Liver-restricted Repin1 Deficiency Improves Whole Body Insulin Sensitivity, Alters
|
|
|
- Lynette Baldwin
- 6 years ago
- Views:
Transcription
1 Page 1 of 60 Diabetes Liver-restricted Repin1 Deficiency Improves Whole Body Insulin Sensitivity, Alters Lipid Metabolism and Causes Secondary Changes in Adipose Tissue in Mice Matthias Kern 1*, Joanna Kosacka 1*, Nico Hesselbarth 1, Julia Brückner 1, John T. Heiker 1, Gesine Flehmig 2, Ingrid Klöting 3, Peter Kovacs 2, Madlen Matz-Soja 4, Rolf Gebhardt 4, Knut Krohn 5, Susanne Sales 6, Kerstin Abshagen 7, Andrej Shevchenko 6, Michael Stumvoll 1,2, Matthias Blüher 1,2 and Nora Klöting 1,2 *shared authorship 1 Department of Medicine, University of Leipzig, Leipzig, Germany 2 IFB AdiposityDiseases, University of Leipzig, Leipzig, Germany 3 Department of Laboratory Animal Science, University of Greifswald, Karlsburg, Germany 4 Institute of Biochemistry, Medical Faculty, University of Leipzig, Leipzig, Germany 5 Interdisciplinary Center for Clinical Research, Core-Unit DNA Technologies, University of Leipzig, Leipzig, Germany 6 Max Planck Institute of Molecular Cell Biology and Genetics, Dresden, Germany 7 Institute for Experimental Surgery, Rostock University Medical School, Rostock, Germany Contact information: Nora Klöting, PhD IFB AdiposityDiseases University of Leipzig Liebigstr Leipzig, Germany Tel. (+49) Fax (+49) nora.kloeting@medizin.uni-leipzig.de Word count: 6119 Figures: 5 Tables: 2 Running title: Repin1 deficiency in liver 1 Diabetes Publish Ahead of Print, published online April 23, 2014
2 Diabetes Page 2 of 60 Abstract Replication initiator 1 (Repin1) is a zinc finger protein highly expressed in liver and adipose tissue and maps within a quantitative trait locus (QTL) for body weight and triglyceride levels in the rat. The QTL has further been supported as a susceptibility locus for dyslipidemia and related metabolic disorders in congenic and subcongenic rat strains. Here, we elucidated the role of Repin1 in lipid metabolism in vivo. We generated a liver-specific Repin1 knockout mouse (LRep1 -/- ) and systematically characterized the consequences of Repin1 deficiency in the liver on body weight, glucose and lipid metabolism, liver lipid patterns and protein/mrna expression. Hyperinsulinemiceuglycemic clamp studies revealed significantly improved whole body insulin sensitivity in LRep1 -/- mice, which may be due to significantly lower triglyceride content in the liver. Repin1 deficiency causes significant changes in potential downstream target molecules including Cd36, Pparg, Glut2 protein, Akt phosphorylation, lipocalin2, Vamp4, and Snap23 mrna expression. Mice with hepatic deletion of Repin1 display secondary changes in adipose tissue function, which may be mediated by altered hepatic expression of lipocalin2 or chemerin. Our findings indicate that Repin1 plays a role in insulin sensitivity and lipid metabolism by regulating key genes of glucose and lipid metabolism. Key words: Repin1, insulin sensitivity, liver gene expression, liver steatosis, dyslipidemia, obesity, hypertriglyceridemia Abbreviations: Rep1, Repin1; LRep1; hepatocyte specific Rep1 knockout mice; ITT, insulin tolerance test; GTT, glucose tolerance test; HFD, high fat diet 2
3 Page 3 of 60 Diabetes Previously, we identified a quantitative trait locus (QTL) for body weight, serum fasting insulin and triglycerides on rat chromosome 4 (1-3). Replication initiator 1 (Repin1) emerged as a potential positional candidate gene within the QTL region considering associations of metabolic alterations in rats with a single nucleotide polymorphism (SNP;449C/T) in the Repin1 coding region and with the size of a triplet repeat in the 3 -untranslated region of the Repin1 gene (4). Repin1 was initially discovered as replication initiation-region protein 60 kda (RIP60) in a study investigating DNA-binding proteins involved in replication activation of the Chinese hamster dihydrofolate reductase gene (dhfr) (5). Repin1 binds to two ATT-rich sites in oriβ, a short region 3 to the dhfr gene, acting as an enhancer of DNA bending during initiation of DNA synthesis (6,7). Plasmid replication assays demonstrated only weak replication enhancing activity and thus Repin1 may act as an accessory factor in origin recognition prior to the assembly of pre-initiation complexes (8). After it was first cloned in 2000, characterization of DNA binding and bending properties revealed the first structural insight into Repin1/RIP60 function as a polydactyl zinc finger protein of the Cys 2 -His 2- type (8). Repin1 is ubiquitously expressed with the highest expression levels in adipose tissue and the liver (4). Moreover, hepatic expression levels of Repin1 are significantly associated with genotype and serum lipid profiles of different rat strains (4). Recently, we showed that Repin1 plays a role in the regulation of lipid accumulation in 3T3-L1 adipocytes as knockdown of Repin1 by sirna resulted in reduced palmitate uptake and altered mrna expression of fatty acid transporter Cd36 and genes involved in lipid droplet formation (Vamp4, Snap23), adipogenesis and glucose transport (9). A balance between storage and release of lipids by adipose tissue is essential for maintenance of normal energy homeostasis and prevention of ectopic lipid accumulation in peripheral tissues, such as liver, pancreas or skeletal muscle (10). 3
4 Diabetes Page 4 of 60 Based on our previous findings, we hypothesize that Repin1 is involved in glucose homeostasis, in hepatic lipid storage and may contribute to alterations in glucose homeostasis and lipid metabolism associated with obesity. We therefore tested the in vivo consequences of Repin1 gene disruption in mice with a liver-restricted, Cre-loxP-mediated Repin1 deletion (LRep1 -/- ). RESEARCH DESIGN AND METHODS Animal studies All animal studies were approved by the local authorities of the state of Saxony, Germany as recommended by the responsible local animal ethics review board (Regierungspräsidium Leipzig, TVV20/08, Germany). All mice were housed in pathogen-free facilities in groups of three to five at 22 ± 2 C on a 12-h light/dark cycle. Animals were bred and kept in the Animal Laboratories at the University of Leipzig, and were fed a standard chow diet (Altromin GmbH, Lage, Germany). A subgroup of eight LRep1 -/- and eight wild-type mice were kept on a high-fat diet (HFD) containing 55.2% of calories from fat (C1057, Altromin). Animals had ad libitum access to water at all times and food was only withdrawn if required for an experiment. Generation of liver-specific Repin1 (LRep1 -/- ) knockout mice The Repin1 gene in the liver was inactivated using conditional gene targeting strategies. Floxed Rep1 -/- mice were generated by Artemis Pharmaceutical (ARTEMIS Pharmaceutical GmbH, Köln, Germany). Exon 2 was flanked by loxp sites and two positive selection markers were used in order to increase co-recombination frequency of both loxp sites (Fig.1A). The selection markers are flanked by frt (Neo) or F3 sites (Puro). The conditional KO occurs after in vivo Flp-mediated removal of selection markers and constitutive KO by Cre-mediated deletion of exon 2. The deletion of exon 2 resulted in loss of function by removing the complete open reading frame. Vector Construction ET (SIS17): Mouse genomic fragments 4
5 Page 5 of 60 Diabetes were ET subcloned using RP23 BAC library and recloned into the basic targeting vector harbouring the indicated features. Mice homozygous for the loxp-flanked Rep1 allele (Rep1 flox/flox ) were crossed with mice expressing a Cre-recombinase under control of the albumin (Alb) promoter (C57BL/6-TgN(Alb-Cre)21Mgn; stock# from Jackson Laboratory). In the liver, Cre-recombinase mediates the deletion of all floxed alleles. LRep1 mice were on C57BL/6N background. Molecular characterization and genotyping of LRep1 AlbCre mice Genotyping was performed by PCR using genomic DNA isolated from the tail tip. Genomic DNA was prepared by using the DNeasy kit (Qiagen, Hilden, Germany). The following two primer pairs were used to genotype LRep1 loxp sites, 5 CCCAACACTGATTACAGATCC 3 (forward) and 5 GTGGGATCAGATAG AACTTAGC 3 (reverse), as well as the AlbCre recombinase 5 GCGGTCTGGCAGTAAAAACTATC 3 (forward) and 5 GTGAAA CAGCATTGCTGTCACTT 3 (reverse). PCR was performed for 35 cycles of 95 C (loxp sites), or 94 C (AlbCre), 60 C (30 seconds, loxp sites) or 51 C (60seconds, AlbCre) and 72 C (60seconds each) using the Fermentas Dream Taq Polymerase (Fermentas GmbH, St.Leon-Rot, Germany) and a Peltier Thermal Cycler PTC-200 (Bio-Rad, Hercules, CA). DNA from wildtype (WT) mice produced a 292-bp band, and a 484-bp band was detected in LRep1 AlbCre lox mice. Phenotypic characterization 12 male mice of each genotype (LRep1 -/-, control littermates (Rep1 flox/flox, Rep1 flox/+, WT) were studied from the age of 6 up to 40 weeks. Body weight was recorded weekly and body length (naso-anal length) was measured once at an age of 32 weeks (n=10 per genotype). Intraperitoneal glucose tolerance tests (ipgtts) and insulin tolerance tests (ipitts) were performed in males at the age of 12, 24 and 40 weeks as described previously (11). Briefly, GTT was performed after an overnight fast of 14 hours by injecting of 2g glucose per kg body 5
6 Diabetes Page 6 of 60 weight into LRep1 -/- and littermate controls. Blood samples for glucose measurements were taken at different time points after 0, 15, 30, 60, and 120 minutes as described previously (11). ITT was performed in random-fed animals by injecting 0.75unit/kg body weight human regular insulin (40 units Insuman Rapid, Sanofi-Aventis, Frankfurt/Main, Germany). Glucose levels were determined in blood collected from tail tip immediately before and 15, 30 and 60 minutes after the intraperitoneal injection. Indirect calorimetry was assessed by a Calorimetry Module (CaloSys V2.1, TSE Systems, Bad Homburg, Germany) at an age of 30 weeks. After two hours of acclimatization, mean oxygen consumption (VO 2 ) as well as spontaneous activity (XYZ cage movement) and ability to run on a treadmill were recorded for 72h. At an age of 16 weeks, a subgroup of 20 (n=10 per genotype) mice underwent a food intake measurement over a time period of 1 week. The daily food intake was calculated as the average intake of chow within the time stated. Rectal body temperature was measures at an age of 32 weeks. Whole body composition (fat mass, lean mass and total body water) was determined in awake mice by using nuclear magnetic resonance technology with EchoMRI700 instrument (Echo Medical Systems, Houston, TX, USA) in control and LRep1 -/- mice at 3, 8, 16, 24, and 80 weeks of age. At least 4 animals per genotype and time point were measured. Data were analyzed by the manufacturer s software. Mice were sacrificed at the age of 32 weeks by an overdose of anesthetic (Isofluran, Baxter, Unterschleißheim, Germany). Liver, heart, brain, lung, spleen, pancreas, kidney, muscle, subcutaneous and epigonadal adipose tissue were immediately removed. The organs (liver, BAT, epigonadal adipose tissue) were weighed and organ mass was related to whole body mass to obtain relative organ weights. Analytical procedures 6
7 Page 7 of 60 Diabetes Blood glucose values were determined from whole venous blood samples using an automated glucose monitor (FreeStyle mini, Abbott GmbH, Ludwigshafen, Germany). Insulin, leptin and adiponectin serum concentrations were measured by ELISA using mouse standards according to the manufacturer s guidelines (Mouse/Rat Insulin ELISA and Mouse Leptin ELISA; CrystalChem. Inc, Downers Grove, IL) and (Mouse Adiponectin ELISA; AdipoGen Inc, Incheon, Korea). Serum concentrations of ALAT, ASAT, Albumin, FFA, triglycerides, LDL-, HDL- cholesterol were analyzed by an automatic chemical analyzer in our Institute of Laboratory Medicine and Clinical Chemistry. Serum glycerol concentration was measured using Adipolysis Assay Kit (Merck-Millipore, Billerica, MA, US) in male LRep1 -/- and controls at an age of 30 weeks. Western blot analysis For Western blot analysis, tissues were removed and homogenized in homogenization buffer with tissue-mill homogenizer (MM400 Retsch GmbH, Haan, Germany), proteins were isolated using standard techniques, and western blot analysis was performed with antibodies raised against Repin1 (1:1,000; Santa Cruz Biotechnology; N-20, Santa Cruz, CA), Pparg (1:100, #351540, antikörper online), Irs1 and pirs1 (1:1000; Cell signalling), Akt and pakt (1:1000; Cell Signaling), Glut2 (1:200, ab85715, abcam, Cambridge, UK), Pparg (1:1000; Cell Signaling), ACC (1:1000; Cell Signaling), Lipocalin 2 (1:1000; abcam), Cd36 (1:500 antibodies online) and D-glyceraldehyde-3-phosphate dehydrogenase antibody (GAPDH, 1:3.000; Research Diagnostics, Flanders, Netherlands) as loading control. For Cd 36 protein detection supernatant was centrifuged at g for 45 minutes at 4 C. To get the membrane and cytosol fractions, the pellet was suspended in ice-cold sucrose buffer and taken to Cd36-analysis in western blot. Hyperinsulinemic-euglycemic clamp studies 7
8 Diabetes Page 8 of 60 Catheters were implanted in the left jugular vein and hyperinsulinemic-euglycemic clamps of 6 male of each genotype at the age of 20 weeks. Clamp was performed as described previously (12-14). Liver lipidomics Lipids were extracted from mouse hepatocytes using Folch et al protocol (15) with minor modification (16). Molecular lipid species were identified and quantified using LipidXplorer software (17) developed in MPI CBG, Dresden. Species were quantified by comparing the intensities of their peaks to peaks of spiked internal standards; lipid quantities determined in individual samples were normalized by the total protein content determined by Bradford assay. Cholesterol was quantified as previously described (18). RNA isolation and quantitative real-time PCR analysis RNA isolation and quantitative real-time PCR was performed as previously described (11). mrna expression of genes listed in supplemental table 3 were determined. Specific mrna expression was calculated relative to 18sRNA which was used as an internal control due to its resistance to glucose-dependent regulation (19). In vivo lipogenese in liver, in vivo VLDL-triglyceride production, fat load test. In vivo lipogenesis was performed as previously described in detail (20, 21). To measure hepatic TG production rate, mice were injected i.p. with Poloxamer 407 (P-407, Sigma Aldrich) in saline approximately 4 h into the light cycle, and plasma TG was measured over a 4 h period as described elsewhere (22). At an age of 16 weeks, following an overnight fast, 200µl olive oil was administrated by intragastic gavage feeding tube. Blood samples were 8
9 Page 9 of 60 Diabetes taken by submandibular bleeding at 0, 1, 2, 3, 4 h after fat load for triglyceride measurements. Eight male mice per genotype were studied. Ex-vivo glucose transport, ex-vivo lipolysis and palmitate uptake into adipocytes. For the determination of glucose transport, lipolysis, palmitate uptake, adipocyte size distribution was performed as previously described (9, 23). Western blot analysis. For Western blot analysis, tissues were removed and homogenized in homogenization buffer with tissue-mill homogenizer (MM400 Retsch GmbH, Haan, Germany), proteins were isolated using standard techniques, and western blot analysis was performed with antibodies raised against Repin1 (1:1,000; Santa Cruz Biotechnology; N-20, Santa Cruz, CA), Pparg (1:100, #351540, antikörper online), Irs1 and pirs1 (1:1000; Cell signalling), Akt and pakt (1:1000; Cell Signaling), Glut2 (1:200, ab85715, abcam, Cambridge, UK), Pparg (1:1000; Cell Signaling), ACC (1:1000; Cell Signaling), Lipocalin 2 (1:1000; abcam), Cd36 (1:500 antibodies online) and D-glyceraldehyde-3-phosphate dehydrogenase antibody (GAPDH, 1:3.000; Research Diagnostics, Flanders, Netherlands) as loading control. For Cd 36 protein detection supernatant was centrifuged at g for 45 minutes at 4 C. To get the membrane and cytosol fractions, the pellet was suspended in ice-cold sucrose buffer and taken to Cd36-analysis in western blot. Insulin signaling. Mice were anesthetized by intraperitoneal (ip) injection and adequacy of the anesthesia was ensured by loss of pedal reflexes. The abdominal cavity of the mice was opened and 125 µl samples containing 5U regular human insulin diluted in 0.9% saline were injected into the Vena cava inferior. Sham injections were performed with 125 µl of 0.9% saline. Samples of liver tissue were harvested ten minutes after injection, respectively, and proteins (Akt, pakt, Irs1, pirs1) were extracted from tissues for Western blot analysis. 9
10 Diabetes Page 10 of 60 Liver Affymetrix GeneChip analysis RNA from liver samples of 3 male wildtype and 3 male LRep1 -/- mice were used for microarray RNA analysis. Analysis of RNA integrity and RNA concentration as well as probe synthesis, hybridization and scanning was done as previously described (24). Histology and Immunohistochemistry. Histology and immunohistochemistry was performed as previously described (23, 25). Statistical analysis Data are given as mean ± SE. Data sets were analyzed for statistical significance using a twotailed unpaired t test or Man Whitney test. P values <0.05 were considered significant. RESULTS Generation of liver specific Repin1 (LRep1 -/- ) mice In order to generate liver specific Rep1 (LRep1 -/- ) knockout mice, mice homozygous for the loxp-flanked Rep1 allele (Rep1 flox/flox ) were crossed with mice expressing the Crerecombinase under control of the liver-specific albumin (alb) promoter. The targeting strategy is shown in Fig.1A. LRep1 -/- mice were obtained with the expected Mendelian frequency were fertile. Efficiency and specificity of the Repin1 knockout were examined in tissue lysates from control and LRep1 mice by Western blot analyses (Fig.1B-1C). Western blot analysis of liver lysates from LRep1 -/- mice confirmed reduced Repin1 expression by ~85% in livers (Fig.1C). Since the Cre recombinase is only active in hepatocytes, and since hepatocytes make up ~85% of the total number of cells in the liver, it is likely that the minimal Repin1 protein in LRep1 -/- mice is derived from vascular endothelial cells, Kupffer and other nonparenchymal cells. Immunohistochemistry of Repin1 in the liver was performed to test whether non-parenchymal cells are responsible for the remaining Repin1 expression in 10
11 Page 11 of 60 Diabetes LRep1 -/- mice. The immunohistochemical images of the liver of LRep1 -/- mice demonstrate the absence of Repin1 in hepatocytes, but positive Repin1-staining for endothelial cells, Kupffer cells and hepatic stellate cells (Fig.1D). In contrast, hepatocellular cytoplasm of wildtype mice showed positive staining for Repin1 (Fig.1D). Repin1 expression in all other tissues were indistinguishable between LRep1 -/- and control littermates (Fig.1B). Growth, tissue mass and energy expenditure of LRep1 -/- mice LRep1 -/- mice had normal body weight compared to control littermates (Rep1 flox/flox,rep1 flox/+, WT) up to an age of 28 weeks when LRep1 -/- mice become significantly leaner with significant less total body fat content up to an age of 40 weeks (Fig.1E-F). This may be due to significantly higher VO 2 consumption and spontaneously higher activity (Z-axis day) in LRep1 -/- mice (Fig.1E-1G). Homozygous deficiency of the Repin1 had no significant influence on body length (Fig. S4A), daily food intake (Fig. S4B) nor relative tissue weights of livers (WT: 5.2%; LRep1 -/- : 5.3%). Repin1 deficiency in liver improves insulin sensitivity To further investigate the role of Repin1 on glucose homeostasis, we characterized the physiological consequences of reduced liver Repin1 expression on insulin action and glucose metabolism. Hyperinsulinemic-euglycemic clamp studies revealed significantly higher whole body insulin sensitivity in LRep1 -/- compared to control mice (Fig.2A). At the steady state, glucose infusion rate was ~60% higher in LRep1 -/- compared to control mice (Fig.2A). LRep1 -/- mice showed a higher rate of hepatic glucose production during the basal period as compared to control animals (Fig.2B), whereas insulin suppressed hepatic glucose production significantly better in LRep1 -/- (-98%) compared to control mice (-51%) (Fig.2C). Circulating insulin concentrations achieved in the steady state of the clamp were not significantly different between WT (9.1 ± 1.4ng/ml) and LRep1 -/- (8.3 ± 1.6ng/ml) mice. 11
12 Diabetes Page 12 of 60 In addition, we monitored blood glucose, insulin as well as adiponectin serum concentrations (Table1) and performed serial GTTs and ITTs over an age range from 12 to 40 weeks (Fig.2D-E, Fig.S4C-D). Independent of age, intraperitoneal GTTs revealed normal glucose tolerance in LRep1 -/- mice (Fig.2D). At an age of 24 weeks, LRep1 -/- mice showed significantly improved insulin sensitivity compared with controls (Fig.2E), which was even more pronounced at a higher age (Fig.2F-H). Significantly lower HbA1c level confirmed the long term glucose metabolism in LRep1 -/- compared to control mice (Fig.2I). Moreover, insulin secretion in response to the ip glucose load in 40 weeks old LRep1 -/- mice showed an improved insulin secretion response to glucose reflected by a higher insulin peak at 15min and a faster decline in insulin serum concentrations than in controls (Fig.2G). Taken together reduced Repin1 expression in liver results in improved whole body insulin sensitivity subsequently contributing to better glycemic control in LRep1 -/- mice. Repin1 affects insulin signaling in the liver We further sought to investigate the consequences of disrupted Repin1 in the liver and improved insulin sensitivity by analyzing key insulin signaling molecules in livers of LRep1 -/- and control mice after insulin stimulation. Supporting a role of Repin1 in the regulation of hepatic insulin sensitivity at the molecular level, we find a significant up-regulation of phosphorylated Akt, and Pparγ expression in LRep1 -/- compared to control mice (Fig.3A). These molecular changes may underlie significantly higher Glut2 expression (Fig.3A) which may explain higher basal glucose uptake in the hyperinsulinemic- euglycemic clamp in LRep1 -/- compared to control mice. Liver-specific Repin1 deficiency leads to dyslipidemia, altered liver lipid transport/storage and liver lipidomic profile 12
13 Page 13 of 60 Diabetes To determine the physiological consequences of reduced liver tissue Repin1 expression, we monitored total serum cholesterol, triglyceride serum concentrations, free fatty acids (FFA), liver function tests such as circulating serum alanin-aminotransferase (ALAT), aspartat aminotransferase (ASAT), glutamate dehydrogenase (GLDH) and albumin concentrations. Fasted triglycerides and FFA were significantly higher LRep1 -/- mice at an age of 32 weeks (Table1). ALAT, ASAT and albumin, were not affected by Repin1 knockout suggesting that lack of Repin1 has no adverse effect on liver function (Table1). Also H&E staining of control and LRep1 -/- mice showed normal hepatic architecture without signs of hepatocyte injury (Fig.3D, Fig. S2-S3). Liver lipidomics analyses revealed significantly lower liver content triacylglycerides (TAG) in LRep1 -/- compared to control mice (Table2). The amount of TAG in liver was significantly decreased in LRep1 -/- mice by ~40% compared with controls (Table2, Fig.3C). Hepatic cholesterol and cholesterolester were unchanged (Table2). Lower lipid accumulation in the liver of LRep1 -/- mice may at least in part explain improved whole body insulin sensitivity in these mice. To identify potential mechanisms underlying reduced triglyceride storage in the liver and elevated circulating triglycerides and free fatty acids in LRep1 -/- mice, we examined hepatic expression of fatty acid transporters Cd36, Fatp1, Fatp2, Fatp4 and carnitine palmitoyltransferase 1 (Ctp1) as the first component and rate-limiting step in beta-oxidation and Cpt2 (Supplemental Table 3). We performed in vivo VLDL production assays, in vivo lipogenesis and an oral fat load tests. Injection of p407 resulted in a linear increase in serum triglyceride concentration without significant differences between both experimental groups, which suggest no influence of hepatic VLDL synthesis on altered lipid content in LRep1 -/- mice (Fig.3G). Further, to investigate de novo lipogenesis, we injected mice both with tritiated water and [U- 14 C]-glucose (Fig.3J). Total rate of hepatic fatty acid synthesis was comparable between controls and LRep1 -/- mice and the incorporation of [U- 14 C]-glucose into de-novo 13
14 Diabetes Page 14 of 60 fatty acids was similar as well (Fig.3I-J). Expression of key lipogenic enzyme, acetyl-coa carboxylase (ACC, pacc) was not changed in livers of LRep1 -/- mice compared to littermates controls (Fig.3K). Cd36 was significantly reduced in LRep1 -/- mice by about 50% both at protein and mrna level (Fig.3E-F). LRep1 -/- mice are protected against development of HFD induced adipocyte hypertrophy In a subgroup of 8 animals of each genotype we performed a high fat diet (HFD) study starting at an age of 6 weeks until 16 weeks of age. Weight gain after HFD was not different between all groups (Fig.4A). Relative liver weight was slightly reduced in LRep1 -/- mice compared with controls (data not shown). Also ALAT and ASAT as well as albumin levels did not differ among genotypes (data not shown) and epigonadal adipose tissue mass was comparable between LRep1 -/- and control mice (data not shown). Despite indistinguishable relative adipose tissue mass, LRep1 -/- mice showed decreased maximal adipocyte diameters compared with controls in response to HFD (Fig.4B-F). Maximal epigonadal adipocyte diameter is significantly larger in controls compared to LRep1 -/- mice (Fig.4E-F). Adipocyte frequency size distribution confirmed these findings (Fig.4C-D). These differences are more pronounced in epigonadal than in subcutaneous fat. To elucidate in more detail adipocyte function, we analyzed glucose uptake and glycerol release in isolated adipocytes. Here, we detected a significant elevated glucose uptake and glycerol release under basal conditions in subcutaneous adipocytes of LRep1 -/- mice (Fig.4I-J). Insulin-stimulated glucose uptake was comparable between the experimental groups (Fig.4J). To elucidate in more detail elevated basal glycerol release we performed gene expression analysis in adipose tissue. Here, we detected an elevation of all lipolysis enzymes (Lpl, Atgl, Hsl) in SC adipose tissue in LRep1 -/- mice (TableS5), indicating enhanced lipolysis. Taken together, LRep1 -/- mice were protected against HFD-induced adipocyte hypertrophy. 14
15 Page 15 of 60 Diabetes Target genes of Repin1 To identify Repin1 regulated target genes, we measured mrna expression in the liver of LRep1 -/- and control mice using a microarray approach. Lipocalin2 (Lcn2), also known as neutrophil gelatinase associated lipocalin (Ngal) was the strongest regulated gene in response to reduced Repin1 expression (TableS2). We further found reduced levels of clathrin coated vesicle transport protein (ap3m2), vesicle-associated membrane proteins (vamps), oxidative stress genes such as metallothein1 (Mt1) and 2 (Mt2) as well as low density lipoprotein receptor (Ldlr), Rarres 2 (Chemerin), whereas exocytosis factor titin, mitochondrial protein, Letm2, and kallikrein inhibitor (serpina4-ps1) were higher expressed in livers of LRep1 -/- mice (TableS2). In addition to these genes, we detected a number of Repin1-regulated genes including various biological processes and molecular functions (Tables S1-S2). We could confirm the expression micro array data for lipocalin2 and chemerin in a bigger liver tissue cohort (see table S4). Circulating lipcalin2 concentrations and liver lipocalin 2 expressions were lower in LRep1 -/- compared to littermate control mice (p=0.2 serum; p=0.06 liver protein; Fig. S5). Altered expression of genes involved in accumulation of cytosolic lipids and lipid droplet fusion in liver Array mrna expression changes together with the results of the hepatic lipidomic screen indicated an alteration in lipid droplet fusion and formation. We therefore investigated mrna levels of proteins involved in the fusion process of lipid droplets in more detail. Vesicleassociated membrane protein 4 (Vamp4) and synaptosomal-associated protein, 23kDa (Snap23) were significantly decreased in livers of Repin1 deficient mice. Vamp4 was reduced by 80% and Snap23 by about 40% in LRep1 -/- (Table S3). Model for the role of Repin1 in insulin sensitivity 15
16 Diabetes Page 16 of 60 We propose the following model how liver restricted knockdown of Repin1 may cause the observed phenotype (Fig. 5). Hepatic Repin1 deficiency causes improved liver and whole body insulin sensitivity most likely through transcriptional regulation of genes involved in insulin signaling (Pparg, Akt), glucose transport (Glut2), lipid uptake (Cd36) and lipid droplet formation (Vamp4, Snap23). These changes result in less body weight gain with aging, higher energy expenditure and reduced liver fat accumulation in LRep1 -/- compared to controls. Lower liver lipid content may be primarily caused by significantly reduced Cd36 expression in LRep1 -/- mice. Improved whole body insulin sensitivity of LRep1 -/- mice is likely a consequence of reduced liver fat, decreased hepatic glucose production, and eventually lower expression of hepatokines such as lipocalin2, which are associated with insulin resistance. Discussion Our data provide first in vivo evidence that Repin1 deletion in liver leads to lower body weight, reduced hepatic steatosis, increased energy expenditure and physical activity, improved insulin sensitivity both at the organ and whole body level. Lower body weight in LRep1 -/- compared to control mice may be due to several mechanisms. LRep1 -/- mice are characterized by higher VO 2 consumption and at least in one dimension higher spontaneous activity with unaltered food intake compared to WT mice. We tested the hypothesis the increased energy expenditure in LRep1 -/- compared to WT mice is a result of increased brown adipose tissue (BAT) mass or activity. Since mean body temperature, relative and absolute BAT mass as well as expression of BAT marker genes in white adipose tissue (WAT) is not different between LRep1 -/- and control mice, we exclude an effect of liver Repin1 deficiency on BAT or browning of WAT. Taken together, increased energy expenditure and activity of LRep1 -/- mice contributes to lower body weight. Importantly, deletion of Repin1 in liver does not cause an increase in inflammatory marker genes or immune cell infiltration into livers of LRep1 -/- mice. Moreover, ASAT, ALAT, and 16
17 Page 17 of 60 Diabetes GLDH serum concentrations were not significantly different between wildtype and LRep1 -/- mice excluding a hepatotoxic effect of Repin1 deletion. Reduced liver fat is most likely due to significant changes in the expression of Repin1 target genes Cd36, fatty acid binding proteins. In the context of these (beneficial) metabolic consequences of reduced liver-specific Repin1 expression, elevated circulating triglycerides and FFAs in LRep1 -/- mice seem to be contradictory to the phenotype. We therefore propose a model (Fig. 5) how these changes in circulating lipids maybe the result of changes in expression of key molecules in fatty acid uptake, transport and lipid droplet formation in the liver. Elevated TGs and FFAs are most likely due to decreased hepatic lipid uptake capacity as a result of lower Cd36 expression in LRep1 -/- mice. In addition to reduced Cd36 expression, several mechanisms may contribute to the observed reduction in TG storage in LRep1 -/- mice. Both de novo lipogenesis and VLDL production were not significantly different between LRep1 -/- and control mice suggesting that these processes do not cause reduced TG storage in livers of LRep1 -/- mice. Moreover, we did not find differences in 14 C palmitate oxidation or the expression of key enzymes of beta-oxidation between the genotypes, suggesting that Repin1 KO in liver does not significantly affect fatty acid oxidation in liver. Key gene expressions of TG synthesis (Fasn, Scd1, Scd2) were not altered by Repin1 deletion in liver. However, since we did not directly assess triglyceride synthesis in hepatocytes, we can not exclude that this mechanism may contribute to reduced TG storage in livers of LRep1 -/- mice. In accordance with altered liver lipid transporter expression, we detected lower concentrations of triacylglycerides (TAG) in the liver of LRep1 -/- mice. These in vivo results strongly support our previous data on sirna mediated Repin1 knockdown in 3T3-L1 cells, which caused significantly reduced mrna expression of fatty acid transporter Cd36 and lipid droplet genes (Snap23 and Vamp4) in 3T3-L1 cells (9). Moreover, the circulating lipid profile of LRep1 -/- mice closely reflects that of CD36 knockout mice, which are also characterized by elevated fasting free fatty acids and triacylglycerol, as well as 17
18 Diabetes Page 18 of 60 cholesterol serum concentrations (26). Interestingly, both LRep1 -/- and CD36 KO mice are protected against impaired glucose homeostasis, despite these alterations in lipid metabolism (26). Therefore Repin1 deficiency in the liver provides a model to dissect the effects of liver fat accumulation from those of increased circulating lipids on whole body insulin sensitivity. The insulin sensitive phenotype of LRep1 -/- mice further suggests that hepatic steatosis may represent a crucial mechanism in the development of insulin resistance independently of the increased circulating free fatty acids and triglycerides. Noteworthy, in mice with liver-specific insulin resistance due to a targeted disruption of the hepatic insulin receptor (LIRKO mice) circulating triglycerides and free fatty acids are ~40-50% lower compared to controls (27). This opposite phenotype further suggests that improved insulin sensitivity in the liver of LRep1 -/- mice is the primary effect of reduced Repin1 expression and changes in circulating lipids are secondary to that. Kennedy et al. demonstrated that adipose tissue from Cd36 knockout mice was more insulin sensitive and had lower levels of inflammatory markers (i.e. IFN-g and MCP-1) as compared to wildtype mice (28). By several measures, we found beneficial effects of Repin1 deletion in liver on insulin sensitivity both under chow and HFD fed conditions. Noteworthy, insulin secretion dynamic in 40 weeks old LRep1 -/- mice suggests that Repin1 KO in the liver may cause secondary changes in islets which are in line with the observed improvements in whole body insulin sensitivity. Whether insulin secretion profile of LRep1 -/- mice is due to improved insulin sensitivity or the result of a hepatic factor directly affecting islets needs to be explored in subsequent studies. Improved insulin sensitivity in LRep1 -/- mice could be the result of improved activation of insulin signaling cascade (e.g. Akt phosphorylation), increased Pparγ expression, lower liver fat content mediated by reduced expression of fatty acid transport proteins in LRep1 -/- mice, lower total body fat content and lower expression of insulin resistance associated hepatokines (e.g. lipocalin2, chemerin). 18
19 Page 19 of 60 Diabetes With regard to the latter mechanism, mrna expression array identified Lipocalin 2 (Lcn2) as strongest potential candidate. Elevated lipocalin2 serum concentrations are associated with obesity, dyslipidemia and insulin resistance (29). In livers of the LRep1 -/- mice, we detected a 60-fold reduction of Lcn2 mrna level and reduced liver protein levels (p=0.06) compared with controls. There was a trend for lower circulating Lcn2 in LRep1 -/- mice. Lcn2, also known as neutrophil gelatinase-associated lipocalin (NGAL), is a lipocalin subfamily member and has been recently identified as an adipose tissue-derived cytokine (30). Lcn2 is an extracellular lipocalin and has structural similarity with fatty acid binding proteins (FABPs), and both are members of the multigene family of up and down β-barrel proteins (31). Both, intracellular FABPs and the extracellular lipocalins, have a clearly defined β-barrel motif that forms either an interior cavity (FABP) or a deep pit (lipocalin) that constitutes the lipid binding domain (31). Because of the unique structure, the lipocalin function as efficient transporters for a number of different hydrophobic ligands in extracellular milieus, including a variety of retinoids, fatty acids, biliverdin, pheromones, porphyrins, odorants, steroids and iron (32). In vitro it was shown that exogenous Lcn2 promotes insulin resistance in cultured hepatocytes (33). It is likely that Lcn2 as retinoic acid could potentially be involved in or mediate lipid storage effects in both liver and adipose tissue. Moreover, reduced Lcn2 in LRep1 -/- mice may contribute, to the observed alterations in adipose tissue with a higher number of smaller adipocytes in LRep1 -/- compared to control mice upon HFD. Liver Repin1 deficiency leads to secondary changes in adipose tissue with a reduction in adipocyte size under HFD in homozygous LRep1 -/- mice, indicating a protection against hypertrophy of adipocytes. However, since we did not biopsy adipose tissue during HFD, we do not provide direct evidence for increased adipogenesis in LRep1 -/- mice. Protection of LRep1 -/- mice against adipocyte hypertrophy could contribute to significantly better insulin sensitivity as measured by ITT after 10 weeks of HFD. 19
20 Diabetes Page 20 of 60 Our previous in vitro studies demonstrated that Repin1 expression increases during adipogenesis and that RNA interference based Repin1 downregulation in mature adipocytes significantly reduces adipocyte size (9). We found significant correlations between Repin1 mrna expression and total body fat mass as well as adipocyte size in human paired visceral and subcutaneous adipose tissue suggesting a potential role for Repin1 in the regulation of adipocyte size (9). However, it is not clear how a lack of Repin1 in liver might influence adipocyte size. One possibility is that absence of Repin1 in liver generates signals that either restrict adipocyte lipid load or increases adipogenesis. For the latter mechanism we consider reduced lipocalin2 expression in livers of LRep1 -/- mice as a candidate molecule. This hypothesis is supported by data that lipocalin2 deficiency protects mice from developing aging- and obesity-induced insulin resistance by modulating insulin resistance factors in adipose tissue (34). Lipocalin2 disrupted mice are partly protected from HFD induced insulin resistance. In this context, it has been shown that other members of the lipocalin family; in particular lipocalin-type prostaglandin D synthase (L-PGDS) may impair the adipogenesis program (35). Although, we do not have direct evidence for increased adipogenesis in LRep1 - /- mice, we speculate that changes in adipose tissue morphology may be due to reduced Lcn2 levels with subsequent disinhibition of adipogenesis. In contrast to our observation marked lipocalin-2 deficiency leads to enlarged adipocytes and improved adipose tissue function has been related to reduce inflammation in adipose tissue of these mice (34). We can not exclude that changes in adipose tissue of LRep1 -/- mice are at least in part mediated by reduced inflammatory activation in adipose tissue. In addition, reduced chemerin expression may lead to smaller adipocyte size. Indirect evidence from insulin sensitive obese individuals supports the hypothesis that lower circulating chemerin (in insulin sensitive, healthy obese) is associated with smaller adipocyte size (36). Whether additional mediators contribute to the specific response to HFD in adipose tissue of LRep1 -/- mice should be further studied. Adipocyte-specific insulin sensitivity was not altered in 20
21 Page 21 of 60 Diabetes LRep1 -/- mice. Hepatocyte-derived factors may have further contributed to increased lipolysis in adipocytes of LRep1 -/- mice, which has been suggested by higher basal glycerol release and higher expression of key lipolysis enzymes (Hsl, Atgl, Lpl) in LRep1 -/- adipocytes. To further elucidate potential mechanisms for improved liver insulin sensitivity and reduced liver fat content in LRep1 -/- mice, we performed a hepatic lipidomics screen as well as mrna expression array analysis in liver samples. The liver lipidomics data demonstrate that Repin1 modulates the overall profile of liver lipid species. Here, the main finding of the screen was that hepatic deletion of Repin1 had a significant effect on TAG amount in livers, suggesting altered lipid storage in liver. Thus, reduced liver lipid storage ability could explain significantly higher serum triglyceride levels in LRep1 -/- mice. Since LRep1 -/- hepatocytes did not undergo typical ballooning under HFD conditions, we examined whether genes involved in lipid droplet fusion as well as lipid transport proteins are regulated by Repin1 deficiency. We detected significantly reduced levels of Vamp4, and Snap23 in livers of Repin1 deficient mice suggesting a disturbance in lipid vesicle formation and hepatic lipid upload. Boström et al. demonstrated that Snap23 and Vamp4 are functional in the fusion between lipid droplets and are essential for the growth of lipid droplets (37). Our results are in accordance with our previous finding that downregulation of Repin1 in 3T3-L1 adipocytes by sirna leads to significantly reduced lipid droplet size (9). In parallel to the mrna expression data in the liver of Repin1 deficient mice, we found reduced Snap23 and Vamp4 expression in 3T3-L1 cells upon downregulation of Repin1 (9). Knockdown of VAMP4 and SNAP23 has been recently shown to decrease the rate of lipid droplet fusion and the size of the lipid droplets (33). Noteworthy, decreased VAMP4 and SNAP23 gene expression in response to Repin1 knockdown seems to be specific, since other lipid droplet proteins including perilipin and syntaxin5 were not regulated by Repin1 (9). In conclusion, we provide a model how Repin1 may through regulation of gene expression lead to lower body weight, improved liver and whole body insulin sensitivity and alterations 21
22 Diabetes Page 22 of 60 lipid metabolism. We propose that hepatic Repin1 deficiency primarily leads to improved insulin sensitivity and reduced liver fat content with secondary changes in serum lipid profile due to alterations in hepatic lipid transport. Furthermore, hepatic deletion of Repin1 causes altered expression of molecules including lipocalin2 and chemerin, which may contribute to reduced adipocyte size in response to HFD, which may subsequently further contribute to beneficial effects on whole body insulin sensitivity in LRep1 -/- mice. Our findings indicate that Repin1 contributes to insulin sensitivity, glucose and lipid metabolism by regulating the expression of key molecules of these processes. Therefore alterations in Repin1 expression may contribute the pathogenesis of insulin resistance and dyslipidemia and subsequent impairment of glucose homeostasis. ACKNOWLEDGEMENTS We would like to thank Eva Böge, Manuela Prellberg, Viola Döbel, Anne Kunath, and Daniela Kern of University of Leipzig for technical assistance. Furthermore, we would like to thank Professor Fitzl at University of Leipzig, Institute of Anatomy, for help with liver staining. The authors have declared that no competing interests exist. This work was supported by the clinical research group Atherobesity (KFO 152, project KL-2346) and the SFB1052: B1 (to MB); B4 (to NK) founded by Deutsche Forschungsgemeinschaft and supported by the Federal Ministry of Education and Research (BMBF), Germany, FKZ: 01EO1001 (N. Klöting). Peter Kovacs is funded by Boehringer Ingelheim Foundation. J.T. Heiker is funded by the European Union and the Free State of Saxony. Author contributions: N.K. conceived the research ideas, supervised the project and wrote manuscript. M.B. reviewed/edited the manuscript. P.K. reviewed manuscript and contributed to discussion. M.S. conceived the research ideas and reviewed/edited the manuscript. M.K. researched data and wrote manuscript. J.K., I.K., N.H. researched data and performed western 22
23 Page 23 of 60 Diabetes blots. J.B. and J.T.H. performed western blots and histological experiments. G.F. measured serum concentrations. R.G., M.M.S., S.S. and A.S. performed lipidomics studies. K.K. performed Affymetrix GeneChip analysis. K.A. researched data and performed immunostaining. Nora Klöting is the guarantor of this work, had full access to all data in the study, and takes responsibility for the integrity of the data and data analysis. 23
24 Diabetes Page 24 of 60 REFERENCES 1. Kovacs P, Kloting I. Quantitative trait loci on chromosomes 1 and 4 affect lipid phenotypes in the rat. Arch Biochem Biophys 1998; 354: Kloting I, Kovacs P, van den Brandt J. Sex-specific and sex-independent quantitative trait loci for facets of the metabolic syndrome in WOKW rats. Biochem Biophys Res Commun 2001; 284: Kovacs P, van den Brandt J, Bonne AC, van Zutphen LF, van Lith HA, Kloting I. Congenic BB.SHR rat provides evidence for effects of a chromosome 4 segment (D4Mit6- Npy approximately 1 cm) on total serum and lipoprotein lipid concentration and composition after feeding a high-fat, high-cholesterol diet. Metabolism 2001; 50: Kloting N, Wilke B, Kloting I. Triplet repeat in the Repin1 3'-untranslated region on rat chromosome 4 correlates with facets of the metabolic syndrome. Diabetes Metab Res Rev 2007; 23: Dailey L, Caddle MS, Heintz N, Heintz NH. Purification of RIP60 and RIP100, mammalian proteins with origin-specific DNA-binding and ATP-dependent DNA helicase activities. Mol Cell Biol 1990; 10: Caddle MS, Dailey L, Heintz NH. RIP60, a mammalian origin-binding protein, enhances DNA bending near the dihydrofolate reductase origin of replication. Mol Cell Biol 1990; 10: Schroll AL, Heintz NH. Chemical footprinting of structural and functional elements of dhfr oribeta during the CHOC 400 cell cycle. Gene 2004; 332: Houchens CR, Montigny W, Zeltser L, Dailey L, Gilbert JM, Heintz NH. The dhfr oribeta-binding protein RIP60 contains 15 zinc fingers: DNA binding and looping by the central three fingers and an associated proline-rich region. Nucleic Acids Res 2000; 28:
25 Page 25 of 60 Diabetes 9. Ruschke K, Illes M, Kern M, Kloting I, Fasshauer M, Schon MR, Kosacka J, Fitzl G, Kovacs P, Stumvoll M, Bluher M, Kloting N. Repin1 maybe involved in the regulation of cell size and glucose transport in adipocytes. Biochem Biophys Res Commun 2010; 400: Saltiel AR, Kahn CR. Insulin signalling and the regulation of glucose and lipid metabolism. Nature 2001; 414: Kloting N, Koch L, Wunderlich T, Kern M, Ruschke K, Krone W, Brüning JC, Blüher M. Autocrine IGF-1 action in adipocytes controls systemic IGF-1 concentrations and growth. Diabetes 2008; 57: Fisher SJ, Kahn CR. Insulin signaling is required for insulin's direct and indirect action on hepatic glucose production. J Clin Invest 2003; 111: Youn JH, Buchanan TA. Fasting does not impair insulin-stimulated glucose uptake but alters intracellular glucose metabolism in conscious rats. Diabetes 1993; 42: Kern M, Kloting N, Niessen HG, Thomas L, Stiller D, Mark M, Klein T, Bluher M. Linagliptin improves insulin sensitivity and hepatic steatosis in diet-induced obesity. PLoS One 2012; 7: e Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem 1957; 226: Carvalho M 1, Sampaio JL, Palm W, Brankatschk M, Eaton S, Shevchenko A. Effects of diet and development on the Drosophila lipidome. Mol Syst Biol. 2012;8:600. doi: /msb Herzog R, Schwudke D, Schuhmann K, Sampaio JL, Bornstein SR, Schroeder M, et al. A novel informatics concept for high-throughput shotgun lipidomics based on the molecular fragmentation query language. Genome Biol 2011; 12:R8 18. Sandhoff R, Brugger B, Jeckel D, Lehmann WD, Wieland FT. Determination of cholesterol at the low picomole level by nano-electrospray ionization tandem mass spectrometry. J Lipid Res 1999; 40:
26 Diabetes Page 26 of Krowczynska AM, Coutts M, Makrides S, Brawerman G. The mouse homologue of the human acidic ribosomal phosphoprotein PO: a highly conserved polypeptide that is under translational control. Nucleic Acids Res 1989; 17: Kotani K, Peroni OD, Minokoshi Y, Boss O, Kahn BB. GLUT4 glucose transporter deficiency increases hepatic lipid production and peripheral lipid utilization. J Clin Invest 2004; 114: Minokoshi Y, Saito M, and Shimazu T. Sympatic denervation impaired response of brown adipose tissue to VMH stimulation. Am J Physiol 1986; 251:R1005-R Millar JS, Cromley DA, McCoy MG, Rader DJ, Billheimer JT. Determining hepatic triglyceride production in mice: comparison of poloxamer 407 with Triton WR J Lipid Res 2005; 46: Klöting N, Blüher M, Klöting I. The polygenetically inherited metabolic syndrome of WOKW rats is associated with insulin resistance and altered gene expression in adipose tissue. Diabetes Metab Res Rev 2006; 22: Lorenz S, Eszlinger M, Paschke R, Aust G, Weick M, Führer D, Krohn K. Calcium signaling of thyrocytes is modulated by TSH through calcium binding protein expression. Biochim Biophys Acta 2010; 1803: Le Minh K, Klemm K, Abshagen K, Eipel C, Menger MD, Vollmar B. Attenuation of inflammation and apoptosis by pre- and posttreatment of darbepoetin-alpha in acute liver failure of mice. Am J Pathol 2007; 170: Febbraio M, Abumrad NA, Hajjar DP, Sharma K, Cheng W, Pearce SF, Silverstein RL. A null mutation in murine CD36 reveals an important role in fatty acid and lipoprotein metabolism. J Biol Chem 1999; 274: Michael MD, Kulkarni RN, Postic C, Previs SF, Shulman GI, Magnuson MA, Kahn CR. Loss of insulin signaling in hepatocytes leads to severe insulin resistance and progressive hepatic dysfunction. Mol Cell 2000; 6:
27 Page 27 of 60 Diabetes 28. Kennedy DJ, Kuchibhotla SD, Guy E, Park YM, Nimako G, Vanegas D, Morton RE, Febbraio M. Dietary cholesterol plays a role in CD36-mediated atherogenesis in LDLRknockout mice. Arterioscler Thromb Vasc Biol 2009; 29: Wang Y, Lam KS, Kraegen EW, Sweeney G, Zhang J, Tso AW, Chow WS, Wat NM, Xu JY, Hoo RL, Xu A. Lipocalin-2 is an inflammatory marker closely associated with obesity, insulin resistance, and hyperglycemia in humans. Clin Chem 2007, 53: Guo H, Jin D, Zhang Y, Wright W, Bazuine M, Brockman DA, Bernlohr DA, Chen X. Lipocalin-2 deficiency impairs thermogenesis and potentiates diet-induced insulin resistance in mice. Diabetes 2010; 59: LaLonde JM, Bernlohr DA, Banaszak LJ. The up-and-down beta-barrel proteins. FASEB J 1994; 8: Yang Q, Graham TE, Mody N, Preitner F, Peroni OD, Zabolotny JM, Kotani K, Quadro L, Kahn BB. Serum retinol binding protein 4 contributes to insulin resistance in obesity and type 2 diabetes. Nature 2005; 436: Yan QW, Yang Q, Mody N, Graham TE, Hsu CH, Xu Z, Houstis NE, Kahn BB, Rosen ED. The adipokine lipocalin 2 is regulated by obesity and promotes insulin resistance. Diabetes 2007; 56: Law IK, Xu A, Lam KS, Berger T, Mak TW, Vanhoutte PM, Liu JT, Sweeney G, Zhou M, Yang B, Wang Y. Lipocalin-2 deficiency attenuates insulin resistance associated with aging and obesity. Diabetes 2010; 59: Hossain MS, Chowdhury AA, Rahman MS, Nishimura K, Jisaka M, Nagaya T, Shono F, Yokota K. Stable expression of lipocalin-type prostaglandin D synthase in cultured preadipocytes impairs adipogenesis program independently of endogenous prostanoids. Exp Cell Res 2012; 318: Klöting N, Fasshauer M, Dietrich A, Kovacs P, Schön MR, Kern M, Stumvoll M, Blüher M. Insulin-sensitive obesity. Am J Physiol Endocrinol Metab 2010;299:E
28 Diabetes Page 28 of Bostrom P, Andersson L, Rutberg M, Perman J, Lidberg U, Johansson BR, Fernandez-Rodriguez J, Ericson J, Nilsson T, Boren J, Olofsson SO. SNARE proteins mediate fusion between cytosolic lipid droplets and are implicated in insulin sensitivity. Nat Cell Biol 2007; 9:
29 Page 29 of 60 Diabetes Figure legends. FIGURE 1. Targeting strategy, assessment of Repin1 recombination and Repin1 expression. (A) Schematic representation of the loxp-flanked Repin1 allele before and after recombination (Cre-expression). The knockout allele is shown below the floxed allele, indicating the deletion of exon 2 in the event of recombination of the Repin1 gene. (B) Western blot analysis showing the expression of Repin1 and GAPDH as loading control of liver, lung, heart, brain, pancreas, kidney, spleen, skeletal muscle, brown adipose tissue (BAT), subcutaneous (sc) and epigonadal (epi) adipose tissue of control and LRep1 -/- mice. (C) Knockdown efficiency in liver from wildtype (Controls), heterozygous (LRep1 +/- ) and homozygous (LRep1 -/- ) mice. (D) Representative images (original magnification x200) of Repin1 immunohistochemistry (positive staining of the Repin1 protein with DAB as chromogen in brown, arrows) of liver tissue 5µm paraffin sections of wildtype control mice (left) and LRep1 -/- mice (right) at an age of 12 weeks. The cytoplasmic staining of hepatocytes for Repin1 in wild-type control mice, whereas in livers of LRep1 -/- mice exclusively nonparenchymal cells are Repin1-positive. (E) Growth phenotype of LRep1 -/- mice (N=12) and controls (N=12) up to an age of 40 weeks was determined. LRep1 -/- mice are significant lighter at an age of 28 weeks up to an age of 40 weeks. (F) Whole body fat mass was determined in awake mice by using nuclear magnetic resonance technology with EchoMRI700 instrument (Echo Medical Systems, Houston, TX, USA) in control and LRep1 -/- mice at 3, 8, 16, 24, and 80 weeks of age. Data are presented as percentage of total body fat from body weight. Results are expressed as means ± SE from at least 4 animals per genotype and time point. ** p-value <0.01 (G) Mean oxygen consumption (VO 2 ) measured by indirect calorimetry in a Calorimetry Module (CaloSys V2.1, TSE Systems, Bad Homburg, Germany) at an age of 30 weeks. After two hours of acclimatization, mean oxygen consumption (VO 2 ) was recorded from LRep1 -/- 29
30 Diabetes Page 30 of 60 (N=10) and control mice (N=8) over a period of 72h (p=0.0008). Results are expressed as means ± SE. (H) Spontaneous activity (counts) measured as cage movement (XYZ axis) was determined over 72h. Data represents means of counts from LRep1 -/- (N=10) and control mice (N=8) of 12 h. (I) Mean running distance (km) of LRep1 -/- (N=10) mice compared to control (N=8) animals over a period of 72h at an age of 30 weeks. Results are expressed as means ± SE. The different degrees of significance was indicated as follows in the graphs-*p<0.05; **P<0.01; ***P< FIGURE. 2. Insulin sensitivity, glucose metabolism and effects in mice with liver specific Repin1 deficiency. (A) Glucose infusion rate (GIR) during a hyperinsulinemic-euglycemic clamp was increased in LRep1 -/- knockout mice (N=8) compared with control mice (N=6) at an age of 20 weeks. Experiments started after an overnight fast. A 120-minute hyperinsulinemic-euglycemic clamp was conducted with a continuous infusion of human insulin at a rate of 20 mu/kg/min. Insulin is infused at a constant rate resulting in a drop in blood glucose. To maintain blood glucose at a constant level, exogenous glucose (D20%) is infused into the circulation. Variable glucose infusion rate (GIR) is determined by measuring blood glucose at brief intervals throughout the experiment and adjusting the GIR accordingly. Glucose infusion rate was calculated as mg/kg/min. Results are expressed as means ± SE. (B) Basal hepatic glucose production (HPG) during the clamp in LRep1 -/- mice is up-regulated compared to littermate controls (N=8). Hepatic glucose production (mg kg-1 min-1) was calculated as the difference between the rate of glucose appearance and glucose infusion rate (GIR). Results are expressed as means ± SE from 6 animals per genotype (C) Percentage suppression of hepatic glucose production by insulin during the clamp in control (N=6) and LRep1 -/- mice (N=8). Results are expressed as means ± SE from 6 animals per genotype. (D) ipgtts performed on 12-h-fasted 24-week-old control (N=12) and LRep1 -/- mice (N=12) on a chow diet. LRep1 -/- mice have normal glucose tolerance. (E) Insulin tolerance test (ITT) of 30
31 Page 31 of 60 Diabetes LRep1 -/- showed improved insulin sensitivity at an age of 24 and 40 weeks. ITTs were performed in a fed state. Results are expressed as means ± SE from 12 animals per genotype. Results are expressed as means ± SE from 12 animals per genotype. (F) Glucose tolerance test in LRep1 -/- mice compared to control animals at an age of 30 weeks (per genotype 8 animals). (G) Insulin levels measures during glucose tolerance test at an age of 30 weeks in male LRep1 -/- mice. (H) Insulin tolerance test in 40-week old control animals and LRep1 -/- mice indicating a significant improvement in insulin sensitivity of LRep1 -/- mice. Results are expressed as means ± SE from 6 animals per genotype. (I) Significant lower HbA1c (%) levels in LRep1 -/- mice (N=8) compared to controls (N=8) at an age of 40 weeks. The different degrees of significance was indicated as follows in the graphs-*p<0.05; **P<0.01; ***P< FIGURE. 3. Insulin signaling, VLDL-triglyceride production and hepatic de novo lipogenese in livers of LRep1 -/- mice. (A) Up-regulated insulin pathway protein expression measurements with quantification by Western Blot analysis (B) in livers of LRep1 -/- (N=5) and control (N=5) animals after injection of 5U regular insulin into the Vena cava inferior. (C) Representative images of hepatic tissue fat staining of liver section using sudan-black staining indicates less fat storage in LRep1 -/- mice compared with control animals. (D) Representative images of hepatic tissue for H&E-staining of wildtype and LRep1 -/- mice showing no morphological changes in livers of LRep1 -/- mice. (E) Reduction of Cd36 mrna expression and (F) Representative Cd36 protein expression and analysis in livers LRep1 -/- mice (N=5) compared to controls (N=5) at an age of 32 weeks. Gapdh protein served as loading control. Results are expressed as means ± SE. (G) Determination of VLDLtriglyceride production rates after p407 i.p. treatment and serum samples were taken over a period of 4 h and assay for triglycerides in LRep1 -/- (grey) and control (black) littermates (n=4 per genotype). (H) Fat load test after oral application of 200µl olive oil in LRep1 -/- (grey) and 31
32 Diabetes Page 32 of 60 control (black) littermates (n=6 per genotype). (I) Rates of 3 H 2 O and (J) 14 C incorporation into liver fatty acids in fed state. n=6 per genotype. K) ACC and phosphorylated ACC (pacc) protein expression analysis with representative images in livers of control (N=5) and LRep1 -/- mice (N=5). Gapdh protein served as loading control. Western blot analysis results are expressed as means ± SE. The different degrees of significance (t-test with Welch correction) was indicated as follows in the graphs *P<0.05; **P<0.01; ***P< FIGURE. 4. Effects of a 10 weeks high fat diet (HFD) on fat accumulation and glucose metabolism in LRep1 -/- mice. (A) Under high fat diet conditions LRep1 -/- mice (N=8) show identical growth compared with controls (N=8). (B) No hypertrophy of visceral adipocytes in LRep1 -/- compared with control mice. Maximal adipocyte size measured with multisizer (Beckman Coulter) after 48-h osmium fixation. Data represent means ± SE of 8 mice per genotype. The different degrees of significance was indicated as follows in the graphs- *P<0.05. (C-D) Frequency of adipocyte size distribution after adipocyte isolation and osmium fixation measured with multisizer under chow and HFD conditions indicating a shift in adipocyte size distribution under high fat feeding conditions. (E-F) Hematoxylin-eosin staining (HE) of white adipose tissue (epigonadal) adipocytes after feeding standard chow or HFD from LRep1 -/- mice. Initial magnification, 20x. Adipocyte size is increased after HFD in control mice only. (G) Insulin tolerance test in 16-week old control animals and LRep1 -/- mice indicating a significant improvement in insulin sensitivity of LRep1 -/- mice under high fat diet conditions. Results are expressed as means ± SE from 6 animals per genotype. (H) Glucose tolerance test in 16-week old control animals and LRep1 -/- male mice under high fat diet conditions. Results are expressed as means ± SE from 6 animals per genotype. (I) Lipolysis and (J) [U- 14 C]-glucose uptake in isolated adipocytes from subcutaneous fat depots of LRep1-32
33 Page 33 of 60 Diabetes /- and controls mice. (I) Lipolysis measured as basal glycerol release assayed over 20 min as an indicator of lipolysis. Results are expressed as nmol of glycerol released per cell in a 20- min period. (J) Insulin-stimulated [U 14 C]-glucose uptake in isolated adipocytes after 20 minutes incubation in the absence (basal) or presence of 100 nm insulin. Insulin stimulation is presented as n-fold change relative to basal from 4 animals per genotype. Significant differences between the groups are indicated: *p < 0.05, **p < 0.01, ***p < Values are means ± SE. FIGURE. 5. Model for the phenotype resulting from hepatic Repin1 deletion. Hepatic Repin1 deficiency leads to expression changes of genes involved in insulin signaling, glucose transport, lipid uptake and lipid droplet formation. Lower liver lipid content may be caused significantly reduced Cd36 expression and the inability of the liver to take up plasma lipids normally. Reduced liver lipid content leads to improved whole body insulin sensitivity. 33
34 Diabetes Page 34 of 60 Table 1. Serum concentrations of parameters of lipid metabolism, glucose homeostasis and liver function at an age of 32 week (N=12 per group). All values were obtained after 14 h overnight fast except for FFA and glycerol levels which were tested in the fed state (N=6 per genotype). *significantly different between wildtype (WT) and LRep1 -/- mice at * p<0.05, **p< Serum lipids WT (N=12) LRep1 -/- (N=12) Triglycerides (mmol/l) 1.51 ± ± 0.40* Cholesterol (mmol/l) 2.44 ± ± 0.28 HDL-cholesterol (mmol/l) 1.96 ± ± 0.26 LDL-cholesterol (mmol/l) 0.18 ± ± 0.07 FFA (mmol/l), fasted 1.50 ± ± 0.20** FFA (mmol/l), fed 1.41 ± ± 0.26 Glycerol (nmol/ml), fed 3184 ± ± 471 Glucose homeostasis Insulin (ng/ml) 0.15 ± ± 0.23 Adiponectin (µg/ml) 77 ± ± 17 Leptin (ng/ml) 139 ± ± 50 Fasting glucose (mmol/l) 4.9 ± ± 1.5 Non-fasting glucose (mmol/l) 6.6 ± ± 0.8 Liver function ALAT (ukat/l) 0.68 ± ± 0.12 ASAT (ukat/l) 3.86 ± ± 1.15 GLDH (U/L) 60.9 ± ± 2.7 Serum Albumin (g/l) ± ±
35 Page 35 of 60 Diabetes Table 2. Liver lipid profile analysis of male wildtype (WT) and LRep -/- mice at an age of 32 weeks. *significant different between wildtype (WT) and LRep1 -/- mice at * 0.05 level. Parameter (pmol/µg) WT (N=5) LRep1 -/- (N=6) Triacylglyceride (TAG) 22.1 ± ± 2.0* Diacylglyceride (DAG) 6.0 ± ± 4.2 Cholesterolester 3.5 ± ± 0.7 Cholesterol 24.8 ± ±
36 Diabetes Page 36 of 60 FIGURE 1. Targeting strategy, assessment of Repin1 recombination and Repin1 expression. (A) Schematic representation of the loxp-flanked Repin1 allele before and after recombination (Cre-expression). The knockout allele is shown below the floxed allele, indicating the deletion of exon 2 in the event of recombination of the Repin1 gene. (B) Western blot analysis showing the expression of Repin1 and GAPDH as loading control of liver, lung, heart, brain, pancreas, kidney, spleen, skeletal muscle, brown adipose tissue (BAT), subcutaneous (sc) and epigonadal (epi) adipose tissue of control and LRep1-/- mice. (C) Knockdown efficiency in liver from wildtype (Controls), heterozygous (LRep1+/-) and homozygous (LRep1-/- ) mice. (D) Representative images (original magnification x200) of Repin1 immunohistochemistry (positive staining of the Repin1 protein with DAB as chromogen in brown, arrows) of liver tissue 5µm paraffin sections of wildtype control mice (left) and LRep1-/- mice (right) at an age of 12 weeks. The cytoplasmic staining of hepatocytes for Repin1 in wild-type control mice, whereas in livers of LRep1-/- mice exclusively nonparenchymal cells are Repin1-positive. (E) Growth phenotype of LRep1-/- mice (N=12) and controls (N=12) up to an age of 40 weeks was determined. LRep1-/- mice are significant lighter at an age of 28 weeks up to an age of 40 weeks. (F) Whole body fat mass was determined in awake mice by using nuclear magnetic resonance technology with EchoMRI700 instrument (Echo Medical Systems, Houston, TX, USA) in control and LRep1-/- mice at 3, 8, 16, 24, and 80 weeks of age. Data are presented as percentage of total body fat from body weight. Results are expressed as means ± SE from at least 4 animals per genotype and time point. ** p-value <0.01 (G) Mean oxygen consumption (VO2) measured by indirect calorimetry in a Calorimetry Module (CaloSys V2.1, TSE Systems, Bad Homburg, Germany) at an age of 30 weeks. After two hours of acclimatization, mean oxygen consumption (VO2) was recorded from LRep1-/- (N=10) and control mice (N=8) over a period of 72h (p=0.0008). Results are expressed as means ± SE. (H) Spontaneous activity (counts) measured as cage movement (XYZ axis) was determined over 72h. Data represents means of counts from LRep1-/- (N=10) and control mice (N=8) of 12h. (I) Mean running distance (km) of LRep1-/- (N=10) mice compared to control (N=8) animals over a period of 72h at an age of 30 weeks. Results are expressed as means ± SE. The different degrees of significance was indicated as follows in the graphs-*p<0.05; **P<0.01; ***P< x190mm (96 x 96 DPI)
37 Page 37 of 60 Diabetes
38 Diabetes Page 38 of 60 FIGURE 1. Targeting strategy, assessment of Repin1 recombination and Repin1 expression. (A) Schematic representation of the loxp-flanked Repin1 allele before and after recombination (Cre-expression). The knockout allele is shown below the floxed allele, indicating the deletion of exon 2 in the event of recombination of the Repin1 gene. (B) Western blot analysis showing the expression of Repin1 and GAPDH as loading control of liver, lung, heart, brain, pancreas, kidney, spleen, skeletal muscle, brown adipose tissue (BAT), subcutaneous (sc) and epigonadal (epi) adipose tissue of control and LRep1-/- mice. (C) Knockdown efficiency in liver from wildtype (Controls), heterozygous (LRep1+/-) and homozygous (LRep1-/- ) mice. (D) Representative images (original magnification x200) of Repin1 immunohistochemistry (positive staining of the Repin1 protein with DAB as chromogen in brown, arrows) of liver tissue 5µm paraffin sections of wildtype control mice (left) and LRep1-/- mice (right) at an age of 12 weeks. The cytoplasmic staining of hepatocytes for Repin1 in wild-type control mice, whereas in livers of LRep1-/- mice exclusively nonparenchymal cells are Repin1-positive. (E) Growth phenotype of LRep1-/- mice (N=12) and controls (N=12) up to an age of 40 weeks was determined. LRep1-/- mice are significant lighter at an age of 28 weeks up to an age of 40 weeks. (F) Whole body fat mass was determined in awake mice by using nuclear magnetic resonance technology with EchoMRI700 instrument (Echo Medical Systems, Houston, TX, USA) in control and LRep1-/- mice at 3, 8, 16, 24, and 80 weeks of age. Data are presented as percentage of total body fat from body weight. Results are expressed as means ± SE from at least 4 animals per genotype and time point. ** p-value <0.01 (G) Mean oxygen consumption (VO2) measured by indirect calorimetry in a Calorimetry Module (CaloSys V2.1, TSE Systems, Bad Homburg, Germany) at an age of 30 weeks. After two hours of acclimatization, mean oxygen consumption (VO2) was recorded from LRep1-/- (N=10) and control mice (N=8) over a period of 72h (p=0.0008). Results are expressed as means ± SE. (H) Spontaneous activity (counts) measured as cage movement (XYZ axis) was determined over 72h. Data represents means of counts from LRep1-/- (N=10) and control mice (N=8) of 12h. (I) Mean running distance (km) of LRep1-/- (N=10) mice compared to control (N=8) animals over a period of 72h at an age of 30 weeks. Results are expressed as means ± SE. The different degrees of significance was indicated as follows in the graphs-*p<0.05; **P<0.01; ***P< x190mm (96 x 96 DPI)
39 Page 39 of 60 Diabetes
40 Diabetes Page 40 of 60 FIGURE. 2. Insulin sensitivity, glucose metabolism and effects in mice with liver specific Repin1 deficiency. (A) Glucose infusion rate (GIR) during a hyperinsulinemic-euglycemic clamp was increased in LRep1-/- knockout mice (N=8) compared with control mice (N=6) at an age of 20 weeks. Experiments started after an overnight fast. A 120-minute hyperinsulinemic-euglycemic clamp was conducted with a continuous infusion of human insulin at a rate of 20 mu/kg/min. Insulin is infused at a constant rate resulting in a drop in blood glucose. To maintain blood glucose at a constant level, exogenous glucose (D20%) is infused into the circulation. Variable glucose infusion rate (GIR) is determined by measuring blood glucose at brief intervals throughout the experiment and adjusting the GIR accordingly. Glucose infusion rate was calculated as mg/kg/min. Results are expressed as means ± SE. (B) Basal hepatic glucose production (HPG) during the clamp in LRep1-/- mice is up-regulated compared to littermate controls (N=8). Hepatic glucose production (mg kg-1 min-1) was calculated as the difference between the rate of glucose appearance and glucose infusion rate (GIR). Results are expressed as means ± SE from 6 animals per genotype (C) Percentage suppression of hepatic glucose production by insulin during the clamp in control (N=6) and LRep1-/- mice (N=8). Results are expressed as means ± SE from 6 animals per genotype. (D) ipgtts performed on 12-hfasted 24-week-old control (N=12) and LRep1-/- mice (N=12) on a chow diet. LRep1-/- mice have normal glucose tolerance. (E) Insulin tolerance test (ITT) of LRep1-/- showed improved insulin sensitivity at an age of 24 and 40 weeks. ITTs were performed in a fed state. Results are expressed as means ± SE from 12 animals per genotype. Results are expressed as means ± SE from 12 animals per genotype. (F) Glucose tolerance test in LRep1-/- mice compared to control animals at an age of 30 weeks (per genotype 8 animals). (G) Insulin levels measures during glucose tolerance test at an age of 30 weeks in male LRep1-/- mice. (H) Insulin tolerance test in 40-week old control animals and LRep1-/- mice indicating a significant improvement in insulin sensitivity of LRep1-/- mice. Results are expressed as means ± SE from 6 animals per genotype. (I) Significant lower HbA1c (%) levels in LRep1-/- mice (N=8) compared to controls (N=8) at an age of 40 weeks. The different degrees of significance was indicated as follows in the graphs-*p<0.05; **P<0.01; ***P< x190mm (96 x 96 DPI)
41 Page 41 of 60 Diabetes FIGURE. 2. Insulin sensitivity, glucose metabolism and effects in mice with liver specific Repin1 deficiency. (A) Glucose infusion rate (GIR) during a hyperinsulinemic-euglycemic clamp was increased in LRep1-/- knockout mice (N=8) compared with control mice (N=6) at an age of 20 weeks. Experiments started after an overnight fast. A 120-minute hyperinsulinemic-euglycemic clamp was conducted with a continuous infusion of human insulin at a rate of 20 mu/kg/min. Insulin is infused at a constant rate resulting in a drop in blood glucose. To maintain blood glucose at a constant level, exogenous glucose (D20%) is infused into the circulation. Variable glucose infusion rate (GIR) is determined by measuring blood glucose at brief intervals throughout the experiment and adjusting the GIR accordingly. Glucose infusion rate was calculated as mg/kg/min. Results are expressed as means ± SE. (B) Basal hepatic glucose production (HPG) during the clamp in LRep1-/- mice is up-regulated compared to littermate controls (N=8). Hepatic glucose production (mg kg-1 min-1) was calculated as the difference between the rate of glucose appearance and glucose infusion rate (GIR). Results are expressed as means ± SE from 6 animals per genotype (C) Percentage suppression of hepatic glucose production by insulin during the clamp in control (N=6) and LRep1-/- mice (N=8). Results are expressed as means ± SE from 6 animals per genotype. (D) ipgtts performed on 12-hfasted 24-week-old control (N=12) and LRep1-/- mice (N=12) on a chow diet. LRep1-/- mice have normal glucose tolerance. (E) Insulin tolerance test (ITT) of LRep1-/- showed improved insulin sensitivity at an age of 24 and 40 weeks. ITTs were performed in a fed state. Results are expressed as means ± SE from 12 animals per genotype. Results are expressed as means ± SE from 12 animals per genotype. (F) Glucose tolerance test in LRep1-/- mice compared to control animals at an age of 30 weeks (per genotype 8 animals). (G) Insulin levels measures during glucose tolerance test at an age of 30 weeks in male LRep1-/- mice. (H) Insulin tolerance test in 40-week old control animals and LRep1-/- mice indicating a significant improvement in insulin sensitivity of LRep1-/- mice. Results are expressed as means ± SE from 6 animals per genotype. (I) Significant lower HbA1c (%) levels in LRep1-/- mice (N=8) compared to controls (N=8) at an age of 40 weeks. The different degrees of significance was indicated as follows in the graphs-*p<0.05; **P<0.01; ***P< x190mm (96 x 96 DPI)
42 Diabetes Page 42 of 60 FIGURE. 3. Insulin signaling, VLDL-triglyceride production and hepatic de novo lipogenese in livers of LRep1- /-mice. (A) Up-regulated insulin pathway protein expression measurements with quantification by Western blot analysis (B) in livers of LRep1-/- (N=5) and control (N=5) animals after injection of 5U regular insulin into the Vena cava inferior. (C) Representative images of hepatic tissue fat staining of liver section using sudan-black staining indicates less fat storage in LRep1-/- mice compared with control animals. (D) Representative images of hepatic tissue for H&E-staining of wildtype and LRep1-/- mice showing no morphological changes in livers of LRep1-/- mice. (E) Reduction of Cd36 mrna expression and (F) Cd36 protein reduction in livers LRep1-/- mice (N=5) compared to controls (N=5) at an age of 32 weeks. Gapdh protein served as loading control. Results are expressed as means ± SE. (G) Determination of VLDLtriglyceride production rates after p407 i.p. treatment and serum samples were taken over a period of 4 h and assay for triglycerides in LRep1-/- (grey) and control (black) littermates (n=4 per genotype). (H) Fat load test after oral application of 200µl olive oil in LRep1-/- (grey) and control (black) littermates (n=6 per genotype). (I) Rates of 3H2O and (J)14C incorporation into liver fatty acids in fed state. n=6 per genotype. K) ACC and phosphorylated ACC (pacc) protein expression analysis with representative images in livers of control (N=5) and LRep1-/- mice (N=5). Gapdh protein served as loading control. Western blot analysis results are expressed as means ± SE. The different degrees of significance was indicated as follows in the graphs *P<0.05; **P<0.01; ***P< x190mm (96 x 96 DPI)
43 Page 43 of 60 Diabetes FIGURE. 3. Insulin signaling, VLDL-triglyceride production and hepatic de novo lipogenese in livers of LRep1- /-mice. (A) Up-regulated insulin pathway protein expression measurements with quantification by Western blot analysis (B) in livers of LRep1-/- (N=5) and control (N=5) animals after injection of 5U regular insulin into the Vena cava inferior. (C) Representative images of hepatic tissue fat staining of liver section using sudan-black staining indicates less fat storage in LRep1-/- mice compared with control animals. (D) Representative images of hepatic tissue for H&E-staining of wildtype and LRep1-/- mice showing no morphological changes in livers of LRep1-/- mice. (E) Reduction of Cd36 mrna expression and (F) Cd36 protein reduction in livers LRep1-/- mice (N=5) compared to controls (N=5) at an age of 32 weeks. Gapdh protein served as loading control. Results are expressed as means ± SE. (G) Determination of VLDLtriglyceride production rates after p407 i.p. treatment and serum samples were taken over a period of 4 h and assay for triglycerides in LRep1-/- (grey) and control (black) littermates (n=4 per genotype). (H) Fat load test after oral application of 200µl olive oil in LRep1-/- (grey) and control (black) littermates (n=6 per genotype). (I) Rates of 3H2O and (J)14C incorporation into liver fatty acids in fed state. n=6 per genotype. K) ACC and phosphorylated ACC (pacc) protein expression analysis with representative images in livers of control (N=5) and LRep1-/- mice (N=5). Gapdh protein served as loading control. Western blot analysis results are expressed as means ± SE. The different degrees of significance was indicated as follows in the graphs *P<0.05; **P<0.01; ***P< x190mm (96 x 96 DPI)
44 Diabetes Page 44 of 60 FIGURE. 3. Insulin signaling, VLDL-triglyceride production and hepatic de novo lipogenese in livers of LRep1- /-mice. (A) Up-regulated insulin pathway protein expression measurements with quantification by Western blot analysis (B) in livers of LRep1-/- (N=5) and control (N=5) animals after injection of 5U regular insulin into the Vena cava inferior. (C) Representative images of hepatic tissue fat staining of liver section using sudan-black staining indicates less fat storage in LRep1-/- mice compared with control animals. (D) Representative images of hepatic tissue for H&E-staining of wildtype and LRep1-/- mice showing no morphological changes in livers of LRep1-/- mice. (E) Reduction of Cd36 mrna expression and (F) Cd36 protein reduction in livers LRep1-/- mice (N=5) compared to controls (N=5) at an age of 32 weeks. Gapdh protein served as loading control. Results are expressed as means ± SE. (G) Determination of VLDLtriglyceride production rates after p407 i.p. treatment and serum samples were taken over a period of 4 h and assay for triglycerides in LRep1-/- (grey) and control (black) littermates (n=4 per genotype). (H) Fat load test after oral application of 200µl olive oil in LRep1-/- (grey) and control (black) littermates (n=6 per genotype). (I) Rates of 3H2O and (J)14C incorporation into liver fatty acids in fed state. n=6 per genotype. K) ACC and phosphorylated ACC (pacc) protein expression analysis with representative images in livers of control (N=5) and LRep1-/- mice (N=5). Gapdh protein served as loading control. Western blot analysis results are expressed as means ± SE. The different degrees of significance was indicated as follows in the graphs *P<0.05; **P<0.01; ***P< x190mm (96 x 96 DPI)
45 Page 45 of 60 Diabetes FIGURE. 4. Effects of a 10 weeks high fat diet (HFD) on fat accumulation and glucose metabolism in LRep1- /- mice. (A) Under high fat diet conditions LRep1-/- mice (N=8) show identical growth compared with controls (N=8). (B) No hypertrophy of visceral adipocytes in LRep1-/- compared with control mice. Maximal adipocyte size measured with multisizer (Beckman Coulter) after 48-h osmium fixation. Data represent means ± SE of 8 mice per genotype. The different degrees of significance was indicated as follows in the graphs-*p<0.05. (C-D) Frequency of adipocyte size distribution after adipocyte isolation and osmium fixation measured with multisizer under chow and HFD conditions indicating a shift in adipocyte size distribution under high fat feeding conditions. (E-F) Hematoxylin-eosin staining (HE) of white adipose tissue (epigonadal) adipocytes after feeding standard chow or HFD from LRep1-/- mice. Initial magnification, 20x. Adipocyte size is increased after HFD in control mice only. (G) Insulin tolerance test in 16-week old control animals and LRep1-/- mice indicating a significant improvement in insulin sensitivity of LRep1-/- mice under high fat diet conditions. Results are expressed as means ± SE from 6 animals per genotype. (H) Glucose tolerance test in 16-week old control animals and LRep1-/- male mice under high fat diet conditions. Results are expressed as means ± SE from 6 animals per genotype. (I) Lipolysis and (J) [U-14C]-glucose uptake in isolated adipocytes from subcutaneous fat depots of LRep1-/- and controls mice. (I) Lipolysis measured as basal glycerol release assayed over 20 min as an indicator of lipolysis. Results are expressed as nmol of glycerol released per cell in a 20-min period. (J) Insulin-stimulated [U 14C]-glucose uptake in isolated adipocytes after 20 minutes incubation in the absence (basal) or presence of 100 nm insulin. Insulin stimulation is presented as n-fold change relative to basal from 4 animals per genotype. Significant differences between the groups are indicated: *p < 0.05, **p < 0.01, ***p < Values are means ± SE. 254x190mm (96 x 96 DPI)
46 Diabetes Page 46 of 60 FIGURE. 4. Effects of a 10 weeks high fat diet (HFD) on fat accumulation and glucose metabolism in LRep1- /- mice. (A) Under high fat diet conditions LRep1-/- mice (N=8) show identical growth compared with controls (N=8). (B) No hypertrophy of visceral adipocytes in LRep1-/- compared with control mice. Maximal adipocyte size measured with multisizer (Beckman Coulter) after 48-h osmium fixation. Data represent means ± SE of 8 mice per genotype. The different degrees of significance was indicated as follows in the graphs-*p<0.05. (C-D) Frequency of adipocyte size distribution after adipocyte isolation and osmium fixation measured with multisizer under chow and HFD conditions indicating a shift in adipocyte size distribution under high fat feeding conditions. (E-F) Hematoxylin-eosin staining (HE) of white adipose tissue (epigonadal) adipocytes after feeding standard chow or HFD from LRep1-/- mice. Initial magnification, 20x. Adipocyte size is increased after HFD in control mice only. (G) Insulin tolerance test in 16-week old control animals and LRep1-/- mice indicating a significant improvement in insulin sensitivity of LRep1-/- mice under high fat diet conditions. Results are expressed as means ± SE from 6 animals per genotype. (H) Glucose tolerance test in 16-week old control animals and LRep1-/- male mice under high fat diet conditions. Results are expressed as means ± SE from 6 animals per genotype. (I) Lipolysis and (J) [U-14C]-glucose uptake in isolated adipocytes from subcutaneous fat depots of LRep1-/- and controls mice. (I) Lipolysis measured as basal glycerol release assayed over 20 min as an indicator of lipolysis. Results are expressed as nmol of glycerol released per cell in a 20-min period. (J) Insulin-stimulated [U 14C]-glucose uptake in isolated adipocytes after 20 minutes incubation in the absence (basal) or presence of 100 nm insulin. Insulin stimulation is presented as n-fold change relative to basal from 4 animals per genotype. Significant differences between the groups are indicated: *p < 0.05, **p < 0.01, ***p < Values are means ± SE. 254x190mm (96 x 96 DPI)
47 Page 47 of 60 Diabetes FIGURE. 4. Effects of a 10 weeks high fat diet (HFD) on fat accumulation and glucose metabolism in LRep1- /- mice. (A) Under high fat diet conditions LRep1-/- mice (N=8) show identical growth compared with controls (N=8). (B) No hypertrophy of visceral adipocytes in LRep1-/- compared with control mice. Maximal adipocyte size measured with multisizer (Beckman Coulter) after 48-h osmium fixation. Data represent means ± SE of 8 mice per genotype. The different degrees of significance was indicated as follows in the graphs-*p<0.05. (C-D) Frequency of adipocyte size distribution after adipocyte isolation and osmium fixation measured with multisizer under chow and HFD conditions indicating a shift in adipocyte size distribution under high fat feeding conditions. (E-F) Hematoxylin-eosin staining (HE) of white adipose tissue (epigonadal) adipocytes after feeding standard chow or HFD from LRep1-/- mice. Initial magnification, 20x. Adipocyte size is increased after HFD in control mice only. (G) Insulin tolerance test in 16-week old control animals and LRep1-/- mice indicating a significant improvement in insulin sensitivity of LRep1-/- mice under high fat diet conditions. Results are expressed as means ± SE from 6 animals per genotype. (H) Glucose tolerance test in 16-week old control animals and LRep1-/- male mice under high fat diet conditions. Results are expressed as means ± SE from 6 animals per genotype. (I) Lipolysis and (J) [U-14C]-glucose uptake in isolated adipocytes from subcutaneous fat depots of LRep1-/- and controls mice. (I) Lipolysis measured as basal glycerol release assayed over 20 min as an indicator of lipolysis. Results are expressed as nmol of glycerol released per cell in a 20-min period. (J) Insulin-stimulated [U 14C]-glucose uptake in isolated adipocytes after 20 minutes incubation in the absence (basal) or presence of 100 nm insulin. Insulin stimulation is presented as n-fold change relative to basal from 4 animals per genotype. Significant differences between the groups are indicated: *p < 0.05, **p < 0.01, ***p < Values are means ± SE. 254x190mm (96 x 96 DPI)
48 Diabetes Page 48 of 60 FIGURE. 5. Model for the phenotype resulting from hepatic Repin1 deletion. Hepatic Repin1 deficiency leads to expression changes of genes involved in insulin signaling, glucose transport, lipid uptake and lipid droplet formation. Lower liver lipid content may be caused significantly reduced Cd36 expression and the inability of the liver to take up plasma lipids normally. Reduced liver lipid content leads to improved whole body insulin sensitivity. 254x190mm (96 x 96 DPI)
49 Page 49 of 60 Diabetes Supplementary Table 1. Results of functional annotation tool using Database for Annotation, Visualization and Integrated Discovery (DAVID). For data analysis probe set fluorescence intensities were extracted for approximately 39,000 transcripts with complete Mouse Genome coverage and were scaled in order to normalize data for inter-array comparison using MAS 5.0 software according to instructions of the manufacturer (Affymetrix, Santa Clara, CA, USA). GOTERM_BP_FAT biological process Category Term Gene count % PValue GOTERM_BP_FAT GO: ~translation 66 4, ,73E-13 GOTERM_BP_FAT GO: ~generation of precursor metabolites and energy 46 2, ,69E-07 GOTERM_CC_FAT cellular component Category Term Gene count % PValue GOTERM_CC_FAT GO: ~ribosome 52 3, ,15E-15 GOTERM_CC_FAT GO: ~ribonucleoprotein complex 85 5, ,52E-13 GOTERM_CC_FAT GO: ~non-membrane-bounded organelle , ,53E-11 GOTERM_CC_FAT GO: ~intracellular non-membrane-bounded organelle , ,53E-11 GOTERM_CC_FAT GO: ~mitochondrial inner membrane 59 3, ,90E-11 GOTERM_CC_FAT GO: ~mitochondrial part 86 5, ,99E-11 GOTERM_CC_FAT GO: ~mitochondrial membrane 67 4, ,29E-10 GOTERM_CC_FAT GO: ~organelle inner membrane 60 3, ,47E-10 GOTERM_CC_FAT GO: ~organelle membrane 115 6, ,65E-10 GOTERM_CC_FAT GO: ~mitochondrial envelope 69 4, ,67E-10 GOTERM_CC_FAT GO: ~organelle envelope 82 4, ,68E-09 GOTERM_CC_FAT GO: ~envelope 82 4, ,04E-09 GOTERM_CC_FAT GO: ~mitochondrion 158 9, ,44E-08 GOTERM_CC_FAT GO: ~proton-transporting ATP synthase complex 10 0, ,88E-06
50 Diabetes Page 50 of 60 GOTERM_CC_FAT GO: ~actin cytoskeleton 35 2, ,34E-05 GOTERM_MF_FAT molecular function Category Term Gene count % PValue GOTERM_MF_FAT GO: ~structural constituent of ribosome 47 2, ,17E-16 GOTERM_MF_FAT GO: ~structural molecule activity 71 4, ,04E-08 GOTERM_MF_FAT GO: ~monovalent inorganic cation transmembrane transporter activity 23 1, ,34E-07 GOTERM_MF_FAT GO: ~hydrogen ion transmembrane transporter activity 22 1, ,07E-07 GOTERM_MF_FAT GO: ~inorganic cation transmembrane transporter activity 27 1, ,84E-06 GOTERM_MF_FAT GO: ~actin binding 46 2, ,25E-06 KEGG_PATHWAY Category Term Gene count % PValue KEGG_PATHWAY mmu03010:ribosome 47 2, ,94E-25 KEGG_PATHWAY mmu05016:huntington's disease 39 2, ,66E-07 KEGG_PATHWAY mmu00190:oxidative phosphorylation 29 1, ,20E-05 KEGG_PATHWAY mmu05012:parkinson's disease 29 1, ,88E-05
51 Page 51 of 60 Diabetes Supplementary Table 2. Regulated genes identified by liver Affymetrix GeneChip analysis. Genes up-regulated in LRep1 -/- n-fold regulation Genes down-regulated in LRep1 -/- n-fold regulation 1 RIKEN cdna N22 gene 30.1 Lipocalin 2* NLR family, pyrin domain containing 4F 27.8 Predicted gene, ENSMUSG Kelch-like 28 (Drosophila) 23.1 Metallothionein 1 (MT1) RIKEN cdna K02 gene 21.3 Transmembrane protein Ubiquitin specific peptidase 15 (Usp15) 20.9 Necdin Methionine sulfoxide reductase B Metallothionein 2 (MT2)* Insulin receptor substrate (Irs3)* 15.3 Serum amyloid A 2 (Saa2)* Granzyme D 13.3 Phosphatidylinositol 4-kinase (PI4Ka)* Collagen, type XIX, alpha 1 (Col19a1) 12.6 Monooxygenase, DBH-like Toll like receptor 3 (Tlr3)* 12.5 ADP-ribosyltransferase 2a (Art2a) Titin (Tnt)* 13.9 Adaptor-related protein complex 3, mu 2 (Ap3m2) Leucine zipper-ef-hand containing transmembrane 13.1 Acsl3 (acyl-coa synthetase long-chain family member 3) 5.6 protein 2 (Letm2) 13 Serine (or cysteine) peptidase inhibitor, clade A, 11.4 Junction adhesion molecule 3 (Jam3) 4.6 member 4 (Serpina-ps1) 14 Calcium-sensing receptor (Casr) 11.9 T-box Serine threonine kinase 31 (Stk31) 11.8 Vesicle associated membrane protein (Vamp5)* Transmembrane protein Low Density Receptor (Ldlr)* zinc finger and AT hook domain containing (Zfat) 8.6 Vesicle associated membrane protein (Vamp4)* Extracellular matrix protein 2, female organ and adipocyte specific (Ecm2) 7.9 Rarres 2 (Chemerin)* 1.6 * liver expression has been validate by qpcr in 10 animals per genotype
52 Diabetes Page 52 of 60 Supplementary Table 3. Relative gene expression in liver (N=12 per group) at an age of 32 weeks. Gene WT LRep1 -/- Vesicle lipid formation Snap ± ± 0.42* Vamp ± ± 0.10*** Caveolin 1.00 ± ± 0.34* Lipolysis enzymes Hsl iso ± ± 0.74 Hsl iso ± ± 0.21 Hsl iso ± ± 0.21 Lpl 1.00 ± ± 0.34 Ldlr 1.00 ± ± 0.28 Synthesis Fasn 1.00 ± ± 0.54 ApoA ± ± 0.33 ApoD 1.00 ± ± 0.97 Mdr ± ± 0.20 Pemt 1.00 ± ± 0.23 Synthesis of unsaturated FA Scd ± ± 0.47 Scd ± ± 0.36 Alt 1.00 ± ± 0.36
53 Page 53 of 60 Diabetes Acsl ± ± 0.54 Fat transporter and fatty oxidation Cd ± ± 0.22** Fatp ± ± 0.21 Fatp ± ± 0.21 Fatp ± ± 0.30 Cpt ± ± 0.29 Cpt ± ± 0.30 Gluconeogenesis &Glucosetransporter Pepck 1.00 ± ± 0.40 Glut ± ± 0.36 Ide 1.00 ± ± 0.63 Target genes Chemerin 1.00 ± ± 0.44* Lipocalin ± ± 0.02** * significant different between Controls (WT) and LRep1 -/- mice at * 0.05, ** 0.01 and ***0.001 level
54 Diabetes Page 54 of 60 Supplementary Table 4. Genexpression analysis in adipose tissue fat depots, epigonadal (EPI) and subcutaneous (SC) of control and LRep1 - /- mice. Gene EPI SC WT (N=7) LRep1 -/- (N=7) WT (N=7) LRep1 -/- (N=7) Lipolysis enzymes Hsl iso ± ± ± ± 0.17 Hsl iso ± ± ± ± 0.32 Hsl iso ± ± ± ± 0.23 Atgl 1.00 ± ± ± ± 0.33 Lpl 1.00 ± ± ± ± 0.12 Glucosetransporter Glut ± ± ± ± 0.56 Glut ± ± ± ± 0.34* Fat transporter and oxidation enzymes Cd ± ± ± ± 0.82** Fasn 1.00 ± ± ± ± 1.02 BAT genes Ucp ± ± ± ± 1.10 Prdm ± ± ± ± 0.32
55 Page 55 of 60 Diabetes Target genes Chemerin 1.00 ± ± ± ± 0.27* Lipocalin ± ± 0.14** 1.00 ± ± 0.12*
56 Diabetes Page 56 of 60 Figure S1: Representative images (original magnification x200) of Repin1 immunohistochemistry (positive staining of the Repin1 protein with DAB as chromogen in brown, arrows) of liver tissue of wild-type mice (left) and LRep1 -/- mice (right). Please note the cytoplasmic staining of hepatocytes for Repin1 in wild-type mice, whereas in livers of LRep1 -/- mice exclusively non-parenchymal cells are Repin1-positive. Sections were stained with hematoxylin and eosin (H&E) for routine examination. For immunohistochemical staining of Repin1 sections of liver tissue were incubated with primary antibody overnight at 4 C (polyclonal anti-repin1, 1:500, Abcam, Cambridge, UK), followed by horseradishperoxidase (HRP)-conjugated rabbit anti-mouse immunoglobin (1:100, Dako Cytomation, Hamburg, Germany) as secondary antibody. Sites of peroxidase-binding were detected by 3,3`-diaminobenzidine (Dako).
57 Page 57 of 60 Diabetes Figure S2: Representative images of hepatic tissue for F4/80 antigen immunostaining of wild-type (left) and LRep1 -/- mice (right) (original magnification x200, upper panel; x400, lower panel) (positive staining of the F4/80 extracellular membrane protein with permanent red as chromogen in red). To evaluate the cellular inflammatory response, the numbers of resident liver macrophages were analyzed using the F4/80 antigen (1:10; Serotec, Oxford, UK). Overnight incubation (4 C) with the first antibody (polyclonal rat anti-f4/80) was followed by conjugated mouse anti-rat immunoglobulin (1:200; Santa Cruz Biotechnology, Santa Cruz, CA, USA). The sites of alkaline phosphatase binding were detected using the chromogen permanent red (Dako). The sections were counterstained with hemalaun and analyzed with a light microscope (Olympus BX51, Hamburg, Germany).
58 Diabetes Page 58 of 60 Figure S3: Representative images of hepatic tissue for CAE-staining of wild-type (left) and LRep1 -/- mice (right) (original magnification x200, upper panel; x400, lower panel) (CAE-positive cells are in red-brown) showing no increased infiltration of inflammatory cells to the liver of LRep1 - /- mice. Sections were stained with hematoxylin and eosin (H&E) for routine examination and with naphthol AS-D chloroacetate esterase (CAE) for detection of liver tissue-infiltrating leukocytes.
59 Page 59 of 60 Diabetes Figure S4: Body length (A) and food intake (B) as well as ipgtt (C) and ipitt (D) in control and LRep1 -/- mice. A) Body length (naso-anal length) was measured once at week 32 (per genotype 10 animals). Results are expressed as means ± SE from 10 animals per genotype. B) At an age of 16 weeks, in a subgroup of 20 (10 LRep1 -/- and 10 controls) underwent a food intake measurement over a time period of 1 week. The daily food intake was calculated as the average intake of chow within the time stated. Results are expressed as means ± SE from 10 animals per genotype. C) GTTs performed on 12-h-fasted 12-week old male wild-type (control) and LRep1 -/- mice. Results are expressed as means ± SE from 10 animals per genotype. D) ITTs on random fed 12-week-old male wild-type (control) and LRep1 -/- mic. Results are expressed as means ± SE from 10 animals per genotype. (E) Rectal body temperature measures of 32-week old male wild-type (control) and LRep1 -/- mice. (F) Relative brown adipose tissue weight (BAT) from 6 animals per genotype. Results are expressed as means ± SE. A B body length (cm) 10 9 food intake (g/day) Controls 3.0 Controls LRep1-/- LRep1-/- C D
60 Diabetes Page 60 of 60 GTT (12 weeks) ITT (12 weeks) blood glucose (mmol/l) Controls LRep1 -/ blood glucose (mmol/l) Controls LRep1 -/ minutes minutes E F body temperature ( C) relative BAT weight (% from BW) Controls 0.00 Controls LRep1-/- LRep1-/-
61 Page 61 of 60 Diabetes Figure S5. Lipocalin 2 measurements (A) Serum concentration measured with ELISA (R&D Systems) in male LRep1 -/- mice and controls at an age of 32 weeks (8 animals per genotype). (B) Representative Lcn2 protein expression in liver lysates and quantification in male LRep1 -/- mice and controls using western blot analysis (5 animals per genotype). Data are given as mean ± SE. circulating lipocalin2 level (ng/ ml) 900 p= Controls WT LRep1-/- LRep1-/- Lcn2/Gapdh 2.0 p= WT LRep1 Lcn2 Actin
OBESITY STUDIES. Diabetes Volume 63, October Diabetes 2014;63: DOI: /db
 Diabetes Volume 63, October 2014 3295 Matthias Kern, 1 Joanna Kosacka, 1 Nico Hesselbarth, 1 Julia Brückner, 1 John T. Heiker, 1 Gesine Flehmig, 2 Ingrid Klöting, 3 Peter Kovacs, 2 Madlen Matz-Soja, 4
Diabetes Volume 63, October 2014 3295 Matthias Kern, 1 Joanna Kosacka, 1 Nico Hesselbarth, 1 Julia Brückner, 1 John T. Heiker, 1 Gesine Flehmig, 2 Ingrid Klöting, 3 Peter Kovacs, 2 Madlen Matz-Soja, 4
SUPPLEMENTARY INFORMATION
 DOI: 10.1038/ncb3461 In the format provided by the authors and unedited. Supplementary Figure 1 (associated to Figure 1). Cpeb4 gene-targeted mice develop liver steatosis. a, Immunoblot displaying CPEB4
DOI: 10.1038/ncb3461 In the format provided by the authors and unedited. Supplementary Figure 1 (associated to Figure 1). Cpeb4 gene-targeted mice develop liver steatosis. a, Immunoblot displaying CPEB4
SUPPLEMENTARY DATA. Supplementary Table 1. Primers used in qpcr
 Supplementary Table 1. Primers used in qpcr Gene forward primer (5'-3') reverse primer (5'-3') β-actin AGAGGGAAATCGTGCGTGAC CAATAGTGATGACCTGGCCGT Hif-p4h-2 CTGGGCAACTACAGGATAAAC GCGTCCCAGTCTTTATTTAGATA
Supplementary Table 1. Primers used in qpcr Gene forward primer (5'-3') reverse primer (5'-3') β-actin AGAGGGAAATCGTGCGTGAC CAATAGTGATGACCTGGCCGT Hif-p4h-2 CTGGGCAACTACAGGATAAAC GCGTCCCAGTCTTTATTTAGATA
Supplementary Materials for
 www.sciencesignaling.org/cgi/content/full/8/407/ra127/dc1 Supplementary Materials for Loss of FTO in adipose tissue decreases Angptl4 translation and alters triglyceride metabolism Chao-Yung Wang,* Shian-Sen
www.sciencesignaling.org/cgi/content/full/8/407/ra127/dc1 Supplementary Materials for Loss of FTO in adipose tissue decreases Angptl4 translation and alters triglyceride metabolism Chao-Yung Wang,* Shian-Sen
Supplementary Figure 1. DJ-1 modulates ROS concentration in mouse skeletal muscle.
 Supplementary Figure 1. DJ-1 modulates ROS concentration in mouse skeletal muscle. (a) mrna levels of Dj1 measured by quantitative RT-PCR in soleus, gastrocnemius (Gastroc.) and extensor digitorum longus
Supplementary Figure 1. DJ-1 modulates ROS concentration in mouse skeletal muscle. (a) mrna levels of Dj1 measured by quantitative RT-PCR in soleus, gastrocnemius (Gastroc.) and extensor digitorum longus
SUPPLEMENTARY INFORMATION
 doi:10.1038/nature12652 Supplementary Figure 1. PRDM16 interacts with endogenous EHMT1 in brown adipocytes. Immunoprecipitation of PRDM16 complex by flag antibody (M2) followed by Western blot analysis
doi:10.1038/nature12652 Supplementary Figure 1. PRDM16 interacts with endogenous EHMT1 in brown adipocytes. Immunoprecipitation of PRDM16 complex by flag antibody (M2) followed by Western blot analysis
Males- Western Diet WT KO Age (wks) Females- Western Diet WT KO Age (wks)
 Relative Arv1 mrna Adrenal 33.48 +/- 6.2 Skeletal Muscle 22.4 +/- 4.93 Liver 6.41 +/- 1.48 Heart 5.1 +/- 2.3 Brain 4.98 +/- 2.11 Ovary 4.68 +/- 2.21 Kidney 3.98 +/-.39 Lung 2.15 +/-.6 Inguinal Subcutaneous
Relative Arv1 mrna Adrenal 33.48 +/- 6.2 Skeletal Muscle 22.4 +/- 4.93 Liver 6.41 +/- 1.48 Heart 5.1 +/- 2.3 Brain 4.98 +/- 2.11 Ovary 4.68 +/- 2.21 Kidney 3.98 +/-.39 Lung 2.15 +/-.6 Inguinal Subcutaneous
1.5 ASK1KO fed. fasted 16 hrs w/o water. Fed. 4th. 4th WT ASK1KO N=29, 11(WT), ,5(ASK1KO) ASK1KO ASK1KO **** Time [h]
![1.5 ASK1KO fed. fasted 16 hrs w/o water. Fed. 4th. 4th WT ASK1KO N=29, 11(WT), ,5(ASK1KO) ASK1KO ASK1KO **** Time [h] 1.5 ASK1KO fed. fasted 16 hrs w/o water. Fed. 4th. 4th WT ASK1KO N=29, 11(WT), ,5(ASK1KO) ASK1KO ASK1KO **** Time [h]](/thumbs/87/97258189.jpg) 7: 13: 19: 1: 7: 151117 a 151117 4th 4th b c RQ.95 KO.9.85.8.75.7 light dark light dark.65 7: 19: 7: 19: 7: Means ± SEM, N=6 RQ 1..9.8.7.6.6 KO CL (-) CL (+) ibat weight ratio (/body weight) [%].5.4.3.2.1
7: 13: 19: 1: 7: 151117 a 151117 4th 4th b c RQ.95 KO.9.85.8.75.7 light dark light dark.65 7: 19: 7: 19: 7: Means ± SEM, N=6 RQ 1..9.8.7.6.6 KO CL (-) CL (+) ibat weight ratio (/body weight) [%].5.4.3.2.1
AAV-TBGp-Cre treatment resulted in hepatocyte-specific GH receptor gene recombination
 AAV-TBGp-Cre treatment resulted in hepatocyte-specific GH receptor gene recombination Supplementary Figure 1. Generation of the adult-onset, liver-specific GH receptor knock-down (alivghrkd, Kd) mouse
AAV-TBGp-Cre treatment resulted in hepatocyte-specific GH receptor gene recombination Supplementary Figure 1. Generation of the adult-onset, liver-specific GH receptor knock-down (alivghrkd, Kd) mouse
General Laboratory methods Plasma analysis: Gene Expression Analysis: Immunoblot analysis: Immunohistochemistry:
 General Laboratory methods Plasma analysis: Plasma insulin (Mercodia, Sweden), leptin (duoset, R&D Systems Europe, Abingdon, United Kingdom), IL-6, TNFα and adiponectin levels (Quantikine kits, R&D Systems
General Laboratory methods Plasma analysis: Plasma insulin (Mercodia, Sweden), leptin (duoset, R&D Systems Europe, Abingdon, United Kingdom), IL-6, TNFα and adiponectin levels (Quantikine kits, R&D Systems
Supplementary Figure 1
 VO (ml kg - min - ) VCO (ml kg - min - ) Respiratory exchange ratio Energy expenditure (cal kg - min - ) Locomotor activity (x count) Body temperature ( C) Relative mrna expression TA Sol EDL PT Heart
VO (ml kg - min - ) VCO (ml kg - min - ) Respiratory exchange ratio Energy expenditure (cal kg - min - ) Locomotor activity (x count) Body temperature ( C) Relative mrna expression TA Sol EDL PT Heart
Supplementary Fig. 1 eif6 +/- mice show a reduction in white adipose tissue, blood lipids and normal glycogen synthesis. The cohort of the original
 Supplementary Fig. 1 eif6 +/- mice show a reduction in white adipose tissue, blood lipids and normal glycogen synthesis. The cohort of the original phenotypic screening was n=40. For specific tests, the
Supplementary Fig. 1 eif6 +/- mice show a reduction in white adipose tissue, blood lipids and normal glycogen synthesis. The cohort of the original phenotypic screening was n=40. For specific tests, the
control kda ATGL ATGLi HSL 82 GAPDH * ** *** WT/cTg WT/cTg ATGLi AKO/cTg AKO/cTg ATGLi WT/cTg WT/cTg ATGLi AKO/cTg AKO/cTg ATGLi iwat gwat ibat
 body weight (g) tissue weights (mg) ATGL protein expression (relative to GAPDH) HSL protein expression (relative to GAPDH) ### # # kda ATGL 55 HSL 82 GAPDH 37 2.5 2. 1.5 1..5 2. 1.5 1..5.. Supplementary
body weight (g) tissue weights (mg) ATGL protein expression (relative to GAPDH) HSL protein expression (relative to GAPDH) ### # # kda ATGL 55 HSL 82 GAPDH 37 2.5 2. 1.5 1..5 2. 1.5 1..5.. Supplementary
Supplementary Figure S1. Effect of Glucose on Energy Balance in WT and KHK A/C KO
 Supplementary Figure S1. Effect of Glucose on Energy Balance in WT and KHK A/C KO Mice. WT mice and KHK-A/C KO mice were provided drinking water containing 10% glucose or tap water with normal chow ad
Supplementary Figure S1. Effect of Glucose on Energy Balance in WT and KHK A/C KO Mice. WT mice and KHK-A/C KO mice were provided drinking water containing 10% glucose or tap water with normal chow ad
BIOL212 Biochemistry of Disease. Metabolic Disorders - Obesity
 BIOL212 Biochemistry of Disease Metabolic Disorders - Obesity Obesity Approx. 23% of adults are obese in the U.K. The number of obese children has tripled in 20 years. 10% of six year olds are obese, rising
BIOL212 Biochemistry of Disease Metabolic Disorders - Obesity Obesity Approx. 23% of adults are obese in the U.K. The number of obese children has tripled in 20 years. 10% of six year olds are obese, rising
Requires Signaling though Akt2 Independent of the. Transcription Factors FoxA2, FoxO1, and SREBP1c
 Cell Metabolism, Volume 14 Supplemental Information Postprandial Hepatic Lipid Metabolism Requires Signaling though Akt2 Independent of the Transcription Factors FoxA2, FoxO1, and SREBP1c Min Wan, Karla
Cell Metabolism, Volume 14 Supplemental Information Postprandial Hepatic Lipid Metabolism Requires Signaling though Akt2 Independent of the Transcription Factors FoxA2, FoxO1, and SREBP1c Min Wan, Karla
Role of fatty acids in the development of insulin resistance and type 2 diabetes mellitus
 Emerging Science Role of fatty acids in the development of insulin resistance and type 2 diabetes mellitus George Wolf Insulin resistance is defined as the reduced responsiveness to normal circulating
Emerging Science Role of fatty acids in the development of insulin resistance and type 2 diabetes mellitus George Wolf Insulin resistance is defined as the reduced responsiveness to normal circulating
Supporting Information
 Supporting Information Charalambous et al. 10.1073/pnas.1406119111 SI Experimental Procedures Serum and Tissue Biochemistry. Enzymatic assay kits were used for determination of plasma FFAs (Roche), TAGs
Supporting Information Charalambous et al. 10.1073/pnas.1406119111 SI Experimental Procedures Serum and Tissue Biochemistry. Enzymatic assay kits were used for determination of plasma FFAs (Roche), TAGs
GPR120 *** * * Liver BAT iwat ewat mwat Ileum Colon. UCP1 mrna ***
 a GPR120 GPR120 mrna/ppia mrna Arbitrary Units 150 100 50 Liver BAT iwat ewat mwat Ileum Colon b UCP1 mrna Fold induction 20 15 10 5 - camp camp SB202190 - - - H89 - - - - - GW7647 Supplementary Figure
a GPR120 GPR120 mrna/ppia mrna Arbitrary Units 150 100 50 Liver BAT iwat ewat mwat Ileum Colon b UCP1 mrna Fold induction 20 15 10 5 - camp camp SB202190 - - - H89 - - - - - GW7647 Supplementary Figure
SUPPLEMENTARY INFORMATION
 DOI: 10.1038/ncb2211 a! mir-143! b! mir-103/107! let-7a! mir-144! mir-122a! mir-126-3p! mir-194! mir-27a! mir-30c! Figure S1 Northern blot analysis of mir-143 expression dependent on feeding conditions.
DOI: 10.1038/ncb2211 a! mir-143! b! mir-103/107! let-7a! mir-144! mir-122a! mir-126-3p! mir-194! mir-27a! mir-30c! Figure S1 Northern blot analysis of mir-143 expression dependent on feeding conditions.
Metabolic Syndrome. DOPE amines COGS 163
 Metabolic Syndrome DOPE amines COGS 163 Overview - M etabolic Syndrome - General definition and criteria - Importance of diagnosis - Glucose Homeostasis - Type 2 Diabetes Mellitus - Insulin Resistance
Metabolic Syndrome DOPE amines COGS 163 Overview - M etabolic Syndrome - General definition and criteria - Importance of diagnosis - Glucose Homeostasis - Type 2 Diabetes Mellitus - Insulin Resistance
AdPLA ablation increases lipolysis and prevents obesity induced by high fat feeding or leptin deficiency
 AdPLA AdPLA ablation increases lipolysis and prevents obesity induced by high fat feeding or leptin deficiency Kathy Jaworski, Maryam Ahmadian, Robin E. Duncan, Eszter Sarkadi-Nagy, Krista A. Va rady,
AdPLA AdPLA ablation increases lipolysis and prevents obesity induced by high fat feeding or leptin deficiency Kathy Jaworski, Maryam Ahmadian, Robin E. Duncan, Eszter Sarkadi-Nagy, Krista A. Va rady,
Central insulin action regulates peripheral glucose and fat metabolism in mice
 Research article Central insulin action regulates peripheral glucose and fat metabolism in mice Linda Koch, 1 F. Thomas Wunderlich, 1 Jost Seibler, 2 A. Christine Könner, 1 Brigitte Hampel, 1 Sigrid Irlenbusch,
Research article Central insulin action regulates peripheral glucose and fat metabolism in mice Linda Koch, 1 F. Thomas Wunderlich, 1 Jost Seibler, 2 A. Christine Könner, 1 Brigitte Hampel, 1 Sigrid Irlenbusch,
Supplementary Table 2. Plasma lipid profiles in wild type and mutant female mice submitted to a HFD for 12 weeks wt ERα -/- AF-1 0 AF-2 0
 Supplementary Table 1. List of specific primers used for gene expression analysis. Genes Primer forward Primer reverse Hprt GCAGTACAGCCCCAAAATGG AACAAAGTCTGGCCTGTATCCA Srebp-1c GGAAGCTGTCGGGGTAGCGTC CATGTCTTCAAATGTGCAATCCAT
Supplementary Table 1. List of specific primers used for gene expression analysis. Genes Primer forward Primer reverse Hprt GCAGTACAGCCCCAAAATGG AACAAAGTCTGGCCTGTATCCA Srebp-1c GGAAGCTGTCGGGGTAGCGTC CATGTCTTCAAATGTGCAATCCAT
In The Name Of God. In The Name Of. EMRI Modeling Group
 In The Name Of God In The Name Of God EMRI Modeling Group Cells work together in functionally related groups called tissues Types of tissues: Epithelial lining and covering Connective support Muscle movement
In The Name Of God In The Name Of God EMRI Modeling Group Cells work together in functionally related groups called tissues Types of tissues: Epithelial lining and covering Connective support Muscle movement
Role of the ventromedial hypothalamic Steroidogenic Factor 1/ Adrenal 4. glucose metabolism in mice.
 Role of the ventromedial hypothalamic Steroidogenic Factor 1/ Adrenal 4 Binding Protein neurons in the regulation of whole body energy and glucose metabolism in mice. Eulalia Coutinho Department of Physiological
Role of the ventromedial hypothalamic Steroidogenic Factor 1/ Adrenal 4 Binding Protein neurons in the regulation of whole body energy and glucose metabolism in mice. Eulalia Coutinho Department of Physiological
Supplementary Figure 1. DNA methylation of the adiponectin promoter R1, Pparg2, and Tnfa promoter in adipocytes is not affected by obesity.
 Supplementary Figure 1. DNA methylation of the adiponectin promoter R1, Pparg2, and Tnfa promoter in adipocytes is not affected by obesity. (a) Relative amounts of adiponectin, Ppar 2, C/ebp, and Tnf mrna
Supplementary Figure 1. DNA methylation of the adiponectin promoter R1, Pparg2, and Tnfa promoter in adipocytes is not affected by obesity. (a) Relative amounts of adiponectin, Ppar 2, C/ebp, and Tnf mrna
Supplementary Figure 1
 Supplementary Figure 1 how HFD how HFD Epi WT p p Hypothalamus p p Inguinal WT T Liver Lean mouse adipocytes p p p p p p Obese mouse adipocytes Kidney Muscle Spleen Heart p p p p p p p p Extracellular
Supplementary Figure 1 how HFD how HFD Epi WT p p Hypothalamus p p Inguinal WT T Liver Lean mouse adipocytes p p p p p p Obese mouse adipocytes Kidney Muscle Spleen Heart p p p p p p p p Extracellular
Supplementary Materials
 Supplementary Materials 1 Supplementary Table 1. List of primers used for quantitative PCR analysis. Gene name Gene symbol Accession IDs Sequence range Product Primer sequences size (bp) β-actin Actb gi
Supplementary Materials 1 Supplementary Table 1. List of primers used for quantitative PCR analysis. Gene name Gene symbol Accession IDs Sequence range Product Primer sequences size (bp) β-actin Actb gi
A Hepatocyte Growth Factor Receptor (Met) Insulin Receptor hybrid governs hepatic glucose metabolism SUPPLEMENTARY FIGURES, LEGENDS AND METHODS
 A Hepatocyte Growth Factor Receptor (Met) Insulin Receptor hybrid governs hepatic glucose metabolism Arlee Fafalios, Jihong Ma, Xinping Tan, John Stoops, Jianhua Luo, Marie C. DeFrances and Reza Zarnegar
A Hepatocyte Growth Factor Receptor (Met) Insulin Receptor hybrid governs hepatic glucose metabolism Arlee Fafalios, Jihong Ma, Xinping Tan, John Stoops, Jianhua Luo, Marie C. DeFrances and Reza Zarnegar
Gene Polymorphisms and Carbohydrate Diets. James M. Ntambi Ph.D
 Gene Polymorphisms and Carbohydrate Diets James M. Ntambi Ph.D Fatty Acids that Flux into Tissue Lipids are from Dietary Sources or are Made De novo from Glucose or Fructose Glucose Fructose Acetyl-CoA
Gene Polymorphisms and Carbohydrate Diets James M. Ntambi Ph.D Fatty Acids that Flux into Tissue Lipids are from Dietary Sources or are Made De novo from Glucose or Fructose Glucose Fructose Acetyl-CoA
3-Thia Fatty Acids A New Generation of Functional Lipids?
 Conference on Food Structure and Food Quality 3-Thia Fatty Acids A New Generation of Functional Lipids? Rolf K. Berge rolf.berge@med.uib.no Fatty acids- Essential cellular metabolites Concentrations must
Conference on Food Structure and Food Quality 3-Thia Fatty Acids A New Generation of Functional Lipids? Rolf K. Berge rolf.berge@med.uib.no Fatty acids- Essential cellular metabolites Concentrations must
Supplemental Information. Increased 4E-BP1 Expression Protects. against Diet-Induced Obesity and Insulin. Resistance in Male Mice
 Cell Reports, Volume 16 Supplemental Information Increased 4E-BP1 Expression Protects against Diet-Induced Obesity and Insulin Resistance in Male Mice Shih-Yin Tsai, Ariana A. Rodriguez, Somasish G. Dastidar,
Cell Reports, Volume 16 Supplemental Information Increased 4E-BP1 Expression Protects against Diet-Induced Obesity and Insulin Resistance in Male Mice Shih-Yin Tsai, Ariana A. Rodriguez, Somasish G. Dastidar,
SUPPLEMENTARY INFORMATION
 SUPPLEMENTARY INFORMATION doi:10.1038/nature11464 Supplemental Figure S1. The expression of Vegfb is increased in obese and diabetic mice as compared to lean mice. a-b, Body weight and postprandial blood
SUPPLEMENTARY INFORMATION doi:10.1038/nature11464 Supplemental Figure S1. The expression of Vegfb is increased in obese and diabetic mice as compared to lean mice. a-b, Body weight and postprandial blood
Supporting Information
 Supporting Information Franco et al. 10.1073/pnas.1015557108 SI Materials and Methods Drug Administration. PD352901 was dissolved in 0.5% (wt/vol) hydroxyl-propyl-methylcellulose, 0.2% (vol/vol) Tween
Supporting Information Franco et al. 10.1073/pnas.1015557108 SI Materials and Methods Drug Administration. PD352901 was dissolved in 0.5% (wt/vol) hydroxyl-propyl-methylcellulose, 0.2% (vol/vol) Tween
Supplementary Figure S1
 Lipidomic-based investigation into the regulatory effect of Schisandrin B on palmitic acid level in non-alcoholic steatotic livers Hiu Yee Kwan 1,2, Xuyan Niu 3, Wenlin Dai 4, Tiejun Tong 4, Xiaojuan Chao
Lipidomic-based investigation into the regulatory effect of Schisandrin B on palmitic acid level in non-alcoholic steatotic livers Hiu Yee Kwan 1,2, Xuyan Niu 3, Wenlin Dai 4, Tiejun Tong 4, Xiaojuan Chao
18s AAACGGCTACCACATCCAAG CCTCCAATGGATCCTCGTTA. 36b4 GTTCTTGCCCATCAGCACC AGATGCAGCAGATCCGCAT. Acc1 AGCAGATCCGCAGCTTG ACCTCTGCTCGCTGAGTGC
 Supplementary Table 1. Quantitative PCR primer sequences Gene symbol Sequences (5 to 3 ) Forward Reverse 18s AAACGGCTACCACATCCAAG CCTCCAATGGATCCTCGTTA 36b4 GTTCTTGCCCATCAGCACC AGATGCAGCAGATCCGCAT Acc1
Supplementary Table 1. Quantitative PCR primer sequences Gene symbol Sequences (5 to 3 ) Forward Reverse 18s AAACGGCTACCACATCCAAG CCTCCAATGGATCCTCGTTA 36b4 GTTCTTGCCCATCAGCACC AGATGCAGCAGATCCGCAT Acc1
Supplementary Information
 Supplementary Information Akt regulates hepatic metabolism by suppressing a Foxo1 dependent global inhibition of adaptation to nutrient intake Mingjian Lu 1, Min Wan 1, Karla F. Leavens 1, Qingwei Chu
Supplementary Information Akt regulates hepatic metabolism by suppressing a Foxo1 dependent global inhibition of adaptation to nutrient intake Mingjian Lu 1, Min Wan 1, Karla F. Leavens 1, Qingwei Chu
Supplementary Information
 Supplementary Information Overexpression of Fto leads to increased food intake and results in obesity Chris Church, Lee Moir, Fiona McMurray, Christophe Girard, Gareth T Banks, Lydia Teboul, Sara Wells,
Supplementary Information Overexpression of Fto leads to increased food intake and results in obesity Chris Church, Lee Moir, Fiona McMurray, Christophe Girard, Gareth T Banks, Lydia Teboul, Sara Wells,
Expanded View Figures
 Expanded View Figures A B C D E F G H I J K L Figure EV1. The dysregulated lipid metabolic phenotype of mouse models of metabolic dysfunction is most pronounced in the fasted state. A L Male 12-weeks-old
Expanded View Figures A B C D E F G H I J K L Figure EV1. The dysregulated lipid metabolic phenotype of mouse models of metabolic dysfunction is most pronounced in the fasted state. A L Male 12-weeks-old
Supplemental Information Supplementary Table 1. Tph1+/+ Tph1 / Analyte Supplementary Table 2. Tissue Vehicle LP value
 Supplemental Information Supplementary Table. Urinary and adipose tissue catecholamines in Tph +/+ and Tph / mice fed a high fat diet for weeks. Tph +/+ Tph / Analyte ewat ibat ewat ibat Urine (ng/ml)
Supplemental Information Supplementary Table. Urinary and adipose tissue catecholamines in Tph +/+ and Tph / mice fed a high fat diet for weeks. Tph +/+ Tph / Analyte ewat ibat ewat ibat Urine (ng/ml)
Fig. S1. REGN1500 reduces plasma levels of cholesterol, TG and NEFA in WT and Ldlr -/- mice. (A) WT
 Figure Legends for Supplementary Figures. Fig. S1. REGN15 reduces plasma levels of cholesterol, TG and NEF in WT and Ldlr -/- mice. () WT and Ldlr -/- mice were injected with control IgG or REGN15 (1 mg/kg)
Figure Legends for Supplementary Figures. Fig. S1. REGN15 reduces plasma levels of cholesterol, TG and NEF in WT and Ldlr -/- mice. () WT and Ldlr -/- mice were injected with control IgG or REGN15 (1 mg/kg)
Reviewer #1 (Remarks to the Author)
 Reviewer #1 (Remarks to the Author) The authors provide an interesting data set concerning the effects of an ATGL inhibitor on energy balance and indices of insulin action in mice fed a high fat diet.
Reviewer #1 (Remarks to the Author) The authors provide an interesting data set concerning the effects of an ATGL inhibitor on energy balance and indices of insulin action in mice fed a high fat diet.
SUPPLEMENTARY INFORMATION
 SUPPLEMENTARY INFORMATION FOR Liver X Receptor α mediates hepatic triglyceride accumulation through upregulation of G0/G1 Switch Gene 2 (G0S2) expression I: SUPPLEMENTARY METHODS II: SUPPLEMENTARY FIGURES
SUPPLEMENTARY INFORMATION FOR Liver X Receptor α mediates hepatic triglyceride accumulation through upregulation of G0/G1 Switch Gene 2 (G0S2) expression I: SUPPLEMENTARY METHODS II: SUPPLEMENTARY FIGURES
Supporting Information Table of content
 Supporting Information Table of content Supporting Information Fig. S1 Supporting Information Fig. S2 Supporting Information Fig. S3 Supporting Information Fig. S4 Supporting Information Fig. S5 Supporting
Supporting Information Table of content Supporting Information Fig. S1 Supporting Information Fig. S2 Supporting Information Fig. S3 Supporting Information Fig. S4 Supporting Information Fig. S5 Supporting
FH- FH+ DM. 52 Volunteers. Oral & IV Glucose Tolerance Test Hyperinsulinemic Euglycemic Clamp in Non-DM Subjects ACADSB MYSM1. Mouse Skeletal Muscle
 A 52 Volunteers B 6 5 4 3 2 FH- FH+ DM 1 Oral & IV Glucose Tolerance Test Hyperinsulinemic Euglycemic Clamp in Non-DM Subjects ZYX EGR2 NR4A1 SRF target TPM1 ACADSB MYSM1 Non SRF target FH- FH+ DM2 C SRF
A 52 Volunteers B 6 5 4 3 2 FH- FH+ DM 1 Oral & IV Glucose Tolerance Test Hyperinsulinemic Euglycemic Clamp in Non-DM Subjects ZYX EGR2 NR4A1 SRF target TPM1 ACADSB MYSM1 Non SRF target FH- FH+ DM2 C SRF
Inflammasome-mediated caspase-1 activity Gatekeeper of inflammation in the adipose tissue. Rinke Stienstra
 Inflammasome-mediated caspase-1 activity Gatekeeper of inflammation in the adipose tissue Rinke Stienstra Obesity promotes the development of insulin resistance and type 2 diabetes County-level Estimates
Inflammasome-mediated caspase-1 activity Gatekeeper of inflammation in the adipose tissue Rinke Stienstra Obesity promotes the development of insulin resistance and type 2 diabetes County-level Estimates
SUPPLEMENTARY DATA. Nature Medicine: doi: /nm.4171
 SUPPLEMENTARY DATA Supplementary Figure 1 a b c PF %Change - -4-6 Body weight Lean mass Body fat Tissue weight (g).4.3.2.1. PF GC iwat awat BAT PF d e f g week 2 week 3 NEFA (mmol/l) 1..5. PF phsl (Ser565)
SUPPLEMENTARY DATA Supplementary Figure 1 a b c PF %Change - -4-6 Body weight Lean mass Body fat Tissue weight (g).4.3.2.1. PF GC iwat awat BAT PF d e f g week 2 week 3 NEFA (mmol/l) 1..5. PF phsl (Ser565)
1Why lipids cannot be transported in blood alone? 2How we transport Fatty acids and steroid hormones?
 1Why lipids cannot be transported in blood alone? 2How we transport Fatty acids and steroid hormones? 3How are dietary lipids transported? 4How lipids synthesized in the liver are transported? 5 Lipoprotien
1Why lipids cannot be transported in blood alone? 2How we transport Fatty acids and steroid hormones? 3How are dietary lipids transported? 4How lipids synthesized in the liver are transported? 5 Lipoprotien
Control 7 d cold 7 d CL
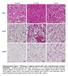 Control 7 d cold 7 d ibat iwat gwat Supplementary Figure 1. Histology of adipose tissues after cold or 3-adrenergic receptor stimulation. C57BL/6J wild-type mice were housed at 4 C or injected daily with
Control 7 d cold 7 d ibat iwat gwat Supplementary Figure 1. Histology of adipose tissues after cold or 3-adrenergic receptor stimulation. C57BL/6J wild-type mice were housed at 4 C or injected daily with
Supplemental Table 1. Plasma NEFA and liver triglyceride levels in ap2-hif1ako and ap2-hif2ako mice under control and high fat diets.
 Supplemental Table 1. Plasma NEFA and liver triglyceride levels in Hif1aKO and Hif2aKO mice under control and high fat diets. Hif1a (n=6) Hif1aK O (n=6) Hif2a Hif2aK O Hif1a (n=5) Hif1aKO (n=5) Hif2a Hif2aK
Supplemental Table 1. Plasma NEFA and liver triglyceride levels in Hif1aKO and Hif2aKO mice under control and high fat diets. Hif1a (n=6) Hif1aK O (n=6) Hif2a Hif2aK O Hif1a (n=5) Hif1aKO (n=5) Hif2a Hif2aK
The antiparasitic drug ivermectin is a novel FXR ligand that regulates metabolism
 Supplementary Information The antiparasitic drug ivermectin is a novel FXR ligand that regulates metabolism Address correspondence to Yong Li (yongli@xmu.edu.cn, Tel: 86-592-218151) GW464 CDCA Supplementary
Supplementary Information The antiparasitic drug ivermectin is a novel FXR ligand that regulates metabolism Address correspondence to Yong Li (yongli@xmu.edu.cn, Tel: 86-592-218151) GW464 CDCA Supplementary
Up-Regulation of Mitochondrial Activity and Acquirement of Brown Adipose Tissue-Like Property in the White Adipose Tissue of Fsp27 Deficient Mice
 Up-Regulation of Mitochondrial Activity and Acquirement of Brown Adipose Tissue-Like Property in the White Adipose Tissue of Fsp27 Deficient Mice Shen Yon Toh 1,2,3., Jingyi Gong 2., Guoli Du 2., John
Up-Regulation of Mitochondrial Activity and Acquirement of Brown Adipose Tissue-Like Property in the White Adipose Tissue of Fsp27 Deficient Mice Shen Yon Toh 1,2,3., Jingyi Gong 2., Guoli Du 2., John
Metabolic defects underlying dyslipidemia in abdominal obesity
 Metabolic defects underlying dyslipidemia in abdominal obesity Professor Marja-Riitta Taskinen Department of Medicine, Division of Cardiology Helsinki University Hospital, Finland Disclosures: Honorariums/
Metabolic defects underlying dyslipidemia in abdominal obesity Professor Marja-Riitta Taskinen Department of Medicine, Division of Cardiology Helsinki University Hospital, Finland Disclosures: Honorariums/
A Central Role of MG53 in Metabolic Syndrome. and Type-2 Diabetes
 A Central Role of MG53 in Metabolic Syndrome and Type-2 Diabetes Yan Zhang, Chunmei Cao, Rui-Ping Xiao Institute of Molecular Medicine (IMM) Peking University, Beijing, China Accelerated Aging in China
A Central Role of MG53 in Metabolic Syndrome and Type-2 Diabetes Yan Zhang, Chunmei Cao, Rui-Ping Xiao Institute of Molecular Medicine (IMM) Peking University, Beijing, China Accelerated Aging in China
Supplemental methods. Total RNA was extracted from the stomach, liver, pancreas, pituitary, and
 Supplemental methods Real-time quantitative RT-PCR and Semi-quantitative PCR Total RNA was extracted from the stomach, liver, pancreas, pituitary, and hypothalamus as previously described (). Primers and
Supplemental methods Real-time quantitative RT-PCR and Semi-quantitative PCR Total RNA was extracted from the stomach, liver, pancreas, pituitary, and hypothalamus as previously described (). Primers and
Effect of BI-1 on insulin resistance through regulation of CYP2E1
 Effect of BI-1 on insulin resistance through regulation of CYP2E1 Geum-Hwa Lee 1, Kyoung-Jin Oh 2, 3, Hyung-Ryong Kim 4, Hye-Sook Han 2, Hwa-Young Lee 1, Keun-Gyu Park 5, Ki-Hoan Nam 6, Seung-Hoi Koo 2
Effect of BI-1 on insulin resistance through regulation of CYP2E1 Geum-Hwa Lee 1, Kyoung-Jin Oh 2, 3, Hyung-Ryong Kim 4, Hye-Sook Han 2, Hwa-Young Lee 1, Keun-Gyu Park 5, Ki-Hoan Nam 6, Seung-Hoi Koo 2
Cornstarch
 Electronic Supplementary Material (ESI) for Food & Function. This journal is The Royal Society of Chemistry 2018 Supplementary data : Supplementary Table 1: Diet composition (g/kg) on the basis of the
Electronic Supplementary Material (ESI) for Food & Function. This journal is The Royal Society of Chemistry 2018 Supplementary data : Supplementary Table 1: Diet composition (g/kg) on the basis of the
Pathogenesis of Diabetes Mellitus
 Pathogenesis of Diabetes Mellitus Young-Bum Kim, Ph.D. Associate Professor of Medicine Harvard Medical School Definition of Diabetes Mellitus a group of metabolic diseases characterized by hyperglycemia
Pathogenesis of Diabetes Mellitus Young-Bum Kim, Ph.D. Associate Professor of Medicine Harvard Medical School Definition of Diabetes Mellitus a group of metabolic diseases characterized by hyperglycemia
Leptin Intro/Signaling. ATeamP: Angelo, Anthony, Charlie, Gabby, Joseph
 Leptin Intro/Signaling ATeamP: Angelo, Anthony, Charlie, Gabby, Joseph Overview Intro to Leptin Definition & Sources Physiology Bound vs. Free Receptors Signaling JAK/STAT MAPK PI3K ACC Experimental findings
Leptin Intro/Signaling ATeamP: Angelo, Anthony, Charlie, Gabby, Joseph Overview Intro to Leptin Definition & Sources Physiology Bound vs. Free Receptors Signaling JAK/STAT MAPK PI3K ACC Experimental findings
PKCδ regulates hepatic insulin sensitivity and hepatosteatosis in mice and humans
 Research article PKCδ regulates hepatic insulin sensitivity and hepatosteatosis in mice and humans Olivier Bezy, 1 Thien T. Tran, 1 Jussi Pihlajamäki, 2 Ryo Suzuki, 1 Brice Emanuelli, 1 Jonathan Winnay,
Research article PKCδ regulates hepatic insulin sensitivity and hepatosteatosis in mice and humans Olivier Bezy, 1 Thien T. Tran, 1 Jussi Pihlajamäki, 2 Ryo Suzuki, 1 Brice Emanuelli, 1 Jonathan Winnay,
Plasma lipoproteins & atherosclerosis by. Prof.Dr. Maha M. Sallam
 Biochemistry Department Plasma lipoproteins & atherosclerosis by Prof.Dr. Maha M. Sallam 1 1. Recognize structures,types and role of lipoproteins in blood (Chylomicrons, VLDL, LDL and HDL). 2. Explain
Biochemistry Department Plasma lipoproteins & atherosclerosis by Prof.Dr. Maha M. Sallam 1 1. Recognize structures,types and role of lipoproteins in blood (Chylomicrons, VLDL, LDL and HDL). 2. Explain
Effects of growth hormone secretagogue receptor agonist and antagonist in nonobese type 2 diabetic MKR mice
 Effects of growth hormone secretagogue receptor agonist and antagonist in nonobese type 2 diabetic MKR mice Rasha Mosa (MBCHC, M.D, PhD candidate) School of Biomedical Sciences University of Queensland
Effects of growth hormone secretagogue receptor agonist and antagonist in nonobese type 2 diabetic MKR mice Rasha Mosa (MBCHC, M.D, PhD candidate) School of Biomedical Sciences University of Queensland
HIV VPR alters fat metabolism. Dorothy E Lewis PhD/Ashok Balasubramanyam MD
 HIV VPR alters fat metabolism Dorothy E Lewis PhD/Ashok Balasubramanyam MD Old Dogma for HIV associated lipodystrophy Differentiation Block (PI) Lipoatrophy Apoptosis (NRTI) Stem cell Preadipocyte Adipocyte
HIV VPR alters fat metabolism Dorothy E Lewis PhD/Ashok Balasubramanyam MD Old Dogma for HIV associated lipodystrophy Differentiation Block (PI) Lipoatrophy Apoptosis (NRTI) Stem cell Preadipocyte Adipocyte
Supplementary Materials for
 www.sciencetranslationalmedicine.org/cgi/content/full/5/213/213ra164/dc1 Supplementary Materials for HIV-1 Vpr Induces Adipose Dysfunction in Vivo Through Reciprocal Effects on PPAR/GR Co-Regulation Neeti
www.sciencetranslationalmedicine.org/cgi/content/full/5/213/213ra164/dc1 Supplementary Materials for HIV-1 Vpr Induces Adipose Dysfunction in Vivo Through Reciprocal Effects on PPAR/GR Co-Regulation Neeti
Adipose tissue dysfunction in obesity. Gijs Goossens, PhD
 Adipose tissue dysfunction in obesity -The role of adipose tissue oxygenation - Gijs Goossens, PhD NUTRIM School of Nutrition and Translational Research in Metabolism Maastricht University Medical Centre
Adipose tissue dysfunction in obesity -The role of adipose tissue oxygenation - Gijs Goossens, PhD NUTRIM School of Nutrition and Translational Research in Metabolism Maastricht University Medical Centre
Male 30. Female. Body weight (g) Age (weeks) Age (weeks) Atg7 f/f Atg7 ΔCD11c
 ody weight (g) ody weight (g) 34 3 Male 3 27 Female 26 24 22 18 7 9 11 13 15 17 19 21 23 21 18 15 7 9 11 13 15 17 19 21 23 Age (weeks) Age (weeks) Supplementary Figure 1. Lean phenotypes in mice regardless
ody weight (g) ody weight (g) 34 3 Male 3 27 Female 26 24 22 18 7 9 11 13 15 17 19 21 23 21 18 15 7 9 11 13 15 17 19 21 23 Age (weeks) Age (weeks) Supplementary Figure 1. Lean phenotypes in mice regardless
Supporting Information. Supporting Tables. S-Table 1 Primer pairs for RT-PCR. Product size. Gene Primer pairs
 Supporting Information Supporting Tables S-Table 1 Primer pairs for RT-PCR. Gene Primer pairs Product size (bp) FAS F: 5 TCTTGGAAGCGATGGGTA 3 429 R: 5 GGGATGTATCATTCTTGGAC 3 SREBP-1c F: 5 CGCTACCGTTCCTCTATCA
Supporting Information Supporting Tables S-Table 1 Primer pairs for RT-PCR. Gene Primer pairs Product size (bp) FAS F: 5 TCTTGGAAGCGATGGGTA 3 429 R: 5 GGGATGTATCATTCTTGGAC 3 SREBP-1c F: 5 CGCTACCGTTCCTCTATCA
Leptin deficiency suppresses progression of atherosclerosis in apoe-deficient mice
 Leptin deficiency suppresses progression of atherosclerosis in apoe-deficient mice Atherosclerosis, 2007 Chiba T, Shinozaki S, Nakazawa T, et al. Present by Sudaporn Pummoung Apolipoprotein E (apoe( apoe)
Leptin deficiency suppresses progression of atherosclerosis in apoe-deficient mice Atherosclerosis, 2007 Chiba T, Shinozaki S, Nakazawa T, et al. Present by Sudaporn Pummoung Apolipoprotein E (apoe( apoe)
FOR REVIEW. BMB Reports - Manuscript Submission. Manuscript Draft. Manuscript Number: BMB
 BMB Reports - Manuscript Submission Manuscript Draft Manuscript Number: BMB-18-095 Title: Insulin Receptor Substrate 2:A Bridge between Hippo and AKT Pathways Article Type: Perspective (Invited Only) Keywords:
BMB Reports - Manuscript Submission Manuscript Draft Manuscript Number: BMB-18-095 Title: Insulin Receptor Substrate 2:A Bridge between Hippo and AKT Pathways Article Type: Perspective (Invited Only) Keywords:
Implications of mitochondrial skeletal muscle metabolism on diabetes and obesity before and after weight loss
 GG2 Implications of mitochondrial skeletal muscle metabolism on diabetes and obesity before and after weight loss Dr Giacomo Gastaldi CHRU Montpellier Folie 1 GG2 19.10.2009 GG_PC; 12.10.2009 Plan Introduction
GG2 Implications of mitochondrial skeletal muscle metabolism on diabetes and obesity before and after weight loss Dr Giacomo Gastaldi CHRU Montpellier Folie 1 GG2 19.10.2009 GG_PC; 12.10.2009 Plan Introduction
ZL ZDF ZDF + E2 *** Visceral (g) ZDF
 Body Weight (g) 4 3 2 1 ** * ZL ZDF 6 8 1 12 14 16 Age (weeks) B * Sub-cutaneous (g) 16 12 8 4 ZL ZDF Visceral (g) 25 2 15 1 5 ZL ZDF Total fat pad weight (g) 4 3 2 1 ZDF ZL Supplemental Figure 1: Effect
Body Weight (g) 4 3 2 1 ** * ZL ZDF 6 8 1 12 14 16 Age (weeks) B * Sub-cutaneous (g) 16 12 8 4 ZL ZDF Visceral (g) 25 2 15 1 5 ZL ZDF Total fat pad weight (g) 4 3 2 1 ZDF ZL Supplemental Figure 1: Effect
Niacin Metabolism: Effects on Cholesterol
 Niacin Metabolism: Effects on Cholesterol By Julianne R. Edwards For Dr. William R. Proulx, PhD, RD Associate Professor of Nutrition and Dietetics In partial fulfillments for the requirements of NUTR342
Niacin Metabolism: Effects on Cholesterol By Julianne R. Edwards For Dr. William R. Proulx, PhD, RD Associate Professor of Nutrition and Dietetics In partial fulfillments for the requirements of NUTR342
ab Adipogenesis Assay Kit (Cell-Based)
 ab133102 Adipogenesis Assay Kit (Cell-Based) Instructions for Use For the study of induction and inhibition of adipogenesis in adherent cells. This product is for research use only and is not intended
ab133102 Adipogenesis Assay Kit (Cell-Based) Instructions for Use For the study of induction and inhibition of adipogenesis in adherent cells. This product is for research use only and is not intended
THE GLUCOSE-FATTY ACID-KETONE BODY CYCLE Role of ketone bodies as respiratory substrates and metabolic signals
 Br. J. Anaesth. (1981), 53, 131 THE GLUCOSE-FATTY ACID-KETONE BODY CYCLE Role of ketone bodies as respiratory substrates and metabolic signals J. C. STANLEY In this paper, the glucose-fatty acid cycle
Br. J. Anaesth. (1981), 53, 131 THE GLUCOSE-FATTY ACID-KETONE BODY CYCLE Role of ketone bodies as respiratory substrates and metabolic signals J. C. STANLEY In this paper, the glucose-fatty acid cycle
LIPOCALIN 2 DEFICIENCY INFLUENCES TRANSFORMING GROWTH FACTOR-β EFFECT ON INFLAMMATION AND EXTRACELLULAR MATRIX REMODELING IN INGUINAL ADIPOCYTES
 LIPOCALIN 2 DEFICIENCY INFLUENCES TRANSFORMING GROWTH FACTOR-β EFFECT ON INFLAMMATION AND EXTRACELLULAR MATRIX REMODELING IN INGUINAL ADIPOCYTES A THESIS SUBMITTED TO THE FACULTY OF THE UNIVERSITY OF MINNESOTA
LIPOCALIN 2 DEFICIENCY INFLUENCES TRANSFORMING GROWTH FACTOR-β EFFECT ON INFLAMMATION AND EXTRACELLULAR MATRIX REMODELING IN INGUINAL ADIPOCYTES A THESIS SUBMITTED TO THE FACULTY OF THE UNIVERSITY OF MINNESOTA
TBP (H) CACAGTGAATCTTGGTTGTAAACTTGA AAACCGCTTGGGATTATATTCG ANGPTL8 (H) CTGGGCCCTGCCTACCGAGA CCGATGCTGCTGTGCCACCA [1]
![TBP (H) CACAGTGAATCTTGGTTGTAAACTTGA AAACCGCTTGGGATTATATTCG ANGPTL8 (H) CTGGGCCCTGCCTACCGAGA CCGATGCTGCTGTGCCACCA [1] TBP (H) CACAGTGAATCTTGGTTGTAAACTTGA AAACCGCTTGGGATTATATTCG ANGPTL8 (H) CTGGGCCCTGCCTACCGAGA CCGATGCTGCTGTGCCACCA [1]](/thumbs/89/99233308.jpg) ESM Table 1. Immunoblot antibodies. Primary Supplier Dilution Antibody Akt Cell Signaling 1:1000 Technology Phosphorylated Cell Signaling 1:1000 Akt (Ser 473) Technology PKCε Cell Signaling 1:1000 Technology
ESM Table 1. Immunoblot antibodies. Primary Supplier Dilution Antibody Akt Cell Signaling 1:1000 Technology Phosphorylated Cell Signaling 1:1000 Akt (Ser 473) Technology PKCε Cell Signaling 1:1000 Technology
Protection against doxorubicin-induced myocardial dysfunction in mice by cardiac-specific expression of carboxyl terminus of hsp70-interacting protein
 Protection against doxorubicin-induced myocardial dysfunction in mice by cardiac-specific expression of carboxyl terminus of hsp70-interacting protein Lei Wang 1, Tian-Peng Zhang 1, Yuan Zhang 2, Hai-Lian
Protection against doxorubicin-induced myocardial dysfunction in mice by cardiac-specific expression of carboxyl terminus of hsp70-interacting protein Lei Wang 1, Tian-Peng Zhang 1, Yuan Zhang 2, Hai-Lian
Chapter 2. The Physiology of Fat
 Chapter 2 The Physiology of Fat Obesity, generalized and localized collections of adiposity, has become endemic in the United States. It is estimated that 25-40% of US adult females and 20-25% of adult
Chapter 2 The Physiology of Fat Obesity, generalized and localized collections of adiposity, has become endemic in the United States. It is estimated that 25-40% of US adult females and 20-25% of adult
Regulation of Lipid Homeostasis: Lipid Droplets
 Regulation of Lipid Homeostasis: Lipid Droplets Bernd Helms Article Brand Recter Hendrik Mertens 1 The Basics I: FAs The Basics II: FA Activation 2 Basics III: TG-FA Interplay. Why? Adipocytes 3 Foam cells
Regulation of Lipid Homeostasis: Lipid Droplets Bernd Helms Article Brand Recter Hendrik Mertens 1 The Basics I: FAs The Basics II: FA Activation 2 Basics III: TG-FA Interplay. Why? Adipocytes 3 Foam cells
Supplementary Figures
 Supplementary Figures mir-150 regulates obesityassociated insulin resistance by controlling B cell functions Wei Ying, Alexander Tseng, Richard Cheng-An Chang, Haiqing Wang, Yu-lieh Lin, Srikanth Kanameni,
Supplementary Figures mir-150 regulates obesityassociated insulin resistance by controlling B cell functions Wei Ying, Alexander Tseng, Richard Cheng-An Chang, Haiqing Wang, Yu-lieh Lin, Srikanth Kanameni,
IL METABOLISMO EPATICO DEI CARBOIDRATI IN FISIOLOGIA E PATOLOGIA
 UNIGASTRO Il fegato come centrale metabolica e i fattori di danno oltre ai virus epatitici IL METABOLISMO EPATICO DEI CARBOIDRATI IN FISIOLOGIA E PATOLOGIA Dr Elisabetta Bugianesi Divisione di Gastro-Epatologia
UNIGASTRO Il fegato come centrale metabolica e i fattori di danno oltre ai virus epatitici IL METABOLISMO EPATICO DEI CARBOIDRATI IN FISIOLOGIA E PATOLOGIA Dr Elisabetta Bugianesi Divisione di Gastro-Epatologia
Energy metabolism - the overview
 Energy metabolism - the overview Josef Fontana EC - 40 Overview of the lecture Important terms of the energy metabolism The overview of the energy metabolism The main pathways of the energy metabolism
Energy metabolism - the overview Josef Fontana EC - 40 Overview of the lecture Important terms of the energy metabolism The overview of the energy metabolism The main pathways of the energy metabolism
Development of an RNA Interference Therapeutic Targeting Angiopoietin-Like Protein 3 for Treatment of Hyperlipidemia
 Development of an RNA Interference Therapeutic Targeting Angiopoietin-Like Protein 3 for Treatment of Hyperlipidemia November 12 th, 2018 So C. Wong Arrowhead Pharmaceuticals Inc. Disclosures All authors
Development of an RNA Interference Therapeutic Targeting Angiopoietin-Like Protein 3 for Treatment of Hyperlipidemia November 12 th, 2018 So C. Wong Arrowhead Pharmaceuticals Inc. Disclosures All authors
Metabolic integration and Regulation
 Metabolic integration and Regulation 109700: Graduate Biochemistry Trimester 2/2016 Assistant Prof. Dr. Panida Khunkaewla kpanida@sut.ac.th School of Chemistry Suranaree University of Technology 1 Overview
Metabolic integration and Regulation 109700: Graduate Biochemistry Trimester 2/2016 Assistant Prof. Dr. Panida Khunkaewla kpanida@sut.ac.th School of Chemistry Suranaree University of Technology 1 Overview
Supplementary Figure 1: Additional metabolic parameters of obesity mouse models and controls. (a) Body weight, (b) blood glucose and (c) insulin
 Supplementary Figure 1: Additional metabolic parameters of obesity mouse models and controls. (a) Body weight, (b) blood glucose and (c) insulin resistance index of homeostatic model assessment (HOMA IR)
Supplementary Figure 1: Additional metabolic parameters of obesity mouse models and controls. (a) Body weight, (b) blood glucose and (c) insulin resistance index of homeostatic model assessment (HOMA IR)
Supplementary Information. MicroRNA-33b knock-in mice for an intron of sterol regulatory
 Supplementary Information MicroRNA-33b knock-in mice for an intron of sterol regulatory element-binding factor 1 (Srebf1) exhibit reduced HDL-C in vivo Takahiro Horie, Tomohiro Nishino, Osamu Baba, Yasuhide
Supplementary Information MicroRNA-33b knock-in mice for an intron of sterol regulatory element-binding factor 1 (Srebf1) exhibit reduced HDL-C in vivo Takahiro Horie, Tomohiro Nishino, Osamu Baba, Yasuhide
Figure S1. Generation of inducible PTEN deficient mice and the BMMCs (A) B6.129 Pten loxp/loxp mice were mated with B6.
 Figure S1. Generation of inducible PTEN deficient mice and the BMMCs (A) B6.129 Pten loxp/loxp mice were mated with B6.129-Gt(ROSA)26Sor tm1(cre/ert2)tyj /J mice. To induce deletion of the Pten locus,
Figure S1. Generation of inducible PTEN deficient mice and the BMMCs (A) B6.129 Pten loxp/loxp mice were mated with B6.129-Gt(ROSA)26Sor tm1(cre/ert2)tyj /J mice. To induce deletion of the Pten locus,
A 10.8 kb ghr fragment containing exons 4 and 5 was PCR amplified with Pfu Turbo. DNA polymerase (Stratagene) and cloned into pcr Blunt II-TOPO vector
 Supplemental Method: Generation of a conditional GHR knockout mice. A 1.8 kb ghr fragment containing exons 4 and 5 was PCR amplified with Pfu Turbo DNA polymerase (Stratagene) and cloned into pcr Blunt
Supplemental Method: Generation of a conditional GHR knockout mice. A 1.8 kb ghr fragment containing exons 4 and 5 was PCR amplified with Pfu Turbo DNA polymerase (Stratagene) and cloned into pcr Blunt
Supplementary Figure 1: Hsp60 / IEC mice are embryonically lethal (A) Light microscopic pictures show mouse embryos at developmental stage E12.
 Supplementary Figure 1: Hsp60 / IEC mice are embryonically lethal (A) Light microscopic pictures show mouse embryos at developmental stage E12.5 and E13.5 prepared from uteri of dams and subsequently genotyped.
Supplementary Figure 1: Hsp60 / IEC mice are embryonically lethal (A) Light microscopic pictures show mouse embryos at developmental stage E12.5 and E13.5 prepared from uteri of dams and subsequently genotyped.
Master class Biomolecular Sciences Molecular Cell Biology.
 Master class Biomolecular Sciences Molecular Cell Biology. 04-09-08: Paul van Bergen en Henegouwen. Clathrin-indep Endocytosis 11-09-08: Willem Stoorvogel. Endocytosis and MHC classii 18-09-08: X-track
Master class Biomolecular Sciences Molecular Cell Biology. 04-09-08: Paul van Bergen en Henegouwen. Clathrin-indep Endocytosis 11-09-08: Willem Stoorvogel. Endocytosis and MHC classii 18-09-08: X-track
Effects of sitagliptin on cardiac metabolism in mice
 Effects of sitagliptin on cardiac metabolism in mice M. Lenski, J.-C. Reil, M. Böhm, U. Laufs Saarland University Hospital Department of Internal Medicine III, Cardiology Homburg - Germany Disclosures
Effects of sitagliptin on cardiac metabolism in mice M. Lenski, J.-C. Reil, M. Böhm, U. Laufs Saarland University Hospital Department of Internal Medicine III, Cardiology Homburg - Germany Disclosures
Fructose in Insulin Resistance- Focused on Diabetes 순천향대학교부천병원 내분비내과 정찬희
 Fructose in Insulin Resistance- Focused on Diabetes 순천향대학교부천병원 내분비내과 정찬희 Introduction Unique characteristics of Fructose Metabolism Mechanism for Fructose-Induced Insulin Resistance Epidemiological Studies
Fructose in Insulin Resistance- Focused on Diabetes 순천향대학교부천병원 내분비내과 정찬희 Introduction Unique characteristics of Fructose Metabolism Mechanism for Fructose-Induced Insulin Resistance Epidemiological Studies
Altered Mouse Adipose Tissue IGF-1 Expression Influences Glucose Control
 Altered Mouse Adipose Tissue IGF-1 Expression Influences Glucose Control Jan Trost Prof. Gudrun A. Brockmann Humboldt Universität zu Berlin Department of Crop and Animal Sciences Breeding Biology and Molecular
Altered Mouse Adipose Tissue IGF-1 Expression Influences Glucose Control Jan Trost Prof. Gudrun A. Brockmann Humboldt Universität zu Berlin Department of Crop and Animal Sciences Breeding Biology and Molecular
SUPPLEMENTARY INFORMATION
 doi: 1.138/nature7221 Brown fat selective genes 12 1 Control Q-RT-PCR (% of Control) 8 6 4 2 Ntrk3 Cox7a1 Cox8b Cox5b ATPase b2 ATPase f1a1 Sirt3 ERRα Elovl3/Cig3 PPARα Zic1 Supplementary Figure S1. stimulates
doi: 1.138/nature7221 Brown fat selective genes 12 1 Control Q-RT-PCR (% of Control) 8 6 4 2 Ntrk3 Cox7a1 Cox8b Cox5b ATPase b2 ATPase f1a1 Sirt3 ERRα Elovl3/Cig3 PPARα Zic1 Supplementary Figure S1. stimulates
Dietary α-linolenic acid-rich flaxseed oil prevents against alcoholic hepatic steatosis
 Dietary α-linolenic acid-rich flaxseed oil prevents against alcoholic hepatic steatosis via ameliorating lipid homeostasis at adipose tissue-liver axis in mice Meng Wang a, Xiao-Jing Zhang a, Kun Feng
Dietary α-linolenic acid-rich flaxseed oil prevents against alcoholic hepatic steatosis via ameliorating lipid homeostasis at adipose tissue-liver axis in mice Meng Wang a, Xiao-Jing Zhang a, Kun Feng
Ct=28.4 WAT 92.6% Hepatic CE (mg/g) P=3.6x10-08 Plasma Cholesterol (mg/dl)
 a Control AAV mtm6sf-shrna8 Ct=4.3 Ct=8.4 Ct=8.8 Ct=8.9 Ct=.8 Ct=.5 Relative TM6SF mrna Level P=.5 X -5 b.5 Liver WAT Small intestine Relative TM6SF mrna Level..5 9.6% Control AAV mtm6sf-shrna mtm6sf-shrna6
a Control AAV mtm6sf-shrna8 Ct=4.3 Ct=8.4 Ct=8.8 Ct=8.9 Ct=.8 Ct=.5 Relative TM6SF mrna Level P=.5 X -5 b.5 Liver WAT Small intestine Relative TM6SF mrna Level..5 9.6% Control AAV mtm6sf-shrna mtm6sf-shrna6
Insulin Resistance. Biol 405 Molecular Medicine
 Insulin Resistance Biol 405 Molecular Medicine Insulin resistance: a subnormal biological response to insulin. Defects of either insulin secretion or insulin action can cause diabetes mellitus. Insulin-dependent
Insulin Resistance Biol 405 Molecular Medicine Insulin resistance: a subnormal biological response to insulin. Defects of either insulin secretion or insulin action can cause diabetes mellitus. Insulin-dependent
Supplementary Figure 1
 Supplementary Figure 1 a Percent of body weight! (%) 4! 3! 1! Epididymal fat Subcutaneous fat Liver SD Percent of body weight! (%) ** 3! 1! SD Percent of body weight! (%) 6! 4! SD ** b Blood glucose (mg/dl)!
Supplementary Figure 1 a Percent of body weight! (%) 4! 3! 1! Epididymal fat Subcutaneous fat Liver SD Percent of body weight! (%) ** 3! 1! SD Percent of body weight! (%) 6! 4! SD ** b Blood glucose (mg/dl)!
Modifications of Pyruvate Handling in Health and Disease Prof. Mary Sugden
 Modifications of Handling Modifications of Handling Centre for Diabetes and Metabolic Medicine Institute of Cell and Molecular Science Barts and the London School of Medicine and Dentistry 1 Potential
Modifications of Handling Modifications of Handling Centre for Diabetes and Metabolic Medicine Institute of Cell and Molecular Science Barts and the London School of Medicine and Dentistry 1 Potential
