MET inhibition overcomes radiation resistance of glioblastoma stem-like cells
|
|
|
- Matthew Bond
- 5 years ago
- Views:
Transcription
1 Published online: April 4, 26 Research Article MET inhibition overcomes radiation resistance of glioblastoma stem-like cells Francesca De Bacco, Antonio D Ambrosio,2, Elena Casanova, Francesca Orzan, Roberta Neggia, Raffaella Albano, Federica Verginelli, Manuela Cominelli 3, Pietro L Poliani 3, Paolo Luraghi, Gigliola Reato,2, Serena Pellegatta 4, Gaetano Finocchiaro 4, Timothy Perera 5, Elisabetta Garibaldi 6, Pietro Gabriele 6, Paolo M Comoglio,2, & Carla Boccaccio,2, Abstract Glioblastoma (GBM) contains stem-like cells (GSCs) known to be resistant to ionizing radiation and thus responsible for therapeutic failure and rapidly lethal tumor recurrence. It is known that GSC radioresistance relies on efficient activation of the DNA damage response, but the mechanisms linking this response with the stem status are still unclear. Here, we show that the MET receptor kinase, a functional marker of GSCs, is specifically expressed in a subset of radioresistant GSCs and overexpressed in human GBM recurring after radiotherapy. We elucidate that MET promotes GSC radioresistance through a novel mechanism, relying on AKT activity and leading to (i) sustained activation of Aurora kinase A, ATM kinase, and the downstream effectors of DNA repair, and (ii) phosphorylation and cytoplasmic retention of p2, which is associated with anti-apoptotic functions. We show that MET pharmacological inhibition causes DNA damage accumulation in irradiated GSCs and their depletion in vitro and in GBMs generated by GSC xenotransplantation. Preclinical evidence is thus provided that MET inhibitors can radiosensitize tumors and convert GSC-positive selection, induced by radiotherapy, into GSC eradication. Keywords glioblastoma; glioblastoma stem-like cells; MET oncogene; radiosensitization; radiotherapy Subject Categories Cancer; Neuroscience DOI.5252/emmm Received 6 October 25 Revised 26 February 26 Accepted 2 March 26 Published online 4 April 26 EMBO Mol Med (26) 8: Introduction Treatment of GBM, a rapidly lethal primary brain tumor, has little advanced in the last decade. Although the genetic and molecular features of the disease have been elucidated, the high degree of inter- and intratumor heterogeneity restrains the use of targeted therapies (Brennan et al, 23; Ene & Holland, 25). As a result, GBM standard of care is still based on maximal but often incomplete surgical resection, combined with chemoradiotherapy (Stupp et al, 29). The short-lived therapeutic response has been associated with the finding that ionizing radiations (IR) destroy the tumor bulk, but not GSCs, which drive tumor recurrence (Bao et al, 26). The higher radioresistance of GSCs, as compared with non-stem cells, correlates with hyperactivation of the DNA damage response (DDR), and escape from apoptosis (Bao et al, 26; Cheng et al, 2). Moreover, it has been envisaged that radioresistance can rely on signaling from tyrosine kinase receptors, such as the frequently amplified EGFR (Squatrito & Holland, 2), but the issue has not been addressed in GSCs. Recently, we and others showed that MET, the HGF receptor, sustains radioresistance of conventional cell lines (Lal et al, 25; De Bacco et al, 2). We then showed that MET is expressed in ~4% of GSC lineages, derived as neurospheres (i.e. in vitro cultures enriched in stem and progenitor cells) from GBM patients (De Bacco et al, 22). Interestingly, these MET-pos-NS usually lack EGFR amplification and display a mesenchymal or proneural transcriptional profile (De Bacco et al, 22), according to Verhaak et al (2). We also showed that, although clonal, MET-pos-NS contain cells expressing different levels of MET. The sorted MET high and MET neg subpopulations display opposite features, with MET high retaining GSC properties such as (i) long-term self-propagating and multi-potential differentiation ability in vitro; (ii) tumorigenic property in vivo; and (iii) ability to reconstitute a mixed MET high /MET neg Laboratory of Cancer Stem Cell Research, Candiolo Cancer Institute, FPO-IRCCS, Candiolo, Italy 2 Department of Oncology, University of Torino, Candiolo, Italy 3 Department of Molecular and Translational Medicine, Pathology Unit, University of Brescia, Brescia, Italy 4 Unit of Molecular Neuro-Oncology, Fondazione IRCCS Istituto Neurologico C. Besta, Milan, Italy 5 Octimet Oncology Ltd., Oxford, UK 6 Unit of Radiotherapy, Candiolo Cancer Institute, FPO-IRCCS, Candiolo, Italy Corresponding author. Tel: ; Fax: ; direzione.scientifica@ircc.it Corresponding author. Tel: ; Fax: ; carla.boccaccio@ircc.it 55 EMBO Molecular Medicine Vol 8 No5 26 ª 26 The Authors. Published under the terms of the CC BY 4. license
2 Published online: April 4, 26 Francesca De Bacco et al MET inhibition in glioblastoma stem-like cells EMBO Molecular Medicine population in vitro and in vivo. In contrast, the MET neg subpopulation lacks the above properties and displays prodifferentiating features (De Bacco et al, 22). Thus, we concluded that MET marks a cell subpopulation that regenerates self-renewing METpositive cells, and a progeny losing GSC properties along with MET expression. Finally, we showed that MET is a functional marker, as HGF sustains GSC self-renewal (De Bacco et al, 22). Accordingly, other studies showed that MET is a marker for prospective GSC isolation from patients (Joo et al, 22) and sustains the GSC phenotype by upregulating reprogramming transcription factors (Li et al, 2). On these premises, we investigated whether MET is a functional marker of GSC radioresistance, and whether MET could be exploited as a therapeutic target to achieve radiosensitization of GBM by depletion of its GSC component. Results Genetic and phenotypic characterization of a large panel of neurospheres and correlations with radioresistance We investigated whether patient-derived GSCs, propagated as NS, displayed greater radioresistance than non-stem/differentiated cells of the same lineage and whether radioresistance could be preferentially associated with specific genetic alterations or transcriptional profiles. A panel of 6 NS derived from primary GBM underwent analysis of the most frequent GBM genetic alterations such as EGFR amplification (EGFR amp ), PTEN inactivation by deletion or mutation (PTEN loss ), TP53 mutation, and NFKBIA deletion. NS were then classified as proneural, classical, or mesenchymal by transcriptional profiling, according to the Verhaak s signature (Verhaak et al, 2). A panel of 2 NS equally distributed among the three subtypes was chosen (Appendix Tables S and S2). The response of these NS to therapeutic doses of IR was assessed and compared with that of astrocytes (SVG, NHA) and GBM cell lines (U25, SNB9), and the same NS cultured in prodifferentiating conditions (Galli et al, 24; De Bacco et al, 22). In dose response and time course viability experiments, all NS invariably displayed higher radioresistance than astrocytes or GBM cell lines, or the NS pseudodifferentiated progeny (Figs A and B and EVA and Appendix Fig SA). No significant viability change was observed among different NS (Fig A), nor it was associated with any of the genetic alterations tested or any transcriptional subtype (Fig EVB and C). Next, NS radioresistance was correlated with the ability to evoke DDR. Phosphorylation of histone H2AX (ch2ax), a key step in the nucleation of DNA repair complex at double-strand breaks (DSBs), was measured by flow cytometry (Olive, 24). Early after irradiation, ch2ax levels were comparable in NS, astrocytes, GBM cell lines, and NS-derived pseudodifferentiated cells (~-fold over basal levels). Within 6 h, ch2ax resolution occurred in NS, indicating DSB repair, while ch2ax persisted, or even increased in the NSderived pseudodifferentiated cells, suggesting DSB persistence (Fig C and Appendix Fig SB). Consistently, comet assays showed that, while a low level of DNA damage was detectable in NS 24 h after irradiation, heavy damage remained in astrocytes, GBM cell lines, and, mainly, in the NS pseudodifferentiated progeny (Fig D). The greater ability of NS to repair DSBs, as compared with differentiated cells, was found to correlate with increased activation of two crucial DDR signal transducers: (i) ATM, the apical kinase unleashed by DNA damage sensors, responsible for H2AX phosphorylation (Shiloh & Ziv, 23); and (ii) the ATM substrate Chk2, whose activation correlates with GSC radioresistance (Bao et al, 26) (Fig E and Appendix Fig SC). Accordingly, the DNA recombinase RAD5, upregulated at the DDR cascade end and critical for homologous recombination repair (van Gent et al, 2), was significantly increased in NS, but not in their pseudodifferentiated progeny or astrocytes (protein: Fig E and Appendix Fig SC; mrna: Fig F). As a result, 24 h after irradiation, GBM cell lines or NS-derived pseudodifferentiated cells displayed cell cycle arrest and accumulation in G2-M phase, while NS resumed proliferation, as indicated by the increased number of S-phase cells (Appendix Fig SD F). Collectively, these findings indicate that GSCs activate DDR more rapidly and effectively than cells endowed with differentiated features, resulting in efficient DNA repair, survival, and proliferation after irradiation. The neurosphere GSC subpopulation is selected by irradiation By definition, NS are clones deriving from expansion of single selfrenewing GSCs. However, each NS entails a certain degree of phenotypic heterogeneity, which arises within the progeny of the founder GSC, and implies loss of stem properties, including radioresistance. We thus investigated whether, within the whole heterogeneous NS, the GSC subpopulation was more radioresistant than its non-stem progeny and whether it was positively selected by IR. It is known that stem-like cells from GBM and other tumors can be identified by retention of PKH-26 dye (Pece et al, 2; Richichi et al, 23). Figure. GSCs are radioresistant. A Cell viability of NS, GBM, and astrocyte cell lines measured 72 h after irradiation (fold versus non-irradiated cells, ). : one-way ANOVA, P =.. n = 2. B Cell viability of BT38NS and their pseudodifferentiated progeny (BT38 diff) measured 24 h after irradiation (fold versus non-irradiated cells, ). : t-test, P =.6. n = 2. C Flow cytometric analysis of phosphorylated histone H2AX (ch2ax) at the indicated time points after IR (, fold versus non-irradiated cells, ). : one-way ANOVA, P =.3. n = 2. D Neutral comet assay performed 24 h after IR (). Ctrl: non-irradiated cells. Comet tail s length is proportional to DSB extent. E Western blot showing phosphorylation of ATM (patm) and Chk2 (pchk2) and accumulation of RAD5 after IR (). Total ATM protein is also shown. Vinculin was used as a loading control. F qpcr of RAD5 expression measured in NS, BT38 diff, and astrocytes at the indicated time points after IR (, fold versus non-irradiated cells, ). n = 3. Data information: Data are represented as mean SEM. ª 26 The Authors EMBO Molecular Medicine Vol 8 No
3 Published online: April 4, 26 EMBO Molecular Medicine MET inhibition in glioblastoma stem-like cells Francesca De Bacco et al A viable cells (fold vs ) C relative γh2ax (fold vs ) Gy 35 BT38 NS 3 NHA U87MG 25 BT38 diff h U25 SNB9 SVG NHA BT273 BT275 BT287 BT32 BT38 BT34 BT328 BT334 BT337 BT347 BT358 BT37 BT373 BT398 BT42 BT437 BT443 BT452 BT463 astrocytes/lines NS D number of cells (%) B.4 viable cells (fold vs ) BT38 NS BT38 diff 2 5 Gy no tail short tail long tail BT38 NS BT38 diff NHA U87MG E BT38 NS BT38 diff NHA h patm ATM pchk2 RAD5 vinculin F 3 RAD5 expression (fold vs ) h 6 h 24 h BT38 NS BT463 NS BT452 NS BT25 NS BT38 diff NHA SVG Figure. 552 EMBO Molecular Medicine Vol 8 No 5 26 ª 26 The Authors
4 Published online: April 4, 26 Francesca De Bacco et al MET inhibition in glioblastoma stem-like cells EMBO Molecular Medicine A B C D E F G H Figure 2. ª 26 The Authors EMBO Molecular Medicine Vol 8 No
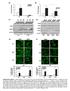
5 Published online: April 4, 26 EMBO Molecular Medicine MET inhibition in glioblastoma stem-like cells Francesca De Bacco et al Figure 2. GSCs are positively selected by irradiation. A Flow cytometric analysis of PKH-26 staining in irradiated BT38NS, 24 h after the last irradiation. The percentage of PKH-26 pos cells is indicated. Ctrl: non-irradiated cells. The mean fluorescence intensity (MFI) was as follow:, 58.2;. 4, 69.35; 2 Gy 3, 68.2;, B qpcr of SOX2 expression measured in NS 6 h after IR (, fold versus non-irradiated cells, ). : t-test, P <.2. n = 3. C LDA (sphere-forming) measuring the enrichment of GSCs after IR (). Ctrl: non-irradiated cells : v 2 test, P <.5. n = 2. D Schematic representation of serial xenotransplantation. Control (NS-) and irradiated (NS-IR) BT38NS were tested for tumor-initiating ability by serial subcutaneous injection of the indicated number of cells (p, n = 4; p2, n = 6; p3, n = 6 for each condition). E Tumor incidence in the LDA performed in p3 mice, as described in (D). F Histogram showing the in vivo GSC frequency measured by LDA in p3 tumors. GSC frequency (95% CI) was as follows: NS-, /258.2 ( ); NS-IR /22. ( ). : v 2 test, P =.6 4. n = 6. G LDA (sphere-forming) measuring the in vitro frequency of GSCs in cells derived from p3 tumors. : v 2 test, P =.6. n = 3. H LDA (sphere-forming) measuring the in vitro frequency of GSCs in cells derived from intracranial tumors generated by BT463NS and irradiated in vivo (2 Gy 3 days) (n = 3). Ctrl: non-irradiated tumors. : v 2 test, P = 2.4. Data information: Data are represented as mean CI in (C, G, H) or SEM in (B). A panel of NS was stained with PKH-26, showing dye retention in a variable percentage of cells in each NS (on average ~3%) (Fig 2A and Appendix Fig S2A). Next, NS were irradiated with a single () or an equivalent fractionated dose (. 4 days or 2 Gy 3 days). After irradiation, the percentage of PKH-26- retaining cells significantly increased, as well as their mean fluorescence intensity (MFI), as compared with non-irradiated cells, indicating that the stem-like NS subpopulation was enriched (Fig 2A and Appendix Fig S2A). A stem-like cell enrichment after NS irradiation was also indicated by (i) qpcr evaluation of SOX2 transcription, previously associated with the GSC phenotype (Fig 2B) (Li et al, 2); (ii) flow cytometric analysis of Olig2, a marker previously used to characterize mesenchymal/proneural radioresistant NS (Appendix Fig S2B) (Bhat et al, 23). We then showed that irradiation raised the frequency of cells displaying GSC functional properties, as measured by limiting dilution assays (LDA) performed in vitro and in vivo. In a representative panel of NS, irradiation with significantly increased the frequency of sphere-forming cells (Fig 2C). In a representative NS (BT38NS), irradiation increased the tumorigenic potential. As depicted in Fig 2D, NS were irradiated in vitro (NS-IR, p) and, after 24 h, transplanted subcutis in the mouse (p). In parallel, an equal number of non-irradiated NS cells (NS-) were transplanted as control. Both NS-IR and NS- generated tumors (p) that were serially passaged by further transplantation of an equal number of cells (p2). Finally, tumors generated in p2 were passaged as a limiting dilution assay, by transplanting 4 cells in p3 mice. The calculated GSC frequency was ~-fold higher in tumors originated from NS-IR, as compared with tumors from NS- (Fig 2E and F). In addition, cells were derived from p3 tumors and assessed in an in vitro LDA, showing that the sphere-forming ability significantly increased in cells from tumors that originated from NS-IR, as compared with controls (Fig 2G). In accordance with in vivo and in vitro evidence of GSC enrichment associated with irradiation, the median volume of tumors generated by NS-IR, comparable to those generated by NS- at p, increased through serial passages to a greater extent, as compared with control tumors (Fig EV2A and B). Finally, an increased GSC frequency was also observed in a second GBM model. This tumor was established by intracranic injection of NS, treated in vivo with IR (2 Gy 3 days), and assessed by in vitro LDA 62 days after treatment (Fig 2H). Collectively, these results show that the cell subpopulation endowed with the clonogenic and tumorigenic properties that qualify GSCs is positively selected by IR. MET-expressing GSCs are selected by irradiation in experimental models We have previously shown that (i) MET is expressed in a subset of NS (~4%) sequentially derived from primary GBM (MET-pos-NS); Figure 3. MET-expressing GSCs are selected by irradiation. A In MET-pos-NS, the MET high subpopulation retains GSC properties and generates a heterogeneous progeny including also MET neg pseudodifferentiated cells. B LDA (sphere-forming) measuring the GSC frequency after IR () in MET high and MET neg subpopulations sorted from BT38NS. : v 2 test, P =.. n = 2. C Flow cytometric analysis of MET in irradiated BT38NS, 24 h after the last irradiation (2 Gy 3 days or a single dose of ). Dotted lines: threshold to define the percentage of MET-expressing cells. Ctrl: non-irradiated cells. The MFI was as follows:, 8; 2 Gy 3, 22.77;, D Flow cytometric analysis of MET in BT38NS weekly treated with IR (2 Gy) for 6 weeks. Dotted lines: threshold to define the percentage of MET-expressing cells. Ctrl: non-irradiated cells. E Representative immunofluorescence staining of MET (red) on tumors generated by subcutaneous injection of BT38NS irradiated (2 Gy 3) and explanted 72 h after the last irradiation. Nuclei are counterstained with DAPI (blue). Ctrl: non-irradiated tumors. Scale bar, µm (63 magnification). F Left: Quantification of the percentage of MET-expressing cells in tumor sections represented in (E) (n = 2 HPF/group). HPF: high-power field. : t-test, P =.. Right: Quantification of MET mean fluorescence intensity in tumor sections represented in (E) (n = 2 HPF/group, fold versus non-irradiated cells, ). : t-test, P =.6. G Representative MET immunohistochemical staining of matched primary and recurrent tumors. Scale bar, 5 µm (4 magnification). H Histogram representing the number of patient displaying different percentages of MET-expressing cells in their primary or recurrent tumors: negative, 5%; low, 6 29%; moderate, 3 69%; high, 7 % (see also Appendix Table S4). I Dot plot representing the MET cumulative score (MET-positive cell score + MET staining intensity score) in 9 primary and recurrent tumors (see Appendix Table S4)., Wilcoxon, P <.2. Data information: Data are represented as mean SEM in (F, I) or CI in (B). 554 EMBO Molecular Medicine Vol 8 No 5 26 ª 26 The Authors
6 Published online: April 4, 26 Francesca De Bacco et al MET inhibition in glioblastoma stem-like cells EMBO Molecular Medicine Figure 3. ª 26 The Authors EMBO Molecular Medicine Vol 8 No
7 Published online: April 4, 26 EMBO Molecular Medicine MET inhibition in glioblastoma stem-like cells Francesca De Bacco et al (ii) MET is a marker of the GSC subpopulation (MET high ) (De Bacco et al, 22) (Fig 3A); and (iii) MET promotes radioresistance of conventional cancer cell lines (De Bacco et al, 2). Therefore, in the subset of MET-pos-NS (Appendix Table S3), we investigated the relationship between MET expression and GSC radioresistance. Accordingly with our previous data, in vitro LDA (sphere-forming assay) showed that the MET high subpopulation, sorted from representative MET-pos-NS, was enriched in GSCs (Fig 3B and Appendix Fig S3A). As assessed by flow cytometry, in MET-pos-NS, the number of MET-expressing cells, and their MFI, significantly increased 24 h after irradiation (Fig 3C and Appendix Fig S3B). An even higher enrichment of MET-expressing cells was observed after a chronic IR treatment (Fig 3D). Accordingly, in tumors established by subcutaneous transplantation of MET-pos-NS, the number of MET-expressing cells and the intensity of staining were significantly increased 72 h after the last in vivo irradiation (Fig 3E and F). As MET expression is inducible by IR through NF-jB activation (De Bacco et al, 2), we asked whether MET upregulation could contribute to the enrichment of MET-expressing cells observed after irradiation. Indeed, MET expression was induced by IR in MET high cells sorted from MET-pos-NS, but not in the remaining MET neg subpopulation (Fig EV3A D). Lack of MET mrna accumulation in irradiated MET neg cells could be explained by the abundant presence of mirna targeting MET (such as mir34a and 23b), which was previously associated with this subpopulation (De Bacco et al, 22). Collectively, these data indicate that MET-expressing GSCs are radioresistant, likely as a result of concomitant mechanisms, involving both cell-positive selection and MET transcriptional upregulation. MET-expressing cells are enriched in recurrent human GBMs We asked whether radioresistance of MET-expressing GSCs could operate in patients tumors and be associated with positive selection under therapeutic pressure. We collected a panel of 9 pairs of GBMs, each including a primary tumor removed by surgery without prior treatment, and the matched recurrent tumor, arising after adjuvant chemoradiotherapy, and again surgically resected (Appendix Table S4). Remarkably, immunohistochemical analysis of whole tissues showed that significant MET expression (MET cumulative score 2) was detectable in 36% of primary tumors (7/9), but in 84% of recurrent tumors (6/9) (Appendix Table S5). In the majority of cases, the percentage of MET-positive cells and/or the intensity of MET staining (summed in the cumulative score) increased in the recurrent as compared with the matched primary tumor (Fig 3G I and Appendix Table S5). These data suggest that, in primary GBMs, cells expressing MET, being resistant to the standard therapeutic regimen invariably including radiotherapy, can drive tumor recurrence. Although we do not provide functional proofs, we reason that the MET-positive cell population expanded in the recurrent tumor can largely correspond to stem-like cells because: (i) In MET-pos-NS, the MET-negative cell subpopulation is devoid of stem properties, as previously shown (De Bacco et al, 22), and further investigated by in vitro LDA (Fig 3B and Appendix Fig S3A); and (ii) GSC differentiation is characterized by loss of MET expression, as shown in vitro (De Bacco et al, 22). The limited size of this cohort did not allow to establish statistically significant correlations between MET expression and clinical outcomes such as disease-free or overall survival (Appendix Table S4). However, in the TCGA dataset, encompassing 4 GBMs analyzed by whole tissue transcriptional profiling (Cerami et al, 22; Gao et al, 23), high MET expression in the primary tumor was significantly associated with decreased disease-free and overall survival (Appendix Fig S3C and D). MET high GSCs efficiently activate DDR Radioresistance of the MET-expressing stem-like subpopulation was further assessed by several experimental approaches. First, in irradiated MET-pos-NS, the cell death response was evaluated by measuring Annexin V incorporation by flow cytometry (Fig 4A). About 36 h after IR, the MET-expressing subpopulation (red) mostly contained viable cells (Annexin V/DAPI-negative cells, ~7%). Conversely, the MET-negative subpopulation (blue) displayed a significant decrease in viable cells as compared with non-irradiated controls (~25% versus 74%), and a cell redistribution in early and, mostly, late apoptotic phases (Annexin V pos /DAPI neg : early apoptotic; Annexin V pos /DAPI pos : late apoptotic cells) (Fig 4A). Cell death of the MET-negative subpopulation was likely due to apoptosis, as indicated by analysis of PARP and caspase-3 activation, which was evident in sorted and irradiated MET neg cells, but not in MET high cells (Fig 4B). The biological response to IR was further evaluated in sorted MET high and MET neg cells. Viability, measured in dose response experiments 24 h after IR, was significantly higher in MET high than in MET neg cells at all doses tested (Fig 4C). Consistently, in radiobiological clonogenic assays, the surviving fraction of MET high cells remained almost unchanged even after irradiation with the highest dose ( Gy), while that of MET neg cells significantly decreased, tending to exhaust after Gy (Fig 4D and Appendix Fig S4A C). Finally, in comet assays performed 24 h after irradiation, MET high cells displayed modest DSB accumulation, as compared with non-irradiated cells, while MET neg displayed severe damage (Fig 4E and Appendix Fig S4D and E). The radioresistance of the MET high subpopulation was then correlated with constitutive DDR activation, as previously assessed in GSCs prospectively isolated with the CD33 marker (Bao et al, 26). Indeed, untreated MET high, but not MET neg cells, displayed constitutive phosphorylation of ATM and Chk2 kinases. After irradiation, ATM phosphorylation was significantly increased in MET high cells, but only weakly induced in MET neg (Fig 4F and Appendix Fig S4F). Consistently, RAD5 expression was significantly more abundant in irradiated MET high than MET neg cells (Fig 4G). Accordingly, 24 h after irradiation, histone H2AX phosphorylation was still detectable only in MET neg cells (Fig 4F). Collectively, these findings indicate that the positive selection of MET high GSC by IR resides on intrinsic radioresistance of this subpopulation, likely conferred by enhanced basal activity, and efficient hyperactivation of DDR effectors after irradiation. MET inhibition radiosensitizes GSCs The above results indicate that MET is a marker of a radioresistant GSC subpopulation. We thus investigated whether MET signaling 556 EMBO Molecular Medicine Vol 8 No 5 26 ª 26 The Authors
8 Published online: April 4, 26 Francesca De Bacco et al MET inhibition in glioblastoma stem-like cells EMBO Molecular Medicine A B C D E F G Figure 4. MET high GSCs efficiently activate DDR. A Flow cytometric analysis of Annexin V/DAPI incorporation and MET expression in BT38NS 36 h after IR (). Ctrl: non-irradiated cells. Blue: MET-negative cells. Red: MET-expressing cells. B Western blot of MET neg and MET high subpopulations sorted from BT452NS, showing activation of PARP (cleaved PARP) and caspase-3 (cleaved casp-3) after IR (). Vinculin was used as a loading control. C Cell viability of MET high and MET neg subpopulations sorted from BT38NS, measured 96 h after IR ( Gy, fold versus non-irradiated cells, ). : t-test, P <.. n = 2. D Clonogenic assay with MET high and MET neg subpopulations sorted from BT38NS and irradiated ( Gy, fold versus non-irradiated cells, ). : t-test, P <.5. n = 3. E Neutral comet assay in MET high and MET neg subpopulations sorted from BT38NS, performed 24 h after IR (). Ctrl: non-irradiated cells. Comet tail s length is proportional to DSB extent. F Western blot of MET neg and MET high subpopulations sorted from BT38NS, showing constitutive () and/or IR-induced phosphorylation of ATM (patm), Chk2 (pchk2), and H2AX (ch2ax), and accumulation of RAD5, 24 h after IR (). Total ATM protein is also shown. b-actin was used as a loading control. G qpcr of RAD5 expression in MET high and MET neg subpopulations sorted from BT38NS 24 h after IR (, fold versus non-irradiated control cells, ). : t-test, P =.2. n = 3. Data information: Data are represented as mean SEM. Source data are available online for this figure. ª 26 The Authors EMBO Molecular Medicine Vol 8 No
9 Published online: April 4, 26 EMBO Molecular Medicine MET inhibition in glioblastoma stem-like cells Francesca De Bacco et al Figure 5. MET inhibition radiosensitizes GSCs. A Cell viability of BT38NS, measured 48 h after IR (2 or ) in the absence (mock) or in the presence of ng/ml HGF (fold versus non-irradiated mock treated cells, mock). Ctrl: non-irradiated cells. : t-test ( + HGF) versus (), P <.. n = 2. B Flow cytometric analysis of Annexin V/DAPI incorporation in BT38NS irradiated () in the absence (mock) or in the presence of HGF. Histograms show the percentage of Annexin V-positive cells 48 h after IR. Ctrl: non-irradiated cells. C Cell viability of BT38NS, irradiated in the absence () or in the presence ( + ) of the MET inhibitor (5 nm) and analyzed at the indicated time points. Vehicle: non-irradiated cells (fold versus -treated cells at time, mock). : t-test, P <.. n = 2. D Cell viability as in C, measured 5 days after IR ( Gy, fold versus -treated cells, mock). : t-test, P <.3. n = 2. E Flow cytometric analysis of Annexin V/DAPI incorporation in BT38NS irradiated () in the absence () or in the presence () of Histograms show the percentage of Annexin V-positive cells 36 h after IR. Ctrl: non-irradiated cells. : t-test ( + ) versus (), P =.. n = 3. F Western blot of BT38NS showing caspase-3 activation (cleaved casp-3), at the indicated time points after IR in the absence ( ) or in the presence (+) of Vinculin was used as a loading control. G Clonogenic assay with MET neg and MET high subpopulations sorted from BT38NS, irradiated () in the absence () or in the presence of MET inhibitors ( and Crizotinib, 5 nm). Ctrl: non-irradiated cells. : t-test, P <.4. n = 3. H LDA (sphere-forming assay) with MET neg and MET high subpopulations sorted from BT38NS, measuring GSC frequency after irradiation in the absence () or in the presence ( + ) of Vehicle: non-irradiated -treated cells. : v 2 test, P =.2. n = 2. I Flow cytometric analysis of phosphorylated histone H2AX (ch2ax) in the whole BT38NS at the indicated time points after IR in the absence () or in the presence ( + ) of (fold versus non-irradiated cells at time, mock). : t-test, P <.5. n = 2. J Neutral comet assay with BT38NS performed 24 h after IR in the absence () or in the presence ( + ) of Vehicle: non-irradiated treated cells. Comet tail s length is proportional to DSB extent. Data information: Data are represented as mean SEM in (A, C E, G, I) or CI in (H). Source data are available online for this figure. has a functional role in sustaining radioresistance, and whether its inhibition can radiosensitize GSCs. MET-pos-NS were kept in a standard EGF/bFGF medium, supplied with HGF to better mimick the brain (tumor) microenvironment (Kunkel et al, 2; Xie et al, 22). In this condition, after irradiation, MET gets hyperphosphorylated (Fig EV4A and B), consistently with previous results obtained in cell lines (Lal et al, 25; De Bacco et al, 2). Addition of HGF to standard culture medium increased radioresistance of irradiated MET-pos-NS, by preserving viability (Fig 5A and Appendix Fig S5A and B) and preventing apoptosis (Fig 5B). We then showed that association of the specific MET inhibitor to IR significantly reduced cell viability and increased the percentage of apoptotic (Annexin V-positive) cells, as compared with treatment with IR alone (Fig 5C E and Appendix Fig S5C and D). Consistently, caspase-3 activation was barely detectable in cells treated with IR alone, to become well evident in those treated with IR and the MET inhibitor (Fig 5F). The effect of MET targeting was also assessed by the use of alternative inhibitors, including the MET-specific small-molecule PHA665752, the monovalent form of the MET monoclonal antibody DN3 (MvDN3) (Pacchiana et al, 2), and the promiscuous, clinically approved, small-molecule Crizotinib (targeting MET and ALK kinases). All the above inhibitors combined with IR similarly reduced cell viability and increased caspase-3/7 activation, as compared with IR alone (Appendix Fig S5E H). These outcomes, observed in whole NS, were obviously due to the activity of the inhibitors in MET-expressing GSCs and not in the MET-negative non-stem subpopulation, which, by definition, lacks the target. Consistently, in radiobiological clonogenic assays performed with the sorted MET neg and MET high subpopulations, the surviving fraction of MET neg cells treated with IR, already very low, was not further decreased by MET inhibitors. Conversely, the surviving fraction of the irradiated MET high subpopulation was reduced by MET inhibitors to the levels of the MET neg cells (Fig 5G and Appendix Fig S5I). Finally, in LDA (sphere-forming assay) with sorted MET high and MET neg subpopulations, the GSC frequency of the MET high subpopulation was abated by combination of IR with the MET inhibitor, while, as expected, the low GSC frequency of the MET neg subpopulation was further decreased only by IR (Fig 5H). Interestingly, a significant decrease of MET high GSC frequency was also observed after treatment with the MET inhibitor alone, indicating that HGF present in the culture medium plays a role in sustaining self-renewal, as previously shown (Li et al, 2; De Bacco et al, 22) (Fig 5H). However, no apoptosis was expected, and indeed observed, by treating NS with MET inhibitors alone (Fig 5E and F and Appendix Fig S5E H). The radiosensitizing activity of MET inhibitors was then correlated with the ability to prevent DNA repair. In whole MET-pos-NS, treatment with IR and the MET inhibitor increased the levels of histone H2AX phosphorylation and the extent of DSBs, as compared with IR alone (Fig 5I and J and Appendix Fig S5J and K). These findings show that MET behaves as a functional marker of radioresistance, whose inhibition efficiently radiosensitizes MET high GSCs. MET inhibition impairs GSC DDR by preventing AKT-dependent ATM and p2 activity We thus asked whether MET inhibition directly impinges on the molecular mechanisms regulating DDR. We previously showed that, after irradiation of conventional cell lines, ATM upregulates MET expression through NF-jB transcription factor (De Bacco et al, 2). We now show that MET, in turn, unleashes a signal transduction cascade that sustains ATM activity and DDR. We found that, in irradiated MET-pos-NS kept in the standard EGF/bFGF medium, addition of HGF increased ATM and Chk2 phosphorylation, and RAD5 expression (Fig 6A and Appendix Fig S6A). Conversely, in irradiated MET-pos-NS kept in the medium supplied with HGF, addition of the MET inhibitor significantly reduced ATM and Chk2 phosphorylation, and RAD5 expression (Fig 6B and C). This effect depended specifically on MET inhibition, as shown by complementary experimental approaches such as: (i) the use of additional MET inhibitors such as the above-described PHA665752, Crizotinib, and MvDN3 (Appendix Fig S6B D), which invariably 558 EMBO Molecular Medicine Vol 8 No 5 26 ª 26 The Authors
10 Published online: April 4, 26 Francesca De Bacco et al MET inhibition in glioblastoma stem-like cells EMBO Molecular Medicine A viable cells (fold vs mock) Gy mock HGF B Annexin V-positive cells (%) mock HGF C viable cells (fold vs mock) H GSC frequency (%) h 2 5 Gy F cleaved casp-3 vinculin MET neg MET high + I relative γh2ax (fold vs mock) D viable cells (fold vs mock) G surviving fraction (%) h MET neg J number of cells (%) Crizotinib E Annexin V-positive cells (%) MET high Crizotinib no tail short tail long tail Figure 5. ª 26 The Authors EMBO Molecular Medicine Vol 8 No
11 Published online: April 4, 26 EMBO Molecular Medicine MET inhibition in glioblastoma stem-like cells Francesca De Bacco et al Figure 6. MET inhibition impairs AKT-dependent ATM activity. A Western blot of BT463NS showing phosphorylation of ATM (patm) and Chk2 (pchk2), and accumulation of RAD5 at the indicated time points after IR in the absence () or in the presence ( + HGF) of HGF ( ng/ml). Total ATM is also shown. H3 was used as a loading control. B Western blot of BT38NS showing phosphorylation of ATM (patm) and Chk2 (pchk2) and accumulation of RAD5 at the indicated time points after IR in the absence () or in the presence ( + ) of the MET inhibitor (5 nm). Total ATM is also shown. b-actin was used as a loading control. Veh: non-irradiated -treated cells. C Densitometric analysis of ATM phosphorylation shown in (B). : t-test, P <.. n = 3. D Western blot of BT38NS showing phosphorylation of ATM, Chk2, and AKT (pakt) and accumulation of RAD5 24 h after IR () in the absence (, veh) or in the presence of MAPK (PD9859, 2 lm), AKT (Ly2942, 2 lm), MET ( ), or ATM (CGK733, lm) inhibitors. Total ATM and AKT are also shown. b-actin was used as a loading control. Ctrl: non-irradiated cells. E Western blot of BT463NS showing phosphorylation of ATM, Chk2, and Aurora kinase A (paurora A) at the indicated time points after IR in the absence () or in the presence ( + Ly) of the AKT inhibitor Ly2942. Total ATM and Aurora kinase A are also shown. H3 was used as a loading control. Veh: non-irradiated treated cells. F Western blot of BT38NS showing phosphorylation of Aurora kinase A at the indicated time points after IR in the absence () or in the presence ( + ) of Total Aurora kinase A is also shown. H3 was used as a loading control. Veh: non-irradiated -treated cells. G Western blot of BT463NS showing phosphorylation of Aurora kinase A at the indicated time points after IR in the absence () or in the presence ( + HGF) of HGF. Total Aurora kinase A is also shown. H3 was used as a loading control (the same control applies to both panel A and G). H Western blot of BT38NS showing phosphorylation of ATM and Aurora kinase A at the indicated time points after IR in the absence () or in the presence ( + MLN) of the Aurora kinase A inhibitor MLN8237 (2 lm). Total ATM and Aurora kinase A are also shown. H3 was used as a loading control. Veh: nonirradiated -treated cells. I Cell viability of BT38NS, measured 72 h after IR () in the absence () or in the presence of MET ( ), Aurora kinase A (MLN8237), or AKT (Ly2942) inhibitors (fold versus non-irradiated -treated cells, mock). : t-test ( + inhibitor) versus ( + ), P <.. n = 2. Data information: Data are represented as mean SEM. Source data are available online for this figure. decreased ATM phosphorylation in irradiated MET-pos-NS; and (ii) the use of a MET inhibitor in MET-neg-NS (lacking MET protein expression, Appendix Table S3), which failed to impair ATM phosphorylation, thus ruling out off-target effects (Appendix Fig S6E). We then investigated the mechanistic link between MET and ATM phosphorylation. In MET-pos-NS, IR induced activation of MET (see above, Fig EV4A and B) and its two main downstream signaling pathways, AKT and MAP kinases (MAPK). Phosphorylation of both AKT and MAPK was dependent of MET, as it was fully prevented by combining IR with the MET inhibitor (Appendix Fig S6F). We further showed that AKT was responsible to connect MET to ATM, as an AKT but not a MAPK inhibitor decreased ATM phosphorylation, and activation of its downstream effectors Chk2 and RAD5 after irradiation (Fig 6D and Appendix Fig S6G). The pathway linking AKT to ATM phosphorylation is currently unknown. A likely candidate to investigate was Aurora kinase A, known to phosphorylate ATM (Yang et al, 2). Indeed, in irradiated MET-pos-NS, we showed that (i) Aurora kinase A phosphorylation on Thr288, required for enzymatic activation (Walter et al, 2), was prevented by the AKT inhibitor LY2942 (Fig 6E and Appendix Fig S6H), as well as by the MET inhibitor (Fig 6F and Appendix Fig S6I); (ii) Aurora kinase A phosphorylation was increased by HGF (Fig 6G and Appendix Fig S6J); and (iii) Aurora kinase A inhibition by its specific small-molecule inhibitor MLN8237 prevented ATM phosphorylation (Fig 6H and Appendix Fig S6K). Consistently, AKT or Aurora kinase A inhibitors mimicked the radiosensitizing activity of the MET inhibitor, as shown in viability experiments (Fig 6I and Appendix Fig S6L and M). The role of the AKT pathway as a crucial player of MET-induced radioresistance was further supported by p2 analysis. It is known that, when it is phosphorylated by AKT on Thr45, p2 is retained in the cytoplasm and exerts anti-apoptotic functions; in the absence of this phosphorylation, p2 translocates into the nucleus to block the cell cycle (Abbas & Dutta, 29). Indeed, we showed that, in irradiated MET-pos-NS kept in the standard EGF/bFGF medium, addition of HGF induced p2 phosphorylation on Thr45 (Fig 7A) and its cytoplasmic accumulation (Fig 7A). In a complementary experiment, in irradiated MET-pos-NS kept in the medium supplied with HGF, addition of the MET inhibitor prevented p2 phosphorylation on Thr45 (Fig 7B and Appendix Fig S7A), and induced its nuclear translocation, as shown by Western blot and immunofluorescence analysis (Fig 7C E and Appendix Fig S7B and C). Consistently, when MET-pos-NS were irradiated in the presence of the MET inhibitor, cell cycle was arrested in the G2-M phase (Fig 7F and Appendix Fig S7D) and apoptosis was unleashed (see above, Fig 5E and F and Appendix Fig S5E H). Collectively, these data indicate that MET supports DDR via AKT activation, which, on its turn, sustains the ATM pathway for DNA repair, and p2 cytoplasmic retention, connected with anti-apoptotic functions (Fig 7G). Impairment of these signaling circuits provides a mechanistic explanation for the radiosensitizing effect of MET inhibitors. MET inhibition radiosensitizes GSC-derived GBMs by GSC depletion We then generated GBMs by in vivo transplantation of MET-pos-NS, to investigate whether combination with MET inhibitors could increase the efficacy of radiotherapy by contributing to deplete GSCs. As assessed, the MET inhibitor crosses the blood brain barrier (Appendix Fig S8A). GBMs were then established by intracranial xenotransplantation of BT463NS. Ten days after NS injection, mice were randomized into four treatment groups: (i), (ii) IR (2 Gy 3 days), (iii) , supplied for 3 days, and (iv) combination therapy (combo, IR and as above). Approximately 6 days after the beginning of treatment, at the onset of severe neurological symptoms in controls, mice were sacrificed and brains were analyzed by epifluorescence imaging (Fig 8A). Combination therapy dramatically reduced tumor growth, measured as GFP intensity, as compared with IR or MET inhibitor alone (Fig 8B). In a second model, tumors were established by subcutaneous 56 EMBO Molecular Medicine Vol 8 No 5 26 ª 26 The Authors
12 Published online: April 4, 26 Francesca De Bacco et al MET inhibition in glioblastoma stem-like cells EMBO Molecular Medicine A patm ATM pchk2 RAD5 H3 D patm ATM pchk2 RAD5 pakt AKT β -actin 3 6 HGF +HGF B 3 6 h patm ATM pchk2 RAD5 β -actin veh PD Ly CGK veh PD Ly CGK veh h E patm pchk2 paurora A Aurora A H3 veh 3 6 Ly C patm/atm densitometry (fold vs ) +Ly h h F paurora A Aurora A veh 5Gy Gy h G paurora A Aurora A H3 3 6 HGF +HGF 3 6 h H3 H patm ATM paurora A Aurora A H3 veh 3 6 MLN +MLN 3 6 h I viable cells (fold vs ) veh MLN8237 Ly2942 Figure 6. ª 26 The Authors EMBO Molecular Medicine Vol 8 No
13 Published online: April 4, 26 EMBO Molecular Medicine MET inhibition in glioblastoma stem-like cells Francesca De Bacco et al A +HGF HGF h C veh h pp2 p2 Thr45 IP on cytoplasmic fraction p2 vinculin cytoplasmic fraction B veh h p2 laminb nuclear fraction pp2 p2 D Thr45 p2 DAPI IP on cytoplasmic fraction E positive cells/hpf (%) nuclear cytoplasmic G F number of cells (%) sub-g G - G S G - M 2 cytoplasm Aurora A MET P P P P AKT P p2 Caspase-3 + nucleus ATM P p2 APOPTOSIS DNA REPAIR CELL CYCLE ARREST Figure 7. MET inhibition promotes p2 nuclear translocation. A Western blot of BT37NS showing p2 phosphorylation (pp2 Thr45 ) in cytoplasmic lysates immunoprecipitated with p2 antibodies at the indicated time points after IR in the absence () or in the presence ( + HGF) of HGF ( ng/ml). B Western blot of BT463NS showing p2 phosphorylation (pp2 Thr45 ) in cytoplasmic lysates immunoprecipitated with p2 antibodies at the indicated time points after IR in the absence () or in the presence ( + ) of the MET inhibitor (5 nm). C Western blot of BT38NS showing p2 localization in cytoplasmic/nuclear extracts at the indicated time points after IR in the absence () or in the presence ( + ) of Vinculin and lamin B were used as loading controls for cytoplasmic and nuclear fractions, respectively. D Representative immunofluorescence staining of p2 (red) on BT38NS cells 3 h after IR () in the absence () or in the presence () of Ctrl: non-irradiated cells. Nuclei are counterstained with DAPI (blue). Scale bar, µm (63 magnification). E Quantification of the percentage of cells showing p2 cytoplasmic or nuclear localization in BT38NS represented in (D) (n = HPF/group). HPF: high-power field. : t-test ( + ) versus ( or ), P <.. F Cell cycle analysis of BT34NS, performed 24 h after IR in the absence () or in the presence ( + ) of Vehicle: non-irradiated cells. : one-way ANOVA, P =.8. n = 3. G Schematic representation of the MET-driven signaling pathways that sustain DNA repair and prevent apoptosis and cell cycle arrest. Data information: Data are represented as mean SEM. Source data are available online for this figure. 562 EMBO Molecular Medicine Vol 8 No 5 26 ª 26 The Authors
14 Published online: April 4, 26 Francesca De Bacco et al MET inhibition in glioblastoma stem-like cells EMBO Molecular Medicine transplantation of BT38NS, displaying a GBM-like morphology (Appendix Fig S8B), and were treated as above ( was administered for 5 days). Tumors treated with, or the MET inhibitor alone, reached the experimental endpoint (tumor volume =,6 mm 3 ) within 8 or 32 days, respectively. Tumors treated with IR alone remained stable for 4 days after beginning of the treatment, leading to the experimental endpoint within 63 days. Remarkably, tumors treated with the combination therapy showed clinical regression (volume reduction > 5% as compared with day ), which persisted until 4 days, leading to the experimental endpoint 9 days after beginning of the treatment (Fig 8C and D). A significant growth inhibition (~2-fold) by combination therapy as compared with radiotherapy alone was observed also in tumors generated by transplantation of BT37NS, although these tumors were not arrested by any therapy (Appendix Fig S8B D). Next, we investigated whether combination treatment led to decreased GSC frequency. As depicted in Fig 8E, in an independent experiment, tumors were generated by BT38NS subcutaneous transplantation, treated as above, explanted days after beginning of the treatment, that is, when regression was observed (Appendix Fig S8E), and analyzed by LDA. Both in vitro clonogenic (Fig 8F) and in vivo tumorigenic (Fig 8G I) LDA indicated a significant GSC frequency decrease in tumors treated with combination therapy, as compared with controls or radiotherapy (or the MET inhibitor) alone. Of note, in vitro but not in vivo LDA (p) allowed to detect an increased GSC frequency after radiotherapy alone. This is expected, as in vivo LDA, measuring tumorigenic potential, requires at least two serial passages (p2) to highlight positive selection of GBM stem-like cells by irradiation, as shown above (Figs 2F and EV2A and B). However, in this experiment, further passaging was prevented by the minimal volume, and poor cell viability, of tumors generated by the first passage (p) after combination therapy (Appendix Fig S8F). Collectively, these data indicate that radiosensitization of GBM by MET inhibitors not only impairs tumor growth, but also depletes the GSC subpopulation responsible for tumor generation and recurrence. Discussion Radioresistance is a well-known pitfall of GBM therapy (Squatrito & Holland, 2). The responsible cell subpopulation(s), as well as the underlying genetic and molecular mechanisms, are still poorly understood. Pioneering work has associated radioresistance of GBM and other tumors with the cancer stem cell phenotype, particularly with the intrinsic ability to efficiently activate DDR (Bao et al, 26; Phillips et al, 26b; Diehn et al, 29; Pajonk et al, 2; Cheng et al, 2; Vlashi & Pajonk, 24; Ahmed et al, 25). In GBM, this property is likely inherited from the cell of origin, a neural stem/ progenitor cell (Blanpain et al, 2). More recently, GSC radioresistance has been associated with the GBM mesenchymal profile, implying a causal role for the subtype-specific genetic lesions and/or signaling circuits (Bhat et al, 23). The latter findings are consistent with the evidence that the GBM mesenchymal profile and the epithelial mesenchymal transition in general overlap with the stem cell phenotype (Phillips et al, 26a; Scheel & Weinberg, 22) and thus with inherent radioresistance. In the panel of twenty NS analyzed in this study, radioresistance was found to be a common property, displayed by mesenchymal as well as by classical or proneural NS. In classical NS, the typical EGFR amplification could play a prominent role in conferring radioresistance, as indicated by previous studies (Squatrito & Holland, 2). In mesenchymal and proneural NS, usually lacking EGFR amplification, we investigated whether a prominent role could be played by the wild-type MET oncogene, which was previously associated with the GBM mesenchymal profile (Phillips et al, 26a; Verhaak et al, 2; De Bacco et al, 22), and is usually expressed in a mutually exclusive fashion with EGFR (Verhaak et al, 2; De Bacco et al, 22). Building on previous studies showing that MET is a marker of a GSC subtype, which identifies and sustains the stem phenotype (Li et al, 2; De Bacco et al, 22; Joo et al, 22), we now show that MET is a functional marker of GSC radioresistance. Indeed, we found that IR positively selects MET high GSCs and that the radioresistant phenotype of these cells correlates with high basal expression levels of ATM, Chk2, and RAD5, which likely prime cells to efficiently activate DDR. As a result, MET high GSCs, but not MET neg non-stem cells, can rapidly repair DNA damage and successfully survive IR. The protective role of MET likely operates in human GBMs treated with the standard combination of radio- and chemotherapy with the alkylating agent temozolomide (Stupp et al, 29). Indeed, in the patients cohort analyzed, MET expression is enriched in the recurrent tumors, as compared with the matched primary. This indicates the expansion of MET-expressing (stem-like) cells, likely benefitting of a selective advantage under therapeutic pressure and Figure 8. MET inhibition radiosensitizes GSC-derived GBMs by GSC depletion. A Timeline of therapeutic treatments undergone by mice intracranially injected with BT463NS. B Left: Representative images of ex vivo epifluorescent GFP signal of intracranial tumors, generated by injection of BT463NS, and explanted 62 days after IR in the absence (2 Gy 3 days) or in the presence (combo) of the MET inhibitor (5 mg/kg). Right: Quantification of epifluorescent GFP signal from tumors represented on the left (n = 4/condition). Vehicle: non-irradiated -treated tumors. : one-way ANOVA, P = C Growth curves of tumors, generated by subcutaneous injection of BT38NS, irradiated in the absence (2 Gy 3 days) or in the presence (combo) of , which was administered for 5 days as indicated (n = 7/condition). Vehicle: non-irradiated -treated tumors. : one-way ANOVA, P =.6. D Survival analysis of mice bearing tumors generated and treated as in (C). Black dot: censored mouse. Log-rank (Mantel-Cox) test (combo) versus (2 Gy 3 days), P =.9. E Experimental design to measure GSC frequency in tumors treated as in (C) ( was administered for days). Explanted tumors were dissociated, and viable cells were tested by in vitro sphere-forming LDA or in vivo single-passage (p) LDA. F In vitro LDA (sphere-forming) measuring GSC frequencies as described in (E) (n = 3/condition). : v 2 test, P < 5. G Tumor incidence in the in vivo LDA performed on tumors generated and treated as described in (E). H Histogram showing GSC frequencies measured by in vivo LDA, as described in (E). : v 2 test, P = I Table showing GSC frequencies measured by in vivo LDA, represented in (H). Data information: Data are represented as mean SEM in (B, C) or CI in (F, H, I). ª 26 The Authors EMBO Molecular Medicine Vol 8 No
CURRICULUM VITAE. January 2014-now: PhD student in Molecular Medicine. Turin Medical School University, Italy.
 First name and surname: Antonio D Ambrosio. CURRICULUM VITAE Place and date of birth: Torino, Italy. September 17, 1981. Citizenship: Italian. Work Adress: Institute for Cancer Research at Candiolo (IRCC),
First name and surname: Antonio D Ambrosio. CURRICULUM VITAE Place and date of birth: Torino, Italy. September 17, 1981. Citizenship: Italian. Work Adress: Institute for Cancer Research at Candiolo (IRCC),
Supplementary Figure 1: si-craf but not si-braf sensitizes tumor cells to radiation.
 Supplementary Figure 1: si-craf but not si-braf sensitizes tumor cells to radiation. (a) Embryonic fibroblasts isolated from wildtype (WT), BRAF -/-, or CRAF -/- mice were irradiated (6 Gy) and DNA damage
Supplementary Figure 1: si-craf but not si-braf sensitizes tumor cells to radiation. (a) Embryonic fibroblasts isolated from wildtype (WT), BRAF -/-, or CRAF -/- mice were irradiated (6 Gy) and DNA damage
Chapter 2. Aims & Objectives
 2.1. Statement of the problem: Earlier reports have shown ambiguous alteration of histone marks in response to DNA damage in asynchronized population of cells. These histone marks not only undergo dynamic
2.1. Statement of the problem: Earlier reports have shown ambiguous alteration of histone marks in response to DNA damage in asynchronized population of cells. These histone marks not only undergo dynamic
Supplemental Figure 1. Intracranial transduction of a modified ptomo lentiviral vector in the mouse
 Supplemental figure legends Supplemental Figure 1. Intracranial transduction of a modified ptomo lentiviral vector in the mouse hippocampus targets GFAP-positive but not NeuN-positive cells. (A) Stereotaxic
Supplemental figure legends Supplemental Figure 1. Intracranial transduction of a modified ptomo lentiviral vector in the mouse hippocampus targets GFAP-positive but not NeuN-positive cells. (A) Stereotaxic
Pharmacologic inhibition of histone demethylation as a therapy for pediatric brainstem glioma
 Supplementary information for: Pharmacologic inhibition of histone demethylation as a therapy for pediatric brainstem glioma Rintaro Hashizume 1, Noemi Andor 2, Yuichiro Ihara 2, Robin Lerner 2, Haiyun
Supplementary information for: Pharmacologic inhibition of histone demethylation as a therapy for pediatric brainstem glioma Rintaro Hashizume 1, Noemi Andor 2, Yuichiro Ihara 2, Robin Lerner 2, Haiyun
Supplementary Figure 1: High-throughput profiling of survival after exposure to - radiation. (a) Cells were plated in at least 7 wells in a 384-well
 Supplementary Figure 1: High-throughput profiling of survival after exposure to - radiation. (a) Cells were plated in at least 7 wells in a 384-well plate at cell densities ranging from 25-225 cells in
Supplementary Figure 1: High-throughput profiling of survival after exposure to - radiation. (a) Cells were plated in at least 7 wells in a 384-well plate at cell densities ranging from 25-225 cells in
Supplementary Figure 1. A. Bar graph representing the expression levels of the 19 indicated genes in the microarrays analyses comparing human lung
 Supplementary Figure 1. A. Bar graph representing the expression levels of the 19 indicated genes in the microarrays analyses comparing human lung immortalized broncho-epithelial cells (AALE cells) expressing
Supplementary Figure 1. A. Bar graph representing the expression levels of the 19 indicated genes in the microarrays analyses comparing human lung immortalized broncho-epithelial cells (AALE cells) expressing
Supplementary Appendix
 Supplementary Appendix This appendix has been provided by the authors to give readers additional information about their work. Supplement to: Bredel M, Scholtens DM, Yadav AK, et al. NFKBIA deletion in
Supplementary Appendix This appendix has been provided by the authors to give readers additional information about their work. Supplement to: Bredel M, Scholtens DM, Yadav AK, et al. NFKBIA deletion in
Supplementary Figure 1 IMQ-Induced Mouse Model of Psoriasis. IMQ cream was
 Supplementary Figure 1 IMQ-Induced Mouse Model of Psoriasis. IMQ cream was painted on the shaved back skin of CBL/J and BALB/c mice for consecutive days. (a, b) Phenotypic presentation of mouse back skin
Supplementary Figure 1 IMQ-Induced Mouse Model of Psoriasis. IMQ cream was painted on the shaved back skin of CBL/J and BALB/c mice for consecutive days. (a, b) Phenotypic presentation of mouse back skin
Supplementary Figure 1. Basal level EGFR across a panel of ESCC lines. Immunoblots demonstrate the expression of phosphorylated and total EGFR as
 Supplementary Figure 1. Basal level EGFR across a panel of ESCC lines. Immunoblots demonstrate the expression of phosphorylated and total EGFR as well as their downstream effectors across a panel of ESCC
Supplementary Figure 1. Basal level EGFR across a panel of ESCC lines. Immunoblots demonstrate the expression of phosphorylated and total EGFR as well as their downstream effectors across a panel of ESCC
Tumour growth environment modulates Chk1 signalling pathways and sensitivity to Chk1 inhibition
 Tumour growth environment modulates Chk1 signalling pathways and sensitivity to Chk1 inhibition Andrew J Massey Supplementary Information Supplementary Figure S1. Related to Fig. 1. (a) HT29 or U2OS cells
Tumour growth environment modulates Chk1 signalling pathways and sensitivity to Chk1 inhibition Andrew J Massey Supplementary Information Supplementary Figure S1. Related to Fig. 1. (a) HT29 or U2OS cells
Supplemental Figure S1. RANK expression on human lung cancer cells.
 Supplemental Figure S1. RANK expression on human lung cancer cells. (A) Incidence and H-Scores of RANK expression determined from IHC in the indicated primary lung cancer subgroups. The overall expression
Supplemental Figure S1. RANK expression on human lung cancer cells. (A) Incidence and H-Scores of RANK expression determined from IHC in the indicated primary lung cancer subgroups. The overall expression
Nature Neuroscience: doi: /nn Supplementary Figure 1
 Supplementary Figure 1 EGFR inhibition activates signaling pathways (a-b) EGFR inhibition activates signaling pathways (a) U251EGFR cells were treated with erlotinib (1µM) for the indicated times followed
Supplementary Figure 1 EGFR inhibition activates signaling pathways (a-b) EGFR inhibition activates signaling pathways (a) U251EGFR cells were treated with erlotinib (1µM) for the indicated times followed
Introduction: 年 Fas signal-mediated apoptosis. PI3K/Akt
 Fas-ligand (CD95-L; Fas-L) Fas (CD95) Fas (apoptosis) 年 了 不 度 Fas Fas-L 力 不 Fas/Fas-L T IL-10Fas/Fas-L 不 年 Fas signal-mediated apoptosis 度降 不 不 力 U-118, HeLa, A549, Huh-7 MCF-7, HepG2. PI3K/Akt FasPI3K/Akt
Fas-ligand (CD95-L; Fas-L) Fas (CD95) Fas (apoptosis) 年 了 不 度 Fas Fas-L 力 不 Fas/Fas-L T IL-10Fas/Fas-L 不 年 Fas signal-mediated apoptosis 度降 不 不 力 U-118, HeLa, A549, Huh-7 MCF-7, HepG2. PI3K/Akt FasPI3K/Akt
Self-inflicted DNA double-strand breaks sustain tumorigenicity and stemness of cancer cells
 ORIGINAL ARTICLE Cell Research (2017) 27:764-783. www.nature.com/cr Self-inflicted DNA double-strand breaks sustain tumorigenicity and stemness of cancer cells Xinjian Liu 1, Fang Li 1, Qian Huang 2, Zhengxiang
ORIGINAL ARTICLE Cell Research (2017) 27:764-783. www.nature.com/cr Self-inflicted DNA double-strand breaks sustain tumorigenicity and stemness of cancer cells Xinjian Liu 1, Fang Li 1, Qian Huang 2, Zhengxiang
Author's personal copy
 Apoptosis (214) 19:1736 1754 DOI 1.17/s1495-14-14-x ORIGINAL PAPER Radiation-induced glioblastoma signaling cascade regulates viability, apoptosis and differentiation of neural stem cells (NSC) Vladimir
Apoptosis (214) 19:1736 1754 DOI 1.17/s1495-14-14-x ORIGINAL PAPER Radiation-induced glioblastoma signaling cascade regulates viability, apoptosis and differentiation of neural stem cells (NSC) Vladimir
Supplementary Materials
 Supplementary Materials Figure S1. MTT Cell viability assay. To measure the cytotoxic potential of the oxidative treatment, the MTT [3-(4,5-dimethylthiazol- 2-yl)-2,5-diphenyl tetrazolium bromide] assay
Supplementary Materials Figure S1. MTT Cell viability assay. To measure the cytotoxic potential of the oxidative treatment, the MTT [3-(4,5-dimethylthiazol- 2-yl)-2,5-diphenyl tetrazolium bromide] assay
Type of file: PDF Size of file: 0 KB Title of file for HTML: Supplementary Information Description: Supplementary Figures
 Type of file: PDF Size of file: 0 KB Title of file for HTML: Supplementary Information Description: Supplementary Figures Supplementary Figure 1 mir-128-3p is highly expressed in chemoresistant, metastatic
Type of file: PDF Size of file: 0 KB Title of file for HTML: Supplementary Information Description: Supplementary Figures Supplementary Figure 1 mir-128-3p is highly expressed in chemoresistant, metastatic
Supplementary Fig. 1: ATM is phosphorylated in HER2 breast cancer cell lines. (A) ATM is phosphorylated in SKBR3 cells depending on ATM and HER2
 Supplementary Fig. 1: ATM is phosphorylated in HER2 breast cancer cell lines. (A) ATM is phosphorylated in SKBR3 cells depending on ATM and HER2 activity. Upper panel: Representative histograms for FACS
Supplementary Fig. 1: ATM is phosphorylated in HER2 breast cancer cell lines. (A) ATM is phosphorylated in SKBR3 cells depending on ATM and HER2 activity. Upper panel: Representative histograms for FACS
Prolonged mitotic arrest induces a caspase-dependent DNA damage
 SUPPLEMENTARY INFORMATION Prolonged mitotic arrest induces a caspase-dependent DNA damage response at telomeres that determines cell survival Karolina O. Hain, Didier J. Colin, Shubhra Rastogi, Lindsey
SUPPLEMENTARY INFORMATION Prolonged mitotic arrest induces a caspase-dependent DNA damage response at telomeres that determines cell survival Karolina O. Hain, Didier J. Colin, Shubhra Rastogi, Lindsey
Supplementary Table; Supplementary Figures and legends S1-S21; Supplementary Materials and Methods
 Silva et al. PTEN posttranslational inactivation and hyperactivation of the PI3K/Akt pathway sustain primary T cell leukemia viability Supplementary Table; Supplementary Figures and legends S1-S21; Supplementary
Silva et al. PTEN posttranslational inactivation and hyperactivation of the PI3K/Akt pathway sustain primary T cell leukemia viability Supplementary Table; Supplementary Figures and legends S1-S21; Supplementary
TEB. Id4 p63 DAPI Merge. Id4 CK8 DAPI Merge
 a Duct TEB b Id4 p63 DAPI Merge Id4 CK8 DAPI Merge c d e Supplementary Figure 1. Identification of Id4-positive MECs and characterization of the Comma-D model. (a) IHC analysis of ID4 expression in the
a Duct TEB b Id4 p63 DAPI Merge Id4 CK8 DAPI Merge c d e Supplementary Figure 1. Identification of Id4-positive MECs and characterization of the Comma-D model. (a) IHC analysis of ID4 expression in the
SUPPLEMENTARY INFORMATION
 DOI: 1.138/ncb3355 a S1A8 + cells/ total.1.8.6.4.2 b S1A8/?-Actin c % T-cell proliferation 3 25 2 15 1 5 T cells Supplementary Figure 1 Inter-tumoral heterogeneity of MDSC accumulation in mammary tumor
DOI: 1.138/ncb3355 a S1A8 + cells/ total.1.8.6.4.2 b S1A8/?-Actin c % T-cell proliferation 3 25 2 15 1 5 T cells Supplementary Figure 1 Inter-tumoral heterogeneity of MDSC accumulation in mammary tumor
Supplementary Figure 1: Expression of NFAT proteins in Nfat2-deleted B cells (a+b) Protein expression of NFAT2 (a) and NFAT1 (b) in isolated splenic
 Supplementary Figure 1: Expression of NFAT proteins in Nfat2-deleted B cells (a+b) Protein expression of NFAT2 (a) and NFAT1 (b) in isolated splenic B cells from WT Nfat2 +/+, TCL1 Nfat2 +/+ and TCL1 Nfat2
Supplementary Figure 1: Expression of NFAT proteins in Nfat2-deleted B cells (a+b) Protein expression of NFAT2 (a) and NFAT1 (b) in isolated splenic B cells from WT Nfat2 +/+, TCL1 Nfat2 +/+ and TCL1 Nfat2
Supplementary Figure 1: Tissue of Origin analysis on 152 cell lines. (a) Heatmap representation of the 30 Tissue scores for the 152 cell lines.
 Supplementary Figure 1: Tissue of Origin analysis on 152 cell lines. (a) Heatmap representation of the 30 Tissue scores for the 152 cell lines. The scores summarize the global expression of the tissue
Supplementary Figure 1: Tissue of Origin analysis on 152 cell lines. (a) Heatmap representation of the 30 Tissue scores for the 152 cell lines. The scores summarize the global expression of the tissue
Effective activity of cytokine-induced killer cells against autologous metastatic melanoma including cells with stemness features
 Effective activity of cytokine-induced killer cells against autologous metastatic melanoma including cells with stemness features Loretta Gammaitoni, Lidia Giraudo, Valeria Leuci, et al. Clin Cancer Res
Effective activity of cytokine-induced killer cells against autologous metastatic melanoma including cells with stemness features Loretta Gammaitoni, Lidia Giraudo, Valeria Leuci, et al. Clin Cancer Res
Supplementary Figure 1
 Supplementary Figure 1 a γ-h2ax MDC1 RNF8 FK2 BRCA1 U2OS Cells sgrna-1 ** 60 sgrna 40 20 0 % positive Cells (>5 foci per cell) b ** 80 sgrna sgrna γ-h2ax MDC1 γ-h2ax RNF8 FK2 MDC1 BRCA1 RNF8 FK2 BRCA1
Supplementary Figure 1 a γ-h2ax MDC1 RNF8 FK2 BRCA1 U2OS Cells sgrna-1 ** 60 sgrna 40 20 0 % positive Cells (>5 foci per cell) b ** 80 sgrna sgrna γ-h2ax MDC1 γ-h2ax RNF8 FK2 MDC1 BRCA1 RNF8 FK2 BRCA1
Appendix Figure S1 A B C D E F G H
 ppendix Figure S1 C D E F G H ppendix Figure S1. RT and chemotherapy alter PD-L1 expression in PDC cells. Flow cytometric analysis of PD-L1 expression in () KPC and () Pan02 cells following treatment with
ppendix Figure S1 C D E F G H ppendix Figure S1. RT and chemotherapy alter PD-L1 expression in PDC cells. Flow cytometric analysis of PD-L1 expression in () KPC and () Pan02 cells following treatment with
Supplementary Figure 1
 Supplementary Figure 1 14 12 SEM4C PLXN2 8 SEM4C C 3 Cancer Cell Non Cancer Cell Expression 1 8 6 6 4 log2 ratio Expression 2 1 4 2 2 p value.1 D Supplementary Figure 1. Expression of Sema4C and Plexin2
Supplementary Figure 1 14 12 SEM4C PLXN2 8 SEM4C C 3 Cancer Cell Non Cancer Cell Expression 1 8 6 6 4 log2 ratio Expression 2 1 4 2 2 p value.1 D Supplementary Figure 1. Expression of Sema4C and Plexin2
Figure S1. B % of Phosphorylation 32H. 32ss
 Figure S1 8H 32ss 32H 32Hc % of Phosphorylation 3 32H 2 1 32ss 1 2 3 4 Extract (μg) C % of Phosphorylation 18 12 6-32H 32Hc 8H 32ss Dbait Figure S1. List of the Dbait molecules and activation of DN-PK
Figure S1 8H 32ss 32H 32Hc % of Phosphorylation 3 32H 2 1 32ss 1 2 3 4 Extract (μg) C % of Phosphorylation 18 12 6-32H 32Hc 8H 32ss Dbait Figure S1. List of the Dbait molecules and activation of DN-PK
Supplementary Figure 1. Identification of tumorous sphere-forming CSCs and CAF feeder cells. The LEAP (Laser-Enabled Analysis and Processing)
 Supplementary Figure 1. Identification of tumorous sphere-forming CSCs and CAF feeder cells. The LEAP (Laser-Enabled Analysis and Processing) platform with laser manipulation to efficiently purify lung
Supplementary Figure 1. Identification of tumorous sphere-forming CSCs and CAF feeder cells. The LEAP (Laser-Enabled Analysis and Processing) platform with laser manipulation to efficiently purify lung
SUPPLEMENTARY INFORMATION
 doi:10.1038/nature12215 Supplementary Figure 1. The effects of full and dissociated GR agonists in supporting BFU-E self-renewal divisions. BFU-Es were cultured in self-renewal medium with indicated GR
doi:10.1038/nature12215 Supplementary Figure 1. The effects of full and dissociated GR agonists in supporting BFU-E self-renewal divisions. BFU-Es were cultured in self-renewal medium with indicated GR
Hunk is required for HER2/neu-induced mammary tumorigenesis
 Research article Hunk is required for HER2/neu-induced mammary tumorigenesis Elizabeth S. Yeh, 1 Thomas W. Yang, 1 Jason J. Jung, 1 Heather P. Gardner, 1 Robert D. Cardiff, 2 and Lewis A. Chodosh 1 1 Department
Research article Hunk is required for HER2/neu-induced mammary tumorigenesis Elizabeth S. Yeh, 1 Thomas W. Yang, 1 Jason J. Jung, 1 Heather P. Gardner, 1 Robert D. Cardiff, 2 and Lewis A. Chodosh 1 1 Department
(A) SW480, DLD1, RKO and HCT116 cells were treated with DMSO or XAV939 (5 µm)
 Supplementary Figure Legends Figure S1. Tankyrase inhibition suppresses cell proliferation in an axin/β-catenin independent manner. (A) SW480, DLD1, RKO and HCT116 cells were treated with DMSO or XAV939
Supplementary Figure Legends Figure S1. Tankyrase inhibition suppresses cell proliferation in an axin/β-catenin independent manner. (A) SW480, DLD1, RKO and HCT116 cells were treated with DMSO or XAV939
Supplementary Table 1. Characterization of HNSCC PDX models established at MSKCC
 Supplementary Table 1. Characterization of HNSCC PDX models established at MSKCC Supplementary Table 2. Drug content and loading efficiency estimated with F-NMR and UV- Vis Supplementary Table 3. Complete
Supplementary Table 1. Characterization of HNSCC PDX models established at MSKCC Supplementary Table 2. Drug content and loading efficiency estimated with F-NMR and UV- Vis Supplementary Table 3. Complete
Antibody-Drug Conjugates in Glioblastoma Multiforme: Finding Ways Forward
 Transcript Details This is a transcript of a continuing medical education (CME) activity accessible on the ReachMD network. Additional media formats for the activity and full activity details (including
Transcript Details This is a transcript of a continuing medical education (CME) activity accessible on the ReachMD network. Additional media formats for the activity and full activity details (including
Supplementary Fig. 1. GPRC5A post-transcriptionally down-regulates EGFR expression. (a) Plot of the changes in steady state mrna levels versus
 Supplementary Fig. 1. GPRC5A post-transcriptionally down-regulates EGFR expression. (a) Plot of the changes in steady state mrna levels versus changes in corresponding proteins between wild type and Gprc5a-/-
Supplementary Fig. 1. GPRC5A post-transcriptionally down-regulates EGFR expression. (a) Plot of the changes in steady state mrna levels versus changes in corresponding proteins between wild type and Gprc5a-/-
Supplementary Materials for
 www.sciencesignaling.org/cgi/content/full/7/322/ra38/dc1 Supplementary Materials for Dynamic Reprogramming of Signaling Upon Met Inhibition Reveals a Mechanism of Drug Resistance in Gastric Cancer Andrea
www.sciencesignaling.org/cgi/content/full/7/322/ra38/dc1 Supplementary Materials for Dynamic Reprogramming of Signaling Upon Met Inhibition Reveals a Mechanism of Drug Resistance in Gastric Cancer Andrea
X P. Supplementary Figure 1. Nature Medicine: doi: /nm Nilotinib LSK LT-HSC. Cytoplasm. Cytoplasm. Nucleus. Nucleus
 a b c Supplementary Figure 1 c-kit-apc-eflu780 Lin-FITC Flt3-Linc-Kit-APC-eflu780 LSK Sca-1-PE-Cy7 d e f CD48-APC LT-HSC CD150-PerCP-cy5.5 g h i j Cytoplasm RCC1 X Exp 5 mir 126 SPRED1 SPRED1 RAN P SPRED1
a b c Supplementary Figure 1 c-kit-apc-eflu780 Lin-FITC Flt3-Linc-Kit-APC-eflu780 LSK Sca-1-PE-Cy7 d e f CD48-APC LT-HSC CD150-PerCP-cy5.5 g h i j Cytoplasm RCC1 X Exp 5 mir 126 SPRED1 SPRED1 RAN P SPRED1
hexahistidine tagged GRP78 devoid of the KDEL motif (GRP78-His) on SDS-PAGE. This
 SUPPLEMENTAL FIGURE LEGEND Fig. S1. Generation and characterization of. (A) Coomassie staining of soluble hexahistidine tagged GRP78 devoid of the KDEL motif (GRP78-His) on SDS-PAGE. This protein was expressed
SUPPLEMENTAL FIGURE LEGEND Fig. S1. Generation and characterization of. (A) Coomassie staining of soluble hexahistidine tagged GRP78 devoid of the KDEL motif (GRP78-His) on SDS-PAGE. This protein was expressed
Results and Discussion of Receptor Tyrosine Kinase. Activation
 Results and Discussion of Receptor Tyrosine Kinase Activation To demonstrate the contribution which RCytoscape s molecular maps can make to biological understanding via exploratory data analysis, we here
Results and Discussion of Receptor Tyrosine Kinase Activation To demonstrate the contribution which RCytoscape s molecular maps can make to biological understanding via exploratory data analysis, we here
SHREE ET AL, SUPPLEMENTAL MATERIALS. (A) Workflow for tumor cell line derivation and orthotopic implantation.
 SHREE ET AL, SUPPLEMENTAL MATERIALS SUPPLEMENTAL FIGURE AND TABLE LEGENDS Supplemental Figure 1. Derivation and characterization of TS1-TGL and TS2-TGL PyMT cell lines and development of an orthotopic
SHREE ET AL, SUPPLEMENTAL MATERIALS SUPPLEMENTAL FIGURE AND TABLE LEGENDS Supplemental Figure 1. Derivation and characterization of TS1-TGL and TS2-TGL PyMT cell lines and development of an orthotopic
(A) RT-PCR for components of the Shh/Gli pathway in normal fetus cell (MRC-5) and a
 Supplementary figure legends Supplementary Figure 1. Expression of Shh signaling components in a panel of gastric cancer. (A) RT-PCR for components of the Shh/Gli pathway in normal fetus cell (MRC-5) and
Supplementary figure legends Supplementary Figure 1. Expression of Shh signaling components in a panel of gastric cancer. (A) RT-PCR for components of the Shh/Gli pathway in normal fetus cell (MRC-5) and
(a) Significant biological processes (upper panel) and disease biomarkers (lower panel)
 Supplementary Figure 1. Functional enrichment analyses of secretomic proteins. (a) Significant biological processes (upper panel) and disease biomarkers (lower panel) 2 involved by hrab37-mediated secretory
Supplementary Figure 1. Functional enrichment analyses of secretomic proteins. (a) Significant biological processes (upper panel) and disease biomarkers (lower panel) 2 involved by hrab37-mediated secretory
Supplementary Materials for
 www.sciencesignaling.org/cgi/content/full/2/1/ra81/dc1 Supplementary Materials for Delivery of MicroRNA-126 by Apoptotic Bodies Induces CXCL12- Dependent Vascular Protection Alma Zernecke,* Kiril Bidzhekov,
www.sciencesignaling.org/cgi/content/full/2/1/ra81/dc1 Supplementary Materials for Delivery of MicroRNA-126 by Apoptotic Bodies Induces CXCL12- Dependent Vascular Protection Alma Zernecke,* Kiril Bidzhekov,
Supplementary Figure 1. Validation of astrocytes. Primary astrocytes were
 Supplementary Figure 1. Validation of astrocytes. Primary astrocytes were separated from the glial cultures using a mild trypsinization protocol. Anti-glial fibrillary acidic protein (GFAP) immunofluorescent
Supplementary Figure 1. Validation of astrocytes. Primary astrocytes were separated from the glial cultures using a mild trypsinization protocol. Anti-glial fibrillary acidic protein (GFAP) immunofluorescent
Irf1 fold changes (D) 24h 48h. p-p65. t-p65. p-irf3. t-irf3. β-actin SKO TKO 100% 80% 60% 40% 20%
 Irf7 Fold changes 3 1 Irf1 fold changes 3 1 8h h 8h 8h h 8h p-p6 p-p6 t-p6 p-irf3 β-actin p-irf3 t-irf3 β-actin TKO TKO STKO (E) (F) TKO TKO % of p6 nuclear translocation % % 1% 1% % % p6 TKO % of IRF3
Irf7 Fold changes 3 1 Irf1 fold changes 3 1 8h h 8h 8h h 8h p-p6 p-p6 t-p6 p-irf3 β-actin p-irf3 t-irf3 β-actin TKO TKO STKO (E) (F) TKO TKO % of p6 nuclear translocation % % 1% 1% % % p6 TKO % of IRF3
Meeting Report. From December 8 to 11, 2012 at Atlanta, GA, U.S.A
 Meeting Report Affiliation Department of Transfusion Medicine and Cell Therapy Name Hisayuki Yao Name of the meeting Period and venue Type of your presentation Title of your presentation The 54 th Annual
Meeting Report Affiliation Department of Transfusion Medicine and Cell Therapy Name Hisayuki Yao Name of the meeting Period and venue Type of your presentation Title of your presentation The 54 th Annual
SUPPLEMENTARY INFORMATION
 Supplementary Discussion The cell cycle machinery and the DNA damage response network are highly interconnected and co-regulated in assuring faithful duplication and partition of genetic materials into
Supplementary Discussion The cell cycle machinery and the DNA damage response network are highly interconnected and co-regulated in assuring faithful duplication and partition of genetic materials into
SUPPLEMENTARY INFORMATION
 SUPPLEMENTARY INFORMATION doi:10.1038/nature11429 S1a 6 7 8 9 Nlrc4 allele S1b Nlrc4 +/+ Nlrc4 +/F Nlrc4 F/F 9 Targeting construct 422 bp 273 bp FRT-neo-gb-PGK-FRT 3x.STOP S1c Nlrc4 +/+ Nlrc4 F/F casp1
SUPPLEMENTARY INFORMATION doi:10.1038/nature11429 S1a 6 7 8 9 Nlrc4 allele S1b Nlrc4 +/+ Nlrc4 +/F Nlrc4 F/F 9 Targeting construct 422 bp 273 bp FRT-neo-gb-PGK-FRT 3x.STOP S1c Nlrc4 +/+ Nlrc4 F/F casp1
Differential Connexin Function Enhances Self-Renewal in Glioblastoma
 CSU ID: 2558414 Name: Sonal Patel Differential Connexin Function Enhances Self-Renewal in Glioblastoma Cancer is one of the most common disease in the US with more than a million people affected every
CSU ID: 2558414 Name: Sonal Patel Differential Connexin Function Enhances Self-Renewal in Glioblastoma Cancer is one of the most common disease in the US with more than a million people affected every
SUPPLEMENTARY FIGURES
 SUPPLEMENTARY FIGURES Figure S1. Clinical significance of ZNF322A overexpression in Caucasian lung cancer patients. (A) Representative immunohistochemistry images of ZNF322A protein expression in tissue
SUPPLEMENTARY FIGURES Figure S1. Clinical significance of ZNF322A overexpression in Caucasian lung cancer patients. (A) Representative immunohistochemistry images of ZNF322A protein expression in tissue
SUPPLEMENTARY INFORMATION
 DOI:.38/ncb2822 a MTC02 FAO cells EEA1 b +/+ MEFs /DAPI -/- MEFs /DAPI -/- MEFs //DAPI c HEK 293 cells WCE N M C P AKT TBC1D7 Lamin A/C EEA1 VDAC d HeLa cells WCE N M C P AKT Lamin A/C EEA1 VDAC Figure
DOI:.38/ncb2822 a MTC02 FAO cells EEA1 b +/+ MEFs /DAPI -/- MEFs /DAPI -/- MEFs //DAPI c HEK 293 cells WCE N M C P AKT TBC1D7 Lamin A/C EEA1 VDAC d HeLa cells WCE N M C P AKT Lamin A/C EEA1 VDAC Figure
Figure S1, related to Figure 1. Escaper p38a-expressing cancer cells repopulate the tumors (A) Scheme of the mt/mg reporter that expresses a
 Cancer Cell, Volume 33 Supplemental Information Targeting p38a Increases DNA Damage, Chromosome Instability, and the Anti-tumoral Response to Taxanes in Breast Cancer Cells Begoña Cánovas, Ana Igea, Alessandro
Cancer Cell, Volume 33 Supplemental Information Targeting p38a Increases DNA Damage, Chromosome Instability, and the Anti-tumoral Response to Taxanes in Breast Cancer Cells Begoña Cánovas, Ana Igea, Alessandro
Effective Targeting of Quiescent Chronic Myelogenous
 Cancer Cell, Volume 7 Supplemental Information Effective Targeting of Quiescent Chronic Myelogenous Leukemia Stem Cells by Histone Deacetylase Inhibitors in Combination with Imatinib Mesylate Bin Zhang,
Cancer Cell, Volume 7 Supplemental Information Effective Targeting of Quiescent Chronic Myelogenous Leukemia Stem Cells by Histone Deacetylase Inhibitors in Combination with Imatinib Mesylate Bin Zhang,
UNIVERSITY OF MEDICINE AND PHARMACY CRAIOVA PhD SCHOOL. PhD THESIS
 UNIVERSITY OF MEDICINE AND PHARMACY CRAIOVA PhD SCHOOL PhD THESIS THE IMPORTANCE OF TUMOR ANGIOGENESIS IN CEREBRAL TUMOR DIAGNOSIS AND THERAPY ABSTRACT PhD COORDINATOR: Prof. univ. dr. DRICU Anica PhD
UNIVERSITY OF MEDICINE AND PHARMACY CRAIOVA PhD SCHOOL PhD THESIS THE IMPORTANCE OF TUMOR ANGIOGENESIS IN CEREBRAL TUMOR DIAGNOSIS AND THERAPY ABSTRACT PhD COORDINATOR: Prof. univ. dr. DRICU Anica PhD
Figure S1. The PDE5 inhibitor sildenafil interacts with celecoxib to kill cancer cell lines. (A) Hepatoma
 Figure S1. The PDE5 inhibitor sildenafil interacts with celecoxib to kill cancer cell lines. (A) Hepatoma cells were treated with celecoxib ( 5.0 M) and/or sildenafil (, 2.0 M). ls were isolated after
Figure S1. The PDE5 inhibitor sildenafil interacts with celecoxib to kill cancer cell lines. (A) Hepatoma cells were treated with celecoxib ( 5.0 M) and/or sildenafil (, 2.0 M). ls were isolated after
Supplementary Figure 1. BMS enhances human T cell activation in vitro in a
 Supplementary Figure 1. BMS98662 enhances human T cell activation in vitro in a concentration-dependent manner. Jurkat T cells were activated with anti-cd3 and anti-cd28 antibody in the presence of titrated
Supplementary Figure 1. BMS98662 enhances human T cell activation in vitro in a concentration-dependent manner. Jurkat T cells were activated with anti-cd3 and anti-cd28 antibody in the presence of titrated
Supplementary Figure 1.TRIM33 binds β-catenin in the nucleus. a & b, Co-IP of endogenous TRIM33 with β-catenin in HT-29 cells (a) and HEK 293T cells
 Supplementary Figure 1.TRIM33 binds β-catenin in the nucleus. a & b, Co-IP of endogenous TRIM33 with β-catenin in HT-29 cells (a) and HEK 293T cells (b). TRIM33 was immunoprecipitated, and the amount of
Supplementary Figure 1.TRIM33 binds β-catenin in the nucleus. a & b, Co-IP of endogenous TRIM33 with β-catenin in HT-29 cells (a) and HEK 293T cells (b). TRIM33 was immunoprecipitated, and the amount of
Supplemental Information. Induction of Expansion and Folding. in Human Cerebral Organoids
 Cell Stem Cell, Volume 20 Supplemental Information Induction of Expansion and Folding in Human Cerebral Organoids Yun Li, Julien Muffat, Attya Omer, Irene Bosch, Madeline A. Lancaster, Mriganka Sur, Lee
Cell Stem Cell, Volume 20 Supplemental Information Induction of Expansion and Folding in Human Cerebral Organoids Yun Li, Julien Muffat, Attya Omer, Irene Bosch, Madeline A. Lancaster, Mriganka Sur, Lee
Page 39 of 44. 8h LTA & AT h PepG & AT h LTA
 Page 39 of 44 Fig. S1 A: B: C: D: 8h LTA 8h LTA & AT7519 E: F: 8h PepG G: 8h PepG & AT7519 Fig. S1. AT7519 overrides the survival effects of lipoteichoic acid (LTA) and peptidoglycan (PepG). (A) Human
Page 39 of 44 Fig. S1 A: B: C: D: 8h LTA 8h LTA & AT7519 E: F: 8h PepG G: 8h PepG & AT7519 Fig. S1. AT7519 overrides the survival effects of lipoteichoic acid (LTA) and peptidoglycan (PepG). (A) Human
CANCER STEM CELLS. IGF1 Receptor Signaling Regulates Adaptive Radioprotection in Glioma Stem Cells INTRODUCTION
 CANCER STEM CELLS IGF1 Receptor Signaling Regulates Adaptive Radioprotection in Glioma Stem Cells SATORU OSUKA, a,b OLTEA SAMPETREAN, b,c TAKATSUNE SHIMIZU, b,d ISAKO SAGA, b,e NOBUYUKI ONISHI, b EIJI
CANCER STEM CELLS IGF1 Receptor Signaling Regulates Adaptive Radioprotection in Glioma Stem Cells SATORU OSUKA, a,b OLTEA SAMPETREAN, b,c TAKATSUNE SHIMIZU, b,d ISAKO SAGA, b,e NOBUYUKI ONISHI, b EIJI
Table S1. New colony formation 7 days after stimulation with doxo and VCR in JURKAT cells
 Table S1. New colony formation 7 days after stimulation with and in JURKAT cells drug co + number of colonies 7±14 4±7 48±11 JURKAT cells were stimulated and analyzed as in Table 1. Drug concentrations
Table S1. New colony formation 7 days after stimulation with and in JURKAT cells drug co + number of colonies 7±14 4±7 48±11 JURKAT cells were stimulated and analyzed as in Table 1. Drug concentrations
T H E J O U R N A L O F C E L L B I O L O G Y
 T H E J O U R N A L O F C E L L B I O L O G Y Supplemental material Krenn et al., http://www.jcb.org/cgi/content/full/jcb.201110013/dc1 Figure S1. Levels of expressed proteins and demonstration that C-terminal
T H E J O U R N A L O F C E L L B I O L O G Y Supplemental material Krenn et al., http://www.jcb.org/cgi/content/full/jcb.201110013/dc1 Figure S1. Levels of expressed proteins and demonstration that C-terminal
Chronic variable stress activates hematopoietic stem cells
 SUPPLEMENTARY INFORMATION Chronic variable stress activates hematopoietic stem cells Timo Heidt *, Hendrik B. Sager *, Gabriel Courties, Partha Dutta, Yoshiko Iwamoto, Alex Zaltsman, Constantin von zur
SUPPLEMENTARY INFORMATION Chronic variable stress activates hematopoietic stem cells Timo Heidt *, Hendrik B. Sager *, Gabriel Courties, Partha Dutta, Yoshiko Iwamoto, Alex Zaltsman, Constantin von zur
Dysregulation of Blimp1 transcriptional repressor unleashes p130cas/erbb2 breast cancer invasion (SREP T) Supplementary File
 Dysregulation of Blimp1 transcriptional repressor unleashes p130cas/erbb2 breast cancer invasion (SREP-16-42496-T) Marianna Sciortino 1, Maria del Pilar Camacho Leal 1, Francesca Orso 1, Elena Grassi 1,
Dysregulation of Blimp1 transcriptional repressor unleashes p130cas/erbb2 breast cancer invasion (SREP-16-42496-T) Marianna Sciortino 1, Maria del Pilar Camacho Leal 1, Francesca Orso 1, Elena Grassi 1,
Mathematical Modeling of PDGF-Driven Glioblastoma Reveals Optimized Radiation Dosing Schedules
 Mathematical Modeling of PDGF-Driven Glioblastoma Reveals Optimized Radiation Dosing Schedules Kevin Leder, Ken Pittner, Quincey LaPlant, Dolores Hambardzumyan, Brian D. Ross, Timothy A. Chan, Eric C.
Mathematical Modeling of PDGF-Driven Glioblastoma Reveals Optimized Radiation Dosing Schedules Kevin Leder, Ken Pittner, Quincey LaPlant, Dolores Hambardzumyan, Brian D. Ross, Timothy A. Chan, Eric C.
number Done by Corrected by Doctor Maha Shomaf
 number 19 Done by Waseem Abo-Obeida Corrected by Abdullah Zreiqat Doctor Maha Shomaf Carcinogenesis: the molecular basis of cancer. Non-lethal genetic damage lies at the heart of carcinogenesis and leads
number 19 Done by Waseem Abo-Obeida Corrected by Abdullah Zreiqat Doctor Maha Shomaf Carcinogenesis: the molecular basis of cancer. Non-lethal genetic damage lies at the heart of carcinogenesis and leads
Supplementary Figure 1. IDH1 and IDH2 mutation site sequences on WHO grade III
 Supplementary Materials: Supplementary Figure 1. IDH1 and IDH2 mutation site sequences on WHO grade III patient samples. Genomic DNA samples extracted from punch biopsies from either FFPE or frozen tumor
Supplementary Materials: Supplementary Figure 1. IDH1 and IDH2 mutation site sequences on WHO grade III patient samples. Genomic DNA samples extracted from punch biopsies from either FFPE or frozen tumor
Supplementary Material
 Supplementary Material The Androgen Receptor is a negative regulator of eif4e Phosphorylation at S209: Implications for the use of mtor inhibitors in advanced prostate cancer Supplementary Figures Supplemental
Supplementary Material The Androgen Receptor is a negative regulator of eif4e Phosphorylation at S209: Implications for the use of mtor inhibitors in advanced prostate cancer Supplementary Figures Supplemental
Supplementary Figure S1: Defective heterochromatin repair in HGPS progeroid cells
 Supplementary Figure S1: Defective heterochromatin repair in HGPS progeroid cells Immunofluorescence staining of H3K9me3 and 53BP1 in PH and HGADFN003 (HG003) cells at 24 h after γ-irradiation. Scale bar,
Supplementary Figure S1: Defective heterochromatin repair in HGPS progeroid cells Immunofluorescence staining of H3K9me3 and 53BP1 in PH and HGADFN003 (HG003) cells at 24 h after γ-irradiation. Scale bar,
Radiobiology of fractionated treatments: the classical approach and the 4 Rs. Vischioni Barbara MD, PhD Centro Nazionale Adroterapia Oncologica
 Radiobiology of fractionated treatments: the classical approach and the 4 Rs Vischioni Barbara MD, PhD Centro Nazionale Adroterapia Oncologica Radiobiology It is fundamental in radiation oncology Radiobiology
Radiobiology of fractionated treatments: the classical approach and the 4 Rs Vischioni Barbara MD, PhD Centro Nazionale Adroterapia Oncologica Radiobiology It is fundamental in radiation oncology Radiobiology
marker. DAPI labels nuclei. Flies were 20 days old. Scale bar is 5 µm. Ctrl is
 Supplementary Figure 1. (a) Nos is detected in glial cells in both control and GFAP R79H transgenic flies (arrows), but not in deletion mutant Nos Δ15 animals. Repo is a glial cell marker. DAPI labels
Supplementary Figure 1. (a) Nos is detected in glial cells in both control and GFAP R79H transgenic flies (arrows), but not in deletion mutant Nos Δ15 animals. Repo is a glial cell marker. DAPI labels
IKK-dependent activation of NF-κB contributes to myeloid and lymphoid leukemogenesis by BCR-ABL1
 Supplemental Figures BLOOD/2014/547943 IKK-dependent activation of NF-κB contributes to myeloid and lymphoid leukemogenesis by BCR-ABL1 Hsieh M-Y and Van Etten RA Supplemental Figure S1. Titers of retroviral
Supplemental Figures BLOOD/2014/547943 IKK-dependent activation of NF-κB contributes to myeloid and lymphoid leukemogenesis by BCR-ABL1 Hsieh M-Y and Van Etten RA Supplemental Figure S1. Titers of retroviral
SUPPLEMENTARY INFORMATION
 DOI: 10.1038/ncb2607 Figure S1 Elf5 loss promotes EMT in mammary epithelium while Elf5 overexpression inhibits TGFβ induced EMT. (a, c) Different confocal slices through the Z stack image. (b, d) 3D rendering
DOI: 10.1038/ncb2607 Figure S1 Elf5 loss promotes EMT in mammary epithelium while Elf5 overexpression inhibits TGFβ induced EMT. (a, c) Different confocal slices through the Z stack image. (b, d) 3D rendering
Supplementary Figure 1 Induction of cellular senescence and isolation of exosome. a to c, Pre-senescent primary normal human diploid fibroblasts
 Supplementary Figure 1 Induction of cellular senescence and isolation of exosome. a to c, Pre-senescent primary normal human diploid fibroblasts (TIG-3 cells) were rendered senescent by either serial passage
Supplementary Figure 1 Induction of cellular senescence and isolation of exosome. a to c, Pre-senescent primary normal human diploid fibroblasts (TIG-3 cells) were rendered senescent by either serial passage
Crizotinib in addition to Radiotherapy and TMZ in newly diagnosed GBM
 Crizotinib in addition to Radiotherapy and TMZ in newly diagnosed GBM Juan M Sepúlveda Sánchez Unidad Multidisciplinar de Neurooncología Hospital 12 de Octubre Madrid GEINO 1402. Phase Ib, open-label,
Crizotinib in addition to Radiotherapy and TMZ in newly diagnosed GBM Juan M Sepúlveda Sánchez Unidad Multidisciplinar de Neurooncología Hospital 12 de Octubre Madrid GEINO 1402. Phase Ib, open-label,
Nature Immunology: doi: /ni Supplementary Figure 1. Transcriptional program of the TE and MP CD8 + T cell subsets.
 Supplementary Figure 1 Transcriptional program of the TE and MP CD8 + T cell subsets. (a) Comparison of gene expression of TE and MP CD8 + T cell subsets by microarray. Genes that are 1.5-fold upregulated
Supplementary Figure 1 Transcriptional program of the TE and MP CD8 + T cell subsets. (a) Comparison of gene expression of TE and MP CD8 + T cell subsets by microarray. Genes that are 1.5-fold upregulated
A dual PI3 kinase/mtor inhibitor reveals emergent efficacy in glioma
 Supplemental data A dual PI3 kinase/mtor inhibitor reveals emergent efficacy in glioma Qi-Wen Fan, Zachary A. Knight, David D. Goldenberg, Wei Yu, Keith E. Mostov, David Stokoe, Kevan M. Shokat, and William
Supplemental data A dual PI3 kinase/mtor inhibitor reveals emergent efficacy in glioma Qi-Wen Fan, Zachary A. Knight, David D. Goldenberg, Wei Yu, Keith E. Mostov, David Stokoe, Kevan M. Shokat, and William
ImmunoTools special Award 2014
 ImmunoTools special Award 2014 Davide Ferrari, PhD University of Ferrara, Department of Morphology, Surgery and Experimental Medicine. Section of Pathology, Oncology and Exper. Biology via L. Borsari 46,
ImmunoTools special Award 2014 Davide Ferrari, PhD University of Ferrara, Department of Morphology, Surgery and Experimental Medicine. Section of Pathology, Oncology and Exper. Biology via L. Borsari 46,
SUPPLEMENTARY FIGURES
 SUPPLEMENTARY FIGURES 1 Supplementary Figure 1, Adult hippocampal QNPs and TAPs uniformly express REST a-b) Confocal images of adult hippocampal mouse sections showing GFAP (green), Sox2 (red), and REST
SUPPLEMENTARY FIGURES 1 Supplementary Figure 1, Adult hippocampal QNPs and TAPs uniformly express REST a-b) Confocal images of adult hippocampal mouse sections showing GFAP (green), Sox2 (red), and REST
Figure S1. ERBB3 mrna levels are elevated in Luminal A breast cancers harboring ERBB3
 Supplemental Figure Legends. Figure S1. ERBB3 mrna levels are elevated in Luminal A breast cancers harboring ERBB3 ErbB3 gene copy number gain. Supplemental Figure S1. ERBB3 mrna levels are elevated in
Supplemental Figure Legends. Figure S1. ERBB3 mrna levels are elevated in Luminal A breast cancers harboring ERBB3 ErbB3 gene copy number gain. Supplemental Figure S1. ERBB3 mrna levels are elevated in
Rational targeting of very high-risk medulloblastoma of childhood - 1 st progress report
 Rational targeting of very high-risk medulloblastoma of childhood - 1 st progress report year Purpose of the research Medulloblastoma is the most common malignant brain tumor of childhood and treatments
Rational targeting of very high-risk medulloblastoma of childhood - 1 st progress report year Purpose of the research Medulloblastoma is the most common malignant brain tumor of childhood and treatments
7SK ChIRP-seq is specifically RNA dependent and conserved between mice and humans.
 Supplementary Figure 1 7SK ChIRP-seq is specifically RNA dependent and conserved between mice and humans. Regions targeted by the Even and Odd ChIRP probes mapped to a secondary structure model 56 of the
Supplementary Figure 1 7SK ChIRP-seq is specifically RNA dependent and conserved between mice and humans. Regions targeted by the Even and Odd ChIRP probes mapped to a secondary structure model 56 of the
Brian T Burgess, DO, PhD, GYN Oncology Fellow Rachel W. Miller, MD, GYN Oncology
 Brian T Burgess, DO, PhD, GYN Oncology Fellow Rachel W. Miller, MD, GYN Oncology Epithelial Ovarian Cancer - Standard Current Treatment: Surgery with De-bulking + Platinum-Taxane based Chemotherapy - No
Brian T Burgess, DO, PhD, GYN Oncology Fellow Rachel W. Miller, MD, GYN Oncology Epithelial Ovarian Cancer - Standard Current Treatment: Surgery with De-bulking + Platinum-Taxane based Chemotherapy - No
(a) Schematic diagram of the FS mutation of UVRAG in exon 8 containing the highly instable
 Supplementary Figure 1. Frameshift (FS) mutation in UVRAG. (a) Schematic diagram of the FS mutation of UVRAG in exon 8 containing the highly instable A 10 DNA repeat, generating a premature stop codon
Supplementary Figure 1. Frameshift (FS) mutation in UVRAG. (a) Schematic diagram of the FS mutation of UVRAG in exon 8 containing the highly instable A 10 DNA repeat, generating a premature stop codon
The HSP90 Inhibitor Ganetespib Radiosensitizes Human Lung Adenocarcinoma Cells
 The HSP90 Inhibitor Ganetespib Radiosensitizes Human Lung Adenocarcinoma Cells The Harvard community has made this article openly available. Please share how this access benefits you. Your story matters
The HSP90 Inhibitor Ganetespib Radiosensitizes Human Lung Adenocarcinoma Cells The Harvard community has made this article openly available. Please share how this access benefits you. Your story matters
Supplementary Information Titles Journal: Nature Medicine
 Supplementary Information Titles Journal: Nature Medicine Article Title: Corresponding Author: Supplementary Item & Number Supplementary Fig.1 Fig.2 Fig.3 Fig.4 Fig.5 Fig.6 Fig.7 Fig.8 Fig.9 Fig. Fig.11
Supplementary Information Titles Journal: Nature Medicine Article Title: Corresponding Author: Supplementary Item & Number Supplementary Fig.1 Fig.2 Fig.3 Fig.4 Fig.5 Fig.6 Fig.7 Fig.8 Fig.9 Fig. Fig.11
Osamu Tetsu, MD, PhD Associate Professor Department of Otolaryngology-Head and Neck Surgery School of Medicine, University of California, San
 Osamu Tetsu, MD, PhD Associate Professor Department of Otolaryngology-Head and Neck Surgery School of Medicine, University of California, San Francisco Lung Cancer Classification Pathological Classification
Osamu Tetsu, MD, PhD Associate Professor Department of Otolaryngology-Head and Neck Surgery School of Medicine, University of California, San Francisco Lung Cancer Classification Pathological Classification
SUPPLEMENTARY FIGURES AND TABLE
 SUPPLEMENTARY FIGURES AND TABLE Supplementary Figure S1: Characterization of IRE1α mutants. A. U87-LUC cells were transduced with the lentiviral vector containing the GFP sequence (U87-LUC Tet-ON GFP).
SUPPLEMENTARY FIGURES AND TABLE Supplementary Figure S1: Characterization of IRE1α mutants. A. U87-LUC cells were transduced with the lentiviral vector containing the GFP sequence (U87-LUC Tet-ON GFP).
BIO360 Quiz #1. September 14, Name five of the six Hallmarks of Cancer (not emerging hallmarks or enabling characteristics ): (5 points)
 Name: BIO360 Quiz #1 September 14, 2012 1. Name five of the six Hallmarks of Cancer (not emerging hallmarks or enabling characteristics ): (5 points) 2. The controversial hypothesis that only a small subset
Name: BIO360 Quiz #1 September 14, 2012 1. Name five of the six Hallmarks of Cancer (not emerging hallmarks or enabling characteristics ): (5 points) 2. The controversial hypothesis that only a small subset
Supplementary Figure 1. Deletion of Smad3 prevents B16F10 melanoma invasion and metastasis in a mouse s.c. tumor model.
 A B16F1 s.c. Lung LN Distant lymph nodes Colon B B16F1 s.c. Supplementary Figure 1. Deletion of Smad3 prevents B16F1 melanoma invasion and metastasis in a mouse s.c. tumor model. Highly invasive growth
A B16F1 s.c. Lung LN Distant lymph nodes Colon B B16F1 s.c. Supplementary Figure 1. Deletion of Smad3 prevents B16F1 melanoma invasion and metastasis in a mouse s.c. tumor model. Highly invasive growth
AP VP DLP H&E. p-akt DLP
 A B AP VP DLP H&E AP AP VP DLP p-akt wild-type prostate PTEN-null prostate Supplementary Fig. 1. Targeted deletion of PTEN in prostate epithelium resulted in HG-PIN in all three lobes. (A) The anatomy
A B AP VP DLP H&E AP AP VP DLP p-akt wild-type prostate PTEN-null prostate Supplementary Fig. 1. Targeted deletion of PTEN in prostate epithelium resulted in HG-PIN in all three lobes. (A) The anatomy
609G: Concepts of Cancer Genetics and Treatments (3 credits)
 Master of Chemical and Life Sciences Program College of Computer, Mathematical, and Natural Sciences 609G: Concepts of Cancer Genetics and Treatments (3 credits) Text books: Principles of Cancer Genetics,
Master of Chemical and Life Sciences Program College of Computer, Mathematical, and Natural Sciences 609G: Concepts of Cancer Genetics and Treatments (3 credits) Text books: Principles of Cancer Genetics,
Supplementary Figure S1 Expression of mir-181b in EOC (A) Kaplan-Meier
 Supplementary Figure S1 Expression of mir-181b in EOC (A) Kaplan-Meier curves for progression-free survival (PFS) and overall survival (OS) in a cohort of patients (N=52) with stage III primary ovarian
Supplementary Figure S1 Expression of mir-181b in EOC (A) Kaplan-Meier curves for progression-free survival (PFS) and overall survival (OS) in a cohort of patients (N=52) with stage III primary ovarian
Muse Assays for Cell Analysis
 Muse Assays for Cell Analysis Multiple Assay Outputs for Cell Analysis Cell Health Cell Signalling Immunology Muse Count & Viability Kit Muse Cell Cycle Kit Muse Annexin V & Dead Cell Kit Muse Caspase
Muse Assays for Cell Analysis Multiple Assay Outputs for Cell Analysis Cell Health Cell Signalling Immunology Muse Count & Viability Kit Muse Cell Cycle Kit Muse Annexin V & Dead Cell Kit Muse Caspase
Supplemental Figure 1. Western blot analysis indicated that MIF was detected in the fractions of
 Supplemental Figure Legends Supplemental Figure 1. Western blot analysis indicated that was detected in the fractions of plasma membrane and cytosol but not in nuclear fraction isolated from Pkd1 null
Supplemental Figure Legends Supplemental Figure 1. Western blot analysis indicated that was detected in the fractions of plasma membrane and cytosol but not in nuclear fraction isolated from Pkd1 null
Off-Label Treatments. Clinical Trials for Recurrent GBM UCSF Radiation Oncology Course: Management of Recurrent Disease. Outline
 Off-Label Treatments Clinical Trials for Recurrent GBM UCSF Radiation Oncology Course: Management of Recurrent Disease Jennifer Clarke, MD, MPH Assistant Professor Division of Neuro-Oncology Depts of Neurological
Off-Label Treatments Clinical Trials for Recurrent GBM UCSF Radiation Oncology Course: Management of Recurrent Disease Jennifer Clarke, MD, MPH Assistant Professor Division of Neuro-Oncology Depts of Neurological
Layered-IHC (L-IHC): A novel and robust approach to multiplexed immunohistochemistry So many markers and so little tissue
 Page 1 The need for multiplex detection of tissue biomarkers. There is a constant and growing demand for increased biomarker analysis in human tissue specimens. Analysis of tissue biomarkers is key to
Page 1 The need for multiplex detection of tissue biomarkers. There is a constant and growing demand for increased biomarker analysis in human tissue specimens. Analysis of tissue biomarkers is key to
5K ALDEFLUOR-positive/ CXCR1-negative. 5K ALDEFLUOR-positive/ CXCR1-positive BAAA BAAA CXCR1-APC BAAA BAAA CXCR1-APC
 A +DEAB -DEAB K ALDEFLUOR-positive/ CXCR-negative BAAA BAAA CXCR-APC B +DEAB -DEAB K ALDEFLUOR-positive/ CXCR-positive BAAA BAAA CXCR-APC C Supplemental Figure. Tumorigenicity of the ALDEFLUOR-positive/CXCR-positive
A +DEAB -DEAB K ALDEFLUOR-positive/ CXCR-negative BAAA BAAA CXCR-APC B +DEAB -DEAB K ALDEFLUOR-positive/ CXCR-positive BAAA BAAA CXCR-APC C Supplemental Figure. Tumorigenicity of the ALDEFLUOR-positive/CXCR-positive
