Atherosclerosis, the major cause of morbidity and mortality
|
|
|
- Theodora Mitchell
- 6 years ago
- Views:
Transcription
1 National Cholesterol Awareness Month Article Basic Sciences Coenzyme Q10 Promotes Macrophage Cholesterol Efflux by Regulation of the Activator Protein-1/miR-378/ ATP-Binding Cassette Transporter G1 Signaling Pathway Dongliang Wang,* Xiao Yan,* Min Xia, Yan Yang, Dan Li, Xinrui Li, Fenglin Song, Wenhua Ling Downloaded from by guest on April 7, 2018 Objective Recent studies have shown the role of mirnas in macrophage reverse cholesterol transport and atherogenesis. We hypothesized that coenzyme Q10 (CoQ10) may increase macrophage reverse cholesterol transport by regulating mirna expression that contributes to the prevention of atherosclerosis. Approach and Results CoQ10 treatment suppressed oxidized low-density lipoprotein induced macrophage foam cell formation by ameliorating the binding of activator protein-1 to the putative promoter region of mir-378 primary transcript, thus decreasing the mir-378 level and enhancing the ATP-binding cassette transporter G1 mediated macrophage cholesterol efflux to high-density lipoprotein. Subsequently, the axis of activator protein-1/mir-378/atp-binding cassette transporter G1 cholesterol efflux was confirmed in peritoneal macrophages isolated from CoQ10-treated apolipoprotein E deficient mice. Finally, CoQ10 consumption promoted macrophage reverse cholesterol transport and inhibited the progression of atherosclerosis in apolipoprotein E deficient mice. Conclusions This study identified activator protein-1/mir-378/atp-binding cassette transporter G1 as a novel cascade for CoQ10 in facilitating macrophage cholesterol efflux in vitro and in vivo. Our data thus imply that both CoQ10 and mir-378 are promising candidates for atherosclerosis prevention and treatment. (Arterioscler Thromb Vasc Biol. 2014;34: ) Key Words: atherosclerosis coenzyme Q10 macrophages mirnas Atherosclerosis, the major cause of morbidity and mortality in Western societies, initiates and develops with the accumulation of circulating monocyte-derived macrophage foam cells in the arterial wall. Cholesterol homeostasis in macrophages is tightly regulated by cholesterol influx, endogenous synthesis, and efflux. Promoting cholesterol efflux from macrophages to high-density lipoprotein (HDL) to be transported to the liver for excretion, a process known as macrophage reverse cholesterol transport (RCT), has been shown to prevent or even regress atherosclerosis. 1 Several transcription factors (TFs) involved in regulating ATP-binding cassette transporters A1 (ABCA1) and G1 (ABCG1), 2 key transporters that facilitate cellular cholesterol efflux, have been discovered. 2 Among them, activation of liver X receptor α (LXRα) remarkably induces ABCA1 and ABCG1 expression and promotes macrophage RCT and established atherosclerosis regression, although it induces excess triglyceride accumulation in blood and liver. 2 Additional levels of regulatory control by small RNAs are now emerging in macrophage cholesterol homeostasis. 3 Mammalian mirnas, highly conserved noncoding small RNAs of 19 to 26 nucleotides, are key posttranscriptional regulators that contribute to the maintenance of differentiated cell phenotypes. 4 mirnas usually bind to the 3 -untranslated regions (3 -UTRs) of target mrnas, promoting mrna degradation and inhibiting translation of the protein-coding genes. 5 Recently, several mirnas that mediate macrophage cholesterol efflux have been demonstrated using cell culture and animal models 3 ; mir-33 is prominent because it promotes macrophage RCT and the regression of established atherosclerosis in low-density lipoprotein receptor deficient mice, 6 thus exemplifying mirna control of macrophage cholesterol homeostasis and atherosclerosis development. However, the identities of the mirnas mediating macrophage cholesterol efflux with natural nutrients in vivo are largely unknown, except we recently reported that protocatechuic acid promotes macrophage RCT partially by repressing mir-10b in apolipoprotein E deficient (apoe / ) mice. 7 See accompanying editorial on page 1795 Coenzyme Q10 (CoQ10), a well-known player in cellular bioenergetics, 8 has been intensively implicated in protecting Received on: November 12, 2013; final version accepted on: March 17, From the Department of Nutrition, School of Public Health, Sun Yat-Sen University (Northern Campus), Guangzhou, Guangdong Province, People s Republic of China (D.W., X.Y., M.X., Y.Y., D.L., X.L., F.S., W.L.); and Guangdong Provincial Key Laboratory of Food, Nutrition and Health, Department of Nutrition, School of Public Health, Sun Yat-Sen University (Northern Campus), Guangzhou, Guangdong Province, People s Republic of China (D.W., M.X., Y.Y., D.L., W.L.). *These authors contributed equally. The online-only Data Supplement is available with this article at Correspondence to Wenhua Ling, PhD, Department of Nutrition, School of Public Health, Sun Yat-sen University, Guangzhou , PR China. lingwh@mail.sysu.edu.cn 2014 American Heart Association, Inc. Arterioscler Thromb Vasc Biol is available at DOI: /ATVBAHA
2 Wang et al CoQ10 Promotes Macrophage Cholesterol Efflux 1861 Downloaded from by guest on April 7, 2018 Nonstandard Abbreviations and Acronyms ABCA1 ABCG1 AP apoa-i apoe / ATF CoQ10 HDL-C LXRα MPM OxLDL PKC RCT TF UTR VLDL ATP-binding cassette transporter A1 ATP-binding cassette transporter G1 activator protein apolipoprotein A-I apolipoprotein E deficient activating transcription factor coenzyme Q10 high-density lipoprotein cholesterol liver X receptor α mouse peritoneal macrophage oxidized low-density lipoprotein protein kinase C reverse cholesterol transport transcription factor untranslated region very-low-density lipoprotein against chronic diseases, especially atherosclerosis. 9 It has been proposed that antioxidation and inhibition of inflammation contribute to CoQ10-induced antiatherosclerotic effects. 10,11 Because a recent study shows that CoQ10 can reduce mir- 146a expression in human THP-1 monocytes and mouse liver, 12 we hypothesized that CoQ10 exerts its atheroprotective effects partially by promoting mirnas-mediated macrophage cholesterol efflux. We showed that CoQ10 transcriptionally suppresses mir-378 expression through activator protein-1 (AP-1), upregulates ABCG1 expression, and then promotes macrophage cholesterol efflux in vitro and in vivo. Materials and Methods Materials and Methods are available in the online-only Supplement. Results CoQ10 Promotes Macrophage Cholesterol Efflux To evaluate the inhibitory effect of CoQ10 on macrophage foam cell formation, mouse J774.A1 and human THP-1 macrophages were loaded with oxidized low-density lipoprotein (OxLDL) as cell culture models. Compared with control cells, CoQ10 significantly inhibited OxLDL-induced macrophage foam cell formation as determined by Oil Red O (Figure 1A 1D) and filipin staining (Figure IA in the online-only Data Supplement), as well as by analyzing cellular cholesterol content (Figure 1E and 1F). Importantly, CoQ10 had no obvious effect on cholesterol influx assessed by microscopic analysis and measurement of cellular 1,1 -dioctadecyl-3,3,3 3 -tetramethylindocarbocyanine perchlorate-oxldl, as well as quantitative real-time polymerase chain reaction and Western blotting analyses of scavenger receptor class A and CD36 (Figure IB and IC in the Figure 1. Coenzyme Q10 (CoQ10) modulates macrophage cholesterol homeostasis by enhancing cholesterol efflux. J774.A1 (A) and THP-1 macrophages (B) were incubated with the vehicle dimethyl sulfoxide (DMSO; control), oxidized low-density lipoprotein (OxLDL; 50 μg/ml), CoQ10, or a combination of CoQ10 and OxLDL for 24 hours. After fixation by 4% paraformaldehyde, cells were stained with Oil Red O to detect lipid accumulation, and hematoxylin was used for counterstaining. The figure is representative of 3 independent experiments (magnification 400). C and D, The density of lipid content was evaluated by alcohol extraction after staining. The absorbance at 540 nm was measured using a microplate reader. E and F, The intracellular levels of total cholesterol were analyzed by colorimetric assay kits. J774.A1 (G) and THP-1 macrophages (H) preloaded with 1.0 μci/ml 3 H-cholesterol and OxLDL were treated with CoQ10 or DMSO for 24 hours and subsequently incubated with apolipoprotein A-I (apoa-i; 15 μg/ml) or high-density lipoprotein (HDL; 100 μg/ml) for another 24 hours. Cholesterol efflux was measured as described in Materials and Methods in the online-only Data Supplement. C H, The data are the mean±sem (n=3). C F, *P<0.05 vs (CoQ10 [ ] and OxLDL [+]). G and H, *P<0.05 vs control. The data were analyzed with 1-way ANOVA and the Bonferroni Dunn post hoc test.
3 1862 Arterioscler Thromb Vasc Biol September 2014 Downloaded from by guest on April 7, 2018 online-only Data Supplement). In addition, CoQ10 cannot obviously alter the expression of genes critical to cholesterol synthesis and influx gauged by quantitative real-time polymerase chain reaction analyses of related genes (Figure ID and IE in the online-only Data Supplement). Instead, CoQ10 promoted cholesterol efflux from OxLDL-loaded macrophages to HDL but not apolipoprotein A-I (apoa-i) in a dose-dependent manner (Figure 1G and 1H). These results imply that CoQ10 inhibits macrophage foam cell formation by promoting macrophage cholesterol efflux. CoQ10 Induces ABCG1 Expression by Repressing mir-378 in Macrophages As shown in Figure 2A to 2D, CoQ10 dose dependently increased the expression of ABCG1 but not ABCA1 and scavenger receptor class B type I at the mrna and protein (quantification in Figure IIA and IIB in the online-only Data Supplement) levels in OxLDL-loaded J774.A1 and THP-1 macrophages. Because macrophages can release cellular free cholesterol to extracellular HDL by diffusion, 13 the specific role of ABCG1 in CoQ10-mediated cholesterol efflux was tested. Western blotting analysis showed that ABCG1 protein expression was effectively suppressed by small interfering RNA, but scramble small interfering RNA had no such effect (inset of Figure 2E and 2F; quantification in Figure IIC in the online-only Data Supplement). ABCG1 silencing abolished CoQ10-induced cholesterol efflux to HDL (Figure 2E and 2F). Importantly, CoQ10 had no significant effect on the transcriptional activity of ABCG1 in OxLDL-loaded macrophages (Figure IIIA and IIIB in the online-only Data Supplement). Furthermore, CoQ10 did not alter LXRα expression and transcriptional activity (Figure IIIC IIIH in the online-only Data Supplement). In contrast, CoQ10 markedly prolonged the halflives of ABCG1 mrna and restored the diminished luciferase reporter activities induced by the presence of mouse and human ABCG1 3 -UTR in 2 cells (Figure 2G 2J). Collectively, these Figure 2. Coenzyme Q10 (CoQ10) post-transcriptionally regulates ATP-binding cassette transporter G1 (ABCG1). A D, Oxidized low-density lipoprotein (OxLDL) loaded J774.A1 and THP-1 macrophages were treated with CoQ10 (1, 10, 100 μmol/l) or the vehicle (dimethyl sulfoxide [DMSO]) for 24 hours. mrna and protein expression of ATP-binding cassette transporter A1 (ABCA1), ABCG1, and scavenger receptor class B type I (SR-BI) were then determined by quantitative real-time polymerase chain reaction (qrt-pcr; A and C) and Western blotting (B and D), respectively. E and F, Cholesterol efflux to high-density lipoprotein (HDL) in OxLDL-loaded J774.A1 (E) and THP-1 macrophages (F) transfected with scrambled or ABCG1 small interfering RNA (sirna) with or without CoQ10 (10 μmol/l). Inset, Western blotting for ABCG1. OxLDL-loaded J774.A1 (G) and THP-1 macrophages (I) were treated with CoQ10 (10 μmol/l) or the vehicle (DMSO) for 24 hours. Cells were then treated with 10 μg/ml of actinomycin D, and ABCG1 mrna expression at indicated time points was quantified by qrt-pcr. OxLDL-loaded J774.A1 (H) and THP-1 macrophages (J) were cotransfected with mouse (m) and human (h) ABCG1 promoter and Renilla (for internal normalization) for 6 hours, respectively. Cells were then treated with CoQ10 (10 μmol/l) or DMSO for 12 hours, and the activity of the luciferase reporter was measured by the Dual-Luciferase Reporter Assay System. B, D, and inset of E and F are representative images of 3 independent assays. For the other panels, data are the mean±sem (n=3 6). A and C, *P<0.05 vs control. The data were analyzed with 1-way ANOVA and the Bonferroni Dunn post hoc test. E, F, H, and J, #P<0.05. The data were analyzed with Student t test.
4 Wang et al CoQ10 Promotes Macrophage Cholesterol Efflux 1863 Downloaded from by guest on April 7, 2018 results suggest that CoQ10 promotes macrophage cholesterol efflux in an ABCG1-dependent manner that is associated with enhanced ABCG1 mrna stability by its 3 -UTR sequence. To test whether mirnas, well-known regulators of mrnas destabilization by binding to 3 -UTRs of their target genes, 4 are involved in CoQ10-mediated ABCG1 mrna stability, we undertook an unbiased mirna microarray analysis. Among the 1891 mouse individual mirnas represented on the microarrays, 40 mirnas were 2-fold differentially expressed between CoQ10- and vehicle-treated J774.A1 cells (Figure IVA in the online-only Data Supplement). The quantitative real-time polymerase chain reaction analyses confirmed the upregulation and downregulation of candidate mirnas by CoQ10 in OxLDL-loaded mouse peritoneal macrophages (MPMs), J774.A1, and THP-1 macrophages (Figure IVB IVD in the online-only Data Supplement). Using several mirna target prediction databases, 4 we found that the mouse or human ABCG1 3 -UTR has 3 or 1 computationally predicted mir-378 binding sites, respectively (Figure VA and VB in the online-only Data Supplement). Sites 2 and 3 in mouse ABCG1 3 -UTR are conserved in mouse and rat, whereas site 1 in human is conserved in human and nonhuman primates (Figure VA and VB in the online-only Data Supplement). Notably, mir-378 levels were higher in aortas isolated from apoe / mice aged 30 weeks than in mice aged 15 weeks, suggesting that mir-378 plays a role in the development of atherosclerosis (Figure VC in the online-only Data Supplement). To gain insight into the effect of mir-378 on ABCG1 expression, macrophages with overexpression or inhibition of mir-378 were established by transfection with a mir-378 mimic or an antisense inhibitor of mir-378 (anti mir-378), respectively. As expected, the mir-378 mimic dose dependently increased cellular mir-378 levels in J774A.1 and THP-1 macrophages, and the concentration of the mir-378 mimic at 150 nmol/l produced a maximal increase of 14-fold (Figure VD and VE in the online-only Data Supplement). In contrast, anti mir-378 dose dependently reduced cellular mir-378 levels in 2 cells (Figure VF and VG in the online-only Data Supplement). Importantly, mir-378 repressed the expression of ABCG1 at the mrna and protein levels in a dose-dependent manner, and conversely, the inhibition of this mirna increased ABCG1 levels (Figure 3A 3H; protein quantification in Figure IID and IIE in the online-only Data Supplement). To determine the effects of mir-378 on the 3 -UTR of mouse and human ABCG1, a luciferase reporter containing the ABCG1 3 -UTR fragment with the mir-378 binding sites (wild type) or the mutant mir-378 binding sites (mutation type) was used. The mir-378 mimic dose dependently repressed mouse and human ABCG1 3 -UTR activities (Figure VH in the online-only Data Supplement). Moreover, overexpression of mir-378 suppressed the wild-type but not the mutant ABCG1 3 -UTR activity in mouse or human macrophages (Figure 3I and 3J). These results demonstrated that mir-378 directly targets ABCG1. Functional studies further showed that mir- 378 overexpression inhibited macrophage cholesterol efflux to HDL, whereas mir-378 inhibition enhanced macrophage cholesterol efflux (Figure 3K 3N). To test the role of mir-378 in CoQ10-induced macrophage cholesterol efflux (Figure 1G and 1H) and ABCG1 expression (Figure 2A 2D), OxLDL-loaded J774.A1 and THP-1 macrophages with mir-378 overexpression or inhibition were studied. As shown in Figure 4A to 4C, mir- 378 mimic could block the increase of ABCG1 expression (protein quantification in Figure IIF in the online-only Data Supplement) and HDL-mediated cholesterol efflux induced by CoQ10. Moreover, anti mir-378 could not further enhance CoQ10-induced ABCG1 expression at the protein (Figure 4A; quantification in Figure IIF in the onlineonly Data Supplement) and mrna (Figure 4D) levels and HDL-mediated cholesterol efflux (Figure 4E). These results thus suggest that CoQ10 induces ABCG1 expression by downregulating mir-378 expression and thus promotes macrophage cholesterol efflux to HDL. CoQ10 Negatively Regulates mir-378 via AP-1 To explore how CoQ10 modulates mir-378 expression, we examined a region 2 kb upstream of the transcription start site of the mir-378 primary transcript (pri-mir-378) in silico using mirgen AP-1 and the other 4 TFs, hepatocyte nuclear factor 4, Elk-1, NK2 homeobox 5, and c-rel (Table II in the online-only Data Supplement), were expected to bind to the putative promoters of mouse and human primir-378. AP-1 is composed of protein products of members of fos and jun families, which form homodimeric or heterodimeric complexes; the predominant forms of AP-1 in most cells are c-fos/c-jun heterodimers. 15 CoQ10 had no obvious effect on the expression of Elk-1, phosphorylated Elk-1, and c-rel, whereas hepatocyte nuclear factor 4 and NK2 homeobox 5 were undetectable in OxLDL-loaded J774. A1 and THP-1 macrophages treated with or without CoQ10 (data not shown). However, CoQ10 remarkably reduced the expression of the AP-1 component c-jun (but not c-fos) at the protein and mrna levels (left panels of Figure 5A and 5B; Figure VIA VID in the online-only Data Supplement; protein quantification in Figure IIG and IIH in the onlineonly Data Supplement). Activation of AP-1 by 12-O-tetradecanoylphorbol-13-acetate, a known stimulator for AP-1, increased mir-378 expression (Figure 5A and 5B, right), and conversely, AP-1 inhibition by c-jun small interfering RNA reduced mir-378 expression (Figure VIE and VIF in the online-only Data Supplement). Furthermore, c-jun silencing increased ABCG1 protein expression in macrophages, whereas this was not observed in macrophages cotransfected with 150 nmol/l mir-378 (Figure 5C and 5D; protein quantification in Figure III in the online-only Data Supplement). Importantly, CoQ10 restored 12-O-tetra-decanoylphorbol- 13-acetate induced mir-378 expression (Figure 5A and 5B, right), whereas CoQ10 had no obvious effect on the mir-378 level in macrophages knockdown of c-jun (Figure VIE and VIF in the online-only Data Supplement). These results suggest that CoQ10 reduces mir-378 expression by inhibiting AP-1, thus leading to increment of its target gene ABCG1. We next performed chromatin immunoprecipitation and luciferase reporter assays to assess the potential transactivation of the mir-378 gene by AP-1. Chromatin immunoprecipitation assays showed that AP-1 directly bound to the putative-binding sites of mouse and human pri-mir-378 at 37 and 531, respectively (Figure 5E and 5F). Luciferase
5 1864 Arterioscler Thromb Vasc Biol September 2014 Downloaded from by guest on April 7, 2018 Figure 3. mir-378 directly targets the 3 -untranslated region (3 -UTR) of ATP-binding cassette transporter G1 (ABCG1). A D, Oxidized low-density lipoprotein (OxLDL) loaded J774.A1 and THP-1 macrophages were transfected with control mir (Con mir; 150 nmol/l) or the indicated concentrations of mir-378 for 48 hours. ABCG1 mrna and protein expression were then determined by quantitative realtime polymerase chain reaction (qrt-pcr; A and C) and Western blotting (B and D). qrt-pcr (E and G) and Western blotting (F and H) analyses of mouse and human ABCG1 in OxLDL-loaded J774.A1 and THP-1 macrophages transfected with Con mir (150 nmol/l) or mir-378 (150 nmol/l) in the presence or absence of a control inhibitor (Con Inh,150 nmol/l) or anti mir-378 (150 nmol/l). I and J, The mir-378 target sites in mouse and human ABCG1 are shown in the right panels of I and J. Mutants were generated in the mouse and human ABCG1 3 -UTR seed regions, as indicated. HEK293 cells were cotransfected with Con mir (40 nmol/l) or mir-378 (40 nmol/l), either 50 ng of pgl-3-wild-type (WT) or pgl3-mutant and 50 ng of prl-tk for 48 hours, as described in Materials and Methods in the online-only Data Supplement. Luciferase reporter activities were then measured by the Dual-Luciferase Reporter Assay System. Cholesterol efflux to HDL from OxLDL-loaded J774.A1 (K and L) and THP-1 macrophages (M and N) transfected with the indicated concentrations of Con mir or mir-378, or Con Inh or anti mir-378. B, D, F, and H, Representative images of 3 independent assays. A, C, E, and G, The data are expressed as the mean±sem of fold of either Con mir (A and C) or Con mir and Con Inh (E and G; n=3). *P<0.05 vs Con mir or Con mir and Con Inh, 1-way ANOVA coupled with the Bonferroni Dunn post hoc test. I and J, The data are expressed as the mean percentage of the 3 -UTR activity of Con mir±sem (n=3). #P<0.05, Student t test. K N, The data are the mean±sem (n=3 6). *P<0.05 vs Con mir or Con Inh, 1-way ANOVA coupled with the Bonferroni Dunn post hoc test. reporter assays showed that AP-1 activation induced by 12-O-tetra-decanoylphorbol-13-acetate increased transactivation of the mir-378 gene with the putative-binding sites of mouse and human pri-mir-378 at 37 and 531, respectively (Figure 5G and 5H, left). After c-jun silencing in macrophages, AP-1 mediated transactivation of the mir-378 gene was remarkably attenuated (Figure 5G and 5H, right), which is consistent with the concept that AP-1 directly targeted the downstream mir-378. In addition, CoQ10 reduced mouse and human mir-378-promoter driven luciferase activity in cells treated with 12-O-tetra-decanoylphorbol-13-acetate or not but not with mutant or deleted AP-1 binding sites (Figure 5G and 5H, left). These data suggest that CoQ10 ameliorates the binding of AP-1 to the putative promoter region of pri-mir-378, thus decreases the mir-378 level. CoQ10 Enhances Macrophage RCT In Vivo To test whether the in vitro findings on macrophage cholesterol efflux by CoQ10 could be extended into in vivo circumstances, 30-week-old male apoe / mice were orally gavaged once daily with CoQ10 (600 mg/kg body weight [BW]) for 14 days. CoQ10 treatment resulted in higher serum CoQ10 concentrations by 50-fold (Figure 6A). CoQ10 treatment decreased c-jun (Figure 6B; left panel of Figure VIIA in the online-only Data Supplement; protein quantification in Figure IIJ in the online-only Data Supplement) and mir-378 (Figure 6C) expression and increased ABCG1 expression (Figure 6D; right panel of Figure VIIA in the online-only Data Supplement; protein quantification in Figure IIJ in the online-only Data Supplement) in mouse aortas. Furthermore, MPMs isolated from mice treated with CoQ10 for 14 days
6 Wang et al CoQ10 Promotes Macrophage Cholesterol Efflux 1865 Downloaded from by guest on April 7, 2018 Figure 4. mir-378 regulates coenzyme Q10 (CoQ10) induced macrophage cholesterol efflux. Oxidized low-density lipoprotein (OxLDL) loaded J774.A1 and THP-1 macrophages were transfected with either a control mir (Con mir, 150 nmol/l) or mir-378 (40 or 150 nmol/l), or a control inhibitor (Con Inh, 150 nmol/l) or anti mir-378 (150 nmol/l) for 24 hours. Cells were then treated with CoQ10 (10 μmol/l) or the vehicle (dimethyl sulfoxide [DMSO]) for another 24 hours. ATP-binding cassette transporter G1 (ABCG1) protein and mrna expression were determined by Western blotting (A) and quantitative real-time polymerase chain reaction (B and D), respectively. The 3 H-cholesterol labeled and OxLDL-loaded J774.A1 and THP-1 macrophages were transfected with either Con mir or mir-378 (C), or Con Inh or anti mir-378 (E) for 24 hours. Cells were then treated with CoQ10 or DMSO for another 24 hours, and cholesterol efflux to high-density lipoprotein (HDL) was determined. A, Representative images of 6 independent assays. B E, The data are expressed as the mean±sem of fold of either Con mir or Con Inh (n=6). *P<0.05 vs CoQ10, Student t test. NS indicates not significant. had lower levels of c-jun (Figure 6E; left panel of Figure VIIB in the online-only Data Supplement; protein quantification in Figure IIK in the online-only Data Supplement) and mir-378 (Figure 6F), which were associated with increased ABCG1 expression (Figure 6G; right panel of Figure VIIB in the online-only Data Supplement; protein quantification in Figure IIK in the online-only Data Supplement) and enhanced cholesterol efflux ability to HDL (Figure 6H). In keeping with ex vivo studies, CoQ10-treated apoe / mice had increased 3 H tracer levels in feces at 48 hours and in plasma at 6 and 24 hours but showed no significant effect on 3 H tracer recovery in the liver or plasma at 48 hours (Figure 6I). To identify whether alteration of ABCG1 expression is modulated by mir-378 in vivo, we performed a time-course experiment in isolated MPMs correlating with the pharmacokinetics of CoQ10 in apoe / mice. Within 0 to 24 hours of CoQ10 intervention, we observed that the peak serum CoQ10 level occurred at 4 hours; however, the mir-378 level started to decline at 6 hours, with the strongest reduction at 10 hours, and retained the lower level until 24 hours. Conversely, ABCG1 mrna expression began to increase at 8 hours, reached its peak at 12 hours, and sustained that level for 24 hours (Figure 6J). Together, the lag response of ABCG1 to mir-378 indicates that CoQ10 may attenuate the mir-378 level and subsequently induce ABCG1 expression. CoQ10 Inhibits the Progression of Atherosclerosis In Vivo To examine the effect of CoQ10 on atherogenesis, male apoe / mice at 30 weeks were either euthanized (baseline) or subjected to treatments of the vehicle, CoQ10, or T (a known agonist for LXR) for 4 weeks. As demonstrated by Oil Red O staining (Figure 7A) and total cellular lipid (Figure 7B) and cholesterol (Figure 7C) assays, isolated MPMs from CoQ10-fed mice had a decreased level of macrophage foam cell formation compared with control mice. Both the atherosclerotic plaque size in the aortic sinus and whole aorta cholesterol levels showed decline in CoQ10-treated mice (Figure 7D 7F) compared with control mice, whereas no significant changes were observed compared with testing mice before CoQ10 intervention (baseline). Furthermore, CoQ10- treated apoe / mice displayed elevated protein (Figure 7G; quantification in Figure IIL and IIM in the online-only Data Supplement) and mrna (Figure VIIC and VIID in the online-only Data Supplement) expression of ABCG1 in aortas and MPMs. The plasma lipid profile revealed that
7 1866 Arterioscler Thromb Vasc Biol September 2014 Downloaded from by guest on April 7, 2018 Figure 5. Coenzyme Q10 (CoQ10) regulates mir-378 through activator protein-1 (AP-1). A and B, Left, Oxidized low-density lipoprotein (OxLDL) loaded J774.A1 (A) and THP-1 macrophages (B) were treated with CoQ10 or the vehicle (dimethyl sulfoxide [DMSO]) for 24 hours. The protein expression of c-jun and c-fos was then determined by Western blotting. Right, OxLDL-loaded J774.A1 (A) and THP-1 macrophages (B) were treated with 12-O-tetra-decanoylphorbol-13-acetate (TPA; 50 nmol/l) or PBS control in the presence or absence of CoQ10 (10 μmol/l) for 24 hours. The mir-378 contents were then determined by quantitative real-time polymerase chain reaction. C and D, Western blotting analysis of ATP-binding cassette transporter G1 (ABCG1) expression in OxLDL-loaded J774.A1 (C) and THP-1 macrophages (D) transfected with scrambled or c-jun small interfering RNA (sirna) in the presence or absence of control mir (Con mir; 150 nmol/l) or mir-378 (150 nmol/l) for 48 hours. E and F, Top, mouse and human putative promoter elements of the mir-378 gene. The schematic diagram represents 2 potential binding sites. Bottom, Densitometry analysis of chromatin immunoprecipitation gels in OxLDL-loaded J774.A1 (E) and THP-1 macrophages (F) treated with TPA or PBS in the presence or absence of CoQ10 for 24 hours. Left, Luciferase reporter activities in J774.A1 (G) and THP-1 macrophages (H) transfected with a series of luciferase constructs containing fulllength or truncated promoter elements of mouse or human mir-378 with wild-type putative AP-1 binding sites or mutant-binding sites. After transfection, cells were treated with TPA or PBS in the presence or absence of CoQ10 for 24 hours. Right, Luciferase activities in J774.A1 (G) and THP-1 macrophages (H) cotransfected with c-jun sirna and the luciferase reporter construct bearing the mouse or human mir-378 promoter. Left panels of A and B, as well as C and D, are representative images of 3 independent assays. Right panels of A and B, The data are the mean±sem (n=3). #P<0.05, Student t test. Lower panels of E and F, and left panels of G and H, The data are the mean±sem (n=3). *P<0.05 vs control, #P<0.05 vs (control+tpa), Student t test. Right panels of G and H, The data are the mean±sem (n=3). *P<0.05 vs scramble sirna, Student t test. CoQ10 decreased plasma total cholesterol and total triglyceride, as well as elevated HDL-C and apoa-i levels (Table III in the online-only Data Supplement). Furthermore, fast protein liquid chromatography analyses showed that CoQ10 reduced cholesterol content in the very LDL (VLDL) fraction (Figure VIIE in the online-only Data Supplement). In addition, T treated mice showed promoted regression of endogenous foam cell formation and established atherosclerosis (Figure 7A 7F). Moreover, T increased ABCG1 protein (Figure 7G; quantification in Figure IIL and IIM in the online-only Data Supplement) and mrna (Figure VIIC and VIID in the online-only Data Supplement) expression in aortas and MPMs, as well as in plasma triglyceride level (Table III in the online-only Data Supplement), which were consistent with recent studies. 16 Together, these observations indicate that CoQ10 reduces the progression but does not promote the regression of atherosclerosis in vivo. Discussion Although both antioxidative and anti-inflammatory functions contribute to the antiatherosclerotic effect of CoQ10, it is still unknown whether the promotion of macrophage RCT is involved. Here, we provide novel findings that CoQ10 promotes macrophage RCT through a previously undescribed
8 Wang et al CoQ10 Promotes Macrophage Cholesterol Efflux 1867 Downloaded from by guest on April 7, 2018 Figure 6. Coenzyme Q10 (CoQ10) promotes macrophage reverse cholesterol transport (RCT) in vivo. A G, Thirtyweek-old male apolipoprotein E deficient (apoe / ) mice were orally gavaged with CoQ10 (600 mg/kg BW) or the vehicle (normal saline) once daily for 14 days. The blood samples were collected 4 hours after CoQ10 treatment at day 14. Serum CoQ10 concentrations were then determined by high-performance liquid chromatography with electrochemical detection (A). c-jun and c-fos protein expression (B), mir-378 content (C), and protein expression of ATP-binding cassette transporter A1 (ABCA1), ATPbinding cassette transporter G1 (ABCG1), and scavenger receptor class B type I (SR-BI; D) in aortas isolated from CoQ10- or the vehicle-treated apoe / mice were determined by Western blotting and quantitative real-time polymerase chain reaction (qrt-pcr). The aortas were pooled with 4 mice in each group. Thioglycollate-elicited mouse peritoneal macrophages (MPMs) were obtained for the qrt-pcr and Western blotting analyses of cellular c-jun and c-fos protein (E) expression, mir-378 contents (F), ABCA1, ABCG1, and SR-BI protein expression levels (G), and cholesterol efflux to apolipoprotein A-I (apoa-i) and high-density lipoprotein (HDL; H). Isolated MPMs were pooled with 4 mice in each group. The method of injection of thioglycollate to obtain MPMs is described in Materials and Methods in the online-only Data Supplement. I, Thirty-week-old male apoe / mice were orally gavaged with CoQ10 (600 mg/kg BW) or the vehicle once daily for 14 days. On day 12, 3 H-cholesterol labeled and oxidized low-density lipoprotein (OxLDL) loaded MPMs (typically cells at cpm per mouse) were intraperitoneally injected into the mice. After cell injection, blood at 6, 24, and 48 hours, liver at 48 hours (after the animals were euthanized), and feces within the 48-hour experimental period were collected for the analysis of macrophage RCT. 3 H-cholesterol recovery in the feces, liver, and plasma were measured. J, Four-hour fasted apoe / mice that were intraperitoneally injected with thioglycollate were orally treated with CoQ10 (600 mg/kg BW) for 0 to 24 hours. Serum CoQ10 concentrations, cellular mir-378, and ABCG1 mrna expression levels in isolated MPMs were quantified (n=6). The results of serum CoQ10 levels are the mean±sem. The results for mir-378 contents and ABCG1 mrna levels are the mean±sem of fold of the control (untreated mice served as controls), which was set to 1. B, D, E, and G, Representative images of 3 independent assays. A, The data are the mean±sem (n=12). *P<0.05 vs control, Student t test. C, F, and H, The data are the mean±sem (n=3). *P<0.05 vs control, #P<0.05, Student t test. I, The data are the mean±sem (n=12). #P<0.05, Student t test. AP-1/miR-378/ABCG1 pathway in apoe / mice, thus contributing to the inhibition of established atherosclerosis progression. Because the atheroprotective or proatherosclerotic role of ABCG1 in atherosclerosis was observed in animal studies, 17,18 it is hard to tell whether the atheroprotective effect of CoQ10 in humans is partially explained by its capacity for accelerating ABCG1-mediated macrophage cholesterol efflux. Nevertheless, these observations suggest that CoQ10 is a promising candidate for atherosclerotic therapy in humans. The concept that the enhancement of macrophage RCT could prevent the progression or even induce regression of atherosclerosis is attractive. Among several potential therapeutic approaches, LXR agonism is most conceptually attractive because it increases both ABCA1 and ABCG1 expression, promotes macrophage cholesterol efflux in vitro 19 and macrophage RCT in vivo, 20 and thus induces the regression of atherosclerosis in mice. 16 However, LXR agonists contribute to hepatic steatosis, as well as elevated plasma triglyceride in animal models. 2 In this regard, it is still difficult to generalize this approach to humans. The current study demonstrated that CoQ10 could significantly promote macrophage RCT and inhibit the progression of established atherosclerosis without adverse effects. Moreover, CoQ10 decreased plasma VLDL cholesterol levels in mice. Although increased plasma VLDL cholesterol is positively related with atherosclerosis development, whether reduction of plasma VLDL cholesterol enables to inhibit the progression of the formed atherosclerotic plaque remained unclear. 21 Consistent with other studies, 16 we evidenced that the LXR agonist T promotes RCT leading to atherosclerosis regression accompanied with hypertriglyceridemia. Reduction of VLDL cholesterol in mice treated with CoQ10 might partially contribute to the inhibition of atherosclerosis. However, the promotion of macrophage RCT might be a significant factor responsible for the inhibitory effect on the formed atherosclerosis progression. Because CoQ10 is highly safe for use as a dietary supplement, 22 it is reasonable to expand the clinical usage of CoQ10 for patients with atherosclerotic lesions in addition to those with heart failure. 9 We found that CoQ10 induces ABCG1 expression in an LXRα-independent manner. Instead, CoQ10 regulates the cholesterol efflux from murine- and human-derived macrophages by downregulating mir-378, which directly targets the 3 -UTRs of ABCG1 mrna. Numerous studies have shown that mirna expression is tightly spatial and temporal. 4 In that context, it is necessary to explore whether mir-378 has a physiological role in atherosclerotic development. We found that mir-378 levels are elevated in aortas during the progression of atherosclerosis in apoe / mice. This finding indicated that mir-378 plays a role in atherosclerosis development. Consistent with this speculation, CoQ10 inhibited the progression of atherosclerosis concomitantly with reduced aortic mir-378 levels.
9 1868 Arterioscler Thromb Vasc Biol September 2014 Downloaded from by guest on April 7, 2018 Figure 7. Coenzyme Q10 (CoQ10) inhibits endogenous foam cell formation and the progression of atherosclerosis in vivo. Male 30-week-old apolipoprotein E deficient (apoe / ) mice were euthanized (baseline) or subjected to oral gavage of CoQ10 (600 mg/kg BW), T (10 mg/kg BW), or the vehicle (normal saline; control) once daily for 4 weeks. Four days before euthanasia, mice (n=12 per group) were intraperitoneally injected with thioglycollate to obtain mouse peritoneal macrophages (MPMs) for Oil Red O staining (A), analyses of total lipid content (B), and total cholesterol content (C). D and E, Representative Oil Red O staining of aortic sinus lesion (D) and quantification of the aortic sinus lesion area (E). F, Total cholesterol content in the thoracic and abdominal aorta. G, Western blotting analyses of ATP-binding cassette transporter G1 (ABCG1) protein expression in aortas and MPMs isolated from baseline, the vehicle-, CoQ10-, or T treated apoe / mice. The aortas and MPMs were pooled with 4 mice in each group, respectively. H, A unified paradigm depicting the role of CoQ10 in promoting macrophage cholesterol efflux in vitro and in vivo. A and G, Representative images of 3 independent assays. D, Representative images of 12 independent experiments. B, C, and F, The data are the mean±sem (n=3). E, The data are the mean±sem (n=12). *P<0.05 vs control, #P<0.05 vs baseline, 1-way ANOVA coupled with the Bonferroni Dunn post hoc test. NS indicates not significant. Moreover, using a time-course experiment assay in mice, we found sequential changes: first, CoQ10 appeared in the serum, then the macrophage mir-378 level decreased; last, ABCG1 expression increased. These phenomena imply that mir-378 might be a novel candidate for promoting macrophage cholesterol efflux and inhibiting atherosclerosis development in vivo. Recent studies have suggested that a single mirna may target >100 mrnas, whereas a single mrna could be regulated by different mirnas. 4 This may be the case of ABCG1, which has >100 potential mirna candidates located in its 3 -UTR (3.7 kb for mouse and 0.9 kb for human) according to mirna target prediction databases. 4 To date, several mirnas, including mir-33 and mir-10b, have been experimentally shown to regulate ABCG1 expression. 7,23 Accordingly, we showed that CoQ10 selectively inhibits mir-378 via downregulation of ABCG1 expression in macrophages. Given a recent study showing that mir-33 and mir-144 cooperatively mediate ABCA1 activity by binding to their respective locations in the ABCA1 3 -UTR, 24 it is possible that other mirnas are required for CoQ10-induced ABCG1 expression. We found that CoQ10 could remarkably upregulate and downregulate the expression of 40 different mirnas by 2-fold. Further studies are needed to discriminate the role of mir-378 from the other mirnas in CoQ10-induced ABCG1 expression and macrophage cholesterol efflux. Although functional studies indicate that mirnas participate in the regulation of cholesterol efflux and the development of atherosclerosis, there is still little known about the molecular mechanisms for mirna expression. Understanding mirna transcription is important for developing new antiatherosclerotic drugs that modulate mirna expression. It has been proposed that 90% of reported TF-binding sites are embedded within 800-bp regions upstream of the transcription start site for pri-mirnas. 25 In the current study, we examined a region 2 kb upstream of the transcription start site of pri-mir-378 in silico 14 and showed that 5 TFs are predicted to bind to the putative promoters of mouse and human pri-mir-378. Subsequently, chromatin immunoprecipitation
10 Wang et al CoQ10 Promotes Macrophage Cholesterol Efflux 1869 Downloaded from by guest on April 7, 2018 and luciferase reporter assays showed that only c-jun, a component of AP-1, is involved in CoQ10-induced regulation of mir-378. Our findings thus provide a third TF regulating mir-378 expression in addition to peroxisome proliferator activated receptor γ and myogenic differentiation 1. 26,27 However, it should be noted that mirna expression usually reflects an integrated consequence of interrelated signals on mirna transcription, 25 thus raising the possibility that other TFs may also be involved in CoQ10-mediated transcriptional modulation of the mir-378 gene. How CoQ10 inhibits c-jun expression in OxLDL-loaded macrophages is still unclear. Previous studies have shown that c-jun belongs to an immediate-early gene and could be rapidly induced at the transcriptional level. 15,28 Specifically, c-jun/activating TF 2 (ATF-2) heterodimers are the major positive transcriptional complex for c-jun transcription. 29 c-jun N-terminal kinase and p38 (2 subfamilies of mitogen-activated protein kinase families) are known to phosphorylate c-jun and ATF-2, which enhances the transcriptional activity of these heterodimers, thereby promoting c-jun transcription. 30 However, it seems to be unlikely that CoQ10 reduces c-jun expression by this manner. For example, JNK and p38 have been shown to participate in OxLDL-induced macrophage-derived foam cell formation by increasing OxLDL uptake and increasing CD36 expression, respectively. 31,32 In contrast, our current findings revealed that CoQ10 cannot alter OxLDL uptake or increasing CD36 expression in either mouse- or human-derived macrophages. Recently, Lau et al 33 reported that the transcriptional activity of ATF-2 can be mediated by protein kinase C-ε (PKC-ε), 1 novel isozymes of PKC. Activation of PKC-ε phosphorylates ATF-2 and increases its transcriptional activity, whereas inhibition of PKC-ε attenuates its transcriptional activity through enabling ATF-2 nuclear export and localization at the mitochondria. Those novel findings suggest that alerting PKC-ε activity and the structure and function of mitochondria might mediate the transcriptional activity of ATF-2. Because CoQ10 is a known factor in maintaining mitochondrion function, 9 it is possible that CoQ10 inhibits c-jun expression through attenuating the transcriptional activity of ATF-2 by altering mitochondrion function. Tsai et al 34 demonstrated that CoQ10 could inhibit PKC-α/β, 2 other isozymes of PKC, in OxLDL-loaded human umbilical vein endothelial cells via regulating AMP-activated protein kinase activity. Therefore, it is also possible that PKC-ε inhibition might be responsible for CoQ10-induced effect on c-jun expression in OxLDL-loaded macrophages. Nevertheless, further investigations are needed to explore the precise mechanisms for the inhibitory effect of CoQ10 on c-jun expression. Atherosclerosis is characterized by excessive cholesterol accumulation in the arterial wall with a strong inflammatory component. 35 Consequently, strategies aimed at both modulating cholesterol removal and inflammation have been brought forward as reasonable targets in limiting the development of atherosclerosis. 35 In addition to the anti-inflammatory property of CoQ10, our present study showed that CoQ10 initially alters AP-1, a known TF-inducing inflammation response, 36 and then regulates macrophage cholesterol efflux by reducing mir-378 expression. Likewise, McGillicuddy et al 37 reported that inflammation could impair macrophage RCT. These data indicate that AP-1 might be a potential molecule that mediates the interaction of inflammation response and macrophage cholesterol efflux. Because the integrated rate of macrophage RCT is mediated by several factors, the increased ABCG1 expression in macrophages may not be wholly responsible for CoQ10-induced effect. Other factors in blood, liver, intestine, and even lymphatic vasculature also might be involved. 1,38 CoQ10 treatment increased the levels of plasma HDL-C. However, plasma HDL-C levels may not always faithfully reflect the rate of macrophage RCT. 1 Moreover, apoe / mice have little HDL-C to begin with, and the subtle changes to HDL-C levels are thus likely not meaningful evidence of increased macrophage RCT. 7 Notably, CoQ10 treatment increased plasma apoa-i levels. If the effect of CoQ10 on plasma apoa-i levels results from the increment in its biosynthesis and secretion from the liver and small intestine, it would be expected that apoa-i is another factor facilitating CoQ10-induced effect on macrophage RCT. 39 Nevertheless, the precise mechanisms for the stimulatory effect of CoQ10 on macrophage RCT require further investigations. In conclusion, we propose the following model for CoQ10-induced promotion of macrophage RCT in vivo (Figure 7H): CoQ10 inhibits AP-1 and further reduces mir-378 expression, which increases the expression of ABCG1 in mouse and human macrophages and thus facilitates macrophage cholesterol efflux in vitro and in vivo. This contributes to reduced progression of atherosclerosis in mice. Because other studies suggested that mir-378 is implicated in lipid metabolism and angiogenesis, 40,41 our findings thus suggest that CoQ10 and mir-378 are promising candidates for atherosclerosis prevention and treatment. Acknowledgments mirna microarray experiments were performed by KangChen Biotech, Shanghai, China. Sources of Funding This work was supported by grants from the National Natural Science Foundation of China ( and ), Natural Science Foundation of Guangdong Province (S ), Research Fund for the Doctoral Program of Higher Education of China ( ), and the Nutrition Research Foundation of By-Health (TY ). None. Disclosures References 1. Rosenson RS, Brewer HB Jr, Davidson WS, Fayad ZA, Fuster V, Goldstein J, Hellerstein M, Jiang XC, Phillips MC, Rader DJ, Remaley AT, Rothblat GH, Tall AR, Yvan-Charvet L. Cholesterol efflux and atheroprotection: advancing the concept of reverse cholesterol transport. Circulation. 2012;125: Beaven SW, Tontonoz P. Nuclear receptors in lipid metabolism: targeting the heart of dyslipidemia. Annu Rev Med. 2006;57: Moore KJ, Rayner KJ, Suárez Y, Fernández-Hernando C. micrornas and cholesterol metabolism. Trends Endocrinol Metab. 2010;21: Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell. 2009;136: Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:
11 1870 Arterioscler Thromb Vasc Biol September 2014 Downloaded from by guest on April 7, Rayner KJ, Sheedy FJ, Esau CC, Hussain FN, Temel RE, Parathath S, van Gils JM, Rayner AJ, Chang AN, Suarez Y, Fernandez-Hernando C, Fisher EA, Moore KJ. Antagonism of mir-33 in mice promotes reverse cholesterol transport and regression of atherosclerosis. J Clin Invest. 2011;121: Wang D, Xia M, Yan X, Li D, Wang L, Xu Y, Jin T, Ling W. Gut microbiota metabolism of anthocyanin promotes reverse cholesterol transport in mice via repressing mirna-10b. Circ Res. 2012;111: Bentinger M, Brismar K, Dallner G. The antioxidant role of coenzyme Q. Mitochondrion. 2007;7(Suppl):S41 S Littarru GP, Tiano L. Clinical aspects of coenzyme Q10: an update. Curr Opin Clin Nutr Metab Care. 2005;8: Lee BJ, Huang YC, Chen SJ, Lin PT. Coenzyme Q10 supplementation reduces oxidative stress and increases antioxidant enzyme activity in patients with coronary artery disease. Nutrition. 2012;28: Lee BJ, Huang YC, Chen SJ, Lin PT. Effects of coenzyme Q10 supplementation on inflammatory markers (high-sensitivity C-reactive protein, interleukin-6, and homocysteine) in patients with coronary artery disease. Nutrition. 2012;28: Schmelzer C, Kitano M, Rimbach G, Niklowitz P, Menke T, Hosoe K, Döring F. Effects of ubiquinol-10 on microrna-146a expression in vitro and in vivo. Mediators Inflamm. 2009;2009: Rothblat GH, Phillips MC. High-density lipoprotein heterogeneity and function in reverse cholesterol transport. Curr Opin Lipidol. 2010;21: Alexiou P, Vergoulis T, Gleditzsch M, Prekas G, Dalamagas T, Megraw M, Grosse I, Sellis T, Hatzigeorgiou AG. mirgen 2.0: a database of microrna genomic information and regulation. Nucleic Acids Res. 2010;38(Database issue):d137 D Shaulian E, Karin M. AP-1 as a regulator of cell life and death. Nat Cell Biol. 2002;4:E131 E Verschuren L, de Vries-van der Weij J, Zadelaar S, Kleemann R, Kooistra T. LXR agonist suppresses atherosclerotic lesion growth and promotes lesion regression in apoe*3leiden mice: time course and mechanisms. J Lipid Res. 2009;50: Tarling EJ. Expanding roles of ABCG1 and sterol transport. Curr Opin Lipidol. 2013;24: Meurs I, Lammers B, Zhao Y, Out R, Hildebrand RB, Hoekstra M, Van Berkel TJ, Van Eck M. The effect of ABCG1 deficiency on atherosclerotic lesion development in LDL receptor knockout mice depends on the stage of atherogenesis. Atherosclerosis. 2012;221: Venkateswaran A, Laffitte BA, Joseph SB, Mak PA, Wilpitz DC, Edwards PA, Tontonoz P. Control of cellular cholesterol efflux by the nuclear oxysterol receptor LXR alpha. Proc Natl Acad Sci U S A. 2000;97: Naik SU, Wang X, Da Silva JS, Jaye M, Macphee CH, Reilly MP, Billheimer JT, Rothblat GH, Rader DJ. Pharmacological activation of liver X receptors promotes reverse cholesterol transport in vivo. Circulation. 2006;113: Nicholls SJ, Tuzcu EM, Brennan DM, Tardif JC, Nissen SE. Cholesteryl ester transfer protein inhibition, high-density lipoprotein raising, and progression of coronary atherosclerosis: insights from ILLUSTRATE (Investigation of Lipid Level Management Using Coronary Ultrasound to Assess Reduction of Atherosclerosis by CETP Inhibition and HDL Elevation). Circulation. 2008;118: Hidaka T, Fujii K, Funahashi I, Fukutomi N, Hosoe K. Safety assessment of coenzyme Q10 (CoQ10). Biofactors. 2008;32: Rayner KJ, Suárez Y, Dávalos A, Parathath S, Fitzgerald ML, Tamehiro N, Fisher EA, Moore KJ, Fernández-Hernando C. MiR-33 contributes to the regulation of cholesterol homeostasis. Science. 2010;328: Ramírez CM, Rotllan N, Vlassov AV, et al. Control of cholesterol metabolism and plasma high-density lipoprotein levels by microrna-144. Circ Res. 2013;112: Megraw M, Baev V, Rusinov V, Jensen ST, Kalantidis K, Hatzigeorgiou AG. MicroRNA promoter element discovery in Arabidopsis. RNA. 2006;12: John E, Wienecke-Baldacchino A, Liivrand M, Heinäniemi M, Carlberg C, Sinkkonen L. Dataset integration identifies transcriptional regulation of microrna genes by PPARγ in differentiating mouse 3T3-L1 adipocytes. Nucleic Acids Res. 2012;40: Gagan J, Dey BK, Layer R, Yan Z, Dutta A. MicroRNA-378 targets the myogenic repressor MyoR during myoblast differentiation. J Biol Chem. 2011;286: Angel P, Hattori K, Smeal T, Karin M. The jun proto-oncogene is positively autoregulated by its product, Jun/AP-1. Cell. 1988;55: Hai T, Curran T. Cross-family dimerization of transcription factors Fos/ Jun and ATF/CREB alters DNA binding specificity. Proc Natl Acad Sci U S A. 1991;88: Whitmarsh AJ, Davis RJ. Transcription factor AP-1 regulation by mitogen-activated protein kinase signal transduction pathways. J Mol Med (Berl). 1996;74: Zhao M, Liu Y, Wang X, New L, Han J, Brunk UT. Activation of the p38 MAP kinase pathway is required for foam cell formation from macrophages exposed to oxidized LDL. APMIS. 2002;110: Rahaman SO, Lennon DJ, Febbraio M, Podrez EA, Hazen SL, Silverstein RL. A CD36-dependent signaling cascade is necessary for macrophage foam cell formation. Cell Metab. 2006;4: Lau E, Kluger H, Varsano T, Lee K, Scheffler I, Rimm DL, Ideker T, Ronai ZA. PKCε promotes oncogenic functions of ATF2 in the nucleus while blocking its apoptotic function at mitochondria. Cell. 2012;148: Tsai KL, Chen LH, Chiou SH, Chiou GY, Chen YC, Chou HY, Chen LK, Chen HY, Chiu TH, Tsai CS, Ou HC, Kao CL. Coenzyme Q10 suppresses oxldl-induced endothelial oxidative injuries by the modulation of LOX-1-mediated ROS generation via the AMPK/PKC/ NADPH oxidase signaling pathway. Mol Nutr Food Res. 2011;55(Suppl 2):S227 S Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med. 2005;352: Schonthaler HB, Guinea-Viniegra J, Wagner EF. Targeting inflammation by modulating the Jun/AP-1 pathway. Ann Rheum Dis. 2011;70(Suppl 1):i109 i McGillicuddy FC, de la Llera Moya M, Hinkle CC, Joshi MR, Chiquoine EH, Billheimer JT, Rothblat GH, Reilly MP. Inflammation impairs reverse cholesterol transport in vivo. Circulation. 2009;119: Martel C, Li W, Fulp B, Platt AM, Gautier EL, Westerterp M, Bittman R, Tall AR, Chen SH, Thomas MJ, Kreisel D, Swartz MA, Sorci-Thomas MG, Randolph GJ. Lymphatic vasculature mediates macrophage reverse cholesterol transport in mice. J Clin Invest. 2013;123: Zhang Y, Zanotti I, Reilly MP, Glick JM, Rothblat GH, Rader DJ. Overexpression of apolipoprotein A-I promotes reverse transport of cholesterol from macrophages to feces in vivo. Circulation. 2003;108: Gerin I, Bommer GT, McCoin CS, Sousa KM, Krishnan V, MacDougald OA. Roles for mirna-378/378* in adipocyte gene expression and lipogenesis. Am J Physiol Endocrinol Metab. 2010;299:E198 E Lee DY, Deng Z, Wang CH, Yang BB. MicroRNA-378 promotes cell survival, tumor growth, and angiogenesis by targeting SuFu and Fus-1 expression. Proc Natl Acad Sci U S A. 2007;104: Significance Coenzyme Q10 is often prescribed for patients with heart failure. Although antioxidative, anti-inflammatory, and cellular bioenergetic functions of coenzyme Q10 contribute to its beneficial effect, it remains unclear whether other mechanisms are involved. In the present study, we demonstrated that coenzyme Q10 greatly inhibits established atherosclerosis progression and enhances macrophage reverse cholesterol transport, a critical pathway in combating atherosclerosis, through a previously undescribed activator protein-1/microrna-378/atp-binding cassette transporter G1 signaling pathway in apolipoprotein E deficient mice. Our novel data support the notion that coenzyme Q10 could be implicated in protection of atherosclerotic cardiovascular diseases not limited to heart failure. Furthermore, microrna-378 seems to be a promising candidate for the therapy of atherosclerosis.
12 Downloaded from by guest on April 7, 2018 Coenzyme Q10 Promotes Macrophage Cholesterol Efflux by Regulation of the Activator Protein-1/miR-378/ATP-Binding Cassette Transporter G1 Signaling Pathway Dongliang Wang, Xiao Yan, Min Xia, Yan Yang, Dan Li, Xinrui Li, Fenglin Song and Wenhua Ling Arterioscler Thromb Vasc Biol. 2014;34: ; originally published online March 27, 2014; doi: /ATVBAHA Arteriosclerosis, Thrombosis, and Vascular Biology is published by the American Heart Association, 7272 Greenville Avenue, Dallas, TX Copyright 2014 American Heart Association, Inc. All rights reserved. Print ISSN: Online ISSN: The online version of this article, along with updated information and services, is located on the World Wide Web at: Data Supplement (unedited) at: Permissions: Requests for permissions to reproduce figures, tables, or portions of articles originally published in Arteriosclerosis, Thrombosis, and Vascular Biology can be obtained via RightsLink, a service of the Copyright Clearance Center, not the Editorial Office. Once the online version of the published article for which permission is being requested is located, click Request Permissions in the middle column of the Web page under Services. Further information about this process is available in the Permissions and Rights Question and Answer document. Reprints: Information about reprints can be found online at: Subscriptions: Information about subscribing to Arteriosclerosis, Thrombosis, and Vascular Biology is online at:
13 Supplemental Material Supplemental Table I. Sequences of primers for real-time quantitative PCR Gene Species Forward primer (5' to 3') Reverse primer (5' to 3') ABCA1 mouse GGACTTGCCTTGTTCCGAGAG GCTGCCACATAACTGATAGCGA ABCA1 human ACCCACCCTATGAACAACATGA GAGTCGGGTAACGGAAACAGG ABCG1 mouse CTCCATCGTCTGTACCATCC CTCCATCGTCTGTACCATCC ABCG1 human ATTCAGGGACCTTTCCTATTCGG CTCACCACTATTGAACTTCCCG SR-BI mouse GGCTGCTGTTTGCTGCG GCTGCTTGATGAGGGAGGG SR-BI human ACTTCTGGCATTCCGATCAGT ACGAAGCGATAGGTGGGGAT SR-A mouse ATGACAGAGAATCAGAGG CCCTCTGTCTCCCTTTTC SR-A human CCAGGGACATGGAATGCAA CCAGTGGGACCTCGATCTCC CD-36 mouse CAGCCCAATGGAGCCATC CAGCGTAGATAGACCTGC CD-36 human GAGAACTGTTATGGGGCTAT TTCAACTGGAGAGGCAAAGG SREBP1 mouse ACTTCCCTGGCCTATTTGACC GGCATGGACGGGTACATCTT SREBP1 human CAAGGCCATCGACTACATT TTGCTTTTGTGGACAGCAGT SREBP2 mouse GTGGAGCAGTCTCAACGTCA TGGTAGGTCTCACCCAGGAG SREBP2 human AGGAGAACATGGTGCTGA TAAAGGAGAGGCACAGGA HMGCR mouse CTTGTGGAATGCCTTGTGATTG AGCCGAAGCAGCACATGAT HMGCR human GTCATTCCAGCCAAGGTTGT GGGACCACTTGCTTCCATTA LDLR mouse GGTACTGGCACAACAACTTGGG GCCAATCGACTCACGGGTTCAG LDLR human CAGATATCATCAACGAAGC CCTCTCACACCAGTTCACTCC LXRα mouse GAGCCGACAGAGCTTCGTC GCGTGCTCCCTTGATGACA LXRα human ACACCTACATGCGTCGCAAG GACGAGCTTCTCGATCATGCC c-jun mouse AGCCTACCAACGTGAGTGCT AGAACGGTCCGTCACTTCAC c-jun human TGACTGCAAAGATGGAAACG CAGGGTCATGCTCTGTTTCA c-fos mouse GCCCAGTGAGGAATATCTGGA ATCGCAGATGAAG CTCTGGT c-fos human GAGAGCTGGTAGTTAGTAGCATGTT GA AATTCCAATAATGAACCCAATAGAT TAGTTA mouse GCAGGCTTAAGAAGTGCTTC GGCTGCTGTCCTCGTAGCTT human TGTCCCGACAGATCACCTC CACTCAACGAGAACCAGCAG ELK-1 mouse TGCTCCCCACACATACCTTGA ACTGGACGGAAACTGGAAGGA ELK-1 human TTGGAGGCCTGTCTGGAGGCTGAA AGCTCTTCCGATTTCAGGTTTGGG NKX2-5 mouse GACAGGTACCGCTGTTGCTT AGCCTACGGTGACCCTGAC NKX2-5 human AAGAGCTGTGCGCGCTGCAGAA ATCTTGACCTGCGTGGACGTG c-rel mouse CTGGAGCCTGTGACAGTGAA GAGTGTTCCCGCTGAGAAAG c-rel human GGAAAAGACTGCAGAGACGG ATTGGGTTCGAGACAACAGG GAPDH mouse GGCAGCTTCGGCACATATTTC CCAGGGTTATAGTCCTTCTCGT GAPDH human CTCACCGGATGCACCAATGTT CGCGTTGCTCACAATGTTCAT 1
14 Supplemental Table II. Potential promoter binding of TFs in the transactivation of mir-378 genes in J774 and THP-1 macrophages Mature mirnas Chromosome (strand) TFs Transfac id TF motif and distance from TSS mmu-mir (-) AP-1 V$AP1_Q2 tgtgagtcacc (-37) HNF-4 V$HNF4_01 ctaagagaaaagttcatag (-157) ELK-1 V$ELK1_01 aatacaggatgttcca (-1463) NKX2-5 V$NKX25_01 tcaagtg (-1018) c-rel V$CREL_01 GGAAAtccca (-203) hsa-mir (+) AP-1 V$AP1_Q4 aattagtcact (-531) aattagtcact (-579) HNF-4 V$HNF4_01 ctaagagaaaagttcatag (-160) ctaagagaaaagttcatag (-181) ctaagagaaaagttcatag (-229) ctaagagaaaagttcatag (-360) ELK-1 V$ELK1_02 cagaccggaaatac (-122) NKX2-5 V$NKX25_02 caattaag (-670) V$NKX25_01 tcaagtg (-751) tcaagtg (-897) c-rel V$CREL_01 GGAAAtccca (-95) 2
15 Supplemental Table III. Plasma lipid profile and ApoA-I levels in ApoE / mice from different groups Groups n TC (mg/dl) TG (mg/dl) HDL-C (mg/dl) ApoA-I (mg/dl) Control ± ± ± ± 1.2 CoQ ± 34 * 87 ± 3.3 * 36.3 ± 3.5 * 16.4 ± 0.9 * T ± ± 7.4 * 29.4 ± ± 1.4 The data were the means ± SEM from the indicated numbers of male ApoE / mice in each group. * The means in the same column as an asterisk differ from the control group, P <
16 Supplemental Figure I. CoQ10 reduces OxLDL-induced lipid accumulation in macrophages. A, Mouse J774.A1 macrophages were treated with the vehicle (DMSO), OxLDL (50 g/ml), CoQ10 (10 M), or a combination of CoQ10 and OxLDL for 24 hours. Cells were then fixed with paraformaldehyde and stained with filipin. The figure is representative of 4 independent experiments (magnification 400, upper panel). The density of lipid content was evaluated by alcohol extraction after staining, and fluorescent signals were quantified (lower panel). B, J774.A1 macrophages were treated with CoQ10 or DMSO in the presence or absence of OxLDL for 24 hours. Cells were then incubated with DiI-OxLDL for another 4 hours. Representative images under fluorescence microscopy are shown (magnification 400, upper panel). DiI was extracted by isopropanol, and the fluorescence was determined at 520/564 nm (lower panel). C, Western blotting (upper panel) and qrt-pcr (lower panel) analyses of SR-A and CD36 protein and mrna expression in J774.A1 macrophages treated with DMSO, CoQ10, OxLDL, or a combination of CoQ10 and OxLDL for 24 hours. D and E, qrt-pcr analyses of ACAT1 and HSL (D), SREBP1, SREBP2, HMGCR, and LDLR (E) in J774.A1 macrophages treated with CoQ10 or DMSO in the presence of OxLDL for 24 hours. The upper panels of C are representative images of 3 independent assays. Lower panels of A to C, as well as D and E, The results are the mean ± SEM. n = 3. * P < 0.05 vs. [CoQ10 (-) and OxLDL (+)], one-way ANOVA coupled with the Bonferroni-Dunn post hoc test. 4
17 Supplemental Figure II. Quantification of protein expression in vitro and in vivo. A and B, Quantification of ABCA1, ABCG1, and SR-BI protein (relative to -actin) in OxLDL-loaded J774.A1 (A) and THP-1 macrophages (B) treated with CoQ10 (1, 10, 100 M) or the vehicle (DMSO) for 24 hours. Data correspond to Western blotting in Figure 2B and 2D. C, Quantification of ABCG1 protein in OxLDL-loaded J774.A1 and THP-1 macrophages transfected with scrambled or ABCG1 sirna with or without CoQ10 (10 M). Data correspond to Western blotting in the inset of Figure 2E and 2F. D, Quantification of mouse and human ABCG1 protein in OxLDL-loaded J774.A1 and THP-1 macrophages transfected with Con mir (150 nm) or the indicated concentrations of mir-378 for 48 hours. Data correspond to Western blotting in Figure 3B and 3D. E, Quantification of ABCG1 protein in OxLDL-loaded J774.A1 and THP-1 macrophages transfected with Con mir (150 nm) or mir-378 (150 nm) in the presence or absence of a control inhibitor (Con Inh,150 nm) or anti-mir-378 (150 nm). Data correspond to Western blotting in Figure 3F and 3H. F, OxLDL-loaded J774.A1 and THP-1 macrophages were transfected with either a control mir (Con mir, 150 nm) or mir-378 (40 or 150 nm), or a control inhibitor (Con Inh, 150 nm) or anti-mir-378 (150 nm) for 24 hours. Cells were then treated with CoQ10 (10 M) or the vehicle (DMSO) for another 24 hour. The ABCG1 protein expression was then quantified. Data correspond to Western blotting in Figure 4A. G and H, Quantification of ABCG1 protein in OxLDL-loaded J774.A1 (G) and THP-1 macrophages (H) treated with CoQ10 or the vehicle (DMSO) for 24 hours. Data correspond to Western blotting in left panels of Figure 5A and B. I, Quantification of ABCG1 protein in OxLDL-loaded J774.A1 and THP-1 macrophages transfected with scrambled or c-jun sirna in the presence or absence of Con mir (150 nm) or mir-378 (150 nm) for 48 hours. Data correspond to Western blotting in left panels of Figure 5C and D. J and K, 5
18 Thirty-week-old male ApoE / mice were orally gavaged with CoQ10 (600 mg/kg BW) or the vehicle (normal saline) once daily for 14 days. The protein expressions of c-jun, c-fos, ABCA1, ABCG1, and SR-BI in aortas and thioglycollate-elicited MPMs isolated from CoQ10- or the vehicle-treated ApoE / mice were then quantified. The aortas and MPMs were pooled with 4 mice in each group. Data correspond to Western blotting in Figure 6B, D, E, and G. L and M, Male 30-week-old ApoE / mice were sacrificed (Baseline) or subjected to oral gavage of CoQ10 (600 mg/kg BW), T (10 mg/kg BW), or the vehicle (normal saline) (Control) once daily for 4 weeks. The ABCG1 protein expressions in aortas and MPMs isolated from Baseline, the vehicle-, CoQ10-, or T treated ApoE / mice were then quantified. The aortas and MPMs were pooled with 4 mice in each group, respectively. Data correspond to Western blotting in Figure 7G. F, The data are the mean ± SEM. n = 6. * P < 0.05 vs. CoQ10, Student s t test. NS indicates not significant. For the other panels, data are the mean ± SEM. n = 3. A, B, G, and H, * P < 0.05 vs. Control, one-way ANOVA coupled with the Bonferroni-Dunn post hoc test. C, * P < 0.05 vs. Ctr si, Student s t test. D and E, * P < 0.05 vs. Con mir or Con mir and Con Inh, one-way ANOVA coupled with the Bonferroni-Dunn post hoc test. I, * P < 0.05 vs. Ctr si, # P < 0.05 vs. (Con mir + c-jun si), Student s t test. J and K, * P < 0.05 vs. Control, Student s t test. L and M, * P < 0.05 vs. Control, one-way ANOVA coupled with Bonferroni-Dunn post hoc test. 6
19 Supplemental Figure III. CoQ10 does not transcriptionally induce ABCG1 in macrophages. A and B, OxLDL-loaded J774.A1 (A) and THP-1 macrophages (B) were cotransfected with human ABCG1 promoter and Renilla (for internal normalization) followed by the vehicle (DMSO), CoQ10 (1, 10, 100 M) or T (1 M) treatment for 24 hours. Cellular lysate was then used to determine the activity of firefly and Renilla luciferases. C to F, qrt-pcr and Western blot analyses of LXR mrna (C and D) and protein (E and F) levels in nuclear extract in OxLDL-loaded 7
20 J774.A1 and THP-1 macrophages treated with CoQ10 or DMSO for 24 hours. G and H, OxLDL-loaded J774.A1 (G) and THP-1 macrophages (H) were transfected with a luciferase reporter plasmid containing the liver X receptor element upstream of the thymidine kinase promoter (LXRE-tk-Luc) (kindly provided by David J. Mangelsdorf, University of Texas Southwestern Medical Center) in the presence of -galactosidase ( -gal) as a reference plasmid. Twelve hours after transfection, cells were treated with CoQ10 or DMSO for 24 h. Luciferase and -gal activities were then determined in the cell lysates. E and F, Representative images of 3 independent assays. For the other panels, results are the mean ± SEM. n = 3 to 6. The data were analyzed by one-way ANOVA and the Bonferroni-Dunn post hoc test. 8
21 Supplemental Figure IV. CoQ10 decreases mir-378 expression in macrophages. A, Heat map of 40 mirnas differentially expressed in OxLDL-loaded J774.A1 macrophages treated with CoQ10 (10 M) or the vehicle (DMSO) for 24 hours. Scale of relative intensity (log 2 ) is shown, upper heat map. Green indicates expression levels below control macrophages and red indicates expression levels greater than control macrophages. B to D, qrt-pcr analysis of mirnas differentially expressed in OxLDL-loaded MPMs (B), J774.A1 (C), and THP-1 (D) macrophages treated with CoQ10 or DMSO for 24 hours. The data are the mean ± SEM. n = 3 to 6. * P < 0.05 vs. Control, Student s t test. 9
Molecular Medicine. Gut Microbiota Metabolism of Anthocyanin Promotes Reverse Cholesterol Transport in Mice Via Repressing mirna-10b
 Molecular Medicine Gut Microbiota Metabolism of Anthocyanin Promotes Reverse Cholesterol Transport in Mice Via Repressing mirna-10b Dongliang Wang, Min Xia, Xiao Yan, Dan Li, Lei Wang, Yuxuan Xu, Tianru
Molecular Medicine Gut Microbiota Metabolism of Anthocyanin Promotes Reverse Cholesterol Transport in Mice Via Repressing mirna-10b Dongliang Wang, Min Xia, Xiao Yan, Dan Li, Lei Wang, Yuxuan Xu, Tianru
Strathprints Institutional Repository
 Strathprints Institutional Repository Tate, Rothwelle and Rotondo, Dino and Davidson, Jillian (2015) Regulation of lipid metabolism by micrornas. Current Opinion in Lipidology, 26 (3). pp. 243-244. ISSN
Strathprints Institutional Repository Tate, Rothwelle and Rotondo, Dino and Davidson, Jillian (2015) Regulation of lipid metabolism by micrornas. Current Opinion in Lipidology, 26 (3). pp. 243-244. ISSN
Materials and Methods Cell culture Foam cell formation Cellular uptake of DiI-OxLDL In vitro cholesterol efflux assays
 Materials and Methods Cell culture Mouse peritoneal macrophages (MPMs) were harvested from adult C57BL/6J mice (Jackson Laboratories, Sacramento, CA) and cultured as previously described. 1 J774.A1, THP-1,
Materials and Methods Cell culture Mouse peritoneal macrophages (MPMs) were harvested from adult C57BL/6J mice (Jackson Laboratories, Sacramento, CA) and cultured as previously described. 1 J774.A1, THP-1,
Supplementary Figure S I: Effects of D4F on body weight and serum lipids in apoe -/- mice.
 Supplementary Figures: Supplementary Figure S I: Effects of D4F on body weight and serum lipids in apoe -/- mice. Male apoe -/- mice were fed a high-fat diet for 8 weeks, and given PBS (model group) or
Supplementary Figures: Supplementary Figure S I: Effects of D4F on body weight and serum lipids in apoe -/- mice. Male apoe -/- mice were fed a high-fat diet for 8 weeks, and given PBS (model group) or
Summary and concluding remarks
 Summary and concluding remarks This thesis is focused on the role and interaction of different cholesterol and phospholipid transporters. Cholesterol homeostasis is accomplished via a tightly regulated
Summary and concluding remarks This thesis is focused on the role and interaction of different cholesterol and phospholipid transporters. Cholesterol homeostasis is accomplished via a tightly regulated
Atherosclerosis is the underlying cause of cardiovascular
 RP5-833A20.1/miR-382-5p/NFIA Dependent Signal Transduction Pathway Contributes to the Regulation of Cholesterol Homeostasis and Inflammatory Reaction Yan-Wei Hu, Jia-Yi Zhao, Shu-Fen Li, Jin-Lan Huang,
RP5-833A20.1/miR-382-5p/NFIA Dependent Signal Transduction Pathway Contributes to the Regulation of Cholesterol Homeostasis and Inflammatory Reaction Yan-Wei Hu, Jia-Yi Zhao, Shu-Fen Li, Jin-Lan Huang,
2.5. AMPK activity
 Supplement Fig. A 3 B phos-ampk 2.5 * Control AICAR AMPK AMPK activity (Absorbance at 45 nm) 2.5.5 Control AICAR Supplement Fig. Effects of AICAR on AMPK activation in macrophages. J774. macrophages were
Supplement Fig. A 3 B phos-ampk 2.5 * Control AICAR AMPK AMPK activity (Absorbance at 45 nm) 2.5.5 Control AICAR Supplement Fig. Effects of AICAR on AMPK activation in macrophages. J774. macrophages were
High density lipoprotein metabolism
 High density lipoprotein metabolism Lipoprotein classes and atherosclerosis Chylomicrons, VLDL, and their catabolic remnants Pro-atherogenic LDL HDL Anti-atherogenic Plasma lipid transport Liver VLDL FC
High density lipoprotein metabolism Lipoprotein classes and atherosclerosis Chylomicrons, VLDL, and their catabolic remnants Pro-atherogenic LDL HDL Anti-atherogenic Plasma lipid transport Liver VLDL FC
Table S1. Quantitative RT-PCR primers
 Table S1. Quantitative RT-PCR primers Gene Forward Primer Reverse Primer Human ApoB gcaagcagaagccagaagta ccatttggagaagcagtttgg Human ApoA1 gaaagctgcggtgctgac agtggccaggtccttcact Human MTP acggccattcccattgtg
Table S1. Quantitative RT-PCR primers Gene Forward Primer Reverse Primer Human ApoB gcaagcagaagccagaagta ccatttggagaagcagtttgg Human ApoA1 gaaagctgcggtgctgac agtggccaggtccttcact Human MTP acggccattcccattgtg
 Glossary For TheFatNurse s For All Ages Series Adipocytes, also known as lipocytes and fat cells, are the cells that primarily compose adipose tissue, specialized in storing energy as fat. Apolipoprotein
Glossary For TheFatNurse s For All Ages Series Adipocytes, also known as lipocytes and fat cells, are the cells that primarily compose adipose tissue, specialized in storing energy as fat. Apolipoprotein
Supplementary Figure 1. Confocal immunofluorescence showing mitochondrial translocation of Drp1. Cardiomyocytes treated with H 2 O 2 were prestained
 Supplementary Figure 1. Confocal immunofluorescence showing mitochondrial translocation of Drp1. Cardiomyocytes treated with H 2 O 2 were prestained with MitoTracker (red), then were immunostained with
Supplementary Figure 1. Confocal immunofluorescence showing mitochondrial translocation of Drp1. Cardiomyocytes treated with H 2 O 2 were prestained with MitoTracker (red), then were immunostained with
G. Chinetti-Gbaguidi and B. Staels, UR 545 INSERM, Institut Pasteur de Lille and Université de Lille 2, Lille, France
 LIVER X RECEPTORS (LXRS): TRANSCRIPTIONAL REGULATORS OF MACROPHAGE CHOLESTEROL METABOLISM G. Chinetti-Gbaguidi and B. Staels, UR 545 INSERM, Institut Pasteur de Lille and Université de Lille 2, Lille,
LIVER X RECEPTORS (LXRS): TRANSCRIPTIONAL REGULATORS OF MACROPHAGE CHOLESTEROL METABOLISM G. Chinetti-Gbaguidi and B. Staels, UR 545 INSERM, Institut Pasteur de Lille and Université de Lille 2, Lille,
Potential Atheroprotective Effects of Ixmyelocel-T Cellular Therapy. Kelly J. Ledford, Nikki Murphy, Frank Zeigler, Ronnda L.
 Potential Atheroprotective Effects of Ixmyelocel-T Cellular Therapy Kelly J. Ledford, Nikki Murphy, Frank Zeigler, Ronnda L. Bartel 1 Ixmyelocel-T, an expanded, autologous multicellular therapy cultured
Potential Atheroprotective Effects of Ixmyelocel-T Cellular Therapy Kelly J. Ledford, Nikki Murphy, Frank Zeigler, Ronnda L. Bartel 1 Ixmyelocel-T, an expanded, autologous multicellular therapy cultured
RNA interference induced hepatotoxicity results from loss of the first synthesized isoform of microrna-122 in mice
 SUPPLEMENTARY INFORMATION RNA interference induced hepatotoxicity results from loss of the first synthesized isoform of microrna-122 in mice Paul N Valdmanis, Shuo Gu, Kirk Chu, Lan Jin, Feijie Zhang,
SUPPLEMENTARY INFORMATION RNA interference induced hepatotoxicity results from loss of the first synthesized isoform of microrna-122 in mice Paul N Valdmanis, Shuo Gu, Kirk Chu, Lan Jin, Feijie Zhang,
SUPPLEMENTARY INFORMATION
 DOI: 10.1038/ncb3461 In the format provided by the authors and unedited. Supplementary Figure 1 (associated to Figure 1). Cpeb4 gene-targeted mice develop liver steatosis. a, Immunoblot displaying CPEB4
DOI: 10.1038/ncb3461 In the format provided by the authors and unedited. Supplementary Figure 1 (associated to Figure 1). Cpeb4 gene-targeted mice develop liver steatosis. a, Immunoblot displaying CPEB4
Niacin Metabolism: Effects on Cholesterol
 Niacin Metabolism: Effects on Cholesterol By Julianne R. Edwards For Dr. William R. Proulx, PhD, RD Associate Professor of Nutrition and Dietetics In partial fulfillments for the requirements of NUTR342
Niacin Metabolism: Effects on Cholesterol By Julianne R. Edwards For Dr. William R. Proulx, PhD, RD Associate Professor of Nutrition and Dietetics In partial fulfillments for the requirements of NUTR342
Regulating Hepatic Cellular Cholesterol
 Under circumstances of cholesterol deficiency, Sterol Regulatory Element Binding Proteins (SREBPs) via binding to DNA nuclear response elements set off genomic production of proteins and enzymes that induce
Under circumstances of cholesterol deficiency, Sterol Regulatory Element Binding Proteins (SREBPs) via binding to DNA nuclear response elements set off genomic production of proteins and enzymes that induce
Stewart et al. CD36 ligands promote sterile inflammation through assembly of a TLR 4 and 6 heterodimer
 NFκB (fold induction) Stewart et al. ligands promote sterile inflammation through assembly of a TLR 4 and 6 heterodimer a. mrna (fold induction) 5 4 3 2 1 LDL oxldl Gro1a MIP-2 RANTES mrna (fold induction)
NFκB (fold induction) Stewart et al. ligands promote sterile inflammation through assembly of a TLR 4 and 6 heterodimer a. mrna (fold induction) 5 4 3 2 1 LDL oxldl Gro1a MIP-2 RANTES mrna (fold induction)
Supplementary Figure 1:
 Supplementary Figure 1: (A) Whole aortic cross-sections stained with Hematoxylin and Eosin (H&E), 7 days after porcine-pancreatic-elastase (PPE)-induced AAA compared to untreated, healthy control aortas
Supplementary Figure 1: (A) Whole aortic cross-sections stained with Hematoxylin and Eosin (H&E), 7 days after porcine-pancreatic-elastase (PPE)-induced AAA compared to untreated, healthy control aortas
Soft Agar Assay. For each cell pool, 100,000 cells were resuspended in 0.35% (w/v)
 SUPPLEMENTARY MATERIAL AND METHODS Soft Agar Assay. For each cell pool, 100,000 cells were resuspended in 0.35% (w/v) top agar (LONZA, SeaKem LE Agarose cat.5004) and plated onto 0.5% (w/v) basal agar.
SUPPLEMENTARY MATERIAL AND METHODS Soft Agar Assay. For each cell pool, 100,000 cells were resuspended in 0.35% (w/v) top agar (LONZA, SeaKem LE Agarose cat.5004) and plated onto 0.5% (w/v) basal agar.
mirna Dr. S Hosseini-Asl
 mirna Dr. S Hosseini-Asl 1 2 MicroRNAs (mirnas) are small noncoding RNAs which enhance the cleavage or translational repression of specific mrna with recognition site(s) in the 3 - untranslated region
mirna Dr. S Hosseini-Asl 1 2 MicroRNAs (mirnas) are small noncoding RNAs which enhance the cleavage or translational repression of specific mrna with recognition site(s) in the 3 - untranslated region
Novel Reduction of PCSK9 Expression: Mechanistic Insights into the Anti-Atherosclerotic & Hypolipidemic Effects of Heat Shock Protein 27
 Novel Reduction of PCSK9 Expression: Mechanistic Insights into the Anti-Atherosclerotic & Hypolipidemic Effects of Heat Shock Protein 27 Ed O Brien, Jean-Claude Bakala-N Goma, Chunhua Shi Cumming School
Novel Reduction of PCSK9 Expression: Mechanistic Insights into the Anti-Atherosclerotic & Hypolipidemic Effects of Heat Shock Protein 27 Ed O Brien, Jean-Claude Bakala-N Goma, Chunhua Shi Cumming School
Endotoxin Induces Toll-Like Receptor 4 Expression in Vascular Cells: A Novel Mechanism Involved in Vascular Inflammation
 Endotoxin Induces Toll-Like Receptor 4 Expression in Vascular Cells: A Novel Mechanism Involved in Vascular Inflammation Introduction Feng-Yen Lin, Ph.D. 1, and Shing-Jong Lin, M.D., PhD. 2, 1 Department
Endotoxin Induces Toll-Like Receptor 4 Expression in Vascular Cells: A Novel Mechanism Involved in Vascular Inflammation Introduction Feng-Yen Lin, Ph.D. 1, and Shing-Jong Lin, M.D., PhD. 2, 1 Department
Differential inhibition of macrophage foam-cell formation and atherosclerosis in mice by PPARα, β/δ, and γ
 Research article Related Commentary, page 1538 Differential inhibition of macrophage foam-cell formation and atherosclerosis in mice by PPARα, β/δ, and γ Andrew C. Li, 1 Christoph J. Binder, 2 Alejandra
Research article Related Commentary, page 1538 Differential inhibition of macrophage foam-cell formation and atherosclerosis in mice by PPARα, β/δ, and γ Andrew C. Li, 1 Christoph J. Binder, 2 Alejandra
BIO360 Fall 2013 Quiz 1
 BIO360 Fall 2013 Quiz 1 1. Examine the diagram below. There are two homologous copies of chromosome one and the allele of YFG carried on the light gray chromosome has undergone a loss-of-function mutation.
BIO360 Fall 2013 Quiz 1 1. Examine the diagram below. There are two homologous copies of chromosome one and the allele of YFG carried on the light gray chromosome has undergone a loss-of-function mutation.
Berberine Sensitizes Human Ovarian Cancer Cells to Cisplatin Through mir-93/ PTEN/Akt Signaling Pathway
 Chen Accepted: et al.: February Berberine 24, Sensitizes 2015 Ovarian Cancer Cells to Cisplatin www.karger.com/cpb 956 1421-9778/15/0363-0956$39.50/0 Original Paper This is an Open Access article licensed
Chen Accepted: et al.: February Berberine 24, Sensitizes 2015 Ovarian Cancer Cells to Cisplatin www.karger.com/cpb 956 1421-9778/15/0363-0956$39.50/0 Original Paper This is an Open Access article licensed
Original Article Regulation of macrophage cholesterol efflux and liver X receptor α activation by nicotine
 Int J Clin Exp Med 2015;8(9):16374-16378 www.ijcem.com /ISSN:1940-5901/IJCEM0010359 Original Article Regulation of macrophage cholesterol efflux and liver X receptor α activation by nicotine Hongming Zhang
Int J Clin Exp Med 2015;8(9):16374-16378 www.ijcem.com /ISSN:1940-5901/IJCEM0010359 Original Article Regulation of macrophage cholesterol efflux and liver X receptor α activation by nicotine Hongming Zhang
HDL and Its Galaxy of Proteins: What Do they Do? Jay Heinecke, University of Washington
 HDL and Its Galaxy of Proteins: What Do they Do? Jay Heinecke, University of Washington Disclosures NIH AHA Insilicos Consultant Merck Research Support GSK Research Support Pfizer Research Support Corcept
HDL and Its Galaxy of Proteins: What Do they Do? Jay Heinecke, University of Washington Disclosures NIH AHA Insilicos Consultant Merck Research Support GSK Research Support Pfizer Research Support Corcept
High AU content: a signature of upregulated mirna in cardiac diseases
 https://helda.helsinki.fi High AU content: a signature of upregulated mirna in cardiac diseases Gupta, Richa 2010-09-20 Gupta, R, Soni, N, Patnaik, P, Sood, I, Singh, R, Rawal, K & Rani, V 2010, ' High
https://helda.helsinki.fi High AU content: a signature of upregulated mirna in cardiac diseases Gupta, Richa 2010-09-20 Gupta, R, Soni, N, Patnaik, P, Sood, I, Singh, R, Rawal, K & Rani, V 2010, ' High
Supplementary Table I - Primers used for real-time quantitative PCR and RT-PCR
 Supplement Supplementary Table I - Primers used for real-time quantitative PCR and RT-PCR Gene Forward Primer (5-3 ) Reverse primer (5-3 ) Reference Human ST2 CTTGATTGATAAACAGAATG CTGATCCAGATACTGTTGAA
Supplement Supplementary Table I - Primers used for real-time quantitative PCR and RT-PCR Gene Forward Primer (5-3 ) Reverse primer (5-3 ) Reference Human ST2 CTTGATTGATAAACAGAATG CTGATCCAGATACTGTTGAA
Table S1. Primer sequences used for qrt-pcr. CACCATTGGCAATGAGCGGTTC AGGTCTTTGCGGATGTCCACGT ACTB AAGTCCATGTGCTGGCAGCACT ATCACCACTCCGAAGTCCGTCT LCOR
 Table S1. Primer sequences used for qrt-pcr. ACTB LCOR KLF6 CTBP1 CDKN1A CDH1 ATF3 PLAU MMP9 TFPI2 CACCATTGGCAATGAGCGGTTC AGGTCTTTGCGGATGTCCACGT AAGTCCATGTGCTGGCAGCACT ATCACCACTCCGAAGTCCGTCT CGGCTGCAGGAAAGTTTACA
Table S1. Primer sequences used for qrt-pcr. ACTB LCOR KLF6 CTBP1 CDKN1A CDH1 ATF3 PLAU MMP9 TFPI2 CACCATTGGCAATGAGCGGTTC AGGTCTTTGCGGATGTCCACGT AAGTCCATGTGCTGGCAGCACT ATCACCACTCCGAAGTCCGTCT CGGCTGCAGGAAAGTTTACA
SUPPLEMENTARY INFORMATION
 doi:10.1038/nature12652 Supplementary Figure 1. PRDM16 interacts with endogenous EHMT1 in brown adipocytes. Immunoprecipitation of PRDM16 complex by flag antibody (M2) followed by Western blot analysis
doi:10.1038/nature12652 Supplementary Figure 1. PRDM16 interacts with endogenous EHMT1 in brown adipocytes. Immunoprecipitation of PRDM16 complex by flag antibody (M2) followed by Western blot analysis
Print: ISSN Online: ISSN
 Review Article Interplay between Cholesterol, SREBPs, MicroRNA-33 in Dyslipidemia Abdul Hafeez Kandhro Abdul Hafeez Kandhro, Healthcare Molecular & Diagnostic Laboratory, Hyderabad, Pakistan, Email of
Review Article Interplay between Cholesterol, SREBPs, MicroRNA-33 in Dyslipidemia Abdul Hafeez Kandhro Abdul Hafeez Kandhro, Healthcare Molecular & Diagnostic Laboratory, Hyderabad, Pakistan, Email of
Supplementary Figure S1. Venn diagram analysis of mrna microarray data and mirna target analysis. (a) Western blot analysis of T lymphoblasts (CLS)
 Supplementary Figure S1. Venn diagram analysis of mrna microarray data and mirna target analysis. (a) Western blot analysis of T lymphoblasts (CLS) and their exosomes (EXO) in resting (REST) and activated
Supplementary Figure S1. Venn diagram analysis of mrna microarray data and mirna target analysis. (a) Western blot analysis of T lymphoblasts (CLS) and their exosomes (EXO) in resting (REST) and activated
Supplemental Figure 1. Western blot analysis indicated that MIF was detected in the fractions of
 Supplemental Figure Legends Supplemental Figure 1. Western blot analysis indicated that was detected in the fractions of plasma membrane and cytosol but not in nuclear fraction isolated from Pkd1 null
Supplemental Figure Legends Supplemental Figure 1. Western blot analysis indicated that was detected in the fractions of plasma membrane and cytosol but not in nuclear fraction isolated from Pkd1 null
Supplementary Materials for
 www.sciencesignaling.org/cgi/content/full/2/1/ra81/dc1 Supplementary Materials for Delivery of MicroRNA-126 by Apoptotic Bodies Induces CXCL12- Dependent Vascular Protection Alma Zernecke,* Kiril Bidzhekov,
www.sciencesignaling.org/cgi/content/full/2/1/ra81/dc1 Supplementary Materials for Delivery of MicroRNA-126 by Apoptotic Bodies Induces CXCL12- Dependent Vascular Protection Alma Zernecke,* Kiril Bidzhekov,
Supplementary Materials for
 www.sciencesignaling.org/cgi/content/full/8/407/ra127/dc1 Supplementary Materials for Loss of FTO in adipose tissue decreases Angptl4 translation and alters triglyceride metabolism Chao-Yung Wang,* Shian-Sen
www.sciencesignaling.org/cgi/content/full/8/407/ra127/dc1 Supplementary Materials for Loss of FTO in adipose tissue decreases Angptl4 translation and alters triglyceride metabolism Chao-Yung Wang,* Shian-Sen
SR-BI inhibits ABCG1-stimulated net cholesterol efflux from cells to plasma HDL
 SR-BI inhibits ABCG1-stimulated net cholesterol efflux from cells to plasma HDL Laurent Yvan-Charvet, 1, * Tamara A. Pagler,* Nan Wang,* Takafumi Senokuchi,* May Brundert, Hongna Li,* Franz Rinninger,
SR-BI inhibits ABCG1-stimulated net cholesterol efflux from cells to plasma HDL Laurent Yvan-Charvet, 1, * Tamara A. Pagler,* Nan Wang,* Takafumi Senokuchi,* May Brundert, Hongna Li,* Franz Rinninger,
 Acetyl CoA HMG CoA Mevalonate (C6) Dimethylallyl Pyrophosphate isopentenyl Pyrophosphate (C5) Geranyl Pyrophosphate (C10) FarnesylPyrophosphate (C15) Squalene (C30) Lanosterol (C30) 7 Dehydrocholesterol
Acetyl CoA HMG CoA Mevalonate (C6) Dimethylallyl Pyrophosphate isopentenyl Pyrophosphate (C5) Geranyl Pyrophosphate (C10) FarnesylPyrophosphate (C15) Squalene (C30) Lanosterol (C30) 7 Dehydrocholesterol
Integrative Physiology. Control of Cholesterol Metabolism and Plasma High-Density Lipoprotein Levels by microrna-144
 Integrative Physiology Control of Cholesterol Metabolism and Plasma High-Density Lipoprotein Levels by microrna-144 Cristina M. Ramírez, Noemi Rotllan, Alexander V. Vlassov, Alberto Dávalos, Mu Li, Leigh
Integrative Physiology Control of Cholesterol Metabolism and Plasma High-Density Lipoprotein Levels by microrna-144 Cristina M. Ramírez, Noemi Rotllan, Alexander V. Vlassov, Alberto Dávalos, Mu Li, Leigh
PPAR history of research
 PPAR Rubens, 1640 PPAR history of research number of publications 3000 2000 1000 0 till now: : 16 296 publications 1985 1990 1995 2000 2005 year liver, brown adipocytes, kidney, heart, skeletal muscles,
PPAR Rubens, 1640 PPAR history of research number of publications 3000 2000 1000 0 till now: : 16 296 publications 1985 1990 1995 2000 2005 year liver, brown adipocytes, kidney, heart, skeletal muscles,
BIOFENOLI ED INIBIZIONE DELL UPTAKE DI oxldl DA PARTE DI CELLULE MACROFAGICHE UMANE E MURINE
 BIOFENOLI ED INIBIZIONE DELL UPTAKE DI oxldl DA PARTE DI CELLULE MACROFAGICHE UMANE E MURINE Roberta Di Benedetto Centro Nazionale per la Qualità degli Alimenti e per i Rischi Alimentari Istituto Superiore
BIOFENOLI ED INIBIZIONE DELL UPTAKE DI oxldl DA PARTE DI CELLULE MACROFAGICHE UMANE E MURINE Roberta Di Benedetto Centro Nazionale per la Qualità degli Alimenti e per i Rischi Alimentari Istituto Superiore
Supplementary Figure 1 IMQ-Induced Mouse Model of Psoriasis. IMQ cream was
 Supplementary Figure 1 IMQ-Induced Mouse Model of Psoriasis. IMQ cream was painted on the shaved back skin of CBL/J and BALB/c mice for consecutive days. (a, b) Phenotypic presentation of mouse back skin
Supplementary Figure 1 IMQ-Induced Mouse Model of Psoriasis. IMQ cream was painted on the shaved back skin of CBL/J and BALB/c mice for consecutive days. (a, b) Phenotypic presentation of mouse back skin
Cholesterol Metabolism
 Cholesterol Metabolism Lippincott s Illustrated Review Chapter 18 Steroid Nucleus 1 2 Cholesterol was isolated from gall bladder stones in 1774 3 Sources and Elimination of Cholesterol Synthesis: 1000
Cholesterol Metabolism Lippincott s Illustrated Review Chapter 18 Steroid Nucleus 1 2 Cholesterol was isolated from gall bladder stones in 1774 3 Sources and Elimination of Cholesterol Synthesis: 1000
The inhibition of CETP: From simply raising HDL-c to promoting cholesterol efflux and lowering of atherogenic lipoproteins Prof Dr J Wouter Jukema
 The inhibition of CETP: From simply raising HDL-c to promoting cholesterol efflux and lowering of atherogenic lipoproteins Prof Dr J Wouter Jukema Dept Cardiology, Leiden University Medical Center, Leiden,
The inhibition of CETP: From simply raising HDL-c to promoting cholesterol efflux and lowering of atherogenic lipoproteins Prof Dr J Wouter Jukema Dept Cardiology, Leiden University Medical Center, Leiden,
The role of reverse cholesterol transport in animals and humans and relationship to atherosclerosis
 The role of reverse cholesterol transport in animals and humans and relationship to atherosclerosis Daniel J. Rader, 1, *, Eric T. Alexander,*, Ginny L. Weibel, Jeffrey Billheimer,* and George H. Rothblat
The role of reverse cholesterol transport in animals and humans and relationship to atherosclerosis Daniel J. Rader, 1, *, Eric T. Alexander,*, Ginny L. Weibel, Jeffrey Billheimer,* and George H. Rothblat
Circular RNAs (circrnas) act a stable mirna sponges
 Circular RNAs (circrnas) act a stable mirna sponges cernas compete for mirnas Ancestal mrna (+3 UTR) Pseudogene RNA (+3 UTR homolgy region) The model holds true for all RNAs that share a mirna binding
Circular RNAs (circrnas) act a stable mirna sponges cernas compete for mirnas Ancestal mrna (+3 UTR) Pseudogene RNA (+3 UTR homolgy region) The model holds true for all RNAs that share a mirna binding
Hepatic ABC transporters and triglyceride metabolism
 University of Nebraska - Lincoln DigitalCommons@University of Nebraska - Lincoln Nutrition and Health Sciences -- Faculty Publications Nutrition and Health Sciences, Department of 2012 Hepatic ABC transporters
University of Nebraska - Lincoln DigitalCommons@University of Nebraska - Lincoln Nutrition and Health Sciences -- Faculty Publications Nutrition and Health Sciences, Department of 2012 Hepatic ABC transporters
Supplementary Material
 Supplementary Material Summary: The supplementary information includes 1 table (Table S1) and 4 figures (Figure S1 to S4). Supplementary Figure Legends Figure S1 RTL-bearing nude mouse model. (A) Tumor
Supplementary Material Summary: The supplementary information includes 1 table (Table S1) and 4 figures (Figure S1 to S4). Supplementary Figure Legends Figure S1 RTL-bearing nude mouse model. (A) Tumor
Supplementary Figure 1
 Supplementary Figure 1 Asymmetrical function of 5p and 3p arms of mir-181 and mir-30 families and mir-142 and mir-154. (a) Control experiments using mirna sensor vector and empty pri-mirna overexpression
Supplementary Figure 1 Asymmetrical function of 5p and 3p arms of mir-181 and mir-30 families and mir-142 and mir-154. (a) Control experiments using mirna sensor vector and empty pri-mirna overexpression
Integrative Physiology
 Integrative Physiology Acute Psychological Stress Accelerates Reverse Cholesterol Transport via Corticosterone-Dependent Inhibition of Intestinal Cholesterol Absorption Reija Silvennoinen, Joan Carles
Integrative Physiology Acute Psychological Stress Accelerates Reverse Cholesterol Transport via Corticosterone-Dependent Inhibition of Intestinal Cholesterol Absorption Reija Silvennoinen, Joan Carles
Chapter 2. Investigation into mir-346 Regulation of the nachr α5 Subunit
 15 Chapter 2 Investigation into mir-346 Regulation of the nachr α5 Subunit MicroRNA s (mirnas) are small (< 25 base pairs), single stranded, non-coding RNAs that regulate gene expression at the post transcriptional
15 Chapter 2 Investigation into mir-346 Regulation of the nachr α5 Subunit MicroRNA s (mirnas) are small (< 25 base pairs), single stranded, non-coding RNAs that regulate gene expression at the post transcriptional
MicroRNA and Male Infertility: A Potential for Diagnosis
 Review Article MicroRNA and Male Infertility: A Potential for Diagnosis * Abstract MicroRNAs (mirnas) are small non-coding single stranded RNA molecules that are physiologically produced in eukaryotic
Review Article MicroRNA and Male Infertility: A Potential for Diagnosis * Abstract MicroRNAs (mirnas) are small non-coding single stranded RNA molecules that are physiologically produced in eukaryotic
Reviewers' comments: Reviewer #1 (Remarks to the Author):
 Reviewers' comments: Reviewer #1 (Remarks to the Author): In this manuscript, Song et al. identified FBXW7 as a new positive regulator for RIG-Itriggered type I IFN signaling pathway. The authors observed
Reviewers' comments: Reviewer #1 (Remarks to the Author): In this manuscript, Song et al. identified FBXW7 as a new positive regulator for RIG-Itriggered type I IFN signaling pathway. The authors observed
Journal of the American College of Cardiology Vol. 50, No. 24, by the American College of Cardiology Foundation ISSN /07/$32.
 Journal of the American College of Cardiology Vol. 50, No. 24, 2007 2007 by the American College of Cardiology Foundation ISSN 0735-1097/07/$32.00 Published by Elsevier Inc. doi:10.1016/j.jacc.2007.07.081
Journal of the American College of Cardiology Vol. 50, No. 24, 2007 2007 by the American College of Cardiology Foundation ISSN 0735-1097/07/$32.00 Published by Elsevier Inc. doi:10.1016/j.jacc.2007.07.081
Lipid/Lipoprotein Structure and Metabolism (Overview)
 Lipid/Lipoprotein Structure and Metabolism (Overview) Philip Barter President, International Atherosclerosis Society Centre for Vascular Research University of New South Wales Sydney, Australia Disclosures
Lipid/Lipoprotein Structure and Metabolism (Overview) Philip Barter President, International Atherosclerosis Society Centre for Vascular Research University of New South Wales Sydney, Australia Disclosures
ESC Congress 2011 Wednesday August 31st 2011
 ESC Congress 211 Wednesday August 31st 211 stimulates endothelial CAT-1 expression and L-arginine uptake: a novel mechanism leading to endothelial-protective effects of that is profoundly impaired in patients
ESC Congress 211 Wednesday August 31st 211 stimulates endothelial CAT-1 expression and L-arginine uptake: a novel mechanism leading to endothelial-protective effects of that is profoundly impaired in patients
Original Article Adiponectin upregulates ABCA1 expression through liver X receptor alpha signaling pathway in RAW 264.
 Int J Clin Exp Pathol 2015;8(1):450-457 www.ijcep.com /ISSN:1936-2625/IJCEP0003356 Original Article Adiponectin upregulates ABCA1 expression through liver X receptor alpha signaling pathway in RAW 264.7
Int J Clin Exp Pathol 2015;8(1):450-457 www.ijcep.com /ISSN:1936-2625/IJCEP0003356 Original Article Adiponectin upregulates ABCA1 expression through liver X receptor alpha signaling pathway in RAW 264.7
Curriculum Vitae. Research Officer in Baker IDI Heart & Diabetes Institute from Aug
 Curriculum Vitae Yi Fu ( 付毅 ) (PhD, MBBS) Baker IDI Heart and Diabetes Institute 75 Commercial Road, Prahran, Melbourne, VIC 3004 Australia Phone: +61 3 8532 1288 Email: yi.fu@bakeridi.edu.au PROFILE Research
Curriculum Vitae Yi Fu ( 付毅 ) (PhD, MBBS) Baker IDI Heart and Diabetes Institute 75 Commercial Road, Prahran, Melbourne, VIC 3004 Australia Phone: +61 3 8532 1288 Email: yi.fu@bakeridi.edu.au PROFILE Research
Part-4. Cell cycle regulatory protein 5 (Cdk5) A novel target of ERK in Carb induced cell death
 Part-4 Cell cycle regulatory protein 5 (Cdk5) A novel target of ERK in Carb induced cell death 95 1. Introduction The process of replicating DNA and dividing cells can be described as a series of coordinated
Part-4 Cell cycle regulatory protein 5 (Cdk5) A novel target of ERK in Carb induced cell death 95 1. Introduction The process of replicating DNA and dividing cells can be described as a series of coordinated
Child born in year /3 will die before parents in US (diabetes)
 Child born in year 2000-1/3 will die before parents in US (diabetes) ATP III identified 6 components of the metabolic syndrome that relate to CVD 1. Abdominal obesity 2. Atherogenic dyslipidemia (elevated
Child born in year 2000-1/3 will die before parents in US (diabetes) ATP III identified 6 components of the metabolic syndrome that relate to CVD 1. Abdominal obesity 2. Atherogenic dyslipidemia (elevated
Supplemental Material. Results
 Supplemental Material Results Fractionation of mouse plasma by high-resolution SEC. APOA1 eluted as a single major peak in fractions 16 of plasma (the apparent size of mature, lipidated HDL) when it was
Supplemental Material Results Fractionation of mouse plasma by high-resolution SEC. APOA1 eluted as a single major peak in fractions 16 of plasma (the apparent size of mature, lipidated HDL) when it was
Lipoproteins Metabolism Reference: Campbell Biochemistry and Lippincott s Biochemistry
 Lipoproteins Metabolism Reference: Campbell Biochemistry and Lippincott s Biochemistry Learning Objectives 1. Define lipoproteins and explain the rationale of their formation in blood. 2. List different
Lipoproteins Metabolism Reference: Campbell Biochemistry and Lippincott s Biochemistry Learning Objectives 1. Define lipoproteins and explain the rationale of their formation in blood. 2. List different
Supplementary Fig. 1. Delivery of mirnas via Red Fluorescent Protein.
 prfp-vector RFP Exon1 Intron RFP Exon2 prfp-mir-124 mir-93/124 RFP Exon1 Intron RFP Exon2 Untransfected prfp-vector prfp-mir-93 prfp-mir-124 Supplementary Fig. 1. Delivery of mirnas via Red Fluorescent
prfp-vector RFP Exon1 Intron RFP Exon2 prfp-mir-124 mir-93/124 RFP Exon1 Intron RFP Exon2 Untransfected prfp-vector prfp-mir-93 prfp-mir-124 Supplementary Fig. 1. Delivery of mirnas via Red Fluorescent
TITLE: MiR-146-SIAH2-AR Signaling in Castration-Resistant Prostate Cancer
 AWARD NUMBER: W81XWH-14-1-0387 TITLE: MiR-146-SIAH2-AR Signaling in Castration-Resistant Prostate Cancer PRINCIPAL INVESTIGATOR: Dr. Goberdhan Dimri, PhD CONTRACTING ORGANIZATION: George Washington University,
AWARD NUMBER: W81XWH-14-1-0387 TITLE: MiR-146-SIAH2-AR Signaling in Castration-Resistant Prostate Cancer PRINCIPAL INVESTIGATOR: Dr. Goberdhan Dimri, PhD CONTRACTING ORGANIZATION: George Washington University,
Supplementary Figure 1
 Supplementary Figure 1 YAP negatively regulates IFN- signaling. (a) Immunoblot analysis of Yap knockdown efficiency with sh-yap (#1 to #4 independent constructs) in Raw264.7 cells. (b) IFN- -Luc and PRDs
Supplementary Figure 1 YAP negatively regulates IFN- signaling. (a) Immunoblot analysis of Yap knockdown efficiency with sh-yap (#1 to #4 independent constructs) in Raw264.7 cells. (b) IFN- -Luc and PRDs
Supplemental Information. Menin Deficiency Leads to Depressive-like. Behaviors in Mice by Modulating. Astrocyte-Mediated Neuroinflammation
 Neuron, Volume 100 Supplemental Information Menin Deficiency Leads to Depressive-like Behaviors in Mice by Modulating Astrocyte-Mediated Neuroinflammation Lige Leng, Kai Zhuang, Zeyue Liu, Changquan Huang,
Neuron, Volume 100 Supplemental Information Menin Deficiency Leads to Depressive-like Behaviors in Mice by Modulating Astrocyte-Mediated Neuroinflammation Lige Leng, Kai Zhuang, Zeyue Liu, Changquan Huang,
Roles of Flow Mechanics in Vascular Cell Biology in Health and Disease
 Roles of Flow Mechanics in Vascular Cell Biology in Health and Disease Shu Chien Dept. of Bioengineering & Medicine UC, San Diego Presented by Ming-Shaung Ju Dept. of Mech. Eng., NCKU, Tainan Background
Roles of Flow Mechanics in Vascular Cell Biology in Health and Disease Shu Chien Dept. of Bioengineering & Medicine UC, San Diego Presented by Ming-Shaung Ju Dept. of Mech. Eng., NCKU, Tainan Background
Cancer Problems in Indonesia
 mirna and Cancer : mirna as a Key Regulator in Cancer Sofia Mubarika 2 nd Symposium Biomolecular Update in Cancer PERABOI Padang 18 Mei 2013 Cancer Problems in Indonesia 1. Chemoresistency / recurrency
mirna and Cancer : mirna as a Key Regulator in Cancer Sofia Mubarika 2 nd Symposium Biomolecular Update in Cancer PERABOI Padang 18 Mei 2013 Cancer Problems in Indonesia 1. Chemoresistency / recurrency
MicroRNAs, RNA Modifications, RNA Editing. Bora E. Baysal MD, PhD Oncology for Scientists Lecture Tue, Oct 17, 2017, 3:30 PM - 5:00 PM
 MicroRNAs, RNA Modifications, RNA Editing Bora E. Baysal MD, PhD Oncology for Scientists Lecture Tue, Oct 17, 2017, 3:30 PM - 5:00 PM Expanding world of RNAs mrna, messenger RNA (~20,000) trna, transfer
MicroRNAs, RNA Modifications, RNA Editing Bora E. Baysal MD, PhD Oncology for Scientists Lecture Tue, Oct 17, 2017, 3:30 PM - 5:00 PM Expanding world of RNAs mrna, messenger RNA (~20,000) trna, transfer
Role of apolipoprotein B-containing lipoproteins in the development of atherosclerosis Jan Borén MD, PhD
 Role of apolipoprotein B-containing lipoproteins in the development of atherosclerosis Jan Borén MD, PhD Our laboratory focuses on the role of apolipoprotein (apo) B- containing lipoproteins in normal
Role of apolipoprotein B-containing lipoproteins in the development of atherosclerosis Jan Borén MD, PhD Our laboratory focuses on the role of apolipoprotein (apo) B- containing lipoproteins in normal
Utility of Circulating micrornas in Cardiovascular Disease
 Utility of Circulating micrornas in Cardiovascular Disease Pil-Ki Min, MD, PhD Cardiology Division, Gangnam Severance Hospital, Yonsei University College of Medicine Introduction Biology of micrornas Circulating
Utility of Circulating micrornas in Cardiovascular Disease Pil-Ki Min, MD, PhD Cardiology Division, Gangnam Severance Hospital, Yonsei University College of Medicine Introduction Biology of micrornas Circulating
MinimallyModifiedLDLInducesActinPolymerization Macrophages viacd14signalingpathway.
 MinimallyModifiedLDLInducesActinPolymerization Macrophages viacd14signalingpathway. *Randon T. Hall I, Joseph L. Witztum2, Yury I. Miller2 From the IMSTP-SURF Program and 2Division of Endocrinology and
MinimallyModifiedLDLInducesActinPolymerization Macrophages viacd14signalingpathway. *Randon T. Hall I, Joseph L. Witztum2, Yury I. Miller2 From the IMSTP-SURF Program and 2Division of Endocrinology and
It is generally accepted that lipoproteins carry cholesterol
 Red Blood Cells Play a Role in Reverse Cholesterol Transport Kimberly T. Hung, Stela Z. Berisha, Brian M. Ritchey, Jennifer Santore, Jonathan D. Smith Objective Reverse cholesterol transport (RCT) involves
Red Blood Cells Play a Role in Reverse Cholesterol Transport Kimberly T. Hung, Stela Z. Berisha, Brian M. Ritchey, Jennifer Santore, Jonathan D. Smith Objective Reverse cholesterol transport (RCT) involves
1Why lipids cannot be transported in blood alone? 2How we transport Fatty acids and steroid hormones?
 1Why lipids cannot be transported in blood alone? 2How we transport Fatty acids and steroid hormones? 3How are dietary lipids transported? 4How lipids synthesized in the liver are transported? 5 Lipoprotien
1Why lipids cannot be transported in blood alone? 2How we transport Fatty acids and steroid hormones? 3How are dietary lipids transported? 4How lipids synthesized in the liver are transported? 5 Lipoprotien
Supplementary Figure 1
 Supplementary Figure 1 Supplementary Figure 1 Schematic depiction of the tandem Fc GDF15. Supplementary Figure 2 Supplementary Figure 2 Gfral mrna levels in the brains of both wild-type and knockout Gfral
Supplementary Figure 1 Supplementary Figure 1 Schematic depiction of the tandem Fc GDF15. Supplementary Figure 2 Supplementary Figure 2 Gfral mrna levels in the brains of both wild-type and knockout Gfral
Attenuating Effect of Curcumin on Diet-induced Hypercholesterolemia in Mice
 International Journal of Medical Laboratory 2017;4(4):299-306. Original Article Attenuating Effect of Curcumin on Diet-induced Hypercholesterolemia in Mice Sahar Farzaneh 1 M.Sc., Masoud Salehipour 1*
International Journal of Medical Laboratory 2017;4(4):299-306. Original Article Attenuating Effect of Curcumin on Diet-induced Hypercholesterolemia in Mice Sahar Farzaneh 1 M.Sc., Masoud Salehipour 1*
Ebrahim Abbasi Oshaghi 1,2
 1 2 Flaxseed normalized antioxidant status and also changed ABCG5 and ABCG8 genes expression in diabetic rat Fatemeh Mirzaei 1,Mona Pourjafarr 1, Seyyed Alireza Vafaei 1, Rezvan Mostoli 1, Ebrahim Abbasi
1 2 Flaxseed normalized antioxidant status and also changed ABCG5 and ABCG8 genes expression in diabetic rat Fatemeh Mirzaei 1,Mona Pourjafarr 1, Seyyed Alireza Vafaei 1, Rezvan Mostoli 1, Ebrahim Abbasi
Supplementary Fig. 1 eif6 +/- mice show a reduction in white adipose tissue, blood lipids and normal glycogen synthesis. The cohort of the original
 Supplementary Fig. 1 eif6 +/- mice show a reduction in white adipose tissue, blood lipids and normal glycogen synthesis. The cohort of the original phenotypic screening was n=40. For specific tests, the
Supplementary Fig. 1 eif6 +/- mice show a reduction in white adipose tissue, blood lipids and normal glycogen synthesis. The cohort of the original phenotypic screening was n=40. For specific tests, the
MicroRNA-mediated incoherent feedforward motifs are robust
 The Second International Symposium on Optimization and Systems Biology (OSB 8) Lijiang, China, October 31 November 3, 8 Copyright 8 ORSC & APORC, pp. 62 67 MicroRNA-mediated incoherent feedforward motifs
The Second International Symposium on Optimization and Systems Biology (OSB 8) Lijiang, China, October 31 November 3, 8 Copyright 8 ORSC & APORC, pp. 62 67 MicroRNA-mediated incoherent feedforward motifs
Nature Medicine doi: /nm.3150
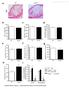 Nature Medicine doi:10.1038/nm.3150 Supplementary Table 1 Primer sequences used for q-pcr analysis Gene Forward primer Reverse primer Abca1 CAGCTTCCATCCTCCTTGTC CCACATCCACAACTGTCTGG Abcg1 GTACCATGACATCGCTGGTG
Nature Medicine doi:10.1038/nm.3150 Supplementary Table 1 Primer sequences used for q-pcr analysis Gene Forward primer Reverse primer Abca1 CAGCTTCCATCCTCCTTGTC CCACATCCACAACTGTCTGG Abcg1 GTACCATGACATCGCTGGTG
Cardiology, CardioVascular Center University Hospital Zurich Zurich, Switzerland
 Modification of by the lipoxidation product malondialdehyde leads to LOX-1 dependent activation of endothelial PKCbeta-2 and adverse endothelial effects of in patients with coronary artery disease Christian
Modification of by the lipoxidation product malondialdehyde leads to LOX-1 dependent activation of endothelial PKCbeta-2 and adverse endothelial effects of in patients with coronary artery disease Christian
902 Biomed Environ Sci, 2014; 27(11):
 902 Biomed Environ Sci, 2014; 27(11): 902-906 Letter to the Editor Curcuminoids Target Decreasing Serum Adipocyte-fatty Acid Binding Protein Levels in Their Glucose-lowering Effect in Patients with Type
902 Biomed Environ Sci, 2014; 27(11): 902-906 Letter to the Editor Curcuminoids Target Decreasing Serum Adipocyte-fatty Acid Binding Protein Levels in Their Glucose-lowering Effect in Patients with Type
Figure S1. Reduction in glomerular mir-146a levels correlate with progression to higher albuminuria in diabetic patients.
 Supplementary Materials Supplementary Figures Figure S1. Reduction in glomerular mir-146a levels correlate with progression to higher albuminuria in diabetic patients. Figure S2. Expression level of podocyte
Supplementary Materials Supplementary Figures Figure S1. Reduction in glomerular mir-146a levels correlate with progression to higher albuminuria in diabetic patients. Figure S2. Expression level of podocyte
Functional characterisation of hepatitis B viral X protein/microrna-21 interaction in HBVassociated hepatocellular carcinoma
 RESEARCH FUND FOR THE CONTROL OF INFECTIOUS DISEASES Functional characterisation of hepatitis B viral X protein/microrna-21 interaction in HBVassociated hepatocellular carcinoma CH Li, SC Chow, DL Yin,
RESEARCH FUND FOR THE CONTROL OF INFECTIOUS DISEASES Functional characterisation of hepatitis B viral X protein/microrna-21 interaction in HBVassociated hepatocellular carcinoma CH Li, SC Chow, DL Yin,
RAW264.7 cells stably expressing control shrna (Con) or GSK3b-specific shrna (sh-
 1 a b Supplementary Figure 1. Effects of GSK3b knockdown on poly I:C-induced cytokine production. RAW264.7 cells stably expressing control shrna (Con) or GSK3b-specific shrna (sh- GSK3b) were stimulated
1 a b Supplementary Figure 1. Effects of GSK3b knockdown on poly I:C-induced cytokine production. RAW264.7 cells stably expressing control shrna (Con) or GSK3b-specific shrna (sh- GSK3b) were stimulated
The functional investigation of the interaction between TATA-associated factor 3 (TAF3) and p53 protein
 THESIS BOOK The functional investigation of the interaction between TATA-associated factor 3 (TAF3) and p53 protein Orsolya Buzás-Bereczki Supervisors: Dr. Éva Bálint Dr. Imre Miklós Boros University of
THESIS BOOK The functional investigation of the interaction between TATA-associated factor 3 (TAF3) and p53 protein Orsolya Buzás-Bereczki Supervisors: Dr. Éva Bálint Dr. Imre Miklós Boros University of
Males- Western Diet WT KO Age (wks) Females- Western Diet WT KO Age (wks)
 Relative Arv1 mrna Adrenal 33.48 +/- 6.2 Skeletal Muscle 22.4 +/- 4.93 Liver 6.41 +/- 1.48 Heart 5.1 +/- 2.3 Brain 4.98 +/- 2.11 Ovary 4.68 +/- 2.21 Kidney 3.98 +/-.39 Lung 2.15 +/-.6 Inguinal Subcutaneous
Relative Arv1 mrna Adrenal 33.48 +/- 6.2 Skeletal Muscle 22.4 +/- 4.93 Liver 6.41 +/- 1.48 Heart 5.1 +/- 2.3 Brain 4.98 +/- 2.11 Ovary 4.68 +/- 2.21 Kidney 3.98 +/-.39 Lung 2.15 +/-.6 Inguinal Subcutaneous
Supplementary Figure 1 IL-27 IL
 Tim-3 Supplementary Figure 1 Tc0 49.5 0.6 Tc1 63.5 0.84 Un 49.8 0.16 35.5 0.16 10 4 61.2 5.53 10 3 64.5 5.66 10 2 10 1 10 0 31 2.22 10 0 10 1 10 2 10 3 10 4 IL-10 28.2 1.69 IL-27 Supplementary Figure 1.
Tim-3 Supplementary Figure 1 Tc0 49.5 0.6 Tc1 63.5 0.84 Un 49.8 0.16 35.5 0.16 10 4 61.2 5.53 10 3 64.5 5.66 10 2 10 1 10 0 31 2.22 10 0 10 1 10 2 10 3 10 4 IL-10 28.2 1.69 IL-27 Supplementary Figure 1.
PREPARED FOR: U.S. Army Medical Research and Materiel Command Fort Detrick, Maryland
 AD Award Number: W81XWH-10-1-1029 TITLE: PRINCIPAL INVESTIGATOR: Mu-Shui Dai, M.D., Ph.D. CONTRACTING ORGANIZATION: Oregon Health Science niversity, Portland, Oregon 97239 REPORT DATE: October 2013 TYPE
AD Award Number: W81XWH-10-1-1029 TITLE: PRINCIPAL INVESTIGATOR: Mu-Shui Dai, M.D., Ph.D. CONTRACTING ORGANIZATION: Oregon Health Science niversity, Portland, Oregon 97239 REPORT DATE: October 2013 TYPE
Mechanisms underlying adverse effects of HDL on enos-activating pathways in patients with coronary artery disease
 Related Commentary, page 2545 Research article Mechanisms underlying adverse effects of HDL on enos-activating pathways in patients with coronary artery disease Christian Besler, 1,2,3 Kathrin Heinrich,
Related Commentary, page 2545 Research article Mechanisms underlying adverse effects of HDL on enos-activating pathways in patients with coronary artery disease Christian Besler, 1,2,3 Kathrin Heinrich,
Doctoral Degree Program in Marine Biotechnology, College of Marine Sciences, Doctoral Degree Program in Marine Biotechnology, Academia Sinica, Taipei,
 Cyclooxygenase 2 facilitates dengue virus replication and serves as a potential target for developing antiviral agents Chun-Kuang Lin 1,2, Chin-Kai Tseng 3,4, Yu-Hsuan Wu 3,4, Chih-Chuang Liaw 1,5, Chun-
Cyclooxygenase 2 facilitates dengue virus replication and serves as a potential target for developing antiviral agents Chun-Kuang Lin 1,2, Chin-Kai Tseng 3,4, Yu-Hsuan Wu 3,4, Chih-Chuang Liaw 1,5, Chun-
Role of reverse cholesterol transport in animals and humans and relationship to atherosclerosis
 Role of reverse cholesterol transport in animals and humans and relationship to atherosclerosis Daniel J. Rader 1,2, Eric T. Alexander 1,2, Ginny L. Weibel 2, Jeffrey Billheimer 1, George H. Rothblat 2
Role of reverse cholesterol transport in animals and humans and relationship to atherosclerosis Daniel J. Rader 1,2, Eric T. Alexander 1,2, Ginny L. Weibel 2, Jeffrey Billheimer 1, George H. Rothblat 2
Reviewers' comments: Reviewer #1 (Remarks to the Author):
 Reviewers' comments: Reviewer #1 (Remarks to the Author): The manuscript by Wu et al describes critical role of RNA binding protein CUGBP1 in the development of TGF-beta-mediated liver fibrosis. The activation
Reviewers' comments: Reviewer #1 (Remarks to the Author): The manuscript by Wu et al describes critical role of RNA binding protein CUGBP1 in the development of TGF-beta-mediated liver fibrosis. The activation
Potential mechanisms for the resolution of atherosclerosis by conjugated linoleic acid (CLA)
 HRB IMDA Oct HRB 2010 IMDA Seminar 28 th Oct 2010 Potential mechanisms for the resolution of atherosclerosis by conjugated linoleic acid (CLA) Dr. Orina Belton, School of Biomolecular and Biomedical Science,
HRB IMDA Oct HRB 2010 IMDA Seminar 28 th Oct 2010 Potential mechanisms for the resolution of atherosclerosis by conjugated linoleic acid (CLA) Dr. Orina Belton, School of Biomolecular and Biomedical Science,
Lipid metabolism in familial hypercholesterolemia
 Lipid metabolism in familial hypercholesterolemia Khalid Al-Rasadi, BSc, MD, FRCPC Head of Biochemistry Department, SQU Head of Lipid and LDL-Apheresis Unit, SQUH President of Oman society of Lipid & Atherosclerosis
Lipid metabolism in familial hypercholesterolemia Khalid Al-Rasadi, BSc, MD, FRCPC Head of Biochemistry Department, SQU Head of Lipid and LDL-Apheresis Unit, SQUH President of Oman society of Lipid & Atherosclerosis
Lipids digestion and absorption, Biochemistry II
 Lipids digestion and absorption, blood plasma lipids, lipoproteins Biochemistry II Lecture 1 2008 (J.S.) Triacylglycerols (as well as free fatty acids and both free and esterified cholesterol) are very
Lipids digestion and absorption, blood plasma lipids, lipoproteins Biochemistry II Lecture 1 2008 (J.S.) Triacylglycerols (as well as free fatty acids and both free and esterified cholesterol) are very
Very low-density lipoproteins (VLDLs) are triacylglyceride-rich
 Molecular Medicine Control of Very Low-Density Lipoprotein Secretion by N-Ethylmaleimide-Sensitive Factor and mir-33 Ryan M. Allen, Tyler J. Marquart, Jordan J. Jesse, Ángel Baldán Rationale: Several reports
Molecular Medicine Control of Very Low-Density Lipoprotein Secretion by N-Ethylmaleimide-Sensitive Factor and mir-33 Ryan M. Allen, Tyler J. Marquart, Jordan J. Jesse, Ángel Baldán Rationale: Several reports
Supplementary Figure 1: Digitoxin induces apoptosis in primary human melanoma cells but not in normal melanocytes, which express lower levels of the
 Supplementary Figure 1: Digitoxin induces apoptosis in primary human melanoma cells but not in normal melanocytes, which express lower levels of the cardiac glycoside target, ATP1A1. (a) The percentage
Supplementary Figure 1: Digitoxin induces apoptosis in primary human melanoma cells but not in normal melanocytes, which express lower levels of the cardiac glycoside target, ATP1A1. (a) The percentage
