Pancreatic beta-cell response to increased metabolic demand and to pharmacologic secretagogues requires EPAC2A
|
|
|
- Julian Berry
- 5 years ago
- Views:
Transcription
1 Page 1 of 48 Pancreatic beta-cell response to increased metabolic demand and to pharmacologic secretagogues requires EPAC2A Woo-Jin Song 1 *, Prosenjit Mondal 1, Yuanyuan Li 1, Suh Eun Lee 1, Mehboob A. Hussain 1,2,3 Departments of Pediatrics 1, Medicine 2 and Biological Chemistry 3, Johns Hopkins University. Address of Correspondence Mehboob A. Hussain, M.D. Departments of Pediatrics, Medicine and Biological Chemistry Johns Hopkins University 6 N. Wolfe Street Baltimore MD Tel: Fax mhussai4@jhmi.edu Running Title: Universal role for EPAC2A in GSIS potentiation Publish Ahead of Print, published online April 11, 213
2 Page 2 of 48 Abstract Incretin hormone action on ß-cells stimulates in parallel two different intracellular cyclic AMP-dependent signaling branches mediated by PKA and EPAC2A. Both pathways contribute towards potentiation of glucose-stimulated insulin secretion (GSIS). However, the overall functional role of EPAC2A in ß-cells as it relates to in vivo glucose homeostasis remains incompletely understood. Therefore, we have examined in vivo GSIS in global EPAC2A knockout mice. Additionally, we have conducted in vitro studies of GSIS and calcium dynamics in isolated EPAC2A-deficient islets. EPAC2A deficiency does not impact glucose-stimulated insulin secretion in mice under basal conditions. However, when mice are exposed to diet-induced insulin resistance, pharmacologic secretagogue stimulation of ß-cells with an incretin hormone glucagon-like peptide-1 analogue or with a Fatty Acid Receptor 1/GPR4 selective activator, EPAC2A is required for the increased ß-cell response to secretory demand. Under these circumstances, EPAC2A is required for potentiating the early dynamic increase in islet calcium levels after glucose stimulation, which is reflected in potentiated first phase insulin secretion. These studies broaden our understanding of EPAC2A function and highlight its significance during increased secretory demand or drive on ß-cells. Our findings advance the rationale for developing EPAC2A selective pharmacologic activators for ß-cell targeted pharmacotherapy in type 2 diabetes mellitus.
3 Page 3 of 48 Introduction Pancreatic ß-cells secrete insulin by regulated exocytosis to tightly control glucose homeostasis. In conditions of increased metabolic demand on insulin action, such as obesity-related insulin resistance, ß-cells are capable of increasing by several-fold glucose-stimulated insulin secretion (GSIS), thereby maintaining tight glycemic regulation [1]. Failure of ß-cells to adequately respond to increased metabolic demand on insulin secretion (ß-cell dysfunction) results in diabetes mellitus [1,2]. Glucose-stimulated insulin secretion is potentiated by activation of the incretin hormone glucagon-like peptide-1 (GLP1R) receptor, which is expressed at high density on pancreatic ß-cells. GLP1R activation potentiates GSIS when blood glucose surpasses a threshold of physiologic fasting glycemia. Pharmacologic activation of GLP1R with the GLP1 agonist Exendin-4 can reverse pancreatic ß- cell dysfunction in humans with T2DM and increases GSIS, leading to improved glycemic control while also avoiding hypoglycemia [3]. Ligand-induced GLP1R activation stimulates intracellular cyclic AMP (camp) synthesis, which in turn engages two distinct intracellular signaling pathways: protein kinase A (PKA) and the exchange protein activated by camp islet/brain isoform 2A (EPAC2A) [3]. Selective disinhibition of PKA activity in murine ß-cells greatly augments GSIS [4]. Conversely, genetic EPAC2A ablation in mice has little effect on glucose homeostasis [5]. However, in contrast to these in vivo observations, in vitro studies using pharmacologic GLP1R activation and EPAC2A activation using EPAC2A-selective camp analogs (ESCA) suggest that upon a glucose stimulus, to ß-cells EPAC2A is important for potentiating a rise in intracellular Ca 2+ (ica 2+ ) [6,7,8] - a prerequisite for insulin vesicle exocytosis [9]. In addition, EPAC2A is activated by camp levels, that are significantly higher than those required to activate PKA [1,11]. Furthermore, pharmacologic stimulation of ß-cells with the sulfonylurea class of antidiabetic drugs activate EPAC2A in a camp-independent manner to stimulate insulin secretion [5]. Based on these considerations, we reasoned that EPAC2A function may be of general importance in ß-cells, particularly during circumstances which demand increased insulin secretion either in response to insulin resistance or to pharmacologic stimuli. We therefore exposed EPAC2A-deficient mice and their isolated islets to a variety of conditions, that provoke augmented ß-cell insulin secretory response to glucose. Our findings indicate that EPAC2A is required for increased GSIS in the face of diet-induced insulin resistance, during pharmacologic stimulation with the GLP1 analogue exendin-4 (E4), as well as during stimulation with a selective fatty acid receptor 1/GPR4 activator [12,13]. In a genetic mouse model of ß-cell autonomous GSIS potentiation, EPAC2A is required for achieving maximum insulin secretion. In all of these circumstances, EPAC2A is important for potentiating the initial increase in ß-cell ica 2+ after glucose stimulation.
4 Page 4 of 48 Our findings suggest that while under basal conditions EPAC2A plays a minor role in regulating GSIS, EPAC2A function occupies an important role in augmenting GSIS under conditions of increased ß-cell secretory activity. In particular, our studies suggest a role for EPAC2A in the adaptation of ß-cell secretory response to insulin resistance. Our findings broaden the role of EPAC2A beyond its currently appreciated function in incretin hormone stimulated camp signaling. These observations provide further rationale for developing EPAC2A-selective activators for individually tailored pharmacotherapy in humans with ß-cell dysfunction [2,14].
5 Page 5 of 48 Materials and Methods Animal studies were approved by the institutional animal care and use committee at Johns Hopkins University. Mice globally lacking the brain/ß-cell EPAC2 isoform EPAC2A (= EPAC2A KO), prkar1a fl/fl and PDX-1 CRE mice and their corresponding PCR-based genotyping protocols have been previously described [8,15,16]. Interbreeding PDX1-CRE and prkar1a fl/fl generated mice lacking pancreas prkar1a ( prkar1a) [8]. prkar1a have disinhibited ß-cell autonomous PKA catalytic activity and augmented glucose-dependent insulin secretion without changes in EPAC2A levels [8]. Glucagon levels in prkar1a and controls are similar [8]. Compound EPAC2A KO/ prkar1a mice were generated by interbreeding. In vivo dynamic tests: oral and intraperitoneal glucose tolerance (ogtt, ipgtt) and insulin tolerance (ITT) tests were performed according to standard protocols [4,17]. After 9 am 3 pm fasting, animals were administered 2% D-glucose (2g/kg orally via gavage or via ip injection) or insulin.5 U/kg i.p. rh insulin (Novolin 1 U/ml), respectively. Tail-vein blood was collected at the indicated times in figures for glucose and insulin measurements. To examine in vivo ß-cell GSIS after acute E4 (Sigma) administration, mice were treated with 1 nmol/kg E4 or PBS (control) ip 3 minutes prior to an ipgtt. Plasma glucose was measured using a glucometer (Johnson and Johnson). Serum insulin was measured using mouse magnetic bead panel (Millipore, Luminex). Immunohistochemistry and pancreas morphometrical analysis were performed as previously described [4,17]. At least 3 Bouin s-fixed and paraffin embedded sections/mouse 15 µm apart were immuno-stained for insulin and analyzed for islet/ß-cell mass [4,17]. Islet and ß-cell mass in WT, EPAC2 KO, prkar1a, and compound prkar1a/epac2 KO mice were similar (data not shown), excluding differences in islet mass as a mechanism underlying any differences in GSIS. Islet isolation was performed by collagenase digestion, gradient centrifugation and three rounds of microscope-assisted manual picking of islets [18]. Islets were cultured in RPMI 164 medium (Invitrogen) containing 5 mm D-glucose and supplemented with 1% bovine serum albumin, 1% HEPES buffer, and 1% penicillin/streptomycin. Islet perifusion was performed on batches of 3 islets with perifusate (1ml/min) containing.2% bovine serum albumin [4,18]. After equilibration for 3 minutes at 3 mm glucose, perifusate glucose was increased to 1 mm. Insulin was measured in effluent at 3,35,4,42,45,5,55,6 min (Alpco ELISA). Where indicated, 3 mm KCl was injected at the end of the perifusion protocol to verify that depolarization-induced insulin exocytosis was operational. AUC of insulin secretion during perifusion was calculated for the first phase GSIS: -1 min and second phase GSIS: 1-2 min after raising glucose from 3 to 1 mm. Because
6 Page 6 of 48 of the rapid dynamic changes in insulin secretion with an early nadir at 5 min after glucose stimulus in prakar1a and prkar1a/epac2a KO, first phase GSIS was considered -5 min after raising glucose with subsequent time points considered as second phase. For pharmacologic treatment, islets were exposed to, respectively, E4 1 nm (Sigma), the EPAC-selective activator 8-(4-cholorophenylthio)-2 -Omethyladenosine 3 5 -cyclic monophosphate acetoxymethyl ester (EPAC specific camp analog = ESCA, 1 µm Biolog Life Science Institute), the PKA-specific camp analog activator N6-benzoyladenosine-3,5 cyclic monophosphate, acetomethyl ester (6BNZ, 1 µm, Biolog Life Science Institute), the Fatty Acid Receptor FFA1/GPR4-selective agonist 3-(4-(((3- (Phenoxy)phenyl)methyl)amino)phenyl) propionoic acid (PMAP 5 nm, EMD Biosciences), or the protein kinase A specific inhibitor myristolated PKA inhibitor (PKI 1 µm, Invitrogen). Whole islet calcium dynamics and glucokinase activity. Accounting for heterogenic responses among individual ß-cells, we measured the integrated response of glucose stimulated calcium dynamics in intact cultured islets using a Fura-2 acetoxymethyl ester-based method. 3 equal-sized islets were incubated in optical micro-well dishes (Nunc #16536) with signal-enhanced ratiometric calcium assay reagents (BD Biosciences) and kept at 3 mm glucose for 1 hour (37 C). For treatment with E4 (1 nm) or PMAP (5 nm), islets were exposed to either compound or corresponding vehicle for 1 hour before measurements. Measurements were performed in quadruplicate for each islet genotype and/or treatment. Intracellular calcium signals were determined after automated injection of glucose (.1 M) directly into the culture wells to rapidly increase glucose to 1 mm (Biotek Synergy). Readings (excitation: 34 and 38, emission: 58 nm) were performed at two-minute intervals from the bottom plane. For Fura-2/Ca 2+ - specific signal, 34/38 nm ratio was calculated. Islet glucokinase (GK) activity was measured in islet extracts as previously described [4]. Islet protein analysis. Immunoblots (IB) were performed with 4-5 µg protein taken in lysis buffer (Cell Signaling). Protein-protein interaction was assessed by co-immunoprecipitation (Co-IP) followed by IB (Co-IP/IB) of 4-5 µg islet protein lysate. To examine time course of exocytosis related protein interactions [9] cultured islets were pretreated for 3 min with E4 (1 nm) at 3 mm glucose before acutely increasing the glucose concentration to 1 mm. Thereafter, islets were taken at indicated time points for Co-IP/IB to assess interactions between vesicle associated soluble N-ethylmaleimide-sensitive factor attachment receptor (v-snare) protein VAMP2 and target cell membrane associated SNARE (t- SNARE) protein SNAP25 [4]. SNAP25-Syntaxin 1A interaction was also analyzed to assess SNARE ternary complex assembly. Lysate was incubated at 4 C overnight with antibodies for target cell membrane protein (t-snare) SNAP25 before adding 25 µl protein A agarose bead slurry (Sigma) for additional 3 hours. Beads were gently spun (pull-down), washed and taken into
7 Page 7 of 48 5 µl SDS buffer to detect VAMP2 by IB. For input control 1% of total protein of corresponding islet lysates served as input controls. Actin served as control for overall protein loading. Densitometric analysis of Co-IP/IB was performed for individual time ponts. shrna knockdown studies. Scrambled and murine EPAC1-specific (TR512817, Origene) shrna-expressing, purified (Virabind, Invitrogen) lentiviral particles (1 5 ) were spinoculated [41] twice in polybrene (2 mg/ml, Sigma) with islets After 3 days in culture, islet insulin secretion assays were performed followed by protein extractions for IB [8]. Quantitative RT-qPCR EPAC1 and EPAC2 isoform mrna expression was determined by qpcr of islet cdna using SYBRgreen mastermix (BioRad). Primers were EPAC1 FW:GGACAAAGTCCCCTACGACA; RV:CTTGGTCCAGTGGTCCTCAT; EPAC2A FW:TGGAACCAACTGGTATGCTG; RV:CCAATTCCCAGAGTGCAGAT; EPAC2B FW:TCTTTGCTACCTGGGACTGG; RV:AGCAGCCAGCCTTTATCTGA. Expression levels were calculated using the 2- CT method [42] with 18S rrna as internal control (FW:GCAATTATTCCCCATGAACG; RV:GGCCTCACTAAACCATCCAA). Statistical analysis was performed with Prism software (GraphPad). Results are shown as averages and standard errors of the mean (SEM). Where appropriate, Student s t test or analysis of variance (ANOVA) and post-test Bonferroni correction were used to calculate differences between groups. P<.5 was considered significant.
8 Page 8 of 48 Results EPAC1 has little role in GSIS potentiation. Of the two different EPAC genes, EPAC1 mrna is ubiquitously expressed [19,2,21]. Neurons and ß-cells express the EPAC2A splice variant [19,2,21]. Quantitative PCR of mouse islets revealed predominant islet expression of EPAC2A, while 1 and 2B isoform mrna expression levels were minimal (Figure 1A), confirming previous observations [8,19,22,23]. EPAC2A KO islets lack any EPAC2 detectable signal on immunoblot (Figure 1B), further confirming that EPAC2B is not expressed in pancreatic islets [8,19,22]. However, EPAC1 protein was detectable on immunoblot of islet protein extracts (Figure 1B). To examine whether EPAC1 participates in GSIS and incretin signaling, we reduced EPAC1 expression by shrna-mediated knockdown in WT control and EPAC2A KO islets (Figure 1B). EPAC1 knockdown did not significantly influence GSIS (Figure 1C). EPAC1 knockdown in EPAC2A KO islets had no effect on E4 potentiated GSIS as compared to scrambled shrna treated islets (Figure 1C). These results suggest that in ß-cells EPAC1 does not regulate GSIS. Islet insulin content was not different between wild-type or EPAC2A KO islets, and EPAC1 knockdown had no affect on insulin content (Table 1) Absence of GSIS augmentation by the EPAC-selective camp agonist (ESCA) confirmed lack of EPAC2A function in EPAC2A KO islets [24] (Figure 1E). E4, 6BNZ, and PMAP all potentiated GSIS in WT islets. As expected, E4-stimulated GSIS was blunted in EPAC2A KO islets (Figure 1E). Interestingly, EPAC2A deficiency impaired GSIS response to PMAP (Figure 1E), indicating an interplay between GPR4 signaling and EPAC2A (see also below). EPAC2A KO and WT islets exhibited similar E4-stimulated camp generation (Supplemental Figure S1A), while PMAP did not stimulate camp (Supplemental Figure S1B). Islet glucokinase activity was similar in WT and EPAC2A KO islets (Supplemental Figure S1C). EPAC2A deficiency does not influence ß-cell mass and results in muted E4- induced GSIS potentiation Pancreas morphometric analyses of 6 week old WT and EPAC2A KO littermates showed no differences in pancreas weight, ß-cell mass, ß-cell size, or insulin content (Table 1). Both ogtt and ipgtt were similar in WT and EPAC2A KO littermates kept on regular rodent diet, consistent with previous observations [5] (Figure 2A, B). Under these basal conditions, insulin secretion during ipgtt was similar in EPAC2A KO and WT littermates (Figure 2C). A slight increase in insulin sensitivity was noted in EPAC2A-deficient mice as compared to controls (Figure 2D). Isolated islets from EPAC2A KO and WT littermates exhibited similar insulin secretion patterns during perifusion studies (Figure 2E; Supplemental Table S1) as well as similar glucose stimulated changes in ica 2+ (Figure 2F, G).
9 Page 9 of 48 In contrast to the above baseline findings, when stimulated with intraperitoneal E4 administration during ipgtt, EPAC2A KO mice exhibited a slower decline of serum glucose levels (Figure 3A). The impaired GTT in EPAC2A KO mice was reflected by a diminished response to E4-mediated GSIS potentiation in EPAC2A KO vs control mice (Figure 3B). Perifusion studies of control and EPAC2A KO islets mirrored the in vivo observations on insulin secretion. During pharmacologic stimulation with E4, EPAC2A KO islets exhibited significantly reduced GSIS potentiation, which was most pronounced during the first phase of the secretory response (Figure 3C; Supplemental Table S1). Integrated islet calcium dynamics of E4 stimulated EPAC2A KO and WT islets were similar at low glucose levels (Figure 3D). When stimulated with glucose plus E4, WT islets exhibited a robust response of ica 2+ primarily in the initial phases after glucose stimulation (Figure 3E). In contrast, EPAC2A KO islets failed to exhibit the dramatic response in ica 2+ observed in control islets (Figure 3E). These results suggest that while EPAC2A function appears dispensible during basal GSIS, EPAC2A is required when ß-cell insulin secretion is potentiated by pharmacologic (i.e. supraphysiologic; in contrast to physiologic) GLP1R activation. Co-immunoprecipitation studies of proteins involved in insulin vesicle exocytosis [9] showed increased interaction of the cell membrane-bound target SNARE (t- SNARE) protein SNAP25 with Syntaxin1A and with the vesicle bound (v-snare) protein VAMP2 in E4 pre-treated WT islets within minutes after exposure to elevated glucose levels (1 mm). In contrast, EPAC2A KO islets showed during identical studies a weaker and delayed SNAP25-VAMP2 and SNAP-25- Syntaxin1A interactions. (Supplemental Figure S2). The time course of this protein interaction correlates with the observations made in intracellular calcium dynamics as well as insulin secretion during perifusion studies. These findings are consistent with previous observations that insulin exocytosis requires an increase in intracellular calcium [9], which - as found here - is delayed in glucose and E4-stimulated EPAC2A KO islets. EPAC2A is required for increased ß-cell functional response to diet-related insulin resistance Based on these observations, we reasoned that EPAC2A function may be required for enhancing ß-cell secretory response not only when pharmacologically stimulated with GLP1R agonist but also under conditions when metabolic demands on insulin secretion are increased. We exposed mice to 4 weeks of HFD in order to achieve diet-induced insulin resistance. During HFD feeding, EPAC2A KO and WT mice did not exhibit any differences in weight gain (data not shown), indicating that EPAC2A deficiency has no effect on weight regulation during this short-term caloric surfeit. Interestingly, HFD fed EPAC2A KO, as compared to controls, exhibited impaired glucose tolerance and diminished insulin secretion during ipgtt (Figure 4A, B). As with the normal diet fed mice, ITT revealed slightly increased insulin sensitivity in EPAC2A KO mice
10 Page 1 of 48 as compared to controls (Figure 4C), Despite increased insulin sensitivity, EPAC2A KO mice exhibited impaired glucose tolerance as compared to WT, indicating a limitation in their insulin secretion (Figure 4A, B). Perifusion studies confirmed a reduction in GSIS in HFD-fed EPAC2A KO islets relative to controls (Figure 4D; Supplemental Table S1). EPAC2A deficiency mainly resulted in reduction of the first phase of GSIS, while the second phase of GSIS was remarkably similar in HFD-fed EPAC2A KO and WT mouse islets (Figure 4D). The observations made during perifusion studies were mirrored in integrated islet calcium dynamics. HFD-fed EPAC2A KO mouse islets exhibited, as compared to controls, diminished glucose-induced ica 2+ increase primarily during the initial rise after glucose stimulation (Figure 4 E, F). EPAC2A is required for enhancing insulin secretion in a model of ß-cell autonomous GSIS potentiation Besides inducing insulin resistance, HFD may cause additional confounding effects on ß-cell function [25,26]. We therefore sought to examine EPAC2A function in a genetic model of ß-cell autonomous GSIS potentiation. Mice with disinihibited PKA in their islets ( prkar1a mice) exhibit dramatically (5-1 fold) enhanced GSIS without hypoglycemia or change in islet mass [4]. We interbred prkar1a and EPAC2A KO mice to generate compound prkar1a/epac2a KO mice. As expected, islet prkar1a ablation resulted in significantly improved glucose tolerance (Figure 5A). GSIS was significantly reduced in prkar1a/epac2a KO as compared to prkar1a mice (Figure 5B). Albeit, the very high insulin secretory response resulting from prkar1a ablation was sufficient to keep glucose tolerance similar in both prkar1a and prkar1a/epac2a KO mice (Figure 5A, B). Insulin sensitivity as assessed by ipitt did not differ between prkar1a and prkar1a/epac2a KO mice (Figure 5C). Perifusion studies showed dramatically augmented GSIS in prkar1a islets (Figure 5D, Supplemental Table S1) reflecting in vivo observations. prkar1a/epac2a KO islets also showed increased GSIS as compared to EPAC2A KO islets (Figure 2E). However, as compared to prkar1a islets, compound prkar1a/epac2a KO islets exhibited a blunted first phase GSIS. This finding suggests that EPAC2A function while not critical, is required for the camp-pka signaling branch to be fully effective in GSIS augmentation. prkar1a islets exhibited increased islet ica 2+ in response to a glucose stimulus, which paralleled the dynamics in insulin secretion found during perifusion. Compared to controls, prkar1a/epac2a KO islets exhibited muted ica 2+ excursions, primarily in the initial phase after glucose stimulation (Figure 5E, F). When additionally exposed to 4 weeks of HFD, prkar1a mice responded with further enhanced in vivo GSIS, whereas prkar1a/epac2a KO counterparts exhibited blunted insulin secretion with a near complete absence of first-phase insulin secretion (Supplemental Figure S3).
11 Page 11 of 48 Taken together, these findings suggest that EPAC2A is required for augmenting GSIS either in response to diet-related insulin resistance, to pharmacologic ß-cell secretagogue stimulation, or in conditions of ß-cell autonomous GSIS potentiation. PKA activity is essential for overall GSIS and EPAC2A predominantly augments first phase of E4 stimulated GSIS To further dissect the interplay between PKA- and EPAC2A-dependent signaling in GSIS, we introduced pharmacologic manipulation of camp-pka and camp- EPAC2 pathways during perifusion studies. Stimulation with the PKA-selective camp agonist 6BZN increased GSIS in both WT and EPAC2A KO islets. However, the immediate early rise in GSIS after the glucose stimulus was significantly blunted in EPAC2A KO islets (Supplemental Figure S4A). EPAC2A-selective activation with ESCA was met with a blunted GSIS in EPAC2A KO islets (Supplemental Figure S4B). This was pronounced during the first phase, and persisted during the second phase of insulin secretion (Supplemental Figure S4B; Supplemental Table S1). In contrast, PKI treatment abolished all GSIS except for a small residual insulin release early after glucose exposure (Supplemental Figure S4C). Stimulation with E4 or with ESCA did not reverse the inhibitory effects of PKI (Supplemental Figure S4D, E), confirming previous observations that PKA activity is required for EPAC2A-mediated GSIS potentiation [6]. PMAP stimulation had a clear but modest effect on GSIS despite PKI inhibition of PKA in WT islets. In contrast, PMAP potentiation of GSIS was absent in PKI treated EPAC2A KO islets suggesting that PMAP and EPAC2A may in part interact in a PKA-independent manner (Supplemental Figure S4F) (see also below). Depolarizing PKI treated islets with KCl stimulated insulin release, indicating that despite PKI inhibition islets maintained exocytosis capability. (Supplemental Figure S4C, D). The mechanism underlying the small, albeit distinct early burst of insulin output seen during PKI treatment remains unclear and is likely directly stimulated by glucose. Taken together, these studies indicate that in ß-cells, while the camp-pka branch is sufficient to augment GSIS, it requires EPAC2A for a maximal response. EPAC2A absence diminishes GPR4-mediated GSIS. GPR4 is expressed at high density on ß-cells. Pharmacologic activation of GPR4 - similarly to incretin hormone receptor activation potentiates GSIS, with no insulin secretion when glucose is below physiologic fasting levels [12,13,27,28]. Initial pharmacologic trials in humans with GPR4-selective activators indicate their effectiveness in ameliorating insulin secretion and glycemia in T2DM [27]. We examined whether EPAC2A function may also be relevant in GPR4- mediated GSIS augmentation, which is distinct from GLP1R activation and camp signaling [12,13]. During perifusion, WT islets exposed to the GPR4-selective agonist PMAP potentiated GSIS, confirming previous observations GPR4 action in the islet [12,13]. In contrast, GPR4 activation in EPAC2A KO islets resulted in
12 Page 12 of 48 significantly reduced first phase GSIS (Figure 6A). PMAP stimulated islet ica 2+ in a glucose dependent manner as previously described [12,13]. Interestingly, EPAC2A KO islets, as compared to controls, exhibited reduced PMAP induced rise in ica 2+ levels (Figure 6B, C), Together with the observation that PMAP does not stimulate camp synthesis in islets (Supplemental Figure S1), these observations suggest that EPAC2A function may only in part be related to its incretin-stimulated camp-dependent activation, and that EPAC2A has a wider role in potentiating GSIS in response to other stimuli. To examine a potential interplay between GPR4- and PKA-mediated GSIS augmentation, we inhibited PKA activity with the selective inhibitor PKI in islets exposed to PMAP (Supplemental Figure S4E). PKA inhibition did not influence PMAP potentiated GSIS. PKI-mediated inhibition in EPAC2A KO islets completely abolished PMAP-induced GSIS stimulation (Supplemental Figure S4F), suggesting a dependency of GPR4 mediated GSIS stimulation on overall intact camp-dependent signaling. Taken together, these findings suggest that GPR4 mediated GSIS potentiation requires EPAC2A function to be fully effective.
13 Page 13 of 48 Discussion The present in vivo studies of genetically defined mouse models combined with in vitro examination of their islets suggest an important role for EPAC2A function during increased demand on insulin secretion by the ß-cell. Our studies expand the conceptual understanding of EPAC2A function beyond its role in mediating camp-dependent effects of pharmacological GLP1R activators (i.e E4). While EPAC2A appears to have little significance during basal conditions (Figure 3A- E), EPAC2A function becomes relevant in circumstances of increased demand on ß-cell GSIS. To our knowledge, this is the first report of a direct in vivo examination of pharmacologic incretin analog E4 action in EPAC2A KO mice. In EPAC2A deficient mice as compared to controls, E4 effects on GSIS potentiation are markedly reduced (Figure 3A, B). Perifusion and (Figure 3D) islet ica 2+ studies (Figure 3 E, F) reveal that during pharmacologic E4 stimulation, EPAC2A is required predominantly for augmenting the initial burst of ica 2+, which is associated with a corresponding GSIS potentiation. Furthermore, EPAC2A deficiency is associated with delayed interaction of insulin vesicle exocytosis associated proteins SNAP25 and VAMP2 (Supplemental Figure S2). These findings are consistent with the observation that a rise in ica 2+ is required for exocytosis to occur [9]. EPAC2A deficiency delays the increase in ica 2+ (Figure 3F) after glucose stimulation, which compromises insulin exocytosis (i.e. secretion) (Figure 3D) The unexpected observation of slightly increased insulin sensitivity in EPAC2A mice may confound in vivo observations on insulin secretion. This prompted us to include in vitro studies in isolated islets, thereby ensuring a direct assessment of EPAC2A function in ß-cells. Of note, EPAC2A may exert a role in non-ß pancreatic islet cells [29], which may additionally influence ß-cell function. In this context, future studies with conditional cell-specific EPAC2A ablation will be necessary to specifically separate in vivo ß-cell from non-ß-cell islet effects or from peripheral tissue effects of EPAC2A. The role for EPAC2A in potentiating GSIS was also observed in the diet-induced obesity and insulin resistance model. When kept on a normal laboratory mouse diet, EPAC2A deficient mice and their islets, as compared to normal littermates, showed no differences in glucose homeostasis, insulin secretion and islet ica 2+ dynamics (Figure 2). Conversely, HFD-fed insulin resistant mice exhibited augmented GSIS during GTT, reflecting the ß-cell response to increased metabolic demand. Under HFD conditions EPAC2A KO mice exhibited defective GSIS and glucose tolerance (Figure 4 A-C), indicating that EPAC2A function becomes relevant under increased demand on ß-cell secretion. These dynamic changes in insulin secretion were reflected in EPAC2A KO islets mainly by a reduction of the initial increase in intracellular calcium levels after glucose
14 Page 14 of 48 stimulation. Thus, EPAC2A absence aggravates ß-cell dysfunction in the face of diet-induced insulin resistance. How ß-cells sense increased insulin resistance and respond to the increased metabolic demand on insulin secretion remains unclear [1]. To this end, the present studies suggest that EPAC2A occupies an important role in the molecular mechanisms of ß-cell adaptation to increased secretory demand. Conversely, we speculate that in T2DM, EPAC2A function may be compromised and contribute towards failure of ß-cells to compensate for increased demand [3]. When examining the interplay of the camp-pka and camp-epac2a branches of GLP1R signaling, ß-cell autonomous disinhibition of the camp-pka branch ( prkar1a mice) is sufficient to potentiate GSIS (Figure 5B). However, EPAC2A function is required for PKA activity to reach optimal stimulation of ica 2+ dynamics and GSIS (Figure 5B, D, E). When ß-cells are further stimulated to augment GSIS by a combination of islet PKA disinhibition plus HFD-induced insulin resistance, EPAC2A absence significantly blunts first phase insulin secretion ( prkar1a/epac2a KO mice; Supplemental Figure S3C). Conversely, inhibition of PKA catalytic activity with the PKA-selective inhibitor PKI, abolishes GSIS elicited by E4 or by the EPAC2A-selective activator (ESCA, Supplemental Figure S4D, E). Based on these studies, we conclude that PKA activity is essential for GSIS. Further, while EPAC2A cannot function independently of PKA, EPAC2A is required and permissive for optimal GSIS potentiation when PKA-dependent signaling is activated. The signal transduction pathways engaged by the G-protein coupled GPR4 receptor and mediated by Gaq/11, while resulting in increased in ica 2+ [28], have thus far not been considered to interplay with EPAC2A. However, the present studies suggest that GPR4 agonist-mediated GSIS potentiation requires functional EPAC2A (Figure 6A, Supplemental Figure S4F). Islet EPAC2A function is required, at least in part, to amplify the rise in ica 2+ levels stimulated by GP4 action (Figure 6C). Both GPR4 and EPAC2A activate different phospholipase C isoforms [28,31,32], which in turn hydrolyze phosphoinositol 4,5, bisphosphate (PIP 2 ) to diacylglycerol (DAG) and inositol trisphosphate (IP 3 ). IP3 engages the endoplasmatic reticulum (EPR) IP3 receptor leading to the opening of Ca 2+ release channels. The Ca 2+ release channels allow EPR-stored Ca 2+ to exit into the cytoplasm, thereby potentiating an increase in cytoplasmic ica 2+ levels [32]. It is thus conceivable that GPR4 and EPAC2A-dependent signaling converge at Ca 2+ release channels in a complementary manner. Our observations suggest that EPAC2A potentiates GPR4-dependent Ca 2+ mobilization early after glucose-stimulated ß-cells (Figure 6C). GPR4 stimulation does not increase camp levels in ß-cells (Supplemental Figure S1) [12,13], making it unlikely that GPR4 activation directly stimulates EPAC2A. The present findings do not exclude EPAC2A-independent mechanisms in dynamic changes in ß-cell cytoplasmic ica 2+. Our findings also do not exclude
15 Page 15 of 48 additional functions of EPAC2A related to insulin secretion such as regulating the pool of vesicles destined for exocytosis [33,34]. Detailed future studies will be necessary to elucidate role of EPAC2A on subcellular events within the ß-cell. Nevertheless, our studies suggest that EPAC2A plays an important role in augmenting ica 2+ levels when ß-cells face an increased demand to secrete insulin. This model is consistent with and extends the models of EPAC2A mediated closure of the K-ATP channel, resulting in voltage-dependent influx of extracellular Ca 2+ into ß-cells [35,36,37] as well as EPAC2A-mediated calciuminduced calcium release (CICR) from intracellular compartments [7,38,39]. Our observations are also compatible with findings that EPAC2A is activated at camp concentrations, which are several-fold higher than those that activate PKA [1,11]. Thus, during physiologic incretin stimulation of ß-cells, the camp-pka signaling branch may be predominantly activated without significantly engaging the camp-epac2a branch. In contrast, during pharmacologic ß-cell GLP-1 receptor activation (i.e. E4 treatment), larger concentrations of camp may be generated, which activate EPAC2A in addition to stimulating PKA. In a broader context, our findings may guide development and tailored administration of pharmacologic therapy for human T2DM. EPAC2A-specific stimulation may improve dynamic GSIS in insulin resistant and pre-diabetic patients [33]. Furthermore, combination of GPR4 agonists with EPAC2Aselective or GLP1R activators tailored to specific patients needs may show synergistic effects on reversing ß-cell dysfunction [2].
16 Page 16 of 48 Acknowledgements M.A.H. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. The authors have no conflict of interest. W-J.S., P.M., Y.L. and E.L. researched data, M.A.H. designed study, researched data and wrote the manuscript. We acknowledge editorial assistance of A. Wolfe and F Wondisford, Johns Hopkins University. We thank C. Stratakis (NICHD) and L. Kirschner (Columbus University) and for prkar1a fl/fl and S. Seino (Kobe University) for EPAC2A KO mice. Supported by grants from the National Institutes of Health (DK9245, DK9816, DK84949, DK79637).
17 Page 17 of 48 Table Legends Table 1 Morphometric analysis and islet insulin content, and EPAC isoform expression of WT and EPAC2A KO littermates. Pancreas weight, ß-cell mass, and size are similar in 6 week old male WT and EPAC2A KO mice in C57Bl/6 background. Islet insulin content (5 islets/assay) normalized to total islet protein is similar in WT and EPAC2A KO mice with or without EPAC1 shrna mediated knockdown. (mean+sem, n=3, NS indicates no statistical significant difference. Table 1 Wild-type EPAC2A KO Morphometry Pancreas weight (mg) NS ß-cell mass (mg) NS ß-cell size (µm 2 ) NS Islet insulin content (ng/µg protein) Scrambled RNA NS EPAC1 shrna NS
18 Page 18 of 48 Figure Legends Figure 1. EPAC1 his little role in GSIS potentiation. EPAC2A KO islets lack GSIS potentiation in response to pharmacologic secretagogue stimulation A Quantitive RT-PCR of mouse islets reveals predominant expression of EPAC2A in islets. EPAC1 and EPAC2B isoforms are expressed at low levels and are similar in WT and EPAC2A deficient islets. B shrna mediated EPAC1 knockdown in wild-type and EPAC2A KO islets. Immunoblot of islet protein samples shows EPAC1 immunoreactivity in both WT (left) and EPAC2A KO (right) islets. EPAC2A is present in WT controls and lacking in EPAC2A KO islets. Specific shrna knockdown specifically reduces EPAC1 immunoreactivity, while no changes were detected with scrambled shrna. Signals on immunoblot were detected at the expected position corresponding to the molecular weight (Supplemental Table S2). Representative immunoblot is shown with triplicate samples from 3 different mice for each group. C. Insulin secretion rates during static incubation of EPAC2A KO islets. shrnamediated EPAC1 knockdown in EPAC2A KO islets does not impair E4- potentiated GSIS. Left: E4 has no effect on insulin secretion at low (3 mm) glucose levels. Right: At 1 mm glucose, both scrambled and EPAC1 shrna treated islets show similar insulin secretion rates and similarly augment GSIS under E4 (1 nm) exposure. D. Wild-type islets secrete insulin in a glucose-dependent manner. Stimulation with incretin hormone analogue E4, PKA-selective activator 6BZN, EPAC2- selective activator ESCA, as well as GPR4-selective activator PMAP augment GSIS at 1 mm but not 3 mm glucose. E. EPAC2A KO islets secrete insulin in a glucose-dependent manner. Stimulation with incretin hormone analogue E4, PKA-selective activator 6BZN, as well as GPR4-selective activator PMAP augment GSIS. E4 and PMAP augment GSIS to a lesser degree in EPAC2A KO islets (D) as compared to WT islets (C). EPAC2-selective activator ESCA fails to augment GSIS in EPAC2A KO islets. Vehicle controls: for E4: PBS, for ESCA and PMAP: DMSO. Results are shown as mean+sem of triplicate studies. * p<.5 vs vehicle, # p<.5 vs E4;! p<.5 for respective treatment EPAC2A KO vs WT islet (D vs C) as determined by 2- way ANOVA and Bonferroni post-test analysis.
19 Page 19 of 48 Figure 2. EPAC2A ablation does not influence GSIS. A-D In vivo studies; E-F in vitro studies EPAC2A KO mice and WT controls have similar glucose excursions during ogtt (A) and ipgtt (B). C: Serum insulin levels during ipgtt are not different in WT and EPAC2A KO mice. D: ipitt shows slightly increased insulin sensitivity in EPAC2A KO mice as compared to controls. E: WT and EPAC2A KO islets show similar insulin secretion rates during perifusion studies. F, G: Islet Intracellular calcium dynamic changes detected with the FURA-2 method in EPAC2A KO and WT islets are similar. F: Islets maintained in 3 mm glucose show no differences in intracellular calcium levels. G: Increasing glucose levels from 5 to 1 mm at time min elicits an increase in intracellular calcium signal, which is similar in WT and EPAC2A KO islets. WT is represented in gray circles; EPAC2A KO is represented in black circles. Results are shown as mean+sem of studies performed at least in triplicate. * indicates p<.5.
20 Page 2 of 48 Figure 3. EPAC2A ablation impairs dynamic insulin response to E4 mediated GSIS potentiation. A, B In vivo studies; C-E in vitro studies A: Acute E4 administration prior to ipgtt reveals relatively improved glucose tolerance in WT as compared to EPAC2A KO mice. B: Acute E4 administration prior to ipgtt elicits augmented GSIS in WT mice, while E4 stimulated GSIS augmentation is lacking in EPAC2A KO counterparts. C: Perifusion studies show E4 stimulated GSIS augmentation in WT islets, which is muted in EPAC2A KO islets. D, E: Islet Intracellular calcium dynamic changes detected with the FURA-2 method in EPAC2A KO and WT islets. D: Islets maintained in 3 mm glucose show no differences in intracellular calcium levels. E: Increasing glucose levels from 5 to 1 mm at time min elicits an increase in intracellular calcium signal, which is reduced in EPAC2A KO islets as compared to WT control islets. WT is represented in gray circles; EPAC2A KO is represented in black circles. Results are shown as mean+sem of studies performed at least in triplicate. * indicates p<.5.
21 Page 21 of 48 Figure 4. EPAC2A ablation impairs dynamic insulin response in diet induced obesity and insulin resistance model A-C In vivo studies; D-F in vitro studies A: HFD fed WT mice show impaired ip glucose tolerance, which is more pronounced in HFD fed EPAC2A KO mice. B: HFD fed WT mice show augmented serum insulin excursions in response to ip glucose. In comparison, EPAC2A KO mice show a delayed and impaired insulin response to ip glucose. C: HFD fed EPAC2A KO mice, as compared to WT controls, show slightly increased insulin sensitivity, which does not compensate their impaired glucose tolerance (A). D: Perifusion studies of islets from HFD fed mice show GSIS augmentation in WT islets, which is blunted in EPAC2A KO islets mainly in the early phases (min 4-45) after glucose stimulus to the islets E, F: Islet Intracellular calcium dynamic changes detected with the FURA-2 method in EPAC2A KO and WT islets of HFD fed mice. E: Islets maintained in 3 mm glucose show no differences in intracellular calcium levels. F: Increasing glucose levels from 3 to 1 mm at time min elicits an increase in intracellular calcium signal. EPAC2A KO islets show a blunted early (-1 min) rise in glucose-stimulated intracellular calcium, which, at later time points (1-2 min) is similar in WT and EPAC2A KO islets. WT is represented in gray circles; EPAC2A KO is represented in black circles. Results are shown as mean+sem of studies performed at least in triplicate. * indicates p<.5.
22 Page 22 of 48 Figure 5. EPAC2A ablation impairs dynamic insulin response in model of ß-cell autonomous GSIS augmentation ( prkar1a mice) A-C In vivo studies; D-F in vitro studies A: During ipgtt, prkar1a and prkar1a/epac2a KO mice exhibit similar glucose excursions. B: During ipgtt, prkar1a show as compared to WT mice (Figure 3) dramatically increased GSIS. prkar1a/epac2a KO mice exhibit reduced serum insulin levels as compared to prkar1a mice. C: During ipitt, prkar1a/epac2a KO, as compared to prkar1a mice, show slightly increased insulin sensitivity, which does not reach statistically significance. D: Perifusion studies of islets from prkar1a and prkar1a/epac2a KO mice. prkar1a islets exhibit a dramatically augmented GSIS. In contrast, prkar1a/epac2a KO islets exhibit a relatively blunted first phase GSIS, while second phase GSIS is similar in both groups. E, F: Islet Intracellular calcium dynamic changes detected with the FURA-2 method in prkar1a and prkar1a/epac2a KO islets. E: Islets maintained in 3 mm glucose show no differences in intracellular calcium levels. F: Increasing glucose levels from 3 to 1 mm at time min elicits a dramatic increase in intracellular calcium signal in prkar1a islets. prkar1a/epac2a KO islets show a blunted early (-1 min) rise in glucose-stimulated intracellular calcium, which, at later time points (1-2 min) is similar in both groups. prkar1a is represented in gray circles; prkar1a/epac2a KO is represented in black circles. Results are shown as mean+sem of studies performed at least in triplicate. * indicates p<.5.
23 Page 23 of 48 Figure 6. EPAC2A ablation impairs GSIS augmentation in response to FFA1/GRP4 activation A: Perifusion studies of PMAP treated islets GSIS augmentation in WT islets, which is blunted in EPAC2A KO islets mainly in the acute phase (min 4-42) after glucose stimulus to the islets, while later time points show similar insulin secretion in WT and EPAC2A KO islets B, C: Islet Intracellular calcium dynamic changes detected with the FURA-2 method in PMAP treated WT and EPAC2A KO islets. B: Islets maintained in 3 mm glucose show no differences in intracellular calcium levels. C: Increasing glucose levels from 3 to 1 mm at time min elicits an increase in intracellular calcium signal in WT islets. EPAC2A KO islets show a blunted early (-1 min) rise in glucose-stimulated intracellular calcium, which, at later time points (1-2 min) is similar in both groups. WT is represented in gray circles; EPAC2A KO is represented in black circles. Results are shown as mean+sem of studies performed at least in triplicate. * indicates p<.5.
24 Page 24 of 48 Supplemental Appendix Supplemental Table S1. Area under the curve (AUC) of perifusion studies on Top: isolated WT and EPAC2A KO islets, First phase: -1 min after glucose stimulation (glucose increases from 3 to 1 mm); Second phase: 1-2 min after glucose stimulation. Bottom: prkar1a and prkar1a/eapac2a KO islets. N=3 in each group. First phase: -5 min after glucose stimulation (glucose increases from 3 to 1 mm); Second phase: 5-2 min after glucose stimulation. Treatment Phase of insulin secretion Insulin AUC WT islets pg/islet (mean+sem) Insulin AUC EPAC2A KO pg/islet (mean+sem) P - 1 st phase NS - 2 nd phase NS E4 1 nm 1 st phase <.5 2 nd phase <.5 High fat diet 1 st phase <.5 2 nd phase NS PMAP 5 nm 1 st phase <.5 2 nd phase NS 6BNZ 1 µm 1 st phase NS 2 nd phase NS ESCA 1 µm 1 st phase <.5 2 nd phase <.5 Phase of insulin secretion Insulin AUC prkar1a pg/islet (mean+sem) Insulin AUC prkar1a/epac2a KO pg/islet (mean+sem) P - 1 st phase <.5-2 nd phase NS
25 Page 25 of 48 Supplemental Table S2 Antibodies used for co-immunoprecipitation assays and immunoblots Detected antigen Antibody source Catalog number Band size on immunoblot EPAC1 Abcam ab kd EPAC2 Santa Cruz sc kd Rim1/2 Santa Cruz sc kd SNAP25 Santa Cruz sc kd Syntaxin1A Abcam ab kd VAMP2 Santa Cruz sc kd Actin Millipore MAB151 4 kd
26 Page 26 of 48 Supplemental Figure S1 Incretin receptor activation but not GPR4 receptor activation increases Islet camp levels similarly in WT EPAC2A KO islets. WT and EPAC2A KO islets have similar glucokinase activity A: E4 stimulation generates camp production to similar degrees in both cultured WT (grey bars) and EPAC2A KO (black bars) islets. EPAC2A ablation does not influence E4 stimulated camp generation. B: PMAP does not stimulate camp production in WT (grey bars) or EPAC2A (black bars) islets. C: E4 stimulation generates camp production to similar degrees in prkar1a (grey bars) and prkar1a/epac2a KO (black bars) islets. prkar1a islets do not exhibit higher E4 stimulated camp production as compared to WT islets (shown panel A). D: Glucokinase (GK) activity is similar in WT (grey bars) and EPAC2A KO (black bars) islets and is not changed by acute (3 min) E4 treatment Values are normalized to WT islets Results are shown as mean+sem of studies performed at least in triplicate. * indicates p<.5. For details see text.
27 Page 27 of 48 Supplemental Figure S2 Co-immunoprecipitation studies of WT and EPAC2A KO islets Delayed interaction of SNARE protein components in EPAC2A KO islets stimulated with E4 A. WT and EPAC2A KO islets exposed to E4 for 3 minutes at 3 mm glucose were stimulated with 1 mm glucose. Protein extracts were obtained at indicated time points after glucose simulation for Co-IP/IB studies to assess SNAP25-VAMP2 and SNAP25-Syntaxin1A SNARE protein interaction. Input into IP studies (1% of protein extract) and SNAP25- specific IP indicate similar amounts of proteins were used at each time point during the time-course. Representative blots from 3 separate Co-IP studies are shown. B. Densitometric analysis of VAMP2 Co-IP. Band intensity is normalized to actin of respective time point. Time = min was defined as 1 arbitrary units C. Densitometric analysis of Syntaxin1A Co-IP. Band intensity is normalized to actin of respective time point. Time = min was defined as 1 arbitrary units.
28 Page 28 of 48 Supplemental Figure S3 EPAC2A ablation impairs dynamic insulin response in HFD fed mice of model of ß-cell autonomous GSIS augmentation ( prkar1a mice) A: During ipgtt, HFD- prkar1a and HFD- prkar1a/epac2a KO mice exhibit similar glucose excursions. B: During ITT, HFD- prkar1a and HFD- prkar1a/epac2a KO show similar insulin sensitivity C: ipgtt, HFD- prkar1a exhibit dramatically increased GSIS. HFD- prkar1a/epac2a KO show increased GSIS, with a blunted first-phase insulin secretory response and a relatively increased second phase insulin secretory response. prkar1a is represented in gray squares; HFD- prkar1a/epac2a KO is represented in black squares. Results are shown as mean+sem of studies performed at least in triplicate. * indicates p<.5. For details see text.
29 Page 29 of 48 Supplemental Figure S4 Perifusion studies of PKA, EPAC2A and GPR4 interplay A: EPAC2A is required for full effect of PKA mediated GSIS stimulation. Stimulation with the PKA-selective camp agonist 6BZN augments GSIS in WT islets. EPAC2A KO islets also exhibit augmented GSIS under 6BZN stimulation. However, the immediate early (4-42 min) insulin secretory response is diminished in EPAC2A KO islets. B: Stimulation with the EPAC2-selective camp analog (ESCA) augments GSIS in WT islets. ESCA mediated GSIS augmentation is abolished in EPAC2A KO islets, which exhibit GSIS similar to WT islets without any pharmacologic secretagogue stimulation (see Figure 2E) C: PKA inhibition with PKI abolishes GSIS in WT and EPAC2A KO islets. A small burst of insulin secretion is detectable early (4-45 min) after stimulation with 1 mm glucose. KCL depolarization at completion of perifusion results in insulin release. D: PKA inhibition with PKI abolishes GSIS in E4 treated WT and EPAC2A KO islets. A small burst of insulin secretion is detectable early (4-45 min) after stimulation with 1 mm glucose. KCL depolarization at completion of perifusion results in insulin release. E: PKA inhibition with PKI abolishes GSIS in ESCA treated WT and EPAC2A KO islets. A small burst of insulin secretion is detectable early (4-45 min) after stimulation with 1 mm glucose. KCL depolarization at completion of perifusion results in insulin release. F: PKA inhibition with PKI does not affect GSIS in (GPR4 activated) PMAP treated islets (see Figure 6A). PKA inhibition in EPAC2A KO islets treated with PMAP show blunted GSIS. KCL depolarization at completion of perifusion results in insulin release. Results are shown as mean+sem of studies performed at least in triplicate. * indicates p<.5. For details see text.
30 Page 3 of 48 References 1. Cavaghan MK, Ehrmann DA, Polonsky KS (2) Interactions between insulin resistance and insulin secretion in the development of glucose intolerance. J Clin Invest 16: DeFronzo RA, Abdul-Ghani MA (211) Preservation of beta-cell function: the key to diabetes prevention. J Clin Endocrinol Metab 96: Drucker DJ (26) The biology of incretin hormones. Cell Metab 3: Song WJ, Seshadri M, Ashraf U, Mdluli T, Mondal P, et al. (211) Snapin mediates incretin action and augments glucose-dependent insulin secretion. Cell Metab 13: Zhang CL, Katoh M, Shibasaki T, Minami K, Sunaga Y, et al. (29) The camp sensor Epac2 is a direct target of antidiabetic sulfonylurea drugs. Science 325: Chepurny OG, Kelley GG, Dzhura I, Leech CA, Roe MW, et al. (21) PKAdependent potentiation of glucose-stimulated insulin secretion by Epac activator 8-pCPT-2'-O-Me-cAMP-AM in human islets of Langerhans. Am J Physiol Endocrinol Metab 298: E Kang G, Chepurny OG, Rindler MJ, Collis L, Chepurny Z, et al. (25) A camp and Ca2+ coincidence detector in support of Ca2+-induced Ca2+ release in mouse pancreatic beta cells. J Physiol 566: Shibasaki T, Takahashi H, Miki T, Sunaga Y, Matsumura K, et al. (27) Essential role of Epac2/Rap1 signaling in regulation of insulin granule dynamics by camp. Proc Natl Acad Sci U S A 14: Sudhof TC, Rothman JE (29) Membrane fusion: grappling with SNARE and SM proteins. Science 323: Doskeland SO, Ogreid D (1981) Binding proteins for cyclic AMP in mammalian tissues. Int J Biochem 13: Ekanger R, Sand TE, Ogreid D, Christoffersen T, Doskeland SO (1985) The separate estimation of camp intracellularly bound to the regulatory subunits of protein kinase I and II in glucagon-stimulated rat hepatocytes. J Biol Chem 26: Salehi A, Flodgren E, Nilsson NE, Jimenez-Feltstrom J, Miyazaki J, et al. (25) Free fatty acid receptor 1 (FFA(1)R/GPR4) and its involvement in fatty-acid-stimulated insulin secretion. Cell Tissue Res 322: Tan CP, Feng Y, Zhou YP, Eiermann GJ, Petrov A, et al. (28) Selective small-molecule agonists of G protein-coupled receptor 4 promote glucose-dependent insulin secretion and reduce blood glucose in mice. 57: Seino S, Takahashi H, Takahashi T, Shibasaki T (212) Treating diabetes today: a matter of selectivity of sulphonylureas. Obes Metab 14 Suppl 1: Lammert E, Cleaver O, Melton D (21) Induction of pancreatic differentiation by signals from blood vessels. Science 294:
31 Page 31 of Kirschner LS, Kusewitt DF, Matyakhina L, Towns WH, 2nd, Carney JA, et al. (25) A mouse model for the Carney complex tumor syndrome develops neoplasia in cyclic AMP-responsive tissues. Cancer Res 65: Song WJ, Schreiber WE, Zhong E, Liu FF, Kornfeld BD, et al. (28) Exendin-4 stimulation of cyclin A2 in beta-cell proliferation. 57: Hussain MA, Porras DL, Rowe MH, West JR, Song WJ, et al. (26) Increased pancreatic beta-cell proliferation mediated by CREB binding protein gene activation. Mol Cell Biol 26: Ozaki N, Shibasaki T, Kashima Y, Miki T, Takahashi K, et al. (2) camp- GEFII is a direct target of camp in regulated exocytosis. Nat Cell Biol 2: de Rooij J, Zwartkruis FJ, Verheijen MH, Cool RH, Nijman SM, et al. (1998) Epac is a Rap1 guanine-nucleotide-exchange factor directly activated by cyclic AMP. Nature 396: Kawasaki H, Springett GM, Mochizuki N, Toki S, Nakaya M, et al. (1998) A family of camp-binding proteins that directly activate Rap1. Science 282: Kashima Y, Miki T, Shibasaki T, Ozaki N, Miyazaki M, et al. (21) Critical role of camp-gefii--rim2 complex in incretin-potentiated insulin secretion. J Biol Chem 276: Kelley GG, Chepurny OG, Schwede F, Genieser HG, Leech CA, et al. (29) Glucose-dependent potentiation of mouse islet insulin secretion by Epac activator 8-pCPT-2'-O-Me-cAMP-AM. Islets 1: Dzhura I, Chepurny OG, Leech CA, Roe MW, Dzhura E, et al. (211) Phospholipase C-epsilon links Epac2 activation to the potentiation of glucose-stimulated insulin secretion from mouse islets of Langerhans. Islets 3: Nolan CJ, Madiraju MS, Delghingaro-Augusto V, Peyot ML, Prentki M (26) Fatty acid signaling in the beta-cell and insulin secretion. 55 Suppl 2: S Peyot ML, Pepin E, Lamontagne J, Latour MG, Zarrouki B, et al. (21) Betacell failure in diet-induced obese mice stratified according to body weight gain: secretory dysfunction and altered islet lipid metabolism without steatosis or reduced beta-cell mass. 59: Araki T, Hirayama M, Hiroi S, Kaku K (212) GPR4-induced insulin secretion by the novel agonist TAK-875: first clinical findings in patients with type 2 diabetes. Obes Metab 14: Ferdaoussi M, Bergeron V, Zarrouki B, Kolic J, Cantley J, et al. (212) G protein-coupled receptor (GPR)4-dependent potentiation of insulin secretion in mouse islets is mediated by protein kinase D1. Diabetologia 55: Islam D, Zhang N, Wang P, Li H, Brubaker PL, et al. (29) Epac is involved in camp-stimulated proglucagon expression and hormone production but not hormone secretion in pancreatic alpha- and intestinal L-cell lines. Am J Physiol Endocrinol Metab 296: E
32 Page 32 of Rorsman P, Braun M (212) Regulation of Insulin Secretion in Human Pancreatic Islets. Annu Rev Physiol. 31. Leech CA, Dzhura I, Chepurny OG, Schwede F, Genieser HG, et al. (21) Facilitation of ss-cell K(ATP) channel sulfonylurea sensitivity by a camp analog selective for the camp-regulated guanine nucleotide exchange factor Epac. Islets 2: Leech CA, Dzhura I, Chepurny OG, Kang G, Schwede F, et al. (211) Molecular physiology of glucagon-like peptide-1 insulin secretagogue action in pancreatic beta cells. Prog Biophys Mol Biol 17: Seino S (212) Cell signalling in insulin secretion: the molecular targets of ATP, camp and sulfonylurea. Diabetologia 55: Seino S, Shibasaki T, Minami K (21) Pancreatic beta-cell signaling: toward better understanding of diabetes and its treatment. Proc Jpn Acad Ser B Phys Biol Sci 86: Holz GGt, Kuhtreiber WM, Habener JF (1993) Pancreatic beta-cells are rendered glucose-competent by the insulinotropic hormone glucagon-like peptide-1(7-37). Nature 361: Kang G, Chepurny OG, Malester B, Rindler MJ, Rehmann H, et al. (26) camp sensor Epac as a determinant of ATP-sensitive potassium channel activity in human pancreatic beta cells and rat INS-1 cells. J Physiol 573: Kang G, Leech CA, Chepurny OG, Coetzee WA, Holz GG (28) Role of the camp sensor Epac as a determinant of KATP channel ATP sensitivity in human pancreatic beta-cells and rat INS-1 cells. J Physiol 586: Holz GG, Kang G, Harbeck M, Roe MW, Chepurny OG (26) Cell physiology of camp sensor Epac. J Physiol 577: Lemmens R, Larsson O, Berggren PO, Islam MS (21) Ca2+-induced Ca2+ release from the endoplasmic reticulum amplifies the Ca2+ signal mediated by activation of voltage-gated L-type Ca2+ channels in pancreatic beta-cells. J Biol Chem 276: Fernandez-Mejia C, Vega-Allende J, Rojas-Ochoa A, Rodriguez-Dorantes M, Romero-Navarro G, et al. (21) Cyclic adenosine 3',5'-monophosphate increases pancreatic glucokinase activity and gene expression. Endocrinology 142: O'Doherty U, Swiggard WJ, Malim MH (2) Human immunodeficiency virus type 1 spinoculation enhances infection through virus binding. J Virol 74: Livak KJ, Schmittgen TD (21) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25:
33 Page 33 of 48 Song et al Figure 1 A EPAC isoform mrna in islets fold expression normalized to EPAC2A in WT (mean±sem; n=4) 1.5 p<.5 1. wild-type EPAC2A KO B IB IB IB IB IB shrna EPAC1 EPAC2 SNAP25 VAMP2 RIM1/2 wild-type islets EPAC2 KO islets scrambled EPAC1 scrambled EPAC1.5 NS EPAC1 EPAC2A NS EPAC2B IB IB Syntaxin 1A actin C EPAC1 knockdown in EPAC2A KO islets insulin release (ng/ml*3 min) (mean±sem; n=3 all points) 3 scr shrna E4 (1 nm) EPAC1 shrna vehicle 2 1 vehicle E4 (1 nm) glucose 3 mm 1 mm D WT islets insulin release (% of islet content *3 min) (mean±sem; n=3 all points) 3 vehicle E4 1 nm 6BNZ 1 µm 2 ESCA 1 µm PMAP 5 nm * p<.5 vs vehicle 1 # p<.5 vs E4 * # * * * E EPAC2A KO islets insulin release (% of islet content *3 min) (mean±sem; n=3 all points) 3 vehicle E4 1 nm 6BNZ 1 µm 2 ESCA 1 µm PMAP 5 nm 1 * p<.5 vs vehicle! p<.5 vs same treatment in WT islet * *,! *,!! glucose 3 mm 1 mm glucose 3 mm 1 mm
34 Page 34 of 48 Song et al Figure 2 A B oral glucose tolerance test plasma glucose(mg/dl) (mean±sem) 6 WT (n=5) 5 EPAC2A KO (n=5) intraperitoneal glucose tolerance test plasma glucose(mg/dl) (mean±sem) WT (n=6) 6 EPAC2A KO (n=5) E F Islet perifusion studies WT (n=3) EPAC2A KO (n=3) insulin(mg/min/islet; mean±sem) mM 1mM glucose intracellular islet calcium (Fura-2) 34/38 nm ratio (mean±sem) WT (n=4) EPAC2A KO (n=4).3 3 mm glucose C intraperitoneal glucose tolerance test serum insulin (pg/ml) (mean±sem) WT (n=3) EPAC2A KO (n=3) G intracellular islet calcium (Fura-2) 34/38 nm ratio (mean±sem) WT (n=8) EPAC2A KO (n=8) (* p<.5).6 1 mm glucose D intraperitoneal insulin tolerance test plasma glucose(mg/dl) (mean±sem, * p<.5) 2 15 WT (n=7) EPAC2A KO (n=12) * 1 5 * * *
35 Page 35 of 48 Song et al Figure 3 A single ip E4 followed by ip glucose tolerance test plasma glucose(mg/dl) (mean±sem) Glucose E4 WT (n=4) EPAC2A KO (n=5) 1 * p<.5 * * * * * C Islet perifusion studies insulin(mg/min/islet; mean±sem) mM E4 1 nm * p<.5 1mM glucose WT (n=3) EPAC2A KO (n=3) * * * * * B single ip E4 followed by ip glucose tolerance test serum insulin (pg/ml) (mean±sem) WT (n=3) EPAC2A KO (n=3) D intracellular islet calcium (Fura-2) 34/38 nm ratio (mean±sem) WT (n=4) EPAC2A KO (n=4).3 3 mm glucose + 1 nm E4 4 3 Glucose E *** * p< E intracellular islet calcium (Fura-2) 34/38 nm ratio (mean±sem) WT (n=4) EPAC2A KO (n=4) (* p<.5) 1 mm glucose + 1 nm E * * * * * * * * *
36 Page 36 of 48 Song et al Figure 4 A B intraperitoneal glucose tolerance test WT on HFD (n=5) EPAC2A KO on HFD (n=4) plasma glucose (mg/dl) (mean±sem,* p<.5) Glucose * * intraperitoneal glucose tolerance test serum insulin (mean±sem) Glucose (pg/ml) WT on HFD (n=3) EPAC2A KO on HFD (n=3) D E Islet perifusion studies insulin (mg/min/islet; mean±sem) mM * p<.5 * 1mM glucose * WT on HFD (n=3) EPAC2A KO on HFD (n=3) intracellular islet calcium (Fura-2) 34/38 nm ratio (mean±sem).3.25 WT on HFD (n=4) EPAC2A KO on HFD (n=4) 3 mm glucose 1 ** * * p< C intraperitoneal insulin tolerance test plasma glucose(mg/dl) (mean±sem, * p<.5) 2 15 WT (n=5) EPAC2A KO (n=6) F intracellular islet calcium (Fura-2) 34/38 nm ratio (mean±sem) WT on HFD (n=4) EPAC2A KO on HFD (n=4) (* p<.5) 1 mm glucose * * * * * * * *
37 Page 37 of 48 Song et al Figure 5 A B C intraperitoneal glucose tolerance test plasma glucose(mg/dl) (mean±sem) prkar1a (n=8) 6 prkar1a/epac2a KO (n=3) intraperitoneal glucose tolerance test serum insulin (pg/ml) (mean±sem) Glucose * * intraperitoneal insulin tolerance test plasma glucose(mg/dl) (mean±sem) prkar1a (n=3) prkar1a/epac2a KO (n=3) * p< * * D E Islet perifusion studies insulin(mg/min/islet; mean±sem) intracellular islet calcium (Fura-2) 34/38 nm ratio (mean±sem) prkar1a (n=4) prkar1a/epac2a KO (n=4) (* p<.5) 3 mm glucose mM * p<.5 * 1mM glucose prkar1a (n=3) prkar1a/epac2a KO (n=3) 2 15 prkar1a (n=8) prkar1a/epac2a KO (n=3) F intracellular islet calcium (Fura-2) 34/38 nm ratio (mean±sem) prkar1a (n=4) prkar1a/epac2a KO (n=4) (* p<.5) 1 mm glucose * * * * *
38 Page 38 of 48 Song et al. Figure 6 A Islet perifusion studies insulin(mg/min/islet; mean±sem) mM 1mM glucose PMAP 5 nm * p<.5 * WT (n=3) EPAC2A KO (n=3) B intracellular islet calcium (Fura-2) 34/38 nm ratio (mean±sem).3 WT (n=4) EPAC2A KO (n=4) 3 mm glucose + 5 nm PMAP C intracellular islet calcium (Fura-2) 34/38 nm ratio (mean±sem) WT (n=4) EPAC2A KO (n=4) (* p<.5) 1 mm glucose + 5 nm PMAP * * * *
39 Page 39 of 48 Supplemental Appendix Supplemental Table S1. Area under the curve (AUC) of perifusion studies on Top: isolated WT and EPAC2A KO islets, First phase: -1 min after glucose stimulation (glucose increases from 3 to 1 mm); Second phase: 1-2 min after glucose stimulation. Bottom: prkar1a and prkar1a/eapac2a KO islets. N=3 in each group. First phase: -5 min after glucose stimulation (glucose increases from 3 to 1 mm); Second phase: 5-2 min after glucose stimulation. Treatment Phase of insulin secretion Insulin AUC WT islets pg/islet (mean+sem) Insulin AUC EPAC2A KO pg/islet (mean+sem) P - 1 st phase NS - 2 nd phase NS E4 1 nm 1 st phase <.5 2 nd phase <.5 High fat diet 1 st phase <.5 2 nd phase NS PMAP 5 nm 1 st phase <.5 2 nd phase NS 6BNZ 1 µm 1 st phase NS 2 nd phase NS ESCA 1 µm 1 st phase <.5 2 nd phase <.5 Phase of insulin secretion Insulin AUC prkar1a pg/islet (mean+sem) Insulin AUC prkar1a/epac2a KO pg/islet (mean+sem) P - 1 st phase <.5-2 nd phase NS
40 Page 4 of 48 Supplemental Table S2 Antibodies used for co-immunoprecipitation assays and immunoblots Detected antigen Antibody source Catalog number Band size on immunoblot EPAC1 Abcam ab kd EPAC2 Santa Cruz sc kd Rim1/2 Santa Cruz sc kd SNAP25 Santa Cruz sc kd Syntaxin1A Abcam ab kd VAMP2 Santa Cruz sc kd Actin Millipore MAB151 4 kd
41 Page 41 of 48 Supplemental Figure S1 Incretin receptor activation but not GPR4 receptor activation increases Islet camp levels similarly in WT EPAC2A KO islets. WT and EPAC2A KO islets have similar glucokinase activity A: E4 stimulation generates camp production to similar degrees in both cultured WT (grey bars) and EPAC2A KO (black bars) islets. EPAC2A ablation does not influence E4 stimulated camp generation. B: PMAP does not stimulate camp production in WT (grey bars) or EPAC2A (black bars) islets. C: E4 stimulation generates camp production to similar degrees in prkar1a (grey bars) and prkar1a/epac2a KO (black bars) islets. prkar1a islets do not exhibit higher E4 stimulated camp production as compared to WT islets (shown panel A). D: Glucokinase (GK) activity is similar in WT (grey bars) and EPAC2A KO (black bars) islets and is not changed by acute (3 min) E4 treatment Values are normalized to WT islets Results are shown as mean+sem of studies performed at least in triplicate. * indicates p<.5. For details see text.
42 Page 42 of 48 Supplemental Figure S2 Co-immunoprecipitation studies of WT and EPAC2A KO islets Delayed interaction of SNARE protein components in EPAC2A KO islets stimulated with E4 A. WT and EPAC2A KO islets exposed to E4 for 3 minutes at 3 mm glucose were stimulated with 1 mm glucose. Protein extracts were obtained at indicated time points after glucose simulation for Co-IP/IB studies to assess SNAP25-VAMP2 and SNAP25-Syntaxin1A SNARE protein interaction. Input into IP studies (1% of protein extract) and SNAP25-specific IP indicate similar amounts of proteins were used at each time point during the time-course. Representative blots from 3 separate Co-IP studies are shown. B. Densitometric analysis of VAMP2 Co-IP. Band intensity is normalized to actin of respective time point. Time = min was defined as 1 arbitrary units C. Densitometric analysis of Syntaxin1A Co-IP. Band intensity is normalized to actin of respective time point. Time = min was defined as 1 arbitrary units.
43 Page 43 of 48 Supplemental Figure S3 EPAC2A ablation impairs dynamic insulin response in HFD fed mice of model of ß-cell autonomous GSIS augmentation ( prkar1a mice) A: During ipgtt, HFD- prkar1a and HFD- prkar1a/epac2a KO mice exhibit similar glucose excursions. B: During ITT, HFD- prkar1a and HFD- prkar1a/epac2a KO show similar insulin sensitivity C: ipgtt, HFD- prkar1a exhibit dramatically increased GSIS. HFD- prkar1a/epac2a KO show increased GSIS, with a blunted first-phase insulin secretory response and a relatively increased second phase insulin secretory response. prkar1a is represented in gray squares; HFD- prkar1a/epac2a KO is represented in black squares. Results are shown as mean+sem of studies performed at least in triplicate. * indicates p<.5. For details see text.
44 Page 44 of 48 Supplemental Figure S4 Perifusion studies of PKA, EPAC2A and GPR4 interplay A: EPAC2A is required for full effect of PKA mediated GSIS stimulation. Stimulation with the PKA-selective camp agonist 6BZN augments GSIS in WT islets. EPAC2A KO islets also exhibit augmented GSIS under 6BZN stimulation. However, the immediate early (4-42 min) insulin secretory response is diminished in EPAC2A KO islets. B: Stimulation with the EPAC2-selective camp analog (ESCA) augments GSIS in WT islets. ESCA mediated GSIS augmentation is abolished in EPAC2A KO islets, which exhibit GSIS similar to WT islets without any pharmacologic secretagogue stimulation (see Figure 2E) C: PKA inhibition with PKI abolishes GSIS in WT and EPAC2A KO islets. A small burst of insulin secretion is detectable early (4-45 min) after stimulation with 1 mm glucose. KCL depolarization at completion of perifusion results in insulin release. D: PKA inhibition with PKI abolishes GSIS in E4 treated WT and EPAC2A KO islets. A small burst of insulin secretion is detectable early (4-45 min) after stimulation with 1 mm glucose. KCL depolarization at completion of perifusion results in insulin release. E: PKA inhibition with PKI abolishes GSIS in ESCA treated WT and EPAC2A KO islets. A small burst of insulin secretion is detectable early (4-45 min) after stimulation with 1 mm glucose. KCL depolarization at completion of perifusion results in insulin release. F: PKA inhibition with PKI does not affect GSIS in (GPR4 activated) PMAP treated islets (see Figure 6A). PKA inhibition in EPAC2A KO islets treated with PMAP show blunted GSIS. KCL depolarization at completion of perifusion results in insulin release. Results are shown as mean+sem of studies performed at least in triplicate. * indicates p<.5. For details see text.
45 Page 45 of 48 Song et al Figure S1 A islet camp (pmol/ml) after E4 (1 nm) (n=3 each group; mean±sem) WT EPAC2A KO B islet camp (pmol/ml) after PMAP (5 nm) (n=3 each group; mean±sem) WT EPAC2A KO 25 NS 25 2 p< p< NS NS 5 5 PBS E4 PBS E4 DMSO PMAP DMSOPMAP C islet camp (pmol/ml) after E4 (1 nm) (n=3 each group; mean±sem) prkar1a prkar1a/epac2a KO NS 25 p<.5 2 p<.5 D islet glucokinase activity relative to untreated WT islets (n=4 each group; mean±sem) WT EPAC2A KO PBS E4 PBS E4 PBS E4 PBS E4 glucose mm
46 Page 46 of 48 Song, Mondal Figure S2 minutes after glucose 1 mm stimulation IB: SNAP25 Input IB: VAMP2 IB: Syntaxin1A Co-immunoprecipitation: IP: SNAP25 IB: SNAP25 Input pretreatment: E4 1 nm IP: SNAP25 IB: VAMP2 IP: SNAP25 IB: Syntaxin1A IB: actin a b c a b c A WT EPAC2A KO densitometry WT EPAC2A KO Co-IP VAMP2 (relative to actin AU) (meansem, n=3, *p<.5) * * 3 2 B * * C Co-IP Syntaxin1A (relative to actin AU) (meansem, n=3, *p<.5) * * * 1 Time min min
Supplementary Figure 1: Steviol and stevioside potentiate TRPM5 in a cell-free environment. (a) TRPM5 currents are activated in inside-out patches
 Supplementary Figure 1: Steviol and stevioside potentiate TRPM5 in a cell-free environment. (a) TRPM5 currents are activated in inside-out patches during application of 500 µm Ca 2+ at the intracellular
Supplementary Figure 1: Steviol and stevioside potentiate TRPM5 in a cell-free environment. (a) TRPM5 currents are activated in inside-out patches during application of 500 µm Ca 2+ at the intracellular
General Laboratory methods Plasma analysis: Gene Expression Analysis: Immunoblot analysis: Immunohistochemistry:
 General Laboratory methods Plasma analysis: Plasma insulin (Mercodia, Sweden), leptin (duoset, R&D Systems Europe, Abingdon, United Kingdom), IL-6, TNFα and adiponectin levels (Quantikine kits, R&D Systems
General Laboratory methods Plasma analysis: Plasma insulin (Mercodia, Sweden), leptin (duoset, R&D Systems Europe, Abingdon, United Kingdom), IL-6, TNFα and adiponectin levels (Quantikine kits, R&D Systems
(a) Significant biological processes (upper panel) and disease biomarkers (lower panel)
 Supplementary Figure 1. Functional enrichment analyses of secretomic proteins. (a) Significant biological processes (upper panel) and disease biomarkers (lower panel) 2 involved by hrab37-mediated secretory
Supplementary Figure 1. Functional enrichment analyses of secretomic proteins. (a) Significant biological processes (upper panel) and disease biomarkers (lower panel) 2 involved by hrab37-mediated secretory
Defects in glucose utilization & GLP-1
 Defects in glucose utilization & GLP-1 Song, Dae-Kyu Department of Physiology & Chronic Disease Research Center Keimyung University School of Medicine 2800 dalgubeol-daero, Dalseo-gu, Daegu, 704-701, Korea
Defects in glucose utilization & GLP-1 Song, Dae-Kyu Department of Physiology & Chronic Disease Research Center Keimyung University School of Medicine 2800 dalgubeol-daero, Dalseo-gu, Daegu, 704-701, Korea
Online Appendix Material and Methods: Pancreatic RNA isolation and quantitative real-time (q)rt-pcr. Mice were fasted overnight and killed 1 hour (h)
 Online Appendix Material and Methods: Pancreatic RNA isolation and quantitative real-time (q)rt-pcr. Mice were fasted overnight and killed 1 hour (h) after feeding. A small slice (~5-1 mm 3 ) was taken
Online Appendix Material and Methods: Pancreatic RNA isolation and quantitative real-time (q)rt-pcr. Mice were fasted overnight and killed 1 hour (h) after feeding. A small slice (~5-1 mm 3 ) was taken
Glucose Homeostasis. Liver. Glucose. Muscle, Fat. Pancreatic Islet. Glucose utilization. Glucose production, storage Insulin Glucagon
 Glucose Homeostasis Liver Glucose Glucose utilization Glucose production, storage Insulin Glucagon Muscle, Fat Pancreatic Islet Classification of Diabetes Type 1 diabetes Type 2 diabetes Other types of
Glucose Homeostasis Liver Glucose Glucose utilization Glucose production, storage Insulin Glucagon Muscle, Fat Pancreatic Islet Classification of Diabetes Type 1 diabetes Type 2 diabetes Other types of
SUPPLEMENTARY DATA. Supplementary Table 1. Primers used for PCR and qpcr Primer Name
 Supplementary Table. Primers used for PCR and qpcr Primer Name ccession Number Fwd Rev Type of PCR Cre NC_8 GGCGTCTTCCGC GTGCCCCTCGTTTG Standard PCR LoUcp CCGGGCTGTCTCCGCGG GGCTGTTCGCCCGGCC Standard PCR
Supplementary Table. Primers used for PCR and qpcr Primer Name ccession Number Fwd Rev Type of PCR Cre NC_8 GGCGTCTTCCGC GTGCCCCTCGTTTG Standard PCR LoUcp CCGGGCTGTCTCCGCGG GGCTGTTCGCCCGGCC Standard PCR
GPR120 *** * * Liver BAT iwat ewat mwat Ileum Colon. UCP1 mrna ***
 a GPR120 GPR120 mrna/ppia mrna Arbitrary Units 150 100 50 Liver BAT iwat ewat mwat Ileum Colon b UCP1 mrna Fold induction 20 15 10 5 - camp camp SB202190 - - - H89 - - - - - GW7647 Supplementary Figure
a GPR120 GPR120 mrna/ppia mrna Arbitrary Units 150 100 50 Liver BAT iwat ewat mwat Ileum Colon b UCP1 mrna Fold induction 20 15 10 5 - camp camp SB202190 - - - H89 - - - - - GW7647 Supplementary Figure
Cells and reagents. Synaptopodin knockdown (1) and dynamin knockdown (2)
 Supplemental Methods Cells and reagents. Synaptopodin knockdown (1) and dynamin knockdown (2) podocytes were cultured as described previously. Staurosporine, angiotensin II and actinomycin D were all obtained
Supplemental Methods Cells and reagents. Synaptopodin knockdown (1) and dynamin knockdown (2) podocytes were cultured as described previously. Staurosporine, angiotensin II and actinomycin D were all obtained
Nature Immunology: doi: /ni.3631
 Supplementary Figure 1 SPT analyses of Zap70 at the T cell plasma membrane. (a) Total internal reflection fluorescent (TIRF) excitation at 64-68 degrees limits single molecule detection to 100-150 nm above
Supplementary Figure 1 SPT analyses of Zap70 at the T cell plasma membrane. (a) Total internal reflection fluorescent (TIRF) excitation at 64-68 degrees limits single molecule detection to 100-150 nm above
Reviewers' comments: Reviewer #1 Expert in EAE and IL-17a (Remarks to the Author):
 Reviewers' comments: Reviewer #1 Expert in EAE and IL-17a (Remarks to the Author): This study shows that the inducible camp early repressor (ICER) is involved in development of Th17 cells that are pathogenic
Reviewers' comments: Reviewer #1 Expert in EAE and IL-17a (Remarks to the Author): This study shows that the inducible camp early repressor (ICER) is involved in development of Th17 cells that are pathogenic
A novel role for vitamin D: modulation of expression and function of the local renin angiotensin system in mouse pancreatic islets
 Diabetologia () 5:77 DOI.7/s5--- SHORT COMMUNICATION A novel role for vitamin D: modulation of expression and function of the local renin angiotensin system in mouse pancreatic islets Q. Cheng & Y. C.
Diabetologia () 5:77 DOI.7/s5--- SHORT COMMUNICATION A novel role for vitamin D: modulation of expression and function of the local renin angiotensin system in mouse pancreatic islets Q. Cheng & Y. C.
Abbreviations: P- paraffin-embedded section; C, cryosection; Bio-SA, biotin-streptavidin-conjugated fluorescein amplification.
 Supplementary Table 1. Sequence of primers for real time PCR. Gene Forward primer Reverse primer S25 5 -GTG GTC CAC ACT ACT CTC TGA GTT TC-3 5 - GAC TTT CCG GCA TCC TTC TTC-3 Mafa cds 5 -CTT CAG CAA GGA
Supplementary Table 1. Sequence of primers for real time PCR. Gene Forward primer Reverse primer S25 5 -GTG GTC CAC ACT ACT CTC TGA GTT TC-3 5 - GAC TTT CCG GCA TCC TTC TTC-3 Mafa cds 5 -CTT CAG CAA GGA
Supporting Information
 Supporting Information Pang et al. 10.1073/pnas.1322009111 SI Materials and Methods ELISAs. These assays were performed as previously described (1). ELISA plates (MaxiSorp Nunc; Thermo Fisher Scientific)
Supporting Information Pang et al. 10.1073/pnas.1322009111 SI Materials and Methods ELISAs. These assays were performed as previously described (1). ELISA plates (MaxiSorp Nunc; Thermo Fisher Scientific)
Supplementary Figure 1
 VO (ml kg - min - ) VCO (ml kg - min - ) Respiratory exchange ratio Energy expenditure (cal kg - min - ) Locomotor activity (x count) Body temperature ( C) Relative mrna expression TA Sol EDL PT Heart
VO (ml kg - min - ) VCO (ml kg - min - ) Respiratory exchange ratio Energy expenditure (cal kg - min - ) Locomotor activity (x count) Body temperature ( C) Relative mrna expression TA Sol EDL PT Heart
SUPPLEMENTARY INFORMATION
 DOI: 10.1038/ncb3461 In the format provided by the authors and unedited. Supplementary Figure 1 (associated to Figure 1). Cpeb4 gene-targeted mice develop liver steatosis. a, Immunoblot displaying CPEB4
DOI: 10.1038/ncb3461 In the format provided by the authors and unedited. Supplementary Figure 1 (associated to Figure 1). Cpeb4 gene-targeted mice develop liver steatosis. a, Immunoblot displaying CPEB4
Supplementary Figure 1. DJ-1 modulates ROS concentration in mouse skeletal muscle.
 Supplementary Figure 1. DJ-1 modulates ROS concentration in mouse skeletal muscle. (a) mrna levels of Dj1 measured by quantitative RT-PCR in soleus, gastrocnemius (Gastroc.) and extensor digitorum longus
Supplementary Figure 1. DJ-1 modulates ROS concentration in mouse skeletal muscle. (a) mrna levels of Dj1 measured by quantitative RT-PCR in soleus, gastrocnemius (Gastroc.) and extensor digitorum longus
Use of a camp BRET Sensor to Characterize a Novel Regulation of camp by the Sphingosine-1-phosphate/G 13 Pathway
 Use of a camp BRET Sensor to Characterize a Novel Regulation of camp by the Sphingosine-1-phosphate/G 13 Pathway SUPPLEMENTAL DATA Characterization of the CAMYEL sensor and calculation of intracellular
Use of a camp BRET Sensor to Characterize a Novel Regulation of camp by the Sphingosine-1-phosphate/G 13 Pathway SUPPLEMENTAL DATA Characterization of the CAMYEL sensor and calculation of intracellular
Figure S1 (A) Comparison of body weight and blood glucose levels in male wild-type (WT) and Cre littermates. Mice were maintained on a normal chow
 Figure S1 (A) Comparison of body weight and blood glucose levels in male wild-type (WT) and Cre littermates. Mice were maintained on a normal chow diet (n = 7 per genotype). Body weight and fed blood glucose
Figure S1 (A) Comparison of body weight and blood glucose levels in male wild-type (WT) and Cre littermates. Mice were maintained on a normal chow diet (n = 7 per genotype). Body weight and fed blood glucose
SUPPLEMENTARY DATA. Supplementary experimental procedures
 Supplementary experimental procedures Glucose tolerance, insulin secretion and insulin tolerance tests Glucose tolerance test (GTT) and glucose stimulated insulin secretion (GSIS) was measured in over-night
Supplementary experimental procedures Glucose tolerance, insulin secretion and insulin tolerance tests Glucose tolerance test (GTT) and glucose stimulated insulin secretion (GSIS) was measured in over-night
Lecture 9: Cell Communication I
 02.05.10 Lecture 9: Cell Communication I Multicellular organisms need to coordinate cellular functions in different tissues Cell-to-cell communication is also used by single celled organisms to signal
02.05.10 Lecture 9: Cell Communication I Multicellular organisms need to coordinate cellular functions in different tissues Cell-to-cell communication is also used by single celled organisms to signal
Supporting Information Table of Contents
 Supporting Information Table of Contents Supporting Information Figure 1 Page 2 Supporting Information Figure 2 Page 4 Supporting Information Figure 3 Page 5 Supporting Information Figure 4 Page 6 Supporting
Supporting Information Table of Contents Supporting Information Figure 1 Page 2 Supporting Information Figure 2 Page 4 Supporting Information Figure 3 Page 5 Supporting Information Figure 4 Page 6 Supporting
Supplementary Figure 1.
 Supplementary Figure 1. Increased β cell mass and islet diameter in βtsc2 -/- mice up to 35 weeks A: Reconstruction of multiple anti-insulin immunofluorescence images showing differences in β cell mass
Supplementary Figure 1. Increased β cell mass and islet diameter in βtsc2 -/- mice up to 35 weeks A: Reconstruction of multiple anti-insulin immunofluorescence images showing differences in β cell mass
hexahistidine tagged GRP78 devoid of the KDEL motif (GRP78-His) on SDS-PAGE. This
 SUPPLEMENTAL FIGURE LEGEND Fig. S1. Generation and characterization of. (A) Coomassie staining of soluble hexahistidine tagged GRP78 devoid of the KDEL motif (GRP78-His) on SDS-PAGE. This protein was expressed
SUPPLEMENTAL FIGURE LEGEND Fig. S1. Generation and characterization of. (A) Coomassie staining of soluble hexahistidine tagged GRP78 devoid of the KDEL motif (GRP78-His) on SDS-PAGE. This protein was expressed
Cellular Messengers. Intracellular Communication
 Cellular Messengers Intracellular Communication Most common cellular communication is done through extracellular chemical messengers: Ligands Specific in function 1. Paracrines Local messengers (neighboring
Cellular Messengers Intracellular Communication Most common cellular communication is done through extracellular chemical messengers: Ligands Specific in function 1. Paracrines Local messengers (neighboring
Supplementary Materials for
 www.sciencesignaling.org/cgi/content/full/8/407/ra127/dc1 Supplementary Materials for Loss of FTO in adipose tissue decreases Angptl4 translation and alters triglyceride metabolism Chao-Yung Wang,* Shian-Sen
www.sciencesignaling.org/cgi/content/full/8/407/ra127/dc1 Supplementary Materials for Loss of FTO in adipose tissue decreases Angptl4 translation and alters triglyceride metabolism Chao-Yung Wang,* Shian-Sen
Supplementary Figure 1) GABAergic enhancement by leptin hyperpolarizes POMC neurons A) Representative recording samples showing the membrane
 Supplementary Figure 1) GABAergic enhancement by leptin hyperpolarizes POMC neurons A) Representative recording samples showing the membrane potential recorded from POMC neurons following treatment with
Supplementary Figure 1) GABAergic enhancement by leptin hyperpolarizes POMC neurons A) Representative recording samples showing the membrane potential recorded from POMC neurons following treatment with
BMP6 treatment compensates for the molecular defect and ameliorates hemochromatosis in Hfe knockout mice
 SUPPLEMENTARY MATERIALS BMP6 treatment compensates for the molecular defect and ameliorates hemochromatosis in Hfe knockout mice Elena Corradini, Paul J. Schmidt, Delphine Meynard, Cinzia Garuti, Giuliana
SUPPLEMENTARY MATERIALS BMP6 treatment compensates for the molecular defect and ameliorates hemochromatosis in Hfe knockout mice Elena Corradini, Paul J. Schmidt, Delphine Meynard, Cinzia Garuti, Giuliana
SUPPLEMENTARY INFORMATION
 SUPPLEMENTARY INFORMATION FOR Liver X Receptor α mediates hepatic triglyceride accumulation through upregulation of G0/G1 Switch Gene 2 (G0S2) expression I: SUPPLEMENTARY METHODS II: SUPPLEMENTARY FIGURES
SUPPLEMENTARY INFORMATION FOR Liver X Receptor α mediates hepatic triglyceride accumulation through upregulation of G0/G1 Switch Gene 2 (G0S2) expression I: SUPPLEMENTARY METHODS II: SUPPLEMENTARY FIGURES
Discussion & Conclusion
 Discussion & Conclusion 7. Discussion DPP-4 inhibitors augment the effects of incretin hormones by prolonging their half-life and represent a new therapeutic approach for the treatment of type 2 diabetes
Discussion & Conclusion 7. Discussion DPP-4 inhibitors augment the effects of incretin hormones by prolonging their half-life and represent a new therapeutic approach for the treatment of type 2 diabetes
General Principles of Endocrine Physiology
 General Principles of Endocrine Physiology By Dr. Isabel S.S. Hwang Department of Physiology Faculty of Medicine University of Hong Kong The major human endocrine glands Endocrine glands and hormones
General Principles of Endocrine Physiology By Dr. Isabel S.S. Hwang Department of Physiology Faculty of Medicine University of Hong Kong The major human endocrine glands Endocrine glands and hormones
Supplementary Table 1. Primer sequences for conventional RT-PCR on mouse islets
 Supplementary Table 1. Primer sequences for conventional RT-PCR on mouse islets Gene 5 Forward 3 5 Reverse 3.T. Product (bp) ( C) mnox1 GTTCTTGGGCTGCCTTGG GCTGGGGCGGCGG 60 300 mnoxa1 GCTTTGCCGCGTGC GGTTCGGGTCCTTTGTGC
Supplementary Table 1. Primer sequences for conventional RT-PCR on mouse islets Gene 5 Forward 3 5 Reverse 3.T. Product (bp) ( C) mnox1 GTTCTTGGGCTGCCTTGG GCTGGGGCGGCGG 60 300 mnoxa1 GCTTTGCCGCGTGC GGTTCGGGTCCTTTGTGC
Propagation of the Signal
 OpenStax-CNX module: m44452 1 Propagation of the Signal OpenStax College This work is produced by OpenStax-CNX and licensed under the Creative Commons Attribution License 3.0 By the end of this section,
OpenStax-CNX module: m44452 1 Propagation of the Signal OpenStax College This work is produced by OpenStax-CNX and licensed under the Creative Commons Attribution License 3.0 By the end of this section,
SUPPLEMENTARY INFORMATION
 doi:10.1038/nature12652 Supplementary Figure 1. PRDM16 interacts with endogenous EHMT1 in brown adipocytes. Immunoprecipitation of PRDM16 complex by flag antibody (M2) followed by Western blot analysis
doi:10.1038/nature12652 Supplementary Figure 1. PRDM16 interacts with endogenous EHMT1 in brown adipocytes. Immunoprecipitation of PRDM16 complex by flag antibody (M2) followed by Western blot analysis
Ghrelin Stimulates Porcine Somatotropes
 Animal Industry Report AS 650 ASL R1957 2004 Ghrelin Stimulates Porcine Somatotropes Aleksandra Glavaski-Joksimovic Ksenija Jeftinija Colin G. Scanes Lloyd L. Anderson Srdija Jeftinija Recommended Citation
Animal Industry Report AS 650 ASL R1957 2004 Ghrelin Stimulates Porcine Somatotropes Aleksandra Glavaski-Joksimovic Ksenija Jeftinija Colin G. Scanes Lloyd L. Anderson Srdija Jeftinija Recommended Citation
Cell Communication. Local and Long Distance Signaling
 Cell Communication Cell to cell communication is essential for multicellular organisms Some universal mechanisms of cellular regulation providing more evidence for the evolutionary relatedness of all life
Cell Communication Cell to cell communication is essential for multicellular organisms Some universal mechanisms of cellular regulation providing more evidence for the evolutionary relatedness of all life
Asma Karameh Omar Sami
 5 Asma Karameh Omar Sami Mohammad khatatbeh Happy day friends! This lecture will be discussing what we have said in the previous lectures relating to different mechanisms of transport across a biological
5 Asma Karameh Omar Sami Mohammad khatatbeh Happy day friends! This lecture will be discussing what we have said in the previous lectures relating to different mechanisms of transport across a biological
Supplemental information
 Carcinoemryonic antigen-related cell adhesion molecule 6 (CEACAM6) promotes EGF receptor signaling of oral squamous cell carcinoma metastasis via the complex N-glycosylation y Chiang et al. Supplemental
Carcinoemryonic antigen-related cell adhesion molecule 6 (CEACAM6) promotes EGF receptor signaling of oral squamous cell carcinoma metastasis via the complex N-glycosylation y Chiang et al. Supplemental
Supplementary information. MARCH8 inhibits HIV-1 infection by reducing virion incorporation of envelope glycoproteins
 Supplementary information inhibits HIV-1 infection by reducing virion incorporation of envelope glycoproteins Takuya Tada, Yanzhao Zhang, Takayoshi Koyama, Minoru Tobiume, Yasuko Tsunetsugu-Yokota, Shoji
Supplementary information inhibits HIV-1 infection by reducing virion incorporation of envelope glycoproteins Takuya Tada, Yanzhao Zhang, Takayoshi Koyama, Minoru Tobiume, Yasuko Tsunetsugu-Yokota, Shoji
Electron micrograph of phosphotungstanic acid-stained exosomes derived from murine
 1 SUPPLEMENTARY INFORMATION SUPPLEMENTARY FIGURES Supplementary Figure 1. Physical properties of murine DC-derived exosomes. a, Electron micrograph of phosphotungstanic acid-stained exosomes derived from
1 SUPPLEMENTARY INFORMATION SUPPLEMENTARY FIGURES Supplementary Figure 1. Physical properties of murine DC-derived exosomes. a, Electron micrograph of phosphotungstanic acid-stained exosomes derived from
SUPPLEMENTARY INFORMATION
 SUPPLEMENTARY INFORMATION doi:10.1038/nature11464 Supplemental Figure S1. The expression of Vegfb is increased in obese and diabetic mice as compared to lean mice. a-b, Body weight and postprandial blood
SUPPLEMENTARY INFORMATION doi:10.1038/nature11464 Supplemental Figure S1. The expression of Vegfb is increased in obese and diabetic mice as compared to lean mice. a-b, Body weight and postprandial blood
Supplementary Table 1. The primers used for quantitative RT-PCR. Gene name Forward (5 > 3 ) Reverse (5 > 3 )
 770 771 Supplementary Table 1. The primers used for quantitative RT-PCR. Gene name Forward (5 > 3 ) Reverse (5 > 3 ) Human CXCL1 GCGCCCAAACCGAAGTCATA ATGGGGGATGCAGGATTGAG PF4 CCCCACTGCCCAACTGATAG TTCTTGTACAGCGGGGCTTG
770 771 Supplementary Table 1. The primers used for quantitative RT-PCR. Gene name Forward (5 > 3 ) Reverse (5 > 3 ) Human CXCL1 GCGCCCAAACCGAAGTCATA ATGGGGGATGCAGGATTGAG PF4 CCCCACTGCCCAACTGATAG TTCTTGTACAGCGGGGCTTG
Goals and Challenges of Communication. Communication and Signal Transduction. How Do Cells Communicate?
 Goals and Challenges of Communication Reaching (only) the correct recipient(s) Imparting correct information Timeliness Causing the desired effect Effective termination Communication and Signal Transduction
Goals and Challenges of Communication Reaching (only) the correct recipient(s) Imparting correct information Timeliness Causing the desired effect Effective termination Communication and Signal Transduction
Supplementary Fig. 1 eif6 +/- mice show a reduction in white adipose tissue, blood lipids and normal glycogen synthesis. The cohort of the original
 Supplementary Fig. 1 eif6 +/- mice show a reduction in white adipose tissue, blood lipids and normal glycogen synthesis. The cohort of the original phenotypic screening was n=40. For specific tests, the
Supplementary Fig. 1 eif6 +/- mice show a reduction in white adipose tissue, blood lipids and normal glycogen synthesis. The cohort of the original phenotypic screening was n=40. For specific tests, the
Potentiation of Glucose-stimulated Insulin Secretion by the GPR40 PLC TRPC
 Supplementary information Potentiation of Glucose-stimulated Insulin Secretion by the GPR40 PLC TRPC Pathway in Pancreatic -Cells Authors: Hodaka Yamada 1,*, Masashi Yoshida 1,*, Kiyonori Ito 1, Katsuya
Supplementary information Potentiation of Glucose-stimulated Insulin Secretion by the GPR40 PLC TRPC Pathway in Pancreatic -Cells Authors: Hodaka Yamada 1,*, Masashi Yoshida 1,*, Kiyonori Ito 1, Katsuya
SUPPLEMENTARY FIGURES
 SUPPLEMENTARY FIGURES 1 Supplementary Figure 1, Adult hippocampal QNPs and TAPs uniformly express REST a-b) Confocal images of adult hippocampal mouse sections showing GFAP (green), Sox2 (red), and REST
SUPPLEMENTARY FIGURES 1 Supplementary Figure 1, Adult hippocampal QNPs and TAPs uniformly express REST a-b) Confocal images of adult hippocampal mouse sections showing GFAP (green), Sox2 (red), and REST
Supplementary Figures
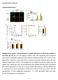 Supplementary Figures Supplementary Figure 1 Characterization of stable expression of GlucB and sshbira in the CT26 cell line (a) Live cell imaging of stable CT26 cells expressing green fluorescent protein
Supplementary Figures Supplementary Figure 1 Characterization of stable expression of GlucB and sshbira in the CT26 cell line (a) Live cell imaging of stable CT26 cells expressing green fluorescent protein
F-actin VWF Vinculin. F-actin. Vinculin VWF
 a F-actin VWF Vinculin b F-actin VWF Vinculin Supplementary Fig. 1. WPBs in HUVECs are located along stress fibers and at focal adhesions. (a) Immunofluorescence images of f-actin (cyan), VWF (yellow),
a F-actin VWF Vinculin b F-actin VWF Vinculin Supplementary Fig. 1. WPBs in HUVECs are located along stress fibers and at focal adhesions. (a) Immunofluorescence images of f-actin (cyan), VWF (yellow),
marker. DAPI labels nuclei. Flies were 20 days old. Scale bar is 5 µm. Ctrl is
 Supplementary Figure 1. (a) Nos is detected in glial cells in both control and GFAP R79H transgenic flies (arrows), but not in deletion mutant Nos Δ15 animals. Repo is a glial cell marker. DAPI labels
Supplementary Figure 1. (a) Nos is detected in glial cells in both control and GFAP R79H transgenic flies (arrows), but not in deletion mutant Nos Δ15 animals. Repo is a glial cell marker. DAPI labels
Supplementary Figure 1. DNA methylation of the adiponectin promoter R1, Pparg2, and Tnfa promoter in adipocytes is not affected by obesity.
 Supplementary Figure 1. DNA methylation of the adiponectin promoter R1, Pparg2, and Tnfa promoter in adipocytes is not affected by obesity. (a) Relative amounts of adiponectin, Ppar 2, C/ebp, and Tnf mrna
Supplementary Figure 1. DNA methylation of the adiponectin promoter R1, Pparg2, and Tnfa promoter in adipocytes is not affected by obesity. (a) Relative amounts of adiponectin, Ppar 2, C/ebp, and Tnf mrna
Cell Communication. Chapter 11. Biology Eighth Edition Neil Campbell and Jane Reece. PowerPoint Lecture Presentations for
 Chapter 11 Cell Communication PowerPoint Lecture Presentations for Biology Eighth Edition Neil Campbell and Jane Reece Lectures by Chris Romero, updated by Erin Barley with contributions from Joan Sharp
Chapter 11 Cell Communication PowerPoint Lecture Presentations for Biology Eighth Edition Neil Campbell and Jane Reece Lectures by Chris Romero, updated by Erin Barley with contributions from Joan Sharp
Receptor mediated Signal Transduction
 Receptor mediated Signal Transduction G-protein-linked receptors adenylyl cyclase camp PKA Organization of receptor protein-tyrosine kinases From G.M. Cooper, The Cell. A molecular approach, 2004, third
Receptor mediated Signal Transduction G-protein-linked receptors adenylyl cyclase camp PKA Organization of receptor protein-tyrosine kinases From G.M. Cooper, The Cell. A molecular approach, 2004, third
BIOLOGY. Cell Communication CAMPBELL. Reece Urry Cain Wasserman Minorsky Jackson. Lecture Presentation by Nicole Tunbridge and Kathleen Fitzpatrick
 CAMPBELL BIOLOGY TENTH EDITION Reece Urry Cain Wasserman Minorsky Jackson 11 Cell Communication Lecture Presentation by Nicole Tunbridge and Kathleen Fitzpatrick Cellular Messaging Cells can signal to
CAMPBELL BIOLOGY TENTH EDITION Reece Urry Cain Wasserman Minorsky Jackson 11 Cell Communication Lecture Presentation by Nicole Tunbridge and Kathleen Fitzpatrick Cellular Messaging Cells can signal to
Supplementary Figure S1. Effect of Glucose on Energy Balance in WT and KHK A/C KO
 Supplementary Figure S1. Effect of Glucose on Energy Balance in WT and KHK A/C KO Mice. WT mice and KHK-A/C KO mice were provided drinking water containing 10% glucose or tap water with normal chow ad
Supplementary Figure S1. Effect of Glucose on Energy Balance in WT and KHK A/C KO Mice. WT mice and KHK-A/C KO mice were provided drinking water containing 10% glucose or tap water with normal chow ad
Supplementary Figure 1: si-craf but not si-braf sensitizes tumor cells to radiation.
 Supplementary Figure 1: si-craf but not si-braf sensitizes tumor cells to radiation. (a) Embryonic fibroblasts isolated from wildtype (WT), BRAF -/-, or CRAF -/- mice were irradiated (6 Gy) and DNA damage
Supplementary Figure 1: si-craf but not si-braf sensitizes tumor cells to radiation. (a) Embryonic fibroblasts isolated from wildtype (WT), BRAF -/-, or CRAF -/- mice were irradiated (6 Gy) and DNA damage
ENERGY FROM INGESTED NUTREINTS MAY BE USED IMMEDIATELY OR STORED
 QUIZ/TEST REVIEW NOTES SECTION 1 SHORT TERM METABOLISM [METABOLISM] Learning Objectives: Identify primary energy stores of the body Differentiate the metabolic processes of the fed and fasted states Explain
QUIZ/TEST REVIEW NOTES SECTION 1 SHORT TERM METABOLISM [METABOLISM] Learning Objectives: Identify primary energy stores of the body Differentiate the metabolic processes of the fed and fasted states Explain
Lipids and Membranes
 Lipids and Membranes Presented by Dr. Mohammad Saadeh The requirements for the Pharmaceutical Biochemistry I Philadelphia University Faculty of pharmacy Membrane transport D. Endocytosis and Exocytosis
Lipids and Membranes Presented by Dr. Mohammad Saadeh The requirements for the Pharmaceutical Biochemistry I Philadelphia University Faculty of pharmacy Membrane transport D. Endocytosis and Exocytosis
MTC-TT and TPC-1 cell lines were cultured in RPMI medium (Gibco, Breda, The Netherlands)
 Supplemental data Materials and Methods Cell culture MTC-TT and TPC-1 cell lines were cultured in RPMI medium (Gibco, Breda, The Netherlands) supplemented with 15% or 10% (for TPC-1) fetal bovine serum
Supplemental data Materials and Methods Cell culture MTC-TT and TPC-1 cell lines were cultured in RPMI medium (Gibco, Breda, The Netherlands) supplemented with 15% or 10% (for TPC-1) fetal bovine serum
islets scored 1 week month months
 Supplementary Table 1. Sampling parameters for the morphometrical analyses Time (post- DT) Control mice (age-matched) α-cells mice pancreatic surface (mm 2 ) scored DT-treated mice islets scored mice pancreatic
Supplementary Table 1. Sampling parameters for the morphometrical analyses Time (post- DT) Control mice (age-matched) α-cells mice pancreatic surface (mm 2 ) scored DT-treated mice islets scored mice pancreatic
Supplementary Materials for
 www.sciencesignaling.org/cgi/content/full/6/283/ra57/dc1 Supplementary Materials for JNK3 Couples the Neuronal Stress Response to Inhibition of Secretory Trafficking Guang Yang,* Xun Zhou, Jingyan Zhu,
www.sciencesignaling.org/cgi/content/full/6/283/ra57/dc1 Supplementary Materials for JNK3 Couples the Neuronal Stress Response to Inhibition of Secretory Trafficking Guang Yang,* Xun Zhou, Jingyan Zhu,
Islet viability assay and Glucose Stimulated Insulin Secretion assay RT-PCR and Western Blot
 Islet viability assay and Glucose Stimulated Insulin Secretion assay Islet cell viability was determined by colorimetric (3-(4,5-dimethylthiazol-2-yl)-2,5- diphenyltetrazolium bromide assay using CellTiter
Islet viability assay and Glucose Stimulated Insulin Secretion assay Islet cell viability was determined by colorimetric (3-(4,5-dimethylthiazol-2-yl)-2,5- diphenyltetrazolium bromide assay using CellTiter
Identification of GLP1R agonists using a novel high throughput screening assay Wan Namkung, Ph.D.
 Identification of GLP1R agonists using a novel high throughput screening assay Wan Namkung, Ph.D. College of Pharmacy, Yonsei University Contents High-throughput screening (HTS) HTS assays for identification
Identification of GLP1R agonists using a novel high throughput screening assay Wan Namkung, Ph.D. College of Pharmacy, Yonsei University Contents High-throughput screening (HTS) HTS assays for identification
Kirschner, 2005). Briefly, parallel MEF cultures were isolated from single littermate
 SUPPLEMENTAL MATERIALS AND METHODS Generation of MEFs, osteoblasts and cell culture Prkar1a -/- and control MEFs were generated and cultured as described (Nadella and Kirschner, 2005). Briefly, parallel
SUPPLEMENTAL MATERIALS AND METHODS Generation of MEFs, osteoblasts and cell culture Prkar1a -/- and control MEFs were generated and cultured as described (Nadella and Kirschner, 2005). Briefly, parallel
Cell Communication. Chapter 11. Biology Eighth Edition Neil Campbell and Jane Reece. PowerPoint Lecture Presentations for
 Chapter 11 Cell Communication PowerPoint Lecture Presentations for Biology Eighth Edition Neil Campbell and Jane Reece Lectures by Chris Romero, updated by Erin Barley with contributions from Joan Sharp
Chapter 11 Cell Communication PowerPoint Lecture Presentations for Biology Eighth Edition Neil Campbell and Jane Reece Lectures by Chris Romero, updated by Erin Barley with contributions from Joan Sharp
Receptors Families. Assistant Prof. Dr. Najlaa Saadi PhD Pharmacology Faculty of Pharmacy University of Philadelphia
 Receptors Families Assistant Prof. Dr. Najlaa Saadi PhD Pharmacology Faculty of Pharmacy University of Philadelphia Receptor Families 1. Ligand-gated ion channels 2. G protein coupled receptors 3. Enzyme-linked
Receptors Families Assistant Prof. Dr. Najlaa Saadi PhD Pharmacology Faculty of Pharmacy University of Philadelphia Receptor Families 1. Ligand-gated ion channels 2. G protein coupled receptors 3. Enzyme-linked
Sarah Jaar Marah Al-Darawsheh
 22 Sarah Jaar Marah Al-Darawsheh Faisal Mohammad Receptors can be membrane proteins (for water-soluble hormones/ligands) or intracellular (found in the cytosol or nucleus and bind to DNA, for lipid-soluble
22 Sarah Jaar Marah Al-Darawsheh Faisal Mohammad Receptors can be membrane proteins (for water-soluble hormones/ligands) or intracellular (found in the cytosol or nucleus and bind to DNA, for lipid-soluble
Figure legends Supplemental Fig.1. Glucose-induced insulin secretion and insulin content of islets. Supplemental Fig. 2.
 Figure legends Supplemental Fig.. Glucose-induced insulin secretion and insulin content of islets. Insulin secretory responses to.,., and. mm glucose (A) (n = 7-), and the insulin content in the islets
Figure legends Supplemental Fig.. Glucose-induced insulin secretion and insulin content of islets. Insulin secretory responses to.,., and. mm glucose (A) (n = 7-), and the insulin content in the islets
SUPPLEMENTARY INFORMATION. Supplementary Figures S1-S9. Supplementary Methods
 SUPPLEMENTARY INFORMATION SUMO1 modification of PTEN regulates tumorigenesis by controlling its association with the plasma membrane Jian Huang 1,2#, Jie Yan 1,2#, Jian Zhang 3#, Shiguo Zhu 1, Yanli Wang
SUPPLEMENTARY INFORMATION SUMO1 modification of PTEN regulates tumorigenesis by controlling its association with the plasma membrane Jian Huang 1,2#, Jie Yan 1,2#, Jian Zhang 3#, Shiguo Zhu 1, Yanli Wang
Endocannabinoid-activated Nlrp3 inflammasome in infiltrating macrophages mediates β- cell loss in type 2 diabetes
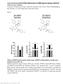 Endocannabinoid-activated Nlrp3 inflammasome in infiltrating macrophages mediates β- cell loss in type 2 diabetes T Jourdan, G Godlewski, R Cinar, A Bertola, G Szanda, J Liu, J Tam, T Han, B Mukhopadhyay,
Endocannabinoid-activated Nlrp3 inflammasome in infiltrating macrophages mediates β- cell loss in type 2 diabetes T Jourdan, G Godlewski, R Cinar, A Bertola, G Szanda, J Liu, J Tam, T Han, B Mukhopadhyay,
Lecture 15. Signal Transduction Pathways - Introduction
 Lecture 15 Signal Transduction Pathways - Introduction So far.. Regulation of mrna synthesis Regulation of rrna synthesis Regulation of trna & 5S rrna synthesis Regulation of gene expression by signals
Lecture 15 Signal Transduction Pathways - Introduction So far.. Regulation of mrna synthesis Regulation of rrna synthesis Regulation of trna & 5S rrna synthesis Regulation of gene expression by signals
Table S1. Sequence of human and mouse primers used for RT-qPCR measurements.
 Table S1. Sequence of human and mouse primers used for RT-qPCR measurements. Ca9, carbonic anhydrase IX; Ndrg1, N-myc downstream regulated gene 1; L28, ribosomal protein L28; Hif1a, hypoxia inducible factor
Table S1. Sequence of human and mouse primers used for RT-qPCR measurements. Ca9, carbonic anhydrase IX; Ndrg1, N-myc downstream regulated gene 1; L28, ribosomal protein L28; Hif1a, hypoxia inducible factor
Effect of macronutrients and mixed meals on incretin hormone secretion and islet cell function
 Effect of macronutrients and mixed meals on incretin hormone secretion and islet cell function Background. Following meal ingestion, several hormones are released from the gastrointestinal tract. Some
Effect of macronutrients and mixed meals on incretin hormone secretion and islet cell function Background. Following meal ingestion, several hormones are released from the gastrointestinal tract. Some
Cell Signaling part 2
 15 Cell Signaling part 2 Functions of Cell Surface Receptors Other cell surface receptors are directly linked to intracellular enzymes. The largest family of these is the receptor protein tyrosine kinases,
15 Cell Signaling part 2 Functions of Cell Surface Receptors Other cell surface receptors are directly linked to intracellular enzymes. The largest family of these is the receptor protein tyrosine kinases,
Supplementary Figure S1. Venn diagram analysis of mrna microarray data and mirna target analysis. (a) Western blot analysis of T lymphoblasts (CLS)
 Supplementary Figure S1. Venn diagram analysis of mrna microarray data and mirna target analysis. (a) Western blot analysis of T lymphoblasts (CLS) and their exosomes (EXO) in resting (REST) and activated
Supplementary Figure S1. Venn diagram analysis of mrna microarray data and mirna target analysis. (a) Western blot analysis of T lymphoblasts (CLS) and their exosomes (EXO) in resting (REST) and activated
Supplemental Information. Ca V 2.2 Gates Calcium-Independent. but Voltage-Dependent Secretion. in Mammalian Sensory Neurons
 Neuron, Volume 96 Supplemental Information Ca V 2.2 Gates Calcium-Independent but Voltage-Dependent Secretion in Mammalian Sensory Neurons Zuying Chai, Changhe Wang, Rong Huang, Yuan Wang, Xiaoyu Zhang,
Neuron, Volume 96 Supplemental Information Ca V 2.2 Gates Calcium-Independent but Voltage-Dependent Secretion in Mammalian Sensory Neurons Zuying Chai, Changhe Wang, Rong Huang, Yuan Wang, Xiaoyu Zhang,
18s AAACGGCTACCACATCCAAG CCTCCAATGGATCCTCGTTA. 36b4 GTTCTTGCCCATCAGCACC AGATGCAGCAGATCCGCAT. Acc1 AGCAGATCCGCAGCTTG ACCTCTGCTCGCTGAGTGC
 Supplementary Table 1. Quantitative PCR primer sequences Gene symbol Sequences (5 to 3 ) Forward Reverse 18s AAACGGCTACCACATCCAAG CCTCCAATGGATCCTCGTTA 36b4 GTTCTTGCCCATCAGCACC AGATGCAGCAGATCCGCAT Acc1
Supplementary Table 1. Quantitative PCR primer sequences Gene symbol Sequences (5 to 3 ) Forward Reverse 18s AAACGGCTACCACATCCAAG CCTCCAATGGATCCTCGTTA 36b4 GTTCTTGCCCATCAGCACC AGATGCAGCAGATCCGCAT Acc1
Supplementary Figure 1 CD4 + T cells from PKC-θ null mice are defective in NF-κB activation during T cell receptor signaling. CD4 + T cells were
 Supplementary Figure 1 CD4 + T cells from PKC-θ null mice are defective in NF-κB activation during T cell receptor signaling. CD4 + T cells were isolated from wild type (PKC-θ- WT) or PKC-θ null (PKC-θ-KO)
Supplementary Figure 1 CD4 + T cells from PKC-θ null mice are defective in NF-κB activation during T cell receptor signaling. CD4 + T cells were isolated from wild type (PKC-θ- WT) or PKC-θ null (PKC-θ-KO)
IL-6Rα IL-6RαT-KO KO. IL-6Rα f/f bp. f/f 628 bp deleted 368 bp. 500 bp
 STD H 2 O WT KO IL-6Rα f/f IL-6Rα IL-6RαT-KO KO 1000 bp 500 bp f/f 628 bp deleted 368 bp Supplementary Figure 1 Confirmation of T-cell IL-6Rα deficiency. (a) Representative histograms and (b) quantification
STD H 2 O WT KO IL-6Rα f/f IL-6Rα IL-6RαT-KO KO 1000 bp 500 bp f/f 628 bp deleted 368 bp Supplementary Figure 1 Confirmation of T-cell IL-6Rα deficiency. (a) Representative histograms and (b) quantification
Inflammation & Type 2 Diabetes Prof. Marc Y. Donath
 Inflammation & Type 2 Diabetes 1, MD Chief Endocrinology, Diabetes & Metabolism University Hospital Basel Petersgraben 4 CH-431 Basel, Switzerland MDonath@uhbs.ch Innate immunity as a sensor of metabolic
Inflammation & Type 2 Diabetes 1, MD Chief Endocrinology, Diabetes & Metabolism University Hospital Basel Petersgraben 4 CH-431 Basel, Switzerland MDonath@uhbs.ch Innate immunity as a sensor of metabolic
Fig. S1. High K+ increases intracellular calcium level.
 Fig. S1. High K + increases intracellular calcium level. (A) Neuronal activation measured by calcium imaging using Fura-2. Intracellular calcium levels were continuously monitored by the fura-2 florescence
Fig. S1. High K + increases intracellular calcium level. (A) Neuronal activation measured by calcium imaging using Fura-2. Intracellular calcium levels were continuously monitored by the fura-2 florescence
Autonomic regulation of islet hormone secretion
 Autonomic regulation of islet hormone secretion Implications for health and disease Billy & Bree Paper 1: Autonomic regulation of islet hormone secretion : Implications for health and disease By Team BBB
Autonomic regulation of islet hormone secretion Implications for health and disease Billy & Bree Paper 1: Autonomic regulation of islet hormone secretion : Implications for health and disease By Team BBB
Optimization of a LanthaScreen Kinase assay for ZAP70
 Optimization of a LanthaScreen Kinase assay for ZAP70 Overview This protocol describes how to develop a LanthaScreen kinase assay designed to detect and characterize kinase inhibitors. The development
Optimization of a LanthaScreen Kinase assay for ZAP70 Overview This protocol describes how to develop a LanthaScreen kinase assay designed to detect and characterize kinase inhibitors. The development
TGF-β Signaling Regulates Neuronal C1q Expression and Developmental Synaptic Refinement
 Supplementary Information Title: TGF-β Signaling Regulates Neuronal C1q Expression and Developmental Synaptic Refinement Authors: Allison R. Bialas and Beth Stevens Supplemental Figure 1. In vitro characterization
Supplementary Information Title: TGF-β Signaling Regulates Neuronal C1q Expression and Developmental Synaptic Refinement Authors: Allison R. Bialas and Beth Stevens Supplemental Figure 1. In vitro characterization
Supporting Information
 Supporting Information Gerasimenko et al..73/pnas.39 SI Materials and Methods Reagents used in this study include Fluo-4/Fura- (Invitrogen), thapsigargin (albiochem), collagenase (Worthington), palmitoleic
Supporting Information Gerasimenko et al..73/pnas.39 SI Materials and Methods Reagents used in this study include Fluo-4/Fura- (Invitrogen), thapsigargin (albiochem), collagenase (Worthington), palmitoleic
Physiology Unit 1 CELL SIGNALING: CHEMICAL MESSENGERS AND SIGNAL TRANSDUCTION PATHWAYS
 Physiology Unit 1 CELL SIGNALING: CHEMICAL MESSENGERS AND SIGNAL TRANSDUCTION PATHWAYS In Physiology Today Cell Communication Homeostatic mechanisms maintain a normal balance of the body s internal environment
Physiology Unit 1 CELL SIGNALING: CHEMICAL MESSENGERS AND SIGNAL TRANSDUCTION PATHWAYS In Physiology Today Cell Communication Homeostatic mechanisms maintain a normal balance of the body s internal environment
The Schedule and the Manual of Basic Techniques for Cell Culture
 The Schedule and the Manual of Basic Techniques for Cell Culture 1 Materials Calcium Phosphate Transfection Kit: Invitrogen Cat.No.K2780-01 Falcon tube (Cat No.35-2054:12 x 75 mm, 5 ml tube) Cell: 293
The Schedule and the Manual of Basic Techniques for Cell Culture 1 Materials Calcium Phosphate Transfection Kit: Invitrogen Cat.No.K2780-01 Falcon tube (Cat No.35-2054:12 x 75 mm, 5 ml tube) Cell: 293
Optimization of a LanthaScreen Kinase assay for NTRK1 (TRKA)
 Optimization of a LanthaScreen Kinase assay for NTRK1 (TRKA) Overview This protocol describes how to develop a LanthaScreen kinase assay designed to detect and characterize kinase inhibitors. The development
Optimization of a LanthaScreen Kinase assay for NTRK1 (TRKA) Overview This protocol describes how to develop a LanthaScreen kinase assay designed to detect and characterize kinase inhibitors. The development
Last updated Glycogen synthesis, glycogenolysis, and gluconeogenesis in primary mouse hepatocytes
 Last updated 2011-03-02 Glycogen synthesis, glycogenolysis, and gluconeogenesis in primary mouse hepatocytes Media Formulations A. Media for glycogen synthesis: Culture medium base: DMEM-Low (Mediatech/Cellgro
Last updated 2011-03-02 Glycogen synthesis, glycogenolysis, and gluconeogenesis in primary mouse hepatocytes Media Formulations A. Media for glycogen synthesis: Culture medium base: DMEM-Low (Mediatech/Cellgro
Protocol for Gene Transfection & Western Blotting
 The schedule and the manual of basic techniques for cell culture Advanced Protocol for Gene Transfection & Western Blotting Schedule Day 1 26/07/2008 Transfection Day 3 28/07/2008 Cell lysis Immunoprecipitation
The schedule and the manual of basic techniques for cell culture Advanced Protocol for Gene Transfection & Western Blotting Schedule Day 1 26/07/2008 Transfection Day 3 28/07/2008 Cell lysis Immunoprecipitation
Expression of acid base transporters in the kidney collecting duct in Slc2a7 -/-
 Supplemental Material Results. Expression of acid base transporters in the kidney collecting duct in Slc2a7 -/- and Slc2a7 -/- mice. The expression of AE1 in the kidney was examined in Slc26a7 KO mice.
Supplemental Material Results. Expression of acid base transporters in the kidney collecting duct in Slc2a7 -/- and Slc2a7 -/- mice. The expression of AE1 in the kidney was examined in Slc26a7 KO mice.
Psych 181: Dr. Anagnostaras
 Psych 181: Dr. Anagnostaras Lecture 5 Synaptic Transmission Introduction to synaptic transmission Synapses (Gk., to clasp or join) Site of action of most psychoactive drugs 6.5 1 Synapses Know basic terminology:
Psych 181: Dr. Anagnostaras Lecture 5 Synaptic Transmission Introduction to synaptic transmission Synapses (Gk., to clasp or join) Site of action of most psychoactive drugs 6.5 1 Synapses Know basic terminology:
SUPPLEMENTARY INFORMATION
 Supplemental Figure 1. Furin is efficiently deleted in CD4 + and CD8 + T cells. a, Western blot for furin and actin proteins in CD4cre-fur f/f and fur f/f Th1 cells. Wild-type and furin-deficient CD4 +
Supplemental Figure 1. Furin is efficiently deleted in CD4 + and CD8 + T cells. a, Western blot for furin and actin proteins in CD4cre-fur f/f and fur f/f Th1 cells. Wild-type and furin-deficient CD4 +
SUPPLEMENTARY INFORMATION
 Supplementary Figure 1. Normal AMPAR-mediated fepsp input-output curve in CA3-Psen cdko mice. Input-output curves, which are plotted initial slopes of the evoked fepsp as function of the amplitude of the
Supplementary Figure 1. Normal AMPAR-mediated fepsp input-output curve in CA3-Psen cdko mice. Input-output curves, which are plotted initial slopes of the evoked fepsp as function of the amplitude of the
Plasma exposure levels from individual mice 4 hours post IP administration at the
 Supplemental Figure Legends Figure S1. Plasma exposure levels of MKC-3946 in mice. Plasma exposure levels from individual mice 4 hours post IP administration at the indicated dose mg/kg. Data represent
Supplemental Figure Legends Figure S1. Plasma exposure levels of MKC-3946 in mice. Plasma exposure levels from individual mice 4 hours post IP administration at the indicated dose mg/kg. Data represent
Supporting Information. Supporting Tables. S-Table 1 Primer pairs for RT-PCR. Product size. Gene Primer pairs
 Supporting Information Supporting Tables S-Table 1 Primer pairs for RT-PCR. Gene Primer pairs Product size (bp) FAS F: 5 TCTTGGAAGCGATGGGTA 3 429 R: 5 GGGATGTATCATTCTTGGAC 3 SREBP-1c F: 5 CGCTACCGTTCCTCTATCA
Supporting Information Supporting Tables S-Table 1 Primer pairs for RT-PCR. Gene Primer pairs Product size (bp) FAS F: 5 TCTTGGAAGCGATGGGTA 3 429 R: 5 GGGATGTATCATTCTTGGAC 3 SREBP-1c F: 5 CGCTACCGTTCCTCTATCA
Requires Signaling though Akt2 Independent of the. Transcription Factors FoxA2, FoxO1, and SREBP1c
 Cell Metabolism, Volume 14 Supplemental Information Postprandial Hepatic Lipid Metabolism Requires Signaling though Akt2 Independent of the Transcription Factors FoxA2, FoxO1, and SREBP1c Min Wan, Karla
Cell Metabolism, Volume 14 Supplemental Information Postprandial Hepatic Lipid Metabolism Requires Signaling though Akt2 Independent of the Transcription Factors FoxA2, FoxO1, and SREBP1c Min Wan, Karla
Supplemental Table 1. Plasma NEFA and liver triglyceride levels in ap2-hif1ako and ap2-hif2ako mice under control and high fat diets.
 Supplemental Table 1. Plasma NEFA and liver triglyceride levels in Hif1aKO and Hif2aKO mice under control and high fat diets. Hif1a (n=6) Hif1aK O (n=6) Hif2a Hif2aK O Hif1a (n=5) Hif1aKO (n=5) Hif2a Hif2aK
Supplemental Table 1. Plasma NEFA and liver triglyceride levels in Hif1aKO and Hif2aKO mice under control and high fat diets. Hif1a (n=6) Hif1aK O (n=6) Hif2a Hif2aK O Hif1a (n=5) Hif1aKO (n=5) Hif2a Hif2aK
Supplemental Figure 1. Western blot analysis indicated that MIF was detected in the fractions of
 Supplemental Figure Legends Supplemental Figure 1. Western blot analysis indicated that was detected in the fractions of plasma membrane and cytosol but not in nuclear fraction isolated from Pkd1 null
Supplemental Figure Legends Supplemental Figure 1. Western blot analysis indicated that was detected in the fractions of plasma membrane and cytosol but not in nuclear fraction isolated from Pkd1 null
Primer sequences Target Sequence F Sequence R TNF-α (Tnfa) TCAGCCGATTTGCTATCTCAT A
 Supplementary Table 1. Q- and RT-PR primers used in this study. Primer sequences Target Sequence F Sequence R TNF-α (Tnfa) TGGTTTGTTTT GTTTGGGGTTG T hemokine (- motif) ligand 5 (cl5) GTGTTTGTTT TGGTGGTG
Supplementary Table 1. Q- and RT-PR primers used in this study. Primer sequences Target Sequence F Sequence R TNF-α (Tnfa) TGGTTTGTTTT GTTTGGGGTTG T hemokine (- motif) ligand 5 (cl5) GTGTTTGTTT TGGTGGTG

 Supplementary Figure 1 (Mu) SBP (mmhg) 2 18 16 p
Supplementary Figure 1 (Mu) SBP (mmhg) 2 18 16 p