Reduced expression of the murine p85α subunit of phosphoinositide 3-kinase improves insulin signaling and ameliorates diabetes
|
|
|
- Aron Marshall
- 5 years ago
- Views:
Transcription
1 Reduced expression of the murine p85α subunit of phosphoinositide 3-kinase improves insulin signaling and ameliorates diabetes Franck Mauvais-Jarvis, 1 Kohjiro Ueki, 1 David A. Fruman, 2 Michael F. Hirshman, 1 Kei Sakamoto, 1 Laurie J. Goodyear, 1 Matteo Iannacone, 1 Domenico Accili, 3 Lewis C. Cantley, 2 and C. Ronald Kahn 1 1 Research Division, Joslin Diabetes Center and Department of Medicine, Harvard Medical School, Boston, Massachusetts, USA 2 Division of Signal Transduction, Beth Israel Deaconess Medical Center, Department of Cell Biology, Harvard Medical School, Boston, Massachusetts, USA 3 Diabetes Research Unit, Columbia University, New York, New York, USA Address correspondence to: C. Ronald Kahn, Research Division, Joslin Diabetes Center, One Joslin Place, Boston, Massachusetts 02215, USA. Phone: (617) ; Fax: (617) ; c.ronald.kahn@joslin.harvard.edu. Franck Mauvais-Jarvis and Kohjiro Ueki contributed equally to this work. Received for publication May 18, 2001, and accepted in revised form October 29, A critical component of insulin action is the enzyme phosphoinositide (PI) 3-kinase. The major regulatory subunits of PI 3-kinase, p85α and its splice variants, are encoded by the Pik3r1 gene. Heterozygous disruption of Pik3r1 improves insulin signaling and glucose homeostasis in normal mice and mice made insulin-resistant by heterozygous deletion of the Insulin receptor and/or insulin receptor substrate-1 (IRS1) genes. Reduced expression of p85 modulates the molecular balance between this protein, the p110 catalytic subunit of PI 3-kinase, and the IRS proteins. Thus, despite the decrease in p85α, PI 3-kinase activation is normal, insulin-stimulated Akt activity is increased, and glucose tolerance and insulin sensitivity are improved. Furthermore, Pik3r1 heterozygosity protects mice with genetic insulin resistance from developing diabetes. These data suggest that regulation of p85α levels may provide a novel therapeutic target for the treatment of type 2 diabetes. J. Clin. Invest. 109: (2002). DOI: /JCI Introduction Insulin resistance is a major pathophysiologic feature of type 2 diabetes, obesity, polycystic ovarian disease, and the metabolic syndrome (1, 2). Unraveling the molecular determinants of insulin resistance is therefore essential to understanding of the pathogenesis of these diseases and development of therapies for them. Over the past decade, evidence has accumulated indicating that class I A phosphoinositide (PI) 3-kinase plays a central role in the metabolic actions of insulin (3). Class I A PI 3-kinase is a heterodimer composed of catalytic and regulatory subunits. Most tissues express two forms of regulatory subunit, p85α and p85β, and two forms of catalytic subunit, p110α and p110β (3). p85α and p85β share the highest degree of homology in the C-terminal half of the molecules, which contains two SH2 domains that bind to tyrosine-phosphorylated proteins and an inter-sh2 domain that interacts with the catalytic subunit. The N-terminal halves of p85α and p85β contain an SH3 domain, a BCR homology region, and two proline-rich domains, but these domains are less well conserved between the two molecules. Two isoforms of p85α truncated in the N-terminal region, identified as AS53 (or p55α) (4, 5) and p50α (6, 7), as well as p85α itself, are derived from a single gene (Pik3r1). p85β and another short isoform with limited tissue distribution termed p55γ/p55 PIK are encoded by separate genes (8). Insulin stimulation of PI 3-kinase activity involves docking of tyrosine-phosphorylated insulin receptor substrate (IRS) proteins to SH2 domains of the regulatory subunit via pyxxm motifs. This increases the intracellular levels of PI(3,4)P 2 and PI(3,4,5)P 3, which are presumed to act as second messengers of insulin actions (9). A large body of evidence in cultured cells indicates that PI 3-kinase is central to the metabolic effects of insulin on glucose transport (10 12), glycogen synthesis (13), hepatic glucose production (14, 15), and regulation of β cell function (16). Although it is known that changes in the regulatory subunits of PI 3-kinase are observed in animals with insulin resistance and diabetes (17, 18), suggesting that expression levels of the regulatory subunits might modulate insulin sensitivity in vivo, the precise role of PI 3-kinase in glucose homeostasis in vivo is still poorly understood. To directly clarify the role of PI 3-kinase in vivo, knockout mice with several types of disruption of PI 3-kinase have been engineered. Of these, disruption of The Journal of Clinical Investigation January 2002 Volume 109 Number 1 141
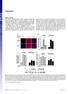
2 Figure 1 Creation of mutant mice. (a) Mice were bred and genotyped using PCR. The presence of the IR mutant allele results in a 240-bp product, while there is no product in the wild-type (WT) allele. The presence of the wild-type allele for the IRS-1 gene results in a 440-bp product; insertion of the neomycin cassette in the targeted allele results in a band of 1.25 kb. In the case of the Pik3r1 gene, the mutant allele results in a band at 600 bp, whereas the wild-type allele yields a 750-bp product. (b) Body weight of the various subgroups of mice was determined at the indicated ages. Results are expressed as mean ± SEM (n = mice per genotype). p110α results in embryonic lethality, presumably through deficiency of PI 3-kinase signaling for normal growth, such that it is impossible to address the role of PI 3-kinase in glucose homeostasis (19). Disrupting the regulatory subunits was also expected to result in an impaired PI 3-kinase signaling in insulin actions, since these subunits function as a bridge between the p110 subunit and IRS proteins. Surprisingly, however, mice carrying a disruption of only the long form of p85α but still expressing the alternatively spliced forms of this gene, p55α and p50α, exhibit increased insulin sensitivity (20). This led to the suggestion that the improvement of insulin sensitivity was due to an increase in the p50α isoform, which seems to be a more potent mediator of PI 3-kinase dependent signaling (20). On the other hand, homozygous disruption of all products of the Pik3r1 gene results in perinatal lethality, presumably via a marked reduction in PI 3-kinase signaling (21, 22). Indeed, in the knockout mice, there is a significant decrease in the p85-p110 dimer (21, 22) by the primary reduction in the regulatory subunits and the secondary reduction in the p110 catalytic subunits. The secondary reduction is caused by a deficiency of the regulatory subunits to stabilize p110 (23). Although this indicates the indispensable role of the regulatory subunits in normal development, with regard to glucose homeostasis the homozygous knockout mice show hypoglycemia with decreased plasma insulin concentrations (22), suggesting that insulin signaling is preserved, at least in part, despite the marked reduction of the p85- p110 dimer. Recent studies suggest that the regulatory subunit plays a dual role in regulation of the p110 catalytic subunit of PI 3-kinase. Binding of p85 to p110 stabilizes p110 but also maintains p110 in a low activity state by an allosteric mechanism (23, 24). Association of the SH2 domains of p85 with tyrosine-phosphorylated IRS proteins recruits PI 3-kinase to the membrane and reduces the negative effect on the catalytic activity. Moreover, overexpression or pharmacological induction of the p85α subunit inhibits insulin or IGF-1 signaling (24, 25), by both this allosteric effect and by increasing the monomeric form of the regulatory subunit that can interfere with the p85-p110 heterodimer for binding phosphorylated IRS proteins. These findings raise a possibility that a reduction in the regulatory subunits may reduce these inhibitory effects and enhance insulin signaling by affecting the balance between the regulatory subunits, catalytic subunits, and phosphorylated IRS proteins. This would be most likely if the regulatory subunits are more abundant than the p110 subunits and IRS proteins. To assess this possibility and better define the physiological role of the regulatory subunit, we have investigated the effects of heterozygous deletion of the Pik3r1 gene on glucose homeostasis in normal mice as well as mice with insulin resistance by heterozygous deletion of the insulin receptor (IR) and/or IRS-1 (26). In this study, we demonstrate that reduction in all isoforms derived from the Pik3r1 gene results in improved insulin signaling and glucose homeostasis and can prevent development of diabetes in mice with genetic insulin resistance. Methods Creation of mutant mice and PCR genotyping. Mice heterozygous for the IR allele (IR +/ ), double heterozygous for the IR- and IRS-1 null alleles (IR/IRS-1 +/ ), and heterozygous for the Pik3r1-null allele (p85 +/ ) were obtained as previously described (21, 26). Double heterozygous IR/p85 +/ and triple heterozygous IR/IRS-1/ p85 +/ mice were generated by breeding the IR/IRS-1 +/ with the p85 +/ mice. This yielded eight different genotypes with the expected mendelian frequency of 12.5% per genotype: wild-type, p85 +/, IR +/, IR/p85 +/, IRS-1 +/, IRS-1/p85 +/, IR/IRS-1 +/, and IR/IRS-1/p85 +/. All mice had a mixed genetic background consisting of 129Sv and C57BL/6. For all studies, we used the 6-month-old male mice of the same generation and mixed background. Genotyping was performed by PCR using DNA from tail biopsies of 3- to 4-week-old mice, as previously described (21, 26). All animals were housed on a 12-hour light/dark cycle and were fed a standard rodent chow (Purina Mills Inc., St. Louis, Missouri, USA). All protocols for animal use and euthanasia were approved by the Animal Care Use Committee of the Joslin Diabetes Center and Harvard Medical School in accordance with NIH guidelines. 142 The Journal of Clinical Investigation January 2002 Volume 109 Number 1
3 Metabolic studies. For glucose tolerance testing (GTT), blood samples were obtained at 0, 15, 30, 60, and 120 minutes after intraperitoneal injection of 2 g/kg dextrose. For stimulated insulin secretion, blood was obtained at 0, 2, 5, 15, and 30 minutes after intraperitoneal injection of 3 g/kg of dextrose. For insulin and IGF-1 tolerance tests, blood samples were obtained at 0, 30, 60, and 120 minutes after intraperitoneal injection of 1 U/kg insulin (Lilly Research Laboratories, Indianapolis, Indiana, USA) or 1 mg/kg IGF-1 (Peprotech Inc., Rocky Hill, New Jersey, USA). Whole blood glucose values were determined using an automatic glucose monitor (One Touch II; Lifescan Inc., Milipitas, California, USA). Plasma insulin levels were measured by ELISA using mouse insulin as a standard (Crystal Chem Inc., Chicago, Illinois, USA). Triglyceride levels in serum from fasted animals were measured by a colorimetric enzyme assay using the GPO-Trinder Assay (Sigma Diagnostics Inc., St. Louis, Missouri, USA). FFA levels were analyzed in serum from fasted animals using the NESCAUTO NEFA-Kit-U (Azwell Inc., Osaka, Japan). Plasma leptin levels were measured by ELISA using mouse leptin as a standard (Crystal Chem). In vivo insulin stimulation and analysis of insulin signaling. Six-month-old male mice were starved overnight, anesthetized with pentobarbital, and injected with 5 U of regular human insulin (Lilly Research Laboratories) into the inferior vena cava. Liver and muscle were removed 5 minutes after injection and frozen in liquid nitrogen. Immunoprecipitation and Western blot analysis of insulin signaling molecules were performed on tissue homogenates as previously described (26). Glucose transport in isolated muscles. Mice were foodrestricted overnight before they were sacrificed, and the soleus and extensor digitorum longus (EDL) muscles were removed. Uptake of 2-deoxy-D-[1,2-3 H]-glucose was measured in the presence or absence of 2.2 nm insulin, as previously described (27). Antibodies and enzyme assays. Anti-p85α antibodies (αp85pan), which recognize all variants of p85α and (to a lesser extent) p85β, p85α-specific antibodies (αp85α), and anti-phosphotyrosine antibodies (4G10) were purchased from Upstate Biotechnology Inc. (Lake Placid, New York, USA). p85β-specific antibodies (αp85β) were generated as described (21). Anti-p55 PIK antibodies (αp55 PIK ) were kindly provided by Morris White (Joslin Diabetes Center) (8). Anti IRS-1 antibodies (αirs-1) were generated against a glutathione-s transferase fusion (GST-fusion) protein corresponding to amino acids of mouse IRS-1. Anti IRS-2 Figure 2 Signaling molecules involved in activation of PI 3-kinase by insulin. (a) Expression levels of Pik3r1 gene products were determined in lysates from liver (left) and skeletal muscle (right) by Western blotting with an anti-p85pan antibody (αp85pan). (b) Tyrosine phosphorylation of IRS proteins and association with p85 were determined using lysates from liver of the indicated animals. Proteins were immunoprecipitated with anti IRS-1 antibody (αirs-1) (top two panels) or anti IRS-2 antibody (αirs-2) (bottom two panels), and blotted with antiphosphotyrosine antibody (αpy) or αp85pan. (c) To determine the molecular balance between p85 and p110, the liver lysates were subjected to three sequential rounds of immunodepletion using αp110, followed by Western blotting with αp110 (left top panel) or αp85pan (left bottom panel). The amount of the p85-p110 dimer and the p85 monomer was expressed as the ratio to the value of the amount of the p85 monomer in the wild-type cells. In the graph, each bar represents the ratio to the total p85 in wild-type cells (open bar, p85-p110 dimer; dotted bar, p85 monomer). The Journal of Clinical Investigation January 2002 Volume 109 Number 1 143
4 Figure 3 PI 3-kinase activation in liver and muscle of Pik3r1 mutant mice. Lysates from the liver of animals were immunoprecipitated with the indicated antibodies and subjected to a PI 3-kinase assay as described in Methods. PI 3-kinase activities associated with total regulatory subunit (αp85pan) (a), associated with p85α regulatory subunit (αp85α) (b), associated with the p110 catalytic subunit (αp110) (c), associated with tyrosinephosphorylated proteins (αpy) (d), and associated with p85β regulatory subunit (αp85β) (e) were assessed. The upper panels shows representative PI 3-kinase assays, while each bar in the lower panels represents the mean ± SEM of the relative PI 3-kinase activity (% of unstimulated wildtype) calculated from at least three independent experiments. *P < 0.05, wild-type vs. p85 +/. (f) Activation of PI 3-kinase in muscle. Lysates were immunoprecipitated with the p85pan antibody (αp85pan, top panel), tyrosine-phosphorylated proteins (αpy, middle panel), or the p85β regulatory subunit (αp85β, bottom panel), and subjected to PI 3-kinase assay as above. antibodies (αirs-2) were generated against a GSTfusion protein corresponding to a plekstrin homology domain of mouse IRS-2. Anti-p110α antibodies (αp110α) and anti-akt antibodies (αakt), which recognize both Akt1 and Akt2, were purchased from Santa Cruz Biotechnology Inc. (Santa Cruz, California, USA). Phosphospecific Akt (Ser473) antibodies (αp-akt) were purchased from New England Biolabs Inc. (Beverly, Massachusetts, USA). PI 3-kinase activities in liver and muscle were determined using immunoprecipitates in vitro as previously described (28). Akt kinase activities were determined in the basal state and after insulin stimulation in immunoprecipitates with αakt antibody using Crosstide, a synthetic peptide (Gly-Arg-Pro-Arg-Thr- Ser-Ser-Phe-Ala-Glu-Gly), corresponding to the region surrounding in vivo phosphorylation site of glycogen synthase kinase 3 by Akt(29). 144 The Journal of Clinical Investigation January 2002 Volume 109 Number 1
5 Results Generation and characterization of mutant mice. Mutant mice were generated as described in Methods and genotyped as outlined in Figure 1a. The p85 +/, IR/p85 +/, and IR/IRS-1/p85 +/ mice were born alive and were indistinguishable from their controls. IR/IRS-1 +/ and IR/IRS- 1/p85 +/ mice showed a stable 20% decrease in body weight compared with the other groups (Figure 1b), consistent with the mild growth retardation previously observed after heterozygous disruption of the IRS-1 gene (26). Immunoblotting revealed an approximately 40% decrease in total p85 protein in both liver and muscle of p85 +/ mice compared with their control group (39.3% ± 1.4% and 42.0% ± 3.6% in p85 +/, 35.4% ± 5.4% and 43.7% ± 13.1% in IR/p85 +/, and 40.9% ± 8.2% and 40.2% ± 4.7% in IR/IRS-1/p85 +/ ; n = 3) (Figure 2a). In addition, there was an approximately 60% reduction in expression of p50α in liver and AS53/p55α in muscle from the three genotypes of mice carrying the heterozygous Pik3r1 allele (Figure 2a). The levels of p50α were 41.1% ± 6.1% in p85 +/, 37.3% ± 1.8% in IR/p85 +/, and 35.5% ± 5.6% in IR/IRS-1/p85 +/ compared with their controls, and the levels of AS53 were 44.7% ± 4.8% in p85 +/, 39.1% ± 4.2% in IR/p85 +/, and 46.5% ± 4.1% in IR/IRS-1/p85 +/ compared with their controls (n = 3). As expected, heterozygous deletion of IR or IRS-1 resulted in about 50% reduction of each of these proteins (data not shown). Study of early insulin signaling. As previously reported (26), IRS-1 phosphorylation and IRS-1 binding to p85 were reduced by about 50% in IR heterozygous mice and by 75 80% in the IR/IRS-1 double heterozygotes (Figure 2b). The levels of IRS-2 phosphorylation and binding to p85 were also reduced by about 40% in IR +/ mice, but were unchanged in IR/IRS-1 +/ mice, probably due to a compensatory increase in IRS-2 phosphorylation (26). Heterozygosity for the Pik3r1 gene did not appreciably affect the level of IR, IRS-1, or IRS-2 phosphorylation (Figure 2b). Interestingly, despite the decrease in p85α expression in mice heterozygous for the Pik3r1 gene, the amount of p85 associated with phosphorylated IRS-1 and IRS-2 in livers of p85 +/, IR/p85 +/, and IR/IRS-1/p85 +/ mice was unchanged compared with controls (Figure 2b). This appears to be due to the fact that in normal cells the p85 regulatory subunits are more abundant than the phosphorylated IRS proteins (24). In normal cells in culture, the p85 regulatory subunits are also more abundant than the p110 catalytic subunits (24). To assess the balance between p85 monomer and p85-p110 dimer in the tissues of the mouse, we performed immunodepletion studies using anti-p110 antibody and liver lysates (Figure 2c, left, top). Under these conditions, p85 protein remaining in the lysates after immunodepletion represents the p85 monomer, whereas the amount of p85 depleted corre- Figure 4 Insulin-stimulated Akt activity in Pik3r1 mutant mice. Lysates from liver and muscle were subjected to Western blotting with αp-akt (top panels). Akt activity was assessed following immunoprecipitation with an anti-akt antibody in an immune complex kinase assay (bottom panels). The results are expressed as percent of unstimulated wild-type. Each bar represents the mean ± SEM of at least four independent experiments. *P < 0.05, wild-type vs. p85 +/ and IR/IRS-1 +/ vs. IR/IRS-1/p85 +/. The Journal of Clinical Investigation January 2002 Volume 109 Number 1 145
6 Figure 5 Improved glucose homeostasis in the Pik3r1 mutant groups. Fasting blood glucose (a) and insulin levels (b), and random-fed blood glucose (c) and insulin levels (d), were determined in 6-month-old male mice of the indicated genotype. For random-fed glucose levels, a scattered plot is represented; bars represent the mean ± SEM (n = mice per genotype). Glucose (e) and insulin (f) concentrations were determined during a glucose tolerance test (2 g/kg body weight, intraperitoneally) at the indicated time points in 6-month-old male mice of the indicated genotypes. Results are expressed as mean ± SEM (n = mice). *P < 0.05, wild-type vs. p85 +/ ; IR +/ vs. IR/p85 +/ ; IR/IRS-1 +/ vs. IR/IRS-1/p85 +/. sponds to the p85-p110 dimer. Using this approach, the ratio of p85-p110 dimer to p85 monomer in liver of wild-type mice was about 2:1 (Figure 2c, left, bottom). By comparison, in the p85 +/ mice, this ratio was increased to 4:1, due to a preferential reduction in p85 monomer as compared with p85-p110 dimer. Similar results were obtained in muscle, where the ratio of p85-p110 dimer to p85 monomer was 3:1 in wild-type mice and 5:1 in p85 +/ mice (data not shown). Insulin-dependent activation of PI 3-kinase and Akt in liver and muscle. The 50% reduction in products of Pik3r1 did not result in a decrease in p85-associated PI 3-kinase activity. Thus, PI 3-kinase activity in liver detected by an anti-p85pan antibody, which recognizes p85α, p55α, p50α, and, to a lesser extent, p85β, or by an antip85α specific antibody, was comparable in mice of all genotypes (Figure 3, a and b). Likewise, heterozygosity for Pik3r1 had no effect on PI 3-kinase activity associated with p110α, consistent with the findings that heterozygosity for Pik3r1 does not reduce the amount of p85-p110 dimer (Figure 3c). PI 3-kinase activity associated with tyrosine-phosphorylated IRS proteins was decreased by 40% in IR +/ and by 50% in IR/IRS-1 +/ mice compared with wild-type, but it was actually increased to some extent by heterozygosity for Pik3r1 (Figure 3d). This is most likely due to the increased ratio of p85- p110 dimer to p85 monomer produced by heterozygous disruption of Pik3r1, since p85 monomer can compete with p85-p110 dimer in binding to IRS proteins and inhibit PI 3-kinase dependent signaling (24). In muscle, PI 3-kinase activity associated with antip85α and anti-phosphotyrosine (anti-py) immunoprecipitates was not affected by the heterozygosity for Pik3r1 (Figure 3f). By contrast, PI 3-kinase activity associated with p85β in liver was increased in the basal and insulin-stimulated states by % in p85 +/, IR/p85 +/, and IR/IRS-1/p85 +/ mice as compared with their controls (Figure 3e), while PI 3-kinase activity associated with p85β in muscle showed no significant difference between the different groups of animals (Figure 3f). PI 3-kinase activity associated with p55 PIK was very low and not stimulated by insulin in all groups of animals in both liver and muscle. Akt is a Ser/Thr kinase that is phosphorylated and activated by PI 3-kinase upon insulin stimulation and is believed to mediate certain metabolic actions of insulin (30). In livers of wild-type mice, insulin induced a threefold increase in Akt phosphorylation and activity. This was unchanged in IR +/ and reduced by about 50% in IR/IRS-1 +/ mice (Figure 4). Surprisingly, decreasing the expression of p85α and its splice variants led to an increase in Akt phosphorylation and activity in livers of p85 +/ mice and IR/IRS-1/p85 +/ mice (P < 0.05) as compared with their controls (Figure 4). In muscle, the effect of insulin on Akt was small, and no modification of Akt activity or phosphorylation was detected by the heterozygous knockouts (Figure 4). Disrupting one allele of the p85α gene improves insulin sensitivity. With respect to glucose homeostasis, by 6 months of age, although fasting glucose concentrations in the IR +/ and the IR/IRS-1 +/ mice were still normal, random-fed glucose levels indicated that about 10% of IR +/ and about 50% of IR/IRS-1 +/ mice had developed diabetes (glucose > 250 mg/dl) (Figure 5, a and c). In addition, GTT revealed impaired glucose tolerance in both the IR +/ and the IR/IRS-1 +/ mice (Figure 5e). In all three genotypes of mice, heterozygosity for Pik3r1 was associated with lower blood glucose in both the fasting and the fed states (Figure 5a). In addition, p85 +/ mice, IR/p85 +/ mice, and IR/IRS-1/p85 +/ mice showed significantly improved glucose tolerance compared with their respective controls (Figure 5e). All p85 heterozygote mice also maintained significantly lower insulin concentrations than did their respective controls (Figure 5, b, d, and f). Consistent with this, p85 +/, 146 The Journal of Clinical Investigation January 2002 Volume 109 Number 1
7 not change either body weight or fat pad mass and resulted in a modest but significant increase in FFA, which is usually associated with increased rather than decreased insulin resistance (31). Thus, it is unlikely that these changes in lipid metabolism contribute to increased insulin sensitivity in p85 +/ mice. Recently, a number of factors secreted from adipocytes have been shown to affect insulin sensitivity (32). Of these factors, leptin is best characterized, and its deficiency is known to lead to insulin resistance in some types of obese mice (33) and lipoatrophic mice (34). Again, there was no change in plasma leptin levels by heterozygous deletion of the Pik3r1 gene that might contribute to increased insulin sensitivity (Table 1). Figure 6 Increased insulin sensitivity in the Pik3r1 mutant groups. (a) Insulin tolerance tests (1 U/kg, intraperitoneally) and (b) IGF-1 tolerance tests (1 mg/kg, intraperitoneally) were performed on 6-month-old male mice of the indicated genotypes. Results represent the blood glucose concentration as a percentage of the starting glucose value and are expressed as mean ± SEM (n = mice per genotype). *P < 0.05, wild-type vs. p85 +/ ; IR +/ vs. IR/p85 +/ ; IR/IRS-1 +/ vs. IR/IRS-1/p85 +/. (c) Glucose transport activity in isolated skeletal muscle of the Pik3r1 mutant mice was estimated using 2-deoxyglucose uptake in isolated EDL and soleus muscles as described in Methods. Results are expressed as mean ± SEM (n = 4). *P < 0.05, wild-type vs. p85 +/. IR/p85 +/, and IR/IRS-1/p85 +/ mice showed improved sensitivity to insulin injection as compared with their respective controls (Figure 6, a and b). Most importantly, the incidence of diabetes in the IR/IRS-1/p85 +/ subgroup was reduced by half (Figure 5c). Although the improved insulin sensitivity is present in several tissues, the most important effect is probably at the liver. Glucose uptake in isolated soleus and EDL muscles was increased in the basal state, but not after insulin stimulation (Figure 6c). Likewise, heterozygosity of p85α did not affect either insulin secretion or islet size (data not shown). In addition to the direct effects of the heterozygous deletion of the Pik3r1 gene on insulin signaling, it is possible that a reduction in the regulatory subunits may affect glucose metabolism in an indirect fashion, such as alteration of lipid metabolism. However, as shown in Table 1, heterozygous disruption of the Pik3r1 gene did Discussion PI 3-kinase activation is central to most of the metabolic actions of insulin (3). The major regulatory subunits of PI 3-kinase in most cells are p85α and its alternatively spliced isoforms AS53/p55α and p50α encoded by the Pik3r1 gene. In the present study, we have shown that reducing the level of p85α, AS53, and p50α by heterozygous disruption of the Pik3r1 gene results in increased insulin sensitivity, lower fasting and postprandial glucose levels, and a significant decrease in the incidence of diabetes related to genetically induced insulin resistance in mice. Recently, Terauchi et al. reported that selective homozygous disruption of the p85α full-length form of regulatory subunit results in hypoglycemia and increased sensitivity to insulin (20). The authors suggested that this was a result of an isoform switch from p85α to p50α or AS53 in insulin-sensitive tissues and that p50α is more potent in activation of PI 3-kinase dependent insulin signaling than is p85α. Since the expression levels of p85α, p50α, and AS53 are all reduced in the Pik3r1 knockout mouse, this mechanism cannot be responsible for the increased insulin sensitivity observed in the present study. Rather, changes in the molecular balance between p85 and p110 by heterozygous knockout of Pik3r1 appear to play a key role in this phenomenon. Indeed, immunodepletion studies reveal that in liver of wild-type mouse, 30% of p85 exits as a monomer that may compete with p85-p110 dimer in binding to phosphorylated IRS proteins, thereby inhibiting PI 3-kinase dependent signaling. Heterozygous disruption of Pik3r1 Table 1 Characteristics of lipid metabolism in the Pik3r1 +/ mice Wild-type p85 +/ (n = 7 11) (n = 11 18) Body weight (g) 37.7 ± ± 1.1 NS Perigonadal fat (g) 1.59 ± ± 0.18 NS Triglyceride (mg/dl) 141 ± ± 11 NS FFA (meq/l) 1665 ± ± 111 P < 0.05 Leptin (ng/ml) 9.4 ± ± 1.5 NS NS, not significant. The Journal of Clinical Investigation January 2002 Volume 109 Number 1 147
8 decreases p85 monomer with only slight reduction of the amount of p85-p110 dimer. As a consequence, p85 +/ mice group exhibits PI 3-kinase activities almost equal to those of mice with normal levels of p85α. Thus, heterozygous disruption of Pik3r1 increases the ratio of p85-p110 dimer to p85 monomer. This could improve insulin-induced PI 3-kinase signaling, depending on the balance between p85 and phosphorylated IRS proteins. For instance, if phosphorylated IRS proteins are less abundant than total p85 monomer and p85-p110 dimer in a p85 +/ tissue, the increase in the ratio of p85-p110 dimer to p85 monomer by heterozygous disruption of Pik3r1 could improve PI 3-kinase signaling by increasing the amount of p85/p110/irs complexes. However, if phosphorylated IRS proteins are more abundant than total p85 in wild-type, the increase of the ratio by heterozygous disruption of Pik3r1 might not affect PI 3-kinase dependent signaling, because all of the p85-p110 dimers can bind IRS proteins even in the wild-type. Following insulin injection, the improvement of PI 3-kinase dependent signaling in mice with a heterozygous disruption of Pik3r1 was confirmed by increased Akt activity, at least in wild-type and IR/IRS-1 +/ mice. Since under normal feeding conditions, phosphorylation levels of IRS proteins should be much lower than those caused by pharmacological insulin injection, the improvement of PI 3-kinase dependent signaling leading to improved insulin sensitivity would be even more pronounced. Heterozygous disruption of Pik3r1 also results in an increase in the PI 3-kinase activity associated with p85β, at least in liver of the mouse, and this may also contribute to the increased insulin sensitivity. We (24) and others (23, 35) have shown that p85α inhibits the lipid kinase activity of the p110 catalytic subunit in vitro. p85β appears to be less potent in this negative regulation, as evidenced by the finding that in Pik3r1- null mice PI 3-kinase activity associated with p85β maintains Akt activation, even though p85α is completely absent and p110 is markedly decreased (22). In addition, p85α may modulate PI 3-kinase dependent signaling by effects that are independent of its regulation of p110. For example, we find that in cultured cells derived from p85 +/ mice, PI(3,4,5)P 3 production is increased and the levels more sustained as compared with those in the wild-type cells, even though PI 3-kinase activity associated with phosphotyrosine complexes is equal in these two cell types (36). This suggests that p85α protein may have some effect to modulate PI(3,4,5)P 3 clearance independent of its effect on PI 3-kinase activity (36). This could account for the 30% increase in Akt activity in livers of p85 +/ and IR/IRS-1/p85 +/ mice, since Akt activity depends on PI(3,4,5)P 3 levels. Similarly, Terauchi et al. demonstrated that disrupting the full-length p85α alone, results in an increased and sustained PI(3,4,5)P 3 production, although PI 3-kinase activity associated with phosphotyrosine complex is markedly decreased (20). While the effect of reducing p85α levels on insulin sensitivity is clear, it remains to be determined which tissue is most important in this physiological response. In both isolated skeletal muscle (this study) and cultured brown adipocyte cell lines (K. Ueki et al., unpublished data), basal glucose uptake is increased in the p85 +/ tissues as compared with controls, although no significant difference is observed in insulin-stimulated glucose uptake. This could contribute to lower fasting glucose levels. Lower fasting glucose levels may also reflect increased sensitivity to insulin-induced suppression of hepatic glucose output, since the enzyme phosphoenolpyruvate carboxykinase (PEPCK) is negatively regulated by insulin via a PI 3-kinase/Akt mediated pathway (14, 15, 37), and increasing Akt activity in liver has been shown to lower glucose levels in insulinresistant mice (28). Thus, insulin-induced inhibition of hepatic glucose production seems to be important for whole-body insulin sensitivity observed in p85 +/ mice. However, in preliminary studies, we found no change in mrna levels of PEPCK in the p85α heterozygous mice (data not shown). It is possible that any differences in PEPCK are too small to be detected or that the increased Akt activity in liver modifies other enzymes involved in hepatic glucose production, such as 6-phospho-fructose 2-kinase (38) and glycogen synthase kinase 3 (39). Further studies using cultured cells heterozygous for the Pik3r1-null allele would help characterize these issues. In summary, reducing the expression level of p85α by 50% significantly improves insulin sensitivity and results in a decrease in the incidence of diabetes in mouse models of insulin resistance. This appears to be due to an improved stoichiometry of the p85/p110/irs complex and enhanced PI 3-kinase dependent signaling. Thus p85α can play a dual role in insulin action mediating PI 3-kinase activation by bridging IRS proteins and the catalytic p110 subunit, but it can also act as a competitive inhibitor in PI 3-kinase signaling in its monomeric state. Pharmacological modulation of p85α expression in insulin-sensitive tissues appears to be a novel strategy to improve insulin sensitivity and may serve as a new therapeutic target in the treatment of type 2 diabetes. Acknowledgments We are grateful to Karrie E. Miller for excellent technical assistance and Sandra Paquette for assistance in preparing this manuscript. This work was supported by NIH grants DK and DK to C.R. Kahn and GM to L.C. Cantley. D.A. Fruman was supported by fellowships from Damon Runyon-Walter Winchell Cancer Research Fund and the Leukemia Society of America. 1. Kahn, C.R Banting Lecture. Insulin action, diabetogenes, and the cause of type II diabetes. Diabetes. 43: Dunaif, A Insulin action in the polycystic ovary syndrome. Endocrinol. Metab. Clin. North Am. 28: Shepherd, P.R., Withers, D.J., and Siddle, K Phosphoinositide 3- kinase: the key switch mechanism in insulin signaling. Biochem. J. 333: The Journal of Clinical Investigation January 2002 Volume 109 Number 1
9 4. Inukai, K., et al A novel 55-kDa regulatory subunit for phosphatidylinositol 3-kinase structurally similar to p55pik is generated by alternative splicing of the p85a gene. J. Biol. Chem. 271: Antonetti, D.A., Algenstaedt, P., and Kahn, C.R Insulin receptor substrate 1 binds two novel splice variants of the regulatory subunit of phosphatidylinositol 3-kinase in muscle and brain. Mol. Cell. Biol. 16: Fruman, D.A., Cantley, L.C., and Carpenter, C.L Structural organization and alternative splicing of the murine phosphoinositide 3- kinase p85a gene. Genomics. 37: Inukai, K., et al p85a gene generates three isoforms of regulatory subunit for phosphatidylinositol 3-kinase (PI 3-kinase), p50a, p55a, and p85a, with different PI 3-kinase activity elevating responses to insulin. J. Biol. Chem. 272: Pons, S., et al The structure and function of p55pik reveal a new regulatory subunit for phosphatidylinositol 3-kinase. Mol. Cell. Biol. 15: Rameh, L.E., and Cantley, L.C The role of phosphoinositide 3- kinase lipid products in cell function. J. Biol. Chem. 274: Hara, K., et al Phosphatidylinositol 3-kinase activity is required for insulin-stimulated glucose transport but not for RAS activation in CHO cells. Proc. Natl. Acad. Sci. USA. 91: Cheatham, B., et al Phosphatidylinositol 3-kinase activation is required for insulin stimulation of pp70 S6 kinase, DNA synthesis, and glucose transporter translocation. Mol. Cell. Biol. 14: Okada, T., Kawano, Y., Sakakibara, T., Hazeki, O., and Ui, M Essential role of phosphatidylinositol 3-kinase in insulin-induced glucose transport and antilipolysis in rat adipocytes. Studies with a selective inhibitor wortmannin. J. Biol. Chem. 269: Cross, D.A., Alessi, D.R., Cohen, P., Andjelkovich, M., and Hemmings, B.A Inhibition of glycogen synthase kinase-3 by insulin mediated by protein kinase B. Nature. 378: Gabbay, R.A., et al Insulin regulation of phosphoenolpyruvate carboxykinase gene expression does not require activation of the Ras/mitogen-activated protein kinase signaling pathway. J. Biol. Chem. 271: Agati, J.M., Yeagley, D., and Quinn, P.G Assessment of the roles of mitogen-activated protein kinase, phosphatidylinositol 3-kinase, protein kinase B, and protein kinase C in insulin inhibition of campinduced phosphoenolpyruvate carboxykinase gene transcription. J. Biol. Chem. 273: Leibiger, I.B., Leibiger, B., Moede, T., and Berggren, P.O Exocytosis of insulin promotes insulin gene transcription via the insulin receptor/pi-3 kinase/p70 s6 kinase and CaM kinase pathways. Mol. Cell. 1: Anai, M., et al Altered expression levels and impaired steps in the pathway to phosphatidylinositol 3-kinase activation via insulin receptor substrates 1 and 2 in Zucker fatty rats. Diabetes. 47: Kerouz, N.J., Horsch, D., Pons, S., and Kahn, C.R Differential regulation of insulin receptor substrates-1 and -2 (IRS-1 and IRS-2) and phosphatidylinositol 3-kinase isoforms in liver and muscle of the obese diabetic (ob/ob) mouse. J. Clin. Invest. 100: Bi, L., Okabe, I., Bernard, D.J., Wynshaw-Boris, A., and Nussbaum, R.L Proliferative defect and embryonic lethality in mice homozygous for a deletion in the p110alpha subunit of phosphoinositide 3-kinase. J. Biol. Chem. 274: Terauchi, Y., et al Increased insulin sensitivity and hypoglycaemia in mice lacking the p85a subunit of phosphoinositide 3-kinase. Nat. Genet. 21: Fruman, D.A., et al Impaired B cell development and proliferation in absence of phosphoinositide 3-kinase p85a. Science. 283: Fruman, D.A., et al Hypoglycaemia, liver necrosis and perinatal death in mice lacking all isoforms of phosphoinositide 3-kinase p85a. Nat. Genet. 26: Yu, J., et al Regulation of the p85/p110 phosphatidylinositol 3 - kinase: stabilization and inhibition of the p110alpha catalytic subunit by the p85 regulatory subunit. Mol. Cell. Biol. 18: Ueki, K., Algenstaedt, P., Mauvais-Jarvis, F., and Kahn, C.R Positive and negative regulation of phosphoinositide 3-kinase-dependent signaling pathways by three different gene products of the p85a regulatory subunit. Mol. Cell. Biol. 20: Giorgino, F., Pedrini, M.T., Matera, L., and Smith, R.J Specific increase in p85alpha expression in response to dexamethasone is associated with inhibition of insulin-like growth factor-i stimulated phosphatidylinositol 3-kinase activity in cultured muscle cells. J. Biol. Chem. 272: Bruning, J.C., et al Development of a novel polygenic model of NIDDM in mice heterozygous for IR and IRS-1 null alleles. Cell. 88: Bruning, J.C., et al A muscle-specific insulin receptor knockout exhibits features of the metabolic syndrome of NIDDM without altering glucose tolerance. Mol. Cell. 2: Ueki, K., et al Restored insulin-sensitivity in IRS-1-deficient mice treated by adenovirus-mediated gene therapy. J. Clin. Invest. 105: Ueki, K., et al Potential role of protein kinase B in insulininduced glucose transport, glycogen synthesis, and protein synthesis. J. Biol. Chem. 273: Coffer, P.J., Jin, J., and Woodgett, J.R Protein kinase B (c-akt): a multifunctional mediator of phosphatidylinositol 3-kinase activation. Biochem. J. 335: Boden, G Role of fatty acids in the pathogenesis of insulin resistance and NIDDM. Diabetes. 46: Flier, J.S Diabetes. The missing link with obesity? Nature. 409: Zhang, Y., et al Positional cloning of the mouse obese gene and its human homologue. Nature. 372: Shimomura, I., Hammer, R.E., Ikemoto, S., Brown, M.S., and Goldstein, J.L Leptin reverses insulin resistance and diabetes mellitus in mice with congenital lipodystrophy. Nature. 401: Yu, J., Wjasow, C., and Backer, J.M Regulation of the p85/p110alpha phosphatidylinositol 3 -kinase. Distinct roles for the n- terminal and c-terminal SH2 domains. J. Biol. Chem. 273: Ueki, K., et al Molecular balance between the regulatory and catalytic subunits of phosphoinositide 3-kinase regulates cell signaling and survival. Mol. Cell. Biol. In press. 37. Michael, M.D., et al Loss of insulin signaling in hepatocytes leads to severe insulin resistance and progressive hepatic dysfunction. Mol. Cell. 6: Lefebvre, V., Mechin, M.C., Louckx, M.P., Rider, M.H., and Hue, L Signaling pathway involved in the activation of heart 6-phosphofructo-2-kinase by insulin. J. Biol. Chem. 271: Peak, M., Rochford, J.J., Borthwick, A.C., Yeaman, S.J., and Agius, L Signaling pathways involved in the stimulation of glycogen synthesis by insulin in rat hepatocytes. Diabetologia. 41: The Journal of Clinical Investigation January 2002 Volume 109 Number 1 149
Restored insulin-sensitivity in IRS-1 deficient mice treated by adenovirus-mediated gene therapy
 Restored insulin-sensitivity in IRS-1 deficient mice treated by adenovirus-mediated gene therapy Kohjiro Ueki, 1 Toshimasa Yamauchi, 1 Hiroyuki Tamemoto, 1 Kazuyuki Tobe, 1 Ritsuko Yamamoto-Honda, 1 Yasushi
Restored insulin-sensitivity in IRS-1 deficient mice treated by adenovirus-mediated gene therapy Kohjiro Ueki, 1 Toshimasa Yamauchi, 1 Hiroyuki Tamemoto, 1 Kazuyuki Tobe, 1 Ritsuko Yamamoto-Honda, 1 Yasushi
Insulin Resistance. Biol 405 Molecular Medicine
 Insulin Resistance Biol 405 Molecular Medicine Insulin resistance: a subnormal biological response to insulin. Defects of either insulin secretion or insulin action can cause diabetes mellitus. Insulin-dependent
Insulin Resistance Biol 405 Molecular Medicine Insulin resistance: a subnormal biological response to insulin. Defects of either insulin secretion or insulin action can cause diabetes mellitus. Insulin-dependent
Role of fatty acids in the development of insulin resistance and type 2 diabetes mellitus
 Emerging Science Role of fatty acids in the development of insulin resistance and type 2 diabetes mellitus George Wolf Insulin resistance is defined as the reduced responsiveness to normal circulating
Emerging Science Role of fatty acids in the development of insulin resistance and type 2 diabetes mellitus George Wolf Insulin resistance is defined as the reduced responsiveness to normal circulating
Supplementary Information
 Supplementary Information Akt regulates hepatic metabolism by suppressing a Foxo1 dependent global inhibition of adaptation to nutrient intake Mingjian Lu 1, Min Wan 1, Karla F. Leavens 1, Qingwei Chu
Supplementary Information Akt regulates hepatic metabolism by suppressing a Foxo1 dependent global inhibition of adaptation to nutrient intake Mingjian Lu 1, Min Wan 1, Karla F. Leavens 1, Qingwei Chu
Insights into insulin resistance and type 2 diabetes from knockout mouse models
 Insights into insulin resistance and type 2 diabetes from knockout mouse models Takashi Kadowaki Department of Metabolic Diseases, Graduate School of Medicine, University of Tokyo, Tokyo 113-8655, Japan.
Insights into insulin resistance and type 2 diabetes from knockout mouse models Takashi Kadowaki Department of Metabolic Diseases, Graduate School of Medicine, University of Tokyo, Tokyo 113-8655, Japan.
SUPPLEMENTARY INFORMATION
 Supplementary Figures Supplementary Figure S1. Binding of full-length OGT and deletion mutants to PIP strips (Echelon Biosciences). Supplementary Figure S2. Binding of the OGT (919-1036) fragments with
Supplementary Figures Supplementary Figure S1. Binding of full-length OGT and deletion mutants to PIP strips (Echelon Biosciences). Supplementary Figure S2. Binding of the OGT (919-1036) fragments with
Supplementary Fig. 1 eif6 +/- mice show a reduction in white adipose tissue, blood lipids and normal glycogen synthesis. The cohort of the original
 Supplementary Fig. 1 eif6 +/- mice show a reduction in white adipose tissue, blood lipids and normal glycogen synthesis. The cohort of the original phenotypic screening was n=40. For specific tests, the
Supplementary Fig. 1 eif6 +/- mice show a reduction in white adipose tissue, blood lipids and normal glycogen synthesis. The cohort of the original phenotypic screening was n=40. For specific tests, the
KEY CONCEPT QUESTIONS IN SIGNAL TRANSDUCTION
 Signal Transduction - Part 2 Key Concepts - Receptor tyrosine kinases control cell metabolism and proliferation Growth factor signaling through Ras Mutated cell signaling genes in cancer cells are called
Signal Transduction - Part 2 Key Concepts - Receptor tyrosine kinases control cell metabolism and proliferation Growth factor signaling through Ras Mutated cell signaling genes in cancer cells are called
A Hepatocyte Growth Factor Receptor (Met) Insulin Receptor hybrid governs hepatic glucose metabolism SUPPLEMENTARY FIGURES, LEGENDS AND METHODS
 A Hepatocyte Growth Factor Receptor (Met) Insulin Receptor hybrid governs hepatic glucose metabolism Arlee Fafalios, Jihong Ma, Xinping Tan, John Stoops, Jianhua Luo, Marie C. DeFrances and Reza Zarnegar
A Hepatocyte Growth Factor Receptor (Met) Insulin Receptor hybrid governs hepatic glucose metabolism Arlee Fafalios, Jihong Ma, Xinping Tan, John Stoops, Jianhua Luo, Marie C. DeFrances and Reza Zarnegar
BIOL212 Biochemistry of Disease. Metabolic Disorders - Obesity
 BIOL212 Biochemistry of Disease Metabolic Disorders - Obesity Obesity Approx. 23% of adults are obese in the U.K. The number of obese children has tripled in 20 years. 10% of six year olds are obese, rising
BIOL212 Biochemistry of Disease Metabolic Disorders - Obesity Obesity Approx. 23% of adults are obese in the U.K. The number of obese children has tripled in 20 years. 10% of six year olds are obese, rising
Supplementary Figure S1. Effect of Glucose on Energy Balance in WT and KHK A/C KO
 Supplementary Figure S1. Effect of Glucose on Energy Balance in WT and KHK A/C KO Mice. WT mice and KHK-A/C KO mice were provided drinking water containing 10% glucose or tap water with normal chow ad
Supplementary Figure S1. Effect of Glucose on Energy Balance in WT and KHK A/C KO Mice. WT mice and KHK-A/C KO mice were provided drinking water containing 10% glucose or tap water with normal chow ad
The Role of Glycogen Synthase Kinase-3 in Insulin-resistant Skeletal Muscle
 The Role of Glycogen Synthase Kinase-3 in Insulin-resistant Skeletal Muscle Item Type text; Electronic Dissertation Authors Dokken, Betsy B. Publisher The University of Arizona. Rights Copyright is held
The Role of Glycogen Synthase Kinase-3 in Insulin-resistant Skeletal Muscle Item Type text; Electronic Dissertation Authors Dokken, Betsy B. Publisher The University of Arizona. Rights Copyright is held
SUPPLEMENTARY INFORMATION
 doi:10.1038/nature12652 Supplementary Figure 1. PRDM16 interacts with endogenous EHMT1 in brown adipocytes. Immunoprecipitation of PRDM16 complex by flag antibody (M2) followed by Western blot analysis
doi:10.1038/nature12652 Supplementary Figure 1. PRDM16 interacts with endogenous EHMT1 in brown adipocytes. Immunoprecipitation of PRDM16 complex by flag antibody (M2) followed by Western blot analysis
Supplementary Materials for
 www.sciencesignaling.org/cgi/content/full/8/364/ra18/dc1 Supplementary Materials for The tyrosine phosphatase (Pez) inhibits metastasis by altering protein trafficking Leila Belle, Naveid Ali, Ana Lonic,
www.sciencesignaling.org/cgi/content/full/8/364/ra18/dc1 Supplementary Materials for The tyrosine phosphatase (Pez) inhibits metastasis by altering protein trafficking Leila Belle, Naveid Ali, Ana Lonic,
Insulin Receptor Substrate 3 (IRS-3) and IRS-4 Impair IRS-1- and IRS-2-Mediated Signaling
 MOLECULAR AND CELLULAR BIOLOGY, Jan. 2001, p. 26 38 Vol. 21, No. 1 0270-7306/01/$04.00 0 DOI: 10.1128/MCB.21.1.26 38.2001 Copyright 2001, American Society for Microbiology. All Rights Reserved. Insulin
MOLECULAR AND CELLULAR BIOLOGY, Jan. 2001, p. 26 38 Vol. 21, No. 1 0270-7306/01/$04.00 0 DOI: 10.1128/MCB.21.1.26 38.2001 Copyright 2001, American Society for Microbiology. All Rights Reserved. Insulin
A high-fructose diet induces changes in pp185 phosphorylation in muscle and liver of rats
 Fructose Brazilian diet Journal induces of Medical changes and in Biological rat pp185 Research () 33: 1421-1427 ISSN -879X Short Communication 1421 A high-fructose diet induces changes in pp185 phosphorylation
Fructose Brazilian diet Journal induces of Medical changes and in Biological rat pp185 Research () 33: 1421-1427 ISSN -879X Short Communication 1421 A high-fructose diet induces changes in pp185 phosphorylation
The functional investigation of the interaction between TATA-associated factor 3 (TAF3) and p53 protein
 THESIS BOOK The functional investigation of the interaction between TATA-associated factor 3 (TAF3) and p53 protein Orsolya Buzás-Bereczki Supervisors: Dr. Éva Bálint Dr. Imre Miklós Boros University of
THESIS BOOK The functional investigation of the interaction between TATA-associated factor 3 (TAF3) and p53 protein Orsolya Buzás-Bereczki Supervisors: Dr. Éva Bálint Dr. Imre Miklós Boros University of
Pathogenesis of Diabetes Mellitus
 Pathogenesis of Diabetes Mellitus Young-Bum Kim, Ph.D. Associate Professor of Medicine Harvard Medical School Definition of Diabetes Mellitus a group of metabolic diseases characterized by hyperglycemia
Pathogenesis of Diabetes Mellitus Young-Bum Kim, Ph.D. Associate Professor of Medicine Harvard Medical School Definition of Diabetes Mellitus a group of metabolic diseases characterized by hyperglycemia
Metformin inhibits hepatic gluconeogenesis in mice independently of the LKB1/AMPK pathway via a decrease in hepatic energy state
 Related Commentary, page 2267 Research article Metformin inhibits hepatic gluconeogenesis in mice independently of the LKB1/AMPK pathway via a decrease in hepatic energy state Marc Foretz, 1,2 Sophie Hébrard,
Related Commentary, page 2267 Research article Metformin inhibits hepatic gluconeogenesis in mice independently of the LKB1/AMPK pathway via a decrease in hepatic energy state Marc Foretz, 1,2 Sophie Hébrard,
The PI3K/AKT axis. Dr. Lucio Crinò Medical Oncology Division Azienda Ospedaliera-Perugia. Introduction
 The PI3K/AKT axis Dr. Lucio Crinò Medical Oncology Division Azienda Ospedaliera-Perugia Introduction Phosphoinositide 3-kinase (PI3K) pathway are a family of lipid kinases discovered in 1980s. They have
The PI3K/AKT axis Dr. Lucio Crinò Medical Oncology Division Azienda Ospedaliera-Perugia Introduction Phosphoinositide 3-kinase (PI3K) pathway are a family of lipid kinases discovered in 1980s. They have
Phospho-AKT Sampler Kit
 Phospho-AKT Sampler Kit E 0 5 1 0 0 3 Kits Includes Cat. Quantity Application Reactivity Source Akt (Ab-473) Antibody E021054-1 50μg/50μl IHC, WB Human, Mouse, Rat Rabbit Akt (Phospho-Ser473) Antibody
Phospho-AKT Sampler Kit E 0 5 1 0 0 3 Kits Includes Cat. Quantity Application Reactivity Source Akt (Ab-473) Antibody E021054-1 50μg/50μl IHC, WB Human, Mouse, Rat Rabbit Akt (Phospho-Ser473) Antibody
General Laboratory methods Plasma analysis: Gene Expression Analysis: Immunoblot analysis: Immunohistochemistry:
 General Laboratory methods Plasma analysis: Plasma insulin (Mercodia, Sweden), leptin (duoset, R&D Systems Europe, Abingdon, United Kingdom), IL-6, TNFα and adiponectin levels (Quantikine kits, R&D Systems
General Laboratory methods Plasma analysis: Plasma insulin (Mercodia, Sweden), leptin (duoset, R&D Systems Europe, Abingdon, United Kingdom), IL-6, TNFα and adiponectin levels (Quantikine kits, R&D Systems
Supplementary Figure 1
 Supplementary Figure 1 how HFD how HFD Epi WT p p Hypothalamus p p Inguinal WT T Liver Lean mouse adipocytes p p p p p p Obese mouse adipocytes Kidney Muscle Spleen Heart p p p p p p p p Extracellular
Supplementary Figure 1 how HFD how HFD Epi WT p p Hypothalamus p p Inguinal WT T Liver Lean mouse adipocytes p p p p p p Obese mouse adipocytes Kidney Muscle Spleen Heart p p p p p p p p Extracellular
Complementary roles of IRS-1 and IRS-2 in the hepatic regulation of metabolism
 Research article Complementary roles of IRS-1 and IRS-2 in the hepatic regulation of metabolism Cullen M. Taniguchi, 1 Kohjiro Ueki, 1,2 and C. Ronald Kahn 1 1 Research Division, Joslin Diabetes Center,
Research article Complementary roles of IRS-1 and IRS-2 in the hepatic regulation of metabolism Cullen M. Taniguchi, 1 Kohjiro Ueki, 1,2 and C. Ronald Kahn 1 1 Research Division, Joslin Diabetes Center,
G-Protein Signaling. Introduction to intracellular signaling. Dr. SARRAY Sameh, Ph.D
 G-Protein Signaling Introduction to intracellular signaling Dr. SARRAY Sameh, Ph.D Cell signaling Cells communicate via extracellular signaling molecules (Hormones, growth factors and neurotransmitters
G-Protein Signaling Introduction to intracellular signaling Dr. SARRAY Sameh, Ph.D Cell signaling Cells communicate via extracellular signaling molecules (Hormones, growth factors and neurotransmitters
Supplementary Figure 1. DJ-1 modulates ROS concentration in mouse skeletal muscle.
 Supplementary Figure 1. DJ-1 modulates ROS concentration in mouse skeletal muscle. (a) mrna levels of Dj1 measured by quantitative RT-PCR in soleus, gastrocnemius (Gastroc.) and extensor digitorum longus
Supplementary Figure 1. DJ-1 modulates ROS concentration in mouse skeletal muscle. (a) mrna levels of Dj1 measured by quantitative RT-PCR in soleus, gastrocnemius (Gastroc.) and extensor digitorum longus
SUPPLEMENTARY INFORMATION
 SUPPLEMENTARY INFORMATION doi:10.1038/nature11464 Supplemental Figure S1. The expression of Vegfb is increased in obese and diabetic mice as compared to lean mice. a-b, Body weight and postprandial blood
SUPPLEMENTARY INFORMATION doi:10.1038/nature11464 Supplemental Figure S1. The expression of Vegfb is increased in obese and diabetic mice as compared to lean mice. a-b, Body weight and postprandial blood
Expanded View Figures
 Expanded View Figures A B C D E F G H I J K L Figure EV1. The dysregulated lipid metabolic phenotype of mouse models of metabolic dysfunction is most pronounced in the fasted state. A L Male 12-weeks-old
Expanded View Figures A B C D E F G H I J K L Figure EV1. The dysregulated lipid metabolic phenotype of mouse models of metabolic dysfunction is most pronounced in the fasted state. A L Male 12-weeks-old
Receptor mediated Signal Transduction
 Receptor mediated Signal Transduction G-protein-linked receptors adenylyl cyclase camp PKA Organization of receptor protein-tyrosine kinases From G.M. Cooper, The Cell. A molecular approach, 2004, third
Receptor mediated Signal Transduction G-protein-linked receptors adenylyl cyclase camp PKA Organization of receptor protein-tyrosine kinases From G.M. Cooper, The Cell. A molecular approach, 2004, third
ANSC/NUTR 601 GENERAL ANIMAL NUTRITION Stearoyl-CoA desaturase, VLDL metabolism, and obesity
 ANSC/NUTR 601 GENERAL ANIMAL NUTRITION Stearoyl-CoA desaturase, VLDL metabolism, and obesity I. Stearoyl coenzyme A desaturase and obesity in rodents A. Stearoyl coenzyme A desaturase (SCD) is the 9 desaturase.
ANSC/NUTR 601 GENERAL ANIMAL NUTRITION Stearoyl-CoA desaturase, VLDL metabolism, and obesity I. Stearoyl coenzyme A desaturase and obesity in rodents A. Stearoyl coenzyme A desaturase (SCD) is the 9 desaturase.
Signal transduction of insulin
 Signal transduction of insulin Diabetes mellitus is a severe chronic disease, affecting 6-11 percent of the populations aged 30-64 and about 20 percent of those older than age 65 throughout the world.
Signal transduction of insulin Diabetes mellitus is a severe chronic disease, affecting 6-11 percent of the populations aged 30-64 and about 20 percent of those older than age 65 throughout the world.
Requires Signaling though Akt2 Independent of the. Transcription Factors FoxA2, FoxO1, and SREBP1c
 Cell Metabolism, Volume 14 Supplemental Information Postprandial Hepatic Lipid Metabolism Requires Signaling though Akt2 Independent of the Transcription Factors FoxA2, FoxO1, and SREBP1c Min Wan, Karla
Cell Metabolism, Volume 14 Supplemental Information Postprandial Hepatic Lipid Metabolism Requires Signaling though Akt2 Independent of the Transcription Factors FoxA2, FoxO1, and SREBP1c Min Wan, Karla
18s AAACGGCTACCACATCCAAG CCTCCAATGGATCCTCGTTA. 36b4 GTTCTTGCCCATCAGCACC AGATGCAGCAGATCCGCAT. Acc1 AGCAGATCCGCAGCTTG ACCTCTGCTCGCTGAGTGC
 Supplementary Table 1. Quantitative PCR primer sequences Gene symbol Sequences (5 to 3 ) Forward Reverse 18s AAACGGCTACCACATCCAAG CCTCCAATGGATCCTCGTTA 36b4 GTTCTTGCCCATCAGCACC AGATGCAGCAGATCCGCAT Acc1
Supplementary Table 1. Quantitative PCR primer sequences Gene symbol Sequences (5 to 3 ) Forward Reverse 18s AAACGGCTACCACATCCAAG CCTCCAATGGATCCTCGTTA 36b4 GTTCTTGCCCATCAGCACC AGATGCAGCAGATCCGCAT Acc1
Protein protein interaction in insulin signaling and the molecular mechanisms of insulin resistance
 Protein protein interaction in insulin signaling and the molecular mechanisms of insulin resistance Antti Virkamäki,, Kohjiro Ueki, C. Ronald Kahn J Clin Invest. 1999;103(7):931-943. https://doi.org/10.1172/jci6609.
Protein protein interaction in insulin signaling and the molecular mechanisms of insulin resistance Antti Virkamäki,, Kohjiro Ueki, C. Ronald Kahn J Clin Invest. 1999;103(7):931-943. https://doi.org/10.1172/jci6609.
A dual PI3 kinase/mtor inhibitor reveals emergent efficacy in glioma
 Supplemental data A dual PI3 kinase/mtor inhibitor reveals emergent efficacy in glioma Qi-Wen Fan, Zachary A. Knight, David D. Goldenberg, Wei Yu, Keith E. Mostov, David Stokoe, Kevan M. Shokat, and William
Supplemental data A dual PI3 kinase/mtor inhibitor reveals emergent efficacy in glioma Qi-Wen Fan, Zachary A. Knight, David D. Goldenberg, Wei Yu, Keith E. Mostov, David Stokoe, Kevan M. Shokat, and William
Supplementary Figure 1
 VO (ml kg - min - ) VCO (ml kg - min - ) Respiratory exchange ratio Energy expenditure (cal kg - min - ) Locomotor activity (x count) Body temperature ( C) Relative mrna expression TA Sol EDL PT Heart
VO (ml kg - min - ) VCO (ml kg - min - ) Respiratory exchange ratio Energy expenditure (cal kg - min - ) Locomotor activity (x count) Body temperature ( C) Relative mrna expression TA Sol EDL PT Heart
Brief Critical Review
 Brief Critical Review May 2007: 251 256 Serum Retinol-Binding Protein: A Link Between Obesity, Insulin Resistance, and Type 2 Diabetes George Wolf, DPhil Insulin resistance occurs under conditions of obesity,
Brief Critical Review May 2007: 251 256 Serum Retinol-Binding Protein: A Link Between Obesity, Insulin Resistance, and Type 2 Diabetes George Wolf, DPhil Insulin resistance occurs under conditions of obesity,
Signaling. Dr. Sujata Persad Katz Group Centre for Pharmacy & Health research
 Signaling Dr. Sujata Persad 3-020 Katz Group Centre for Pharmacy & Health research E-mail:sujata.persad@ualberta.ca 1 Growth Factor Receptors and Other Signaling Pathways What we will cover today: How
Signaling Dr. Sujata Persad 3-020 Katz Group Centre for Pharmacy & Health research E-mail:sujata.persad@ualberta.ca 1 Growth Factor Receptors and Other Signaling Pathways What we will cover today: How
Growth and Differentiation Phosphorylation Sampler Kit
 Growth and Differentiation Phosphorylation Sampler Kit E 0 5 1 0 1 4 Kits Includes Cat. Quantity Application Reactivity Source Akt (Phospho-Ser473) E011054-1 50μg/50μl IHC, WB Human, Mouse, Rat Rabbit
Growth and Differentiation Phosphorylation Sampler Kit E 0 5 1 0 1 4 Kits Includes Cat. Quantity Application Reactivity Source Akt (Phospho-Ser473) E011054-1 50μg/50μl IHC, WB Human, Mouse, Rat Rabbit
Supplementary Table 1. Metabolic parameters in GFP and OGT-treated mice
 Supplementary Table 1. Metabolic parameters in GFP and OGT-treated mice Fasted Refed GFP OGT GFP OGT Liver G6P (mmol/g) 0.03±0.01 0.04±0.02 0.60±0.04 0.42±0.10 A TGs (mg/g of liver) 20.08±5.17 16.29±0.8
Supplementary Table 1. Metabolic parameters in GFP and OGT-treated mice Fasted Refed GFP OGT GFP OGT Liver G6P (mmol/g) 0.03±0.01 0.04±0.02 0.60±0.04 0.42±0.10 A TGs (mg/g of liver) 20.08±5.17 16.29±0.8
Supplemental Information. Increased 4E-BP1 Expression Protects. against Diet-Induced Obesity and Insulin. Resistance in Male Mice
 Cell Reports, Volume 16 Supplemental Information Increased 4E-BP1 Expression Protects against Diet-Induced Obesity and Insulin Resistance in Male Mice Shih-Yin Tsai, Ariana A. Rodriguez, Somasish G. Dastidar,
Cell Reports, Volume 16 Supplemental Information Increased 4E-BP1 Expression Protects against Diet-Induced Obesity and Insulin Resistance in Male Mice Shih-Yin Tsai, Ariana A. Rodriguez, Somasish G. Dastidar,
MBB317. Dr D MANGNALL OBESITY. Lecture 2
 MBB317 Dr D MANGNALL OBESITY Lecture 2 When the structure of the insulin receptor was first discovered it was assumed that the active beta subunit tyrosine kinase would phosphorylate some intracellular
MBB317 Dr D MANGNALL OBESITY Lecture 2 When the structure of the insulin receptor was first discovered it was assumed that the active beta subunit tyrosine kinase would phosphorylate some intracellular
GPR120 *** * * Liver BAT iwat ewat mwat Ileum Colon. UCP1 mrna ***
 a GPR120 GPR120 mrna/ppia mrna Arbitrary Units 150 100 50 Liver BAT iwat ewat mwat Ileum Colon b UCP1 mrna Fold induction 20 15 10 5 - camp camp SB202190 - - - H89 - - - - - GW7647 Supplementary Figure
a GPR120 GPR120 mrna/ppia mrna Arbitrary Units 150 100 50 Liver BAT iwat ewat mwat Ileum Colon b UCP1 mrna Fold induction 20 15 10 5 - camp camp SB202190 - - - H89 - - - - - GW7647 Supplementary Figure
Supplementary Figure 1. Normal T lymphocyte populations in Dapk -/- mice. (a) Normal thymic development in Dapk -/- mice. Thymocytes from WT and Dapk
 Supplementary Figure 1. Normal T lymphocyte populations in Dapk -/- mice. (a) Normal thymic development in Dapk -/- mice. Thymocytes from WT and Dapk -/- mice were stained for expression of CD4 and CD8.
Supplementary Figure 1. Normal T lymphocyte populations in Dapk -/- mice. (a) Normal thymic development in Dapk -/- mice. Thymocytes from WT and Dapk -/- mice were stained for expression of CD4 and CD8.
AdPLA ablation increases lipolysis and prevents obesity induced by high fat feeding or leptin deficiency
 AdPLA AdPLA ablation increases lipolysis and prevents obesity induced by high fat feeding or leptin deficiency Kathy Jaworski, Maryam Ahmadian, Robin E. Duncan, Eszter Sarkadi-Nagy, Krista A. Va rady,
AdPLA AdPLA ablation increases lipolysis and prevents obesity induced by high fat feeding or leptin deficiency Kathy Jaworski, Maryam Ahmadian, Robin E. Duncan, Eszter Sarkadi-Nagy, Krista A. Va rady,
Over-expression of MKP-3 and knockdown of MKP-3 and FOXO1 in primary rat. day, cells were transduced with adenoviruses expressing GFP, MKP-3 or shgfp,
 SUPPLEMENTAL METHODS Over-expression of MKP-3 and knockdown of MKP-3 and FOXO1 in primary rat hepatocytes Primary rat hepatocytes were seeded as described in experimental procedures. The next day, cells
SUPPLEMENTAL METHODS Over-expression of MKP-3 and knockdown of MKP-3 and FOXO1 in primary rat hepatocytes Primary rat hepatocytes were seeded as described in experimental procedures. The next day, cells
SUPPLEMENTARY INFORMATION
 DOI: 10.1038/ncb3461 In the format provided by the authors and unedited. Supplementary Figure 1 (associated to Figure 1). Cpeb4 gene-targeted mice develop liver steatosis. a, Immunoblot displaying CPEB4
DOI: 10.1038/ncb3461 In the format provided by the authors and unedited. Supplementary Figure 1 (associated to Figure 1). Cpeb4 gene-targeted mice develop liver steatosis. a, Immunoblot displaying CPEB4
Supplementary Materials
 Supplementary Materials 1 Supplementary Table 1. List of primers used for quantitative PCR analysis. Gene name Gene symbol Accession IDs Sequence range Product Primer sequences size (bp) β-actin Actb gi
Supplementary Materials 1 Supplementary Table 1. List of primers used for quantitative PCR analysis. Gene name Gene symbol Accession IDs Sequence range Product Primer sequences size (bp) β-actin Actb gi
Redistribution of substrates to adipose tissue promotes obesity in mice with selective insulin resistance in muscle
 Redistribution of substrates to adipose tissue promotes obesity in mice with selective insulin resistance in muscle Jason K. Kim, 1 M. Dodson Michael, 2 Stephen F. Previs, 1 Odile D. Peroni, 3 Franck Mauvais-Jarvis,
Redistribution of substrates to adipose tissue promotes obesity in mice with selective insulin resistance in muscle Jason K. Kim, 1 M. Dodson Michael, 2 Stephen F. Previs, 1 Odile D. Peroni, 3 Franck Mauvais-Jarvis,
Amino acids. Side chain. -Carbon atom. Carboxyl group. Amino group
 PROTEINS Amino acids Side chain -Carbon atom Amino group Carboxyl group Amino acids Primary structure Amino acid monomers Peptide bond Peptide bond Amino group Carboxyl group Peptide bond N-terminal (
PROTEINS Amino acids Side chain -Carbon atom Amino group Carboxyl group Amino acids Primary structure Amino acid monomers Peptide bond Peptide bond Amino group Carboxyl group Peptide bond N-terminal (
Signal Transduction: G-Protein Coupled Receptors
 Signal Transduction: G-Protein Coupled Receptors Federle, M. (2017). Lectures 4-5: Signal Transduction parts 1&2: nuclear receptors and GPCRs. Lecture presented at PHAR 423 Lecture in UIC College of Pharmacy,
Signal Transduction: G-Protein Coupled Receptors Federle, M. (2017). Lectures 4-5: Signal Transduction parts 1&2: nuclear receptors and GPCRs. Lecture presented at PHAR 423 Lecture in UIC College of Pharmacy,
Introduction! Introduction! Introduction! Chem Lecture 10 Signal Transduction & Sensory Systems Part 2
 Chem 452 - Lecture 10 Signal Transduction & Sensory Systems Part 2 Questions of the Day: How does the hormone insulin trigger the uptake of glucose in the cells that it targets. Introduction! Signal transduction
Chem 452 - Lecture 10 Signal Transduction & Sensory Systems Part 2 Questions of the Day: How does the hormone insulin trigger the uptake of glucose in the cells that it targets. Introduction! Signal transduction
Reduced glucose uptake precedes insulin signaling defects in adipocytes from heterozygous GLUT4 knockout mice
 Reduced glucose uptake precedes insulin signaling defects in adipocytes from heterozygous GLUT4 knockout mice JING LI,* KAREN L. HOUSEKNECHT, ANTINE E. STENBIT,* ELLEN B. KATZ,* AND MAUREEN J. CHARRON*,1
Reduced glucose uptake precedes insulin signaling defects in adipocytes from heterozygous GLUT4 knockout mice JING LI,* KAREN L. HOUSEKNECHT, ANTINE E. STENBIT,* ELLEN B. KATZ,* AND MAUREEN J. CHARRON*,1
Supplementary Figure 1. DNA methylation of the adiponectin promoter R1, Pparg2, and Tnfa promoter in adipocytes is not affected by obesity.
 Supplementary Figure 1. DNA methylation of the adiponectin promoter R1, Pparg2, and Tnfa promoter in adipocytes is not affected by obesity. (a) Relative amounts of adiponectin, Ppar 2, C/ebp, and Tnf mrna
Supplementary Figure 1. DNA methylation of the adiponectin promoter R1, Pparg2, and Tnfa promoter in adipocytes is not affected by obesity. (a) Relative amounts of adiponectin, Ppar 2, C/ebp, and Tnf mrna
Part-4. Cell cycle regulatory protein 5 (Cdk5) A novel target of ERK in Carb induced cell death
 Part-4 Cell cycle regulatory protein 5 (Cdk5) A novel target of ERK in Carb induced cell death 95 1. Introduction The process of replicating DNA and dividing cells can be described as a series of coordinated
Part-4 Cell cycle regulatory protein 5 (Cdk5) A novel target of ERK in Carb induced cell death 95 1. Introduction The process of replicating DNA and dividing cells can be described as a series of coordinated
Final Review Sessions. 3/16 (FRI) 126 Wellman (4-6 6 pm) 3/19 (MON) 1309 Surge 3 (4-6 6 pm) Office Hours
 Final Review Sessions 3/16 (FRI) 126 Wellman (4-6 6 pm) 3/19 (MON) 1309 Surge 3 (4-6 6 pm) Office ours 3/14 (WED) 9:30 11:30 am (Rebecca) 3/16 (FRI) 9-11 am (Abel) Final ESSENTIALS Posted Lecture 20 ormonal
Final Review Sessions 3/16 (FRI) 126 Wellman (4-6 6 pm) 3/19 (MON) 1309 Surge 3 (4-6 6 pm) Office ours 3/14 (WED) 9:30 11:30 am (Rebecca) 3/16 (FRI) 9-11 am (Abel) Final ESSENTIALS Posted Lecture 20 ormonal
SUPPLEMENTARY INFORMATION
 SUPPLEMENTARY INFORMATION doi:10.1038/nature11429 S1a 6 7 8 9 Nlrc4 allele S1b Nlrc4 +/+ Nlrc4 +/F Nlrc4 F/F 9 Targeting construct 422 bp 273 bp FRT-neo-gb-PGK-FRT 3x.STOP S1c Nlrc4 +/+ Nlrc4 F/F casp1
SUPPLEMENTARY INFORMATION doi:10.1038/nature11429 S1a 6 7 8 9 Nlrc4 allele S1b Nlrc4 +/+ Nlrc4 +/F Nlrc4 F/F 9 Targeting construct 422 bp 273 bp FRT-neo-gb-PGK-FRT 3x.STOP S1c Nlrc4 +/+ Nlrc4 F/F casp1
Insulin/IGF-1 and TNF-α stimulate phosphorylation of IRS-1 at inhibitory Ser 307 via distinct pathways
 Insulin/IGF-1 and TNF-α stimulate phosphorylation of IRS-1 at inhibitory Ser 307 via distinct pathways Liangyou Rui, 1 Vincent Aguirre, 1 Jason K. Kim, 2 Gerald I. Shulman, 2 Anna Lee, 3 Anne Corbould,
Insulin/IGF-1 and TNF-α stimulate phosphorylation of IRS-1 at inhibitory Ser 307 via distinct pathways Liangyou Rui, 1 Vincent Aguirre, 1 Jason K. Kim, 2 Gerald I. Shulman, 2 Anna Lee, 3 Anne Corbould,
Supplementary Information. Glycogen shortage during fasting triggers liver-brain-adipose. neurocircuitry to facilitate fat utilization
 Supplementary Information Glycogen shortage during fasting triggers liver-brain-adipose neurocircuitry to facilitate fat utilization Supplementary Figure S1. Liver-Brain-Adipose neurocircuitry Starvation
Supplementary Information Glycogen shortage during fasting triggers liver-brain-adipose neurocircuitry to facilitate fat utilization Supplementary Figure S1. Liver-Brain-Adipose neurocircuitry Starvation
Modifications of Pyruvate Handling in Health and Disease Prof. Mary Sugden
 Modifications of Handling Modifications of Handling Centre for Diabetes and Metabolic Medicine Institute of Cell and Molecular Science Barts and the London School of Medicine and Dentistry 1 Potential
Modifications of Handling Modifications of Handling Centre for Diabetes and Metabolic Medicine Institute of Cell and Molecular Science Barts and the London School of Medicine and Dentistry 1 Potential
Supplementary Figure 1 CD4 + T cells from PKC-θ null mice are defective in NF-κB activation during T cell receptor signaling. CD4 + T cells were
 Supplementary Figure 1 CD4 + T cells from PKC-θ null mice are defective in NF-κB activation during T cell receptor signaling. CD4 + T cells were isolated from wild type (PKC-θ- WT) or PKC-θ null (PKC-θ-KO)
Supplementary Figure 1 CD4 + T cells from PKC-θ null mice are defective in NF-κB activation during T cell receptor signaling. CD4 + T cells were isolated from wild type (PKC-θ- WT) or PKC-θ null (PKC-θ-KO)
AAV-TBGp-Cre treatment resulted in hepatocyte-specific GH receptor gene recombination
 AAV-TBGp-Cre treatment resulted in hepatocyte-specific GH receptor gene recombination Supplementary Figure 1. Generation of the adult-onset, liver-specific GH receptor knock-down (alivghrkd, Kd) mouse
AAV-TBGp-Cre treatment resulted in hepatocyte-specific GH receptor gene recombination Supplementary Figure 1. Generation of the adult-onset, liver-specific GH receptor knock-down (alivghrkd, Kd) mouse
FOR REVIEW. BMB Reports - Manuscript Submission. Manuscript Draft. Manuscript Number: BMB
 BMB Reports - Manuscript Submission Manuscript Draft Manuscript Number: BMB-18-095 Title: Insulin Receptor Substrate 2:A Bridge between Hippo and AKT Pathways Article Type: Perspective (Invited Only) Keywords:
BMB Reports - Manuscript Submission Manuscript Draft Manuscript Number: BMB-18-095 Title: Insulin Receptor Substrate 2:A Bridge between Hippo and AKT Pathways Article Type: Perspective (Invited Only) Keywords:
Supplementary Materials for
 www.sciencesignaling.org/cgi/content/full/8/407/ra127/dc1 Supplementary Materials for Loss of FTO in adipose tissue decreases Angptl4 translation and alters triglyceride metabolism Chao-Yung Wang,* Shian-Sen
www.sciencesignaling.org/cgi/content/full/8/407/ra127/dc1 Supplementary Materials for Loss of FTO in adipose tissue decreases Angptl4 translation and alters triglyceride metabolism Chao-Yung Wang,* Shian-Sen
Supplemental Figure 1. Western blot analysis indicated that MIF was detected in the fractions of
 Supplemental Figure Legends Supplemental Figure 1. Western blot analysis indicated that was detected in the fractions of plasma membrane and cytosol but not in nuclear fraction isolated from Pkd1 null
Supplemental Figure Legends Supplemental Figure 1. Western blot analysis indicated that was detected in the fractions of plasma membrane and cytosol but not in nuclear fraction isolated from Pkd1 null
Lecture: CHAPTER 13 Signal Transduction Pathways
 Lecture: 10 17 2016 CHAPTER 13 Signal Transduction Pathways Chapter 13 Outline Signal transduction cascades have many components in common: 1. Release of a primary message as a response to a physiological
Lecture: 10 17 2016 CHAPTER 13 Signal Transduction Pathways Chapter 13 Outline Signal transduction cascades have many components in common: 1. Release of a primary message as a response to a physiological
AT1 RECEPTOR BLOCKADE ATTENUATES INSULIN RESISTANCE AND MYOCARDIAL REMODELING IN RATS WITH DIET-INDUCED OBESITY
 AT1 RECEPTOR BLOCKADE ATTENUATES INSULIN RESISTANCE AND MYOCARDIAL REMODELING IN RATS WITH DIET-INDUCED OBESITY SA Oliveira Jr, MP Okoshi, PF Martinez, DM Guizoni, BP Torres, M Dal Pai-Silva, K Okoshi,
AT1 RECEPTOR BLOCKADE ATTENUATES INSULIN RESISTANCE AND MYOCARDIAL REMODELING IN RATS WITH DIET-INDUCED OBESITY SA Oliveira Jr, MP Okoshi, PF Martinez, DM Guizoni, BP Torres, M Dal Pai-Silva, K Okoshi,
Signal Transduction Pathway Smorgasbord
 Molecular Cell Biology Lecture. Oct 28, 2014 Signal Transduction Pathway Smorgasbord Ron Bose, MD PhD Biochemistry and Molecular Cell Biology Programs Washington University School of Medicine Outline 1.
Molecular Cell Biology Lecture. Oct 28, 2014 Signal Transduction Pathway Smorgasbord Ron Bose, MD PhD Biochemistry and Molecular Cell Biology Programs Washington University School of Medicine Outline 1.
Nature Biotechnology: doi: /nbt Supplementary Figure 1. Analysis of hair bundle morphology in Ush1c c.216g>a mice at P18 by SEM.
 Supplementary Figure 1 Analysis of hair bundle morphology in Ush1c c.216g>a mice at P18 by SEM. (a-c) Heterozygous c.216ga mice displayed normal hair bundle morphology at P18. (d-i) Disorganized hair bundles
Supplementary Figure 1 Analysis of hair bundle morphology in Ush1c c.216g>a mice at P18 by SEM. (a-c) Heterozygous c.216ga mice displayed normal hair bundle morphology at P18. (d-i) Disorganized hair bundles
ZL ZDF ZDF + E2 *** Visceral (g) ZDF
 Body Weight (g) 4 3 2 1 ** * ZL ZDF 6 8 1 12 14 16 Age (weeks) B * Sub-cutaneous (g) 16 12 8 4 ZL ZDF Visceral (g) 25 2 15 1 5 ZL ZDF Total fat pad weight (g) 4 3 2 1 ZDF ZL Supplemental Figure 1: Effect
Body Weight (g) 4 3 2 1 ** * ZL ZDF 6 8 1 12 14 16 Age (weeks) B * Sub-cutaneous (g) 16 12 8 4 ZL ZDF Visceral (g) 25 2 15 1 5 ZL ZDF Total fat pad weight (g) 4 3 2 1 ZDF ZL Supplemental Figure 1: Effect
Exercise modulates postreceptor insulin signaling and glucose transport in muscle-specific insulin receptor knockout mice
 Exercise modulates postreceptor insulin signaling and glucose transport in muscle-specific insulin receptor knockout mice Jørgen F.P. Wojtaszewski, Yasuki Higaki, Michael F. Hirshman, M. Dodson Michael,
Exercise modulates postreceptor insulin signaling and glucose transport in muscle-specific insulin receptor knockout mice Jørgen F.P. Wojtaszewski, Yasuki Higaki, Michael F. Hirshman, M. Dodson Michael,
Cell Signaling part 2
 15 Cell Signaling part 2 Functions of Cell Surface Receptors Other cell surface receptors are directly linked to intracellular enzymes. The largest family of these is the receptor protein tyrosine kinases,
15 Cell Signaling part 2 Functions of Cell Surface Receptors Other cell surface receptors are directly linked to intracellular enzymes. The largest family of these is the receptor protein tyrosine kinases,
Amajor pathway for the metabolic effects of insulin and other
 Characterization of the Met326Ile variant of phosphatidylinositol 3-kinase p85 Katrine Almind*, Laurent Delahaye, Torben Hansen, Emmanuel Van Obberghen, Oluf Pedersen, and C. Ronald Kahn* *Research Division,
Characterization of the Met326Ile variant of phosphatidylinositol 3-kinase p85 Katrine Almind*, Laurent Delahaye, Torben Hansen, Emmanuel Van Obberghen, Oluf Pedersen, and C. Ronald Kahn* *Research Division,
Muscle-specific knockout of PKC-λ impairs glucose transport and induces metabolic and diabetic syndromes
 Research article Muscle-specific knockout of PKC-λ impairs glucose transport and induces metabolic and diabetic syndromes Robert V. Farese, 1,2,3 Mini P. Sajan, 1,2,3 Hong Yang, 1,2,3 Pengfei Li, 1,3 Steven
Research article Muscle-specific knockout of PKC-λ impairs glucose transport and induces metabolic and diabetic syndromes Robert V. Farese, 1,2,3 Mini P. Sajan, 1,2,3 Hong Yang, 1,2,3 Pengfei Li, 1,3 Steven
Correction. E3588 PNAS June 21, 2016 vol. 113 no. 25
 Correction Correction for Phosphoinositide 3-kinase regulatory subunit p85α suppresses insulin action via positive regulation of PTEN, by Cullen M. Taniguchi, Thien T. Tran, Tatsuya Kondo, Ji Luo, Kohjiro
Correction Correction for Phosphoinositide 3-kinase regulatory subunit p85α suppresses insulin action via positive regulation of PTEN, by Cullen M. Taniguchi, Thien T. Tran, Tatsuya Kondo, Ji Luo, Kohjiro
BCR-ABL uncouples canonical JAK2-STAT5 signaling in chronic myeloid
 Supplementary Results BCR-ABL uncouples canonical JAK2-STAT5 signaling in chronic myeloid leukemia Oliver Hantschel*, Wolfgang Warsch*, Eva Eckelhart*, Ines Kaupe, Florian Grebien, Kay-Uwe Wagner, Giulio
Supplementary Results BCR-ABL uncouples canonical JAK2-STAT5 signaling in chronic myeloid leukemia Oliver Hantschel*, Wolfgang Warsch*, Eva Eckelhart*, Ines Kaupe, Florian Grebien, Kay-Uwe Wagner, Giulio
Characterization of selective resistance to insulin signaling in the vasculature of obese Zucker (fa/fa) rats
 Characterization of selective resistance to insulin signaling in the vasculature of obese Zucker (fa/fa) rats Zhen Y. Jiang, You-Wei Lin, Allen Clemont, Edward P. Feener, Katherine D. Hein, Masahiko Igarashi,
Characterization of selective resistance to insulin signaling in the vasculature of obese Zucker (fa/fa) rats Zhen Y. Jiang, You-Wei Lin, Allen Clemont, Edward P. Feener, Katherine D. Hein, Masahiko Igarashi,
A particular set of insults induces apoptosis (part 1), which, if inhibited, can switch to autophagy. At least in some cellular settings, autophagy se
 A particular set of insults induces apoptosis (part 1), which, if inhibited, can switch to autophagy. At least in some cellular settings, autophagy serves as a defence mechanism that prevents or retards
A particular set of insults induces apoptosis (part 1), which, if inhibited, can switch to autophagy. At least in some cellular settings, autophagy serves as a defence mechanism that prevents or retards
Critical role for peptide YY in protein-mediated satiation and bodyweight
 Cell Metabolism, Volume 4 Supplemental data Critical role for peptide YY in protein-mediated satiation and bodyweight regulation Rachel L. Batterham, Helen Heffron, Saloni Kapoor, Joanna E. Chivers, Keval
Cell Metabolism, Volume 4 Supplemental data Critical role for peptide YY in protein-mediated satiation and bodyweight regulation Rachel L. Batterham, Helen Heffron, Saloni Kapoor, Joanna E. Chivers, Keval
HSP72 HSP90. Quadriceps Muscle. MEF2c MyoD1 MyoG Myf5 Hsf1 Hsp GLUT4/GAPDH (AU)
 Supplementary Figure 1. Impaired insulin action in HSP72 deficient muscle and myotubes in culture cannot be explained by altered myogenesis or reduced total GLUT4 expression. Genes associated with myogenesis
Supplementary Figure 1. Impaired insulin action in HSP72 deficient muscle and myotubes in culture cannot be explained by altered myogenesis or reduced total GLUT4 expression. Genes associated with myogenesis
Disruption of IRS-2 causes type 2 diabetes in mice
 27. Banfi, S. et al. Identification and mapping of human cdnas homologous to Drosophila mutant genes through EST database searches. Nature Genet. 13, 167 174 (1996). 28. Bernal, J., Lee, J.-H., Cribbs,
27. Banfi, S. et al. Identification and mapping of human cdnas homologous to Drosophila mutant genes through EST database searches. Nature Genet. 13, 167 174 (1996). 28. Bernal, J., Lee, J.-H., Cribbs,
Supplementary Figure 1
 Supplementary Figure 1 Supplementary Figure 1 Schematic depiction of the tandem Fc GDF15. Supplementary Figure 2 Supplementary Figure 2 Gfral mrna levels in the brains of both wild-type and knockout Gfral
Supplementary Figure 1 Supplementary Figure 1 Schematic depiction of the tandem Fc GDF15. Supplementary Figure 2 Supplementary Figure 2 Gfral mrna levels in the brains of both wild-type and knockout Gfral
Supplementary Materials for
 www.sciencesignaling.org/cgi/content/full/9/439/ra78/dc1 Supplementary Materials for Small heterodimer partner mediates liver X receptor (LXR) dependent suppression of inflammatory signaling by promoting
www.sciencesignaling.org/cgi/content/full/9/439/ra78/dc1 Supplementary Materials for Small heterodimer partner mediates liver X receptor (LXR) dependent suppression of inflammatory signaling by promoting
DETAIL EXPERIMENTAL PROCEDURES
 DETAIL EXPERIMENTAL PROCEDURES Generation of knockout animals and genotyping Heterozygous PIKE -/- C57BL/6 mice with a targeted deletion of exon 3 to 6 of CENTG1 were generated under contract by Ozgene
DETAIL EXPERIMENTAL PROCEDURES Generation of knockout animals and genotyping Heterozygous PIKE -/- C57BL/6 mice with a targeted deletion of exon 3 to 6 of CENTG1 were generated under contract by Ozgene
PKC-θ knockout mice are protected from fat-induced insulin resistance
 Research article PKC-θ knockout mice are protected from fat-induced insulin resistance Jason K. Kim, 1 Jonathan J. Fillmore, 1 Mary Jean Sunshine, 2 Bjoern Albrecht, 2 Takamasa Higashimori, 1 Dong-Wook
Research article PKC-θ knockout mice are protected from fat-induced insulin resistance Jason K. Kim, 1 Jonathan J. Fillmore, 1 Mary Jean Sunshine, 2 Bjoern Albrecht, 2 Takamasa Higashimori, 1 Dong-Wook
William C. Comb, Jessica E. Hutti, Patricia Cogswell, Lewis C. Cantley, and Albert S. Baldwin
 Molecular Cell, Volume 45 Supplemental Information p85 SH2 Domain Phosphorylation by IKK Promotes Feedback Inhibition of PI3K and Akt in Response to Cellular Starvation William C. Comb, Jessica E. Hutti,
Molecular Cell, Volume 45 Supplemental Information p85 SH2 Domain Phosphorylation by IKK Promotes Feedback Inhibition of PI3K and Akt in Response to Cellular Starvation William C. Comb, Jessica E. Hutti,
A Central Role of MG53 in Metabolic Syndrome. and Type-2 Diabetes
 A Central Role of MG53 in Metabolic Syndrome and Type-2 Diabetes Yan Zhang, Chunmei Cao, Rui-Ping Xiao Institute of Molecular Medicine (IMM) Peking University, Beijing, China Accelerated Aging in China
A Central Role of MG53 in Metabolic Syndrome and Type-2 Diabetes Yan Zhang, Chunmei Cao, Rui-Ping Xiao Institute of Molecular Medicine (IMM) Peking University, Beijing, China Accelerated Aging in China
Signaling pathways in insulin action: molecular targets of insulin resistance
 Signaling pathways in insulin action: molecular targets of insulin resistance Jeffrey E. Pessin, Alan R. Saltiel J Clin Invest. 2000;106(2):165-169. https://doi.org/10.1172/jci10582. Perspective Insulin
Signaling pathways in insulin action: molecular targets of insulin resistance Jeffrey E. Pessin, Alan R. Saltiel J Clin Invest. 2000;106(2):165-169. https://doi.org/10.1172/jci10582. Perspective Insulin
Chem Lecture 10 Signal Transduction
 Chem 452 - Lecture 10 Signal Transduction 111130 Here we look at the movement of a signal from the outside of a cell to its inside, where it elicits changes within the cell. These changes are usually mediated
Chem 452 - Lecture 10 Signal Transduction 111130 Here we look at the movement of a signal from the outside of a cell to its inside, where it elicits changes within the cell. These changes are usually mediated
Effects of growth hormone secretagogue receptor agonist and antagonist in nonobese type 2 diabetic MKR mice
 Effects of growth hormone secretagogue receptor agonist and antagonist in nonobese type 2 diabetic MKR mice Rasha Mosa (MBCHC, M.D, PhD candidate) School of Biomedical Sciences University of Queensland
Effects of growth hormone secretagogue receptor agonist and antagonist in nonobese type 2 diabetic MKR mice Rasha Mosa (MBCHC, M.D, PhD candidate) School of Biomedical Sciences University of Queensland
Chapter 11. B cell generation, Activation, and Differentiation. Pro-B cells. - B cells mature in the bone marrow.
 Chapter B cell generation, Activation, and Differentiation - B cells mature in the bone marrow. - B cells proceed through a number of distinct maturational stages: ) Pro-B cell ) Pre-B cell ) Immature
Chapter B cell generation, Activation, and Differentiation - B cells mature in the bone marrow. - B cells proceed through a number of distinct maturational stages: ) Pro-B cell ) Pre-B cell ) Immature
The Novel Role of Interleukin-1 Receptor-Associated Kinase 1 in the Signaling. Process Controlling Innate Immunity and Inflammation
 The Novel Role of Interleukin-1 Receptor-Associated Kinase 1 in the Signaling Process Controlling Innate Immunity and Inflammation By Youjia Fang Thesis submitted to the faculty of the Virginia Polytechnic
The Novel Role of Interleukin-1 Receptor-Associated Kinase 1 in the Signaling Process Controlling Innate Immunity and Inflammation By Youjia Fang Thesis submitted to the faculty of the Virginia Polytechnic
Insulin Signaling After Exercise in Insulin Receptor Substrate-2 Deficient Mice
 Insulin Signaling After Exercise in Insulin Receptor Substrate-2 Deficient Mice Kirsten F. Howlett, Kei Sakamoto, Michael F. Hirshman, William G. Aschenbach, Matthew Dow, Morris F. White, and Laurie J.
Insulin Signaling After Exercise in Insulin Receptor Substrate-2 Deficient Mice Kirsten F. Howlett, Kei Sakamoto, Michael F. Hirshman, William G. Aschenbach, Matthew Dow, Morris F. White, and Laurie J.
Signal-Transduction Cascades - 2. The Phosphoinositide Cascade
 Signal-Transduction Cascades - 2 The Phosphoinositide Cascade Calcium ion as a second messenger Tyrosine kinase and receptor dimerization scribd.com Faisal Khatib JU The Phosphoinositide Cascade Used by
Signal-Transduction Cascades - 2 The Phosphoinositide Cascade Calcium ion as a second messenger Tyrosine kinase and receptor dimerization scribd.com Faisal Khatib JU The Phosphoinositide Cascade Used by
Chapter 10. Introduction to Nutrition and Metabolism, 3 rd edition David A Bender Taylor & Francis Ltd, London 2002
 Chapter 10 Introduction to Nutrition and Metabolism, 3 rd edition David A Bender Taylor & Francis Ltd, London 2002 Chapter 10: Integration and Control of Metabolism Press the space bar or click the mouse
Chapter 10 Introduction to Nutrition and Metabolism, 3 rd edition David A Bender Taylor & Francis Ltd, London 2002 Chapter 10: Integration and Control of Metabolism Press the space bar or click the mouse
AP VP DLP H&E. p-akt DLP
 A B AP VP DLP H&E AP AP VP DLP p-akt wild-type prostate PTEN-null prostate Supplementary Fig. 1. Targeted deletion of PTEN in prostate epithelium resulted in HG-PIN in all three lobes. (A) The anatomy
A B AP VP DLP H&E AP AP VP DLP p-akt wild-type prostate PTEN-null prostate Supplementary Fig. 1. Targeted deletion of PTEN in prostate epithelium resulted in HG-PIN in all three lobes. (A) The anatomy
Supplementary Figure 1: Additional metabolic parameters of obesity mouse models and controls. (a) Body weight, (b) blood glucose and (c) insulin
 Supplementary Figure 1: Additional metabolic parameters of obesity mouse models and controls. (a) Body weight, (b) blood glucose and (c) insulin resistance index of homeostatic model assessment (HOMA IR)
Supplementary Figure 1: Additional metabolic parameters of obesity mouse models and controls. (a) Body weight, (b) blood glucose and (c) insulin resistance index of homeostatic model assessment (HOMA IR)
Supplementary Materials for
 www.sciencesignaling.org/cgi/content/full/6/283/ra57/dc1 Supplementary Materials for JNK3 Couples the Neuronal Stress Response to Inhibition of Secretory Trafficking Guang Yang,* Xun Zhou, Jingyan Zhu,
www.sciencesignaling.org/cgi/content/full/6/283/ra57/dc1 Supplementary Materials for JNK3 Couples the Neuronal Stress Response to Inhibition of Secretory Trafficking Guang Yang,* Xun Zhou, Jingyan Zhu,
Interplay between FGF21 and insulin action in the liver regulates metabolism
 Research article Interplay between FGF21 and insulin action in the liver regulates metabolism Brice Emanuelli, 1 Sara G. Vienberg, 1 Graham Smyth, 1 Christine Cheng, 2 Kristin I. Stanford, 1 Manimozhiyan
Research article Interplay between FGF21 and insulin action in the liver regulates metabolism Brice Emanuelli, 1 Sara G. Vienberg, 1 Graham Smyth, 1 Christine Cheng, 2 Kristin I. Stanford, 1 Manimozhiyan
SH2B1 Regulates Insulin Sensitivity and Glucose Homeostasis by Multiple Mechanisms. David L. Morris
 SH2B1 Regulates Insulin Sensitivity and Glucose Homeostasis by Multiple Mechanisms by David L. Morris A dissertation submitted in partial fulfillment of the requirements for the degree of Doctor of Philosophy
SH2B1 Regulates Insulin Sensitivity and Glucose Homeostasis by Multiple Mechanisms by David L. Morris A dissertation submitted in partial fulfillment of the requirements for the degree of Doctor of Philosophy
