Atrogin-1 inhibits Akt-dependent cardiac hypertrophy in mice via ubiquitin-dependent coactivation of Forkhead proteins
|
|
|
- Beverly Todd
- 5 years ago
- Views:
Transcription
1 Atrogin-1 inhibits Akt-dependent cardiac hypertrophy in mice via ubiquitin-dependent coactivation of Forkhead proteins Hui-Hua Li,, David J. Glass, Cam Patterson J Clin Invest. 2007;117(11): Research Article Cardiology Cardiac hypertrophy is a major cause of human morbidity and mortality. Although much is known about the pathways that promote hypertrophic responses, mechanisms that antagonize these pathways have not been as clearly defined. Atrogin-1, also known as muscle atrophy F-box, is an F-box protein that inhibits pathologic cardiac hypertrophy by participating in a ubiquitin ligase complex that triggers degradation of calcineurin, a factor involved in promotion of pathologic hypertrophy. Here we demonstrated that atrogin-1 also disrupted Akt-dependent pathways responsible for physiologic cardiac hypertrophy. Our results indicate that atrogin-1 does not affect the activity of Akt itself, but serves as a coactivator for members of the Forkhead family of transcription factors that function downstream of Akt. This coactivator function of atrogin-1 was dependent on its ubiquitin ligase activity and the deposition of polyubiquitin chains on lysine 63 of Foxo1 and Foxo3a. Transgenic mice expressing atrogin-1 in the heart displayed increased Foxo1 ubiquitylation and upregulation of known Forkhead target genes concomitant with suppression of cardiac hypertrophy, while mice lacking atrogin-1 displayed the opposite physiologic phenotype. These experiments define a role for lysine 63 linked ubiquitin chains in transcriptional coactivation and demonstrate that atrogin-1 uses this mechanism to disrupt physiologic cardiac hypertrophic signaling through its effects on Forkhead transcription factors. Find the latest version:
2 Research article Atrogin-1 inhibits Akt-dependent cardiac hypertrophy in mice via ubiquitin-dependent coactivation of Forkhead proteins Hui-Hua Li, 1,2 Monte S. Willis, 1 Pamela Lockyer, 1 Nathaniel Miller, 1 Holly McDonough, 1 David J. Glass, 3 and Cam Patterson 1,4 1 Carolina Cardiovascular Biology Center, University of North Carolina, Chapel Hill, North Carolina, USA. 2 Institute of Basic Medical Sciences, Chinese Academy of Medical Sciences, Beijing, People s Republic of China. 3 Novartis Institutes for Biomedical Research Inc., Cambridge, Massachusetts, USA. 4 Department of Medicine, Department of Pharmacology, and Department of Cell and Developmental Biology, University of North Carolina, Chapel Hill, North Carolina, USA. Cardiac hypertrophy is a major cause of human morbidity and mortality. Although much is known about the pathways that promote hypertrophic responses, mechanisms that antagonize these pathways have not been as clearly defined. Atrogin-1, also known as muscle atrophy F-box, is an F-box protein that inhibits pathologic cardiac hypertrophy by participating in a ubiquitin ligase complex that triggers degradation of calcineurin, a factor involved in promotion of pathologic hypertrophy. Here we demonstrated that atrogin-1 also disrupted Akt-dependent pathways responsible for physiologic cardiac hypertrophy. Our results indicate that atrogin-1 does not affect the activity of Akt itself, but serves as a coactivator for members of the Forkhead family of transcription factors that function downstream of Akt. This coactivator function of atrogin-1 was dependent on its ubiquitin ligase activity and the deposition of polyubiquitin chains on lysine 63 of Foxo1 and Foxo3a. Transgenic mice expressing atrogin-1 in the heart displayed increased Foxo1 ubiquitylation and upregulation of known Forkhead target genes concomitant with suppression of cardiac hypertrophy, while mice lacking atrogin-1 displayed the opposite physiologic phenotype. These experiments define a role for lysine 63 linked ubiquitin chains in transcriptional coactivation and demonstrate that atrogin-1 uses this mechanism to disrupt physiologic cardiac hypertrophic signaling through its effects on Forkhead transcription factors. Introduction Factors that increase LV afterload such as hypertension, aortic stenosis, and age-related arterial stiffness elicit cardiac hypertrophy as an adaptive mechanism to normalize wall stress. The shortterm hemodynamic benefits of hypertrophy occur at a cost: cardiac hypertrophy leads to diastolic dysfunction and heart failure and is a powerful predictor of cardiovascular mortality even in the absence of symptoms (1, 2). At the cellular level, cardiac hypertrophy is a consequence of increased cardiomyocyte cell volume (1, 2), a process that requires coordination of cellular signaling cascades, activation of fetal cardiac gene expression programs, increased protein synthesis, sarcomere assembly, and modulation of cellular energy sources. At the present time, no specific pharmacologic strategies to reverse cardiac hypertrophy have been approved for clinical use, so the delineation of hypertrophic mechanisms (especially those that prevent or reverse hypertrophy) remains a priority. Although complexity and redundancy exist in the signaling pathways that activate cardiac hypertrophy, 2 independent circuits that elicit distinct manifestations of hypertrophy are now recognized. Hypertrophy in response to stimuli such as pressure overload and adrenergic stimulation activates the calcineurin/ nuclear factor of activated T cell dependent signaling pathway, resulting in so-called pathological hypertrophy that is associated with maladaptive features such as fibrosis, chamber dilatation, Nonstandard abbreviations used: Ad, adenovirus; GH, growth hormone; GSK-3β, glycogen synthase kinase 3β; GST, glutathione-s-transferase. Conflict of interest: The authors have declared that no conflict of interest exists. Citation for this article: J. Clin. Invest. 117: (2007). doi: /jci and hemodynamic decompensation (3). A second, autonomous pathway activated by exercise or insulin/igf-1 signaling mediates hypertrophy of the heart under more physiologic conditions that occur during development or conditioning. This physiologic form of hypertrophy depends on regulated activation of the kinase Akt, which in turn coordinates protein synthesis and cardiac gene expression to increase cardiomyocyte size and heart mass. Hypertrophy under these circumstances occurs without the propensity to chamber enlargement or heart failure. Recent studies have identified several downstream targets of Akt activation including mammalian target of rapamycin (mtor) and glycogen synthase kinase 3β (GSK-3β) (4 7) that participate in Akt-dependent cardiac hypertrophy in the setting of exercise or IGF-1 stimulation. We recently found that atrogin-1 (also known as muscle atrophy F-box) blocks pathologic hypertrophy in vivo and in vitro by binding to calcineurin A and inducing its ubiquitylation and degradation (8). Atrogin-1 is a cardiac- and skeletal muscle specific F-box protein that binds to Skp1, Cul1, and Roc1, the common components of SCF ubiquitin ligase complexes. Atrogin-1 was first identified as a crucial participant in skeletal muscle atrophy programs (9, 10). Interestingly, atrogin-1 expression is carefully regulated at the transcriptional level in skeletal myocytes and in cardiomyocytes by members of the Forkhead family of transcription factors (11, 12). In the setting of cardiomyocyte hypertrophy, atrogin-1 decorates calcineurin with lysine 48 linked ubiquitin chains that target calcineurin for proteasome-dependent degradation. This in turn leads to potent suppression of agonist-induced cardiomyocyte hypertrophy in vitro and increased heart muscle mass after aortic banding in vivo (8), indicating that atrogin-1 plays a criti- The Journal of Clinical Investigation Volume 117 Number 11 November
3 3212 The Journal of Clinical Investigation Volume 117 Number 11 November 2007
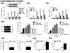
4 Figure 1 Overexpression of atrogin-1 represses insulin- and IGF-1 induced cardiomyocyte hypertrophy and Foxo1 and Foxo3a dephosphorylation. (A) Cardiomyocytes were infected with Ad-GFP or Ad atrogin-1 GFP (MOI 10), and cells were stimulated with insulin (20 μg/ml 1 ), IGF-1 (20 ng/ml 1 ), or PE (100 μm) for 24 h and stained with α-actinin antibody (red). Quantitation of cell surface area was performed on 100 cells per group. *P < vs. serum-free control and Ad-GFP. (B) Protein levels of endogenous Akt pathway members were determined in cardiomyocytes expressing atrogin-1 ectopically by IB with the indicated antibodies. (C) Foxo1 and Foxo3a expression and phosphorylation in cytoplasmic and nuclear fractions from cardiomyocytes were assessed by IB using anti-total or phospho-foxo1 and -Foxo3a antibodies after the indicated treatments. *P < vs. Ad-GFP + insulin or IGF-1; # P < 0.01 vs. Ad-GFP + IGF-1. (D) Cardiomyocytes were infected with increasing MOIs of Ad atrogin-1 GFP and Ad-GFP with IGF-1 (20 μg/ml 1 ) stimulation for 24 h. Endogenous Foxo1 and Foxo3a levels were determined by IB with the indicated antibodies. Maximal suppression of Foxo1 and Foxo3a occurred at MOI 30. *P < vs. Ad-GFP + IGF-1 at MOI 30. (E) Cardiomyocytes were infected with Ad-siRNA control or Ad-siRNA atrogin-1 and treated with insulin (20 μg/ml 1 ) or PE (100 μm) for 24 h. (F) Cardiomyocytes treated as indicated were stained with α-actinin antibody. Quantitation of cell surface area was performed on 100 cells per group. Original magnification, 200. *P < 0.01 vs. Ad-siRNA control + insulin or PE. Scale bars: 50 μm. cal role in regulating calcineurin-dependent cardiac hypertrophy under pathologic circumstances. To extend these previous studies, we have examined the role atrogin-1 plays in modulating hypertrophic responses that do not depend on calcineurin signaling. Results Suppression of IGF-1 and insulin-dependent cardiomyocyte hypertrophy by atrogin-1. We used an adenovirus-dependent gene delivery system (8) to examine the effects of atrogin-1 on rat neonatal cardiomyocyte hypertrophy elicited by both calcineurin-dependent and calcineurin-independent stimuli. Consistent with our previous observations, phenylephrine stimulation of cardiomyocytes induced a 2-fold increase in cardiomyocyte area, and this hypertrophy was completely blocked by enforced atrogin-1 expression (Figure 1A). Both insulin and IGF-1 similarly increased cardiomyocyte size, and these effects were also efficiently blocked in cardiomyocytes infected with the atrogin-1 expressing adenovirus (Ad atrogin-1). To explore the specific signaling events downstream of insulin and IGF-1 stimulation that are restrained by atrogin-1 in cardiomyocytes, we examined the activation status of Akt and its downstream targets S6 kinase and GSK-3β. Each of these known modulators of cardiomyocyte size was efficiently phosphorylated after insulin and IGF-1 stimulation, but atrogin-1 did not suppress their activation (Figure 1B). In contrast, when we examined the phosphorylation status of the Forkhead transcription factors Foxo1 and Foxo3a, which are downstream targets of Akt that also mediate hypertrophic responses in cardiomyocytes (11 13), we found that atrogin-1 efficiently suppressed insulin- and IGF-1 dependent phosphorylation of both proteins by 55% 60% (Figure 1, C and D). To test the contributions of endogenous atrogin-1 to Forkhead phosphorylation and cardiomyocyte hypertrophy, we knocked down endogenous atrogin-1 expression using an adenoviral sirna approach that suppresses atrogin-1 mrna by about 80% (8). Atrogin-1 knockdown augmented Foxo3a phosphorylation in unstimulated cardiomyocytes and increased both Foxo1 and Foxo3a phosphorylation when Akt was activated by insulin (Figure 1E), but had no effect on phosphorylation of Akt itself or on the Forkhead transcription factors when cardiomyocytes were stimulated with phenylephrine. As we have shown previously (8), suppression of atrogin-1 mrna led to an enhanced hypertrophic response of cardiomyocytes after phenylephrine treatment, and similar consequences were also observed in cells stimulated with insulin to activate Akt (Figure 1F), indicating that endogenous atrogin-1 regulates both Forkhead activation and insulin-dependent hypertrophy in cardiomyocytes. Interaction of atrogin-1 with Foxo1 and Foxo3a. The Forkhead proteins are activated in their dephosphorylated state and trans-activate a transcriptional program that suppresses cardiomyocyte hypertrophy (11 13). To explore whether atrogin-1 mediates its effects on Forkhead phosphorylation via direct interactions, we tested whether mutual interactions existed between these proteins. Using tagged forms of atrogin-1 and Foxo1 or Foxo3a transfected into 293 cells, both Foxo1 and Foxo3a were efficiently coimmunoprecipitated with atrogin-1, but not by similarly tagged α-actinin as a control (Figure 2A). These observations likely reflect direct interactions, because recombinant glutathione-s-transferase tagged (GST-tagged) atrogin-1 efficiently coprecipitated both Foxo1 and Foxo3a from cell lysates (Figure 2B). Using a specific anti atrogin-1 antibody, we also found that endogenous atrogin-1 participated in complexes with Foxo1 and Foxo3a in cultured cardiomyocytes (Figure 2C). We mapped the sites of Foxo1 and Foxo3a interactions with atrogin-1 using GST-tagged atrogin-1 fragments and found that both proteins required a 60 amino acid sequence immediately adjacent to the F-box of atrogin-1 for binding (Figure 2, D and E), although our experiments do not exclude the possibility that additional sequences of atrogin-1 also participate in interactions with Foxo proteins. Interestingly, this site is distinct from the calcineurin-binding site of atrogin-1 (8). Conversely, we found that an intact Forkhead domain within HA-tagged Foxo1 fragments was required for atrogin-1 interactions (Figure 2, F and G). Atrogin-1 is required for optimal transcriptional activity of Forkhead transcription factors in cardiomyocytes. Because atrogin-1 exists in both a sarcomere-associated and a nuclear pool in cardiomyocytes (8), we tested whether the effects of atrogin-1 on Foxo1 phosphorylation coincide with changes in Forkhead localization. Foxo1 and Foxo3a reside primarily in the cytoplasm of insulin-stimulated cardiomyocytes; however, when atrogin-1 was coexpressed, both Foxo1 and Foxo3a relocalized into the nucleus (Figure 3, A and B, and Supplemental Figure 1). It is possible that this enforced nuclear localization prevents Akt-dependent Foxo phosphorylation, accounting for our observations in Figure 1. Coexpression of Foxo1 and Foxo3a with promoter/reporter constructs responsive to Foxo activity (p27 kip1, DBE, and Bim; ref. 14) resulted in increased luciferase activity, and in each case coexpression of atrogin-1 with Foxo1 and Foxo3a markedly increased promoter activity (Figure 3C and Supplemental Figure 2). The transcriptional effects of atrogin-1 on reporter activity were even more marked when atrogin-1 was coexpressed with Foxo3aA3 or Foxo1A3, which are constitutively active, nuclearlocalized mutants (Figure 3C and Supplemental Figure 2). The ability of atrogin-1 to enhance the constitutively active Forkhead mutants indicates that the transcriptional effects of atrogin-1 do not depend only on increased nuclear accumulation of Forkhead proteins by atrogin-1, which strongly endorses a coactivator role for atrogin-1 on Forkhead activity within the nucleus. To determine whether endogenous atrogin-1 is required for The Journal of Clinical Investigation Volume 117 Number 11 November
5 Figure 2 Atrogin-1 interacts with Foxo1 and Foxo3a in vivo and in vitro. (A) We transfected 293 cells with the indicated plasmids. Equal amounts of protein lysates were immunoprecipitated with anti-ha or anti-myc antibody and analyzed by IB with the indicated antibodies. WCE, whole cell extract. (B) The ability of Foxo1 and Foxo3a expressed in 293 cells to be retained by GST or a GST atrogin-1 fusion protein was analyzed by IB after GST pulldown. (C) Endogenous protein interactions were examined in cardiomyocyte cell lysates immunoprecipitated with rabbit IgG or anti atrogin-1 antibody and analyzed by IB with antibodies to detect Foxo1, Foxo3a, and atrogin-1. (D) Domains of atrogin-1 involved in binding to Foxo1 and Foxo3a expressed in 293 cells were mapped with GST pulldown assays. GST atrogin-1 proteins were affinity purified and analyzed by SDS- PAGE and Coomassie blue staining. The ability of the truncated atrogin-1 fusion proteins to bind to Foxo1 and Foxo3a from whole-cell lysates was determined by blotting with antibodies against Flag and HA, respectively. (E) Deletion constructs of atrogin-1 in D that bind to Foxo1 and Foxo3a. NLS, nuclear localization sequence; PDZ, PDZ-binding domain. (F) Interaction domains of Foxo1 required for binding to atrogin-1 were mapped by co-ip. We transfected 293 cells with the indicated Flag-Foxo1 and HA atrogin-1 plasmids. Equal amounts of lysates were prepared for IP with a Flag antibody and immunoblotted with antibodies for HA or Flag. (G) Deletion constructs of Foxo1 in F that bind to atrogin-1. FK, Forkhead domain; NES, nuclear export sequence; LxxLL, nuclear receptor interacting domain or LxxLL motif; TAD, transactivation domain The Journal of Clinical Investigation Volume 117 Number 11 November 2007
6 Figure 3 Atrogin-1 induces Foxo1 and Foxo3a nuclear localization and increased transcriptional activity. (A) Cardiomyocytes were transiently transfected with expression vectors encoding HA-Foxo3a or Myc atrogin-1. At 24 h after transfection, cells were treated without or with insulin for 12 h and immunostained with antibodies against HA (green) and Myc (red). Staining was assessed by confocal microscopy; representative images are shown. Scale bars: 50 μm. (B) Cardiomyocytes (100 random cells per condition) expressing the indicated proteins were scored for Foxo1 or Foxo3a localization. Data are mean ± SEM of 2 independent experiments. Original magnification, 200. (C) To assess the role of atrogin-1 on Foxo3a-dependent transcription, cardiomyocytes were transfected with luciferase expression plasmids driven by the indicated promoters and vectors expressing Foxo3a or Foxo3aA3 (a constitutively active form) and atrogin-1. Data were normalized by cotransfection with a plasmid expressing β-gal. Cells were harvested 24 h later for measurement of luciferase activities. Results are expressed relative to the level of expression with the reporter gene alone and representative of 3 independent experiments. Error bars indicate SEM. (D) Cardiomyocytes were transfected with a luciferase reporter driven by the p27 kip1 promoter along with Foxo1 or Foxo3a and plasmids expressing sirna atrogin-1 or sirna-control. Luciferase activity was measured 24 h after transfection. *P < 0.01 vs. control sirna. maximal transcriptional activity of Foxo1 and Foxo3a, we tested the activity of these proteins in cardiomyocytes when atrogin-1 expression is knocked down (8). Transcriptional activation of the p27 kip1 promoter by Foxo1 and Foxo3a was almost completely suppressed to baseline levels by atrogin-1 knockdown, but not by a scrambled sirna construct (Figure 3D), indicating that endogenous atrogin-1 is required for optimal Forkhead transactivation of these reporters in cardiomyocytes. Noncanonical ubiquitylation of Forkhead transcription factors by atrogin-1. Transcriptional activation of Forkhead transcription factors by atrogin-1 raised the question of whether the ubiquitin ligase activity of atrogin-1 participates in the coactivation of Foxo1 and Foxo3a. In pulse-chase experiments using ectopically expressed proteins in 293 cells, both Foxo1 and Foxo3a were stable proteins with t 1/2 well in excess of 4 h, and coexpression of atrogin-1 had no effect on the stability of either protein (Figure 4A). Similarly, inhibi- The Journal of Clinical Investigation Volume 117 Number 11 November
7 Figure 4 Atrogin-1 induces lysine 63 dependent ubiquitylation of Foxo3a. (A) Cultured 293 cells were transfected with the indicated plasmids, and pulsechase analysis was performed. Extracts were subjected to IP using either anti-flag or anti-ha antibodies. Immunoprecipitates were analyzed by SDS PAGE followed by autoradiography. (B) The indicated plasmids were cotransfected, and 293 cells were treated for 4 h with DMSO or MG132 (20 μm). The expression levels of the respective proteins were analyzed by IB with anti-flag, anti-ha, or anti-myc antibodies. (C) We transfected 293 cells with vectors expressing HA-ubiquitin (HA-Ub), HA-Foxo3a, atrogin-1, or atrogin-1 ΔF-box mutant lacking the E3-ligase activity. Cell extracts were immunoprecipitated with Foxo3a antibody and analyzed by IB with the indicated antibodies. An aliquot of the cell extracts was subjected to direct IB analysis using anti-foxo3a or anti-myc antibodies. (D) In vitro ubiquitylation reactions were performed with purified ubiquitin, E1, the E2 UBC13, GST-Foxo3a, and the SCF atrogin-1 complex. Reactions were resolved by SDS-PAGE followed by IB with anti-ubiquitin antibody. (E) We cotransfected 293 cells with the indicated plasmids. At 24 h after transfection, ubiquitin-conjugated proteins were prepared for IP with anti-foxo3a. Immunoprecipitates were subject to SDS PAGE followed by IB with anti-ubiquitin, anti-foxo3a, or anti-myc antibodies. An aliquot of the cell extracts was subjected to direct IB analysis using anti-foxo3a or anti-myc antibodies The Journal of Clinical Investigation Volume 117 Number 11 November 2007
8 Figure 5 Lysine 63 linked ubiquitin chains are required for transcriptional coactivation of Foxo3a by atrogin-1. (A) Cultured cardiomyocytes were transfected with vectors expressing HA-Foxo3a, Myc atrogin-1, and/ or atrogin-1 ΔF-box together with the p27 kip1 luciferase reporter and β-gal constructs. At 24 h after transfection, cells were lysed and luciferase activity was measured. Lysates were immunoblotted with anti-ha and Myc. *P < 0.01 vs. atrogin-1 WT. (B) We transfected 293 cells with the indicated plasmids. Equal amounts of protein lysates were immunoprecipitated with anti-myc or anti-ha antibodies to detect atrogin-1 and Foxo3a, respectively, followed by IB to detect proteinprotein interactions. (C) Reporter assays were performed using the p27 kip1 promoter in cardiomyocytes cotransfected with plasmids expressing Foxo3a and/or atrogin-1, along with vectors expressing WT ubiquitin or mutant ubiquitins containing mutations of lysine 48 (K48R) or lysine 63 (K63R). At 24 h after transfection, cells were lysed and luciferase activity was measured. Lysates were immunoblotted with anti-ha and Myc. *P < vs. WT ubiquitin. Results are expressed relative to the level of expression with the reporter gene alone and representative of 3 independent experiments. Error bars indicate SEM. tion of the proteasome with MG-132 had no effect on steady-state levels of Foxo1 or Foxo3a in the presence or absence of atrogin-1 (Figure 4B). Interestingly, steady-state levels of atrogin-1 were consistently higher after MG-132 treatment, suggesting that atrogin-1 expression is itself determined in a proteasome-dependent manner. Collectively, these data indicate that atrogin-1 does not target the Forkhead transcription factors for proteasomal degradation. We next asked whether atrogin-1 is capable of adding either mono- or polyubiquitin chains to the Forkhead transcription factors. We performed assays in 293 cells (so we could recover proteins efficiently) after determining that atrogin-1 had similar effects on Forkhead transcriptional activity in 293 cells compared with cardiomyocytes (data not shown). After IP of Foxo3a, we probed immunoprecipitates with an antibody recognizing tagged ubiquitin. Ubiquitylated species of Foxo3a were detected as a high molecular weight smear only when atrogin-1 was coexpressed with Foxo3a, and the effect of atrogin-1 was abolished by deletion of its F-box (Figure 4C). Similar results were obtained in experiments examining atrogin-1 dependent ubiquitylation of Foxo1 (Supplemental Figure 3A). To determine whether the effects of atrogin-1 on Forkhead multiubiquitylation are direct, we reassembled the components of ubiquitylation in vitro (8) with immunopurified Skp1, Cul1, and Roc1 and recombinant atrogin-1 and Foxo3a or Foxo1. We found that multiubiquitylation of Foxo3a (Figure 4D) and Foxo1 (Supplemental Figure 3B) proceeded efficiently in the presence of atrogin-1, was dependent on the F-box of atrogin-1, and was abolished by deletion of atrogin-1 or any other component of the reaction. Taken together, these observations indicate that atrogin-1 induces multiubiquitylation of Foxo1 and Foxo3a, yet does not target either protein for proteasome-dependent degradation and instead increases their activity. This suggested that atrogin-1 may not be decorating the Forkhead transcription factors with canonical, lysine 48 linked polyubiquitin chains. To test this, we examined the ubiquitylation of Foxo3a induced by atrogin-1 in the presence of WT ubiquitin or ubiquitin variants that cannot assemble lysine 48 or lysine 63 chains. Mutation of lysine 48 in ubiquitin actually enhanced the polyubiquitylation of Foxo3a by atrogin-1, indicating that canonical ubiquitin chains are not assembled on Foxo3a by atrogin-1 (Figure 4E). In contrast, ubiquitylation of Foxo3a (and also Foxo1; Supplemental Figure 3C) was markedly suppressed by the lysine 63 ubiquitin mutant, indicating that lysine 63 linked chains were the predominant species assembled on Forkhead transcription factors by atrogin-1. Although lysine 63 linked ubiquitin chains participate in a number of cellular events, such as cell signaling and DNA damage repair (15, 16), they have not yet been clearly associated with transcriptional regulation in mammalian cells, and there are few descriptions of noncanonical ubiquitylation in the context of cardiomyocyte biology. We therefore performed experiments to confirm or refute the importance of lysine 63 linked chains in atrogin-1 dependent coactivation of Foxo1 and Foxo3a. We first asked whether the F-box of atrogin-1 is necessary for coactivation. Indeed, when coexpressed with Foxo3a, deletion of the F-box of atrogin-1 totally abolished its ability to coactivate Foxo3a in transcriptional assays using the p27 kip1 promoter (Figure 5A). The F-box requirement in these experiments could indicate that either F-box dependent ubiquitylation or F-box dependent binding (independent of ubiquitylation) mediates the transcriptional The Journal of Clinical Investigation Volume 117 Number 11 November
9 Figure 6 Enhanced Forkhead protein ubiquitylation and activity and suppressed IGF-1/GH-dependent cardiac hypertrophy in cardiac-specific atrogin-1 Tg mice. (A) Cardiac hypertrophy was induced in 8-wk-old mice with IGF-1/GH injections. Atrogin-1 Tg and WT mice were sacrificed 14 d later; their hearts were freshly isolated; and levels of the indicated total and phospho-proteins were determined by IB. Results were normalized to relative expression levels of phospho-foxo1, Foxo3a, and Akt (n = 6). *P < vs. WT. (B) Equal amounts of lysates from WT and Tg hearts were immunoprecipitated with Foxo3a antibody and analyzed by IB with antibodies against ubiquitin or Foxo3a to detect ubiquitylated forms of Foxo3a in vivo before and after IGF-1/GH treatment. (C) RT-PCR was performed to measure the expression of known Foxo1 and Foxo3a target genes in WT and Tg mouse hearts before and after IGF-1/GH treatment. Total RNA was isolated from mouse hearts, and expression of transcripts for Bim, p27 Kip1, GADD45, SOD2, and GAPDH was determined and normalized to GAPDH (n = 6). *P < vs. WT. A representative analysis is shown. (D) M-mode echocardiographic analysis of hearts from Tg and WT mice after 2 wk IGF-1/GH injection. (E) Representative macroscopic histologic analysis of H&E-stained hearts from indicated mice after 2 wk IGF-1/GH injection. Histologic sections were also stained with wheat germ agglutinin TRITC conjugate to determine cell size. Scale bars: 1 mm (top); 50 μm (bottom). (F) Cardiomyocyte size from WT and Tg hearts after IGF-1/GH treatment. effects of atrogin-1 on the Forkhead transcription factors. To exclude the latter possibility, we assayed the interactions of Foxo3a and atrogin-1 with and without an intact F-box. The presence of the F-box was not required for Foxo3a interactions (Figure 5B), which is consistent with our in vitro binding assays (Figure 2D) and suggests that the role of the F-box of atrogin-1 in Foxo3a coactivation is to recruit the Skp- Cul1-Roc1 components to atrogin-1 for ubiquitin ligase assembly. If this model is true, then prevention of lysine 63 linked ubiquitin chain assembly on Foxo3a should abolish the coactivation of Foxo3a by atrogin-1. To test this, we examined the transcriptional effects of atrogin-1 on Foxo3a under conditions in which lysine 63 linked ubiquitin linkages are suppressed in reporter gene assays. Under conditions in which atrogin-1 potently coactivates Foxo3a transcription of the p27 kip1 promoter, overexpression of lysine 63 ubiquitin completely blocked the transcriptional effects of atrogin-1, whereas lysine 48 ubiquitin had no effect (Figure 5C). Similar results were obtained in studies examining Foxo1 (Supplemental Figure 4). Deletion of the F-box of atrogin-1, or truncation to prevent binding to Foxo proteins, abolished the transcriptional activity of atrogin-1 (Supplemental Figure 5). Collectively, these experiments indicate that atrogin-1 suppresses Akt-dependent cardiomyocyte hypertrophy pathways by favoring nuclear retention of unphosphorylated Forkhead species and that noncanonical ubiquitylation of Foxo1 and Foxo3a via atrogin-1 dependent lysine 63 linked chains is required for transcriptional coactivation. Atrogin-1 increases Forkhead protein activity and suppresses IGF-1 dependent cardiac hypertrophy in vivo. To test the relevance of our observations in vivo, we examined the effects of atrogin-1 on Akt-dependent hypertrophy in Tg mice that overexpress atrogin The Journal of Clinical Investigation Volume 117 Number 11 November 2007
10 Table 1 Echocardiography of dimensions and function in WT and atrogin-1 Tg mice at baseline and after 2 wk IGF/GH treatment WT baseline Tg baseline WT 2 wk Tg 2 wk BW (g) 19.2 ± ± ± ± 1.5 HW/TL (mg/mm) ND ND 8.66 ± ± 0.35 LV mass index (mg) ± ± ± 12.3 A ± 6.9 A,B HR (bpm) ± ± ± ± IVSTD (mm) 0.93 ± ± ± 0.06 A 1.01 ± 0.05 A,B IVSTS (mm) 1.58 ± ± ± 0.09 A 1.64 ± 0.05 B PWTD (mm) 1.01 ± ± ± 0.05 A 0.95 ± 0.04 A,B PWTS (mm) 1.50 ± ± ± 0.06 A 1.50 ± 0.03 B LVEDD (mm) 3.2 ± ± ± ± 0.13 A,B LVESD (mm) 1.60 ± ± ± ± 0.09 FS (%) 50.17± ± ± ± 1.99 Transthoracic echocardiography on unanesthetized mice. Data are mean ± SEM. BW, body wt; HW/TL, heart wt/tibia length; HR, heart rate; IVSTD, interventricular septal thickness in diastole; IVSTS, interventricular septal thickness in systole; PWTD, posterior wall thickness in diastole; PWTS, posterior wall thickness in systole; LVEDD, LV end-diastolic dimension; LVESD, LV end-systolic dimension; ND, not determined. LV mass index was calculated as (external LV diameter in diastole 3 LV end-diastolic dimension 3 ) x Fractional shortening (FS) was calculated as (LV end-diastolic dimension LV end-systolic dimension)/lv enddiastolic dimension. A P < vs. baseline. B P < vs. WT 2 wk. under control of the α myosin heavy chain promoter (8). We used injections of IGF-1 and growth hormone (GH) for 14 d to stimulate Akt-dependent cardiac hypertrophy (17 19). Akt phosphorylation was increased by IGF-1/GH treatment in ventricles from both WT and nontransgenic littermates (Figure 6A), as has been previously described (17). However, whereas Foxo1 and Foxo3a phosphorylation were increased after IGF-1/GH treatment in WT mouse hearts, this phosphorylation was suppressed in atrogin-1 Tg mice. To establish whether ubiquitylation of Forkhead proteins is regulated by atrogin-1 in vivo, we immunoprecipitated Foxo3a from heart lysates and probed these precipitates for ubiquitylated species. We found that Foxo3a ubiquitylation was increased in atrogin-1 Tg hearts after IGF-1/GH treatment (Figure 6B). We next measured the mrna expression of known Forkhead target genes Bim, p27 kip1, GADD45, and SOD2 to determine whether the transcriptional activity of Foxo1 and Foxo3a was concomitantly increased in atrogin-1 Tg hearts. Quantitative analysis indicated that the expression of each of these target genes was increased by 2-fold or more in hearts from atrogin-1 Tg mice after IGF-1/GH treatment compared with hearts from WT mice (Figure 6C). Echocardiography was performed on mice at baseline and after 14 d of IGF-1/GH treatment to determine the physiologic consequences of atrogin-1 on IGF-1/GH dependent hypertrophy. LV mass increased by 44% in WT hearts and by only 17% in atrogin-1 Tg hearts after IGF-1/GH treatment (Table 1 and Figure 6D). The parameters of hypertrophy, interventricular and posterior wall thickness, also failed to increase in atrogin-1 Tg mouse hearts. Heart weight/tibia length ratios, determined as an additional index of cardiac hypertrophy, were also significantly lower (P < 0.02) in atrogin-1 Tg mice after IGF-1/GH treatment. Cardiac function, as estimated by fractional shortening, was not affected in atrogin-1 Tg mice before or after IGF-1/GH treatment (Table 1), in spite of the lack of a hypertrophic response in these mice. Histologically, cardiomyocyte size increased by 96% in WT mouse hearts but only by 27% in atrogin-1 Tg hearts after IGF-1/GH treatment (Figure 6, E and F). Our analyses using Tg mice allowed us to assess the activity of ectopically expressed atrogin-1 on cardiac responses to IGF-1/GH treatment and to perform biochemical analyses of Forkhead protein activation. However, the role of endogenous atrogin-1 in Akt-dependent hypertrophy, especially under more physiologic conditions, is not addressed by these studies. To answer these questions, we examined the physiologic induction of hypertrophy after 3 wk voluntary wheel exercise in WT and Atrogin-1 / mice. Run times, total mileage, and average speed were not different between groups (Table 2). Hearts from Atrogin-1 / mice were grossly larger than those from WT mice after running (Figure 7A and Table 2), and the area of cardiomyocytes from Atrogin-1 / hearts was 64% greater than control after exercise (Figure 7B). These observations correspond to the increased LV mass index and wall thickness in Atrogin-1 / mice noted by echocardiography after, but not before, running (Table 2, Figure 7C). Because amounts of ubiquitylated Foxo proteins were at the limits of detection in WT hearts, it was technically not feasible to measure decreases in their modification in Atrogin-1 / hearts. Nevertheless, our overexpression and underexpression data in vivo collectively correspond closely to our in vitro observations and indicate that atrogin-1 enhances the activation of Forkhead transcription factors Foxo1 and Foxo3a and concomitantly suppresses Akt-dependent cardiomyocyte hypertrophy, indicating what we believe to be a new role for noncanonical ubiquitylation in a pathophysiologically relevant context. Discussion Because cardiac hypertrophy eventually plateaus even in the setting of continued prohypertrophic stimulation and is reversible when these stimuli abate, it is evident that the regulation of cardiomyocyte size depends on the balance between factors that either promote or antagonize cellular enlargement. Our previous studies identified a role for atrogin-1 in limiting pathological cardiomyocyte hypertrophic responses in vivo and in vitro by targeting calcineurin for proteasome-dependent degradation (8), which provides a regulatory mechanism to counterbalance pro- and antigrowth mechanisms in cardiac hypertrophy (12). The results of our present studies indicate that this counterregulatory role for atrogin-1 exists for calcineurin-independent cardiac growth as well. Akt is a proximal component of the physiologic, calcineurin-independent hypertrophic pathway (1). We found using a combination of knockdown and overexpression approaches that atrogin-1 has no effect on Akt activation in response to IGF-1 or insulin challenge in cardiomyocytes, but nevertheless represses Akt-dependent hypertrophy by activating the Forkhead transcription factors. The transcriptional coactivation of Foxo1 and Foxo3a by atrogin-1 and upregulation of Forkhead-dependent transcriptional targets allows the heart to uncouple the inhibition of Akt-dependent hypertrophic signaling from other essential effects of Akt on cell survival and metabolism. Activated Forkhead transcription factors participate in repression of both Akt-dependent and calcineurin-dependent hypertrophy (12, 13). Thus, whereas prohypertrophic signaling is segregated The Journal of Clinical Investigation Volume 117 Number 11 November
11 Figure 7 Lack of atrogin-1 expression results in exaggerated cardiac hypertrophy in response to voluntary running exercise. (A) Representative macroscopic histologic analysis of H&E-stained mouse hearts after 3 wk voluntary wheel exercise. Scale bar: 1 mm. Histologic sections were also stained with wheat germ agglutinin-tritc to determine cell size. Scale bars: 1 mm (top); 50 μm (bottom). (B) Quantitation of cardiomyocyte size from WT and Atrogin-1 / heart after 3 wk voluntary wheel exercise and sham controls. (C) M-mode echocardiographic analysis of hearts from WT and Atrogin-1 / mice after 3 wk voluntary wheel exercise. exquisitely in physiologic and pathologic circumstances (1), the pathways that antagonize cardiac hypertrophic responses through transcriptional (Forkhead proteins) and protein stability (atrogin-1) mechanisms are common to both types of cardiac hypertrophy. This shared role is consistent with studies implicating both protein synthesis and protein degradation in antigrowth mechanisms in the heart in vivo (20). Importantly, because Forkhead proteins regulate atrogin-1 expression in skeletal and cardiac muscle (11, 12), our results indicate the presence of a feed-forward mechanism in which atrogin-1 is activated by, and in turn coactivates, Foxo3a and Foxo1. The presence of such a mechanism suggests that postnatal cardiac growth is carefully regulated at several levels through inhibitory processes, with atrogin-1 acting as both a transcriptional coactivator and ubiquitin ligase to coordinate multiple steps in this process. These studies also suggest the existence of additional layers of regulation that suppress this feed-forward pathway, and the elucidation of these mechanisms remains a fruitful topic for further inquiry. One potential mechanism to regulate this feedforward loop is through Foxo-dependent activation of Akt itself, which has recently been described (13, 21). Because canonical ubiquitylation of proteins via lysine 48 linked chains targets proteins for degradation via the proteasome, the observation that atrogin-1 binds and activates the Forkhead transcription factors raised the possibility of non proteasomedependent activities for atrogin-1. The Forkhead transcription factors are regulated through several well-defined posttranslational mechanisms. When phosphorylated by Akt, these proteins are retained in the cytoplasm, where they are transcriptionally inactive and susceptible to proteasome-dependent degradation that is triggered by a Skp2-containing ubiquitin ligase (22). In contrast, the dephosphorylated forms of Forkhead proteins are nuclear localized and transcriptionally active, and p300-dependent acetylation additionally enhances their activity (14, 23). Atrogin-1 blocks Akt-dependent Foxo1 and Foxo3a phosphorylation and enforces their localization in the nucleus, which may account in part for the transcriptional effects of atrogin-1 on these proteins. However, atrogin-1 also enhances the transcriptional activity of phosphorylation-defective constitutively active mutants of Foxo1 and Foxo3a that spontaneously localize to the nucleus, indicating that atrogin-1 modifies the activity of Forkhead proteins within the nucleus directly and not simply as a consequence of altered subcellular trafficking. The coactivation of transcriptionally active proteins by ubiquitin ligases may seem paradoxical, but there is precedent for ubiquitin modifications regulating transcription. Monoubiquitylation of histones, transcription factors, and components of the core RNA polymerase machinery is a well-described mechanism for regulation of transcription (24 27). Indeed, the Forkhead protein Foxo4 was recently shown to be monoubiquitylated in response to hydrogen peroxide stimulation (28). However, the effects of atrogin-1 on Foxo1 and Foxo3a we observed here are distinctive in that atrogin-1 regulation is dependent on assembly of lysine 63 linked ubiquitin chains. Lysine 63 linked ubiquitin chains have typically been associated with intracellular signaling events by serving as a signal for recruitment of accessory proteins (15), but a clear role for this noncanonical ubiquitylation event as a regulatory mechanism for mammalian transcription factors has not been established. The promiscuity of atrogin-1 with respect to chain linkage topology is not unprecedented for a ubiquitin ligase (29, 30) and provides an elegant means for a ubiquitin ligase to 3220 The Journal of Clinical Investigation Volume 117 Number 11 November 2007
12 Table 2 Echocardiography and run statistics of Atrogin-1 / and littermate Atrogin-1 +/+ mice at baseline and after 3 wk voluntary running WT baseline Atrogin-1 / baseline WT 3 wk running Atrogin-1 / 3 wk running Atrogin-1 / 3 wk sham Run time/24 h (h) ND ND 4.45 ± ± Distance/24 h (miles) ND ND 1.89 ± ± Speed (MPH) ND ND 0.35 ± ± BW (g) 22.9 ± ± ± ± ± 1.3 HW/TL (mg/mm) ND ND 7.4 ± ± 0.5 A,B 7.1 ± 0.5 LV mass index (mg) 95.4 ± ± ± 6.8 C ± 8.0 A,B,C 97.0 ± 3.3 HR (bpm) ± ± ± ± ± 24 IVSTD (mm) 0.87 ± ± ± 0.01 C 1.24 ± 0.03 A,B,C 0.86 ± 0.01 IVSTS (mm) 1.51 ± ± ± 0.02 C 1.89 ± 0.16 A,B,C 1.51 ± 0.14 PWTD (mm) 0.86 ± ± ± 0.04 C 1.25 ± 0.05 A,B,C 0.82 ± 0.01 PWTS (mm) 1.53 ± ± ± 0.04 C 1.7 ± 0.05 A,B,C 1.48 ± 0.06 LVEDD (mm) 2.95 ± ± ± ± ± 0.06 LVESD (mm) 1.31 ± ± ± ± ± 0.07 FS% 54.1 ± ± ± ± ± 2.4 Transthoracic echocardiography on unanesthetized mice. Data are mean ± SEM. MPH, miles per h; BW, body wt; HW/TL, heart wt/tibia length; HR, heart rate; IVSTD, interventricular septal thickness in diastole; IVSTS, interventricular septal thickness in systole; PWTD, posterior wall thickness in diastole; PWTS, posterior wall thickness in systole; LVEDD, LV end-diastolic dimension; LVESD, LV end-systolic dimension; ND, not determined.lv mass index was calculated as (external LV diameter in diastole 3 LV end-diastolic dimension 3 ) x Fractional shortening (FS) was calculated as (LV end-diastolic dimension LV endsystolic dimension)/lv end-diastolic dimension. A P < vs. WT 3 wk running. B P < 0.01 vs. Atrogin-1 / 3 wk sham. C P < 0.01 vs. baseline. mediate activities that are both dependent on and independent of proteasomes. In the case of atrogin-1, the ability to assemble ubiquitin chains of different linkages permits the inactivation of calcineurin through targeting to the proteasome at the same time that transcriptional activation of Forkhead transcription factors is favored. The assembly of noncanonical ubiquitin chains by atrogin-1 provides what we believe to be a new muscle-specific regulatory modification that regulates nuclear functions to suppress hypertrophic signaling within the heart. Methods Plasmids and antibodies. The WT and mutants of atrogin-1, His- or HAubiquitin, Flag-Foxo1, and HA-Foxo1A3 have been described previously (8, 14). Flag-Foxo1 mutants (1 593, 1 517, 1 417, 1 261, and S256A) were gifts from D.J. Tindall (Mayo Clinic, Rochester, Minnesota, USA). HA- Foxo3a and Foxo3aA3, 6xDBE- and Bim-luciferase reporters were gifts from L. Guarente (Massachusetts Institute of Technology, Cambridge, Massachusetts, USA), and pgl2-p27 kip1 luciferase reporter was a gift from T. Sakai (Kyoto Prefectural University of Medicine, Kyoto, Japan). sirna sequences for atrogin-1 were previously described (8). Antibodies used were as follows: anti-flag (Sigma-Aldrich); anti-myc (clone 9E10; Santa Cruz Biotechnology Inc.); anti-ha (clone 12CA5; Roche Diagnostics); anti-akt, anti phospho-akt (Ser473), anti phospho-akt (308), antimtor, anti phospho-mtor (Ser2448), anti-p70s6k, phospho-p70s6k (Thr389), anti GSK-3β, anti phospho-gsk-3β (Ser9), anti-foxo1, anti phospho-foxo1 (Ser256), and anti phospho-foxo3 (Thr32; all from Cell Signaling Technology); anti-foxo3a (06-951; Upstate); anti-ubiquitin and anti-gapdh (Chemicon International Inc.); and anti-mouse or anti-rabbit conjugated antibodies (Invitrogen). Cell culture and adenovirus infection. We cultured 293 cells in DMEM supplemented with 10% FBS at 37 C. Neonatal rat cardiomyocytes were isolated by enzymatic disassociation of 1- to 2-d-old neonatal rat hearts and infected with adenoviruses expressing vectors as previously described (8). Recombinant adenoviruses expressing GFP alone (Ad-GFP), Myc-tagged atrogin-1 and GFP (Ad atrogin-1 GFP), sirna-control, or sirna atrogin-1 driven by the cytomegalovirus promoter were generated using the AdEasy system (MP Biomedicals Inc.) as described previously (31). To induce the hypertrophic response, IGF-1, insulin, or PE was added to cultures at concentrations indicated in serum-free media for 24 h. IP, Western blotting, and GST pulldown assays. IP and IB were performed as described previously (8). Briefly, 293 cells were cotransfected with expression vectors for HA- or Myc-tagged atrogin-1, Flag-tagged Foxo1, or HAtagged Foxo3 using FuGENE 6 (Roche Diagnostics). Tagged proteins were immunoprecipitated for 2 h at 4 C with the appropriate antibody. Beads were washed and analyzed by IB. GST pulldown assays were performed as described previously (8). Briefly, 293 cells were transfected with a Flag- Foxo1 or HA-Foxo3a expression plasmid for 36 h, and cells were lysed for 30 min in lysis buffer. Lysates were precleared with GST beads for 1 h and incubated with GST or GST atrogin-1 fusion proteins for 1 h at 4 C. The bound beads were washed 4 times with lysis buffer and analyzed by IB. Luciferase assays. Luciferase activity assays were performed using the Luciferase Assay System (Promega) as described previously (8). Neonatal cardiomyocytes were transiently cotransfected with expression vectors carrying atrogin-1 or ΔF-box mutant, Foxo1 or Foxo3a, atrogin-1 sirna or control vector, and luciferase reporter constructs using FuGENE 6 (Roche Diagnostics) or Lipofectamine 2000 (Invitrogen). Cells were lysed and assayed for luciferase activity h after transfection. Data represent mean ± SEM of 3 independent experiments performed in duplicate and normalization for β-gal activity. Immunofluorescence. Cardiomyocytes were processed for immunofluorescence as described previously (8). Visualization of cardiomyocyte size was performed by immunostaining with anti α-actinin antibodies. Fluorescent images were collected on an epifluorescence microscope (Eclipse E800; Nikon Inc.). Quantitation of cardiomyocyte cell surface area was performed on digitized images using NIH Image software. We examined 100 cardiomyocytes in fields in 3 independent experiments. In vivo ubiquitylation assays. To assess ubiquitylation in vivo, 293 cells were transfected with expression vectors containing His-ubiquitin or HA-ubiquitin, Flag-Foxo1, HA-Foxo3a, and Myc atrogin-1 using FuGENE 6. Lysate proteins were precipitated and analyzed by IB using appreciate antibodies as previously described (8). In vitro ubiquitylation reactions. For atrogin-1 mediated ubiquitylation, Skp1, Cul1, and Roc1 immunocomplexes were precipitated from trans- The Journal of Clinical Investigation Volume 117 Number 11 November
13 fected 293 cells with 2 μg anti-myc antibody as described previously (8). Purified Foxo1 or Foxo3a and immunocomplexes were incubated in 30 μl ubiquitin reaction buffer, 60 ng E1, 600 ng Ubc13, and 10 μg ubiquitin in the presence of bacterially purified GST only, GST atrogin-1, or GST atrogin-1 ΔF-box (1 μg). After incubation at 30 C for 2 h, reaction products were analyzed by IB using the indicated antibodies. Pulse-chase analysis. Pulse-chase analysis was performed as described previously (25). Briefly, 293 cells were transfected with the indicated plasmid and then incubated for 2 h at 37 C in methionine- and cysteine-free media, followed by pulse-labeling for 15 min with 1 mci/ml 1 35 S-methionine-cysteine (Trans label; ICN Biochemicals). At the indicated times, cells were washed twice in PBS and lysed in IP lysis buffer. Lysates were subjected to IP using the anti-flag or anti-ha antibodies. Immunoprecipitates were analyzed by SDS-PAGE followed by autoradiography. Unloaded voluntary wheel exercise induced cardiac hypertrophy. Atrogin-1 / mice backcrossed approximately 7 generations onto C57BL/6 mice were used as previously described (9). Heterozygous mice were used to generate Atrogin-1 / and littermate Atrogin-1 +/+ mice for the experiments described. All animal experiments were approved by the Institutional Animal Care and Use Committee of the University of North Carolina at Chapel Hill. Physiologic hypertrophy was induced by unloaded voluntary wheel running in female mice as previously described (32). Briefly, Atrogin-1 / mice were assigned to either no-resistance wheel running (n = 6) or sedentary control (n = 3). WT littermates (n = 5) were assigned to no-resistance wheel running. Exercise wheels were equipped with a Mity 8 Cyclocomputer (model CC-MT400), which recorded distance, average speed, running time, and maximum speed. Voluntary running began at an average age of about 12 wk for all groups. Mice underwent echocardiography at baseline, ran voluntarily for 21 d, and underwent a final echocardiography before harvest. After 21 d of nonloaded, voluntary wheel running, mice underwent echocardiography followed by sacrifice via isoflurane inhalation overdose and subsequent cervical dislocation. IGF-1/GH-induced cardiac hypertrophy. The IGF-1/GH infusion model of hypertrophy was previously described (17). GH (Nutropin AQ; Genentech) and Long R 3 -IGF-1 (JRH Biosciences) were infused at 4 mg/kg each twice daily via subcutaneous injection. Echocardiography was performed with a Vevo 660 ultrasound system (VisualSonics Inc.) equipped with a 30-MHz transducer. All procedures were approved by and performed in accordance with the University of North Carolina Institutional Animal Care and Use Committee. Histologic analyses of heart tissues were performed according to standard protocols (8). Samples were stained with H&E for routine histologic examination and wheat germ agglutinin TRITC conjugate to identify sarcolemmal membranes so that myofiber diameter could be quantified. Total RNA was purified from cultured cells or intact hearts with TRIzol (Invitrogen). For RT-PCR, the expression levels of Bim, p27 kip1, GADD45, SOD2, and GAPDH (as a control) were measured with primers designed to detect mouse gene products as described previously (33). Statistics. Data are presented as mean ± SEM. Differences between groups were evaluated for statistical significance using 2-tailed Student s t test. A P value less than 0.05 was considered significant. A 1-way analysis of variance was used to determine significant differences between groups in the voluntary running data. A pairwise multiple comparison was employed using the Holm-Sidak method to determine statistical significance between groups (Sigma Stat 3.5; Systat Inc.). Acknowledgments This study was supported by grants from the NIH (HL65619 and HL072347; to C. Patterson) and the China Natural Science Foundation ( ; to H.-H. Li). C. Patterson is an Established Investigator of the American Heart Association and a Burroughs Wellcome Fund Clinical Scientist in Translational Research. Received for publication February 8, 2007, and accepted in revised form August 29, Address correspondence to: Cam Patterson, Division of Cardiology and Carolina Cardiovascular Biology Center, 8200 Medical Biomolecular Research Building, Chapel Hill, North Carolina , USA. Phone: (919) ; Fax: (919) ; cpatters@med.unc.edu. Or to: Hui-Hua Li, Department of Pathology, Institute of Basic Medical Sciences, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, , People s Republic of China. Phone and Fax: ; hhli1995@yahoo.com. Hui-Hua Li and Monte S. Willis contributed equally to this work. 1. Heineke, J., and Molkentin, J.D Regulation of cardiac hypertrophy by intracellular signalling pathways. Nat. Rev. Mol. Cell Biol. 7: Hardt, S.E., and Sadoshima, J Negative regulators of cardiac hypertrophy. Cardiovasc. Res. 63: Wilkins, B.J., et al Calcineurin/NFAT coupling participates in pathological, but not physiological, cardiac hypertrophy. Circ. Res. 94: Taniyama, Y., et al Akt3 overexpression in the heart results in progression from adaptive to maladaptive hypertrophy. J. Mol. Cell. Cardiol. 38: Matsui, T., Nagoshi, T., and Rosenzweig, A Akt and PI 3-kinase signaling in cardiomyocyte hypertrophy and survival. Cell Cycle. 2: Hardt, S.E., and Sadoshima, J Glycogen synthase kinase-3beta: a novel regulator of cardiac hypertrophy and development. Circ. Res. 90: Kim, Y.B., Peroni, O.D., Franke, T.F., and Kahn, B.B Divergent regulation of Akt1 and Akt2 isoforms in insulin target tissues of obese Zucker rats. Diabetes. 49: Li, H.H., et al Atrogin-1/muscle atrophy F-box inhibits calcineurin-dependent cardiac hypertrophy by participating in an SCF ubiquitin ligase complex. J. Clin. Invest. 114: doi: /jci Bodine, S.C., et al Identification of ubiquitin ligases required for skeletal muscle atrophy. Science. 294: Gomes, M.D., Lecker, S.H., Jagoe, R.T., Navon, A., and Goldberg, A.L Atrogin-1, a muscle-specific F-box protein highly expressed during muscle atrophy. Proc. Natl. Acad. Sci. U. S. A. 98: Sandri, M., et al Foxo transcription factors induce the atrophy-related ubiquitin ligase atrogin-1 and cause skeletal muscle atrophy. Cell. 117: Ni, Y.G., et al Foxo transcription factors blunt cardiac hypertrophy by inhibiting calcineurin signaling. Circulation. 114: Skurk, C., et al The FOXO3a transcription factor regulates cardiac myocyte size downstream of AKT signaling. J. Biol. Chem. 280: Motta, M.C., et al Mammalian SIRT1 represses forkhead transcription factors. Cell. 116: Wu, C.J., Conze, D.B., Li, T., Srinivasula, S.M., and Ashwell, J.D Sensing of Lys 63-linked polyubiquitination by NEMO is a key event in NF-kappaB activation [corrected]. Nat. Cell Biol. 8: Kirkpatrick, D.S., et al Quantitative analysis of in vitro ubiquitinated cyclin B1 reveals complex chain topology. Nat. Cell Biol. 8: Tanaka, N., et al Effects of growth hormone and IGF-I on cardiac hypertrophy and gene expression in mice. Am. J. Physiol. 275:H393 H DeBosch, B., et al Akt1 is required for physiological cardiac growth. Circulation. 113: Muslin, A.J., and DeBosch, B Role of Akt in cardiac growth and metabolism. Novartis Found. Symp. 274: ; discussion , , Razeghi, P., et al Atrophic remodeling of the heart in vivo simultaneously activates pathways of protein synthesis and degradation. Circulation. 108: Matsumoto, M., Han, S., Kitamura, T., and Accili, D Dual role of transcription factor FoxO1 in controlling hepatic insulin sensitivity and lipid metabolism. J. Clin. Invest. 116: doi: / JCI Huang, H., et al Skp2 inhibits FOXO1 in tumor suppression through ubiquitin-mediated degradation. Proc. Natl. Acad. Sci. U. S. A. 102: Matsuzaki, H., et al Acetylation of Foxo1 alters its DNA-binding ability and sensitivity to phosphorylation. Proc. Natl. Acad. Sci. U. S. A The Journal of Clinical Investigation Volume 117 Number 11 November 2007
Atrogin-1/muscle atrophy F-box inhibits calcineurin-dependent cardiac hypertrophy by participating in an SCF ubiquitin ligase complex
 Research article Atrogin-1/muscle atrophy F-box inhibits calcineurin-dependent cardiac hypertrophy by participating in an SCF ubiquitin ligase complex Hui-Hua Li, 1 Vishram Kedar, 1 Chunlian Zhang, 1 Holly
Research article Atrogin-1/muscle atrophy F-box inhibits calcineurin-dependent cardiac hypertrophy by participating in an SCF ubiquitin ligase complex Hui-Hua Li, 1 Vishram Kedar, 1 Chunlian Zhang, 1 Holly
SUPPLEMENTARY INFORMATION
 a c e doi:10.1038/nature10407 b d f Supplementary Figure 1. SERCA2a complex analysis. (a) Two-dimensional SDS-PAGE gels of SERCA2a complexes. A silver-stained SDSPAGE gel is shown, which reveals a 12 kda
a c e doi:10.1038/nature10407 b d f Supplementary Figure 1. SERCA2a complex analysis. (a) Two-dimensional SDS-PAGE gels of SERCA2a complexes. A silver-stained SDSPAGE gel is shown, which reveals a 12 kda
Atrogin-1, a muscle-specific F-box protein highly expressed during muscle atrophy
 (Courtesy of Magda Stumpfova. Used with permission.) Atrogin-1, a muscle-specific F-box protein highly expressed during muscle atrophy M.D. Gomes, S.H. Lecker, R.T. Jagoe, A. Navon, and A.L. Goldberg PNAS,
(Courtesy of Magda Stumpfova. Used with permission.) Atrogin-1, a muscle-specific F-box protein highly expressed during muscle atrophy M.D. Gomes, S.H. Lecker, R.T. Jagoe, A. Navon, and A.L. Goldberg PNAS,
Supplementary Figure 1. Normal T lymphocyte populations in Dapk -/- mice. (a) Normal thymic development in Dapk -/- mice. Thymocytes from WT and Dapk
 Supplementary Figure 1. Normal T lymphocyte populations in Dapk -/- mice. (a) Normal thymic development in Dapk -/- mice. Thymocytes from WT and Dapk -/- mice were stained for expression of CD4 and CD8.
Supplementary Figure 1. Normal T lymphocyte populations in Dapk -/- mice. (a) Normal thymic development in Dapk -/- mice. Thymocytes from WT and Dapk -/- mice were stained for expression of CD4 and CD8.
SUPPLEMENTARY INFORMATION
 Supplementary Figures Supplementary Figure S1. Binding of full-length OGT and deletion mutants to PIP strips (Echelon Biosciences). Supplementary Figure S2. Binding of the OGT (919-1036) fragments with
Supplementary Figures Supplementary Figure S1. Binding of full-length OGT and deletion mutants to PIP strips (Echelon Biosciences). Supplementary Figure S2. Binding of the OGT (919-1036) fragments with
Supplementary Figure 1. Confocal immunofluorescence showing mitochondrial translocation of Drp1. Cardiomyocytes treated with H 2 O 2 were prestained
 Supplementary Figure 1. Confocal immunofluorescence showing mitochondrial translocation of Drp1. Cardiomyocytes treated with H 2 O 2 were prestained with MitoTracker (red), then were immunostained with
Supplementary Figure 1. Confocal immunofluorescence showing mitochondrial translocation of Drp1. Cardiomyocytes treated with H 2 O 2 were prestained with MitoTracker (red), then were immunostained with
Supplementary Figure 1.TRIM33 binds β-catenin in the nucleus. a & b, Co-IP of endogenous TRIM33 with β-catenin in HT-29 cells (a) and HEK 293T cells
 Supplementary Figure 1.TRIM33 binds β-catenin in the nucleus. a & b, Co-IP of endogenous TRIM33 with β-catenin in HT-29 cells (a) and HEK 293T cells (b). TRIM33 was immunoprecipitated, and the amount of
Supplementary Figure 1.TRIM33 binds β-catenin in the nucleus. a & b, Co-IP of endogenous TRIM33 with β-catenin in HT-29 cells (a) and HEK 293T cells (b). TRIM33 was immunoprecipitated, and the amount of
c Ischemia (30 min) Reperfusion (8 w) Supplementary Figure bp 300 bp Ischemia (30 min) Reperfusion (4 h) Dox 20 mg/kg i.p.
 a Marker Ripk3 +/ 5 bp 3 bp b Ischemia (3 min) Reperfusion (4 h) d 2 mg/kg i.p. 1 w 5 w Sacrifice for IF size A subset for echocardiography and morphological analysis c Ischemia (3 min) Reperfusion (8
a Marker Ripk3 +/ 5 bp 3 bp b Ischemia (3 min) Reperfusion (4 h) d 2 mg/kg i.p. 1 w 5 w Sacrifice for IF size A subset for echocardiography and morphological analysis c Ischemia (3 min) Reperfusion (8
SUPPLEMENTARY INFORMATION
 doi:10.1038/nature12652 Supplementary Figure 1. PRDM16 interacts with endogenous EHMT1 in brown adipocytes. Immunoprecipitation of PRDM16 complex by flag antibody (M2) followed by Western blot analysis
doi:10.1038/nature12652 Supplementary Figure 1. PRDM16 interacts with endogenous EHMT1 in brown adipocytes. Immunoprecipitation of PRDM16 complex by flag antibody (M2) followed by Western blot analysis
Supplementary Figure 1 Role of Raf-1 in TLR2-Dectin-1-mediated cytokine expression
 Supplementary Figure 1 Supplementary Figure 1 Role of Raf-1 in TLR2-Dectin-1-mediated cytokine expression. Quantitative real-time PCR of indicated mrnas in DCs stimulated with TLR2-Dectin-1 agonist zymosan
Supplementary Figure 1 Supplementary Figure 1 Role of Raf-1 in TLR2-Dectin-1-mediated cytokine expression. Quantitative real-time PCR of indicated mrnas in DCs stimulated with TLR2-Dectin-1 agonist zymosan
(a) Significant biological processes (upper panel) and disease biomarkers (lower panel)
 Supplementary Figure 1. Functional enrichment analyses of secretomic proteins. (a) Significant biological processes (upper panel) and disease biomarkers (lower panel) 2 involved by hrab37-mediated secretory
Supplementary Figure 1. Functional enrichment analyses of secretomic proteins. (a) Significant biological processes (upper panel) and disease biomarkers (lower panel) 2 involved by hrab37-mediated secretory
Supplementary Figure 1. Spatial distribution of LRP5 and β-catenin in intact cardiomyocytes. (a) and (b) Immunofluorescence staining of endogenous
 Supplementary Figure 1. Spatial distribution of LRP5 and β-catenin in intact cardiomyocytes. (a) and (b) Immunofluorescence staining of endogenous LRP5 in intact adult mouse ventricular myocytes (AMVMs)
Supplementary Figure 1. Spatial distribution of LRP5 and β-catenin in intact cardiomyocytes. (a) and (b) Immunofluorescence staining of endogenous LRP5 in intact adult mouse ventricular myocytes (AMVMs)
SUPPLEMENTARY INFORMATION. Supplementary Figures S1-S9. Supplementary Methods
 SUPPLEMENTARY INFORMATION SUMO1 modification of PTEN regulates tumorigenesis by controlling its association with the plasma membrane Jian Huang 1,2#, Jie Yan 1,2#, Jian Zhang 3#, Shiguo Zhu 1, Yanli Wang
SUPPLEMENTARY INFORMATION SUMO1 modification of PTEN regulates tumorigenesis by controlling its association with the plasma membrane Jian Huang 1,2#, Jie Yan 1,2#, Jian Zhang 3#, Shiguo Zhu 1, Yanli Wang
Supplementary Fig. 1. GPRC5A post-transcriptionally down-regulates EGFR expression. (a) Plot of the changes in steady state mrna levels versus
 Supplementary Fig. 1. GPRC5A post-transcriptionally down-regulates EGFR expression. (a) Plot of the changes in steady state mrna levels versus changes in corresponding proteins between wild type and Gprc5a-/-
Supplementary Fig. 1. GPRC5A post-transcriptionally down-regulates EGFR expression. (a) Plot of the changes in steady state mrna levels versus changes in corresponding proteins between wild type and Gprc5a-/-
Protection against doxorubicin-induced myocardial dysfunction in mice by cardiac-specific expression of carboxyl terminus of hsp70-interacting protein
 Protection against doxorubicin-induced myocardial dysfunction in mice by cardiac-specific expression of carboxyl terminus of hsp70-interacting protein Lei Wang 1, Tian-Peng Zhang 1, Yuan Zhang 2, Hai-Lian
Protection against doxorubicin-induced myocardial dysfunction in mice by cardiac-specific expression of carboxyl terminus of hsp70-interacting protein Lei Wang 1, Tian-Peng Zhang 1, Yuan Zhang 2, Hai-Lian
Supplementary Materials
 Supplementary Materials Supplementary Figure S1 Regulation of Ubl4A stability by its assembly partner A, The translation rate of Ubl4A is not affected in the absence of Bag6. Control, Bag6 and Ubl4A CRISPR
Supplementary Materials Supplementary Figure S1 Regulation of Ubl4A stability by its assembly partner A, The translation rate of Ubl4A is not affected in the absence of Bag6. Control, Bag6 and Ubl4A CRISPR
SUPPLEMENTARY INFORMATION
 SUPPLEMENTARY INFORMATION doi:1.138/nature9814 a A SHARPIN FL B SHARPIN ΔNZF C SHARPIN T38L, F39V b His-SHARPIN FL -1xUb -2xUb -4xUb α-his c Linear 4xUb -SHARPIN FL -SHARPIN TF_LV -SHARPINΔNZF -SHARPIN
SUPPLEMENTARY INFORMATION doi:1.138/nature9814 a A SHARPIN FL B SHARPIN ΔNZF C SHARPIN T38L, F39V b His-SHARPIN FL -1xUb -2xUb -4xUb α-his c Linear 4xUb -SHARPIN FL -SHARPIN TF_LV -SHARPINΔNZF -SHARPIN
Supplementary Figure 1
 Supplementary Figure 1 Supplementary Figure 1. Neither the activation nor suppression of the MAPK pathway affects the ASK1/Vif interaction. (a, b) HEK293 cells were cotransfected with plasmids encoding
Supplementary Figure 1 Supplementary Figure 1. Neither the activation nor suppression of the MAPK pathway affects the ASK1/Vif interaction. (a, b) HEK293 cells were cotransfected with plasmids encoding
Fetal gene upregulation by 1-wk TAC is significantly increased in mice lacking RGS2.
 3562-RG-1 Supplementary Figure 1 Fetal gene upregulation by 1-wk is significantly increased in mice lacking RGS2. ANP(Nppa) /BNP(Nppb) A-type and B-type natriuretic peptide; β-mhc (Myh7) beta myosin heavy
3562-RG-1 Supplementary Figure 1 Fetal gene upregulation by 1-wk is significantly increased in mice lacking RGS2. ANP(Nppa) /BNP(Nppb) A-type and B-type natriuretic peptide; β-mhc (Myh7) beta myosin heavy
condition. Left panel, the HCT-116 cells were lysed with RIPA buffer containing 0.1%
 FIGURE LEGENDS Supplementary Fig 1 (A) sumoylation pattern detected under denaturing condition. Left panel, the HCT-116 cells were lysed with RIPA buffer containing 0.1% SDS in the presence and absence
FIGURE LEGENDS Supplementary Fig 1 (A) sumoylation pattern detected under denaturing condition. Left panel, the HCT-116 cells were lysed with RIPA buffer containing 0.1% SDS in the presence and absence
Supplemental Information
 Supplemental Information Tobacco-specific Carcinogen Induces DNA Methyltransferases 1 Accumulation through AKT/GSK3β/βTrCP/hnRNP-U in Mice and Lung Cancer patients Ruo-Kai Lin, 1 Yi-Shuan Hsieh, 2 Pinpin
Supplemental Information Tobacco-specific Carcinogen Induces DNA Methyltransferases 1 Accumulation through AKT/GSK3β/βTrCP/hnRNP-U in Mice and Lung Cancer patients Ruo-Kai Lin, 1 Yi-Shuan Hsieh, 2 Pinpin
A Hepatocyte Growth Factor Receptor (Met) Insulin Receptor hybrid governs hepatic glucose metabolism SUPPLEMENTARY FIGURES, LEGENDS AND METHODS
 A Hepatocyte Growth Factor Receptor (Met) Insulin Receptor hybrid governs hepatic glucose metabolism Arlee Fafalios, Jihong Ma, Xinping Tan, John Stoops, Jianhua Luo, Marie C. DeFrances and Reza Zarnegar
A Hepatocyte Growth Factor Receptor (Met) Insulin Receptor hybrid governs hepatic glucose metabolism Arlee Fafalios, Jihong Ma, Xinping Tan, John Stoops, Jianhua Luo, Marie C. DeFrances and Reza Zarnegar
hemodynamic stress. A. Echocardiographic quantification of cardiac dimensions and function in
 SUPPLEMENTAL FIGURE LEGENDS Supplemental Figure 1. Fbn1 C1039G/+ hearts display normal cardiac function in the absence of hemodynamic stress. A. Echocardiographic quantification of cardiac dimensions and
SUPPLEMENTAL FIGURE LEGENDS Supplemental Figure 1. Fbn1 C1039G/+ hearts display normal cardiac function in the absence of hemodynamic stress. A. Echocardiographic quantification of cardiac dimensions and
A Central Role of MG53 in Metabolic Syndrome. and Type-2 Diabetes
 A Central Role of MG53 in Metabolic Syndrome and Type-2 Diabetes Yan Zhang, Chunmei Cao, Rui-Ping Xiao Institute of Molecular Medicine (IMM) Peking University, Beijing, China Accelerated Aging in China
A Central Role of MG53 in Metabolic Syndrome and Type-2 Diabetes Yan Zhang, Chunmei Cao, Rui-Ping Xiao Institute of Molecular Medicine (IMM) Peking University, Beijing, China Accelerated Aging in China
S1a S1b S1c. S1d. S1f S1g S1h SUPPLEMENTARY FIGURE 1. - si sc Il17rd Il17ra bp. rig/s IL-17RD (ng) -100 IL-17RD
 SUPPLEMENTARY FIGURE 1 0 20 50 80 100 IL-17RD (ng) S1a S1b S1c IL-17RD β-actin kda S1d - si sc Il17rd Il17ra rig/s15-574 - 458-361 bp S1f S1g S1h S1i S1j Supplementary Figure 1. Knockdown of IL-17RD enhances
SUPPLEMENTARY FIGURE 1 0 20 50 80 100 IL-17RD (ng) S1a S1b S1c IL-17RD β-actin kda S1d - si sc Il17rd Il17ra rig/s15-574 - 458-361 bp S1f S1g S1h S1i S1j Supplementary Figure 1. Knockdown of IL-17RD enhances
SUPPLEMENTAL FIGURE LEGENDS
 SUPPLEMENTAL FIGURE LEGENDS Supplemental Figure S1: Endogenous interaction between RNF2 and H2AX: Whole cell extracts from 293T were subjected to immunoprecipitation with anti-rnf2 or anti-γ-h2ax antibodies
SUPPLEMENTAL FIGURE LEGENDS Supplemental Figure S1: Endogenous interaction between RNF2 and H2AX: Whole cell extracts from 293T were subjected to immunoprecipitation with anti-rnf2 or anti-γ-h2ax antibodies
SUPPLEMENTARY INFORMATION
 Supplementary Table 1. Cell sphingolipids and S1P bound to endogenous TRAF2. Sphingolipid Cell pmol/mg TRAF2 immunoprecipitate pmol/mg Sphingomyelin 4200 ± 250 Not detected Monohexosylceramide 311 ± 18
Supplementary Table 1. Cell sphingolipids and S1P bound to endogenous TRAF2. Sphingolipid Cell pmol/mg TRAF2 immunoprecipitate pmol/mg Sphingomyelin 4200 ± 250 Not detected Monohexosylceramide 311 ± 18
p47 negatively regulates IKK activation by inducing the lysosomal degradation of polyubiquitinated NEMO
 Supplementary Information p47 negatively regulates IKK activation by inducing the lysosomal degradation of polyubiquitinated NEMO Yuri Shibata, Masaaki Oyama, Hiroko Kozuka-Hata, Xiao Han, Yuetsu Tanaka,
Supplementary Information p47 negatively regulates IKK activation by inducing the lysosomal degradation of polyubiquitinated NEMO Yuri Shibata, Masaaki Oyama, Hiroko Kozuka-Hata, Xiao Han, Yuetsu Tanaka,
RAW264.7 cells stably expressing control shrna (Con) or GSK3b-specific shrna (sh-
 1 a b Supplementary Figure 1. Effects of GSK3b knockdown on poly I:C-induced cytokine production. RAW264.7 cells stably expressing control shrna (Con) or GSK3b-specific shrna (sh- GSK3b) were stimulated
1 a b Supplementary Figure 1. Effects of GSK3b knockdown on poly I:C-induced cytokine production. RAW264.7 cells stably expressing control shrna (Con) or GSK3b-specific shrna (sh- GSK3b) were stimulated
Sestrin2 and BNIP3 (Bcl-2/adenovirus E1B 19kDa-interacting. protein3) regulate autophagy and mitophagy in renal tubular cells in. acute kidney injury
 Sestrin2 and BNIP3 (Bcl-2/adenovirus E1B 19kDa-interacting protein3) regulate autophagy and mitophagy in renal tubular cells in acute kidney injury by Masayuki Ishihara 1, Madoka Urushido 2, Kazu Hamada
Sestrin2 and BNIP3 (Bcl-2/adenovirus E1B 19kDa-interacting protein3) regulate autophagy and mitophagy in renal tubular cells in acute kidney injury by Masayuki Ishihara 1, Madoka Urushido 2, Kazu Hamada
(Stratagene, La Jolla, CA) (Supplemental Fig. 1A). A 5.4-kb EcoRI fragment
 SUPPLEMENTAL INFORMATION Supplemental Methods Generation of RyR2-S2808D Mice Murine genomic RyR2 clones were isolated from a 129/SvEvTacfBR λ-phage library (Stratagene, La Jolla, CA) (Supplemental Fig.
SUPPLEMENTAL INFORMATION Supplemental Methods Generation of RyR2-S2808D Mice Murine genomic RyR2 clones were isolated from a 129/SvEvTacfBR λ-phage library (Stratagene, La Jolla, CA) (Supplemental Fig.
Gallic acid prevents isoproterenol-induced cardiac hypertrophy and fibrosis through regulation of JNK2 signaling and Smad3 binding activity
 Gallic acid prevents isoproterenol-induced cardiac hypertrophy and fibrosis through regulation of JNK2 signaling and Smad3 binding activity Yuhee Ryu 1,+, Li Jin 1,2+, Hae Jin Kee 1,, Zhe Hao Piao 3, Jae
Gallic acid prevents isoproterenol-induced cardiac hypertrophy and fibrosis through regulation of JNK2 signaling and Smad3 binding activity Yuhee Ryu 1,+, Li Jin 1,2+, Hae Jin Kee 1,, Zhe Hao Piao 3, Jae
TRAF6 ubiquitinates TGFβ type I receptor to promote its cleavage and nuclear translocation in cancer
 Supplementary Information TRAF6 ubiquitinates TGFβ type I receptor to promote its cleavage and nuclear translocation in cancer Yabing Mu, Reshma Sundar, Noopur Thakur, Maria Ekman, Shyam Kumar Gudey, Mariya
Supplementary Information TRAF6 ubiquitinates TGFβ type I receptor to promote its cleavage and nuclear translocation in cancer Yabing Mu, Reshma Sundar, Noopur Thakur, Maria Ekman, Shyam Kumar Gudey, Mariya
Supplementary Materials for
 www.sciencesignaling.org/cgi/content/full/6/283/ra57/dc1 Supplementary Materials for JNK3 Couples the Neuronal Stress Response to Inhibition of Secretory Trafficking Guang Yang,* Xun Zhou, Jingyan Zhu,
www.sciencesignaling.org/cgi/content/full/6/283/ra57/dc1 Supplementary Materials for JNK3 Couples the Neuronal Stress Response to Inhibition of Secretory Trafficking Guang Yang,* Xun Zhou, Jingyan Zhu,
Probe. Hind III Q,!?R'!! /0!!!!D1"?R'! vector. Homologous recombination
 Supple-Zhang Page 1 Wild-type locus Targeting construct Targeted allele Exon Exon3 Exon Probe P1 P P3 FRT FRT loxp loxp neo vector amh I Homologous recombination neo P1 P P3 FLPe recombination Q,!?R'!!
Supple-Zhang Page 1 Wild-type locus Targeting construct Targeted allele Exon Exon3 Exon Probe P1 P P3 FRT FRT loxp loxp neo vector amh I Homologous recombination neo P1 P P3 FLPe recombination Q,!?R'!!
SUPPLEMENTARY INFORMATION
 DOI: 10.1038/ncb2566 Figure S1 CDKL5 protein expression pattern and localization in mouse brain. (a) Multiple-tissue western blot from a postnatal day (P) 21 mouse probed with an antibody against CDKL5.
DOI: 10.1038/ncb2566 Figure S1 CDKL5 protein expression pattern and localization in mouse brain. (a) Multiple-tissue western blot from a postnatal day (P) 21 mouse probed with an antibody against CDKL5.
293T cells were transfected with indicated expression vectors and the whole-cell extracts were subjected
 SUPPLEMENTARY INFORMATION Supplementary Figure 1. Formation of a complex between Slo1 and CRL4A CRBN E3 ligase. (a) HEK 293T cells were transfected with indicated expression vectors and the whole-cell
SUPPLEMENTARY INFORMATION Supplementary Figure 1. Formation of a complex between Slo1 and CRL4A CRBN E3 ligase. (a) HEK 293T cells were transfected with indicated expression vectors and the whole-cell
Supplementary Figure 1: si-craf but not si-braf sensitizes tumor cells to radiation.
 Supplementary Figure 1: si-craf but not si-braf sensitizes tumor cells to radiation. (a) Embryonic fibroblasts isolated from wildtype (WT), BRAF -/-, or CRAF -/- mice were irradiated (6 Gy) and DNA damage
Supplementary Figure 1: si-craf but not si-braf sensitizes tumor cells to radiation. (a) Embryonic fibroblasts isolated from wildtype (WT), BRAF -/-, or CRAF -/- mice were irradiated (6 Gy) and DNA damage
Tel: ; Fax: ;
 Tel.: +98 216 696 9291; Fax: +98 216 696 9291; E-mail: mrasadeghi@pasteur.ac.ir Tel: +98 916 113 7679; Fax: +98 613 333 6380; E-mail: abakhshi_e@ajums.ac.ir A Soluble Chromatin-bound MOI 0 1 5 0 1 5 HDAC2
Tel.: +98 216 696 9291; Fax: +98 216 696 9291; E-mail: mrasadeghi@pasteur.ac.ir Tel: +98 916 113 7679; Fax: +98 613 333 6380; E-mail: abakhshi_e@ajums.ac.ir A Soluble Chromatin-bound MOI 0 1 5 0 1 5 HDAC2
Supplementary Figure 1. PD-L1 is glycosylated in cancer cells. (a) Western blot analysis of PD-L1 in breast cancer cells. (b) Western blot analysis
 Supplementary Figure 1. PD-L1 is glycosylated in cancer cells. (a) Western blot analysis of PD-L1 in breast cancer cells. (b) Western blot analysis of PD-L1 in ovarian cancer cells. (c) Western blot analysis
Supplementary Figure 1. PD-L1 is glycosylated in cancer cells. (a) Western blot analysis of PD-L1 in breast cancer cells. (b) Western blot analysis of PD-L1 in ovarian cancer cells. (c) Western blot analysis
MicroRNA-208a is a regulator of cardiac hypertrophy and conduction in mice
 Research article MicroRNA-208a is a regulator of cardiac hypertrophy and conduction in mice Thomas E. Callis, 1,2 Kumar Pandya, 3 Hee Young Seok, 1,2 Ru-Hang Tang, 1,2,4 Mariko Tatsuguchi, 1,2 Zhan-Peng
Research article MicroRNA-208a is a regulator of cardiac hypertrophy and conduction in mice Thomas E. Callis, 1,2 Kumar Pandya, 3 Hee Young Seok, 1,2 Ru-Hang Tang, 1,2,4 Mariko Tatsuguchi, 1,2 Zhan-Peng
T H E J O U R N A L O F C E L L B I O L O G Y
 T H E J O U R N A L O F C E L L B I O L O G Y Supplemental material Krenn et al., http://www.jcb.org/cgi/content/full/jcb.201110013/dc1 Figure S1. Levels of expressed proteins and demonstration that C-terminal
T H E J O U R N A L O F C E L L B I O L O G Y Supplemental material Krenn et al., http://www.jcb.org/cgi/content/full/jcb.201110013/dc1 Figure S1. Levels of expressed proteins and demonstration that C-terminal
supplementary information
 DOI: 10.1038/ncb2153 Figure S1 Ectopic expression of HAUSP up-regulates REST protein. (a) Immunoblotting showed that ectopic expression of HAUSP increased REST protein levels in ENStemA NPCs. (b) Immunofluorescent
DOI: 10.1038/ncb2153 Figure S1 Ectopic expression of HAUSP up-regulates REST protein. (a) Immunoblotting showed that ectopic expression of HAUSP increased REST protein levels in ENStemA NPCs. (b) Immunofluorescent
William C. Comb, Jessica E. Hutti, Patricia Cogswell, Lewis C. Cantley, and Albert S. Baldwin
 Molecular Cell, Volume 45 Supplemental Information p85 SH2 Domain Phosphorylation by IKK Promotes Feedback Inhibition of PI3K and Akt in Response to Cellular Starvation William C. Comb, Jessica E. Hutti,
Molecular Cell, Volume 45 Supplemental Information p85 SH2 Domain Phosphorylation by IKK Promotes Feedback Inhibition of PI3K and Akt in Response to Cellular Starvation William C. Comb, Jessica E. Hutti,
Kidney. Heart. Lung. Sirt1. Gapdh. Mouse IgG DAPI. Rabbit IgG DAPI
 a e Na V 1.5 Ad-LacZ Ad- 110KD b Scn5a/ (relative to Ad-LacZ) f 150 100 50 0 p = 0.65 Ad-LacZ Ad- c Heart Lung Kidney Spleen 110KD d fl/fl c -/- DAPI 20 µm Na v 1.5 250KD fl/fl Rabbit IgG DAPI fl/fl Mouse
a e Na V 1.5 Ad-LacZ Ad- 110KD b Scn5a/ (relative to Ad-LacZ) f 150 100 50 0 p = 0.65 Ad-LacZ Ad- c Heart Lung Kidney Spleen 110KD d fl/fl c -/- DAPI 20 µm Na v 1.5 250KD fl/fl Rabbit IgG DAPI fl/fl Mouse
The functional investigation of the interaction between TATA-associated factor 3 (TAF3) and p53 protein
 THESIS BOOK The functional investigation of the interaction between TATA-associated factor 3 (TAF3) and p53 protein Orsolya Buzás-Bereczki Supervisors: Dr. Éva Bálint Dr. Imre Miklós Boros University of
THESIS BOOK The functional investigation of the interaction between TATA-associated factor 3 (TAF3) and p53 protein Orsolya Buzás-Bereczki Supervisors: Dr. Éva Bálint Dr. Imre Miklós Boros University of
Nature Immunology doi: /ni.3268
 Supplementary Figure 1 Loss of Mst1 and Mst2 increases susceptibility to bacterial sepsis. (a) H&E staining of colon and kidney sections from wild type and Mst1 -/- Mst2 fl/fl Vav-Cre mice. Scale bar,
Supplementary Figure 1 Loss of Mst1 and Mst2 increases susceptibility to bacterial sepsis. (a) H&E staining of colon and kidney sections from wild type and Mst1 -/- Mst2 fl/fl Vav-Cre mice. Scale bar,
SUPPLEMENTARY INFORMATION
 Figure S1 Treatment with both Sema6D and Plexin-A1 sirnas induces the phenotype essentially identical to that induced by treatment with Sema6D sirna alone or Plexin-A1 sirna alone. (a,b) The cardiac tube
Figure S1 Treatment with both Sema6D and Plexin-A1 sirnas induces the phenotype essentially identical to that induced by treatment with Sema6D sirna alone or Plexin-A1 sirna alone. (a,b) The cardiac tube
Supporting Information
 Supporting Information Palmisano et al. 10.1073/pnas.1202174109 Fig. S1. Expression of different transgenes, driven by either viral or human promoters, is up-regulated by amino acid starvation. (A) Quantification
Supporting Information Palmisano et al. 10.1073/pnas.1202174109 Fig. S1. Expression of different transgenes, driven by either viral or human promoters, is up-regulated by amino acid starvation. (A) Quantification
supplementary information
 DOI: 10.1038/ncb1875 Figure S1 (a) The 79 surgical specimens from NSCLC patients were analysed by immunohistochemistry with an anti-p53 antibody and control serum (data not shown). The normal bronchi served
DOI: 10.1038/ncb1875 Figure S1 (a) The 79 surgical specimens from NSCLC patients were analysed by immunohistochemistry with an anti-p53 antibody and control serum (data not shown). The normal bronchi served
HEK293FT cells were transiently transfected with reporters, N3-ICD construct and
 Supplementary Information Luciferase reporter assay HEK293FT cells were transiently transfected with reporters, N3-ICD construct and increased amounts of wild type or kinase inactive EGFR. Transfections
Supplementary Information Luciferase reporter assay HEK293FT cells were transiently transfected with reporters, N3-ICD construct and increased amounts of wild type or kinase inactive EGFR. Transfections
Supplementary Figure 1 Expression of Crb3 in mouse sciatic nerve: biochemical analysis (a) Schematic of Crb3 isoforms, ERLI and CLPI, indicating the
 Supplementary Figure 1 Expression of Crb3 in mouse sciatic nerve: biochemical analysis (a) Schematic of Crb3 isoforms, ERLI and CLPI, indicating the location of the transmembrane (TM), FRM binding (FB)
Supplementary Figure 1 Expression of Crb3 in mouse sciatic nerve: biochemical analysis (a) Schematic of Crb3 isoforms, ERLI and CLPI, indicating the location of the transmembrane (TM), FRM binding (FB)
supplementary information
 DOI: 1.138/ncb1 Control Atg7 / NAC 1 1 1 1 (mm) Control Atg7 / NAC 1 1 1 1 (mm) Lamin B Gstm1 Figure S1 Neither the translocation of into the nucleus nor the induction of antioxidant proteins in autophagydeficient
DOI: 1.138/ncb1 Control Atg7 / NAC 1 1 1 1 (mm) Control Atg7 / NAC 1 1 1 1 (mm) Lamin B Gstm1 Figure S1 Neither the translocation of into the nucleus nor the induction of antioxidant proteins in autophagydeficient
Supplementary Figure 1 CD4 + T cells from PKC-θ null mice are defective in NF-κB activation during T cell receptor signaling. CD4 + T cells were
 Supplementary Figure 1 CD4 + T cells from PKC-θ null mice are defective in NF-κB activation during T cell receptor signaling. CD4 + T cells were isolated from wild type (PKC-θ- WT) or PKC-θ null (PKC-θ-KO)
Supplementary Figure 1 CD4 + T cells from PKC-θ null mice are defective in NF-κB activation during T cell receptor signaling. CD4 + T cells were isolated from wild type (PKC-θ- WT) or PKC-θ null (PKC-θ-KO)
Supporting Information
 Supporting Information Muraski et al. 10.1073/pnas.0709135105 SI Text Generation of Transgenic Animals. Pim-WT and Pim-DN cdnas were subcloned NheI/SmaI from pegfp-c1 Pim-1 and pegfp-c1 Pim-DN plasmids
Supporting Information Muraski et al. 10.1073/pnas.0709135105 SI Text Generation of Transgenic Animals. Pim-WT and Pim-DN cdnas were subcloned NheI/SmaI from pegfp-c1 Pim-1 and pegfp-c1 Pim-DN plasmids
FOXO Reporter Kit PI3K/AKT Pathway Cat. #60643
 Data Sheet FOXO Reporter Kit PI3K/AKT Pathway Cat. #60643 Background The PI3K/AKT signaling pathway is essential for cell growth and survival. Disruption of this pathway or its regulation has been linked
Data Sheet FOXO Reporter Kit PI3K/AKT Pathway Cat. #60643 Background The PI3K/AKT signaling pathway is essential for cell growth and survival. Disruption of this pathway or its regulation has been linked
MicroRNA sponges: competitive inhibitors of small RNAs in mammalian cells
 MicroRNA sponges: competitive inhibitors of small RNAs in mammalian cells Margaret S Ebert, Joel R Neilson & Phillip A Sharp Supplementary figures and text: Supplementary Figure 1. Effect of sponges on
MicroRNA sponges: competitive inhibitors of small RNAs in mammalian cells Margaret S Ebert, Joel R Neilson & Phillip A Sharp Supplementary figures and text: Supplementary Figure 1. Effect of sponges on
The clathrin adaptor Numb regulates intestinal cholesterol. absorption through dynamic interaction with NPC1L1
 The clathrin adaptor Numb regulates intestinal cholesterol absorption through dynamic interaction with NPC1L1 Pei-Shan Li 1, Zhen-Yan Fu 1,2, Ying-Yu Zhang 1, Jin-Hui Zhang 1, Chen-Qi Xu 1, Yi-Tong Ma
The clathrin adaptor Numb regulates intestinal cholesterol absorption through dynamic interaction with NPC1L1 Pei-Shan Li 1, Zhen-Yan Fu 1,2, Ying-Yu Zhang 1, Jin-Hui Zhang 1, Chen-Qi Xu 1, Yi-Tong Ma
Appendix. Table of Contents
 Appendix Table of Contents Appendix Figures Figure S1: Gp78 is not required for the degradation of mcherry-cl1 in Hela Cells. Figure S2: Indel formation in the MARCH6 sgrna targeted HeLa clones. Figure
Appendix Table of Contents Appendix Figures Figure S1: Gp78 is not required for the degradation of mcherry-cl1 in Hela Cells. Figure S2: Indel formation in the MARCH6 sgrna targeted HeLa clones. Figure
Genetic ablation of Acp1 (Lmptp) in mice prevents heart failure
 Genetic ablation of Acp1 (Lmptp) in mice prevents heart failure Coralie Poizat, Ph.D. Director, Cardiovascular Research Program KFSHRC-Riyadh Saudi Heart Failure Working Group Jeddah, 5 December 2015 Cardiovascular
Genetic ablation of Acp1 (Lmptp) in mice prevents heart failure Coralie Poizat, Ph.D. Director, Cardiovascular Research Program KFSHRC-Riyadh Saudi Heart Failure Working Group Jeddah, 5 December 2015 Cardiovascular
Supplementary Figure S1: Defective heterochromatin repair in HGPS progeroid cells
 Supplementary Figure S1: Defective heterochromatin repair in HGPS progeroid cells Immunofluorescence staining of H3K9me3 and 53BP1 in PH and HGADFN003 (HG003) cells at 24 h after γ-irradiation. Scale bar,
Supplementary Figure S1: Defective heterochromatin repair in HGPS progeroid cells Immunofluorescence staining of H3K9me3 and 53BP1 in PH and HGADFN003 (HG003) cells at 24 h after γ-irradiation. Scale bar,
Supplemental information
 Carcinoemryonic antigen-related cell adhesion molecule 6 (CEACAM6) promotes EGF receptor signaling of oral squamous cell carcinoma metastasis via the complex N-glycosylation y Chiang et al. Supplemental
Carcinoemryonic antigen-related cell adhesion molecule 6 (CEACAM6) promotes EGF receptor signaling of oral squamous cell carcinoma metastasis via the complex N-glycosylation y Chiang et al. Supplemental
Predictive PP1Ca binding region in BIG3 : 1,228 1,232aa (-KAVSF-) HEK293T cells *** *** *** KPL-3C cells - E E2 treatment time (h)
 Relative expression ERE-luciferase activity activity (pmole/min) activity (pmole/min) activity (pmole/min) activity (pmole/min) MCF-7 KPL-3C ZR--1 BT-474 T47D HCC15 KPL-1 HBC4 activity (pmole/min) a d
Relative expression ERE-luciferase activity activity (pmole/min) activity (pmole/min) activity (pmole/min) activity (pmole/min) MCF-7 KPL-3C ZR--1 BT-474 T47D HCC15 KPL-1 HBC4 activity (pmole/min) a d
Tumor suppressor Spred2 interaction with LC3 promotes autophagosome maturation and induces autophagy-dependent cell death
 www.impactjournals.com/oncotarget/ Oncotarget, Supplementary Materials 2016 Tumor suppressor Spred2 interaction with LC3 promotes autophagosome maturation and induces autophagy-dependent cell death Supplementary
www.impactjournals.com/oncotarget/ Oncotarget, Supplementary Materials 2016 Tumor suppressor Spred2 interaction with LC3 promotes autophagosome maturation and induces autophagy-dependent cell death Supplementary
T H E J O U R N A L O F C E L L B I O L O G Y
 Supplemental material Chairoungdua et al., http://www.jcb.org/cgi/content/full/jcb.201002049/dc1 T H E J O U R N A L O F C E L L B I O L O G Y Figure S1. Expression of CD9 and CD82 inhibits Wnt/ -catenin
Supplemental material Chairoungdua et al., http://www.jcb.org/cgi/content/full/jcb.201002049/dc1 T H E J O U R N A L O F C E L L B I O L O G Y Figure S1. Expression of CD9 and CD82 inhibits Wnt/ -catenin
Reviewers' comments: Reviewer #1 (Remarks to the Author):
 Reviewers' comments: Reviewer #1 (Remarks to the Author): In this manuscript, Song et al. identified FBXW7 as a new positive regulator for RIG-Itriggered type I IFN signaling pathway. The authors observed
Reviewers' comments: Reviewer #1 (Remarks to the Author): In this manuscript, Song et al. identified FBXW7 as a new positive regulator for RIG-Itriggered type I IFN signaling pathway. The authors observed
Supplementary Information
 Supplementary Information Supplementary Figure 1. CD4 + T cell activation and lack of apoptosis after crosslinking with anti-cd3 + anti-cd28 + anti-cd160. (a) Flow cytometry of anti-cd160 (5D.10A11) binding
Supplementary Information Supplementary Figure 1. CD4 + T cell activation and lack of apoptosis after crosslinking with anti-cd3 + anti-cd28 + anti-cd160. (a) Flow cytometry of anti-cd160 (5D.10A11) binding
(A) RT-PCR for components of the Shh/Gli pathway in normal fetus cell (MRC-5) and a
 Supplementary figure legends Supplementary Figure 1. Expression of Shh signaling components in a panel of gastric cancer. (A) RT-PCR for components of the Shh/Gli pathway in normal fetus cell (MRC-5) and
Supplementary figure legends Supplementary Figure 1. Expression of Shh signaling components in a panel of gastric cancer. (A) RT-PCR for components of the Shh/Gli pathway in normal fetus cell (MRC-5) and
Supplementary Figure 1
 Supplementary Figure 1 YAP negatively regulates IFN- signaling. (a) Immunoblot analysis of Yap knockdown efficiency with sh-yap (#1 to #4 independent constructs) in Raw264.7 cells. (b) IFN- -Luc and PRDs
Supplementary Figure 1 YAP negatively regulates IFN- signaling. (a) Immunoblot analysis of Yap knockdown efficiency with sh-yap (#1 to #4 independent constructs) in Raw264.7 cells. (b) IFN- -Luc and PRDs
Supplementary Table 1. Metabolic parameters in GFP and OGT-treated mice
 Supplementary Table 1. Metabolic parameters in GFP and OGT-treated mice Fasted Refed GFP OGT GFP OGT Liver G6P (mmol/g) 0.03±0.01 0.04±0.02 0.60±0.04 0.42±0.10 A TGs (mg/g of liver) 20.08±5.17 16.29±0.8
Supplementary Table 1. Metabolic parameters in GFP and OGT-treated mice Fasted Refed GFP OGT GFP OGT Liver G6P (mmol/g) 0.03±0.01 0.04±0.02 0.60±0.04 0.42±0.10 A TGs (mg/g of liver) 20.08±5.17 16.29±0.8
Supplementary information
 Supplementary information Human Cytomegalovirus MicroRNA mir-us4-1 Inhibits CD8 + T Cell Response by Targeting ERAP1 Sungchul Kim, Sanghyun Lee, Jinwook Shin, Youngkyun Kim, Irini Evnouchidou, Donghyun
Supplementary information Human Cytomegalovirus MicroRNA mir-us4-1 Inhibits CD8 + T Cell Response by Targeting ERAP1 Sungchul Kim, Sanghyun Lee, Jinwook Shin, Youngkyun Kim, Irini Evnouchidou, Donghyun
Supplementary data Supplementary Figure 1 Supplementary Figure 2
 Supplementary data Supplementary Figure 1 SPHK1 sirna increases RANKL-induced osteoclastogenesis in RAW264.7 cell culture. (A) RAW264.7 cells were transfected with oligocassettes containing SPHK1 sirna
Supplementary data Supplementary Figure 1 SPHK1 sirna increases RANKL-induced osteoclastogenesis in RAW264.7 cell culture. (A) RAW264.7 cells were transfected with oligocassettes containing SPHK1 sirna
TFEB-mediated increase in peripheral lysosomes regulates. Store Operated Calcium Entry
 TFEB-mediated increase in peripheral lysosomes regulates Store Operated Calcium Entry Luigi Sbano, Massimo Bonora, Saverio Marchi, Federica Baldassari, Diego L. Medina, Andrea Ballabio, Carlotta Giorgi
TFEB-mediated increase in peripheral lysosomes regulates Store Operated Calcium Entry Luigi Sbano, Massimo Bonora, Saverio Marchi, Federica Baldassari, Diego L. Medina, Andrea Ballabio, Carlotta Giorgi
Supplementary Table 1. Characterization of HNSCC PDX models established at MSKCC
 Supplementary Table 1. Characterization of HNSCC PDX models established at MSKCC Supplementary Table 2. Drug content and loading efficiency estimated with F-NMR and UV- Vis Supplementary Table 3. Complete
Supplementary Table 1. Characterization of HNSCC PDX models established at MSKCC Supplementary Table 2. Drug content and loading efficiency estimated with F-NMR and UV- Vis Supplementary Table 3. Complete
Brd4 Coactivates Transcriptional Activation of NF- B via Specific Binding to Acetylated RelA
 MOLECULAR AND CELLULAR BIOLOGY, Mar. 2009, p. 1375 1387 Vol. 29, No. 5 0270-7306/09/$08.00 0 doi:10.1128/mcb.01365-08 Copyright 2009, American Society for Microbiology. All Rights Reserved. Brd4 Coactivates
MOLECULAR AND CELLULAR BIOLOGY, Mar. 2009, p. 1375 1387 Vol. 29, No. 5 0270-7306/09/$08.00 0 doi:10.1128/mcb.01365-08 Copyright 2009, American Society for Microbiology. All Rights Reserved. Brd4 Coactivates
(A) SW480, DLD1, RKO and HCT116 cells were treated with DMSO or XAV939 (5 µm)
 Supplementary Figure Legends Figure S1. Tankyrase inhibition suppresses cell proliferation in an axin/β-catenin independent manner. (A) SW480, DLD1, RKO and HCT116 cells were treated with DMSO or XAV939
Supplementary Figure Legends Figure S1. Tankyrase inhibition suppresses cell proliferation in an axin/β-catenin independent manner. (A) SW480, DLD1, RKO and HCT116 cells were treated with DMSO or XAV939
Supplementary Materials for
 www.sciencesignaling.org/cgi/content/full/10/471/eaah5085/dc1 Supplementary Materials for Phosphorylation of the exocyst protein Exo84 by TBK1 promotes insulin-stimulated GLUT4 trafficking Maeran Uhm,
www.sciencesignaling.org/cgi/content/full/10/471/eaah5085/dc1 Supplementary Materials for Phosphorylation of the exocyst protein Exo84 by TBK1 promotes insulin-stimulated GLUT4 trafficking Maeran Uhm,
Intracellular MHC class II molecules promote TLR-triggered innate. immune responses by maintaining Btk activation
 Intracellular MHC class II molecules promote TLR-triggered innate immune responses by maintaining Btk activation Xingguang Liu, Zhenzhen Zhan, Dong Li, Li Xu, Feng Ma, Peng Zhang, Hangping Yao and Xuetao
Intracellular MHC class II molecules promote TLR-triggered innate immune responses by maintaining Btk activation Xingguang Liu, Zhenzhen Zhan, Dong Li, Li Xu, Feng Ma, Peng Zhang, Hangping Yao and Xuetao
Supplementary Figure 1. MAT IIα is Acetylated at Lysine 81.
 IP: Flag a Mascot PTM Modified Mass Error Position Gene Names Score Score Sequence m/z [ppm] 81 MAT2A;AMS2;MATA2 35.6 137.28 _AAVDYQK(ac)VVR_ 595.83-2.28 b Pre-immu After-immu Flag- WT K81R WT K81R / Flag
IP: Flag a Mascot PTM Modified Mass Error Position Gene Names Score Score Sequence m/z [ppm] 81 MAT2A;AMS2;MATA2 35.6 137.28 _AAVDYQK(ac)VVR_ 595.83-2.28 b Pre-immu After-immu Flag- WT K81R WT K81R / Flag
SUPPLEMENTARY INFORMATION
 doi: 10.1038/nature05732 SUPPLEMENTARY INFORMATION Supplemental Data Supplement Figure Legends Figure S1. RIG-I 2CARD undergo robust ubiquitination a, (top) At 48 h posttransfection with a GST, GST-RIG-I-2CARD
doi: 10.1038/nature05732 SUPPLEMENTARY INFORMATION Supplemental Data Supplement Figure Legends Figure S1. RIG-I 2CARD undergo robust ubiquitination a, (top) At 48 h posttransfection with a GST, GST-RIG-I-2CARD
Supplementary Figure 1. Baf60c and baf180 are induced during cardiac regeneration in zebrafish. RNA in situ hybridization was performed on paraffin
 Supplementary Figure 1. Baf60c and baf180 are induced during cardiac regeneration in zebrafish. RNA in situ hybridization was performed on paraffin sections from sham-operated adult hearts (a and i) and
Supplementary Figure 1. Baf60c and baf180 are induced during cardiac regeneration in zebrafish. RNA in situ hybridization was performed on paraffin sections from sham-operated adult hearts (a and i) and
BNP mrna expression in DR and DS rat left ventricles (n = 5). (C) Plasma norepinephrine
 Kanazawa, et al. Supplementary figure legends Supplementary Figure 1 DS rats had congestive heart failure. (A) DR and DS rat hearts. (B) QRT-PCR analysis of BNP mrna expression in DR and DS rat left ventricles
Kanazawa, et al. Supplementary figure legends Supplementary Figure 1 DS rats had congestive heart failure. (A) DR and DS rat hearts. (B) QRT-PCR analysis of BNP mrna expression in DR and DS rat left ventricles
Live cell imaging of trafficking of the chaperone complex vaccine to the ER. BMDCs were incubated with ER-Tracker Red (1 M) in staining solution for
 Live cell imaging of trafficking of the chaperone complex vaccine to the ER. BMDCs were incubated with ER-Tracker Red (1 M) in staining solution for 15 min at 37 C and replaced with fresh complete medium.
Live cell imaging of trafficking of the chaperone complex vaccine to the ER. BMDCs were incubated with ER-Tracker Red (1 M) in staining solution for 15 min at 37 C and replaced with fresh complete medium.
Supplement Material. chain (MLC2v) promoter to generate cardiac-specific MKK4 knockout mice (referred to
 Supplement Material Materials and Methods Generation of MKK Mutant Mice The mkk-flox mice (referred to as ) were previously generated and characterised. mice were mated with mice expressing Cre under a
Supplement Material Materials and Methods Generation of MKK Mutant Mice The mkk-flox mice (referred to as ) were previously generated and characterised. mice were mated with mice expressing Cre under a
Supplementary Figure 1
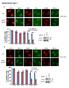 Supplementary Figure 1 a γ-h2ax MDC1 RNF8 FK2 BRCA1 U2OS Cells sgrna-1 ** 60 sgrna 40 20 0 % positive Cells (>5 foci per cell) b ** 80 sgrna sgrna γ-h2ax MDC1 γ-h2ax RNF8 FK2 MDC1 BRCA1 RNF8 FK2 BRCA1
Supplementary Figure 1 a γ-h2ax MDC1 RNF8 FK2 BRCA1 U2OS Cells sgrna-1 ** 60 sgrna 40 20 0 % positive Cells (>5 foci per cell) b ** 80 sgrna sgrna γ-h2ax MDC1 γ-h2ax RNF8 FK2 MDC1 BRCA1 RNF8 FK2 BRCA1
E3 ligase TRIM72 negatively regulates myogenesis by IRS-1 ubiquitination
 E3 ligase negatively regulates myogenesis by IRS-1 ubiquitination Young-Gyu Ko College of Life Science and Biotechnology Korea University MyHC immunofluorescence Lipid rafts are plasma membrane compartments
E3 ligase negatively regulates myogenesis by IRS-1 ubiquitination Young-Gyu Ko College of Life Science and Biotechnology Korea University MyHC immunofluorescence Lipid rafts are plasma membrane compartments
SUPPLEMENTAL MATERIAL. Supplementary Methods
 SUPPLEMENTAL MATERIAL Supplementary Methods Culture of cardiomyocytes, fibroblasts and cardiac microvascular endothelial cells The isolation and culturing of neonatal rat ventricular cardiomyocytes was
SUPPLEMENTAL MATERIAL Supplementary Methods Culture of cardiomyocytes, fibroblasts and cardiac microvascular endothelial cells The isolation and culturing of neonatal rat ventricular cardiomyocytes was
Supporting Information
 Supporting Information Kuroda et al. 10.1073/pnas.1002178107 SI Methods Monoclonal Antibodies Against Nox4. Generation of the anti-nox4 mouse monoclonal antibody (3D2), which detects Nox4 and does not
Supporting Information Kuroda et al. 10.1073/pnas.1002178107 SI Methods Monoclonal Antibodies Against Nox4. Generation of the anti-nox4 mouse monoclonal antibody (3D2), which detects Nox4 and does not
Supplemental Figure 1
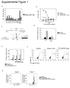 Supplemental Figure 1 1a 1c PD-1 MFI fold change 6 5 4 3 2 1 IL-1α IL-2 IL-4 IL-6 IL-1 IL-12 IL-13 IL-15 IL-17 IL-18 IL-21 IL-23 IFN-α Mut Human PD-1 promoter SBE-D 5 -GTCTG- -1.2kb SBE-P -CAGAC- -1.kb
Supplemental Figure 1 1a 1c PD-1 MFI fold change 6 5 4 3 2 1 IL-1α IL-2 IL-4 IL-6 IL-1 IL-12 IL-13 IL-15 IL-17 IL-18 IL-21 IL-23 IFN-α Mut Human PD-1 promoter SBE-D 5 -GTCTG- -1.2kb SBE-P -CAGAC- -1.kb
Supplementary Fig. 1 eif6 +/- mice show a reduction in white adipose tissue, blood lipids and normal glycogen synthesis. The cohort of the original
 Supplementary Fig. 1 eif6 +/- mice show a reduction in white adipose tissue, blood lipids and normal glycogen synthesis. The cohort of the original phenotypic screening was n=40. For specific tests, the
Supplementary Fig. 1 eif6 +/- mice show a reduction in white adipose tissue, blood lipids and normal glycogen synthesis. The cohort of the original phenotypic screening was n=40. For specific tests, the
Soft Agar Assay. For each cell pool, 100,000 cells were resuspended in 0.35% (w/v)
 SUPPLEMENTARY MATERIAL AND METHODS Soft Agar Assay. For each cell pool, 100,000 cells were resuspended in 0.35% (w/v) top agar (LONZA, SeaKem LE Agarose cat.5004) and plated onto 0.5% (w/v) basal agar.
SUPPLEMENTARY MATERIAL AND METHODS Soft Agar Assay. For each cell pool, 100,000 cells were resuspended in 0.35% (w/v) top agar (LONZA, SeaKem LE Agarose cat.5004) and plated onto 0.5% (w/v) basal agar.
hexahistidine tagged GRP78 devoid of the KDEL motif (GRP78-His) on SDS-PAGE. This
 SUPPLEMENTAL FIGURE LEGEND Fig. S1. Generation and characterization of. (A) Coomassie staining of soluble hexahistidine tagged GRP78 devoid of the KDEL motif (GRP78-His) on SDS-PAGE. This protein was expressed
SUPPLEMENTAL FIGURE LEGEND Fig. S1. Generation and characterization of. (A) Coomassie staining of soluble hexahistidine tagged GRP78 devoid of the KDEL motif (GRP78-His) on SDS-PAGE. This protein was expressed
Electron micrograph of phosphotungstanic acid-stained exosomes derived from murine
 1 SUPPLEMENTARY INFORMATION SUPPLEMENTARY FIGURES Supplementary Figure 1. Physical properties of murine DC-derived exosomes. a, Electron micrograph of phosphotungstanic acid-stained exosomes derived from
1 SUPPLEMENTARY INFORMATION SUPPLEMENTARY FIGURES Supplementary Figure 1. Physical properties of murine DC-derived exosomes. a, Electron micrograph of phosphotungstanic acid-stained exosomes derived from
SUPPLEMENTARY INFORMATION
 doi:10.1038/nature11095 Supplementary Table 1. Summary of the binding between Angptls and various Igdomain containing receptors as determined by flow cytometry analysis. The results were summarized from
doi:10.1038/nature11095 Supplementary Table 1. Summary of the binding between Angptls and various Igdomain containing receptors as determined by flow cytometry analysis. The results were summarized from
SUPPLEMENTARY FIGURES AND TABLE
 SUPPLEMENTARY FIGURES AND TABLE Supplementary Figure S1: Characterization of IRE1α mutants. A. U87-LUC cells were transduced with the lentiviral vector containing the GFP sequence (U87-LUC Tet-ON GFP).
SUPPLEMENTARY FIGURES AND TABLE Supplementary Figure S1: Characterization of IRE1α mutants. A. U87-LUC cells were transduced with the lentiviral vector containing the GFP sequence (U87-LUC Tet-ON GFP).
SUPPLEMENTARY INFORMATION
 SUPPLEMENTARY INFORMATION FOR Liver X Receptor α mediates hepatic triglyceride accumulation through upregulation of G0/G1 Switch Gene 2 (G0S2) expression I: SUPPLEMENTARY METHODS II: SUPPLEMENTARY FIGURES
SUPPLEMENTARY INFORMATION FOR Liver X Receptor α mediates hepatic triglyceride accumulation through upregulation of G0/G1 Switch Gene 2 (G0S2) expression I: SUPPLEMENTARY METHODS II: SUPPLEMENTARY FIGURES
Tcf21 MCM ; R26 mtmg Sham GFP Col 1/3 TAC 8W TAC 2W. Postn MCM ; R26 mtmg Sham GFP Col 1/3 TAC 8W TAC 2W
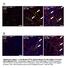 A Tcf21 MCM ; R26 mtmg Sham GFP Col 1/3 Tcf21 MCM ; R26 mtmg TAC 2W Tcf21 MCM ; R26 mtmg TAC 8W B Postn MCM ; R26 mtmg Sham GFP Col 1/3 Postn MCM ; R26 mtmg TAC 2W Postn MCM ; R26 mtmg TAC 8W Supplementary
A Tcf21 MCM ; R26 mtmg Sham GFP Col 1/3 Tcf21 MCM ; R26 mtmg TAC 2W Tcf21 MCM ; R26 mtmg TAC 8W B Postn MCM ; R26 mtmg Sham GFP Col 1/3 Postn MCM ; R26 mtmg TAC 2W Postn MCM ; R26 mtmg TAC 8W Supplementary
Supplemental Materials. STK16 regulates actin dynamics to control Golgi organization and cell cycle
 Supplemental Materials STK16 regulates actin dynamics to control Golgi organization and cell cycle Juanjuan Liu 1,2,3, Xingxing Yang 1,3, Binhua Li 1, Junjun Wang 1,2, Wenchao Wang 1, Jing Liu 1, Qingsong
Supplemental Materials STK16 regulates actin dynamics to control Golgi organization and cell cycle Juanjuan Liu 1,2,3, Xingxing Yang 1,3, Binhua Li 1, Junjun Wang 1,2, Wenchao Wang 1, Jing Liu 1, Qingsong
Supplementary Materials for
 www.sciencesignaling.org/cgi/content/full/9/439/ra78/dc1 Supplementary Materials for Small heterodimer partner mediates liver X receptor (LXR) dependent suppression of inflammatory signaling by promoting
www.sciencesignaling.org/cgi/content/full/9/439/ra78/dc1 Supplementary Materials for Small heterodimer partner mediates liver X receptor (LXR) dependent suppression of inflammatory signaling by promoting
In vitro functional study of Cardiomyocyte
 In vitro functional study of Cardiomyocyte Gwang Hyeon Eom Department of Pharmacology and Medical Research Center for Gene Regulation Chonnam National University Medical School, Gwangju, South Korea Heart
In vitro functional study of Cardiomyocyte Gwang Hyeon Eom Department of Pharmacology and Medical Research Center for Gene Regulation Chonnam National University Medical School, Gwangju, South Korea Heart
