Intestine- specific expression of Apobec- 1 rescues apolipoprotein B. RNA editing and alters chylomicron production in Apobec1 / mice
|
|
|
- Derick Craig
- 5 years ago
- Views:
Transcription
1 Intestine- specific expression of Apobec- 1 rescues apolipoprotein B RNA editing and alters chylomicron production in Apobec1 / mice Valerie Blanc, Yan Xie, Jianyang Luo, Susan Kennedy and Nicholas O. Davidson* Department of Medicine, Washington University School of Medicine, St. Louis, MO *To whom communication should be addressed Division of Gastroenterology, Box 8124, Washington University School of Medicine St. Louis, 8/27/12 nod@wustl.edu Running title: Intestine- specific Apobec- 1 rescue
2 ABSTRACT (198 WORDS) Intestinal apolipoprotein B (apo B) mrna undergoes C- to- U editing, mediated by the catalytic deaminase apobec- 1, which results in translation of apob48. Apobec1 / mice produce only apob100 and secrete larger chylomicron particles than observed in wild type (WT) mice. Here we show that transgenic rescue of intestinal apobec- 1 expression (Apobec1 Int/O ) restores C- to- U RNA editing of apob mrna in vivo including the canonical site at position 6666 and also at approximately 20 other newly identified downstream sites present in WT mice. The small intestine of Apobec1 Int/O mice produces only apob48, while the liver produces only apob100. Serum chylomicron particles were smaller in Apobec1 Int/O mice, compared to those from Apobec1 / mice and the predominant fraction of serum apob48 in Apobec1 Int/O mice migrated in lipoproteins smaller than chylomicrons, even when these mice were fed a high fat diet. Since apob48 arises exclusively from the intestine in Apobec1 Int/O mice and intestinal apob48 synthesis and secretion rates were comparable to WT mice, we were able to infer the major sites of origin of serum apob48 in WT mice. Our findings imply that less than 25% of serum apob48 in WT mice arises from the intestine, with the majority originating from the liver. Supplementary Key words: RNA binding; Cytidine Deaminase; Hyperediting; apob48; lipoprotein assembly 2
3 The continued epidemic of obesity has focused attention on the metabolic pathways and key players that regulate dietary lipid absorption from the mammalian small intestine. Lipoprotein biogenesis and bulk lipid transport from enterocytes of the small intestine is coordinated through interactions between the structural protein apolipoprotein B (apob) and a resident endoplasmic reticulum (ER) chaperone, microsomal triglyceride transfer protein (Mttp), that together facilitate complex lipid delivery into the secretory pathway and orchestrate chylomicron assembly and secretion (reviewed in (1, 2)). Adult mammalian enterocytes synthesize and secrete a truncated apob isoform, apob48, produced as a result of post- transcriptional cytidine to uridine (C to U) RNA editing of the nascent nuclear apob transcript, via a heteromeric enzymatic complex that contains apobec- 1 (the catalytic deaminase) (3) and its obligate RNA binding subunit, apobec- 1 complementation factor (ACF) (4, 5). There is a tissue- specific pattern of apob isoform distribution, in which tissues that express apobec- 1 (such as the intestine) produce apob48 while tissues that express apob, but not apobec- 1 (such as the human liver) produce only apob100 (6). Mouse and rat liver express apobec- 1 and as a result synthesize and secrete both apob48 and apob100, but there is wide variation in the extent of C to U editing from tissues of other mammalian species (7). For all practical purposes, however, adult murine and human small intestine secretes virtually only apob48 (8). 3
4 Apobec1 / mice are unable to edit apob RNA in any tissue and as a result synthesize and secrete lipoproteins from both the liver and small intestine that contain exclusively apob100 (9, 10). Nascent intestinal lipoproteins isolated from Apobec1 / mice were larger than those from wild type mice and, as inferred from lymphatic transport studies, Apobec1 / mice secrete fewer triglyceride- rich particles (11). These findings imply a subtle functional advantage to intestinal apob48 (versus apob100) in terms of accommodating efficient lipid transport by optimizing chylomicron assembly (11). However, several features of mammalian intestinal apob RNA editing and the functional significance of apobec- 1 remain incompletely understood. Earlier studies indicated that transgenic overexpression of apobec- 1 caused C to U hyperediting at multiple sites (including the canonical nucleotide) in hepatic apob RNA both in vivo (12), and also in hepatoma cell lines (13). These findings raised questions of how C to U RNA editing at the canonical site is regulated physiologically and whether hyperediting might be demonstrated upon apobec- 1 overexpression in non- hepatic tissues (14, 15). In addition, as alluded to above, the importance of intestinal apob48 and its contribution to serum apob48 levels in the mouse has been difficult to discern, at least in part because mouse liver secretes both isoforms (7). Here we use a strategy of transgenic rescue to reveal unanticipated sites of C to U editing in intestinal apob RNA and to characterize apob- containing lipoproteins in mice expressing only apob48 in the small intestine. 4
5 METHODS Generation of transgenic animals. The coding region of human apobec- 1 was cloned into pgem- AclI- villin- EGFP- KpnI- BGHpolyA- AclI vector (a gift from Dr. J. Turner, University of Chicago). The 711 bp coding region was inserted into the unique KpnI site downstream of EGFP motif, resulting in the expression of a N- terminal EGFP- tagged human Apobec- 1. The expression of the transgene was under the control of a 9 kb villin promoter (16). This construct (Figure 1A) was microinjected into fertilized eggs from C57BL/6 X CBA mice. Apobec- 1 transgenic mice were then mated with C57BL/6 Apobec1 / mice to generate lines of Apobec- 1 Int/O animals expressing Apobec- 1 only in the intestine. Northern blot analysis and semi- quantitative PCR. Intestinal segments were flushed and scraped mucosa flash- frozen in liquid nitrogen for total RNA extraction. Total RNA (20 µg) prepared in 1X MOPS, 6.7% formaldehyde, 72 % formamide and 40 mg/ml ethidium bromide was subjected to denaturing formaldehyde/ 1.2 % agarose electrophoresis and transferred to Imobilon- NY+ membrane (Millipore, Billerica, MA). Membranes were pre- hybridized and hybridized in ExpressHyb hybridization solution (Clontech Laboratories, Inc Mountain View, CA) at 70 C when probing for apobec- 1 or 76 C when probing with GFP. Apobec- 1 and GFP probes (445 nt) generated by PCR were labeled by random priming using [ 32 P]- datp and Klenow (NEB Biolabs Inc. Ipswich, MA). Apobec- 1 probe was generated using the following primers: Fwd H/M34 5-5
6 CCCACTCTGAGGAGAAG- 3 and Rev H/M GGTAGTTGACGAAATTCCTCCAGCAG- 3. Washes were performed at room temperature for 40 minutes in 2X SSC, 0.05% SDS, followed by 2 washes at 50 C in 0.1X SSC, 0.1 % SDS. Membranes were exposed to Phosphorimager (SI, Molecular Dynamics, Sunnyvale, CA). Integrity and loading were monitored by visualization of ribosomal subunits. For semi- quantitative evaluation of human Apobec- 1 RNA expression total RNA from wild- type, Apobec- 1 - /-, Apobec- 1 Int/O high (h) and low (l) expressor animals was treated with DNase (Ambion, Foster City, CA) and used to synthesize cdnas. A 220- nt motif targeting exon 6 of Apobec- 1 (Supplemental Figure 1) was amplified using the following primers: Fwd H/M 64 Apobec- 1: 5 - GAGTTTGAC/AGTCTTCTA/TTGACCC- 3 ; Rev H/M 284 Apobec- 1: 5 - TCCCC/AGCAGGGACTCCAGGAC- 3. Semiquantitative RT- PCR was optimized (Supplemental Figure 2) and performed as follows: 94 C 2 min, 29 cycles [95 C 30 sec, 55 C 45 sec, 72 C 1 min], 72 C 10 min. PCR products were resolved on a 1 % agarose gel and quantitated using Kodak/Image Digital Science and Carestream Molecular Imaging System software. Apobec- 1 PCR products were normalized to Gapdh. Quantitative PCR was performed using individual sample (3-4 per genotype). For each sample, reactions were performed in triplicate on an ABI Prism 7000 sequence detection system using SYBR GreenER PCR Master Mix (Invitrogen, Grand Island, NY) and primers designed by Primer Express software (Applied Biosystems). Murine Apobec- 1- specific primers: Fwd: 5 - TGTTTATTTACATAGCACGGCTTTATC- 3 ; Rev : 5 - TGCTAATAAGGTCCCTGAGTCCTT- 3. Degenerate human Apobec- 1 primers: H/M Fwd: 5-6
7 AGAATC/TGAA/GCCCT/CG/AG/CGAGTTT- 3 ; H/M Rev: 5 - GATC/TTCA/GTAC/GAGCAGACAGGT/CCTCTT- 3. RNA levels are expressed as fold change compared to WT intestinal mrna, normalized to Gapdh RNA. Protein extraction, Western blot and histologic analysis. Scraped mucosa was homogenized in 0.5 ml of tissue lysis buffer containing 20 mm Tris PH 8; 0.15 M NaCl; 2 mm EDTA; 1 mm sodium vanadate; 0.1 M sodium fluoride; 50 mm beta- glycerophosphate; 5% glycerol, 2X protease Inhibitor (Roche Applied Science, Indianapolis, IN); 1% Triton; 0.1% SDS. Aliquots of homogenate (100 µg protein) were resolved by 10% SDS- PAGE, transferred to PVDF membrane and probed with anti- GFP antibody (Santa Cruz Biotechnology, Inc. Santa Cruz, CA). Equal loading was verified using anti- Hsp40 antibody (Assay Designs, Ann Arbor, MI). For detection of isoforms of apob, homogenates were resolved through a 4-15% gradient SDS- PAGE and probed with rabbit anti- murine apob IgG using antisera previously characterized in our laboratory (17). Histological analysis was conducted on formalin- fixed tissues (proximal, mid, distal intestine) in which 4- micron sections were stained with anti- GFP antibody (Santa Cruz Biotechnology Inc. Santa Cruz, CA). ApoB RNA editing analysis by primer extension and sequencing. RNA was isolated from scraped mucosa using TRIzol (Invitrogen, Grand Island, NY). DNAse- treated RNAs (1µg) were used for primer extension analysis of apob RNA editing as previously detailed (17). Sequence analysis of apob RNA hyperediting 7
8 was conducted on a 738- nt region spanning nucleotides 6508 to 7246, amplified by RT- PCR using total RNA isolated from scraped mucosa of WT, Apobec- 1 / and Apobec- 1 Int/O mice. PCR products were purified and subcloned into ppcr- Script SK(+) plasmid (Agilent Technologies / Stratagene, La Jolla, CA). Twenty individual clones were sequenced using Big Dye terminator mix. Sequences were aligned with SerialCloner 3-1 software. Enterocyte isolation and pulse chase analysis of apob secretion. Enterocytes were isolated using EDTA chelation from 4 individual mice per genotype and used for pulse- chase experiments as described (18). In brief, freshly isolated enterocytes were pulse labeled for 30 minutes with 250 µci [ 35 S]- protein labeling mix (Cat# NEG072 PerkinElmer, Boston, MA) and chased for 60 min in DMEM supplemented with 10 mm methionine and 5 mm cysteine. A mixed micellar lipid solution [0.4 mm sodium taurocholate, 0.54 mm sodium taurodeoxycholate, 0.3 mm phosphatidycholine, 0.45 mm oleic acid, 0.26 mm monoolein] was added to both pulse and chase medium for all the experiments (18). At the end of chase, medium and cells were collected and aliquots of lysate and medium immunoprecipitated with rabbit anti- mouse apob IgG. Quantitation of [ 35 S]- incorporation into apob48 was conducted using 4 15% SDS- PAGE gel separation of the immunoprecipitation reactions, which were then quantified using PhosphorImager analysis (Molecular Dynamics, Sunnydale,CA). Lipoprotein fractionation by fast pressure liquid chromatography (FPLC). 8
9 Animals were fed chow or a Western diet (Harlan Teklad #TD 88137, Madison, WI) as indicated for weeks before study. After an overnight fast animals were weighed and injected intravenously with Pluronic F127 (19), followed 30 minutes later by an intragastric bolus of 0.5 ml of a lipid mixture containing 100 µl corn oil and 400 µl 20% intralipid. Blood was collected before Pluronic F127 injection (time 0) and 4 hr after lipid gavage. Serum was recovered by centrifugation at 4000 rpm for 20 min at 4 C. Pooled serum (100 µl) was analyzed by FPLC using two Superose 6 columns connected in series (GE Healthcare Life Sciences, Pittsburgh, PA). Each FPLC run yielded 56 fractions of 500 µl each. Aliquots of each individual fraction (30 µl) were resolved by 4-15 % SDS- PAGE and the distribution of apob, apoa1, apoa4 and apoe was determined by Western blot. Cholesterol and triglyceride content in each fraction was determined enzymatically using Cholesterol E and L- Type Triglyceride M kits (Wako Chemicals, Richmond, VA). Electron microscopy Pooled serum (200 µl) collected 4 hr after lipid gavage was layered under 600 µl 0.15 M NaCl and centrifuged at 50,000 rpm for 30 min in the MLA- 130 rotor in a table- top ultracentrifuge (Beckman Instruments). Chylomicrons were collected and submitted to negative staining electron microscopy and visualized using a Zeiss 902 electron microscope. At the time of sacrifice (4 hr post lipid gavage), 2 mm segments of the jejunum were flushed with PBS followed by immersion in fixative solution containing 3% Glutaradehyde (EM Grade); 1% formaldehyde (EM Grade) in 0.1 M sodium cacodylate ph 7.4; 2 mm Cacl2; 2 mm MgCl2. Specimens were kept at 9
10 4 C in 1.5 ml fixative, stained in 2% aqueous uranyl acetate and viewed on a Hitachi H- 600 electron microscope (Hitachi High Technologies, Schaumburg, IL). Three animals per genotype were examined to obtain representative images. Statistical comparisons All data are shown as mean ± standard error (SE) unless otherwise noted. Statistical comparisons were made using GraphPad Prism 4.0 (GraphPad, San Diego, CA) with one way ANOVA for multiple group comparisons and t testing as a post- hoc test for comparing different pairs. A value of p< 0.05 was considered significant. 10
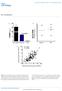
11 RESULTS Generation of Intestine only- Apobec- 1 (Apobec- 1 Int/O ) transgenic mice- Three founder transgenic lines were generated using the construct shown in Figure 1A. For the purposes of this report, we focused on two lines in which human apobec- 1 was expressed homogeneously in the small intestine at either relatively high or low levels (defined below) when compared to endogenous murine apobec- 1. The apobec- 1 intestinal transgenic founders were then crossed into the Apobec1 / line to generate lines of Apobec1 Int/O mice. Northern blot analysis revealed a strong signal only with the high expressor line (Figure 1B). The relative expression levels of transgenic apobec- 1 mrna were determined in comparison to endogenous murine apobec- 1 by semiquantitative PCR targeting a conserved region within murine exon 6 (Supplemental Figures 1, 2). These data indicate a range of ~5- fold overexpression of the high overexpressor line and ~2- fold overexpression with the low overexpressor line, both compared to WT mice (Supplemental Figure 2C). Quantitative RT- PCR using species- specific primers suggested a much greater range of overexpression within the lines (Supplemental Figure 2D). However, relative expression levels of the GFP- apobec- 1 fusion protein in extracts from these lines of mice (Figure 1C) indicated ~8- fold higher levels in the high expressor line compared to the low expressor line (Figure 1D), which was more in line with the semiquantitative RT- PCR data. Transgenic rescue in these lines was confirmed by immunohistochemical localization of the fusion protein, which indicated homogeneous expression in villus enterocytes in both nuclear and cytoplasmic compartments (Figure 1E). Electron micrographs of jejunal enterocytes from chow 11
12 fed mice revealed comparable morphology between WT, Apobec1 / and Apobec1 Int/O mice without changes in lipid droplet accumulation (Figure 1F). Other parameters of intestinal lipid metabolism are detailed in Table 1 and discussed in a later section. ApoB C to U RNA editing in the intestine of Apobec- 1 / mice- Intestinal RNA was examined by primer extension assay (17) to determine the extent of C to U editing of endogenous murine apob RNA at the canonical site. WT mice showed >90% C to U editing while Apobec1 / mice demonstrated no RNA editing, as expected (Figure 2A, B). Transgenic rescue of apobec- 1, in both lines of Apobec1 Int/O mice, also revealed >90% C to U editing at the canonical site indicating that the transgene is fully functional in regard to its physiological target (Figure 2A, B). We next asked if transgenic rescue of apobec- 1 was associated with changes in cytidine deamination of other targets in apob RNA, since prior studies in transgenic mouse and rabbit liver had indicated that overexpression of apobec- 1 induced promiscuous hyperediting (12, 20). We undertook Sanger sequencing of cdna clones from a 738 nt fragment spanning nt , including the region from nt examined in that earlier study (20). Our findings demonstrate that C to U RNA editing is detectable in many sites 3 to the canonical target at position 6666, and, importantly, these RNA modifications were found in both WT and Apobec1 Int/O mice (high expressor), (Figure 2C). The data show that the extent of C to U editing varies across the sites, with a maximum of 30% editing at 6987 and generally lower 12
13 levels (~10%) at other sites (Figure 2C). The findings reveal sites at which C to U RNA editing was found only in the Apobec1 Int/O mice (for example 6933, 6990, and 7187), but also sites in which C to U RNA editing was only found in the WT intestine (6998, 7036 and 7120). However, for the most part, there was concordance in the patterns observed between WT and Apobec1 Int/O mice. The observed pattern of C to U RNA editing of these additional sites does not appear to be processive (5 - >3 or 3 - >5 ) based on the finding that none of the sites appear linked to one another. One additional editing site (1/20 clones) was identified (6639) 5 to the canonical base, changing a CUC (Leu) to UUC (Phe), but all the other changes within the 738 nucleotides examined were found 3 to the canonical site, which was quantitatively edited. Whether additional sites for C to U RNA editing exist in exon 26 upstream of nucleotide 6508 or in other 5 exons remains to be determined, but we will return to this question in a later section. The findings in regard to the sites of hyperediting of intestinal apob RNA contrast with those demonstrated earlier for hepatic apob RNA in liver specific apobec- 1 transgenic mice (20), in which a major cluster of cytidine residues at positions and another cluster at were each observed to undergo C to U hyperediting. None of those sites were found to undergo C to U editing in mouse intestine either in WT or Apobec1 Int/O mice (Figure 2C). These findings strongly suggest that site selectivity of apob RNA modifications is influenced in a tissue- specific fashion. We checked whether targeting of these additional sites was mooring sequence- dependent (13, 20). When compared with the canonical 13
14 mooring sequence, we identified only one site (7017) with an almost perfect match with the consensus motif (Figure 2D). However, editing efficiency at this site (~10%) was similar to other sites (6933, 6990, 7085, 7187) with more mismatches to the mooring sequence suggesting that the presence of this motif is not a critical determinant of site selection (Figure 2D). We also examined the nearest neighbor nucleotide preference surrounding editing sites with frequency higher than 10%. The nucleotide immediately upstream of the targeted cytidine (position - 1) is an adenosine (70%) or guanosine (28%), compared to the immediate downstream nucleotides, adenosine or uridine (43% each) or guanosine (14%) (Figure 2E). These findings contrast with the nearest neighbor preferences identified for liver hyperediting in which the upstream preference was for thymidine (70%) or adenosine (30%) (20). The findings together suggest that sequence specific (contextual) elements interact with tissue- specific factors in the specificity of apobec- 1 mediated C to U RNA editing of apob. We also examined these patterns of C to U RNA editing in the low expressor line of Apobec1 Int/O mice since earlier work indicated a dose response for hyperediting in apobec- 1 transgenic liver (21, 22). Our findings demonstrate that low expressor Apobec1 Int/O mice exhibit reduced levels of hyperediting compared to both WT and the high expressor Apobec1 Int/O line with the dominant location again at the 6987 site (Figure 2F). 14
15 Taken together, the findings reveal an unanticipated array of targets for C to U RNA editing in WT murine intestine which is eliminated in Apobec1 / mice (data not shown) and largely restored in a dose dependent manner by transgenic rescue in Apobec1 Int/O mice. For the purposes of the functional characterization (detailed below), we used Apobec1 Int/O mice expressing high levels of the transgene. Intestinal apob48 production in Apobec1 Int/O mice- We examined apob isoform expression in mice of the indicated genotypes, which revealed that apob48 was the dominant intestinal isoform expressed in Apobec1 Int/O mice and that there were no other truncated species detectable either in serum or intestinal mucosa (Figure 3A, B). We next examined apob48 synthesis and secretion in isolated enterocytes from WT and Apobec1 Int/O mice, the findings demonstrating comparable synthesis and secretion rates with no differences in the overall recovery of apob (Figure 4 A- D). These findings demonstrate that intestine specific apobec- 1 transgenic expression rescues the patterns of synthesis and secretion of intestinal apob48 in Apobec1 Int/O mice. In addition, there was no discernable difference between WT and Apobec1 Int/O mice in terms of weight gain or lipid absorption (data not shown), intestinal or fasting serum cholesterol or triglyceride concentrations either on a chow or western diet (Table 1). These findings would be predicted based on the predominant pattern of intestinal apob48 production in WT mice. Serum apob isoform and lipoprotein distribution in Apobec1 Int/O mice- 15
16 We next turned our attention to the distribution pattern of apob isoforms in Apobec1 Int/O mice since these mice produce apob48 exclusively in the intestine and apob100 exclusively in the liver. Following Pluronic F127 administration, we found that the net (representing both hepatic plus intestinal) secretion rate of triglyceride rich lipoproteins was comparable, but with reduced abundance of apob48 in Apobec1 Int/O mice compared to WT 4h following a lipid bolus (Figure 5A, B and see also Figure 3B). The distribution of isoforms across the lipoprotein profile, revealed a subtle change in apob48 distribution by genotype. ApoB48 fractionated into chylomicron/vldl- sized particles in both WT and Apobec- 1 Int/O mice (Figure 5C, E), but apob48 distributed into a more homogeneous population of LDL particles in Apobec- 1 Int/O mice (fractions 24-28, Figure 5E) compared to WT mice, where apob48 appears in broad population of LDL- sized particles (fractions 18 to 29, Figure 5C). Triglyceride distribution was indistinguishable between the genotypes, almost exclusively within chylomicron and VLDL fractions, as expected (Figure 5 D, F). We next evaluated the size distribution of chylomicron and VLDL particles from the indicated genotypes using negative stain electron microscopy, which revealed a shift to a smaller population of particles in Apobec1 Int/O mice (Figure 6), again suggesting that intestinal lipoprotein production is altered upon rescue of intestinal apob48 production. The findings to this point suggest that there is a shift in the profile of intestinal lipoproteins in Apobec1 Int/O mice. We extended this characterization to mice fed a 16
17 high fat western diet (see Table 1 for lipid data) in which the FPLC profile again revealed that only a fraction of apob48 from Apobec1 Int/O mice distributes into chylomicron/vldl sized particles. (Figure 7 A- C). As noted above in chow fed mice, triglycerides distributed virtually exclusively in chylomicron and VLDL fractions. We also examined the distribution of apoe and apoa- 1, which revealed comparable distribution in chylomicron and VLDL fractions of both genotypes (Figure 7D). By contrast, apoe and apoa- 1 was found in the small LDL/large HDL size range in Apobec1 Int/O mice (Figure 7D, bracketed fractions), suggesting again a distinctive pattern of particle distribution in association with intestinal apob48 in these mice. Finally, because apob48 arises exclusively from the intestine of Apobec1 Int/O mice and since intestinal apob48 synthesis and secretion rates (as inferred from our in- vitro studies in isolated enterocytes, Figure 4, above) were comparable between the genotypes, we reasoned that it would be feasible to infer the contribution of intestinal apob48 production to serum apob48 levels in WT mice under conditions where clearance of intestinal and hepatic apob48 particles was eliminated, i.e. following Pluronic F127 administration. Accordingly, we constructed a semi- quantitative standard curve of serum apob48 in both chow (Figure 8A) and western diet fed mice (Figure 8B) using serial dilutions to obtain comparable apob48 abundance under each dietary condition. The findings (Figure 8 A, B) imply that the intestine contributes ~10-25% of total serum apob48, with the majority of apob48 production reflecting hepatic secretion. 17
18 DISCUSSION The key findings from this report demonstrate that transgenic expression of human apobec- 1 in the intestine of Apobec1 Int/O mice rescues apob RNA editing at the canonical site (6666), restores intestinal apob48 production and produces a shift in the spectrum of intestinal lipoproteins. The findings also reveal an unanticipated number of additional sites of C to U RNA editing of intestinal apob, including a newly identified cluster of sites which were present not only in Apobec1 Int/O mice where apobec- 1 was overexpressed, but also in WT animals. These features together raise important implications for our understanding of the regulation of posttranscriptional C to U RNA editing and the functional importance of apob RNA editing in the assembly and secretion of intestinal lipoproteins. Each of these features merit additional discussion. Considerable effort has been devoted to understanding the cis- acting elements and trans- acting factors that regulate C to U RNA editing of apob (12, 13, 20, 23). Historically, this work focused predominantly on C to U editing of synthetic apob RNA templates flanking the canonical site and indicated both nearest neighbor preferences (23, 24) and also distinct structural context requirements (25, 26). These RNA elements, together with regulated abundance of apobec- 1 and its auxiliary components including ACF, were viewed as sufficient and necessary to constrain C to U editing of apob to a physiological site at position 6666 (6). However, the finding that forced overexpression of apobec- 1 induced hyperediting 18
19 of apob RNA at multiple sites suggested that these constraints could be overcome (13, 20). The current findings illustrate several important contrasts from those earlier studies. First, almost none of the sites identified as targets of hyperediting in apobec- 1 transgenic mouse and rabbit liver apob RNA by Yamanaka et al (20) were found to be edited in intestinal apob RNA in Apobec1 Int/O mice. This includes the 6802 site, which was identified earlier in human intestinal cdna as an example of non- canonical C to U RNA editing (27). These findings demonstrate that target specificity for C to U RNA editing of apob is regulated in a tissue- specific manner and that distinctive factors constrain and regulate the extent and efficiency of hepatic versus intestinal apob RNA editing, particularly under conditions where apobec- 1 expression may be altered. The second important feature is that non- canonical sites for C to U editing, in particular the cluster of targets between , were found in both WT and Apobec1 Int/O mice albeit at lower efficiency (up to 30%) compared to the canonical site. These findings imply that C to U RNA editing of murine intestinal apob is not confined to a single site, even under circumstances where apobec- 1 is expressed at physiological levels. These findings contrast with earlier observations in murine liver, where none of the cdna clones spanning nt sequenced from endogenous (murine) or transgenic human apob were edited (with the exception of 6806) (20). It will be of interest to extend those findings and include the 3 cluster of C to U editing sites identified here in intestinal apob cdnas. Those studies are currently in progress and will be reported elsewhere. 19
20 The predominant overlap in hyperediting site selection and efficiency noted in WT and the high expressing Apobec1 Int/O mice suggest that simply overexpressing human (versus murine) apobec- 1 in murine intestine is unlikely to account for the findings. The observation that hyperediting of apob RNA involved fewer non- canonical sites in the low expressor line of Apobec1 Int/O mice (again largely overlapping sites observed in WT mice) further supports the conclusion that human and murine apobec- 1 exhibit similar functional characteristics in regard to murine intestinal apob RNA editing in vivo. These conclusions are broadly consistent with the findings from rabbit apobec- 1 transgenic overexpression in murine liver and from in- vitro studies in human and rat hepatoma cells (20, 24). However, recent findings using more sensitive amplification methods to detect C to U RNA editing events, have demonstrated that adenoviral expression of rat and human apobec- 1 in hepatoma cell lines exhibit different specificity for introducing hypermutations, suggesting that tissue- specific cofactor interactions may be an important consideration in understanding how site selection is regulated (28). It is also worth pointing out that virtually all the sites of C to U RNA editing were 3 of the canonical site and that the additional RNA editing events described occur in the 3 untranslated region (3 UTR) of the apob transcript. Recent findings using deep sequencing of intestinal RNA (RNA seq) from WT mice found 32 new (i.e. non- apob) targets of C to U editing, all of which were in the 3 UTR and all of which were eliminated in the intestine of Apobec1 / mice (29). These findings, taken together with the current results, indicate that there is a wider range of C to U editing targets 20
21 than previously thought and that these targets reside within the 3 UTR, raising questions regarding the functional impact of these modifications. It will be important to undertake a comprehensive analysis of the entire apob transcript from mice of the three genotypes examined in the current study to determine if there are additional sites of C to U editing beyond those identified in the region of As alluded to above, this analysis will extend to regions both 5 and 3 of the regions examined in the current report and those studies are currently in progress. However, RNA seq studies reported earlier indicated only a low level (up to 10%) of hyperediting of cytidine residues in apob RNA, downstream and in close proximity to the canonical site (6676 and 6708) (29). It is important to emphasize that Western blots of intestinal mucosal extracts and serum from Apobec1 Int/O mice each indicated only apob48 and no other truncated apob species, suggesting that there are no new functional translational termination codons created through alternative C to U RNA editing. That being said, it is possible that these additional RNA editing events within the 3 UTR of apob might influence other aspects of apob mrna biology including transport and translational efficiency. Those possibilities await formal examination. The current studies demonstrate that intestinal apob48 synthesis and secretion are restored in Apobec1 Int/O mice and reveal no obvious impact on the efficiency of lipid absorption. The findings indicate a subtle shift in apob48 distribution that includes a population of smaller chylomicron particles (identified by electron microscopy) whose significance remains to be elucidated. In addition, the findings demonstrate 21
22 that the distribution of apob48 in Apobec1 Int/O mice also includes LDL and HDL sized fractions. This observation raises the question of the functional relevance of apob48 within these fractions. It is worth noting that earlier studies in apob48- only versus apob100- only mice demonstrated that cholesterol distributed into predominantly HDL- sized particles while TG distributed predominantly into chylomicron/vldl sized particles (30). However, those studies left open the relative contribution of intestinal versus hepatic apob48 in the apob48- only mice. The current findings demonstrate that following a lipid bolus, virtually all the triglyceride in serum is transported in the apob48 containing fractions of chylomicron and VLDL size in both WT and Apobec1 Int/O mice. On the other hand, a large fraction of the apob48 in Apobec1 Int/O mice cofractionates with smaller particles, raising the possibility that these apob48 containing particles in the LDL and HDL range are underlipidated, precursor lipoprotein particles. This possibility will require further experimental validation but is broadly consistent with the two- step hypothesis for triglyceride- rich lipoprotein assembly and secretion as inferred from studies in the liver (31). The lipid cargo of these smaller, apob48- containing intestinal lipoproteins remains to be defined more completely but our findings (Figure 5E, 7C) indicate that they contain cholesterol. The possibility that intestinal lipid transport utilizes different secretory pathways (i.e. chylomicron and HDL) is consistent with other work examining intestinal vitamin E (32) and cholesterol secretion (33). 22
23 The strategy for generating Apobec1 Int/O mice also provided the opportunity to examine the contribution of intestinal apob48 production to the overall serum apob48 pool in WT mice. We used the assumption that since apob48 synthesis and secretion rates were similar, as inferred from studies in isolated enterocytes, the abundance of serum apob48 in the respective genotypes would reflect intestinal secretion under circumstances where apob uptake was eliminated. The findings suggest that under conditions of either chow or high fat western diet feeding, the intestinal contribution of apob48 in WT mice is only 10-25% of the total. In other words, under both dietary conditions, the majority of apob48 in WT mice originates from the liver. Following intake of a high fat western diet, the contribution of intestinal apob48 was lower than in chow fed mice, but these findings should be interpreted with caution since hepatic apob48 synthesis and secretion rates were not formally analyzed in these experiments. Nevertheless, the results suggest that the intestine accommodates chylomicron assembly and triglyceride mobilization through apob48 production and secretion of particles in the size range of chylomicrons, but in addition secretes a range of smaller particles containing apob48 whose lipid transport function and metabolic implications are yet to be fully understood. In summary, the findings from this report illustrate an unanticipated range of targets of C to U apob RNA editing, findings that extend discussion regarding the diversity of DNA versus RNA differences (34-37). Further questions, both in relation to C to U RNA editing as a biological process and in relation to apob48 23
24 assembly and secretion as a functional pathway for intestinal lipid export, are the focus of ongoing investigation. 24
25 ACKNOWLEDGEMENTS This work was supported by grants (HL , DK and Digestive Disease Research Core Center DK , particularly the ARAC, murine and morphology cores) to NOD. The authors are very grateful to all members of the Davidson lab for their input and to Dr. Steven Farber, Carnegie Institution for Science, Baltimore, for helpful suggestions and comments. 25
26 REFERENCES 1. Chen, Z., and Davidson, N.O Genetic Regulation of Intestinal Lipid Transport and Metabolism. Physiology of the Gastrointestinal Tract: Hussain, M.M., Rava, P., Walsh, M., Rana, M., and Iqbal, J Multiple functions of microsomal triglyceride transfer protein. Nutr Metab (Lond) 9: Teng, B., Burant, C.F., and Davidson, N.O Molecular cloning of an apolipoprotein B messenger RNA editing protein. Science 260: Mehta, A., Kinter, M.T., Sherman, N.E., and Driscoll, D.M Molecular cloning of apobec- 1 complementation factor, a novel RNA- binding protein involved in the editing of apolipoprotein B mrna. Mol Cell Biol 20: Lellek, H., Kirsten, R., Diehl, I., Apostel, F., Buck, F., and Greeve, J Purification and molecular cloning of a novel essential component of the apolipoprotein B mrna editing enzyme- complex. J Biol Chem 275: Blanc, V., and Davidson, N.O C- to- U RNA editing: mechanisms leading to genetic diversity. J Biol Chem 278: Greeve, J., Altkemper, I., Dieterich, J.H., Greten, H., and Windler, E Apolipoprotein B mrna editing in 12 different mammalian species: hepatic expression is reflected in low concentrations of apob- containing plasma lipoproteins. J Lipid Res 34: Powell, L.M., Wallis, S.C., Pease, R.J., Edwards, Y.H., Knott, T.J., and Scott, J A novel form of tissue- specific RNA processing produces apolipoprotein- B48 in intestine. Cell 50: Hirano, K., Young, S.G., Farese, R.V., Jr., Ng, J., Sande, E., Warburton, C., Powell- Braxton, L.M., and Davidson, N.O Targeted disruption of the mouse apobec- 1 gene abolishes apolipoprotein B mrna editing and eliminates apolipoprotein B48. J Biol Chem 271: Nakamuta, M., Chang, B.H., Zsigmond, E., Kobayashi, K., Lei, H., Ishida, B.Y., Oka, K., Li, E., and Chan, L Complete phenotypic characterization of apobec- 1 knockout mice with a wild- type genetic background and a human apolipoprotein B transgenic background, and restoration of apolipoprotein B mrna editing by somatic gene transfer of Apobec- 1. J Biol Chem 271: Lo, C.M., Nordskog, B.K., Nauli, A.M., Zheng, S., Vonlehmden, S.B., Yang, Q., Lee, D., Swift, L.L., Davidson, N.O., and Tso, P Why does the gut choose apolipoprotein B48 but not B100 for chylomicron formation? Am J Physiol Gastrointest Liver Physiol 294:G Yamanaka, S., Balestra, M.E., Ferrell, L.D., Fan, J., Arnold, K.S., Taylor, S., Taylor, J.M., and Innerarity, T.L Apolipoprotein B mrna- editing protein induces hepatocellular carcinoma and dysplasia in transgenic animals. Proc Natl Acad Sci U S A 92:
27 13. Sowden, M., Hamm, J.K., and Smith, H.C Overexpression of APOBEC- 1 results in mooring sequence- dependent promiscuous RNA editing. J Biol Chem 271: Siddiqui, J.F., Van Mater, D., Sowden, M.P., and Smith, H.C Disproportionate relationship between APOBEC- 1 expression and apolipoprotein B mrna editing activity. Exp Cell Res 252: Galloway, C.A., and Smith, H.C The expression of apob mrna editing factors is not the sole determinant for the induction of editing in differentiating Caco- 2 cells. Biochem Biophys Res Commun 391: Pinto, D., Robine, S., Jaisser, F., El Marjou, F.E., and Louvard, D Regulatory sequences of the mouse villin gene that efficiently drive transgenic expression in immature and differentiated epithelial cells of small and large intestines. J Biol Chem 274: Blanc, V., and Davidson, N.O Mouse and other rodent models of C to U RNA editing. Methods Mol Biol 718: Xie, Y., Nassir, F., Luo, J., Buhman, K., and Davidson, N.O Intestinal lipoprotein assembly in apobec- 1- /- mice reveals subtle alterations in triglyceride secretion coupled with a shift to larger lipoproteins. Am J Physiol Gastrointest Liver Physiol 285:G Millar, J.S., Cromley, D.A., McCoy, M.G., Rader, D.J., and Billheimer, J.T Determining hepatic triglyceride production in mice: comparison of poloxamer 407 with Triton WR J Lipid Res 46: Yamanaka, S., Poksay, K.S., Driscoll, D.M., and Innerarity, T.L Hyperediting of multiple cytidines of apolipoprotein B mrna by APOBEC- 1 requires auxiliary protein(s) but not a mooring sequence motif. J Biol Chem 271: Qian, X., Balestra, M.E., Yamanaka, S., Boren, J., Lee, I., and Innerarity, T.L Low expression of the apolipoprotein B mrna- editing transgene in mice reduces LDL levels but does not cause liver dysplasia or tumors. Arterioscler Thromb Vasc Biol 18: Hersberger, M., Patarroyo- White, S., Qian, X., Arnold, K.S., Rohrer, L., Balestra, M.E., and Innerarity, T.L Regulatable liver expression of the rabbit apolipoprotein B mrna- editing enzyme catalytic polypeptide 1 (APOBEC- 1) in mice lacking endogenous APOBEC- 1 leads to aberrant hyperediting. Biochem J 369: Sowden, M., Hamm, J.K., Spinelli, S., and Smith, H.C Determinants involved in regulating the proportion of edited apolipoprotein B RNAs. Rna 2: Sowden, M.P., Eagleton, M.J., and Smith, H.C Apolipoprotein B RNA sequence 3' of the mooring sequence and cellular sources of auxiliary factors determine the location and extent of promiscuous editing. Nucleic Acids Res 26: Backus, J.W., and Smith, H.C Three distinct RNA sequence elements are required for efficient apolipoprotein B (apob) RNA editing in vitro. Nucleic Acids Res 20:
28 26. Smith, H.C Apolipoprotein B mrna editing: the sequence to the event. Semin Cell Biol 4: Navaratnam, N., Patel, D., Shah, R.R., Greeve, J.C., Powell, L.M., Knott, T.J., and Scott, J An additional editing site is present in apolipoprotein B mrna. Nucleic Acids Res 19: Chen, Z., Eggerman, T.L., Bocharov, A.V., Baranova, I.N., Vishnyakova, T.G., Kurlander, R.J., Csako, G., and Patterson, A.P Hypermutation of ApoB mrna by rat APOBEC- 1 overexpression mimics APOBEC- 3 hypermutation. J Mol Biol 418: Rosenberg, B.R., Hamilton, C.E., Mwangi, M.M., Dewell, S., and Papavasiliou, F.N Transcriptome- wide sequencing reveals numerous APOBEC1 mrna- editing targets in transcript 3' UTRs. Nat Struct Mol Biol 18: Farese, R.V., Jr., Veniant, M.M., Cham, C.M., Flynn, L.M., Pierotti, V., Loring, J.F., Traber, M., Ruland, S., Stokowski, R.S., Huszar, D., et al Phenotypic analysis of mice expressing exclusively apolipoprotein B48 or apolipoprotein B100. Proc Natl Acad Sci U S A 93: Sundaram, M., and Yao, Z Recent progress in understanding protein and lipid factors affecting hepatic VLDL assembly and secretion. Nutr Metab (Lond) 7: Reboul, E., Trompier, D., Moussa, M., Klein, A., Landrier, J.F., Chimini, G., and Borel, P ATP- binding cassette transporter A1 is significantly involved in the intestinal absorption of alpha- and gamma- tocopherol but not in that of retinyl palmitate in mice. Am J Clin Nutr 89: Iqbal, J., and Hussain, M.M Evidence for multiple complementary pathways for efficient cholesterol absorption in mice. J Lipid Res 46: Li, M., Wang, I.X., Li, Y., Bruzel, A., Richards, A.L., Toung, J.M., and Cheung, V.G Widespread RNA and DNA sequence differences in the human transcriptome. Science 333: Pickrell, J.K., Gilad, Y., and Pritchard, J.K Comment on "Widespread RNA and DNA sequence differences in the human transcriptome". Science 335:1302; author reply Lin, W., Piskol, R., Tan, M.H., and Li, J.B Comment on "Widespread RNA and DNA sequence differences in the human transcriptome". Science 335:1302; author reply Gu, T., Buaas, F.W., Simons, A.K., Ackert- Bicknell, C.L., Braun, R.E., and Hibbs, M.A Canonical A- to- I and C- to- U RNA editing is enriched at 3'UTRs and microrna target sites in multiple mouse tissues. PLoS One 7:e
29 FIGURE LEGENDS Figure 1. Generation of Intestine only- Apobec- 1 (Apobec- 1 Int/O ) transgenic mice. A. Schematic of transgenic construct. Human apobec- 1 was cloned downstream of the coding sequence of green fluorescent protein (EGFP) and expressed as a GFP fusion. The expression of the transgene is under control of the Villin promoter (black box). B. Northern blot analysis. Total RNA was extracted from proximal scraped mucosa of individual WT, Apobec- 1 - /-, Apobec- 1 Int/O, low (L) and high expressor (H) mice was resolved by electrophoresis and hybridized with either a 445- nt 32 P- labeled DNA probe recognizing both murine and human apobec- 1 or a 445- nt 32 P- labeled DNA probe recognizing the GFP coding sequence. C. Western blot analysis of transgene expression. Equal amounts of protein extracted from scraped proximal mucosa processed from individual animals were resolved by SDS- PAGE and probed with anti- GFP antibody and Hsp40 for loading control. D. Histogram representing protein expression of GFP- Apobec- 1 relative to Hsp40. The high expressor line (Apobec- 1 Int/Oh ) shows ~8- fold higher level of transgene expression compared to the low expressor (Apobec- 1 Int/Ol ) line. E. Immunohistochemical analysis. Proximal, mid and distal sections of the small intestine from Apobec- 1 Int/O and Apobec- 1 / mice were stained with anti- GFP antibody. Note that transgene distributes between nucleus and cytoplasm of epithelial cells. F. Electron microscopy of small intestines harvested from chow- fed WT, Apobec- 1 / and Apobec- 1 Int/O 4 hr after lipid gavage pictures were analyzed per mouse using 3 mice per genotype. The arrows indicate lipid droplets. 29
30 Note that there is no significant difference in the size of lipid droplets between the 3 genotypes. Figure 2. Transgenic rescue of apobec- 1 in the intestine of Apobec- 1 / mice restores ApoB C to U RNA editing. A. Endogenous apob mrna editing was determined by primer extension. The relative mobility of the unedited (C) and edited (U) products is indicated on the left. B. Bar graph representation of % C to U editing for each group as mean ± SE. (n=3-5). C- D. Hyperediting of apob mrna analyzed by DNA sequencing. A 738- bp fragment ( nt) of apob mrna overlapping the C6666 canonical editing site was amplified by RT- PCR subcloned into ppcr- Script vector and sequenced. Twenty clones per genotype (from 2 mice per genotype) were analyzed. Targeted cytidines identified in Apobec- 1 Int/O clones are indicated by blue circles, aligned with the nucleotide position. Edited cytidines identified in WT clones are indicated with black circles, aligned with the nucleotide position. The bracket on the left side of the vertical panel indicates the region of apob mrna found by Innerarity and colleagues to be promiscuously edited in hepatic apobec- 1 transgenic mice (20). D. Alignment of a 14 nt sequence downstream of C to U editing sites with 10% editing identified in RNA from Apobec- 1 Int/Oh mice (see figure 2C). The sequences were compared to the apob RNA canonical mooring sequence shown on the top. W: A/U, R: A/G, Y: U/C. The edited C is indicated in bold font at the beginning of each sequence. The nucleotides matching the mooring sequence are indicated in bold/italic. Below is shown the frequency plot of nucleotides in the 10 nt motif containing matching residue aligned 30
31 with the consensus mooring sequence (blue box). E. Alignment and frequency plot of nearest neighbor nucleotides flanking each C to U RNA editing site. Positions are indicated relative to the targeted cytidine. Frequency plots were created using Weblogo software (Berkeley, CA). F. Hyperediting of intestinal apob RNA isolated from Apobec- 1 Int/O low expressor mice and analyzed by sequencing as in panel C. Edited cytidines identified in twenty independent clones are indicated by red circles. Figure 3. Intestinal and serum apob 48 protein expression is restored in Apobec- 1 Int/O mice. A. Protein from scraped proximal mucosa was resolved by 4-15% SDS- PAGE and probed with anti- apob and anti- GAPDH antibody. B. Serum from chow- fed mice was separated by 4-15% SDS- PAGE and analyzed with anti- apob antibody. Figure 4. ApoB 48 synthesis and secretion in Apobec- 1 Int/O and WT enterocytes. Enterocytes from WT and Apobec- 1 Int/O chow fed mice were pulse labeled for 30 min and chased for 30 to 60 minutes. Lysates and media were collected at the indicated times. [ 35 S]- ApoB isoforms were immunoprecipitated from cell lysates and media, separated by SDS- PAGE and quantitated by PhosphorImager (A- D). [ 35 S]- ApoB 48 is expressed as a percentage of the initial label incorporated at the beginning of the chase. Four separate experiments were performed for each genotype using enterocytes isolated from 4 individual animals. Data are represented as mean ± SE. 31
32 Figure 5. Serum apob 48 is distributed in smaller LDL and HDL- size particles in Apobec- 1 Int/O mice. Following an overnight fast, chow fed Apobec- 1 Int/O and WT mice were administered Pluronic F127 and received an intragastric bolus of lipid. Serum was collected before (time 0) and 4 hr after gavage. A. 2 µl of serum were separated by 4-15% SDS- PAGE and probed with apob antibody. B. Serum triglyceride levels were evaluated enzymatically at indicated times. Data represent mean ± SE from 3 WT and 6 Apobec- 1 Int/O mice. (C and E). Pooled serum from 3 WT and 6 Apobec- 1 Int/O animals, collected 4 hr after lipid gavage were fractionated by FPLC and 56 (500 µl) fractions collected. Aliquots of individual fractions were separated by SDS- PAGE and probed with apob antibody. This is a representative of two separate experiments. Cholesterol (C and E) and triglycerides (D and F) content was enzymatically determined in individual fractions. The reactive bands migrating faster than apob48 are non- specific. Figure 6. Electron microscopy of serum chylomicrons. Chylomicrons (density <1.006) were isolated from pooled serum collected 4 hr after Pluronic F127 injection and lipid gavage and submitted for negative staining electron microscopy. The histograms (A- C) representing the size distribution were generated by measuring 400 particles per sample. Representative images from 3 animals per genotype are shown (D, WT; E, Apobec- 1 / ; F, Apobec- 1 Int/O ). 32
33 Figure 7. Serum lipoprotein distribution of apob 48 in Western diet- fed Apobec- 1 Int/O and WT mice. Animals were fed a Western diet for 12 weeks and overnight fasted mice received Pluronic 127 followed by an intragastric lipid bolus. Animals were sacrificed 4 hr later. A. Serum was collected and 2 µl was separated by SDS- PAGE and analyzed with apob antibody. (B- C). Pooled serum from 5 WT and 6 Apobec- 1 Int/O mice were fractionated by FPLC and individual fractions were resolved by SDS- PAGE. Distribution of apob 48 was determined by Western blot using anti- apob antibody. Cholesterol and triglycerides content were determined enzymatically. Note that HDL- size particles from Apobec- 1 Int/O mice are enriched in apob 48 compared to WT controls. D. Individual FPLC fractions were resolved by SDS- PAGE and lipoprotein distribution of apoe, apoa4 and apoa1 was analyzed by Western blot. Note that small LDL- size particles are enriched in apoe and apoai in Apobec- 1 Int/O mice as indicated by the horizontal brackets underlining fractions 24 and 26. Figure 8. Relative contribution of liver and intestine to serum apob 48. Pooled serum collected 4 hr following lipid gavage from chow- fed (A) and Western diet- fed animals (B) were diluted 1:4 to 1:128 (A) and 1:2 to 1:32 in PBS (B). One µl of diluted serum was resolved by 4-15% SDS- PAGE and probed using anti- apob antibody. ApoB48 band intensity was quantitated using Kodak/Image Digital Science and the Carestream Molecular Imaging System software. Band intensities from WT apob48 were used to create a standard curve. ApoB48 band intensity within the WT standard curve was used to evaluate the relative contribution of 33
34 intestine to apob48 production. As example in panel A, the intensity of the band from Apobec- 1 Int/O serum diluted 1:16 represents 25% of the band intensity from WT serum of the same dilution. 34
35 Table 1. Basic physiological parameters in WT and Apobec- 1 Int/O fed chow or western diet. Chow Western diet Body Weight (g) WT 24.4 ± ± 1.9 Apobec- 1 Int/O 21.8 ± ± 1.6 Serum Cholesterol (mg/dl) WT 79 ± ± 6 Apobec- 1 Int/O 79 ± ± 19 Serum Triglycerides (mg/dl) WT 65 ± ± 1 Apobec- 1 Int/O 52 ± 7 11 ± 4 Intestinal Cholesterol (µg/mg protein) WT 64 ± ± 6 Apobec- 1 Int/O 52 ± 9 68 ± 12 Intestinal Triglycerides (µg/mg protein) WT 118 ± ± 104 Apobec- 1 Int/O 141 ± ± 121 n= 3 WT and 6 Apobec- 1 Int/O on Chow diet. n = 5 WT and 6 Apobec- 1 Int/O on Western diet. Data represent mean ± SE. No significant difference was observed between genotypes for any parameter. 35
36
37
38
39
40
41
42
43
44
45
46
47
Mark P. Sowden 1,2, Matthew J. Eagleton 3 and Harold C. Smith 1,2,4, * Nucleic Acids Research, 1998, Vol. 26, No. 7
 1644 1652 Nucleic Acids Research, 1998, Vol. 26, No. 7 1998 Oxford University Press Apolipoprotein B RNA sequence 3 of the mooring sequence and cellular sources of auxiliary factors determine the location
1644 1652 Nucleic Acids Research, 1998, Vol. 26, No. 7 1998 Oxford University Press Apolipoprotein B RNA sequence 3 of the mooring sequence and cellular sources of auxiliary factors determine the location
Intestinal lipoprotein assembly in apobec-1 / mice reveals subtle alterations in triglyceride secretion coupled with a shift to larger lipoproteins
 Am J Physiol Gastrointest Liver Physiol 285: G735 G746, 2003. First published June 19, 2003; 10.1152/ajpgi.00202.2003. Intestinal lipoprotein assembly in apobec-1 / mice reveals subtle alterations in triglyceride
Am J Physiol Gastrointest Liver Physiol 285: G735 G746, 2003. First published June 19, 2003; 10.1152/ajpgi.00202.2003. Intestinal lipoprotein assembly in apobec-1 / mice reveals subtle alterations in triglyceride
SUPPLEMENTARY INFORMATION
 SUPPLEMENTARY INFORMATION FOR Liver X Receptor α mediates hepatic triglyceride accumulation through upregulation of G0/G1 Switch Gene 2 (G0S2) expression I: SUPPLEMENTARY METHODS II: SUPPLEMENTARY FIGURES
SUPPLEMENTARY INFORMATION FOR Liver X Receptor α mediates hepatic triglyceride accumulation through upregulation of G0/G1 Switch Gene 2 (G0S2) expression I: SUPPLEMENTARY METHODS II: SUPPLEMENTARY FIGURES
RNA interference induced hepatotoxicity results from loss of the first synthesized isoform of microrna-122 in mice
 SUPPLEMENTARY INFORMATION RNA interference induced hepatotoxicity results from loss of the first synthesized isoform of microrna-122 in mice Paul N Valdmanis, Shuo Gu, Kirk Chu, Lan Jin, Feijie Zhang,
SUPPLEMENTARY INFORMATION RNA interference induced hepatotoxicity results from loss of the first synthesized isoform of microrna-122 in mice Paul N Valdmanis, Shuo Gu, Kirk Chu, Lan Jin, Feijie Zhang,
Online Appendix Material and Methods: Pancreatic RNA isolation and quantitative real-time (q)rt-pcr. Mice were fasted overnight and killed 1 hour (h)
 Online Appendix Material and Methods: Pancreatic RNA isolation and quantitative real-time (q)rt-pcr. Mice were fasted overnight and killed 1 hour (h) after feeding. A small slice (~5-1 mm 3 ) was taken
Online Appendix Material and Methods: Pancreatic RNA isolation and quantitative real-time (q)rt-pcr. Mice were fasted overnight and killed 1 hour (h) after feeding. A small slice (~5-1 mm 3 ) was taken
MicroRNA sponges: competitive inhibitors of small RNAs in mammalian cells
 MicroRNA sponges: competitive inhibitors of small RNAs in mammalian cells Margaret S Ebert, Joel R Neilson & Phillip A Sharp Supplementary figures and text: Supplementary Figure 1. Effect of sponges on
MicroRNA sponges: competitive inhibitors of small RNAs in mammalian cells Margaret S Ebert, Joel R Neilson & Phillip A Sharp Supplementary figures and text: Supplementary Figure 1. Effect of sponges on
(a) Significant biological processes (upper panel) and disease biomarkers (lower panel)
 Supplementary Figure 1. Functional enrichment analyses of secretomic proteins. (a) Significant biological processes (upper panel) and disease biomarkers (lower panel) 2 involved by hrab37-mediated secretory
Supplementary Figure 1. Functional enrichment analyses of secretomic proteins. (a) Significant biological processes (upper panel) and disease biomarkers (lower panel) 2 involved by hrab37-mediated secretory
Supplemental Figure 1 ELISA scheme to measure plasma total, mature and furin-cleaved
 1 Supplemental Figure Legends Supplemental Figure 1 ELISA scheme to measure plasma total, mature and furin-cleaved PCSK9 concentrations. 4 Plasma mature and furin-cleaved PCSK9s were measured by a sandwich
1 Supplemental Figure Legends Supplemental Figure 1 ELISA scheme to measure plasma total, mature and furin-cleaved PCSK9 concentrations. 4 Plasma mature and furin-cleaved PCSK9s were measured by a sandwich
Supplementary Figure 1: Hsp60 / IEC mice are embryonically lethal (A) Light microscopic pictures show mouse embryos at developmental stage E12.
 Supplementary Figure 1: Hsp60 / IEC mice are embryonically lethal (A) Light microscopic pictures show mouse embryos at developmental stage E12.5 and E13.5 prepared from uteri of dams and subsequently genotyped.
Supplementary Figure 1: Hsp60 / IEC mice are embryonically lethal (A) Light microscopic pictures show mouse embryos at developmental stage E12.5 and E13.5 prepared from uteri of dams and subsequently genotyped.
Free Glycerol Assay Kit (Colorimetric)
 Product Manual Free Glycerol Assay Kit (Colorimetric) Catalog Number STA-398 100 assays FOR RESEARCH USE ONLY Not for use in diagnostic procedures Introduction Glycerol is the backbone of Triglycerides
Product Manual Free Glycerol Assay Kit (Colorimetric) Catalog Number STA-398 100 assays FOR RESEARCH USE ONLY Not for use in diagnostic procedures Introduction Glycerol is the backbone of Triglycerides
Expression of acid base transporters in the kidney collecting duct in Slc2a7 -/-
 Supplemental Material Results. Expression of acid base transporters in the kidney collecting duct in Slc2a7 -/- and Slc2a7 -/- mice. The expression of AE1 in the kidney was examined in Slc26a7 KO mice.
Supplemental Material Results. Expression of acid base transporters in the kidney collecting duct in Slc2a7 -/- and Slc2a7 -/- mice. The expression of AE1 in the kidney was examined in Slc26a7 KO mice.
Supplemental Data Tamoxifen administration to Vil-Scap- mice.
 Supplemental Data FIGURE S1. Tamoxifen administration to Vil-Scap - mice. In the experiments shown in Fig. 1 to Fig. 5, tamoxifen (2 mg per dose) was dissolved in corn oil and administered by orogastric
Supplemental Data FIGURE S1. Tamoxifen administration to Vil-Scap - mice. In the experiments shown in Fig. 1 to Fig. 5, tamoxifen (2 mg per dose) was dissolved in corn oil and administered by orogastric
TFEB-mediated increase in peripheral lysosomes regulates. Store Operated Calcium Entry
 TFEB-mediated increase in peripheral lysosomes regulates Store Operated Calcium Entry Luigi Sbano, Massimo Bonora, Saverio Marchi, Federica Baldassari, Diego L. Medina, Andrea Ballabio, Carlotta Giorgi
TFEB-mediated increase in peripheral lysosomes regulates Store Operated Calcium Entry Luigi Sbano, Massimo Bonora, Saverio Marchi, Federica Baldassari, Diego L. Medina, Andrea Ballabio, Carlotta Giorgi
Two Distinct TATA-less Promoters Direct Tissue-specific Expression of the Rat Apo-B Editing Catalytic Polypeptide 1 Gene*
 THE JOURNAL OF BIOLOGICAL CHEMISTRY Vol. 272, No. 29, Issue of July 18, pp. 18060 18070, 1997 1997 by The American Society for Biochemistry and Molecular Biology, Inc. Printed in U.S.A. Two Distinct TATA-less
THE JOURNAL OF BIOLOGICAL CHEMISTRY Vol. 272, No. 29, Issue of July 18, pp. 18060 18070, 1997 1997 by The American Society for Biochemistry and Molecular Biology, Inc. Printed in U.S.A. Two Distinct TATA-less
Materials and Methods , The two-hybrid principle.
 The enzymatic activity of an unknown protein which cleaves the phosphodiester bond between the tyrosine residue of a viral protein and the 5 terminus of the picornavirus RNA Introduction Every day there
The enzymatic activity of an unknown protein which cleaves the phosphodiester bond between the tyrosine residue of a viral protein and the 5 terminus of the picornavirus RNA Introduction Every day there
SUPPLEMENTARY INFORMATION
 doi:10.1038/nature12652 Supplementary Figure 1. PRDM16 interacts with endogenous EHMT1 in brown adipocytes. Immunoprecipitation of PRDM16 complex by flag antibody (M2) followed by Western blot analysis
doi:10.1038/nature12652 Supplementary Figure 1. PRDM16 interacts with endogenous EHMT1 in brown adipocytes. Immunoprecipitation of PRDM16 complex by flag antibody (M2) followed by Western blot analysis
The subcortical maternal complex controls symmetric division of mouse zygotes by
 The subcortical maternal complex controls symmetric division of mouse zygotes by regulating F-actin dynamics Xing-Jiang Yu 1,2, Zhaohong Yi 1, Zheng Gao 1,2, Dan-dan Qin 1,2, Yanhua Zhai 1, Xue Chen 1,
The subcortical maternal complex controls symmetric division of mouse zygotes by regulating F-actin dynamics Xing-Jiang Yu 1,2, Zhaohong Yi 1, Zheng Gao 1,2, Dan-dan Qin 1,2, Yanhua Zhai 1, Xue Chen 1,
A Hepatocyte Growth Factor Receptor (Met) Insulin Receptor hybrid governs hepatic glucose metabolism SUPPLEMENTARY FIGURES, LEGENDS AND METHODS
 A Hepatocyte Growth Factor Receptor (Met) Insulin Receptor hybrid governs hepatic glucose metabolism Arlee Fafalios, Jihong Ma, Xinping Tan, John Stoops, Jianhua Luo, Marie C. DeFrances and Reza Zarnegar
A Hepatocyte Growth Factor Receptor (Met) Insulin Receptor hybrid governs hepatic glucose metabolism Arlee Fafalios, Jihong Ma, Xinping Tan, John Stoops, Jianhua Luo, Marie C. DeFrances and Reza Zarnegar
Table S1. Quantitative RT-PCR primers
 Table S1. Quantitative RT-PCR primers Gene Forward Primer Reverse Primer Human ApoB gcaagcagaagccagaagta ccatttggagaagcagtttgg Human ApoA1 gaaagctgcggtgctgac agtggccaggtccttcact Human MTP acggccattcccattgtg
Table S1. Quantitative RT-PCR primers Gene Forward Primer Reverse Primer Human ApoB gcaagcagaagccagaagta ccatttggagaagcagtttgg Human ApoA1 gaaagctgcggtgctgac agtggccaggtccttcact Human MTP acggccattcccattgtg
Supplementary Figure 1: Additional metabolic parameters of obesity mouse models and controls. (a) Body weight, (b) blood glucose and (c) insulin
 Supplementary Figure 1: Additional metabolic parameters of obesity mouse models and controls. (a) Body weight, (b) blood glucose and (c) insulin resistance index of homeostatic model assessment (HOMA IR)
Supplementary Figure 1: Additional metabolic parameters of obesity mouse models and controls. (a) Body weight, (b) blood glucose and (c) insulin resistance index of homeostatic model assessment (HOMA IR)
(A) PCR primers (arrows) designed to distinguish wild type (P1+P2), targeted (P1+P2) and excised (P1+P3)14-
 1 Supplemental Figure Legends Figure S1. Mammary tumors of ErbB2 KI mice with 14-3-3σ ablation have elevated ErbB2 transcript levels and cell proliferation (A) PCR primers (arrows) designed to distinguish
1 Supplemental Figure Legends Figure S1. Mammary tumors of ErbB2 KI mice with 14-3-3σ ablation have elevated ErbB2 transcript levels and cell proliferation (A) PCR primers (arrows) designed to distinguish
Supplemental Figure S1. Expression of Cirbp mrna in mouse tissues and NIH3T3 cells.
 SUPPLEMENTAL FIGURE AND TABLE LEGENDS Supplemental Figure S1. Expression of Cirbp mrna in mouse tissues and NIH3T3 cells. A) Cirbp mrna expression levels in various mouse tissues collected around the clock
SUPPLEMENTAL FIGURE AND TABLE LEGENDS Supplemental Figure S1. Expression of Cirbp mrna in mouse tissues and NIH3T3 cells. A) Cirbp mrna expression levels in various mouse tissues collected around the clock
Analysis of small RNAs from Drosophila Schneider cells using the Small RNA assay on the Agilent 2100 bioanalyzer. Application Note
 Analysis of small RNAs from Drosophila Schneider cells using the Small RNA assay on the Agilent 2100 bioanalyzer Application Note Odile Sismeiro, Jean-Yves Coppée, Christophe Antoniewski, and Hélène Thomassin
Analysis of small RNAs from Drosophila Schneider cells using the Small RNA assay on the Agilent 2100 bioanalyzer Application Note Odile Sismeiro, Jean-Yves Coppée, Christophe Antoniewski, and Hélène Thomassin
Males- Western Diet WT KO Age (wks) Females- Western Diet WT KO Age (wks)
 Relative Arv1 mrna Adrenal 33.48 +/- 6.2 Skeletal Muscle 22.4 +/- 4.93 Liver 6.41 +/- 1.48 Heart 5.1 +/- 2.3 Brain 4.98 +/- 2.11 Ovary 4.68 +/- 2.21 Kidney 3.98 +/-.39 Lung 2.15 +/-.6 Inguinal Subcutaneous
Relative Arv1 mrna Adrenal 33.48 +/- 6.2 Skeletal Muscle 22.4 +/- 4.93 Liver 6.41 +/- 1.48 Heart 5.1 +/- 2.3 Brain 4.98 +/- 2.11 Ovary 4.68 +/- 2.21 Kidney 3.98 +/-.39 Lung 2.15 +/-.6 Inguinal Subcutaneous
Islet viability assay and Glucose Stimulated Insulin Secretion assay RT-PCR and Western Blot
 Islet viability assay and Glucose Stimulated Insulin Secretion assay Islet cell viability was determined by colorimetric (3-(4,5-dimethylthiazol-2-yl)-2,5- diphenyltetrazolium bromide assay using CellTiter
Islet viability assay and Glucose Stimulated Insulin Secretion assay Islet cell viability was determined by colorimetric (3-(4,5-dimethylthiazol-2-yl)-2,5- diphenyltetrazolium bromide assay using CellTiter
Pair-fed % inkt cells 0.5. EtOH 0.0
 MATERIALS AND METHODS Histopathological analysis Liver tissue was collected 9 h post-gavage, and the tissue samples were fixed in 1% formalin and paraffin-embedded following a standard procedure. The embedded
MATERIALS AND METHODS Histopathological analysis Liver tissue was collected 9 h post-gavage, and the tissue samples were fixed in 1% formalin and paraffin-embedded following a standard procedure. The embedded
Soft Agar Assay. For each cell pool, 100,000 cells were resuspended in 0.35% (w/v)
 SUPPLEMENTARY MATERIAL AND METHODS Soft Agar Assay. For each cell pool, 100,000 cells were resuspended in 0.35% (w/v) top agar (LONZA, SeaKem LE Agarose cat.5004) and plated onto 0.5% (w/v) basal agar.
SUPPLEMENTARY MATERIAL AND METHODS Soft Agar Assay. For each cell pool, 100,000 cells were resuspended in 0.35% (w/v) top agar (LONZA, SeaKem LE Agarose cat.5004) and plated onto 0.5% (w/v) basal agar.
MicroRNAs, RNA Modifications, RNA Editing. Bora E. Baysal MD, PhD Oncology for Scientists Lecture Tue, Oct 17, 2017, 3:30 PM - 5:00 PM
 MicroRNAs, RNA Modifications, RNA Editing Bora E. Baysal MD, PhD Oncology for Scientists Lecture Tue, Oct 17, 2017, 3:30 PM - 5:00 PM Expanding world of RNAs mrna, messenger RNA (~20,000) trna, transfer
MicroRNAs, RNA Modifications, RNA Editing Bora E. Baysal MD, PhD Oncology for Scientists Lecture Tue, Oct 17, 2017, 3:30 PM - 5:00 PM Expanding world of RNAs mrna, messenger RNA (~20,000) trna, transfer
Supplementary Information Titles Journal: Nature Medicine
 Supplementary Information Titles Journal: Nature Medicine Article Title: Corresponding Author: Supplementary Item & Number Supplementary Fig.1 Fig.2 Fig.3 Fig.4 Fig.5 Fig.6 Fig.7 Fig.8 Fig.9 Fig. Fig.11
Supplementary Information Titles Journal: Nature Medicine Article Title: Corresponding Author: Supplementary Item & Number Supplementary Fig.1 Fig.2 Fig.3 Fig.4 Fig.5 Fig.6 Fig.7 Fig.8 Fig.9 Fig. Fig.11
Serum Triglyceride Quantification Kit (Colorimetric)
 Product Manual Serum Triglyceride Quantification Kit (Colorimetric) Catalog Number STA-396 100 assays FOR RESEARCH USE ONLY Not for use in diagnostic procedures Introduction Triglycerides (TAG) are a type
Product Manual Serum Triglyceride Quantification Kit (Colorimetric) Catalog Number STA-396 100 assays FOR RESEARCH USE ONLY Not for use in diagnostic procedures Introduction Triglycerides (TAG) are a type
Luminescent platforms for monitoring changes in the solubility of amylin and huntingtin in living cells
 Electronic Supplementary Material (ESI) for Molecular BioSystems. This journal is The Royal Society of Chemistry 2016 Contents Supporting Information Luminescent platforms for monitoring changes in the
Electronic Supplementary Material (ESI) for Molecular BioSystems. This journal is The Royal Society of Chemistry 2016 Contents Supporting Information Luminescent platforms for monitoring changes in the
Supplementary Figure S1. Venn diagram analysis of mrna microarray data and mirna target analysis. (a) Western blot analysis of T lymphoblasts (CLS)
 Supplementary Figure S1. Venn diagram analysis of mrna microarray data and mirna target analysis. (a) Western blot analysis of T lymphoblasts (CLS) and their exosomes (EXO) in resting (REST) and activated
Supplementary Figure S1. Venn diagram analysis of mrna microarray data and mirna target analysis. (a) Western blot analysis of T lymphoblasts (CLS) and their exosomes (EXO) in resting (REST) and activated
Mechanisms of alternative splicing regulation
 Mechanisms of alternative splicing regulation The number of mechanisms that are known to be involved in splicing regulation approximates the number of splicing decisions that have been analyzed in detail.
Mechanisms of alternative splicing regulation The number of mechanisms that are known to be involved in splicing regulation approximates the number of splicing decisions that have been analyzed in detail.
Product Manual. Omni-Array Sense Strand mrna Amplification Kit, 2 ng to 100 ng Version Catalog No.: Reactions
 Genetic Tools and Reagents Universal mrna amplification, sense strand amplification, antisense amplification, cdna synthesis, micro arrays, gene expression, human, mouse, rat, guinea pig, cloning Omni-Array
Genetic Tools and Reagents Universal mrna amplification, sense strand amplification, antisense amplification, cdna synthesis, micro arrays, gene expression, human, mouse, rat, guinea pig, cloning Omni-Array
BMP6 treatment compensates for the molecular defect and ameliorates hemochromatosis in Hfe knockout mice
 SUPPLEMENTARY MATERIALS BMP6 treatment compensates for the molecular defect and ameliorates hemochromatosis in Hfe knockout mice Elena Corradini, Paul J. Schmidt, Delphine Meynard, Cinzia Garuti, Giuliana
SUPPLEMENTARY MATERIALS BMP6 treatment compensates for the molecular defect and ameliorates hemochromatosis in Hfe knockout mice Elena Corradini, Paul J. Schmidt, Delphine Meynard, Cinzia Garuti, Giuliana
(Stratagene, La Jolla, CA) (Supplemental Fig. 1A). A 5.4-kb EcoRI fragment
 SUPPLEMENTAL INFORMATION Supplemental Methods Generation of RyR2-S2808D Mice Murine genomic RyR2 clones were isolated from a 129/SvEvTacfBR λ-phage library (Stratagene, La Jolla, CA) (Supplemental Fig.
SUPPLEMENTAL INFORMATION Supplemental Methods Generation of RyR2-S2808D Mice Murine genomic RyR2 clones were isolated from a 129/SvEvTacfBR λ-phage library (Stratagene, La Jolla, CA) (Supplemental Fig.
SUPPLEMENTARY INFORMATION
 DOI: 10.1038/ncb3461 In the format provided by the authors and unedited. Supplementary Figure 1 (associated to Figure 1). Cpeb4 gene-targeted mice develop liver steatosis. a, Immunoblot displaying CPEB4
DOI: 10.1038/ncb3461 In the format provided by the authors and unedited. Supplementary Figure 1 (associated to Figure 1). Cpeb4 gene-targeted mice develop liver steatosis. a, Immunoblot displaying CPEB4
The clathrin adaptor Numb regulates intestinal cholesterol. absorption through dynamic interaction with NPC1L1
 The clathrin adaptor Numb regulates intestinal cholesterol absorption through dynamic interaction with NPC1L1 Pei-Shan Li 1, Zhen-Yan Fu 1,2, Ying-Yu Zhang 1, Jin-Hui Zhang 1, Chen-Qi Xu 1, Yi-Tong Ma
The clathrin adaptor Numb regulates intestinal cholesterol absorption through dynamic interaction with NPC1L1 Pei-Shan Li 1, Zhen-Yan Fu 1,2, Ying-Yu Zhang 1, Jin-Hui Zhang 1, Chen-Qi Xu 1, Yi-Tong Ma
ANSC/NUTR 618 LIPIDS & LIPID METABOLISM Lipoprotein Metabolism
 ANSC/NUTR 618 LIPIDS & LIPID METABOLISM Lipoprotein Metabolism I. Chylomicrons (exogenous pathway) A. 83% triacylglycerol, 2% protein, 8% cholesterol plus cholesterol esters, 7% phospholipid (esp. phosphatidylcholine)
ANSC/NUTR 618 LIPIDS & LIPID METABOLISM Lipoprotein Metabolism I. Chylomicrons (exogenous pathway) A. 83% triacylglycerol, 2% protein, 8% cholesterol plus cholesterol esters, 7% phospholipid (esp. phosphatidylcholine)
SUPPLEMENTAL DATA RESULTS
 SUPPLEMENTAL DATA RESULTS Ded1-mediated ribosomal scanning is less leaky than scanning promoted by eifs 4A/4B/4F. The efficiency of leaky scanning in the presence of Ded1 or of eifs 4A/4B/4F was investigated
SUPPLEMENTAL DATA RESULTS Ded1-mediated ribosomal scanning is less leaky than scanning promoted by eifs 4A/4B/4F. The efficiency of leaky scanning in the presence of Ded1 or of eifs 4A/4B/4F was investigated
Online Data Supplement. Anti-aging Gene Klotho Enhances Glucose-induced Insulin Secretion by Upregulating Plasma Membrane Retention of TRPV2
 Online Data Supplement Anti-aging Gene Klotho Enhances Glucose-induced Insulin Secretion by Upregulating Plasma Membrane Retention of TRPV2 Yi Lin and Zhongjie Sun Department of physiology, college of
Online Data Supplement Anti-aging Gene Klotho Enhances Glucose-induced Insulin Secretion by Upregulating Plasma Membrane Retention of TRPV2 Yi Lin and Zhongjie Sun Department of physiology, college of
Doctor of Philosophy
 Regulation of Gene Expression of the 25-Hydroxyvitamin D la-hydroxylase (CYP27BI) Promoter: Study of A Transgenic Mouse Model Ivanka Hendrix School of Molecular and Biomedical Science The University of
Regulation of Gene Expression of the 25-Hydroxyvitamin D la-hydroxylase (CYP27BI) Promoter: Study of A Transgenic Mouse Model Ivanka Hendrix School of Molecular and Biomedical Science The University of
Supplementary Materials for
 www.sciencesignaling.org/cgi/content/full/8/407/ra127/dc1 Supplementary Materials for Loss of FTO in adipose tissue decreases Angptl4 translation and alters triglyceride metabolism Chao-Yung Wang,* Shian-Sen
www.sciencesignaling.org/cgi/content/full/8/407/ra127/dc1 Supplementary Materials for Loss of FTO in adipose tissue decreases Angptl4 translation and alters triglyceride metabolism Chao-Yung Wang,* Shian-Sen
Fig. S1. REGN1500 reduces plasma levels of cholesterol, TG and NEFA in WT and Ldlr -/- mice. (A) WT
 Figure Legends for Supplementary Figures. Fig. S1. REGN15 reduces plasma levels of cholesterol, TG and NEF in WT and Ldlr -/- mice. () WT and Ldlr -/- mice were injected with control IgG or REGN15 (1 mg/kg)
Figure Legends for Supplementary Figures. Fig. S1. REGN15 reduces plasma levels of cholesterol, TG and NEF in WT and Ldlr -/- mice. () WT and Ldlr -/- mice were injected with control IgG or REGN15 (1 mg/kg)
Effec<ve Use of PI3K and MEK Inhibitors to Treat Mutant K Ras G12D and PIK3CA H1047R Murine Lung Cancers
 Effec
Effec
A complete next-generation sequencing workfl ow for circulating cell-free DNA isolation and analysis
 APPLICATION NOTE Cell-Free DNA Isolation Kit A complete next-generation sequencing workfl ow for circulating cell-free DNA isolation and analysis Abstract Circulating cell-free DNA (cfdna) has been shown
APPLICATION NOTE Cell-Free DNA Isolation Kit A complete next-generation sequencing workfl ow for circulating cell-free DNA isolation and analysis Abstract Circulating cell-free DNA (cfdna) has been shown
LDL/VLDL Purification Kit (Ultracentrifugation Free)
 Product Manual LDL/VLDL Purification Kit (Ultracentrifugation Free) Catalog Number STA- 606 10 preps FOR RESEARCH USE ONLY Not for use in diagnostic procedures Introduction Lipoproteins are submicroscopic
Product Manual LDL/VLDL Purification Kit (Ultracentrifugation Free) Catalog Number STA- 606 10 preps FOR RESEARCH USE ONLY Not for use in diagnostic procedures Introduction Lipoproteins are submicroscopic
 DOI: 10.1038/ncb2210 b. ICAM1 ng ml -1 P = 0.0001 Small RNA (15-30nts) ng ml -1 Cell Lysate Exosome HDL Plasma HDL Normal Human HDL mirnas R = 0.45 P < 0.0001 Normal Human Exosome mirnas Figure S1. Characterization
DOI: 10.1038/ncb2210 b. ICAM1 ng ml -1 P = 0.0001 Small RNA (15-30nts) ng ml -1 Cell Lysate Exosome HDL Plasma HDL Normal Human HDL mirnas R = 0.45 P < 0.0001 Normal Human Exosome mirnas Figure S1. Characterization
Cells and reagents. Synaptopodin knockdown (1) and dynamin knockdown (2)
 Supplemental Methods Cells and reagents. Synaptopodin knockdown (1) and dynamin knockdown (2) podocytes were cultured as described previously. Staurosporine, angiotensin II and actinomycin D were all obtained
Supplemental Methods Cells and reagents. Synaptopodin knockdown (1) and dynamin knockdown (2) podocytes were cultured as described previously. Staurosporine, angiotensin II and actinomycin D were all obtained
Antibodies: LB1 buffer For 50 ml For 10ml For 30 ml Final 1 M HEPES, ph 2.5 ml 0.5 ml 1.5 ml 50mM. 5 M NaCl 1.4 ml 280 µl 0.
 Experiment: Date: Tissue: Purpose: ChIP-Seq Antibodies: 11x cross-link buffer: Regent Stock Solution Final Vol for 10 ml of 11xstock concentration 5 M NaCl 0.1M 0.2 ml 0.5 M EDTA 1 mm 20 ul 0.5 M EGTA,
Experiment: Date: Tissue: Purpose: ChIP-Seq Antibodies: 11x cross-link buffer: Regent Stock Solution Final Vol for 10 ml of 11xstock concentration 5 M NaCl 0.1M 0.2 ml 0.5 M EDTA 1 mm 20 ul 0.5 M EGTA,
HEK293FT cells were transiently transfected with reporters, N3-ICD construct and
 Supplementary Information Luciferase reporter assay HEK293FT cells were transiently transfected with reporters, N3-ICD construct and increased amounts of wild type or kinase inactive EGFR. Transfections
Supplementary Information Luciferase reporter assay HEK293FT cells were transiently transfected with reporters, N3-ICD construct and increased amounts of wild type or kinase inactive EGFR. Transfections
Supplemental Information. Increased 4E-BP1 Expression Protects. against Diet-Induced Obesity and Insulin. Resistance in Male Mice
 Cell Reports, Volume 16 Supplemental Information Increased 4E-BP1 Expression Protects against Diet-Induced Obesity and Insulin Resistance in Male Mice Shih-Yin Tsai, Ariana A. Rodriguez, Somasish G. Dastidar,
Cell Reports, Volume 16 Supplemental Information Increased 4E-BP1 Expression Protects against Diet-Induced Obesity and Insulin Resistance in Male Mice Shih-Yin Tsai, Ariana A. Rodriguez, Somasish G. Dastidar,
Mammalian Membrane Protein Extraction Kit
 Mammalian Membrane Protein Extraction Kit Catalog number: AR0155 Boster s Mammalian Membrane Protein Extraction Kit is a simple, rapid and reproducible method to prepare cellular protein fractions highly
Mammalian Membrane Protein Extraction Kit Catalog number: AR0155 Boster s Mammalian Membrane Protein Extraction Kit is a simple, rapid and reproducible method to prepare cellular protein fractions highly
Supplementary Information
 Supplementary Information Supplementary Figures and Figure Legends Supplementary Figure 1 A. HDL-C (mg/dl) 14 12 1 8 6 4 2 Noncarriers HDL Cholesterol **** Carriers B. apoa-i (mg/dl) 325 3 275 25 225 2
Supplementary Information Supplementary Figures and Figure Legends Supplementary Figure 1 A. HDL-C (mg/dl) 14 12 1 8 6 4 2 Noncarriers HDL Cholesterol **** Carriers B. apoa-i (mg/dl) 325 3 275 25 225 2
SUPPLEMENTAL MATERIALS AND METHODS. Puromycin-synchronized metabolic labelling - Transfected HepG2 cells were depleted of
 SUPPLEMENTAL MATERIALS AND METHODS Puromycin-synchronized metabolic labelling - Transfected HepG2 cells were depleted of cysteine and methionine and then treated with 10 μm puromycin in depletion medium
SUPPLEMENTAL MATERIALS AND METHODS Puromycin-synchronized metabolic labelling - Transfected HepG2 cells were depleted of cysteine and methionine and then treated with 10 μm puromycin in depletion medium
Life Sciences 1A Midterm Exam 2. November 13, 2006
 Name: TF: Section Time Life Sciences 1A Midterm Exam 2 November 13, 2006 Please write legibly in the space provided below each question. You may not use calculators on this exam. We prefer that you use
Name: TF: Section Time Life Sciences 1A Midterm Exam 2 November 13, 2006 Please write legibly in the space provided below each question. You may not use calculators on this exam. We prefer that you use
Supplementary information
 Supplementary information Human Cytomegalovirus MicroRNA mir-us4-1 Inhibits CD8 + T Cell Response by Targeting ERAP1 Sungchul Kim, Sanghyun Lee, Jinwook Shin, Youngkyun Kim, Irini Evnouchidou, Donghyun
Supplementary information Human Cytomegalovirus MicroRNA mir-us4-1 Inhibits CD8 + T Cell Response by Targeting ERAP1 Sungchul Kim, Sanghyun Lee, Jinwook Shin, Youngkyun Kim, Irini Evnouchidou, Donghyun
Unit IV Problem 3 Biochemistry: Cholesterol Metabolism and Lipoproteins
 Unit IV Problem 3 Biochemistry: Cholesterol Metabolism and Lipoproteins - Cholesterol: It is a sterol which is found in all eukaryotic cells and contains an oxygen (as a hydroxyl group OH) on Carbon number
Unit IV Problem 3 Biochemistry: Cholesterol Metabolism and Lipoproteins - Cholesterol: It is a sterol which is found in all eukaryotic cells and contains an oxygen (as a hydroxyl group OH) on Carbon number
Supplementary Figure 1 Role of Raf-1 in TLR2-Dectin-1-mediated cytokine expression
 Supplementary Figure 1 Supplementary Figure 1 Role of Raf-1 in TLR2-Dectin-1-mediated cytokine expression. Quantitative real-time PCR of indicated mrnas in DCs stimulated with TLR2-Dectin-1 agonist zymosan
Supplementary Figure 1 Supplementary Figure 1 Role of Raf-1 in TLR2-Dectin-1-mediated cytokine expression. Quantitative real-time PCR of indicated mrnas in DCs stimulated with TLR2-Dectin-1 agonist zymosan
Figure S1. Generation of inducible PTEN deficient mice and the BMMCs (A) B6.129 Pten loxp/loxp mice were mated with B6.
 Figure S1. Generation of inducible PTEN deficient mice and the BMMCs (A) B6.129 Pten loxp/loxp mice were mated with B6.129-Gt(ROSA)26Sor tm1(cre/ert2)tyj /J mice. To induce deletion of the Pten locus,
Figure S1. Generation of inducible PTEN deficient mice and the BMMCs (A) B6.129 Pten loxp/loxp mice were mated with B6.129-Gt(ROSA)26Sor tm1(cre/ert2)tyj /J mice. To induce deletion of the Pten locus,
Supplemental Materials and Methods Plasmids and viruses Quantitative Reverse Transcription PCR Generation of molecular standard for quantitative PCR
 Supplemental Materials and Methods Plasmids and viruses To generate pseudotyped viruses, the previously described recombinant plasmids pnl4-3-δnef-gfp or pnl4-3-δ6-drgfp and a vector expressing HIV-1 X4
Supplemental Materials and Methods Plasmids and viruses To generate pseudotyped viruses, the previously described recombinant plasmids pnl4-3-δnef-gfp or pnl4-3-δ6-drgfp and a vector expressing HIV-1 X4
Supplementary Material and Methods
 Online Supplement Kockx et al, Secretion of Apolipoprotein E from Macrophages 1 Supplementary Material and Methods Cloning of ApoE-GFP Full-length human apoe3 cdna (pcdna3.1/zeo + -apoe) was kindly provided
Online Supplement Kockx et al, Secretion of Apolipoprotein E from Macrophages 1 Supplementary Material and Methods Cloning of ApoE-GFP Full-length human apoe3 cdna (pcdna3.1/zeo + -apoe) was kindly provided
General Laboratory methods Plasma analysis: Gene Expression Analysis: Immunoblot analysis: Immunohistochemistry:
 General Laboratory methods Plasma analysis: Plasma insulin (Mercodia, Sweden), leptin (duoset, R&D Systems Europe, Abingdon, United Kingdom), IL-6, TNFα and adiponectin levels (Quantikine kits, R&D Systems
General Laboratory methods Plasma analysis: Plasma insulin (Mercodia, Sweden), leptin (duoset, R&D Systems Europe, Abingdon, United Kingdom), IL-6, TNFα and adiponectin levels (Quantikine kits, R&D Systems
Supplementary data Supplementary Figure 1 Supplementary Figure 2
 Supplementary data Supplementary Figure 1 SPHK1 sirna increases RANKL-induced osteoclastogenesis in RAW264.7 cell culture. (A) RAW264.7 cells were transfected with oligocassettes containing SPHK1 sirna
Supplementary data Supplementary Figure 1 SPHK1 sirna increases RANKL-induced osteoclastogenesis in RAW264.7 cell culture. (A) RAW264.7 cells were transfected with oligocassettes containing SPHK1 sirna
Lipoprotein Clearance Mechanisms in LDL Receptor Deficient Apo-B48-only and Apo-B100-only Mice
 Lipoprotein Clearance Mechanisms in LDL Receptor Deficient Apo-B48-only and Apo-B100-only Mice Murielle M. Véniant,* Constance H. Zlot,* Rosemary L. Walzem, Vincenzo Pierotti,* Robert Driscoll,* David
Lipoprotein Clearance Mechanisms in LDL Receptor Deficient Apo-B48-only and Apo-B100-only Mice Murielle M. Véniant,* Constance H. Zlot,* Rosemary L. Walzem, Vincenzo Pierotti,* Robert Driscoll,* David
Protocol for Gene Transfection & Western Blotting
 The schedule and the manual of basic techniques for cell culture Advanced Protocol for Gene Transfection & Western Blotting Schedule Day 1 26/07/2008 Transfection Day 3 28/07/2008 Cell lysis Immunoprecipitation
The schedule and the manual of basic techniques for cell culture Advanced Protocol for Gene Transfection & Western Blotting Schedule Day 1 26/07/2008 Transfection Day 3 28/07/2008 Cell lysis Immunoprecipitation
Selective depletion of abundant RNAs to enable transcriptome analysis of lowinput and highly-degraded RNA from FFPE breast cancer samples
 DNA CLONING DNA AMPLIFICATION & PCR EPIGENETICS RNA ANALYSIS Selective depletion of abundant RNAs to enable transcriptome analysis of lowinput and highly-degraded RNA from FFPE breast cancer samples LIBRARY
DNA CLONING DNA AMPLIFICATION & PCR EPIGENETICS RNA ANALYSIS Selective depletion of abundant RNAs to enable transcriptome analysis of lowinput and highly-degraded RNA from FFPE breast cancer samples LIBRARY
Chapter 4. Estrogen receptor expression in human macrophages
 Chapter 4 Estrogen receptor expression in human macrophages 4.1. Introduction Macrophages respond to estrogen present in their microenvironment and hence should express functional estrogen receptors unless
Chapter 4 Estrogen receptor expression in human macrophages 4.1. Introduction Macrophages respond to estrogen present in their microenvironment and hence should express functional estrogen receptors unless
The Interaction of Alcohol and Iron-Overload in the in-vivo Regulation of Iron Responsive Genes
 Cantaurus, Vol. 5, -, May 7 McPherson College Division of Science and Technology The Interaction of Alcohol and Iron-Overload in the in-vivo Regulation of Iron Responsive Genes Callie Crist, Elizabeth
Cantaurus, Vol. 5, -, May 7 McPherson College Division of Science and Technology The Interaction of Alcohol and Iron-Overload in the in-vivo Regulation of Iron Responsive Genes Callie Crist, Elizabeth
Supplementary Figure 1.TRIM33 binds β-catenin in the nucleus. a & b, Co-IP of endogenous TRIM33 with β-catenin in HT-29 cells (a) and HEK 293T cells
 Supplementary Figure 1.TRIM33 binds β-catenin in the nucleus. a & b, Co-IP of endogenous TRIM33 with β-catenin in HT-29 cells (a) and HEK 293T cells (b). TRIM33 was immunoprecipitated, and the amount of
Supplementary Figure 1.TRIM33 binds β-catenin in the nucleus. a & b, Co-IP of endogenous TRIM33 with β-catenin in HT-29 cells (a) and HEK 293T cells (b). TRIM33 was immunoprecipitated, and the amount of
Supplementary Figure 1. AdipoR1 silencing and overexpression controls. (a) Representative blots (upper and lower panels) showing the AdipoR1 protein
 Supplementary Figure 1. AdipoR1 silencing and overexpression controls. (a) Representative blots (upper and lower panels) showing the AdipoR1 protein content relative to GAPDH in two independent experiments.
Supplementary Figure 1. AdipoR1 silencing and overexpression controls. (a) Representative blots (upper and lower panels) showing the AdipoR1 protein content relative to GAPDH in two independent experiments.
GPR120 *** * * Liver BAT iwat ewat mwat Ileum Colon. UCP1 mrna ***
 a GPR120 GPR120 mrna/ppia mrna Arbitrary Units 150 100 50 Liver BAT iwat ewat mwat Ileum Colon b UCP1 mrna Fold induction 20 15 10 5 - camp camp SB202190 - - - H89 - - - - - GW7647 Supplementary Figure
a GPR120 GPR120 mrna/ppia mrna Arbitrary Units 150 100 50 Liver BAT iwat ewat mwat Ileum Colon b UCP1 mrna Fold induction 20 15 10 5 - camp camp SB202190 - - - H89 - - - - - GW7647 Supplementary Figure
Supplementary Appendix
 Supplementary Appendix This appendix has been provided by the authors to give readers additional information about their work. Supplement to: Choi YL, Soda M, Yamashita Y, et al. EML4-ALK mutations in
Supplementary Appendix This appendix has been provided by the authors to give readers additional information about their work. Supplement to: Choi YL, Soda M, Yamashita Y, et al. EML4-ALK mutations in
review Genetically modified mice for the study of apolipoprotein B Edward Kim 1 and Stephen G. Young
 review Genetically modified mice for the study of apolipoprotein B Edward Kim 1 and Stephen G. Young Gladstone Institute of Cardiovascular Disease, San Francisco, CA 94141-9100, and Cardiovascular Research
review Genetically modified mice for the study of apolipoprotein B Edward Kim 1 and Stephen G. Young Gladstone Institute of Cardiovascular Disease, San Francisco, CA 94141-9100, and Cardiovascular Research
Supplementary Information
 Supplementary Information Supplementary Figure 1. CD4 + T cell activation and lack of apoptosis after crosslinking with anti-cd3 + anti-cd28 + anti-cd160. (a) Flow cytometry of anti-cd160 (5D.10A11) binding
Supplementary Information Supplementary Figure 1. CD4 + T cell activation and lack of apoptosis after crosslinking with anti-cd3 + anti-cd28 + anti-cd160. (a) Flow cytometry of anti-cd160 (5D.10A11) binding
行政院國家科學委員會補助專題研究計畫成果報告
 NSC892314B002270 898 1 907 31 9010 23 1 Molecular Study of Type III Hyperlipoproteinemia in Taiwan β β ε E Abstract β Type III hyperlipoproteinemia (type III HLP; familial dysbetalipoproteinemia ) is a
NSC892314B002270 898 1 907 31 9010 23 1 Molecular Study of Type III Hyperlipoproteinemia in Taiwan β β ε E Abstract β Type III hyperlipoproteinemia (type III HLP; familial dysbetalipoproteinemia ) is a
Chapter VIII: Dr. Sameh Sarray Hlaoui
 Chapter VIII: Dr. Sameh Sarray Hlaoui Lipoproteins a Lipids are insoluble in plasma. In order to be transported they are combined with specific proteins to form lipoproteins: Clusters of proteins and lipids.
Chapter VIII: Dr. Sameh Sarray Hlaoui Lipoproteins a Lipids are insoluble in plasma. In order to be transported they are combined with specific proteins to form lipoproteins: Clusters of proteins and lipids.
2.5. AMPK activity
 Supplement Fig. A 3 B phos-ampk 2.5 * Control AICAR AMPK AMPK activity (Absorbance at 45 nm) 2.5.5 Control AICAR Supplement Fig. Effects of AICAR on AMPK activation in macrophages. J774. macrophages were
Supplement Fig. A 3 B phos-ampk 2.5 * Control AICAR AMPK AMPK activity (Absorbance at 45 nm) 2.5.5 Control AICAR Supplement Fig. Effects of AICAR on AMPK activation in macrophages. J774. macrophages were
Genome-editing via Oviductal Nucleic Acids Delivery (GONAD) system: a novel microinjection-independent genome engineering method in mice
 Supplementary Information Genome-editing via Oviductal Nucleic Acids Delivery (GONAD) system: a novel microinjection-independent genome engineering method in mice Gou Takahashi, Channabasavaiah B Gurumurthy,
Supplementary Information Genome-editing via Oviductal Nucleic Acids Delivery (GONAD) system: a novel microinjection-independent genome engineering method in mice Gou Takahashi, Channabasavaiah B Gurumurthy,
Single Cell Quantitative Polymer Chain Reaction (sc-qpcr)
 Single Cell Quantitative Polymer Chain Reaction (sc-qpcr) Analyzing gene expression profiles from a bulk population of cells provides an average profile which may obscure important biological differences
Single Cell Quantitative Polymer Chain Reaction (sc-qpcr) Analyzing gene expression profiles from a bulk population of cells provides an average profile which may obscure important biological differences
HDL Purification Kit (Ultracentrifugation Free)
 Product Manual HDL Purification Kit (Ultracentrifugation Free) Catalog Number STA- 607 10 preps FOR RESEARCH USE ONLY Not for use in diagnostic procedures Introduction Lipoproteins are submicroscopic particles
Product Manual HDL Purification Kit (Ultracentrifugation Free) Catalog Number STA- 607 10 preps FOR RESEARCH USE ONLY Not for use in diagnostic procedures Introduction Lipoproteins are submicroscopic particles
Figure S1 Time-dependent down-modulation of HER3 by EZN No Treatment. EZN-3920, 2 μm. Time, h
 Figure S1 Time-dependent down-modulation of HER3 by EZN-392 HE ER3 mrna A, %Contr rol 12 No Treatment EZN-392, 2 μm 1 8 6 4 2 2 8 24 Time, h Figure S2. Specific target down-modulation by HER3 (EZN-392)
Figure S1 Time-dependent down-modulation of HER3 by EZN-392 HE ER3 mrna A, %Contr rol 12 No Treatment EZN-392, 2 μm 1 8 6 4 2 2 8 24 Time, h Figure S2. Specific target down-modulation by HER3 (EZN-392)
SUPPLEMENTARY INFORMATION
 DOI: 10.1038/ncb2566 Figure S1 CDKL5 protein expression pattern and localization in mouse brain. (a) Multiple-tissue western blot from a postnatal day (P) 21 mouse probed with an antibody against CDKL5.
DOI: 10.1038/ncb2566 Figure S1 CDKL5 protein expression pattern and localization in mouse brain. (a) Multiple-tissue western blot from a postnatal day (P) 21 mouse probed with an antibody against CDKL5.
Supplementary Materials for
 www.sciencesignaling.org/cgi/content/full/7/308/ra4/dc1 Supplementary Materials for Antipsychotics Activate mtorc1-dependent Translation to Enhance Neuronal Morphological Complexity Heather Bowling, Guoan
www.sciencesignaling.org/cgi/content/full/7/308/ra4/dc1 Supplementary Materials for Antipsychotics Activate mtorc1-dependent Translation to Enhance Neuronal Morphological Complexity Heather Bowling, Guoan
SHORT COMMUNICATION. Human Papillomavirus Type 11 E1 Ú E4 and L1 Proteins Colocalize in the Mouse Xenograft System at Multiple Time Points
 VIROLOGY 214, 259 263 (1995) SHORT COMMUNICATION Human Papillomavirus Type 11 E1 Ú E4 and L1 Proteins Colocalize in the Mouse Xenograft System at Multiple Time Points DARRON R. BROWN,*,,1 JANINE T. BRYAN,
VIROLOGY 214, 259 263 (1995) SHORT COMMUNICATION Human Papillomavirus Type 11 E1 Ú E4 and L1 Proteins Colocalize in the Mouse Xenograft System at Multiple Time Points DARRON R. BROWN,*,,1 JANINE T. BRYAN,
CHAPTER 4 RESULTS. showed that all three replicates had similar growth trends (Figure 4.1) (p<0.05; p=0.0000)
 CHAPTER 4 RESULTS 4.1 Growth Characterization of C. vulgaris 4.1.1 Optical Density Growth study of Chlorella vulgaris based on optical density at 620 nm (OD 620 ) showed that all three replicates had similar
CHAPTER 4 RESULTS 4.1 Growth Characterization of C. vulgaris 4.1.1 Optical Density Growth study of Chlorella vulgaris based on optical density at 620 nm (OD 620 ) showed that all three replicates had similar
reads observed in trnas from the analysis of RNAs carrying a 5 -OH ends isolated from cells induced to express
 Supplementary Figure 1. VapC-mt4 cleaves trna Ala2 in E. coli. Histograms representing the fold change in reads observed in trnas from the analysis of RNAs carrying a 5 -OH ends isolated from cells induced
Supplementary Figure 1. VapC-mt4 cleaves trna Ala2 in E. coli. Histograms representing the fold change in reads observed in trnas from the analysis of RNAs carrying a 5 -OH ends isolated from cells induced
Plasma lipoproteins & atherosclerosis by. Prof.Dr. Maha M. Sallam
 Biochemistry Department Plasma lipoproteins & atherosclerosis by Prof.Dr. Maha M. Sallam 1 1. Recognize structures,types and role of lipoproteins in blood (Chylomicrons, VLDL, LDL and HDL). 2. Explain
Biochemistry Department Plasma lipoproteins & atherosclerosis by Prof.Dr. Maha M. Sallam 1 1. Recognize structures,types and role of lipoproteins in blood (Chylomicrons, VLDL, LDL and HDL). 2. Explain
Supplementary Figure 1: si-craf but not si-braf sensitizes tumor cells to radiation.
 Supplementary Figure 1: si-craf but not si-braf sensitizes tumor cells to radiation. (a) Embryonic fibroblasts isolated from wildtype (WT), BRAF -/-, or CRAF -/- mice were irradiated (6 Gy) and DNA damage
Supplementary Figure 1: si-craf but not si-braf sensitizes tumor cells to radiation. (a) Embryonic fibroblasts isolated from wildtype (WT), BRAF -/-, or CRAF -/- mice were irradiated (6 Gy) and DNA damage
SUPPLEMENTAL INFORMATION
 SUPPLEMENTAL INFORMATION EXPERIMENTAL PROCEDURES Tryptic digestion protection experiments - PCSK9 with Ab-3D5 (1:1 molar ratio) in 50 mm Tris, ph 8.0, 150 mm NaCl was incubated overnight at 4 o C. The
SUPPLEMENTAL INFORMATION EXPERIMENTAL PROCEDURES Tryptic digestion protection experiments - PCSK9 with Ab-3D5 (1:1 molar ratio) in 50 mm Tris, ph 8.0, 150 mm NaCl was incubated overnight at 4 o C. The
Supporting Online Material Material and Methods References Supplemental Figures S1, S2, and S3
 Supporting Online Material Material and Methods References Supplemental Figures S1, S2, and S3 Sarbassov et al. 1 Material and Methods Materials Reagents were obtained from the following sources: protein
Supporting Online Material Material and Methods References Supplemental Figures S1, S2, and S3 Sarbassov et al. 1 Material and Methods Materials Reagents were obtained from the following sources: protein
Nature Methods: doi: /nmeth Supplementary Figure 1
 Supplementary Figure 1 Subtiligase-catalyzed ligations with ubiquitin thioesters and 10-mer biotinylated peptides. (a) General scheme for ligations between ubiquitin thioesters and 10-mer, biotinylated
Supplementary Figure 1 Subtiligase-catalyzed ligations with ubiquitin thioesters and 10-mer biotinylated peptides. (a) General scheme for ligations between ubiquitin thioesters and 10-mer, biotinylated
Taylor Yohe. Project Advisor: Dr. Martha A. Belury. Department of Human Nutrition at the Ohio State University
 Atherosclerosis Development and the Inflammatory Response of Hepatocytes to Sesame Oil Supplementation Taylor Yohe Project Advisor: Dr. Martha A. Belury Department of Human Nutrition at the Ohio State
Atherosclerosis Development and the Inflammatory Response of Hepatocytes to Sesame Oil Supplementation Taylor Yohe Project Advisor: Dr. Martha A. Belury Department of Human Nutrition at the Ohio State
Nature Structural & Molecular Biology: doi: /nsmb Supplementary Figure 1. Generation and validation of mtef4-knockout mice.
 Supplementary Figure 1 Generation and validation of mtef4-knockout mice. (a) Alignment of EF4 (E. coli) with mouse, yeast and human EF4. (b) Domain structures of mouse mtef4 compared to those of EF4 (E.
Supplementary Figure 1 Generation and validation of mtef4-knockout mice. (a) Alignment of EF4 (E. coli) with mouse, yeast and human EF4. (b) Domain structures of mouse mtef4 compared to those of EF4 (E.
T H E J O U R N A L O F C E L L B I O L O G Y
 T H E J O U R N A L O F C E L L B I O L O G Y Supplemental material Stelter et al., http://www.jcb.org/cgi/content/full/jcb.201105042/dc1 S1 Figure S1. Dyn2 recruitment to edid-labeled FG domain Nups.
T H E J O U R N A L O F C E L L B I O L O G Y Supplemental material Stelter et al., http://www.jcb.org/cgi/content/full/jcb.201105042/dc1 S1 Figure S1. Dyn2 recruitment to edid-labeled FG domain Nups.
SUPPLEMENTARY FIGURES
 SUPPLEMENTARY FIGURES Figure S1. Clinical significance of ZNF322A overexpression in Caucasian lung cancer patients. (A) Representative immunohistochemistry images of ZNF322A protein expression in tissue
SUPPLEMENTARY FIGURES Figure S1. Clinical significance of ZNF322A overexpression in Caucasian lung cancer patients. (A) Representative immunohistochemistry images of ZNF322A protein expression in tissue
Product Contents. 1 Specifications 1 Product Description. 2 Buffer Preparation... 3 Protocol. 3 Ordering Information 4
 INSTRUCTION MANUAL Quick-RNA Midiprep Kit Catalog No. R1056 Highlights 10 minute method for isolating RNA (up to 1 mg) from a wide range of cell types and tissue samples. Clean-Spin column technology allows
INSTRUCTION MANUAL Quick-RNA Midiprep Kit Catalog No. R1056 Highlights 10 minute method for isolating RNA (up to 1 mg) from a wide range of cell types and tissue samples. Clean-Spin column technology allows
Supplementary Figure 1
 Supplementary Figure 1 Asymmetrical function of 5p and 3p arms of mir-181 and mir-30 families and mir-142 and mir-154. (a) Control experiments using mirna sensor vector and empty pri-mirna overexpression
Supplementary Figure 1 Asymmetrical function of 5p and 3p arms of mir-181 and mir-30 families and mir-142 and mir-154. (a) Control experiments using mirna sensor vector and empty pri-mirna overexpression
SUPPLEMENTARY INFORMATION
 Supplementary Figures Supplementary Figure S1. Binding of full-length OGT and deletion mutants to PIP strips (Echelon Biosciences). Supplementary Figure S2. Binding of the OGT (919-1036) fragments with
Supplementary Figures Supplementary Figure S1. Binding of full-length OGT and deletion mutants to PIP strips (Echelon Biosciences). Supplementary Figure S2. Binding of the OGT (919-1036) fragments with
MTC-TT and TPC-1 cell lines were cultured in RPMI medium (Gibco, Breda, The Netherlands)
 Supplemental data Materials and Methods Cell culture MTC-TT and TPC-1 cell lines were cultured in RPMI medium (Gibco, Breda, The Netherlands) supplemented with 15% or 10% (for TPC-1) fetal bovine serum
Supplemental data Materials and Methods Cell culture MTC-TT and TPC-1 cell lines were cultured in RPMI medium (Gibco, Breda, The Netherlands) supplemented with 15% or 10% (for TPC-1) fetal bovine serum
