Conventional PKC-α/β Negatively Regulate RIG-I Antiviral Signal Transduction
|
|
|
- Todd McCoy
- 5 years ago
- Views:
Transcription
1 JVI Accepts, published online ahead of print on 23 November 2011 J. Virol. doi: /jvi Copyright 2011, American Society for Microbiology and/or the Listed Authors/Institutions. All Rights Reserved. PKC- / inhibit RIG-I antiviral activity Conventional PKC-α/β Negatively Regulate RIG-I Antiviral Signal Transduction Natalya P. Maharaj, Effi Wies, Andrej Stoll, and Michaela U. Gack* Department of Microbiology and Molecular Genetics, New England Primate Research Center, Harvard Medical School, 1 Pine Hill Drive, Southborough, MA , USA. Running title: PKC- / inhibit RIG-I antiviral activity *Corresponding author: Michaela U. Gack, Ph.D. Department of Microbiology and Molecular Genetics Harvard Medical School New England Primate Research Center One Pine Hill Drive, Box 9102 Southborough, MA , USA Phone: (508) Fax: (508) michaela_gack@hms.harvard.edu Word Count: Abstract (250 words); Entire text (6,135 words). 1
2 ABSTRACT Retinoic acid-inducible gene-i (RIG-I) is a key sensor for viral RNA in the cytosol and initiates a signaling cascade leading to the establishment of an interferon (IFN)-mediated antiviral state. Because of its integral role in immune signaling, RIG-I activity must be precisely controlled. Recent studies have shown that RIG-I CARD-dependent signaling function is regulated by the dynamic balance between phosphorylation and TRIM25-induced K 63 -linked ubiquitination: While ubiquitination of RIG-I is critical for RIG-I ability to induce an antiviral IFN response, phosphorylation of RIG-I at S 8 or T 170 suppresses RIG-I signal transducing activity under normal conditions. Here, we not only further define the roles of S 8 and T 170 phosphorylation for controlling RIG-I activity, but also identify conventional PKC- / as important negative regulators of the RIG-I signaling pathway. Mutational analysis indicated that while phosphorylation of either S 8 or T 170 potently inhibits RIG-I downstream signaling, dephosphorylation of RIG-I at both residues is necessary for optimal TRIM25 binding and ubiquitination-mediated RIG-I activation. Furthermore, exogenous expression as well as gene silencing and specific inhibitor treatment demonstrated that PKC- / are primary kinases responsible for RIG-I S 8 and T 170 phosphorylation. Co-Immunoprecipitation showed that PKC- / interact with RIG-I under normal conditions leading to its phosphorylation, which suppresses TRIM25 binding, RIG-I CARD ubiquitination and thereby RIG-I-mediated IFN induction. PKC- / double knockdown cells exhibited markedly decreased S 8 /T 170 phosphorylation levels of RIG-I and resistance to infection by vesicular stomatitis virus. Thus, these findings demonstrate that PKC- / -induced RIG-I phosphorylation is a critical regulatory mechanism for controlling RIG-I antiviral signal transduction under normal conditions. 2
3 INTRODUCTION Key to a successful innate immune response to viral infections is the rapid detection of invading viruses by pattern-recognition receptors (PRRs) and subsequent induction of type I interferons (IFN- / ). For antiviral IFN responses, hosts have evolved at least two main classes of PRRs that sense nucleic acids or other conserved molecular components of viral pathogens: Toll-like receptors (TLRs) and RIG-I-like receptors (RLRs) (15, 21). While TLRs play a major role in the detection of incoming virions in specialized immune cells, viral RNA sensing in the cytosol of most cells is carried out by retinoic acid-inducible gene-i (RIG-I) and melanoma differentiation-associated gene 5 (MDA5) (1, 29, 31). RIG-I and MDA5 are composed of two N- terminal caspase recruitment domains (CARDs), a central DExD/H box ATPase/helicase, and a C-terminal regulatory/repressor domain (RD) (3, 27, 31). Binding of viral RNA to the RD/helicase results in a conformational change that demasks the N-terminal CARDs. The exposed CARDs of RIG-I and MDA5 then interact with the CARD-containing adaptor protein MAVS/VISA/IPS-1/Cardif to trigger downstream signaling, resulting in type I IFN production (16, 20, 28, 30). While RIG-I recognizes the 5 triphosphate-containing RNA of paramyxoviruses, influenza virus and vesicular stomatitis virus (VSV) as well as of hepatitis C virus (HCV), MDA5 is a key sensor of picornaviruses (14, 19, 24). In addition, MDA5 was recently shown to play a critical role in the innate immune response to paramyxovirus infections in vivo (9). Tight regulation of innate immune sensing and initiation of antiviral signaling is crucial for eliciting an effective immune response. Whereas positive regulatory loops lead to rapid induction of IFNs and proinflammatory cytokines upon viral infection, multiple negative regulatory checkpoints must be in place to prevent unwanted or excessive cytokine production 3
4 and autoimmune reactions. A recent series of studies has identified ubiquitination as an important cellular mechanism for regulating or fine-tuning RIG-I signal transducing ability. RIG- I activity is negatively regulated by K 48 -linked ubiquitination, leading to its proteasomal degradation (2). Furthermore, K 63 -linked ubiquitination of the N-terminal CARDs of RIG-I as well as its C-terminal region is critical for RIG-I ability to initiate antiviral IFN responses (8, 23). Specifically, ubiquitination of RIG-I at K 172 induced by tripartite motif 25 (TRIM25) is essential for efficient RIG-I-MAVS interaction and thereby for RIG-I ability to elicit host surveillance against RNA virus infections (8). The necessity of TRIM25-mediated ubiquitination for RIG-I signaling was evidenced by a RIG-I splice variant which is unable to bind TRIM25 and thereby completely loses CARD ubiquitination and antiviral signaling ability (6). Furthermore, a recent study showed that TRIM25 can induce RIG-I signaling in an in vitro reconstituted cell-free system (32). In addition to ubiquitination, we have recently shown that serine/threonine phosphorylation of the RIG-I CARDs represents an important mechanism to regulate RIG-I antiviral activity (7, 22). The RIG-I CARDs undergo phosphorylation at S 8 and T 170 under normal conditions, which suppresses RIG-I downstream signaling by inhibiting RIG-I-TRIM25 binding and thereby the TRIM25-mediated RIG-I ubiquitination. Despite the wealth of knowledge resulting from recent studies on RIG-I regulation through ubiquitination, the precise molecular mechanisms by which phosphorylation modulates RIG-I antiviral activity remain elusive. Here, we further characterize the role of S 8 and T 170 phosphorylation for regulating RIG-I downstream signaling and identify conventional PKC- / as important negative regulators of the RIG-I-mediated type I IFN response. 4
5 MATERIALS AND METHODS Plasmid Construction. GST-RIG 2CARD, pires-rig-i-2card-flag, pires-rig-i- 2CARD-Flag, pires-mavs-card-prd-flag, and pires-trim25-v5 were previously described (8). GST-RIG-I 2CARD and RIG-I full length mutants were generated by PCR using site-directed mutagenesis or overlapping PCR using GST-RIG-I 2CARD or pef-bos-flag-rig-i (provided by James Chen, University of Texas) as template, respectively. Introduced mutations were confirmed by DNA sequence analysis. Myc-tagged PKC isozymes and PKG were subcloned into pef-ires-puro encoding a C-terminal Myc tag between AflII and NotI. RIG-I helicase (aa ) or RIG-I-RD (aa ) were subcloned into pef-ires-puro containing a C-terminal Flag tag. DNA fragments corresponding to the coding sequence of PKC- and PKC- II genes were amplified from template DNA by PCR and subcloned into plasmid pebg between KpnI and NotI. Flag-tagged PKA was provided by Jiuyong Xie, University of Manitoba. The construct for pcdna-ha-pkb T308D S473D constitutively active mutant (Addgene) was a gift from Ellen Cahir-McFarland (Harvard). The plasmids encoding GFP-PKC- and GFP-PKC- II were kindly provided by Yusuf Hannun, Medical University of South Carolina. The psuperretro vectors encoding human PKC- shrna or non-silencing control shrna were provided by Debra Tonetti (University of Illinois at Chicago) and have been previously described (18). Cell Culture and Transfection. HEK293T, A549, HeLa, Vero, NHLF (Normal Human Lung Fibroblasts) and HPdlF (Human Periodontal Ligament Fibroblasts) were cultured in Dulbecco's Modified Eagle's Medium (DMEM) supplemented with 10% fetal bovine serum, 2 mm l-glutamine, and 1% penicillin-streptomycin (Gibco-BRL). Transient transfections were performed with calcium phosphate (Clontech), FuGENE 6 (Roche) or Lipofectamine 2000 (Invitrogen) according to the manufacturers instructions. For shrna transfection, Arrest-In 5
6 Transfection Reagent (Open Biosystems) was used. To obtain stable HEK293T cells, cells were transfected with pires-puro-vector, pires-puro-pkc- -Myc, pires-puro-pkc- II -Myc, or pires-puro-pkc- -Myc, followed by selection using 2 μg/ml of puromycin Knockdown of PKC- and PKC- using shrna or sirna. For transient knockdown of endogenous PKC- and/or PKC-, HEK293T cells were transfected with retroviral psm2 encoding human PKC- -specific shrna and/or psm2 encoding PKC- -specific shrna (Open Biosystems). As control, retroviral psm2 encoding non-silencing control shrna was transfected. To generate HEK293T cells in which PKC- or PKC- is stably silenced, cells were transfected with retroviral psm2-pkc- -specific shrna or psm2-pkc- -specific shrna, followed by selection using 2 μg/ml of puromycin. To generate PKC- /PKC- -double knockdown cells, HEK293T cells were transfected with retroviral psm2-pkc- -shrna (Open Biosystems) together with psuper-retro-pkc- -shrna (provided by Debra Tonetti, University of Illinois at Chicago), followed by selection using 2 μg/ml puromycin and 400 μg/ml G418. As controls, stable HEK293T cells expressing retroviral psm2 or psuper encoding non-silencing control shrna were generated. Transient knockdown of endogenous PKC- and PKC- in NHLF cells was achieved by transfection of sigenome SMARTpool sirnas specific for PKC- and PKC- (Dharmacon) with Lipofectamine and plus reagent (Invitrogen) according to the manufacturer s instructions. A final concentration of 300 nm of each PKC- - and PKC- specific SMARTpool sirna or 600 nm of sigenome non-targeting sirna (Dharmacon) was used. 6
7 Viruses. VSV-eGFP was provided by Sean Whelan (Harvard). NDV-GFP and NS1 recombinant A/PR/8/34 (H1N1) influenza virus were a gift from Adolfo García-Sastre (Mount Sinai). Sendai virus (SeV; Cantell strain) was obtained from Charles River Laboratories Antibodies and Reagents. For immunoblotting the following primary antibodies were used: anti-myc (1:2,000) (Covance), anti-v5 (1:5,000) (Invitrogen), anti-flag (M2; 1:2,000) (Sigma), anti-ha (1:2,000) (Clone HA-7; Sigma), anti-glutathione S-transferase (anti-gst) (1:2,000) (Sigma), anti-ubiquitin (P4D1; 1:500) (Santa Cruz), anti-polyubiquitin (Lys 63 -linkagespecific) (1:200) (Biomol), monoclonal anti-trim25 (1:2,000) (BD Biosciences), monoclonal anti-rig-i (Alme-1; 1:1,000) (Alexis), anti-irf3 (1:1,000) (Santa Cruz), anti-pkc- (clone M4; 1:1,000) (Millipore), anti-pkc- I (1:1,000) (Santa Cruz), anti-pkc- II (1:1,000) (Santa Cruz), anti-pkc- II (1:200) (Abcam), anti-phospho-s 657 -PKC- (1:500) (Millipore), anti-phospho-t 500 PKC- I&II (1:500) (Upstate), anti-pkc- (1:1,000) (Millipore), anti-pkc- (1:1,000) (Millipore), anti- -actin (Abcam), and anti-phospho-threonine (1:500) (Cell Signaling Technology). The phospho-specific ps 8 -RIG-I and pt 170 -RIG-I antibodies were generated by immunizing rabbits with phospho-peptides as previously described (7, 22). Protein Kinase C inhibitors Bisindolylmaleimide I, Gö6976, and Ro were purchased from Calbiochem. Poly(U/UC)-RNA of HCV J4L6 strain was a gift from Lee Gehrke (Harvard/M.I.T.). GST Pull-Down Assay, Immunoprecipitation, and Immunoblot Analysis. HEK293T cells were lysed in NP-40 buffer (50 mm HEPES, ph 7.4, 150 mm NaCl, 1% [vol/vol] NP-40, protease inhibitor cocktail [Roche], and Ser/Thr phosphatase inhibitor cocktail [Sigma]), 7
8 followed by centrifugation at 13,000 rpm for 20 min. GST pull-down, immunoprecipitation and westernblot analysis were performed as previously described (7-8) VSV-eGFP Replication and NDV-GFP Bioassay. Stable HEK293T or NHLF cells were seeded into 6-well plates and infected with VSV-eGFP at the indicated MOI. At h postinfection, the culture medium was harvested and the virus yield determined by standard plaque assay on Vero cells. For the NDV-GFP bioassay, supernatants from transfected HEK293T cells were diluted 1:2 in fresh DMEM complete medium and then added to Vero cells. Eighteen hours later, cells were infected with NDV-GFP (MOI of 3). At 24 h postinfection, GFP expression was monitored by epifluorescence and percentage of GFP-positive cells determined by FACS analysis. IFN- ELISA. NHLF cells, that had been transfected with sigenome non-targeting sirna or with PKC- - and PKC- -specific sirnas, were mock-treated or infected with SeV (40 HA units/ml) at 40 h after transfection. 30 h later, the supernatants were collected and analyzed for IFN- production by using enzyme-linked immunosorbent assays (PBL Biomedical Laboratories). RNA Pull-Down Experiments. Biotinylated 5 -triphosphate containing rabies virus leader RNA (5 ppprvl) was transcribed using the T7 Megashortscript kit (Ambion) and biotin- 16-uridine-5 ppp (Roche). For transcription, the annealed oligonucleotides 5 CGCGTAATACGACTCACTATA 3 and 5'ACA TTT TTG CTT TGC AAT TGA CAA TGT CTG TTT TTT CTT TGA TCT GGT TGT TAA GCG TTA TAG TGA GTC GTA TTA CGC G 3' were used as template. The DNA template was removed by DNase I treatment, and RNA was purified using Microspin G-25 Columns (GE Healthcare). For RNA binding assay, 1μg of 8
9 biotinylated RNA was incubated for 1 h at 25 C with 25 μg of cell extract prepared from HEK293T cells transfected with pef-bos-flag-rig-i plasmids. Following the incubation, the mixture was transferred into 400 μl of wash buffer (50 mm Tris, ph 7.5, 150 mm NaCl, 1 mm EDTA, 1% NP-40) containing 30 μl streptavidin agarose affinity gel (Sigma), and rocked at 4 C for 2 h. The RNA-protein complexes were collected by centrifugation, washed three times with wash buffer, followed by SDS-PAGE and immunoblotting with anti-flag antibody. Protein Purification and In Vitro Phosphorylation Assay. RIG-I 2CARD was cloned into pgex-4t-1 vector. XL1 blue E. coli were transformed with the plasmid and RIG-I 2CARD fusion protein was purified using Glutathione Sepharose 4B resin (GE Healthcare) according to the manufacturer s instruction. For the in vitro phosphorylation assay, purified GST-RIG-I 2CARD was incubated with 25 ng of purified PKC- / / (Millipore) in PKC Assay Dilution Buffer II (Millipore) at 30 C for 30 min. The reaction was stopped by adding 2x SDS-Laemmli buffer, followed by SDS-PAGE. RIG-I phosphorylation was detected by immunoblotting with phospho-specific ps 8 - or pt 170 -RIG-I antibody. Luciferase Reporter Assay. HEK293T cells were seeded into 6-well plates. 24 h later, the cells were transfected with 500 ng IFN- luciferase reporter plasmid together with 800 ng constitutive -gal-expressing pgk- -gal. In addition, 2 ng of plasmid encoding RIG-I 2CARD- GST fusions was transfected. At h posttransfection, WCLs were prepared and subjected to a luciferase assay (Promega). Luciferase values were normalized to -galactosidase to measure transfection efficiency. To test the effect of PKC inhibitors on the SeV-induced IFN- promoter activation, HEK293T cells, transfected with IFN- luciferase construct and constitutive -gal-expressing pgk- -gal, were treated with PKC inhibitors (Bisindolylmaleimide I 4 μm; 9
10 Gö μm; and Ro μm) at 20 h posttransfection. Six hours later, cells were infected with SeV (30 HA units/ml). At h postinfection, WCLs were prepared and subjected to a luciferase assay (Promega) Bioinformatic analysis of RIG-I Ser-8 and Thr-170. Potential protein kinase phosphorylation consensus sites for RIG-I Ser-8 and Thr-170 residues were determined by using EMBL-EBI and Uniprot/GPS2.1 softwares, using the settings high threshold and low threshold for Ser-8 and Thr-170, respectively. This identified the motif QRRS 8 LQ as putative PKA or PKC consensus site; whereas the motif WPKT 170 LK was identified as potential PKC site. Native PAGE. Native PAGE was performed as described previously (6). Confocal Immunofluorescence Microscopy. HeLa cells grown on chamber slides were transfected with pbos-flag-rig-i and GFP-PKC- or GFP-PKC- II. At 20 h posttransfection, cells were fixed with 4% paraformaldehyde for 20 min, and permeabilized with 0.2% (vol/vol) Triton. Cell preparation and confocal microscopy analysis were performed as previously described (7-8). For immunostaining Flag-RIG-I, anti-flag M2 antibody (Sigma) was used, followed by incubation with anti-mouse Alexa-fluor-594 (Invitrogen). Laser scanning images were taken on a Leica TCS SP5 confocal microscope (Leica Microsystems). 10
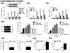
11 RESULTS Dephosphorylation of RIG-I at both S 8 and T 170 is necessary for efficient TRIM25- mediated RIG-I ubiquitination and optimal RIG-I signaling activity Mutation of either S 8 or T 170 to phospho-mimetic D or E (aspartic/glutamic acid), but not to A (alanine), strongly suppressed TRIM25-mediated RIG-I ubiquitination and antiviral signaling, indicating that RIG-I phosphorylation and TRIM25-mediated RIG-I ubiquitination functionally antagonize each other (7, 22). To define the distinct roles of S 8 and T 170 phosphorylation in RIG-I regulation, we generated additional point mutants of the RIG-I N- terminal CARDs fused to mammalian glutathione S-transferase (GST-RIG-I 2CARD), as well as of full-length RIG-I: S 8 E/T 170 E and S 8 A/T 170 A mutants, mimicking constitutive phosphorylation or non-phosphorylation at both residues, as well as S 8 A/T 170 E and S 8 E/T 170 A mutants, in which one site is non-phosphorylated while the other mimics constitutive phosphorylation. These RIG-I mutants were then tested for a series of biochemical activities: CARD ubiquitination, TRIM25 and MAVS binding, as well as RNA binding and downstream signaling activity. Like GST-RIG- I 2CARD S 8 E and T 170 E mutants, GST-RIG-I 2CARD S 8 A/T 170 E, S 8 E/T 170 A and S 8 E/T 170 E showed a near-complete loss of ubiquitination compared to GST-RIG-I 2CARD WT (Fig. 1A). In striking contrast, the GST-RIG-I 2CARD S 8 A/T 170 A mutant, mimicking non-phosphorylation at both sites, exhibited a markedly increased level of ubiquitination compared to WT GST-RIG-I 2CARD, while T 170 A and S 8 A showed a similar or slightly enhanced ubiquitination level to WT (Fig. 1A). In line with this, Sendai virus (SeV)-induced K 63 -linked ubiquitination of RIG-I S 8 A/T 170 A mutant was strongly enhanced compared to WT RIG-I, whereas S 8 A/T 170 E, S 8 E/T 170 A and S 8 E/T 170 E RIG-I mutants were minimally ubiquitinated (Fig. 1B). Given that S 8 E or T 170 E mutation strongly suppressed RIG-I binding to TRIM25 (7, 22), which apparently 11
12 abolished RIG-I ubiquitination, we tested Flag-tagged RIG-I S 8 A/T 170 E, S 8 E/T 170 A, S 8 A/T 170 A, and S 8 E/T 170 E mutants for their TRIM25 binding abilities (Fig. 1C). RIG-I S 8 A/T 170 E, S 8 E/T 170 A and S 8 E/T 170 E mutants showed no detectable interaction with V5-TRIM25; in contrast, the S 8 A/T 170 A mutant exhibited an increased binding activity for TRIM25 compared to WT RIG-I (Fig. 1C). K 63 -linked ubiquitination of the RIG-I CARDs is essential for efficient RIG-I-MAVS interaction, and thereby for RIG-I ability to induce downstream signal transduction (8). GST- RIG-I 2CARD S 8 A/T 170 A, which exhibited elevated ubiquitination levels compared to WT, also showed a stronger binding to the MAVS-CARD-proline-rich domain (PRD) as well as signal transducing activity than GST-RIG-I 2CARD WT (Fig. 1D and E). Furthermore, GST-RIG-I 2CARD S 8 E/T 170 E did not bind the MAVS-CARD under the same conditions and did not detectably induce IFN- promoter activation, whereas S 8 A/T 170 E and S 8 E/T 170 A mutants showed minimal MAVS binding and IFN inducing activities (Fig. 1D and E). The inability of RIG-I S 8 E/T 170 E mutant to induce IFN- promoter activation was not due to an abolished RNA binding activity, since S 8 E/T 170 E RIG-I bound in vitro-transcribed 5 triphosphate rabies virus leader RNA (5 ppprvl) with similar affinity as WT or S 8 A/T 170 A RIG-I (Fig. 1F). In contrast, a RIG-I mutant in which K 858, a critical residue for viral RNA binding to the C-terminal RD (3), is mutated (K 858 A RIG-I) did not bind 5 ppprvl under the same conditions (Fig. 1F). Finally, we tested the phosphorylation of endogenous RIG-I at S 8 and T 170 under normal conditions, following viral infection or upon stimulation with viral RNA by using a phospho-specific ps 8 - or pt 170 -RIG-I antibody (Fig. 1G). This showed that RIG-I underwent robust phosphorylation at S 8 and T 170 in mock-treated cells, and that phosphorylation at both sites declined following infection with SeV or delta NS1 PR8 influenza virus ( NS1), as well as upon stimulation with HCV poly(u/uc) RNA (Fig. 1G). In summary, these results indicate that RIG-I is robustly 12
13 phosphorylated at S 8 and T 170 under normal conditions, and that phosphorylation of either S 8 or T 170 is sufficient to potently suppress RIG-I ubiquitination by TRIM25, thereby preventing RIG-I downstream signaling. Furthermore, our results indicate that stimulus-induced dephosphorylation of RIG-I at both residues is necessary for optimal RIG-I-TRIM25 binding, RIG-I CARD ubiquitination, and RIG-I-mediated signal transduction. PKC- / phosphorylate the S 8 and T 170 residue of RIG-I, thereby suppressing RIG-I K 63 - linked ubiquitination Bioinformatic analysis reveals that the S 8 and T 170 residue of RIG-I are potential consensus phosphorylation sites for protein kinase A (PKA) and protein kinase C (PKC), respectively. To test the potential role of PKA and PKC in phosphorylation-dependent RIG-I regulation, we examined the effect of exogenous expression of PKA or PKC on the phosphorylation and ubiquitination of GST-RIG-I 2CARD (Fig. 2A). Protein kinase B (PKB) and protein kinase G (PKG) were included in this assay. PKC expression markedly increased the S 8 and T 170 phosphorylation of GST-RIG-I 2CARD and suppressed its ubiquitination, whereas PKA, PKB and PKG showed no effect under the same conditions (Fig. 2A). PKC represents a family of serine/threonine kinases comprising at least 11 isozymes that are classified into three subfamilies based on structural similarities and cofactor dependence: conventional PKCs (, I, II, and ), novel PKCs (,,,, and ) and atypical PKCs ( and ) (25). We thus tested the effect of ectopic expression of conventional isozyme PKC- or PKC- II, novel PKC-, or atypical PKC- on the phosphorylation and ubiquitination of GST-RIG-I 2CARD (Fig. 2B). Specifically, co-expression of PKC- or PKC- II enhanced the S 8 and T 170 phosphorylation of GST-RIG-I 2CARD and suppressed its ubiquitination, whereas PKC- and PKC- did not have 13
14 any effect (Fig. 2B). Accordingly, exogenous PKC- and PKC- II but not PKC- or PKC- strongly increased the phosphorylation of endogenous RIG-I at S 8 and T 170, and suppressed its K 63 -linked polyubiquitination (Fig. 2C and 2D). To test the physiological relevance of conventional PKC- and PKC- for RIG-I phosphorylation, we examined the phosphorylation of endogenous RIG-I in HEK293T cells, in which PKC-, PKC-, or both were stably silenced by using PKC- - and/or PKC- -specific small hairpin RNAs (shrnas) (Fig. 3A). Cells stably expressing a non-silencing, scrambled shrna served as control. This showed that stable knockdown of PKC- had little or no effect on endogenous RIG-I S 8 and T 170 phosphorylation. In contrast, silencing of PKC- or both PKC- and PKC-, markedly reduced the RIG-I phosphorylations compared to those of cells expressing non-silencing shrna, with the PKC- / -double knockdown having the strongest effect (Fig. 3A). Consistently, transient knockdown of endogenous PKC- and PKC- strongly decreased the T 170 phosphorylation of endogenous RIG-I and enhanced its K 63 -linked ubiquitination in mocktreated cells as well as SeV-infected cells in a dose-dependent manner (Fig. 3B). Conventional Gö6976 PKC inhibitor treatment also reduced the S 8 and T 170 phosphorylation of GST-RIG-I 2CARD and increased its ubiquitination (Fig. 3C). In addition, Gö6976 PKC inhibitor treatment profoundly decreased the S 8 and T 170 phosphorylation of endogenous RIG-I in HEK293T cells as well as primary NHLF and HPdlF fibroblasts compared to that of mock-treated cells (Fig. 3D, 3E, and data not shown). In correlation with its decreasing effect on RIG-I phosphorylation, Gö6976 inhibitor increased the RIG-I K 63 -linked ubiquitination (Fig. 3D and Suppl. Fig. 1). Finally, conventional PKC robustly phosphorylated bacterially purified GST-RIG-I 2CARD at S 8 and T 170 in an in vitro phosphorylation assay (Fig. 3F). These results collectively indicate that 14
15 conventional PKC- and PKC- phosphorylate RIG-I at S 8 and T 170, which inhibits the K 63 - linked ubiquitination of RIG-I PKC- / interact with RIG-I We next tested the potential interaction between Myc-tagged PKC- or PKC- II and Flag-tagged RIG-I or GST-RIG-I 2CARD in HEK293T cells (Fig. 4A and B). PKG-Myc was included as control. Coimmunoprecipitation (Co-IP) revealed that PKC- and PKC- II but not PKG efficiently interacted with Flag-RIG-I and, albeit more weakly, also with GST-RIG-I 2CARD (Fig. 4A and B). In addition, we readily observed an interaction of endogenous PKC-, PKC- I, and PKC- II with RIG-I in HEK293T, A549 and HeLa cells (Fig. 4C). In contrast, PKC- and PKC- did not bind endogenous RIG-I under the same conditions (Fig. 4C). Confocal microscopy also showed that GFP-PKC- or GFP-PKC- II extensively co-localized with Flag- RIG-I in the cytoplasm (data not shown). Furthermore, we examined the interaction of endogenous PKC- or PKC- II with RIG-I in primary normal human lung fibroblasts (NHLF) under normal conditions and at different time points after SeV infection (Fig. 4D). Consistent with the results in HEK293T, A549 and HeLa cell lines, PKC- and PKC- II efficiently interacted with endogenous RIG-I in mock-infected NHLF cells. In contrast, viral infection rapidly led to a strongly decreased binding of PKC- or PKC- II to RIG-I (Fig. 4D). In line with this, while PKC- and PKC- II strongly bound to endogenous RIG-I in HEK293T cells under normal conditions, only a very weak RIG-I-PKC- /RIG-I-PKC- II interaction was observed upon SeV or NS1 influenza virus infection (Fig. 4E). Activation loop phosphorylation of PKC- at S 657 and PKC- at T 500 has been shown to render PKC- / catalytically competent (10). As shown in Fig. 4E, endogenous PKC- and PKC- II that bound to RIG-I, were robustly 15
16 phosphorylated at S 657 and T 500, respectively, in non-infected cells; in contrast, no phosphorylation of PKC- or PKC- II was detected in cells infected with SeV or NS1 influenza virus (Fig. 4E). In summary, these results indicate that PKC- and PKC- efficiently interact with RIG-I under normal conditions, and that they are enzymatically active in the RIG-I-PKCcomplex. To define the molecular architecture of the RIG-I-PKC complex, we tested Myc-tagged PKC- and PKC- II for their ability to interact with Flag-tagged RIG-I 2CARD or with a RIG-I mutant in which the CARDs were deleted (RIG-I 2CARD) (Fig. 5B). PKG-Myc was included as control. PKC- and PKC- II strongly interacted with both RIG-I 2CARD and RIG-I 2CARD; in contrast, PKG did not bind either under the same conditions (Fig. 5B). As with other PKC family members, PKC- and PKC- are comprised of four domains: an N-terminal pseudo-substrate (PS) domain, a central tandem C1 domain, a C2 domain, and a C-terminal catalytic domain (CR) (Fig. 5A). Binding studies using N-terminal GST-fused PKC- or PKC- II polypeptides corresponding to PS, PS/C1, C1, C2, and CR domains, showed that the C-terminal CR domain of PKC- / II bound to Flag-tagged RIG-I 2CARD as well as RIG-I 2CARD and the helicase of RIG-I (Fig. 5C and 5D). Furthermore, the regulatory PS/C1 and C1 domain of PKC- / II strongly and specifically interacted with RIG-I 2CARD and RIG-I helicase (Fig. 5C lower panel, Fig. 5E and 5F). These results demonstrate that the N-terminal CARDs of RIG-I interact with the C-terminal CR domain of PKC- / II, whereas the helicase domain of RIG-I binds both the CR and regulatory C1 domain of PKC- / II. PKC- / inhibit the RIG-I-TRIM25 interaction, RIG-I-MAVS binding and the RIG-Imediated antiviral IFN induction 16
17 To test the role of PKC- / in RIG-I signal transduction, HEK293T cells were transfected with GST-RIG-I 2CARD and IFN- promoter luciferase together with vector, Myctagged PKC-, PKC- II, PKC- or PKC- (Fig. 6A). PKC- or PKC- II expression markedly inhibited the IFN- promoter activation induced by GST-RIG-I 2CARD, whereas PKC- or PKC- had little or no effect on the RIG-I 2CARD-mediated IFN- promoter activation (Fig. 6A). Furthermore, exogenous PKC- and PKC- II but not PKC- or PKC- potently suppressed the SeV-induced IFN- promoter activation (Fig. 6B). Conversely, treatment of HEK293T with conventional PKC inhibitor Bisindolylmaleimide I (BIMI), Gö6976, or Ro increased the SeV-induced IFN- promoter activation compared to mock-treatment (Fig. 6C). However, BIMI, Gö6976 or Ro treatment was not sufficient to detectably induce IFN- promoter activation in mock-infected cells (Fig. 6D). To further delineate the inhibitory effect of PKC- / on the RIG-I-mediated downstream signaling cascade, we examined virus-induced dimerization of IFN-regulatory factor 3 (IRF3) (Fig. 6E). For this, primary NHLF fibroblasts were transfected with vector, Myc-tagged PKC-, PKC- II, or PKC-, followed by SeV infection. Native PAGE showed that SeV led to an efficient dimerization of endogenous IRF3 in vector or PKC- transfected cells; in contrast, IRF3-dimer formation was strongly suppressed in cells expressing exogenous PKC- or PKC- II (Fig. 6E). To trigger downstream signaling, RIG-I binding to TRIM25 and to MAVS downstream partner is essential. We thus examined the effect of exogenous PKC- or PKC- II expression on the interaction between RIG-I and the MAVS- CARD by Co-IP (Fig. 6F). As control, Myc-tagged PKC- was included. Ectopic expression of PKC- or PKC- II potently inhibited the SeV-induced interaction of endogenous RIG-I with Flag-tagged MAVS-CARD-proline rich domain (PRD); in contrast, PKC- did not have any effect on the binding of RIG-I to the MAVS-CARD (Fig. 6F). Consistent with this, exogenous 17
18 PKC- or PKC- II, but not PKC- or PKC-, strongly inhibited the interaction of GST-RIG-I 2CARD and the MAVS-CARD (data not shown). Finally, in correlation with our results indicating that phosphorylation of RIG-I at S 8 and T 170 inhibits the RIG-I binding to TRIM25 (Fig. 1C), exogenously expressed PKC- or PKC- II, but not PKC- and PKC-, markedly suppressed the interaction between endogenous RIG-I and TRIM25 (Fig. 6G). Collectively, these results indicate that PKC- / -mediated phosphorylation of RIG-I inhibits the RIG-I-TRIM25- interaction and RIG-I ubiquitination, which subsequently suppresses RIG-I-MAVS binding and, thereby RIG-I-induced IFN gene expression. PKC- / suppress the IFN-mediated antiviral activity of RIG-I To examine the effect of PKC- / on RIG-I-mediated antiviral IFN production, we performed a bioassay using recombinant Newcastle disease virus (NDV) expressing green fluorescent protein (GFP) (NDV-GFP) (Fig. 7A). For this, Vero cells were incubated with supernatants from HEK293T cells that had been transfected with GST, GST-RIG-I 2CARD alone, or GST-RIG-I 2CARD together with PKC-, PKC- II or PKC-, followed by infection with NDV-GFP (Fig. 7A). Supernatants from 293T cells transfected with GST-RIG-I 2CARD alone or GST-RIG-I 2CARD together with PKC- substantially suppressed the replication of NDV-GFP compared to supernatants from cells transfected with GST. In contrast, supernatants from cells transfected with GST-RIG-I 2CARD together with PKC- or PKC- II blocked NDV- GFP replication to a far lesser extent than supernatants from transfections with GST-RIG-I 2CARD alone (Fig. 7A). We also performed the same NDV-bioassay using the RIG-I S 8 A/T 170 A mutant, which cannot be phosphorylated, and observed that supernatants from cells cotransfected with GST-RIG-I S 8 A/T 170 A together with PKC- or PKC- II suppressed the 18
19 replication of NDV-GFP to a comparable extent as supernatants from cells transfected with GST-RIG-I S 8 A/T 170 A alone (data not shown). This corroborates that the inhibitory effect of PKC- / on the RIG-I 2CARD-mediated IFN production is due to PKC- / -dependent RIG-I phosphorylation at S 8 and T 170. We next examined the effect of PKC- / on the replication of recombinant vesicular stomatitis virus (VSV) expressing enhanced-gfp (VSV-eGFP) in HEK293T cells stably expressing PKC- or PKC- II (Fig. 7B). This showed that stable expression of PKC- or PKC- II detectably increased the replication of VSV-eGFP compared with vector or PKC- expression (Fig. 7B). To determine the physiological role of PKC- / in regulating the antiviral activity of RIG-I, we examined the replication of VSV-eGFP in HEK293T cells, in which PKC-, PKC- II, or both have been stably silenced using specific shrnas (Fig. 7C). While knockdown of either PKC- or PKC- II had little or no effect on the replication of VSV-eGFP, stable silencing of both markedly suppressed the replication of VSV-eGFP compared to expression of non-silencing control shrna (Fig. 7C). To confirm these results in primary human cells, we tested the replication of VSV-eGFP in normal human lung fibroblasts (NHLF) in which endogenous PKC- and PKC- have been depleted using specific sirnas (Fig.7D). Cells transfected with nonsilencing control sirna served as control. In line with our results in HEK293T cells, NHLF cells in which both PKC- and PKC- were silenced exhibited a markedly reduced VSV-eGFP replication; VSV-eGFP titers were ~10 fold reduced compared to cells transfected with control sirna (Fig. 7D). Finally, we examined the effect of silencing PKC- and PKC- on the SeVinduced IFN- production in NHLF cells (Fig. 7E). For this, NHFL cells were transiently transfected with non-silencing control sirna or with PKC- - and PKC- -specific sirnas, followed by mock-treatment or infection with SeV. IFN- production was then determined by 19
20 ELISA. This showed that PKC- / knockdown cells exhibited detectably increased IFN- protein levels in the supernatant compared to cells transfected with non-silencing control sirna (Fig. 7E). In summary, these results indicate that RIG-I phosphorylation by PKC- / suppresses the RIG-I ability to induce IFN production and thereby its antiviral activity
21 DISCUSSION Dynamic interplay between phosphorylation and ubiquitination is an emerging paradigm for regulating the activities of key signaling molecules (11). Our study reveals that conventional PKC- / -induced RIG-I phosphorylation and TRIM25-mediated RIG-I ubiquitination functionally antagonize each other to tightly regulate the RIG-I CARD-mediated antiviral signal transduction. Our results showed that PKC- and PKC- interact with RIG-I under normal conditions in various different cell lines including primary fibroblasts. Furthermore, ectopic expression, gene silencing and in vitro phosphorylation assay indicated that conventional PKC- / phosphorylate RIG-I at S 8 and T 170, which keeps RIG-I inactive by suppressing RIG-I- TRIM25 binding, RIG-I CARD ubiquitination, and thereby RIG-I-MAVS interaction. Upon viral infection, however, PKC- / apparently dissociate from RIG-I, which potentially allows phosphatase-dependent RIG-I dephosphorylation, leading to efficient RIG-I-TRIM25 interaction and RIG-I ubiquitination, ultimately initiating antiviral IFN responses. Mutational analysis indicated that whereas phosphorylation of RIG-I at either S 8 or T 170 prevents TRIM25-mediated RIG-I ubiquitination and RIG-I downstream signaling, dephosphorylation of RIG-I at both residues is necessary for optimal ubiquitination-mediated RIG-I activation. This kind of dynamic balance between constitutive phosphorylation for cytosolic PRR inactivation, and phosphatase-dependent activation represents a novel type of regulation in antiviral innate immunity. Whereas kinases, such as IKK- or TBK-1, are well known to activate innate immune signaling, this study strongly suggests that phosphatases play key roles in the initiation of the RIG-I signal transduction pathway. Thus, future studies directed toward the identification of the phosphatase(s) responsible for RIG-I dephosphorylation promise 21
22 to decipher the impact of protein dephosphorylation for orchestrating antiviral innate immune responses. PKC comprises a family of more than 10 isoenzymes divided into conventional, novel, and atypical subgroups (10, 25). Our study showed that specifically conventional PKC- / interacted with RIG-I, but neither novel PKC- nor atypical PKC- did. Furthermore, coexpression of PKC- or PKC- robustly increased the S 8 and T 170 phosphorylation of endogenously and exogenously expressed RIG-I, while PKC- and PKC- had no effect or only marginally enhanced RIG-I phosphorylation when grossly overexpressed. Furthermore, we employed shrna-mediated knockdown techniques, specific inhibitor treatment as well as PKC in vitro phosphorylation assay to examine the physiological role of PKC- / for phosphorylating RIG-I. This clearly showed that PKC- and PKC- are primary kinases responsible for RIG-I S 8 and T 170 phosphorylation, indicating an important role of conventional PKC- / isoforms for regulating RIG-I antiviral activity. Furthermore, whereas ectopic expression of either PKC- or PKC- profoundly inhibited RIG-I downstream signaling, knockdown of both, but not either of them alone, markedly enhanced RIG-I antiviral activity (Fig. 7C), suggesting a partially redundant function of these two conventional isozymes in regulating RIG-I. PKC activation typically requires membrane association as well as binding of cofactors such as phospholids or Ca 2+ (25). In addition, it is well established that binding of proteins to the regulatory domain of PKC can alter its subcellular localization as well as cofactor-dependence, and, in some cases, even bypass the need of allosteric inputs for PKC activation (13). Our detailed interaction studies showed that PKC- / bind with their regulatory C1 and catalytic CR domain to the RIG-I CARD and helicase. Confocal microscopy analysis indicated that the RIG-I- PKC complex resides in the cytoplasm (data not shown); and in this complex, PKC- / are 22
23 apparently enzymatically active. However, the molecular details of how PKC- / bound to RIG- I are activated and whether cofactors of PKC- / play any role for RIG-I phosphorylation remains to be elucidated. Future structural analyses of the RIG-I-PKC-complex will not only elucidate the detailed mechanism of RIG-I regulation through phosphorylation but may also provide further insights into the exact mode of PKC activation. A recent series of studies has suggested the following model of RIG-I activation. Under normal conditions, RIG-I is kept inactive through autorepression by the C-terminal RD (27), which interacts with both CARD and helicase domains of RIG-I. Binding of 5 triphosphate containing viral RNA to the RD and subsequent ATPase activity induces a conformational change in RIG-I, leading to the exposure of the N-terminal CARDs. Our previous study showed that the ubiquitin E3 ligase TRIM25 binds to the first CARD of RIG-I, leading to K 63 -linked ubiquitination of the second CARD (6, 8). Ubiquitination of K 172 in RIG-I is essential for efficient RIG-I binding to MAVS, thereby initiating downstream signaling. The data presented here reveal that, in addition to RIG-I autoinhibition mediated by the RD, PKC- / -dependent phosphorylation of the CARDs plays a crucial role in keeping RIG-I in an inactive state under normal condition. Phosphorylation of S 8 and T 170 did not affect RIG-I binding to viral RNA, but it markedly suppressed RIG-I-TRIM25 binding and thereby RIG-I CARD ubiquitination (Fig. 1F). This suggests that the negative charge provided by the RIG-I S 8 and T 170 phosphorylation may induce structural alterations within the tandem CARD which interfere with TRIM25 binding. Furthermore, it has been recently shown that the RIG-I CARDs can bind unanchored polyubiquitin-chains in an in vitro reconstituted cell-free system, and that TRIM25 was able to catalyze these free ubiquitin chains for RIG-I binding (32). Thus, future studies will be required 23
24 to address whether RIG-I S 8 /T 170 phosphorylation by PKC- / also affects the free ubiquitin binding capability of the RIG-I CARDs. Our study further showed that viral infection markedly decreased RIG-I-PKC- / binding and the RIG-I S 8 and T 170 phosphorylation, suggesting that, upon viral RNA binding, conformational changes in RIG-I not only cause dissociation of the RIG-I/PKC-complex but may also allow the recruitment of a yet-to-be-identified phosphatase for RIG-I S 8 and T 170 dephosphorylation. In fact, our previous studies demonstrated that RIG-I S 8 /T 170 phosphorylation is robustly enhanced by phosphatase inhibitor treatment (7, 22), suggesting that S 8 and T 170 phosphorylations are tightly regulated by phosphatase-dependent dephosphorylation. The data presented here further indicate that dephosphorylation of both S 8 and T 170 ultimately allows efficient TRIM25 binding, RIG-I CARD ubiquitination and MAVS-mediated downstream signaling. During the submission of this manuscript, Zhu et al. reported that PKC- enhances IRF3- mediated gene induction upon viral infection through activation of HDAC6 and -catenin (33). Our study indicates that PKC- / act as negative regulators of RIG-I by phosphorylating its N- terminal CARDs, which keeps RIG-I in a latent, inactive state. Consistent with this model, we observed an inhibitory effect of exogenous PKC- / expression on RIG-I-CARD mediated IFN induction, and enhanced IFN- production in primary lung fibroblasts in which PKC- and PKC- were silenced using specific sirnas. Our biochemical analyses also showed that PKC- / efficiently bind to RIG-I in non-infected cells, and that this interaction leads to RIG-I phosphorylation, which ultimately suppresses the RIG-I signal transducing activity. It is thus conceivable that conventional PKC may have two distinct roles in type I IFN induction: inhibition of RIG-I downstream signaling by PKC- / prior to viral infection; and activation of 24
25 IRF3-mediated transcription by PKC- upon viral infection. This also suggests that specific substrate binding and subcellular localization of conventional PKCs determine their functional roles in IFN induction before and after viral infection. Alternatively, it is possible that the different experimental systems used may account for the discrepancies between our results and the findings by Zhu et al. (33). Host has developed numerous checkpoints to regulate viral RNA sensing and IFN signaling initiated by the cytosolic viral RNA receptor RIG-I. Otherwise, excessive production of type I IFNs or proinflammatory cytokines can potentially be detrimental to the host cell rather than beneficial. RIG-I activity is negatively regulated by K 48 -linked ubiquitination and the CARD-lacking helicase LGP2 (2, 17, 26). In addition, alternative splicing and deubiquitination by CYLD have been shown to represent effective inhibitory mechanisms of the RIG-I antiviral signaling function (4, 6). Furthermore, it has been recently shown that influenza A virus nonstructural protein 1 (NS1) and cellular HOIL-1L-HOIP linear ubiquitin assembly complex (LUBAC) antagonize TRIM25 activity to ubiquitinate the RIG-I CARDs, thereby leading to RIG-I inhibition (5, 12). In this study, we show that PKC- / function as endogenous suppressors to prevent the RIG-I CARD-dependent signal transduction under normal conditions, unveiling PKC- / -induced RIG-I phosphorylation as an important mechanism in modulating host IFN-mediated antiviral immune responses. 25
26 515 ACKNOWLEDGEMENTS We greatly thank Jae Jung, Lee Gehrke, Debra Tonetti, Ellen Cahir-McFarland, Jiuyong Xie, Yusuf Hannun, Sean Whelan, and Adolfo García-Sastre for providing reagents. This work was supported by U.S. Public Health Service grants RO1AI and RR00168 (M.U.G.) and by the German Science Foundation (E.W.). Downloaded from on August 13, 2018 by guest 26
27 521 FIGURE LEGENDS 522 FIG. 1. Dephosphorylation of RIG-I at both S 8 and T 170 is necessary for optimal TRIM binding, K 63 -linked ubiquitination and signaling activity of RIG-I. (A and B) S 8 A/T 170 A mutation enhances RIG-I ubiquitination. (A) HEK293T cells were transfected with GST-RIG-I 2CARD WT or the indicated mutants. Whole cell lysates (WCLs) were subjected to GSTpulldown (GST-PD) followed by immunoblotting (IB) with anti-gst or anti-ub antibody. Arrows indicate the ubiquitinated bands. (B) HEK293T transfected with vector, Flag-RIG-I WT or mutants together with an HA-ubiquitin (Ub) mutant in which all lysines except K 63 are mutated (HA-K 63only -Ub), were infected with SeV (50 HA units/ml) for 14 h. WCLs were subjected to IP with anti-flag, followed by IB with anti-ha or anti-flag antibody. (C) S 8 A/T 170 A mutation enhances RIG-I binding to TRIM25. At 48 h posttransfection with vector, Flag-RIG-I WT or the indicated mutants together with V5-tagged TRIM25, HEK293T WCLs were used for IP with anti-flag antibody, followed by IB with anti-v5 antibody. TRIM25 expression was determined by IB with an anti-v5 antibody. (D) S 8 A/T 170 A mutation increases RIG-I CARD binding to MAVS. At 48 h posttransfection with GST, GST-RIG-I 2CARD WT or the indicated mutants together with Flag-tagged MAVS-CARD-PRD, HEK293T WCLs were used for GST- PD followed by IB with anti-flag, anti-gst, or anti-ub antibody. MAVS-CARD-PRD expression was determined by IB with an anti-flag antibody. Arrows indicate the ubiquitinated bands. (E) S 8 A/T 170 A mutation increases the RIG-I 2CARD-mediated IFN- promoter activation. GST or GST-RIG-I fusion constructs together with IFN- luciferase and constitutive -gal-expressing pgk- -gal were expressed in HEK293T. Luciferase and -galactosidase values were determined as previously described (8). Data represent the mean + SD (n=3). (F) RIG-I phosphorylation at S 8 and T 170 does not affect its RNA binding ability. Cell extracts from 27
28 HEK293T cells, transfected with vector, Flag-RIG-I WT or the indicated mutants, were incubated with in vitro-transcribed, biotinylated 5 -triphosphate containing rabies virus leader RNA (5 ppprvl). RNA-protein-complexes were recovered by pull-down assay using streptavidin affinity gel, followed by SDS-PAGE and immunoblotting using an anti-flag antibody. (G) Viral infection or RIG-I stimulation with viral RNA decreases the S 8 and T 170 phosphorylation of endogenous RIG-I. HEK293T cells were infected with SeV (25 HA units/ml) or NS1 PR8 virus (MOI 4) for 10 h, or transfected with HCV poly(u/uc). WCLs were subjected to IP with an anti-rig-i antibody, followed by IB with anti-pt 170 -RIG-I, anti-ps 8 -RIG- I, anti-ub, or anti-rig-i antibody. FIG. 2. Ectopic PKC-α/ expression enhances RIG-I phosphorylation at S 8 and T 170, thereby suppressing its K 63 -linked ubiquitination. (A and B) Exogenous expression of PKC-α or PKC- II increases the S 8 and T 170 phosphorylation of RIG-I 2CARD and inhibits its ubiquitination. At 48 h posttransfection with GST-RIG-I 2CARD together with vector, Flagtagged PKA, HA-tagged PKB, Myc-tagged PKC-α, or Myc-tagged PKG (A), or vector, Myctagged PKC-α, PKC- II, PKC- or PKC- (B), WCLs were used for GST-PD followed by IB with anti-pt 170 -RIG-I, anti-ps 8 -RIG-I, anti-gst, or anti-ub antibody. Arrows indicate the ubiquitinated bands. (C) Ectopic expression of PKC-α/ increases the phosphorylation of endogenous RIG-I at T 170 and suppresses its K 63 -linked ubiquitination. HEK293T cells transfected with vector or the indicated Myc-tagged PKC isozymes, were infected with SeV (50 HA units/ml) for 6 h. WCLs were used for IP with an anti-rig-i antibody, followed by IB with anti-pt 170 -RIG-I, anti-k 63 -Ub, or anti-rig-i antibody. (D) Ectopic expression of PKC-α or 28
29 PKC- increases the S 8 phosphorylation of endogenous RIG-I. HEK293T cells were transfected with vector, Myc-tagged PKC-α, PKC- II, PKC- or PKC-, followed by infection with SeV (50 HA units/ml) for 6 h. At 48 h posttransfection, WCLs were used for IP with an anti-rig-i antibody, followed by IB with anti-ps 8 -RIG-I or anti-rig-i antibody. Expression of Myc-tagged PKC isozymes was determined in the WCLs by IB with an anti-myc antibody. FIG. 3. ShRNA-mediated depletion or inhibition of PKC-α/ using specific inhibitors decreases RIG-I S 8 and T 170 phosphorylations and enhances the RIG-I K 63 -linked ubiquitination. (A) ShRNA-mediated knockdown of endogenous PKC-α and PKC- decreases RIG-I phosphorylation at S 8 and T 170. WCLs of HEK293T cells stably expressing psm2-non-silencing (ns) shrna, psm2-pkc-α-shrna, psm2-pkc- -shrna, or psuper-retro-pkc-α-shrna and psm2-pkc- -shrna, were used for IP with an anti-rig-i antibody, followed by IB with anti-ps 8 -RIG-I, anti-pt 170 -RIG-I, or anti-rig-i antibody. Endogenous PKC-α, PKC- I and PKC- II expressions were determined in the WCLs by IB with anti-pkc-α, anti-pkc- I or anti-pkc- II antibodies. Loading control was determined by using an anti-actin antibody. (B) ShRNAmediated knockdown of PKC-α and PKC- decreases RIG-I phosphorylation, thereby enhancing RIG-I K 63 -linked ubiquitination. At 36 h posttransfection with non-silencing (ns) shrna, or PKC-α- and PKC- -specific shrnas, HEK293T were either mock-treated or infected with SeV for 8 h. WCLs were used for IP with anti-rig-i, followed by IB anti-pt 170 -RIG-I, anti-k 63 -Ub, or anti-rig-i antibody. (C) Conventional PKC inhibitor Gö6976 decreases the S 8 and T 170 phosphorylation of RIG-I 2CARD. HEK293T cells transfected with GST-RIG-I 2CARD were either treated with DMSO or 10 μm Gö6976 for 16 h. WCLs were subjected to GST-PD, 29
30 followed by IB with anti-pt 170 -RIG-I, anti-ps 8 -RIG-I, or anti-gst antibody. Arrows indicate the ubiquitinated bands. (D) Conventional PKC inhibitor Gö6976 decreases the S 8 and T 170 phosphorylation of endogenous RIG-I and enhances its K 63 -ubiquitination. Primary NHLF cells were either treated with DMSO or 10 μm Gö6976 for 16 h. WCLs were used for IP with an anti- RIG-I antibody, followed by IB with anti-pt 170 -RIG-I, anti-ps 8 -RIG-I, anti-k 63 -Ub, or anti-rig- I antibody. WCLs were further used for immunoblotting with anti-pthr, anti-ub, or anti-actin antibody. (E) PKC inhibitor Gö6976 decreases the S 8 and T 170 phosphorylation of endogenous RIG-I in mock-treated and SeV-infected cells. HEK293T cells were either treated with DMSO or 10 μm Gö6976 for 12 h, followed by mock-treatment or infection with SeV (50 HA units/ml) for 10 h. WCLs were used for IP with an anti-rig-i antibody, followed by IB with anti-pt 170 -RIG-I, anti-ps 8 -RIG-I, or anti-rig-i antibody. (F) In vitro phosphorylation of RIG-I 2CARD by conventional PKC. Bacterially purified GST-RIG-I 2CARD was subjected to an in vitro phosphorylation assay using purified PKC-α/ /, followed by IB with anti-pt 170 -RIG-I, anti-ps 8 - RIG-I, or anti-gst antibody. FIG. 4. PKC- / interact with RIG-I. (A and B) Interaction between PKC- / and RIG-I or RIG-I 2CARD. At 48 h posttransfection with Myc-tagged PKC-α, PKC- II or PKG together with vector or Flag-RIG-I (A), or GST or GST-RIG-I 2CARD (B), WCLs were used for IP with anti-flag (A) or GST-PD (B), followed by IB with anti-myc (A, B), anti-flag (A) or anti-gst antibody (B). (C) Interaction between endogenous RIG-I and PKC- /. WCLs of HEK293T, A549 or HeLa cells were subjected to IP with an anti-rig-i antibody, followed by IB with anti- PKC-α, anti-pkc- I, anti-pkc- II, anti-pkc-, anti-pkc- or anti-rig-i antibody. Input (2%) for 293T is shown. (D) RIG-I-PKC- / interaction in primary NHLF. NHLF cells were mock- 30
31 infected or infected with SeV (50 HA units/ml) for the indicated hours. WCLs were subjected to IP with an anti-rig-i antibody, followed by IB with anti-pkc-α, anti-pkc- II, or anti-rig-i antibody. (E) PKC- and PKC- that interact with RIG-I are phosphorylated at S 657 or T 500, respectively. HEK293T cells were mock-treated or infected with SeV (50 HA units/ml) or NS1 PR8 virus (MOI 2) for 14 h. WCLs were used for IP with an anti-rig-i antibody, followed by IB with anti-pkc-α, anti-ps 657 -PKC-α, anti-pkc- II, or anti-pt 500 -PKC- antibodies. FIG. 5. Biochemical mapping of the RIG-I-PKC- / interaction. (A) Domain structures of RIG-I and PKC- as well as schematic representation of Flag-tagged RIG-I or GST-fused PKC- truncation constructs. Numbers indicate amino acid. GST-PKC- II fusion constructs were constructed corresponding to PKC- constructs. (B) PKC- / interact with the RIG-I 2CARD and RIG-I 2CARD. At 48 h posttransfection with Myc-tagged PKC-α, PKC- II or PKG together with vector, RIG-I 2CARD-Flag or RIG-I 2CARD-Flag, WCLs were used for IP with an anti-flag antibody, followed by IB with anti-myc or anti-flag antibodies. WCLs were further used for IB with an anti-myc antibody to determine the expression of PKC-α, PKC- II or PKG. (C) The CR and PS/C1 domains of PKC- / interact with RIG-I 2CARD and RIG-I 2CARD. HEK293T cells were transfected with RIG-I 2CARD-Flag (upper) or RIG-I 2CARD-Flag (lower) together with GST or the indicated GST-PKC-α or GST-PKC- II fusion constructs. WCLs were subjected to GST-PD, followed by IB with anti-flag or anti-gst antibody. (D and E) The CR and PS/C1 domains of PKC- / bind to the helicase of RIG-I. HEK293T cells were transfected with GST, GST-PKC-α-CR, or GST-PKC- II -CR (D), or GST, GST-PKC-α-PS/C1, or GST-PKC- II -PS/C1 (E) together with RIG-I-helicase-Flag or RIG-I- 31
32 RD-Flag. WCLs were used for GST-PD followed by IB with anti-flag or anti-gst antibody. Expression of Flag-tagged RIG-I-helicase and RIG-I-RD was determined by IB with an anti- Flag antibody. (F) The C1 domain of PKC-α/ is sufficient for RIG-I helicase binding. HEK293T cells were transfected with GST, GST-PKC-α-PS/C1, GST-PKC-α-C1, GST-PKC- II -PS/C1, or GST-PKC- II -C1 together with Flag-tagged RIG-I-helicase. WCLs were used for GST-PD followed by IB with anti-flag or anti-gst antibody. Expression of Flag-tagged RIG-Ihelicase was determined by IB with an anti-flag antibody. FIG. 6. PKC-α/ inhibit the RIG-I-TRIM25 interaction, RIG-I-MAVS binding, and thereby RIG-I downstream signaling. (A) Ectopic PKC-α/ expression inhibits the RIG-I 2CARD-induced IFN-β promoter activation. HEK293T were transfected with GST or GST- RIG-I 2CARD together with vector, Myc-tagged PKC-α, PKC- II, PKC- or PKC- as well as IFN- -luciferase and pgk- -gal. At 40 h posttransfection luciferase activity was determined as described in Fig 1E. Data represent the mean + SD (n=3). (B) Exogenous expression of PKCα/ suppresses SeV-induced IFN- promoter activation. HEK293T cells were transfected with vector, Myc-tagged PKC-α, PKC- II, PKC- or PKC- together with IFN- -luciferase and pgk- -gal. At 24 h after transfection, cells were mock-treated or infected with SeV. Luciferase activity was determined 18 h later as described in Fig 1E. Data represent the mean + SD (n=3). (C) Conventional PKC inhibitors enhance SeV-induced IFN- promoter activation. At 20 h posttransfection with IFN- -luciferase and pgk- -gal, HEK293T cells were treated with DMSO or the indicated PKC inhibitors [Bisindolylmaleimide I (BIM I) 4 μm; Gö μm; and Ro μm], followed by mock-treatment or infection with SeV (30 HA units/ml). 20 h 32
33 later, luciferase and -galactosidase values were determined and IFN- luciferase values normalized to -galactosidase activity for transfection efficiency control. Data represent the mean + SD (n=3). Statistical analysis was performed by Student s t test. ns, statistically not significant; ** indicates P< D) PKC inhibitor treatment is not sufficient to induce IFN- promoter activation in mock-infected cells. At 20 h posttransfection with IFN- -luciferase and pgk- -gal, HEK293T cells were treated with DMSO or the indicated PKC inhibitors as described in Fig. 6C. IFN- -luciferase and pgk- -gal were determined 20 h later. Data represent the mean + SD (n=3). (E) PKC-α/ inhibit virus-induced IRF3 dimerization in primary NHLF cells. At 24 h posttransfection with vector, Myc-tagged PKC-α, PKC- II, or PKC-, NHLF cells were mock-treated or infected with SeV (80 HA units/ml) for 22 h. WCLs were used for native PAGE followed by IB with an anti-irf3 antibody, or subjected to SDS-PAGE followed by IB with an anti-myc antibody. (F) PKC-α/ suppress the RIG-I-MAVS interaction. HEK293T cells were cotransfected with MAVS-CARD-PRD-Flag together with vector or Myc-tagged PKC isozymes, and subsequently either mock-treated or infected with SeV (50 HA units/ml) for 5 h. WCLs were subjected to IP with an anti-rig-i antibody, followed by IB with anti-flag or anti- RIG-I antibody. WCLs were further used for immunoblotting with anti-flag or anti-myc antibody. (G) PKC-α/ inhibit RIG-I binding to TRIM25. At 20 h after transfection with vector, Myc-tagged PKC-α, PKC- II, PKC- or PKC-, HEK293T cells were infected with SeV (50 HA units/ml) for 8 h. WCLs were subjected to IP with an anti-rig-i antibody, followed by IB with anti-trim25 or anti-rig-i antibody FIG. 7. PKC-α/ suppress RIG-I antiviral activity. (A) PKC-α/ inhibit RIG-I 2CARD- 33
34 mediated antiviral IFN production. Vero cells were incubated for 18 h with supernatants from HEK293T that had been transfected with GST or GST-RIG-I 2CARD together with vector, PKC-α, PKC- II, or PKC-, followed by NDV-GFP infection (MOI 3). At 24 h after infection, GFP expression was detected by epifluorescence and GFP-positive cells were determined by FACS analysis. (B) Ectopic PKC-α or PKC- expression increases VSV-eGFP replication. HEK293T stably expressing vector, Myc-tagged PKC-α, PKC- II, or PKC- were infected with VSV-eGFP at an MOI of 0.2. At 38 h after infection, virus titer and replication were determined by plaque assay and GFP expression, respectively. Pfu, plaque-forming unit. (C) ShRNAmediated PKC- /PKC- double knockdown inhibits VSV-eGFP replication. HEK293T cells stably transfected with non-silencing (n.s.) shrna, PKC-α-specific shrna, PKC- -specific shrna, or PKC-α- and PKC- -specific shrnas, were infected with VSV-eGFP at an MOI of 0.5. Thirty hours after infection, virus titer and GFP expression was determined by plaque assay or microscopy, respectively. Pfu, plaque-forming unit. (D) Knockdown of endogenous PKC- and PKC- in primary normal human lung fibroblasts (NHLF) suppresses VSV-eGFP replication. NHLF cells were transiently transfected with non-silencing control sirna (n.s.) or with both PKC-α- and PKC- -specific sirna. Forty hours after transfection, cells were infected with VSV-eGFP at an MOI of 0.1. Thirty hours after infection, virus titer and GFP expression was determined by plaque assay or microscopy, respectively. Knockdown of endogenous PKC-, PKC- I and PKC- II was confirmed by immunoblotting using the indicated antibodies. Pfu, plaque-forming unit. (E) Increased IFN- production in primary NHLF cells in which PKC- and PKC- gene expressions have been silenced. NHLF cells, that had been transiently transfected with non-silencing control sirna (n.s.) or with both PKC-α- and PKC- -specific 34
35 sirna, were either mock-treated or infected with SeV (40 HA units/ml). Thirty hours after infection, IFN- production in the supernatants was determined by ELISA. Statistical analysis was performed by Student s t test. ** indicates P< KD, knock-down. ND, not detected
36 703 REFERENCES Andrejeva, J., K. S. Childs, D. F. Young, T. S. Carlos, N. Stock, S. Goodbourn, and R. E. Randall The V proteins of paramyxoviruses bind the IFN-inducible RNA helicase, mda-5, and inhibit its activation of the IFN-beta promoter. Proc Natl Acad Sci U S A 101: Arimoto, K., H. Takahashi, T. Hishiki, H. Konishi, T. Fujita, and K. Shimotohno Negative regulation of the RIG-I signaling by the ubiquitin ligase RNF125. Proc Natl Acad Sci U S A 104: Cui, S., K. Eisenacher, A. Kirchhofer, K. Brzozka, A. Lammens, K. Lammens, T. Fujita, K. K. Conzelmann, A. Krug, and K. P. Hopfner The C-terminal regulatory domain is the RNA 5'-triphosphate sensor of RIG-I. Mol Cell 29: Friedman, C. S., M. A. O'Donnell, D. Legarda-Addison, A. Ng, W. B. Cardenas, J. S. Yount, T. M. Moran, C. F. Basler, A. Komuro, C. M. Horvath, R. Xavier, and A. T. Ting The tumour suppressor CYLD is a negative regulator of RIG-I-mediated antiviral response. EMBO Rep 9: Gack, M. U., R. A. Albrecht, T. Urano, K. S. Inn, I. C. Huang, E. Carnero, M. Farzan, S. Inoue, J. U. Jung, and A. Garcia-Sastre Influenza A virus NS1 targets the ubiquitin ligase TRIM25 to evade recognition by the host viral RNA sensor RIG-I. Cell Host Microbe 5: Gack, M. U., A. Kirchhofer, Y. C. Shin, K. S. Inn, C. Liang, S. Cui, S. Myong, T. Ha, K. P. Hopfner, and J. U. Jung Roles of RIG-I N-terminal tandem CARD and splice variant in TRIM25-mediated antiviral signal transduction. Proc Natl Acad Sci 725 U S A 105:
37 Gack, M. U., E. Nistal-Villan, K. S. Inn, A. Garcia-Sastre, and J. U. Jung Phosphorylation-mediated negative regulation of RIG-I antiviral activity. J Virol 84: Gack, M. U., Y. C. Shin, C. H. Joo, T. Urano, C. Liang, L. Sun, O. Takeuchi, S. Akira, Z. Chen, S. Inoue, and J. U. Jung TRIM25 RING-finger E3 ubiquitin ligase is essential for RIG-I-mediated antiviral activity. Nature 446: Gitlin, L., L. Benoit, C. Song, M. Cella, S. Gilfillan, M. J. Holtzman, and M. Colonna Melanoma differentiation-associated gene 5 (MDA5) is involved in the innate immune response to Paramyxoviridae infection in vivo. PLoS Pathog 6:e Gould, C. M., and A. C. Newton The life and death of protein kinase C. Curr Drug Targets 9: Hunter, T The age of crosstalk: phosphorylation, ubiquitination, and beyond. Mol Cell 28: Inn, K. S., M. U. Gack, F. Tokunaga, M. Shi, L. Y. Wong, K. Iwai, and J. U. Jung Linear ubiquitin assembly complex negatively regulates RIG-I- and TRIM25- mediated type I interferon induction. Mol Cell 41: Jaken, S., and P. J. Parker Protein kinase C binding partners. Bioessays 22: Kato, H., O. Takeuchi, S. Sato, M. Yoneyama, M. Yamamoto, K. Matsui, S. Uematsu, A. Jung, T. Kawai, K. J. Ishii, O. Yamaguchi, K. Otsu, T. Tsujimura, C. S. Koh, C. Reis e Sousa, Y. Matsuura, T. Fujita, and S. Akira Differential roles of MDA5 and RIG-I helicases in the recognition of RNA viruses. Nature 441:
38 Kawai, T., and S. Akira Toll-like receptor and RIG-I-like receptor signaling. Ann N Y Acad Sci 1143: Kawai, T., K. Takahashi, S. Sato, C. Coban, H. Kumar, H. Kato, K. J. Ishii, O. Takeuchi, and S. Akira IPS-1, an adaptor triggering RIG-I- and Mda5-mediated type I interferon induction. Nat Immunol 6: Komuro, A., and C. M. Horvath RNA- and virus-independent inhibition of antiviral signaling by RNA helicase LGP2. J Virol 80: Lin, X., Y. Yu, H. Zhao, Y. Zhang, J. Manela, and D. A. Tonetti Overexpression of PKCalpha is required to impart estradiol inhibition and tamoxifenresistance in a T47D human breast cancer tumor model. Carcinogenesis 27: Loo, Y. M., J. Fornek, N. Crochet, G. Bajwa, O. Perwitasari, L. Martinez-Sobrido, S. Akira, M. A. Gill, A. Garcia-Sastre, M. G. Katze, and M. Gale, Jr Distinct RIG-I and MDA5 signaling by RNA viruses in innate immunity. J Virol 82: Meylan, E., J. Curran, K. Hofmann, D. Moradpour, M. Binder, R. Bartenschlager, and J. Tschopp Cardif is an adaptor protein in the RIG-I antiviral pathway and is targeted by hepatitis C virus. Nature 437: Nakhaei, P., P. Genin, A. Civas, and J. Hiscott RIG-I-like receptors: sensing and responding to RNA virus infection. Semin Immunol 21: Nistal-Villan, E., M. U. Gack, G. Martinez-Delgado, N. P. Maharaj, K. S. Inn, H. Yang, R. Wang, A. K. Aggarwal, J. U. Jung, and A. Garcia-Sastre Negative role of RIG-I serine 8 phosphorylation in the regulation of interferon-beta production. J Biol Chem 285:
39 Oshiumi, H., M. Matsumoto, S. Hatakeyama, and T. Seya Riplet/RNF135, a RING finger protein, ubiquitinates RIG-I to promote interferon-beta induction during the early phase of viral infection. J Biol Chem 284: Pichlmair, A., O. Schulz, C. P. Tan, T. I. Naslund, P. Liljestrom, F. Weber, and C. Reis e Sousa RIG-I-mediated antiviral responses to single-stranded RNA bearing 5'-phosphates. Science 314: Rosse, C., M. Linch, S. Kermorgant, A. J. Cameron, K. Boeckeler, and P. J. Parker PKC and the control of localized signal dynamics. Nat Rev Mol Cell Biol 11: Rothenfusser, S., N. Goutagny, G. DiPerna, M. Gong, B. G. Monks, A. Schoenemeyer, M. Yamamoto, S. Akira, and K. A. Fitzgerald The RNA helicase Lgp2 inhibits TLR-independent sensing of viral replication by retinoic acidinducible gene-i. J Immunol 175: Saito, T., R. Hirai, Y. M. Loo, D. Owen, C. L. Johnson, S. C. Sinha, S. Akira, T. Fujita, and M. Gale, Jr Regulation of innate antiviral defenses through a shared repressor domain in RIG-I and LGP2. Proc Natl Acad Sci U S A 104: Seth, R. B., L. Sun, C. K. Ea, and Z. J. Chen Identification and characterization of MAVS, a mitochondrial antiviral signaling protein that activates NF-kappaB and IRF 3. Cell 122: Uematsu, S., and S. Akira Toll-like receptors and Type I interferons. J Biol Chem 282: Xu, L. G., Y. Y. Wang, K. J. Han, L. Y. Li, Z. Zhai, and H. B. Shu VISA is an adapter protein required for virus-triggered IFN-beta signaling. Mol Cell 19:
40 Yoneyama, M., M. Kikuchi, T. Natsukawa, N. Shinobu, T. Imaizumi, M. Miyagishi, K. Taira, S. Akira, and T. Fujita The RNA helicase RIG-I has an essential function in double-stranded RNA-induced innate antiviral responses. Nat Immunol 5: Zeng, W., L. Sun, X. Jiang, X. Chen, F. Hou, A. Adhikari, M. Xu, and Z. J. Chen Reconstitution of the RIG-I pathway reveals a signaling role of unanchored polyubiquitin chains in innate immunity. Cell 141: Zhu, J., C. B. Coyne, and S. N. Sarkar PKC alpha regulates Sendai virusmediated interferon induction through HDAC6 and beta-catenin. Embo J. Downloaded from on August 13, 2018 by guest 40
41
42
43
44
45
46
47
SUPPLEMENTARY INFORMATION
 doi: 10.1038/nature05732 SUPPLEMENTARY INFORMATION Supplemental Data Supplement Figure Legends Figure S1. RIG-I 2CARD undergo robust ubiquitination a, (top) At 48 h posttransfection with a GST, GST-RIG-I-2CARD
doi: 10.1038/nature05732 SUPPLEMENTARY INFORMATION Supplemental Data Supplement Figure Legends Figure S1. RIG-I 2CARD undergo robust ubiquitination a, (top) At 48 h posttransfection with a GST, GST-RIG-I-2CARD
Supporting Information
 Supporting Information Gack et al. 1.173/pnas.8494715 SI Text Cell Culture, Transfection, and Reagents. HEK293T, MEF, Vero, EcoPack2-293 (D iosciences), HeLa, HCT116, Huh7, NHLF, 2fTGH wild-type, U3A,
Supporting Information Gack et al. 1.173/pnas.8494715 SI Text Cell Culture, Transfection, and Reagents. HEK293T, MEF, Vero, EcoPack2-293 (D iosciences), HeLa, HCT116, Huh7, NHLF, 2fTGH wild-type, U3A,
Species-Specific Inhibition of RIG-I Ubiquitination and IFN Induction by the Influenza A Virus NS1 Protein
 Species-Specific Inhibition of RIG-I Ubiquitination and IFN Induction by the Influenza A Virus NS1 Protein Ricardo Rajsbaum 1,2, Randy A. Albrecht 1,2, May K. Wang 3, Natalya P. Maharaj 3, Gijs A. Versteeg
Species-Specific Inhibition of RIG-I Ubiquitination and IFN Induction by the Influenza A Virus NS1 Protein Ricardo Rajsbaum 1,2, Randy A. Albrecht 1,2, May K. Wang 3, Natalya P. Maharaj 3, Gijs A. Versteeg
SUPPLEMENTARY INFORMATION
 Supplementary Figures Supplementary Figure S1. Binding of full-length OGT and deletion mutants to PIP strips (Echelon Biosciences). Supplementary Figure S2. Binding of the OGT (919-1036) fragments with
Supplementary Figures Supplementary Figure S1. Binding of full-length OGT and deletion mutants to PIP strips (Echelon Biosciences). Supplementary Figure S2. Binding of the OGT (919-1036) fragments with
RAW264.7 cells stably expressing control shrna (Con) or GSK3b-specific shrna (sh-
 1 a b Supplementary Figure 1. Effects of GSK3b knockdown on poly I:C-induced cytokine production. RAW264.7 cells stably expressing control shrna (Con) or GSK3b-specific shrna (sh- GSK3b) were stimulated
1 a b Supplementary Figure 1. Effects of GSK3b knockdown on poly I:C-induced cytokine production. RAW264.7 cells stably expressing control shrna (Con) or GSK3b-specific shrna (sh- GSK3b) were stimulated
Supplementary Figure 1
 Supplementary Figure 1 YAP negatively regulates IFN- signaling. (a) Immunoblot analysis of Yap knockdown efficiency with sh-yap (#1 to #4 independent constructs) in Raw264.7 cells. (b) IFN- -Luc and PRDs
Supplementary Figure 1 YAP negatively regulates IFN- signaling. (a) Immunoblot analysis of Yap knockdown efficiency with sh-yap (#1 to #4 independent constructs) in Raw264.7 cells. (b) IFN- -Luc and PRDs
Innate Immunity & Inflammation
 Innate Immunity & Inflammation The innate immune system is an evolutionally conserved mechanism that provides an early and effective response against invading microbial pathogens. It relies on a limited
Innate Immunity & Inflammation The innate immune system is an evolutionally conserved mechanism that provides an early and effective response against invading microbial pathogens. It relies on a limited
Supplementary Figure 1.TRIM33 binds β-catenin in the nucleus. a & b, Co-IP of endogenous TRIM33 with β-catenin in HT-29 cells (a) and HEK 293T cells
 Supplementary Figure 1.TRIM33 binds β-catenin in the nucleus. a & b, Co-IP of endogenous TRIM33 with β-catenin in HT-29 cells (a) and HEK 293T cells (b). TRIM33 was immunoprecipitated, and the amount of
Supplementary Figure 1.TRIM33 binds β-catenin in the nucleus. a & b, Co-IP of endogenous TRIM33 with β-catenin in HT-29 cells (a) and HEK 293T cells (b). TRIM33 was immunoprecipitated, and the amount of
SUPPLEMENTARY INFORMATION. Supplementary Figures S1-S9. Supplementary Methods
 SUPPLEMENTARY INFORMATION SUMO1 modification of PTEN regulates tumorigenesis by controlling its association with the plasma membrane Jian Huang 1,2#, Jie Yan 1,2#, Jian Zhang 3#, Shiguo Zhu 1, Yanli Wang
SUPPLEMENTARY INFORMATION SUMO1 modification of PTEN regulates tumorigenesis by controlling its association with the plasma membrane Jian Huang 1,2#, Jie Yan 1,2#, Jian Zhang 3#, Shiguo Zhu 1, Yanli Wang
Supplementary Figure 1 Role of Raf-1 in TLR2-Dectin-1-mediated cytokine expression
 Supplementary Figure 1 Supplementary Figure 1 Role of Raf-1 in TLR2-Dectin-1-mediated cytokine expression. Quantitative real-time PCR of indicated mrnas in DCs stimulated with TLR2-Dectin-1 agonist zymosan
Supplementary Figure 1 Supplementary Figure 1 Role of Raf-1 in TLR2-Dectin-1-mediated cytokine expression. Quantitative real-time PCR of indicated mrnas in DCs stimulated with TLR2-Dectin-1 agonist zymosan
Reviewers' comments: Reviewer #1 (Remarks to the Author):
 Reviewers' comments: Reviewer #1 (Remarks to the Author): In this manuscript, Song et al. identified FBXW7 as a new positive regulator for RIG-Itriggered type I IFN signaling pathway. The authors observed
Reviewers' comments: Reviewer #1 (Remarks to the Author): In this manuscript, Song et al. identified FBXW7 as a new positive regulator for RIG-Itriggered type I IFN signaling pathway. The authors observed
SUPPLEMENTAL FIGURE LEGENDS
 SUPPLEMENTAL FIGURE LEGENDS Supplemental Figure S1: Endogenous interaction between RNF2 and H2AX: Whole cell extracts from 293T were subjected to immunoprecipitation with anti-rnf2 or anti-γ-h2ax antibodies
SUPPLEMENTAL FIGURE LEGENDS Supplemental Figure S1: Endogenous interaction between RNF2 and H2AX: Whole cell extracts from 293T were subjected to immunoprecipitation with anti-rnf2 or anti-γ-h2ax antibodies
p47 negatively regulates IKK activation by inducing the lysosomal degradation of polyubiquitinated NEMO
 Supplementary Information p47 negatively regulates IKK activation by inducing the lysosomal degradation of polyubiquitinated NEMO Yuri Shibata, Masaaki Oyama, Hiroko Kozuka-Hata, Xiao Han, Yuetsu Tanaka,
Supplementary Information p47 negatively regulates IKK activation by inducing the lysosomal degradation of polyubiquitinated NEMO Yuri Shibata, Masaaki Oyama, Hiroko Kozuka-Hata, Xiao Han, Yuetsu Tanaka,
Supplementary Figure 1. Normal T lymphocyte populations in Dapk -/- mice. (a) Normal thymic development in Dapk -/- mice. Thymocytes from WT and Dapk
 Supplementary Figure 1. Normal T lymphocyte populations in Dapk -/- mice. (a) Normal thymic development in Dapk -/- mice. Thymocytes from WT and Dapk -/- mice were stained for expression of CD4 and CD8.
Supplementary Figure 1. Normal T lymphocyte populations in Dapk -/- mice. (a) Normal thymic development in Dapk -/- mice. Thymocytes from WT and Dapk -/- mice were stained for expression of CD4 and CD8.
Brd4 Coactivates Transcriptional Activation of NF- B via Specific Binding to Acetylated RelA
 MOLECULAR AND CELLULAR BIOLOGY, Mar. 2009, p. 1375 1387 Vol. 29, No. 5 0270-7306/09/$08.00 0 doi:10.1128/mcb.01365-08 Copyright 2009, American Society for Microbiology. All Rights Reserved. Brd4 Coactivates
MOLECULAR AND CELLULAR BIOLOGY, Mar. 2009, p. 1375 1387 Vol. 29, No. 5 0270-7306/09/$08.00 0 doi:10.1128/mcb.01365-08 Copyright 2009, American Society for Microbiology. All Rights Reserved. Brd4 Coactivates
Supplementary data Supplementary Figure 1 Supplementary Figure 2
 Supplementary data Supplementary Figure 1 SPHK1 sirna increases RANKL-induced osteoclastogenesis in RAW264.7 cell culture. (A) RAW264.7 cells were transfected with oligocassettes containing SPHK1 sirna
Supplementary data Supplementary Figure 1 SPHK1 sirna increases RANKL-induced osteoclastogenesis in RAW264.7 cell culture. (A) RAW264.7 cells were transfected with oligocassettes containing SPHK1 sirna
Intrinsic cellular defenses against virus infection
 Intrinsic cellular defenses against virus infection Detection of virus infection Host cell response to virus infection Interferons: structure and synthesis Induction of antiviral activity Viral defenses
Intrinsic cellular defenses against virus infection Detection of virus infection Host cell response to virus infection Interferons: structure and synthesis Induction of antiviral activity Viral defenses
S1a S1b S1c. S1d. S1f S1g S1h SUPPLEMENTARY FIGURE 1. - si sc Il17rd Il17ra bp. rig/s IL-17RD (ng) -100 IL-17RD
 SUPPLEMENTARY FIGURE 1 0 20 50 80 100 IL-17RD (ng) S1a S1b S1c IL-17RD β-actin kda S1d - si sc Il17rd Il17ra rig/s15-574 - 458-361 bp S1f S1g S1h S1i S1j Supplementary Figure 1. Knockdown of IL-17RD enhances
SUPPLEMENTARY FIGURE 1 0 20 50 80 100 IL-17RD (ng) S1a S1b S1c IL-17RD β-actin kda S1d - si sc Il17rd Il17ra rig/s15-574 - 458-361 bp S1f S1g S1h S1i S1j Supplementary Figure 1. Knockdown of IL-17RD enhances
MicroRNA sponges: competitive inhibitors of small RNAs in mammalian cells
 MicroRNA sponges: competitive inhibitors of small RNAs in mammalian cells Margaret S Ebert, Joel R Neilson & Phillip A Sharp Supplementary figures and text: Supplementary Figure 1. Effect of sponges on
MicroRNA sponges: competitive inhibitors of small RNAs in mammalian cells Margaret S Ebert, Joel R Neilson & Phillip A Sharp Supplementary figures and text: Supplementary Figure 1. Effect of sponges on
Predictive PP1Ca binding region in BIG3 : 1,228 1,232aa (-KAVSF-) HEK293T cells *** *** *** KPL-3C cells - E E2 treatment time (h)
 Relative expression ERE-luciferase activity activity (pmole/min) activity (pmole/min) activity (pmole/min) activity (pmole/min) MCF-7 KPL-3C ZR--1 BT-474 T47D HCC15 KPL-1 HBC4 activity (pmole/min) a d
Relative expression ERE-luciferase activity activity (pmole/min) activity (pmole/min) activity (pmole/min) activity (pmole/min) MCF-7 KPL-3C ZR--1 BT-474 T47D HCC15 KPL-1 HBC4 activity (pmole/min) a d
Plasmids Western blot analysis and immunostaining Flow Cytometry Cell surface biotinylation RNA isolation and cdna synthesis
 Plasmids psuper-retro-s100a10 shrna1 was constructed by cloning the dsdna oligo 5 -GAT CCC CGT GGG CTT CCA GAG CTT CTT TCA AGA GAA GAA GCT CTG GAA GCC CAC TTT TTA-3 and 5 -AGC TTA AAA AGT GGG CTT CCA GAG
Plasmids psuper-retro-s100a10 shrna1 was constructed by cloning the dsdna oligo 5 -GAT CCC CGT GGG CTT CCA GAG CTT CTT TCA AGA GAA GAA GCT CTG GAA GCC CAC TTT TTA-3 and 5 -AGC TTA AAA AGT GGG CTT CCA GAG
316 Cell 141, , April 16, 2010 ª2010 Elsevier Inc.
 Figure 1. In Vitro Reconstitution of the RIG-I Pathway and Regulation of RIG-I by RNA and Ubiquitination (A) Purification of RIG-I protein from Sendai virus-infected (+SeV) or untreated ( SeV) HEK293T
Figure 1. In Vitro Reconstitution of the RIG-I Pathway and Regulation of RIG-I by RNA and Ubiquitination (A) Purification of RIG-I protein from Sendai virus-infected (+SeV) or untreated ( SeV) HEK293T
Appendix. Table of Contents
 Appendix Table of Contents Appendix Figures Figure S1: Gp78 is not required for the degradation of mcherry-cl1 in Hela Cells. Figure S2: Indel formation in the MARCH6 sgrna targeted HeLa clones. Figure
Appendix Table of Contents Appendix Figures Figure S1: Gp78 is not required for the degradation of mcherry-cl1 in Hela Cells. Figure S2: Indel formation in the MARCH6 sgrna targeted HeLa clones. Figure
supplementary information
 DOI: 10.1038/ncb1875 Figure S1 (a) The 79 surgical specimens from NSCLC patients were analysed by immunohistochemistry with an anti-p53 antibody and control serum (data not shown). The normal bronchi served
DOI: 10.1038/ncb1875 Figure S1 (a) The 79 surgical specimens from NSCLC patients were analysed by immunohistochemistry with an anti-p53 antibody and control serum (data not shown). The normal bronchi served
SUPPLEMENTARY INFORMATION
 SUPPLEMENTARY INFORMATION doi:1.138/nature9814 a A SHARPIN FL B SHARPIN ΔNZF C SHARPIN T38L, F39V b His-SHARPIN FL -1xUb -2xUb -4xUb α-his c Linear 4xUb -SHARPIN FL -SHARPIN TF_LV -SHARPINΔNZF -SHARPIN
SUPPLEMENTARY INFORMATION doi:1.138/nature9814 a A SHARPIN FL B SHARPIN ΔNZF C SHARPIN T38L, F39V b His-SHARPIN FL -1xUb -2xUb -4xUb α-his c Linear 4xUb -SHARPIN FL -SHARPIN TF_LV -SHARPINΔNZF -SHARPIN
Trim29 gene-targeting strategy. (a) Genotyping of wildtype mice (+/+), Trim29 heterozygous mice (+/ ) and homozygous mice ( / ).
 Supplementary Figure 1 Trim29 gene-targeting strategy. (a) Genotyping of wildtype mice (+/+), Trim29 heterozygous mice (+/ ) and homozygous mice ( / ). (b) Immunoblot analysis of TRIM29 in lung primary
Supplementary Figure 1 Trim29 gene-targeting strategy. (a) Genotyping of wildtype mice (+/+), Trim29 heterozygous mice (+/ ) and homozygous mice ( / ). (b) Immunoblot analysis of TRIM29 in lung primary
SUPPLEMENTARY INFORMATION
 Figure S1. Silver staining and immunoblotting of the purified TAK1 kinase complex. The TAK1 kinase complex was purified through tandem affinity methods (Protein A and FLAG), and aliquots of the purified
Figure S1. Silver staining and immunoblotting of the purified TAK1 kinase complex. The TAK1 kinase complex was purified through tandem affinity methods (Protein A and FLAG), and aliquots of the purified
The early interferon response to rotavirus is regulated by PKR and depends on
 JVI Accepts, published online ahead of print on February 0 J. Virol. doi:./jvi.0- Copyright 0, American Society for Microbiology and/or the Listed Authors/Institutions. All Rights Reserved. 1 The early
JVI Accepts, published online ahead of print on February 0 J. Virol. doi:./jvi.0- Copyright 0, American Society for Microbiology and/or the Listed Authors/Institutions. All Rights Reserved. 1 The early
SUPPLEMENT. Materials and methods
 SUPPLEMENT Materials and methods Cell culture and reagents Cell media and reagents were from Invitrogen unless otherwise indicated. Antibiotics and Tet-certified serum were from Clontech. In experiments
SUPPLEMENT Materials and methods Cell culture and reagents Cell media and reagents were from Invitrogen unless otherwise indicated. Antibiotics and Tet-certified serum were from Clontech. In experiments
Supplementary Figure S1. Venn diagram analysis of mrna microarray data and mirna target analysis. (a) Western blot analysis of T lymphoblasts (CLS)
 Supplementary Figure S1. Venn diagram analysis of mrna microarray data and mirna target analysis. (a) Western blot analysis of T lymphoblasts (CLS) and their exosomes (EXO) in resting (REST) and activated
Supplementary Figure S1. Venn diagram analysis of mrna microarray data and mirna target analysis. (a) Western blot analysis of T lymphoblasts (CLS) and their exosomes (EXO) in resting (REST) and activated
Supplementary Material
 Supplementary Material Nuclear import of purified HIV-1 Integrase. Integrase remains associated to the RTC throughout the infection process until provirus integration occurs and is therefore one likely
Supplementary Material Nuclear import of purified HIV-1 Integrase. Integrase remains associated to the RTC throughout the infection process until provirus integration occurs and is therefore one likely
TRAF6 ubiquitinates TGFβ type I receptor to promote its cleavage and nuclear translocation in cancer
 Supplementary Information TRAF6 ubiquitinates TGFβ type I receptor to promote its cleavage and nuclear translocation in cancer Yabing Mu, Reshma Sundar, Noopur Thakur, Maria Ekman, Shyam Kumar Gudey, Mariya
Supplementary Information TRAF6 ubiquitinates TGFβ type I receptor to promote its cleavage and nuclear translocation in cancer Yabing Mu, Reshma Sundar, Noopur Thakur, Maria Ekman, Shyam Kumar Gudey, Mariya
The Coxsackievirus B 3C pro Protease Cleaves MAVS and TRIF to Attenuate Host Type I Interferon and Apoptotic Signaling
 The Coxsackievirus B 3C pro Protease Cleaves MAVS and TRIF to Attenuate Host Type I Interferon and Apoptotic Signaling Amitava Mukherjee 1, Stefanie A. Morosky 2, Elizabeth Delorme-Axford 1, Naomi Dybdahl-Sissoko
The Coxsackievirus B 3C pro Protease Cleaves MAVS and TRIF to Attenuate Host Type I Interferon and Apoptotic Signaling Amitava Mukherjee 1, Stefanie A. Morosky 2, Elizabeth Delorme-Axford 1, Naomi Dybdahl-Sissoko
The Early Interferon Response to Rotavirus Is Regulated by PKR and Depends on MAVS/IPS-1, RIG-I, MDA-5, and IRF3
 JOURNAL OF VIROLOGY, Apr. 2011, p. 3717 3732 Vol. 85, No. 8 0022-538X/11/$12.00 doi:10.1128/jvi.02634-10 Copyright 2011, American Society for Microbiology. All Rights Reserved. The Early Interferon Response
JOURNAL OF VIROLOGY, Apr. 2011, p. 3717 3732 Vol. 85, No. 8 0022-538X/11/$12.00 doi:10.1128/jvi.02634-10 Copyright 2011, American Society for Microbiology. All Rights Reserved. The Early Interferon Response
Tel: ; Fax: ;
 Tel.: +98 216 696 9291; Fax: +98 216 696 9291; E-mail: mrasadeghi@pasteur.ac.ir Tel: +98 916 113 7679; Fax: +98 613 333 6380; E-mail: abakhshi_e@ajums.ac.ir A Soluble Chromatin-bound MOI 0 1 5 0 1 5 HDAC2
Tel.: +98 216 696 9291; Fax: +98 216 696 9291; E-mail: mrasadeghi@pasteur.ac.ir Tel: +98 916 113 7679; Fax: +98 613 333 6380; E-mail: abakhshi_e@ajums.ac.ir A Soluble Chromatin-bound MOI 0 1 5 0 1 5 HDAC2
Nature Immunology doi: /ni.3268
 Supplementary Figure 1 Loss of Mst1 and Mst2 increases susceptibility to bacterial sepsis. (a) H&E staining of colon and kidney sections from wild type and Mst1 -/- Mst2 fl/fl Vav-Cre mice. Scale bar,
Supplementary Figure 1 Loss of Mst1 and Mst2 increases susceptibility to bacterial sepsis. (a) H&E staining of colon and kidney sections from wild type and Mst1 -/- Mst2 fl/fl Vav-Cre mice. Scale bar,
Soft Agar Assay. For each cell pool, 100,000 cells were resuspended in 0.35% (w/v)
 SUPPLEMENTARY MATERIAL AND METHODS Soft Agar Assay. For each cell pool, 100,000 cells were resuspended in 0.35% (w/v) top agar (LONZA, SeaKem LE Agarose cat.5004) and plated onto 0.5% (w/v) basal agar.
SUPPLEMENTARY MATERIAL AND METHODS Soft Agar Assay. For each cell pool, 100,000 cells were resuspended in 0.35% (w/v) top agar (LONZA, SeaKem LE Agarose cat.5004) and plated onto 0.5% (w/v) basal agar.
Supporting Online Material Material and Methods References Supplemental Figures S1, S2, and S3
 Supporting Online Material Material and Methods References Supplemental Figures S1, S2, and S3 Sarbassov et al. 1 Material and Methods Materials Reagents were obtained from the following sources: protein
Supporting Online Material Material and Methods References Supplemental Figures S1, S2, and S3 Sarbassov et al. 1 Material and Methods Materials Reagents were obtained from the following sources: protein
Supplementary Materials for
 www.sciencesignaling.org/cgi/content/full/8/366/ra25/dc1 Supplementary Materials for Viral entry route determines how human plasmacytoid dendritic cells produce type I interferons Daniela Bruni, Maxime
www.sciencesignaling.org/cgi/content/full/8/366/ra25/dc1 Supplementary Materials for Viral entry route determines how human plasmacytoid dendritic cells produce type I interferons Daniela Bruni, Maxime
Supplementary Figure 1 CD4 + T cells from PKC-θ null mice are defective in NF-κB activation during T cell receptor signaling. CD4 + T cells were
 Supplementary Figure 1 CD4 + T cells from PKC-θ null mice are defective in NF-κB activation during T cell receptor signaling. CD4 + T cells were isolated from wild type (PKC-θ- WT) or PKC-θ null (PKC-θ-KO)
Supplementary Figure 1 CD4 + T cells from PKC-θ null mice are defective in NF-κB activation during T cell receptor signaling. CD4 + T cells were isolated from wild type (PKC-θ- WT) or PKC-θ null (PKC-θ-KO)
Supplementary Figure 1. Prevalence of U539C and G540A nucleotide and E172K amino acid substitutions among H9N2 viruses. Full-length H9N2 NS
 Supplementary Figure 1. Prevalence of U539C and G540A nucleotide and E172K amino acid substitutions among H9N2 viruses. Full-length H9N2 NS nucleotide sequences (a, b) or amino acid sequences (c) from
Supplementary Figure 1. Prevalence of U539C and G540A nucleotide and E172K amino acid substitutions among H9N2 viruses. Full-length H9N2 NS nucleotide sequences (a, b) or amino acid sequences (c) from
SUPPLEMENTAL INFORMATION
 SUPPLEMENTAL INFORMATION EXPERIMENTAL PROCEDURES Tryptic digestion protection experiments - PCSK9 with Ab-3D5 (1:1 molar ratio) in 50 mm Tris, ph 8.0, 150 mm NaCl was incubated overnight at 4 o C. The
SUPPLEMENTAL INFORMATION EXPERIMENTAL PROCEDURES Tryptic digestion protection experiments - PCSK9 with Ab-3D5 (1:1 molar ratio) in 50 mm Tris, ph 8.0, 150 mm NaCl was incubated overnight at 4 o C. The
Supplementary Information POLO-LIKE KINASE 1 FACILITATES LOSS OF PTEN-INDUCED PROSTATE CANCER FORMATION
 Supplementary Information POLO-LIKE KINASE 1 FACILITATES LOSS OF PTEN-INDUCED PROSTATE CANCER FORMATION X. Shawn Liu 1, 3, Bing Song 2, 3, Bennett D. Elzey 3, 4, Timothy L. Ratliff 3, 4, Stephen F. Konieczny
Supplementary Information POLO-LIKE KINASE 1 FACILITATES LOSS OF PTEN-INDUCED PROSTATE CANCER FORMATION X. Shawn Liu 1, 3, Bing Song 2, 3, Bennett D. Elzey 3, 4, Timothy L. Ratliff 3, 4, Stephen F. Konieczny
Supplementary Figure 1
 Supplementary Figure 1 Supplementary Figure 1. Neither the activation nor suppression of the MAPK pathway affects the ASK1/Vif interaction. (a, b) HEK293 cells were cotransfected with plasmids encoding
Supplementary Figure 1 Supplementary Figure 1. Neither the activation nor suppression of the MAPK pathway affects the ASK1/Vif interaction. (a, b) HEK293 cells were cotransfected with plasmids encoding
HDAC6 regulates cellular viral RNA sensing by deacetylation of RIG-I
 Article HDAC6 regulates cellular viral RNA sensing by deacetylation of RIG-I Su Jin Choi 1,, Hyun-Cheol Lee 2,, Jae-Hoon Kim 2, Song Yi Park 1, Tae-Hwan Kim 2, Woon-Kyu Lee 3, Duk-Jae Jang 2, Ji-Eun Yoon
Article HDAC6 regulates cellular viral RNA sensing by deacetylation of RIG-I Su Jin Choi 1,, Hyun-Cheol Lee 2,, Jae-Hoon Kim 2, Song Yi Park 1, Tae-Hwan Kim 2, Woon-Kyu Lee 3, Duk-Jae Jang 2, Ji-Eun Yoon
The signaling lifetime of protein kinase C (PKC) 4 is under the control of multiple mechanisms. Phosphorylation controls the
 THE JOURNAL OF BIOLOGICAL CHEMISTRY VOL. 282, NO. 46, pp. 33776 33787, November 16, 2007 2007 by The American Society for Biochemistry and Molecular Biology, Inc. Printed in the U.S.A. Amplitude Control
THE JOURNAL OF BIOLOGICAL CHEMISTRY VOL. 282, NO. 46, pp. 33776 33787, November 16, 2007 2007 by The American Society for Biochemistry and Molecular Biology, Inc. Printed in the U.S.A. Amplitude Control
CA Tel.: ; Fax: ; ucsd.edu.
 THE JOURNAL OF BIOLOGICAL CHEMISTRY VOL. 283, NO. 10, pp. 6300 6311, March 7, 2008 2008 by The American Society for Biochemistry and Molecular Biology, Inc. Printed in the U.S.A. The Phosphatase PHLPP
THE JOURNAL OF BIOLOGICAL CHEMISTRY VOL. 283, NO. 10, pp. 6300 6311, March 7, 2008 2008 by The American Society for Biochemistry and Molecular Biology, Inc. Printed in the U.S.A. The Phosphatase PHLPP
Supplementary information. MARCH8 inhibits HIV-1 infection by reducing virion incorporation of envelope glycoproteins
 Supplementary information inhibits HIV-1 infection by reducing virion incorporation of envelope glycoproteins Takuya Tada, Yanzhao Zhang, Takayoshi Koyama, Minoru Tobiume, Yasuko Tsunetsugu-Yokota, Shoji
Supplementary information inhibits HIV-1 infection by reducing virion incorporation of envelope glycoproteins Takuya Tada, Yanzhao Zhang, Takayoshi Koyama, Minoru Tobiume, Yasuko Tsunetsugu-Yokota, Shoji
c Tuj1(-) apoptotic live 1 DIV 2 DIV 1 DIV 2 DIV Tuj1(+) Tuj1/GFP/DAPI Tuj1 DAPI GFP
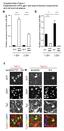 Supplementary Figure 1 Establishment of the gain- and loss-of-function experiments and cell survival assays. a Relative expression of mature mir-484 30 20 10 0 **** **** NCP mir- 484P NCP mir- 484P b Relative
Supplementary Figure 1 Establishment of the gain- and loss-of-function experiments and cell survival assays. a Relative expression of mature mir-484 30 20 10 0 **** **** NCP mir- 484P NCP mir- 484P b Relative
Supplementary Materials for
 www.sciencesignaling.org/cgi/content/full/6/283/ra57/dc1 Supplementary Materials for JNK3 Couples the Neuronal Stress Response to Inhibition of Secretory Trafficking Guang Yang,* Xun Zhou, Jingyan Zhu,
www.sciencesignaling.org/cgi/content/full/6/283/ra57/dc1 Supplementary Materials for JNK3 Couples the Neuronal Stress Response to Inhibition of Secretory Trafficking Guang Yang,* Xun Zhou, Jingyan Zhu,
SUPPLEMENTARY INFORMATION
 Supplementary Table 1. Cell sphingolipids and S1P bound to endogenous TRAF2. Sphingolipid Cell pmol/mg TRAF2 immunoprecipitate pmol/mg Sphingomyelin 4200 ± 250 Not detected Monohexosylceramide 311 ± 18
Supplementary Table 1. Cell sphingolipids and S1P bound to endogenous TRAF2. Sphingolipid Cell pmol/mg TRAF2 immunoprecipitate pmol/mg Sphingomyelin 4200 ± 250 Not detected Monohexosylceramide 311 ± 18
Impact of hyper-o-glcnacylation on apoptosis and NF-κB activity SUPPLEMENTARY METHODS
 SUPPLEMENTARY METHODS 3D culture and cell proliferation- MiaPaCa-2 cell culture in 3D was performed as described previously (1). Briefly, 8-well glass chamber slides were evenly coated with 50 µl/well
SUPPLEMENTARY METHODS 3D culture and cell proliferation- MiaPaCa-2 cell culture in 3D was performed as described previously (1). Briefly, 8-well glass chamber slides were evenly coated with 50 µl/well
Received 3 September 2002/Accepted 15 January 2003
 JOURNAL OF VIROLOGY, Apr. 2003, p. 4646 4657 Vol. 77, No. 8 0022-538X/03/$08.00 0 DOI: 10.1128/JVI.77.8.4646 4657.2003 Copyright 2003, American Society for Microbiology. All Rights Reserved. Ability of
JOURNAL OF VIROLOGY, Apr. 2003, p. 4646 4657 Vol. 77, No. 8 0022-538X/03/$08.00 0 DOI: 10.1128/JVI.77.8.4646 4657.2003 Copyright 2003, American Society for Microbiology. All Rights Reserved. Ability of
West Nile Virus-Induced Interferon Production Is Mediated by the Double-Stranded RNA-Dependent Protein Kinase PKR
 JOURNAL OF VIROLOGY, Oct. 2007, p. 11148 11158 Vol. 81, No. 20 0022-538X/07/$08.00 0 doi:10.1128/jvi.00446-07 Copyright 2007, American Society for Microbiology. All Rights Reserved. West Nile Virus-Induced
JOURNAL OF VIROLOGY, Oct. 2007, p. 11148 11158 Vol. 81, No. 20 0022-538X/07/$08.00 0 doi:10.1128/jvi.00446-07 Copyright 2007, American Society for Microbiology. All Rights Reserved. West Nile Virus-Induced
Eukaryotic transcription (III)
 Eukaryotic transcription (III) 1. Chromosome and chromatin structure Chromatin, chromatid, and chromosome chromatin Genomes exist as chromatins before or after cell division (interphase) but as chromatids
Eukaryotic transcription (III) 1. Chromosome and chromatin structure Chromatin, chromatid, and chromosome chromatin Genomes exist as chromatins before or after cell division (interphase) but as chromatids
supplementary information
 DOI: 10.1038/ncb2153 Figure S1 Ectopic expression of HAUSP up-regulates REST protein. (a) Immunoblotting showed that ectopic expression of HAUSP increased REST protein levels in ENStemA NPCs. (b) Immunofluorescent
DOI: 10.1038/ncb2153 Figure S1 Ectopic expression of HAUSP up-regulates REST protein. (a) Immunoblotting showed that ectopic expression of HAUSP increased REST protein levels in ENStemA NPCs. (b) Immunofluorescent
The Ubiquitin E3 Ligase RAUL Negatively Regulates Type I Interferon through Ubiquitination of the Transcription Factors IRF7 and IRF3
 Article The Ubiquitin E3 Ligase Negatively Regulates Type I Interferon through Ubiquitination of the Transcription Factors and Yanxing Yu 1,2, * and Gary S. Hayward 1, * 1 Viral Oncology Program, The Sidney
Article The Ubiquitin E3 Ligase Negatively Regulates Type I Interferon through Ubiquitination of the Transcription Factors and Yanxing Yu 1,2, * and Gary S. Hayward 1, * 1 Viral Oncology Program, The Sidney
Supplementary Materials for
 www.sciencesignaling.org/cgi/content/full/9/439/ra78/dc1 Supplementary Materials for Small heterodimer partner mediates liver X receptor (LXR) dependent suppression of inflammatory signaling by promoting
www.sciencesignaling.org/cgi/content/full/9/439/ra78/dc1 Supplementary Materials for Small heterodimer partner mediates liver X receptor (LXR) dependent suppression of inflammatory signaling by promoting
Tbk1-TKO! DN cells (%)! 15! 10!
 a! T Cells! TKO! B Cells! TKO! b! CD4! 8.9 85.2 3.4 2.88 CD8! Tbk1-TKO! 1.1 84.8 2.51 2.54 c! DN cells (%)! 4 3 2 1 DP cells (%)! 9 8 7 6 CD4 + SP cells (%)! 5 4 3 2 1 5 TKO! TKO! TKO! TKO! 15 1 5 CD8
a! T Cells! TKO! B Cells! TKO! b! CD4! 8.9 85.2 3.4 2.88 CD8! Tbk1-TKO! 1.1 84.8 2.51 2.54 c! DN cells (%)! 4 3 2 1 DP cells (%)! 9 8 7 6 CD4 + SP cells (%)! 5 4 3 2 1 5 TKO! TKO! TKO! TKO! 15 1 5 CD8
TRIM25 Is Required for the Antiviral Activity of Zinc-finger Antiviral Protein
 JVI Accepted Manuscript Posted Online 15 February 2017 J. Virol. doi:10.1128/jvi.00088-17 Copyright 2017 American Society for Microbiology. All Rights Reserved. 1 2 TRIM25 Is Required for the Antiviral
JVI Accepted Manuscript Posted Online 15 February 2017 J. Virol. doi:10.1128/jvi.00088-17 Copyright 2017 American Society for Microbiology. All Rights Reserved. 1 2 TRIM25 Is Required for the Antiviral
a b G75 G60 Sw-2 Sw-1 Supplementary Figure 1. Structure predictions by I-TASSER Server.
 a b G75 2 2 G60 Sw-2 Sw-1 Supplementary Figure 1. Structure predictions by I-TASSER Server. a. Overlay of top 10 models generated by I-TASSER illustrates the potential effect of 7 amino acid insertion
a b G75 2 2 G60 Sw-2 Sw-1 Supplementary Figure 1. Structure predictions by I-TASSER Server. a. Overlay of top 10 models generated by I-TASSER illustrates the potential effect of 7 amino acid insertion
~Lentivirus production~
 ~Lentivirus production~ May 30, 2008 RNAi core R&D group member Lentivirus Production Session Lentivirus!!! Is it health threatening to lab technician? What s so good about this RNAi library? How to produce
~Lentivirus production~ May 30, 2008 RNAi core R&D group member Lentivirus Production Session Lentivirus!!! Is it health threatening to lab technician? What s so good about this RNAi library? How to produce
Ebola Virus VP35 Protein Binds Double-Stranded RNA and Inhibits Alpha/Beta Interferon Production Induced by RIG-I Signaling
 JOURNAL OF VIROLOGY, June 2006, p. 5168 5178 Vol. 80, No. 11 0022-538X/06/$08.00 0 doi:10.1128/jvi.02199-05 Copyright 2006, American Society for Microbiology. All Rights Reserved. Ebola Virus VP35 Protein
JOURNAL OF VIROLOGY, June 2006, p. 5168 5178 Vol. 80, No. 11 0022-538X/06/$08.00 0 doi:10.1128/jvi.02199-05 Copyright 2006, American Society for Microbiology. All Rights Reserved. Ebola Virus VP35 Protein
Supplementary Figure 1. MAT IIα is Acetylated at Lysine 81.
 IP: Flag a Mascot PTM Modified Mass Error Position Gene Names Score Score Sequence m/z [ppm] 81 MAT2A;AMS2;MATA2 35.6 137.28 _AAVDYQK(ac)VVR_ 595.83-2.28 b Pre-immu After-immu Flag- WT K81R WT K81R / Flag
IP: Flag a Mascot PTM Modified Mass Error Position Gene Names Score Score Sequence m/z [ppm] 81 MAT2A;AMS2;MATA2 35.6 137.28 _AAVDYQK(ac)VVR_ 595.83-2.28 b Pre-immu After-immu Flag- WT K81R WT K81R / Flag
Supplementary Data Table of Contents:
 Supplementary Data Table of Contents: - Supplementary Methods - Supplementary Figures S1(A-B) - Supplementary Figures S2 (A-B) - Supplementary Figures S3 - Supplementary Figures S4(A-B) - Supplementary
Supplementary Data Table of Contents: - Supplementary Methods - Supplementary Figures S1(A-B) - Supplementary Figures S2 (A-B) - Supplementary Figures S3 - Supplementary Figures S4(A-B) - Supplementary
TFEB-mediated increase in peripheral lysosomes regulates. Store Operated Calcium Entry
 TFEB-mediated increase in peripheral lysosomes regulates Store Operated Calcium Entry Luigi Sbano, Massimo Bonora, Saverio Marchi, Federica Baldassari, Diego L. Medina, Andrea Ballabio, Carlotta Giorgi
TFEB-mediated increase in peripheral lysosomes regulates Store Operated Calcium Entry Luigi Sbano, Massimo Bonora, Saverio Marchi, Federica Baldassari, Diego L. Medina, Andrea Ballabio, Carlotta Giorgi
William C. Comb, Jessica E. Hutti, Patricia Cogswell, Lewis C. Cantley, and Albert S. Baldwin
 Molecular Cell, Volume 45 Supplemental Information p85 SH2 Domain Phosphorylation by IKK Promotes Feedback Inhibition of PI3K and Akt in Response to Cellular Starvation William C. Comb, Jessica E. Hutti,
Molecular Cell, Volume 45 Supplemental Information p85 SH2 Domain Phosphorylation by IKK Promotes Feedback Inhibition of PI3K and Akt in Response to Cellular Starvation William C. Comb, Jessica E. Hutti,
(a) Schematic diagram of the FS mutation of UVRAG in exon 8 containing the highly instable
 Supplementary Figure 1. Frameshift (FS) mutation in UVRAG. (a) Schematic diagram of the FS mutation of UVRAG in exon 8 containing the highly instable A 10 DNA repeat, generating a premature stop codon
Supplementary Figure 1. Frameshift (FS) mutation in UVRAG. (a) Schematic diagram of the FS mutation of UVRAG in exon 8 containing the highly instable A 10 DNA repeat, generating a premature stop codon
Tumor suppressor Spred2 interaction with LC3 promotes autophagosome maturation and induces autophagy-dependent cell death
 www.impactjournals.com/oncotarget/ Oncotarget, Supplementary Materials 2016 Tumor suppressor Spred2 interaction with LC3 promotes autophagosome maturation and induces autophagy-dependent cell death Supplementary
www.impactjournals.com/oncotarget/ Oncotarget, Supplementary Materials 2016 Tumor suppressor Spred2 interaction with LC3 promotes autophagosome maturation and induces autophagy-dependent cell death Supplementary
Supplements. Figure S1. B Phalloidin Alexa488
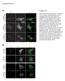 Supplements A, DMSO, PP2, PP3 Crk-myc Figure S1. (A) Src kinase activity is necessary for recruitment of Crk to Nephrin cytoplasmic domain. Human podocytes expressing /7-NephrinCD () were treated with
Supplements A, DMSO, PP2, PP3 Crk-myc Figure S1. (A) Src kinase activity is necessary for recruitment of Crk to Nephrin cytoplasmic domain. Human podocytes expressing /7-NephrinCD () were treated with
(a) Significant biological processes (upper panel) and disease biomarkers (lower panel)
 Supplementary Figure 1. Functional enrichment analyses of secretomic proteins. (a) Significant biological processes (upper panel) and disease biomarkers (lower panel) 2 involved by hrab37-mediated secretory
Supplementary Figure 1. Functional enrichment analyses of secretomic proteins. (a) Significant biological processes (upper panel) and disease biomarkers (lower panel) 2 involved by hrab37-mediated secretory
Identification of Host Cytosolic Sensors and Bacterial Factors Regulating the Type I Interferon Response to Legionella pneumophila
 Identification of Host Cytosolic Sensors and Bacterial Factors Regulating the Type I Interferon Response to Legionella pneumophila Kathryn M. Monroe, Sarah M. McWhirter, Russell E. Vance* Division of Immunology
Identification of Host Cytosolic Sensors and Bacterial Factors Regulating the Type I Interferon Response to Legionella pneumophila Kathryn M. Monroe, Sarah M. McWhirter, Russell E. Vance* Division of Immunology
Supplementary Figure 1. PD-L1 is glycosylated in cancer cells. (a) Western blot analysis of PD-L1 in breast cancer cells. (b) Western blot analysis
 Supplementary Figure 1. PD-L1 is glycosylated in cancer cells. (a) Western blot analysis of PD-L1 in breast cancer cells. (b) Western blot analysis of PD-L1 in ovarian cancer cells. (c) Western blot analysis
Supplementary Figure 1. PD-L1 is glycosylated in cancer cells. (a) Western blot analysis of PD-L1 in breast cancer cells. (b) Western blot analysis of PD-L1 in ovarian cancer cells. (c) Western blot analysis
HEK293FT cells were transiently transfected with reporters, N3-ICD construct and
 Supplementary Information Luciferase reporter assay HEK293FT cells were transiently transfected with reporters, N3-ICD construct and increased amounts of wild type or kinase inactive EGFR. Transfections
Supplementary Information Luciferase reporter assay HEK293FT cells were transiently transfected with reporters, N3-ICD construct and increased amounts of wild type or kinase inactive EGFR. Transfections
Pre-made Reporter Lentivirus for NF-κB Signal Pathway
 Pre-made Reporter for NF-κB Signal Pathway Cat# Product Name Amounts LVP965-P or: LVP965-P-PBS NFKB-GFP (Puro) LVP966-P or: LVP966-P-PBS NFKB-RFP (Puro) LVP967-P or: LVP967-P-PBS NFKB-Luc (Puro) LVP968-P
Pre-made Reporter for NF-κB Signal Pathway Cat# Product Name Amounts LVP965-P or: LVP965-P-PBS NFKB-GFP (Puro) LVP966-P or: LVP966-P-PBS NFKB-RFP (Puro) LVP967-P or: LVP967-P-PBS NFKB-Luc (Puro) LVP968-P
SUPPLEMENTARY INFORMATION
 a c e doi:10.1038/nature10407 b d f Supplementary Figure 1. SERCA2a complex analysis. (a) Two-dimensional SDS-PAGE gels of SERCA2a complexes. A silver-stained SDSPAGE gel is shown, which reveals a 12 kda
a c e doi:10.1038/nature10407 b d f Supplementary Figure 1. SERCA2a complex analysis. (a) Two-dimensional SDS-PAGE gels of SERCA2a complexes. A silver-stained SDSPAGE gel is shown, which reveals a 12 kda
Supplementary Figure 1. SC35M polymerase activity in the presence of Bat or SC35M NP encoded from the phw2000 rescue plasmid.
 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 Supplementary Figure 1. SC35M polymerase activity in the presence of Bat or SC35M NP encoded from the phw2000 rescue plasmid. HEK293T
1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 Supplementary Figure 1. SC35M polymerase activity in the presence of Bat or SC35M NP encoded from the phw2000 rescue plasmid. HEK293T
T H E J O U R N A L O F C E L L B I O L O G Y
 T H E J O U R N A L O F C E L L B I O L O G Y Supplemental material Krenn et al., http://www.jcb.org/cgi/content/full/jcb.201110013/dc1 Figure S1. Levels of expressed proteins and demonstration that C-terminal
T H E J O U R N A L O F C E L L B I O L O G Y Supplemental material Krenn et al., http://www.jcb.org/cgi/content/full/jcb.201110013/dc1 Figure S1. Levels of expressed proteins and demonstration that C-terminal
A Hepatocyte Growth Factor Receptor (Met) Insulin Receptor hybrid governs hepatic glucose metabolism SUPPLEMENTARY FIGURES, LEGENDS AND METHODS
 A Hepatocyte Growth Factor Receptor (Met) Insulin Receptor hybrid governs hepatic glucose metabolism Arlee Fafalios, Jihong Ma, Xinping Tan, John Stoops, Jianhua Luo, Marie C. DeFrances and Reza Zarnegar
A Hepatocyte Growth Factor Receptor (Met) Insulin Receptor hybrid governs hepatic glucose metabolism Arlee Fafalios, Jihong Ma, Xinping Tan, John Stoops, Jianhua Luo, Marie C. DeFrances and Reza Zarnegar
Supplementary information
 Supplementary information Human Cytomegalovirus MicroRNA mir-us4-1 Inhibits CD8 + T Cell Response by Targeting ERAP1 Sungchul Kim, Sanghyun Lee, Jinwook Shin, Youngkyun Kim, Irini Evnouchidou, Donghyun
Supplementary information Human Cytomegalovirus MicroRNA mir-us4-1 Inhibits CD8 + T Cell Response by Targeting ERAP1 Sungchul Kim, Sanghyun Lee, Jinwook Shin, Youngkyun Kim, Irini Evnouchidou, Donghyun
Supplementary Figure 1
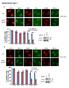 Supplementary Figure 1 a γ-h2ax MDC1 RNF8 FK2 BRCA1 U2OS Cells sgrna-1 ** 60 sgrna 40 20 0 % positive Cells (>5 foci per cell) b ** 80 sgrna sgrna γ-h2ax MDC1 γ-h2ax RNF8 FK2 MDC1 BRCA1 RNF8 FK2 BRCA1
Supplementary Figure 1 a γ-h2ax MDC1 RNF8 FK2 BRCA1 U2OS Cells sgrna-1 ** 60 sgrna 40 20 0 % positive Cells (>5 foci per cell) b ** 80 sgrna sgrna γ-h2ax MDC1 γ-h2ax RNF8 FK2 MDC1 BRCA1 RNF8 FK2 BRCA1
Supplementary Material
 Supplementary Material accompanying the manuscript Interleukin 37 is a fundamental inhibitor of innate immunity Marcel F Nold, Claudia A Nold-Petry, Jarod A Zepp, Brent E Palmer, Philip Bufler & Charles
Supplementary Material accompanying the manuscript Interleukin 37 is a fundamental inhibitor of innate immunity Marcel F Nold, Claudia A Nold-Petry, Jarod A Zepp, Brent E Palmer, Philip Bufler & Charles
Multiple Anti-Interferon Actions of the Influenza A Virus NS1 Protein
 JOURNAL OF VIROLOGY, July 2007, p. 7011 7021 Vol. 81, No. 13 0022-538X/07/$08.00 0 doi:10.1128/jvi.02581-06 Copyright 2007, American Society for Microbiology. All Rights Reserved. Multiple Anti-Interferon
JOURNAL OF VIROLOGY, July 2007, p. 7011 7021 Vol. 81, No. 13 0022-538X/07/$08.00 0 doi:10.1128/jvi.02581-06 Copyright 2007, American Society for Microbiology. All Rights Reserved. Multiple Anti-Interferon
EBV essential antigen EBNA3C attenuates H2AX expression
 JVI Accepts, published online ahead of print on 15 January 2014 J. Virol. doi:10.1128/jvi.03568-13 Copyright 2014, American Society for Microbiology. All Rights Reserved. 1 EBV essential antigen EBNA3C
JVI Accepts, published online ahead of print on 15 January 2014 J. Virol. doi:10.1128/jvi.03568-13 Copyright 2014, American Society for Microbiology. All Rights Reserved. 1 EBV essential antigen EBNA3C
Toll-like Receptor Signaling
 Toll-like Receptor Signaling 1 Professor of Medicine University of Massachusetts Medical School, Worcester, MA, USA Why do we need innate immunity? Pathogens multiply very fast We literally swim in viruses
Toll-like Receptor Signaling 1 Professor of Medicine University of Massachusetts Medical School, Worcester, MA, USA Why do we need innate immunity? Pathogens multiply very fast We literally swim in viruses
Antagonism of the Phosphatase PP1 by the Measles Virus V Protein Is Required for Innate Immune Escape of MDA5
 Article Antagonism of the Phosphatase PP1 by the Measles Virus V Protein Is Required for Innate Immune Escape of MDA5 Meredith E. Davis, 1,2 May K. Wang, 1,2 Linda J. Rennick, 3 Florian Full, 1 Sebastian
Article Antagonism of the Phosphatase PP1 by the Measles Virus V Protein Is Required for Innate Immune Escape of MDA5 Meredith E. Davis, 1,2 May K. Wang, 1,2 Linda J. Rennick, 3 Florian Full, 1 Sebastian
Supplementary Materials
 Supplementary Materials Supplementary Figure S1 Regulation of Ubl4A stability by its assembly partner A, The translation rate of Ubl4A is not affected in the absence of Bag6. Control, Bag6 and Ubl4A CRISPR
Supplementary Materials Supplementary Figure S1 Regulation of Ubl4A stability by its assembly partner A, The translation rate of Ubl4A is not affected in the absence of Bag6. Control, Bag6 and Ubl4A CRISPR
Sestrin2 and BNIP3 (Bcl-2/adenovirus E1B 19kDa-interacting. protein3) regulate autophagy and mitophagy in renal tubular cells in. acute kidney injury
 Sestrin2 and BNIP3 (Bcl-2/adenovirus E1B 19kDa-interacting protein3) regulate autophagy and mitophagy in renal tubular cells in acute kidney injury by Masayuki Ishihara 1, Madoka Urushido 2, Kazu Hamada
Sestrin2 and BNIP3 (Bcl-2/adenovirus E1B 19kDa-interacting protein3) regulate autophagy and mitophagy in renal tubular cells in acute kidney injury by Masayuki Ishihara 1, Madoka Urushido 2, Kazu Hamada
SUPPLEMENTARY INFORMATION
 DOI: 10.1038/ncb3076 Supplementary Figure 1 btrcp targets Cep68 for degradation during mitosis. a) Cep68 immunofluorescence in interphase and metaphase. U-2OS cells were transfected with control sirna
DOI: 10.1038/ncb3076 Supplementary Figure 1 btrcp targets Cep68 for degradation during mitosis. a) Cep68 immunofluorescence in interphase and metaphase. U-2OS cells were transfected with control sirna
Live cell imaging of trafficking of the chaperone complex vaccine to the ER. BMDCs were incubated with ER-Tracker Red (1 M) in staining solution for
 Live cell imaging of trafficking of the chaperone complex vaccine to the ER. BMDCs were incubated with ER-Tracker Red (1 M) in staining solution for 15 min at 37 C and replaced with fresh complete medium.
Live cell imaging of trafficking of the chaperone complex vaccine to the ER. BMDCs were incubated with ER-Tracker Red (1 M) in staining solution for 15 min at 37 C and replaced with fresh complete medium.
NLRC5 Negatively Regulates the NF-kB and Type I Interferon Signaling Pathways
 NLRC5 Negatively Regulates the NF-kB and Type I Interferon Signaling Pathways Jun Cui, 1,2,5 Liang Zhu, 1,3,5 Xiaojun Xia, 1,5 Helen Y. Wang, 1 Xavier Legras, 1 Jun Hong, 1 Jiabing Ji, 1 Pingping Shen,
NLRC5 Negatively Regulates the NF-kB and Type I Interferon Signaling Pathways Jun Cui, 1,2,5 Liang Zhu, 1,3,5 Xiaojun Xia, 1,5 Helen Y. Wang, 1 Xavier Legras, 1 Jun Hong, 1 Jiabing Ji, 1 Pingping Shen,
Supplementary Figure 1: si-craf but not si-braf sensitizes tumor cells to radiation.
 Supplementary Figure 1: si-craf but not si-braf sensitizes tumor cells to radiation. (a) Embryonic fibroblasts isolated from wildtype (WT), BRAF -/-, or CRAF -/- mice were irradiated (6 Gy) and DNA damage
Supplementary Figure 1: si-craf but not si-braf sensitizes tumor cells to radiation. (a) Embryonic fibroblasts isolated from wildtype (WT), BRAF -/-, or CRAF -/- mice were irradiated (6 Gy) and DNA damage
The functional investigation of the interaction between TATA-associated factor 3 (TAF3) and p53 protein
 THESIS BOOK The functional investigation of the interaction between TATA-associated factor 3 (TAF3) and p53 protein Orsolya Buzás-Bereczki Supervisors: Dr. Éva Bálint Dr. Imre Miklós Boros University of
THESIS BOOK The functional investigation of the interaction between TATA-associated factor 3 (TAF3) and p53 protein Orsolya Buzás-Bereczki Supervisors: Dr. Éva Bálint Dr. Imre Miklós Boros University of
Supporting Information
 Supporting Information Palmisano et al. 10.1073/pnas.1202174109 Fig. S1. Expression of different transgenes, driven by either viral or human promoters, is up-regulated by amino acid starvation. (A) Quantification
Supporting Information Palmisano et al. 10.1073/pnas.1202174109 Fig. S1. Expression of different transgenes, driven by either viral or human promoters, is up-regulated by amino acid starvation. (A) Quantification
HIV-1 Virus-like Particle Budding Assay Nathan H Vande Burgt, Luis J Cocka * and Paul Bates
 HIV-1 Virus-like Particle Budding Assay Nathan H Vande Burgt, Luis J Cocka * and Paul Bates Department of Microbiology, Perelman School of Medicine at the University of Pennsylvania, Philadelphia, USA
HIV-1 Virus-like Particle Budding Assay Nathan H Vande Burgt, Luis J Cocka * and Paul Bates Department of Microbiology, Perelman School of Medicine at the University of Pennsylvania, Philadelphia, USA
Construction of a hepatocellular carcinoma cell line that stably expresses stathmin with a Ser25 phosphorylation site mutation
 Construction of a hepatocellular carcinoma cell line that stably expresses stathmin with a Ser25 phosphorylation site mutation J. Du 1, Z.H. Tao 2, J. Li 2, Y.K. Liu 3 and L. Gan 2 1 Department of Chemistry,
Construction of a hepatocellular carcinoma cell line that stably expresses stathmin with a Ser25 phosphorylation site mutation J. Du 1, Z.H. Tao 2, J. Li 2, Y.K. Liu 3 and L. Gan 2 1 Department of Chemistry,
Nature Methods: doi: /nmeth Supplementary Figure 1
 Supplementary Figure 1 Subtiligase-catalyzed ligations with ubiquitin thioesters and 10-mer biotinylated peptides. (a) General scheme for ligations between ubiquitin thioesters and 10-mer, biotinylated
Supplementary Figure 1 Subtiligase-catalyzed ligations with ubiquitin thioesters and 10-mer biotinylated peptides. (a) General scheme for ligations between ubiquitin thioesters and 10-mer, biotinylated
The rabbit femoral artery was prepared and each arterial ring was permeabilized
 Online Supplement Nakmura et al. cgmp-dependent relaxation of smooth muscle Materials and Methods Measurement of tension The rabbit femoral artery was prepared and each arterial ring was permeabilized
Online Supplement Nakmura et al. cgmp-dependent relaxation of smooth muscle Materials and Methods Measurement of tension The rabbit femoral artery was prepared and each arterial ring was permeabilized
SUPPLEMENTARY INFORMATION
 SUPPLEMENTARY INFORMATION doi:10.1038/nature11429 S1a 6 7 8 9 Nlrc4 allele S1b Nlrc4 +/+ Nlrc4 +/F Nlrc4 F/F 9 Targeting construct 422 bp 273 bp FRT-neo-gb-PGK-FRT 3x.STOP S1c Nlrc4 +/+ Nlrc4 F/F casp1
SUPPLEMENTARY INFORMATION doi:10.1038/nature11429 S1a 6 7 8 9 Nlrc4 allele S1b Nlrc4 +/+ Nlrc4 +/F Nlrc4 F/F 9 Targeting construct 422 bp 273 bp FRT-neo-gb-PGK-FRT 3x.STOP S1c Nlrc4 +/+ Nlrc4 F/F casp1
