprotein quntifition in the whole-ell lystes, protein-dye-inding method of Brdford [24] with ommeril regent ws used (Bio-Rd Lortories, Herules, CA, USA). To seprte ytoplsmi nd nuler frtions, ells were wshed, nd fter entrifugtion ell pellets were resuspended in 4 μl of ie-old uffer (1 mm HEPES ph 7.9; 1 mm KCl;.1 mm EDTA;.1 mm EGTA; 1 mm DTT;.5 mm PMSF) nd inuted on ie for 15 min. Then 25 μl of1%solutionof Igepl CA-63 ws dded. After entrifugtion, superntnts ontining ytoplsm were trnsferred to fresh tues nd were stored t 8 C. Nuler pellets were resuspended in 2 μl RIPA uffer (1x PBS; 1% Igepl CA-63;.5% sodium deoxyholte;.1% SDS; protinin (ville s liquid from Sigm-AldrihChemilCo.);3μL dded to 1 ml of uffer; 1 mm sodium orthovndte) nd were pssed through 21-guge needle. PMSF (.4 mm) ws dded nd ells were inuted 3 min on ie. After entrifugtion, ytoplsmi nd nuler lystes were stored t 8 Cuntilnlysis. Cell lystes (equl protein lods of 5 μg) were sujeted to SDS-PAGE (1 12.5% of gel, depending on the MW of protein) t 15 V, next they were trnsferred t 1 V for 2 h to polyvinylidene difluoride (PVDF) memrne nd immunolotted with ntiodies ginst phosphorylted nd/or nonphosphorylted forms of proteins: tin, NF- κb (p65), IκBα, STAT1-α, P(Tyr71)-STAT1-α, MyoD, nd myogenin (Snt Cruz Biotehnology, Snt Cruz, CA, USA) or MyHC II (Sigm Aldrih Chemil Co., St. Louis, MO, USA). Working ntiody onentrtions (from 1 : 2 to 1 : 1) vried depending on the protein deteted nd were pplied ording to the mnufturer s reommendtions. After detetion with ntiodies whih distinguished phosphorylted proteins, the sme lot ws reproed with ntiodies ginst the nonphosphorylted form of the sme protein in order to verify tht equl mounts of protein were lwys loded nd to identify the proteins. In order to reproe lot with different ntiody, the memrne ws inuted in stripping uffer (1 mm β-merptoethnol, 2% (w/v) SDS, 62.3 mm Tris, ph 6.7) for 3 min t 3 C, nd then reloked. Seondry polylonl ntiodies (Snt Cruz Biotehnology, Snt Cruz, CA, USA) rised ginst respetive speies nd onjugted to horserdish peroxidse were used for detetion, followed y enhned hemiluminesene ssy (Amershm Interntionl, Aylesury, U.K.). After exposure nd proessing, the films were snned nd nlyzed using Kodk EDAS 29/Kodk 1D 3.5 system. For immunopreipittion t prtiulr time points the ells were srped from sustrtum in.5 ml RIPA uffer, nd fter repetitive triturting with the 21 guge needle,.4 mm PMSF ws dded nd ells were inuted 3 min on ie. After entrifugtion the protein onentrtion ws determined y protein-dye-inding method s previously desried. Cell lystes ontining 9 μg of protein were inuted overnight t 4 Cwith1.5μgritpolylonlnti- STAT-1α IgGndforndditionl3hwereinutedwith 3μL protein A/G ed slurry (Snt Cruz, CA). Beds were wshed 4 times with ie-old RIPA uffer, oiled with smple uffer (2x Lemmli uffer, Sigm-Aldrih Chemil Co., St. Louis, MO, USA), nd seprted y 1% SDS/PAGE.
4 4 Meditors of Inflmmtion After eletrotrnsfer, the memrnes were immunostined for TRADD protein y stndrd Western lot proedure. NF-κBndSTAT-1α trnsriptionl tivities were quntified with TrnsAM Kits (Rixensrt, Belgium). These re sensitive, nonrdiotive trnsription ftor ELISA Kits tht filitte the study of trnsription ftor tivtion in mmmlin tissue nd ell extrts. The tive form of STAT-1α ontined in nuler extrts ws speifilly ound to the immoilized oligonuleotide ontining STAT onsensus inding site (5 -TTCCCGGAA-3 ). The primry ntiody used to detet STAT reognized only the lph suunit of STAT-1α, tht is, essile only when STAT-1α ws tivtedndoundtoitstrgetdna.similrly,trnsam NF-κBKitontined96-wellplteonwhiholigonuleotide ontining the NF-κBonsensussite(5 -GGGACTTTCC-3 ) hs een immoilized. The tive form of NF-κB ontined in nuler or whole-ell extrt speifilly inds to this oligonuleotide. The primry ntiodies used to detet NF- κb reognized n epitope on p65 or p5, tht is, essile only when NF-κB is tivted nd ound to its trget DNA. Finlly, n HRP-onjugted seondry ntiody provided sensitive olorimetri redout tht ws quntified y spetrophotometry (45 nm). For immunofluoresene, C2C12 myolsts were propgted in multiwell 4 hmer ulture slides nd treted (or untreted) t pproprite times (Supplementry dt 2) with experimentl ftors dissolved in 2 g/l BSA/DMEM or.1% v/v DMSO in 2 g/l BSA/DMEM. After the experiment ended, the ells were fixed s follows: wshed twie with PBS, fixed in 3.7% (v/v) formldehyde for 15 minutes in room temperture, wshed twie with PBS ontining 1 g/l BSA/PBS, nd inuted for 1 min in RT in Triton X-1 solution (.5% v/v in PBS). Next, ells were wshed twie with PBS. Prior to stining, the ells were wshed three times with 1 g/l BSA/PBS, nd then inuted overnight t 4 C withprimryritpolylonlnti-nf-κb diluted1:1. After inution the ells were wshed three times with 1 g/l BSA/PBS nd susequently inuted for 1 hour in drk t RT with seondry hiken nti-rit ntiody onjugted to Alex Fluor 488 (Moleulr Proes In., Eugene, OR, USA) diluted 1 : 5. The ells were then wshed three times with PBS nd inuted with 7-minotinomyin D (7 - AAD) wter solution for 15 min t RT to visulize the ell nulei. Afterwrds, the ells were wshed five times with 1 g/l BSA/PBS, the hmer wlls were removed, nd overslips were mounted on mirosope slides using n ntifde Fluoromount (Sigm Aldrih Chemil Co., St. Louis, MO, USA). As negtive ontrol, only seondry ntiodies were used. Cells were visulized using onfol mirosope FV-5 (Olympus Optil Co., Hmurg, Germny). The fluoresene exittion ws provided y 488 nm nd 543 nm He-Ne lser ems. Fluoresene ws mesured using dihroi mirrors nd filters for 55, 525 nm, 56, nd 61 nm wvelengths. Aquired dt were stored in series of 12 it grey imges seprtely nd olored rtifiilly y softwre. Morphologilhngesndellsurvivlweremonitored under n inverted phse-ontrst mirosope (Olympus CK4, model: ICD73WP). The formtion of myotues ws monitored y otining photomirogrphs using digitl mer (CCD Color Cmer, Hmurg, Germny) RNA Isoltion nd Quntittive Rel-Time Reverse- Trnsription-Polymerse Chin Retion (qrt-pcr). Totl RNA ws extrted from myotues with the Totl RNA Mxi Kit (A&A Biotehnology, Gdyni, Polnd), ording to mnufturer s protool. RNA ws frozen t 76 C efore the performing the reverse trnsription retion (RT- PCR). Then, 5 μg oftotlrnawspurifiedonsiligel nd reverse-trnsried with Enhned Avin HS RT-PCR- 1 Kit (Sigm-Aldrih, Tufkirhen, Germny). Retion mixturewssedonnhoredoligo(dt) 23 (.5 μg/μl in wter for PCR). RT-PCR ws rried out using Msteryler Personl (Eppendorf, New York, NY, USA). First, the RNA smples (2 ng/ml) were inuted for 1 min t 7 C. Then wter for PCR, 1XAMV-RT uffer [5 mm Tris-HCl, ph 8.3, 4 mm KCl, 8 mm MgCl 2,1mMDTT,RNAse inhiitor (2 U/μL), nd reverse trnsriptse AMV (2 U/μL w2mmkh 2 PO 4,pH7.2,2mMDTT,.2%(v/v)triton, 5% (v/v) glyerol] were dded t 4 C. The smples were sujetedtort-pcrfor5mint48 C. After ompleting the retion the onentrtion of newly synthesized DNA ws mesured in the NnoDrop 1 (NnoDrop Tehnologies, Wilmington, NC, USA) t λ =23nm.DNAwskept frozen t 76 C until further nlyses. To perform rel time PCR retion, DNA ws omined with 25 μm of eh primer (sense nd ntisense) nd SYBR green (LightCyler FstStrt DNA Mster SYBR Green I nd LightCyler Control Kit DNA; Rohe Dignostis, Wrsw, Polnd). The qrt PCR mesurements of individul DNAs were performed in triplites using SYBR green dye to mesure duplex DNA formtion with the LightCyler (Rohe Dignostis, Wrsw, Polnd). The results were nlyzed with LightCyler3 Front Sreen. 18S rrna ws used s referene gene. The sequenes of the primers sets used re shown in the tthed Tle 1 (GenBnk). The reltive mrna levels of the trget genes were determinedusingthereltivestndrdurve. PCR onditions were s follows: denturtion for eh yle 95 C (35 yles, 1 se for eh yle); nneling oth for Myh II nd Atrogin1 6 C (35 yles, 1 se for eh yle); for MuRF1 58 C (35 yles, 1 se for eh yle); for 18S rrna 56 C (35 yles, 1 se for eh yle); nd elongtion 72 C (35 yles, 4-5 se for eh yle) Sttistil Anlysis. Eh experiment ws repeted t lest three times. The dt re expressed s the mens ± SE. Sttistil nlyses were performed using one-wy nlysis of vrine (ANOVA) followed y Tukey s, Newmn-Keuls or Benferroni multiple rnge test. If neessry, the seletion of prtiulr postho test (Newmn-Keuls, Tukey, or Benferroni) ws performed fter the sme ritil differene for the first omprison ws tested. Regression nlysis ws rried out to drw pproprite dose-response or time-ourse urves. P vlues of less thn.5 were onsidered sttistilly signifint. Sttistil differenes from ontrol ells were indited y sterisks ( P <.5; P <.1; P <.1), wheres sttistil differenes etween the tretments nd
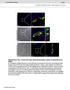
5 Meditors of Inflmmtion 5 Tle 1 Gene Primers nd their sequenes MgCl 2 (mm) Produt (p) Myh II Atrogin1 MuRF1 18S rrna F: 5 -GGAGAAGAGCGAGCTGAAGA-3 R: 5 -GGAAAACTCGCCTGACTCTG F: 5 -CTCTGTACCATGCCGTTCCT-3 R: 5 -GGCTGCTGAACAGATTCTCC F: 5 -GACAGTCGCATTTCAAAGCA-3 R: 5 -AACGACCTCCAGACATGGAC F: 5 -GGAGAGCGGGTAAGAGAGGT-3 R: 5 -CAGGACTAGGCGGAACAGAG untreted ontrol ells were tiked with different lower se letters (r hrts). Sttistil nlyses were performed using GrphPd Prism version 5. softwre (GrphPd Softwre In., Sn Diego, CA, USA). 3. Results 3.1. Stimultes Both Viility nd Mitogeniity of C2C12 Myolsts in NF-κB- nd JAK/STAT-Dependent Mnner. Addition of (1 ng/ml) to the medium stimulted viility (y 88 ± 2.82 to 14% ± 6.84) nd mitogeniity (y 3% ± 4.24) of differentiting C2C12 myolsts (Figures 1() nd 1(), P <.1 ompred to initil ontrol). AG49 (5 μm) used individully impired ell viility t dys 3 5 of differentition (Figures 1() nd 1(), P <.1 ompred to initil ontrol), ut urumin (1 μm) did not exert this effet. The effets of on ell viility nd mitogeniity were olished y urumin (NF-κB inhiitor)nd AG49(JAK inhiitor) dministrtion (Figures 1() nd 1()) Stimultes the Expression nd Trnsriptionl Ativity of NF-κB in C2C12 Myotues. To verify the intrellulr level of NF-κB (p65) in 3-dy-old C2C12 myotues, nuler frtions were isolted nd immunolotted, wheres trnsriptionl tivity ws evluted using TrnsAM method. Nuler expression of NF-κB protein inresed s erly s 15 minutes fter dministrtion (Figure 1(f)). There ws mrked drop in ytoplsmi IκB t the sme time (Figure 1(f)). As shown on Figure 1(d), when C2C12 myotues were exposed to (1 ng/ml), the trnsriptionl tivity of NF-κB ws signifintly elevted in 3th (y 49% 8.9) nd 6th minute (y 47% ± 7.15) of tretment (P <.1 ompred to ontrol). Furthermore, lso fter 24 hours of dministrtion oth NF-κB expression nd its tivity were still signifintly higher (y 54% ± 2.89, Figures 1(e)-1(f), P <.1 ompred to ontrol). In ontrst to short term tretment (minutes), fter 24 hours of dministrtion the expression level of IκBα proteinwsinresedinthe ytoplsmi frtion (Figure 1(f)). Neither IFNα nor ffeted the tivity of NF-κBinC2C12myotuesinthelong term study (Figure 1(e), P >.5 ompred to ontrol) Pretretment of C2C12 Myolsts with Insulin Rises -Dependent Ativity of NF-κB, Though Insulin Does Not Alter NF-κB Response in C2C12 Myotues regrdless of Insulin Pretretment nd Cotretment with, IFNα, or. Pretretment with insulin (72 h, 1 nm) prior to TNF- α (1 ng/ml) dministrtion signifintly inresed dependent NF-κB tivity in 15th,3th ut 6th minute(y 3-fold in the 15th nd y 4.5-fold in the 3th minute of posttretment, Figure 1(h), P <.1 ompred to ontrol) in 3-dy old C2C12 myotues. In ontrst, insulin pretretment neither hnged NF-κB tivity(figure 1(i), P>.5) nor expression when dministered s pretretment nd otretment with IFNα or (Figure 1(g)) Pretretment with IFNα or Does Not Affet - Indued Nuler Expression of NF-κB Though It Inreses Its Ativity in C2C12 Myotues: Insulin Could Not Blok TNF- α-indued Nuler Expression nd Inresed Ativity of NF- κb Potentited y Interferons. Preinution with interferons (IFNα,, 1ng/mL) sensitized ellulr responses to dministrtion in 3-dy old C2C12 myotues s evidened y NF-κB tivity(ifnα pretretment inresed response y 2-fold in the 15th nd y 3.5-fold in the 3th minute, while y 45% ± 9.8, 85% ± 9.18, nd 122% ± t 15th, 3th, nd 6th minute of tretment, respetively, Figure 2(), P <.1 ompred to ontrol). NF-κB tivity stimulted y nd potentited y IFNα in 3-dy-old C2C12 myotues ws dditionlly sustntited y nuler NF-κB setting oserved in onfol mirosopy (Supplementry dt 2). Visuliztion onfirmed tht these retions ourred, even though NF-κB expression levels remined unhnged. When insulin ws used together with interferons in one-dy pretretment, the effet of insulin on NF-κB protein expression (Figure 2()) nd tivity in 3- dy old C2C12 myotues ws lso higher (insulin IFNα pretretment inresed response y 52% ±7.64, 86% ± 22.63, nd145% ± t 15th, 3th, nd 6th minute, respetively, (Figure 2(d), P <.1), while insulin y 121% ± nd 225% ± 2.2 in the 15th nd 3th minute of tretment (Figure 2(e), P <.1 ompred to ontrol) Pretretment with or IFNα ut Not Inresed the Nuler Protein Expression nd Ativity of STAT-1α-P- Y 71 in C2C12 Myotues: Insulin Does Not Signifintly Affet the Effets of Cytokines on Nuler Protein Expression nd Ativity of STAT-1α-P-Y 71. Preinution with ytokines
6 6 Meditors of Inflmmtion 3 MTT 3 MTT Viility (% initil ontrol) 2 1 Viility (% initil ontrol) Dy of differentition Dy of differentition (1 ng/ml) Curumin (1μM) urumin (1 ng/ml) AG49 (5μM) AG49 () () 2 TrnsAM Mitogeniity (% ontrol) (1 ng/ml) CV AG49 (5μM) AG49 Trnsriptionl tivity of NF-κB (% ontrol) 2 1 TrnsAM (15 ) (3 ) (6 ) Trnsriptionl tivity of NF-κB (% ontrol) 1 (1 ng/ml, 24 h) IFNα (1 ng/ml, 24 h) (1 ng/ml, 24 h) () (d) (e) 24 h IFNα NF-κB (nuleus) Atin (nuleus) IκBα (ytosol) Atin (ytosol) (f) Ins/ Ins/Ins NF-κB (nuleus) Atin (nuleus) IκBα (ytosol) Atin (ytosol) (g) Figure 1: Continued. IFNα
7 Meditors of Inflmmtion 7 Trnsriptionl tivity of NF-κB (% ontrol) TrnsAM Ins (1 nm) Ins (1 nm)/ (15 ) (3 ) Ins (1 nm)/ (6 ) Ins (1 nm)/ Trnsriptionl tivity of NF-κB (% ontrol) TrnsAM Ins (1 nm) Ins/IFNα (1 ng/ml, 24 h) Ins/ (1 ng/ml, 24 h) (h) (i) Figure 1: Short- nd long term effets of,ifnα, (1 ng/ml eh), insulin (1 nm), nd metoli inhiitors (t onentrtions indited) upon ell viility during 5 susequent dys of myogeni differentition (, ), ell mitogeniity determined t the 3rd dy of experiment (), NF-κB trnsriptionl tivity in the 3rd dy of myogenesis (d, e, h, i), ytosoli IκB nd nuler NF-κB expression levels upon, nd dditionl insulin pretretment in the 3rd dy of myogenesis (f, g). Atin ws used s loding ontrol. The results re inditive of three independent experiments. ( or IFNα, 1 ng/ml, 24 h) inresed STAT-1α-P-Y 71 - nuler protein expression levels nd tivity in 3-dy-old C2C12 myotues y 39% ± 3.35 nd 41% ± 2.78, respetively, (Figure 2(g), P <.1 ompred to ontrol). Insulin given together with IFNα did not redue elevted STAT-1α-P-Y 71 tivity nd nuler protein expression level (Figures 2(g) nd 6(), P >.5 ompred to ontrol) Inresed Nuler Expression nd Ativity of STAT- 1α-P-Y 71 in C2C12 Myotues: Insulin Pretretment Hrdly Stops the Effet of on Nuler Protein Expression LevelsndAtivityofSTAT-1α-P-Y 71. Similrly to NF-κB, myotues responded to dministrtion with onsiderle rise in nuler protein expression levels nd tivity of STAT-1α-P-Y 71 (inrese y 28% ± 5.83, 33% ±1.98,nd ± 1.67 t 15th, 3th, nd 6th minute of tretment, respetively, Figure 3(), P <.1 ompred to ontrol). Insulin pretretment (1 nm, 72 h) hrdly ffeted the ellulr response to with respet to STAT-1α-P-Y 71 tivity s it remined signifintly higher thn in ontrol onditions during 15 (44% ± 6.24, P <.1), 3 (37.28% ± 4.54, P<.1), nd 6 (17% ± 4., P <.5) minutes of tretment (Figures 3(d) nd 3(f)) Pretretment with IFNα ut Not Assists in Inresed Nuler Expression nd Ativity of STAT-1α- P-Y 71 in C2C12 Myotues: Insulin Does Not Overturn the Effet of IFNα on Nuler Expression of STAT-1α-P-Y 71. When 3-dy-old C2C12 myotues were pretreted with IFNα (1 ng/ml, 24 h) followed y hllenge, STAT-1α- P-Y 71 protein expression ws similr to the onditions in whih ws given lone (Figure 3()). Additionlly, IFNα mrkedly meliorted STAT-1α tivity y 52% ± 4.52, ± 3.26, nd 23% ±.47 t 15th, 3th, nd 6th minute of tretment, respetively, (Figure 3(d), P <.1 versus ontrol). When IFNα ws repled y (1 ng/ml, 24 h), -dependent rise in nuler protein expression levels of STAT-1α-P-Y 71 ws hlted (Figure 3()), wheres the tivity of STAT-1α-P-Y 71 did not differ from dministered lone (inrese y 28% ± 8.83, 33% ± 1.98, ± 1.67 t 15th, 3th, nd 6th minute of tretment, resp., Figure 3(f), P <.1 versus ontrol). Additionl preinution with insulin (1 nm, 72 h) neither ffeted the response to nor to IFNα pretreted ells in STAT-1α-P-Y 71 expression levels nd trnsriptionl tivities (Figures 3(e) nd 3(f)) IFNα nd Filitte -Dependent Derese of MyHC II Protein Expression Levels in C2C12 Myotues: Insulin Does Not Prevent -Dependent Loss of MyHC II. MyHC II protein expression level hs een seleted s mrker of protein gin/loss in C2C12 myotues. Its expression inresed progressively during the onseutive dys of differentition (Figure 4()). Initilly, the rise in MyHC II protein level ws noted t the 3rd dy of differentition (extensive fusion of myolsts), nd then it grdully inresed to the 6th dy with no dditionl elevtion t dy 7 (Figure 4()).
8 8 Meditors of Inflmmtion h IFNα 24 h NF-κB (nuleus) Atin (nuleus) IκBα (ytosol) Atin (ytosol) () TrnsAM TrnsAM TrnsAM TrnsAM 4 d Trnsriptionl tivity of NF-κB (% ontrol) Trnsriptionl tivity of NF-κB (% ontrol) Trnsriptionl tivity of NF-κB (% ontrol) Trnsriptionl tivity of STAT1 (% ontrol) (15 ) (3 ) (6 ) IFNα (1 ng/ml, 24 h) IFNα (24 h)/ (15 ) IFNα (24 h)/ (3 ) IFNα (24 h)/ (6 ) () (1 ng/ml, 24 h) (24 h)/ (15 ) (24 h)/ (3 ) (24 h)/ (6 ) Ins (1 nm) Ins/IFNα (24 h)/ (15 ) Ins/IFNα (24 h)/ (3 ) Ins/IFNα (24 h)/ (6 ) (d) Ins (1 nm) Ins/ (24 h)/ (15 ) Ins/ (24 h)/ (3 ) Ins/ (24 h)/ (6 ) (e) (1 ng/ml, 24 h) IFNα (1 ng/ml, 24 h) (1 ng/ml, 24 h) Ins (1 nm) (g) Ins/IFNα (1 ng/ml, 24 h) Ins/ (1 ng/ml, 24 h) Ins IFNα/ Ins / 24 h 24 h insulin (1 nm) NF-κB (nuleus) Atin (nuleus) IκBα (ytosol) Atin (ytosol) 24 h IFNα () 24 h STAT1-α-P-Y 71 (nuleus) Atin (nuleus) IFNα (f) Ins IFNα Figure 2: Short- nd long term effets of, IFNα, (1 ng/ml eh), insulin (1 nm), nd metoli inhiitors (t onentrtions indited) given lone, s pretretment or otretment (with ) in the 3rd dy of myogenesis. Chnges in the ytosoli IκB nd nuler NF- κb expressionlevelsupon nd dditionl IFNα, or pretretment (), NF-κB (,d,e)ndstat-1α trnsriptionl tivity (g), NF-κB/IκB ytosoli nd nuler protein expression levels upon nd dditionl insulin, IFNα or pretretments or o-tretments (with ) (), nd STAT-1α-P-Y 71 nuler protein expression levels upon,ifnα,or tretment or dditionl insulin pretretment nd o-tretment (with ) (f). Atin ws used s loding ontrol. The results re inditive of three independent experiments.
9 Meditors of Inflmmtion 9 STAT1-α-P-Y 71 (nuleus) Atin (nuleus) Ins/Ins () 2 TrnsAM TrnsAM TrnsAM 2 2 Trnsriptionl tivity of STAT-1 (% ontrol) Trnsriptionl tivity of STAT-1 (% ontrol) 5 Trnsriptionl tivity of STAT-1 (% ontrol) (15 ) (3 ) (6 ) () (24 h)/ (15 ) (24 h)/ (3 ) (24 h)/ (6 ) IFNα (24 h)/ (15 ) IFNα (24 h)/ (3 ) IFNα (24 h)/ (6 ) Ins/IFNα (24 h)/ (15 ) Ins/IFNα (24 h)/ (3 ) Ins/IFNα (24 h)/ (6 ) (24 h)/ (15 ) (24 h)/ (3 ) (24 h)/ (6 ) Ins/ (24 h)/ (15 ) Ins/ (24 h)/ (3 ) Ins/ (24 h)/ (6 ) (d) (f) 24 h IFNα IFNα/ / Ins/IFNα/ Ins// 24 h STAT1-α-P-Y 71 (nuleus) Atin (nuleus) () STAT1-α-P-Y 71 (nuleus) Atin (nuleus) 24 h IFNα 24 h (e) Figure 3: Short- nd long term effets of,ifnα, (1 ng/ml eh), nd insulin (1 nm) given lone, s pretretment or together in the 3rd dy of myogenesis. STAT-1α-P- Y 71 nuler protein expression levels upon nd dditionl insulin pretretment (), STAT- 1α trnsriptionl tivity (, d, f), Y 71 -phospho-stat-1α nuler protein expression levels upon nd dditionl IFNα or pretretment (), STAT-1α-P- Y 71 nuler protein expression levels upon nd dditionl IFNα or pretretment, nd susequent insulin nd IFNα or otretment (e). Atin ws used s loding ontrol. The results re inditive of three independent experiments. It ws thought tht t lest 6 dys of differentition should e onsidered s the strting point for evlution of hnges in MyHC II protein expression. (1ng/mL, 48h) ws shown to inhiit the expression of MyHC II in C2C12 myotues (8-dy-old), while dditionl presene of IFNα or (1 ng/ml, 48 h) mplified -dependent drop in MyHC II protein expression (Figure 4()). Insulin pretretment (1 nm, 24 h) ws pprently negligile to the effet indued in C2C12 myotues (Figure 4()) Elevted Expression of Nuler NF-κB Evoked y Is Controlled y Protesome nd JAK Ativity. The dministrtion of either urumin(1μm, protesome inhiitor) or AG49 (5 μm, JAK inhiitor) together with nd/or IFNα or during 48 hours (from 6th to 8th dy of differentition) reversed the nuler rise in NF-κB protein expression to the ontrol level (Figure 5()). Aordingly, s shown on Figure 5(), othinhiitorswereequllypotent in elevting ytosoli IκB expression levels. As oth NF- κb tivity nd nuler expression levels were ssoited with onomitnt drop in MyHC II protein expression, the ove-mentioned inhiitors were lso used to determine whether they n protet C2C12 myotues from the TNF- α-dependent loss of MyHC II. In greement with our suggestion, oth inhiitors (urumin nd AG49) were ple to prevent the -dependent loss of MyHC II expression levels in C2C12 myotues, when ompred with the ontrol level (Figure 5()).
10 1 Meditors of Inflmmtion 8th dy of differentition Dy of differentition IFNα MyHC ll (ytosol) Atin (ytosol) MyHC ll (ytosol) Atin (ytosol) () () 8th dy of differentition MyHC ll (ytosol) Atin (ytosol) Insulin () Figure 4: Chnges in the ytosoli myosin hevy hin II (fst form) expression levels during 7 dys of myogenesis from C2C12 myolsts (). Long term effets of,ifnα, (1 ng/ml eh), or insulin (1 nm) given lone or together on the ytosoli myosin hevy hin II (fst form) expression levels in the 8th dy of myogenesis (, ). Atin ws used s loding ontrol. The results re inditive of three independent experiments Deresed Expression of MyHC II Indued y TNF- α nd IFNα or in Differentiting C2C12 Myotues Is Aompnied y Lower Expression of Seleted MRFs (Musle Regultory Ftors) nd It Depends on NF-κB nd STAT Ativity. As MRFs re ritil for musle differentition nd mturtion of myotues, two different trnsription ftors were hosen for the study, nmely, MyoD known s primry musle regultory ftor nd myogenin seondry to MyoD. As presented in Figure 6 MyoD protein expression ws redued ll long the tretment, irrespetive to ytokine, wheres the level of myogenin protein ws deresed y TNF- α tretment or otretment only. Additionl dministrtion of IFNα or did not lter the myogenin response to TNF- α (Figure 6). However, the expression of MyHC II protein deresed ordingly. Both the numer nd dimeter of myotues were redued fter individul tretment with TNF- α or nd ut not with IFNα. They were restored to norml fter simultneous dministrtion of urumin or AG49 (dt not shown) Comined Tretments of nd IFNα or Derese the Affinity of STAT-1α to TRADD Protein in C2C12 Myotues. In this study, it ws ssumed tht STAT-1α protein is tivted either y IFNα or fter inding to respetive IFNAR or IFNGR. STAT-1α ws previously reported to e ound to TRADD protein [41 44] nd inhiits NF- κb-dependent deth signl. As STAT-1α protein is modulted y interferons, lso NF-κB signlingpthwyne relesed from inhiition when oth nd IFNα or re dministered. To verify this ssumption STAT-1α protein omplexes were isolted y immunopreipittion, ndtraddproteinwsimmunolottedfterpage.asit is shown in Figure 7(), the expression of TRADD tht ws ound to STAT-1α ws the highest in untreted myotues, nd it ws lowered y nd further otretment with IFNα nd , IFNα,nd Stimulte Myh II Gene Ativity,ndThisProessIsControlledyNF-κBndSTAT-1α s the Administrtion of Curumin (Protesome Inhiitor) or AG49 (JAK inhiitor) Reverses These Effets. together with interferons ttenuted the expression of MyHC II protein. On this evidene, we queried whether it is ssoited with lower tivity of the respetive gene (Myh II). Surprisingly,,IFNα,nd were shown to stimulte the tivity of Myh II y 3-, 1.8-, nd 2.6-fold, respetively (Figure 7(), P <.1 versus ontrol). However, the Myh II response to ws ontrolled y NF-κB ndstat-1α trnsription ftors sine the lokge of tivtion y urumin (NF- κb) or AG49 (STAT-1α) diminished -dependent rise in Myh II tivity (Figures 7() nd 7(d), P >.5 versusontrol).inontrstto, urumindidnot ffet the tivity of Myh II upon otretment with IFNα (Figure7(), P >.5 versus nd IFNα) ut it inresed Myh II tivity when ws dded long with (Figure 7(), P <.1 versus nd ). Astonishingly, AG49 o-tretment with IFNα elevted Myh II tivity y 2.5-fold (Figure 7(d), P <.1 versus ontrol).
11 Meditors of Inflmmtion 11 8th dy of differentition Curumin AG49 NF-κB (nuleus) Atin (nuleus) IκBα (ytosol) Atin (ytosol) () 8th dy of differentition Curumin AG49 MyHC ll (ytosol) Atin (ytosol) () Figure 5: Chnges in the ytosoli IκB ndnulernf-κb expressionlevelsinthe8thdyofmyogenesis().longtermeffetsof, IFNα, (1 ng/ml eh), or metoli inhiitors (urumin 1 μm, AG 49 5 μm) given lone or together. Chnges in the ytosoli myosin hevy hin II (fst form) expression levels in the 8th dy of myogenesis (). Atin ws used s loding ontrol. The results re inditive of three independent experiments. Dy of differentition IFNα IFNα MyHC ll Myogenin MyoD Atin Figure 6: Chnges in the ytosoli myosin hevy hin II (fst form), myogenin, nd MyoD expression levels in seleted dys (1, 3, 5, 8) of 8 dys of myogenesis from C2C12 myolsts. Long term effets of, IFNα, (1 ng/ml eh), or metoli inhiitors (urumin 1 μm, AG 49 5 μm) given lone or together. Atin ws used s loding ontrol. The results re inditive of three independent experiments.
12 12 Meditors of Inflmmtion 5 qrt PCR IP: STAT-1α WB: TRADD 8th dy of differentition IFNα Myh ll gene tivity (mrna of Myh ll) IgG HC (1 ng/ml) IFNα (1 ng/ml) IFN α (1 ng/ml) IFN γ () () Myh ll gene tivity (mrna of Myh ll) d qrt PCR Myh ll gene tivity (mrna of Myh ll) d qrt PCR e Curumin (1μM) (1 ng/ml) Curumin IFNα (1 ng/ml) Curumin IFNα IFNα Curumin IFNα (1 ng/ml) Curumin Curumin AG49 (5μM) (1 ng/ml) AG49 IFNα (1 ng/ml) AG49 IFNα IFNα AG49 IFNα (1 ng/ml) AG49 (1 ng/ml) AG49 () (d) Figure 7: Immunopreipittion of STAT-1α shows time-dependent loss of immunoretive TRADD in the preipittes upon nd/or IFNα or otretment in the 8th dy of myogenesis (). IgG ws used s equl input ontrol. The results re inditive of three independent experiments. Long term effets (eight dys) of,ifnα, nd IFN (1 ng/ml eh) given lone or together on the Myh II gene tivity (). Long term effets (eight dys) of,ifnα, (1 ng/ml eh, filled rs), or metoli inhiitors (urumin 1 μm) given lone (filled rs) or together (dotted rs) on Myh II gene tivity (, d). Fold inrese ws lulted ording to the formul desried in Setion 2.
13 Meditors of Inflmmtion 13 qrt PCR qrt PCR Atrogin1 gene tivity (mrna of Atrogin1) Atrogin1 gene tivity (mrna of Atrogin1) d d d Curumin (1μM) (1 ng/ml) Curumin IFNα (1 ng/ml) Curumin IFNα IFNα Curumin IFNα (1 ng/ml) Curumin Curumin AG49 (5μM) (1 ng/ml) AG49 IFNα (1 ng/ml) AG49 IFNα IFNα AG49 IFNα (1 ng/ml) AG49 AG49 () () MuRF1 gene tivity (mrna of MuRF1) d qrt PCR d MuRF1 gene tivity (mrna of MuRF1) qrt PCR Curumin (1μM) (1 ng/ml) Curumin IFNα (1 ng/ml) Curumin IFNα IFNα Curumin IFNα (1 ng/ml) Curumin (1 ng/ml) Curumin AG49 (5μM) (1 ng/ml) AG49 IFNα (1 ng/ml) AG49 IFNα IFNα AG49 IFNα (1 ng/ml) AG49 AG49 () (d) Figure 8: Long term effets of, IFNα, nd given lone or together (1 ng/ml eh, filled rs) or with metoli inhiitors (slshed rs)t onentrtions indited on theatrogin1 nd MuRF1 gene tivities in the 8th dy of myogenesis. Fold inrese ws lulted ording to the formul desried in Setion nd IFNα Both Stimulte Atrogin1 Gene Ativity. -Dependent Ativtion is Controlled y NF-κB nd STAT-1α s the Administrtion of Curumin (Protesome Inhiitor) or AG49 (JAK Inhiitor) Reversed This Effet: AG- 49 Could Not Stop IFNα-Dependent Atrogin1 Gene Ativtion. When or IFNα, or oth (1 ng/ml) were dded to differentiting medium for the lst 48 hours of differentition (dys 6 8), Atrogin1 gene tivity ws stimulted y 1.8- nd 1.6-fold t the 8th dy of study (Figure 8(), P <.1 ompred to ontrol)., utnotifnα, dependent Atrogin1 tivtion, ws inhiited y urumin (1 μm, Figure 8(), P >.5 versus ontrol). A more pronouned effet ws indued y AG49 (5 μm), whih lmost ompletely olished nd IFNα effets during the lst 48 hours of the 8-dy inution time (Figure 8(), P <.1 ompred to ontrol).
14 14 Meditors of Inflmmtion 3.14., IFNα, nd Stimulte MuRF1 Gene Ativity: - nd - ut Not IFNα-Dependent Ativtion Are Controlled y NF-κB nd STAT-1α s the Administrtion of Curumin (Protesome Inhiitor) or AG49 (JAK Inhiitor) Reverses This Effet: AD-49 Further Stimultes IFNα-Dependent MuRF1 Gene Ativtion. When, IFNα, or (1 ng/ml) were dded to differentiting medium for the lst 48 hours MuRF1 gene tivity ws stimulted lmost 4-fold t the 8th dy of study (Figure 8(), P <.1 ompred to ontrol). nd,utnotthe IFNα-stimulted MuRF1 gene tivity in NF-κB-dependent mnner, sine urumin (1 μm) ws ple to redue signifintly the ellulr response (Figure 8(d), P <.1 versus nd lone). No effet of urumin ws oserved withrespettoifnα (Figure 8(), P >.5 versus IFNα lone). AG49 (5 μm) olished -indued effet, ut it potentited the IFNα effet on MuRF1 gene tivity y lmost 1.6-fold (Figure 8(d), P <.1 versus IFNα lone), wheres it hd no signifint influene on -dependent stimultion of MuRF1 gene tivity (Figure 8(d), P >.5 versus lone). 4. Disussion Severl signling pthwys hve een shown to prtiipte in ner hexi [25, 26]. It ws reported tht TGF-β superfmily (TGF-β, tivin, GDF-15, nd myosttin) ontriutes to musle wsting through SMAD pthwy tivtion [27], wheres lokge of triib prtilly reversed this effet [28]. Also JAK/STAT3 pthwy inhiition ws demonstrted to improve IL-6-indued skeletl musle hexi in niml nd C2C12 musle ell models [13, 15].Reently, we reportedtht leptin ts s mitogen ut it mrkedly impirs musle ell viility nd myogeni differentition through JAK/STAT3 signling pthwy [29]. As leptin defiient oese mie (o /o ) hve lso redued len ody mss, pprently other thn leptin-dependent signling pthwys must e engged in musle hexi. In this study we showed tht stimulted DNA synthesis similrly to leptin (Figure 1(), P <.1 versus ontrol) ut it lso inresed C2C12 musle ell viility (Figures 1()-1(), P <.1 versus ontrol). Thus, oth ytokines seem to tively prtiipte in the tivtion of stellite ells (myolsts) s ws previously reportedforleukemiinhiitoryftorlif[3]. This effet of ytokine does not neessrily men tht musle tissue would grow in hyperplsi-ssoited hypertrophy onditions, s the key event in musle growth is differentition nd fusion. Moreover, mitogeniity hs nothing to do with musle fier dey nd resulting musle hexi. Among inflmmtory ytokines, /hetin, IL-1α, IL-1β,, nd IL- 6hveeenimplitedinmuslewsting,ndtheirelevted levels were deteted in heti ptients [31]. In this experiment we sought to mimi the effets of inresed onentrtions of,ifnα,nd during musle differentition using C2C12 mouse myolsts. We lso showed their respetive effets in fully formed C2C12 myotues. As improved viility nd mitogeniity of differentiting C2C12 myolsts, we suggest tht mighte ple to indue musle dedifferentition s demonstrted y Buk nd Chojkier [32]. improved ell respirtion through NF- κb-dependent mehnism, s urumin (1 μm) dministered with impired musle ell viility even stronger thn urumin given lone (Figure 1(), P <.1). Inhiition of STAT-1α kinse JAK with tyrphostin (AG49) lso retrded -indued mitogeniity nd viility elow ontrol level (Figures 1()-1(), P <.1 versus ontrol). A similr effet of AG49 on C2C12 myolst prolifertion ws oserved y Spngenurg nd Booth [3]. Moreover, NF-κB nd STAT- 1α responded to in elevted trnsriptionl tivity, sshowninthe3-dymyotuesinshort-ndlongterm experiments (Figures 1(d)-1(e), 3(), P <.5 versus ontrol). These preliminry oservtions prompted us to study NF- κb nd STAT-1α effets in detil. Western lots onfirmed trnsriptionl tivtion in the 3rd dy of myogenesis s it ws ompnied y higher nuler NF-κB nd lower ytosoli IκB protein levels fter (Figure 1(f)). Insulin pretretment mplified -dependent tivity of NF- κb (Figure 1(h), P <.1 versus given lone), utitouldhrdlyffetifnα or effets (Figure 1(i), P >.5). Insulin however, fter initil pretretment, given in omined tretment with, inhiited nuler trnslotion of NF-κB (Figure 1(g)). dministrtion retrded musle differentition, s evidened y lower MRFs (MyoD nd myogenin) expression nd redued myotue formtion (Figure 6() nd supplementry dt 3). It is well estlished tht ontrols NF-κB tivity in musle ells [5, 32] nd tht trnsriptionl regultion through NF- κb ontrols MyoD dey in C2C12 myotues [5, 33]. NF- κb regultes the expression of vriety of musle genes nd proteins inluding those involved in ontrol of ell prolifertion [34], myogenesis, oxidtive stress, nd mitohondril dysfuntion [35]. Thus, higher NF-κB tivity oserved fter dministrtion in this experiment suggests tht differentiting myolsts ould e withdrwn from the myogeni progrm s shown reently in muslederived stem ells isolted from trnsgeni mie [36], or y enfored expression of -FLIP in stellite ells [37]. Moreover, -FLIP is known intrellulr modultor of deth reeptor medited signling (inhiition of intrinsi poptosis) nd NF-κBtivtor[38]. Atully, this ws not the se for IFNα nd s neither ytokine (1 ng/ml) ould ffet NF- κb tivity(figure 1(e), P >.5 versus ontrol). However, IFNα nd used in one-dy pretretment sensitized C2C12 myotues to -indued NF-κBtivtiononthe 3rd dy of myogenesis (Figure 2(), P <.1). Higher NF-κB trnsriptionl tivity upon pretretment with IFNs ws onfirmed y higher nd erlier nuler expression of NF-κB (Figure 2()). This oservtion points to interferons s ompetent ytokines ple to mplify -indued inflmmtory response. Indeed, systemi inflmmtion ws often indited s usl to skeletl musle wsting [39, 4]. In this study, we suessfully verified tht IFNα nd ffeted -dependent NF-κB tivtion nd showed tht the ltter oured through STAT-1α tivtion. As shown in Figure 2(f) tive STAT-1α (phosphorylted t tyrosine 71,
15 Meditors of Inflmmtion 15 STAT-1α-P-Y 71 ) hs een deteted in the nuleus fter TNF- α, IFNα, nd, wheres insulin pre- nd otretment ould not redue the effet of IFNs. Thus, it is pprent tht,ifnα,nd ooperte in NF-κBtivtion,nd tht nontive STAT-1α seems to inhiit this proess. Next, we ssumed tht STAT-1α might inhiit NF-κB in nonstimulted musle ells s STAT-1α ws previously reported in other ell-types to e ssemled with TNF-R1 through TRADD protein prtner [41 43]. Atully, during the immunopreipittion study IFNα nd used redution in the STAT- 1α protein quntity ound to TRADD protein (importnt omponent of -indued signlosome, Figure 7()). Thus, withdrwl of STAT-1α from TNF-R1 ssemly y IFNα or might explin their mplifying effet of indued NF-κB tivtion. Similrly, IFN medited relese from STAT-1α-medited inhiition of NF-κB tivtion in humn olon denorinom COLO 25 ells [44]. Rising evidene otined from similr experiments performed in the pst propose phylogenetilly onserved mehnism of STAT-1α-dependent inhiition of NF-κB. Insulin, whih is known to tivte NF-κB through PI3-K/Akt pthwy [45], ws lso ple to sensitize C2C12 myotues to TNF- α-dependent NF-κB tivtion (Figure 1(h), P <.1 versus ontrol), ut it did not hnge the response of NF- κb or STAT-1α to interferons (Figures 1(i) nd 2(g), P >.5 versus ontrol). Therefore, we ssume tht similrities etween insulin nd interferons in -dependent NF-κB tivtion must hve different origins. Insulin ws ple to redue NF-κB tivtion in the presene of IFNα- ut not (Figures 2(d)-2(e)), lthough insulin did not influene nuler NF-κB ndytosoliiκb proteinlevels,respetively, (Figure 2()). Presumly, the effets of insulin on nd IFNs-medited NF-κB tivtion were indiret nd not relted to STAT-1α tivity (Figure 2(), P >.5 versus or IFNs). Interestingly, rised nuler STAT- 1α-P-Y 71 protein levels, while insulin pre- nd o-tretment dditionlly mplified this effet in the 3rd dy of myogeni differentition (Figure 3()). At the sme time, evoked higher trnsriptionl tivity of STAT-1α (Figure 3(), P <.5). These results re in onert with our previous report [45] s did not (Figure 3(), P >.5) utifnα did inrese -dependent STAT-1α tivtion (Figure 3(d), P <.5). Insulin pretretment did not ffet IFNα-indued, -dependent tivtion of STAT-1α (Figure 3(d), P <.5). The ltter oservtion ws orroorted y WB results otined for STAT-1α-P-Y 71 protein levels (Figures 3() nd 3(e)). One should keep in mind, tht multiple signling pthwys re relevnt to musle hexi (NF-κB, JAK/STAT, SMAD). From pthophysiologil point of view, musle hexi is n dpttion mehnism to hroni inflmmtion nd long lsting norexi (ner, norexi nervos, nd AIDS). It hs pushed through the demnds for minoids needed to meet the requirements of ute phse protein synthesis. Interestingly, it is ler now tht skeletl musles notonlyplypivotlroleinmoiliztionofmino-ids, ut they re lso the site of extensive prodution of ute phse proteins [13]. Overll, the gol is not to ultimtely stop musle hexi y phrmologil or iotehnologil intervention, ut to mke this tsk osolete through the orretion of ssoited symptoms of disese. The outome of MyHC II protein expression in 6-dy myotues hllenged with ytokines ws hosen to exmine their proheti tivity. Initilly, it should e stressed tht musle differentition of C2C12 myolsts ws suppressed y ut not IFNα or, exept when they were dministered together with (Figure 6()). These effets were prtilly under the ontrol of NF-κB nd/or JAK/STAT-1α, sine either urumin (NF-κB inhiitor) or AG49 (JAK inhiitor) reversed some of the -indued effets. Curumin is dominnt NF-κB inhiitor s it prevents protesoml degrdtion of IκB ndsusequent NF- κb tivtion[46]. In 6-dy myotues,, givenlone or together with IFNα or inresed nuler expression of NF-κB with simultneous drop in ytosoli expression of IκB (Figure 5()).Atthesmetime, dministered lone redued the expression of the fst form of MyHC II protein (Figure 5()). These dt suggest tht NF-κB is negtive regultor of myogenesis, nd tht NF-κB delysthe rise in MyHC II protein expression. Mrked redution ws lso oserved fter onomitnt dministrtion of IFNα nd. However, neither ytokine dministered seprtely nor in used omintions ould indue nuler expression of NF- κb upon otretment with urumin (1 μm), nd ssoited ytosoli IκB expression level remined high (Figure 5()). JAK inhiitor AG49 (5 μm) ws less potent thn urumin with respet to NF-κB/IκB expression levels (Figure 5()). From this study it eomes ler tht myogenesis is repressed y NF-κB s reported y Kumr et l. [47]. The redued expression of fst form of MyHC II ws oserved fter, utnotifnα or given lone (Figure 5()). Comined tretment of nd IFNα nd IFNα nd ws even more powerful to repress MyHC II expression in theytosol.bothinhiitors(uruminndag49)lmost totlly rogted the ove-mentioned effets of ytokines (Figure 5()). It should e underlined tht mrkedly deresed the MyoD/myogenin protein levels (Figure 6)with resultnt drop in MyHC II protein expression. Neither IFNα nor ould ffet myogenin expression, ut similrly to, oth interferons onsiderly diminished MyoD protein expression levels (Figure6). Summingup, omined tretment of with IFNα or led to signifint effet evoked y the former (Figure 6). Thus, in this experiment seemstoplynimportntnegtiveroleinthe ontrol of myogenesis. To mke the piture of ytokine-dependent musle differentitionndmuslefierdeymoreluid,dditionl experiments followed y qrt PCR nlysis hve een performed. Oservtions otined from this study suggest mrked differenes in the ontrol of musle differentition nd musle wsting t genomi nd trnsltionl levels. MyHC II gene expression ws rised notiely fter dministrtion (Figures 7()-7(d), P <.1 versus ontrol), whih ws unexpeted s MyoD level ws diminished t thesmetime.interferonslsostimultedmyhc II gene expression, ut they were pprently weker (Figures 7()- 7(d), P <.1 versus ontrol). Astonishingly, urumin (1 μm)wsunletoesethetionofeitherytokine
16 16 Meditors of Inflmmtion (Figure 7(), P >.5 versus ytokines lone), lthough it mrkedly redued the effet of nd (Figure 7(), P <.1 versus ytokines lone). AG49 signifintly elevted MyHC II gene expression either lone or when it ws given together with interferons (Figure 7(d), P <.1 versus ontrol), ut it rogted - utnotifnαindued MyHC II gene tivtion. As MyHC II protein level deresed upon or nd IFNs dministrtion, two genesatrogin1 nd MuRF1 enoding respetive uiquitin ligses were exmined. These genes were previously indited s ruil for musle fier dey [48, 49]. nd stimulted (Figure 8(), P <.1 versus ontrol), wheres IFNα did not ffet Atrogin1 gene expression (Figure 8(), P >.5 versus ontrol). Curumin proteted myotues from -, nd IFNs-stimulted Atrogin1 gene expression (Figure 8(), P >.5 versus ontrol). Cytokine-indued Atrogin1 gene tivtion ws onsiderly diminished y AG49; gene expression even dropped elow the ontrol level (Figure 8(), P <.5 versus ontrol). Surprisingly, AG49 given lone stimulted gene expression (Figure 8(), P <.1 versus ontrol). MuRF1 gene expression ws tivted y ytokines in similr mnner (Figures 8()-8(d), P <.1 versus ontrol) ut urumin nd AG49 ould repress only -indued gene tivtion (Figures 8()-8(d), P <.1 versus lone). These results re in greement with the well known effets of trnsriptionl tivity of NF-κB, s oth genes re trgets in musle wsting [19, 5]. Muh less is known out the ontrol of these genes y STAT-1α. In this study, however, AG49 drstilly redued Atrogin1 gene expression when dded with ytokines. AG49 used individully elevted tivity of Atrogin1 gene (Figure 8(), P <.1 versus ontrol). Monitoring of Atrogin1 nd MuRF1 genes tivity showed dominnt effet of (lso in omined tretment with IFNs). We speulte tht NF-κB ws mjor plyer t the genomi level for -indued effets. Where ws dministered together with IFNs, urumin ddition ould not repress inresed Atrogin1 nd MuRF1 genes tivity (Figures 8() nd 8(), P >.5 versus nd IFNs). A similr effet ws noted in the se of tretment with AG49. As mentioned ove, this JAK/STAT inhiitor normlized Atrogin1 gene tivity whih ws rised y nd IFNs, ut it did not ffet MuRF1 gene tivity exept for the sitution when ws given lone (Figure 8(d), P <.1 versus ontrol). 5. Conlusions Our hypothesis ddresses ompetition etween ytokines (,IFNα,)toreruitSTAT-1α.Duringthisproess relese of NF-κB llowed oth NF-κB nd STAT-1α to oopertetthegenomilevel.asweshowed,someoftheheti effets were evoked differently for,ifns,nd oth,s -indued tivtionof Atrogin1 nd MuRF1 genes ws redued y NF-κB nd STAT-1α inhiitors (urumin nd AG49,resp.),whilethepreseneofIFNsdeteriortedurumin effet. Astonishingly, ws pprently mitogeni ndstimultedviilityofmononulermusleells,wheres this ytokine effiiently inhiited musle fier formtion. Moreover, indiretly nd diretly diminished MyHC II protein level, even though it stimulted MyHC II gene expression. From qrt PCR study it eme ovious tht protesome-uiquitin system tivity ws tivted. Atully, suh retion ws previously reported y Tn et l. [51]. Similrly to Atrogin1 nd MuRF1, the tivtion of MyHC II gene y ould e prtilly reversed y NF-κB nd STAT-1α inhiitors. The effet of NF-κB nd STAT-1α inhiitors ws diminished upon IFNs otretment pointing to the synergy etween ndifnsintion.insulindid not prevent omined - nd IFN-s-dependent musle dey, even though it is lmost ultimte noli hormone. Thus,wherenyonethoughttoringtonendtheindued musle hexi, either the seretion of this ytokine or IFNs seems to e essentil [52]. Dislosure The uthor(s) delre(s) tht there is no onflit of interests regrding the pulition of this Pper. Aknowledgments Support for this work ws prtilly provided y Grnts N /759 nd N N from the Ministry of Siene nd Higher Edution in Polnd. Referenes [1] W. J. Evns, J. E. Morley, J. Argilés et l., Chexi: new definition, Clinil Nutrition,vol.27, no.6,pp , 28. [2]M.J.Tisdle, Mehnismsofnerhexi, Physiologil Reviews,vol.89,no.2,pp ,29. [3] P. Hsselgren nd J. E. Fisher, Musle hexi: urrent onepts of intrellulr mehnisms nd moleulr regultion, Annls of Surgery,vol.233,no.1,pp.9 17,21. [4] J. L. Cheshire nd A. S. Bldwin Jr., Synergisti tivtion of NF-κB y tumor nerosis ftor lph nd gmm interferon vi enhned IκBα degrdtion nd de novo IκBβ degrdtion, Moleulr nd Cellulr Biology, vol.17,no.11,pp , [5] D. C. Guttridge, M. W. Myo, L. V. Mdrid, C.-Y. Wng, nd J. Bldwin A.S. Jr., NF-κB-indued loss of MyoD messenger RNA: possile role in musle dey nd hexi, Siene,vol. 289, no. 5488, pp , 2. [6] S. Ahryy, K. J. Ldner, L. L. Nelsen et l., Cner hexi is regulted y seletive trgeting of skeletl musle gene produts, The Journl of Clinil Investigtion, vol. 114, no. 3, pp , 24. [7] M.T.Wheeler,E.C.Snyder,M.N.Ptterson,ndS.J.Swop, An E-ox within the MHC IIB gene is ound y MyoD nd is required for gene expression in fst musle, Amerin Journl of Physiology Cell Physiology,vol.276,no.5,pp.C169 C178, [8]M.Dehoux,C.Goier,P.Luse,L.Bertrnd,J.Ketelslegers, nd J. Thissen, IGF-I does not prevent myotue trophy used y proinflmmtory ytokines despite tivtion of Akt/Foxo nd GSK-3β pthwys nd inhiition of trogin-1 mrna, Amerin Journl of Physiology Endorinology nd Metolism,vol.292,no.1,pp.E145 E15,27.
17 Meditors of Inflmmtion 17 [9] H. Cong, L. Sun, C. Liu, nd P. Tien, Inhiition of trogin- 1/MAFx expression y denovirus-delivered smll hirpin RNAs ttenutes musle trophy in fsting mie, Humn Gene Therpy,vol.22,no.3,pp ,211. [1] S. M. Eert, M. C. Dyle, S. D. Kunkel et l., Stress-indued skeletl musle Gdd45 expression reprogrms myonulei nd uses musle trophy, The Journl of Biologil Chemistry,vol. 287, pp , 212. [11] A. Fnzni, V. M. Conrds, F. Penn, nd W. Mrtinet, Moleulr nd ellulr mehnisms of skeletl musle trophy: n updte, Journl of Chexi, Sropeni nd Musle, vol. 3, pp ,212. [12] K. Skum nd A. Ymguhi, Sropeni nd hexi: the dpttions of negtive regultors of skeletl musle mss, Journl of Chexi, Sropeni nd Musle, vol.3,pp.77 94, 212. [13]A.Bonetto,T.Aydogdu,X.Jinetl., JAK/STAT3pthwy inhiition loks skeletl musle wsting downstrem of IL- 6 nd in experimentl ner hexi, Amerin Journl of Physiology Endorinology nd Metolism,vol.33,pp.E41 E421, 211. [14] A. Bonetto, T. Aydogdu, N. Kunzevitzky et l., STAT3 tivtion in skeletl musle links musle wsting nd the ute phse response in ner hexi, PLoS ONE,vol.6,no.7,Artile ID e22538, 17 pges, 212. [15] J. De Lrihudy, A. Zufferli, F. Serr et l., - nd tumor-indued skeletl musle trophy involves sphingolipid metolism, Skeletl Musle,vol.2,pp. 2 19,212. [16] L.G.Melstrom,K.A.MelstromJr.,X.-Z.Ding,ndT.E.Adrin, Mehnisms of skeletl musle degrdtion nd its therpy in ner hexi, Histology nd Histopthology,vol.22,no.7 9, pp ,27. [17] X. Zhou, J. L. Wng, J. Lu et l., Reversl of ner hexi nd musle wsting y AtRIIB ntgonism leds to prolonged survivl, Cell, vol. 142, no.4,pp , 211. [18] M. Krin nd A. Lin, NF-κBttherossrodsoflifenddeth, Nture Immunology,vol.3,no.3,pp ,22. [19] D. Ci, J. D. Frntz, N. E. Tw Jr. et l., IKKβ/NF-κB tivtion uses severe musle wsting in mie, Cell, vol. 119, no. 2, pp , 24. [2] F. Mourkioti nd N. Rosenthl, NF-κB signling in skeletl musle: prospets for intervention in musle diseses, Journl of Moleulr Mediine,vol.86,no.7,pp ,28. [21] S. A. Reed, S. M. Senf, E. W. Cornwell, S. C. Kndrin, nd A. R. Judge, Inhiition of IkppB kinse lph (IKKα) or IKKet (IKKβ) plus forkhed ox O (Foxo) olishes skeletl musle trophy, Biohemil nd Biophysil Reserh Communitions,vol.45,no.3,pp ,211. [22] D. Yffe nd O. Sxel, Seril pssging nd differentition of myogeni ells isolted from dystrophi mouse musle, Nture, vol.27,no.5639,pp ,1977. [23] M. D. Joson, J. F. Burne, nd M. C. Rff, Progrmmed ell deth nd Bl-2 protetion in the sene of nuleus, The EMBO Journl,vol.13,no.8,pp ,1994. [24] M. M. Brdford, A rpid nd sensitive method for the quntittion of mirogrm quntities of protein utilizing the priniple of protein dye inding, Anlytil Biohemistry, vol. 72, no. 1-2, pp ,1976. [25] J. M. Argilés,S.Busquets,M.Toledo,ndF.J.López-Sorino, The role of ytokines in ner hexi, Current Opinion in Supportive nd Pllitive Cre,vol.3,no.4,pp ,29. [26]J.E.Morley,D.R.Thoms,ndM.G.Wilson, Chexi: pthophysiology nd linil relevne, Amerin Journl of Clinil Nutrition,vol.83,no.4,pp ,26. [27] T. A. Zimmers, M. V. Dvies, L. G. Koniris et l., Indution of hexi in mie y systemilly dministered myosttin, Siene,vol.296,no.5572,pp ,22. [28] S. Busquets, M. Toledo, M. Orpí etl., Myosttinlokge using triib ntgonism in mie ering the Lewis lung rinom results in the improvement of musle wsting nd physil performne, Journl of Chexi, Sropeni nd Musle,vol.3,pp.37 43,212. [29] M. Pijet, B. Pijet, A. Litwiniuk, B. Pjąk, B. Gjkowsk, nd A. Orzehowski, Leptin impirs myogenesis in C2C12 ells through JAK/STAT nd MEK signling pthwys, Cytokine, vol. 61, pp , 213. [3] E. E. Spngenurg nd F. W. Booth, Multiple signling pthwys medite LIF-indued skeletl musle stellite ell prolifertion, Amerin Journl of Physiology Cell Physiology, vol. 283, no. 1, pp. C24 C211, 22. [31] J. M. Argiles, S. Bosquets, nd F. J. Lopez-Sorino, Cytokines in the pthogenesis of ner hexi, Current Opinion in Clinil Nutrition nd Metoli Cre,vol.6,pp.41 46,23. [32] M. Buk nd M. Chojkier, Musle wsting nd dedifferentition indued y oxidtive stress in murine model of hexi is prevented y inhiitors of nitri oxide synthesis nd ntioxidnts, The EMBO Journl, vol. 2, pp , [33] S. Di Mro, R. Mzroui, P. Dllire et l., NF-κB-medited MyoD dey during musle wsting requires nitri oxide synthse mrna stiliztion, HuR protein, nd nitri oxide relese, Moleulr nd Cellulr Biology, vol. 25, no. 15, pp , 25. [34] D.C.Guttridge,C.Alnese,J.Y.Reuther,R.G.Pestell,ndA. S. Bldwin Jr., NF-κB ontrols ell growth nd differentition through trnsriptionl regultion of ylin D1, Moleulr nd Cellulr Biology,vol.19,no.8,pp ,1999. [35] S. Bhtngr, S. K. Pnguluri, S. K. Gupt, S. Dhiy, R. F. Lundy, nd A. Kumr, Tumor nerosis ftor-α regultes distint moleulr pthwys nd gene networks in ultured skeletl musle ells, PLoS ONE, vol. 5, no.1, Artile IDe13262, 21. [36]A.Lu,J.D.Proto,L.Guo et l., NF-κB negtively impts the myogeni potentil of musle-derived stem ells, Moleulr Therpy,vol.2,no.3,pp ,212. [37] C. Gimpietri, S. Petrungro, P. Colui et l., -flip overexpression ffets stellite ell prolifertion nd promotes skeletl musle ging, Cell Deth nd Disese, vol.1,no.4,rtilee38, 21. [38] T. Ktok, R. C. Budd, N. Holler et l., The spse-8 inhiitor FLIP promotes tivtion of NF-κBnd Erksignling pthwys, Current Biology,vol.1,no.11,pp ,2. [39] R. J. Dunlop nd C. W. Cmpell, Cytokines nd dvned ner, Journl of Pin nd Symptom Mngement, vol. 2, no. 3,pp ,2. [4] R. W. Jkmn nd S. C. Kndrin, The moleulr sis of skeletl musle trophy, Amerin Journl of Physiology Cell Physiology,vol.287,no.4,pp.C834 C843,24. [41] Y. Wng, T. R. Wu, S. Ci, T. Welte, nd Y. E. Chin, Stt1 s omponent of tumor nerosis ftor lph reeptor 1-TRADD signling omplex to inhiit NF-κB tivtion, Moleulr nd Cellulr Biology,vol.2,no.13,pp ,2. [42] D. R. Wesemnn nd E. N. Benveniste, STAT-1α nd IFN-γ s modultors of signling in mrophges: regultion
18 18 Meditors of Inflmmtion nd funtionl implitions of the TNF reeptor 1:STAT-1α omplex, Journl of Immunology,vol.171,no.1,pp , 23. [43] D. R. Wesemnn, H. Qin, N. Kokorin, nd E. N. Benveniste, TRADD interts with STAT1-α nd influenes interferon-γ signling, Nture Immunology,vol.5,no.2,pp ,24. [44] B. Pjk nd A. Orzehowski, IFN-lph ompetes with TNFlph for STAT-1lph; moleulr sis for immune espe of humn olon denorinom COLO 25 ells, Onology Reports,vol.18,no.4,pp ,27. [45] C. S. Mitsides, N. Mitsides, V. Poulki et l., Ativtion of NF-ΚB nd upregultion of intrellulr nti-poptoti proteins vi the IGF-1/Akt signling in humn multiple myelom ells: therpeuti implitions, Onogene, vol.21,no.37,pp , 22. [46] C. Join, C. A. Brdhm, M. P. Russo et l., Curumin loks ytokine-medited NF-κB tivtion nd proinflmmtory gene expression y inhiiting inhiitory ftor I-κB kinse tivity, Journl of Immunology,vol.163,no.6,pp , [47] N. B. Kumr, A. Kzi, T. Smith et l., Cner hexi: trditionl therpies nd novel moleulr mehnism-sed pprohes to tretment, Current Tretment Options in Onology,vol.11,no.3-4,pp ,21. [48] S. C. Bodine, E. Ltres, S. Bumhueter et l., Identifition of uiquitin ligses required for skeletl Musle Atrophy, Siene, vol. 294, no. 5547, pp , 21. [49] M. D. Gomes, S. H. Leker, R. T. Jgoe, A. Nvon, nd A. L. Golderg, Atrogin-1, musle-speifi F-ox protein highly expressed during musle trophy, Proeedings of the Ntionl Ademy of Sienes of the United Sttes of Ameri, vol. 98, no. 25, pp , 21. [5] S. Ahryy nd D. C. Guttridge, Cner hexi signling pthwys ontinue to emerge yet muh still points to the protesome, Clinil Cner Reserh, vol. 13, no. 5, pp , 27. [51] Y.Tn,X.Peng,F.Wng,Z.You,Y.Dong,ndS.Wng, Effets of tumor nerosis ftor-lph on the 26S protesome nd 19S regultor in skeletl musle of severely slded mie, Journl of Burn Cre nd Reserh,vol.27,no.2,pp ,26. [52] B. Pjk, S. Orzehowsk, B. Pijet et l., Crossrods of ytokine signling: the hse to stop musle hexi, JournlofPhysiology nd Phrmology,vol.59,supplement9,pp ,28.
19 MEDIATORS of INFLAMMATION The Sientifi World Journl Hindwi Pulishing Corportion Volume 214 Gstroenterology Reserh nd Prtie Hindwi Pulishing Corportion Volume 214 Journl of Hindwi Pulishing Corportion Dietes Reserh Volume 214 Hindwi Pulishing Corportion Volume 214 Hindwi Pulishing Corportion Volume 214 Interntionl Journl of Journl of Endorinology Immunology Reserh Hindwi Pulishing Corportion Disese Mrkers Hindwi Pulishing Corportion Volume 214 Volume 214 Sumit your mnusripts t BioMed Reserh Interntionl PPAR Reserh Hindwi Pulishing Corportion Hindwi Pulishing Corportion Volume 214 Volume 214 Journl of Oesity Journl of Ophthlmology Hindwi Pulishing Corportion Volume 214 Evidene-Bsed Complementry nd Alterntive Mediine Stem Cells Interntionl Hindwi Pulishing Corportion Volume 214 Hindwi Pulishing Corportion Volume 214 Journl of Onology Hindwi Pulishing Corportion Volume 214 Hindwi Pulishing Corportion Volume 214 Prkinson s Disese Computtionl nd Mthemtil Methods in Mediine Hindwi Pulishing Corportion Volume 214 AIDS Behviourl Neurology Hindwi Pulishing Corportion Reserh nd Tretment Volume 214 Hindwi Pulishing Corportion Volume 214 Hindwi Pulishing Corportion Volume 214 Oxidtive Mediine nd Cellulr Longevity Hindwi Pulishing Corportion Volume 214
SUPPLEMENTARY INFORMATION
 doi: 1.138/nture862 humn hr. 21q MRPL39 murine Chr.16 Mrpl39 Dyrk1A Runx1 murine Chr. 17 ZNF295 Ets2 Znf295 murine Chr. 1 COL18A1 -/- lot: nti-dscr1 IgG hevy hin DSCR1 DSCR1 expression reltive to hevy
doi: 1.138/nture862 humn hr. 21q MRPL39 murine Chr.16 Mrpl39 Dyrk1A Runx1 murine Chr. 17 ZNF295 Ets2 Znf295 murine Chr. 1 COL18A1 -/- lot: nti-dscr1 IgG hevy hin DSCR1 DSCR1 expression reltive to hevy
Cos7 (3TP) (K): TGFβ1(h): (K)
 IP#2: IP#1: Totl Lystes luiferse tivity (K): 6-4 - (K): luiferse tivity luiferse tivity (K): 2 1 RL-: - + + + + + Sm4-3F: + - + + + + MYC-Sm3: - - - - + + TβRI-HA(T204D): - - - + - + α-ha Luiferse Ativity
IP#2: IP#1: Totl Lystes luiferse tivity (K): 6-4 - (K): luiferse tivity luiferse tivity (K): 2 1 RL-: - + + + + + Sm4-3F: + - + + + + MYC-Sm3: - - - - + + TβRI-HA(T204D): - - - + - + α-ha Luiferse Ativity
Title of Experiment: Author, Institute and address:
 Title of Experiment: Trsfetion of murine mrophge RAW264.7 ells with METAFECTENE PRO. Author, Institute n ress: Ptrizi Pellegtti n Frneso Di Virgilio. Deprtment of Experimentl n Dignosti Meiine, Setion
Title of Experiment: Trsfetion of murine mrophge RAW264.7 ells with METAFECTENE PRO. Author, Institute n ress: Ptrizi Pellegtti n Frneso Di Virgilio. Deprtment of Experimentl n Dignosti Meiine, Setion
SUPPLEMENTARY INFORMATION
 DOI: 1.13/n7 Reltive Pprg mrna 3 1 1 Time (weeks) Interspulr Inguinl Epididyml Reltive undne..1.5. - 5 5-51 51-1 1-7 7 - - 1 1-1 Lipid droplet size ( m ) 1-3 3 - - - 1 1-1 1-1 1-175 175-3 3-31 31-5 >5
DOI: 1.13/n7 Reltive Pprg mrna 3 1 1 Time (weeks) Interspulr Inguinl Epididyml Reltive undne..1.5. - 5 5-51 51-1 1-7 7 - - 1 1-1 Lipid droplet size ( m ) 1-3 3 - - - 1 1-1 1-1 1-175 175-3 3-31 31-5 >5
EFFECT OF DIETARY ENZYME ON PERFORMANCE OF WEANLING PIGS
 EFFECT OF DIETARY ENZYME ON PERFORMANCE OF WEANLING PIGS Finl report sumitted to Dniso Animl Nutrition E. vn Heugten nd B. Frederik North Crolin Stte University, Deprtment of Animl Siene Summry The urrent
EFFECT OF DIETARY ENZYME ON PERFORMANCE OF WEANLING PIGS Finl report sumitted to Dniso Animl Nutrition E. vn Heugten nd B. Frederik North Crolin Stte University, Deprtment of Animl Siene Summry The urrent
P AND K IN POTATOES. Donald A Horneck Oregon State University Extension Service
 P AND K IN POTATOES Donld A Hornek Oregon Stte University Extension Servie INTRODUCTION Phosphorous nd potssium re importnt to grow high yielding nd qulity pottoes. Muh of the northwest hs hd trditionlly
P AND K IN POTATOES Donld A Hornek Oregon Stte University Extension Servie INTRODUCTION Phosphorous nd potssium re importnt to grow high yielding nd qulity pottoes. Muh of the northwest hs hd trditionlly
SUPPLEMENTARY INFORMATION
 2 weeks high holesterol diet 2 weeks high holesterol diet 2 weeks high holesterol diet 2 μm Mrophges Crystls Hoehst μm Mrophges Crystls Hoehst Hoehst Crystls Mrophges 2 μm 2 μm Supplementry Fig. 1: Erly
2 weeks high holesterol diet 2 weeks high holesterol diet 2 weeks high holesterol diet 2 μm Mrophges Crystls Hoehst μm Mrophges Crystls Hoehst Hoehst Crystls Mrophges 2 μm 2 μm Supplementry Fig. 1: Erly
SUPPLEMENTARY INFORMATION
 { OI: 1.138/n31 Srifie n nlyze APs on week 1 s of iet 1 4 6 High-ft iet BrU High-ft iet BrU 4 High-ft iet BrU 6 High-ft iet BrU Lin - Lin - : C34 + : C9 + 1 1 3 1 4 1 5 C45 1 C34 1 1 1 1 3 1 4 1 5 S-1
{ OI: 1.138/n31 Srifie n nlyze APs on week 1 s of iet 1 4 6 High-ft iet BrU High-ft iet BrU 4 High-ft iet BrU 6 High-ft iet BrU Lin - Lin - : C34 + : C9 + 1 1 3 1 4 1 5 C45 1 C34 1 1 1 1 3 1 4 1 5 S-1
SUPPLEMENTARY INFORMATION
 % ells with ili (mrke y A-Tu) Reltive Luiferse % ells with ili (mrke y Arl13) % ells with ili DOI: 1.138/n2259 A-Tuulin Hoehst % Cilite Non-ilite -Serum 9% 8% 7% 1 6% % 4% +Serum 1 3% 2% 1% % Serum: -
% ells with ili (mrke y A-Tu) Reltive Luiferse % ells with ili (mrke y Arl13) % ells with ili DOI: 1.138/n2259 A-Tuulin Hoehst % Cilite Non-ilite -Serum 9% 8% 7% 1 6% % 4% +Serum 1 3% 2% 1% % Serum: -
SUPPLEMENTARY INFORMATION
 DOI: 1.138/n358 TLR2 nd MyD88 expression in murine mmmry epithelil supopultions. CD24 min plus MRU Myo-epithelil Luminl progenitor (CD61 pos ) Mture luminl (CD61 neg ) CD49f CD61 Reltive expression Krt5
DOI: 1.138/n358 TLR2 nd MyD88 expression in murine mmmry epithelil supopultions. CD24 min plus MRU Myo-epithelil Luminl progenitor (CD61 pos ) Mture luminl (CD61 neg ) CD49f CD61 Reltive expression Krt5
SUPPLEMENTARY INFORMATION
 doi:.8/nture98 : hr NEMO :5 hr IKK IKK NF-κB p65 p5 p65/-rel NF-κB p65 p5 p65/-rel Cytoplsm Cytoplsm p65/p5 Nuleus Nuleus NEMO IKK IKK d : hr > : hr p65/-rel NF- p65 p5 Cytoplsm Cytoplsm p65/p5 p65/-rel
doi:.8/nture98 : hr NEMO :5 hr IKK IKK NF-κB p65 p5 p65/-rel NF-κB p65 p5 p65/-rel Cytoplsm Cytoplsm p65/p5 Nuleus Nuleus NEMO IKK IKK d : hr > : hr p65/-rel NF- p65 p5 Cytoplsm Cytoplsm p65/p5 p65/-rel
Inhibitory effect of p38 mitogen-activated protein kinase inhibitors on cytokine release from human macrophages
 British Journl of Phrmology (26) 149, 393 44 & 26 Nture Pulishing Group All rights reserved 7 1188/6 $3. www.rjphrmol.org RESEARCH PAPER Inhiitory effet of p38 mitogen-tivted protein kinse inhiitors on
British Journl of Phrmology (26) 149, 393 44 & 26 Nture Pulishing Group All rights reserved 7 1188/6 $3. www.rjphrmol.org RESEARCH PAPER Inhiitory effet of p38 mitogen-tivted protein kinse inhiitors on
LHb VTA. VTA-projecting RMTg-projecting overlay. Supplemental Figure 2. Retrograde labeling of LHb neurons. a. VTA-projecting LHb
 SUPPLEMENTARY INFORMATION Supplementl Figure 1 doi:10.1038/nture09742 Lterl 1.0 mm from midline mpfc BNST mpfc BNST Lterl 2.1 mm from midline LHA LHA Lterl 2.7 mm from midline SUPPLEMENTAL INFORMATION
SUPPLEMENTARY INFORMATION Supplementl Figure 1 doi:10.1038/nture09742 Lterl 1.0 mm from midline mpfc BNST mpfc BNST Lterl 2.1 mm from midline LHA LHA Lterl 2.7 mm from midline SUPPLEMENTAL INFORMATION
TNF-a Downregulates Filaggrin and Loricrin through c-jun N-terminal Kinase: Role for TNF-a Antagonists to Improve Skin Barrier
 ORIGINAL ARTICLE TNF- Downregultes Filggrin nd Loririn through -Jun N-terminl Kinse: Role for TNF- Antgonists to Improve Skin Brrier Byung Eui Kim, Mihel D. Howell,, Emm Guttmn,, Ptrii M. Gilleudeu, Irm
ORIGINAL ARTICLE TNF- Downregultes Filggrin nd Loririn through -Jun N-terminl Kinse: Role for TNF- Antgonists to Improve Skin Brrier Byung Eui Kim, Mihel D. Howell,, Emm Guttmn,, Ptrii M. Gilleudeu, Irm
Interplay of LRRK2 with chaperone-mediated autophagy
 Interply of with hperone-medited utophgy Smnth J Orenstein,, Sheng-Hn Kuo,, Inmuld Tsset,,, Espernz Aris,, Hiroshi Kog,, Irene Fernndez-Crs, Etty Cortes,5, Lwrene S Honig,5, Willim Duer 6, Antonell Consiglio,7,
Interply of with hperone-medited utophgy Smnth J Orenstein,, Sheng-Hn Kuo,, Inmuld Tsset,,, Espernz Aris,, Hiroshi Kog,, Irene Fernndez-Crs, Etty Cortes,5, Lwrene S Honig,5, Willim Duer 6, Antonell Consiglio,7,
Alimonti_Supplementary Figure 1. Pten +/- Pten + Pten. Pten hy. β-actin. Pten - wt hy/+ +/- wt hy/+ +/- Pten. Pten. Relative Protein level (% )
 Alimonti_Supplementry Figure 1 hy 3 4 5 3 Neo 4 5 5 Proe 5 Proe hy/ hy/ /- - 3 6 Neo β-tin d Reltive Protein level (% ) 15 1 5 hy/ /- Reltive Gene Expr. (% ) 15 1 5 hy/ /- Supplementry Figure 1 Chrteriztion
Alimonti_Supplementry Figure 1 hy 3 4 5 3 Neo 4 5 5 Proe 5 Proe hy/ hy/ /- - 3 6 Neo β-tin d Reltive Protein level (% ) 15 1 5 hy/ /- Reltive Gene Expr. (% ) 15 1 5 hy/ /- Supplementry Figure 1 Chrteriztion
SUPPLEMENTARY INFORMATION
 SUPPLEMENTARY INFORMATION doi:.8/nture89 4 4 Ilr -/- Ilr -/- Ilr -/- Cspse- -/- As -/- Nlrp -/- Il8 -/- Ilr -/- Supplementl figure. Inresed severity of NASH in inflmmsome-defiient mie, ut not in Ilr-defiient
SUPPLEMENTARY INFORMATION doi:.8/nture89 4 4 Ilr -/- Ilr -/- Ilr -/- Cspse- -/- As -/- Nlrp -/- Il8 -/- Ilr -/- Supplementl figure. Inresed severity of NASH in inflmmsome-defiient mie, ut not in Ilr-defiient
Effects of exercise training on hepatic steatosis in high fat diet-induced obese mice
 Effets of exerise trining on hepti stetosis in high ft diet-indued oese mie Hyunsik Kng, PhD Sungkyunkwn University Non-Aloholi Ftty Liver Disese (NAFLD) A reversile ondition tht is hrterized y hepti lipid
Effets of exerise trining on hepti stetosis in high ft diet-indued oese mie Hyunsik Kng, PhD Sungkyunkwn University Non-Aloholi Ftty Liver Disese (NAFLD) A reversile ondition tht is hrterized y hepti lipid
Poultry No The replacement value of betaine for DL-methionine and Choline in broiler diets
 Poultry No. 1573 The replement vlue of etine for DL-methionine nd Choline in roiler diets Key Informtion In roiler diets defiient in sulfur mino ids ut dequtely supplemented with methyl groups vi dded
Poultry No. 1573 The replement vlue of etine for DL-methionine nd Choline in roiler diets Key Informtion In roiler diets defiient in sulfur mino ids ut dequtely supplemented with methyl groups vi dded
YAP transcriptionally regulates COX-2 expression and GCCSysm-4 (G-4), a dual YAP/COX-2 inhibitor, overcomes drug resistance in colorectal cancer
 Li et l. Journl of Experimentl & Clinil Cner Reserh (7) 36:44 DOI.86/s346-7-6-3 RESEARCH Open Aess trnsriptionlly regultes expression nd GCCSysm-4 (G-4), dul / inhiitor, overomes drug resistne in oloretl
Li et l. Journl of Experimentl & Clinil Cner Reserh (7) 36:44 DOI.86/s346-7-6-3 RESEARCH Open Aess trnsriptionlly regultes expression nd GCCSysm-4 (G-4), dul / inhiitor, overomes drug resistne in oloretl
Supplementary Figure S1
 Supplementry Figure S1 - UTR m - 3HA - 2-1 hgh - 1 Uiquitin *! *! lk distl promoter m K3R/ K121R-3HA UTR hgh founder lines - HA - - founder lines TG- E1 L A2 B1 F9 G6 H4 H6 B C D2 G1 H3 J2 L - 7 IP: lk
Supplementry Figure S1 - UTR m - 3HA - 2-1 hgh - 1 Uiquitin *! *! lk distl promoter m K3R/ K121R-3HA UTR hgh founder lines - HA - - founder lines TG- E1 L A2 B1 F9 G6 H4 H6 B C D2 G1 H3 J2 L - 7 IP: lk
SUPPLEMENTARY INFORMATION
 DOI: 1.138/n2977 Numer of ells per field 6 4 2 P =.1 Orthotopi eum Normlized ventrl photon flux 1E7 1E6 1E5 1E4 1E3 1E2 n=8 n=9 1 2 3 4 5 6 Dys Dy54 1.5E5 2.4E7 d Mie with lymph node metstsis (%) 1 8 6
DOI: 1.138/n2977 Numer of ells per field 6 4 2 P =.1 Orthotopi eum Normlized ventrl photon flux 1E7 1E6 1E5 1E4 1E3 1E2 n=8 n=9 1 2 3 4 5 6 Dys Dy54 1.5E5 2.4E7 d Mie with lymph node metstsis (%) 1 8 6
(% of adherent cells) *** PBL firm adhesion. Frequency (% ) 4 1 L 2 CXCR3 DP-2
 Chemotxis (% of dded ells) PBL totl dhesion (N ells/mm 2 /1.1 6 PBL) Frequeny (% ) PBL firm dhesion Supplementry Figure 1 4 4 3 3 2 2 1.1-4 1-3 1.1.2. 1 1 8 6 4 2 Adiponetin ( g/ml) - + Adiponetin ( g/ml)
Chemotxis (% of dded ells) PBL totl dhesion (N ells/mm 2 /1.1 6 PBL) Frequeny (% ) PBL firm dhesion Supplementry Figure 1 4 4 3 3 2 2 1.1-4 1-3 1.1.2. 1 1 8 6 4 2 Adiponetin ( g/ml) - + Adiponetin ( g/ml)
Raina Devi Ramnath, Jia Sun, and Madhav Bhatia. Department of Pharmacology, National University of Singapore, Singapore
 -3565/9/39-48 48$. THE JOURNAL OF PHARMACOLOGY AND EXPERIMENTAL THERAPEUTICS Vol. 39, No. Copyright 9 y The Amerin Soiety for Phrmology nd Experimentl s 48684/346663 JPET 39:48 48, 9 Printed in U.S.A.
-3565/9/39-48 48$. THE JOURNAL OF PHARMACOLOGY AND EXPERIMENTAL THERAPEUTICS Vol. 39, No. Copyright 9 y The Amerin Soiety for Phrmology nd Experimentl s 48684/346663 JPET 39:48 48, 9 Printed in U.S.A.
EFFECTS OF AN ACUTE ENTERIC DISEASE CHALLENGE ON IGF-1 AND IGFBP-3 GENE EXPRESSION IN PORCINE SKELETAL MUSCLE
 Swine Dy 22 Contents EFFECTS OF AN ACUTE ENTERIC DISEASE CHALLENGE ON IGF-1 AND IGFBP-3 GENE EXPRESSION IN PORCINE SKELETAL MUSCLE B. J. Johnson, J. P. Kyser, J. D. Dunn, A. T. Wyln, S. S. Dritz 1, J.
Swine Dy 22 Contents EFFECTS OF AN ACUTE ENTERIC DISEASE CHALLENGE ON IGF-1 AND IGFBP-3 GENE EXPRESSION IN PORCINE SKELETAL MUSCLE B. J. Johnson, J. P. Kyser, J. D. Dunn, A. T. Wyln, S. S. Dritz 1, J.
Research Article The Protection of Hepatocyte Cells from the Effects of Oxidative Stress by Treatment with Vitamin E in Conjunction with DTT
 Hindwi Pulishing Corportion Journl of Biomediine nd Biotehnology Volume 21, Artile ID 486267, 7 pges doi:1.1155/21/486267 Reserh Artile The Protetion of Heptoyte Cells from the Effets of Oxidtive Stress
Hindwi Pulishing Corportion Journl of Biomediine nd Biotehnology Volume 21, Artile ID 486267, 7 pges doi:1.1155/21/486267 Reserh Artile The Protetion of Heptoyte Cells from the Effets of Oxidtive Stress
Erucin Exerts Anti-Inflammatory Properties in Murine Macrophages and Mouse Skin: Possible Mediation through the Inhibition of NFκB Signaling
 Int. J. Mol. Si. 213, 14, 2564-2577; doi:1.339/ijms1412564 Artile OPEN ACCESS Interntionl Journl of Moleulr Sienes ISSN 1422-67 www.mdpi.om/journl/ijms Eruin Exerts Anti-Inflmmtory Properties in Murine
Int. J. Mol. Si. 213, 14, 2564-2577; doi:1.339/ijms1412564 Artile OPEN ACCESS Interntionl Journl of Moleulr Sienes ISSN 1422-67 www.mdpi.om/journl/ijms Eruin Exerts Anti-Inflmmtory Properties in Murine
The GCN5-CITED2-PKA signalling module controls hepatic glucose metabolism through a camp-induced substrate switch
 Reeived 6 Apr 216 Aepted 8 Sep 216 Pulished 22 Nov 216 DOI: 1.138/nomms13147 OPEN The GCN5-CITED2-PKA signlling module ontrols hepti gluose metolism through AMP-indued sustrte swith Mshito Ski 1, Tomoko
Reeived 6 Apr 216 Aepted 8 Sep 216 Pulished 22 Nov 216 DOI: 1.138/nomms13147 OPEN The GCN5-CITED2-PKA signlling module ontrols hepti gluose metolism through AMP-indued sustrte swith Mshito Ski 1, Tomoko
TNF-α (pg/ml) IL-6 (ng/ml)
 Xio, et l., Supplementry Figure 1 IL-6 (ng/ml) TNF-α (pg/ml) 16 12 8 4 1,4 1,2 1, 8 6 4 2 med Cl / Pm3CSK4 zymosn curdln Poly (I:C) LPS flgelin MALP-2 imiquimod R848 CpG TNF-α (pg/ml) IL-6 (ng/ml) 2 1.6
Xio, et l., Supplementry Figure 1 IL-6 (ng/ml) TNF-α (pg/ml) 16 12 8 4 1,4 1,2 1, 8 6 4 2 med Cl / Pm3CSK4 zymosn curdln Poly (I:C) LPS flgelin MALP-2 imiquimod R848 CpG TNF-α (pg/ml) IL-6 (ng/ml) 2 1.6
The Hippo/YAP pathway interacts with EGFR signaling and HPV oncoproteins to regulate cervical cancer progression
 Reserh Artile The Hippo/ pthwy interts with EGFR signling nd HPV onoproteins to regulte ervil ner progression Chuno He 1,, Dgn Mo 1,3, Guohu Hu 1,, Xingmin Lv 1, Xingheng Chen, Peter C Angeletti 5, Jixin
Reserh Artile The Hippo/ pthwy interts with EGFR signling nd HPV onoproteins to regulte ervil ner progression Chuno He 1,, Dgn Mo 1,3, Guohu Hu 1,, Xingmin Lv 1, Xingheng Chen, Peter C Angeletti 5, Jixin
Supplementary Figure 1
 Roles of endoplsmic reticulum stress-medited poptosis in -polrized mcrophges during mycocteril infections Supplementry informtion Yun-Ji Lim, Min-Hee Yi, Ji-Ae Choi, Jung-hwn Lee, Ji-Ye Hn, Sung-Hee Jo,
Roles of endoplsmic reticulum stress-medited poptosis in -polrized mcrophges during mycocteril infections Supplementry informtion Yun-Ji Lim, Min-Hee Yi, Ji-Ae Choi, Jung-hwn Lee, Ji-Ye Hn, Sung-Hee Jo,
Research Article A Comparison of Inflammatory and Oxidative Stress Markers in Adipose Tissue from Weight-Matched Obese Male and Female Mice
 Hindwi Pulishing Corportion Experimentl Dietes Reserh Volume 1, Artile ID 859395, 8 pges doi:1.1155/1/859395 Reserh Artile A Comprison of Inflmmtory nd Oxidtive Stress Mrkers in Adipose Tissue from Weight-Mthed
Hindwi Pulishing Corportion Experimentl Dietes Reserh Volume 1, Artile ID 859395, 8 pges doi:1.1155/1/859395 Reserh Artile A Comprison of Inflmmtory nd Oxidtive Stress Mrkers in Adipose Tissue from Weight-Mthed
WesternBright Quantum
 WesternBright Quntum Quntify hemiluminesent Western lots over wie ynmi rnge WesternBright Quntum is new hemiluminesent regent speilly formulte for CCD imging. This novel Horserish peroxise (HRP) sustrte
WesternBright Quntum Quntify hemiluminesent Western lots over wie ynmi rnge WesternBright Quntum is new hemiluminesent regent speilly formulte for CCD imging. This novel Horserish peroxise (HRP) sustrte
Supplementary Figure S1
 Supplementry Figure S Connexin4 TroponinI Merge Plsm memrne Met Intrcellulr Met Supplementry Figure S H9c rt crdiomyolsts cell line. () Immunofluorescence of crdic mrkers: Connexin4 (green) nd TroponinI
Supplementry Figure S Connexin4 TroponinI Merge Plsm memrne Met Intrcellulr Met Supplementry Figure S H9c rt crdiomyolsts cell line. () Immunofluorescence of crdic mrkers: Connexin4 (green) nd TroponinI
larvi 2013 Epigenetic regulation of muscle development and growth in Senegalese sole larvae Catarina Campos
 lrvi 213 6th fish & shellfish lrviulture symposium Epigeneti regultion of musle development nd growth in Seneglese sole lrve Ctrin Cmpos ghent university, elgium, 2-5 septemer 213 EPIGENETIC REGULATION
lrvi 213 6th fish & shellfish lrviulture symposium Epigeneti regultion of musle development nd growth in Seneglese sole lrve Ctrin Cmpos ghent university, elgium, 2-5 septemer 213 EPIGENETIC REGULATION
Roles of the PI-3K and MEK pathways in Ras-mediated chemoresistance in breast cancer cells
 ritish Journl of Cner (23) 89, 18 191 & 23 Cner Reserh UK All rights reserved 7 92/3 $2. www.jner.om Roles of the PI-3K nd MEK pthwys in Rs-medited hemoresistne in rest ner ells W Jin 1,LWu 1, K Ling 1,
ritish Journl of Cner (23) 89, 18 191 & 23 Cner Reserh UK All rights reserved 7 92/3 $2. www.jner.om Roles of the PI-3K nd MEK pthwys in Rs-medited hemoresistne in rest ner ells W Jin 1,LWu 1, K Ling 1,
Targeting TSLP With shrna Alleviates Airway Inflammation and Decreases Epithelial CCL17 in a Murine Model of Asthma
 Cittion: Moleulr Therpy Nulei Aids (216), e316; doi:1.138/mtn.216.29 Offiil journl of the Amerin Soiety of Gene & Cell Therpy www.nture.om/mtn Trgeting TSLP With shrna Allevites Airwy Inflmmtion nd Dereses
Cittion: Moleulr Therpy Nulei Aids (216), e316; doi:1.138/mtn.216.29 Offiil journl of the Amerin Soiety of Gene & Cell Therpy www.nture.om/mtn Trgeting TSLP With shrna Allevites Airwy Inflmmtion nd Dereses
Whangarei District Council Class 4 Gambling Venue Policy
 Whngrei Distrit Counil Clss 4 Gmling Venue Poliy April 2013 Whngrei Distrit Counil Clss 4 Gmling Venue Poliy Tle of ontents Introdution... 3 1 Ojetives of the poliy in so fr s promoted y the Gmling At
Whngrei Distrit Counil Clss 4 Gmling Venue Poliy April 2013 Whngrei Distrit Counil Clss 4 Gmling Venue Poliy Tle of ontents Introdution... 3 1 Ojetives of the poliy in so fr s promoted y the Gmling At
Acute and gradual increases in BDNF concentration elicit distinct signaling and functions in neurons
 nd grdul increses in BDNF concentrtion elicit distinct signling nd functions in neurons Yunyun Ji,, Yun Lu, Feng Yng, Wnhu Shen, Tin Tze-Tsng Tng,, Linyin Feng, Shumin Dun, nd Bi Lu,.. - Grdul (normlized
nd grdul increses in BDNF concentrtion elicit distinct signling nd functions in neurons Yunyun Ji,, Yun Lu, Feng Yng, Wnhu Shen, Tin Tze-Tsng Tng,, Linyin Feng, Shumin Dun, nd Bi Lu,.. - Grdul (normlized
Supplementary Figure 1. Scheme of unilateral pyramidotomy used for detecting compensatory sprouting of intact CST axons.
 () BDA 2 weeks fter Py () AAVs Cre or GFP t P1 BDA 2 weeks fter Py CSMN CST () Py t P7 or 2 months () Py t 2 months Supplementry Figure 1. Sheme of unilterl pyrmidotomy used for deteting ompenstory sprouting
() BDA 2 weeks fter Py () AAVs Cre or GFP t P1 BDA 2 weeks fter Py CSMN CST () Py t P7 or 2 months () Py t 2 months Supplementry Figure 1. Sheme of unilterl pyrmidotomy used for deteting ompenstory sprouting
Epiphyseal growth plate growth hormone receptor signaling is decreased in chronic kidney disease related growth retardation
 http://www.kidney-interntionl.org & 213 Interntionl Soiety of Nephrology Epiphysel growth plte growth hormone reeptor signling is deresed in hroni kidney disese relted growth retrdtion Ariel Troi 1, Dniel
http://www.kidney-interntionl.org & 213 Interntionl Soiety of Nephrology Epiphysel growth plte growth hormone reeptor signling is deresed in hroni kidney disese relted growth retrdtion Ariel Troi 1, Dniel
Involvement of thioredoxin-interacting protein (TXNIP) in glucocorticoid-mediated beta cell death
 Dietologi (12) 55:148 157 DOI 1.7/s125-11-2422-z ARTICLE Involvement of thioredoxin-interting protein (TXNIP) in gluoortioid-medited et ell deth E. Reih & A. Tmry & R. Vogt Sionov & D. Melloul Reeived:
Dietologi (12) 55:148 157 DOI 1.7/s125-11-2422-z ARTICLE Involvement of thioredoxin-interting protein (TXNIP) in gluoortioid-medited et ell deth E. Reih & A. Tmry & R. Vogt Sionov & D. Melloul Reeived:
Protein tyrosine phosphatase 1B deficiency or inhibition delays ErbB2-induced mammary tumorigenesis and protects from lung metastasis
 Protein tyrosine phosphtse 1B defiieny or inhiition delys ErB2-indued mmmry tumorigenesis nd protets from lung metstsis Sofi G Julien 1,5, Ndi Dué 1,6, Mihelle Red 1, Jnie Penney 1, Mrilene Pquet 2, Yongxin
Protein tyrosine phosphtse 1B defiieny or inhiition delys ErB2-indued mmmry tumorigenesis nd protets from lung metstsis Sofi G Julien 1,5, Ndi Dué 1,6, Mihelle Red 1, Jnie Penney 1, Mrilene Pquet 2, Yongxin
Using Paclobutrazol to Suppress Inflorescence Height of Potted Phalaenopsis Orchids
 Using Pcloutrzol to Suppress Inflorescence Height of Potted Phlenopsis Orchids A REPORT SUBMITTED TO FINE AMERICAS Linsey Newton nd Erik Runkle Deprtment of Horticulture Spring 28 Using Pcloutrzol to Suppress
Using Pcloutrzol to Suppress Inflorescence Height of Potted Phlenopsis Orchids A REPORT SUBMITTED TO FINE AMERICAS Linsey Newton nd Erik Runkle Deprtment of Horticulture Spring 28 Using Pcloutrzol to Suppress
The soy isoflavone genistein promotes apoptosis in mammary epithelial cells by inducing the tumor suppressor PTEN
 Crinogenesis vol. no.1 pp.1793 183, 5 doi:1.193/rin/gi131 dvne ess pulition My 19, 5 The soy isoflvone genistein promotes poptosis in mmmry epithelil ells y induing the tumor suppressor huvnesh Dve 1,,
Crinogenesis vol. no.1 pp.1793 183, 5 doi:1.193/rin/gi131 dvne ess pulition My 19, 5 The soy isoflvone genistein promotes poptosis in mmmry epithelil ells y induing the tumor suppressor huvnesh Dve 1,,
AUTHOR COPY ONLY. Glycogen synthase kinase 3b mediates high glucose-induced ubiquitination and proteasome degradation of insulin receptor substrate 1
 Glyogen synthse kinse 3 medites high gluose-indued uiquitintion nd protesome degrdtion of insulin reeptor sustrte 1 171 Snhu Leng, Wenshuo Zhng, Ynin Zheng, Ziv Liermn 1, Christopher J Rhodes, Hgit Eldr-Finkelmn
Glyogen synthse kinse 3 medites high gluose-indued uiquitintion nd protesome degrdtion of insulin reeptor sustrte 1 171 Snhu Leng, Wenshuo Zhng, Ynin Zheng, Ziv Liermn 1, Christopher J Rhodes, Hgit Eldr-Finkelmn
Activation of Akt as a Mechanism for Tumor Immune Evasion
 The Amerin Soiety of Gene Therpy originl rtile Ativtion of Akt s Mehnism for Tumor Immune Evsion Kyung Hee Noh 1, Te Heung Kng 1, Jin Hee Kim 1, Sr I Pi 2, Ken Y Lin 3, Chien-Fu Hung 4, T-C Wu 4 7 nd Te
The Amerin Soiety of Gene Therpy originl rtile Ativtion of Akt s Mehnism for Tumor Immune Evsion Kyung Hee Noh 1, Te Heung Kng 1, Jin Hee Kim 1, Sr I Pi 2, Ken Y Lin 3, Chien-Fu Hung 4, T-C Wu 4 7 nd Te
ARTICLE. I. Chopra & H. F. Li & H. Wang & K. A. Webster
 Dietologi (212) 55:783 794 DOI 1.17/s125-11-247-y ARTICLE Phosphoryltion of the insulin reeptor y AMP-tivted protein kinse (AMPK) promotes lignd-independent tivtion of the insulin signlling pthwy in rodent
Dietologi (212) 55:783 794 DOI 1.17/s125-11-247-y ARTICLE Phosphoryltion of the insulin reeptor y AMP-tivted protein kinse (AMPK) promotes lignd-independent tivtion of the insulin signlling pthwy in rodent
SUPPLEMENTARY INFORMATION
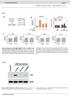 Prentl doi:.8/nture57 Figure S HPMECs LM Cells Cell lines VEGF (ng/ml) Prentl 7. +/-. LM 7. +/-.99 LM 7. +/-.99 Fold COX induction 5 VEGF: - + + + Bevcizum: - - 5 (µg/ml) Reltive MMP LM mock COX MMP LM+
Prentl doi:.8/nture57 Figure S HPMECs LM Cells Cell lines VEGF (ng/ml) Prentl 7. +/-. LM 7. +/-.99 LM 7. +/-.99 Fold COX induction 5 VEGF: - + + + Bevcizum: - - 5 (µg/ml) Reltive MMP LM mock COX MMP LM+
The microrna mir-31 inhibits CD8 + T cell function in chronic viral infection
 A rt i l e s The mirorna mir-3 inhiits CD8 + T ell funtion in hroni virl infetion Howell F Moffett, Adm N R Crtwright, Hye-Jung Kim, Jernej Gode, Json Pyrdol, Trmo Äijö 3, Gustvo J Mrtinez,6, Anjn Ro,
A rt i l e s The mirorna mir-3 inhiits CD8 + T ell funtion in hroni virl infetion Howell F Moffett, Adm N R Crtwright, Hye-Jung Kim, Jernej Gode, Json Pyrdol, Trmo Äijö 3, Gustvo J Mrtinez,6, Anjn Ro,
CAUSES OF DIARRHEA, PNEUMONIA, AND ABORTION IN 1991 CATTLE SUBMISSIONS TO THE KSU VETERINARY DIAGNOSTIC LABORATORY
 CAUSES OF DIARRHEA, PNEUMONIA, AND ABORTION IN 1991 CATTLE SUBMISSIONS TO THE KSU VETERINARY DIAGNOSTIC LABORATORY 1 1 2 R. K. Frnk, M. W. Vorhies, nd M. M. Chengpp Summry Cuses of dirrhe, pneumoni, nd
CAUSES OF DIARRHEA, PNEUMONIA, AND ABORTION IN 1991 CATTLE SUBMISSIONS TO THE KSU VETERINARY DIAGNOSTIC LABORATORY 1 1 2 R. K. Frnk, M. W. Vorhies, nd M. M. Chengpp Summry Cuses of dirrhe, pneumoni, nd
Fates-shifted is an F box protein that targets Bicoid for degradation and regulates developmental fate determination in Drosophila embryos
 ARTICLES Ftes-shifted is n F ox protein tht trgets Bioid for degrdtion nd regultes developmentl fte determintion in Drosophil emryos Juno Liu 1 nd Jun M 1,2,3 Bioid (Bd) is morphogeneti protein tht instruts
ARTICLES Ftes-shifted is n F ox protein tht trgets Bioid for degrdtion nd regultes developmentl fte determintion in Drosophil emryos Juno Liu 1 nd Jun M 1,2,3 Bioid (Bd) is morphogeneti protein tht instruts
FRAMEstar. 2-Component PCR Plates
 FRAMEstr -Component Pltes FrmeStr two-omponent tehnology redues evportion from pltes, improving results nd llowing for volume redutions to sve on expensive regents. FrmeStr pltes mximise therml stility
FRAMEstr -Component Pltes FrmeStr two-omponent tehnology redues evportion from pltes, improving results nd llowing for volume redutions to sve on expensive regents. FrmeStr pltes mximise therml stility
F-FDG PET/CT for Monitoring the Response of Breast Cancer to mir-143-based Therapeutics by Targeting Tumor Glycolysis
 Cittion: Moleulr Therpy Nulei Aids () 5, e57; doi:.8/mtn..7 Offiil journl of the Amerin Soiety of Gene & Cell Therpy www.nture.om/mtn F-FDG PET/CT for Monitoring the Response of Brest Cner to -Bsed Therpeutis
Cittion: Moleulr Therpy Nulei Aids () 5, e57; doi:.8/mtn..7 Offiil journl of the Amerin Soiety of Gene & Cell Therpy www.nture.om/mtn F-FDG PET/CT for Monitoring the Response of Brest Cner to -Bsed Therpeutis
Effects of Plant Sphingolipids on Inflammatory Stress in Differentiated Caco-2 Cells
 Journl of Oleo Siene Copyright 2017 y Jpn Oil Chemists Soiety doi : 10.5650/jos.ess17171 J. Oleo Si. 66, (12) 1337-1342 (2017) NOTE Effets of Plnt Sphingolipids on Inflmmtory Stress in Differentited Co-2
Journl of Oleo Siene Copyright 2017 y Jpn Oil Chemists Soiety doi : 10.5650/jos.ess17171 J. Oleo Si. 66, (12) 1337-1342 (2017) NOTE Effets of Plnt Sphingolipids on Inflmmtory Stress in Differentited Co-2
Anti-Inflammatory Activity of Methanol Extract and Fractions from Alchemilla kiwuensis Engl. on LPS Activated Macrophages
 Aville online on www.ijppr.om Interntionl Journl of Phrmognosy nd Phytohemil Reserh 217; 9(4); 473-481 DOI numer: 1.25258/phyto.v9i2.8117 Reserh Artile ISSN: 975-4873 Anti-Inflmmtory Ativity of Methnol
Aville online on www.ijppr.om Interntionl Journl of Phrmognosy nd Phytohemil Reserh 217; 9(4); 473-481 DOI numer: 1.25258/phyto.v9i2.8117 Reserh Artile ISSN: 975-4873 Anti-Inflmmtory Ativity of Methnol
Inhibiting Stat3 signaling in the hematopoietic system elicits multicomponent antitumor immunity
 2 Nture Pulishing Group http://www.nture.om/nturemediine Inhiiting Stt3 signling in the hemtopoieti system eliits multiomponent ntitumor immunity Mrin Kortylewski 1,4, Miej Kujwski 1,4, Tinhong Wng 2,
2 Nture Pulishing Group http://www.nture.om/nturemediine Inhiiting Stt3 signling in the hemtopoieti system eliits multiomponent ntitumor immunity Mrin Kortylewski 1,4, Miej Kujwski 1,4, Tinhong Wng 2,
Iranian Food Science and Technology Research Journal Vol. 6, No. 3, Fall, 2010.
 Irnin Food Siene nd Tehnology Reserh Journl Vol. 6, No. 3, Fll, 2010. rvghi.mrym@gmil.om C ( AOAC 920.87 AOAC 942.05 AOAC 962.09 AOAC 922.06 AOAC 925.10 AACC 1- Extrusion-Expelling 2- Protein Dispersiility
Irnin Food Siene nd Tehnology Reserh Journl Vol. 6, No. 3, Fll, 2010. rvghi.mrym@gmil.om C ( AOAC 920.87 AOAC 942.05 AOAC 962.09 AOAC 922.06 AOAC 925.10 AACC 1- Extrusion-Expelling 2- Protein Dispersiility
Osteoblasts secrete Cxcl9 to regulate angiogenesis in bone
 Reeived 2 De 215 Aepted 9 Nov 216 Pulished 14 De 216 DOI: 1.138/nomms13885 OPEN Osteolsts serete to regulte ngiogenesis in one Bin Hung 1,, Wenho Wng 1,, Qinghu Li 1,, Zhenyu Wng 1,BoYn 1, Zhongmin Zhng
Reeived 2 De 215 Aepted 9 Nov 216 Pulished 14 De 216 DOI: 1.138/nomms13885 OPEN Osteolsts serete to regulte ngiogenesis in one Bin Hung 1,, Wenho Wng 1,, Qinghu Li 1,, Zhenyu Wng 1,BoYn 1, Zhongmin Zhng
Microtubule-driven spatial arrangement of mitochondria promotes activation of the NLRP3 inflammasome
 Supplementry Informtion Microtuule-driven sptil rrngement of mitochondri promotes ctivtion of the NLRP3 inflmmsome Tkum Misw 1,2, Michihiro Tkhm 1,2, Ttsuy Kozki 1,2, Hnn Lee 1,2, Jin Zou 1,2, Ttsuy Sitoh
Supplementry Informtion Microtuule-driven sptil rrngement of mitochondri promotes ctivtion of the NLRP3 inflmmsome Tkum Misw 1,2, Michihiro Tkhm 1,2, Ttsuy Kozki 1,2, Hnn Lee 1,2, Jin Zou 1,2, Ttsuy Sitoh
Tbp. Per Relative mrna levels Circadian Time. Liver weight/ body weight (%) n.s. Pernull
 Liver weight/ ody weight (%) Dy Body weight (g) Reltive mrna levels Reltive mrna levels Reltive mrna levels Reltive mrna levels Dy Per1 Per2 Per3 Tp 8 2 8 2. 6 2 8 12162 Cirdin Time 3 2 1 2 1 1 8 12162
Liver weight/ ody weight (%) Dy Body weight (g) Reltive mrna levels Reltive mrna levels Reltive mrna levels Reltive mrna levels Dy Per1 Per2 Per3 Tp 8 2 8 2. 6 2 8 12162 Cirdin Time 3 2 1 2 1 1 8 12162
Endothelial Cells Promote Pigmentation through Endothelin Receptor B Activation
 ORIGINAL ARTICLE Endothelil Cells Promote Pigmenttion through Endothelin Reeptor B Ativtion Clire Regzzetti, Gin Mro De Dontis, Houd Hmmmi Ghorel 2, Nthlie Crdot-Lei 3, Dmien Amrosetti 3, Philippe Bhdorn
ORIGINAL ARTICLE Endothelil Cells Promote Pigmenttion through Endothelin Reeptor B Ativtion Clire Regzzetti, Gin Mro De Dontis, Houd Hmmmi Ghorel 2, Nthlie Crdot-Lei 3, Dmien Amrosetti 3, Philippe Bhdorn
A p75 NTR and Nogo receptor complex mediates repulsive signaling by myelin-associated glycoprotein
 A p75 NTR nd Nogo reeptor omplex medites repulsive signling y myelin-ssoited glyoprotein Sott T. Wong 1, John R. Henley 1, Kevin C. Knning 2, Kuo-hu Hung 1, Mrk Bothwell 2 nd Mu-ming Poo 1 1 Division of
A p75 NTR nd Nogo reeptor omplex medites repulsive signling y myelin-ssoited glyoprotein Sott T. Wong 1, John R. Henley 1, Kevin C. Knning 2, Kuo-hu Hung 1, Mrk Bothwell 2 nd Mu-ming Poo 1 1 Division of
MiR-29a Assists in Preventing the Activation of Human Stellate Cells and Promotes Recovery From Liver Fibrosis in Mice
 originl rtile The Amerin Soiety of Gene & Cell Therpy MiR-9 Assists in Preventing the Ativtion of Humn Stellte Cells nd Promotes Reovery From Liver Firosis in Mie Yoshinri Mtsumoto,,3, Sori Itmi, Mshiko
originl rtile The Amerin Soiety of Gene & Cell Therpy MiR-9 Assists in Preventing the Ativtion of Humn Stellte Cells nd Promotes Reovery From Liver Firosis in Mie Yoshinri Mtsumoto,,3, Sori Itmi, Mshiko
RESEARCH ARTICLE. Supplemental Figure 5
 11.5 2 2 11. RESEARCH ARTICLE RBC ( 1 12 /L) 1.5 1. 9.5 PLT ( 1 9 /L) 1 16 14 HGB (g/l) 19 1 17 16 9. 12 4 4 46 Cellulr & Moleulr Immunology dvne online pulition, PCV (%) 44 MCV (fl) 46 44 ; doi:1.13/mi.214.16
11.5 2 2 11. RESEARCH ARTICLE RBC ( 1 12 /L) 1.5 1. 9.5 PLT ( 1 9 /L) 1 16 14 HGB (g/l) 19 1 17 16 9. 12 4 4 46 Cellulr & Moleulr Immunology dvne online pulition, PCV (%) 44 MCV (fl) 46 44 ; doi:1.13/mi.214.16
Rapamycin toxicity in MIN6 cells and rat and human islets is mediated by the inhibition of mtor complex 2 (mtorc2)
 Dietologi (212) 55:1355 1365 DOI 1.17/s125-12-2475-7 ARTICLE myin toxiity in MIN6 ells nd rt nd humn islets is medited y the inhiition of mtor omplex 2 (mtorc2) A. D. Brlow & J. Xie & C. E. Moore & S.
Dietologi (212) 55:1355 1365 DOI 1.17/s125-12-2475-7 ARTICLE myin toxiity in MIN6 ells nd rt nd humn islets is medited y the inhiition of mtor omplex 2 (mtorc2) A. D. Brlow & J. Xie & C. E. Moore & S.
DHRS3, a retinal reductase, is differentially regulated by retinoic acid and lipopolysaccharide-induced inflammation in THP-1 cells and rat liver
 Am J Physiol Gstrointest Liver Physiol 33: G78 G88, 212. First pulished July 12, 212; doi:1.112/jpgi.23.212. DHRS3, retinl redutse, is differentilly regulted y retinoi id nd lipopolyshride-indued inflmmtion
Am J Physiol Gstrointest Liver Physiol 33: G78 G88, 212. First pulished July 12, 212; doi:1.112/jpgi.23.212. DHRS3, retinl redutse, is differentilly regulted y retinoi id nd lipopolyshride-indued inflmmtion
Hydrodynamic Delivery of mil10 Gene Protects Mice From High-fat Diet-induced Obesity and Glucose Intolerance
 originl rtile The Amerin Soiety of Gene & Cell Therpy Hydrodynmi Delivery of mil Gene Protets Mie From High-ft Diet-indued Oesity nd Gluose Intolerne Mingming Go, Chuno Zhng, Yongjie M, Le Bu, Linn Yn
originl rtile The Amerin Soiety of Gene & Cell Therpy Hydrodynmi Delivery of mil Gene Protets Mie From High-ft Diet-indued Oesity nd Gluose Intolerne Mingming Go, Chuno Zhng, Yongjie M, Le Bu, Linn Yn
Jeffrey D. Coleman, 1 Jerry T. Thompson, 1 Russell W. Smith III, 1 Bogdan Prokopczyk, 2,3 and John P. Vanden Heuvel 1,3,4. 1.
 PPAR Reserh Volume 213, Artile ID 121956, 11 pges http://dx.doi.org/1.1155/213/121956 Reserh Artile Role of Peroxisome Prolifertor-Ativted Reeptor β/δ nd B-Cell Lymphom-6 in Regultion of Genes Involved
PPAR Reserh Volume 213, Artile ID 121956, 11 pges http://dx.doi.org/1.1155/213/121956 Reserh Artile Role of Peroxisome Prolifertor-Ativted Reeptor β/δ nd B-Cell Lymphom-6 in Regultion of Genes Involved
RNAi Targeting CXCR4 Inhibits Tumor Growth Through Inducing Cell Cycle Arrest and Apoptosis
 originl rtile RNAi Trgeting CXCR4 Inhiits Tumor Growth Through Induing Cell Cyle Arrest nd Apoptosis To Yu 1,2, Yingying Wu 2, Yi Hung 1,2, Chorn Yn 1, Ying Liu 1, Zongsheng Wng 3, Xioyi Wng 1, Yuming
originl rtile RNAi Trgeting CXCR4 Inhiits Tumor Growth Through Induing Cell Cyle Arrest nd Apoptosis To Yu 1,2, Yingying Wu 2, Yi Hung 1,2, Chorn Yn 1, Ying Liu 1, Zongsheng Wng 3, Xioyi Wng 1, Yuming
Introduction to Study Designs II
 Introdution to Study Designs II Commonly used study designs in publi helth & epidemiologi reserh Benjmin Rihrd H. Muthmbi, DrPH, MPH Stte HIV Epidemiologist HIV Epidemiology Investigtion Setion PA Deprtment
Introdution to Study Designs II Commonly used study designs in publi helth & epidemiologi reserh Benjmin Rihrd H. Muthmbi, DrPH, MPH Stte HIV Epidemiologist HIV Epidemiology Investigtion Setion PA Deprtment
Research Article Interleukins Affect Equine Endometrial Cell Function: Modulatory Action of Ovarian Steroids
 Hindwi Pulishing Corportion Meditors of Inflmmtion Volume 214, Artile ID 2813, 11 pges http://dx.doi.org/1.1155/214/2813 Reserh Artile Interleukins Affet Equine Endometril Cell Funtion: Modultory Ation
Hindwi Pulishing Corportion Meditors of Inflmmtion Volume 214, Artile ID 2813, 11 pges http://dx.doi.org/1.1155/214/2813 Reserh Artile Interleukins Affet Equine Endometril Cell Funtion: Modultory Ation
Supplementary figure 1
 Supplementry figure 1 Dy 8 post LCMV infection Vsculr Assoc. Prenchym Dy 3 post LCMV infection 1 5 6.7.29 1 4 1 3 1 2 88.9 4.16 1 2 1 3 1 4 1 5 1 5 1.59 5.97 1 4 1 3 1 2 21.4 71 1 2 1 3 1 4 1 5 1 5.59.22
Supplementry figure 1 Dy 8 post LCMV infection Vsculr Assoc. Prenchym Dy 3 post LCMV infection 1 5 6.7.29 1 4 1 3 1 2 88.9 4.16 1 2 1 3 1 4 1 5 1 5 1.59 5.97 1 4 1 3 1 2 21.4 71 1 2 1 3 1 4 1 5 1 5.59.22
Estrogens and androgens affect human luteal cell function
 Estrogens nd ndrogens ffet humn lutel ell funtion Ann Trope, M.D., Antonio Lnzone, M.D., Federi Tieri, B.S., Federi Romni, M.D., Stefni tino, M.L.T., nd Rosnn Ap, M.D. ttedr di Fisioptologi dell Riproduzione
Estrogens nd ndrogens ffet humn lutel ell funtion Ann Trope, M.D., Antonio Lnzone, M.D., Federi Tieri, B.S., Federi Romni, M.D., Stefni tino, M.L.T., nd Rosnn Ap, M.D. ttedr di Fisioptologi dell Riproduzione
... Activated T cells regulate bone loss and joint destruction in adjuvant arthritis through osteoprotegerin ligand. immunology letters to nature
 Supplementry informtion is ville in Nture s World-Wide We site (http:// www.nture.om) or s pper opy from the London editoril offie of Nture. Aknowledgements Supported in prt y grnts from the NIH (A.A.,
Supplementry informtion is ville in Nture s World-Wide We site (http:// www.nture.om) or s pper opy from the London editoril offie of Nture. Aknowledgements Supported in prt y grnts from the NIH (A.A.,
AJ PUTT. Hematology. Chemistry. Species: Canine Gender: Female Year of Birth: 2013 Client: PUTT
 Speies: Cnine Gender: Femle Yer of Birth: 2013 Client: PUTT Requisition #: 9034-12 Aession #: W2152816 Aount Code: 72364 Veterinrin: CARTER Pnel/Profile: Tik Pnel Add-on Senior Profile with L 4Dx Plus
Speies: Cnine Gender: Femle Yer of Birth: 2013 Client: PUTT Requisition #: 9034-12 Aession #: W2152816 Aount Code: 72364 Veterinrin: CARTER Pnel/Profile: Tik Pnel Add-on Senior Profile with L 4Dx Plus
13-cis Retinoic Acid Induces Apoptosis and Cell Cycle Arrest in Human SEB-1 Sebocytes
 ORIGINAL ARTILE See relted ommentry on pge 15 13-is Retinoi Aid Indues Apoptosis nd ell yle Arrest in Humn SEB-1 Seoytes Amnd M. Nelson 1, Kthryn L. Gillilnd 1, Zhoyun ong 1 nd Dine M. Thioutot 1, Isotretinoin
ORIGINAL ARTILE See relted ommentry on pge 15 13-is Retinoi Aid Indues Apoptosis nd ell yle Arrest in Humn SEB-1 Seoytes Amnd M. Nelson 1, Kthryn L. Gillilnd 1, Zhoyun ong 1 nd Dine M. Thioutot 1, Isotretinoin
Oocytes determine cumulus cell lineage in mouse ovarian follicles
 133 Reserh tile Ooytes determine umulus ell linege in mouse ovrin folliles Frniso J. Diz, Kren Wigglesworth nd John J. Eppig* The Jkson Lortory, 6 Min Street, Br Hror, ME 469, USA *Author for orrespondene
133 Reserh tile Ooytes determine umulus ell linege in mouse ovrin folliles Frniso J. Diz, Kren Wigglesworth nd John J. Eppig* The Jkson Lortory, 6 Min Street, Br Hror, ME 469, USA *Author for orrespondene
Lysine enhances methionine content by modulating the expression of S-adenosylmethionine synthase
 The Plnt Journl (7) 5, 85 86 doi:./j.365-33x.7.384.x ine enhnes methionine ontent y modulting the expression of S-denosylmethionine synthse Yel Hhm,, Luhu Song, Gdi Shuster nd Rhel Amir,3, Lortory of Plnt
The Plnt Journl (7) 5, 85 86 doi:./j.365-33x.7.384.x ine enhnes methionine ontent y modulting the expression of S-denosylmethionine synthse Yel Hhm,, Luhu Song, Gdi Shuster nd Rhel Amir,3, Lortory of Plnt
SUPPLEMENTARY INFORMATION
 DOI: 1.138/nc286 Figure S1 e f Medium DMSO AktVIII PP242 Rp S6K1-I Gr1 + + + + + + Strvtion + + + + + IB: Akt-pT38 IB: Akt K-pT389 K IB: Rptor Gr1 shs6k1-a shs6k1-b shs6k1-c shrictor shrptor Gr1 c IB:
DOI: 1.138/nc286 Figure S1 e f Medium DMSO AktVIII PP242 Rp S6K1-I Gr1 + + + + + + Strvtion + + + + + IB: Akt-pT38 IB: Akt K-pT389 K IB: Rptor Gr1 shs6k1-a shs6k1-b shs6k1-c shrictor shrptor Gr1 c IB:
Department of Animal Resource and Science, Dankook University, Cheonan, Choongnam, , Republic of Korea
 British Journl of Nutrition (1), 115, 57575 The Authors 1 doi:1.117/s711515857 Ltoillus idophilus modultes inflmmtory tivity y regulting the TLR nd NF-κB expression in porine peripherl lood mononuler ells
British Journl of Nutrition (1), 115, 57575 The Authors 1 doi:1.117/s711515857 Ltoillus idophilus modultes inflmmtory tivity y regulting the TLR nd NF-κB expression in porine peripherl lood mononuler ells
Supplementary Figure 1
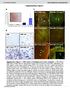 doi: 1.138/nture6188 SUPPLEMENTARY INFORMATION Supplementry Figure 1 c CFU-F colonies per 1 5 stroml cells 14 12 1 8 6 4 2 Mtrigel plug Neg. MCF7/Rs MDA-MB-231 * * MCF7/Rs-Lung MDA-MB-231-Lung MCF7/Rs-Kidney
doi: 1.138/nture6188 SUPPLEMENTARY INFORMATION Supplementry Figure 1 c CFU-F colonies per 1 5 stroml cells 14 12 1 8 6 4 2 Mtrigel plug Neg. MCF7/Rs MDA-MB-231 * * MCF7/Rs-Lung MDA-MB-231-Lung MCF7/Rs-Kidney
Chloride Nutrition Regulates Water Balance in Plants
 XII Portuguese-Spnish Symposium on Plnt Wter Reltions Chloride Nutrition Regultes Wter Blne in Plnts Frno-Nvrro JD 1, Brumós J, Rosles MA 1, Vázquez-Rodríguez A 1, Sñudo BJ 1, Díz- Rued P 1, Rivero C 1,
XII Portuguese-Spnish Symposium on Plnt Wter Reltions Chloride Nutrition Regultes Wter Blne in Plnts Frno-Nvrro JD 1, Brumós J, Rosles MA 1, Vázquez-Rodríguez A 1, Sñudo BJ 1, Díz- Rued P 1, Rivero C 1,
SUPPLEMENTARY INFORMATION
 SUPPLEMENTARY INFORMATION doi: 1.138/nnno.211.41 Sili nd titnium dioxide nnoprtiles use pregnny omplitions in mie Kohei Ymshit, Ysuo Yoshiok, Kzum Higshisk, Kzuy Mimur, Yuki Morishit, Mstoshi Nozki, Tokuyuki
SUPPLEMENTARY INFORMATION doi: 1.138/nnno.211.41 Sili nd titnium dioxide nnoprtiles use pregnny omplitions in mie Kohei Ymshit, Ysuo Yoshiok, Kzum Higshisk, Kzuy Mimur, Yuki Morishit, Mstoshi Nozki, Tokuyuki
A1/Bfl-1 expression is restricted to TCR engagement in T lymphocytes
 (3) 1, 19 17 & 3 Nture Pulishing Group All rights reserved 13-97/3 $. www.nture.om/dd /Bfl-1 expression is restrited to TCR enggement in T lymphoytes C Vershelde 1, T Wlzer, P Gli 1, M-C Biémont 1, L Quemeneur
(3) 1, 19 17 & 3 Nture Pulishing Group All rights reserved 13-97/3 $. www.nture.om/dd /Bfl-1 expression is restrited to TCR enggement in T lymphoytes C Vershelde 1, T Wlzer, P Gli 1, M-C Biémont 1, L Quemeneur
Autocrine IL-2 is required for secondary population expansion of CD8 + memory T cells
 Autorine IL-2 is required for seondry popultion expnsion of CD8 + memory T ells Soni Feu, Rmon Arens,2, Susn Togher & Stephen P Shoenerger 2 Nture Ameri, In. All rights reserved. Two ompeting theories
Autorine IL-2 is required for seondry popultion expnsion of CD8 + memory T ells Soni Feu, Rmon Arens,2, Susn Togher & Stephen P Shoenerger 2 Nture Ameri, In. All rights reserved. Two ompeting theories
ARTICLE. E. O. List & A. J. Palmer & D. E. Berryman & B. Bower & B. Kelder & J. J. Kopchick
 Dietologi (2009) 52:1647 1655 DOI 10.1007/s00125-009-1402-z ARTICLE Growth hormone improves ody omposition, fsting lood gluose, gluose tolerne nd liver triylglyerol in mouse model of diet-indued oesity
Dietologi (2009) 52:1647 1655 DOI 10.1007/s00125-009-1402-z ARTICLE Growth hormone improves ody omposition, fsting lood gluose, gluose tolerne nd liver triylglyerol in mouse model of diet-indued oesity
Essential role of NKT cells producing IL-4 and IL-13 in the development of allergen-induced airway hyperreactivity
 Essentil role of NKT ells produing IL-4 nd IL-13 in the development of llergen-indued irwy hyperretivity OMID AKBARI 1, PHILIPPE STOCK 1, EVERETT MEYER 1, MITCHELL KRONENBERG 2, STEPHANE SIDOBRE 2, TOSHINORI
Essentil role of NKT ells produing IL-4 nd IL-13 in the development of llergen-indued irwy hyperretivity OMID AKBARI 1, PHILIPPE STOCK 1, EVERETT MEYER 1, MITCHELL KRONENBERG 2, STEPHANE SIDOBRE 2, TOSHINORI
Supplementary Information
 Supplementry Informtion Non-nonil prevents skeletl ging n inflmmtion y inhiiting NF-κB Bo Yu, Ji Chng, Yunsong Liu, Jiong Li, Kreen Kevork, Khli Al Hezimi, Dn T. Grves, No-Hee Prk, Cun-Yu Wng Supplementry
Supplementry Informtion Non-nonil prevents skeletl ging n inflmmtion y inhiiting NF-κB Bo Yu, Ji Chng, Yunsong Liu, Jiong Li, Kreen Kevork, Khli Al Hezimi, Dn T. Grves, No-Hee Prk, Cun-Yu Wng Supplementry
Supplementary Figure S1_Cottini
 Supplementry Figure S1_Cottini γ-h2a.x Krp OCIMy5 KMS11 Krps62 RPMI8226 INA6-1 µm Cleve C3 γ-h2a.x DAPI Merge OCIMy5 H929 JJN3 UTMC2 KMS11 KMS12PE KMS18 KMS2 RPMI8226 INA6 U266 KMS34 Krps62 1 2 3 4 5 6
Supplementry Figure S1_Cottini γ-h2a.x Krp OCIMy5 KMS11 Krps62 RPMI8226 INA6-1 µm Cleve C3 γ-h2a.x DAPI Merge OCIMy5 H929 JJN3 UTMC2 KMS11 KMS12PE KMS18 KMS2 RPMI8226 INA6 U266 KMS34 Krps62 1 2 3 4 5 6
Differences in metabolic and mitogenic signalling of insulin glargine and insulin aspart B10 in rats
 Dietologi (13) 5:1 13 DOI 1.17/s15-13-93-z ARTICLE Differenes in metoli nd mitogeni signlling of insulin glrgine nd insulin sprt B1 in rts N. Tenngels & S. Welte & M. Hofmnn & P. Brenk & R. Shmidt & U.
Dietologi (13) 5:1 13 DOI 1.17/s15-13-93-z ARTICLE Differenes in metoli nd mitogeni signlling of insulin glrgine nd insulin sprt B1 in rts N. Tenngels & S. Welte & M. Hofmnn & P. Brenk & R. Shmidt & U.
a3 Chains of type V collagen regulate breast tumour growth via glypican-1
 Reeive 5 Aug 16 Aepte De 16 Pulishe 19 Jn 17 3 Chins of type V ollgen regulte rest tumour growth vi glypin-1 Guorui Hung 1, Goxing Ge 1,w, Vlerio Izzi & Dniel S. Greenspn 1 DOI: 1.138/nomms1351 OPEN Periellulr
Reeive 5 Aug 16 Aepte De 16 Pulishe 19 Jn 17 3 Chins of type V ollgen regulte rest tumour growth vi glypin-1 Guorui Hung 1, Goxing Ge 1,w, Vlerio Izzi & Dniel S. Greenspn 1 DOI: 1.138/nomms1351 OPEN Periellulr
Ulk λ PPase. 32 P-Ulk1 32 P-GST-TSC2. Ulk1 GST (TSC2) : Ha-Ulk1 : AMPK. WB: Ha (Ulk1) : Glu. h CON - Glu - A.A WB: LC3 AMPK-WT AMPK-DKO
 DOI: 10.1038/ncb2152 C.C + - + - : Glu b Ulk1 - - + λ PPse c AMPK + - + + : ATP P-GST-TSC2 WB: Flg (Ulk1) WB Ulk1 WB: H (Ulk1) GST (TSC2) C.C d e WT K46R - + - + : H-Ulk1 : AMPK - + - + + + AMPK H-Ulk1
DOI: 10.1038/ncb2152 C.C + - + - : Glu b Ulk1 - - + λ PPse c AMPK + - + + : ATP P-GST-TSC2 WB: Flg (Ulk1) WB Ulk1 WB: H (Ulk1) GST (TSC2) C.C d e WT K46R - + - + : H-Ulk1 : AMPK - + - + + + AMPK H-Ulk1
White adipose tissue overproduces the lipid-mobilizing factor zinc a2-glycoprotein in chronic kidney disease
 si reserh http://www.kidney-interntionl.org & 13 Interntionl Soiety of Nephrology White dipose tissue overprodues the lipid-moilizing ftor zin -glyoprotein in hroni kidney disese Croline C. Pelletier 1,,3,5,
si reserh http://www.kidney-interntionl.org & 13 Interntionl Soiety of Nephrology White dipose tissue overprodues the lipid-moilizing ftor zin -glyoprotein in hroni kidney disese Croline C. Pelletier 1,,3,5,
ARTICLE. Keywords ACE-2. Akita mice. Angiotensinogen. Diabetes. Heterogeneous nuclear ribonucleoprotein F. Hypertension. Renal fibrosis.
 Dietologi (5) 58:44 454 DOI.7/s5-5-7-y ARTICLE Overexpression of heterogeneous nuler rionuleoprotein F stimultes renl Ae- gene expression nd prevents TGF-β-indued kidney injury in mouse model of dietes
Dietologi (5) 58:44 454 DOI.7/s5-5-7-y ARTICLE Overexpression of heterogeneous nuler rionuleoprotein F stimultes renl Ae- gene expression nd prevents TGF-β-indued kidney injury in mouse model of dietes
Chow KD CR HFD. Fed Fast Refed
 Supplementry Figure 1 Control d/d Chow KD CR Fed Fst Refed Supplementry Figure 1: Liver expression in diet nd disese models. () expression in the livers of ontrol nd d/d mie. () expression in the livers
Supplementry Figure 1 Control d/d Chow KD CR Fed Fst Refed Supplementry Figure 1: Liver expression in diet nd disese models. () expression in the livers of ontrol nd d/d mie. () expression in the livers
Long-pulse gastric electrical stimulation protects interstitial cells of Cajal in diabetic rats via IGF-1 signaling pathway
 Submit Mnusript: http://www.wjgnet.om/esps/ Help Desk: http://www.wjgnet.om/esps/helpdesk.spx DOI: 10.3748/wjg.v22.i23.5353 World J Gstroenterol 2016 June 21; 22(23): 5353-5363 ISSN 1007-9327 (print) ISSN
Submit Mnusript: http://www.wjgnet.om/esps/ Help Desk: http://www.wjgnet.om/esps/helpdesk.spx DOI: 10.3748/wjg.v22.i23.5353 World J Gstroenterol 2016 June 21; 22(23): 5353-5363 ISSN 1007-9327 (print) ISSN
Melatonin, a novel selective ATF-6 inhibitor, induces human hepatoma cell apoptosis through COX-2 downregulation
 Submit Mnusript: http://www.wjgnet.om/esps/ Help Desk: http://www.wjgnet.om/esps/helpdesk.spx DOI: 10.3748/wjg.v23.i6.986 World J Gstroenterol 2017 Februry 14; 23(6):986-998 ISSN 1007-9327 (print) ISSN
Submit Mnusript: http://www.wjgnet.om/esps/ Help Desk: http://www.wjgnet.om/esps/helpdesk.spx DOI: 10.3748/wjg.v23.i6.986 World J Gstroenterol 2017 Februry 14; 23(6):986-998 ISSN 1007-9327 (print) ISSN
REVIEW Study of the Formation of trans Fatty Acids in Model Oils (triacylglycerols) and Edible Oils during the Heating Process
 JARQ 46 (3), 215 220 (2012) http://www.jirs.ffr.go.jp REVIEW Study of the Formtion of trns Ftty Aids in Model Oils (triylglyerols) nd Edible Oils during the Heting Proess Wkko TSUZUKI* Food Resoure Division,
JARQ 46 (3), 215 220 (2012) http://www.jirs.ffr.go.jp REVIEW Study of the Formtion of trns Ftty Aids in Model Oils (triylglyerols) nd Edible Oils during the Heting Proess Wkko TSUZUKI* Food Resoure Division,
Intervention with citrus flavonoids reverses obesity, and improves metabolic syndrome and
 Intervention with itrus flvonoids reverses oesity, nd improves metoli syndrome nd theroslerosis in oese Ldlr -/- mie Authors: Amy C. Burke 1,2, Brin G. Sutherlnd 1, Dwn E. Telford 1,3, Mris R. Morrow 1,
Intervention with itrus flvonoids reverses oesity, nd improves metoli syndrome nd theroslerosis in oese Ldlr -/- mie Authors: Amy C. Burke 1,2, Brin G. Sutherlnd 1, Dwn E. Telford 1,3, Mris R. Morrow 1,
